Abstract
The Chinese medicine formula Tian Xian Liquid (TXL) has been used clinically for cancer therapy in China for more than 25 years. However, the comprehensive and holistic effects of its bioactive fractions for various antitumor therapeutic effects have not been unraveled. This is the first study to scientifically elucidate the holistic effect of Chinese medicine formula for treating colon cancer, hence allowing a better understanding of the essence of Chinese medicine formula, through the comparison of the actions of TXL and its functional constituent fractions, including ethyl acetate (EA), butanol (BU), and aqueous (WA) fractions. Tissue-specific proliferative/antiproliferative effects of these fractions on human colorectal carcinoma HT-29 cells and splenocytes were studied by using the MTT assay. Their modulations on the expression of markers of antiproliferation, antimetastasis, reversion of multidrug resistance in treated HT-29 cells were examined with real-time polymerase chain reaction and Western blot analysis, and their modulations in a xenografted nude mouse model were examined by Western blot analysis. Results revealed that EA fraction slightly inhibited the proliferation of HT-29 cells, but tissue-specifically exerted the most potent antiproliferative effect on splenocytes. On the contrary, only TXL and BU fraction tissue-specifically contributed to the proliferation of splenocytes, but inhibited the proliferation of HT-29 cells. WA fraction exerted the most potent antiproliferative effect on HT-29 cells and also the strongest inhibitory action on tumor size in the nude mouse model in our previous study. In the HT-29 model, TXL and WA fraction exerted the most pronounced effect on upregulation of p21 mRNA and protein; TXL, and EA and WA fractions exerted the effect on downregulation of G1 phase cell cycle protein, cyclin D1 mRNA and protein; EA and BU fractions exerted the most prominent anti-invasive effect on anti-invasion via downregulation of MMP-1 mRNA; TXL potently reversed most multidrug resistance via downregulation of MDR-1 protein. In conclusion, the comprehensive and holistic effects of TXL were demonstrated with (a) mutual accentuation and mutual enhancement, (b) mutual counteraction and mutual suppression, and (c) mutual antagonism among the 3 constituent fractions. Moreover, the design of the present study may lead to further development of more tissue-specific effective drugs with minimal side effects for clinical use in combating carcinoma.
Keywords: Chinese medicinal formula, holistic, Tian Xian Liquid, constituent fractions, antitumorigenicity, colon cancer
Introduction
Colon cancer is the third most common cancer globally nowadays,1 and its mortality rate ranked third worldwide.1 The incidence of colon cancer is increasing especially in Asia and Eastern Europe.1 Hong Kong, Japan, Singapore, and Taiwan are the 4 places in Asia with comparatively high incidence rates of colon cancer.2 The incidence rate in Hong Kong is the highest among all Chinese populations in southeast Asia.3 The incidence rate also ranked first in Hong Kong,4 Singapore,5 and Taiwan6 and second in Japan.7 The mortality rate ranked second in Hong Kong8 and Singapore,5 and third in Japan7 and Taiwan.6 In contemporary medicine, there are 4 types of conventional treatments for colon cancer, namely, surgery, chemotherapy, radiation therapy, and targeted therapy, depending on the stage of colon cancer.9,10 However, there are side effects accompanying these treatments, including low blood cell counts, diarrhea, fatigue, edema, and so on.11,12 Furthermore, and more important, the high lethal rate of colon cancer reveals that the effectiveness of the conventional treatment approaches, namely, surgery, radiotherapy, chemotherapy, and targeted therapy in curing the cancer is less than satisfactory. Because of the adverse effects of conventional therapeutic strategies, scientists have been seeking more effective anticancer agents from natural products, which can reverse the aberrant characteristics of carcinomas, including uncontrolled rapid growth, invasion, metastasis, and multidrug resistance.
Chinese medicine appears to be an attractive alternative. In fact, interest in the anticancer properties of traditional Chinese medicine has been gaining momentum in recent years.13 In daily life, Chinese medicine has been applied in treating various kinds of cancer in Chinese communities. Both clinical and scientific evidence showed that Chinese medicine is efficacious as supportive care for cancer patients.14,15 For colon cancer, clinical findings also showed that Chinese medicine was effective, particularly in adjuvant treatment with Western medicine, to increase the survival rate of patients and reduce the side effects of chemotherapy.16,17
The Chinese medicine formula of Tian Xian liquid (TXL), invented by Prof Wang Zhen Guo, was developed into a commercial product in 1991 as an anticancer dietary supplement without any adverse effect reported.18 Similar to many other Chinese medicinal formulas, TXL is composed of a number of medicinal herbs. It is an aqueous extract prepared from a mixture of Chinese medicinal herbs, which have been shown to possess antitumor activities, mainly Radix Ginseng (Panax ginseng CA Mey), Cordyceps (Cordyceps sinensis (Berk) Sacc), Radix Astragali (Astragalus membranaceus (Fisch) Bge), Radix Glycyrrhizae (Glycyrrhiza uralensis Fisch), Rhizoma Dioscorea (Dioscorea opposita Thunb), Margarita (Pteria martensii (Dunker)), Fructus Lycii (Lycium barbarum L.), Ganoderma Lucidum (Ganoderma lucidum (Leyss Ex Fr) Karst), Fructus Ligustri Lucidi (Ligustrum lucidum Alt), and Herba Scutellariae Barbatae (Scutellaria barbata D Don). It has been reported that TXL exerted an inhibitory effect on various carcinomas.18-22 Moreover, it was demonstrated in a randomized, double blinded, placebo-controlled, parallel group study in patients suffering from refractory metastatic breast cancer that oral administration of TXL was safe without inducing any severe adverse effects on the patients, and was effective in enhancing the quality of life, physical and emotional well-being, and cognitive functioning, reducing fatigue and side effects of systemic therapy, as well as showing immune-modulating effects on lymphocytes in the patients.23 As each herb in a Chinese medicinal formula is known to carry out distinctive functions, the combination of different chemical compounds in the constituent herbs composing TXL may work in a comprehensive and holistic manner, in which they may not only collectively contribute to the diverse pharmacological effects of the formula but also simultaneously offset the adverse effects of some components. However, there are no studies investigating the mechanisms how different chemical components of TXL contribute to the therapeutic effects.
