Abstract
Background. The Chinese herbal mixture, Tien-Hsien liquid (THL), has been used as an anticancer dietary supplement for more than 20 years. Our previous studies have shown that THL can modulate immune responseand inhibit tumor growth. In this study, we further evaluated the effect of THL on anticancer immune response in mice vaccinated with γ-ray-irradiated tumor cells. Methods. The antitumor effect of THL was determined in mice vaccinated with low-tumorigenic CT-26-low colon cancer cells or γ-ray-irradiated high-tumorigenic CT-26-high colon cancer cells. The number of natural killer (NK) cells and T lymphocytes in the spleen was analyzed by flow cytometry. The tumor-killing activities of NK cells and cytotoxic T lymphocytes (CTLs) were analyzed by flow cytometry using YAC-1 and CT-26-high cells, respectively, as target cells. The levels of IFN-γ, IL-2, and TNF-α were determined by ELISA. Results. THL suppressed the growth of CT-26-high tumor in mice previously vaccinated with low-tumorigenic CT-26-low cells or γ-irradiated CT-26-high cells. THL increased the populations of NK cells and CD4+ T lymphocytes in the spleen and enhanced the tumor-killing activities of NK cells and CTL in mice vaccinated with γ-irradiated CT-26-high cells. THL increased the production of IFN-γ, IL-2, and TNF-α in mice vaccinated with γ-irradiated CT-26-high cells. Conclusion. THL can enhance the antitumor immune responses in mice vaccinated with killed tumor cells. These results suggest that THL may be used as a complementary medicine for cancer patients previously treated with killed tumor cell vaccines, radiotherapy, or chemotherapy.
Keywords: Tien-Hsien liquid, herbal mixture, anticancer immunity, tumor suppression, natural killer cell, cytotoxic T lymphocyte, interferon-γ, interleukin-2, tumor necrosis factor α
Introduction
The Chinese herbal cocktail Tien-Hsien liquid (THL; prepared by China-Japan Feida Union Co, Ltd, Hong Kong) has been used as an anti-cancer dietary supplement for more than 20 years and has been used by many cancer patients with favorable outcomes in more than 15 countries. THL is an aqueous preparation of herbal mixture consisting mainly of extracts from 14 Chinese medicinal herbs: Cordyceps sinensis (CS), Oldenlandia diffusa (OD), Indigo pulverata levis (also known as indigo naturalis), Polyporus umbellatus (PU), Radix astragali (RA), Panax ginseng (PG), Solanum nigrum L, Pogostemon cablin, Atractylodis macrocephalae rhizoma (AMR), Trichosanthes radix, Clematis radix, Margarite, Ligustrum lucidum Ait (LLA), and Glycyrrhiza radix (GR). It is believed that a properly formulated herbal cocktail, which takes advantage of synergy and interactions among the myriad phytochemicals present in the different herbs, may achieve better therapeutic efficacy than single herbs. Our previous studies have shown that THL can induce apoptosis and inhibit metastasis and angiogenesis in a wide variety of human cancer cells.1,2 Other studies also showed that THL can induce apoptosis and inhibit the growth and metastasis of cancer cells by targeting various oncogenic signaling pathways and metastatic markers.3-5 More recently, Yao et al3 showed that THL could eliminate the cancer stem-like cells, accompanied by the suppression of stemness genes expression, colony formation, and tumorigenicity.6 Together, these studies suggest that THL has the potential to be used as a therapeutic agent for established tumors.
