This article summarizes the current treatment landscape for metastatic colorectal cancer, reviews the clinical data supporting the roles for targeted therapies in treatment sequencing, examines investigational treatments for third‐line metastatic colorectal cancer, and reviews emerging trends in therapeutic strategies and molecular testing.
Keywords: Colorectal neoplasms, Molecular targeted therapy, Combination drug therapy, Investigational therapies, Patient selection, Antineoplastic protocols
Abstract
The emergence of targeted therapies for the treatment of metastatic colorectal cancer (mCRC) has considerably improved survival, but has also resulted in a dilemma of identifying the optimal sequence and combination of various agents in the mCRC treatment landscape. A number of cytotoxic agents, including irinotecan, oxaliplatin, 5‐fluorouracil, capecitabine, and TAS‐102, are available for treatment of mCRC. Additionally, whereas patients harboring rat sarcoma viral oncogene homolog (RAS)–wild type mCRC can be treated with the anti‐epidermal growth factor receptor antibodies cetuximab and panitumumab or antiangiogenic agents (bevacizumab, ziv‐aflibercept, and ramucirumab), patients with RAS‐mutant mCRC are limited to antiangiogenic agents as biologic options. Regorafenib, a multikinase inhibitor, can be used in both RAS subgroups. As such, the recommended sequence of therapies that should be received by each subgroup must also be considered separately. This review provides an overview of recent clinical data for approved and investigational targeted therapies that have been studied across different mCRC treatment lines and patient subgroups. It also examines emerging trends in the treatment landscape for mCRC, including treatment with immune checkpoint inhibitors and the utilization of genomic profiling.
Implications for Practice.
Currently, there are no established guidelines for optimal sequencing of cytotoxic or targeted agents in metastatic colorectal cancer (mCRC). This review provides a snapshot of the current mCRC treatment paradigm and examines the latest clinical data that support the utilization of several targeted agents alone or in combination with backbone chemotherapy across different lines of treatment and patient populations, highlighting recommendations for their usage. Recent advances in the treatment landscape are also summarized, including genomic profiling and preliminary results with immune checkpoint inhibitors.
摘要
靶向治疗的出现大大提高了转移性结直肠癌(mCRC)的生存率, 但同时也导致难以识别多种mCRC治疗药物的最佳顺序和组合的困境。包括伊立替康、奥沙利铂、5‐氟尿嘧啶、卡培他滨和TAS‐102在内的多种细胞毒性药物可以用于治疗mCRC。此外, 尽管罹患大鼠肉瘤病毒癌基因同源物(RAS)‐野生型mCRC的患者可以接受抗表皮生长因子受体抗体西妥昔单抗和帕尼单抗或抗血管生成药物(贝伐单抗、阿柏西普和雷莫芦单抗)治疗, 但RAS突变mCRC患者只能接受抗血管生成药物作为生物学治疗。两个RAS亚组中均可使用瑞戈非尼(一种多激酶抑制剂)。因此, 也必须单独考虑每个亚组应接受的治疗的推荐顺序。本次回顾提供了已获批准疗法和研究性靶向治疗(已在不同mCRC治疗线和患者亚组中进行过研究)的最新临床研究数据的概览。本次回顾还考察了mCRC治疗领域的新趋势, 包括使用免疫检查点抑制剂治疗和基因组分析。
对临床实践的启示:目前仍然没有关于细胞毒性或靶向药物治疗转移性结直肠癌(mCRC)的最佳顺序的既定指南。本回顾简要介绍了当前mCRC的治疗方法。我们回顾了支持在不同的治疗线和患者人群中单独使用几种靶向药物或靶向药物与核心化疗药物联合给药的最新临床研究数据, 并重点回顾了关于这些药物的使用建议。此外, 我们还总结了治疗领域的最新进展, 包含基因组分析和使用免疫检查点抑制剂的初步结果。
Introduction
Localized colorectal cancer (CRC) is often successfully treated with curative surgery followed by chemotherapy in patients at high risk for recurrence. Overall prognosis for patients with localized CRC is favorable, with a 5‐year survival rate of up to 90% [1]. Because early‐stage CRC can be asymptomatic, screening is often necessary to detect the disease at this stage [1]. However, a minority (∼40%) of CRC cases are diagnosed early, leaving a large number of patients initially diagnosed with metastatic CRC (mCRC), for whom the 5‐year survival rate is poor (13%) [1].
Several targeted therapies that inhibit the angiogenic process or the epidermal growth factor receptor (EGFR) are currently available for the treatment of mCRC, either as monotherapy or in combination with backbone chemotherapies [2]. This article will summarize the current treatment landscape for mCRC, review clinical data supporting the roles for targeted therapies in treatment sequencing, examine investigational treatments for third‐line mCRC, and review emerging trends in therapeutic strategies and molecular testing.
Treating mCRC with Cytotoxic Therapy
The current National Comprehensive Cancer Network (NCCN) guidelines for mCRC recommend FOLFOX (5‐fluorouracil [5‐FU] + oxaliplatin + leucovorin [LV]), FOLFIRI (5‐FU + irinotecan + LV), XELOX (capecitabine + oxaliplatin), infusional 5‐FU/LV or capecitabine, or FOLFOXIRI (5‐FU + oxaliplatin + irinotecan + LV) for patients with mCRC who are appropriate for intensive therapy [2]. The NCCN panel recommends these regimens as equally effective cytotoxic treatment options, and little or no clinical difference is observed when administering intensive therapy as first‐line versus subsequent‐line therapy following less intensive therapy [3], [4], [5], [6]. The European Society for Medical Oncology guidelines similarly recommend a first‐line backbone chemotherapy of a fluoropyrimidine in various schedules and combinations [7], and FOLFOX and FOLFIRI are currently considered the preferred cytotoxic treatment options for first‐line treatment of mCRC [3], [7].
Targeted Therapies for Treatment of mCRC
Since the mid‐1990s, six targeted agents have been approved by the U.S. Food and Drug Administration for treatment of mCRC (Table 1). With the availability of new treatment options and combinations, the median overall survival (OS) has been extended to approximately 36 months in rat sarcoma viral oncogene homolog (RAS)–wild type patients [8], [9]. Some clinical data now provide guidance to clinicians on how to sequence these agents.
Table 1. Approved targeted drugs for mCRC.

Abbreviations: 5‐FU, 5‐fluorouracil; BRAF, B‐Raf proto‐oncogene, serine/threonine kinase; c‐KIT, KIT proto‐oncogene receptor tyrosine kinase; EGFR, epidermal growth factor receptor; FDA, U.S. Food and Drug Administration; FGFR, fibroblast growth factor receptor; FOLFIRI, 5‐fluorouracil + irinotecan + leucovorin; FOLFOX, 5‐fluorouracil + oxaliplatin + leucovorin; KRAS, Kirsten rat sarcoma viral oncogene homolog; mAb, monoclonal antibody; mCRC, metastatic colorectal cancer; PDGFR, platelet‐derived growth factor receptor; RAF‐1, Raf‐1 proto‐oncogene, serine/threonine kinase; RET, ret proto‐oncogene; TIE2, tyrosine kinase with immunoglobulin and epidermal growth factor homology domain 2; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Sequencing of Targeted Agents for mCRC
First‐Line Treatment of mCRC
Bevacizumab (vascular endothelial growth factor [VEGF] monoclonal antibody [mAb]), cetuximab (EGFR mAb), and panitumumab (EGFR mAb) are the only targeted therapies currently indicated as first‐line treatment of mCRC in combination with backbone chemotherapies, with approvals based on findings from their respective pivotal phase III trials [10], [11], [12], [13]. The phase III Cancer and Leukemia Group B (CALGB)/Southwest Oncology Group (SWOG) 80405 [14] and FIRE‐3 [8] studies compared bevacizumab with cetuximab to identify the optimal first‐line targeted agent for use in combination with FOLFIRI (examined CALGB/SWOG 80405 and FIRE‐3) or modified FOLFOX6 (mFOLFOX6; examined in CALGB/SWOG 80405 only) in RAS–wild type mCRC.
