Carcinoid syndrome symptoms, particularly diarrhea and flushing, have a negative effect on patient quality of life. In the ELECT trial, daily patient‐reported data on diarrhea and flushing frequency and intensity were prospectively collected to evaluate the direct impact of lanreotide on patient symptom relief. This article reports the findings of these patient‐reported symptom data.
Keywords: Neuroendocrine tumor, Carcinoid syndrome, Quality of life, Clinical trial, Lanreotide depot/autogel
Abstract
Background.
In the double‐blind (DB) ELECT study, lanreotide depot/autogel significantly reduced versus placebo the need for short‐acting octreotide for symptomatic carcinoid syndrome (CS) control in neuroendocrine tumor (NET) patients. Here we present patient‐reported symptom data during DB and initial open‐label (IOL) treatment.
Materials and Methods.
Adults with NETs and CS history, with/without prior somatostatin analog use, were randomized to 16 weeks’ DB lanreotide 120 mg subcutaneous or placebo every 4 weeks, followed by 32 weeks’ IOL lanreotide. Patients recorded diarrhea and/or flushing frequency and severity daily by Interactive Voice (Web) Response System for 1 month prior to randomization and throughout the study.
Results.
Of 115 patients randomized (n = 59 lanreotide, n = 56 placebo), 56 lanreotide and 45 placebo patients enrolled in the IOL phase. During DB treatment, least square (LS) mean percentages of days with moderate/severe diarrhea and/or flushing were significantly lower for lanreotide (23.4%) versus placebo (35.8%; LS mean difference [95% confidence interval]: −12.4 [−20.73 to −4.07]; p = .004). For DB lanreotide patients, average daily composite (frequency × severity) diarrhea scores improved significantly between DB and IOL treatment (mean difference: −0.71 [−1.20 to −0.22]; p = .005), and remained stable for diarrhea and/or flushing. For DB placebo patients, composite scores for diarrhea, flushing, and diarrhea and/or flushing improved significantly between DB and IOL treatment (mean differences: −1.07 [−1.65 to −0.49]; −1.06 [−1.93 to −0.19]; and −2.13 [−3.35 to −0.91]; all p ≤ .018).
Conclusion.
Improved diarrhea and flushing control in CS patients during 16‐week lanreotide treatment was sustained during maintenance of lanreotide treatment for the 32‐week IOL phase (48 weeks total).
Implications for Practice.
This study prospectively collected daily patient‐reported data on diarrhea and flushing from the ELECT trial to evaluate the direct impact of lanreotide depot on patients’ relief of carcinoid syndrome symptoms. Treatment with lanreotide depot was associated with significant reductions in the percentages of days patients reported symptoms of diarrhea and flushing, as well as reductions in the frequency and severity of daily symptoms compared with placebo during 16 weeks of double‐blind treatment. These improvements were sustained for 32 additional weeks of open‐label lanreotide treatment (i.e., through week 48 of treatment), resulting in clinically meaningful, long‐term symptom reduction.
Introduction
Neuroendocrine tumors (NETs) are a heterogeneous group of often slow‐growing tumors originating from neuroendocrine cells, with most common primary tumor sites occurring in the gastrointestinal tract, pancreas, and lungs [1], [2], [3]. Carcinoid syndrome (CS), which is associated most frequently with metastatic midgut NETs [4], [5], results from the secretion by NETs of serotonin and other vasoactive amines into the systemic circulation and is characterized by flushing, diarrhea, right‐sided cardiac valve fibrosis, and abdominal pain [2], [6], [7]. A recent analysis of the U.S. Surveillance, Epidemiology, and End Results‐Medicare database found that the incidence of CS has increased from 11% in 2000 to 19% in 2011 in patients with NETs, excluding those with pancreatic tumors or small‐ or large‐cell lung cancer [8]. Shorter overall median survival for patients with CS (5 years [95% confidence interval (CI), 4.5–5.4]) was also noted compared with those patients without CS (5.6 years [5.4–5.9]), particularly in patients with metastatic grade I–II small‐bowel NETs, for whom the median overall survival was 4.7 years (4.0–5.4) in those with CS compared with 7.1 years (5.2–8.1) in those without CS.
Long‐acting somatostatin analogs (SSAs) are recommended as first‐line medical therapy for symptomatic CS control [3], [9], [10], [11]. The efficacy of lanreotide treatment to improve CS symptom control was shown in several single‐arm, open‐label prospective [12], [13], [14] and retrospective studies [15], [16]. Based on these findings, the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines recommended lanreotide as a treatment option for CS in patients with metastatic NETs [11]. Lanreotide depot was recently approved in the U.S. for the treatment of adults with carcinoid syndrome; when used, it reduces the frequency of short‐acting SSA rescue therapy.
To date, there have been relatively few randomized, controlled trials conducted in CS treatment [17], [18], [19]. One of these, the recent multinational, double‐blind (DB), placebo‐controlled phase 3 ELECT (Evaluation of Lanreotide depot/autogel Efficacy and safety as a Carcinoid‐syndrome Treatment) trial (NCT00774930), showed that lanreotide depot (also known as lanreotide autogel), 120 mg administered every 4 weeks by deep subcutaneous (SC) injection, significantly reduced the need for short‐acting octreotide rescue medication (primary endpoint) for symptomatic control of CS in patients with NETs compared with placebo [17].
CS symptoms, particularly diarrhea and flushing, have a significant negative impact on patient quality of life [20], [21], and patients may live with these symptoms for years. National Comprehensive Cancer Network guidelines have called for clinical trials in NETs that assess the treatment of CS and include quality of life assessments [11]. In ELECT, daily patient‐reported data on diarrhea and flushing frequency and intensity were prospectively collected to evaluate the direct impact of lanreotide on patient symptom relief. Herein, we report the findings of these patient‐reported symptom data from the ELECT DB and initial open‐label (IOL) phases.
Materials and Methods
Study Design and Participants
Details of the ELECT study design have been previously reported [17]. Briefly, ELECT consisted of a 4‐week screening phase, randomized DB phase (16 weeks), IOL phase (32 weeks), and long‐term open‐label phase (≥2 years).
