Abstract
Background: Osteosarcoma is a malignant bone tumor prevalent in adolescents with poor prognosis. Toona sinensis showed potent antiproliferation effect on lung, melatonin, ovary, colon, and liver cancers. However, the effects of the species on osteosarcoma cells are rarely investigated. Results: In this study, we found fraction 1 of Toona sinensis leaf (TSL-1) resulted in inhibition of cell viability in MG-63, Saos-2, and U2OS osteosarcoma cell lines, while it only caused a moderate suppressive effect on normal osteoblasts. In addition, TSL-1 significantly elevated lactate dehydrogenase leakage and induced apoptosis and necrosis in Saos-2 cells. TSL-1 increased mRNA expression of pro-apoptotic factor Bad. Most important, TSL-1 significantly suppressed Saos-2 xenograft tumor growth in nude mice by increasing caspase-3. The IC-50 of TSL-1 for the 3 tested osteosarcoma cells is around 1/9 of that for lung cancer cells. Conclusion: We demonstrated that TSL-1, a fractionated extract from TSL, caused significant cytotoxicity to osteosarcoma cells due to apoptosis. In vivo xenograft study showed that TSL-1 suppressed the growth of osteosarcoma cells at least in part by inducing apoptosis. Our results indicate that TSL-1 has potential to be a promising anti-osteosarcoma adjuvant functional plant extract.
Keywords: apoptosis, health food, osteosarcoma, Toona sinensis, xenograft
Introduction
Osteosarcoma is the most common sarcoma of bone.1 In spite of the fact that this cancer type only accounts for 5% to 6% of all childhood tumors, they are ranked among the most frequent causes of cancer-related death, because osteosarcomas have a high probability of metastasis.2 At the time of diagnosis, about 10% to 20% of patients have evidence of metastatic disease, most commonly (90%) in the lungs.3 Current management comprises preoperative chemotherapy followed by surgical removal of all detectable disease and postoperative (adjuvant) chemotherapy.4 Most chemotherapy regimens applied for osteosarcoma have been based on the following drugs: high-dose methotrexate with leucovorin rescue, doxorubicin (adriamycin), cisplatin, and ifosfamide.3 Preoperative “neoadjuvant” chemotherapy is generally administered for a period of about 8 to 10 weeks prior to surgery. Postoperative adjuvant chemotherapy is continued for a period of another 12 to 29 weeks after wound healing.5,6 During the chemotherapy period, other functional supplement plant extracts may be beneficial.
Toona sinensis Roem (Meliaceae; TS), which grows mostly in Asia, is widely used as a medicine and especially as a vegetable. The bark is used as an astringent and depurative agent, the root is used as refreshment and diuretic agents, the tender leaves are used as carminative and corrective agent, and its fruit is used as astringent and in treatment of eye infections. Especially leaves and young shoots, which have been used as a vegetable in China for thousands of years, are used as the treatment of enteritis, dysentery, and itch in oriental medicine.7 The aqueous extract of TS leaf (TSL) possesses antioxidant,8-10 antidiabetes,11 antivirus,12 and antiseptic13 activity. In addition, TSL shows potent antiproliferation effect on many types of cancer, including leukemia, lung, oral squamous carcinoma, prostate, and ovary.14-20 Previous studies found that crude extracts from the TSL exerted potent antiproliferative effects on A549 lung cancer cells, H441 cells (lung adenocarcinoma), H661 cells (lung large cell carcinoma), and H520 cells (lung squamous cell carcinoma).16,17,19,21 It was also reported that crude extracts from TSL exert potent antiproliferative effects via decreased Bcl-2 protein accompanied by increased Bax protein level in H441 cells.16 However, the effects of TSL on osteosarcoma cells are rarely investigated. In this study, we tested the effects of TSL-1, an advanced fraction of TSL crude extraction, on cell viability and cytotoxicity in human osteosarcoma cell lines, U2-OS, Saos-2, and MG-63, and on tumor growth in xenograft tumor.
Materials and Methods
Preparation and Fractionation of TSL
The specimen was confirmed by previous studies.21-23 The leaves used in this preparation were obtained from TS grown in Tuku (Yunlin County, Taiwan) and were picked and washed briskly with water. Reverse osmosis water was added to the leaves at a proportion of 4 liters water to 1 kg leaves. The mixture of water and leaves was boiled for 30 minutes and then cooled down slowly for at least 2 hours at room temperature. The debris was then removed and remaining liquid was concentrated by incubating in low heat and filtered with a sieve (70-mesh). The filtered concentrate was lyophilized with a Virtis apparatus to obtain a crude extract. Following this procedure, different fractions of TSL—TSL-1, TSL-5, and TSL-7—were obtained following the aforementioned procedure by high-performance liquid chromatography. Furthermore, the powder was then dissolved in 99.5% ethanol and was centrifuged at 3000 rpm at 4°C (Beckman AvantiTM J-30I) for 12 minute to give a supernatant portion and a precipitate portion. The supernatant portion was further lyophilized with a Virtis apparatus to obtain the lyophilized powder, TSL-2.19
Cell Culture
The human osteosarcoma cell lines U2OS, Saos-2, and MG-63 were obtained from the American Type Culture Collection (Manassas, VA), and Saos-2 and MG-63 were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco BRL, Bethesda, MD) supplemented with 10% fetal bovine serum (FBS; Gibco BRL, Bethesda, MD) and 100 U/mL penicillin. U2-OS was cultured in McCoy’s 5A medium (Sigma, St Louis, MO) supplemented with 10% fetal FBS, 2 mM L-glutamine, and 100 unit/mL penicillin. The cultures were maintained in a humidified atmosphere with 5% CO2 at 37°C. Osteoblast cell lines (MT3T3-E1) were isolate cultured in DMEM with 10% FBS and 100 unit/mL penicillin for 1 week and then discarded. The attached bone cells were cultured in DMEM with 10% FBS, 100 U/mL penicillin, 50 µg/mL ascorbic acid and 100 mg/mL nonessential amino acids solution. Cells were cultured at 37°C in a 5% CO2 incubator.
