Abstract
Purpose
The purpose of this study was to determine the clinical utility of an algorithm-based decision tool designed to assess risk associated with opioid use. Specifically, we sought to assess how physicians were using the profile in patient care and how its use affected patient outcomes.
Patients and methods
A prospective, longitudinal study was conducted to assess the utility of precision medicine testing in 5,397 patients across 100 clinics in the USA. Using a patent-protected, validated algorithm combining specific genetic risk factors with phenotypic traits, patients were categorized into low-, moderate-, and high-risk patients for opioid abuse. Physicians who ordered precision medicine testing were asked to complete patient evaluations and document their actions, decisions, and perceptions regarding the utility of the precision medicine tests. The patient outcomes associated with each treatment action were carefully documented.
Results
Physicians used the profile to guide treatment decisions for over half of the patients. Of those, guided treatment decisions for 24.5% of the patients were opioid related, including changing the opioid prescribed, starting an opioid, or titrating a patient off the opioid. Treatment guidance was strongly influenced by profile-predicted opioid use disorder (OUD) risk. Most importantly, patients whose physicians used the profile to guide opioid-related treatment decisions had improved clinical outcomes, including better pain management by medication adjustments, with an average pain decrease of 3.4 points on a scale of 1–10.
Conclusion
Patients whose physicians used the profile to guide opioid-related treatment decisions had improved clinical outcomes, as measured by decreased pain levels resulting from better pain management with prescribed medications. The clinical utility of the profile is twofold. It provides clinically actionable recommendations that can be used to 1) prevent OUD through limiting initial opioid prescriptions and 2) reduce pain in patients at low risk of developing OUD.
Keywords: precision medicine, personalized medicine, opioid, pain management, opioid use disorder, clinical utility, patient outcomes
Introduction
Pain medications play a critical role in treating more than 100 million Americans suffering from chronic pain.1 However, the prescription opioid abuse epidemic is a real public health issue, with skyrocketing overdose rates and burgeoning health care costs.2–6 Physicians are burdened with treating pain appropriately while simultaneously mitigating the risk of opioid use disorder (OUD).
To help physicians navigate this tightrope, the Centers for Disease Control (CDC) released opioid prescribing guidelines. These guidelines lay out three main foci for physicians: 1) determining “when to initiate or continue pain management with opioids”; 2) managing “opioid selection, dosage, duration, and discontinuation”; and 3) “assessing risk and addressing harms of opioid use”.7 Currently utilized screening tools include the Screener and Opioid Assessment for Patients with Pain-Revised (SOAPP-R),8,9 the Opioid Risk Tool (ORT),10 and the Brief Risk Interview.11,12 These screening assessments, however, are based completely on subjective information. Furthermore, three previous studies demonstrated that the algorithm described herein, referred to as “profile”, performed with greater sensitivity and specificity than either the ORT or the SOAPP-R.13–15
The improved sensitivity and specificity of the profile over other current screening measures stems from its unique algorithm that combines clinical risk factors with objective genotypic information.13–15 The clinical validity of the profile has been examined in multiple clinical settings, including primary care,14 addiction treatment centers,15 pain clinics,13 and orthopedic clinics,13 among others. In these studies, the high accuracy of the profile, ranging from 76% to 97%, demonstrated its diagnostic potential as a predictor of risk to opioid abuse.13–15 The profile can be used clinically to determine patients for whom opioids should be avoided, those who need more frequent monitoring, or those for whom opioids are a low-risk treatment, with regard to OUD.
Previous studies demonstrated the profile is beneficial for physician decision making and patient clinical improvement. This study is focused on specific opioid-related treatment actions and their downstream effect on patient outcomes. We observed that the profile is a clinically actionable tool that can be used to guide opioid-related medication regimens, which result in improved patient outcomes. This study demonstrates the utility of the profile to provide physicians with actionable information that fulfill the three aims of the CDC guidelines.
Patients and methods
Study population
A prospective, longitudinal study was conducted to assess the clinical utility of precision medicine testing in 5,397 patients across 100 clinics in the USA. This study (Protocols 1JAN15-14CR, 1JAN15-20CR) was reviewed, approved, and overseen by Solutions IRB, an institutional review board licensed by the USA Department of Health and Human Services, Office for Human Research Protections. Additionally, patients were enrolled in the study by participating physicians based on medical necessity for assessment of risk to opioid abuse. All participants signed informed consent forms prior to data collection. Per protocol, the exclusion criteria were significantly diminished mental capacity, recent febrile illness that precludes or delays participation by more than 1 month, pregnancy or lactation, incomplete gene report, participation in a clinical study that may interfere with participation in this study, and anything that would place the individual at increased risk or preclude full compliance.
Data collection
Data were collected across three visits: 1) an initial visit (day 0) during which buccal swabs and patient information were collected, 2) a baseline visit (~day 30) during which physicians received and presented profile results to patients and implemented any changes to their medical regimen, and 3) a follow-up visit (~day 60) to evaluate patient clinical status.
Genomic DNA was isolated from buccal swabs obtained from each patient using a proprietary DNA isolation technique and DNA isolation kit (Macherey Nagel GmbH & Co, KG, Germany), according to the manufacturer’s instructions. Genotyping was performed using predesigned TaqMan® assays (Thermo Fisher Scientific, Waltham, MA, USA). Allele-specific fluorescence signals were distinguished by measuring end point 6-fluorescein or VIC fluorescence intensities at 508 and 560 nm, respectively, and genotypes were generated using Genotyper® Software V 1.3 (Thermo Fisher Scientific). The DNA elution buffer was used as a negative control, and K562 cell line DNA (Promega Corporation, Madison, WI, USA) was included in each batch of samples tested as positive control.
Age and phenotypic information were also collected at the initial visit, including family histories of alcoholism, illegal drug abuse, prescription drug abuse; and personal histories of alcoholism, illegal drug abuse, prescription drug abuse, depression, and/or other mental health disorders.
Opioid risk profile
A profile score and its associated risk stratification were calculated for each subject. The profile score is an algorithm-based, validated measure of OUD risk. In short, it combines phenotypic and genotypic information to calculate a risk score that correlates to low-, moderate-, or high-risk stratifications of OUD.13–15 The genetic markers used in the algorithm include 11 different single-nucleotide polymorphisms that have been implicated in opioid abuse, misuse, dependence, or addiction (Table S1). This approach, which focuses on validated genetic variants, as opposed to comprehensive next-generation sequencing, is the preferred approach of many in the field.16 The phenotypic factors tested include age of 16–45 years17,18 and personal histories of alcohol abuse,19,20 illegal drug abuse,10,21 prescription drug abuse, depression,22–24 and other mental health diseases (e.g., attention deficit disorder, obsessive–compulsive disorder, bipolar disorder, and schizophrenia). The algorithm that comprises the profile is 42% genotypic information and 58% phenotypic information.
Assessment of clinical utility
Physicians who ordered precision medicine testing were asked to complete patient evaluations two times in the study: 1) during the baseline visit (~day 30), after the profile results were available for review, and 2) when physicians were conducting a follow-up visit (~day 60) to evaluate patient improvement. The evaluation form consisted of a 15-item checklist of actions or decisions in the patient’s treatment that the physician might have made using profile guidance (Table S2), and was used to document the physician’s assessment of the validity and utility of the profile. The evaluation included eight interventions that would result in a change to the opioid medication regimen of the patient. The physician also rated the benefit of the profile on clinical decision making during the baseline visit and the benefit of the profile to patient outcomes during the follow-up on a scale of 1–5 (1: no benefit, 5: significant benefit). There were 9,057 ratings in total – 4,836 and 4,221 ratings for patients’ baseline and follow-up visits, respectively, which were assessed ~1 month apart.
