Abstract
Background
To provide further information on the clinical and pathological prognostic factors in triple-negative breast cancer (TNBC), for which limited and inconsistent data are available.
Methods
Pathological characteristics and clinical records of 841 TNBCs diagnosed between 1994 and 2015 in four major oncologic centers from Sardinia, Italy, were reviewed. Multivariate hazard ratios (HRs) for mortality and recurrence according to various clinicopathological factors were estimated using Cox proportional hazards models.
Results
After a mean follow-up of 4.3 years, 275 (33.3%) TNBC patients had a progression of the disease and 170 (20.2%) died. After allowance for study center, age at diagnosis, and various clinicopathological factors, all components of the TNM staging system were identified as significant independent prognostic factors for TNBC mortality. The HRs were 3.13, 9.65, and 29.0, for stage II, III and IV, respectively, vs stage I. Necrosis and Ki-67 > 16% were also associated with increased mortality (HR: 1.61 and 1.99, respectively). Patients with tumor histotypes other than ductal invasive/lobular carcinomas had a more favorable prognosis (HR: 0.40 vs ductal invasive carcinoma). No significant associations with mortality were found for histologic grade, tumor infiltrating lymphocytes, and lymphovascular invasion. Among lymph node positive TNBCs, lymph node ratio appeared to be a stronger predictor of mortality than pathological lymph nodes stage (HR: 0.80 for pN3 vs pN1, and 3.05 for >0.65 vs <0.21 lymph node ratio), respectively. Consistent results were observed for cancer recurrence, except for Ki-67 and necrosis that were not found to be significant predictors for recurrence.
Conclusions
This uniquely large study of TNBC patients provides further evidence that, besides tumor stage at diagnosis, lymph node ratio among lymph node positive tumors is an additional relevant predictor of survival and tumor recurrence, while Ki-67 seems to be predictive of mortality, but not of recurrence.
Electronic supplementary material
The online version of this article (10.1186/s12885-017-3969-y) contains supplementary material, which is available to authorized users.
Keywords: Clinicopathologic factors, Prognostic factors, Stage, Survival, Triple-negative breast cancer
Background
With an estimated 1.8 million new patients each year, breast cancer is the most common cancer in women worldwide [1]. In Italy, age-standardized (European standard) incidence and mortality rates in 2012 were 118/100,000 and 23/100,000, respectively, i.e., higher than those from other southern European countries [2].
Since 2005, triple-negative breast cancer (TNBC) identifies a specific subtype of breast cancer, characterized by the lack of expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) [3]. TNBCs include a heterogeneous group of diseases which account for about 10–20% of all breast cancers and are more frequent among African American and Hispanic women, and in women with younger age, higher premenopausal body mass index, earlier age at menarche, and higher parity [3–5]. Moreover, they have higher expression of the Ki-67 antigen, higher mitotic index, and more frequent BRCA1 mutations. TNBCs are generally more aggressive than other breast neoplasms and have limited therapeutic options; therefore, they have usually a high risk of recurrence or death within 5 years since diagnosis [6].
Data on the clinical and pathological prognostic determinants for TNBC tumors are scanty and inconsistent and they generally derive from small hospital cancer registries including around a few hundreds of patients. TNM stage – including in particular the number of axillary lymph nodes involved – is one of the best-established prognostic factors for breast cancer, but its prognostic value in TNBCs, as in other intrinsic subtypes of breast cancer, is less clear [7, 8]. The ratio of positive lymph nodes on the total number of lymph nodes removed has been proposed as an additional and more accurate prognostic factor than the number of lymph nodes involved, although only a few studies have specifically evaluated its role in TNBC survival [9]. Although histologic grade has been shown to be a good predictor of survival for breast cancer, its prognostic role in TNBCs may be more limited given that most of these tumors are of high grade [10]. Furthermore, the findings on the prognostic value of the proliferation marker Ki-67 in TNBCs have been inconsistent [11]. Scantier data exist on tumor histotypes, tumor infiltrating lymphocytes (TIL), necrosis, and lymphovascular invasion (LVI) and survival from TNBC [12–15].
The primary aim of the study was therefore to provide further information on the clinical and pathological factors contributing to its prognosis of this subtype of breast cancer, i.e., survival or cancer progression, taking advantage of data from a uniquely large cohort of TNBC patients enrolled in Sardinia.
Methods
Our study included 1152 women with a new, histologically confirmed diagnosis of TNBC, identified between 1994 and 2015 in Sardinia, an Italian region with around 1.68 million inhabitants and 1500 new breast cancer patients each year (incidence 127/100,000) [16]. TNBC cases were retrospectively selected through a complete review of surgical samples and medical records of breast cancer women treated in the main oncology hospitals of the region.
The study protocol was approved by the local research ethics committee of Sardinia Region (File number 224/CE/12). Informed consent was waived since patients’ information was collected during the routine clinical practice and patients were identified by anonymized investigator-generated code not linkable to their personal data.
Immunohistochemical analysis
TNBC status was evaluated by immunohistochemistry using specific antibodies against monoclonal rabbit ER antibody, Clone SP1 (Neomarker) and monoclonal mouse anti-human PR antibody, Clone PgR 636 (Dako). ER and PgR expression was interpreted as positive if at least 1% immunostained tumor nuclei were detected in the sample, according with ASCO/CAP recommendations for immunohistochemical testing of hormone receptors in breast cancer [17]. HER2 protein expression was determined using FDA approved HercepTest™ (K5206 DAKO) and evaluated according to the manufacturer’s instructions. HER2 gene amplification was determined by ultra-View SISH Detection Kit (Ventana Medical Systems, Tucson, USA). Tumors were classified according to the 2013 ASCO/CAP recommendations [18]. Given that the study included patients diagnosed over almost 20 years in different hospital centers, all surgical specimens of TNBC patients were reviewed independently by three experienced pathologists to achieve a consensus on morphologic criteria and to standardize the results at the current guidelines recommendations for ER, PgR and HER2 immunohistochemistry [17].
Baseline data
For each TNBC patient, personal and medical data were retrospectively collected from medical records and systematically integrated into a comprehensive database. The final database included patients’ information on socio-demographic factors, anthropometric characteristics, obstetric and gynecologic features, lifestyle habits, family history of breast and other cancers, and various comorbidities. Clinicopathological data of TNBC, including tumor site, histologic type and grade, TNM classification (tumor size, T, pathological lymph node status, pN, and distant metastases, M), TIL, necrosis, LVI, and expression of Ki-67 were also collected at cancer diagnosis. Tumor type was determined according to the UICC-WHO criteria [19] and tumor grade was established according to the Nottingham scheme [20]. Breast cancer TNM staging was defined according to the 7th edition of the American Joint Committee on Cancer criteria (AJCC) [21]. TILs were evaluated in the intra-epithelial compartment, in the stroma, and in the tumor periphery. LVI was defined as the presence of tumor emboli in peritumoral lymphatic spaces, capillary or postcapillary venules.
