An expert panel was convened to provide specific, expert consensus guidelines for the use of glucarpidase in patients who develop high‐dose methotrexate (HDMTX)‐induced nephrotoxicity and delayed methotrexate excretion. This guideline provides recommendations to identify the population of patients who would benefit from glucarpidase rescue by more precisely defining the absolute methotrexate concentrations associated with risk for severe or life‐threatening toxicity at several time points after the start of a HDMTX infusion.
Keywords: Methotrexate, Acute kidney injury, Creatinine, Glucarpidase, Leucovorin
Abstract
Acute kidney injury due to high‐dose methotrexate (HDMTX) is a serious, life‐threatening toxicity that can occur in pediatric and adult patients. Glucarpidase is a treatment approved by the Food and Drug Administration for high methotrexate concentrations in the context of kidney dysfunction, but the guidelines for when to use it are unclear. An expert panel was convened to provide specific, expert consensus guidelines for the use of glucarpidase in patients who develop HDMTX‐induced nephrotoxicity and delayed methotrexate excretion. The guideline provides recommendations to identify the population of patients who would benefit from glucarpidase rescue by more precisely defining the absolute methotrexate concentrations associated with risk for severe or life‐threatening toxicity at several time points after the start of an HDMTX infusion. For an HDMTX infusion ≤24 hours, if the 36‐hour concentration is above 30 µM, 42‐hour concentration is above 10 µM, or 48‐hour concentration is above 5 µM and the serum creatinine is significantly elevated relative to the baseline measurement (indicative of HDMTX‐induced acute kidney injury), glucarpidase may be indicated. After a 36‐ to 42‐hour HDMTX infusion, glucarpidase may be indicated when the 48‐hour methotrexate concentration is above 5 µM. Administration of glucarpidase should optimally occur within 48–60 hours from the start of the HDMTX infusion, because life‐threatening toxicities may not be preventable beyond this time point.
Implications for Practice.
Glucarpidase is a rarely used medication that is less effective when given after more than 60 hours of exposure to high‐dose methotrexate, so predicting early which patients will need it is imperative. There are no currently available consensus guidelines for the use of this medication. The indication on the label does not give specific methotrexate concentrations above which it should be used. An international group of experts was convened to develop a consensus guideline that was specific and evidence‐based to identify the population of patients who would benefit from glucarpidase.
Introduction
Nephrotoxicity induced by high‐dose methotrexate (HDMTX) is a medical emergency because the renal excretion of methotrexate (MTX) is subsequently delayed, and the resulting prolonged exposure to high concentrations of the drug can cause severe and life‐threatening toxicity. The recombinant bacterial enzyme glucarpidase, which is approved by the Food and Drug Administration (FDA) for use in patients with delayed MTX excretion, is a rescue agent that cleaves MTX into inactive metabolites, providing an alternative route of elimination for the drug in patients with nephrotoxicity [1], [2], [3], [4], [5], [6]. A task force was assembled to develop specific, evidence‐based guidelines for the use of glucarpidase in patients who develop HDMTX‐induced nephrotoxicity and delayed MTX excretion, and the recommendations of this working group are reported herein.
High‐Dose Methotrexate Pharmacology
Methotrexate is an antifolate that can be administered over a broad dose range (from 20 to 33,600 mg/m2) [7] but is only tolerable at higher doses when followed by the rescue agent leucovorin (5‐formyltetrahydrofolate). HDMTX is commonly regarded as doses exceeding 500 mg/m2 infused over 2–36 hours, requiring supportive care (e.g., hyperhydration) and leucovorin rescue. Doses and infusion times vary by indication (Table 1).
Table 1. Common high‐dose MTX regimens.
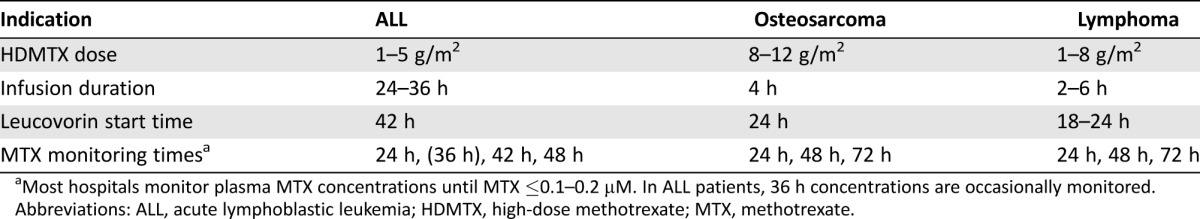
Most hospitals monitor plasma MTX concentrations until MTX ≤0.1–0.2 µM. In ALL patients, 36 h concentrations are occasionally monitored.
Abbreviations: ALL, acute lymphoblastic leukemia; HDMTX, high‐dose methotrexate; MTX, methotrexate.
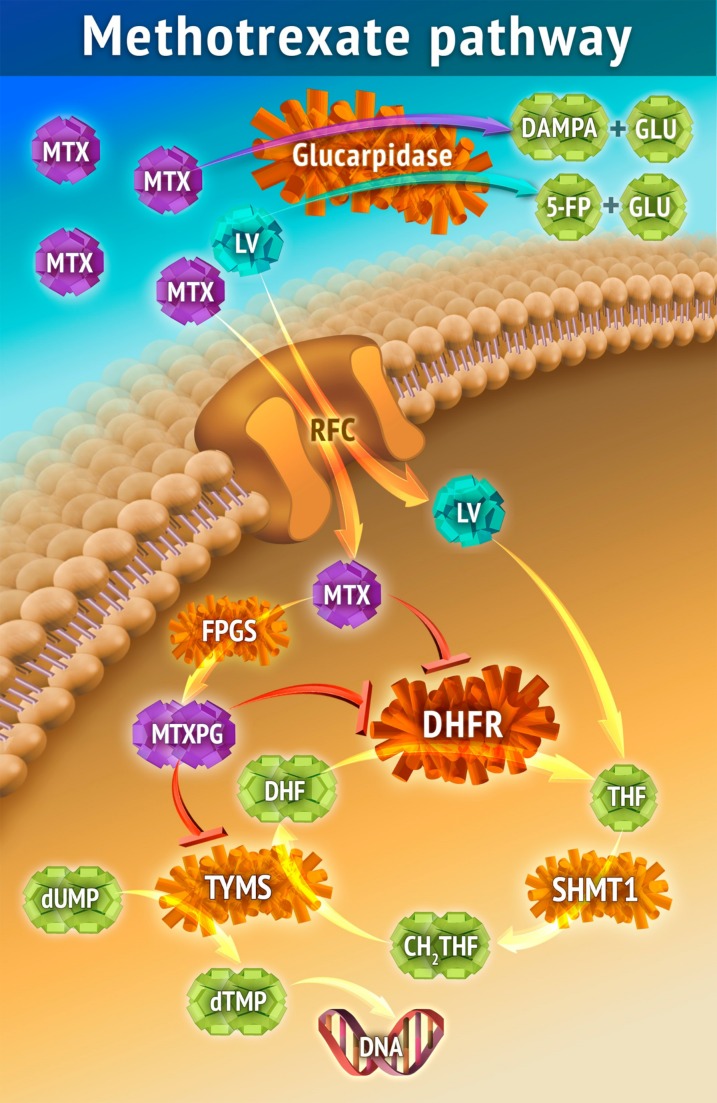
MTX is a competitive inhibitor of the enzyme dihydrofolate reductase (DHFR; Fig. 1) and blocks the conversion of dihydrofolate to its active, chemically reduced tetrahydrofolate form, thus depleting the intracellular pool of tetrahydrofolates, which are required cofactors (single‐carbon donors) for the synthesis of methionine, thymidine, and purines. MTX is polyglutamated intracellularly, and in this form (MTXPG) can also directly inhibit enzymes in the purine/pyrimidine synthetic pathways. Thymidylate synthase is the only folate‐requiring enzyme that oxidizes its tetrahydrofolate cofactor to dihydrofolate, and active synthesis of thymidine is necessary for MTX to deplete intracellular tetrahydrofolates.
Figure 1.