In FIRE‐3 (N = 592), patients were randomized 1:1 to cetuximab plus FOLFIRI versus bevacizumab plus FOLFIRI, and objective response rate (ORR) was assessed as the primary endpoint. Although no statistical differences in ORR (62.0% vs. 58.0%; p = .18) or median progression‐free survival (PFS; 10.0 vs. 10.3 months; hazard ratio [HR] = 1.06 [95% confidence interval (CI), 0.88–1.26]; p = .55) were observed, median OS favored the cetuximab arm (28.7 vs. 25.0 months; HR = 0.77 [95% CI, 0.62–0.96]; p = .017) [8]. A secondary analysis of survival by subsequent lines of therapy showed that patients who started cetuximab versus bevacizumab during first‐line therapy demonstrated longer PFS (6.5 vs. 4.7 months; HR = 0.68 [95% CI, 0.54–0.85]; p < .001) and OS (16.3 vs. 13.2 months; HR = 0.70 [95% CI, 0.55–0.88]; p = .0021) from start of second‐line therapy [15].
In contrast, the much larger CALGB/SWOG 80405 phase III study (n = 1,137) demonstrated no clear differences between the bevacizumab and cetuximab treatment arms in median PFS (10.84 vs. 10.45 months) or OS (29.04 vs. 29.93 months; HR = 0.92 [95% CI, 0.78–1.09]; p = .34) for patients with RAS–wild type mCRC [14]. The inconsistent findings between the two studies are likely due to differences between the primary chemotherapy backbones [16], patient selection, and laboratory techniques [17] as well as perhaps in the biology of responses after first‐line therapy [18], [19]. These differences also highlight the need to better identify underlying biologic and clinical factors that can predict outcome [20].
For example, a recent post hoc analysis of the CALGB/SWOG 80405 study suggested that patients with left‐sided tumors had longer median OS versus those with right‐sided tumors (33.3 vs. 19.4 months; HR = 1.55 [95% CI, 1.32–1.82]; p < .0001) [9]. According to the NCCN panel, the left side of the colon includes the area encompassing the splenic flexure to the rectum, whereas the right side of the colon includes the hepatic flexure to the cecum [2]. Moreover, OS with cetuximab was longer than with bevacizumab when the primary tumor was on the left side (36.0 vs. 31.4 months, respectively), whereas OS with bevacizumab was longer than with cetuximab when the primary tumor location was on the right side (24.2 vs. 16.7 months, respectively) [9]. A post hoc analysis from the FIRE‐3 study concurred with these findings, showing that OS is significantly longer in patients with left‐sided versus right‐sided mCRC, and OS is better with cetuximab in left‐sided tumors and worse in right‐sided tumors [9], [21], [22]. Similarly, a retrospective analysis of the National Cancer Institute of Canada (NCIC) CO.17 trial of cetuximab versus best supportive care (BSC) in chemotherapy‐refractory mCRC demonstrated a PFS advantage with cetuximab compared with BSC in patients with left‐sided but not right‐sided tumors (left: median PFS, 5.4 vs. 1.8 months, respectively; HR = 0.28 [95% CI, 0.18–0.45]; p < .0001; right: median PFS, 1.9 vs. 1.9 months; HR = 0.73 [95% CI, 0.42–1.27]; p = .26) [23]. A population‐based study using Surveillance, Epidemiology, and End Results Program data found a similar effect of sidedness on prognosis, with inferior survival in patients with right‐sided tumors [24]. Moreover, the results from molecular analyses suggest potential biologic underpinnings of the right‐ versus left‐sided phenomenon [25]. Nonetheless, the findings from the CALGB/SWOG 80405 and FIRE‐3 studies suggest front‐line treatment with either cetuximab or bevacizumab, in combination with either FOLFOX or FOLFIRI, is a reasonable option.
Consistent with the findings for cetuximab‐based regimens, a randomized phase III study (PRIME) of panitumumab plus FOLFOX4 versus FOLFOX4 alone in first‐line treatment of RAS–wild type mCRC showed a significant median PFS benefit (10.0 vs. 8.6 months; HR = 0.80 [95% CI, 0.67–0.95]; p = .01) and a trend toward median OS benefit (23.9 vs. 19.7 months; HR = 0.88 [95% CI, 0.73–1.06]; p = .17) following treatment with the panitumumab‐based regimen [13], [26]. A significant OS benefit for panitumumab was observed in an exploratory analysis of updated survival with >80% OS events (23.8 vs. 19.4 months; HR = 0.83 [95% CI, 0.70–0.98]; p = .03) [26]. A retrospective analysis of the PRIME study and the PEAK study, which evaluated first‐line panitumumab plus FOLFOX versus bevacizumab plus FOLFOX, found that patients with left‐sided tumors had an OS advantage with panitumumab versus patients with right‐sided tumors (PRIME: 30.3 vs. 23.6 months, respectively; adjusted HR = 0.73; p = .0112; PEAK: 43.4 vs. 32.0 months; adjusted HR = 0.77; p = .3125) [27].
Based on the collective findings from these studies, the NCCN panel currently recommends cetuximab and panitumumab for the first‐line treatment of left‐sided tumors only, in combination with cytotoxic agents in RAS–wild type mCRC [2]. Bevacizumab may be preferred for right‐sided tumors in this setting because these tumors are unlikely to respond to anti‐EGFR antibodies [2].
Based on the collective findings from these studies, the NCCN panel currently recommends cetuximab and panitumumab for the first‐line treatment of left‐sided tumors only, in combination with cytotoxic agents in RAS–wild type mCRC. Bevacizumab may be preferred for right‐sided tumors in this setting because these tumors are unlikely to respond to anti‐EGFR antibodies.
Recently, two additional phase II studies (MAVERICC and STEAM) were conducted to evaluate the potential role of chemotherapy backbone (mFOLFOX6, FOLFIRI, FOLFOXIRI) as the preferred combination partner for bevacizumab in the first‐line treatment of mCRC and included analyses of patients stratified by expression of the putative predictive biomarker excision repair cross‐complementation group 1 (ERCC1) [28], [29]. In MAVERICC, FOLFIRI was associated with nonsignificant trends toward longer median PFS (12.6 vs. 10.1 months; HR = 0.79; p = .056) and median OS (27.5 vs. 23.9 months; HR = 0.76; p = .086) when compared with FOLFOX [28]. Comparable results were observed in patients with high baseline levels of ERCC1 and in the entire study population; additional analyses are ongoing for patients stratified by VEGF‐A levels. The findings from this study, along with those from CALGB/SWOG 80405, suggest FOLFIRI may be a preferred backbone to combine with bevacizumab. The STEAM study compared concurrent versus sequential FOLFOXIRI (i.e., alternating FOLFOX and FOLFIRI) with FOLFOX as a combination partner for bevacizumab [29]. Trends toward increased ORR (72% vs. 73% vs. 62%) and longer median PFS (11.7 vs. 10.7 vs. 9.3 months) were observed with concurrent and sequential FOLFOXIRI compared with FOLFOX backbone regimen, respectively, with all three regimens demonstrating similar safety profiles.
Other targeted therapies approved for treatment of mCRC in the second‐line setting and beyond are being or have been investigated in combination with chemotherapy in first‐line studies. A randomized, open‐label, phase II study of aflibercept, a VEGF receptor (VEGFR) fusion protein, in combination with mFOLFOX6 versus mFOLFOX6 alone as first‐line treatment of mCRC (AFFIRM) suggest similar response rates (49.1% vs. 45.9%) and median PFS (8.48 vs. 8.77 months) between treatment arms [30], [31]. Comparable results were reported in a single‐arm, multicenter, open‐label, phase II study of regorafenib plus mFOLFOX6 as first‐line treatment of mCRC, with an ORR of 43.9%, disease control rate (DCR) of 85.4%, and a median PFS of 8.5 months [32]. Finally, in an open‐label, phase II study of the VEGFR2 mAb ramucirumab in combination with mFOLFOX6, a median OS of 20.4 months, median PFS of 11.5 months, ORR of 58.3%, and DCR of 93.8% were reported [33].
