Abstract
Purpose
Cough on anesthetic emergence should be prevented considering its dangerous complications. Target-controlled infusion (TCI) of remifentanil can reduce emergence cough effectively, and sex-related differences in effect-site concentration (Ce) of remifentanil have been evaluated in young patients. In this study, we determined the Ce of remifentanil for preventing emergence cough following extubation in male and female elderly patients and evaluated the sex-related difference.
Patients and methods
Twenty-three male and 22 female elderly patients aged between 60 and 75 years were enrolled. Anesthesia was maintained with sevoflurane and remifentanil TCI. The Ce of remifentanil for preventing emergence cough was determined for each sex using isotonic regression method with a bootstrapping approach, following Dixon’s up-and-down method.
Results
The Ce of remifentanil for preventing emergence cough in 50% (EC50) and 95% (EC95) of the population was significantly lower in females than in males. Isotonic regression revealed the EC50 (83% confidence interval [CI]) of remifentanil was 1.67 (1.55–1.83) ng/mL in females and 2.60 (2.29–2.91) ng/mL in males. The EC95 (95% CI) of remifentanil was 2.30 (2.02–2.62) ng/mL in females and 3.41 (3.27–3.58) ng/mL in males. Dixon’s up-and-down method indicated that the mean EC50 in females was lower than in males (1.56±0.26 ng/mL vs 2.56±0.37 ng/mL, P<0.001).
Conclusion
The remifentanil requirement for preventing emergence cough was lower in female than in male elderly patients, indicative of sex-related differences in Ce of remifentanil. Sex should be considered when using remifentanil TCI for preventing emergence cough in elderly patients.
Keywords: effect-site concentration, elderly patients, emergence cough, remifentanil, sex difference
Introduction
Coughing on emergence from general anesthesia is common,1 particularly when an endotracheal tube is utilized. Coughing at extubation may result in serious complications including laryngospasm, negative-pressure pulmonary edema, arterial hypertension, arrhythmia, bleeding, and wound disruption. Based on the American Society of Anesthesiologists recommendation of preformulated strategy for extubation by the anesthesiologist,2 a variety of techniques have been tried to reduce emergence cough including long-acting opioids, lidocaine, dexmedetomidine, and deep extubation.3–6 These may be beneficial, but can delay emergence.
Remifentanil is a potent ultrashort-acting opioid,7 with rapid onset and offset of drug effect. Moreover, it is affected minimally by extremes of age or renal or hepatic dysfunction. Remifentanil allows rapid anesthetic emergence even after a prolonged infusion and decreases the at-risk time during extubation. In addition, cough suppression of remifentanil enables smooth extubation with reduced complications.8,9 Low-dose infusion than bolus dose of remifentanil was determined to decrease the emergence cough;10,11 hence, target-controlled infusion (TCI) has been used to customize infusion rate for individual patients, which maintains a desired target concentration by using pharmacokinetic model.12 To date, several studies have examined the optimal effect-site concentration (Ce) of remifentanil for preventing emergence cough following extubation.13–21
Human studies have indicated that opioid has quantitative and qualitative differences in effect in male and female patients.22–25 Nevertheless, most studies regarding Ce of remifentanil for preventing emergence cough have neglected sex-related difference, except for one study.20 Soh et al reported the occurrence of sex-related differences in the Ce of remifentanil for suppressing emergence cough among young patients aged 20–46 years.20 Although there are little studies regarding differential effect of remifentanil based on sex,26 it may be postulated that the difference is due to the higher mu-opioid receptor availability in females than in males. However, the elderly patient population shows a rapid rise, with 4 times higher surgery rates than young patients.27 High comorbidities of elderly patients may lead to detrimental hemodynamic changes on emergence cough, despite age-related decline of cough reflex.28 In addition, elderly patients have different opioid sensitivity, as compared to young patients.25,29 Thus, there is a need to determine the Ce of remifentanil for smooth extubation in each sex of elderly patients. We hypothesized that there may exist sex-related differences in the Ce of remifentanil for suppressing cough during anesthetic emergence among elderly patients and that male elderly patients may require higher Ce of remifentanil for emergence cough suppression than female elderly patients.
The purpose of this study was to investigate the Ce of remifentanil for preventing emergence cough following extubation during general anesthesia among elderly patients and to evaluate possible sex-based differences.
Patients and methods
This study was approved by Ajou University Hospital Institutional Review Board (protocol number: AJIRB-MED-CT4-15-55, April 16, 2015) and was registered at http://cris.nih.go.kr (registration number: KCT0001910). All data were set and collected in Ajou University School of Medicine. The data collection was performed from September 2015 to June 2016. Twenty-three male and 22 female geriatric patients aged between 60 and 75 years with an ASA physical status I–II who underwent general anesthesia for laparoscopic cholecystectomy were included. Patients were excluded if any of the following criteria were present: Mallampati class 3 or 4, history of difficult intubation or respiratory disease, upper respiratory infection in previous 2 weeks, gastroesophageal reflux disease, uncontrolled hypertension and diabetes mellitus, smoking habit, and body mass index (BMI) >30 kg/m2. Female patients on hormone treatment were also excluded. Written informed consent was obtained from all patients.