From our previous studies on the quality control of TXL, antitumor effect and antimetastatic mechanism,20-22 it has been unveiled that TXL inhibited the proliferation of HT-29 colon cells via G1 phase arrest in the cell cycle. The positive cell cycle regulator, cyclin D1, was downregulated and the negative cell cycle regulator, cyclin-dependent kinase inhibitor p21, was upregulated. The potential of TXL to reduce cancer invasion and metastasis by downregulation of matrix metalloproteinase-1 (MMP-1), MMP-2, and MMP-9 was also demonstrated in our study.24 TXL potently reversed multidrug resistance in a HT-29 xenograft model via downregulation of MDR-1.22 Besides, TXL and its fractions (viz, butanol [BU], ethyl acetate [EA], and aqueous [WA] fractions) have also elicited a significant decrease in tumor size in the nude mouse model of colorectal cancer after 14 to 16 days of treatment in our study. TXL and the fractions all produced a significant inhibition of tumor size after 16 days of treatment when compared with the control (CTL): (CTL vs TXL: *P < .05; **P < .01; CTL vs WA: P < .05; P < .01; CTL vs BU: P < .001; by paired t test). EA showed a gradual increase in tumor size after 14 days of treatment, though its effect was still smaller than that of the control (CTL vs EA: #P < .05; ##P < .01; ###P < .001; by paired t test), indicating that the antitumor effect of EA was only a temporary effect. Besides, there was no significant change in the body weight of all the treated animal bearing colorectal xenografts when compared with the control, implying that all of the drugs had no in vivo toxicity.20
Because of the complexity of chemical profiles in a traditional Chinese medicine (TCM) formula, it is difficult to scientifically understand its comprehensive and holistic effects. This is the first study aimed at elucidating the holistic effect of TCM by unveiling the differentiated actions and mechanisms of 3 functional constituent fractions of TXL (viz TXL–ethyl acetate fraction [EA], TXL-butanol fraction [BU], and TXL-aqueous fraction [WA]). Tissue-specific proliferative or antiproliferative activity of TXL toward human colorectal carcinoma and splenocytes was demonstrated by the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. In order to elucidate the contribution of holistic effects of TXL, transcriptional and translational levels of p21, cyclin D1, MMP1, and MDR-1 in vitro and in vivo after treatment with TXL and its 3 functional constituent fractions were examined by real-time polymerase chain reaction (PCR) and Western blot analysis. Results obtained from this study can provide scientific evidence for understanding the holistic philosophy of a TCM formula that strongly supports the clinical use of the TCM formula for cancer treatment; and establish an antitumorigenicity drug screening platform for further development of functional constituent fractions or/and ingredients derived from TCM as effective anticancer agents.
Materials and Methods
Herbal Materials and Fractionation
The sample of TXL, a commercially available Chinese medicine decoction, was provided by China-Japan Feida Union Company Limited. The quality control study has been reported in our previous publications.20-22 It is an aqueous extract of a Chinese herbal mixture that consists mainly of 10 Chinese medicinal herbs: 12.5% Radix Ginseng, 24% Cordyceps, 15% Radix Astragali, 5% Radix Glycyrrhizae, 11% Rhizoma Dioscorea, 4% Margarita, 9% Fructus Lycii, 17% Ganoderma lucidum, 0.5% Fructus Ligustri Lucidi, and 2% Herba Scutellariae Barbatae. The sample was kept in the School of Chinese Medicine, the University of Hong Kong as previously described.20-22
One hundred milliliters of TXL aqueous sample were partitioned with ethyl acetate and butanol, successively as described in our previous study.20 The TXL–ethyl acetate fraction (EA), TXL-butanol fraction (BU), and TXL-aqueous fraction (WA) were collected separately in different bottles. The solvents in EA, BU, and WA fractions were evaporated and the fractions were then dissolved in 100 mL of milli-Q water, respectively, resulting in the same amount of components in each fraction as that in the same volume of the original TXL. All samples were stored at 4°C for subsequent use.20
Quality Control and High-Performance Liquid Chromatography
To evaluate the quality consistency of TXL and its constituent fractions, 2 batches of 10-mL liquid samples were extracted with 50 mL 20% methanol/H2O. The mixture was mixed well and subjected to sonication for 30 minutes. The extracted mixture was centrifuged at 5000 rpm for 15 minutes and the supernatant was retained. The supernatant was filtered by using 0.45 μm Millex Syringe filter unit and injected in a volume of 10 μL for high-performance liquid chromatography (HPLC). The experiment was repeated 3 times for each batch. A reverse-phase column XBridge C18 Column, 5 µm, 4.6 × 250 mm (part no. 186003117) (Waters Corporation, Milford, MA, USA) was used with XBridge C18 Guard Column, 5 µm, 4.6 × 20 mm (part no. 186003064) (Waters Corporation, Milford, MA, USA). The mobile phase consisted of acetonitrile (solvent A) and 0.2% acetic acid (solvent B) using a gradient program of 1-5% solvent A in 0 to 20 minutes, 5% to 25% solvent A in 20 to 30 minutes, 25% to 60% solvent A in 30 to 50 minutes, 60% to 80% solvent A in 50 to 60 min, and constant 80% A in 60 to 65 minutes. A preequilibration period of 15 minutes was used between individual runs. The flow rate was 1.0 mL/min. A diode array detector was set at 275 nm for obtaining chromatograms with a maximum number of peaks. Ultraviolet spectra were obtained from 200 to 400 nm. Chromatogram and peak integration were analyzed by the Waters Empower Software (Waters Corporation, Milford, MA, USA).
Cell Culture
Human colorectal carcinoma HT-29 cells (HTB-38, ATCC, Rockville, MD, USA) were cultured in RPMI-1640 medium (Hyclone, Logan, UT, USA), supplemented with 10% fetal bovine serum (Hyclone, Logan, UT, USA) 1% bovine serum albumin, 100 IU/mL penicillin and 100 mg/mL streptomycin sulfate (Hyclone, Logan, UT, USA). Mouse splenocytes were obtained from male inbred BALB/c mice as described in our previous publication.25 Spleens of the mice were aseptically removed after they had been sacrificed by cervical dislocation. Spleen cells were isolated by pressing the tissue through a sterilized 100-mesh stainless steel sieve and resuspended to 5 × 106 cells/mL in RPMI-1640 culture medium supplemented with 10% fetal bovine serum, 100 units penicillin/mL, and 100 µg streptomycin/mL. All cells were incubated at 37°C in a humidified 5% CO2 incubator.
Cell Proliferation Assay (MTT Assay)
HT-29 cells were cultured in 96-well plates at the density of 1 × 104 cells/0.1 mL/well. Cells were serum-starved for 24 hours and then incubated with a series of concentrations (0.157-2.5 v/v %) of TXL and its constituent fractions (EA, BU, and WA) in triplicates for an additional 24 hours at 37°C in a humidified 5% CO2 incubator. Mouse splenocytes were cultured at 4 × 105 cells/0.1 mL/well in 96-well culture plates in the presence of a series of concentrations of TXL and its constituent fractions in triplicates for 48 hours at 37°C in a humidified 5% CO2 incubator. The procedure of cell proliferation was carried out in accordance with recommendations of the manufacturer (Cell Proliferation Kit I, Roche Applied Science, Madison, WI, USA). All reported values are the means of triplicate samples. IC50 (half-maximal inhibitory concentration) was calculated by SigmaStat Statistical Analysis Software.
Drug Treatment on HT-29 Cells
HT-29 cells were cultured in 35-mm dishes at the density of 1 × 106 cells/well. Cells were serum-starved for 24 hours26 and then treated with 1% (v/v) TXL, 1% (v/v) EA, 1% (v/v) BU, and 1% (v/v) WA, respectively, for an additional 8, 15, and 24 hours. At the end of each time point, cells were harvested by trypsinization for the subsequent RNA and protein extractions. The selection of time points at 8, 15, and 24 hours was based on the phase distribution at a series of time points analyzed by flow cytometry in our previous publication,22 in which the start of G1 phase, S phase, and G2/M phase in untreated HT-29 cells were at 8, 15, and 24 hours, respectively.
