Abstract
Frontotemporal dementia (FTD) is a highly heritable condition with multiple genetic causes. In this study, similarities and differences of gray matter (GM) atrophy patterns were assessed among 3 common forms of genetic FTD (mutations in C9orf72, GRN, and MAPT). Participants from the Genetic FTD Initiative (GENFI) cohort with a suitable volumetric T1 magnetic resonance imaging scan were included (319): 144 nonmutation carriers, 128 presymptomatic mutation carriers, and 47 clinically affected mutation carriers. Cross-sectional differences in GM volume between noncarriers and carriers were analyzed using voxel-based morphometry. In the affected carriers, each genetic mutation group exhibited unique areas of atrophy but also a shared network involving the insula, orbitofrontal lobe, and anterior cingulate. Presymptomatic GM atrophy was observed particularly in the thalamus and cerebellum in the C9orf72 group, the anterior and medial temporal lobes in MAPT, and the posterior frontal and parietal lobes as well as striatum in GRN. Across all presymptomatic carriers, there were significant decreases in the anterior insula. These results suggest that although there are important differences in atrophy patterns for each group (which can be seen presymptomatically), there are also similarities (a fronto-insula-anterior cingulate network) that help explain the clinical commonalities of the disease.
Keywords: Frontotemporal dementia, Magnetic resonance imaging, Atrophy, Voxel-based morphometry, Preclinical dementia
1. Introduction
Frontotemporal dementia (FTD) is a common cause of dementia with around one-third of cases being familial, most commonly caused by mutations in 1 of 3 genes: chromosome 9 open reading frame 72 (C9orf72), progranulin (GRN), and microtubule-associated protein tau (MAPT) (Rohrer et al., 2009). Studying mutation carriers in the years before any signs of clinical manifestation provides insight into the early stage of the disease process. One biomarker of particular interest in presymptomatic FTD is brain atrophy as measured by structural magnetic resonance imaging (MRI). Although there have been many studies describing the atrophy patterns in FTD (Mahoney et al., 2012, Rohrer et al., 2010, Whitwell et al., 2009, Whitwell et al., 2012), these have commonly been smaller single-site studies of patients who are already symptomatic. The multicenter Genetic FTD Initiative (GENFI) investigates both affected and at-risk FTD family members, with preliminary region of interest (ROI) based cross-sectional analysis indicating that carriers have significantly lower cortical and subcortical volumes a number of years before the expected age at onset (Rohrer et al., 2015). In this study, we use data from GENFI to perform a whole-brain voxel-wise analysis to provide complementary information to the previous ROI study and expand on previous voxel-based morphometry studies in genetic FTD, with a particular focus on comparing and contrasting patterns of atrophy between the mutations and determining the extent of gray matter loss in the presymptomatic phase.
2. Material and methods
2.1. Participants
At the time of the second data freeze in the GENFI study, 365 participants had been recruited across 13 centers in the United Kingdom, Canada, Italy, Netherlands, Sweden, and Portugal, of whom 319 had a usable volumetric T1-weighted MRI scan for analysis (15 participants did not have a scan, and a further 31 participants were excluded as the scans were of unsuitable quality due to motion, other imaging artifacts, or pathology unlikely to be attributed to FTD). All participants were known to be a symptomatic carrier of a pathogenic mutation in C9orf72, GRN, or MAPT or to be an at-risk first-degree relative. Patients were considered symptomatic when the assessing clinician felt that the patient had evidence of progressive cognitive or behavioral change. All participants underwent genetic testing to determine whether they were a carrier or noncarrier: in total, 144 were noncarriers, 128 were presymptomatic mutation carriers, and 47 were affected mutation carriers (Table 1). All participants underwent a standardized clinical assessment as described previously (Rohrer et al., 2015). All aspects of the study were approved by the institutional review boards for each of the GENFI sites, with every participant providing written informed consent.
Table 1.