All patients were not premedicated. On arrival at the operating room, electrocardiogram, pulse oxygen saturation, noninvasive arterial pressure, end-tidal carbon dioxide (EtCO2), and bispectral index (BIS™ Quatro Sensor; Covidien, Dublin, Ireland) were monitored. Anesthesia was induced with 4–5 mg/kg of thiopental sodium and 1–5 ng/mL target concentration of remifentanil (Ultiva; GlaxoSmithKline, Brentford, UK) using TCI for all patients. TCI of remifentanil was administered with a commercial total intravenous (IV) anesthesia pump (Orchestra® Base Primea; Fresenius Kabi, Bad Homburg, Germany) based on the pharmacokinetic model of Minto et al.30 On absence of response to a verbal or eye stimulus, rocuronium 0.6 mg/kg was administered intravenously. Orotracheal intubation was performed in all patients using a 7.0 mm (internal diameter) tracheal tube for females and an 8.0 mm (internal diameter) tracheal tube for males. Cuff pressure was maintained at 20–25 cm H2O using a hand pressure gauge (Hi-Lo™ Hand Pressure Gauge; VBM Medizintechnik GmbH, Sulz am Neckar, Germany).
Anesthesia was maintained with 1.5–2.5 vol% sevoflurane and a remifentanil Ce of 1–5 ng/mL to maintain a BIS value of 40–55, mean arterial pressure (MAP), and heart rate (HR) within 20% of baseline. Mechanical ventilation was maintained with a tidal volume of 8 mL/kg, air/oxygen mixture (FiO2: 0.5, 3 L/min), and EtCO2 at 35–40 mmHg.
At the time of skin suture, sevoflurane was adjusted to an approximate BIS level of 60, and the Ce of remifentanil was titrated to a predetermined concentration. The Ce was maintained for at least 15 min throughout emergence. At the end of surgery, sevoflurane was discontinued, and fresh gas flow was increased up to 10 L/min. Postoperative analgesics and antiemetics included IV ketorolac 30 mg and ramosetron 0.3 mg, respectively, and IV Bridion® (sugammadex) 3 mg/kg was administered for reversal of neuromuscular block, which was reconfirmed as a train-of-4 ratio >90% using nerve stimulator. Manual ventilation was initiated with maintaining EtCO2 of 35–40 mmHg. During this phase, the patients were not stimulated, except for a verbal request to open their eyes. The tracheal tube was extubated on confirming that the patients showed opening of eyes and spontaneous respiration with an adequate tidal volume and respiratory rate. Subsequently, remifentanil infusion was stopped, and oxygen was supplemented via a facemask for at least 3 min. Extubation was performed by a single anesthesiologist with considerable experience with intubation.
Emergence cough was defined as the cough developed during the period of discontinuing sevoflurane and 3 min after extubation. Hemodynamic data, such as HR and MAP, were recorded at baseline (before induction, T0), end of operation (T1), just before extubation (T2), just after extubation (T3), and 3 min after extubation (T4). Bradycardia (HR <40 bpm) and hypotension (MAP <60 mmHg) were treated with IV atropine 0.5 mg and ephedrine at 4 mg, respectively. The sevoflurane concentration at the time of eye opening was recorded. Time to extubation was defined as the time from discontinuing sevoflurane until extubation. Complications such as bradypnea (respiratory rate <8 bpm), laryngospasm, and desaturation (SpO2 <95% despite oxygen supplementation) were evaluated throughout the emergence period. Sedation score was assessed for each patient on arrival at the recovery room. In addition, overall pain score was evaluated. Pain intensity was evaluated using an 11-point numerical rating scale (NRS; 0= no pain and 10= worst pain). Fentanyl 0.5 μg/kg was administered intravenously in patients who reported an NRS score ≥5.
For estimation of the remifentanil Ce, the up-and-down sequential allocation design was used. The initial Ce of remifentanil was 2 ng/mL for the first patient of each sex, and for the subsequent patient, the predetermined Ce of remifentanil was based on the cough response of the previous patient. If the patient did not cough throughout the peri-extubation period, it was considered as “success”, and the predetermined Ce of remifentanil for the subsequent patient was decreased by 0.5 ng/mL. If the patient coughed at any time before, during, or after extubation, it was considered as “failure”, and the predetermined Ce was increased by 0.5 ng/mL.
Statistics
The primary end point of this study was to investigate the effective concentration of remifentanil in 50% of the population (EC50) and 95% of population (EC95) for preventing emergence cough following extubation in patients aged 60–75 years in each sex. Since females show different sensitivity to opioid than males,25 we hypothesized that there is a sex difference in the Ce of remifentanil for preventing emergence cough.
Based on previous studies in which the EC50 was estimated by the Dixon’s method,20 the stopping rule requires at least 6 success/failure pairs in the same direction. Simulation studies for the up-and-down design suggest that at least 20 patients should be included to obtain stable estimates.31 In our study, 23 and 22 patients were included in male and female groups, respectively, and so there were sufficient numbers to satisfy all requirements.