Real-Time PCR of TXL-Treated HT-29 Cells
The total RNA of HT-29 cells treated with 1% (v/v) TXL, 1% (v/v) EA, 1% (v/v) BU, and 1% (v/v) WA as well as that of control cells was extracted after incubation for 8, 15, and 24 hours using a High Pure Isolation kit (Roche Applied Science, Madison, WI, USA). Total RNA (2 μg) of each sample was reverse-transcribed into cDNA with Oligo-dT primers using a Revert First strand cDNA synthesis kit (Fermentas, Canada). Primer pairs and probes for real-time PCR were designed by the Assay Design Centre of the Universal Probe Library (Roche Applied Science, Madison, WI, USA) except those of MMP-1. The target genes included p21 (NM_000389.3; 112nt; Forward: ccgaggcactcagaggag; Reverse: agctgctcgctgtccact; Probe ID: #70, cat. no. 04688937001) and cyclin D1 (ENST00000227507.1|ENSG00000110092.1; 61nt; Forward: gaagatcgtcgccacctg; Reverse: gacctcctcctcgcacttct; Probe ID: #67, cat. no. 04688660001). TaqMan Gene Expression assays (Applied Biosystems, Carlsbad, CA, USA) for MMP-1 were used in a separate experiment (Assay ID: Hs00899658_m1; 64nt). GAPDH (Universal ProbeLibrary Human GAPD Gene Assay, cat. no. 05190541001) or Human GAPD (GAPDH) Endogenous Control (FAM / MGB Probe, Non-Primer Limited, part no. 4333764F) was included as the internal control. Real-time PCR was carried out in a 384-multiwell plate of LightCycler 480 system (Roche Applied Science, Madison, WI, USA). After the experiment, the normalized mRNA level was calculated by LightCycler 480 Software 1.5.0 release SP3.
Drug Administration to Tumor-Bearing Nude Mice and Collection of Tumor Specimens
The experiment had been approved by the Department of Health, Hong Kong SAR and Committee on the Use of Live Animals in Teaching and Research (CULATR) of Li Ka Shing Faculty of Medicine, the University of Hong Kong. Five-week-old female nude mice from the Laboratory Animal Unit, the University of Hong Kong, were employed as the in vivo model. The mice were kept under sterile conditions in isolated pathogen-free ventilation chambers at an ambient temperature of 22°C to 24°C and 50% to 65% relative humidity with automatic 12-hour light:dark illumination cycles. A cell suspension was obtained by trypsinization of confluent HT-29 cells. The HT-29 carcinoma was established subcutaneously in nude mice by injecting 1 × 105 cells into the right thigh of each animal. When the tumors became palpable (size attaining 18 mm3) after xenografting, the mice were randomly divided into 5 groups of 6 animals each. The recommended dosage of TXL in human is 40 mL per day for cancer patients at early and middle stage and 60 mL per day for cancer patients at the terminal stage of cancer patients. Taking into account the average of these 2 recommended dosages of TXL, the human equivalent dose (200 µL) of TXL, EA, BU, and WA was administered orally for 16 consecutive days.20,22 The control group received orally an equal volume of water instead. At the end of the experiment, all animals were sacrificed by cervical dislocation. Their tumor specimens were collected and stored at −80°C for further analysis.
Western Blot Analysis of TXL-Treated HT-29 Cells and Excised Tumor Specimens
HT-29 cells that had been treated with 1% (v/v) TXL (IC50) and 1% of its constituent bioactive fractions EA, BU, and WA, were lysed after 8, 15, and 24 hours of incubation, using 300 µL RIPA (radioimmunoprecipitation assay) buffer (Sigma-Aldrich, St Louis, MO, USA). Protein in the lysate was retained. Tumors excised from the nude mice were homogenized by grinding in liquid nitrogen. Protein was extracted by adding around 300 µL RIPA buffer. Protein concentration was determined using the Bio-Rad protein assay (Bio-Rad, Richmond, CA, USA). The denatured total protein (15 µg) was separated on 12% sodium dodecyl sulfate–polyacrylamide gels, and the resolved proteins were transferred to a polyvinylene difluoride (PVDF) membrane (Roche Diagnostics Corporation, Indianapolis, IN, USA). The membrane was blocked with 5% bovine serum albumin for 1 hour at room temperature, then incubated overnight with a primary antibody specifically recognizing one of the following p21 (cat. no. 05655), cyclin D1 (cat. no. 06-137), MMP-1 (cat. no. MAB3307), or MDR-1 (cat. no. MAB3307) at 4°C. All antibodies were purchased from Millipore. The membrane was then washed with TBS-T 3 times, and incubated with horseradish peroxidase (HRP)–conjugated secondary antibodies (Milipore, Billerica, MA, USA) in TBS-T buffer for 1 hour. Chemiluminescence detection was performed using the Advanced Chemiluminescence Western blotting detection system (GE Healthcare, London, UK). The band intensities were quantified by a Bio-Rad Chemi Doc EQ densitometer and Bio-Rad Quantity One software (Bio-Rad Laboratories, Hercules, CA, USA) and normalized by anti-GADPH (cat. no. MAB374) (Millipore, Billerica, MA, USA). Antibodies were stripped by using the Restore Western Blot Stripping Buffer (Thermo Fisher Scientific Inc, Waltham, MA, USA) and reprobed with other antibodies as described above. For the photograph of ECL detection, the representative insert with its corresponding GAPDH at the same trial that best matches the mean relative intensity have been selected as shown in the result figures.
Statistical Analysis
Comparison of antiproliferative effects of TXL and its fractions at each dosage on HT-29 cells with individual control was carried out after analysis by Student’s t test. Comparison of proliferation of drug-treated mouse splenocytes treated with corresponding control was carried out after analysis by Student’s t test. The normalized intensities of the respective protein markers were reported as mean ± standard error of the mean. All comparisons were statistically analyzed by unpaired t test. For normalized mRNA expression of the respective genes, the data are presented as log (mean fold differences) ± standard error of the mean. Comparison with control at 0 hours was done by using one sample t test. Comparison of treatment groups with the control at the corresponding time point was done by using unpaired t test. Statistical analysis was conducted by GraphPad Prism 4 for Windows version 4.
Results
Quality Control
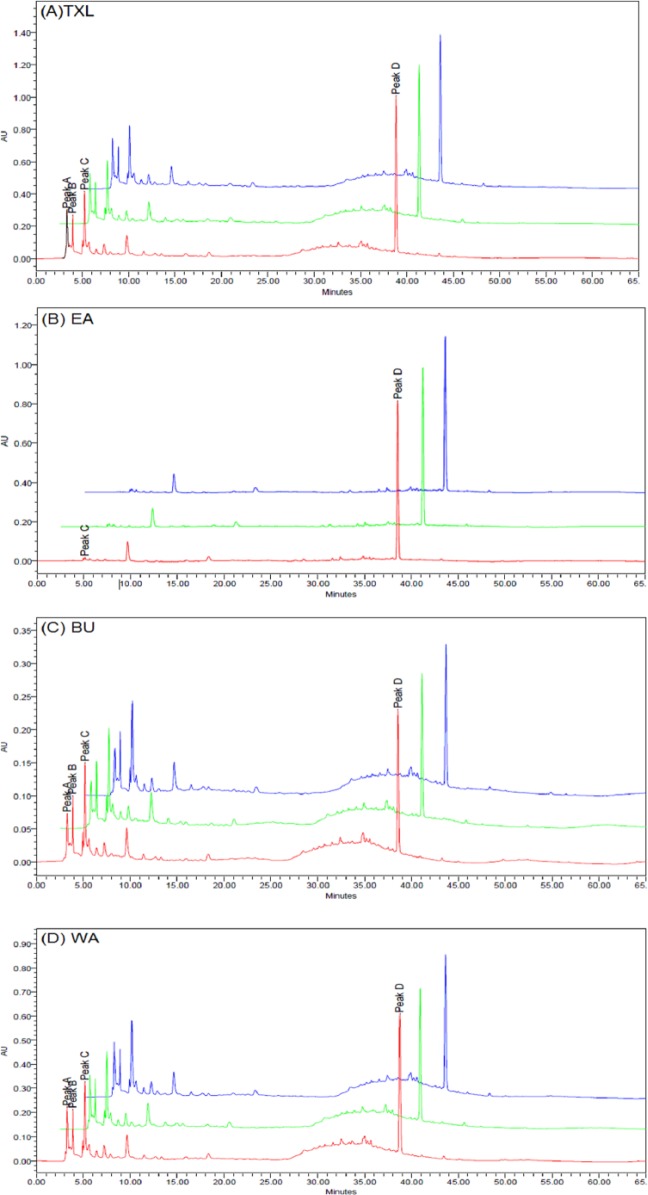
After investigation of the spectra of all eluted peaks with photodiode array detection, chromatograms were generated at the detection wavelength of 275 nm for exploring the most conspicuous peaks. The chromatographic fingerprints showing the elution peaks derived from TXL and the constituent fractions are presented in Figure 1. Two batches (n = 3 each) of samples were examined by HPLC using the aforementioned conditions. The peak areas of 4 common major peaks in the chromatograms of TXL, EA, BU, and WA, were assessed. The results are shown in Table 1. Interassay relative standard deviation (RSD) values were less than 5% for peak area of the peaks selected. This indicated that the composition of each fraction was consistent between injections.