The cellular entry of MTX is mediated primarily by the ubiquitously expressed RFC, encoded by the human solute carrier family 19 member 1 (SLC19A1) gene on chromosome 21. Other influx and efflux mechanisms include ABC transporters (specifically ABCC1–4) and breast cancer resistance protein [79], but their role in MTX clearance and efficacy is less well documented. Passive diffusion plays a significant role during high‐dose MTX (HDMTX) therapy. Most of the MTX that enters the liver re‐enters the blood circulation by enterohepatic circulation via the ABCC transporters, and only a small portion is excreted into the bile. MTX interferes with the natural folate‐homocysteine cycle and inhibits folate‐dependent enzymes and pathways, including DHFR, TYMS, 5,10‐methylene‐tetrahydrofolate reductase, and purine de novo synthesis. This leads to a lack of reduced folate, inhibition of DNA synthesis, increased homocysteine and adenosine concentrations, and potentially life‐threatening toxicities [32], [80], [81]. Leucovorin (folinic acid) is essential for cellular rescue after HDMTX, and insufficient intracellular concentrations of leucovorin after HDMTX may cause life‐threatening toxicities. Intracellularly, the enzyme FPGS polyglutamates MTX by adding 2–6 glutamate residues, creating MTXPG, which increases intracellular retention as well as affinity for its target enzymes proportional to glutamyl chain lengths [82], [83], [84], [85], [86].
Abbreviations: 5‐FP, 5‐formylpteroate; ABC, ATP‐binding cassette; ABCC, ABC subfamily C; CH2THF, 5,10‐Methylenetetrahydrofolate; DAMPA, 4‐deoxy‐4‐amino‐N10‐methylpteroic acid; DHF, dihydrofolate; DHFR, dihydrofolate reductase; dTMP, deoxythymidine monophosphate; dUMP, deoxyuridine monophosphate; FPGS, folylpolyglutamate synthetase; GLU, glutamate; LV, leucovorin, MTX, methotrexate; MTXPG, polyglutamated methotrexate; RFC, reduced folate carrier; SHMT1, serine hydroxymethyltransferase 1; THF, tetrahydrofolate; TYMS, thymidylate synthase.
The anticancer and toxic effects of MTX are highly schedule‐dependent and are determined by the duration of exposure to a threshold concentration of the drug. The threshold concentration is tissue‐ and tumor‐specific. Prolonged continuous exposure to low concentrations of MTX can cause severe myelosuppression and other severe toxicities. Leucovorin, the HDMTX rescue agent, provides an alternative source of tetrahydrofolate and counteracts MTX toxicity if administered, preferably within 42 hours of the start of the HDMTX infusion. Leucovorin must compete with MTX for cell entry and polyglutamation (Fig. 1), so it is less effective as a rescue agent at high MTX concentrations if it is not also present at an equipotent concentration.
Renal excretion of the parent drug is the primary route of elimination for HDMTX, accounting for approximately 70%–90% of MTX clearance [8]. MTX is a weak acid with limited solubility in acidic conditions (2 mM maximum solubility at pH 5). Alkalinization of the urine has a greater impact on MTX solubility in urine than fluid hydration [9]. MTX solubility increases 10‐fold by increasing the pH from 6 to 7 [10]. MTX is secreted and not reabsorbed by renal tubules, but tubular secretion is saturated at higher plasma concentrations and only plays a minor role in MTX elimination during and immediately after the HDMTX infusion [11]. MTX is 60% protein‐bound, and glomerular filtration is limited to free drug. Therefore, MTX clearance at high concentrations is less than the glomerular filtration rate (GFR). MTX is also metabolized in the liver by aldehyde oxidase to 7‐hydroxymethotrexate, which accounts for 5%–10% of MTX elimination [8]. There is substantial interpatient and intrapatient variability in the clearance of MTX, which ranges 10‐fold (depending on dose, age, and infusion time) in patients with normal renal function after an HDMTX infusion [12], [13], [14], [15].
HDMTX‐Induced Acute Kidney Injury and Delayed MTX Clearance
HDMTX‐induced acute kidney injury (AKI) occurs during or shortly after the end of the infusion near the end of the steady‐state plasma concentration (Fig. 2) [16]. The AKI is manifested as a rise in serum creatinine (decrease in GFR), but urine output is usually maintained (nonoliguric) [17]. MTX clearance declines in proportion to the decrease in GFR, resulting in prolonged exposure to high MTX concentrations that may exceed the capacity of standard doses of leucovorin to rescue from the toxicity of MTX. HDMTX‐induced AKI that is associated with substantial reductions in MTX clearance occurs in 0.5%–1.0% of courses of 5 g/m2 over 24 hours in children with acute lymphoblastic leukemia (ALL) [18], [19] and 1.8% of courses of 12 g/m2 over 4 hours in children, adolescents, and young adults with osteosarcoma [20]. Approximately 2%–12% of adults treated with HDMTX develop nephrotoxicity [21]. HDMTX‐induced AKI is reversible, and nearly all patients fully recover renal function. The GFR of most patients with HDMTX‐induced AKI returns to baseline values after AKI, although this can take weeks [22], [23]. The long‐term consequences of HDMTX‐induced AKI have not been fully investigated, but it is possible that nephron loss occurs after such injury, resulting in subsequent chronic kidney disease, as has been shown in other models of AKI [24].
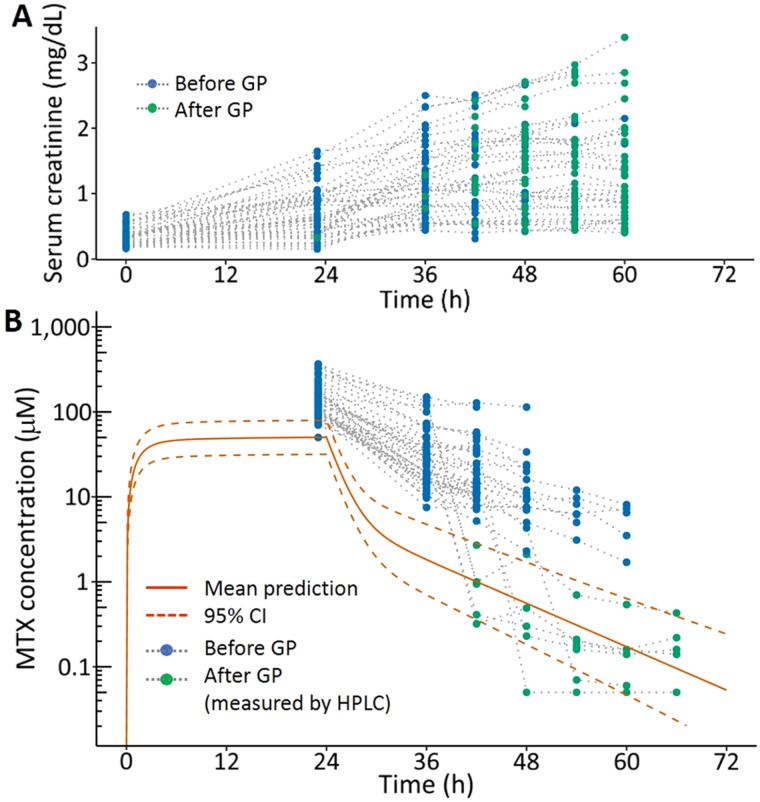
Figure 2.
Change in serum creatinine (A) and plasma methotrexate (B) concentrations relative to the start of the high‐dose methotrexate infusion (time 0) in pediatric patients with acute lymphoblastic leukemia (ALL) receiving 5 g/m2 over 24 hours on the Nordic Society of Paediatric Haematology and Oncology ALL‐2008 protocol who also received GP. Their measurements prior to glucarpidase (blue) and after glucarpidase (green) are shown relative to the predicted mean and 95% CI for patients receiving the same MTX dose (orange lines).
Abbreviations: CI, confidence interval; GP, glucarpidase; HPLC, high pressure liquid chromatography; MTX, methotrexate.
Possible mechanisms of HDMTX‐induced AKI include pH‐dependent precipitation of MTX in urine in the renal tubules [25], [26], [27], reduced renal perfusion from afferent arteriolar vasoconstriction, or uptake of MTX into the renal tubules with direct tubular toxicity [5], [9], [16]. The osteosarcoma HDMTX regimen (8–12 g/m2 over 4 hours), which has a higher incidence of HDMTX‐induced AKI, as noted above, yields an end‐of‐infusion plasma MTX concentration of approximately 1,000 µM (1 mM) [15], [28], [29], and the simultaneous urine MTX concentration is approximately 10,000 µM (10 mM) [8], which approximates the solubility limit of MTX at pH 7. The timing of HDMTX‐induced AKI and the effectiveness of alkalinization and volume expansion (fluid hydration) administered prior to, during, and after a HDMTX infusion at preventing HDMTX‐induced AKI supports the view that MTX precipitation plays a role in this toxicity.