Second‐Line Treatment of mCRC
Both cetuximab and panitumumab are appropriate to use in the second‐line setting in patients with RAS–wild type mCRC following progression on chemotherapy [34], [35], but are not recommended for patients who previously received an EGFR inhibitor [2]. A randomized phase II study (SPIRITT) comparing panitumumab plus FOLFIRI versus bevacizumab plus FOLFIRI among RAS–wild type patients who progressed on an oxaliplatin‐based regimen containing bevacizumab showed no difference in median PFS (7.7 vs. 9.2 months; HR = 1.01 [95% CI, 0.68–1.50]; p = .97) or median OS (18.0 vs. 21.4 months; HR = 1.06 [95% CI, 0.75–1.49]; p = .75) between the two regimens, suggesting that patients who progress on a bevacizumab‐based regimen can be considered for receiving panitumumab or bevacizumab plus FOLFIRI for their second‐line therapy [36]. The phase III 181 study demonstrated a significant improvement in PFS with panitumumab plus FOLFIRI versus FOLFIRI alone in patients with RAS–wild type mCRC following progression on chemotherapy (median PFS, 5.9 vs. 3.9 months, respectively; HR = 0.73 [95% CI, 0.59–0.90]; p = .004) [37]. Objective response rate was 35% versus 10% for the combination versus FOLFIRI alone (descriptive p < .001), and there was a trend toward improved OS with the combination in patients with wild‐type RAS (median OS, 14.5 vs. 12.5 months, respectively; HR = 0.85 [95% CI, 0.70–1.04]; p = .12). Efficacy was not improved with panitumumab in patients with mutant RAS [37].
Use of bevacizumab‐containing regimens for treatment of second‐line mCRC is supported by results from two large phase III trials [38], [39]. The phase III E3200 study compared FOLFOX4 with or without bevacizumab in patients with mCRC who previously received a fluoropyrimidine‐ and irinotecan‐based regimen, but not bevacizumab [38]. The bevacizumab‐containing regimen demonstrated favorable median OS (12.9 vs. 10.8 months; HR = 0.75; p = .0011) and median PFS (7.3 vs. 4.7 months; HR = 0.61; p < .0001), and these results led to a second‐line indication for bevacizumab in 2006. More recently, the open‐label, phase III ML18147 study compared chemotherapy (oxaliplatin‐ or irinotecan‐based; switch from first‐line chemotherapy) with or without bevacizumab in patients with mCRC who had previously received bevacizumab plus chemotherapy as first‐line therapy [39]. Median OS (11.2 vs. 9.8 months; HR = 0.81 [95% CI, 0.69–0.94]; p = .0062) and median PFS (5.7 vs. 4.1 months; HR = 0.68 [95% CI, 0.59–0.78]; p < .0001) favored continuation of bevacizumab beyond progression. Of note, the PFS benefit for the bevacizumab‐containing regimen was maintained regardless of RAS status.
Similar activity has been observed with other antiangiogenic agents. The pivotal phase III trial (VELOUR) of ziv‐aflibercept plus FOLFIRI versus placebo plus FOLFIRI in the second‐line setting showed an OS (13.5 vs. 12.1 months; HR = 0.82 [95% CI, 0.71–0.94]; p = .003) and PFS (6.9 vs. 4.7 months; HR = 0.76 [95% CI, 0.66–0.87]; p < .0001) benefit with ziv‐aflibercept [40]. In a subgroup analysis, the observed benefit between treatment groups was smaller among patients who previously received bevacizumab (12.5 vs. 11.7 months; HR = 0.86) compared with those who did not (13.9 vs. 12.4 months; HR = 0.79) [41]. Another phase III trial (RAISE) evaluated the role of ramucirumab plus FOLFIRI versus placebo plus FOLFIRI and likewise showed an OS (13.3 vs. 11.7 months; HR = 0.84 [95% CI, 0.73–0.98]; p = .02) and PFS (5.7 vs. 4.5 months; HR = 0.79 [95% CI, 0.70–0.90]; p = .0005) benefit with the addition of ramucirumab to FOLFIRI in patients who progressed on first‐line therapy with bevacizumab, fluoropyrimidine, and oxaliplatin [42].
In a phase II randomized trial (BOND) assessing the role of cetuximab plus irinotecan versus cetuximab monotherapy in mCRC patients refractory to irinotecan, higher responses were observed in the combination treatment arm (22.9% vs. 10.8%; p = .007), with significantly longer PFS (4.1 vs. 1.5 months; HR = 0.54 [95% CI, 0.42–0.71]; p < .001) and a trend towards longer OS (8.6 vs. 6.9 months; HR = 0.91 [95% CI, 0.68–1.21]; p = .48) [43]. Results of trials in second‐line mCRC are summarized in Table 2.
Table 2. Results from clinical trials in second‐line mCRC.
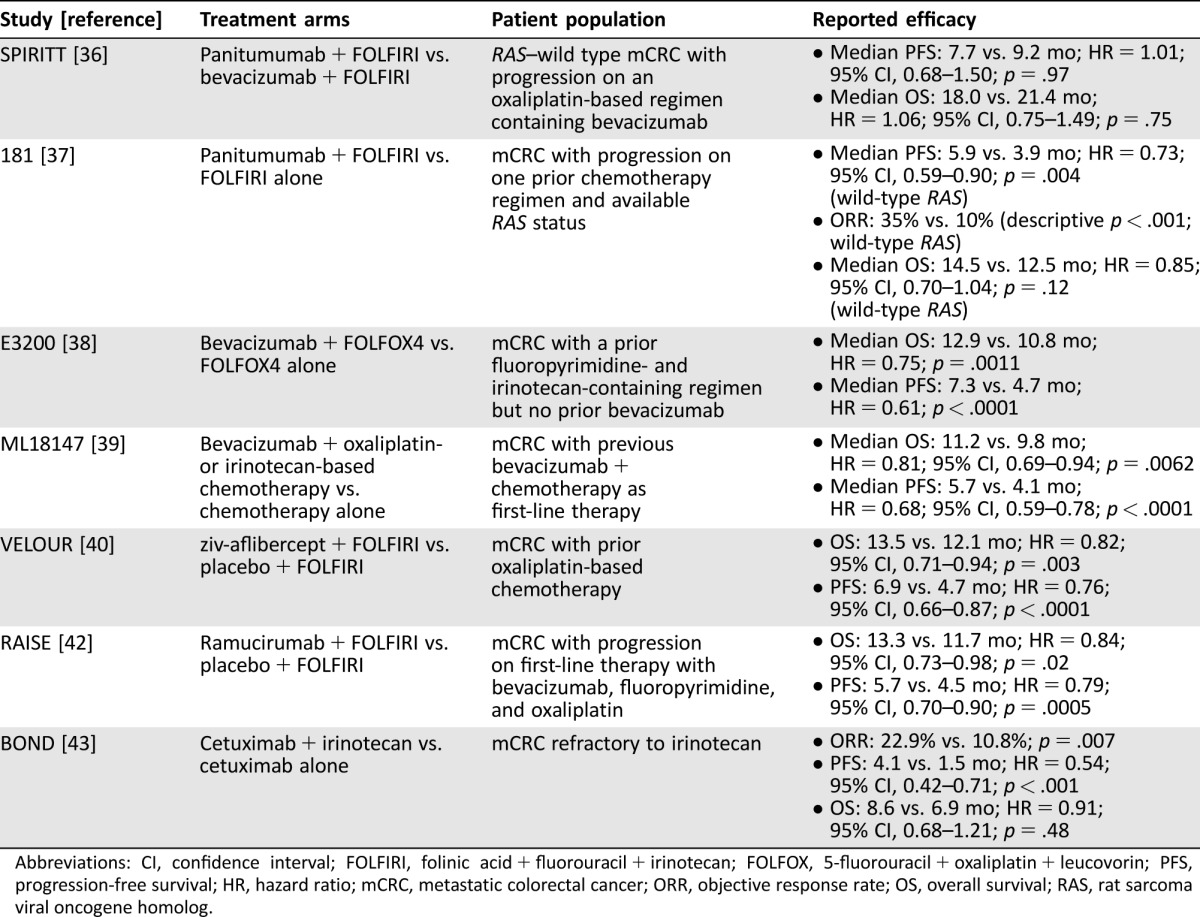
Abbreviations: CI, confidence interval; FOLFIRI, folinic acid + fluorouracil + irinotecan; FOLFOX, 5‐fluorouracil + oxaliplatin + leucovorin; PFS, progression‐free survival; HR, hazard ratio; mCRC, metastatic colorectal cancer; ORR, objective response rate; OS, overall survival; RAS, rat sarcoma viral oncogene homolog.