Cell Viability Assays
Human osteosarcoma cell lines (U2OS, Saos-2, and MG-63), human lung adenocarcinoma epithelial cell line (H441), and normal osteoblasts were seeded in 96-well plates (5000 cells/well) and incubated overnight. The cells were then incubated in 10% FBS media containing different amounts of TSL fractions and gallic acid for 24 hours or 48 hours (TSL-1 treated group). Then, cells were cultured in media containing 2 mg/mL of MTT (3-(4,5-dimethylthiazol-2-yl)]-2,5-diphenyltetrazolium bromide; Sigma-Aldrich, St Louis, MO) for 4 hours. Then, reaction of MTT was terminated by adding 100 µL of dimethyl sulfoxide, and absorbance OD values were measured at 540 nm by using a microplate reader (Molecular Probes Inc, Eugene, OR).
Lactate Dehydrogenase Leakage Assay
Lactate dehydrogenase (LDH) leakage from cells was measured to quantify the cytotoxicity using a cytotoxicity detection kit (Roche, Mannheim, Germany).24 Saos-2 cells were seeded in 24-well plates (5 × 104 cells/well) and were not treated with drugs until 80% confluence. After drug treatment, the supernatants and cell layers of the cultures were collected for assay. According to the manufacturer’s guidelines, cell layers were lysed with 1% Triton X-100. Both cell lysine and supernatant of each sample were transferred to a 96-well plate for assay. Briefly, 100 µL of catalyst solution was added to each assay well for 20 minutes. Absorbance was measured using an ELISA reader with a 490-nm filter. LDH leakage from osteoblasts was calculated using the following formula:
Annexin V-FITC and Propidium Iodide Double-Stained Flow Cytometry
To distinguish between apoptosis and necrosis, Saos-2 cells and normal osteoblasts were seeded in 6-well plates (2 × 105 cells/well). After drug treatment for 6, 12, 24, and 48 hours, cells were collected and stained using the Annexin V-FITC Apoptosis Detection Kit (Roche, Mannheim, Germany) at room temperature in the dark, and then filtered with a 41-µm filter right before analysis. Cells (1 × 104) were counted by laser flow cytometer (EPICS Elite; Coulter, Hialeah, FL) to detect cells undergoing apoptosis or necrosis, and data were analyzed by Winmidi software (EPICS Elite, Coulter Hialeah, FL).
Real-Time Polymerase Chain Reaction (PCR)
Saos-2 cells were seeded in 6-well plates (2 × 105 cells/well) to analyze gene expression of apoptosis. After treating with TSL-1 for 12 hours, total mRNA was isolated using TRIZOL reagent (Invitrogen, Carlsbad, CA). Quantitative real-time PCR was performed with a Bio-Rad iQ5 real-time PCR detection system (Bio-Rad, Hercules, CA) using the iQ SYBR green supermix (Bio-Rad, Hercules, CA).24,25 The cycling conditions were 95°C for 30 seconds and 95°C for 4 minutes, followed by 35 cycles of 95°C for 10 seconds, 61.5°C for 15 seconds, and 72°C for 15 seconds. The primer sequences of Bax, Bcl-2, Bcl-XL, Bad, Bak, and GAPDH were as follows: Bax forward: TTT GCT TCA GGG TTTC ATCC and reverse: TCC TCT GCA GCT CCA TGT TA; Bcl-2 forward: GAG GAT TGT GGC CTT CTT TG and reverse: ACA GTT CCA CAA AGG CAT CC; Bcl-XL forward: CAT GGC AGC AGT AAA GCA AG and reverse: TGC TGC ATT GTT CCC ATA GA; Bad forward: CCA GAT CCC AGA GTT TGA GC and reverse: CTG CTC CTG CTG GTG ACT G; Bak forward: ACC AGC CTG TTT GAG AGT GG and reverse: AGT GAT GCA GCA TGA AGT CG; and GAPDH forward: CAATGACCCCTTCATTGACC and reverse: TTGATTTTGGAGGGATCTCG. The specific PCR products were detected by measuring the fluorescence of SYBR Green, a double-stranded DNA binding dye.26 The relative mRNA expression level was normalized with GAPDH. The mean of the relative value of gene expression in the control group was assigned a value of 1, and the gene expression level of each experimental group was calculated relative to the control.
Animal Experiments
The Animal Care and Use Committee of Kaohsiung Medical University approved all animal experiments. Forty 4-week-old male nude mice (25-30 g) were purchased from BioLASCO Taiwan Co (Taipei, Taiwan) and housed under standard laboratory conditions (temperature 24°C; 12-hour light-dark cycle) with food and water ad libitum. The animals were acclimated to the laboratory environment for 1 week before the experiments were initiated.
Xenograft Tumor Model
Forty nude mice were intraperitoneally injected with 1 million of Saos-2 cells (1 × 106 cells) and then randomly divided into 3 groups: control (double-distilled water [DDW] only), 1 g/kg TSL-1 group, and 5 g/kg TSL-1 group. Nude mice were fed with DDW, 1 g/kg TSL-1, or 5 g/kg TSL-1 every 2 days for 5 weeks. Weight of mice and tumor diameters were measured weekly after the first day of injection. Tumor diameters were measured with digital calipers, and the tumor volume in mm3 is calculated by the following formula: Volume = (width)2 × length/2.