The EC50 of remifentanil by Dixon’s up-and-down method was defined as the mean value of mid-point dose of the independent crossover pairs for each sex (ie, success to failure), and the mean EC50 values of each sex were compared using independent t-test. To specify the precision of the target concentration, the isotonic regression method was also used to estimate the EC50 and the EC95 of remifentanil along with the confidence interval (CI). From the observed response rate, which presents the ratio of the number of successful patients to the number of subjects at each concentration, an adjusted response probability was calculated by a pooled-adjacent-violators algorithm (PAVA) in order to adhere to the assumption that the drug effect increases with increased dosage. The CI was estimated by a bootstrapping approach.31 If the EC50 and EC95 values did not overlap at the level of the 83% and 95% CIs, respectively, the null hypothesis of equal effective concentrations was rejected at an α of 0.05.31,32
All values were expressed as the mean ± standard deviation (SD), the median (range), or the number of patients. The analysis between sexes was performed using an independent t-test (height, weight, BMI, anesthesia time, time to extubation), Mann–Whitney U test (age, operation time, sevoflurane vol% at eye opening, and NRS), Chi-squared test (ASA physical status and fentanyl administration), or Fisher’s exact test (respiratory complications and sedation score). Repeated-measures variables (MAP and HR) were analyzed using repeated-measures ANOVA with the Bonferroni correction. A P-value of <0.05 was considered statistically significant. Statistical analyses were performed using the Statistical Package for Social Sciences (version 23.0 for Windows; IBM Corporation, Armonk, NY, USA) and R for Windows (version 3.2.5; The R foundation for Statistical Computing).
Results
Among the enrolled 47 patients, 2 male patients were excluded due to delayed skin suturing and an incorrect predetermined Ce of remifentanil, and 23 male and 22 female patients were included in the final analysis (Figure 1). Patient characteristics and operation details are summarized in Table 1. Due to sex-related differences, height and weight were significantly lower in females than in males, but BMI showed no significant difference.
Figure 1.
Consort diagram.
Table 1.
Patients characteristics and operation details
| Variables | Male group (n=23) | Female group (n=22) |
|---|---|---|
| Age (years) | 68±5 | 65±6 |
| Height (cm) | 166.9±6.0 | 155.4±5.2 |
| Weight (kg) | 65.4±6.2 | 58.8±7.1 |
| Body mass index (kg/m2) | 23.5±2.4 | 24.4±2.8 |
| ASA physical status (I/II) | 7/16 | 12/10 |
| Operation time (min) | 40 (25–55) | 30 (25–40) |
| Anesthesia time (min) | 83±26 | 73±16 |
| Sevoflurane vol% at eye opening | 0 (0–0) | 0 (0–0.2) |
Note: Values are mean ± standard deviation or median (range).
Abbreviation: ASA, American Society of Anesthesiologists.
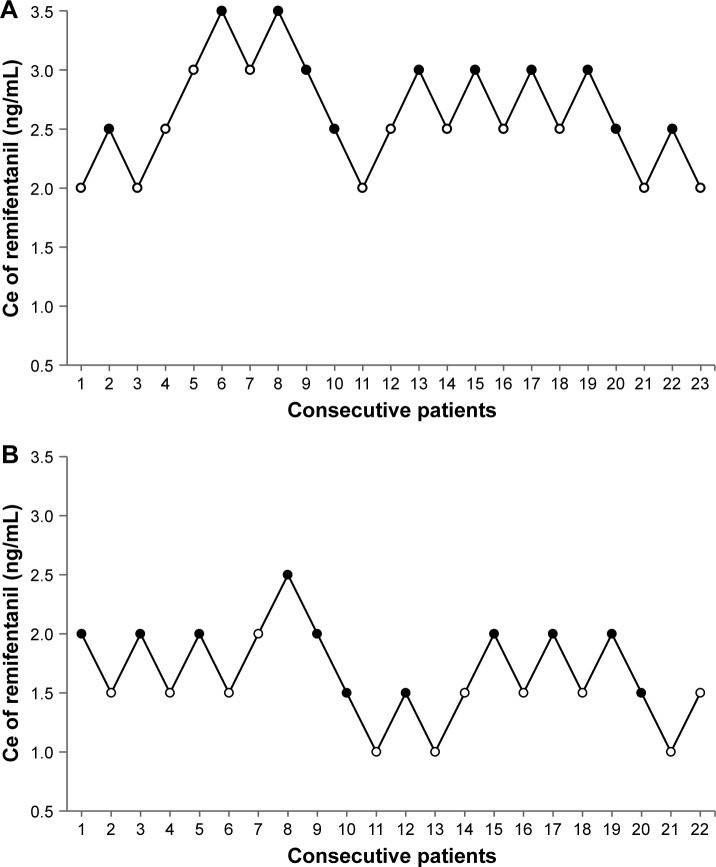
The sequence of successes and failures determined by Dixon’s up-and-down method is shown in Figure 2, and the isotonic regression calculated using the PAVA is shown in Figure 3. The EC50 and EC95 of remifentanil Ce for preventing emergence cough calculated by Dixon’s method or isotonic regression are presented in Table 2. The EC50 of remifentanil Ce estimated by Dixon’s method was lower in females than in males (mean ± SD 1.56±0.26 vs 2.56±0.37 ng/mL; P<0.001). The EC50 (83% CI) of remifentanil Ce estimated by isotonic regression was 1.67 (1.55–1.83) ng/mL in females and 2.60 (2.29–2.91) ng/mL in males. The EC95 (95% CI) of remifentanil Ce estimated by isotonic regression was 2.30 (2.02–2.62) ng/mL in females and 3.41 (3.27–3.58) ng/mL in males. As the EC50 and EC95 values did not overlap at the level of the 83% and 95% CIs, respectively, the Ce of remifentanil for preventing emergence cough was significantly lower in females than in males.
