Abstract
Lessons Learned.
There is no presenting parameter that predicts the success of neoadjuvant therapy for pancreatic cancer.
Despite the images on scans following neoadjuvant therapy, all patients should be evaluated, because inflammation following radiation therapy (RT) may overstate the extent of tumor and vascular involvement.
Background.
In patients presenting with locally advanced pancreatic adenocarcinoma deemed unresectable by two pancreatic cancer surgeons, we analyzed presenting tumor size, extent of vascular involvement, tumor markers, response to neoadjuvant gemcitabine (G), docetaxel (T), and capecitabine (X) with or without additional chemoradiotherapy with GX on R0 resection rates (≥2 mm margins), and survival.
Methods.
All patients had baseline magnetic resonance imaging (MRI) and/or computed tomography (CT) scans and endoscopic ultrasound. A baseline positron emission tomography‐computed tomography (PET‐CT) was performed in 39 patients. The scans were reviewed by two radiologists.
GTX (gemcitabine 750 mg/m2 and docetaxel 30 mg/m2 on days 4 and 11 with capecitabine 1,500 mg/m2 days 1–14) was administered on a 3‐week schedule for 6 cycles to patients with both arterial and venous‐only involvement. Patients in the arterial arm received GX/RT before surgery, and those in the venous arm received GX/RT after R1 resection. Standard‐dose RT was delivered by intensity‐modulated radiation therapy (IMRT) or conformal fields to 5040 cGy along with capecitabine for 5 days and gemcitabine on day 5 of weeks 1, 2, 4, and 5 of RT, starting with the first full week of RT.
A cancer antigen test 19‐9 (CA 19‐9) was obtained at baseline and days 4 and 11 of each cycle. The rate of change in CA 19‐9 was calculated using the formula: (Log10 CA 19‐9 time 0) − (Log10 CA 19‐9 at 9 weeks)/9 weeks. This was derived based on the observation that the fall in CA 19‐9 following effective chemotherapy is a second‐order function.
Results.
Of the 34 patients with arterial involvement and 11 with extensive venous involvement who met the eligibility criteria and began GTX, only 5 patients in the arterial arm did not undergo subsequent resection. The remaining 40 patients were included in this analysis of presenting parameters with respect to R0 resection, disease‐free survival (DFS), and overall survival (OS). R0 resection was achieved in 28 of 40 patients (70%), and R1 resection in the remaining 12 (30%). The OS after R0 resection was a median 37 months (95% confidence interval [CI]: 29.3–44.7) compared with 29 months (95% CI: 28.5–41.5) for those with R1 resection.
Excluding four postoperative deaths, median DFS for the 25 (71%) with R0 resection was 31 months (95% CI: 11.3–51.1), and the median DFS for R1 resection was only 14 months (95% CI: 11.1–17). Eleven of the twenty‐eight (39%) patients achieving R0 resection have not relapsed (median = 45 months, range = 25–71 months).
Conclusion.
R0 resection, the goal of neoadjuvant treatment, can be achieved in 70% of patients presenting with locally advanced pancreatic cancer. The median DFS was 31 months (95% CI: 11. 3–51.1). No relationship was found with tumor size, degree of vascular involvement, carcinoembryonic antigen test (CEA), CA 19‐9, degree of tumor regression on scan, fall in CA 19‐9, or SUV on PET scan and subsequent survival.