Demographics of participants included in the analysis
| Variable | Noncarriers | C9orf72 |
GRN |
MAPT |
p-value |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Presymptomatic | Affected | Presymptomatic | Affected | Presymptomatic | Affected | All groups | At-risk groups (including noncarriers) | Affected groups | ||
| N | 144 | 40 | 25 | 65 | 12 | 23 | 10 | - | - | - |
| Age, mean (SD) | 48.7 (14.3) | 43.5 (10.5) | 65.2 (7.7) | 48.9 (10.7) | 63.2 (6.0) | 38.6 (9.0) | 57.2 (5.9) | <0.001 | <0.001 | 0.011 |
| %Female | 63 | 63 | 28 | 63 | 67 | 61 | 30 | 0.021 | 1.000 | 0.083 |
| EYO, mean (SD) | −10.5 (14.2) | −15.0 (12.5) | 5.8 (5.0) | −10.3 (11.3) | 1.4 (2.1) | −11.8 (10.3) | 5.9 (3.5) | <0.001 | 0.270 | 0.001 |
| Education, y | 13.8 (3.4) | 13.9 (3.0) | 13.1 (4.5) | 14.1 (3.1) | 10 (3.8) | 13.4 (3.4) | 12.2 (4.7) | 0.040 | 0.788 | 0.125 |
| Disease duration, y | N/A | N/A | 6.6 (4.8) | N/A | 2.5 (1.3) | N/A | 4.9 (5.3) | N/A | N/A | 0.010 |
| MMSE (max = 30) | 29.2 (1.3) | 29.2 (1.2) | 24.8 (4.2) | 29.1 (1.4) | 20.7 (6.2) | 29.4 (1.4) | 24.7 (4.9) | <0.001 | 0.359 | 0.139 |
Diagnoses in affected subjects: bvFTD 33 (18 C9orf72, 5 GRN, 10 MAPT), 3 FTD-ALS (all C9orf72), 7 nonfluent variant primary progressive aphasia (PPA) (2 C9orf72, 5, GRN), 1 semantic variant PPA (C9orf72), 1 corticobasal syndrome (GRN), 1 dementia - not otherwise specified (C9orf72).
Bold text indicates a statistically significant difference (p< 0.05) between groups.
2.2. MR image acquisition
Participants underwent a 1.1-mm isotropic resolution volumetric T1 MR imaging on a 3T scanner (10 sites: 5 Siemens Trio, 1 Siemens Skyra, 3 Philips Achieva, 1 GE Discovery MR750) or 1.5T scanner (1–1.25 mm isotropic resolution) when a 3T scanner was not available (3 sites: Siemens Avanto, Siemens Aera, GE Signa HDxt).
All analyses were performed using the Statistical Parametric Mapping toolbox (SPM12) in Matlab. The images were first segmented into maps representing probability of gray matter, white matter, and cerebrospinal fluid at each voxel (Ashburner and Friston, 2005). Next, all images were spatially normalized using geodesic shooting (Ashburner and Friston, 2011) to a study specific template, modulating the probability maps to preserve tissue volumes. The warped and modulated tissue maps were smoothed with a Gaussian kernel of full width at half max of 6 mm to reduce errors caused by misalignment while at the same time allowing for detection of differences over small regions of the brain. Analysis was limited to a gray matter mask that included voxels where the mean probability of the gray matter mask over all subjects was 0.2 or above. Estimates of total intracranial volume were computed by summing the 3 tissue class volumes (Malone et al., 2014).
2.3. Statistical analysis
Baseline demographic variables were compared across groups using a Kruskal-Wallis test for the continuous variables and a Fisher's exact test for gender. Three tests were performed: across all groups, across the at-risk groups (noncarriers and presymptomatic mutation carriers), and across the affected groups.
Data at each voxel of the smoothed, warped, and modulated gray matter (GM) maps were fitted to a general linear model. We implemented 2 models, subdividing the carriers into more distinct subgroups with each level. In model 1, a 3-level factor was used: nonmutation carriers, presymptomatic mutation carriers, and affected carriers. Model 2 further subdivided the carrier subgroup to create a 7-level factor: noncarriers, presymptomatic and affected C9orf72 carriers, presymptomatic and affected GRN carriers, and presymptomatic and affected MAPT carriers. All models included a factor variable for imaging site and covariates for age, gender, and total intracranial volume. As it was expected that members from the same family enrolled in GENFI might have covariance in brain structure, family membership was included in the model as a random effect.