Eligible patients were ≥18 years with a histopathologically‐confirmed NET, or NET of unknown location with biopsy‐confirmed liver metastases, and history of CS (flushing and/or diarrhea), confirmed positive somatostatin receptor (SSTR) status by SSTR scintigraphy, and absence of tumor progression documented on sequential computed tomography or magnetic resonance imaging scans at least 3 months apart and final scan within 6 months of study enrollment. Patients were either SSA‐naïve or responsive to previous SSA therapy with octreotide long‐acting release (LAR) (≤30 mg every 4 weeks) or short‐acting SC octreotide (≤600 μg daily). Patients with prior SSA use did not undergo washout from treatment, which meant that patients previously treated with octreotide LAR must have received their last dose at least 4 weeks before the first study treatment. The study protocol was approved by an Independent Ethics Committee/Institutional Review Board in each country where the study was conducted. Signed informed consent was obtained from each participant before any study‐related activities were conducted.
Procedures
Patients were randomized 1:1 to receive either lanreotide or placebo within strata consisting of prior SSA use (de novo/prior SSA) and region (U.S./ex‐U.S.). Randomization was conducted centrally by the clinical research organization. Treatment allocation was assigned using an Interactive Web Response System, and patients and investigators were blinded to the treatment assignments. During DB treatment, lanreotide depot 120 mg deep SC injection or placebo was administered every 4 weeks. During the open‐label phases, all patients received lanreotide depot 120 mg every 4 weeks. Throughout the screening period, as well as the DB and open‐label phases, patients self‐administered short‐acting SC octreotide as rescue medication when needed for breakthrough symptoms.
Patients recorded in a daily diary the presence (yes/no), frequency (1–50), and severity (mild, moderate, severe) of diarrhea and flushing events using Interactive Voice/Web Response System (IVRS/IWRS) for 1 month prior to randomization and throughout the DB and IOL study duration. Fifty‐two weeks of patient‐reported symptom data were collected and assessed for treatment‐related changes. Composite symptom intensity scores were created post hoc based upon daily patient‐reported symptom frequency and severity data, in which severity was scored on a 0 to 3 scale (0 = none, 1 = mild, 2 = moderate, and 3 = severe). An average daily composite symptom score was calculated for each patient per day by multiplying daily symptom frequency by daily severity, and then dividing by the total number of days that a diary entry was recorded during the DB or IOL phases.
Urinary 5‐hydroxyindoleacetic acid (5‐HIAA) and plasma chromogranin A (CgA) levels were assessed at baseline and every 12 weeks during the DB and IOL phases, for a total of 48 weeks. Patients were instructed to avoid a list of food and drugs known to interact with 5‐HIAA for 24 hours before initiation of the 24‐hour urine collection. Safety assessments included recorded adverse events (AEs) and serious AEs (SAEs) by the investigator. Diarrhea and flushing events were not captured as AEs because they were already included as study assessments. A central laboratory conducted all urinary 5‐HIAA and plasma CgA analyses.
Outcomes
The primary endpoint of the ELECT trial was the percentage of days that short‐acting octreotide was used for symptom control during the DB phase [17]. In this post hoc analysis, mean composite symptom intensity scores were assessed during the DB and IOL phases. In addition to composite symptom scores, mean percentages of days that patients experienced symptoms of diarrhea and flushing during the DB phase were also computed to further characterize symptoms experienced by patients with NETs on study. Biochemical response was defined as a 50% decrease from baseline in 5‐HIAA levels in the IOL phase.
Statistical Analysis
Patient‐reported data from the intention‐to‐treat (ITT) patient population (randomized and open‐label) were evaluated. Analysis of covariance (ANCOVA) was used to analyze patient symptom data in the DB phase, with fixed effect terms for treatment group, prior SSA use, and region (U.S. vs. ex‐U.S.), and baseline diarrhea and flushing events as continuous variables. Comparisons of patient symptom data between the DB and IOL phases were analyzed using paired t tests.
Changes in 5‐HIAA levels from baseline were assessed in all patients with available 5‐HIAA data using ANCOVA with fixed effect terms as stated above, and baseline values as continuous variables. Given the data's skewness, 5‐HIAA values were log‐transformed. Relative ratio and associated 95% CIs (back‐transformed) for the absolute changes from baseline in logarithmic 5‐HIAA levels for lanreotide versus placebo groups during the DB treatment phase were calculated considering fixed effects for treatment group, SSA, region, and categorical 5‐HIAA baseline values (≤upper limit of normal [ULN], >ULN; ULN = 77.927 µmol/d). Absolute median changes in 5‐HIAA values during the DB phase were analyzed using a Wilcoxon‐rank sum test. All statistical tests were two‐tailed, and analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, https://www.sas.com/en_us/home.html). Safety data during the IOL phase were assessed using descriptive statistics.
Results
Patient Characteristics
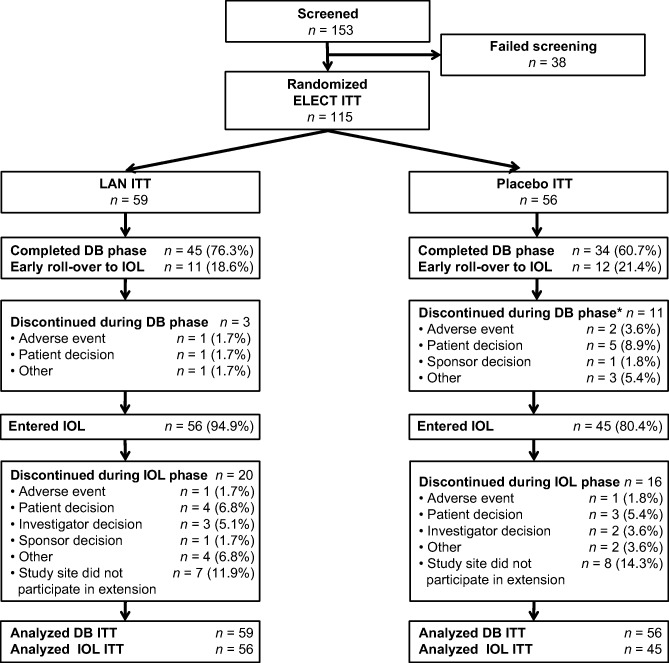
Between June 29, 2009, and January 15, 2013, a total of 115 patients were enrolled and randomized (n = 59 lanreotide, n = 56 placebo) in the primary study, and of those, 101 were enrolled in the IOL phase (n = 56 lanreotide DB/lanreotide IOL, n = 45 placebo DB/lanreotide IOL; Fig. 1). In total, 32,345 patient diary entries were recorded, of which 6 entries were not considered because of discordant results. During the screening phase, 99% of expected patient daily diary entries were recorded, followed by 98% in the DB and 85% in the IOL phase. Baseline data regarding symptoms and biomarkers are shown in Table 1. Mean percentages of patient‐reported days with severe diarrhea and severe flushing at baseline were low in both treatment groups (lanreotide vs. placebo), with no significant differences between groups. Forty‐six percent (46/99) of patients had 5‐HIAA levels >ULN at baseline and 86% (87/101) of patients had CgA levels >ULN. Baseline demographics and clinical characteristics for patients who entered the IOL phase (n = 101; supplemental online Table 1) were similar to those of the DB patient population, which have been previously published [17].