Documenting normal renal function prior to administering HDMTX and serial monitoring of serum creatinine before and at the end of HDMTX infusion are mandatory to detect HDMTX‐induced AKI as early as possible. An increase in serum creatinine of more than 50% within 24–36 hours from the pre‐HDMTX baseline value has a sensitivity of 0.32 and a specificity of 0.99 for predicting delayed MTX elimination [16]. However, serum creatinine is a suboptimal biomarker of AKI, as creatinine rise may lag significantly from the time of the renal insult. Elevated plasma MTX concentration may indicate HDMTX‐induced AKI prior to a significant change in creatinine. Thus, clinicians administering HDMTX should be familiar with the expected plasma MTX concentration at the various time points after infusion (supplemental online Tables 1 and 2). After recovery of renal function (normal GFR), HDMTX therapy can generally be safely administered at full dose in pediatric and adult patients who previously experienced HDMTX‐induced AKI or received glucarpidase [18], [19], [30].
Strategies to Prevent HDMTX‐Induced AKI
In early studies of HDMTX, severe toxicity occurred in approximately 10% of patients with a 6% toxic mortality rate [31]. The incidence of severe, life‐threatening toxicity after HDMTX therapy has been reduced to less than 1% by the implementation of supportive care measures to prevent HDMTX‐induced AKI, including alkalinization of urine, fluid hydration with frequent monitoring of serum creatinine, and serial monitoring of plasma MTX concentrations to determine the dose and duration of leucovorin rescue [19], [32], [33], [34]. Urine pH should be documented to be above 7 prior to the start of a HDMTX infusion and should be maintained at this level until the plasma MTX concentration drops below the solubility threshold. Urinary flow prior to, during, and after a HDMTX infusion should be maintained at least at 2,500 mL/m2 per day (supplemental online Table 3) [35]. If urinary flow drops below 2,000 mL/m2 per day, there is a higher risk for delayed MTX clearance [36].
The purpose of alkalinization of the urine and fluid hydration is to maximize the solubility of MTX in urine. These measures do not enhance MTX clearance, which is largely dependent on glomerular filtration at high plasma MTX concentrations. Once the plasma MTX concentration drops below 10 µM after infusion, the urine concentration is estimated to be an order of magnitude below the drug's solubility limit at pH 6; thus, alkalinization and hydration are less important as the plasma MTX concentration continues to decrease to the target level (typically ≤0.1 or 0.2 µM) at which leucovorin rescue can be stopped in patients with normal renal function.
Leucovorin Rescue
Leucovorin and its primary circulating metabolite, 5‐methyl‐tetrahydrofolate, prevent the potentially severe and life‐threatening toxicities from HDMTX by providing a source of intracellular tetrahydrofolates that enter the folate cycle downstream of DHFR, which is inhibited by MTX. However, these exogenous tetrahydrofolates must compete with MTX for cellular uptake via the reduced folate carrier and, once in the cell, for polyglutamylation, which enhances intracellular retention of folates and affinity for the target enzymes (Fig. 1). Therefore, leucovorin rescue is less effective at high MTX concentrations, especially when the MTX concentration exceeds 10 µM for 48 hours or longer. The leucovorin dose must be increased in proportion to the MTX concentration when MTX clearance is delayed due to HDMTX‐induced AKI.
Timing of MTX measurements and the dose and initiation of leucovorin rescue vary across different treatment regimens (Table 1) [9]. Leucovorin rescue usually starts 24–42 hours after the start of the HDMTX infusion and must not be delayed beyond 42–48 hours, even if the HDMTX infusion does not finish at the planned time. Delaying the start of leucovorin rescue beyond 48 hours after the start of the HDMTX infusion significantly increases the risk for severe MTX toxicity [37]. Standard doses of leucovorin can be administered orally, but higher doses used when the MTX concentration is elevated must be administered intravenously because the absorption of leucovorin and other folates from the intestinal tract is carrier‐mediated and saturable at doses above 40 mg.
Glucarpidase Background
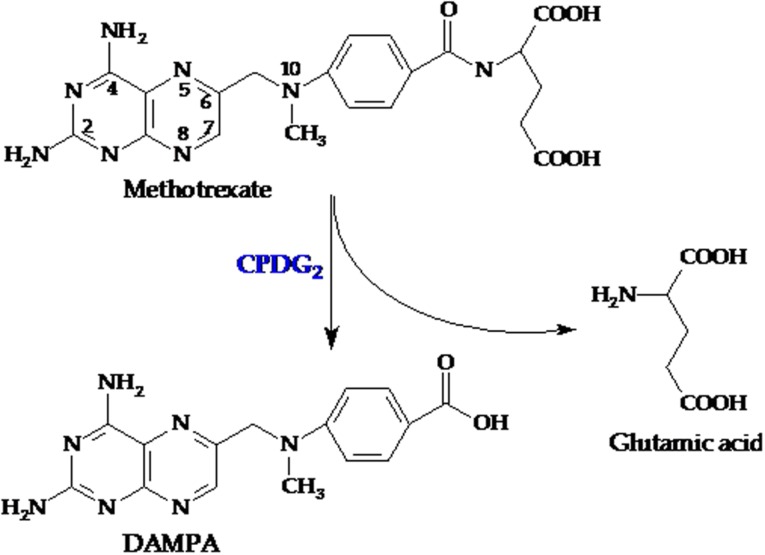
Glucarpidase (carboxypeptidase G2 or Voraxaze, BTG plc, London, UK, www.btgplc.com) is a recombinant bacterial enzyme that inactivates MTX and folates by hydrolyzing the glutamate moiety (Fig. 3). Carboxypeptidase G1 (CPG1) was isolated from Pseudomonas stutzeri in the 1970s. Studies in mice and in a small number of patients revealed that administration of CPG1 after MTX dosing prevented subsequent toxicity [38]; however, the bacterial source was subsequently lost [39]. In the 1980s, carboxypeptidase G2 (CPG2) was isolated and purified from the Pseudomonas strain RS‐16 and cloned into Escherichia coli [40], [41]. Glucarpidase rapidly lowers the MTX concentration by cleaving MTX into two noncytotoxic metabolites, 4‐deoxy‐4‐amino‐N10‐methylpteroic acid (DAMPA) and glutamate, which are eliminated primarily by the liver (through the bile) and not by the kidney [4], [42].
Figure 3.
Methotrexate is cleaved into two noncytotoxic metabolites, DAMPA and glutamic acid, by CPDG2.
Abbreviations: CPDG2, glucarpidase; DAMPA, 4‐deoxy‐4‐amino‐N10‐methylpteroic acid.
Glucarpidase is provided as a lyophilized powder in vials containing 1,000 units and is administered in a single intravenous dose of 50 units/kg. Dose‐finding studies of glucarpidase were not conducted in humans, but this recommended dose has been shown to be safe and effective [43]. Glucarpidase may not be routinely stocked in pharmacies because of its high cost and infrequent use, so the drug should be ordered as soon as the need for its use is anticipated (https://www.btgplc.com/products/specialty-pharmaceuticals/voraxaze-glucarpidase/). Adverse reactions related to glucarpidase, including nausea/vomiting, hypotension, paresthesia, flushing, and headache (mostly grade ≤2, according to the NCI Common Terminology Criteria for Adverse Events, version 3), were recorded each in less than 3% of patients [6]. Despite the potential immunogenicity related to the bacterial source of glucarpidase, hypersensitivity reactions were reported in <1% of patients, but 17% of patients receiving one or two doses of glucarpidase developed antiglucarpidase antibodies [6], [18], [44], [45].
Glucarpidase Pharmacology
The pharmacokinetics of glucarpidase, which is an 83 kDa protein, were studied in the absence of MTX in eight healthy volunteers and in four subjects with severe renal dysfunction [46]. The molecular weight exceeds the threshold for glomerular filtration, so renal excretion is not likely to play a role in drug elimination. In normal volunteers, the clearance was 7.5 mL/min; the volume of distribution was 3.6 L, which is comparable to plasma volume; and the half‐life was 6 or 9 hours (depending on the drug assay method) and was not substantially different in patients with renal dysfunction [43]. Due to the large molecular size of glucarpidase, it does not enter cells or cross the blood‐brain barrier. Because of the limited distribution volume of glucarpidase, metabolism of tissue and intracellular MTX is reliant on diffusion of the drug back into circulation. Glucarpidase cannot directly rescue the intracellular effects of MTXPG, although it can limit further kidney damage. Thus, leucovorin is still required after glucarpidase to protect normal cells from MTX toxicity [47].
