Abstract
Purpose. Doxorubicin has been found to be associated with insulin resistance in animal models. Onion, a so-called functional food, is noted to affect the insulin signaling pathway of diabetes in vitro. To our knowledge, this is the first study to investigate the effects of consuming fresh yellow onions on insulin-related indices compared with a low–onion-containing diet among breast cancer (BC) patients treated with doxorubicin. Methods. This parallel-design, randomized, triple-blind, controlled clinical trial was conducted on 56 eligible BC patients (aged 30-63 years), diagnosed with invasive ductal carcinoma. Following their second cycle of chemotherapy, subjects were assigned in a stratified-random allocation to receive body mass index–dependent 100 to 160 g/d of onion as high onion group (HO; n = 28) or 30 to 40 g/d small onions in low onion group (LO; n = 28) for 8 weeks intervention. Participants, care givers, and those who assessed laboratory analyses were blinded to the assignments (IRCT Registry No.: IRCT2012103111335N1). Results. The compliance level of participants in the analysis was as high as 87.85%. A total of 23 available cases was analyzed in each group. The daily use of HO resulted in a significant decrease in serum fasting blood glucose and insulin levels in comparison with LO, over the period of study (P < .001). Posttreatment with HO showed a significant decrease in homeostasis model of assessment-insulin resistance relative to changes in the LO group (P < .05). A comparison of the changes that occurred throughout pre- and postdose treatments indicated improved quantitative insulin sensitivity check index (P < .05) and controls on C-peptide in the HO group (P < .05). Conclusions. The present study demonstrated the effectiveness of onion to ameliorate hyperglycemia and insulin resistance in BC during doxorubicin-based chemotherapy.
Keywords: breast cancer, onion, insulin resistance, doxorubicin, intervention
Introduction
Breast cancer (BC) is the most common malignancy and the second most lethal cancer type among women worldwide.1 Epidemiological observations have supported several lines of evidence from experimental in vitro studies on dietary factors as lifestyle-related variables to be implicated in breast tumorigenesis.2-4 Findings from the European Prospective Investigation into Cancer and Nutrition and other cohort studies resulted in inverse associations regarding fruit and vegetable intakes with BC risk.5-7 The risk of female breast carcinoma in relation to the consumption of Allium vegetables evaluated in the Netherlands Cohort study related to diet and cancer showed no significant results, whereas a French epidemiological study showed that higher onion (Allium cepa L) intake, as the most frequently consumed Allium vegetable, was correlated with a lower risk of BC.7,8 Evidence from a case-control study undertaken in Switzerland has indicated that a high frequency of onion consumption was associated with a notable decrease in BC risk.9 According to Leviac et al, a descending trend within the range of 40% to 60% BC risk has been observed for the highest versus the lowest tertiles of consumptions.9 Although there is consensus within most observational studies to indicate an inverse association between onion and Allium intake and cancer risk, no clinical trial has to date been undertaken to prove the role of onion consumption on biomarkers related to BC prognosis and metabolic determinants.
Insulin is a peptide hormone synthesized in pancreatic β cells and considered as a potent mitogenic hormone.10 C-peptide is a by-product of cleaved pro-insulin released when insulin is being secreted. Therefore, circulating levels of C-peptide could be interpreted as a sensitive biomarker for insulin changes and taken into account as biomarkers for predicting increased risk of tumorigenesis, especially when pathological lesions of neoplastic transformation exist (particularly in the early stages of BC).11 Insulin resistance (IR) and hyperglycemia are common metabolic features of type 2 diabetes mellitus (T2DM), formerly known as non–insulin-dependent diabetes mellitus; IR has rarely been targeted in intervention studies of cancer patients. T2DM may be incorporated in malignant transformation by several mechanisms, such as hyperinsulinemia, hyperglycemia, and elevated levels of cytokines.12,13 High circulating levels of insulin directly induce the neoplastic phenotypes of growth, being resistant to apoptotic stimuli, propagation, and metastasis in BC patients.13 Hyperinsulinemia also indirectly associates with tumorigenesis through reduction of the hepatic expression levels of insulin-like growth factor (IGF) binding proteins and sex-hormone binding protein to enhance mitogenic effects of free circulating IGF and estradiol, respectively.13,14 In addition, hyperinsulinemia-dependent T2DM and/or obesity could potently promote inflammatory features that interplay in the etiology of cancer-related transformation or tumor progression.12 There is surge of interest in determining whether the possible comorbidity of IR with cancer could be associated with increased mortality and poorer disease-specific survival.13
The homeostasis model assessment (HOMA) has been implicated in identifying insulin resistance (HOMA-IR) and insulin sensitivity (HOMA-β).15 More recently, it has been tempting to study the metabolic behaviors and related physiologic phenotypes of cancer patients involved in dietary interventions. On the other hand, it has been revealed that a doxorubicin (DOX)-contained chemotherapy regimen can potently induce cellular resistance to insulin and predispose individuals to hyperinsulinemia in cancer cases while concurrent administration of high dose of dexamethasone used to prevent nausea and vomiting of DOX play a basic role in inducing IR as well.16 Consequently, this chemotherapy effect may give rise to the possibility of IR at least as short-term changes in metabolism and correlated markedly with adiposity changes in treating cancer patients.17 The effectiveness of adjuvant therapies on cancer prognosis has been speculated to be attributed in part to adiposity status.14,18 In particular, a greater effect on prognosis is observed in younger women.18
Onion contains biologically active constituents that mainly include sulfur compounds and flavonoids such as rutin and quercetin (QR). A significant collection of data from in vitro studies has established the apoptotic, antiproliferative, and immune-enhancing properties of onion products.4,19-21 However, current evidence from animal studies has reported insulinotropic,22 and insulin-sensitizing,23 effects as a result of onion, either in diabetic or hypercholesterolemic conditions. Despite the possible cytotoxic effects of DOX in the regimen of chemotherapy for controlling the proliferation of cancer cells, DOX is bioavailable to the whole body through systematic blood circulation and raises the possibility of adverse toxic effects, including neutropenia as a result of immunosuppressant effects, also having hepatotoxic and cardiotoxic effects in some cases.24,25 Although onion has conventionally been used to enhance the function of the immune system in folk medicine, no data exist to support this hypothesis in BC patients who have received an immunosuppressant such as DOX in previous studies. Therefore, a randomized, triple-blind, placebo-controlled clinical trial was conducted to investigate the effect of 8 weeks of manipulation of dietary habits through the daily ingestion of raw yellow onion, a staple vegetable item in an Iranian diet, on IR-related biomarkers in BC patients receiving chemotherapy.
Materials and Methods
Study Subjects
The randomized, triple-blind, placebo-controlled clinical trial study was conducted at Tabriz University of Medical Sciences (Faculty of Nutrition), Tabriz, Iran. BC patients whose disease had been histopathologically proven following radical or partial mastectomy at the Surgery Ward of Nour-Nejat Hospital, and who were referred to Shahid Ghazi Cancer Research Centre and private cancer clinics (Tabriz, Iran) and aged 30 to 65 years, were recruited among the primary population of BC patients (the complete date range for patient recruitment was October 2012 till June 2013). This multicenter design trial gives the possibility to include patients from Tabriz city, which is the capital of Eastern-Azerbaijan located in the northwest of Iran.
The inclusion criteria included the self-intentions of the patient to participate and a completed consent form prior to the commencement of the study, patient diagnosed with invasive ductal carcinoma (IDC), grade 2 or 3, having no metastasis, and having no history of any cancer in other anatomic sites. The exclusion criteria included the following: energy intake out of the range of 700 to 3500 kcal/day; having severe liver or kidney failure, hyperthyroidism, polycystic ovary syndrome, and gastrointestinal inflammatory disorders (gastritis, peptic ulcer, and inflammatory bowel syndrome); allergy or intolerance to onion; bleeding disorders; asthma; low blood pressure; being pregnant and lactating during the study; any prior history of chemotherapy, radiotherapy, and/or hormone therapy; and medically used methotrexate, aspirin, metformin, cyclosporine, epilepsy-related drugs, contraceptive, hormone replacement therapy, uridine 5-phosphate, or colchicines. The consumption of flaxseed, supplements of vitamin E, and omega-3 for 4 weeks prior to enrollment was considered as an exclusion criterion (changed in initial protocol after trial commencement) and therefore avoided during the whole study period.