Patient-centered outcomes
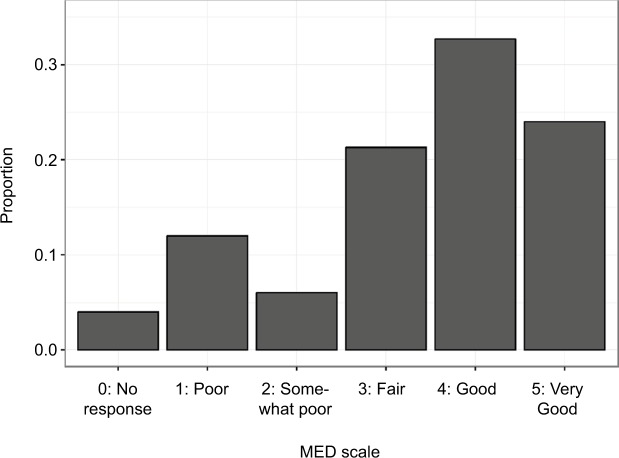
At the baseline and follow-up visits, patients were asked to use the pain numeric rating scale (NRS) to rate the level of their pain before and after taking medications. NRS scores of 7–10 correspond to severe pain, 4–6 to moderate pain, and 1–3 to mild pain.25 The NRS scores before and after taking medication from the follow-up visit were used in analyses to assure the change in pain levels was due to a change the patient’s physician made after receiving the profile test results (Figures 1 and 2). Also, at the follow-up visit, patients were asked to list their individual medications and rate their response to each one using the Medication Efficacy Differentiation scale (MED scale; Figure 3). The MED scale is a numeric scale that indicates how well the medication is working to treat the patients’ condition(s) and/or symptom(s). It ranges from 0 to 5, where 0 is “no response”, 1 is “poor”, 2 is “somewhat poor”, 3 is “fair”, 4 is “good”, and 5 is “very good”.
Figure 1.
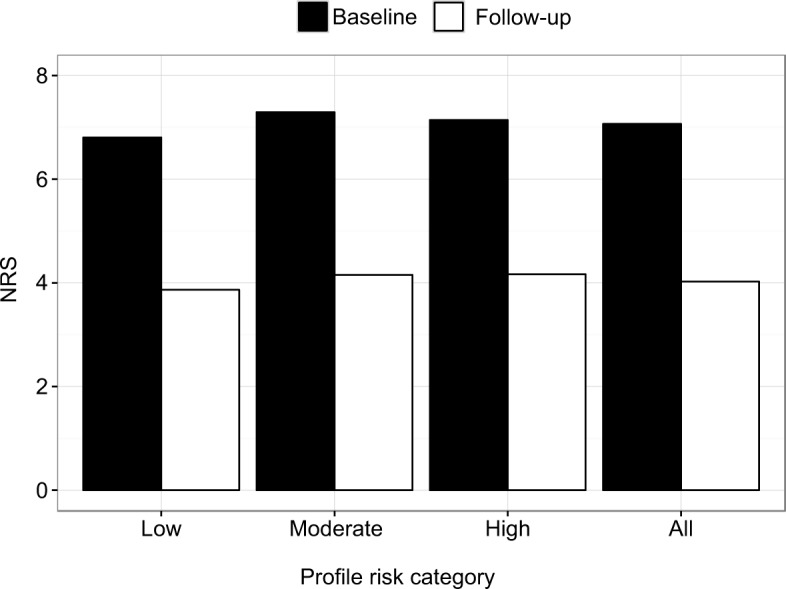
Average pain NRS scores at baseline and follow-up visits by profile risk category.
Note: For patients of each profile risk category, pain NRS at follow-up visit was significantly lower at the follow-up visit than at the baseline visit (p≤10−9).
Abbreviation: NRS, numeric rating scale.
Figure 2.
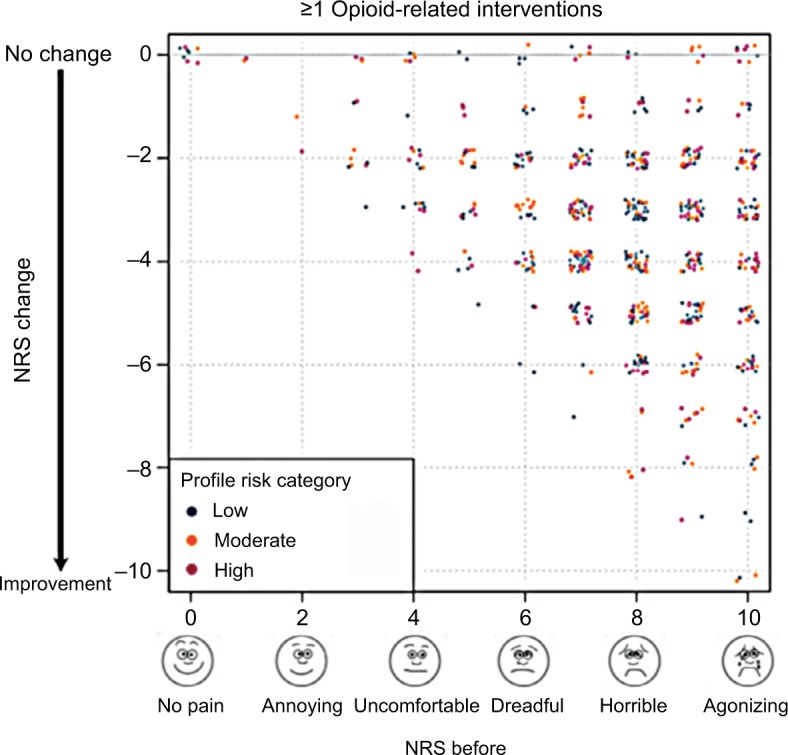
Change in pain NRS scores after patients received opioid-related interventions.
Notes: On average, patients’ pain scores decreased by 3.4 points (p=3.39×10−108) after physicians implemented opioid-related guidance from the profile. There was no obvious difference in NRS change based on profile risk category. For plotting, individual data points were displayed.
Abbreviation: NRS, numeric rating scale.
Figure 3.
Distribution of MED scale among patients whose physicians implemented a change in their opioid medication regimen based on profile results.
Notes: Overall, due to receiving opioid-related interventions guided by the profile, 78% of patients reported a MED scale of ≥3 and 57% reported a MED scale of ≥4. Patients who were given a new opioid prescription based on the profile results – that is, reported taking an opioid for ≤30 days at the follow-up visit (n=150) – indicated fair to good response to their medications (MED scale average rating 3.4). Response to medications was reported to be the lowest (2.6) among individuals who scored as high risk on the profile test.
Abbreviation: MED, Medication Efficacy Differentiation.
Only patients who recently started using opioids, reported their medications, and whose physicians noted a related opioid-related intervention (change in opioid medication or started an opioid) were included in the analysis of the MED scale ratings. If a patient reported more than one opioid medication, MED scale ratings were averaged for patients who reported starting a new opioid within 30 days prior to the follow-up visit.
Statistical analysis
To determine any significant changes in pain levels, the Wilcoxon signed rank sum test for paired data was used to test for significant differences in pain NRS scores before and after their physicians altered their opioid medication regimen. For baseline and follow-up visits, ordinal logistic regression was used to determine the association of ratings with whether or not physicians used the profile to guide patient care decisions, as well as with specific decisions the physicians made, adjusting for age, gender, and race when appropriate. To maintain sufficient sample size, race was categorized as African-American, Caucasian, Hispanic, other, and those who declined to answer. Odds ratios reported for ordinal logistic regression are proportional odds across comparing all possible consecutive ratings (i.e., rating of 5 versus 4, 4 versus 3, and so on). Statistical analysis was performed with R version 3.2.5.
Results
Physicians alter opioid treatment based on profile results
In total, 5,397 patients were included in this study (Table 1). These patients’ physicians received profile results and completed an intervention evaluation (Table S2) describing their clinical decision making based on the results of the profile. In total, physicians took clinical actions based on the profile results (“profile-guided”) for 2,809 (52%) patients (Table 2; Table S3). As a direct consequence of receiving profile results, physicians altered the opioid medication regimen for 691 patients (24.5% of the total profile-guided patients). This included changing the opioid prescribed, starting an opioid rotation, switching from a short-/long-acting to a long-/short-acting opioid, increasing/decreasing total opioid dose or frequency, titrating the patient off opioids, adding a non-opioid, and/or switching from an opioid to a non-opioid (Table S2). The most frequent opioid-based intervention was to change the opioid prescribed (50% of the patients), followed by starting an opioid rotation (19% of the patients; Table 2).