Lymph node ratio was defined as the number of positive lymph nodes divided by the number of lymph nodes evaluated. Lymph node ratio was then categorized according to validated cut-off points, i.e., <0.21, 0.21–0.65, and >0.65 [9].
Follow-up data
During the study period, all clinical data of TNBC patients, including cancer treatments (surgery, radiotherapy and/or chemotherapy), TNBC recurrence, occurrence of other neoplasm(s), metastasis or death, were recorded. A review of all clinical charts allowed us to integrate information on cancer treatment and mortality into the database. Moreover, for each patient vital status was ascertained by enquiring the Sardinian registries of health. Since electronic cause-of-death certificates were available only for a small proportion of women (i.e., those dead at hospital or over most recent calendar years), this information was not used in our analyses. Cancer recurrence or occurrence of other neoplasms was assessed by the medical oncologists.
Overall survival (OS) was defined as the time between the date at diagnosis and the date of death (from any cause); disease-free survival (DFS) was defined as the time from the date at diagnosis to the date of local recurrence of TNBC, occurrence of other primary cancers, clinical metastatic diseases, death, or last follow-up visit, whichever occurred first. For the analysis of recurrence or DFS, 16 patients with missing information on cancer recurrence or with metastasis at baseline were excluded.
Statistical analysis
The multivariate hazard ratios (HRs) for mortality or recurrence according to various clinicopathological characteristics, and the corresponding 95% confidence intervals (CIs), were estimated using Cox proportional hazards models. The models were adjusted for study center and age at diagnosis. HRs further adjusted for clinicopathological factors found to be significantly associated (p < 0.05) to mortality or recurrence in the center and age-adjusted analyses were also estimated. In multivariable models, a complete cases analysis was performed; as a sensitivity analysis, the missing indicator method was also used. The Cox proportional hazards assumptions for each covariate were checked graphically and using the Schoenfeld’s test. Kaplan-Meier method and Log-Rank test were used to describe OS and DFS according to various factors of interest. All the analyses well performed using the SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Among a total of 1152 TNBC patients, 311 (27%) did not have information on vital status at follow-up and had a high proportion of missing for most variables considered. Therefore, overall 841 TNBC patients were included in the analysis of mortality or OS and 825 for the analysis of recurrence or DFS. Of these, 275 (33.3%) had a progression of the disease (i.e., either cancer recurrence, metastasis, or death) and 170 (20.2%) died after a mean follow up of 4.3 years (standard deviation, SD, 3.7). Five-year overall survival (OS) and disease-free survival (DFS) were 75.0% and 63.6%, respectively.
Table 1 shows the distribution of TNBC patients, according to selected patients’ characteristics. Mean age of TNBC patients was 55.8 (SD 13.5); 46.5% of patients had a diagnosis before age 50. Overall, 42.3% of patients had menarche at age 12–13 years. Almost two-thirds of patients (67.4%) have had at least one child and 30.3% of patients were in pre-menopause. With reference to clinical and pathological characteristics of TNBCs at diagnosis, 74.3% of TNBCs were invasive ductal, 7.7% lobular, 3.4% medullary, 2.4% apocrine, 1.7% pleomorphic, 1.4% metaplastic carcinoma, and 3.4% were other carcinomas. 1.3% of TNBCs were of grade 1, while the large majority of TNBCs (71.3%) were grade 3. Overall, 36.7% of TNBCs were classified as T1, 43.3% as T2, 6.5% as T3, and 5.1% as T4. According to number of lymph nodes involved, 52.2% were classified as pN0, 22.0% as pN1, 10.5% as pN2, and 5.6% as pN3. At the time of diagnosis, 10 of TNBC patients (1.2%) presented metastasis, the site of involvement of metastatic disease being mostly liver (80%), followed by bone (50%) and lung (30%), and 50% of patients had a metastasis at one single site (data not shown). Overall, 25.1% of TNBCs were stage I, 42.7% stage II, 19.1% stage III, and 1.2% stage IV (Table 1). TILs were present in more than one third (33.7%) of TNBCs, LVI in 23.0% of tumors, and necrosis in 35.8%. Ki-67 protein was highly expressed (i.e., ≥46%) in 44.7% of TNBCs (median value 40%, range 0–95%). 52.0% of women had a quadrantectomy and 37.9% had a mastectomy; of those who received a quadrantectomy, 94.1% received radiotherapy, while of those who ad a mastectomy, 7.5 received radiotherapy; finally, 4.0% of women had neoadjuvant chemotherapy only, 64.8% adjuvant chemotherapy only, and 8.7% received both treatments.
Table 1.