Abstract
经验总结
• 目前没有发现预测新辅助疗法治疗胰腺癌成功的参数。
• 尽管新辅助治疗后扫描了图像, 但应对所有患者都进行评估, 因为放射治疗(RT)后的炎症可能夸大了肿瘤和血管受累的程度。
摘要
背景. 在患有局部晚期胰腺腺癌且被两个胰腺癌外科医生认为无法切除的患者中, 我们分析了肿瘤大小、血管受累程度、肿瘤标志物、对新辅助疗法吉西他滨(G)、多西他赛(T)和卡培他滨(X)联合或不联合GX放化疗在R0切除率(≥2mm切缘)方面的结局和生存期。
方法. 所有患者均接受了基线磁共振成像(MRI)扫描和/或计算机断层扫描(CT)及超声内镜检查。对39名患者进行基线正电子发射断层扫描‐计算机断层扫描(PET‐CT)。两名放射科医师对扫描结果进行评估。
以3周的时间表给予动脉和静脉均受累及仅静脉受累的患者6个周期的GTX治疗(在第4天和第11天给予吉西他滨750 mg/m2和多西他赛30 mg/m2, 第1‐14天给予卡培他滨1 500 mg/m2)。动脉组患者在手术前接受GX/RT, 静脉组患者在R1切除后接受GX/RT。通过调强放射治疗(IMRT)或适形野给予标准剂量RT直至5 040 cGy, 同时给予卡培他滨5天和吉西他滨(从RT的第一整周开始, 在RT的第1、2、4和5周的第5天)。
在基线时和每个周期的第4天和第11天获取癌症抗原测试19‐9(CA 19‐9)。计算CA 19‐9的变化率的公式为:(Log10起始时间的CA 19‐9)‐(Log10第9周时的CA 19‐9)/9周。此公式的推导基于一个观察结果, 即有效化疗后CA 19‐9的下降过程是二阶函数。
结果. 在符合合格标准并开始进行GTX的34名动脉受累患者和11名广泛静脉受累患者中, 动脉组中只有5名患者未接受随后的手术切除。其余40例患者被纳入本次R0切除、无病生存期(DFS)和总生存期(OS)的参数分析。40例患者中有28例(70%)实现了R0切除, 其余12例(30%)实现了R1切除, R0切除后的中位OS为37个月[95%置信区间(CI):29.3‐44.7], R1切除的患者中位OS为29个月(95% CI:28.5‐41.5)。
排除4例术后死亡, 25例(71%)R0切除患者的中位DFS为31个月(95% CI:11.3‐51.1), R1切除患者的中位DFS仅14个月(95% CI:11.1‐17)。实现R0切除的28例患者中有11例(39%)未复发(中位数 = 45个月, 范围 = 25‐71个月)。
结论. R0切除(新辅助治疗的目标)有70%的局部晚期胰腺癌患者可以实现。中位DFS为31个月(95% CI:11.3‐51.1)。肿瘤大小、血管受累程度、癌胚抗原测定(CEA)、CA 19‐9、扫描肿瘤消退程度、CA19‐9下降程度、PET扫描SUV情况与随后的生存期没有关联性。
Discussion
R0 resection is not possible in arterial presentations without arterial resection or neoadjuvant therapy. For those with arterial involvement, we found no difference in the resection rate for those with greater than or less than 180‐degree involvement of the artery with tumor (p = .5). Twenty‐five percent of patients are alive and disease‐free beyond 32 months, and relapses after 24 months of complete remission are rare.
There was no presenting parameter—tumor size, degree of vascular involvement, CA 19‐9, or PET SUV—that predicted the success of this approach.
The only patient with R1 resection alive beyond 38 months was in the venous‐only arm and received GX/RT following resection.
Analysis of parameters predicting outcome of neoadjuvant therapy is shown in Table 1.
Table 1. Presenting parameters.
Thirty‐four patients were in the arterial arm. Five did not undergo surgery because of lack of significant response (2), refusal of operation (1), death during chemotherapy (1), and error in scan interpretation (1). Four patients died in the postoperative period, so the analysis is limited to the 25 patients with arterial involvement who underwent surgery and survived the postoperative period.
The only item that may be significant is the degree of hepatic artery involvement because only two of the six patients with greater than 180‐degree involvement achieved R0 resections. Although those with more extensive arterial involvement may fare worse, some with >180‐degree arterial involvement, including hepatic artery involvement, still achieved R0 resections.
Kaplan‐Meier.
Statistical test: Mann‐Whitney U test.
Chi‐square test.
Bolded values indicate statistical significance.
Abbreviations: CI, confidence interval; CT, computed tomography; DFS, disease‐free survival; m, months; MRI, magnetic resonance imaging; PET, positron emission tomography; R0, complete resection with ≥ 2 mm margin; R1, complete resection with closer or positive margin; SD, standard deviation; SMA, superior mesenteric artery; SUV, standardized uptake values; Vol, volume.
