A case of fatal anti‐Hu‐associated autoimmune limbic encephalitis in a patient with myxoid chondrosarcoma treated with a PD‐1 inhibitor is reported.
Abstract
Autoimmune encephalitis is an uncommon complication of immune checkpoint inhibitor therapy. This article reports a case of fatal anti‐Hu‐associated autoimmune limbic encephalitis presenting within 8 weeks following anti‐PD1 therapy in a patient with myxoid chondrosarcoma and pre‐existing anti‐Hu antibodies. Although tumor reduction occurred in response to PD‐1 inhibitor therapy, the patient had a rapidly progressive decline in neurologic function despite initial stabilization with immunosuppression. Considering the increasing use of immune checkpoint inhibitors for the treatment of various malignancies, an increase in the occurrence of neurologic adverse events is likely, requiring prompt intervention and enhanced pharmacovigilance in malignancies associated with onconeuronal antibodies.
Introduction
Immune checkpoint inhibitors, including those targeting programmed cell death 1 (PD‐1), programmed cell death ligand 1, and cytotoxic T‐lymphocyte antigen 4, have shown exceptional and durable objective clinical responses in multiple tumor types [1], [2]. These responses are thought to be related in part to the disinhibition of CD8+ T cells that had been suppressed by signaling via the immune checkpoint inhibitory T‐cell pathways. The mechanism of action is not tumor specific and can promote unintended autoimmunity. Neurologic immune‐related adverse events following immune checkpoint inhibitor therapy are uncommon (<1%) but appear to encompass the spectrum of central and peripheral neuropathies [3]. We describe a patient with extraskeletal myxoid chondrosarcoma who had tumor reduction during anti‐PD‐1 therapy but who developed limbic encephalopathy (LE) associated with anti‐Hu (antineuronal nuclear antibodies) and experienced an ultimately fatal progressive neurologic deficit despite immunosuppressive therapy.
Case Report
The patient was a man aged 46 years diagnosed with extraskeletal myxoid chondrosarcoma (fluorescence in situ hybridization +ve EWSR1 (22q12) chromosome rearrangement) arising in the left lower extremity. Following resection of his primary tumor, he underwent radiation therapy, multiple metastectomies, and several systemic therapies without objective response over the course of the preceding 6 years.
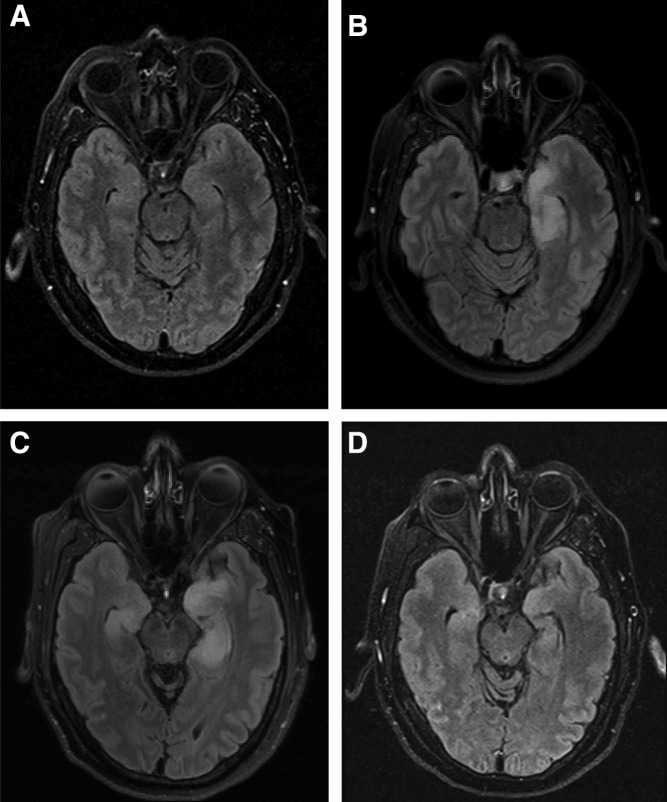
The patient was enrolled in a clinical trial and commenced therapy with the PD‐1 inhibitor REGN2810 (cemiplimab, 3 mg/kg) every 2 weeks (ClinicalTrials.gov identifier NCT02383212). After 4 doses, prior to scheduled computed tomography for tumor assessment at 8 weeks, he reported increasing anxiety and depression. Imaging showed stable disease by RECIST (version 1.1), with 18% reduction in size of metastases (supplemental online Fig. 1). In the week following his fifth infusion, the patient presented with new onset short‐term memory loss. The neurologic examination was otherwise unremarkable. Brain magnetic resonance imaging (MRI) showed prominent abnormalities in the left medial temporal lobe, with lesser involvement of the right temporal and frontal lobes (Fig. 1). Cerebrospinal fluid (CSF) was clear, with lymphocyte‐predominant (83%) pleocytosis (17 cells per microliter; normal ≤5 cells per microliter). Protein (38 mg/dL; normal 15–45 mg/dL) and glucose were normal, and no organisms or malignant cells were present. CSF viral polymerase chain reaction tests were negative for herpes simplex virus 1 and 2. A presumptive diagnosis of autoimmune limbic encephalitis was made and therapy commenced with intravenous (IV) high‐dose methylprednisolone (1 g per day for 5 days) and intravenous immunoglobulin (1 g/kg per day for 2 days). Repeat brain MRI confirmed patchy, T2‐weighted, fluid‐attenuated inversion recovery signal abnormalities involving the temporal lobes, left worse than right, and to a lesser extent the inferior frontal lobe. The patient was continued on oral prednisone 60 mg daily with a slow taper