Figure 1.
Overlaid high-performance liquid chromatography (HPLC) fingerprints of different batches of (A) Tian Xian Liquid (TXL), (B) ethyl acetate fraction of TXL (EA), (C) butanol fraction of TXL (BU), and (D) aqueous fraction of TXL (WA) sample extracts monitored at 275 nm. Four peaks (A-D) were denoted on the chromatograms.
Table 1.
The Contents of Peak A to D in Different Batches of TXL and Its Constituent Fractions (EA, BU, WA).
| Peak Areaa |
||||
|---|---|---|---|---|
| Peak A | Peak B | Peak C | Peak D | |
| TXL-1 | 3236014 | 2308755 | 4535332 | 10842957 |
| TXL-2 | 3283011 | 2153480 | 4311943 | 10881620 |
| TXL-3 | 3271133 | 2186308 | 4387149 | 10146040 |
| Mean | 3263386 | 2216181 | 4411475 | 10623539 |
| RSD (%) | 0.748839 | 3.692589 | 2.576549 | 3.896798 |
| EA-1 | Nil | Nil | 222102 | 8246091 |
| EA-2 | Nil | Nil | 205591 | 8198175 |
| EA-3 | Nil | Nil | 208576 | 8171840 |
| Mean | Nil | Nil | 212089.7 | 8205369 |
| RSD (%) | Nil | Nil | 4.148456 | 0.45878 |
| BU-1 | 795076 | 702134 | 1517039 | 2220761 |
| BU-2 | 789367 | 693180 | 1546567 | 2188597 |
| BU-3 | 771114 | 673635 | 1453866 | 2181869 |
| Mean | 785185.7 | 689649.7 | 1505824 | 2197076 |
| RSD (%) | 1.594052 | 2.113218 | 3.144934 | 0.946081 |
| WA-1 | 2259612 | 1755923 | 3437135 | 7041986 |
| WA-2 | 2143760 | 1626801 | 3243499 | 6968758 |
| WA-3 | 2240035 | 1605498 | 3343815 | 6984375 |
| Mean | 2214469 | 1662741 | 3341483 | 6998373 |
| RSD (%) | 2.800364 | 4.895423 | 2.898086 | 0.551109 |
Abbreviations: TXL, Tian Xian Liquid; RSD, relative standard deviation; EA, ethyl acetate fraction of TXL; BU, butanol fraction of TXL; WA, aqueous fraction of TXL.
The unit of peak area is µV·s.
MTT Assay on HT-29 Cells and Mouse Splenocytes
MTT assay was employed to assess the cytotoxicity of TXL and its constituent fractions on HT-29 cells (Figure 2) as well as mouse splenocytes (Figure 3). The IC50 values of TXL, EA, BU, and WA on HT-29 cells were found at the concentration of 1.0% (v/v), 1.4% (v/v), 0.7% (v/v), and 0.2% (v/v), respectively (Figure 2).
Figure 2.
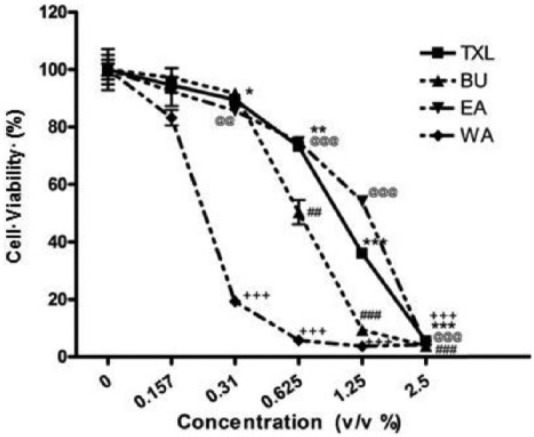
Proliferative Assay of Tian Xian Liquid (TXL) and its constituent fractions on HT-29 cells at different concentrations after 24 hours of treatment as revealed by the MTT assay. Results are presented in mean ± SEM (n = 3). Student’s t test was performed to compare the antiproliferative effect of each dosage with individual control (%). EA, ethyl acetate fraction of TXL; BU, butanol fraction of TXL; WA, aqueous fraction of TXL.
In the TXL-treated cells, corresponding dosage versus control (0%): *P < .05; **P < .01; ***P < .001.
In the BU-treated cells, corresponding dosage versus control (0%): #P < .05; ##P < .01; ###P < .001.
In the EA-treated cells, corresponding dosage versus control (0%): @P < .05; @@P < .01; @@@P < .001.
In the WA-treated cells, corresponding dosage versus control (0%): +P < .05; ++P < .01; +++P < .001.
Figure 3.
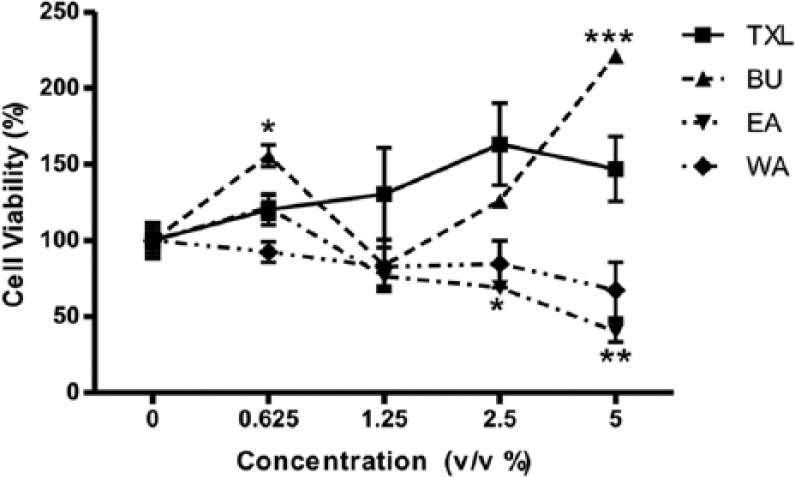
Effect of Tian Xian Liquid (TXL) and its constituent fractions on proliferation of mouse splenocytes at different concentrations after 24 hours of treatment as revealed by the MTT assay. Results are presented in mean ± SEM (n = 3). Student’s t test was performed to compare the proliferative effect of each dosage with individual control (0%). EA, ethyl acetate fraction of TXL; BU, butanol fraction of TXL; WA, aqueous fraction of TXL.