Table 1.
Subject demographics
| Race | Female n (%) | Male n (%) | Total n (%) | Average age (years) |
|---|---|---|---|---|
| African-American | 333 (10) | 155 (7.5) | 488 (9.0) | 56 |
| American-Indian | 10 (<1) | 10 (<1) | 20 (<1) | 60 |
| Asian/Pacific Islander | 41 (1.2) | 26 (1.3) | 67 (1.2) | 53 |
| Caucasian/Non-Hispanic | 2,555 (77) | 1,617 (78) | 4,172 (77) | 58 |
| Hispanic | 168 (5.0) | 114 (5.5) | 282 (5.2) | 57 |
| Mixed | 41 (1.2) | 16 (<1) | 57 (1.1) | 54 |
| Other | 47 (1.4) | 36 (1.7) | 83 (1.5) | 56 |
| Declined to specify | 137 (4.1) | 87 (4.2) | 228 (4.2) | 57 |
| Total | 3,332 (68) | 2,061 (28) | 5,397a | 57 |
Note:
Total number of subjects includes four individuals who declined to specify any demographics.
Table 2.
Profile-guided opioid-related interventions resulted in significant decreases in patient pain
| Opioid-related intervention | Profile risk categorya (% in category)
|
Totala (overall %) | Pain NRS follow-up versus baseline
|
|||
|---|---|---|---|---|---|---|
| Low n (%) | Moderate n (%) | High n (%) | Average change | p-value | ||
| Changed selection | 141 (48) | 182 (51) | 23 (52) | 346 (50) | −3.6 | 1.29×10−55 |
| Opioid initiation | 58 (20) | 68 (19) | 5 (11) | 131 (19) | −3.2 | 1.07×10−22 |
| Dose increase | 63 (22) | 61 (17) | 4 (9) | 128 (19) | −3.4 | 1.96×10−21 |
| Dose decrease | 23 (8) | 39 (11) | 5 (11) | 67 (10) | −3.3 | 1.82×10−12 |
| Short acting to long acting | 19 (6) | 10 (3) | 0 (0) | 29 (4) | −2.9 | 1.19×10−5 |
| Long acting to short acting | 11 (4) | 9 (3) | 0 (0) | 20 (3) | −3.4 | 1.31×10−4 |
| Opioid discontinuation | 28 (10) | 42 (12) | 10 (23) | 80 (12) | −4.0 | 4.44×10−14 |
| Switched to non-opioid | 11 (4) | 10 (3) | 2 (5) | 23 (3) | −3.5 | 1.33×10−4 |
| ≥1 interventions | 291 | 356 | 44 | 691 | −3.4 | 3.39×10−108 |
Notes: Physicians used the profile to guide treatment for 2,809 (52%) patients. Of these, physicians made a change in the opioid medication regimen for 691 (24.5%) subjects. All actions except for implementing a non-opioid therapy resulted in a significant decrease in patient pain, as measured by the NRS. Physicians were less likely to initiate opioid therapy or increase dose and more likely to discontinue opioids for high-risk profile patients. The actions of decreasing the prescription dose and implementing a non-opioid therapy did not differ across risk categories.
Column totals add to more than overall if patients were given more than one intervention.
Abbreviation: NRS, numeric rating scale.
We also observed that physicians alter patient opioid treatment depending on the profile-determined OUD risk. Physicians were more likely to initiate an opioid regimen and/or increase opioid doses for low- and moderate-risk patients as compared to high-risk patients. There were 131 patients (19%) whose physicians initiated an opioid regimen after receiving the results of the profile. The vast majority (96%) of these patients were classified as low- and moderate-risk patients according to the profile, so opioid initiation was within the risk mitigation strategy of the report. There were only five subjects with high-risk results (4% of the total cohort) who were still prescribed an opioid (Table 2). In these cases, opioids were initiated cautiously, patients were monitored more closely for aberrant behavior, and concurrent use of adjuvant non-opioid medications was emphasized. Similarly, physicians were more likely to increase the opioid doses for low- or moderate-risk patients as compared to high-risk patients (63 and 61 versus 4 patients, respectively).
Physicians were also more likely to discontinue opioids for high-risk patients as compared with low- or moderate-risk patients. Among patients who were identified as high risk for OUD by the profile, the two most frequent clinical actions taken by physicians were changing the type of opioid prescribed (52%) and titrating the patient off opioids (23%). Other actions taken by the physicians for patients in the high-risk category included decreasing the opioid dose (11%) and switching from an opioid to a non-opioid medication (5%).
Patient outcomes improved following profile-guided treatment decisions
Information about pain intensity and response to medications was collected from each patient. Every opioid-related intervention that was made based on the profile resulted in significantly improved (p≤0.05) pain levels by the follow-up visit (Figure 1; Figure S1), with an average decrease of 3.4 points in the NRS (p=3.39×10−108; Figures 1 and 2).
Overall, due to receiving opioid-related interventions guided by the profile, 78% of the patients reported a MED scale of ≥3 and 57% reported a MED scale of ≥4 (Figure 3). Patients who were given a new opioid prescription based on profile results – that is, reported taking an opioid for ≤30 days at the follow-up visit (n=150) – indicated fair to good response to their medications (MED scale average rating 3.4). Response to medications was reported to be the lowest (2.6) among individuals who scored as high risk on the profile test.
Physician-rated benefit of the profile
Of the total 5,397 patients, 4,836 (90%) at the baseline visit and 4,221 (78%) at the follow-up visit had physicians who rated the benefit of the profile on their patient’s care at each visit. Benefit ratings ranged from 1 to 5, where 1 was “no benefit” and 5 was “significant benefit”. Baseline visit benefit ratings are cross-sectional in nature and describe the utility of the profile in assisting with clinical decision making. Follow-up visit benefit ratings are prospective evaluations and describe the utility of the profile for patient outcomes. At the baseline visit, physicians who used the profile to guide decisions rated the benefit of the test significantly higher on average than those who did not use the profile to guide decisions (4.0 compared to 3.7; p=7.18×10−18). Furthermore, each action resulted in a benefit rating that was significantly higher than the unguided average rating (p≤0.05; Table 3). At the follow-up visit, only those physicians who changed the opioid medication or dosage of their patients started their patient on an opioid, or increased/decreased drug screens continued to rate the profile significantly higher than the physicians who made other decisions. Opioid initiation, which was predominantly performed for profile low- or moderate-risk patients as per profile guidance (Table 2), was the most significant action that resulted in the most benefit to both clinical decision making and patient improvement (Table 3). Increasing urine drug screen frequency was more frequently performed when the profile results indicated high risk (Table 2), and changing the frequency of urine drug screens was ranked as the second most significant action that resulted in the most benefit to patient improvement. The average benefit rating at the baseline visit was slightly higher than at the follow-up visit (3.9 compared to 3.8), but not significantly so (p=0.07; Figure 4). Regardless, physicians found the profile useful for making treatment decisions and for their benefit on patient outcomes.
Table 3.