The enhancement of host immune response has been considered as an alternative strategy for the prevention and cure of cancers and as a possible means of inhibiting tumor growth without harming the host.7,8 Natural killer (NK) cells and cytotoxic T lymphocyte (CTLs) are the 2 major cytotoxic lymphocytes that are important in the defense against tumors.9,10 CTLs perform the surveillance function by recognizing and killing potentially malignant cells that express peptides derived from mutant cellular protein or oncogenic proteins, which are presented by major histocompatibility complex (MHC) class I molecules. Unlike CTLs, the killing by NK cells is not through antigen/MHC recognition. NK cells kill many types of tumor cells, especially cells that have reduced MHC class I expression and can escape killing by CTLs.11 Many in vitro and in vivo studies have suggested that tumor cells are recognized as NK cell targets.12 NK cells also act as regulatory cells to influence various other cells, such as dendritic cells, helper T-cells, CTLs, and B cells.13 Therefore, many studies for cancer immunotherapy were focused on enhancing the activity of NK cells and CTLs.14
Immunotherapy using whole tumor cell vaccines has become an alternative strategy for cancer treatment.15,16 For example, granulocyte-macrophage colony-stimulating factor-expressing tumor cell vaccines are very efficient in inducing tumor-specific immune response in mice and in preliminary clinical trials.17-19 In addition, γ-ray-irradiated apoptotic tumor cell vaccines can induce a potent immune response in vivo probably through the cross-presentation of tumor antigens to CTLs by dendritic cells.20,21 Our previous studies have shown that THL has immunomodulating activity and can modulate the antigen-stimulated cytokine production by T-cells.22,23 Moreover, several major ingredients of THL have been reported to be able to modulate immune response.24,25 For instance, CS, RA, PG, and GR can increase the cytotoxic activity of murine NK cells. OD can increase the cytotoxic activity of murine CTLs. CS and GR can increase the secretion of interleukin (IL)-1 by murine macrophages. RA, PG, and GR can induce the secretion of interferon-γ (IFN-γ) by mouse spleen cells. CS, OD, PU, RA, PG, AMR, LLA, and GR can induce the secretion of IL-2 by mouse spleen cells. Together, these results suggest that THL can modulate antitumor immunity in tumor-bearing mice. In this study, we used γ-ray-irradiated apoptotic tumor cells as a vaccine to immunize mice and investigate whether THL could enhance the antitumor immunity in tumor cell–vaccinated mice. We found that THL could enhance the tumor-killing activities of NK cells and CTL and increase the production of IFN-γ, IL-2, and TNF-α in mice vaccinated with γ-irradiated tumor cells.
Materials and Methods
Cell Culture
The mouse colon carcinoma cell lines, CT-26 (including CT-26-low and CT-26-high), were established and provided by Dr Sheng-Hong Tseng (Department of Surgery, National Taiwan University Hospital, Taipei, Taiwan). Their tumorigenicity was confirmed, as shown in Table 1. These cells were routinely grown in Dulbecco’s modified Eagle medium (DMEM; GIBCO BRL Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS) in 5% CO2. The mouse lymphoma cell line, YAC-1 was cultured in RPMI-1640 medium (GIBCO BRL Life Technologies) supplemented with 10% FBS in 5% CO2.
Table 1.
The Tumorigenicity of CT-26-Low and CT-26-High Colon Cancer Cells in the Syngeneic BALB/c Mice.
| Number of Injected Cells | Frequency of Tumor Formation |
|
|---|---|---|
| CT-26-Low Cells | CT-26-High Cells | |
| 2 × 104 | ND* | 90% |
| 5 × 104 | ND | 100% |
| 1 × 105 | ND | 100% |
| 3 × 106 | 50% | 100% |
Abbreviations: ND, not determined.
Handling of THL
THL (obtained from China-Japan Feida Union Co, Ltd, Hong Kong) is an aqueous preparation of herbal mixture and consists mainly of extracts from 14 Chinese medicinal herbs as mentioned previously. For the in vivo mice xenograft experiment, THL (200 µL/d) was orally given to mice directly. The dose of THL for each mouse was calculated as follows. The dosage of THL for a cancer patient (60 kg) is 1 mL/kg/d.26 The given dosage (D) of THL in mice was calculated according to the Meeh-Rubner conversion formula between human and mouse: Dmouse = Dhuman × (Kmouse/Khuman), where K is the conversion factor (Kmouse = 1 and Khuman = 0.11). The calculated dosage in mice (0.02 kg) was 9.09 mL/kg/d, which equals 198 µL per day for each mouse. Therefore, a daily dose of 200 µL THL per mouse was used in this study. This dose of THL did not cause toxic effects in the mice in our preliminary study.
Preparation of Tumor Vaccines
CT-26-high cells were irradiated with 72-Gy γ-rays, and the viability of the irradiated cells was analyzed by propidium iodide (PI) staining and flow cytometry. The majority of irradiated CT-26-high cells were found to be dead, and the irradiated cells were promptly inoculated subcutaneously on the back of BALB/c mice (1 × 106 cells, suspended in 100 µL of phosphate-buffered saline [PBS]).
Experimental Animals
All animal experiments in this study were performed following the Guidelines for Animal Experiments in National Taiwan University and were approved by the Institutional Animal Care and Use Committee in College of Medicine, National Taiwan University (IACUC Approval No.: 20110101). BALB/c female mice (6-8 weeks old) were purchased from Laboratory Animal Center at College of Medicine, National Taiwan University (Taipei, Taiwan) and given food and water ad libitum. The mice were randomly divided into 2 groups for oral feeding with THL or water (200 µL; each day) throughout the experimental period. The experimental schedule for assessing the immune-enhancing effect of THL in tumor-bearing mice is summarized in Figures 1A and 2A. The body weight and tumor size of mice were measured at different time points following tumor implantation, and the tumor volume was calculated according to the following formula: 1/2 (Length × Width2).