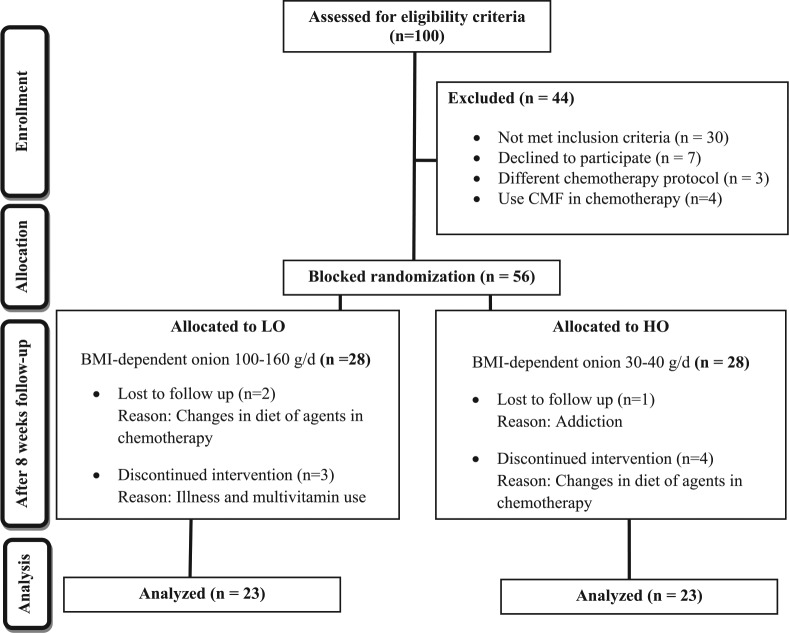
Eligible participants were requested to remain with their common habitual diet and lifestyle within the range of adherence to dietary guidelines. Finally, after obtaining informed consent, 56 women with newly diagnosed BC fulfilled the selection criteria and were randomly assigned into 2 groups via stratified-random allocation, which has been described in detail in an erarlier article.26 The sampling procedure is summarized in the flowchart depicted in Figure 1.26 The number needed to treat was derived from a study in which polycystic ovary syndrome patients were treated with onion for 2 months.2,27 Eventually, 23 participants completed the intervention in each arm of follow-up to be included in analysis (Figure 1).
Figure 1.
CONSORT flow chart diagram of intervention.
Ethics Statement
The study was carried out according to the revised guidelines released in the Declaration of Helsinki.28 A written informed consent form was completed prior to the start of the study by each participant. All procedures were subject to the prior approval of the Ethics Committee at Tabriz University of Medical Sciences (Ethics No.: 5-4-6829). The enrollment of eligible participants was started after the ethics committee approved the study (October 2012). This clinical trial was also registered at the Centre of Iranian Registry of Clinical Trials (IRCT) and linked to the World Health Organization Registry Network (IRCT No.: IRCT2012103111335N1). The authors confirm that all the protocol of trials for this intervention was registered in the framework specified in the IRCT homepage (http://www.irct.ir).
Study Design
The second cycle of chemotherapy was generally considered as the baseline of interventions, and blood sampling was conducted prior to receiving a second cycle of chemotherapy. The second chemotherapy cycle was chosen to rule out a level of adaptation to chemotherapy that might change metabolic and proliferation-related indices in patients. Prior to the baseline of the study, all participants (between the initial and second chemotherapy cycles) were placed in a 2-week run-in (4 days had been planned in initial protocol, to include the ovulation phase of the menses cycle) period in order to obtain information regarding well-tolerated conditions in chemotherapy, lifestyle-related carcinogenic risk factors, and the compliance of patients to follow the basics of treatment. During the run-in period, participants were instructed not to consume any onion and less than half a serving of other Allium species such as spring onion, shallots, garlic, garlic chives, native water-cress leaves, and leek on a daily basis (amount of Allium had to be less than 90 g/d). Dietary assessments and information were provided regarding the concept of treatment to encourage better adherence during the 8-week intervention, as described in detail in previously published data.26 Possible cachexia following chemotherapy might increase the patients’ desire for weight gain, which might have been a potent motivation for overreporting of calorie intake and underreporting of some food intakes.4,29 Therefore, in order to possibly improve the accuracy of data compliance related to onion consumption, based on Goldberg cutoffs, individuals with misreporting were not included for randomization after the run-in period.29,30 The measure of the actual body weight at diagnosis prior to surgery was used for basal metabolic rate calculation.
All participants were individually counselled not to change their habitual diet, with some considerations regarding World Cancer Research Fund International guidelines (specifically, the fat content of their diet) and abstaining from Allium-based products, as well as a limitation to eat less than 90 g/d of Allium vegetables in order to increase their compliance throughout the study.27,31,32 A physical activity record was also obtained from each participant during run-in period, in addition to baseline assessments. Then, BC subjects included in the study were randomly allocated to either the intervention or control group by means of the method of sequence generation of computer-generated randomization software.
In total, 46 patients who completed the trial received 1 of the following 3 chemotherapy arms: (1) 13 patients (28.3%) included in 4 cycles of intravenous (IV) doxorubicin 75 mg/m2 every 3 weeks followed by 3 cycles of cyclophosphamide, methotrexate, and 5-fluorouracil (CMF); (2) 19 (41.3%) patients included in the arm of 4 cycles of IV doxorubicin 60 mg/m2 along with IV cyclophosphamide 600 mg/m2 every 3 weeks, followed by 3 cycles of CMF; (3) 14 patients (30.4%) included in 3 cycles of IV doxorubicin 75 mg/m2 every 3 weeks, followed by 4 cycles of IV docetaxel 100 mg/m2 every 3 weeks, followed by 3 cycles of CMF. The duration of each chemotherapy protocol was estimated around 24 weeks (except for the regimen in arm 3, which was 33 weeks). The present 8-week intervention was carried out within this duration and commenced after the second treatment cycle of DOX. The endpoint of the intervention was determined prior to any prescription of docetaxel. Tamoxifen as a hormonal adjuvant therapy (20 mg/d) was prescribed during chemotherapy for 20 patients out of 46 participants and intended to be used for almost 5 years in patients with estrogen receptor–positive and/or progesterone receptor–positive tumors characterized by immunohistochemistry staining data. Radiation therapy was also set as adjuvant therapy according to institutional guidelines. Dexamethasone was administered in 8 mg IV (Daroo Pakhsh Inc, Iran) for each participant before DOX treatment to prevent nausea and vomiting induced by IV injection of DOX.
Details about the compliance for onion intervention were described in a previous report.26 In summary, weighing the leftover parts of onion and a weekly checklist to estimate the frequency of onion use were employed to monitor the compliance of each participant to the study. This checklist also instructed participants to record in a diary details for all unexpected adverse occurrences, cases of doubtful or accidental consumption, as well as delivering the checklist at each 3-week visit. During the study, all participants provided data concerning a 3-day 24-hour dietary records and a weekly physical activity record once every 2 weeks. As a result, each participant had 3 visits between the baseline and end point of 8 weeks. In addition, physical examinations at these visits were conducted following chemotherapy-related changes such as primary and secondary cachexia. Nonresponse to follow-up was defined for patients who ate less than 85% of the prescribed onions and thus were not included in the study. However, this was clearly not the case. At the beginning and end of the study, fasting blood samples were obtained and sera were stored at −70°C until the analyses were performed.
Interventions
Obese participants with body mass index (BMI) >35 kg/m2 were not included in the randomization and this resulted in less variation regarding calorie-dependent risk factors. We provided a range of total daily amounts of onion consumption, individually based on BMI at diagnosis (time of enrolment) of each participant. The BMI-dependent amount of onion (those with BMI < 24.9 consumed 100-120 g/d and those with BMI > 25 consumed 140-160 g/d) was taken daily by the high onion group (HO) in addition to main meals. Participants in the low onion group (LO; placebo group) took 30 to 40 g/d onion in addition to meals in a BMI-dependent manner (modified to the initial protocol). This range of onion consumption by participants was also followed in other studies. This superiority trial was set to demonstrate that HO treatment could be more effective than LO on outcome measures. Patients did not experience any loss of body weight more than 25%, which would have triggered exclusion from the study. All participants were asked not to consume any onion a day after receiving chemotherapy in order to enhance compliance and adherence to the study. The duration of treatments for both groups was set at 8 weeks. The complete date range for patient follow-up took place between October 2012 and September 2013.