Characteristics of 841 triple-negative breast cancer (TNBC) patients. Sardinia, Italy 1994–2015
| TNBC patients | ||
|---|---|---|
| N | % | |
| Calendar period at diagnosis | ||
| 1994–2004 | 289 | 34.4 |
| 2005–2009 | 294 | 34.9 |
| 2010–2015 | 258 | 30.1 |
| Age at diagnosis (years) | ||
| < 45 | 190 | 22.6 |
| 45–54 | 201 | 23.9 |
| 55–64 | 226 | 26.9 |
| ≥ 65 | 224 | 26.6 |
| Age at menarche (years) | ||
| < 12 | 106 | 12.6 |
| 12 | 166 | 19.7 |
| 13 | 190 | 22.6 |
| ≥ 14 | 193 | 23.0 |
| Missing | 186 | 22.1 |
| Parity | ||
| Parous | 567 | 67.4 |
| Nulliparous | 117 | 13.9 |
| Missing | 157 | 18.7 |
| Menopausal status | ||
| Pre-menopause | 255 | 30.3 |
| Post-menopause | 501 | 59.6 |
| Missing | 85 | 10.1 |
| Tumor histotype | ||
| Invasive ductal carcinoma | 625 | 74.3 |
| Lobular carcinoma | 65 | 7.7 |
| Medullary carcinoma | 29 | 3.4 |
| Apocrine carcinoma | 20 | 2.4 |
| Pleomorphic carcinoma | 14 | 1.7 |
| Metaplastic carcinoma | 12 | 1.4 |
| Others | 29 | 3.4 |
| Missing | 47 | 5.6 |
| Histologic grade | ||
| 1 | 11 | 1.3 |
| 2 | 169 | 20.1 |
| 3 | 600 | 71.3 |
| Missing | 61 | 7.3 |
| Tumor size (T) | ||
| Tx | 15 | 1.8 |
| T1 | 309 | 36.7 |
| T2 | 364 | 43.3 |
| T3 | 55 | 6.5 |
| T4 | 43 | 5.1 |
| Missing | 55 | 6.5 |
| Pathological lymph nodes (pN) | ||
| pNx | 26 | 3.1 |
| pN0 | 439 | 52.2 |
| pN1 | 185 | 22.0 |
| pN2 | 88 | 10.5 |
| pN3 | 46 | 5.6 |
| Missing | 57 | 6.8 |
| Distant metastases (M) | ||
| M0 | 747 | 88.8 |
| M1 | 10 | 1.2 |
| Missing | 84 | 10.0 |
| Tumor stage | ||
| I | 211 | 25.1 |
| II | 359 | 42.7 |
| III | 161 | 19.1 |
| IV | 10 | 1.2 |
| Missing | 100 | 11.9 |
| Tumor infiltrating lymphocytes | ||
| No | 497 | 59.1 |
| Yes | 283 | 33.7 |
| Missing | 61 | 7.2 |
| Lymphovascular invasion | ||
| No | 587 | 69.8 |
| Yes | 193 | 23.0 |
| Missing | 61 | 7.2 |
| Necrosis | ||
| No | 480 | 57.1 |
| Yes | 301 | 35.8 |
| Missing | 60 | 7.1 |
| Ki-67 (%) | ||
| 0–15 | 112 | 13.3 |
| 16–25 | 95 | 11.3 |
| 26–35 | 122 | 14.5 |
| 36–45 | 100 | 11.9 |
| ≥ 46 | 376 | 44.7 |
| Missing | 36 | 4.3 |
| Type of surgery | ||
| No surgery | 6 | 0.7 |
| Quadrantectomy | 437 | 52.0 |
| Mastectomy | 319 | 37.9 |
| Missing | 79 | 9.4 |
| Radiotherapy on residual breasta | ||
| No | 0 | 0.0 |
| Yes | 411 | 94.1 |
| Missing | 26 | 5.9 |
| Post-mastectomy radiotherapyb | ||
| No | 236 | 74.0 |
| Yes | 24 | 7.5 |
| Missing | 59 | 18.5 |
| Chemotherapy | ||
| Neoadjuvant, only | 34 | 4.0 |
| Adjuvant, only | 545 | 64.8 |
| Neoadjuvant and adjuvant | 73 | 8.7 |
| Missing | 189 | 22.5 |
aFor patients who had a quadrantectomy. bFor patients who had a mastectomy
Table 2 shows the multivariate HRs for mortality according to selected clinical and pathological characteristics of TNBCs. After allowance for study center and age at diagnosis, all the components of the TNM staging system were significant independent prognostic factors for TNBC. In particular, compared to T1, HRs for mortality were 2.71 (95% CI 1.74–4.23) for T2, 2.85 (95% CI 1.46–5.55) for T3, and 8.13 (95% CI 4.44–14.9) for T4. Compared to pN0, HRs were 2.63 (95% CI 1.69–4.10) for pN1, 3.54 (95% CI 2.06–6.06) for pN2, and 6.10 (95% CI 3.44–10.8) for pN3. Compared to patients without metastasis at diagnosis, the HR was 6.01 (95% CI 2.72–13.3) for those with metastasis. Compared to tumor stage I, HR was 3.09 (95% CI 1.59–6.00) for stage II, 9.68 (95% CI 5.02–18.7) for stage III, and 19.8 (95% CI 7.54–51.9) for stage IV. Presence of LVI and necrosis was also significantly associated with increased mortality (HR: 2.45, 95% CI 1.71–3.51, and 1.58, 95% CI 1.11–2.24, respectively). Expression of Ki-67 over 16% were associated with increased mortality, although in the absence of a clear trend with increasing expression (HR: 1.77, 95% CI 1.08–2.91, for Ki-67 ≥ 16% compared to 0–15%). Patients with tumor histotypes other than ductal invasive or lobular carcinomas had a more favorable, though not significant, prognosis as compared to ductal invasive carcinoma (HR: 0.56, 95% CI 0.30–1.02). No significant associations were found for histologic grade (HR: 1.12, 95% CI 0.77–1.63, for grade 3 vs grade 1–2), and presence of TIL (HR: 1.24, 95% CI 0.85–1.80). For most clinical and pathological characteristics, the results were consistent when models were further adjusted for TNM-T, TNM-N, TNM-M, LVI, necrosis, and Ki-67. Only the presence of LVI was no more significantly associated to increased mortality after accounting for those clinicopathological factors (HR: 1.49, 95% CI 0.93–2.38).
Table 2.
Hazard ratios (HRs) of mortality, and corresponding 95% confidence intervals (CIs), according to selected clinical and pathological characteristics, among 841 triple-negative breast cancers (TNBCs). Sardinia, Italy 1994–2015
| Number of deaths (%) | HRa (95% CI) | HRb (95% CI) | ||
|---|---|---|---|---|
| Tumor histotype | ||||
| Ductal invasive carcinoma | 625 | 106 (16.9) | 1.00c | 1.00c |
| Lobular carcinoma | 65 | 14 (21.2) | 1.04 (0.59–1.84) | 0.66 (0.31–1.42) |
| Other carcinomasd | 104 | 12 (11.9) | 0.56 (0.30–1.02) | 0.40 (0.21–0.76) |
| Histologic grade | ||||
| 1,2 | 180 | 40 (22.2) | 1.00c | 1.00c |
| 3 | 600 | 102 (17.0) | 1.12 (0.77–1.63) | 0.96 (0.58–1.58) |
| Tumor size (T) | ||||
| T1 | 309 | 30 (9.7) | 1.00c | 1.00c |
| T2 | 364 | 67 (18.4) | 2.71 (1.74–4.23) | 2.41 (1.40–4.15) |
| T3 | 55 | 13 (23.6) | 2.85 (1.46–5.55) | 2.24 (1.00–5.06) |