Figure 1.
Consolidated Standards of Reporting Trials diagram for ELECT DB and IOL study. *Includes one patient who completed the DB phase, but who did not continue into the IOL phase.
Abbreviations: DB, double‐blind; ELECT, evaluation of lanreotide depot/autogel efficacy and safety as a carcinoid‐syndrome treatment [study]; IOL, initial open‐label; ITT, intention‐to‐treat; LAN, lanreotide depot/autogel.
Table 1. Patient‐reported symptoms and biomarker data at baseline (screening).
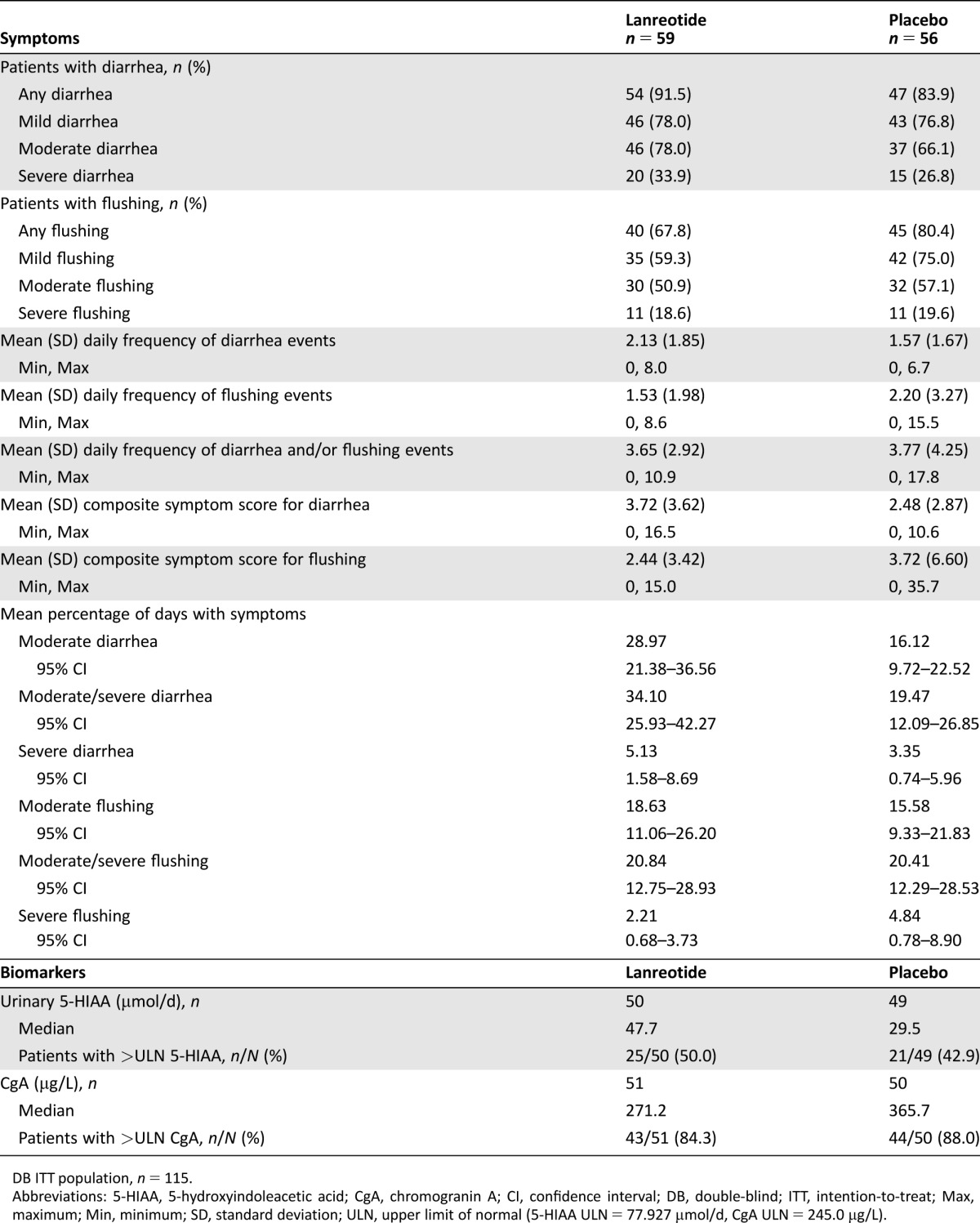
DB ITT population, n = 115.
Abbreviations: 5‐HIAA, 5‐hydroxyindoleacetic acid; CgA, chromogranin A; CI, confidence interval; DB, double‐blind; ITT, intention‐to‐treat; Max, maximum; Min, minimum; SD, standard deviation; ULN, upper limit of normal (5‐HIAA ULN = 77.927 µmol/d, CgA ULN = 245.0 µg/L).