Based on these studies, the following recommendations for second‐line therapy should be considered: (a) If a patient was previously treated with FOLFOX‐ or XELOX‐based regimen with bevacizumab, then cetuximab/panitumumab (RAS–wild type only), bevacizumab, ramucirumab, or ziv‐aflibercept in combination with FOLFIRI is recommended. (b) If the patient was previously treated with FOLFIRI in combination with bevacizumab, then FOLFOX or XELOX with bevacizumab or with cetuximab/panitumumab (RAS–wild type only) is recommended; alternatively, cetuximab/panitumumab can be combined with FOLFIRI or single‐agent irinotecan. (c) If the patient was previously treated with FOLFOXIRI, then cetuximab/panitumumab with or without irinotecan are recommended for patients with RAS–wild type mCRC [2].
The three approved antiangiogenic agents have somewhat different mechanisms of action. Bevacizumab is a VEGF mAb, ramucirumab is a VEGFR‐2 mAb, and ziv‐aflibercept is a fusion protein that inhibits VEGF and placental growth factor. There may be issues associated with toxicity and/or cost, particularly with ramucirumab, where the monthly cost in combination with FOLFIRI was estimated to be more than twice as high as the cost of bevacizumab or ziv‐aflibercept with FOLFIRI [2], [44]. In addition, ramucirumab and ziv‐aflibercept do not appear to provide additional value over bevacizumab in this setting. Of note, although the cost of bevacizumab may be lower compared with other antiangiogenic agents [44], cost‐effectiveness analysis demonstrated high cost with minimal incremental benefit for bevacizumab when used beyond progression [45]. Given the availability of these three antiangiogenic‐based therapies, we strongly favor the use of bevacizumab over ramucirumab or ziv‐aflibercept as a second‐line antiangiogenic agent in patients with previous exposure to bevacizumab. On the other hand, the choice of anti‐EGFR therapy depends on factors related to the risk of hypersensitivity reactions (influenced by factors such as geographical location [46]) and dosing schedules that may favor panitumumab over cetuximab in some instances.
Given the availability of these three antiangiogenic‐based therapies, we strongly favor the use of bevacizumab over ramucirumab or ziv‐aflibercept as a second‐line antiangiogenic agent in patients with previous exposure to bevacizumab.
Third‐Line Treatment and Beyond for mCRC
For patients with RAS–wild type mCRC who were not exposed to prior anti‐EGFR therapy, cetuximab and panitumumab are recommended in the third‐line setting [47]. In a large, open‐label, phase III trial comparing panitumumab plus BSC versus BSC alone in patients who progressed after standard chemotherapy, all patients had received two prior lines of chemotherapy, and 37% had received three prior lines [48]. Although median PFS was significantly prolonged with panitumumab compared with BSC (8 vs. 7.3 weeks; HR = 0.54 [95% CI, 0.44–0.66]; p < .0001), the difference was modest. No significant difference in OS was observed (HR = 1.0 [95% CI, 0.82–1.22]; p = .81), given that patients receiving BSC were allowed to cross over to panitumumab.
The open‐label, noninferiority, phase III ASPECCT trial compared single‐agent cetuximab with single‐agent panitumumab in patients with chemotherapy‐refractory, RAS–wild type mCRC [49]. Median OS was 10.4 months with panitumumab versus 10.0 months with cetuximab (HR = 0.97 [95% CI, 0.84–1.11]), with a similar toxicity profile.
Other agents that have been recently added to our armamentarium include regorafenib and TAS‐102. Regorafenib, an oral multikinase inhibitor, is recommended for patients who have progressed on 5‐FU‐, oxaliplatin‐, and irinotecan‐containing regimens (and an anti‐EGFR agent if RAS–wild type) [47]. In the pivotal phase III CORRECT trial, patients with mCRC who progressed after all approved standard therapies were treated with BSC plus placebo or regorafenib [50]. The primary endpoint of OS was met, with regorafenib showing a benefit in median OS (6.4 vs. 5.0 months; HR = 0.77 [95% CI, 0.64–0.94]; p = .005) and PFS (1.9 vs. 1.7 months; HR = 0.49 [95% CI, 0.42–0.58]; p < .0001) compared with placebo. A similar phase III trial (CONCUR) examined regorafenib plus BSC or placebo plus BSC in Asian patients who received two or more prior lines of standard therapy or were unable to tolerate standard therapy [51]. Before randomization, 40% of patients had not received any targeted biological treatment. The primary endpoint of OS was again met, with regorafenib showing a benefit in median OS (8.8 vs. 6.3 months; HR = 0.55 [95% CI, 0.40–0.77]; one‐sided p = .00016) and PFS (3.2 vs. 1.7 months; HR = 0.31 [95% CI, 0.22–0.44]; one‐sided p < .0001) compared with placebo. Given some of the dose‐limiting toxicities associated with regorafenib and the variability in dosing, an ongoing phase II study (ReDOS; NCT02368886) is investigating optimal dosing of regorafenib with dose‐escalation versus standard‐dose strategies.
TAS‐102 (trifluridine/tipiracil), a more traditional cytotoxic therapy that acts as a thymidine nucleoside analog, was recently approved for treatment of patients with mCRC who have progressed on all standard therapies, including regorafenib [2]. In a pivotal phase III trial (RECOURSE), patients receiving TAS‐102 had a significant improvement in median OS (7.1 vs. 5.3 months; HR = 0.68 [95% CI, 0.58–0.81]; p < .001) and PFS (2.0 vs. 1.7 months; HR = 0.48 [95% CI, 0.41–0.57]; p < .001) versus placebo [52]. The choice to use regorafenib or TAS‐102 first in patients with treatment‐refractory mCRC may be guided by patient characteristics such as performance status, comorbidities, and tolerability of prior therapies. It has been suggested, for example, that regorafenib would be more appropriate for patients with pancytopenias from prior chemotherapy, whereas patients with hand‐foot skin syndrome would be better suited for TAS‐102 [53].