| T4 | 43 | 17 (39.5) | 8.13 (4.44–14.9) | 5.13 (2.21–11.9) |
| Pathological lymph nodes (pN) | ||||
| pN0 | 439 | 39 (8.9) | 1.00c | 1.00c |
| pN1 | 185 | 42 (22.7) | 2.63 (1.69–4.10) | 2.04 (1.22–3.40) |
| pN2 | 88 | 21 (23.9) | 3.54 (2.06–6.06) | 3.11 (1.70–5.68) |
| pN3 | 46 | 19 (41.3) | 6.10 (3.44–10.8) | 3.18 (1.51–6.71) |
| Distant metastases (M) | ||||
| M0 | 747 | 117 (15.7) | 1.00c | 1.00c |
| M1 | 10 | 7 (70.0) | 6.01 (2.72–13.3) | 5.13 (1.69–15.6) |
| Tumor stagee | ||||
| I | 211 | 11 (5.2) | 1.00c | 1.00c |
| II | 359 | 48 (13.4) | 3.09 (1.59–6.00) | 3.13 (1.56–6.27) |
| III | 161 | 52 (32.3) | 9.68 (5.02–18.7) | 9.65 (4.66–20.0) |
| IV | 10 | 7 (70.0) | 19.8 (7.54–51.9) | 29.0 (9.65–86.9) |
| Tumor infiltrating lymphocytes (TIL) | ||||
| No | 497 | 90 (18.1) | 1.00c | 1.00c |
| Yes | 283 | 40 (14.1) | 1.24 (0.85–1.80) | 1.20 (0.76–1.91) |
| Lymphovascular invasion (LVI) | ||||
| No | 587 | 82 (14.0) | 1.00c | 1.00c |
| Yes | 193 | 48 (24.9) | 2.45 (1.71–3.51) | 1.49 (0.93–2.38) |
| Necrosis | ||||
| No | 480 | 71 (14.8) | 1.00c | 1.00c |
| Yes | 301 | 60 (19.9) | 1.58 (1.11–2.24) | 1.61 (1.03–2.51) |
| Ki-67 (%) | ||||
| 0–15 | 112 | 19 (17.0) | 1.00c | 1.00c |
| 16–25 | 95 | 31 (32.6) | 2.15 (1.21–3.83) | 2.19 (1.03–4.66) |
| 26–35 | 122 | 24 (19.7) | 1.45 (0.79–2.65) | 1.65 (0.71–3.84) |
| 36–45 | 100 | 18 (18.0) | 1.46 (0.76–2.80) | 1.69 (0.70–4.09) |
| ≥ 46 | 376 | 57 (15.2) | 2.00 (1.14–3.51) | 2.37 (1.08–5.21) |
aEstimates from multivariate proportional hazard regression models adjusted for study center and age at diagnosis. Estimates in bold are those significant at the 0.05 level. bEstimates further adjusted for TNM-T, TNM-N, TNM-M, necrosis, LVI, and Ki-67. cReference category. dIncluding medullary, apocrine, pleomorphic, and metaplastic carcinomas. eEstimates not adjusted for TNM-T, TNM-N, and TNM-M
The multivariate HRs for cancer recurrence according to selected clinical and pathological characteristics were consistent with those observed for mortality, with the exception of Ki-67 and necrosis which were not found to be a significant predictor of recurrence in TNBC patients, particularly when taking into account for other clinicopathological factors (Additional file 1: Table S1).
When we considered the role of pathological lymph nodes stage and lymph node ratio on mortality among TNBC patients with positive lymph nodes (Table 3), we found that the HR for pN3 versus pN1 was 2.18 (95% CI 1.23–3.84) and that for lymph node ratio > 0.65 versus <0.20 was 3.62 (95% CI 1.96–6.68). Moreover, when we took into account for other clinicopathological factors, as well as for pathological lymph nodes stage and lymph node ratio simultaneously, the HRs became 0.80 (95% CI 0.34–1.87) and 3.05 (95% CI 1.35–6.87) for pN3 and lymph node ratio > 0.65, respectively. Consistent results were found for cancer recurrence (Additional file 1: Table S2).
Table 3.
Hazard ratios (HRs) of mortality, and corresponding 95% confidence intervals (CIs), according to pathological lymph nodes and lymph node ratio among 319 triple-negative breast cancers (TNBCs) with positive lymph nodes. Sardinia, Italy 1994–2015
| Number of deaths (%) | HRa (95% CI) | HRb (95% CI) | ||
|---|---|---|---|---|
| Pathological lymph nodes (pN) | ||||
| pN1 | 185 | 42 (22.7) | 1.00c | 1.00c |
| pN2 | 88 | 21 (23.9) | 1.37 (0.80–2.37) | 1.13 (0.58–2.17) |
| pN3 | 46 | 19 (41.3) | 2.18 (1.23–3.84) | 0.80 (0.34–1.87) |
| Lymph node ratio | ||||
| < 0.20 | 169 | 25 (14.8) | 1.00c | 1.00c |
| 0.21–0.65 | 93 | 29 (31.2) | 2.47 (1.43–4.25) | 2.44 (1.25–4.78) |
| > 0.65 | 48 | 22 (45.8) | 3.62 (1.96–6.68) | 3.05 (1.35–6.87) |
| Missing | 9 | |||
aEstimates from multivariate proportional hazard regression models adjusted for study center and age at diagnosis. Estimates in bold are those significant at the 0.05 level. bEstimates further adjusted for TNM-T, TNM-N, TNM-M, necrosis, LVI, and Ki-67. cReference category
Using the missing indicator method to treat missing data in the multivariable models as a sensitivity analysis, we found HR estimates consistent with those presented above (data not shown).
Figure 1 shows the Kaplan-Meier curves for the association between tumor stage and OS. There was a clear and significant (p < 0.001) reduction in OS according to increasing stage at diagnosis of TNBC. 5-years OS was 93.9% for stage I, 84.5% for stage II, 57.2% for stage III, and 26.7% for stage IV. Results were similar for DFS (Additional file 1: Figure S1).
Fig. 1.
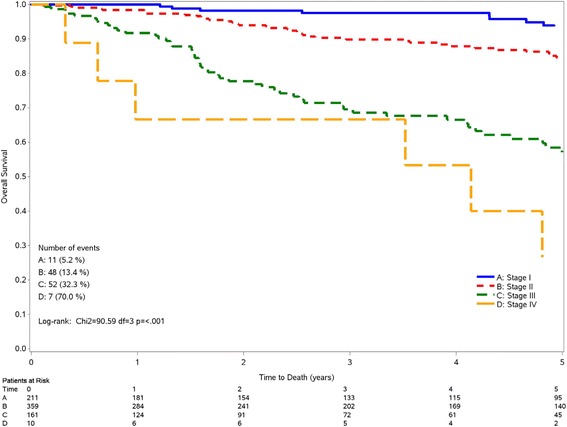
Kaplan-Meir curves for overall survival according to tumor stage among 841 triple-negative breast cancer patients. Sardinia, Italy 1994–2005
Figure 2 shows the Kaplan-Meier curve for OS according to pathological lymph nodes stage (Fig. 1a) and lymph node ratio (Fig. 1b), among patients with positive lymph nodes. For pathological lymph nodes, the survival curves for pN1 and pN2 stage patients overlapped, while pN3 stage patients had a worse survival (p = 0.006). The 5-yrs survival was 71.6%, 68.3%, and 44.1% for pN1, pN2, and pN3, respectively. When considering lymph node ratio, there was significant reduction in OS according to increasing level of lymph node ratio (p < 0.001), 5-yrs survival being 80.7%, 59.4%, and 40.5% for <0.21, 0.21–0.65, and >0.65, respectively. Again, consistent results were found for DFS (Additional file 1: Figure S2).