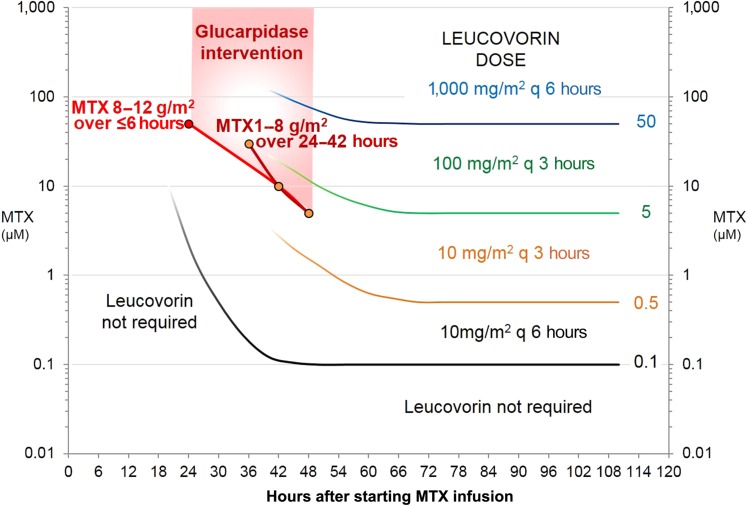
Glucarpidase rapidly metabolizes circulating MTX and reduces plasma MTX concentrations by >95% within 15 minutes of administration [18], [42], [45]. This catalytic effect on circulating MTX persists for 48 hours, but there can be a rebound of plasma MTX concentration as the activity of glucarpidase wanes and MTX redistributes to circulation from tissues [21] (Fig. 4). However, the rebound plasma MTX concentration is typically substantially lower than the preglucarpidase MTX level [23]. Glucarpidase has a higher affinity for MTX (Km 8 mM) than for leucovorin (Km 120 mM) and 5‐methyltetrahydrofolate (Km 35 mM), but in vivo the AUC0–3h of 5‐MeTHF drops by >90% after administration of glucarpidase [40], [41].
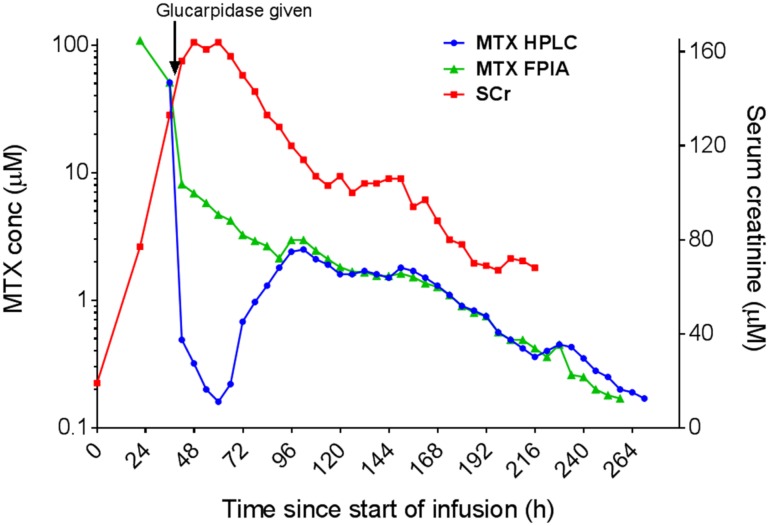
Figure 4.
Concentrations of MTX and SCr in a patient enrolled on the Nordic Society of Paediatric Haematology and Oncology acute lymphoblastic leukemia 2008 protocol treated with glucarpidase following a 5 g/m2 dose of MTX over 24 hours. The HPLC measurement of MTX (blue line) is more accurate than the FPIA measurement of MTX (green line) for the 48 hours following the administration of glucarpidase at hour 41. The HPLC measurement shows a rebound of the MTX concentrations from hour 60 to hour 96. The SCr increased during the 24‐hour infusion and remained high for several days following the glucarpidase administration. The FPIA and HPLC measurements were performed by Stein Bergan at the Department of Pharmacology, Rikshospitalet, Oslo, Norway.
Abbreviations: conc, concentration; FPIA, fluorescence polarization immunoassay; HPLC, high pressure liquid chromatography; MTX, methotrexate; SCr, serum creatinine.
Intravenously administered glucarpidase mediates rapid degradation of MTX and, as such, may have a role in limiting further nephrotoxicity; however, by itself, glucarpidase does not impact the normalization of kidney dysfunction [23]. Successful intrathecal administration of glucarpidase has been reported in a limited number of patients who experienced an accidental intrathecal MTX overdose [44], [48]; in contrast, in this emergency scenario, intrathecal administration of leucovorin should strictly be avoided [49].
Indications for Glucarpidase Therapy
Treatment with glucarpidase is indicated for patients with HDMTX‐induced AKI and delayed MTX elimination leading to potentially toxic plasma MTX concentrations. The FDA has approved a dose of 50 units/kg for the treatment of toxic plasma methotrexate concentrations (>1 micromole per liter) in patients with delayed methotrexate clearance due to impaired renal function. But the limitations of use indicate that it should not be used in patients who exhibit the expected clearance of methotrexate (plasma methotrexate concentrations within two standard deviations of the mean methotrexate excretion curve specific for the dose of methotrexate administered) or those with normal or mildly impaired renal function because of the potential risk of subtherapeutic exposure to MTX [6].
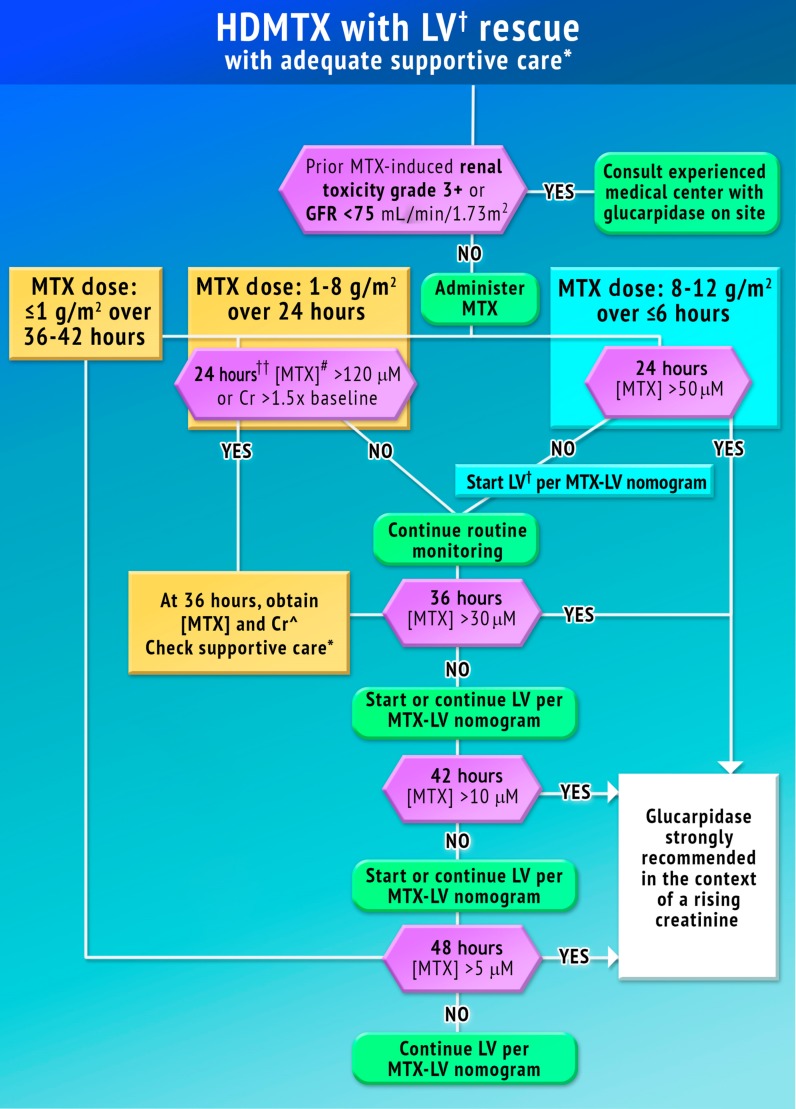
The goal of this task force was to identify the population of patients who would benefit from glucarpidase rescue by more precisely defining the absolute MTX concentrations that put patients at risk for severe or life‐threatening toxicity at specific time points after the start of the HDMTX infusion based on reported experience with HDMTX infusions. The duration of time above the threshold MTX concentration for various tissues was considered when determining the concentration cutoffs at each time point. We provide guidance for many of the time points that are routinely monitored in clinical practice (Fig. 5). Consulting with an experienced oncologist, nephrologist, or clinical pharmacist who is familiar with managing patients treated with HDMTX infusions is recommended if the patient has prior HDMTX‐induced AKI or a GFR <75 mL/min/1.73 m2. Administration of glucarpidase should optimally occur within 48–60 hours from the start of the HDMTX infusion, because life‐threatening toxicities may not be preventable beyond this time point.