Contrasts were performed to look at the following pairwise differences: in model 1, noncarriers with presymptomatic carriers and noncarriers with affected carriers; and in model 2, noncarriers with each of the 6 carrier groups, as well as comparisons between the 3 mutations within the presymptomatic and affected groups separately. In model 2, we combined the 3 contrasts between noncarriers and each of the affected carrier subgroups to construct a compound hypothesis, where the null hypothesis was that 2 or less contrasts were significant. Significant findings from the compound hypothesis indicate GM atrophy patterns that are common to all 3 genetic mutations. To correct for the multiple comparisons problem inherent in mass univariate statistical analysis, we controlled for voxel-level family-wise error (FWE) at p < 0.05. If results did not reach significance after correction for multiple comparisons, we describe patterns at an uncorrected level of p < 0.001. Findings were reported with a cluster extent greater than the empirically determined threshold provided by SPM (the expected voxels per cluster, k = 56 voxels, 190 mm3).
3. Results
3.1. Demographics variables
Baseline demographic variables are shown in Table 1. Across all groups, there are significant differences in all variables. These findings are primarily driven by differences between the at-risk groups and the affected groups. When comparing the at-risk groups, only significant differences in age remained, with the noncarriers and presymptomatic GRN carriers being older than the C9orf72 and MAPT carriers. When comparing only the affected groups, differences remained in age, estimated years to onset, and the disease duration as determined by the participant's actual onset. MAPT carriers were younger than the other 2 mutations, while affected GRN carriers had lower estimated years to onset and disease duration.
3.2. Symptomatic mutation carriers
Comparison of all affected mutation carriers (GRN, MAPT, and C9orf72 combined) with noncarriers shows widespread decrease in GM, with the most significant areas in the orbitofrontal and inferior frontal lobes, temporal lobes (anterior > posterior), insula, anterior cingulate, parietal lobe (around the precuneus), and cerebellum (eTable 1 and eFig. 1).
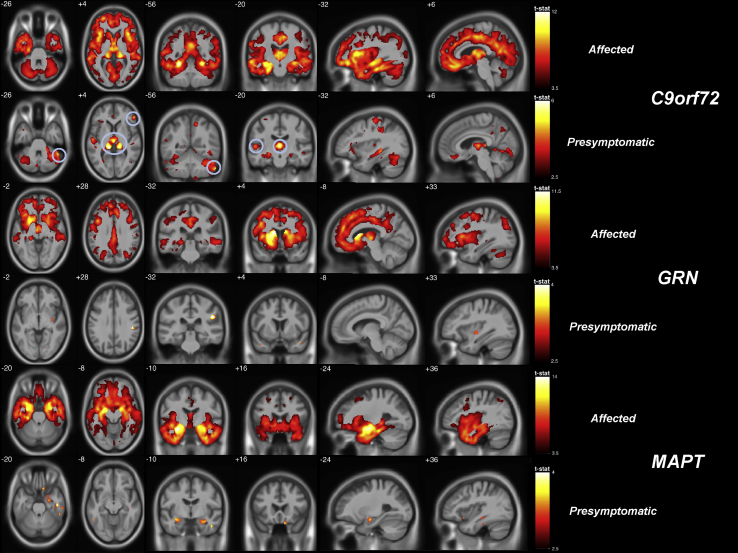
Looking at the affected mutation carriers in each individual mutation, different but overlapping patterns of atrophy were seen (eTable 2, Fig. 1, Fig. 2). In C9orf72 carriers, there were significant areas of GM loss throughout the brain including the frontal (orbitofrontal > dorsolateral/ventromedial prefrontal), temporal (inferior > superior), insula (anterior > posterior), and cingulate (both anterior and posterior) regions as well as more posterior cortical areas (precuneus and inferior > superior parietal regions). Subcortical structures were particularly affected, most significantly the thalamus but also the hippocampus, amygdala, and basal ganglia. GM loss was also seen in the cerebellum, affecting the superior posterior area most significantly (Fig. 1). Significant GM atrophy was seen in the affected GRN carriers in the frontal lobe (particularly in the dorsolateral and ventromedial prefrontal cortices), insula, anterior cingulate, superior and middle temporal gyri and striatum (caudate and putamen) as well as more posteriorly in the lateral and medial parietal lobes (precuneus). Affected MAPT carriers had significant atrophy in the hippocampus, amygdala, and temporal lobes (particularly anterior, medial, and inferior) as well as the insula and orbitofrontal lobe. Direct comparisons between affected mutation groups are shown in eTable 2.