Histological Analysis
After nude mice were sacrificed, the tumors were harvested. Samples for histological studies were collected and fixed with 10% neutral buffered formalin. The tumor samples were then embedded in paraffin, and 5-µm microsections from the coronary plane were prepared. Immunostaining was performed for cleaved caspase-3 (#9661; Cell signaling Technologies, Beverly, MA) in the tissues.
Immunohistochemistry
Tumor sections were rehydrated, and endogenous peroxidase activity in the tissue was blocked by treatment with 3% hydrogen peroxide. For epitope retrieval, sections were digested with a mixture of 2.5% hyaluronidase (Sigma, St Louis, MO) and 1 mg/mL pronase in phosphate-buffered saline (pH 7.4; Sigma, St Louis, MO) as previously described.27,28 Sections were subsequently incubated with the primary antibody against caspase-3 (R&D System, Inc, Minneapolis, MN). The samples were incubated with secondary, biotin-labeled antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and then incubated with horseradish peroxidase–conjugated streptavidin (Dako, Carpinteria, CA). The specific immunoreactivity was confirmed with a secondary antibody-only control. The enzyme substrate (3,3′-diaminobenzidine solution containing 0.01% hydrogen peroxide) was then added, resulting in a brown color, and sections were counterstained with hematoxylin (Santa Cruz Biotechnology) and examined by light microscopy.
Statistical Analysis
Data are presented as mean ± standard deviation for the indicated number of separate experiments. Statistical analysis of the data was performed with one-way analysis of variance (ANOVA), followed by a t test. A P value less than .05 was considered statistically significant.
Results
TSL Fractions Inhibited Human Osteosarcoma Cell Lines Growth
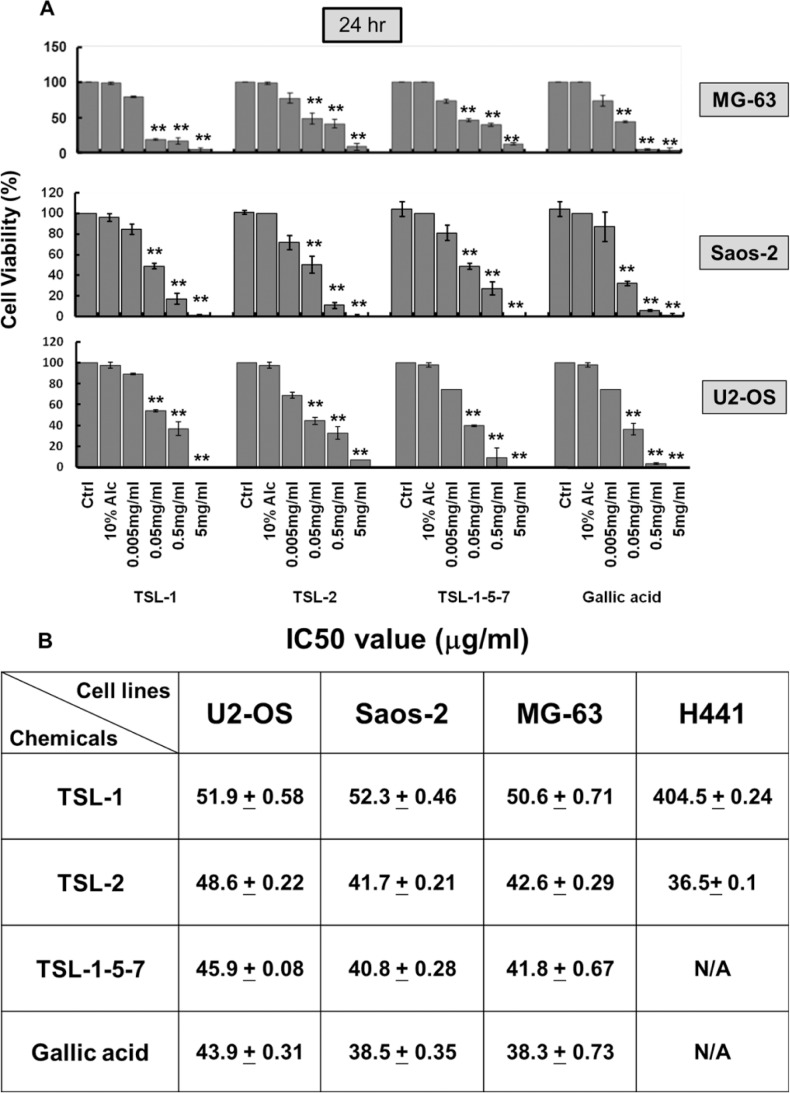
Our results showed that 0.005, 0.05, 0.5, and 5 mg/mL of TSL fractions, including TSL-1, TSL-2, and TSL1-5-7, and gallic acid significantly inhibit cell viability of MG-63, Saos-2, and U2OS at 24 hours (Figure 1A). TSL-2 and TSL1-5-7 fractions do not have higher inhibitory effect on human osteosarcoma cell than TSL-1 (Figure 1A). We further compared human osteosarcoma cells and H441 treated groups: IC50 dosage of TSL-1 on human osteosarcoma cell lines is 10 times lower than that on H441 cells, indicating that TSL-1 reveals a high efficiency on inhibiting cell viability of human osteosarcoma cells (Figure 1B).
Figure 1.
TSL fractions inhibit growth in human osteosarcoma cell lines. Human osteosarcoma cells, MG-63, Saos-2, and U2OS, were treated with 0.005, 0.05, 0.5, and 5mg/mL of TSL fractions for 24 hours. At the end of treatment, the cell viability was measured by MTT assay (A). The IC50 values of MG-63, Saos-2, and U2OS cells after TSL fractions treatment (B). **P < .001.