Figure 5.
Treatment with glucarpidase may be indicated in the case of excessively high MTX concentrations and rising creatinine. Time points indicated are after the start of the infusion and are not required measurement times. Guidance is provided for time points that are routinely measured in clinical practice. Renal toxicity grade 3+ is according to the National Cancer Institute's Common Terminology Criteria for Adverse Events, version 4. Concentrations in µM can be converted to µg/mL by dividing by 2.2005. 120 µM = 54.5 µg/mL, 50 µM = 22.7 µg/mL, 30 µM = 13.6 µg/mL, 10 µM = 4.54 µg/mL, and 5 µM = 2.27 µg/mL.
*Urine pH >7, urine output >2.5L/m2 per day, emesis control.
†Hours are indicated after infusion start.
Abbreviations: Cr, serum creatinine; GFR, glomerular filtration rate; HDMTX, high‐dose methotrexate; LV, leucovorin (folinic acid, citrovorum factor, 5‐methyltetrahydrofolate); MTX, methotrexate; [MTX], plasma methotrexate concentration.
1–8 g/m2 MTX Infused over 24–42 Hours
For a 24‐hour infusion, a plasma MTX concentration >120 µM (54.5 µg/mL) at the end of the infusion or a creatinine increase ≥50% over baseline warrants additional monitoring at 36 hours. If the 36‐hour MTX concentration is above 30 µM (13.6 µg/mL), the 42‐hour MTX concentration is above 10 µM (4.54 µg/mL), or the 48‐hour concentration is above 5 µM (2.27 µg/mL) and the serum creatinine is elevated relative to the baseline measurement (indicative of HDMTX‐induced AKI), glucarpidase may be indicated. After a 36–42 hour HDMTX infusion, administration of glucarpidase may be indicated when the 48‐hour MTX concentration is above 5 µM.
8–12 g/m2 MTX Infused over ≤6 Hours
A plasma MTX concentration >1500 µM (681 µg/mL) at the end of the infusion warrants additional monitoring at 24 hours. If the 24‐hour concentration is above 50 µM (22.7 µg/mL), the 36‐hour concentration is above 30 µM (13.6 µg/mL), the 42‐hour MTX concentration is above 10 µM (4.54 µg/mL), or the 48‐hour concentration is above 5 µM (2.27 µg/mL) and the serum creatinine is elevated relative to the baseline measurement (indicative of HDMTX‐induced AKI), administration of glucarpidase may be indicated.
There is a potential for falsely elevated plasma MTX concentration during or shortly after the end of the HDMTX infusion due to contamination if the specimen is drawn from the same lumen of the central venous catheter through which the drug was infused. If the plasma MTX concentration is elevated, but the serum creatinine is normal, the measurement should be repeated with a new blood sample.
Glucarpidase Administration Recommendations
Leucovorin should be dosed according to the standard guidelines (Fig. 6) until glucarpidase can be given. Some regimens use the following equation to calculate the leucovorin dose (in mg) rather than the leucovorin nomogram in Figure 6 if the plasma MTX concentration is >5 µM: plasma MTX concentration (µM) × body weight (kg). If glucarpidase is administered within 2 hours of the last dose of leucovorin, it will cleave the leucovorin as well as the MTX. Leucovorin should not be administered until 2 hours after a dose of glucarpidase because it may interfere with glucarpidase‐mediated metabolism of MTX.
Figure 6.
Standard leucovorin nomogram used in the U.S. Glucarpidase use may be indicated when MTX concentrations are within the shaded area.
Abbreviation: MTX, methotrexate.
Leucovorin and MTX are racemic mixtures of two stereoisomers, and only the levo (L) enantiomer is recognized by most folate carriers and enzymes that utilize folates as a cofactor or are inhibited by MTX or MTXPG. The active levo enantiomer of leucovorin (levoleucovorin calcium) is FDA‐approved for use as a MTX rescue agent. Glucarpidase cleavage of MTX and tetrahydrofolates is less stereo‐specific because the cleavage site does not involve the chiral carbon. Glucarpidase should be administered according to the package insert. If less than the full dose of 50 units/kg is all that is available, any amount that is available should be given, as lower doses may be effective [22]. Leucovorin rescue should be reinitiated no sooner than 2 hours after glucarpidase administration. Repeat administration of glucarpidase within 48 hours of the first dose during the same MTX course is not recommended due to decreased efficacy.
Measurement of MTX After Glucarpidase Therapy
Most clinical laboratories measure plasma MTX with an immunoassay method and not by a more specific high pressure liquid chromatography (HPLC) method [50]. Immunoassay methods detect MTX and its metabolites DAMPA and, to a varying extent, 7‐hydroxymethotrexate. After glucarpidase administration, essentially all circulating MTX is converted into the nontoxic metabolite DAMPA and other metabolites [4] (Fig. 3), but an immunoassay method that does not distinguish between MTX and DAMPA overestimates the true MTX concentration. With an HPLC method that chromatographically separates MTX from DAMPA, the plasma MTX concentration usually drops by >95% by 15 minutes after glucarpidase. The half‐life of DAMPA is approximately 9–10 hours, resulting in immunoassay interference and an inability to accurately quantify the MTX concentration for approximately 48 hours after glucarpidase. This limitation of the MTX immunoassay methods after glucarpidase therapy should be recognized and communicated between clinical and laboratory personnel. Another factor to consider is the potential rebound of MTX concentrations more than 48 hours after glucarpidase administration (Fig. 4) due to release of MTX from tissue stores; therefore, continued monitoring of MTX concentrations and administration of leucovorin is very important in these patients [23], [42], [51], [52].
Guidelines for Leucovorin Use After Glucarpidase Therapy
Due to the large quantities of calcium, the infusion time of leucovorin at doses >200–500 mg (or the patient's body surface area (BSA) × 50) should be over 1–2 hours. To avoid excessive calcium concentrations, leucovorin can be substituted with calcium levofolinate or disodium levofolinate, which contain the active L‐form of leucovorin, allowing the dose of leucovorin to be reduced by 50% compared with the racemic form. Disodium levofolinate can be given as a bolus dose and thus gives a faster clinical effect. Treatment with leucovorin should be continued until the plasma MTX concentration is below the threshold prescribed by the treatment protocol (e.g., below 0.1–0.2 µM). The minimal single dose of leucovorin is 15 mg/m2 per dose. The minimal single dose of calcium or disodium levofolinate is 7.5 mg/m2 per dose.
Leucovorin rescue should be restarted 2 hours after the administration of glucarpidase at the dose based on the MTX level prior to glucarpidase therapy. Because most institutions do not use an HPLC assay to specifically quantify MTX levels after glucarpidase administration, leucovorin rescue will be based on the MTX plasma concentration measured with the clinical immunoassay method, which measures MTX and its metabolites. Leucovorin should be administered for at least 48 hours after glucarpidase because of the MTX reentering the bloodstream from the tissues (Fig. 4).
Discussion
Alternatives to Glucarpidase Therapy
There are scant data to directly compare the efficacy of glucarpidase with other modalities of lowering MTX plasma concentrations [53], including intermittent and continuous hemodialysis, peritoneal dialysis, charcoal hemoperfusion, and increasing elimination via enterohepatic circulation using enteral binding agents like oral cholestyramine. Hemodialysis and hemodiafiltration can clear MTX that is free in the plasma. Given the relatively high volume of distribution and protein binding of MTX, a rebound of free MTX occurs when dialysis is stopped [54], [55], [56]. In situations in which glucarpidase is not available, when patients have very high MTX concentrations, the risks of hemodialysis and/or high dose continuous veno‐venous hemodiafiltration are low compared with the potential benefit of providing enhanced MTX clearance. Thymidine, which counteracts the effects of MTX through the restoration of DNA synthesis, did show promising results in clinical trials in a small number of patients but has been discontinued and is unavailable for investigational use [5].
High‐dose leucovorin rescue has over the years been used for delayed MTX clearance when CPG1 and CPG2 were not easily available [57]. It is still the best option when glucarpidase is not available within 60 hours from the start of the HDMTX infusion. However, unnecessarily high doses of leucovorin should be avoided, as treatment failures in association with higher leucovorin doses or “over‐rescue” have been reported in pediatric patients with ALL and osteosarcoma [58], [59]. Leucovorin is a storage vitamin, and excessive rescue could thus interfere with the MTX efficacy at the next HDMTX course [60]. Therefore, glucarpidase has increasingly come to the fore in the management and prevention of HDMTX‐induced AKI.