Figure 2.
Assessment of success or failure at preventing emergence cough following extubation under the predetermined remifentanil Ce in consecutive patients determined by Dixon’s up-and-down methods.
Notes: The mean EC50 of remifentanil Ce for preventing emergence cough was calculated from crossover pairs of successes (filled circles) and failures (open circles) in (A) 23 male patients and (B) 22 female patients. The mean ± standard deviation EC50 values of remifentanil Ce values were 2.56±0.37 ng/mL in males and 1.56±0.26 ng/mL in females. EC50 is defined as effective concentration for preventing emergence cough following extubation in 50% of patients.
Abbreviation: Ce, effect-site concentration.
Figure 3.
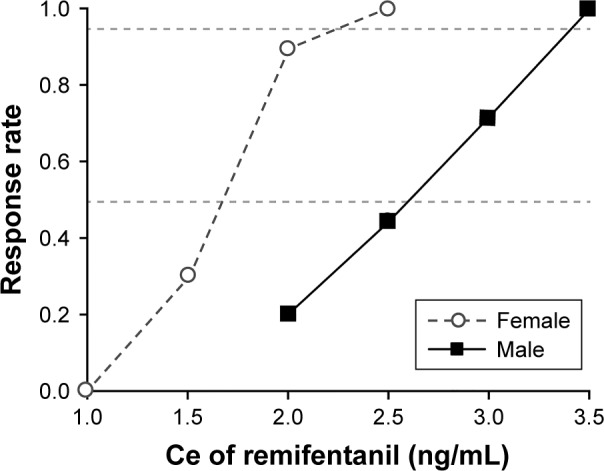
Pooled-adjacent-violators algorithm response rates in females (open circles) and in males (filled circles).
Notes: The EC50 values (83% CI) of the remifentanil Ce for preventing emergence cough following extubation were 2.60 (2.29–2.91) ng/mL in males and 1.67 (1.55–1.83) ng/mL in females. The EC95 values (95% CI) of remifentanil Ce for preventing emergence cough following extubation were 3.41 (3.27–3.58) ng/mL in males and 2.30 (2.02–2.62) ng/mL in females. Both EC50 and EC95 were significantly lower in the female group than in the male group. EC50 is defined as the effective concentration for preventing emergence cough following extubation in 50% of patients. EC95 is defined as the effective concentration for preventing emergence cough following extubation in 95% of patients.
Abbreviations: Ce, effect-site concentration; CI, confidence interval.
Table 2.
Ce of remifentanil for preventing emergence cough following extubation
| Male group (n=23) | Female group (n=22) | |
|---|---|---|
| Dixon’s method (ng/mL) | ||
| EC50 of remifentanil Ce | 2.56±0.37* | 1.56±0.26 |
| Isotonic regression method (ng/mL) | ||
| EC50 of remifentanil Ce | 2.60 (2.29–2.91)# | 1.67 (1.55–1.83) |
| EC95 of remifentanil Ce | 3.41 (3.27–3.58)# | 2.30 (2.02–2.62) |
Notes: Values are expressed as mean ± standard deviation determined by Dixon’s method and the EC50 (83% CI) and EC95 (95% CI) determined by the isotonic regression method.
P<0.001 vs females (independent t-test).
Significantly higher vs females (nonoverlapping CI method).
Abbreviations: Ce, effect-site concentration; CI, confidence interval.
Emergence and recovery profiles are summarized in Table 3. Among respiratory complications, bradypnea was observed in 4 female patients, of which remifentanil Ce was 1, 1.5, 2, and 2 ng/mL. Desaturation was developed in 1 female patient with remifentanil Ce of 1.5 ng/mL. Patients returned to a normal respiratory pattern immediately just by encouragement of deep breathing via facial mask without ventilatory support.
Table 3.
Emergence and recovery profiles
| Variables | Male group (n=23) | Female group (n=22) | P-value |
|---|---|---|---|
| During emergence | |||
| Time to extubation (min) | 13.5±4.1 | 11.1±5.8 | 0.114 |
| Respiratory complications | 0.053 | ||
| Bradypnea | 0 | 4 (18) | |
| Laryngospasm | 0 | 0 | |
| Desaturation | 0 | 1 (5) | |
| At recovery room | |||
| Sedation score (1/2) | 2/21 | 1/21 | >0.999 |
| NRS | 3 (3–5) | 4 (3–7) | 0.160 |
| Patients receiving fentanyl | 5 (22%) | 6 (27%) | 0.932 |
Note: Values are expressed as mean ± standard deviation, median (range), or number (%).