In each type of treated cells, corresponding dosage vs control (0%): *P < .05; **P < .01; ***P < .001.
The results in Figure 3 revealed that the proliferation of BU-treated splenocytes increased significantly compared with the control at a BU dose of 5% (v/v). In contrast to the dose-dependent proliferation of splenocytes in, response to TXL, a decreasing trend was observed in WA-treated splenocytes, whereas the viability of splenocytes started to decrease significantly at a dose of 2.5% (v/v) EA. Besides, a similar effect on the proliferaton of splenocytes was also found at the respective concentration of IC50 for TXL and its fractions. That is to say, splenocytes exposed to TXL (1% v/v) and BU (0.7% v/v) underwent proliferation. On the contrary, there was antiproliferative action of EA (1.4% v/v) and WA (0.2% v/v) on splencoytes.
Quantitative Real-Time PCR Analysis of mRNA in HT-29 Cells
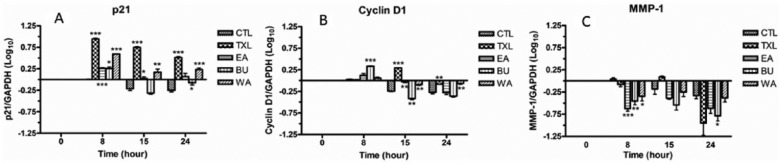
Quantitative real-time PCR analysis was used to evaluate the mRNA expression of p21, cyclin D1, and MMP-1 (Figure 4).
Figure 4.
mRNA expression of p21 (A), cyclin D1 (B), and MMP-1 (C) in treated HT-29 cells at different time points. The quantification is relative to that of control at 0 hours. The results are expressed as mean fold difference ± standard error of the mean. Comparison with control 0 hours was done by one sample t test. Comparison of treated cells with the control of corresponding time point was done by unpaired t test. CTL, control group.
CTL of corresponding time point vs treated cells: *P < .05; **P < .01; ***P < .001.
The mRNA expression of p21 in TXL- and WA-treated HT-29 cells was significantly elevated at all time points, with TXL exhibiting a greater stimulating effect. The mRNA expression of p21 in EA -treated HT-29 cells was significantly higher than the control at 8 and 15 hours. BU demonstrated a significant increase in mRNA of p21 only at 8 hours (Figure 4A).
Regarding the mRNA of cyclin D1, there was a significant decrease in the control at 15 and 24 hours. There was a significant increase in TXL-treated cells at 15 hours and a significant decrease at 24 hours, as compared with the control at the same time points, but a significant decrease at 24 hours, compared with the control at the corresponding time point. The mRNA of cyclin D1 in BU-treated cells increased significantly at 8 hours but decreased significantly at 15 hours. On the other hand, the decrease was to a significantly lesser extent in WA-treated cells at both 15 and 24 hours, compared with the control at the respective time points (Figure 4B).
The mRNA level of MMP-1 in TXL-treated cells decreased at 8 and 24 hours compared with the corresponding control, although the level rose slightly at 15 hours. A decrease was observed in EA-treated cells at all time points compared with the control at corresponding time points, with statistical significance shown at 8 hours. A significant decrease of MMP-1 mRNA was observed only at 8 hours for WA and at 8 and 24 hours for BU compared with the corresponding control, though a decline found at all time points (Figure 4C).
Western Blot Analysis of TXL-Treated HT-29 Cells and Excised Tumor Specimens
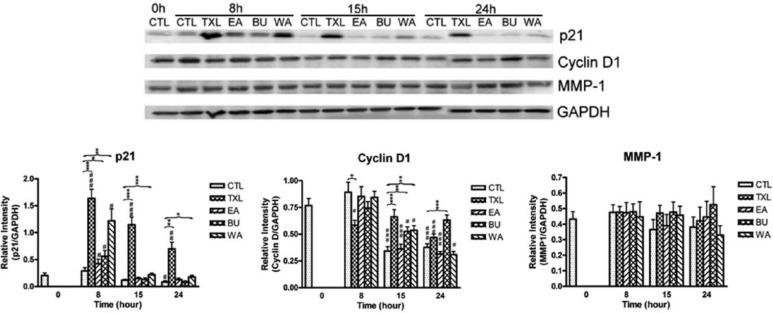
Western blot analyses in vitro and in vivo were carried out with biomarkers p21, cyclin D1 (in vitro only), MMP-1 and MDR-1 (in vivo only). The results are shown in Figures 5 and 6, respectively.
Figure 5.
Protein expression of p21, cyclin D1, and MMP-1 in treated HT-29 cells at different time points. The results are expressed as mean relative intensity ± standard error of the mean (n = 3). Comparison was done by using unpaired t test. CTL, control group.
CTL 0 hours versus treated cells: #P < .05; ##P < .01; ###P < .001.
CTL of corresponding time point versus treated cells: *P < .05; **P < .01; ***P < .001.
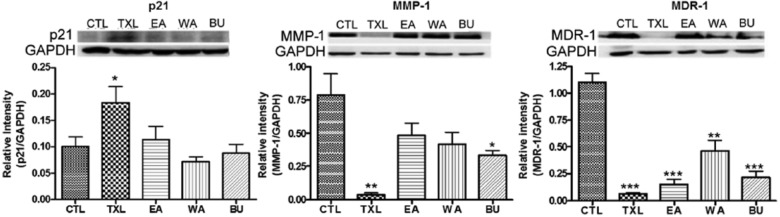
Figure 6.
Protein expression of p21, MMP-1, and MDR-1 in tumor excised from nude mouse model (n ≥ 3). The results are expressed as mean relative intensity ± standard error of the mean. Comparison was done by unpaired t test. CTL, control group.
CTL versus treated group: *P < .05; **P < .01; ***P < .001.
Compared with the corresponding control, TXL treatment significantly upregulated the protein level of the negative cell cycle regulator p21 after 8, 15, and 24 hours of treatment in vitro (Figure 5). The effects of its fractions were less prominent, with a significant upregulation by BU and WA of p21 in vitro observed only at 8 hours after treatment. The upregulation persisted in that of WA up to 24 hours. The protein level of cyclin D1 at 8 hours after TXL treatment decreased significantly in vitro but not after treatment with its constituent fractions. The level of cyclin D1 in TXL-, BU- and WA-treated cells was significantly higher than the control at 15 hours, while the effect persisted in BU-treated cells up to 24 hours. On the other hand, the protein level of MMP-1 showed no significant differences in all treated cells at all time points.
The protein expression of p21 after TXL treatment, but not after treatment with its fractions, was also significantly increased in vivo (Figure 6). TXL could significantly suppress the protein level of MMP-1 in vivo, while a similar effect was observed in the BU fraction–treated group, albeit to a lesser extent. MMP-1 levels in those of EA fraction– and WA fraction–treated groups were reduced but the declines were not statistically significant. For the effect on multidrug resistance in vivo, both TXL and its various constituent fractions could inhibit MDR-1 protein level, and the effect was most obvious after TXL treatment.