Effect of Age on MTX‐Induced Nephrotoxicity
Adults generally do not tolerate HDMTX as well as children, and MTX pharmacokinetic parameters are dependent on age [12], [61], [62]. The available pharmacokinetic data in adults are not as abundant as in children. One protocol reduces the MTX dose based on the GFR in elderly patients with central nervous system lymphoma, which resulted in similar toxicities compared with patients with normal renal function [63]. However, in most contemporary adult ALL protocols, there is no dose reduction. In a retrospective analysis including 649 HDMTX treatment cycles in 194 adult patients, advanced age was significantly associated with delayed MTX elimination and grade 2–4 renal toxicity [64]. In addition, elderly cancer patients are more likely to have some degree of baseline renal dysfunction that can be exacerbated by MTX [65]. Of 749 patients aged 1–45 years old treated by the Nordic Society of Paediatric Haematology and Oncology (NOPHO) ALL‐2008 protocol, the 61 adult standard‐ or intermediate‐risk patients who received 8 courses of HDMTX each and the 31 high‐risk patients who received 6 courses demonstrated similar toxicities to the children treated on the same protocol [19].
Concomitant Medications
As MTX‐induced nephrotoxicity is closely tied to MTX clearance, reducing the risk of AKI from other nephrotoxic medications is imperative. Each potential nephrotoxic medication should be carefully evaluated, and the risk and benefits of stopping this medication should be individualized. Studies in pediatrics have clearly shown that the AKI can nearly be prevented with judicious use of nephrotoxic medications [66]. In addition, increased exposure to 7‐hydroxymethotrexate has been reported in association with pantoprazole [67]. Concomitant use of medications that can interfere with MTX elimination, such as proton pump inhibitors and nonsteroidal anti‐inflammatory drugs, should be avoided [5], [68], [69], [70], [71], [72], [73], [74], [75]. Even food (e.g., licorice [76]) and beverages (soft drinks, often sweetened with licorice extract) with low pH have been suspected to affect the MTX clearance. Since the introduction of 5‐hydroxytryptamine (5HT3) receptor antagonists, emesis is not a problem during HDMTX and nowadays unlikely to be linked to AKI.
Trimethroprim‐sulfamethoxazole is generally used as Pneumocystis jiroveci prophylaxis during ALL therapy [77], and there has been a worry that it could interfere with MTX pharmacokinetics and/or efficacy. However, it does not seem to interfere with HDMTX pharmacokinetics [78].
Further Research Necessary
Given the paucity of published pharmacokinetic data in adults, it is difficult to determine whether the present recommendations, which are primarily based on pediatric experiences, adequately apply to adult patients. Thus, pharmacokinetic data in adult patients should be prospectively collected and analyzed and include patients with HDMTX‐induced AKI and patients receiving glucarpidase treatment. These data are being collected in adults in Denmark (personal communication with Nina Toft, Adult NOPHO ALL‐2008 coordinator). Additional studies on the effect of comedications on HDMTX pharmacokinetics are also needed, because existing data are based mostly on single case reports.
Conclusion
The expert panel was able to come to consensus, providing specific MTX concentrations above which glucarpidase is strongly recommended. Implementation of the recommendations in this guideline may help reduce the incidence of life‐threatening HDMTX‐induced toxicities.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
The authors of this guideline were selected to represent a diverse group of clinical providers with a range of expertise, including adult and pediatric oncologists, nephrologists, pharmacists, clinical pharmacologists, and a nurse. The group met virtually three times and once in person. The organizer, Dr. Ramsey, first provided evidence to the group for consideration and generated a draft flow chart. Changes were made to the guideline iteratively until the group reached consensus. JLP acknowledges support by a Cancer Center Support Grant (CA21765) from the National Cancer Institute (NCI) and by the American Lebanese Syrian Associated Charities (ALSAC). We are grateful to Tomoyuki Mizuno for performing methotrexate pharmacokinetic analysis. We are thankful to Dr. Kenneth Carson and Suhong Luo at Washington University, St. Louis, for providing methotrexate concentrations at specified time points adapted from previously published analyses. We are also thankful to Holly Ward for coordinating the in‐person meeting in Cincinnati. This was an investigator‐initiated project designed and executed by the authors. Funding support was provided in the form of an educational grant from BTG, International (the manufacturer of glucarpidase) to AAV and LBR. Sponsor representatives were not present during any discussions of the algorithm or manuscript development.
Author Contributions
Conception/design: Laura B. Ramsey, Frank M. Balis, Maureen M. O'Brien, Kjeld Schmiegelow, Jennifer L. Pauley, Archie Bleyer, Brigitte C. Widemann, David Askenazi, Sharon Bergeron, Anushree Shirali, Stefan Schwartz, Alexander A. Vinks, Jesper Heldrup
Collection and/or assembly of data: Laura B. Ramsey, Maureen M. O'Brien, Kjeld Schmiegelow, Alexander A. Vinks, Jesper Heldrup
Dana analysis and interpretation: Laura B. Ramsey, Frank M. Balis, Maureen M. O'Brien, Kjeld Schmiegelow, Jennifer L. Pauley, Archie Bleyer, Brigitte C. Widemann, David Askenazi, Sharon Bergeron, Anushree Shirali, Stefan Schwartz, Alexander A. Vinks, Jesper Heldrup
Manuscript writing: Laura B. Ramsey, Frank M. Balis, Maureen M. O'Brien, Kjeld Schmiegelow, Jennifer L. Pauley, Archie Bleyer, Brigitte C. Widemann, David Askenazi, Sharon Bergeron, Anushree Shirali, Stefan Schwartz, Alexander A. Vinks, Jesper Heldrup
Final approval of manuscript: Laura B. Ramsey, Frank M. Balis, Maureen M. O'Brien, Kjeld Schmiegelow, Jennifer L. Pauley, Archie Bleyer, Brigitte C. Widemann, David Askenazi, Sharon Bergeron, Anushree Shirali, Stefan Schwartz, Alexander A. Vinks, Jesper Heldrup
Disclosures
Laura B. Ramsey: BTG International (RF); Maureen M. O'Brien: BTG Pharmaceuticals (RF); Archie Bleyer: Jazz Pharmaceuticals (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Green MR, Chamberlain MC. Renal dysfunction during and after high‐dose methotrexate. Cancer Chemother Pharmacol 2009;63:599–604. [DOI] [PubMed] [Google Scholar]
- 2. Goldman P, Levy CC. The enzymatic hydrolysis of folate analogues. Biochem Pharmacol 1968;17:2265–2270. [DOI] [PubMed] [Google Scholar]
- 3. Levy CC, Goldman P. The enzymatic hydrolysis of methotrexate and folic acid. J Biol Chem 1967;242:2933–2938. [PubMed] [Google Scholar]
- 4. Widemann BC, Sung E, Anderson L et al. Pharmacokinetics and metabolism of the methotrexate metabolite 2, 4‐diamino‐n 10‐methylpteroic acid. J Pharmacol Exp Ther 2000;294:894–901. [PubMed] [Google Scholar]
- 5. Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. The Oncologist 2006;11:694–703. [DOI] [PubMed] [Google Scholar]
- 6.BTG International, Inc . Voraxaze (glucarpidase) package insert. 2012. Brentwood, TN: BTG International, Inc.