Two onions for daily usage were packed in a 5″ × 5″ white foam–hinged container to provide supplies every 3 weeks in order to fulfil the concealment criteria. The weight of the container was adjusted by pieces of wood so that filled containers of the 2 treatments had identical weights, and onions were fixed by surrounding them with cotton (introduced to the protocol). Opaque plastic foam containers were used to pack onions to fulfil the concealment criteria. Participants were asked to store all onions in a refrigerator at 4°C. Subjects were served one fresh raw onion with lunch and another with dinner meals. Only the 2 outer layers of onions were peeled off before consumption, and the onions were sliced in half before being served to the patient. The average weight of this pulled-off skin (20-30 g) was excluded from the computed weight of served raw onion (pure weight). Raw yellow onions were obtained from a local market (one seller, Tabriz), who declared obtaining the onions from a particular cultivated farm.
Sequence generation and allocation concealment were listed and marked by the designer of the study and implemented by clinic personnel unaware of the allocated intervention at the time of enrolment. Participants, clinic personnel, and laboratory assessors were blinded to the treatment assignments. The date of entry to receive the first chemotherapy varied between participants in the study. The multicenter design of the study prevented participants from coming in contact with each other collectively during the course of the intervention, in order to improve the blinding concept. Ultimately, each participant at posttreatment timeline was asked whether she had been aware of assignment in control or intervention group and responded of being unaware of grouping.
Anthropometric Assessments
Measurement of participants’ height without shoes was conducted in the standing position to the nearest 0.1 cm, using a wall-mounted stadiometer (Seca, Hamburg, Germany). Weight was measured by a calibrated Seca scale (Itin Scale, Berlin, Germany) to the nearest 0.1 kg with subjects wearing light clothing without shoes prior to surgery and during other time periods. Information pertaining to BMI (kg/m2) is actual body weight in kilograms divided by squared meter of height at diagnosis as the primary measure of total adiposity. Based on World Health Organization guidelines,11 “overweight” was defined as a BMI between 25 and 29.9 kg/m2, while obesity was defined as a BMI greater than 29.9 kg/m2. To avoid subjective errors, all measurements were made by only one instructor.
Biochemical Assessments
Venous blood samples (8 mL) were taken from subjects at least after 12 hours of fasting in a clot tube (Vacuum Blood Collection Tube—Gel & Clot Activator Tube, AMIS Medical Co, China), prior to the second cycle of chemotherapy at Danesh Laboratory, which is under quality control and verified by the National Reference Laboratory (Tabriz, Iran). The samples were immediately centrifuged (Refrigerated Centrifuge, Sigma, Germany) at 3000×g and at 20°C for 10 minutes to separate serum supernatant. Aliquots were stored at −70°C until laboratory tests were conducted. Commercially available ELISA kits and standards were used as a follow-up to measure prespecified primary outcomes such as fasting blood glucose (FBG) by Pars-Azmoon (coefficient variation [CV] of interassay = 0.90%; Cat. No.: 5825; Tehran, Iran), insulin by Monobind (Cat No.: 5825-300, Lake Forest, CA), and C-peptide using a Monobind kit (Cat No.: 2725-300). Measurements were carried out following the manufacturers’ instructions. The homeostasis model assessment (HOMA-IR) was calculated using the following: HOMA-IR = [glucose (mg/dL) × insulin (µIU)/405] and HOMA-β = [(360 × insulin (µIU)]/[glucose (mg/dL) − 63] × 100. Quantitative insulin sensitivity check index (QUICKI) was estimated using the following: 1/[logarithm of insulin (µIU) + logarithm of glucose (mg/dL)]. The within and between assays’ CVs for all biochemical measures were <10%. For each biomarker, measures were performed at the same time in one laboratory run and in random order to attenuate systematic errors. The name of the patient in each serum sample was labelled with a specific numeric code in order to impart blindness in the laboratory analysis procedures. The National Cancer Institute and the World Health Organization toxicity criteria recommend standard criteria for the assessment of therapy-induced toxicity (added to the initial eligibility criteria).33 Their classification of more severe grades of anemia referring to a grade higher than 3, in which hemoglobin is measured as <7.9 g/dL, was an exclusion criterion.
Statistical Analyses
Analysis was performed using SPSS software (Version 15). A graphed linear histogram was used to test the potential skewness and kurtosis. A Kolmogorov-Smirnov test was also applied to ensure the normality of data distribution in each analyzed subclass. A box plot was used to detect possible outliers. Descriptive results were expressed in mean ± standard deviation (SD; or standard error of the mean [SEM]) and median (95% confidence interval [CI]) for the general characteristics of studied variables. Two independent sample t tests were used to compare variables between the control and intervention groups. Date-dependent within group comparisons in a tail between the baseline and 8 weeks’ intervention were carried out by paired t test.
Absolute treatment effect was calculated by assessing the mean change in a variable between the 2 arms of HO and LO, from baseline to 8 weeks of follow-up. The P value of the treatment effect was tested by repeated-measures linear mixed model (Table 3). Percentage of changes for certain dependent biochemical variables within each interventional group was estimated using the following formula: [(postdose measure) − (predose measure)/predose measure] × 100. Independent sample t test was performed to analyze significance levels of comparisons between HO and LO groups for mean percentage changes. Absolute treatment effect was determined as an estimate of change from predose to follow-up in the HO group minus the absolute change from predose to follow-up in the LO group in a mixed model. Repeated-measures analysis of variance was used to test the statistical levels of significance of absolute treatment effect in terms of comparing mean differences between 2 arms of interventions. Study participants were included in the model as a random effect, and absolute treatment effect was calculated and reported. For each comparison, P < .05 was considered as statistically significant.
Table 3.
The Effects of Onion on Body Mass Index During the Intervention in Women With BC.
| Variable | LO (Placebo; n = 23), Mean ± SD | HO (Intervention; n = 23), Mean ± SD | Mean Differencea | P Value a | Absolute Treatment Effectb |
|
|---|---|---|---|---|---|---|
| Mean [95% CI] | P Valuec | |||||
| BMI (kg/m2) | ||||||
| Predose | 27.87 ± 5.03d | 28.26 ± 3.70 | +0.38 | .788 | 0.339 | .810 |
| Week 8 | 28.22 ± 5.09 | 28.52 ± 3.65 | +0.28 | .835 | [−2.50, 3.18] | |
| Mean difference | +0.35e | +0.27 | ||||
| P valuee | <.001 | .005 | ||||
Abbreviations: BMI, body mass index; BC, breast cancer; LO, low onion group; HO, high onion group.
Independent sample t test was performed between group. The mean difference of between groups was calculated for a variable at (endpoint) − (baseline).
Absolute treatment effect is an estimate of change from predose to follow-up in the HO group minus the absolute change from predose to follow-up in the LO group in a mixed model.
Repeated-measures ANOVA was carried out in the mixed model.
Data are expressed in geometric mean ± SD.
Paired t test was performed to compare within-group changes in intervention group during the study. The mean difference within groups was calculated from averages of variables at (postdose) − (week 8).
Results
Demographic Characteristics
Volunteer subjects with histopathologically confirmed BC (n = 100) were recruited to the primary research population. In this controlled clinical trial, 44 patients were not eligible to participate. Main reasons included not meeting inclusion criteria (n = 30), declining to participate (n = 7), refusing chemotherapy (n = 3), or using CMF as a chemotherapeutic regimen (n = 4). As a result, 56 participants were randomly allocated into either the HO or control (LO) group, and 28 BC volunteers in each group entered the trial; also 10 participants were excluded during the 8-week study. Finally 23 patients in each group completed the study. The minimum, maximum, and median duration of follow-up were 57, 68, and 61 days, respectively. The general and dietary variables of BC patients who were randomly assigned to arms of the interventions are summarized in Table 1. Mean age at diagnosis for participants included in the study was 42.7 ± 5.9 years (range = 32.0-58.0 years) for the LO group and 43.9 ± 8.7 years (range = 30.0-63.0 years) for the intervention group (HO). The 2 groups were similar with respect to demographic variables and well-known risk factors for BC (Table 1). Daily intakes of total energy, carbohydrates, fat, protein, and fiber did not differ significantly between the 2 groups at baseline (Table 1). The habitual dietary and lifestyle-related factors of all the subjects did not differ significantly in terms of the interventions. The BMIs for each group increased but were not significantly different between placebo and HO groups at the completion of the study. The histopathological and therapeutic characteristics of the BC participants at the baseline of the study are also shown in Table 2. The administered adjuvant therapy for participants during the study included analogous protocols of chemotherapy regimens based on DOX in 4 courses with average 3-week intervals.