The Promise of Immunotherapy in mCRC
Immune checkpoint inhibitors represent a new, exciting treatment strategy that stimulates the host's immune response to malignant cells [54]. Pembrolizumab, a programmed death receptor‐1 (PD‐1) mAb, is one of several available immune checkpoint inhibitors and is approved for multiple indications in other tumor types [55]. A recent phase II study of pembrolizumab in heavily pretreated patients with either mismatch repair (MMR)–deficient or MMR‐proficient mCRC suggested a notable difference in ORR (50% vs. 0%) [56] and PFS rate (78% vs. 11%) between the groups [57]. Although median PFS and OS have not yet been reached for MMR‐deficient patients, the median PFS was 2.4 months (HR = 0.135 [95% CI, 0.043–0.191]; p < .0001), and OS was 6.0 months (HR = 0.247 [95% CI, 0.117–0.589]; p = .001) for MMR‐proficient patients [56]. Although preliminary, these results suggest patients with pretreated MMR‐deficient mCRC, who typically have high mutational loads in microsatellite regions (microsatellite instability–high [MSI‐H]), benefit from immune checkpoint inhibition. Further, analyses of the KEYNOTE 164 and 158 studies have demonstrated an ORR of 26.2% and a DCR of 50.8% with pembrolizumab in MSI‐H CRC, although efficacy with pembrolizumab has not yet been established in patients with MMR‐proficient/microsatellite‐stable mCRC [57], [58]. An ongoing phase III study (KEYNOTE‐177) is examining first‐line pembrolizumab versus chemotherapy in patients with MMR‐deficient or MSI‐H advanced CRC (NCT02563002).
The ongoing phase II CheckMate 142 study is evaluating the PD‐1 mAb nivolumab with or without ipilimumab, an mAb targeting cytotoxic T‐lymphocyte–associated protein 4, in MMR‐deficient mCRC or MSI‐H mCRC (NCT02060188). Preliminary results demonstrated an ORR and DCR of 31% and 69%, respectively, with nivolumab, and an ORR and DCR of 41% and 78%, respectively, with the combination of nivolumab plus ipilimumab [59], [60]. The median time to response for both regimens was 2.7 months, and the safety profiles of the two regimens were manageable.
Preclinical studies have demonstrated that mitogen‐activated protein kinase kinase (MEK) inhibition leads to increased expression of major histocompatibility complex molecules and PD‐1 ligand (PD‐L1) on tumor cells, and dual inhibition of MEK and PD‐L1 in vitro had more antitumor activity than either agent alone [61]. Based on these findings, a phase Ib study of atezolizumab, an engineered mAb that inhibits binding of PD‐L1 to its receptors, in combination with cobimetinib, an MEK inhibitor, was undertaken [62]. In 23 patients with microsatellite‐stable mCRC, ORR was 17% (four patients with partial responses, five patients with stable disease). Three responses were ongoing (range, 4.0–7.7 months at time of data cutoff). At the maximum administered doses (atezolizumab 800 mg intravenously every 2 weeks plus cobimetinib 60 mg per day [21 days on and 7 days off]), combination therapy was well tolerated. Based on these promising results, patients are currently being recruited for a phase III study to investigate the efficacy and safety of atezolizumab and atezolizumab plus cobimetinib versus regorafenib in patients with mCRC (NCT02788279).
Future Directions
Recent advances in genomic profiling have led to the identification of patient subgroups that may respond to specific targeted therapies. A study by The Cancer Genome Atlas (TCGA) Network performed a genome‐scale analysis of 224 CRC tumor and normal tissue pair samples and found 24 genes that are commonly mutated in CRC [63]. Nearly all malignant samples had deregulated WNT signaling pathways. There were newly identified recurrent mutations including FAM123B, ARID1A, and SOX9, all of which are involved in oncogenic signaling pathways. In a separate study, gene expression profiling of 1,290 CRC tumors with consensus‐based unsupervised clustering led to the proposal of a CRC classification system consisting of six subtypes based on subtype‐specific gene signatures and differential response to cetuximab [64]. Differences in disease‐free survival were observed between these subtypes following surgical resection, treatment with cetuximab, and treatment with FOLFIRI, with these subtypes shown to be associated with distinctive anatomical regions within the colon crypts. Additionally, candidate biomarkers were identified to help classify CRC samples into proposed subtypes that can be matched to subtype‐guided therapeutic strategies.
Additional comprehensive genomic profiling using DNA extracted from formalin‐fixed, paraffin‐embedded tissue sections from patients with advanced CRC has been used to identify clinically relevant genomic alterations [65]. In one study, the most frequently reported genomic alterations included those in APC (76%), TP53 (75%), and KRAS (53%) [65], at rates higher than those reported by the TCGA [63]. The investigators reported 100% concordance between comprehensive genomic profiling and standard hot‐spot sequencing assays, and additional genomic alterations included phosphatidylinositol‐4,5‐bisphosphate 3‐kinase catalytic subunit alpha (PIK3CA; 18%), phosphatase and tensin homolog (PTEN; 8%), erb‐b2 receptor tyrosine kinase 2 (ERBB2; 5%), SRC (4%), SMAD family member 2 (SMAD2; 3%), neurofibromin 1 (NF1; 3%), EGFR (3%), ERBB3 (2%), met proto‐oncogene, receptor tyrosine kinase (MET; 1%), and KIT (1%). Moreover, a recent large‐scale genomic analysis of >15,000 patients across 50 tumor types, including CRC, showed that patterns of genetic changes detected in blood samples via liquid biopsy were similar to those identified using traditional tumor biopsy [66], suggesting liquid biopsy may provide a highly informative, minimally invasive alternative to tissue biopsy.
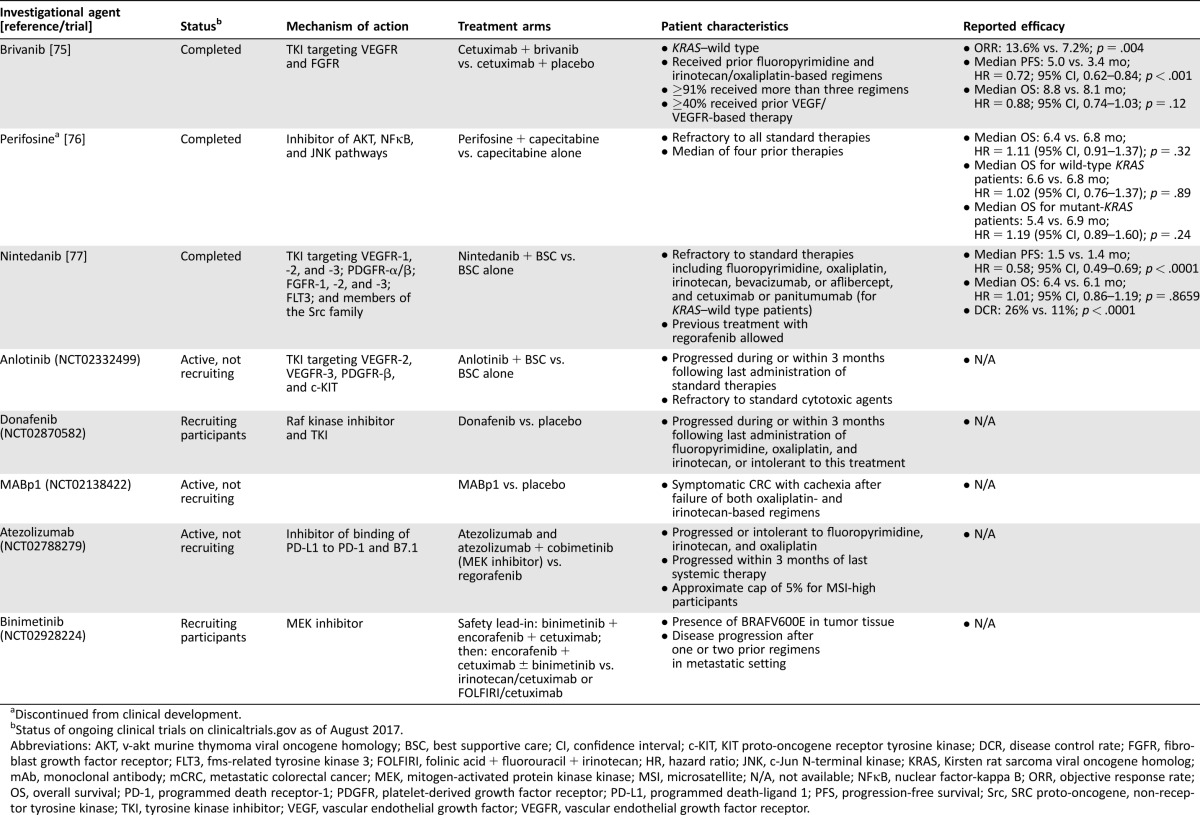
Due to the limited number of treatment options for patients with mCRC refractory to available standard therapies, several investigational agents are undergoing development for this indication (Table 3). Several of these agents are multi‐targeted, including brivanib, a small‐molecule tyrosine kinase inhibitor (TKI) of VEGFR and fibroblast growth factor receptor (FGFR), perifosine, a synthetic alkylphospholipid that targets the v‐akt murine thymoma viral oncogene homology, nuclear factor‐kappa B, and c‐Jun N‐terminal kinase pathways, anlotinib, a TKI that inhibits VEGFR‐2, VEGFR‐3, platelet‐derived growth factor receptor (PDGFR)‐β, and KIT proto‐oncogene receptor tyrosine kinase, and nintedanib, a triple angiokinase inhibitor of VEGFR‐1, ‐2, and ‐3; PDGFR‐α/β; FGFR‐1, ‐2, and ‐3; fms‐related tyrosine kinase 3; and members of the Src family [67]. Additional agents in development for treatment‐refractory mCRC include the Raf kinase inhibitor donafenib and the anti‐interleukin‐1α human mAb MABp1, which interrupts angiogenesis and other inflammatory processes that promote the malignant phenotype (Table 3) [68]. For patients with BRAF‐mutant mCRC, which carries a poor prognosis [69], the MEK inhibitor binimetinib is being evaluated in combination with the BRAF inhibitor encorafenib and cetuximab versus cetuximab and irinotecan‐based therapy (Table 3).