Figure 1.
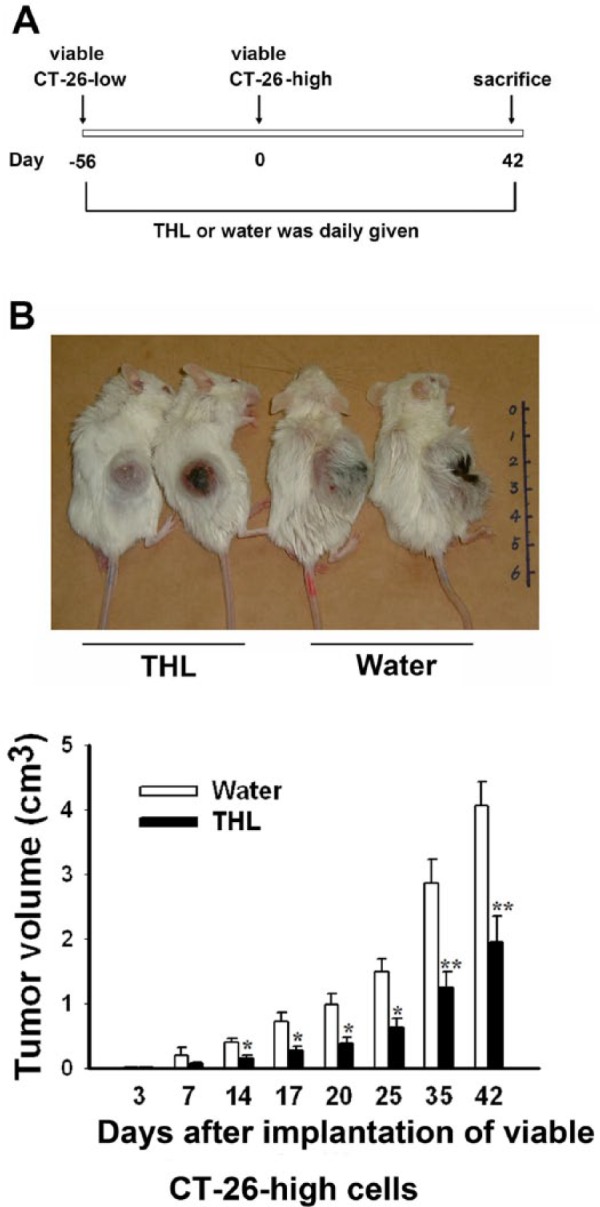
Tien-Hsien liquid (THL) inhibited the growth of CT-26-high tumor xenografts in syngeneic BALB/c mice previously vaccinated with viable CT-26-low colon cancer cells. (A) The experimental schedule for assessing the effect of THL on the growth of CT-26-high tumors in mice vaccinated with viable CT-26-low cancer cells. (B) The effect of THL on the growth of CT-26-high tumors in mice vaccinated with viable CT-26-low cancer cells. Values represent means ± standard error, n = 8 of THL-treated group and n = 4 of water-treated group. *P < .05; **P < .01 versus water-treated group.
Figure 2.