Fig. 1.
Gray-matter (GM) differences by mutation and clinical status. GM differences in affected (odd rows, p < 0.05 FWE-corrected) and presymptomatic (even rows, p < 0.001 uncorrected) carriers compared to noncarriers. Comparisons to the C9orf72 carriers are in the top 2 rows (with findings at p < 0.05, FWE-corrected circled in the presymptomatic group), the GRN carriers in the middle 2 rows, and MAPT carriers in the bottom 2 rows.
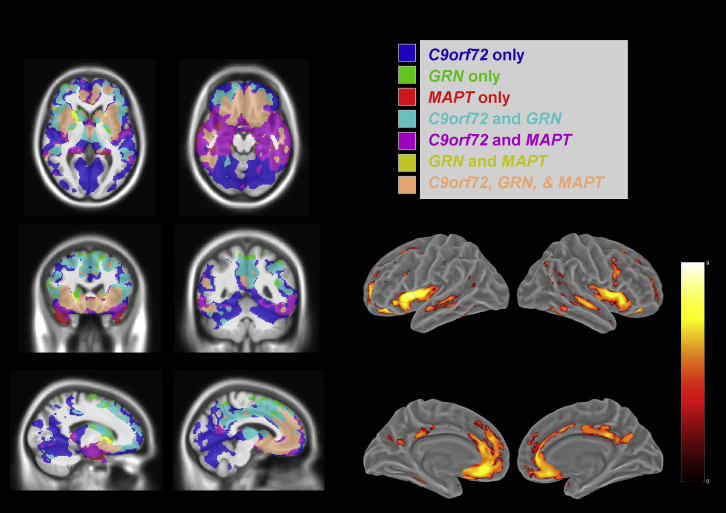
Fig. 2.
Comparison of gray matter atrophy patterns across the 3 genetic mutations. Comparison of atrophy patterns across the 3 genetic groups (symptomatic carriers). On the left hand of the figure, masks of the regions where there are significant differences (p < 0.05, FWE-corrected) are shown, color coded by mutation, along with areas where the patterns intersect within 2 or more mutations. The region satisfying the compound hypothesis of all 3 contrasts being true, indicating the intersection of atrophy in these mutations, is coded in light pink. A surface rendering of this intersection is shown on the right. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 2 summarizes the similarities and differences in GM atrophy within the 3 mutations. The blue (C9orf72), green (GRN), and red (MAPT) regions are areas where only that specific mutation had evidence of significant GM atrophy in the affected carriers (p < 0.05, FWE-corrected). Thalamic, superior cerebellar and very posterior cortical involvement (particularly inferior parietal lobe) is unique to C9orf72; superior aspect of the dorsolateral frontal cortex, dorsolateral caudate, and superior parts of the medial parietal regions are only affected in GRN; and anteroinferior temporal lobe involvement is unique to MAPT. In contrast, the findings from the compound hypothesis indicate the atrophy pattern common to all 3 mutations (light pink), which comprises a network of regions encompassing mainly the insula, orbitofrontal lobe, and anterior cingulate bilaterally.
3.3. Presymptomatic mutation carriers
When comparing the combined group of presymptomatic carriers to noncarriers, significant areas of GM loss are seen in the anterior insula when correcting for multiple comparisons (eTable 1), with further regions (uncorrected for multiple comparisons) of GM loss in the orbitofrontal lobe, anterior temporal lobe, posterior insula, parietal lobe and thalamus.