Fig. 2.
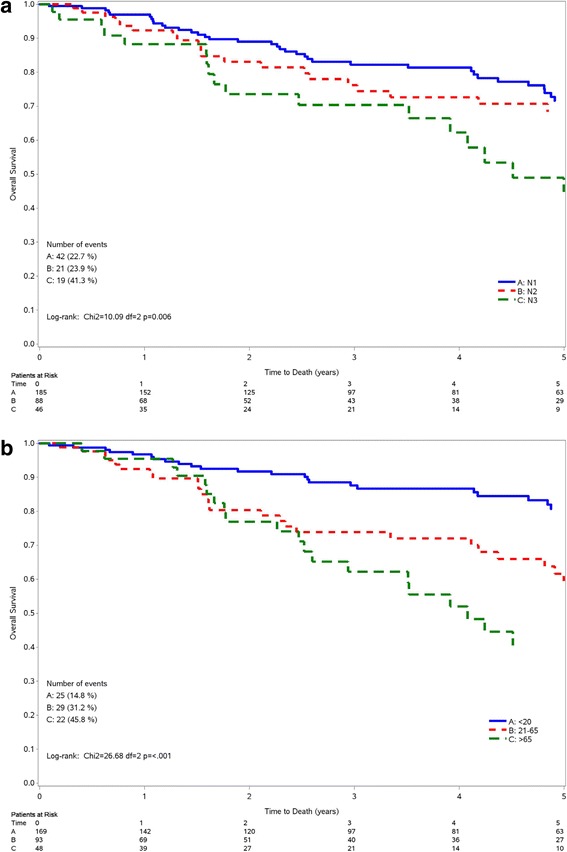
Kaplan-Meir curves for overall survival according to pathological lymph nodes stage (a) and lymph node ratio (b) among 319 triple-negative breast cancer patients with positive lymph nodes. Sardinia, Italy 1994–2005
Discussion
In this uniquely large cohort of TNBC patients, we found that a high tumor stage at diagnosis – as defined by tumor size, pathological lymph nodes, and presence of metastasis – is the most important prognostic factor for cancer progression and mortality. Among other tumor features, necrosis and Ki-67 are also independently associated with increased mortality. Moreover, among lymph node positive tumors, lymph node ratio appears to be a better predictor of mortality than pathological lymph nodes.
The TNM staging system has long been identified as one of the best predictors of long-term survival and an indicator for therapeutic decisions in patients with breast cancer [22]. However, the usefulness of TNM as a prognostic factor for biologically different subtypes of breast cancer, including TNBCs, has been questioned [7, 8]. Indeed, a cohort based on 391 TNBC patients from the Samsung Medical Center, Korea, found no association between tumor stage and recurrence-free survival among TNBC patients with stage 1 to 3A, suggesting that the TNM staging system might not be a good predictor of survival outcomes in TNBC patients [8]. Moreover, a large study from the US suggested that survival in TNBC patients was affected by the presence of positive lymph nodes, but were not greatly influenced by the number of positive lymph nodes [23]. Nevertheless, a few studies found that tumor size and lymph node status have a significant association with both DFS and OS in TNBC patients [24–27]. Consistently, in our study we found a highly significant association between TNM stage and cancer progression or mortality among TNBC patients. Among single TNM staging components, both tumor size and involvement of lymph nodes were independently and significantly associated with recurrence and overall mortality. Moreover, we found that the presence of metastasis at diagnosis increased cancer recurrence and mortality by more than five-fold. Another study also showed that that patients with metastatic TNBC have poorer prognosis as compared to non-metastatic ones [28].
Increasing evidence has shown that the lymph node ratio – which takes into account not only the number of pathological lymph nodes involved but also the number of lymph nodes evaluated – is a more accurate prognostic factor for breast cancer as compared to number of lymph nodes involved [9]. A few studies have also shown that lymph node ratio is an additional independent prognostic factor to the traditional pN stage in the OS and DFS of TNBCs [27, 29, 30]. Consistently, we found that in lymph node positive patients, lymph node ratio appeared to be a stronger predictor of mortality than pN stage, allowing a better discrimination of TNBC patients at high or low risk of mortality.
Many investigations have suggested that the proliferation marker Ki-67 is a valuable prognostic marker in early breast cancer [31]. The prognostic significance of Ki-67 in TNBC patients has also been investigated in several studies providing, however, inconsistent results [24, 32–36]. Some studies did not find any association between Ki-67 and survival outcomes for TNBCs [24, 33, 35], while other larger studies showed that a relatively high Ki-67 expression (≥10%) was inversely associated with TNBC outcomes [32, 34, 36]. These results are in agreement with our findings, showing that TNBC patients with Ki-67 expression over 16% have a poorer prognosis, although the mortality did not increase with increasing expression of Ki-67. The inconsistencies of the results across various studies can be explained either by lack of analytical validity and standardization of this marker or by the use of different cut-off points for the definition of positivity to ki-67 [37]. Given these drawbacks, the use of ki-67 in the clinical practice for patients with TNBCs, as other breast cancers, remains still debatable [11, 31].
Limited information is available on the association between histological subtype of TNBC and survival outcomes. One study conducted on 476 TNBC patients from Belgium suggested some differences in DFS according to tumor histology. However, given the relatively low number of TNBC histotypes other than invasive ductal carcinoma, the study was not able to provide significant estimates [38]. A larger study conducted on 781 TNBC patients from Italy found that, compared with patients with invasive ductal carcinoma, OS and DFS were less favorable in women with metaplastic carcinoma, and more favorable in those with adenoid cystic and medullary subtypes, while no difference was observed for lobular carcinoma [12]. Accordingly, we did not find any difference in terms of recurrence and mortality between lobular and invasive ductal carcinomas, whereas other TNBC subtypes (mostly medullary and apocrine carcinomas) showed significantly better outcomes than invasive ductal carcinomas.
Among the best-established prognostic factors for breast cancer there is histologic grade [10]. This notwithstanding, in our large cohort of TNBC patients – as reported in a few other smaller studies [8, 26] – grade had no role in survival outcomes. This may be at least in part due to the high histological grade of TNBC patients [3], which might make it difficult to disentangle the role of grade on TNBC prognosis. Indeed, in our cohort only 1.5% of patients were G1 and over 3 out of 4 patients were G3.
Various large studies recently found that tumor TILs (mainly stromal) – a surrogate marker of adaptive immune response – is associated with a favorable prognosis in TNBC patients [39–42], although the use of TILs as an additional prognostic factors in TNBCs is not yet recommend giving the lack of standardization and clinical validation of this marker [13]. In our cohort, although death rates were lower among TNBC patients with TILs, no significant association was found between TILs and cancer progression or mortality. However, we had no information on the number/proportion of TILs and we did not specifically measured stromal TILs.