Discussion
To evaluate the consistency in quality of TXL and its constituent fractions, different batches of TXL, and EA, BU, and WA fractions were analyzed by HPLC. The peaks of the chromatograms were detected at 275 nm and the peak area of the selected peaks were assessed (Table 1). Our results showed that the relative standard deviations of the selected peaks were less than 5% among different batches of sample, indicating the consistency in quality among different batches of TXL and its constituent fractions. Thus the influences of the variation in the subsequent bioactivity investigation due to instability of the composition of the extracts can be excluded in this study.
Our previous studies demonstrated that TXL possessed antitumorigenicity by virtue of its antiproliferative and antimetastatic properties, and ability to revert multidrug resistance.20-22 TXL was able to upregulate the negative cell cycle regulator p21, which inhibits the activity of cyclin-dependent kinase/cyclin complex27,28 and downregulates D-type cyclin, resulting in G1 phase arrest.29-33 TXL has also been demonstrated to suppress the enzyme MMP-1 involved in cancer cell invasion and metastasis,34-37 thus exhibiting antimetastatic potency. Reversion of multidrug resistance was also revealed by downregulation of MDR-1 proteins.38-40 This finding adds value to the potential use of TXL as an antitumorigenic supplement since MDR-1 is one of the key mechanisms for impedance of chemotherapy.41 However, up till now, no study demonstrated the comprehensive and holistic effects of TCM formula on antitumorigenicity, including tissue-specific antiproliferative effect, using the TCM theory of “mutual accentuation (相須) and mutual enhancement (相使), mutual counteraction (相殺) and mutual suppression (相畏), and mutual antagonism (相惡) and mutual incompatibility (相反).”42
Result from antiproliferative effects of TXL, BU fraction and EA fraction (Figures 2 and 3) revealed their antiproliferative action in a tissue-specific manner. BU fraction exerted an antiproliferative effect on HT-29 cells but a proliferative effect on mouse splenocytes which facilitates immune function. On the contrary, EA fraction exerted an antiproliferative action on mouse splenocytes but to a much lesser extent on HT-29 cells. WA exerted an antiproliferative action on both types of cells. Therefore, BU fraction may be the most effective bioactive fraction for further studies in order to better understand the tissue-specific action mechanism of TXL.
Mutual Accentuation and Mutual Enhancement
In this study, mutual accentuation and mutual enhancement were evidenced by the expression of p21, MMP-1, and MDR-1 in response to TXL and its functional constituent fractions. Results obtained from TXL and its constituent fractions indicated that the upregulation of in vivo p21 at the translational level after TXL treatment was the most prominent (Figure 6), and a significant increase of in vitro p21 mRNA levels was evoked after 8 hours of treatment, but this upregulation in cells treated with EA, BU, and WA fractions occurred to a much lesser extent compared with TXL-treated cells (Figures 4 and 5), which implies that these 3 fractions may together contribute to the effect of TXL on the expression of p21 in the manner of mutual accentuation and/or mutual enhancement that promote the medicinal efficacy of a medicinal formula.43 Besides p21, the extent of inhibition of in vivo metastatic protein MMP-1 and the suppression of multidrug resistance protein MDR-1 in TXL-treated group was the greatest among all groups (Figure 6). This further supports the mutual accentuation and/or mutual enhancement functions of the action of components in the TXL formula, in which constituents with different properties work in concert to facilitate the medicinal efficacy of the entire medicinal formula.
Mutual Counteraction and Mutual Suppression
Mutual counteraction and mutual suppression were demonstrated in the effects of TXL and its 3 functional constituent fractions on the proliferative effect of mouse splenocyctes (Figure 3). TXL and its BU fraction exerted a proliferative action toward splenocytes in a dose-dependent manner, while treatment with EA and WA fractions showed an antiproliferative effect on splenocytes, which is an adverse effect against the therapeutic purpose toward colorectal carcinoma (Figure 3). According to the holistic concept in TCM, it appears that some constituents in BU fraction interact with those in WA and EA fractions to reduce the cytotoxic effect of WA and EA fractions against splenocytes, so as to bring along an overall proliferative action toward splenocytes in TXL. This is the concept of mutual counteraction and mutual suppression in TCM theory, meaning that some constituents reduce or eliminate the adverse effects or toxicity arising from the other constituents.43 While the effect of BU fraction counteracted those of WA and EA fractions (mutual counteraction), the effect of these 2 fractions were suppressed by that of BU fraction (mutual suppression).
Mutual Antagonism
In this study, mutual antagonism was illustrated in the effects of TXL and its constituent fractions on antiproliferative activity toward HT-29 cells, and downregulation in cyclin D1 and MMP-1 protein expression in vitro. In mutual antagonism, based on TCM theory, constituent herbs in a medicinal formula interact to reduce or eliminate their therapeutic effects.43 Results obtained from MTT assay demonstrated that TXL and all its constituent fractions could inhibit the proliferation of HT-29 in vitro (Figure 2). Treatment with BU and WA fractions more effectively inhibited the proliferation of HT-29 cells in vitro (Figure 2) compared with TXL. Although this overall antiproliferative effect of TXL is statistically significant, it is less potent than the respective antiproliferative effect of WA and BU fractions (Figure 2). Similarly, EA fraction also curtailed the inhibitory effect of BU and WA fractions on tumor size of the nude mouse model in our previous study.20 Mutual antagonism was also shown in the downregulation of cyclin D1 mRNA in 3 fractions-treated HT-29 cells at the 15-hour time point (Figure 4). It was found in the in vitro study that BU fraction downregulated cyclin D1 to a greater extent than the other fractions at the 15-hour time point. It was followed by EA and WA, whereas EA and WA fractions work antagonistically against the effect of BU fraction, through reducing the effect of BU fraction, leading to the effect in the upregulation of cyclin D1 mRNA expression as shown in the treatment with TXL. This may be attributed to mutual antagonism. It is worth noting that the downregulation of cyclin D1 protein expression in vitro was mainly attributed to BU fraction at 24 hours (Figure 5), resulting in an antagonistic action, which brought about downregulation of cyclin D1 protein in the TXL formula. As reported by Musgrove et al,44 cyclin D1 overexpression was found in 55% of colon cancer cases. Several preclinical studies supported that pan-CDK inhibitors work synergistically with cytotoxic agents, such as 5-fluorouracil and cisplatin against cancer cells.44 Since TXL formula and its BU fraction can downregulate cyclin D1, it is worthwhile to further study if TXL and/or its fraction may help develop an alternative adjuvant in chemotherapy. For MMP-1 expression in the in vitro study, treatment with EA, BU, and WA fractions significantly reduced MMP-1 mRNA expression in HT-29 cells after 8 hours of treatment, while the significant reduction persisted till 24 hours only in the treatment with BU fraction (Figure 4). It seems that BU fraction mainly contributed to the effect of TXL in reducing MMP-1 expression, whereas EA as well as WA fractions work antagonistically against the effect of BU fraction, through diminishing the effect of BU fraction, leading to the resultant effect in the reduction of MMP-1 mRNA expression as shown in the treatment with TXL. On the other hand, none of the treatments reduced the protein level of MMP-1 at all time points (Figure 5). It might be due to the fact that protein expression is a later event compared with mRNA expression, which is an earlier event, or due to some posttranslational modification and regulation. However, further study has to be performed to explain such discrepancy. Anyhow, the results indicating the attenuation of MMP-1 mRNA expression in HT-29 cells after treatment for 24 hours illustrates mutual antagonism, in the sense of holistic philosophy in TCM.