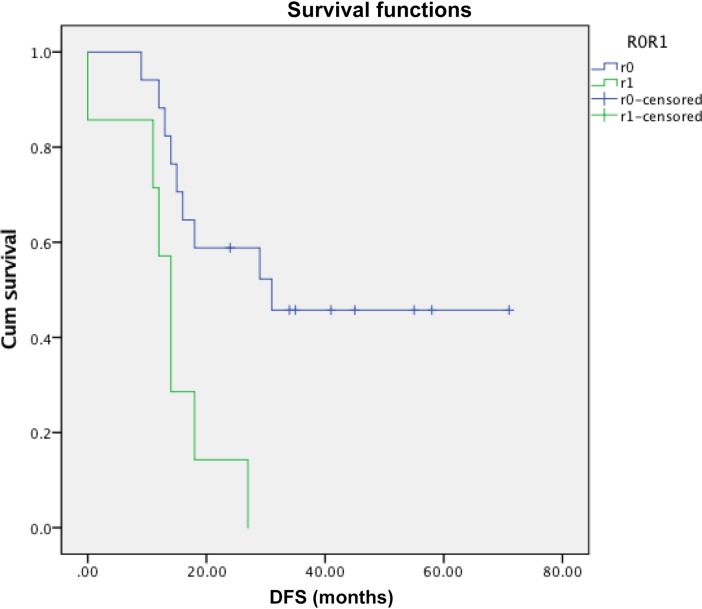
There was a difference in tumor area at initial presentation by MRI or CT scan (p = .02) that correlated with R0 resection (p = .02), but there was significant overlap between the R0 and R1 groups. Neither tumor volume nor SUV by PET analysis distinguished between subsequent R0 and R1 resections or recurrence (p = .52 and p = .68). Standardized uptake value did not predict DFS (p = .85).
There was no statistically significant difference in R0/R1 resection (p = .43), survival (p = .51), or recurrence (p =.79) related to the degree of response on imaging following chemotherapy.
There was no statistically significant difference in R0 resection or DFS between those with less than 180 degrees of encasement and those with greater than 180 degrees of encasement (p = 0.5).
The CA 19‐9 was elevated in 35 patients (median = 501, range = 69–6,363). There was no difference in the CA 19‐9 level between those achieving R0 and R1 resections (p = .74). Although a higher CA 19‐9 at presentation was associated with a shorter DFS (rs = −.42, p = .04), the CA 19‐9 level after 3 cycles (p = .29) or 6 cycles of treatment did not correlate with DFS (p = .29 and p = .10, respectively). By the end of cycle 6, the CA 19‐9 had normalized in 14 of the 35 (40%) patients.
This study supports treating all patients who are surgical candidates and have laparoscopy‐confirmed locally advanced pancreatic adenocarcinoma with neoadjuvant GTX +/− GXRT. With this therapy, the 40% of patients presenting with locally advanced pancreatic cancer can achieve a survival rate similar to that of the 15% of patients presenting with “resectable” cancers. Relapses after 2 years of continuous remission are unusual. Future studies should evaluate longer neoadjuvant chemotherapy or compare this regimen with FOLFIRINOX.