Benefit of profile-specific guidance in patient care at baseline and follow-up visits
| Decision at baseline | Baseline
|
Follow-up
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n=4,836 patients
|
n=4,221 patients
|
|||||||||||
| Count “Yes” | Percent “Yes” | Average rating Yes/no | OR | p-value | Rank | Count “Yes” | Percent “Yes” | Average rating Yes/no | OR | p-value | Rank | |
| No changes made | 2,309 | 48 | 4.0/3.7 | 0.63 | 7.18×10−18 | 8a | 2,050 | 49 | 3.7/3.8 | 0.94 | 0.265 | 7 |
|
| ||||||||||||
| n=2,527 patients (guided only) | n=2,171 patients (guided only) | |||||||||||
| Changed opioid or dosage | 422 | 17 | 4.3/3.8 | 1.77 | 2.56×10−8* | 2a | 354 | 16 | 4.0/3.8 | 1.31 | 0.014* | 3a |
| Started an opioid | 123 | 4.9 | 4.5/3.8 | 3.29 | 3.90×10−10* | 1a | 99 | 4.6 | 4.2/3.8 | 2.36 | 3.44×10−5* | 1a |
| Changed drug screen frequency | 42 | 1.7 | 4.4/3.8 | 2.61 | 0.003* | 4a | 32 | 1.5 | 4.3/3.8 | 2.46 | 0.011* | 2a |
| Advised another provider | 39 | 1.5 | 4.3/3.8 | 2.14 | 0.020* | 5a | 30 | 1.4 | 4.0/3.8 | 1.45 | 0.291 | 5 |
| Discontinued opioids | 48 | 1.9 | 4.4/3.8 | 2.05 | 0.021* | 6a | 44 | 2.0 | 4.1/3.8 | 1.42 | 0.246 | 4 |
| Spent more time with patient | 1,440 | 57 | 4.0/3.8 | 0.70 | 2.09 ×10−6* | 7a | 1,224 | 56 | 3.8/3.8 | 0.86 | 0.065 | 8 |
| Felt more confident with medical regimen | 1,745 | 69 | 4.1/3.7 | 1.38 | 0.002* | 3a | 1,521 | 70 | 3.8/3.8 | 1.03 | 0.669 | 6 |
Notes: Any actions or decisions reported to be made based on profile results (Table S2) were considered as profile guided. As expected, physicians who made any profile-guided decisions rated the benefit of the profile higher than physicians who did not report any guidance. However, physicians who made decisions to change opioid selection or dosage, start an opioid rotation, and/or changed the frequency of urine drug screens based on the profile reported it provided considerable benefit at the follow-up visit as well, indicating both immediate and prospective benefit to these clinical use scenarios. ORs are proportional odds that may be interpreted as the average odds comparing consecutive ratings (i.e., the overall average of the odds of having a rating of 5 versus 4, 4 versus 3, and so on). An OR <1 indicates that the decision correlated with decreased ratings. ORs are adjusted for age, gender, and race. Decisions are ranked by signed −log10 (p-value), where signed is −1 if OR <1 and 1 if OR ≥1. Thus, a rank of 1 would indicate the most significant decision that resulted in higher ratings and a rank of 8 would indicate the most significant decision that resulted in lower ratings.
Decisions p≤0.05 are denoted with*.
Abbreviation: OR, odds ratio.
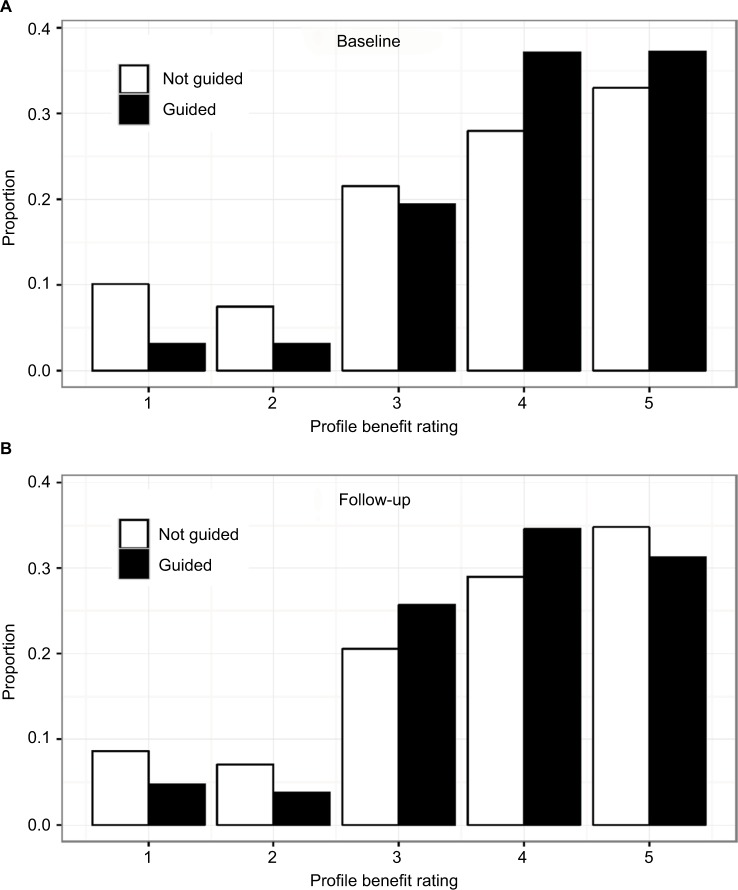
Figure 4.
Distribution of benefit ratings by study visit and profile guidance.
Notes: The majority of physicians reported significant benefit of profile results toward assisting with clinical decision making and improving patient outcomes. (A) At baseline, the benefit rating describes the utility of the profile in clinical decision making. The average benefit rating by physicians was 4.0 for profile-guided patients compared to 3.7 for non-guided patients (p=7.18×10−18), demonstrating the expected increase in benefit when the profile is used for guidance, but suggesting that even those who did not report taking action based on the profile felt the information was at least moderately beneficial. (B) At the follow-up visit, the benefit rating describes the utility of the profile in improving patient outcomes. The average benefit rating by physicians was 3.8 for guided patients compared to 3.7 for non-guided patients (p=0.265). The average benefit rating at baseline was slightly higher than at follow-up (3.9 compared to 3.8), but not significantly so (p=0.07).
Discussion
The prescription opioid epidemic is costly on multiple fronts: from decreasing the quality of life of those suffering from OUD to increasing the demands on physicians trying to balance pain management with the risk of abuse and imposing an astronomical economic burden on the health care system. Drug abuse treatment costs, health care costs, lost productivity, and criminal justice costs result in US$52–$78.3 billion in economic costs stemming from OUD.3,4 Prescription pain killers result in 46 overdose deaths26 and more than 1,000 emergency room visits daily.2 However, the solution is not as simple as eliminating opioid treatment altogether, as there exists an obligation to treat pain and judicious use of opioids can, in part, provide that. Thus, it is imperative to stratify subjects by the risk of OUD to avoid prescribing opioids to those at high risk and to avoid withholding opioids from those at low risk of OUD.
The profile described herein stratifies subjects at low risk, moderate risk, and high risk of OUD with high accuracy,13–15 and performs with greater sensitivity and specificity than either the SOAPP-R or the ORT. This algorithm can be used to fulfill the main recommendations when prescribing opioids: evaluating a patient for treatment of opioids using a systematic method,27 consistent use of a screening method,28–31 and monitoring the patient’s drug use with urine drug testing.28,30–33 The profile was used by physicians in this study to evaluate the selection, dosage, and discontinuation of opioids based on patient risk levels. Physicians altered patient opioid treatments depending on the profile results, decreasing total opioid usage for those at high risk and initiating or increasing dosage of opioid therapy for those who were not.
When physicians used the results of the profile to guide opioid-related treatments, there was a significant reduction in patient pain levels, equivalent to a reduction from severe to moderate or low pain. Beyond the improvement in quality of life for the patient, there is a huge economic benefit from a reduction of this magnitude. Based on the Medical Expenditure Panel Survey, patients with severe pain had the highest health care expenditures, which were US$3,210 higher than for those with moderate pain and US$7,726 higher than for those with no pain.5 Considering that 100 million people in the USA suffer from chronic pain,1 simply a reduction from severe to moderate pain results in astronomical health care savings, reaching hundreds of millions. Private insurers, Medicare, Medicaid, and individuals share this health care burden, although private insurers pay the largest share of costs.5
Beyond direct costs, chronic pain is expensive in terms of indirect costs, such as loss of productivity, decreased hourly wages, and fewer working days.5 The indirect costs for severe pain are higher than those for moderate pain and much higher than those of an individual with no pain.5 Health economists estimate the total costs of pain to be US$560–$635 billion, using 2010 data.5 Pain is the most expensive health condition in the USA, exceeding cardiovascular diseases, respiratory system diseases, and injury.5 This study demonstrates that precision medicine testing results in significant pain reductions when physicians use the results to guide opioid-related treatment.