In summary, the overall antitumor effect of TXL is a combination of mutual accentuation and mutual enhancement, mutual counteractive and mutual suppressive, and mutual antagonistic actions among constituents in EA, BU, and WA fractions. The findings in this study support the holistic philosophy of Chinese medicine. However, evidence supporting mutual incompatibility interaction was not shown in this study, which may not exist in TXL. Moreover, each constituent fraction plays an indispensable role in the therapeutic effects of the entire TXL formula. As shown in Table 2, WA fraction was the most potent in exerting antiproliferative effect on HT-29 cells in vitro, and also inhibiting tumor size as was shown in our previous study.20 BU fraction contributed the most in the proliferation of splenocyctes in vitro, while its action on suppressing the mRNA level of cyclin D in vitro and protein expression of MMP-1 in vivo was the strongest among all constituent fractions. The upregulation of p21 in vivo, and the downregulation of MDR-1 in vivo were mutually attributed to the effect exerted by 3 functional fractions.
Table 2.
Antitumorigenicity of Tian Xian Liquid (TXL) Exhibited as a Result of Interactions Among Different Fractions of TXL.a
| Fraction | Proliferative Effect |
Inhibition of Tumor size In Vivo (Chu et al., 2011) | Upregulation of p21 Protein In Vivo (Figure 6) | Downregulation of MMP-1 Protein In Vivo (Figure 6) | Downregulation of MDR-1 Protein In Vivo (Figure 6) | |
|---|---|---|---|---|---|---|
| HT-29 (IC50) (Figure 2) | Mouse Splenocytes at Concentration 5% (Figure 3) | |||||
| EA | − | − − | − | + | − | − − − |
| BU | − − − | + + + + | − − − | − | − − − | − − |
| WA | − − − − | − | − − − − | − − | − − | − |
| TXL | − − | + + | − | + + + + | − − − − | − − − − |
| Interaction | Mutual antagonism | Mutual suppression /mutual counteraction | Mutual antagonism | Mutual enhancement | Mutual accentuation/mutual enhancement | |
Abbreviations: EA, ethyl acetate fraction of TXL; BU, butanol fraction of TXL; WA, aqueous fraction of TXL; IC50, half-maximal inhibitory concentration.
+, + +, + + +, and + + + + indicate the relative enhancing effect exhibited by the respective fractions of TXL and the whole formula of TXL. −, − −, − − −, and − − − − indicate the relative inhibitory effect exhibited by the respective fractions of TXL and the whole formula of TXL.
In the future, in addition to conducting studies on herbal interaction in TCM formulas based on both holistic philosophy in TCM and the concept in contemporary medicine, it may be necessary to investigate into the bioactive compounds of each fraction of a TCM formula that may give some hints to further development of more effective drugs with minimal side effects for fighting against human colorectal carcinoma. Taking into account that the results in this article were obtained from the experiments carried out in vitro as well as in vivo based on animal model, and there may be physiological discrepancy between mice and human in response to the effect of TXL, it is recommended to obtain clinical evidence to further ascertain the antitumor effect of TXL against human colorectal carcinoma. Nevertheless, through the comparison of the antitumorigenic actions of TXL and its functional constituent fractions (viz EA, BU, WA fractions) in human colorectal carcinoma, the results lay the scientific foundation for the development of novel anticancer agents from bioactive ingredients in the respective fractions or the combination of the respective fractions. The therapeutic potential of TXL can therefore be further developed for clinical use to benefit colon cancer treatment. Moreover, the results from this study would shed light on the science behind the holism philosophy of Chinese medicine formula, with TXL as an illustration, and allow a better understanding of the essence and action mechanism of Chinese medicine.
Conclusion
In this study, the holistic effects of TXL were investigated, demonstrating the science-based (a) mutual accentuation and mutual enhancement, (b) mutual counteraction and mutual suppression, and (c) mutual antagonism. So, there is a better understanding of the comprehensive and holistic effects of Chinese medicine formula. Further research in this area may give hints to the development of a more effective fraction and its ingredients with minimal side effects for fighting against human colorectal carcinoma.
Acknowledgments
We also thank Dr Yong Mei Hu, Mr Keith Wong, Ms Cindy Lee, and Mr Freddy Tsang for expert technical assistance in fractionation and molecular study.
Footnotes
Authors’ contribution: SCWS, YBZ and YT designed and conceived the study. SCWS, ABL and HPC conducted the experiments. SCWS, ABL and TBN wrote the manuscript. LLZ, LXL, YBZ and ZJZ provided the constructive comments and re-wrote parts of manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported in part by a grant from Seed Funding Programme for Applied Research (no. 200807160015), Small Project Funding (no. 200807176239), the University of Hong Kong and by contract research funding from China-Japan Feida Union Company Limited.
References
- 1. American Cancer Society. Global Cancer Facts & Figures. 2nd ed. Atlanta, GA: American Cancer Society; 2011. http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-027766.pdf. Accessed May 10, 2016. [Google Scholar]
- 2. Pfizer Inc. Pfizer Facts: The Burden of Cancer in Asia. New York, NY: Pfizer Inc; 2008. http://www.pfizer.com/files/products/cancer_in_asia.pdf. Accessed May 10, 2016. [Google Scholar]
- 3. TCM Forum, Hong Kong. Treatment and Aftercare for Colon Cancer in TCM. http://www.tcmforum.com/forum2.php?reply=0&forumID=100404&fgroup=expert. Accessed April 6, 2011.
- 4. Cancer Fund, Hong Kong. Latest Cancer Statistics. http://www.cancer-fund.org/en/cancer-statistics.html. Accessed June 27, 2014.
- 5. National Cancer Centre, Singapore. Cancer Statistics. http://www.nccs.com.sg/patientcare/whatiscancer/cancerStatistics/Pages/Home.aspx. Accessed May 15, 2014.
- 6. Taiwan Cancer Registry, Taiwan. Cancer Statistics: Cancer Incidence and Mortality Rates in Taiwan. http://tcr.cph.ntu.edu.tw/main.php?Page=N2. Accessed May 15, 2014.
- 7. Foundation for Promotion of Cancer Research, Japan. Cancer Statistics in Japan ’13. http://ganjoho.jp/en/professional/statistics/brochure/2013_en.html. Accessed May 15, 2014.
- 8. Centre for the Health Protection, Hong Kong SAR. Colorectal Cancer Statistics. http://www.chp.gov.hk/en/content/9/25/51.html. Accessed May 15, 2014.
- 9. National Cancer Institute, National Institutes of Health. Colon Cancer Treatment (PDQ®). http://www.cancer.gov/cancertopics/pdq/treatment/colon/Patient/. Accessed February 19, 2013.
- 10. American Cancer Society. Colorectal Cancer: Treatment by Stage of Colon Cancer. http://www.cancer.org/cancer/colonandrectumcancer/detailedguide/colorectal-cancer-treatment-by-stage-coloncancer/. Accessed February 19, 2013.
- 11. American Cancer Society. Colorectal Cancer Overview. http://www.cancer.org/cancer/colonandrectumcancer/overviewguide/. Accessed February 19, 2013.
- 12. Cancer Research, UK. Side Effects of Bowel Cancer Treatment. http://www.cancerresearchuk.org/cancer-help/type/bowel-cancer/treatment/. Accessed February 19, 2013.