Trial Information
- Disease
Pancreatic cancer
- Stage of Disease/Treatment
Neo‐adjuvant
- Prior Therapy
None
- Type of Study ‐ 1
Phase II
- Type of Study ‐ 2
Single arm
- Primary Endpoint
Overall survival
- Primary Endpoint
Correlative endpoint
- Secondary Endpoint
Overall response rate
- Secondary Endpoint
Tolerability
- Secondary Endpoint
Correlative endpoint
- Additional Details of Endpoints or Study Design
- The primary objective of this study was to determine the effect of neoadjuvant chemotherapy on the 2‐year DFS rate. The secondary objectives were to assess toxicity and efficacy for resection. The standard 2‐year DFS rate for patients with stage II and III pancreatic adenocarcinoma is 5%. We tested the hypothesis that this survival rate is increased to 20% for patients with venous involvement who receive neoadjuvant therapy. To achieve 80% power to detect the difference between 5% using the standard treatment and the 20% using the proposed treatment at the .05 significance level, we planned to accrue 32 patients to receive the proposed treatment. We planned to treat at least 30 patients with arterial involvement (group II) with about the same accrual time (2 years). They were assessed for resectability and followed for survival and sites of relapse. Data reporting the primary outcome of this study have been previously reported [1]. The present analysis was undertaken to determine whether prognostic factors could be identified that would predict the utility or futility of this approach. Parameters analyzed for prediction of outcome were tumor size, degree of vascular involvement, CEA, CA 19‐9, degree of tumor regression on scan, and fall in CA 19‐9 or SUV on PET scan. Note that if the bilirubin was elevated at presentation, treatment was instituted following bile duct stenting, when the bilirubin fell to 1.5 mg/dL. CA 19‐9 was re‐evaluated before treatment started. Statistical Analysis: Comparison and correlation of parameters with OS and DFS were performed using the Mann‐Whitney U test and univariate regression analysis. Kaplan‐Meier analysis with log‐rank test was performed. A p value of <.05 was considered statistically significant. All statistical analysis was performed using a software package (SPSS version 24.0; IBM, Chicago, IL).
- Investigator's Analysis
Active and should be pursued further
Drug Information for Phase II Neoadjuvant GTX
- Drug 1
- Generic/Working name
Gemcitabine
- Trade name
Gemzar
- Company name
Lilly
- Drug type
Small molecule
- Drug class
Anti‐metabolite
- Dose
750 milligrams (mg) per square meter (m2)
- Route
IV
- Schedule of administration
Capecitabine is given for 14 days. Gemcitabine is given over 90 minutes on days 4 and 11, and docetaxel is given over 1 hour on days 4 and 11 for a total of 6 cycles of treatment
- Drug 2
- Generic/Working name
Docetaxel
- Trade name
Taxotere
- Company name
Aventis
- Drug type
Small molecule
- Drug class
Tubulin/Microtubules targeting agent
- Dose
30 mg/m2
- Route
IV
- Schedule of administration
Days 4 and 11 of a 21‐day cycle
- Drug 3
- Generic/Working name
Capecitabine
- Trade name
Xeloda
- Company name
Roche
- Drug type
Small molecule
- Drug class
Anti‐metabolite
- Dose
750 mg/m2
- Route
p.o.
- Schedule of administration
Days 1–14
Drug Information for Phase II GX with Radiation
- Drug 1
- Generic/Working name
Gemcitabine
- Trade name
Gemzar
- Company name
Lilly
- Drug type
Small molecule
- Drug class
Anti‐metabolite
- Dose
750 mg/m2
- Route
IV
- Schedule of administration
Capecitabine is given days 1–5, with gemcitabine on day 5 for weeks 1, 2, 4, and 5 of radiation therapy at the same doses as for the GTX combination
- Drug 2
- Generic/Working name
Capecitabine
- Trade name
Xeloda
- Company name
Roche
- Drug type
Small molecule
- Drug class
Anti‐metabolite
- Dose
750 mg/m2
- Route
p.o.
- Schedule of administration
Capecitabine is given days 1–5, with gemcitabine on day 5 for weeks 1, 2, 4, and 5 of radiation therapy at the same doses as for the GTX combination
Patient Characteristics for Phase II Neoadjuvant GTX
- Number of Patients, Male
18
- Number of Patients, Female
27
- Stage
II/III
- Age
Median (range): median 64 years, range 44–83 years
- Number of Prior Systemic Therapies
Median (range): 0
- Performance Status: ECOG
-
0 —
1 — 45
2 —
3 —
Unknown —
Primary Assessment Method for Phase II Neoadjuvant GTX: Total Patient Population
- Number of Patients Screened
54
- Number of Patients Enrolled
45
- Number of Patients Evaluable for Toxicity
45
- Number of Patients Evaluated for Efficacy
45
- Evaluation Method
Other: Pathology
- Response Assessment CR
n = 3 (6%)
- Response Assessment PR
n = 37 (82%)
- Response Assessment SD
n = 3 (6%)
- Response Assessment PD
n = 2 (6%)
- (Median) Duration Assessments OS
24.8 months, CI: 22–34
- (Median) Duration Assessments Duration of Treatment
5 months
Phase II Neoadjuvant GTX Adverse Events
- Adverse events for this study appeared in the original report [1]. Regarding serious adverse events, one of the patients with a biliary stent presented to the emergency department with hypotension 3 days after treatment. At the time of treatment, the white blood cell count was 3,400. He died of presumed sepsis.