Study limitations
The study was conducted in the USA and we have not demonstrated its utility in countries without a high level of health care infrastructure. It will be imperative to conduct global studies prior to global adoption. However, this study demonstrates the utility of the profile in the USA. Additionally, pain was measured using the NRS, which is a self-reported measure of pain. This measurement is not always reliable, as it is based on subjective information. This is an issue that the medical community needs to address, and the authors suggest the inclusion of objective information, including genetic information, may result in more accurate pain measures. That said, the NRS is the current standard in health care to measure pain and it is reasonable for the authors to use that pain measurement. The study focused on opioid-related treatment decisions, and we did not collect data from physicians on how they treated patients if they did not use the profile to guide treatment. This will be a component of a future study. A randomized controlled study will be conducted to further evaluate the utility of the profile.
Conclusion
The clinical utility of the precision medicine profile is two-fold: it provides clinically actionable recommendations that can be used to 1) prevent OUD through limiting initial opioid prescriptions and 2) reduce pain in patients at low risk of developing OUD. Physicians in this study found the profile to be useful on clinical decision making and patient outcomes. The ramifications are widespread – fewer lives negatively impacted by addiction, reduced health care costs, increased productivity, decreased pain for patients, and increased functionality for individuals.
Supplementary materials
Change in pain NRS scores after patients received specific opioid-related interventions.
Note: The majority of patients experienced significant reduction (2–6 points) for moderate to severe pain.
Abbreviation: NRS, numeric rating scale.
Table S1.
Profile algorithm test panel markers
| Protein name | Gene | SNP marker | Associated neuropsychiatric disorders |
|---|---|---|---|
| Catechol-O-methyltransferase | COMT | rs4680 | Alcohol abuse1,2 |
| Methamphetamine abuse2 | |||
| Heroin dependence3,4 | |||
| Cocaine dependence5,6 | |||
| Anxiety7,8 | |||
| Depression9,10 | |||
| Dopamine beta-hydroxylase | DBH | rs1611115 | Cocaine addiction11,12 |
| ADHD13 | |||
| Methamphetamine abuse14 | |||
| Schizophrenia15 | |||
| Dopamine D1 receptor | DRD1 | rs4532 | Depression16 |
| Heroin addiction17 | |||
| Alcohol dependence17 | |||
| Ankyrin repeat and kinase domain containing 1/dopamine receptor D2 | ANKK1/DRD2 | rs1800497 | Alcohol and cocaine dependence18 |
| Heroin abuse4,17,19 | |||
| Dopamine D4 receptor | DRD4 | rs3758653 | Anxiety20,21 |
| Heroin abuse22–24 | |||
| Dopamine transporter SLC6A3 | DAT | rs27072 | Methamphetamine addiction25 |
| Gamma aminobutyric acid receptor A, gamma2 subunit | GABRG2 | rs211014 | Alcohol abuse26 |
| Heroin abuse27 | |||
| Methamphetamine abuse27 | |||
| Opioid receptor, kappa 1 | OPRK1 | rs1051660 | Mood disorders28 |
| Alcohol dependence29 | |||
| Methylenetetrahydrofolate reductase | MTHFR | rs1801133 | Bipolar disorder, depression30,31 |
| Opioid receptor, mu 1 | OPRM1 | rs1799971 | Heroin addiction32 |
| Opioid use disorder33 | |||
| Substance use disorder34,35 | |||
| Alcoholism29,36,37 | |||
| Serotonin receptor 2A | HTR2A | rs7997012 | Drug abuse1 |
| Depression38 | |||
| Phenotypic traits | Risk factors | ||
| Age | 16–45 years39,43 | ||
| Personal history | Mental health disorders1,40–42 | ||
| Depression48–50 | |||
| Alcoholism43,44 | |||
| Illicit drug use45,46 | |||
| Prescription drug abuse47 | |||
Abbreviations: ADHD, attention-deficit hyperactive disorder; SNP, single-nucleotide polymorphism.
Table S2.
List of treatment decisions taken by physicians based on profile resultsa
| 1. Changed opioid prescribed |
| 2. Started an opioid rotation |
| 3. Switched from a long-acting to a short-acting opioid |
| 4. Switched from a short-acting to a long-acting opioid |
| 5. Increased total opioid dose/frequency |
| 6. Decreased total opioid dose/frequency |
| 7. Increased frequency of urine drug screens |
| 8. Decreased frequency of urine drug screens |
| 9. Decided to titrate the patient off opioids |
| 10. Switched from an opioid to a non-opioid pain medication |
| 11. Advised another provider to make changes in this patient’s prescriptions |
| 12. Spent more time with the patient |
| 13. Felt more confident with your medical regimen after reviewing and implementing the genetic information |
| 14. Other:______________________. |
| 15. No changes were implanted based on this test result |
Notes: Questions 1–6, 9, and 10 were considered to be opioid-related interventions.
Physicians were asked to report all that applied.
Table S3.
Other decisions implemented by physicians based on profile test results
| Decision | Profile risk category (% in category)
|
Totala (overall %) | ||
|---|---|---|---|---|
| Low (%) | Moderate (%) | High (%) | ||
| Increased drug screens | 7 (<1) | 25 (2) | 10 (8) | 42 (2) |
| Decreased drug screens | 1 (<1) | 0 (0) | 1 (<1) | 2 (<1) |
| Advised other provider | 14 (1) | 22 (2) | 6 (5) | 42 (2) |
| Spent more time | 688 (64) | 797 (66) | 91 (72) | 1,576 (66) |
| Felt more confident | 906 (85) | 869 (72) | 78 (62) | 1,853 (77) |
| Other | 37 (3) | 40 (3) | 7 (6) | 84 (3.5) |
| Total patients | 1,072 | 1,200 | 126 | 2,398 |
Notes: In total, 2,809 (52%) patients received profile-guided decisions from their physicians. Shown in this table are the 2,398 (85% of the guided patients) who received profile-guided decisions that did not result in a change in opioid medication regimen.
Column totals add to more than overall if physicians took more than one action/consideration.
References
- 1.Tassin JP. Uncoupling between noradrenergic and serotonergic neurons as a molecular basis of stable changes in behavior induced by repeated drugs of abuse. Biochem Pharmacol. 2008;75(1):85–97. doi: 10.1016/j.bcp.2007.06.038. [DOI] [PubMed] [Google Scholar]
- 2.Jugurnauth SK, Chen CK, Barnes MR, et al. A COMT gene haplotype associated with methamphetamine abuse. Pharmacogenet Genomics. 2011;21(11):731–740. doi: 10.1097/FPC.0b013e32834a53f9. [DOI] [PubMed] [Google Scholar]
- 3.Levran O, Randesi M, da Rosa JC, et al. Overlapping dopaminergic pathway genetic susceptibility to heroin and cocaine addictions in African Americans. Ann Hum Genet. 2015;79(3):188–198. doi: 10.1111/ahg.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vereczkei A, Demetrovics Z, Szekely A, et al. Multivariate analysis of dopaminergic gene variants as risk factors of heroin dependence. PLoS One. 2013;8(6):e66592. doi: 10.1371/journal.pone.0066592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lohoff FW, Weller AE, Bloch PJ, et al. Association between the catechol-O-methyltransferase Val158Met polymorphism and cocaine dependence. Neuropsychopharmacology. 2008;33(13):3078–3084. doi: 10.1038/npp.2008.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ittiwut R, Listman JB, Ittiwut C, et al. Association between polymorphisms in catechol-O-methyltransferase (COMT) and cocaine-induced paranoia in European-American and African-American populations. Am J Med Genet B Neuropsychiatr Genet. 2011;156B(6):651–660. doi: 10.1002/ajmg.b.31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baumann C, Klauke B, Weber H, et al. The interaction of early life experiences with COMT val158met affects anxiety sensitivity. Genes Brain Behav. 2013;12(8):821–829. doi: 10.1111/gbb.12090. [DOI] [PubMed] [Google Scholar]