- 13. Parekh HS, Liu G, Wei MQ. A new dawn for the use of traditional Chinese medicine in cancer therapy. Mol Cancer. 2009;8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wong R, Sagar CM, Sagar SM. Integration of Chinese medicine into supportive cancer care: a modern role for an ancient tradition. Cancer Treat Rev. 2001;27:235-246. [DOI] [PubMed] [Google Scholar]
- 15. Cho WC. Scientific evidence on the supportive cancer care with Chinese medicine. Zhongguo Fei Ai Za Zhi. 2010;13:190-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang SR, He YH. Review on present research situation of TCM in treating colorectal cancers. Hunan Zong Yi Yao Dao Bao. 2001;7:292-294. [Google Scholar]
- 17. Xu H, Li Q. Progress of clinical research in applying Chinese medicine formulas in colon cancer treatment [in Chinese]. Liaoning Zhong Yi Za Zhi. 2009;36:1241-1243. [Google Scholar]
- 18. Sun A, Chia JS, Chiang CP, et al. The Chinese herbal medicine Tien-Hsien liquid inhibits cell growth and induces apoptosis in a wide variety of human cancer cells. J Altern Complement Med. 2005;11:245-256. [DOI] [PubMed] [Google Scholar]
- 19. Yao CJ, Yang CM, Chuang SE, et al. Targeting PML-RARα and oncogenic signaling pathways by Chinese herbal mixture Tien-Hsien liquid in acute promyelocytic leukemia NB4 cells. Evid Based Complement Alternat Med. 2011;2011:984154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chu ES, Sze SC, Cheung HP, et al. Differential effects of anti-metastatic mechanism of Tian-Xian Liquid (TXL) and its bioactive fractions on human colorectal cancer models. J Ethnopharmacol. 2011;137:403-413. [DOI] [PubMed] [Google Scholar]
- 21. Liu Q, Tong Y, Sze SC, et al. Tian Xian Liquid (TXL) induces apoptosis in HT-29 colon cancer cell in vitro and inhibits tumor growth in vivo. Chin Med. 2010;5:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sze SC, Wong KL, Liu WK, et al. Regulatiion of p21, MMP-1, and MDR-1 expression in human colon carcinoma HT29 cells by Tian Xian Liquid, a Chinese medicinal formula, in vitro and in vivo. Integr Cancer Ther. 2011;10:58-69. [DOI] [PubMed] [Google Scholar]
- 23. Kuo WH, Yao CA, Lin CH, Lin CH, Chang KJ. Safety and efficacy of Tien-Hsien liquid practical in patients with refractory metastatic breast cancer: a randomized double-blind, placebo-controlled, parallel-group, phase II trial. Evid Based Complement Alternat Med. 2012;2012:803239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chia JS, Du, Hsu WB, Sun A, Chiang CP, Wang WB. Inhibition of metastasis, angiogenesis and tumor growth by Chinese herbal cocktail Tien-Hsien liquid. BMC Cancer. 2010;10:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ho JC, Sze SC, Shen WZ, Liu WK. Mitogenic activity of edible mushroom lectins. Biochim Biophys Acta. 2004;1671:9-17. [DOI] [PubMed] [Google Scholar]
- 26. Davis PK, Ho A, Dowdy SF. Biological methods for cell-cycle synchronization of mammalian cells. Biotechniques. 2001;30:1322-1326, 1328,, 1330-1321. [DOI] [PubMed] [Google Scholar]
- 27. Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701-704. [DOI] [PubMed] [Google Scholar]
- 28. Rousseau D, Cannella D, Boulaire J, Fitzgerald P, Fotedar A, Fotedar R. Growth inhibition by CDK-cyclin and PCNA binding domains of p21 occurs by distinct mechanisms and is regulated by ubiquitin-proteasome pathway. Oncogene. 1999;18:4313-4325. [DOI] [PubMed] [Google Scholar]
- 29. Baldin V, Lukas, Marcote MJ, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for all cell cycle progression in G1. Genes Dev. 1993;7:812-821. [DOI] [PubMed] [Google Scholar]
- 30. Fukami-Kobayashi J, Mitsui Y. Cyclin D1 inhibits cell proliferation through binding to PCNA and Cdk2. Exp Cell Res. 1999;246:338-347. [DOI] [PubMed] [Google Scholar]
- 31. Yu B, Lane ME, Pestell RG, Albanese C, Wadler S. Downregulation of cyclin D1 alters cdk4- and cdk2-specific phosphorylation of retinoblastoma protein. Mol Cell Biol Res Commun. 2000;3:352-359. [DOI] [PubMed] [Google Scholar]
- 32. Chytil A, Waltner-Law M, West R, et al. Construction of a cyclin D1-Cdk2 fusion protein to model the biological functions of cyclin D1-Cdk2 complexes. J Biol Chem. 2004;279:47688-47698. [DOI] [PubMed] [Google Scholar]
- 33. Shen G, Xu C, Chen C, Hebbar V, Kong AN. p53-independent G1 cell cycle arrest of human colon carcinoma cells HT-29 by sulforaphane is associated with induction of p21CIP1 and inhibition of expression of cyclin D1. Cancer Chemother Pharmacol. 2006;57:317-327. [DOI] [PubMed] [Google Scholar]
- 34. Airola K, Karonen T, Vaalamo M, et al. Expression of collagenases-1 and -3 and their inhibitors TIMP-1 and -3 correlates with the level of invasion in malignant melanomas. Br J Cancer. 1999;80:733-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hofmann UB, Westphal JR, Van Muijen GN, Ruiter DJ. Matrix metalloproteinases in human melanoma. J Invest Dermatol. 2000;115:337-344. [DOI] [PubMed] [Google Scholar]
- 36. Sunami E, Tsuno N, Osada T, et al. MMP-1 is a prognostic marker for hematogenous metastasis of colorectal cancer. Oncologist. 2000;5:108-114. [DOI] [PubMed] [Google Scholar]
- 37. Zucker S, Vacirca J. Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev. 2004;23:101-117. [DOI] [PubMed] [Google Scholar]
- 38. Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP dependent transporters. Nat Rev Cancer. 2002;2:48-58. [DOI] [PubMed] [Google Scholar]
- 39. Hennessy M, Spiers JP. A primer on the mechanics of P-glycoprotein the multidrug transporter. Pharmacol Res. 2007;55:1-15. [DOI] [PubMed] [Google Scholar]
- 40. Li X, Li JP, Yuan HY, et al. Recent advances in P-glyprotein-mediated multidrug resistance reversal mechanisms. Methods Find Exp Clin Pharmacol. 2007;29:607-617. [DOI] [PubMed] [Google Scholar]
- 41. Leitner HM, Kachadourian R, Day BJ. Harnessing drug resistance: using ABC transorter proteins to target cancer cells. Biochem Pharmacol. 2007;74:1677-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yuan R, Lin Y. Traditional Chinese medicine: an approach to scientific proof and clinical validation. Pharmacol Res. 2000;86:191-198. [DOI] [PubMed] [Google Scholar]
- 43. Lei Z, Chen S, Gao X, et al. Pharmaceutics of Chinese Medicine. Shanghai, China: Shanghai Scientific Technology Publishing House; 2002. [Google Scholar]
- 44. Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL. Cyclin D as a therapeutic target in cancer. Nat Rev Cancer. 2011;11:558-572. [DOI] [PubMed] [Google Scholar]