Abbreviation: NRS, numerical rating scale.
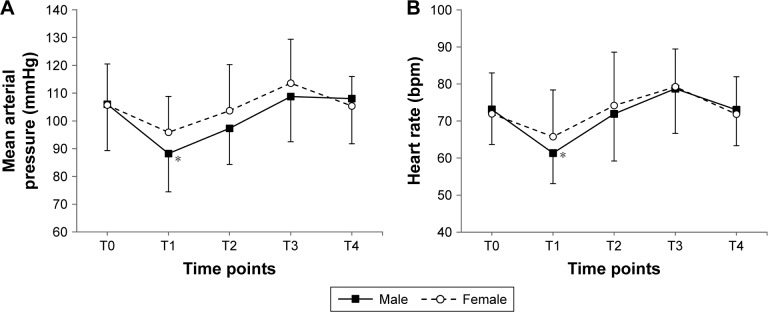
MAP and HR during anesthetic emergence and tracheal extubation are shown in Figure 4. MAP showed no significant difference between sexes (Figure 4A, P=0.271), but MAP in males was significantly lower at the end of operation (T1), as compared with baseline (T0) value (P=0.001). Similarly, there were no significant differences between sexes in HR (Figure 4B, P=0.723), and HR in males was lower at the end of operation (T1), as compared with baseline (T0) value (P<0.001).
Figure 4.
The (A) mean arterial pressure and (B) heart rate during emergence of anesthesia and tracheal extubation.
Notes: Data are expressed as the mean ± standard deviation. *P<0.05 vs T0 for each sex (Bonferroni corrected). T0: before induction; T1: end of operation; T2: just before extubation; T3: just after extubation; T4: 3 min after extubation.
Discussion
In the present study, we implemented balanced anesthesia with sevoflurane and remifentanil TCI in elderly patients undergoing laparoscopic cholecystectomy in order to find the Ce of remifentanil in each sex for smooth extubation during anesthetic emergence. The results indicated that the remifentanil requirement for preventing emergence cough in elderly patients was lower in female subjects (EC50: 1.67 ng/mL) than in male subjects (EC50: 2.60 ng/mL), indicative of sex-related differences.
Cough reflex is mediated by dual-sensory neuron (nociceptor and mechanoreceptor) around trachea and brainstem processing of afferent information.33 While anesthesia affects subcortical processing and regulates a voluntary cough, opioid acts at both brain stem and peripheral nociceptor and reduces a reflexive cough.33 Among opioids for general anesthesia, remifentanil is commonly administered as infusion throughout the anesthesia. It is associated with deeper intraoperative analgesia and anesthesia, faster postoperative recovery, and less respiratory depression compared with short-acting opioids (fentanyl, alfentanil, or sufentanil).34 In addition, remifentanil infusion on anesthetic emergence decreases the emergence cough following extubation.7 Several previous studies, with differing types of surgery and combined anesthetics, have demonstrated reduced severity and incidence of anesthetic emergence cough,14–16,19 or optimal Ce values of remifentanil for preventing emergence cough,13,18,21 with the use of remifentanil TCI. However, most of these studies neglected sex-related differences regarding Ce of remifentanil. Recently, Soh et al reported the Ce of remifentanil for preventing emergence cough in male (EC50: 2.57 ng/mL) and in female (EC50: 1.30 ng/mL) young patients (aged 20–46 years) and existence of sex-related differences.20 To our best knowledge, there has been no report showing sex-related difference in the Ce of remifentanil for preventing emergence cough in elderly patients.
The main result of our study was the sex-related difference in Ce of remifentanil for preventing emergence cough among elderly patients. The sex-related differences in opioid effect may not be restricted to the pain, but have a same direction with other effects such as emesis, respiratory depression, and cough suppression, since they stem from an inherent property of the endogenous opioid receptor system.24 However, sex-related differences in opioid efficacy in elderly patients in prevention of emergence cough remain unclear.
Female elderly patients required lower Ce of remifentanil for preventing emergence cough compared to male elderly patients in the present study. Generally, gender is a significant factor influencing opioid requirement during postoperative period,24,25,35 although the direction of sex-related differences in opioid efficacy is less clear in human studies because of many interacting variables.23 First, sex-related differences in opioid efficacy may contribute to sex-related differences in basal pain perception. There is greater deactivation in pain-related brain regions among females.36 Second, it could result from sex-related differences in sensitivity to opioid. Females differ from males in terms of distribution, expression, or sensitivity of opioid receptors in brain.25 Third, it could be partially mediated by interaction between gonadal hormones and the opioid system.25 Gonadectomy reduces analgesia in males but increases analgesia in females in vivo.37 In a clinical study, Chia et al investigated the influence of age on the requirements for postoperative morphine in approximately 2,300 patients and revealed greater opioid consumption in males than in females.35 This indicates a greater opioid efficacy in female patients.37
The sex-related differences in opioid efficacy are confirmed in young patients, but conflicting in elderly patients.25,38 Clinically, Ce of remifentanil at loss of response to painful stimulus in elderly patients was similar to that in young patients during anesthetic induction.39 In addition, young patients exhibited sex-related differences for postoperative pain and morphine requirement in postanesthesia care unit, but no differences were observed in elderly patients (aged ≥75 years).40 These modulations by aging may be due to the influence of gonadal steroid hormone sensitivity to opioid analgesia.41 In contrast, a meta-analysis concluded persistence of sex-related differences in opioid efficacy among elderly patients, suggesting that pain circuitry of humans is less regulated by sex hormones compared with animals.24 In the present study, elderly patients exhibited sex-related differences in Ce of remifentanil for preventing emergence cough.