Patient‐Reported Symptoms
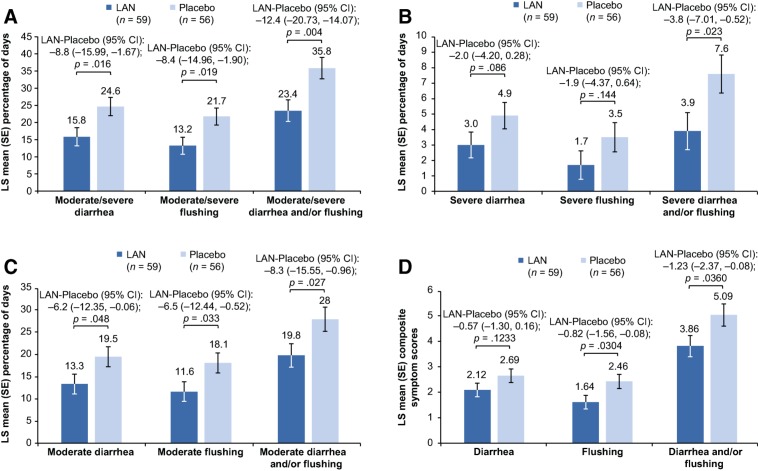
The adjusted least square (LS) mean percentages of days with moderate/severe symptoms (diarrhea, flushing, and diarrhea and/or flushing; all p ≤ .019), moderate symptoms (diarrhea, flushing, and diarrhea and/or flushing; all p ≤ .048), and severe diarrhea and/or flushing (p = .023) during the DB phase were significantly lower for the lanreotide versus placebo group (Fig. 2A–2C). Treatment differences in the adjusted percentages of days that patients experienced moderate/severe, severe, and moderate symptoms all favored the lanreotide group (i.e., greater symptom reduction) over placebo (Fig. 3A–3C). Percentage reductions in days with symptoms ranged from 29.4% for moderate diarrhea and/or flushing up to 53.0% for severe flushing with lanreotide versus placebo.
Figure 2.
Least square means (with SE bars) for percentage of days with moderate/severe symptoms (A), percentage of days with severe symptoms (B), percentage of days with moderate symptoms (C), and daily composite symptom scores (D) during the double‐blind phase (analysis of covariance, intention‐to‐treat population). Note: Figure 2A–2C. Least square means are based on an analysis of covariance model adjusted for prior somatostatin analog use, region, and baseline values. Figure 2D. Least square means based on an analysis of covariance model adjusted for daily average of diarrhea and flushing events at baseline, prior somatostatin analog use, and region.
Abbreviations: CI, confidence interval; LAN, lanreotide depot/autogel; SE, standard error.
Figure 3.
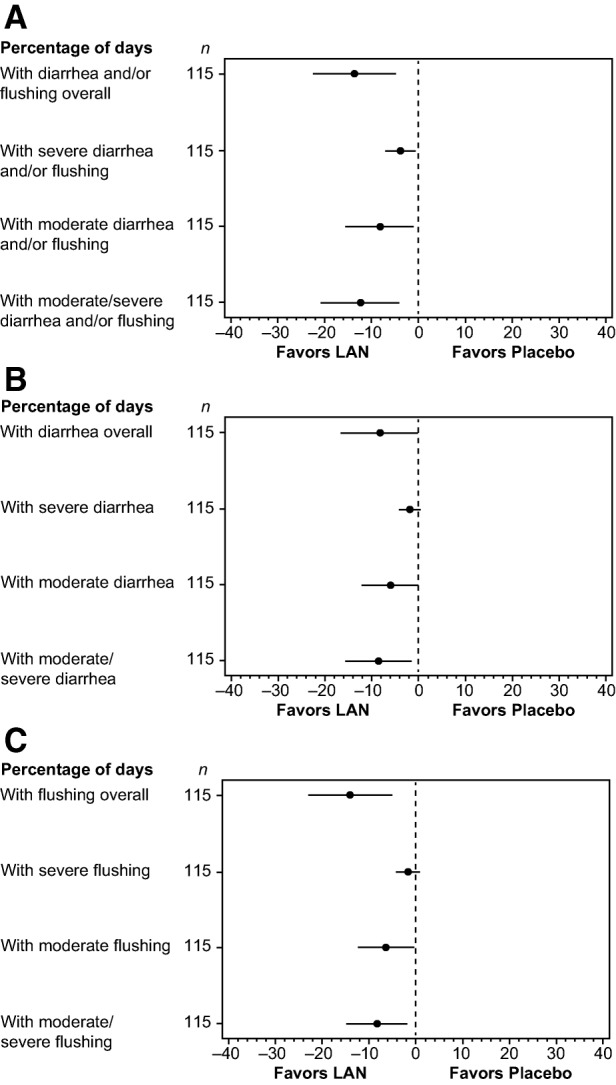
Percentage of days with diarrhea and/or flushing (A), diarrhea (B), or flushing (C) symptoms and their intensity during the double‐blind phase (analysis of covariance, intention‐to‐treat population n = 115). Least square mean difference together with 95% confidence interval adjusted for stratification factors at baseline and respective baseline value based on an analysis of covariance model.
Abbreviation: LAN, lanreotide depot/autogel.
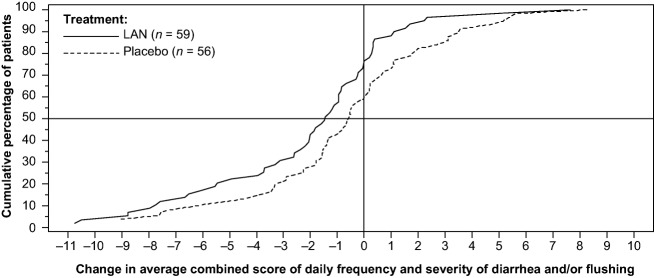
Lanreotide‐treated patients showed greater reductions (i.e., improvement) in average daily composite symptom scores for diarrhea, flushing, and diarrhea and/or flushing compared with placebo‐treated patients during the DB phase (Fig. 2D). Improvements in average daily composite symptom scores were significantly greater for lanreotide‐treated patients versus placebo for flushing (p = .030) and flushing and/or diarrhea (p = .036). Figure 4 shows the cumulative distribution of the change in average daily composite symptom scores for diarrhea and/or flushing. Approximately 75% of patients in the lanreotide group had a decrease in their composite symptom score compared with less than 60% for patients in the placebo group. Median changes in the composite symptom scores were −1.46 and −0.57 for lanreotide and placebo patients, respectively.
Figure 4.
Cumulative distribution of the change in average daily composite symptom score of diarrhea and/or flushing from baseline during double‐blind phase (intention‐to‐treat population n = 115).
Abbreviation: LAN, lanreotide depot/autogel.