TSL-1 Has Lower Inhibitory Effect on Normal Osteoblasts Than Human Osteosarcoma Cells
TSL-1 (0.05 to 1 mg/mL) inhibits cell viability in both Saos-2 and normal osteoblasts but at different levels. Comparing both results, 0.2 mg/mL of TSL-1 inhibits about 60% (24 hours) to 90% (48 hours) of cell viability in Saos-2 cells (Figure 2A), while 0.2 mg/mL of TSL-1 decreases about 30% (24 hours) to 40% (48 hours) of cell viability in normal osteoblasts (Figure 2B).
Figure 2.
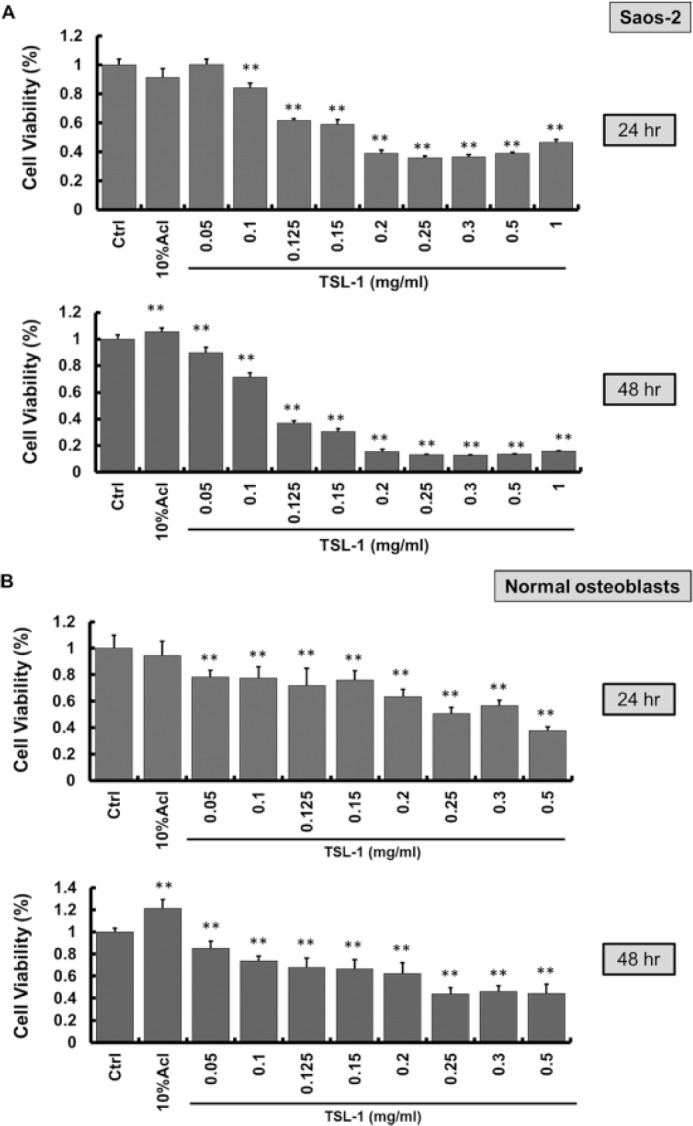
TSL-1 has lower inhibitory effect on normal human osteoblasts than human osteosarcoma cells, Saos-2. Saos-2 cells (A) and normal human osteoblasts (B) were treated with TSL-1 (0.05 to 0.5 mg/mL) for 24 and 48 hours. At the end of treatment, the cell viability was measured by MTT assay. *P < .05, **P < .001.
TSL-1 Induces Apoptosis and Necrosis in Saos-2 and Increases Pro-Apoptotic Factor, Bad, mRNA Expression
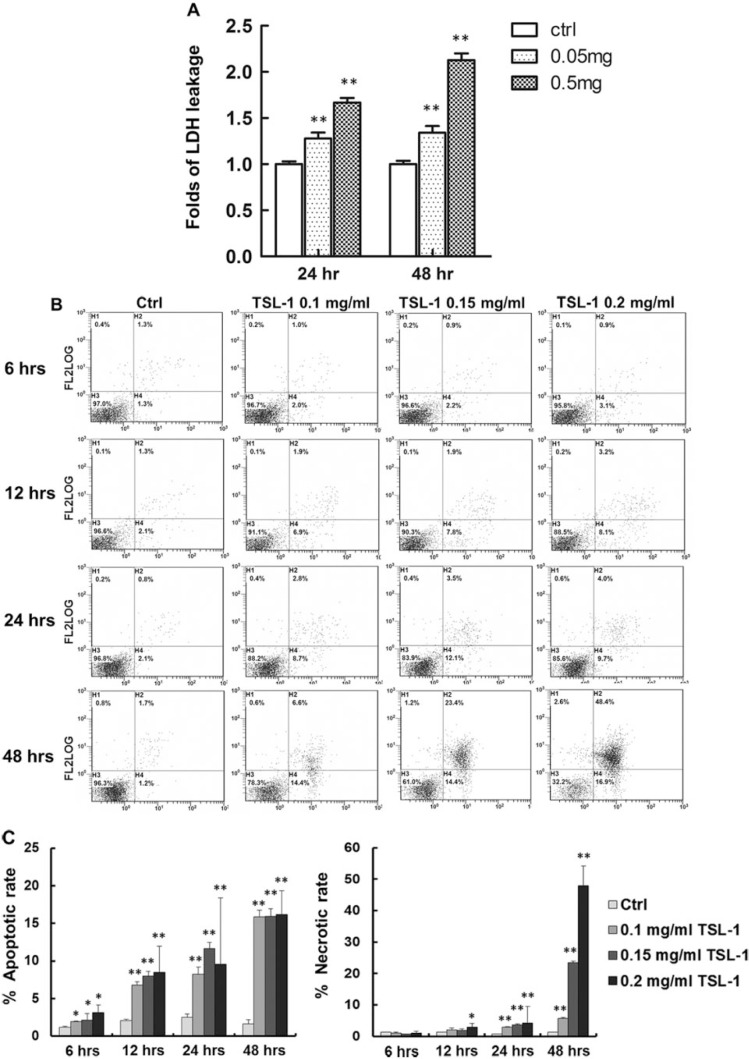
After 24 and 48 hours of treatment with 0.05 and 0.5 mg/mL TSL-1, LDH leakage is significantly elevated in Saos-2 (P < .01; Figure 3A). We further found that 0.1, 0.15, and 0.2 mg/mL TSL-1 significantly increase apoptosis (6 hours P < .05; 12, 24, and 48 hours P < .01) on 6-, 12-, 18-, and 24-hour treatments in Saos-2 (Figure 3B). We also found that 0.1, 0.15, and 0.2 mg/mL TSL-1 significantly induced necrosis at the 24th and 48th hours (P < .01) while 0.2 mg/mL of TSL-1 induces necrosis at the 12th hour in Saos-2 (P < .05; Figure 3B). Notably, at an early time point of TSL-1 treatment, 0.1, 0.15, and 0.2 mg/mL TSL-1 significantly induces apoptosis but not necrosis in Saos-2 (Figure 3C). Furthermore, 0.15 and 0.2 mg/mL TSL-1 increase mRNA expression of a pro-apoptotic factor, Bad (P < .05; Figure 4), in Saos-2, indicating that TSL-1-induced apoptosis may be one of the important mechanisms of cell viability inhibition by TSL-1.