LVI – which refers to the invasion of lymphatic spaces and blood vessels – has long been considered a relevant prognostic marker of breast cancer, although it has not been incorporated in most internationally recognized staging system as the AJCC/TMN one [14, 21]. A few small studies which have investigated the relationship between LVI and DFS or OS in patients with TNBC showed that LVI is an independent predictor of poor outcome [14, 26, 43]. In our large cohort we found that LVI presence has a negative impact on both tumor recurrence and mortality when taking into account only study center and age at diagnosis. However, after allowance for other clinicopathological characteristics, the association between LVI and mortality was no more significant, thus do not supporting a relevant prognostic role of this marker.
Scanty data are available on the role of necrosis on prognostic outcomes in TNBC patients. In a study on 154 TBNCs from China, tumor necrosis was found to be a significant prognostic factor, although only results from univariate analyses were provided [15]. In our large cohort, necrosis at baseline was significantly associated to survival outcomes, even after allowance for other clinicopathological factors.
The results of this study should be interpreted after taking into consideration various limitations, mainly inherent to its retrospective design. Thus, we could not retrieve information on vital status at follow-up for 311 out of 1152 TNBC patients (about 27%) and we had to exclude them from the present analyses. Moreover, for some patients important clinical and pathological data were missing because not originally included in the medical records, and those missing information may have to some extent influenced the associations evaluated. Furthermore, some misclassification of patients may have resulted from the classification of tumors in different laboratories across hospital centers, where clinical and pathological testing practices can vary. However, pathology materials were reviewed centrally by three pathologists following the same national/international breast cancer guidelines in order to uniformely classify TNBCs across hospital centers and standardize ER, PgR and HER2 immunohistochemical results for TNBC samples, according to the ASCO/CAP recommendations [17].
The strengths of our study include its uniquely large sample size – including from one third up to half of all new Sardinian TNBC patients over the study period, the comprehensive and standardized nature of the registry database with patients’ characteristics, pathological tumor features, cancer treatments, and the complete ascertainment of patient status at regular follow-up intervals. This also allowed us to derive multivariate HR estimates for OS and DFS after allowance for a number of potential confounders.
Conclusions
In this uniquely large cohort, we provide further evidence that, besides tumor stage at diagnosis, lymph node ratio among lymph node positive tumors is an additional relevant predictor of mortality and recurrence in TNBCs, while Ki-67 seems to be predictive of mortality, but not of recurrence.
Additional files
Hazard ratios (HRs) of recurrence, and corresponding 95% confidence intervals (CIs), according to selected clinical and pathological characteristics, among 825 triple-negative breast cancers (TNBCs). Sardinia, Italy 1994-2015. Table S2. Hazard ratios (HRs) of recurrence, and corresponding 95% confidence intervals (CIs), according to pathological lymph nodes and lymph node ratio among 311 triple-negative breast cancers (TNBCs) with positive lymph nodes. Sardinia, Italy 1994-2015. Figure S1. Kaplan-Meir curves for disease-free survival according to tumor stage among 825 triple-negative breast cancer patients. Sardinia, Italy 1994–2005. Figure S2. Kaplan-Meir curves for disease-free survival according to pathological lymph nodes stage (a) and lymph node ratio (b) among 311 triple-negative breast cancer patients with positive lymph nodes. Sardinia, Italy 1994–2005 (DOC 658 kb)
Acknowledgements
Authors wish to thank Prof. Andrea Piga ("A. Businco" Oncological Hospital, Caglari, Italy), Università degli Studi di Sassari, for the initial concept of this study, Dr. Eliana Rulli (IRCCS-Istituto di Ricerche Farmacologiche “Mario Negri”, Milan, Italy) for the development of the SAS macro for the Kaplan-Meier curve, and Mrs. Ivana Garimoldi (IRCCS-Istituto di Ricerche Farmacologiche “Mario Negri”, Milan, Italy) for editorial assistance.
Funding
The study was supported by the Regione Autonoma della Sardegna (Legge Regionale 7 Agosto 2007, N. 7, “Promozione della Ricerca Scientifica e dell'Innovazione Tecnologica in Sardegna”). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The dataset analyzed during the current study is available from the corresponding author on reasonable request.
Abbreviations
- AJCC
American Joint Committee on Cancer criteria
- CI
confidence interval
- DFS
Disease-free survival
- ER
estrogen receptor
- HER2
human epidermal growth factor receptor 2
- HR
hazard ratio
- LVI
lymphovascular invasion
- M
metastasis
- OS
Overall survival
- pN
pathological N stage
- PR
progesterone receptor
- SD
standard deviation
- T
tumor stage
- TIL
tumor infiltrating lymphocytes
- TNBC
triple-negative breast cancer
Authors’ contributions
AB, MRD, PCR, SAMU and SO had the original study idea; EV, FA, CC, GS, MG, AMu, AMA, VM, DO, SC, MCS, LC provided materials and/or patients; ES, DP, RM, SAMU, FS, GP, AMa, MF,TM collected and assembled data; SG conducted the statistical analysis; SAMU and SG wrote the manuscript; CB, EV, MDI, MRD, PCR and SO contributed in drafting the manuscript; all authors gave substantial contributions in the conception, design and interpretation of data and approved the final version of the manuscript.
Ethics approval and consent to participate
The study protocol was approved by the local research ethics committee of Sardinia Region (File number 224/CE/12). Informed consent was waived from the local research ethics committee since patients’ information was collected during the routine clinical practice and patients were identified by anonymized investigator-generated code not linkable to their personal data.
Consent for publication
Not required
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12885-017-3969-y) contains supplementary material, which is available to authorized users.
Contributor Information
Silvana Anna Maria Urru, Email: silvanaurru@crs4.it.
Silvano Gallus, Email: silvano.gallus@marionegri.it.
Cristina Bosetti, Phone: +39 0239014526, Email: cristina.bosetti@marionegri.it.
Tiziana Moi, Email: tyzumoi@yahoo.it.
Ricardo Medda, Email: ricard@tiscali.it.
Elisabetta Sollai, Email: elisol81@tiscali.it.
Alma Murgia, Email: alma.murgia@aob.it.
Francesca Sanges, Email: f.sanges@yahoo.it.
Giovanna Pira, Email: gpira@uniss.it.
Alessandra Manca, Email: al_manc@yahoo.it.
Dolores Palmas, Email: dolor64@alice.it.
Matteo Floris, Email: matteo.floris@gmail.com.
Anna Maria Asunis, Email: anna_asunis@yahoo.it.
Francesco Atzori, Email: francescoatzori74@yahoo.it.
Ciriaco Carru, Email: carru@uniss.it.
Maurizio D’Incalci, Email: maurizio.dincalci@marionegri.it.