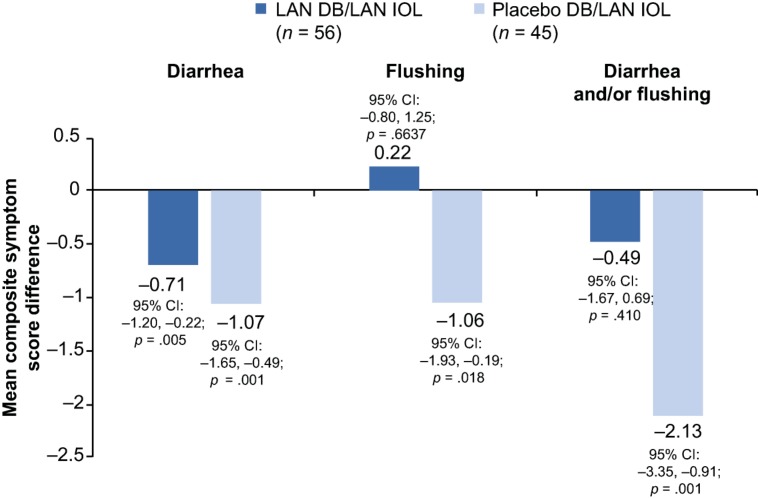
Among lanreotide DB/lanreotide IOL patients, mean composite symptom scores for diarrhea improved significantly from DB to IOL phases (p = .005), whereas mean composite symptom scores for diarrhea and/or flushing remained stable from the DB to IOL phases (p = .410; Fig. 5). Among placebo DB/lanreotide IOL patients, mean differences in composite symptom scores for diarrhea, flushing, and diarrhea and/or flushing (all p ≤ .018) reflected significant improvement from the DB phase to the end of the IOL phase (Fig. 5). The reduction in composite scores in the IOL phase among placebo DB/lanreotide IOL patients was comparable to the reductions observed among lanreotide‐treated patients during the DB phase.
Figure 5.
Mean difference in average daily composite symptom scores for patients between the DB and IOL phases according to initial DB treatment group (paired t test*, intention‐to‐treat population, n = 101). *Paired comparison between DB and IOL phase; within subject difference was calculated as IOL–DB.
Abbreviations: CI, confidence interval; DB, double‐blind; LAN, lanreotide depot/autogel; IOL, initial open‐label.
Biochemical Response
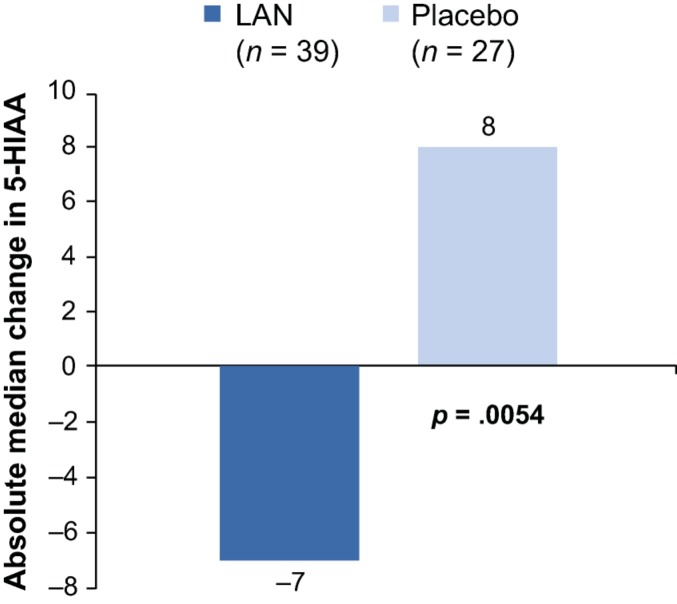
During the DB phase, the lanreotide group (n = 38) had a 36% greater decrease in adjusted absolute logarithmic urinary 5‐HIAA levels from baseline to week 12 versus the placebo group (n = 27; lanreotide LS mean change of 0.7 µmol/d vs. placebo LS mean change of 1.10 µmol/d; LS mean ratio of 0.64 [95% CI: 0.39–1.04]). Greater reductions in 5‐HIAA were observed among patients with baseline levels >ULN (lanreotide LS mean change of 0.56 µmol/d; placebo LS mean change of 0.80 µmol/d). Median absolute changes from baseline in urinary 5‐HIAA levels to week 12 of the DB phase were −7.0 µmol/d for the lanreotide group (n = 39) and 8.0 µmol/d for the placebo group (n = 27; Fig. 6).
Figure 6.
Absolute median change from baseline to week 12 (double‐blind phase) in 5‐HIAA levels (µmol/d; Wilcoxon rank‐sum, intention‐to‐treat population).
Abbreviations: 5‐HIAA, 5‐hydroxyindoleacetic acid; LAN, lanreotide depot/autogel.
Median changes in absolute urinary 5‐HIAA levels between each IOL and DB visit (week 12) for lanreotide DB/lanreotide IOL patients remained relatively stable (supplemental online Table 2), suggesting that 5‐HIAA reductions during DB treatment were maintained during IOL treatment. In placebo DB/lanreotide IOL patients, median changes in absolute urinary 5‐HIAA levels between each IOL and DB visit (week 12) reflected a decrease in 5‐HIAA levels at week 24 (−14.0 µmol/d), followed by smaller decreases at weeks 36 (−8.0 µmol/d) and 48 (−6.0 µmol/d). When considering the last value of the IOL phase, biochemical response (>50% reduction from DB baseline 5‐HIAA levels) was achieved in 31% (15/49) of lanreotide DB/lanreotide IOL patients and in 14.6% (6/41) of placebo DB/lanreotide IOL patients.
Safety During IOL Phase
Mean (standard deviation) durations of treatment in the IOL phase were 212.8 (60.0) days for lanreotide DB/lanreotide IOL patients and 214.4 (59.7) days for placebo DB/lanreotide IOL patients. Adverse‐event frequency during the IOL phase by DB group (lanreotide, placebo) was 69.6% versus 71.1% (supplemental online Table 3). The most common AEs (≥10%) among lanreotide DB/lanreotide IOL patients that occurred during the IOL phase were abdominal pain (14.3% [8/56]), weight loss (10.7% [6/56]), dyspnea (10.7% [6/56]), and hypertension (10.7% [6/56]). The most common AEs among placebo DB/lanreotide IOL patients that occurred during the IOL were nausea (11.1% [5/45]), fatigue (11.1% [5/45]), and headache (11.1% [5/45]). There were no treatment‐related SAEs or deaths during the IOL phase.