There was no great discrepancy in Ce value of remifentanil for preventing emergence cough between elderly patients in the present study and young patients in the previous study.20 Although it is hard to compare between 2 studies because of different conditions including types of surgery, several possibilities may explain the phenomenon. First, elderly patients do not feel less pain than young patients, although the relationship between aging and postoperative pain has not yet been confirmed.38 In Yang et al’s study, remifentanil requirement at loss of response for electrical stimulus using a peripheral nerve stimulator was similar between elderly and young patients.39 Elderly patients tend to have lower pain score and demand less opioid than young patients, despite similar pain intensity.42 Second, the patients in Gerbershagen et al’s and present studies underwent different types of surgery: thyroidectomy and laparoscopic cholecystectomy. Different conditions could require different doses of opioid for the desired effect, even though pain intensities of the surgeries are similar (NRS, 4 [3–6] vs 5 [3–6] in thyroidectomy and laparoscopic cholecystectomy).43
There are some limitations in this study. First, all single cough were considered as “failure” regardless of the severity in this study. But minimal episode of single cough could have been considered as “success” because the single cough may have no clinical impact on practice or the patients. Second, TCI for remifentanil is performed using pharmacokinetic model of Minto et al, which takes into account co-variables such as age and lean body mass (using height and weight). In 1997, Minto et al30 demonstrated that gender has no influence on the pharmacological model. However, the formula for lean body mass used different constants between genders. In addition, the sex-related differences in opioid effect have been raised after then, and there have been some trials about the different effect of remifentanil base on sex.20,26,44
Conclusion
The Ce of remifentanil for preventing emergence cough following extubation was lower in female than in male patients, indicative of sex-related differences in optimal Ce of remifentanil in elderly patients. Sex should be considered when using remifentanil TCI for prevention of emergence cough in elderly patients.
Acknowledgments
This study was presented as a scientific poster at the 2017 Annual Meeting of Euroanesthesia on June 3, 2017, in Switzerland, Geneva.
Footnotes
Author contributions
SYL conceived and designed the experiments, performed the experiments, and wrote the paper. YYJ and BHL contributed to perform the experiments. JEK performed the experiments and wrote the paper. All authors contributed toward data analysis and drafting and critically revising the paper, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kim ES, Bishop MJ. Cough during emergence from isoflurane anesthesia. Anesth Analg. 1998;87(5):1170–1174. doi: 10.1097/00000539-199811000-00036. [DOI] [PubMed] [Google Scholar]
- 2.American Society of Anesthesiologists Task Force on Management of the Difficult Airway Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology. 2003;98(5):1269–1277. doi: 10.1097/00000542-200305000-00032. [DOI] [PubMed] [Google Scholar]
- 3.Lee JY, Lim BG, Park HY, Kim NS. Sufentanil infusion before extubation suppresses coughing on emergence without delaying extubation time and reduces postoperative analgesic requirement without increasing nausea and vomiting after desflurane anesthesia. Korean J Anesthesiol. 2012;62(6):512–517. doi: 10.4097/kjae.2012.62.6.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JH, Koo BN, Jeong JJ, Kim HS, Lee JR. Differential effects of lidocaine and remifentanil on response to the tracheal tube during emergence from general anaesthesia. Br J Anaesth. 2011;106(3):410–415. doi: 10.1093/bja/aeq396. [DOI] [PubMed] [Google Scholar]
- 5.von Ungern-Sternberg BS, Davies K, Hegarty M, Erb TO, Habre W. The effect of deep vs. awake extubation on respiratory complications in high-risk children undergoing adenotonsillectomy: a randomised controlled trial. Eur J Anaesthesiol. 2013;30(9):529–536. doi: 10.1097/EJA.0b013e32835df608. [DOI] [PubMed] [Google Scholar]
- 6.Lee JS, Choi SH, Kang YR, Kim Y, Shim YH. Efficacy of a single dose of dexmedetomidine for cough suppression during anesthetic emergence: a randomized controlled trial. Can J Anaesth. 2015;62(4):392–398. doi: 10.1007/s12630-014-0295-6. [DOI] [PubMed] [Google Scholar]
- 7.Beers R, Camporesi E. Remifentanil update: clinical science and utility. CNS Drugs. 2004;18(15):1085–1104. doi: 10.2165/00023210-200418150-00004. [DOI] [PubMed] [Google Scholar]