Massimo Ghiani, Email: massimoghiani67@gmail.com.
Vincenzo Marras, Email: marrasv@gmail.com.
Daniela Onnis, Email: danielaonnis@aob.it.
Maria Cristina Santona, Email: maricri.san@tiscali.it.
Giuseppina Sarobba, Email: gi.sarobba@tiscali.it.
Enrichetta Valle, Email: enrichettavalle@yahoo.it.
Luisa Canu, Email: luisa.canu@yahoo.it.
Sergio Cossu, Email: sergio.cossu@tiscali.it.
Alessandro Bulfone, Email: alessandro.bulfone@gmail.com.
Paolo Cossu Rocca, Email: pcossurocca@yahoo.it.
Maria Rosaria De Miglio, Email: mrdemiglio@gmail.com.
Sandra Orrù, Email: orrusandra14@gmail.com.
References
- 1.Global Burden of Disease Cancer C. Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, MF MI, Allen C, Hansen G, Woodbrook R, et al. The Global Burden of Cancer 2013. JAMA Oncol. 2015;1:505–527. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 4.Boyle P. Triple-negative breast cancer: epidemiological considerations and recommendations. Ann Oncol. 2012;23(Suppl 6):vi7–v12. doi: 10.1093/annonc/mds187. [DOI] [PubMed] [Google Scholar]
- 5.Lin NU, Vanderplas A, Hughes ME, Theriault RL, Edge SB, Wong YN, Blayney DW, Niland JC, Winer EP, Weeks JC. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the national comprehensive cancer network. Cancer. 2012;118:5463–5472. doi: 10.1002/cncr.27581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 7.Foulkes WD, Grainge MJ, Rakha EA, Green AR, Ellis IO. Tumor size is an unreliable predictor of prognosis in basal-like breast cancers and does not correlate closely with lymph node status. Breast Cancer Res Treat. 2009;117:199–204. doi: 10.1007/s10549-008-0102-6. [DOI] [PubMed] [Google Scholar]
- 8.Park YH, Lee SJ, Cho EY, Choi YL, Lee JE, Nam SJ, Yang JH, Shin JH, Ko EY, Han BK, et al. Clinical relevance of TNM staging system according to breast cancer subtypes. Ann Oncol. 2011;22:1554–1560. doi: 10.1093/annonc/mdq617. [DOI] [PubMed] [Google Scholar]
- 9.Vinh-Hung V, Verkooijen HM, Fioretta G, Neyroud-Caspar I, Rapiti E, Vlastos G, Deglise C, Usel M, Lutz JM, Bouchardy C. Lymph node ratio as an alternative to pN staging in node-positive breast cancer. J Clin Oncol. 2009;27:1062–1068. doi: 10.1200/JCO.2008.18.6965. [DOI] [PubMed] [Google Scholar]
- 10.Rakha EA, Reis-Filho JS, Baehner F, Dabbs DJ, Decker T, Eusebi V, Fox SB, Ichihara S, Jacquemier J, Lakhani SR, et al. Breast cancer prognostic classification in the molecular era: the role of histological grade. Breast Cancer Res. 2010;12:207. doi: 10.1186/bcr2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andre F, Arnedos M, Goubar A, Ghouadni A, Delaloge S. Ki67--no evidence for its use in node-positive breast cancer. Nat Rev Clin Oncol. 2015;12:296–301. doi: 10.1038/nrclinonc.2015.46. [DOI] [PubMed] [Google Scholar]
- 12.Montagna E, Maisonneuve P, Rotmensz N, Cancello G, Iorfida M, Balduzzi A, Galimberti V, Veronesi P, Luini A, Pruneri G, et al. Heterogeneity of triple-negative breast cancer: histologic subtyping to inform the outcome. Clin Breast Cancer. 2013;13:31–39. doi: 10.1016/j.clbc.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, Thurlimann B, Senn HJ, Panel M. Tailoring therapies--improving the management of early breast cancer: St Gallen international expert consensus on the primary therapy of early breast cancer 2015. Ann Oncol. 2015;26:1533–1546. doi: 10.1093/annonc/mdv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rakha EA, Martin S, Lee AH, Morgan D, Pharoah PD, Hodi Z, Macmillan D, Ellis IO. The prognostic significance of lymphovascular invasion in invasive breast carcinoma. Cancer. 2012;118:3670–3680. doi: 10.1002/cncr.26711. [DOI] [PubMed] [Google Scholar]
- 15.Liu YX, Wang KR, Xing H, Zhai XJ, Wang LP, Wang W. Attempt towards a novel classification of triple-negative breast cancer using immunohistochemical markers. Oncol Lett. 2016;12:1240–1256. doi: 10.3892/ol.2016.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Budroni M, Sechi O, Cossu A, Palmieri G, Tanda F, Foschi R, Rossi S. Estimates of cancer burden in Sardinia. Tumori. 2013;99:408–415. doi: 10.1177/030089161309900317. [DOI] [PubMed] [Google Scholar]
- 17.Hammond ME, Hayes DF, Wolff AC, Mangu PB, Temin S. American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract. 2010;6:195–197. doi: 10.1200/JOP.777003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 19.Sobin LH, Gospodarovicz M, Wittekind C. TNM Classificassification of malignant Tumours. 5. New York: Wiley-Liss; 1997. [Google Scholar]
- 20.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 21.Edge SB, Byrd DR, Compton CC, Fritz AG, Green FL, Trotti A. AJCC cancer staging manual. 7. New York: Springer; 2010. [Google Scholar]
- 22.Veronesi U, Zurrida S, Viale G, Galimberti V, Arnone P, Nole F, Rethinking TNM. A breast cancer classification to guide to treatment and facilitate research. Breast J. 2009;15:291–295. doi: 10.1111/j.1524-4741.2009.00719.x. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez-Aya LF, Chavez-Macgregor M, Lei X, Meric-Bernstam F, Buchholz TA, Hsu L, Sahin AA, Do KA, Valero V, Hortobagyi GN, et al. Nodal status and clinical outcomes in a large cohort of patients with triple-negative breast cancer. J Clin Oncol. 2011;29:2628–2634. doi: 10.1200/JCO.2010.32.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JA, Kim KI, Bae JW, Jung YH, An H, Lee ES. Korean breast cancer S. Triple negative breast cancer in Korea-distinct biology with different impact of prognostic factors on survival. Breast Cancer Res Treat. 2010;123:177–187. doi: 10.1007/s10549-010-0998-5. [DOI] [PubMed] [Google Scholar]
- 25.Ovcaricek T, Frkovic SG, Matos E, Mozina B, Borstnar S. Triple negative breast cancer - prognostic factors and survival. Radiol Oncol. 2011;45:46–52. doi: 10.2478/v10019-010-0054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwon J, Eom KY, Koo TR, Kim BH, Kang E, Kim SW, Kim YJ, Park SY, Kim IAA. Prognostic model for patients with triple-negative breast cancer: importance of the modified Nottingham prognostic index and age. J Breast Cancer. 2017;20:65–73. doi: 10.4048/jbc.2017.20.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He M, Zhang JX, Jiang YZ, Chen YL, Yang HY, Tang LC, Shao ZM, Di GH. The lymph node ratio as an independent prognostic factor for node-positive triple-negative breast cancer. Oncotarget. 2017; [DOI] [PMC free article] [PubMed]