Discussion
This analysis of the ELECT study found significant reduction in days with CS symptoms and a reduction in composite symptom intensity scores (assessed both in terms of frequency and severity) observed in patients treated with lanreotide compared with placebo. Clinical symptoms of CS, particularly flushing and diarrhea, negatively impact the quality of life of patients with functional NETs, and because these symptoms can last for many years, they are particularly detrimental to patients’ well‐being. The assessment and control of CS symptoms are critical components of ongoing disease management for these patients [20], as emphasized by NCCN guideline recommendations [11]. We believe the results of this analysis are noteworthy, because they are the first to prospectively document symptom frequency and severity on a daily basis for 1 year using an IVRS/IWRS methodology within a randomized, placebo‐controlled phase 3 trial in CS.
Lanreotide depot was generally well‐tolerated in the ELECT IOL, and no new safety signals were identified. The AEs reported during the IOL phase in ELECT were generally comparable to the AEs reported during the DB phase [17], indicating that lanreotide depot's safety profile was acceptable and sustained with continued use. Current study safety outcomes were also generally comparable to the long‐term safety outcomes reported in antiproliferative studies of lanreotide [22], [23]. Of note, the percentage of days with severe symptoms at baseline was low in this population, which is not surprising because patients were required to have stable disease (no tumor progression or treatment‐refractory CS with conventional SSA therapy) before study entry and there was no washout period for patients previously treated with octreotide. The baseline symptoms may also reflect a selection bias, which is another possible limitation of the study, because investigators may have enrolled patients whose disease would have permitted placebo treatment despite the allowance of SC octreotide in the study design.
Long‐acting SSAs, including lanreotide depot, are well supported as treatment for CS symptoms [12], [13], [14], [15], [16], [24], and lanreotide depot is recommended in CS treatment guidelines, including NCCN [11]. Because of the placebo‐controlled study design of the phase 3 ELECT trial, short‐acting octreotide utilization was used as an objective surrogate endpoint for symptom control [17]. Rescue SC octreotide was also incorporated as the primary endpoint in ELECT with recognition that SSAs are effective in the management of NETs [25], [26], [27]. Regardless of SC octreotide utilization, these additional analyses showed a greater reduction in average daily composite symptom scores for lanreotide‐treated versus placebo‐treated patients. Symptom improvement with lanreotide was sustained in the 32‐week IOL phase for a total of 48 weeks of treatment. This is an important finding, because some patients can become refractory to standard SSA therapy over time. Of note, over half (55.7%) of the patients enrolled in ELECT had received previous treatment with SSAs, which was a stratification factor used in the ELECT primary study design [17]. A recent subanalysis of ELECT symptom data by prior SSA use found lower mean percentages of days with moderate or severe diarrhea and/or flushing in both de novo and prior octreotide groups treated with lanreotide compared with respective placebo groups; treatment differences (lanreotide vs. placebo) in the de novo group were statistically significant [28]. Together, these findings support the clinical benefit of lanreotide on patient symptom relief, using patient‐reported outcomes to assess treatment benefit.
The observational SymNET study previously evaluated diarrhea and flushing experienced by NET patients with CS treated with lanreotide for 6 months [12], [24]. In SymNET, 76% of patients reported being completely/rather satisfied with control of diarrhea and 73% reported being completely/rather satisfied with control of flushing following treatment with lanreotide for >3 months [24]. However, the study utilized a questionnaire conducted on a single study visit to document patient satisfaction, in contrast to the daily collection of patient‐reported symptom and frequency data during ELECT. In addition to ELECT, there have been two other phase 3, randomized, controlled trials for CS symptom treatment [18], [19]. These studies, conducted in patients with refractory CS symptoms, also utilized daily patient‐reported data; however, the data captured only symptom frequency and not severity [18], [19].
Patient‐reported symptoms of diarrhea and flushing were rigorously documented daily for 1 month before randomization in ELECT, providing 30 days of daily diarrhea and flushing frequency and severity as a baseline for each individual patient on study. Using each individual patient's score as his or her baseline value for subsequent daily reports permitted a meaningful characterization of an individual's symptomatology while on study. The composite symptom score, although not a validated outcome measure, provided an important assessment of the intensity of diarrhea and flushing symptoms experienced by patients with NETs via a daily patient‐reported record. In rare diseases such as NETs, validation of symptom‐assessment tools is difficult to accomplish when compared with symptom assessment tools for more common diseases [29]. The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire‐Core 30 (EORTC QLQ‐C30) [30] is a frequently used quality of life assessment in patients with CS [17], [18], [21], [24]. Two other questionnaires developed specifically for patients with NETs are the 21‐item gastrointestinal‐related NET supplement of the EORTC (EORTC QLQ‐GI.NET21) [17], [21], [24] and the Norfolk Quality of Life NET [31], [32]. Both of these quality of life questionnaires have been shown to correlate with tumor burden and biochemical measures (serotonin), and the Norfolk Quality of Life NET, in particular, also measures symptom frequency and severity [31], [32]. Quality of life questionnaires, however, are typically completed once during screening and then again at a specified interval during treatment to detect before and after changes, requiring patients to recall symptom information between intervals.
Although not precisely known, the etiology of CS is thought to be associated with the release of serotonin, tachykinins, prostaglandins, and bradykinins [4], [6]. 5‐HIAA (a metabolite of serotonin) levels can vary widely, however, and many patients with CS may have normal urinary or serum 5‐HIAA levels [12], [18], [33]. Based on the adjusted ANCOVA analyses, there was a 30% reduction in LS mean 5‐HIAA levels observed with lanreotide treatment during DB treatment in patients overall, and a 44% reduction in those with elevated 5‐HIAA levels at baseline. The reduction in 5‐HIAA was generally sustained over the IOL phase among patients initially treated with lanreotide during the DB phase. Although this analysis did not formally assess the relationship between the magnitude of the reduction in 5‐HIAA and improvement in composite symptom scores, these findings support the strong positive correlations previously noted between quality of life and serotonin [31].