to 10 mg daily and remained stable without further deterioration in mental status. Repeat MRI performed 6 weeks from the initial diagnosis of LE showed noticeable improvement. Despite these findings, within a week after the MRI, the patient's condition unexpectedly rapidly worsened, with progressive confusion, dysarthria, and weakness. He was hospitalized and received high‐dose methylprednisolone (1 g per day for 6 days) followed by oral prednisone, 60 mg daily, and rituximab (375 mg/m2 IV) but became obtunded. His clinical course was complicated by pneumonia. His family decided on hospice comfort care, and the patient died 11 weeks after initial presentation. Post‐mortem findings were consistent with limbic encephalitis; the midbrain was found positive for perivascular lymphocytic infiltrate and diffuse macrophage activity, with CD68 and CD45 immunostains showing macrophages and lymphocytes, respectively. The hippocampus showed diffuse loss of neurons and a CD68‐positive macrophage infiltrate in Ammon's horn, with glial fibrillary acidic protein immunostaining showing gliosis. There was extensive bilateral bronchopneumonia with left upper and lower lobe thromboembolism but no macroscopic evidence of residual metastatic chondrosarcoma.
Figure 1.
Brain fluid‐attenuated inversion‐recovery magnetic resonance imaging (MRI) prior to and during course of therapy with programmed cell death 1 (PD‐1) inhibitor REGN2810 and following immunosuppressive therapy. (A): Baseline prior to PD‐1 therapy. (B): Initial diagnosis at week 9. (C): Brief interval follow‐up at week 10. Panels B and C show abnormalities involving the temporal lobes, left worse than right, and to a lesser extent the inferior frontal lobe. (D): Follow‐up at week 15. Repeat MRI performed 6 weeks from initial diagnosis of limbic encephalopathy and following immunosuppressive therapy shows noticeable improvement.
Analysis of CSF at initial presentation showed an anti‐Hu‐positive 1:16 titer, confirmed on Western blot, that was negative for anti‐Yo, anti‐Ri, voltage‐gated K channel, and GAD65. The serum anti‐Hu titers, both at baseline prior to REGN2810 therapy and at onset of neurologic symptoms, were unchanged at 1:5,120. Immunohistochemical staining of tumor with anti‐HuC/HuD (Molecular Probes, Inc., Eugene, OR) confirmed tumor expression of Hu antigen (supplemental online Fig. 2).
Discussion
We report a case of fatal anti‐Hu‐associated autoimmune limbic encephalitis in a patient with myxoid chondrosarcoma treated with a PD‐1 inhibitor. Anti‐Hu antibodies directed against intraneuronal neuron‐specific RNA‐binding nuclear proteins are most commonly associated with paraneoplastic encephalitis and limited‐stage small cell lung cancer (SCLC) but also occur with other malignancies [4]. Paraneoplastic encephalitis usually precedes the diagnosis of cancer; a minority of Hu‐associated encephalitides present following diagnosis of the malignancy, but they usually present within 12 months of diagnosis [5]. Our patient had a prolonged period (6 years) from initial cancer diagnosis with a number of prior ineffective systemic therapies. The temporal onset of symptoms suggests autoimmune limbic encephalitis triggered after administration of PD‐1 inhibitor therapy. High‐level anti‐Hu antibodies were present before initiation of PD‐1 therapy, and the titer remained unchanged despite onset of neurologic symptoms. Although causality cannot be confirmed, a second patient with anti‐Hu antibodies and SCLC, who developed acute memory loss 9 days following the first dose of the PD‐1 inhibitor nivolumab, with MRI findings consistent with autoimmune limbic encephalitis, strengthens the case for the role of PD‐1 inhibition [[6]; Bristol‐Myers Squibb Suspected Unexpected Serious Adverse Reaction report on file]. Rapid‐onset autoimmune encephalitis has also been associated with combination nivolumib and ipilumumab therapy in a patient with SCLC with antiglial nuclear antibodies and a patient with melanoma with neuronal cell surface anti‐N‐methyl‐D‐aspartate receptor antibodies [7].