- 8.Twersky RS, Jamerson B, Warner DS, Fleisher LA, Hogue S. Hemodynamics and emergence profile of remifentanil versus fentanyl prospectively compared in a large population of surgical patients. J Clin Anesth. 2001;13(6):407–416. doi: 10.1016/s0952-8180(01)00292-6. [DOI] [PubMed] [Google Scholar]
- 9.Mandel JE. Considerations for the use of short-acting opioids in general anesthesia. J Clin Anesth. 2014;26(1 Suppl):S1–S7. doi: 10.1016/j.jclinane.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Aouad MT, Al-Alami AA, Nasr VG, Souki FG, Zbeidy RA, Siddik-Sayyid SM. The effect of low-dose remifentanil on responses to the endotracheal tube during emergence from general anesthesia. Anesth Analg. 2009;108(4):1157–1160. doi: 10.1213/ane.0b013e31819b03d8. [DOI] [PubMed] [Google Scholar]
- 11.Shajar MA, Thompson JP, Hall AP, Leslie NA, Fox AJ. Effect of a remifentanil bolus dose on the cardiovascular response to emergence from anaesthesia and tracheal extubation. Br J Anaesth. 1999;83(4):654–656. doi: 10.1093/bja/83.4.654. [DOI] [PubMed] [Google Scholar]
- 12.Albrecht S, Hering W, Schuttler J, Schwilden H. Neue intravenöse Anästhetika Remifentanil, S(+)-Ketamin, Eltanolon und Target Controlled Infusion [New intravenous anesthetics. Remifentanil, S(+)-ketamine, eltanolone and target controlled infusion] Anaesthesist. 1996;45(12):1129–1141. doi: 10.1007/s001010050349. German [with English abstract] [DOI] [PubMed] [Google Scholar]
- 13.Lee B, Lee JR, Na S. Targeting smooth emergence: the effect site concentration of remifentanil for preventing cough during emergence during propofol-remifentanil anaesthesia for thyroid surgery. Br J Anaesth. 2009;102(6):775–778. doi: 10.1093/bja/aep090. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Li W, Wang D, Hu X. The effect of remifentanil on cough suppression after endoscopic sinus surgery: a randomized study. Acta Anaesthesiol Scand. 2010;54(10):1197–1203. doi: 10.1111/j.1399-6576.2010.02303.x. [DOI] [PubMed] [Google Scholar]
- 15.Jun NH, Lee JW, Song JW, Koh JC, Park WS, Shim YH. Optimal effect-site concentration of remifentanil for preventing cough during emergence from sevoflurane-remifentanil anaesthesia. Anaesthesia. 2010;65(9):930–935. doi: 10.1111/j.1365-2044.2010.06450.x. [DOI] [PubMed] [Google Scholar]
- 16.Lim JH, Ryu SJ, Lim YS. The incidence of cough induced by remifentanil during anesthetic induction was decreased by graded escalation of the remifentanil concentration. Korean J Anesthesiol. 2010;58(2):117–121. doi: 10.4097/kjae.2010.58.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi EM, Park WK, Choi SH, Soh S, Lee JR. Smooth emergence in men undergoing nasal surgery: the effect site concentration of remifentanil for preventing cough after sevoflurane-balanced anaesthesia. Acta Anaesthesiol Scand. 2012;56(4):498–503. doi: 10.1111/j.1399-6576.2011.02620.x. [DOI] [PubMed] [Google Scholar]
- 18.Cho HB, Kim JY, Kim DH, Kim DW, Chae YJ. Comparison of the optimal effect-site concentrations of remifentanil for preventing cough during emergence from desflurane or sevoflurane anaesthesia. J Int Med Res. 2012;40(1):174–183. doi: 10.1177/147323001204000118. [DOI] [PubMed] [Google Scholar]
- 19.Chang CH, Lee JW, Choi JR, Shim YH. Effect-site concentration of remifentanil to prevent cough after laryngomicrosurgery. Laryngoscope. 2013;123(12):3105–3109. doi: 10.1002/lary.24199. [DOI] [PubMed] [Google Scholar]
- 20.Soh S, Park WK, Kang SW, Lee BR, Lee JR. Sex differences in remifentanil requirements for preventing cough during anesthetic emergence. Yonsei Med J. 2014;55(3):807–814. doi: 10.3349/ymj.2014.55.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoo JY, Kim JY, Kwak HJ, et al. Effect-site concentration of remifentanil for preventing cough during emergence in elderly patients undergoing nasal surgery: a comparison with adult patients. Clin Interv Aging. 2016;11:1247–1252. doi: 10.2147/CIA.S108705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Craft RM. Sex differences in opioid analgesia: “from mouse to man”. Clin J Pain. 2003;19(3):175–186. doi: 10.1097/00002508-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Dahan A, Kest B, Waxman AR, Sarton E. Sex-specific responses to opiates: animal and human studies. Anesth Analg. 2008;107(1):83–95. doi: 10.1213/ane.0b013e31816a66a4. [DOI] [PubMed] [Google Scholar]