Assessment, Analysis, and Discussion
- Completion
Study completed
- Investigator's Assessment
Active and should be pursued further
Pancreatic cancer is one of the most difficult to treat cancers. It is often discovered too late for surgical resection and historically has been among the most refractory of cancers. A complete surgical resection offers the only prospect of cure, and at the time of diagnosis, only 10% of patients have disease that is considered amenable to resection. This has led to efforts to downstage disease by the administration of combination chemotherapy in the neoadjuvant setting. To date, regimens used in this setting are derived from data obtained in the metastatic setting, and the choice of regimen often depends on the clinician's impression of the patient's ability to tolerate that regimen. The choice is typically made between the two leading regimens for metastatic disease: FOLFIRINOX and gemcitabine/nab‐paclitaxel. The authors, together with investigators at Columbia University Medical Center, developed a regimen, GTX, which combines agents from both regimens. Data for this study were first reported in 2015 [1]; the current report represents an update on the clinical results with longer followup and an analysis of factors that we hypothesized would be predictive of outcome.
Neoadjuvant therapy has become widely accepted in the management of pancreatic cancer. A recent meta‐analysis published in Lancet Oncology reviewed 11 studies with 315 patients with “bordeline resectable” locally advanced pancreatic cancer [2]. The study reported that a quarter of patients treated with FOLFIRINOX for locally advanced pancreatic cancer underwent a surgical resection; three‐quarters had an R0 resection. The study noted that many of the trials reviewed were retrospective studies, and that there was no consensus of opinion about which patients are fit for FOLFIRINOX or which patients could have been resected without neoadjuvant treatment with venous resconstruction [3]. Among the studies reviewed, median overall survival ranged from 10 months to 32.7 months, with a median overall survival of 24.2 months. Progression‐free survival (PFS) ranged from 3 months to 20.4 months, with a median PFS of 15 months. Others have reported lower R0 resection rates [4], [5], which likely reflects differences in what is initially considered “borderline” or “locally advanced” disease and what surgeons are willing to attempt following neoadjuvant therapy.
Our study with GTX demonstrated that R0 resection, the goal of neoadjuvant treatment, can be achieved in 70% of patients presenting with locally advanced pancreatic cancer [1]. The median DFS after R0 resection was 31 m (95% confidence interval [CI]:11.3–51.1). All 10 venous‐only patients were resected, but only 29 of 34 with arterial involvement were resected; however, there was no difference in the median DFS between venous‐only and arterial involvement (p = .82) following R0 resection. Further, 25% of all patients have not relapsed (median = 45 months, range = 25–71 months). We have seen no relapses after 24 months of complete remission. With respect to DFS, four postoperative deaths (liver failure, sepsis, arterial clotting, and pulmonary embolus) and one patient who died following an unrelated fall 15 months after surgery (3 R0, 2 R1) were excluded from analysis. The median DFS for the 25 (71%) with R0 resection was 31 months (95% CI: 11.3–51.1), whereas the median DFS for R1 resection was only 14 months (95% CI: 11.1–17). The difference in DFS between R0 and R1 was significant irrespective of type or degree of vascular involvement (p = .04). For those achieving R0 resection, there was no difference in the median DFS between the 7 patients with venous‐only and the 18 patients with arterial involvement (p = .82).