Table 1.
Demographic and Clinical Characteristics of BC Patients in LO (Placebo; n = 23) and HO (Intervention; n = 23) Groups at Baseline of Intervention.
| Characteristics | LO (Placebo) |
HO (Intervention) |
P Valuea | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Median | 95% CI | Mean ± SD | Median | 95% CI | ||
| Age (years) | |||||||
| At diagnosis | 42.7 ± 5.9 | 43.0 | 40.1-45.2 | 43.9 ± 8.7 | 42.0 | 40.1-47.7 | 0.570 |
| At first delivery | 21.7 ± 3.6 | 21.5 | 20.0-23.3 | 22.1 ± 3.7 | 22.0 | 20.4-23.8 | 0.937 |
| At first menses | 13.9 ± 1.3 | 13.7 | 13.3-14.5 | 13.5 ± 1.7 | 13.0 | 12.7-14.3 | 0.455 |
| Anthropometric indices | |||||||
| BMI (kg/m2) | 27.4 ± 4.8 | 28.1 | 25.1-29.7 | 27.8 ± 3.7 | 28.1 | 26.2-29.5 | 0.832 |
| Waist (cm) | 88.4 ± 11.5 | 89.7 | 83.3-93.5 | 89.8 ± 9.1 | 93.2 | 85.3-94.3 | 0.441 |
| Hip (cm) | 106 ± 10 | 106 | 101-110 | 107 ± 13 | 103 | 101-114 | 0.683 |
| Waist-to-hip ratio | 0.83 ± 0.06 | 0.83 | 0.80-0.85 | 0.8 ± 0.1 | 0.8 | 0.8-0.9 | 0.735 |
| Daily dietary intake | |||||||
| Total calorie intake (kcal/day) | 1893 ± 417 | 1954 | 1712-2073 | 1952 ± 442 | 1789 | 1761-2144 | 0.641 |
| Protein intake (g/day) | 68.6 ± 21.6 | 73.2 | 59.2-77.9 | 67.9 ± 17.5 | 63.3 | 60.4-75.5 | 0.918 |
| Carbohydrate intake (g/day) | 253 ± 81 | 262 | 217-288 | 270 ± 98 | 279 | 228-313 | 0.505 |
| Fat intake (g/day) | 56.5 ± 20.75 | 53.15 | 47.5-65.4 | 56.6 ± 18.8 | 56.2 | 48.5-64.7 | 0.977 |
| Total dietary fiber (g/day) | 4.6 ± 2.9 | 3.8 | 3.3-5.8 | 4.6 ± 2.9 | 3.9 | 3.3-5.8 | 0.956 |
| Soluble fiber (g/day) | 0.6 ± 0.5 | 0.6 | 0.4-0.9 | 0.5 ± 0.7 | 0.3 | 0.2-0.8 | 0.286 |
| Crude fiber (g/day) | 3.9 ± 2.5 | 3.2 | 2.8-5.1 | 4.1 ± 2.4 | 3.5 | 2.9-5.0 | 0.684 |
Abbreviations: BC, breast cancer; LO, low onion group; HO, high onion group; BMI, body mass index.
Independent sample t test was performed.
Table 2.
Histopathological and Therapeutic Characteristics Among BC Patients in Placebo (n = 23) and Intervention (n = 23) Groups at Baseline of Studya.
| Characteristics | LO (Placebo) | HO (Intervention) | P Value |
|---|---|---|---|
| Menopausal status | |||
| Premenopausal | 19 (82.6)b | 17 (73.9) | |
| Postmenopausal | 4 (17.4) | 6 (26.1) | .722c |
| Tumor size (cm) | |||
| Mean ± SD | 3.5 ± 1.4 | 3.3 ± 1.5 | .527d |
| ≤2 | 4 (18.2)b | 5 (22.7)b | |
| 2.1-5.0 | 15 (68.2) | 14 (63.6) | |
| >5 | 3 (13.6) | 3 (13.6) | .930 |
| Nuclear grade | |||
| 1 or 2 | 19 (86.4) | 20 (90.9) | |
| 3 | 3 (13.6) | 2 (9.1) | 1.000c |
| ER | |||
| Negative | 2 (9.1) | 1 (4.5) | |
| Positive | 20 (90.9) | 21 (95.5) | .550c |
| PR | |||
| Negative | 2 (9.1) | 0 (0.0) | |
| Positive | 20 (90.9) | 22 (100.0) | .488c |
| Surgery | |||
| Breast conservation | 4 (17.4) | 2 (8.7) | |
| Mastectomy | 19 (82.6) | 21 (91.3) | .655c |
| Adjuvant endocrine therapy | |||
| No | 6 (27.3) | 7 (31.8) | |
| Tamoxifen | 14 (63.6) | 9 (40.9) | |
| Letrozole | 2 (9.1) | 6 (27.3) | .256 |
| Lymphatic invasion | |||
| Yes | 10 (45.5) | 8 (36.4) | |
| No | 12 (54.5) | 14 (63.6) | .540 |
Abbreviations: BC, breast cancer; LO, low onion group (placebo); HO, high onion group (intervention); ER, estrogen receptor; PR, progesterone receptor.aSome missing data exit in variables.bData are expressed as observed number (%).cFisher exact test was considered.dIndependent sample t test was performed.
Patients’ adjuvant taxane (docetaxel and paclitaxel)-based chemotherapy was not included for possible interaction with onion in the pharmacodynamics of taxane and its toxicity.34,35 DOX-based chemotherapy after 4 courses was in the majority of patients followed by taxane and figured as one of the main reason to explain the 8-week duration of the study.
Compliance
The compliance of participants in the analysis was as high as 87.85%. The average compliance for the LO and HO groups were 87.85 ± 2.37% (82.22% to 91.78%) and 88.29 ± 1.98% (84.44% to 91.44%), respectively, during the 8 weeks of intervention. None of the participants in either group consumed <80% per day in order to be considered as noncompliance.
Erythropenia, which counts as an indicator of chemotherapy-induced blood toxicity, was not observed in blood tests prior to each chemotherapy cycle. The average red blood cell counts did not differ significantly between pre- and postdose in each of the intervention groups. The likelihood of an observed reduction in neutropenic toxicity in HO rather than LO was not observed between the 2 arms of the trial (data not shown). Participants who were highly prone to neutropenic reactions (white blood cell count <4 × 103, neutrophil <50%, and lymphocytes <24%) were not considered for this nutritional intervention.