Conclusion
In addition to the previously reported primary objective finding of reduced short‐acting octreotide use, these further analyses of the ELECT study documented a reduction in the percentage of days patients experienced the moderate/severe symptoms of diarrhea and flushing associated with CS, as well as a reduction in severity of patient‐reported symptoms of diarrhea and flushing associated with CS during the initial 16 weeks (DB phase) of lanreotide treatment. The improvement in diarrhea was sustained for 32 additional weeks (the IOL phase) of lanreotide treatment (i.e., through week 48 of treatment), resulting in clinically meaningful long‐term symptom reduction. The collection of daily patient‐reported symptomatology using IVRS/IWRS technology is a useful method to assess the clinical meaningfulness of treatment in patients with NETs and CS treated in clinical trials. Results from the open‐label extension study (≥2 years) will provide additional long‐term efficacy and safety data for lanreotide depot.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
The authors thank Sarah Mizne, Pharm.D., and Barbara Schwedel, M.S., E.L.S., both of MedVal Scientific Information Services, LLC, for medical writing support and editorial support, respectively, which were funded by Ipsen Biopharmaceuticals, Inc. This manuscript was prepared according to the International Society for Medical Publication Professionals’ “Good Publication Practice for Communicating Company‐Sponsored Medical Research: The GPP3 Guidelines.” The substudy was designed by academic investigators and by representatives of the sponsor, Ipsen Biopharmaceuticals, Inc. Data were collected with the use of the sponsor's data management systems and were analyzed by the sponsor's statistical team, INC Research, Raleigh, North Carolina, USA, funded by Ipsen.
M.S. is now at University of Arizona, Tucson, Arizona, USA.
The members of the ELECT Study Group are as follows: Brazil: A.C. Andrade, J.N. de Matos Neto, G.C. Girotto, A.M. Murad, M.C. Ramos, F.D. Tomasich, R.F. Weschenfelder; Czech Republic: E. Sedláčková; India: S. Aggarwal, S.A. Bondarde, R. Bhojwani, G. Karimundackal, M.V.T. Krishna Mohan, S.S. Nirni, N. Somani; Latvia: G. Purkalne; Poland: M. Bolanowski; Russia: V. Gorbunova, L.D. Roman, A. Severtsev; Serbia: S. Damjanović, D. Jovanović; South Africa: R.E. Frohling, J.K. Harris, C. Jacobs, J.P. Jordaan, B. Robertson; Turkey: F. Bayram; Ukraine: I. Bodarenko, O. Borota, O. Datsenko, Y. Hotko, O. Iemets, I. Kryachok, V. Paramonov, O. Ponomarova, R. Senyutovych, A. Zaychuk; USA: R.J. Auchus, M.H. Fekrazad, G. Fisher, H. Harvey, A. Heaney, D. Metz, R. Pommier, A. Vinik, E.M. Wolin, E.A. Woltering.
Author Contributions
Conception/design: Edward M. Wolin, Susan Pitman Lowenthal, Beloo Mirakhur
Collection and/or assembly of data: George A. Fisher Jr., Edward M. Wolin, Susan Pitman Lowenthal, Beloo Mirakhur, Rodney F. Pommier, Montaser Shaheen
Data analysis and interpretation: George A. Fisher Jr., Edward M. Wolin, Nilani Liyanage, Susan Pitman Lowenthal, Beloo Mirakhur, Rodney F. Pommier, Montaser Shaheen, Aaron Vinik
Manuscript writing: George A. Fisher Jr., Edward M. Wolin, Nilani Liyanage, Susan Pitman Lowenthal, Beloo Mirakhur, Rodney F. Pommier, Montaser Shaheen, Aaron Vinik
Final approval of manuscript: George A. Fisher Jr., Edward M. Wolin, Nilani Liyanage, Susan Pitman Lowenthal, Beloo Mirakhur, Rodney F. Pommier, Montaser Shaheen, Aaron Vinik
Disclosures
George A. Fisher Jr.: Ipsen Genentech/Roche, Merck (C/A), Ipsen (RF); Edward M. Wolin: Ipsen, Advanced Accelerator Applications, Lexicon (SAB); Nilani Liyanage: Ipsen (E, OI); Susan Pitman Lowenthal: Ipsen (C/A, OI, travel expenses), Pfizer (OI); Beloo Mirakhur: Ipsen (E, OI); Rodney F. Pommier: Novartis, Ipsen, Lexicon (C/A); Aaron Vinik: Merck (SAB), Merck, Pfizer, Alnylam, Hydra Biosciences, Astellas, Ipsen, Ionis Pharmaceuticals (C/A), ViroMed, VeroScience, Nestle Health Science‐Pamlab, Novo Nordisk, Matrix Biomed (RF), Ipsen (H). The other author indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Yao JC, Hassan M, Phan A et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063–3072. [DOI] [PubMed] [Google Scholar]
- 2. Kunz PL, Reidy‐Lagunes D, Anthony LB et al. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas 2013;42:557–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu EH, Solorzano CC, Katznelson L et al. Aace/Ace disease state clinical review: Diagnosis and management of midgut carcinoids. Endocr Pract 2015;21:534–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Strosberg J. Neuroendocrine tumours of the small intestine. Best Pract Res Clin Gastroenterol 2012;26:755–773. [DOI] [PubMed] [Google Scholar]
- 5. Janson ET, Holmberg L, Stridsberg M et al. Carcinoid tumors: Analysis of prognostic factors and survival in 301 patients from a referral center. Ann Oncol 1997;8:685–690. [DOI] [PubMed] [Google Scholar]
- 6. Vinik AI, Chaya C. Clinical presentation and diagnosis of neuroendocrine tumors. Hematol Oncol Clin North Am 2016;30:21–48. [DOI] [PubMed] [Google Scholar]
- 7. Kunz PL. Carcinoid and neuroendocrine tumors: Building on success. J Clin Oncol 2015;33:1855–1863. [DOI] [PubMed] [Google Scholar]
- 8. Halperin DM, Shen C, Dasari A et al. Frequency of carcinoid syndrome at neuroendocrine tumour diagnosis: A population‐based study. Lancet Oncol 2017;18:525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pavel M, O'Toole D, Costa F et al. ENETS consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Neuroendocrinology 2016;103:172–185. [DOI] [PubMed] [Google Scholar]
- 10. Oberg K, Knigge U, Kwekkeboom D et al. Neuroendocrine gastro‐entero‐pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2012;23(suppl 7):vii124–vii130. [DOI] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network . NCCN clinical practice guidelines in oncology (NCCN guidelines) neuroendocrine tumors. Version 3.2017. June 13, 2017. Available at: https://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf. Accessed September 22, 2017.