- 8.Stein MB, Fallin MD, Schork NJ, Gelernter J. COMT polymorphisms and anxiety-related personality traits. Neuropsychopharmacology. 2005;30(11):2092–2102. doi: 10.1038/sj.npp.1300787. [DOI] [PubMed] [Google Scholar]
- 9.Illi A, Setala-Soikkeli E, Kampman O, et al. Catechol-O-methyltransferase val108/158met genotype, major depressive disorder and response to selective serotonin reuptake inhibitors in major depressive disorder. Psychiatry Res. 2010;176(1):85–87. doi: 10.1016/j.psychres.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Schosser A, Calati R, Serretti A, et al. The impact of COMT gene polymorphisms on suicidality in treatment resistant major depressive disorder a European multicenter study. Eur Neuropsychopharmacol. 2012;22(4):259–266. doi: 10.1016/j.euroneuro.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Brousse G, Vorspan F, Ksouda K, et al. Could the inter-individual variability in cocaine-induced psychotic effects influence the development of cocaine addiction? Towards a new pharmacogenetic approach to addictions. Med Hypotheses. 2010;75(6):600–604. doi: 10.1016/j.mehy.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Castillo N, Ribases M, Roncero C, Casas M, Gonzalvo B, Cormand B. Association study between the DAT1, DBH and DRD2 genes and cocaine dependence in a Spanish sample. Psychiatr Genet. 2010;20(6):317–320. doi: 10.1097/YPG.0b013e32833b6320. [DOI] [PubMed] [Google Scholar]
- 13.Ji N, Shuai L, Chen Y, et al. Dopamine beta-hydroxylase gene associates with stroop color-word task performance in Han Chinese children with attention deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2011;156B(6):730–736. doi: 10.1002/ajmg.b.31215. [DOI] [PubMed] [Google Scholar]
- 14.Kalayasiri R, Verachai V, Gelernter J, Mutirangura A, Malison RT. Clinical features of methamphetamine-induced paranoia and preliminary genetic association with DBH-1021C–>T in a Thai treatment cohort. Addiction. 2014;109(6):965–976. doi: 10.1111/add.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cubells JF, Sun X, Li W, et al. Linkage analysis of plasma dopamine beta-hydroxylase activity in families of patients with schizophrenia. Hum Genet. 2011;130(5):635–643. doi: 10.1007/s00439-011-0989-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nyman ES, Sulkava S, Soronen P, et al. Interaction of early environment, gender and genes of monoamine neurotransmission in the aetiology of depression in a large population-based Finnish birth cohort. BMJ Open. 2011;1(1):e000087. doi: 10.1136/bmjopen-2011-000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Foll B, Gallo A, Le Strat Y, Lu L, Gorwood P. Genetics of dopamine receptors and drug addiction: a comprehensive review. Behav Pharmacol. 2009;20(1):1–17. doi: 10.1097/FBP.0b013e3283242f05. [DOI] [PubMed] [Google Scholar]
- 18.Noble EP. D2 dopamine receptor gene in psychiatric and neurologic disorders and its phenotypes. Am J Med Genet B Neuropsychiatr Genet. 2003;116B(1):103–125. doi: 10.1002/ajmg.b.10005. [DOI] [PubMed] [Google Scholar]
- 19.Lawford BR, Young RM, Noble EP, et al. The D(2) dopamine receptor A(1) allele and opioid dependence: association with heroin use and response to methadone treatment. Am J Med Genet. 2000;96(5):592–598. doi: 10.1002/1096-8628(20001009)96:5<592::aid-ajmg3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 20.Cao BJ, Rodgers RJ. Dopamine D4 receptor and anxiety: behavioural profiles of clozapine, L-745,870 and L-741,742 in the mouse plus-maze. Eur J Pharmacol. 1997;335(2–3):117–125. doi: 10.1016/s0014-2999(97)01234-x. [DOI] [PubMed] [Google Scholar]
- 21.Navarro JF, Luna G, Garcia F, Pedraza C. Effects of L-741,741, a selective dopamine receptor antagonist, on anxiety tested in the elevated plus-maze in mice. Methods Find Exp Clin Pharmacol. 2003;25(1):45–47. doi: 10.1358/mf.2003.25.1.724862. [DOI] [PubMed] [Google Scholar]
- 22.Kotler M, Cohen H, Segman R, et al. Excess dopamine D4 receptor (D4DR) exon III seven repeat allele in opioid-dependent subjects. Mol Psychiatry. 1997;2(3):251–254. doi: 10.1038/sj.mp.4000248. [DOI] [PubMed] [Google Scholar]
- 23.Li T, Xu K, Deng H, et al. Association analysis of the dopamine D4 exon III VNTR and heoin abuse in Chinese subjects. Mol Psychiatry. 1997;2(5):413–416. doi: 10.1038/sj.mp.4000310. [DOI] [PubMed] [Google Scholar]
- 24.Shao C, Li Y, Jiang K, et al. Dopamine D4 receptor polymorphism modulates cue-elicited heroin craving in Chinese. Psychopharmacology (Berl) 2006;186(2):185–190. doi: 10.1007/s00213-006-0375-6. [DOI] [PubMed] [Google Scholar]
- 25.Gross NB, Duncker PC, Marshall JF. Striatal dopamine D1 and D2 receptors: widespread influences on methamphetamine-induced dopamine and serotonin neurotoxicity. Synapse. 2011;65(11):1144–1155. doi: 10.1002/syn.20952. [DOI] [PubMed] [Google Scholar]
- 26.Han DH, Bolo N, Daniels MA, et al. Craving for alcohol and food during treatment for alcohol dependence: modulation by T allele of 1519T>C GABAAalpha6. Alcohol Clin Exp Res. 2008;32(9):1593–1599. doi: 10.1111/j.1530-0277.2008.00734.x. [DOI] [PubMed] [Google Scholar]
- 27.Li D, Sulovari A, Cheng C, Zhao H, Kranzler HR, Gelernter J. Association of gamma-aminobutyric acid A receptor alpha2 gene (GABRA2) with alcohol use disorder. Neuropsychopharmacology. 2014;39(4):907–918. doi: 10.1038/npp.2013.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carlezon WA, Jr, Beguin C, Knoll AT, Cohen BM. Kappa-opioid ligands in the study and treatment of mood disorders. Pharmacol Ther. 2009;123(3):334–343. doi: 10.1016/j.pharmthera.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones JD, Comer SD, Kranzler HR. The pharmacogenetics of alcohol use disorder. Alcohol Clin Exp Res. 2015;39(3):391–402. doi: 10.1111/acer.12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peerbooms OL, van Os J, Drukker M, et al. Meta-analysis of MTHFR gene variants in schizophrenia, bipolar disorder and unipolar depressive disorder: evidence for a common genetic vulnerability? Brain Behav Immun. 2011;25(8):1530–1543. doi: 10.1016/j.bbi.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Hu CY, Qian ZZ, Gong FF, et al. Methylenetetrahydrofolate reductase (MTHFR) polymorphism susceptibility to schizophrenia and bipolar disorder: an updated meta-analysis. J Neural Transm (Vienna) 2015;122(2):307–320. doi: 10.1007/s00702-014-1261-8. [DOI] [PubMed] [Google Scholar]
- 32.Woodcock EA, Lundahl LH, Burmeister M, Greenwald MK. Functional mu opioid receptor polymorphism (OPRM1 A(118) G) associated with heroin use outcomes in Caucasian males: a pilot study. Am J Addict. 2015;24(4):329–335. doi: 10.1111/ajad.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haerian BS, Haerian MS. OPRM1 rs1799971 polymorphism and opioid dependence: evidence from a meta-analysis. Pharmacogenomics. 2013;14(7):813–824. doi: 10.2217/pgs.13.57. [DOI] [PubMed] [Google Scholar]