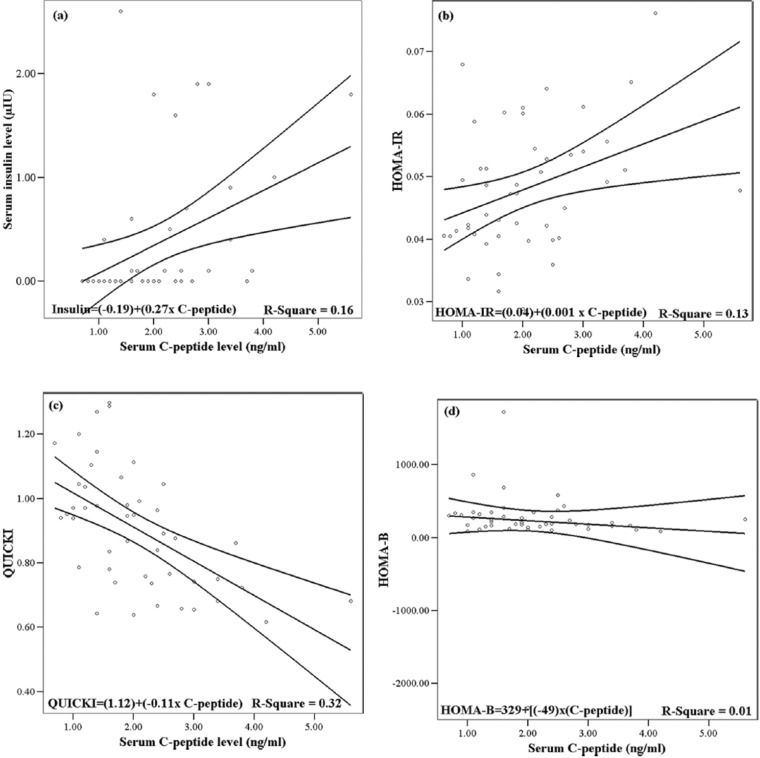
Figure 2 depicts a scatter plot for plasma variables of C-peptide in association with dependent variables in terms of serum insulin and insulin-dependent indices. C-peptide correlated significantly with plasma insulin levels (r = .40, P = .0001). C-peptide was inversely correlated with QUICKI (r = −.56, P = .0001) and also correlated positively with HOMA-IR (r = .36, P = .014). Table 3 showed increases in the average BMI within either HO or LO group during the intervention. No difference was observed regarding to BMI between groups.
Figure 2.
Linear regressions with 95% mean predictive values obtained to show the validity of data based on serum C-peptide concentration (independent variable) in association with insulin-related biomarkers (dependent variable) at baseline compartment of the study (n = 46).
Intervention Effect
Raw yellow onion was administered to adjuvant-treated BC patients consistently and begun in the post-second course of chemotherapy (after 2 days) in a triple-blind state. The BMI-dependent weighed onion in the intervention of HO and LO groups was administered following a 14-day run-in period. Changes in certain metabolic parameters during 8 weeks of intervention for both groups are summarized in Table 4. The HO group experienced a significant decline of FBG at week 8 (P = .014) while the LO group showed a nonsignificant increase. Posttreatment, the HO group showed a significant decrease in serum levels of insulin in comparison to LO (P = .008). The within-group comparison in the LO group showed an increase in HOMA IR (P = .025), while the HO group presented a significant decrease in HOMA-IR levels (P = .021; Table 4). Between-group comparison at posttreatment compartment demonstrated a decline of HOMA-IR in HO group, but the difference failed to reach a significance level (P = .080). The comparable relative changes of FBG and HOMA-IR observed between interventional groups presented significant declines in high onion consumers rather than in the LO group (P = .005; Figure 3). Likewise, there was a negative relative change of FBG obtained in HO rather than a rise of FBG in the LO group (P = .003; Figure 3). Comparing pre- and postdose stages in the group of LO consumers showed a significant decrease in the QUICKI variable (P = .012). Postintervention after 8 weeks resulted in less affected QUICKI levels among HO consumers compared with the postdose serum level of the LO group (P = .002). The absolute treatment effect for the mean changes of QUICKI in LO was estimated to be higher than what was observed in the HO group (P = .028). There existed also a statistically nonsignificant decline in the mean difference of QUICKI within the HO group when LO exhibited greater changes within the group (P = .012). The absolute treatment effect for the mean changes of QUICKI in the LO group was estimated to be higher than what was observed in the HO group (P = .028). There was a significant increase in fasting plasma C-peptide levels in the LO group (P = .026), but no significant changes were demonstrated in the HO group. Absolute treatment effects estimated in the HO group in comparison to LO was −0.27, showing a negative impact of high onion consumption on C-peptide levels (P = .005).
Table 4.
Serum Levels of Glycemic and Insulinemic Biomarkers at Baseline Compartment of Study and 8 Weeks After the Intervention in Women With BC Who Received Onion (HO Group) Versus LO Consumers.
| Variable | LO (Placebo; n = 23), Mean ± SD | HO (Intervention; n = 23), Mean ± SD | Mean Differencea | P valuea | Absolute Treatment Effectb |
|
|---|---|---|---|---|---|---|
| Mean [95% CI] | P Valuec | |||||
| FBG (mg/dL) | ||||||
| Predose | 91.1 ± 16.0d | 106.6 ± 21.2 | +15.5 | .008 | −5.04 | .245 |
| Week 8 | 100.6 ± 21.6 | 95.1 ± 13.6 | −5.3 | .313 | [−3.58, 13.67] | |
| Mean difference | +9.5e | −11.5 | ||||
| P valuee | .079 | .014 | ||||
| Insulin (ng/mL) | ||||||
| Predose | 0.198 ± 0.006 | 0.196 ± 0.005 | −0.002 | .235 | −0.006 | .009 |
| Week 8 | 0.207 ± 0.018 | 0.197 ± 0.006 | −0.010 | .008 | [−0.010, −0.002] | |
| Mean difference | +0.009 | +0.001 | ||||
| P value | 0.123 | 0.523 | ||||
| HOMAIRf | ||||||
| Predose | 0.045 ± 0.01 | 0.052 ± 0.011 | +0.007 | .017 | 0.001 | .655 |
| Week 8 | 0.051 ± 0.01 | 0.046 ± 0.006 | −0.005 | .080 | [−0.003, 0.005] | |
| Mean difference | +0.006 | −0.005 | ||||
| P value | .025 | .021 | ||||
| HOMAβg | ||||||
| Predose | 226.1 ± 643.8 | 229.4 ± 188.6 | 3.2 | .982 | +19.64 | .804 |
| Week 8 | 259.5 ± 140.4 | 295.5 ± 226.6 | 35.9 | .521 | [−138.8, 178.0] | |
| Mean difference | +33.4 | +66.1 | ||||
| P value | .806 | .207 | ||||
| QUICKIh | ||||||
| Predose | 0.90 ± 0.20 | 0.90 ± 0.18 | 0.01 | .938 | +0.063 | .028 |
| Week 8 | 0.76 ± 0.14 | 0.89 ± 0.13 | 0.13 | .002 | [−0.004, 0.131] | |
| Mean difference | −0.15 | −0.011 | ||||
| P value | .012 | .801 | ||||
| C-peptide (ng/mL) | ||||||
| Predose | 2.07 ± 1.10 | 2.09 ± 0.91 | 0.02 | .954 | −0.27 | .005 |
| Week 8 | 3.14 ± 2.17 | 2.59 ± 1.41 | 0.55 | .314 | [−0.965, 0.430] | |
| Mean difference | +1.07 | +0.50 | ||||
| P value | .026 | .096 | ||||
Abbreviations: BC, breast cancer; LO, low onion group; HO, high onion group; FBG, fasting blood glucose.
Independent sample t test was used to compare between 2 interventional groups. The mean difference of between groups was calculated for variables at (endpoint) − (baseline).
Absolute treatment effect is an estimate of change from predose to follow-up in the HO group minus the absolute change from predose to follow-up in the LO group in a mixed model.
Repeated-measures ANOVA was carried out in the mixed model.
Data are expressed in geometric mean ± SD.
Paired t test was performed to compare within-group changes. The mean difference within groups was calculated from averages of variables at (postdose) − (week 8).
Homeostatic model assessment.
Assessment of β-cell functionality.
Quantitative insulin sensitivity check index.
Figure 3.
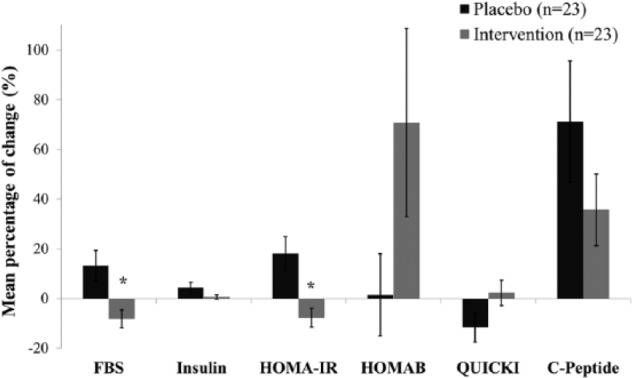
Mean percentage of changes (±SEM) for selected metabolic variables (serum FBG, insulin, C-peptide, and insulin-related variables) between pre- and postdose timelines by which finally compared between LO and HO groups as well. *Independent sample t test: P < .01.