In the individual subgroups of presymptomatic mutation carriers (in comparison with noncarriers) the areas of GM atrophy were similar to those seen in the affected cases but to a lesser extent (Fig. 1, eTable 3). In C9orf72 carriers there were significant areas of GM loss bilaterally in the thalamus, right superior posterior cerebellum (Crus I), superior temporal and inferior frontal regions when correcting for multiple comparisons, with more extensive loss in the same areas as well as the anterior insula, temporal (including hippocampi and amygdala), and parietal (particularly inferiorly) regions at an uncorrected p-value of <0.001 (as shown in Fig. 1). In GRN and MAPT carriers no areas survived correction for multiple comparisons, but at an uncorrected significance level, areas of GM atrophy were seen in GRN carriers in the insula, parietal, posterior frontal and anterior temporal lobes as well as the striatum (Fig. 1, eTable 3), and in MAPT carriers in the anterior and medial temporal lobes (including hippocampus and amygdala) and the orbitofrontal lobe (Fig. 1, eTable 3). Direct comparisons between mutation groups are shown in eTable 3.
As there have been reports in the familial AD literature of increased volume in brain structures prior to atrophy (Fortea et al., 2010, Lee et al., 2012), we performed reverse contrasts looking for areas where any of the carrier groups had more GM than the noncarriers. In each of these tests, there were no findings of GM increase, even at an uncorrected level of p < 0.001, within any affected or presymptomatic group.
4. Discussion
Using a whole-brain voxel-wise analysis of gray matter volume in a genetic FTD cohort, we show evidence of unique areas of atrophy in each mutation with an intersecting region of atrophy affecting all 3 mutation groups in the insula, orbitofrontal lobe, and anterior cingulate. Analysis of presymptomatic carriers shows evidence of atrophy before symptom onset in each of the mutation groups. This work expands on previous studies, which have investigated smaller cohorts, commonly at a single site and primarily focusing on differences in affected patients.
Findings in symptomatic mutation carriers are consistent with atrophy signatures found in previous voxel-based morphometry studies of genetic FTD (Mahoney et al., 2012, Rohrer et al., 2010, Whitwell et al., 2009, Whitwell et al., 2012). In MAPT mutation carriers, atrophy primarily affects the anterior and medial temporal lobes, orbitofrontal lobe and insula; in GRN mutation carriers atrophy is found in the dorsolateral and ventromedial prefrontal, superolateral temporal and lateral parietal lobes as well as the anterior cingulate, insula, precuneus and striatum; whilst in C9orf72 mutation carriers there is relatively widespread cortical atrophy including posterior areas, and particularly affecting the thalamus and superoposterior cerebellum. Importantly, in this study, we show through a compound hypothesis of contrasts in the general linear model that there are common regions of atrophy across all 3 genes in the orbitofrontal lobe, insula, and anterior cingulate. Interestingly, this area overlaps substantially with the so-called salience network (Seeley et al., 2007), an intrinsic connectivity network, described as being fundamentally involved in FTD (Filippi et al., 2013, Seeley et al., 2009, Zhou et al., 2010). It is this neuroanatomical correspondence, which likely accounts for the overlapping behavioral clinical syndrome seen in the majority of cases of genetic FTD.
The findings in the presymptomatic carriers add to the region of interest results in the initial GENFI study and other presymptomatic studies (Bocchetta et al., 2016, Rohrer et al., 2015, Whitwell et al., 2012). The strongest evidence of presymptomatic atrophy was observed in C9orf72 carriers in the thalamus and superoposterior cerebellum. A recent study of 15 presymptomatic C9orf72 carriers (Lee et al., 2017) also found decreased GM compared to noncarriers in the left thalamus but did not show any differences in the cerebellum. Uncorrected findings of atrophy were observed in MAPT mutation carriers in the anteromedial temporal and orbitofrontal lobes, and in striatum, frontal, temporal, and parietal areas of GRN carriers. However, when pooling all presymptomatic carriers in model 1, there was significant evidence of atrophy surviving FWE correction for multiple comparisons in the right anterior insula. These results indicate that there may be some distinct regions in which the disease process starts, but those common sites across all 3 mutations may also be involved during the presymptomatic phase of the disease.