Table 3. Ongoing and completed phase III studies for refractory mCRC.
Discontinued from clinical development.
Status of ongoing clinical trials on clinicaltrials.gov as of August 2017.
Abbreviations: AKT, v‐akt murine thymoma viral oncogene homology; BSC, best supportive care; CI, confidence interval; c‐KIT, KIT proto‐oncogene receptor tyrosine kinase; DCR, disease control rate; FGFR, fibroblast growth factor receptor; FLT3, fms‐related tyrosine kinase 3; FOLFIRI, folinic acid + fluorouracil + irinotecan; HR, hazard ratio; JNK, c‐Jun N‐terminal kinase; KRAS, Kirsten rat sarcoma viral oncogene homolog; mAb, monoclonal antibody; mCRC, metastatic colorectal cancer; MEK, mitogen‐activated protein kinase kinase; MSI, microsatellite; N/A, not available; NFκB, nuclear factor‐kappa B; ORR, objective response rate; OS, overall survival; PD‐1, programmed death receptor‐1; PDGFR, platelet‐derived growth factor receptor; PD‐L1, programmed death‐ligand 1; PFS, progression‐free survival; Src, SRC proto‐oncogene, non‐receptor tyrosine kinase; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Conclusion
The outcomes of patients with mCRC have steadily improved over the past 2 decades due to the availability of an increasing armamentarium of cytotoxic and targeted agents. A number of promising targeted and immunotherapeutic strategies in rationally selected patients are underway. Currently, multiple sequencing strategies are available. In patients with RAS–wild type mCRC, there does not appear to be a clear favorite biologic in the first‐line setting [8], [14]. A recent analysis suggests primary tumor–sidedness may help with the biologic selection, although further molecular characterization is needed before widespread recommendations can be made [9], [21], [22]. Ongoing studies will undoubtedly contribute to the evolving mCRC treatment landscape.
Acknowledgments
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors. The authors received no direct compensation related to the development of the manuscript. Writing and editorial support were provided by Lauren Fink, Ph.D., of MedErgy, which was contracted and funded by Boehringer Ingelheim Pharmaceuticals, Inc. Boehringer Ingelheim Pharmaceuticals, Inc. was given the opportunity to review the manuscript for medical and scientific accuracy, as well as intellectual property considerations.
Author Contributions
Conception/design: Kristen K. Ciombor, Tanios Bekaii‐Saab
Collection and/or assembly of data: Kristen K. Ciombor, Tanios Bekaii‐Saab
Data analysis and interpretation: Kristen K. Ciombor, Tanios Bekaii‐Saab
Manuscript writing: Kristen K. Ciombor, Tanios Bekaii‐Saab
Final approval of manuscript: Kristen K. Ciombor, Tanios Bekaii‐Saab
Disclosures
Tanios Bekaii‐Saab: Amgen, Bayer, Boehringer Ingelheim, Eli Lilly, Genentech, Regeneron, Taiho (C/A). The other author indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.American Cancer Society . Cancer Facts & Figures. 2016. Accessed April 1, 2017. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2016.html
- 2.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Colon Cancer. Version 2.2017. Accessed April 1, 2017. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 3. Tournigand C, Andre T, Achille E et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J Clin Oncol 2004;22:229–237. [DOI] [PubMed] [Google Scholar]
- 4. Ducreux M, Malka D, Mendiboure J et al. Sequential versus combination chemotherapy for the treatment of advanced colorectal cancer (FFCD 2000‐05): An open‐label, randomised, phase 3 trial. Lancet Oncol 2011;12:1032–1044. [DOI] [PubMed] [Google Scholar]
- 5. Koopman M, Antonini NF, Douma J et al. Sequential versus combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer (CAIRO): A phase III randomised controlled trial. Lancet 2007;370:135–142. [DOI] [PubMed] [Google Scholar]
- 6. Seymour MT, Maughan TS, Ledermann JA et al. Different strategies of sequential and combination chemotherapy for patients with poor prognosis advanced colorectal cancer (MRC FOCUS): A randomised controlled trial. Lancet 2007;370:143–152. [DOI] [PubMed] [Google Scholar]
- 7. Van Cutsem E, Cervantes A, Nordlinger B et al. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2014;25(suppl 3):iii1–iii9. [DOI] [PubMed] [Google Scholar]
- 8. Heinemann V, von Weikersthal LF, Decker T et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first‐line treatment for patients with metastatic colorectal cancer (FIRE‐3): A randomised, open‐label, phase 3 trial. Lancet Oncol 2014;15:1065–1075. [DOI] [PubMed] [Google Scholar]
- 9. Venook A, Niedzwiecki D, Innocenti F. Impact of primary tumor location on overall survival and progression free survival in patients with metastatic colorectal cancer: Analysis of CALGB/SWOG 80405 (Alliance). Oral presentation at: the 2016 American Society of Clinical Oncology (ASCO) Annual Meeting; June 3–7, 2016; Chicago, IL.