Figure 3.
TSL-1 induces cell death of human osteosarcoma cells, Saos-2. Saos-2 cells were treated with 0.05 and 0.5 mg/mL TSL-1 to analyze the cell toxicity by measuring lactate dehydrogenase (LDH) leakage at 24 and 48 hours (A). Saos-2 cells treated with 0.1, 0.15, and 0.2 mg/mL TSL-1 were stained with Annexin-V and propidium iodide followed by flow cytometry analysis at 6, 12, 24, and 48 hours (B) and then quantitated for the ratio of apoptosis and necrosis (C). *P < .05, **P < .001.
Figure 4.
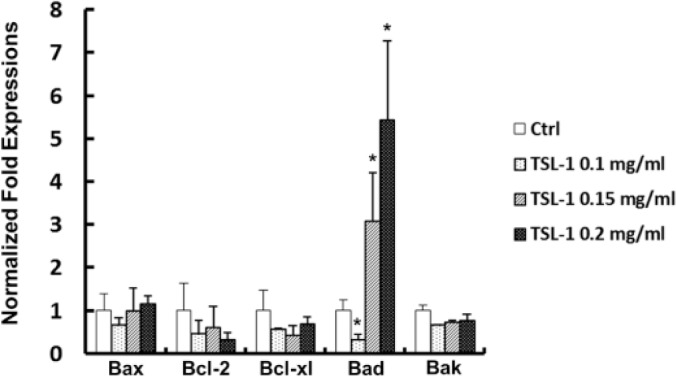
TSL-1 increases mRNA expression of pro-apoptotic factor. Saos-2 cells treated with 0.1, 0.15, and 0.2 mg/mL TSL-1 were analyzed for pro-apoptotic factor, Bax, Bcl-2, Bcl-xl, Bad, and Bak, mRNA expression at 12 hours. *P < .05.
TSL-1 Reduces Tumor Volume and Increases Caspase-3 in Animal Study
In the xenograft tumor model, the treatment group of 5 g/kg TSL-1 but not the 1 g/kg TSL-1 group showed significant decrease in body weight compared with the control group (Figure 5A). However, the gross appearance and activity level of nude mice were good even with less body weight. Both 1 and 5 g/kg TSL-1 significantly inhibit tumor growth at weeks 1 and 2 and decrease tumor volume about 80% at the fifth week (P < .01; Figure 5B). We further found positive stains in 1 and 5 g/kg TSL-1 treated groups while control shows no observable stain in tumor session at the fifth week (Figure 5C). Additionally, we kept 12 mice (control: 5 mice; 1 g/kg: 4 mice; 5 g/kg: 3 mice) for another 4 weeks, without further TSL-1 treatment during these 4 weeks. Our results show that tumors became remarkably larger in all groups, further supporting our finding that TSL-1 is able to slow down the growth of tumor (data not shown).
Figure 5.