There is relatively minimal atrophy in presymptomatic GRN carriers. The explanation for this finding is unclear. It is unlikely to represent a difference in the age or expected proximity to onset of the GRN presymptomatic cohort compared to the other genes but could be indicative of atrophy occurring nearer to symptom onset in GRN-related FTD. A longitudinal study of cortical thickness in 16 presymptomatic GRN carriers (Caroppo et al., 2015) found no cross-sectional differences at baseline compared to 17 noncarriers, but longitudinal changes over 20-month follow-up were observed in 1 cluster in the middle and inferior temporal gyrus. This cluster was not found in our presymptomatic GRN carriers, but it was present in the affected participants. This could be consistent with the pathophysiological process that occurs in GRN, where an additional insult or injury (superimposed on low progranulin levels) is likely to be required to start the neurodegeneration process with more rapid GM loss (and sooner onset of symptoms) subsequently (Martens et al., 2012, Rohrer et al., 2010, Yin et al., 2009). It could also be in part due to the noted asymmetric atrophy in these cases, which can variably affect the right or left hemisphere (Le Ber et al., 2008, Rohrer et al., 2010). In MAPT mutation carriers, the findings in the presymptomatic group closely mirror those found in the affected carriers (Fig. 2, bottom 2 rows), and although not reaching significance once correcting for multiple comparisons, the pattern is similar to that of symptomatic cases but to a lesser extent.
In all 3 at-risk groups, it will be important to investigate the GENFI cohort in more detail when a larger cohort is available who can be further stratified by age (or expected time to symptom onset). The current presymptomatic cohort represents a heterogeneous sample with 39 (9 C9orf72, 22 GRN, 8 MAPT) of 130 participants within 5 years of their expected age at onset, when atrophy is more likely to be present, while 33 participants (12 C9orf72, 15 GRN, and 6 MAPT) are more than 20 years away from expected onset, where atrophy should be minimal.
In summary, we were able to observe distinct but overlapping patterns of GM atrophy between carriers of key mutations known to cause FTD with similar patterns (albeit to a lesser extent) seen during the presymptomatic phase. We found decreases in gray matter in presymptomatic participants that survived stringent correction for multiple comparisons: both within C9orf72 carriers in the thalamus, cerebellar crus, frontal, and temporal lobes, as well as in the anterior insula across all presymptomatic carriers. Further studies are required not only to increasingly stratify the presymptomatic cohort but also of other imaging modalities including diffusion tensor imaging and resting-state functional MRI to understand how the GM regions identified in this study are structurally and functionally connected, allowing insight into the earliest involved areas in genetic FTD and how disease propagates from those regions.
Disclosure statement
The authors report no conflicts of interest.
Acknowledgements
The authors would like to thank the participants of the GENFI study, as well as their caregivers, for participating in this study. This work was funded by the UK Medical Research Council, the Italian Ministry of Health, and the Canadian Institutes of Health Research as part of a Centres of Excellence in Neurodegeneration grant. The Dementia Research Centre is supported by Alzheimer's Research UK, Brain Research Trust, and The Wolfson Foundation. This work was supported by the NIHR Queen Square Dementia Biomedical Research Unit and the NIHR UCL/H Biomedical Research Centre. JDR is supported by an MRC Clinician Scientist Fellowship (MR/M008525/1) and has received funding from the NIHR Rare Disease Translational Research Collaboration. KD is supported by an Alzheimer's Society PhD Studentship. JBR is supported by the Wellcome Trust (103838) and the NIHR Cambridge Biomedical Research Centre. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.neurobiolaging.2017.10.008.