Anti‐Hu‐associated LE is difficult to treat and often resistant to immunosuppression but may respond to treatment of the underlying malignancy in some cases [8]. Anti‐Hu‐mediated immune response can be associated with tumor regression [9]. Anti‐Hu‐associated sensory neuronopathy has been described in two patients with myxoid chondrosarcoma, with improvement after chemotherapy and/or surgical resection of tumor and immunosuppression [10], [11]. Despite a response of his tumor to PD‐1 inhibitor therapy and initial brief stabilization following immunosuppression, our patient had a rapidly progressive decline in neurologic function. Although high‐titer anti‐Hu antibodies in patients with limbic encephalitis suggest a paraneoplastic complication of concurrent malignancy, the anti‐Hu antibody in and of itself does not appear to be pathogenic [4]. Evidence suggests that the pathogenesis of intraneuronal antigen‐associated encephalitis is cytotoxic T cell‐mediated [12]. PD‐1 inhibition potentially allows for expansion of tumor‐ or neoantigen‐specific T‐cell clones that might otherwise be suppressed [13]. Interestingly, as with our patient, the published cases of autoimmune checkpoint inhibitor‐associated encephalitis had responses of their malignancy to treatment [7].
Conclusion
Approval by regulatory agencies worldwide and increasing use of immune checkpoint inhibitors for the treatment of various malignancies is likely to increase occurrence of neurologic adverse events such as autoimmune encephalitis in less familiar contexts. Pre‐existing neuronal autoimmune antibodies, such as anti‐Hu, may predispose patients to a higher risk of autoimmune encephalitis, but neither the extent of this risk nor the level of autoimmune antibody required to constitute a risk is known. Therefore, enhanced pharmacovigilance is required before routine screening in patients with malignancies known to have a higher prevalence for such antibodies can be recommended. Vigilance, prompt discontinuation of the immune checkpoint inhibitor therapy, and initiation of aggressive therapeutic immunosuppression will be essential in managing these potentially lethal complications.
Supplementary Material
Acknowledgments
REGN2810 is being developed by Regeneron Pharmaceuticals, Inc., in partnership with Sanofi Genzyme.
Disclosures
Kyriakos P. Papadopoulos: Regeneron Pharmaceuticals (RF); Rebecca S. Romero: Acorda Therapeutics (C/A); Israel Lowy: Regeneron Pharmaceuticals (E, OI); Matthew Fury: Regeneron Pharmaceuticals (E, OI). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Topalian SL, Hodi FS, Brahmer JR et al. Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. N Engl J Med 2012;366:2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol 2015;33:1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hottinger AF. Neurologic complications of immune checkpoint inhibitors. Curr Opin Neurol 2016;29:806–812. [DOI] [PubMed] [Google Scholar]
- 4. Graus F, Keime‐Guibert F, Reñe R et al. Anti‐Hu‐associated paraneoplastic encephalomyelitis: Analysis of 200 patients. Brain 2001;124:1138–1148. [DOI] [PubMed] [Google Scholar]
- 5. Dalmau J, Graus F, Rosenblum MK et al. Anti‐Hu–associated paraneoplastic encephalomyelitis/sensory neuronopathy. A clinical study of 71 patients. Medicine (Baltimore) 1992;71:59–72. [DOI] [PubMed] [Google Scholar]
- 6. Antonia SJ, López‐Martin JA, Bendell J et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small‐cell lung cancer (CheckMate 032): A multicentre, open‐label, phase 1/2 trial. Lancet Oncol 2016;17:883–895. [DOI] [PubMed] [Google Scholar]
- 7. Williams TJ, Benavides DR, Patrice KA et al. Association of autoimmune encephalitis with combined immune checkpoint inhibitor treatment for metastatic cancer. JAMA Neurol 2016;73:928–933. [DOI] [PubMed] [Google Scholar]
- 8. Gultekin SH, Rosenfeld MR, Voltz R et al. Paraneoplastic limbic encephalitis: Neurological symptoms, immunological findings and tumour association in 50 patients. Brain 2000;123:1481–1494. [DOI] [PubMed] [Google Scholar]
- 9. Darnell RB, DeAngelis LM. Regression of small‐cell lung carcinoma in patients with paraneoplastic neuronal antibodies. Lancet 1993;341:21–22. [DOI] [PubMed] [Google Scholar]
- 10. Hoosien M, Vredenburgh J, Lanfranco J et al. A myxoid chondrosarcoma associated with an anti‐Hu‐positive paraneoplastic encephalomyelitis. J Neurooncol 2011;101:135–139. [DOI] [PubMed] [Google Scholar]
- 11. Verschuuren J, Twijnstra A, De Baets M et al. Hu antigens and anti‐Hu antibodies in a patient with myxoid chondrosarcoma. Neurology 1994;44:1551–1552. [DOI] [PubMed] [Google Scholar]
- 12. Bien CG, Vincent A, Barnett MH et al. Immunopathology of autoantibody‐associated encephalitides: Clues for pathogenesis. Brain 2012;135:1622–1638. [DOI] [PubMed] [Google Scholar]
- 13. McGranahan N, Furness AJ, Rosenthal R et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016;351:1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.