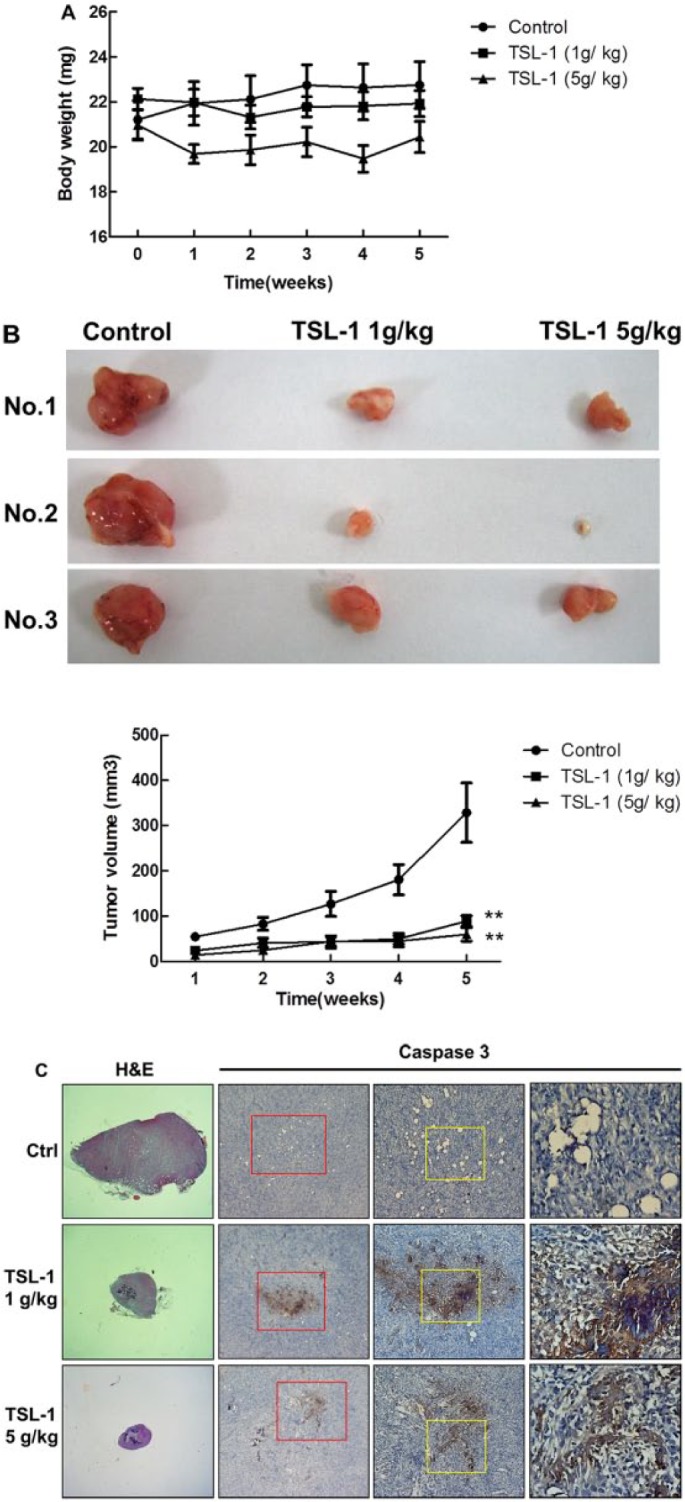
TSL-1 reduces tumor volume and increases caspase-3 in animal study. Nude mice were intraperitoneally injected with 1 million Saos-2 cells and then randomly divided into 3 groups: control (DDW only; n = 5), 1 g/kg TSL-1 group (n = 5), and 5 g/kg TSL-1 group (n = 5). Changes in body weight (A) and tumor volume (B) weekly and at the end of experiment showed the tumor images (B). Immunohistochemistry analysis of the xenografts at the fifth week showed cleaved caspase-3 (C). (Third lanes are magnified areas indicated by red line, and fourth lanes are magnified areas indicated by yellow line.) **P < .001.
Discussion
In this study, we first showed TSL had significant cytotoxic effect on osteosarcoma cells both in vitro and in xenograft studies. We found TSL-1, TSL-2, and TSL-1-5-7 decrease cell viability in a dose-dependent manner in 3 human osteosarcoma cell lines, MG-63, Saos-2, and U2OS. We further studied the anti-osteosarcoma effects of TSL-1 and found TSL-1 induced osteosarcoma cells toward apoptosis and necrosis through increasing Bad expression. The results were further confirmed in vivo by decreasing tumor size in nude mice. Because there was some weight loss in the dose of 5 g/kg group even though the gross appearance and activity level were good, we recommend the dose of 1 g/kg for future treatment, which was nearly as effective as 5 g/kg in vivo.
Among the conventional antitumor cytotoxic chemotherapies, many compounds are derived from natural products.29 Natural compounds have acted as cancer adjuvant agents and therapeutics in cancer treatment.30 More than half of the current anticancer drugs originate from natural sources.31 Modulation of apoptosis signaling pathways by natural compounds is a key point in their antitumor activities.32 A previous study found gallic acid, a major component of TSL, contains a reactive oxygen species (ROS)–mediated anticancer activity through generation of ROS and mitochondria-mediated apoptosis in DU145 human prostate cancer cells.20 In addition to gallic acid, some fractions of TSL were also found to have antitumor activities including TSL-1 and TSL-2. TSL-1 induced 3 different non–small-cell lung cancer cell lines, H441 cells (lung adenocarcinoma), H661 cells (lung large cell carcinoma), and H520 cells (lung squamous cell carcinoma), toward apoptosis by decreasing Bcl-2 and increasing Bax protein levels at the dose of 0.5 and 1 mg/mL.16 The dose was much higher than our study, which was only less than 0.2 mg/mL. Additionally, we only found Bad expression change in Saos-2 cells. Chang et al found TSL-2 also induced apoptosis in ovarian cancer cell lines SKOV3 at a dose 0.5 mg/mL via inducing the expression of Bax at 1 hour after treatment.19 The dose of TSL-2 was also much higher than our study, and the apoptotic gene changes were different to ours. TSL-2 also decreased tumor size in a dose-dependent manner at concentrations of 0.67 and 6.7 µg/g in a nude mouse xenograft model.19 The crude extract of TSL (10-75 µg/mL) was also found to induce apoptosis of human premyelocytic leukemia HL-60 cells in a dose- and time-dependent manner via reducing the protein levels of Bcl-2 and increasing that of Bax.33 The dose for apoptosis of human premyelocytic leukemia HL-60 cells was much higher than that required for Saos-2 cells, and the mechanisms in apoptosis was different. In addition, the crude extract of TSL at the dilution of 64 times induced apoptosis of A549 lung adenocarcinoma cells.21 Some studies also showed similar results with different extraction protocols of TSL. Chia et al found TS extract (0.5 g/mL) induced apoptosis in human oral squamous carcinoma cell lines, including UM1, UM2, SCC-4, and SCC-9, by upregulating pro-apoptotic genes such TNF-α, TP53BP2, and GADD45A, and downregulating the anti-apoptotic genes Survivin and cIAP1.18 The dose required in this study was very high, more than 1000 times than that in our study, and the mechanisms were different from ours. Yang et al reported TS extract, betulonic acid, and 3-oxours-12-en-28-oic acid inhibited the proliferation of MGC-803 and PC3 cells. Both components induced apoptosis at 20 µM by increasing p53, Bax, caspase-9, and caspase-3 protein levels.14 Zhen et al found decoctions from TS induced cervical carcinoma cell line HeLa arrest at S phase without inducing apoptosis.34 Chen et al found TSL-1 (0.375 and 0.5 mg/mL) induces apoptosis through the generation of ROS and activation of intrinsic apoptotic pathways in human renal carcinoma cell lines (786-O and A-498).35 The dose was somewhat higher than ours, and the mechanism was also different. The antitumor effect of TS is not a specific finding of our group. Though the mechanism is not completely same as previous studies, there may be some differences in the pre-apoptotic effect of TSL between different cancer cell lines. In addition, we found an IC50 dosage of TSL-1 on human osteosarcoma cell lines 10 times lower than that on H441 cells in our study; it was also lower than H661 cells, H520 cells,16 SKOV3 cells,19 HL-60 cells,33 UM1 cells, UM2 cells, SCC-4 cells, SCC-9 cells,18 786-O cells, and A-498 cells35 from previous studies. Our results indicates that TSL-1 reveals a high efficiency on inhibiting cell viability of human osteosarcoma cells, which may be a choice as an adjuvant functional plant extract for human osteosarcoma.