- 24.Niesters M, Dahan A, Kest B, et al. Do sex differences exist in opioid analgesia? A systematic review and meta-analysis of human experimental and clinical studies. Pain. 2010;151(1):61–68. doi: 10.1016/j.pain.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 25.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., 3rd Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10(5):447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park SJ, Park HJ, Choi JY, Kang HS, Choi HS. The influence of age and gender on remifentanil EC(50) for preventing rocuronium induced withdrawal movements. Korean J Anesthesiol. 2010;58(3):244–248. doi: 10.4097/kjae.2010.58.3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aubrun F, Marmion F. The elderly patient and postoperative pain treatment. Best Pract Res Clin Anaesthesiol. 2007;21(1):109–127. doi: 10.1016/j.bpa.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Ebihara S, Ebihara T, Kohzuki M. Effect of aging on cough and swallowing reflexes: implications for preventing aspiration pneumonia. Lung. 2012;190(1):29–33. doi: 10.1007/s00408-011-9334-z. [DOI] [PubMed] [Google Scholar]
- 29.Bowie MW, Slattum PW. Pharmacodynamics in older adults: a review. Am J Geriatr Pharmacother. 2007;5(3):263–303. doi: 10.1016/j.amjopharm.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Minto CF, Schnider TW, Egan TD, et al. Influence of age and gender on the pharmacokinetics and pharmacodynamics of remifentanil. I. Model development. Anesthesiology. 1997;86(1):10–23. doi: 10.1097/00000542-199701000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Pace NL, Stylianou MP. Advances in and limitations of up-and-down methodology: a precis of clinical use, study design, and dose estimation in anesthesia research. Anesthesiology. 2007;107(1):144–152. doi: 10.1097/01.anes.0000267514.42592.2a. [DOI] [PubMed] [Google Scholar]
- 32.Payton ME, Greenstone MH, Schenker N. Overlapping confidence intervals or standard error intervals: what do they mean in terms of statistical significance? J Insect Sci. 2003;3:34. doi: 10.1093/jis/3.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Canning BJ, Chang AB, Bolser DC, Smith JA, Mazzone SB, McGarvey L, CHEST Expert Cough Panel Anatomy and neurophysiology of cough: CHEST Guideline and Expert Panel report. Chest. 2014;146(6):1633–1648. doi: 10.1378/chest.14-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Komatsu R, Turan AM, Orhan-Sungur M, McGuire J, Radke OC, Apfel CC. Remifentanil for general anaesthesia: a systematic review. Anaesthesia. 2007;62(12):1266–1280. doi: 10.1111/j.1365-2044.2007.05221.x. [DOI] [PubMed] [Google Scholar]
- 35.Chia YY, Chow LH, Hung CC, Liu K, Ger LP, Wang PN. Gender and pain upon movement are associated with the requirements for postoperative patient-controlled iv analgesia: a prospective survey of 2,298 Chinese patients. Can J Anaesth. 2002;49(3):249–255. doi: 10.1007/BF03020523. [DOI] [PubMed] [Google Scholar]
- 36.Henderson LA, Gandevia SC, Macefield VG. Gender differences in brain activity evoked by muscle and cutaneous pain: a retrospective study of single-trial fMRI data. Neuroimage. 2008;39(4):1867–1876. doi: 10.1016/j.neuroimage.2007.10.045. [DOI] [PubMed] [Google Scholar]
- 37.Bodnar RJ, Kest B. Sex differences in opioid analgesia, hyperalgesia, tolerance and withdrawal: central mechanisms of action and roles of gonadal hormones. Horm Behav. 2010;58(1):72–81. doi: 10.1016/j.yhbeh.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 38.Wilder-Smith OH. Opioid use in the elderly. Eur J Pain. 2005;9(2):137–140. doi: 10.1016/j.ejpain.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 39.Yang N, Zuo MZ, Yue Y, Wang Y, Shi Y, Zhang XN. Comparison of C50 for propofol-remifentanil target-controlled infusion and bispectral index at loss of consciousness and response to painful stimulus in elderly and young patients. Chin Med J (Engl) 2015;128(15):1994–1999. doi: 10.4103/0366-6999.161338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aubrun F, Salvi N, Coriat P, Riou B. Sex- and age-related differences in morphine requirements for postoperative pain relief. Anesthesiology. 2005;103(1):156–160. doi: 10.1097/00000542-200507000-00023. [DOI] [PubMed] [Google Scholar]
- 41.Aloisi AM. Gonadal hormones and sex differences in pain reactivity. Clin J Pain. 2003;19(3):168–174. doi: 10.1097/00002508-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Gagliese L, Weizblit N, Ellis W, Chan VW. The measurement of postoperative pain: a comparison of intensity scales in younger and older surgical patients. Pain. 2005;117(3):412–420. doi: 10.1016/j.pain.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 43.Gerbershagen HJ, Aduckathil S, van Wijck AJ, Peelen LM, Kalkman CJ, Meissner W. Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Anesthesiology. 2013;118(4):934–944. doi: 10.1097/ALN.0b013e31828866b3. [DOI] [PubMed] [Google Scholar]
- 44.Kim SY, Song MK, Kim MS, Kim EH, Han DW. Sex-related differences in the effect-site concentration of remifentanil for preventing QTc interval prolongation following intubation in elderly patients with a normal QTc interval. Drugs Aging. 2014;31(9):695–702. doi: 10.1007/s40266-014-0198-9. [DOI] [PubMed] [Google Scholar]