Discussion
Throughout this randomized controlled clinical trial conducted on newly diagnosed BC patients receiving postoperative chemotherapy, the raw yellow onion intervention concurrently showed favorable effects in terms of controlling insulin-related variables. To the best of our knowledge, this is the first study suggesting that the variable of QUICKI, which is a sensitive determinant for predicting insulin sensitivity,36 declined in LO counterparts as compared to HO participants, who in the meantime had all been exposed to conventional DOX-based chemotherapy.
Onion is a staple vegetable item used in the Iranian habitual diet. Its inclusion in the side dishes accompanying main meals (lunch and dinner) provided an exclusive opportunity for observing the effects of this functional food on hyperinsulinemia and its possible tumor-promoting effects. The role of HOMA-related indices in BC prognosis is well-established.37 Our observations showed that the HO group treatment significantly controlled HOMA-IR and insulin levels. Earlier studies have shown that fasting glucose levels in nondiabetic women with early BC was associated with a modest increase in the risk of distant breast tumor recurrence and relevant lethal outcomes, suggesting insulin-dependent underlying biologic mechanisms.38 The hypoglycemic effect of onion was also determined in an earlier clinical study conducted by Augusti et al,37 on normal healthy individuals. Onion has been consistently and widely used for the treatment of diabetes in traditional Chinese practices.39 A clinical trial conducted on diabetic patients showed that the use of onion slices could cause antidiabetic effects with particularly significant reductions of FBG levels in both insulin-dependent and noninsulin-dependent diabetes.40 Similar antihyperglycemic effects were also shown by very early clinical trials of administered juice, aqueous extract, or oil constituent of onion on healthy volunteers.40-42 Additionally, Sharma et al demonstrated the blood sugar lowering effects of boiled onion extract when used to treat diabetic patients.42 The consumption of cooked onion (150 g onion cake containing 89.7 mg QR) by healthy human subjects caused significant effects on plasma levels of QR; however, there was no remark regarding its ability to reduce oxidative DNA damage in leukocyte, plasma F2 isoprostane concentration, and changes in malodialdehyde-modified low-density lipoprotein cholesterol.43
The findings of this study agree with a considerable number of studies that have indicated the antidiabetic effects of various types of onions.22,44-46 BC participants in the present intervention study generally received DOX as an anthracycline antibiotic reagent used widely in chemotherapeutic approaches for different types of malignancies.47 Substantial follow-up evidence has supported that receiving chemotherapeutic regimens including DOX causes systemic IR.16 In this regard, it is primarily implied that DOX-mediated dysregulation of genes is highly involved in insulin signaling pathways that are mainly involved in skeletal muscle cells.48 Collectively, DOX administration during the course of patients’ chemotherapy regimens might be capable of predisposing BC patients to IR, which may lead to subsequent impaired insulin secretion and signaling in targeted cells.48 Dexamethasone, which is administered as an antivomiting agent before DOX IV injection, could potentially give rise to IR that is correlated to adiposity.49,50 Notably, evidence in colorectal cancer has shown that raised BMI or weight gain during the time period between ongoing adjuvant chemotherapy and 6 months after its completion increased the risk of cancer recurrence and death in patients but none of them addressed clinical relevance to dexamethasone.18,51,52 Results from a cohort of the Nurses’ Health Study reported by Kroenke and colleagues suggested that BC patients who gained weight after diagnosis and had no history of smoking might be at risk of disease relapse, and eventually all-cause mortality.53 A comprehensive pooled analysis on different data sets of well-known cohort-based studies (NHS, Life after Cancer Epidemiology study, and Women’s Healthy Eating and Living study) showed that weight gain after chemotherapy could increase all-cause mortality rates for BC survivors.54 Therefore, we can suggest that the comorbidity of other causes of death, such as being IR, and obesity in cancer patients, can be effective contributors on predicting the overall survival rate. Makari-Judson and colleagues, in a prospective observational study of women with BC who were treated with adjuvant therapy, showed that the variable of HOMA-IR was positively associated with weight gain after diagnosis.55 Of those patients who received chemotherapy, HOMA-IR remained high, even after 6 months.
The administration of crude hydroalcoholic extract of Allium cepa in alloxan-induced diabetic rats produced significant hypoglycemic effects, which can most likely be attributable to improvement in or perhaps remission of functionally disabled pancreatic β-cells.44 In contrast, in a quasi-experimental research study conducted on 26 patients with T2DM, patients ate breakfast containing 60 g/d of onion, which resulted in no significant changes in glucose level between sampling intervals; however, increased serum insulin levels were obvious.56 Despite the limited data pertaining to human intervention trials,56 there are additional experimental animal model studies supporting the effect of onion in controlling hyperglycemia and hyperinsulinemia. Wu et al have shown inhibitory activities against α-glucosidase after onion administration to alloxan-induced diabetic Rattus norvegicus (for 6 weeks).57 Additionally, this study indicated that onions showed strong free radical scavenging capacities, contributing to their antidiabetic properties.58 Onions were observed to improve control of fasting hyperglycemia by enhancing insulin sensitivity, possibly through α-glucosidase inhibition, and subsequently to promote insulin signaling in db/db mice.59 Kannappan and Anuradha showed improvements in insulin signaling and subsequent insulin sensitizing activity in IR-induced rats through the administration of QR.58 Moreover, in support of our findings, an in vivo study performed by Hanasaki et al60 reported that onion enhanced insulin sensitivity and secretion, subsequently ameliorating the manifestation of diabetes. Data from animal model studies have shown that both onion and QR may upregulate insulin receptor (INSR) and glucose tranporter-4 (GLUT4) genes in peripheral tissues.61 The intervening effect of onion could partly be ascribed to the effect of QR on systemic glucose homeostasis. AMP-activated protein kinase (AMPK), an extracellular regulated kinase, functions as an energy sensor related to intracellular transmembrane insulin receptor and has been observed to be influenced potently by QR in experimental in vitro studies.62 In addition, extract of onion can regulate the function of hepatic hexokinase, glucose-6-phosphate, and 3-hydroxy-3-methylglutaryl coenzyme-A reductase to yield hypoglycemic and hypolipidemic effects.21 Insulin also exerts its function through the phosphatidylinositide-3-kinase (PI3K)/Akt signaling pathway, which is suppressed by QR treatment in vitro.63 Among limited evidence on hypoinsulinemic effects of onion in cancer patients, the present study is the first evidence suggesting that regular onion consumption as part of the diet could attenuate QUICKI and control high HOMA-IR in DOX-treated BC patients.
There were some limitations of the present study that should be considered when interpreting the results. The sample size by subgroups of the present interventions was relatively small and small changes in serum levels of biomarkers might not be supported by sufficient power of analysis. The interventional time frame of 8 weeks after the second cycle of chemotherapy was considered limited by the possible interaction of high onion consumption in the detoxification of taxane (docetaxel and paclitaxel), which was administered according to the protocol after 12 weeks in the chemotherapy regimens in this study. We attempted to ensure homogenous adjuvant chemotherapy was given alongside the onion intervention by including only patients on DOX-based regimens in the study. We therefore cannot rule out the possibility of IR changes induced by the DOX-based chemotherapy. Thus, the observed prospective effects of onion consumption on IR changes might be masked to some extent by the profound effects of primary adjuvant therapy. We suggest that at least stratified random allocation could be imperative to attenuate the variable effects that resulted from variant chemotherapy regimens. Potential dietary changes are frequently observed after cancer diagnosis, when patients can become more sensitive about their lifestyle. To overcome this challenge, instructions to patients that they should remain on their habitual diets were administered and verified over the run-in period and throughout the 8 weeks of intervention. Although chemotherapy-related metabolic changes that promote weight loss (eg, cachexia) can be observed after receiving the first cycle of chemotherapy, our intervention, which was administered after the second cycle, was not associated with significantly reduced anthropometric changes in controls. One advantage of the study is that the variables of insulin and insulinemia-related indices were validated by the C-peptide biomarker. Overall, the results of this 8-week intervention suggest that onion favorably alters some markers of insulin in a direction that would be effective during chemotherapy.