There are some limitations in our study. First, the actual active constituents of TSL in this study were not identified. Gallic acid may be one of the active constituents for anti-osteosarcoma effect. Second, we found decreased expression of caspase-3 after TSL-1 treatment in vivo. There is decreased caspase-3 activity as the expression of caspase-3 decreases. Although there is a significant difference in active caspase-3 staining in histology, we did not stain the caspase-3 activity to further confirm the difference in caspase-3 activity.
In summary, we demonstrated that TSL-1, a fractionated extract from the TSL, caused significant cytotoxicity to osteosarcoma cells due to apoptosis 6 hours after treatment and both apoptosis and necrosis 24 hours after treatment. In vivo xenograft study showed that TSL-1 suppressed the growth of osteosarcoma cells at least in part by inducing apoptosis. Our results indicate that TSL-1 has the potential to be a promising anti-osteosarcoma adjuvant functional plant extract.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported in part by grants from the Kaohsiung Medical University (KMU-DK105009), Kaohsiung Medical University Hospital (KMUH102-2R36), Kaohsiung Medical University “Aim for the Top Universities Grant” (KMU-TP103B00, KMU-TP103B01, KMU-TP103B06, KMU-TP104B09), and Minister of Science and Technology of Taiwan (MOST 102-2314-B-037-021-MY2, MOST 102-2314-B-037-023-MY3, and MOST 104-2314-B-037-032-MY3).
References
- 1. Whelan J, McTiernan A, Cooper N, et al. Incidence and survival of malignant bone sarcomas in England 1979-2007. Int J Cancer. 2012;131:E508-E517. [DOI] [PubMed] [Google Scholar]
- 2. Linabery AM, Ross JA. Trends in childhood cancer incidence in the U.S. (1992-2004). Cancer. 2008;112:416-432. [DOI] [PubMed] [Google Scholar]
- 3. Luetke A, Meyers PA, Lewis I, Juergens H. Osteosarcoma treatment—where do we stand? A state of the art review. Cancer Treat Rev. 2014;40:523-532. [DOI] [PubMed] [Google Scholar]
- 4. Chou AJ, Geller DS, Gorlick R. Therapy for osteosarcoma: where do we go from here? Paediatr Drugs. 2008;10:315-327. [DOI] [PubMed] [Google Scholar]
- 5. Geller DS, Gorlick R. Osteosarcoma: a review of diagnosis, management, and treatment strategies. Clin Adv Hematol Oncol. 2010;8:705-718. [PubMed] [Google Scholar]
- 6. Smeland S, Bruland OS, Hjorth L, et al. Results of the Scandinavian Sarcoma Group XIV protocol for classical osteosarcoma: 63 patients with a minimum follow-up of 4 years. Acta Orthop. 2011;82:211-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Edmonds JM, Staniforth M. Toona sinensis: Meliaceae. Curtis’s Bot Mag. 1998;15:186-193. [Google Scholar]
- 8. Yang HL, Chen SC, Lin KY, et al. Antioxidant activities of aqueous leaf extracts of Toona sinensis on free radical-induced endothelial cell damage. J Ethnopharmacol. 2011;137:669-680. [DOI] [PubMed] [Google Scholar]
- 9. Hseu YC, Chang WH, Chen CS, et al. Antioxidant activities of Toona sinensis leaves extracts using different antioxidant models. Food Chem Toxicol. 2008;46:105-114. [DOI] [PubMed] [Google Scholar]
- 10. Yu WJ, Chang CC, Kuo TF, Tsai TC, Chang SJ. Toona sinensis Roem leaf extracts improve antioxidant activity in the liver of rats under oxidative stress. Food Chem Toxicol. 2012;50:1860-1865. [DOI] [PubMed] [Google Scholar]
- 11. Hsieh TJ, Tsai YH, Liao MC, et al. Anti-diabetic properties of non-polar Toona sinensis Roem extract prepared by supercritical-CO2 fluid. Food Chem Toxicol. 2012;50:779-789. [DOI] [PubMed] [Google Scholar]
- 12. Chen CJ, Michaelis M, Hsu HK, et al. Toona sinensis Roem tender leaf extract inhibits SARS coronavirus replication. J Ethnopharmacol. 2008;120:108-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang CJ, Chen YC, Tsai YJ, Huang MS, Wang CC. Toona sinensis leaf aqueous extract displays activity against sepsis in both in vitro and in vivo models. Kaohsiung J Med Sci. 2014;30:279-285. [DOI] [PubMed] [Google Scholar]
- 14. Yang S, Zhao Q, Xiang H, et al. Antiproliferative activity and apoptosis-inducing mechanism of constituents from Toona sinensis on human cancer cells. Cancer Cell Int. 2013;13:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang PJ, Hseu YC, Lee MS, et al. In vitro and in vivo activity of gallic acid and Toona sinensis leaf extracts against HL-60 human premyelocytic leukemia. Food Chem Toxicol. 2012;50:3489-3497. [DOI] [PubMed] [Google Scholar]