In this study, we stratified patients between arterial and venous involvement, and excluded patients with venous‐only disease that could be resected with venous reconstruction. Those with arterial involvement of any degree were included because these patients are not believed to be surgically resectable without prior treatment. This analysis shows that there is no presenting feature that identifies patients with laparoscopically confirmed locally advanced pancreatic cancer (LAPC) who will or will not respond adequately and subsequently undergo an R0 resection with the same chance of long‐term DFS as those presenting with primarily resectable tumors. For LAPC, all patients should have laparoscopic evaluation prior to treatment and 15%–20% will be found to have metastatic disease not seen on imaging. This observation was reconfirmed in our study, making 16% of the patients registered ineligible for the study. Laparoscopy should be considered routine in staging for neoadjuvant treatments given the high incidence of metastatic disease, even with modern imaging techniques [6], [7]. Related to this, no patient developed metastasis while on study.
We did not complete enrollment in the venous‐only arm because almost all patients presenting with venous only‐disease were deemed resectable with venous reconstruction, even though they would fall into the definition of borderline resectable disease. As expected, all our patients with venous‐only disease were resected. Other reported neoadjuvant studies included in Suker's meta‐analysis include primarily patients with venous‐only disease, so the benefit of those therapies is difficult to assess. Because there is no proven effective neoadjuvant regimen, this was not designed as a randomized study. We did not find the degree of arterial involvement to predict subsequent resection or survival; however, all patients screened with 360‐degree encasement had metastatic disease.
There was no statistically significant difference in R0 resection or DFS between those with less than 180 degrees of encasement and those with greater than 180 degrees of encasement (p = .5).
There was no statistically significant difference in R0/R1 resection (p = .43), survival (p = .51), or recurrence (p = .79) related to the degree of response on imaging following chemotherapy, although the two patients who did not have disease response did not undergo resection.
CA 19‐9 is a validated tumor marker in pancreatic cancer, reflecting response to therapy and progression [8]. The CA 19‐9 was elevated in 35 patients (median = 501, range = 69–6,363). There was no difference in the CA 19‐9 level between those achieving R0 and R1 resections (p = .74). Although a higher CA 19‐9 at presentation was associated with a shorter DFS (rs= −0.42, p = .04), the CA 19‐9 level after 3 cycles (p = .29) or 6 cycles of treatment did not correlate with DFS (p = .29 and p = .10, respectively). By the end of cycle 6, the CA 19‐9 had normalized in 14 of the 35 (40%) patients.
Those with elevated CA 19‐9 were just as likely to have an R0 resection as those with normal CA 19‐9 (p = .33) at presentation. Residual elevation of CA 19‐9 did not correlate with DFS. No relationship was found between the rate of decrease in CA 19‐9 and the incidence of R0 resection (p = .44) or DFS (p = .93). Even the patient with CA 19‐9 of 6,363 achieved R0 resection. Of the 13 patients whose CA 19‐9 remained elevated after neoadjuvant treatment, 8 (62%) achieved R0 resection. Of the 14 patients who had normalization of CA 19‐9, 10 (71%) had R0 resections.
Given the data supporting use of FOLFIRINOX in pancreatic cancer, a randomized study between FOLFIRINOX and GTX should be considered in the neoadjuvant setting. Future studies should clearly define respectability [4]. Imaging must be centrally reviewed for venous‐only involvement and arterial involvement, and these patients must be considered separately. Further, stringent criteria for R0 resection (≥2 mm margins) need to be stated [9], [10]. Smaller margins have the same survival as R1 resected patients or unresected patients in large retrospective series of patients with resectable disease. And, finally, an argument can be made for assessing the underlying genetic aberrations in patients enrolled on such studies going forward. In a preliminary analysis of patients treated with GTX, we found that outcome of patients with tumors bearing KRAS G12D and G12V mutations had poorer outcome than patients with G12R or G12C mutations [11]. Similarly, patients with pancreatic cancers bearing BRCA mutations may have different outcomes, depending upon the chemotherapeutic regimen administered [12].