- 28.Lin NU, Claus E, Sohl J, Razzak AR, Arnaout A, Winer EP. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer. 2008;113:2638–2645. doi: 10.1002/cncr.23930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahn SH, Kim HJ, Lee JW, Gong GY, Noh DY, Yang JH, Jung SS, Park HY. Lymph node ratio and pN staging in patients with node-positive breast cancer: a report from the Korean breast cancer society. Breast Cancer Res Treat. 2011;130:507–515. doi: 10.1007/s10549-011-1730-9. [DOI] [PubMed] [Google Scholar]
- 30.Tsai J, Bertoni D, Hernandez-Boussard T, Telli ML, Wapnir IL. Lymph node ratio analysis after neoadjuvant chemotherapy is prognostic in hormone receptor-positive and triple-negative breast cancer. Ann Surg Oncol. 2016;23:3310–3316. doi: 10.1245/s10434-016-5319-8. [DOI] [PubMed] [Google Scholar]
- 31.Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010;11:174–183. doi: 10.1016/S1470-2045(09)70262-1. [DOI] [PubMed] [Google Scholar]
- 32.Keam B, Im SA, Lee KH, Han SW, Oh DY, Kim JH, Lee SH, Han W, Kim DW, Kim TY, et al. Ki-67 can be used for further classification of triple negative breast cancer into two subtypes with different response and prognosis. Breast Cancer Res. 2011;13:R22. doi: 10.1186/bcr2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aleskandarany MA, Green AR, Benhasouna AA, Barros FF, Neal K, Reis-Filho JS, Ellis IO, Rakha EA. Prognostic value of proliferation assay in the luminal, HER2-positive, and triple-negative biologic classes of breast cancer. Breast Cancer Res. 2012;14:R3. doi: 10.1186/bcr3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munzone E, Botteri E, Sciandivasci A, Curigliano G, Nole F, Mastropasqua M, Rotmensz N, Colleoni M, Esposito A, Adamoli L, et al. Prognostic value of Ki-67 labeling index in patients with node-negative, triple-negative breast cancer. Breast Cancer Res Treat. 2012;134:277–282. doi: 10.1007/s10549-012-2040-6. [DOI] [PubMed] [Google Scholar]
- 35.Denkert C, Loibl S, Muller BM, Eidtmann H, Schmitt WD, Eiermann W, Gerber B, Tesch H, Hilfrich J, Huober J, et al. Ki67 levels as predictive and prognostic parameters in pretherapeutic breast cancer core biopsies: a translational investigation in the neoadjuvant GeparTrio trial. Ann Oncol. 2013;24:2786–2793. doi: 10.1093/annonc/mdt350. [DOI] [PubMed] [Google Scholar]
- 36.Abubakar M, Orr N, Daley F, Coulson P, Ali HR, Blows F, Benitez J, Milne R, Brenner H, Stegmaier C, et al. Prognostic value of automated KI67 scoring in breast cancer: a centralised evaluation of 8088 patients from 10 study groups. Breast Cancer Res. 2016;18:104. doi: 10.1186/s13058-016-0765-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dowsett M, Nielsen TO. A'Hern R, Bartlett J, Coombes RC, Cuzick J, Ellis M, Henry NL, Hugh JC, Lively T, et al. Assessment of Ki67 in breast cancer: recommendations from the international Ki67 in breast cancer working group. J Natl Cancer Inst. 2011;103:1656–1664. doi: 10.1093/jnci/djr393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dreyer G, Vandorpe T, Smeets A, Forceville K, Brouwers B, Neven P, Janssens H, Deraedt K, Moerman P, Van Calster B, et al. Triple negative breast cancer: clinical characteristics in the different histological subtypes. Breast. 2013;22:761–766. doi: 10.1016/j.breast.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, Kellokumpu-Lehtinen PL, Bono P, Kataja V, Desmedt C, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25:1544–1550. doi: 10.1093/annonc/mdu112. [DOI] [PubMed] [Google Scholar]
- 40.Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32:2959–2966. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pruneri G, Gray KP, Vingiani A, Viale G, Curigliano G, Criscitiello C, Lang I, Ruhstaller T, Gianni L, Goldhirsch A, et al. Tumor-infiltrating lymphocytes (TILs) are a powerful prognostic marker in patients with triple-negative breast cancer enrolled in the IBCSG phase III randomized clinical trial 22-00. Breast Cancer Res Treat. 2016;158:323–331. doi: 10.1007/s10549-016-3863-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pruneri G, Vingiani A, Bagnardi V, Rotmensz N, De Rose A, Palazzo A, Colleoni AM, Goldhirsch A, Viale G. Clinical validity of tumor-infiltrating lymphocytes analysis in patients with triple-negative breast cancer. Ann Oncol. 2016;27:249–256. doi: 10.1093/annonc/mdv571. [DOI] [PubMed] [Google Scholar]
- 43.Gujam FJ, Going JJ, Mohammed ZM, Orange C, Edwards J, DC MM. Immunohistochemical detection improves the prognostic value of lymphatic and blood vessel invasion in primary ductal breast cancer. BMC Cancer. 2014;14:676. doi: 10.1186/1471-2407-14-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hazard ratios (HRs) of recurrence, and corresponding 95% confidence intervals (CIs), according to selected clinical and pathological characteristics, among 825 triple-negative breast cancers (TNBCs). Sardinia, Italy 1994-2015. Table S2. Hazard ratios (HRs) of recurrence, and corresponding 95% confidence intervals (CIs), according to pathological lymph nodes and lymph node ratio among 311 triple-negative breast cancers (TNBCs) with positive lymph nodes. Sardinia, Italy 1994-2015. Figure S1. Kaplan-Meir curves for disease-free survival according to tumor stage among 825 triple-negative breast cancer patients. Sardinia, Italy 1994–2005. Figure S2. Kaplan-Meir curves for disease-free survival according to pathological lymph nodes stage (a) and lymph node ratio (b) among 311 triple-negative breast cancer patients with positive lymph nodes. Sardinia, Italy 1994–2005 (DOC 658 kb)
Data Availability Statement
The dataset analyzed during the current study is available from the corresponding author on reasonable request.