Appendix A. Supplementary data
References
- Ashburner J., Friston K.J. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Diffeomorphic registration using geodesic shooting and Gauss-Newton optimisation. Neuroimage. 2011;55:954–967. doi: 10.1016/j.neuroimage.2010.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchetta M., Cardoso M.J., Cash D.M., Ourselin S., Warren J.D., Rohrer J.D. Patterns of regional cerebellar atrophy in genetic frontotemporal dementia. Neuroimage Clin. 2016;11:287–290. doi: 10.1016/j.nicl.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroppo P., Habert M.O., Durrleman S., Funkiewiez A., Perlbarg V., Hahn V., Bertin H., Gaubert M., Routier A., Hannequin D., Deramecourt V., Pasquier F., Rivaud-Pechoux S., Vercelletto M., Edouart G., Valabregue R., Lejeune P., Didic M., Corvol J.C., Benali H., Lehericy S., Dubois B., Colliot O., Brice A., Le Ber I. Lateral temporal lobe: an early imaging marker of the presymptomatic GRN disease? J. Alzheimers Dis. 2015;47:751–759. doi: 10.3233/JAD-150270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi M., Agosta F., Scola E., Canu E., Magnani G., Marcone A., Valsasina P., Caso F., Copetti M., Comi G., Cappa S.F., Falini A. Functional network connectivity in the behavioral variant of frontotemporal dementia. Cortex. 2013;49:2389–2401. doi: 10.1016/j.cortex.2012.09.017. [DOI] [PubMed] [Google Scholar]
- Fortea J., Sala-Llonch R., Bartrés-Faz D., Bosch B., Lladó A., Bargalló N., Molinuevo J.L., Sánchez-Valle R. Increased cortical thickness and caudate volume precede atrophy in PSEN1 mutation carriers. J. Alzheimers Dis. 2010;22:909–922. doi: 10.3233/JAD-2010-100678. [DOI] [PubMed] [Google Scholar]
- Le Ber I., Camuzat A., Hannequin D., Pasquier F., Guedj E., Rovelet-Lecrux A., Hahn-Barma V., Van Der Zee J., Clot F., Bakchine S., Puel M., Ghanim M., Lacomblez L., Mikol J., Deramecourt V., Lejeune P., De La Sayette V., Belliard S., Vercelletto M., Meyrignac C., Van Broeckhoven C., Lambert J.C., Verpillat P., Campion D., Habert M.O., Dubois B., Brice A., Clerget-Darpoux F., Didic M., Desnuelle C., Duyckaerts C., Golfier V., Michel B.F., Thomas-Anterion C., Salachas F., Sellal F., Camu W. Phenotype variability in progranulin mutation carriers: a clinical, neuropsychological, imaging and genetic study. Brain. 2008;131:732–746. doi: 10.1093/brain/awn012. [DOI] [PubMed] [Google Scholar]
- Lee G.J., Lu P.H., Medina L.D., Rodriguez-Agudelo Y., Melchor S., Coppola G., Braskie M.N., Hua X., Apostolova L.G., Leow A.D., Thompson P.M., Ringman J.M. Regional brain volume differences in symptomatic and presymptomatic carriers of familial Alzheimer's disease mutations. J. Neurol. Neurosurg. Psychiatry. 2012;84:154–162. doi: 10.1136/jnnp-2011-302087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.E., Sias A.C., Mandelli M.L., Brown J.A., Brown A.B., Khazenzon A.M., Vidovszky A.A., Zanto T.P., Karydas A.M., Pribadi M., Dokuru D., Coppola G., Geschwind D.H., Rademakers R., Gorno-Tempini M.L., Rosen H.J., Miller B.L., Seeley W.W. Network degeneration and dysfunction in presymptomatic C9ORF72 expansion carriers. Neuroimage Clin. 2017;14:286–297. doi: 10.1016/j.nicl.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney C.J., Beck J., Rohrer J.D., Lashley T., Mok K., Shakespeare T., Yeatman T., Warrington E.K., Schott J.M., Fox N.C., Rossor M.N., Hardy J., Collinge J., Revesz T., Mead S., Warren J.D. Frontotemporal dementia with the C9ORF72 hexanucleotide repeat expansion: clinical, neuroanatomical and neuropathological features. Brain. 2012;135:736–750. doi: 10.1093/brain/awr361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone I.B., Leung K.K., Clegg S., Barnes J., Whitwell J.L., Ashburner J., Fox N.C., Ridgway G.R. Accurate automatic estimation of total intracranial volume: a nuisance variable with less nuisance. Neuroimage. 