- 16. Yang CJ, Huang YJ, Wang CY, et al. Antiproliferative effect of Toona sinensis leaf extract on non-small-cell lung cancer. Transl Res. 2010;155:305-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang CJ, Huang YJ, Wang CY, et al. Antiproliferative and antitumorigenic activity of Toona sinensis leaf extracts in lung adenocarcinoma. J Med Food. 2010;13:54-61. [DOI] [PubMed] [Google Scholar]
- 18. Chia YC, Rajbanshi R, Calhoun C, Chiu RH. Anti-neoplastic effects of gallic acid, a major component of Toona sinensis leaf extract, on oral squamous carcinoma cells. Molecules. 2010;15:8377-8389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chang HL, Hsu HK, Su JH, et al. The fractionated Toona sinensis leaf extract induces apoptosis of human ovarian cancer cells and inhibits tumor growth in a murine xenograft model. Gynecol Oncol. 2006;102:309-314. [DOI] [PubMed] [Google Scholar]
- 20. Chen HM, Wu YC, Chia YC, et al. Gallic acid, a major component of Toona sinensis leaf extracts, contains a ROS-mediated anti-cancer activity in human prostate cancer cells. Cancer Lett. 2009;286:161-171. [DOI] [PubMed] [Google Scholar]
- 21. Chang HC, Hung WC, Huang MS, Hsu HK. Extract from the leaves of Toona sinensis roemor exerts potent antiproliferative effect on human lung cancer cells. Am J Chin Med. 2002;30:307-314. [DOI] [PubMed] [Google Scholar]
- 22. Hsu HK, Yang YC, Hwang JH, Hong SJ. Effects of Toona sinensis leaf extract on lipolysis in differentiated 3T3-L1 adipocytes. Kaohsiung J Med Sci. 2003;19:385-390. [DOI] [PubMed] [Google Scholar]
- 23. Su YF, Yang YC, Hsu HK, et al. Toona sinensis leaf extract has antinociceptive effect comparable with non-steroidal anti-inflammatory agents in mouse writhing test. BMC Complement Altern Med. 2015;15:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu SC, Chen CH, Chang JK, et al. Hyaluronan initiates chondrogenesis mainly via CD44 in human adipose-derived stem cells. J Appl Physiol (1985). 2013;114:1610-1618. [DOI] [PubMed] [Google Scholar]
- 25. Lee MJ, Chen HT, Ho ML, et al. PPARgamma silencing enhances osteogenic differentiation of human adipose-derived mesenchymal stem cells. J Cell Mol Med. 2013;17:1188-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morrison TB, Weis JJ, Wittwer CT. Quantification of low-copy transcripts by continuous SYBR Green I monitoring during amplification. Biotechniques. 1998;24:954-958. [PubMed] [Google Scholar]
- 27. Kang L, Chen CH, Wu MH, Chang JK, Chang FM, Cheng JT. 17beta-Estradiol protects against glucosamine-induced pancreatic beta-cell dysfunction. Menopause. 2014;21:1239-1248. [DOI] [PubMed] [Google Scholar]
- 28. Chen CH, Kang L, Lin RW, et al. (−)-Epigallocatechin-3-gallate improves bone microarchitecture in ovariectomized rats. Menopause. 2013;20:687-694. [DOI] [PubMed] [Google Scholar]
- 29. Demain A, Vaishnav P. Natural products for cancer chemotherapy. Microb Biotechnol. 2011;4:687-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nobili S, Lippi D, Witort E, et al. Natural compounds for cancer treatment and prevention. Pharmacol Res. 2009;59:365-378. [DOI] [PubMed] [Google Scholar]
- 31. Cragg G, Newman J. Nature: a vital source of leads for anticancer drug development. Phytochem Rev. 2009;8:313-331. [Google Scholar]
- 32. Fulda S. Modulation of apoptosis by natural products for cancer therapy. Planta Med. 2010;76:1075-1079. [DOI] [PubMed] [Google Scholar]
- 33. Yang HL, Chang WH, Chia YC, et al. Toona sinensis extracts induces apoptosis via reactive oxygen species in human premyelocytic leukemia cells. Food Chem Toxicol. 2006;44:1978-1988. [DOI] [PubMed] [Google Scholar]
- 34. Zhen H, Zhang Y, Fang Z, Huang Z, You C, Shi P. Toona sinensis and Moschus decoction induced cell cycle arrest in human cervical carcinoma HeLa cells. Evid Based Complement Alternat Med. 2014;2014:121276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen YC, Chien LH, Huang BM, Chia YC. Toona sinensis (aqueous leaf extracts) induces apoptosis through the generation of ROS and activation of intrinsic apoptotic pathways in human renal carcinoma cells. J Functional Foods. 2014;7:362-372. [Google Scholar]