- 7. Nathan PC, Whitcomb T, Wolters PL et al. Very high‐dose methotrexate (33.6 g/m2) as central nervous system preventive therapy for childhood acute lymphoblastic leukemia: Results of National Cancer Institute/Children's Cancer Group trials CCG‐191P, CCG‐134P and CCG‐144P. Leuk Lymphoma 2006;47:2488–2504. [DOI] [PubMed] [Google Scholar]
- 8. Fukuhara K, Ikawa K, Morikawa N et al. Population pharmacokinetics of high‐dose methotrexate in Japanese adult patients with malignancies: A concurrent analysis of the serum and urine concentration data. J Clin Pharm Ther 2008;33:677–684. [DOI] [PubMed] [Google Scholar]
- 9. Messmann R, Allegra C. Antifolates. Philadelphia, PA: Lippincott Williams & Wilkins, 2001: 139–184. [Google Scholar]
- 10. Mir O, Ropert S, Babinet A et al. Hyper‐alkalinization without hyper‐hydration for the prevention of high‐dose methotrexate acute nephrotoxicity in patients with osteosarcoma. Cancer Chemother Pharmacol 2010;66:1059–1063. [DOI] [PubMed] [Google Scholar]
- 11. Mikkelsen TS, Thorn CF, Yang JJ et al. PharmGKB summary: Methotrexate pathway. Pharmacogenet Genomics 2011;21:679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ramsey LB, Panetta JC, Smith C et al. Genome‐wide study of methotrexate clearance replicates SLCO1B1. Blood 2013;121:898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ramsey LB, Bruun GH, Yang W et al. Rare versus common variants in pharmacogenetics: SLCO1B1 variation and methotrexate disposition. Genome Res 2012;22:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wall A, Gajjar A, Link A et al. Individualized methotrexate dosing in children with relapsed acute lymphoblastic leukemia. Leukemia 2000;14:221. [DOI] [PubMed] [Google Scholar]
- 15. Pignon T, Lacarelle B, Duffaud F et al. Pharmacokinetics of high‐dose methotrexate in adult osteogenic sarcoma. Cancer Chemother Pharmacol 1994;33:420–424. [DOI] [PubMed] [Google Scholar]
- 16. Skärby T, Jönsson P, Hjorth L et al. High‐dose methotrexate: On the relationship of methotrexate elimination time vs renal function and serum methotrexate levels in 1164 courses in 264 Swedish children with acute lymphoblastic leukaemia (ALL). Cancer Chemother Pharmacol 2003;51:311–320. [DOI] [PubMed] [Google Scholar]
- 17. Yarlagadda SG, Perazella MA. Drug‐induced crystal nephropathy: An update. Expert Opin Drug Saf 2008;7:147–158. [DOI] [PubMed] [Google Scholar]
- 18. Christensen AM, Pauley JL, Molinelli AR et al. Resumption of high‐dose methotrexate after acute kidney injury and glucarpidase use in pediatric oncology patients. Cancer 2012;118:4321–4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Svahn T, Mellgren K, Harila‐Saari A et al. Delayed elimination of high‐dose methotrexate and use of carboxypeptidase G2 in pediatric patients during treatment for acute lymphoblastic leukemia. Pediatr Blood Cancer 2017;64:e26395. [DOI] [PubMed] [Google Scholar]
- 20. Widemann BC, Balis FM, Kempf‐Bielack B et al. High‐dose methotrexate‐induced nephrotoxicity in patients with osteosarcoma. Cancer 2004;100:2222–2232. [DOI] [PubMed] [Google Scholar]
- 21. Widemann BC, Balis FM, Kim A et al. Glucarpidase, leucovorin, and thymidine for high‐dose methotrexate‐induced renal dysfunction: Clinical and pharmacologic factors affecting outcome. J Clin Oncol 2010;28:3979–3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scott JR, Zhou Y, Cheng C et al. Comparable efficacy with varying dosages of glucarpidase in pediatric oncology patients. Pediatr Blood Cancer 2015;62:1518–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Widemann BC, Schwartz S, Jayaprakash N et al. Efficacy of glucarpidase (carboxypeptidase G2) in patients with acute kidney injury after high‐dose methotrexate therapy. Pharmacotherapy 2014;34:427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chawla LS, Eggers PW, Star RA et al. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 2014;371:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jacobs SA, Stoller RG, Chabner B et al. 7‐hydroxymethotrexate as a urinary metabolite in human subjects and rhesus monkeys receiving high dose methotrexate. J Clin Invest 1976;57:534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lankelma J, van der Klein E, Ramaekers F. The role of 7‐hydroxymethotrexate during methotrexate anti‐cancer therapy. Cancer Lett 1980;9:133–142. [DOI] [PubMed] [Google Scholar]
- 27. Garneau AP, Riopel J, Isenring P. Acute methotrexate‐induced crystal nephropathy. N Engl J Med 2015;373:2691–2693. [DOI] [PubMed] [Google Scholar]
- 28. Borsi JD, Schuler D, Moe PJ. Methotrexate administered by 6‐h and 24‐h infusion: A pharmacokinetic comparison. Cancer Chemother Pharmacol 1988;22:33–35. [DOI] [PubMed] [Google Scholar]
- 29. Aquerreta I, Aldaz A, Giráldez J et al. Methotrexate pharmacokinetics and survival in osteosarcoma. Pediatr Blood Cancer 2004;42:52–58. [DOI] [PubMed] [Google Scholar]
- 30. Toft N, Birgens H, Abrahamsson J et al. Risk group assignment differs for children and adults 1–45 yr with acute lymphoblastic leukemia treated by the NOPHO ALL‐2008 protocol. Eur J Haematol 2013;90:404–412. [DOI] [PubMed] [Google Scholar]
- 31. Ahmad S, Shen FH, Bleyer WA. Methotrexate‐induced renal failure and ineffectiveness of peritoneal dialysis. Arch Intern Med 1978;138:1146–1147. [PubMed] [Google Scholar]
- 32. Jürgens H, Beron G, Winkler K. Toxicity associated with combination chemotherapy for osteosarcoma: A report of the cooperative osteosarcoma study (COSS 80). J Cancer Res Clin Oncol 1983;106:14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Allegra CJ, Boarman D. Interaction of methotrexate polyglutamates and dihydrofolate during leucovorin rescue in a human breast cancer cell line (MCF‐7). Cancer Res 1990;50:3574–3578. [PubMed] [Google Scholar]
- 34. Schmiegelow K. Advances in individual prediction of methotrexate toxicity: A review. Br J Haematol 2009;146:489–503. [DOI] [PubMed] [Google Scholar]
- 35. Sasaki K, Tanaka J, Fujimoto T. Theoretically required urinary flow during high‐dose methotrexate infusion. Cancer Chemother Pharmacol 1984;13:9–13. [DOI] [PubMed] [Google Scholar]
- 36. Relling MV, Fairclough D, Ayers D et al. Patient characteristics associated with high‐risk methotrexate concentrations and toxicity. J Clin Oncol 1994;12:1667–1672. [DOI] [PubMed] [Google Scholar]
- 37. Bertino JR. “Rescue” techniques in cancer chemotherapy: Use of leucovorin and other rescue agents after methotrexate treatment. Semin Oncol 1977;4:203–216. [PubMed] [Google Scholar]
- 38. Chabner B, Johns D, Bertino J. Enzymatic cleavage of methotrexate provides a method for prevention of drug toxicity. Nature 1972;239:395–397. [DOI] [PubMed] [Google Scholar]
- 39. Adamson PC, Balis FM, McCully CL et al. Methotrexate pharmacokinetics following administration of recombinant carboxypeptidase‐G2 in rhesus monkeys. J Clin Oncol 1992;10:1359–1364. [DOI] [PubMed] [Google Scholar]
- 40. Minton NP, Atkinson T, Sherwood RF. Molecular cloning of the Pseudomonas carboxypeptidase G2 gene and its expression in Escherichia coli and Pseudomonas putida. J Bacteriol 1983;156:1222–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sherwood RF, Melton RG, Alwan SM et al. Purification and properties of carboxypeptidase G2 from Pseudomonas sp. strain RS‐16. Eur J Biochem 1985;148:447–453. [DOI] [PubMed] [Google Scholar]
- 42. Schwartz S, Borner K, Müller K et al. Glucarpidase (carboxypeptidase g2) intervention in adult and elderly cancer patients with renal dysfunction and delayed methotrexate elimination after high‐dose methotrexate therapy. The Oncologist 2007;12:1299–1308. [DOI] [PubMed] [Google Scholar]
- 43.BTG International, Inc . Summary Review, Application Number 125327Orig1s000. U.S. Food and Drug Administration, Center for Drug Evaluation and Research, January 12, 2012. Available at http://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/125327Orig1s000SumR.pdf. Accessed December 5, 2016.