2014;104:366–372. doi: 10.1016/j.neuroimage.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens L.H., Zhang J., Barmada S.J., Zhou P., Kamiya S., Sun B., Min S.W., Gan L., Finkbeiner S., Huang E.J., Farese R.V. Progranulin deficiency promotes neuroinflammation and neuron loss following toxin-induced injury. J. Clin. Invest. 2012;122:3955–3959. doi: 10.1172/JCI63113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer J.D., Guerreiro R., Vandrovcova J., Uphill J., Reiman D., Beck J., Isaacs A.M., Authier A., Ferrari R., Fox N.C., MacKenzie I.R.A., Warren J.D., De Silva R., Holton J., Revesz T., Hardy J., Mead S., Rossor M.N. The heritability and genetics of frontotemporal lobar degeneration. Neurology. 2009;73:1451–1456. doi: 10.1212/WNL.0b013e3181bf997a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer J.D., Nicholas J.M., Cash D.M., van Swieten J., Dopper E., Jiskoot L., van Minkelen R., Rombouts S.A., Cardoso M.J., Clegg S., Espak M., Mead S., Thomas D.L., De Vita E., Masellis M., Black S.E., Freedman M., Keren R., MacIntosh B.J., Rogaeva E., Tang-Wai D., Tartaglia M.C., Laforce R., Tagliavini F., Tiraboschi P., Redaelli V., Prioni S., Grisoli M., Borroni B., Padovani A., Galimberti D., Scarpini E., Arighi A., Fumagalli G., Rowe J.B., Coyle-Gilchrist I., Graff C., Fallström M., Jelic V., Ståhlbom A.K., Andersson C., Thonberg H., Lilius L., Frisoni G.B., Binetti G., Pievani M., Bocchetta M., Benussi L., Ghidoni R., Finger E., Sorbi S., Nacmias B., Lombardi G., Polito C., Warren J.D., Ourselin S., Fox N.C., Rossor M.N. Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the Genetic Frontotemporal dementia Initiative (GENFI) study: a cross-sectional analysis. Lancet Neurol. 2015;14:253–262. doi: 10.1016/S1474-4422(14)70324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer J.D., Ridgway G.R., Modat M., Ourselin S., Mead S., Fox N.C., Rossor M.N., Warren J.D. Distinct profiles of brain atrophy in frontotemporal lobar degeneration caused by progranulin and tau mutations. Neuroimage. 2010;53:1070–1076. doi: 10.1016/j.neuroimage.2009.12.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Crawford R.K., Zhou J., Miller B.L., Greicius M.D. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell J.L., Jack C.R., Boeve B.F., Senjem M.L., Baker M., Rademakers R., Ivnik R.J., Knopman D.S., Wszolek Z.K., Petersen R.C., Josephs K.A. Voxel-based morphometry patterns of atrophy in FTLD with mutations in MAPT or PGRN. Neurology. 2009;72:813–820. doi: 10.1212/01.wnl.0000343851.46573.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell J.L., Weigand S.D., Boeve B.F., Senjem M.L., Gunter J.L., DeJesus-Hernandez M., Rutherford N.J., Baker M., Knopman D.S., Wszolek Z.K., Parisi J.E., Dickson D.W., Petersen R.C., Rademakers R., Jack C.R., Josephs K.A. Neuroimaging signatures of frontotemporal dementia genetics: C9ORF72, tau, progranulin and sporadics. Brain. 2012;135:794–806. doi: 10.1093/brain/aws001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F., Banerjee R., Thomas B., Zhou P., Qian L., Jia T., Ma X., Ma Y., Iadecola C., Beal M.F., Nathan C., Ding A. Exaggerated inflammation, impaired host defense, and neuropathology in progranulin-deficient mice. J. Exp. Med. 2009;207:117–128. doi: 10.1084/jem.20091568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Greicius M.D., Gennatas E.D., Growdon M.E., Jang J.Y., Rabinovici G.D., Kramer J.H., Weiner M., Miller B.L., Seeley W.W. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer's disease. Brain. 2010;133:1352–1367. doi: 10.1093/brain/awq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.