Figures and Tables
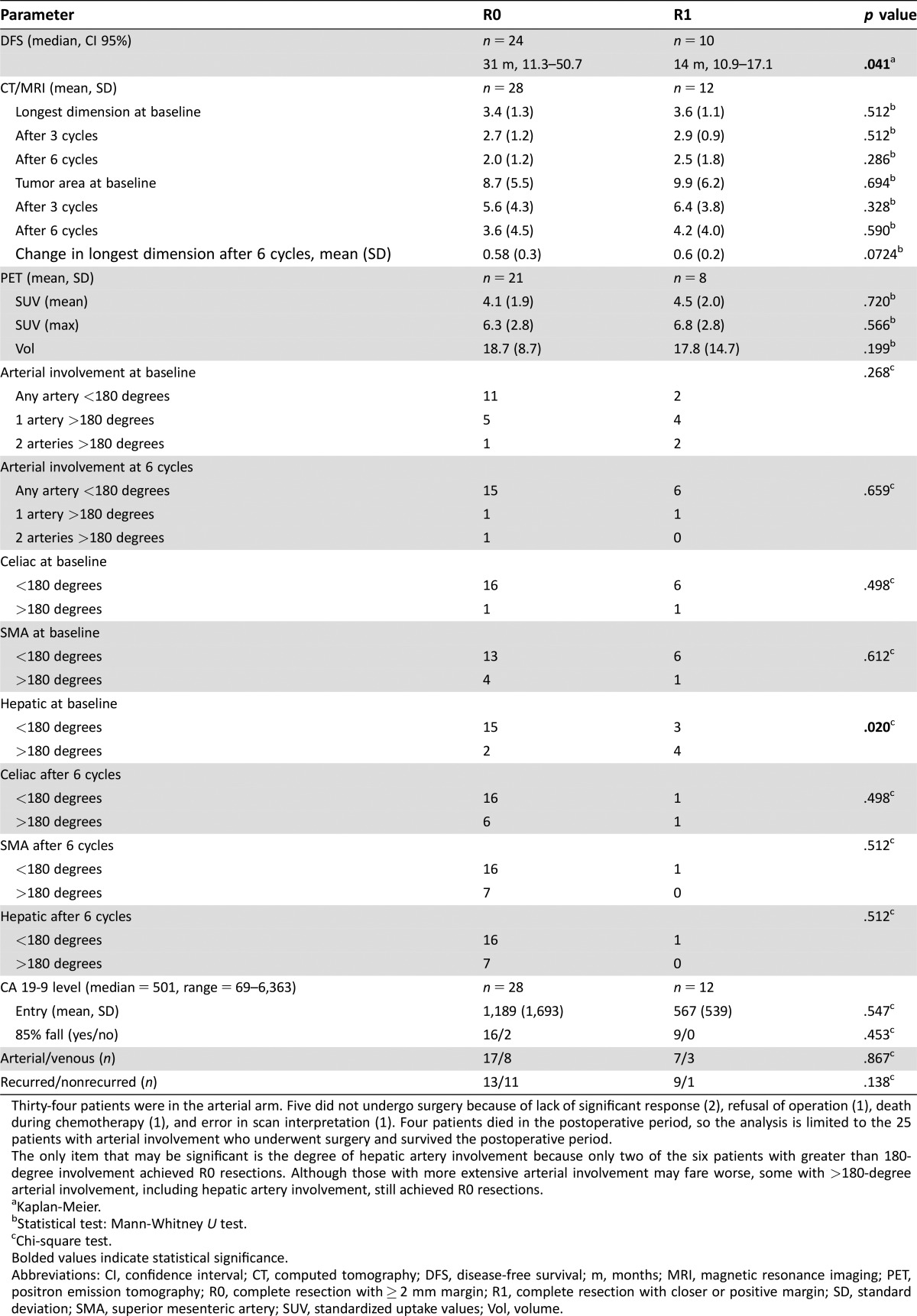
Figure 1.
Disease‐free survival by R0 or R1 resection (p = .04).
Abbreviation: DFS, disease‐free survival.