Conclusions
The present study demonstrated the potential effectiveness of onion in ameliorating hyperglycemia and insulin resistance in BC during DOX-based chemotherapy. Manipulation of diet through high intake of onion is promising for having a synergistic effect with DOX-based chemotherapy. The common use of fresh and raw onion in Iranian dietary habits renders this study important in terms of establishing the possible effects of regular onion consumption associated with DOX among BC survivors. Fresh yellow onion demonstrates promising properties in terms of insulin-related indices in BC patients prone to IR while being treated with DOX.
Acknowledgments
The authors would like to thank all those involved in this study for their participation, and the staff members of Nour-Nejat Hospital, Cancer Clinic, Danesh-2 Laboratory, Shahid Ghazi and Emam Reza Hospitals for providing their valuable time to this research.
Footnotes
Authors’ Note: Authors Ali Adili and Ali Esfehani contributed equally to this work. This article was outlined using a data set obtained from an MSc thesis titled “The Effect of Raw Yellow Onion Consumption on Plasma Levels of Hormonal/Metabolic Parameters in Patients With Primary Breast Cancer: A Randomized Controlled Clinical Trial,” registered at Tabriz University of Medical Sciences, Tabriz Iran (Registration No.: 5/97/803).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the Drug Applied Research Center (5-64403), and Vice Chancellor for Research of Tabriz University of Medical Sciences.
References
- 1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: Cancer J Clin. 2012;62:10-29. [DOI] [PubMed] [Google Scholar]
- 2. Adebamowo CA, Cho E, Sampson L, et al. Dietary flavonols and flavonol-rich foods intake and the risk of breast cancer. Int J Cancer. 2005;114:628-633. [DOI] [PubMed] [Google Scholar]
- 3. Willett WC. Diet and breast cancer. J Intern Med. 2001;249:395-411. [DOI] [PubMed] [Google Scholar]
- 4. Pirouzpanah S, Taleban FA, Atri M, Abadi AR, Mehdipour P. The effect of modifiable potentials on hypermethylation status of retinoic acid receptor-beta2 and estrogen receptor-alpha genes in primary breast cancer. Cancer Cause Control. 2010;21:2101-2111. [DOI] [PubMed] [Google Scholar]
- 5. Bradbury KE, Appleby PN, Key TJ. Fruit, vegetable, and fiber intake in relation to cancer risk: findings from the European Prospective Investigation into Cancer and Nutrition (EPIC). Am J Clin Nutr. 2014;100(suppl 1):394S-398S. [DOI] [PubMed] [Google Scholar]
- 6. Gonzalez CA, Riboli E. Diet and cancer prevention: where we are, where we are going. Nutr Cancer. 2006;56:225-231. [DOI] [PubMed] [Google Scholar]
- 7. Norat T, Aune D, Chan D, Romaguera D. Fruits and vegetables: updating the epidemiologic evidence for the WCRF/AICR lifestyle recommendations for cancer prevention. Cancer Treat Res. 2014;159:35-50. [DOI] [PubMed] [Google Scholar]
- 8. Challier B, Perarnau JM, Viel JF. Garlic, onion and cereal fibre as protective factors for breast cancer: a French case-control study. Eur J Epidemiol. 1998;14:737-747. [DOI] [PubMed] [Google Scholar]
- 9. Leviac F, La Vecchiacd C, Gulieb C, Negri E. Dietary factors and breast cancer risk in Aaud, Switzerland. Nutr Cancer. 1993;19:327-335. [DOI] [PubMed] [Google Scholar]
- 10. Granata R, Settanni F, Trovato L, et al. RFamide peptides 43RFa and 26RFa both promote survival of pancreatic β-cells and human pancreatic islets but exert opposite effects on insulin secretion. Diabetes. 2014;63:2380-2393. [DOI] [PubMed] [Google Scholar]
- 11. Goodwin PJ, Ennis M, Pritchard KI, et al. Insulin- and obesity-related variables in early-stage breast cancer: correlations and time course of prognostic associations. J Clin Oncol. 2012;30:164-171. [DOI] [PubMed] [Google Scholar]
- 12. Akash MS, Rehman K, Chen S. Role of inflammatory mechanisms in pathogenesis of type 2 diabetes mellitus. J Cell Biochem. 2013;114:525-531. [DOI] [PubMed] [Google Scholar]
- 13. Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33:1674-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Novosyadlyy R, Lann DE, Vijayakumar A, et al. Insulin-mediated acceleration of breast cancer development and progression in a nonobese model of type 2 diabetes. Cancer Res. 2010;70:741-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mogul HR, Freeman R, Nguyen K, et al. Carbohydrate modified diet & insulin sensitizers reduce body weight & modulate metabolic syndrome measures in EMPOWIR (enhance the metabolic profile of women with insulin resistance): a randomized trial of normoglycemic women with midlife weight gain. PLoS One. 2014;9:e108264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arunachalam S, Tirupathi Pichiah PB, Achiraman S. Doxorubicin treatment inhibits PPARy and may induce lipotoxicity by mimicking a type 2 diabetes-like condition in rodent models. FEBS Lett. 2013;587:105-110. [DOI] [PubMed] [Google Scholar]
- 17. Edson Alves de Lima J, de Souza CO, de Souza Teixeira AA, Batatinha DA, de Santos Lira F, Rosa Neto JC. Doxorubicin leads to impaired insulin signaling in skeletal muscle. Cancer Metab. 2014;2(suppl 1):P2. [Google Scholar]
- 18. de Azambuja E, McCaskill-Stevens W, Francis P, et al. The effect of body mass index on overall and disease-free survival in node-positive breast cancer patients treated with docetaxel and doxorubicin-containing adjuvant chemotherapy: the experience of the BIG 02-98 trial. Breast Cancer Res Treat. 2010;119:145-153. [DOI] [PubMed] [Google Scholar]
- 19. Park H, Kim HS. Korean traditional natural herbs and plants as immune enhancing, antidiabetic, chemopreventive, and antioxidative agents: a narrative review and perspective. J Med Food. 2014;17:21-27. [DOI] [PubMed] [Google Scholar]
- 20. Pérez-Gregorio MR, Regueiro J, Simal-Gándara AS. Increasing the added-value of onions as a source of antioxidant flavonoids: a critical review. Crit Rev Food Sci Nutr. 2014;54:1050-1062. [DOI] [PubMed] [Google Scholar]
- 21. Akash MS, Rehman K, Chen S. Spice plant Allium cepa: dietary supplement for treatment of type 2 diabetes mellitus. Nutr Cancer. 2014;30:1128-1137. [DOI] [PubMed] [Google Scholar]
- 22. Campos KE, Diniz YS, Cataneo AC, Faine LA, Alves MJ, Novelli EL. Hypoglycemic and antioxidant effects of onion, Allium cepa: dietary onion addition, antioxidant activity and hypoglycemic effects on diabetic rats. Int J Food Sci Nutr. 2003;54:241-246. [DOI] [PubMed] [Google Scholar]
- 23. Sheela CG, Kumud K, Augusti KT. Anti-diabetic effects of onion and garlic sulfoxide amino acids in rats. Planta Med. 1995;61:356-357. [DOI] [PubMed] [Google Scholar]
- 24. Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst. 1999;91:1616-1634. [DOI] [PubMed] [Google Scholar]
- 25. Bulucu F, Ocal R, Karadurmus N, et al. Effects of n-acetylcysteine, deferoxamine and selenium on doxorubicin- induced hepatotoxicity. Biol Trace Elem Res. 2009;132:184-196. [DOI] [PubMed] [Google Scholar]
- 26. Jafarpour-Sadegh F, Montazeri V, Adili A, et al. Effects of fresh yellow onion consumption on CEA, CA125 and hepatic enzymes in breast cancer patients: a double-blind randomized controlled clinical trial. Asian Pac J Cancer Prev. 2015;16:7517-7522. [DOI] [PubMed] [Google Scholar]
- 27. Ebrahimi-Mamaghani M, Saghafi-Asl M, Pirouzpanah S, Asghari-Jafarabadi M. Effects of raw red onion consumption on metabolic features in overweight or obese women with polycystic ovary syndrome: a randomized controlled clinical trial. J Obstet Gynaecol Res. 2014;40:1067-1076. [DOI] [PubMed] [Google Scholar]