- 44. Krause AS, Weihrauch MR, Bode U et al. Carboxypeptidase‐G2 rescue in cancer patients with delayed methotrexate elimination after high‐dose methotrexate therapy. Leuk Lymphoma 2002;43:2139–2143. [DOI] [PubMed] [Google Scholar]
- 45. Buchen S, Ngampolo D, Melton R et al. Carboxypeptidase G2 rescue in patients with methotrexate intoxication and renal failure. Br J Cancer 2005;92:480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Phillips M, Smith W, Balan G et al. Pharmacokinetics of glucarpidase in subjects with normal and impaired renal function. J Clin Pharmacol 2008;48:279–284. [DOI] [PubMed] [Google Scholar]
- 47. DeAngelis LM, Tong WP, Lin S et al. Carboxypeptidase G2 rescue after high‐dose methotrexate. J Clin Oncol 1996;14:2145–2149. [DOI] [PubMed] [Google Scholar]
- 48. Widemann BC, Balis FM, Shalabi A et al. Treatment of accidental intrathecal methotrexate overdose with intrathecal carboxypeptidase G2. J Natl Cancer Inst 2004;96:1557–1559. [DOI] [PubMed] [Google Scholar]
- 49. Jardine LF, Ingram LC, Bleyer WA. Intrathecal leucovorin after intrathecal methotrexate overdose. J Pediatr Hematol Oncol 1996;18:302–304. [DOI] [PubMed] [Google Scholar]
- 50. Albertioni F, Rask C, Eksborg S et al. Evaluation of clinical assays for measuring high‐dose methotrexate in plasma. Clin Chem 1996;42:39–44. [PubMed] [Google Scholar]
- 51. Widemann BC, Balis FM, Murphy RF et al. Carboxypeptidase‐G2, thymidine, and leucovorin rescue in cancer patients with methotrexate‐induced renal dysfunction. J Clin Oncol 1997;15:2125–2134. [DOI] [PubMed] [Google Scholar]
- 52. Wall SM, Johansen MJ, Molony DA et al. Effective clearance of methotrexate using high‐flux hemodialysis membranes. Am J Kidney Dis 1996;28:846–854. [DOI] [PubMed] [Google Scholar]
- 53. Kumar N, Shirali AC. What is the best therapy for toxicity in the setting of methotrexate‐associated acute kidney injury: High‐flux hemodialysis or carboxypeptidase G2? Semin Dial 2014;27:226–226. [DOI] [PubMed] [Google Scholar]
- 54. Gibson TP, Reich SD, Krumlovsky FA et al. Hemoperfusion for methotrexate removal. Clin Pharmacol Ther 1978;23:351–355. [DOI] [PubMed] [Google Scholar]
- 55. Relling MV, Stapleton FB, Ochs J et al. Removal of methotrexate, leucovorin, and their metabolites by combined hemodialysis and hemoperfusion. Cancer 1988;62:884–888. [DOI] [PubMed] [Google Scholar]
- 56. Saland J, Leavey PJ, Bash RO et al. Effective removal of methotrexate by high‐flux hemodialysis. Pediatr Nephrol 2002;17:825–829. [DOI] [PubMed] [Google Scholar]
- 57. Flombaum CD, Meyers PA. High‐dose leucovorin as sole therapy for methotrexate toxicity. J Clin Oncol 1999;17:1589–1589. [DOI] [PubMed] [Google Scholar]
- 58. Cohen IJ. Progression of osteosarcoma after high‐dose methotrexate: Over‐rescue by folinic acid. Pediatr Hematol Oncol 2003;20:579–581. [PubMed] [Google Scholar]
- 59. Skärby TC, Anderson H, Heldrup J et al. High leucovorin doses during high‐dose methotrexate treatment may reduce the cure rate in childhood acute lymphoblastic leukemia. Leukemia 2006;20:1955–1962. [DOI] [PubMed] [Google Scholar]
- 60. Sterba J, Dusek L, Demlova R et al. Pretreatment plasma folate modulates the pharmacodynamic effect of high‐dose methotrexate in children with acute lymphoblastic leukemia and non‐Hodgkin lymphoma: “Folate overrescue” concept revisited. Clin Chem 2006;52:692–700. [DOI] [PubMed] [Google Scholar]
- 61. Csordas K, Hegyi M, Eipel OT et al. Comparison of pharmacokinetics and toxicity after high‐dose methotrexate treatments in children with acute lymphoblastic leukemia. Anticancer Drugs 2013;24:189–197. [DOI] [PubMed] [Google Scholar]
- 62. Bacci G, Ferrari S, Longhi A et al. Delayed methotrexate clearance in osteosarcoma patients treated with multiagent regimens of neoadjuvant chemotherapy. Oncol Rep 2003;10:851–858. [PubMed] [Google Scholar]
- 63. Jahnke K, Korfel A, Martus P et al. High‐dose methotrexate toxicity in elderly patients with primary central nervous system lymphoma. Ann Oncol 2005;16:445–449. [DOI] [PubMed] [Google Scholar]
- 64. May J, Carson KR, Butler S et al. High incidence of methotrexate associated renal toxicity in patients with lymphoma: A retrospective analysis. Leuk Lymphoma 2014;55:1345–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sahni V, Choudhury D, Ahmed Z. Chemotherapy‐associated renal dysfunction. Nat Rev Nephrol 2009;5:450–462. [DOI] [PubMed] [Google Scholar]
- 66.KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012;2:1–138. [Google Scholar]
- 67. Tröger U, Stötzel B, Martens‐Lobenhoffer J et al. Drug points: Severe myalgia from an interaction between treatments with pantoprazole and methotrexate. BMJ 2002;324:1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Treon S, Chabner B. Concepts in use of high‐dose methotrexate therapy. Clin Chem 1996;42:1322–1329. [PubMed] [Google Scholar]
- 69. Suzuki K, Doki K, Homma M et al. Co‐administration of proton pump inhibitors delays elimination of plasma methotrexate in high‐dose methotrexate therapy. Br J Clin Pharmacol 2009;67:44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Joerger M, Huitema AD, van den Bongard HJ et al. Determinants of the elimination of methotrexate and 7‐hydroxy‐methotrexate following high‐dose infusional therapy to cancer patients. Br J Clin Pharmacol 2006;62:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bauters TG, Verlooy J, Robays H et al. Interaction between methotrexate and omeprazole in an adolescent with leukemia: A case report. Pharm World Sci 2008;30:316–318. [DOI] [PubMed] [Google Scholar]
- 72. Ronchera CL, Hernández T, Peris JE et al. Pharmacokinetic interaction between high‐dose methotrexate and amoxycillin. Ther Drug Monit 1993;15:375–379. [DOI] [PubMed] [Google Scholar]
- 73. Thyss A, Milano G, Kubar J et al. Clinical and pharmacokinetic evidence of a life‐threatening interaction between methotrexate and ketoprofen. Lancet 1986;1:256–258. [DOI] [PubMed] [Google Scholar]
- 74. de Miguel D, García‐ Suárez J, Martín Y et al. Severe acute renal failure following high‐dose methotrexate therapy in adults with haematological malignancies: A significant number result from unrecognized co‐administration of several drugs. Nephrol Dial Transplant 2008;23:3762–3766. [DOI] [PubMed] [Google Scholar]
- 75. Loue C, Garnier N, Bertrand Y et al. High methotrexate exposure and toxicity in children with t(9;22) positive acute lymphoblastic leukaemia treated with imatinib. J Clin Pharm Ther 2015;40:599–600. [DOI] [PubMed] [Google Scholar]
- 76. Lin SP, Tsai SY, Hou YC et al. Glycyrrhizin and licorice significantly affect the pharmacokinetics of methotrexate in rats. J Agric Food Chem 2009;57:1854–1859. [DOI] [PubMed] [Google Scholar]
- 77. Schmiegelow K, Attarbaschi A, Barzilai S et al. Consensus definitions of 14 severe acute toxic effects for childhood lymphoblastic leukaemia treatment: A Delphi consensus. Lancet Oncol 2016;17:e231–e239. [DOI] [PubMed] [Google Scholar]
- 78. Watts CS, Sciasci JN, Pauley JL et al. Prophylactic trimethoprim‐sulfamethoxazole does not affect pharmacokinetics or pharmacodynamics of methotrexate. J Pediatr Hematol Oncol 2016;38:449–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mikkelsen TS, Thorn CF, Yang JJ et al. PharmGKB summary: Methotrexate pathway. Pharmacogenet Genomics 2011;21:679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cronstein BN, Merrill JT. Mechanisms of the effects of methotrexate. Bull Rheum Dis 1996;45:6–8. [PubMed] [Google Scholar]
- 81. Davidsen ML, Dalhoff K, Schmiegelow K. Pharmacogenetics influence treatment efficacy in childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol 2008;30:831–849. [DOI] [PubMed] [Google Scholar]
- 82. Chabner BA, Allegra CJ, Curt GA et al. Polyglutamation of methotrexate. Is methotrexate a prodrug? J Clin Invest 1985;76:907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Schrøder H, Clausen N, Ostergaard E et al. Pharmacokinetics of erythrocyte methotrexate in children with acute lymphoblastic leukemia during maintenance treatment. Cancer Chemother Pharmacol 1986;16:190–193. [DOI] [PubMed] [Google Scholar]
- 84. Schrøder H. Methotrexate pharmacokinetics in age‐fractionated erythrocytes. Cancer Chemother Pharmacol 1986;18:203–207. [DOI] [PubMed] [Google Scholar]
- 85. Schrøder H. Methotrexate kinetics in myeloid bone marrow cells and peripheral neutrophils. Cancer Chemother Pharmacol 1987;19:42–46. [DOI] [PubMed] [Google Scholar]
- 86. Fotoohi AK, Albertioni F. Mechanisms of antifolate resistance and methotrexate efficacy in leukemia cells. Leuk Lymphoma 2008;49:410–426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.