- 34.Carpentier PJ, Arias Vasquez A, Hoogman M, et al. Shared and unique genetic contributions to attention deficit/hyperactivity disorder and substance use disorders: a pilot study of six candidate genes. Eur Neuropsychopharmacol. 2013;23(6):448–457. doi: 10.1016/j.euroneuro.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Schwantes-An TH, Zhang J, Chen LS, et al. Association of the OPRM1 Variant rs1799971 (A118G) with non-specific liability to substance dependence in a collaborative de novo meta-analysis of European-Ancestry Cohorts. Behav Genet. 2016;46(2):151–169. doi: 10.1007/s10519-015-9737-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Enoch MA. Genetic influences on response to alcohol and response to pharmacotherapies for alcoholism. Pharmacol Biochem Behav. 2014;123:17–24. doi: 10.1016/j.pbb.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reyes-Gibby CC, Yuan C, Wang J, Yeung SC, Shete S. Gene network analysis shows immune-signaling and ERK1/2 as novel genetic markers for multiple addiction phenotypes: alcohol, smoking and opioid addiction. BMC Syst Biol. 2015;9:25. doi: 10.1186/s12918-015-0167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Celada P, Puig M, Amargos-Bosch M, Adell A, Artigas F. The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. J Psychiatry Neurosci. 2004;29(4):252–265. [PMC free article] [PubMed] [Google Scholar]
- 39.Khazaal Y, Gex-Fabry M, Nallet A, et al. Affective temperaments in alcohol and opiate addictions. Psychiatr Q. 2013;84(4):429–438. doi: 10.1007/s11126-013-9257-3. [DOI] [PubMed] [Google Scholar]
- 40.Compton WM, Volkow ND. Abuse of prescription drugs and the risk of addiction. Drug Alcohol Depend. 2006;83(Suppl 1):S4–S7. doi: 10.1016/j.drugalcdep.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 41.Daigre C, Roncero C, Grau-Lopez L, et al. Attention deficit hyperactivity disorder in cocaine-dependent adults: a psychiatric comorbidity analysis. Am J Addict. 2013;22(5):466–473. doi: 10.1111/j.1521-0391.2013.12047.x. [DOI] [PubMed] [Google Scholar]
- 42.Fontenelle LF, Oostermeijer S, Harrison BJ, Pantelis C, Yücel M. Obsessive-compulsive disorder, impulse control disorders and drug addiction: common features and potential treatments. Drugs. 2011;71(7):827–840. doi: 10.2165/11591790-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 43.Lauschke VM, Ingelman-Sundberg M. Requirements for comprehensive pharmacogenetic genotyping platforms. Pharmacogenomics. 2016;17(8):917–924. doi: 10.2217/pgs-2016-0023. [DOI] [PubMed] [Google Scholar]
- 44.Cleland CM, Rosenblum A, Fong C, Maxwell C. Age differences in heroin and prescription opioid abuse among enrolees into opioid treatment programs. Subst Abuse Treat Prev Policy. 2011;6:11. doi: 10.1186/1747-597X-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sproule B, Brands B, Li S, Catz-Biro L. Changing patterns in opioid addiction: characterizing users of oxycodone and other opioids. Can Fam Physician. 2009;55(1):68–69. [PMC free article] [PubMed] [Google Scholar]
- 46.Edlund MJ, Steffick D, Hudson T, Harris KM, Sullivan M. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain. 2007;129(3):355–362. doi: 10.1016/j.pain.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 47.Institute of Medicine Committee on Advancing Pain Research Care . Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington DC, USA: National Academies Press; 2011. The National Academies Collection: Reports funded by National Institutes of Health. [PubMed] [Google Scholar]
- 48.Manchikanti L, Giordano J, Boswell MV, Fellows B, Manchukonda R, Pampati V. Psychological factors as predictors of opioid abuse and illicit drug use in chronic pain patients. J Opioid Manag. 2007;3(2):89–100. doi: 10.5055/jom.2007.0045. [DOI] [PubMed] [Google Scholar]
- 49.Brooner RK, King VL, Kidorf M, Schmidt CW, Jr, Bigelow GE. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54(1):71–80. doi: 10.1001/archpsyc.1997.01830130077015. [DOI] [PubMed] [Google Scholar]
- 50.Burke JD, Jr, Burke KC, Rae DS. Increased rates of drug abuse and dependence after onset of mood or anxiety disorders in adolescence. Hosp Community Psychiatry. 1994;45(5):451–455. doi: 10.1176/ps.45.5.451. [DOI] [PubMed] [Google Scholar]
Acknowledgments
The study was funded and sponsored by Proove Biosciences, Inc.
Footnotes
Disclosure
AB, CL, SK, and BM are employees of Proove Biosciences. KL and MS are members of the Proove Medical Advisory Board. GAS is a principal investigator on a Proove-sponsored study. The authors report no other conflicts of interest in this work.
References
- 1.Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education . Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 2.SAMHSA . The DAWN Report: Highlights of the 2011 Drug Abuse Warning Network (DAWN) Findings on Drug-Related Emergency Department Visits. 2013. [PubMed] [Google Scholar]
- 3.Hansen RN, Oster G, Edelsberg J, Woody GE, Sullivan SD. Economic costs of nonmedical use of prescription opioids. Clin J Pain. 2011;27(3):194–202. doi: 10.1097/AJP.0b013e3181ff04ca. [DOI] [PubMed] [Google Scholar]
- 4.Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose, abuse, and Dependence in the United States, 2013. Med Care. 2016;54(10):901–906. doi: 10.1097/MLR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaskin DJ, Richard P. Appendix C: the economic costs of pain the United States. J Pain. 2012;13(8):715–724. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Prevention. CfDCa CDC Guideline for Prescribing Opioids for Chronic Pain. 2017. [Accessed December 15, 2017]. Available from: https://www.cdc.gov/mmwr/volumes/65/rr/rr6501e1.htm.
- 7.Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain-United States, 2016. JAMA. 2016;315(15):1624–1645. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butler SF, Budman SH, Fernandez KC, Fanciullo GJ, Jamison RN. Cross-validation of a screener to predict opioid misuse in chronic pain patients (SOAPP-R) J Addict Med. 2009;3(2):66–73. doi: 10.1097/ADM.0b013e31818e41da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butler SF, Zacharoff KL, Budman SH, et al. Spanish translation and linguistic validation of the screener and opioid assessment for patients with pain-revised (SOAPP-R) Pain Med. 2013;14(7):1032–1038. doi: 10.1111/pme.12098. [DOI] [PubMed] [Google Scholar]
- 10.Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the opioid risk tool. Pain Med. 2005;6(6):432–442. doi: 10.1111/j.1526-4637.2005.00072.x. [DOI] [PubMed] [Google Scholar]
- 11.Jones T, Moore T. Preliminary data on a new opioid risk assessment measure: the brief risk interview. J Opioid Manag. 2013;9(1):19–27. doi: 10.5055/jom.2013.0143. [DOI] [PubMed] [Google Scholar]
- 12.Jones T, Lookatch S, Grant P, McIntyre J, Moore T. Further validation of an opioid risk assessment tool: the brief risk interview. J Opioid Manag. 2014;10(5):353–364. doi: 10.5055/jom.2014.0226. [DOI] [PubMed] [Google Scholar]
- 13.Brenton A, Richeimer S, Sharma M, et al. Observational study to calculate addictive risk to opioids: a validation study of a predictive algorithm that detects opioid use disorder. Pharmacogenomics Pers Med. 2017;10:187–195. doi: 10.2147/PGPM.S123376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brenton A, Richeimer S, Sharma M, et al. Observational study to calculate addictive risk to opioids: a validation study of a predictive algorithm to evaluate opioid use disorder in a primary care setting. Pharmgenomics Pers Med. 2017;10:187–195. doi: 10.2147/PGPM.S123376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farah JR, Lee C, Kantorovich S, Smith GA, Meshkin B, Brenton A. Evaluation of a predictive algorithm that detects aberrant use of opioids in an addiction treatment centre. J Addict Res Ther. 2017;8(2) [Google Scholar]
- 16.Lauschke VM, Ingelman-Sundberg M. Requirements for comprehensive pharmacogenetic genotyping platforms. Pharmacogenomics. 2016;17(8):917–924. doi: 10.2217/pgs-2016-0023. [DOI] [PubMed] [Google Scholar]
- 17.Cleland CM, Rosenblum A, Fong C, Maxwell C. Age differences in heroin and prescription opioid abuse among enrolees into opioid treatment programs. Subst Abuse Treat Prev Policy. 2011;6:11. doi: 10.1186/1747-597X-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sproule B, Brands B, Li S, Catz-Biro L. Changing patterns in opioid addiction: characterizing users of oxycodone and other opioids. Can Fam Physician. 2009;55(1):68–69. [PMC free article] [PubMed] [Google Scholar]