- 10. Hurwitz H, Fehrenbacher L, Novotny W et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–2342. [DOI] [PubMed] [Google Scholar]
- 11. Van Cutsem E, Kohne CH, Hitre E et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009;360:1408–1417. [DOI] [PubMed] [Google Scholar]
- 12. Van Cutsem E, Kohne CH, Lang I et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first‐line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 2011;29:2011–2019. [DOI] [PubMed] [Google Scholar]
- 13. Douillard JY, Siena S, Cassidy J et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first‐line treatment in patients with previously untreated metastatic colorectal cancer: The PRIME study. J Clin Oncol 2010;28:4697–4705. [DOI] [PubMed] [Google Scholar]
- 14. Venook AP, Niedzwiecki D, Lenz HJ et al. CALGB/SWOG 80405: Phase III trial of irinotecan/5‐FU/leucovorin (FOLFIRI) or oxaliplatin/5‐FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild‐type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). J Clin Oncol 2014;32(suppl 5s):LBA3a. [Google Scholar]
- 15. Modest DP, Stintzing S, von Weikersthal LF et al. Impact of subsequent therapies on outcome of the FIRE‐3/AIO KRK0306 trial: First‐line therapy with FOLFIRI plus cetuximab or bevacizumab in patients with KRAS wild‐type tumors in metastatic colorectal cancer. J Clin Oncol 2015;33:3718–3726. [DOI] [PubMed] [Google Scholar]
- 16. Maughan TS, Adams RA, Smith CG et al. Addition of cetuximab to oxaliplatin‐based first‐line combination chemotherapy for treatment of advanced colorectal cancer: Results of the randomised phase 3 MRC COIN trial. Lancet 2011;377:2103–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holdhoff M, Schmidt K, Diehl F et al. Detection of tumor DNA at the margins of colorectal cancer liver metastasis. Clin Cancer Res 2011;17:3551–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Formica V, Roselli M. Targeted therapy in first line treatment of RAS wild type colorectal cancer. World J Gastroenterol 2015;21:2871–2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stintzing S, Modest DP, Rossius L et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE‐3): A post‐hoc analysis of tumour dynamics in the final RAS wild‐type subgroup of this randomised open‐label phase 3 trial. Lancet Oncol 2016;17:1426–1434. [DOI] [PubMed] [Google Scholar]
- 20. O'Neil BH, Venook AP. Trying to understand differing results of FIRE‐3 and 80405: Does the first treatment matter more than others? J Clin Oncol 2015;33:3686–3688. [DOI] [PubMed] [Google Scholar]
- 21. Heinemann V, Modest DP, von Weikersthal LF. Gender and tumor location as predictors for efficacy: Influence of endpoints in first‐line treatment with FOLFIRI in combination with cetuximab or bevacizumab in the AIO KRK 0306 (FIRE3) trial. J Clin Oncol 2014;32(suppl 5s):3600a. 25135994 [Google Scholar]
- 22. Tejpar S, Stintzing S, Ciardiello F et al. Prognostic and predictive relevance of primary tumor location in patients with RAS wild‐type metastatic colorectal cancer: Retrospective analyses of the CRYSTAL and FIRE‐3 trials. JAMA Oncol 2016. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brule SY, Jonker DJ, Karapetis CS et al. Location of colon cancer (right‐sided versus left‐sided) as a prognostic factor and a predictor of benefit from cetuximab in NCIC CO.17. Eur J Cancer 2015;51:1405–1414. [DOI] [PubMed] [Google Scholar]
- 24. Schrag D, Weng S, Brooks G et al. The relationship between primary tumor sidedness and prognosis in colorectal cancer. J Clin Oncol 2016;34(suppl):3505a. [Google Scholar]
- 25. Lee MS, Advani SM, Morris J. Association of primary (1°) site and molecular features with progression‐free survival (PFS) and overall survival (OS) of metastatic colorectal cancer (mCRC) after anti‐epidermal growth factor receptor (áEGFR) therapy. J Clin Oncol 2016;34(suppl):3506a. [Google Scholar]
- 26. Douillard JY, Siena S, Cassidy J et al. Final results from PRIME: Randomized phase III study of panitumumab with FOLFOX4 for first‐line treatment of metastatic colorectal cancer. Ann Oncol 2014;25:1346–1355. [DOI] [PubMed] [Google Scholar]
- 27. Boeckx N, Koukakis R, Op de Beeck K et al. Primary tumor sidedness has an impact on prognosis and treatment outcome in metastatic colorectal cancer: Results from two randomized first‐line panitumumab studies. Ann Oncol 2017;28:1862–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lenz HJ, Lee FC, Yau L et al. MAVERICC, a phase 2 study of mFOLFOX6‐bevacizumab (BV) vs FOLFIRI‐BV with biomarker stratification as first‐line (1L) chemotherapy (CT) in patients (pts) with metastatic colorectal cancer (mCRC). J Clin Oncol 2016;34(suppl 4s):3515a. [Google Scholar]
- 29. Bendell JC, Tan BR, Reeves JA et al. Overall response rate (ORR) in STEAM, a randomized, open‐label, phase 2 trial of sequential and concurrent FOLFOXIRI‐bevacizumab (BEV) vs FOLFOX‐BEV for the first‐line (1L) treatment (tx) of patients (pts) with metastatic colorectal cancer (mCRC). J Clin Oncol 2016;34(suppl 4s):492a. [Google Scholar]
- 30. Pericay C, Folprecht G, Saunders M et al. Phase 2 randomized, noncomparative, open‐label study of aflibercept and modified FOLFOX6 in the first‐line treatment of metastatic colorectal cancer (AFFIRM). Ann Oncol 2012;23(suppl 4):iv16. [Google Scholar]
- 31. Folprecht G, Pericay C, Saunders MP et al. Oxaliplatin and 5‐FU/folinic acid (modified FOLFOX6) with or without aflibercept in first‐line treatment of patients with metastatic colorectal cancer: The AFFIRM study. Ann Oncol 2016;27:1273–1279. [DOI] [PubMed] [Google Scholar]
- 32. Argilés G, Saunders MP, Rivera F et al. Regorafenib plus modified FOLFOX6 as first‐line treatment of metastatic colorectal cancer: A phase II trial. Eur J Cancer 2015;51:942–949. [DOI] [PubMed] [Google Scholar]
- 33. Garcia‐Carbonero R, Rivera F, Maurel J et al. An open‐label phase II study evaluating the safety and efficacy of ramucirumab combined with mFOLFOX‐6 as first‐line therapy for metastatic colorectal cancer. The Oncologist 2014;19:350–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.ERBITUX (cetuximab) injection, for intravenous infusion [package insert]. Branchburg, NJ: ImClone LLC; 2016. [Google Scholar]
- 35.VECTIBIX (panitumumab) injection for intravenous infusion [package insert]. Thousand Oaks, CA: Amgen, Inc.; 2015.