- 12. Ruszniewski P, Ish‐Shalom S, Wymenga M et al. Rapid and sustained relief from the symptoms of carcinoid syndrome: Results from an open 6‐month study of the 28‐day prolonged‐release formulation of lanreotide. Neuroendocrinology 2004;80:244–251. [DOI] [PubMed] [Google Scholar]
- 13. Bajetta E, Procopio G, Catena L et al. Lanreotide autogel every 6 weeks compared with Lanreotide microparticles every 3 weeks in patients with well differentiated neuroendocrine tumors: A Phase III Study. Cancer 2006;107:2474–2481. [DOI] [PubMed] [Google Scholar]
- 14. O'Toole D, Ducreux M, Bommelaer G et al. Treatment of carcinoid syndrome: A prospective crossover evaluation of lanreotide versus octreotide in terms of efficacy, patient acceptability, and tolerance. Cancer 2000;88:770–776. [DOI] [PubMed] [Google Scholar]
- 15. Bianchi A, De Marinis L, Fusco A et al. The treatment of neuroendocrine tumors with long‐acting somatostatin analogs: A single center experience with lanreotide autogel. J Endocrinol Invest 2011;34:692–697. [DOI] [PubMed] [Google Scholar]
- 16. Khan MS, El‐Khouly F, Davies P et al. Long‐term results of treatment of malignant carcinoid syndrome with prolonged release lanreotide (somatuline autogel). Aliment Pharmacol Ther 2011;34:235–242. [DOI] [PubMed] [Google Scholar]
- 17. Vinik AI, Wolin EM, Liyanage N et al. Evaluation of lanreotide depot/autogel efficacy and safety as a carcinoid syndrome treatment (ELECT): A randomized, double‐blind, placebo‐controlled trial. Endocr Pract 2016;22:1068–1080. [DOI] [PubMed] [Google Scholar]
- 18. Kulke MH, Horsch D, Caplin ME et al. Telotristat ethyl, a tryptophan hydroxylase inhibitor for the treatment of carcinoid syndrome. J Clin Oncol 2017;35:14–23. [DOI] [PubMed] [Google Scholar]
- 19. Wolin EM, Jarzab B, Eriksson B et al. Phase III study of pasireotide long‐acting release in patients with metastatic neuroendocrine tumors and carcinoid symptoms refractory to available somatostatin analogues. Drug Des Devel Ther 2015;9:5075–5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beaumont JL, Cella D, Phan AT et al. Comparison of health‐related quality of life in patients with neuroendocrine tumors with quality of life in the general US population. Pancreas 2012;41:461–466. [DOI] [PubMed] [Google Scholar]
- 21. Gelhorn HL, Kulke MH, O'Dorisio T et al. Patient‐reported symptom experiences in patients with carcinoid syndrome after participation in a study of telotristat etiprate: A qualitative interview approach. Clin Ther 2016;38:759–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Caplin ME, Pavel M, Cwikla JB et al. Anti‐tumour effects of lanreotide for pancreatic and intestinal neuroendocrine tumours: The CLARINET open‐label extension study. Endocr Relat Cancer 2016;23:191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martin‐Richard M, Massuti B, Pineda E et al. Antiproliferative effects of lanreotide autogel in patients with progressive, well‐differentiated neuroendocrine tumours: A Spanish, multicentre, open‐label, single arm phase II study. BMC Cancer 2013;13:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ruszniewski P, Valle JW, Lombard‐Bohas C et al. Patient‐reported outcomes with lanreotide Autogel/Depot for carcinoid syndrome: An international observational study. Dig Liver Dis 2016;48:552–558. [DOI] [PubMed] [Google Scholar]
- 25. Rinke A, Muller HH, Schade‐Brittinger C et al. Placebo‐controlled, double‐blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID Study Group. J Clin Oncol 2009;27:4656–4663. [DOI] [PubMed] [Google Scholar]
- 26. Caplin ME, Pavel M, Cwikla JB et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 2014;371:224–233. [DOI] [PubMed] [Google Scholar]
- 27. Caplin ME, Ruszniewski PB, Pavel ME et al. Progression‐free survival (PFS) with lanreotide autogel/depot (LAN) in enteropancreatic NETs patients: The CLARINET extension study. J Clin Oncol 2014;32(suppl 15):4107. [Google Scholar]
- 28. Pommier RF, Fisher GA, Wolin EM et al. Efficacy of lanreotide depot (LAN) for symptomatic control of carcinoid syndrome (CS) in patients with neuroendocrine tumor (NET) previously responsive to octreotide (OCT): Subanalysis of patient‐reported symptoms from the phase III ELECT study. J Clin Oncol 2017;35(suppl 4):378. 28095264 [Google Scholar]
- 29.US Food and Drug Administration, US Department of Health and Human Services, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. Rare diseases: common issues in drug development. Guidance for industry. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM458485.pdf. Accessed January 25, 2017.
- 30. Aaronson NK, Ahmedzai S, Bergman B et al. The European Organization for Research and Treatment of Cancer QLQ‐C30: A quality‐of‐life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–376. [DOI] [PubMed] [Google Scholar]
- 31. Vinik E, Silva MP, Vinik AI. Measuring the relationship of quality of life and health status, including tumor burden, symptoms, and biochemical measures in patients with neuroendocrine tumors. Endocrinol Metab Clin North Am 2011;40:97–109, viii. [DOI] [PubMed] [Google Scholar]
- 32. Vinik E, Carlton CA, Silva MP et al. Development of the Norfolk quality of life tool for assessing patients with neuroendocrine tumors. Pancreas 2009;38:e87–e95. [DOI] [PubMed] [Google Scholar]
- 33. Anthony LB, Pavel ME, Hainsworth JD et al. Impact of previous somatostatin analogue use on the activity of everolimus in patients with advanced neuroendocrine tumors: Analysis from the phase III RADIANT‐2 trial. Neuroendocrinology 2015;102:18–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.