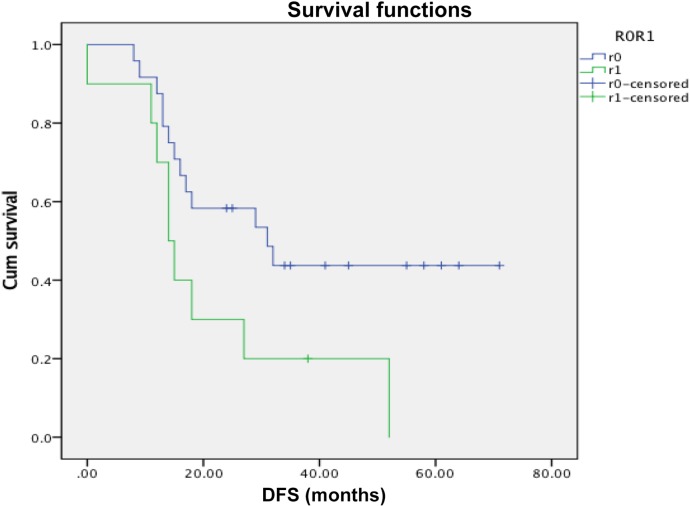
Figure 2.
Disease‐free survival between R0 and R1 in arterial arm only (p = .006).
Abbreviation: DFS, disease‐free survival.
Figure 3.
Difference in R0 resection rates based on tumor area. (A): Baseline magnetic resonance imaging largest dimension. (B): Baseline area and resection. (C): PET mean SUV.
Abbreviations: PET, positron emission tomography; SUV, standardized uptake value.
Footnotes
ClinicalTrials.gov Identifier: NCT00869258
Sponsor(s): None
Principal Investigator: William H. Sherman
IRB Approved: Yes
Disclosures
The authors indicated no financial relationships.
References
- 1. Sherman WH, Chu K, Chabot J et al. Neoadjuvant gemcitabine, docetaxel, and capecitabine followed by gemcitabine and capecitabine/radiation therapy and surgery in locally advanced, unresectable pancreatic adenocarcinoma. Cancer 2015;121:673–680. [DOI] [PubMed] [Google Scholar]
- 2. Suker M, Beumer BR, Sadot E et al. FOLFIRINOX for locally advanced pancreatic cancer: A systematic review and patient‐level meta‐analysis. Lancet Oncol 2016;17:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bockhorn M, Uzunoglu FG, Adham M et al. Borderline resectable pancreatic cancer: A consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2014;155:977–988. [DOI] [PubMed] [Google Scholar]
- 4. Hosein PJ, Macintyre J, Kawamura C et al. A retrospective study of neoadjuvant FOLFIRINOX in unresectable or borderline‐resectable locally advanced pancreatic adenocarcinoma. BMC Cancer 2012;12:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Faris JE, Blaszkowsky LS, McDermott S et al. FOLFIRINOX in locally advanced pancreatic cancer: The Massachusetts General Hospital Cancer Center experience. The Oncologist 2013;18:543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Rosa A, Cameron IC, Gomez D. Indications for staging laparoscopy in pancreatic cancer. HPB (Oxford) 2016;18:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stefanidis D, Grove KD, Schwesinger WH et al. The current role of staging laparoscopy for adenocarcinoma of the pancreas: A review. Ann Oncol 2006;17:189–199. [DOI] [PubMed] [Google Scholar]
- 8. Sperti C, Pasquali C, Catalini S et al. CA 19‐9 as a prognostic index after resection for pancreatic cancer. J Surg Oncol 1993;52:137–141. [DOI] [PubMed] [Google Scholar]
- 9. Campbell F, Smith RA, Whelan P et al. Classification of R1 resections for pancreatic cancer: The prognostic relevance of tumour involvement within 1 mm of a resection margin. Histopathology 2009;55:277–283. [DOI] [PubMed] [Google Scholar]
- 10. Chang DK, Johns AL, Merrett ND et al. Margin clearance and outcome in resected pancreatic cancer. J Clin Oncol 2009;27:2855–2862. [DOI] [PubMed] [Google Scholar]
- 11. Chu K, Sherman WH. Effect of subtype of k‐ras mutation on survival in resected pancreatic adenocarcinoma. J Pancreas 2015;16:569–573. [Google Scholar]
- 12. Golan T, Sella T, O'Reilly EM et al. Overall survival and clinical characteristics of BRCA mutation carriers with stage I/II pancreatic cancer. Br J Cancer 2017;116:697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]