- 36. Hecht JR, Cohn A, Dakhil S et al. SPIRITT: A randomized, multicenter, phase II study of panitumumab with FOLFIRI and bevacizumab with FOLFIRI as second‐line treatment in patients with unresectable wild type KRAS metastatic colorectal cancer. Clin Colorectal Cancer 2015;14:72–80. [DOI] [PubMed] [Google Scholar]
- 37. Peeters M, Price TJ, Cervantes A et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second‐line treatment in patients with metastatic colorectal cancer. J Clin Oncol 2010;28:4706–4713. [DOI] [PubMed] [Google Scholar]
- 38. Giantonio BJ, Catalano PJ, Meropol NJ et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 2007;25:1539–1544. [DOI] [PubMed] [Google Scholar]
- 39. Bennouna J, Sastre J, Arnold D et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): A randomised phase 3 trial. Lancet Oncol 2013;14:29–37. [DOI] [PubMed] [Google Scholar]
- 40. Van Cutsem E, Tabernero J, Lakomy R et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin‐based regimen. J Clin Oncol 2012;30:3499–3506. [DOI] [PubMed] [Google Scholar]
- 41. Tabernero J, Van Cutsem E, Lakomy R et al. Aflibercept versus placebo in combination with fluorouracil, leucovorin and irinotecan in the treatment of previously treated metastatic colorectal cancer: Prespecified subgroup analyses from the VELOUR trial. Eur J Cancer 2014;50:320–331. [DOI] [PubMed] [Google Scholar]
- 42. Tabernero J, Yoshino T, Cohn AL et al. Ramucirumab versus placebo in combination with second‐line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first‐line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): A randomised, double‐blind, multicentre, phase 3 study. Lancet Oncol 2015;16:499–508. [DOI] [PubMed] [Google Scholar]
- 43. Cunningham D, Humblet Y, Siena S et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan‐refractory metastatic colorectal cancer. N Engl J Med 2004;351:337–345. [DOI] [PubMed] [Google Scholar]
- 44. Goldstein DA, El‐Rayes BF. Considering efficacy and cost, where does ramucirumab fit in the management of metastatic colorectal cancer? The Oncologist 2015;20:981–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Goldstein DA, Chen Q, Ayer T et al. First‐ and second‐line bevacizumab in addition to chemotherapy for metastatic colorectal cancer: A United States‐based cost‐effectiveness analysis. J Clin Oncol 2015;33:1112–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. O'Neil BH, Allen R, Spigel DR et al. High incidence of cetuximab‐related infusion reactions in Tennessee and North Carolina and the association with atopic history. J Clin Oncol 2007;25:3644–3648. [DOI] [PubMed] [Google Scholar]
- 47. Foubert F, Matysiak‐Budnik T, Touchefeu Y. Options for metastatic colorectal cancer beyond the second line of treatment. Dig Liver Dis 2014;46:105–112. [DOI] [PubMed] [Google Scholar]
- 48. Van Cutsem E, Peeters M, Siena S et al. Open‐label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy‐refractory metastatic colorectal cancer. J Clin Oncol 2007;25:1658–1664. [DOI] [PubMed] [Google Scholar]
- 49. Price TJ, Peeters M, Kim TW et al. Panitumumab versus cetuximab in patients with chemotherapy‐refractory wild‐type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open‐label, non‐inferiority phase 3 study. Lancet Oncol 2014;15:569–579. [DOI] [PubMed] [Google Scholar]
- 50. Grothey A, Van Cutsem E, Sobrero A et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo‐controlled, phase 3 trial. Lancet 2013;381:303–312. [DOI] [PubMed] [Google Scholar]
- 51. Li J, Qin S, Xu R et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): A randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Oncol 2015;16:619–629. [DOI] [PubMed] [Google Scholar]
- 52. Mayer RJ, Van Cutsem E, Falcone A et al. Randomized trial of TAS‐102 for refractory metastatic colorectal cancer. N Engl J Med 2015;372:1909–1919. [DOI] [PubMed] [Google Scholar]
- 53. Grothey A, Marshall JL, Seery TE. Current options for third‐line treatment of metastatic colorectal cancer. Clin Adv Hematol Oncol 2016;14(suppl 3):1–15. [PubMed] [Google Scholar]
- 54. Barbee MS, Ogunniyi A, Horvat TZ et al. Current status and future directions of the immune checkpoint inhibitors ipilimumab, pembrolizumab, and nivolumab in oncology. Ann Pharmacother 2015;49:907–937. [DOI] [PubMed] [Google Scholar]
- 55.KEYTRUDA (pembrolizumab) for injection, for intravenous use [package insert]. Whitehouse Station, NJ: Merck & Co, Inc; 2016. [Google Scholar]
- 56.Le DT, Uram JN, Wang H. Programmed death‐1 blockade in mismatch repair deficient colorectal cancer. J Clin Oncol 2016;34(suppl 15):103a. 26628472 [Google Scholar]
- 57. Le DT, Uram JN, Wang H et al. PD‐1 blockade in tumors with mismatch‐repair deficiency. N Engl J Med 2015;372:2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Diaz LA, Marabelle A, Delord JP et al. Pembrolizumab therapy for microsatellite instability high (MSI‐H) colorectal cancer (CRC) and non‐CRC. J Clin Oncol 2017;35(suppl 15):3071a. [Google Scholar]
- 59. Overman MJ, Lonardi S, Leone F et al. Nivolumab in patients with DNA mismatch repair deficient/microsatellite instability high metastatic colorectal cancer: Update from CheckMate 142. J Clin Oncol 2017;35(suppl 4):519a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Andre T, Lonardi S, Wong KY et al. Combination of nivolumab (nivo) + ipilimumab (ipi) in the treatment of patients (pts) with deficient DNA mismatch repair (dMMR)/high microsatellite instability (MSIH) metastatic colorectal cancer (mCRC): CheckMate 142 study. J Clin Oncol 2017;35(suppl 15):3531a. [Google Scholar]
- 61. Loi S, Dushyanthen S, Beavis PA et al. RAS/MAPK activation is associated with reduced tumor‐infiltrating lymphocytes in triple‐negative breast cancer: Therapeutic cooperation between MEK and PD‐1/PD‐L1 immune checkpoint inhibitors. Clin Cancer Res 2016;22:1499–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bendell JC, Kim TW, Goh BC, et al. Clinical activity and safety of cobimetinib (cobi) and atezolizumab in colorectal cancer (CRC). J Clin Oncol 2016;34(suppl 15):3502a. 27458302 [Google Scholar]
- 63. Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012;487:330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sadanandam A, Lyssiotis CA, Homicsko K et al. A colorectal cancer classification system that associates cellular phenotype and responses to therapy. Nat Med 2013;19:619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ross JS, Wang K, Khaira D et al. Comprehensive genomic profiling of clinically advanced colorectal carcinoma to reveal frequent opportunities for targeted therapies. J Clin Oncol 2015;33(suppl 15):3553a. [Google Scholar]
- 66. Zill OA, Mortimer S, Banks KC. Somatic genomic landscape of over 15,000 patients with advanced‐stage cancer from clinical next‐generation sequencing analysis of circulating tumor DNA. J Clin Oncol 2016;34(suppl 18):LBA11501a. [Google Scholar]
- 67. Hilberg F, Roth GJ, Krssak M et al. BIBF 1120: Triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res 2008;68:4774–4782. [DOI] [PubMed] [Google Scholar]
- 68. Hong DS, Hui D, Bruera E et al. MABp1, a first‐in‐class true human antibody targeting interleukin‐1alpha in refractory cancers: An open‐label, phase 1 dose‐escalation and expansion study. Lancet Oncol 2014;15:656–666. [DOI] [PubMed] [Google Scholar]
- 69. Scartozzi M, Giampieri R, Aprile G et al. The distinctive molecular, pathological and clinical characteristics of BRAF‐mutant colorectal tumors. Expert Rev Mol Diagn 2015;15:979–987. [DOI] [PubMed] [Google Scholar]
- 70.AVASTIN (bevacizumab) solution for intravenous infusion [package insert]. South San Francisco, CA: Genentech, Inc; 2015. [Google Scholar]
- 71.ZALTRAP (ziv‐aflibercept) injection for intravenous infusion [package insert]. Bridgewater, NJ: Sanofi‐Aventis U.S. LLC; 2016. [Google Scholar]
- 72. Wilhelm SM, Dumas J, Adnane L et al. Regorafenib (BAY 73‐4506): A new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 2011;129:245–255. [DOI] [PubMed] [Google Scholar]
- 73.STIVARGA (regorafenib) tablets, oral [package insert]. Wayne, NJ: Bayer, Inc; 2016. [Google Scholar]
- 74.CYRAMZA (ramucirumab) injection, for intravenous use [package insert]. Indianapolis, IN: Eli Lilly and Company; 2015.
- 75. Siu LL, Shapiro JD, Jonker DJ et al. Phase III randomized, placebo‐controlled study of cetuximab plus brivanib alaninate versus cetuximab plus placebo in patients with metastatic, chemotherapy‐refractory, wild‐type K‐RAS colorectal carcinoma: The NCIC Clinical Trials Group and AGITG CO.20 Trial. J Clin Oncol 2013;31:2477–2484. [DOI] [PubMed] [Google Scholar]
- 76. Bendell JC, Ervin TJ, Senzer NN et al. Results of the X‐PECT study: A phase III randomized double‐blind, placebo‐controlled study of perifosine plus capecitabine (P‐CAP) versus placebo plus capecitabine (CAP) in patients (pts) with refractory metastatic colorectal cancer (mCRC). J Clin Oncol 2012;30(suppl 18):LBA3501a. [Google Scholar]
- 77. Van Cutsem E, Yoshino T, Lenz HJ et al. Nintedanib plus best supportive care (BSC) versus placebo plus BSC for the treatment of patients (pts) with colorectal cancer (CRC) refractory to standard therapies: Results of the phase III LUME‐colon 1 study. Ann Oncol 2016;27(suppl 6):vi552–vi587. [Google Scholar]