- 28. World Medical Association. Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191-2194. [DOI] [PubMed] [Google Scholar]
- 29. Johansson L, Solvoll K, Bjørneboe GE, Drevon CA. Under- and overreporting of energy intake related to weight status and lifestyle in a nationwide sample. Am J Clin Nutr. 1998;68:266-274. [DOI] [PubMed] [Google Scholar]
- 30. Pirouzpanah S, Taleban FA, Mehdipour P, Atri M, Hooshyareh-rad A, Sabour S. The biomarker-based validity of a food frequency questionnaire to assess the intake status of folate, pyridoxine and cobalamin among Iranian primary breast cancer patients. Eur J Clin Nutr. 2014;68:316-323. [DOI] [PubMed] [Google Scholar]
- 31. Pirouzpanah S, Kouhdani F. Nutritional facts about macronutrients in cancer. In: Mehdipour P, ed. Bridging Cell Biology and Genetics to the Cancer Clinic. Kerala, India: Transworld Research Network; 2011. [Google Scholar]
- 32. Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. 2008;67:253-256. [DOI] [PubMed] [Google Scholar]
- 33. Pirouzpanah S, Taleban FA, Abadi AR, Atri M, Mehdipour P. The association of plasma folate, vitamin B12 and homocysteine levels on hypermethylation status of RARβ2 gene in primary breast carcinoma (in Persian). Iran J Epidemiol. 2009;5(2):19-17. [Google Scholar]
- 34. Bun SS, Ciccolini J, Bun H, Aubert C, Catalin J. Drug interactions of paclitaxel metabolism in human liver microsomes. J Chemother. 2003;15:266-274. [DOI] [PubMed] [Google Scholar]
- 35. Meijerman I, Beijnen JH, Schellens JH. Herb-drug interactions in oncology: focus on mechanisms of induction. Oncologist. 2006;11:742-752. [DOI] [PubMed] [Google Scholar]
- 36. Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402-2410. [DOI] [PubMed] [Google Scholar]
- 37. Augusti KT, Benaim ME. Effect of essential oil of onion (allyl propyl disulphide) on blood glucose, free fatty acid and insulin levels of normal subjects. Clin Chim Acta. 1975;60:121-123. [DOI] [PubMed] [Google Scholar]
- 38. Irwin ML, Duggan C, Wang CY, et al. Fasting c-peptide levels and death resulting from all causes and breast cancer: the health, eating, activity, and lifestyle study. J Clin Oncol. 2011;29:147-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu H, Xu B. Inhibitory effects of onion against α-glucosidase activity and its correlation with phenolic antioxidants. Int J Food Properties. 2014;17:599-609. [Google Scholar]
- 40. Eldin IMT, Ahmed EM, Elwahab AHM. Preliminary study of the clinical hypoglycemic effects of Allium cepa (red onion) in type 1 and type 2 diabetic patients. Environ Health Insights. 2010;4:71-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mathew PT, Augusti KT. Hypoglycemic effects of onion, Allium cepa Linn. on diabetes mellitus—a preliminary report. Indian J Physiol Pharmacol. 1975;19:213-217. [PubMed] [Google Scholar]
- 42. Sharma KK, Gupta RK, Gupta S, Samuel KC. Anti-hyperglycaemic effect of onion: effect on fasting blood sugar & induced hyperglycemia in man. Indian J Med Res. 1977;65:422-429. [PubMed] [Google Scholar]
- 43. Beatty ER, O’Reilly JD, England TG, et al. Effect of dietary quercetin on oxidative DNA damage in healthy human subjects. Br J Nutr. 2000;84:919-925. [PubMed] [Google Scholar]
- 44. El-Demerdash FM, Yousef MI, El-Naga NI. Biochemical study on the hypoglycemic effects of onion and garlic in alloxan-induced diabetic rats. Food Chem Toxicol. 2005;43:57-63. [DOI] [PubMed] [Google Scholar]
- 45. Babu PS, Srinivasan K. Influence of dietary capsaicin and onion on the metabolic abnormalities associated with streptozotocin induced diabetes mellitus. Mol Cell Biochem. 1997;175:49-57. [DOI] [PubMed] [Google Scholar]
- 46. Jelodar GA, Maleki M, Motadayen MH, Sirus S. Effect of fenugreek, onion and garlic on blood glucose and histopathology of pancreas of alloxan-induced diabetic rats. Indian J Med Sci. 2005;59:64-69. [PubMed] [Google Scholar]
- 47. Geetha A, Catherine J, Shyamala Devi CS. Effect of a-tocopherol on doxorubicin induced alterations in glucose metabolism—a pilot study. J Biosci. 1989;14:243-247. [Google Scholar]
- 48. Alves de Lima Junior E, Oliveira de Souza C, Abílio de Souza Teixeira A, Angélica Batatinha H, de Santos Lira F, César Rosa Neto J. Doxorubicin leads to impaired insulin signaling in skeletal muscle. Cancer Metab. 2014;2(1):P2. [Google Scholar]
- 49. Jeong Y, Han HS, Lee HD, et al. A pilot study evaluating steroid-induced diabetes after antiemetic dexamethasone therapy in chemotherapy-treated cancer patients. Cancer Res Treat.2016;48(4):1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chow EJ, Pihoker C, Friedman DL, et al. Glucocorticoids and insulin resistance in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013;60:621-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dignam JJ, Polite BN, Yothers G, et al. Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. J Natl Cancer Inst. 2006;98:1647-1654. [DOI] [PubMed] [Google Scholar]
- 52. Meyerhardt JA, Catalano PJ, Haller DG, et al. Influence of body mass index on outcomes and treatment-related toxicity in patients with colon carcinoma. Cancer. 2003;98:484-495. [DOI] [PubMed] [Google Scholar]
- 53. Kroenke CH, Chen WY, Rosner B, Holmes MD. Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol. 2005;23:1370-1378. [DOI] [PubMed] [Google Scholar]
- 54. Caan BJ, Kwan ML, Shu XO, et al. Weight change and survival after breast cancer in the after breast cancer pooling project. Cancer Epidemiol Biomarkers Prev. 2012;21:1260-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Makari-Judson G, Braun B, Jerry DJ, Mertens WC. Weight gain following breast cancer diagnosis: implication and proposed mechanisms. World J Clin Oncol. 2014;5:272-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zamani-pour NN, Ahmadi A, Tahbaz F. Postprandial glucose and insulin responses to onion ingestion with breakfast in patients with type 2 diabetes (in Persian). Shahrekord Univ Med Sci J. 2012;13(6):19-26. [Google Scholar]
- 57. Dai B, Ruan B, Wu J, et al. Insulin-like growth factor binding protein-1 inhibits cancer cell invasion and is associated with poor prognosis in hepatocellular carcinoma. Int J Clin Exp Pathol. 2014;7:5645-5654. [PMC free article] [PubMed] [Google Scholar]
- 58. Kannappan S, Anuradha CV. Insulin sensitizing actions of fenugreek seed polyphenols, quercetin and metformin in a rat model. Indian J Med Res. 2009;129:401-408. [PubMed] [Google Scholar]
- 59. Jevas C. Anti-diabetic effects of allium cepa (onions) aqueous extracts on alloxan-induced diabetic Rattus novergicus. J Med Plant Res. 2011;5:1134-1139. [Google Scholar]
- 60. Hanasaki Y, Ogawa S, Fukui S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Radic Biol Med. 1994;16:845-850. [DOI] [PubMed] [Google Scholar]
- 61. Jung JY, Lim Y, Moon MS, Kim JY, Kwon O. Onion peel extracts ameliorate hyperglycemia and insulin resistance in high fat diet/streptozotocin-induced diabetic rats. Nutr Metab (Lond). 2011;8(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang P, Phan T, Gordon D, Chung S, Henning SM, Vadgama JV. Arctigenin in combination with quercetin synergistically enhances the antiproliferative effect in prostate cancer cells. Mol Nutr Food Res. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bhat FA, Sharmila G, Balakrishnan S, et al. Quercetin reverses EGF-induced epithelial to mesenchymal transition and invasiveness in prostate cancer (PC-3) cell line via EGFR/PI3K/Akt pathway. J Nutr Biochem. 2014. 25(11):1132-1139. [DOI] [PubMed] [Google Scholar]