- 19.Jang KL, Livesley WJ, Vernon PA. Alcohol and drug problems: a multivariate behavioural genetic analysis of co-morbidity. Addiction. 1995;90(9):1213–1221. doi: 10.1046/j.1360-0443.1995.90912136.x. [DOI] [PubMed] [Google Scholar]
- 20.Edlund MJ, Steffick D, Hudson T, Harris KM, Sullivan M. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain. 2007;129(3):355–362. doi: 10.1016/j.pain.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 21.Institute of Medicine Committee on Advancing Pain Research Care . Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington DC, USA: National Academies Press; 2011. The National Academies Collection: Reports funded by National Institutes of Health. [PubMed] [Google Scholar]
- 22.Manchikanti L, Giordano J, Boswell MV, Fellows B, Manchukonda R, Pampati V. Psychological factors as predictors of opioid abuse and illicit drug use in chronic pain patients. J Opioid Manag. 2007;3(2):89–100. doi: 10.5055/jom.2007.0045. [DOI] [PubMed] [Google Scholar]
- 23.Brooner RK, King VL, Kidorf M, Schmidt CW, Jr, Bigelow GE. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54(1):71–80. doi: 10.1001/archpsyc.1997.01830130077015. [DOI] [PubMed] [Google Scholar]
- 24.Burke JD, Jr, Burke KC, Rae DS. Increased rates of drug abuse and dependence after onset of mood or anxiety disorders in adolescence. Hosp Community Psychiatry. 1994;45(5):451–455. doi: 10.1176/ps.45.5.451. [DOI] [PubMed] [Google Scholar]
- 25.McCaffery M, Beebe A. Pain: Clinical Manual for Nursing Practice. 1993 [Google Scholar]
- 26.Prevention CfDCa . CDC VitalSigns – Opioid Painkiller Prescribing. Centers for Disease Control and Prevention; 2016. [Accessed December 15, 2017]. Available from: https://www.cdc.gov/vitalsigns/opioid-prescribing/index.html. [Google Scholar]
- 27.Agarin T, Trescot AM, Agarin A, Lesanics D, Decastro C. Reducing opioid analgesic deaths in America: what health providers can do. Pain Physician. 2015;18(3):E307–E322. [PubMed] [Google Scholar]
- 28.Jamison RN, Sheehan KA, Scanlan E, et al. Beliefs and attitudes about opioid prescribing and chronic pain management: survey of primary care providers. J Opioid Manag. 2014;10(6):375–382. doi: 10.5055/jom.2014.0234. [DOI] [PubMed] [Google Scholar]
- 29.Chou R, Fanciullo GJ, Fine PG, et al. American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113–130. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krashin D, Murinova N, Ballantyne J. Management of pain with comorbid substance abuse. Curr Psychiatry Rep. 2012;14(5):462–468. doi: 10.1007/s11920-012-0298-3. [DOI] [PubMed] [Google Scholar]
- 31.Miotto K, Kaufman A, Kong A, Jun G, Schwartz J. Managing co-occurring substance use and pain disorders. Psychiatr Clin North Am. 2012;35(2):393–409. doi: 10.1016/j.psc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Cheatle MD, O’Brien CP. Opioid therapy in patients with chronic non-cancer pain: diagnostic and clinical challenges. Adv Psychosom Med. 2011;30:61–91. doi: 10.1159/000324067. [DOI] [PubMed] [Google Scholar]
- 33.Atluri S, Akbik H, Sudarshan G. Prevention of opioid abuse in chronic non-cancer pain: an algorithmic, evidence based approach. Pain Physician. 2012;15(3 Suppl):ES177–ES189. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Change in pain NRS scores after patients received specific opioid-related interventions.
Note: The majority of patients experienced significant reduction (2–6 points) for moderate to severe pain.
Abbreviation: NRS, numeric rating scale.
Table S1.
Profile algorithm test panel markers
| Protein name | Gene | SNP marker | Associated neuropsychiatric disorders |
|---|---|---|---|
| Catechol-O-methyltransferase | COMT | rs4680 | Alcohol abuse1,2 |
| Methamphetamine abuse2 | |||
| Heroin dependence3,4 | |||
| Cocaine dependence5,6 | |||
| Anxiety7,8 | |||
| Depression9,10 | |||
| Dopamine beta-hydroxylase | DBH | rs1611115 | Cocaine addiction11,12 |
| ADHD13 | |||
| Methamphetamine abuse14 | |||
| Schizophrenia15 | |||
| Dopamine D1 receptor | DRD1 | rs4532 | Depression16 |
| Heroin addiction17 | |||
| Alcohol dependence17 | |||
| Ankyrin repeat and kinase domain containing 1/dopamine receptor D2 | ANKK1/DRD2 | rs1800497 | Alcohol and cocaine dependence18 |
| Heroin abuse4,17,19 | |||
| Dopamine D4 receptor | DRD4 | rs3758653 | Anxiety20,21 |
| Heroin abuse22–24 | |||
| Dopamine transporter SLC6A3 | DAT | rs27072 | Methamphetamine addiction25 |
| Gamma aminobutyric acid receptor A, gamma2 subunit | GABRG2 | rs211014 | Alcohol abuse26 |
| Heroin abuse27 | |||
| Methamphetamine abuse27 | |||
| Opioid receptor, kappa 1 | OPRK1 | rs1051660 | Mood disorders28 |
| Alcohol dependence29 | |||
| Methylenetetrahydrofolate reductase | MTHFR | rs1801133 | Bipolar disorder, depression30,31 |
| Opioid receptor, mu 1 | OPRM1 | rs1799971 | Heroin addiction32 |
| Opioid use disorder33 | |||
| Substance use disorder34,35 | |||
| Alcoholism29,36,37 | |||
| Serotonin receptor 2A | HTR2A | rs7997012 | Drug abuse1 |
| Depression38 | |||
| Phenotypic traits | Risk factors | ||
| Age | 16–45 years39,43 | ||
| Personal history | Mental health disorders1,40–42 | ||
| Depression48–50 | |||
| Alcoholism43,44 | |||
| Illicit drug use45,46 | |||
| Prescription drug abuse47 | |||
Abbreviations: ADHD, attention-deficit hyperactive disorder; SNP, single-nucleotide polymorphism.
Table S2.
List of treatment decisions taken by physicians based on profile resultsa
| 1. Changed opioid prescribed |
| 2. Started an opioid rotation |
| 3. Switched from a long-acting to a short-acting opioid |
| 4. Switched from a short-acting to a long-acting opioid |
| 5. Increased total opioid dose/frequency |
| 6. Decreased total opioid dose/frequency |
| 7. Increased frequency of urine drug screens |
| 8. Decreased frequency of urine drug screens |
| 9. Decided to titrate the patient off opioids |
| 10. Switched from an opioid to a non-opioid pain medication |
| 11. Advised another provider to make changes in this patient’s prescriptions |
| 12. Spent more time with the patient |
| 13. Felt more confident with your medical regimen after reviewing and implementing the genetic information |
| 14. Other:______________________. |
| 15. No changes were implanted based on this test result |
Notes: Questions 1–6, 9, and 10 were considered to be opioid-related interventions.
Physicians were asked to report all that applied.
Table S3.
Other decisions implemented by physicians based on profile test results
| Decision | Profile risk category (% in category)
|
Totala (overall %) | ||
|---|---|---|---|---|
| Low (%) | Moderate (%) | High (%) | ||
| Increased drug screens | 7 (<1) | 25 (2) | 10 (8) | 42 (2) |
| Decreased drug screens | 1 (<1) | 0 (0) | 1 (<1) | 2 (<1) |
| Advised other provider | 14 (1) | 22 (2) | 6 (5) | 42 (2) |
| Spent more time | 688 (64) | 797 (66) | 91 (72) | 1,576 (66) |
| Felt more confident | 906 (85) | 869 (72) | 78 (62) | 1,853 (77) |
| Other | 37 (3) | 40 (3) | 7 (6) | 84 (3.5) |
| Total patients | 1,072 | 1,200 | 126 | 2,398 |
Notes: In total, 2,809 (52%) patients received profile-guided decisions from their physicians. Shown in this table are the 2,398 (85% of the guided patients) who received profile-guided decisions that did not result in a change in opioid medication regimen.
Column totals add to more than overall if physicians took more than one action/consideration.