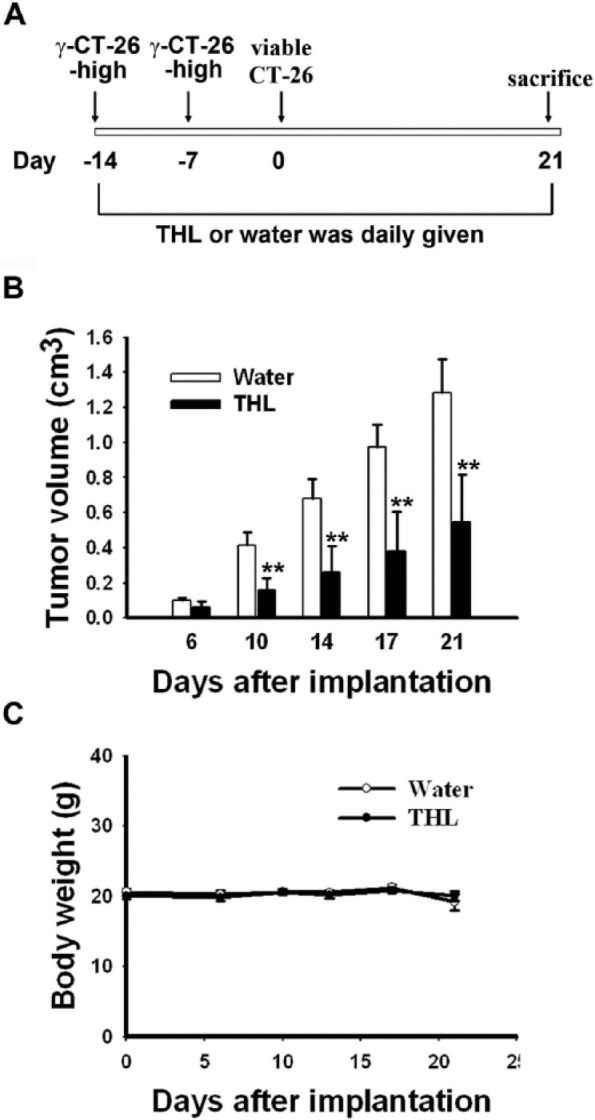
Tien-Hsien liquid (THL) inhibited the growth of CT-26-high tumor xenografts in syngeneic BALB/c mice previously vaccinated with γ-irradiated CT-26-high colon cancer cells. (A) The experimental schedule for assessing the effect of THL on the growth of CT-26-high tumors in mice vaccinated with γ-irradiated CT-26-high cancer cells. The effect of THL on the growth of CT-26-high tumors (B) and body weight (C) in mice vaccinated with γ-irradiated CT-26-high cancer cells. Values represent means ± standard error; n = 10 mice per group. **P < .01 versus water-treated group.
Preparation of Splenocytes
Spleens were removed aseptically, placed in RPMI medium, gently homogenized, and passed through a 200-µm-mesh (Becton Dickinson Bioscience, San Jose, CA) to generate single-cell suspension. Erythrocytes were rapidly washed and lysed by the RBC lysis buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM EDTA), and the splenocytes were resuspended at a density of 1 × 107 cells/mL in RPMI medium.
Flow Cytometry Analysis of NK Cells and T Lymphocytes in Spleen
The lymphocytes from spleens were stained with fluorescence-labeled antibodies in PBS for 30 minutes, followed by 3 washes with PBS, and then analyzed by flow cytometry (Becton Dickinson Bioscience). Fluorescence-labeled antibodies against CD4, CD8a, and CD49b (pan-NK) were all purchased from Becton Dickinson Bioscience.
Assay of NK Cell– and CTL-Mediated Killing Activity
The mouse lymphoma YAC-1 cells and CT-26-high cells were used as the target cells of NK cells and CTL, respectively. Before the coincubation of effector and target cells, YAC-1 cells and CT-26-high cells were labeled with 200 nM CFSE (Molecular Probes, Eugene, OR) for 15 minutes in serum-free RPMI medium, washed once, and diluted in complete RPMI or DMEM medium. In the NK killing assay, lymphocytes and CFSE-labeled YAC-1 cells were cocultured in 5-mL Falcon tubes (Becton Dickinson Bioscience) at 25:1 or 50:1 of the effector cell versus target cell (E:T) ratio. After incubation at 37°C for 4 hours, the samples were resuspended in PI solution (0.25 µg/mL PI and 1% bovine serum albumin (BSA) in PBS) and analyzed by flow cytometry. The CFSE+/PI+ YAC-1 cells were considered as killed target cells. In the CTL-killing assay, lymphocytes were stimulated in vitro with 72-Gy γ-irradiated CT-26-high cells at a 40:1 ratio (lymphocytes vs γ-irradiated CT-26-high cells) and 1 ng/mL of IL-2. After incubation for 5 days, lymphocytes and CFSE-labeled viable CT-26-high cells were cocultured in 5-mL Falcon tubes at 10:1, 25:1, or 50:1 E:T ratios. After incubation at 37°C for 4 hours, the samples were resuspended in PI solution and analyzed by flow cytometry. The CFSE+/PI+ CT-26-high cells were considered as killed target cells.
Cytokine Production by Cultured Splenocytes and Tumor Xenografts
Isolated splenocytes (1 × 106/mL) from water- or THL-treated mice were ex vivo stimulated with concanavalin A (Con A; Sigma-Aldrich, Inc, St Louis, MO) at 5 µg/mL for 24 hours. The levels of IFN-γ , IL-2, and tumor necrosis factor α (TNF-α) secreted in the culture supernatant were measured by ELISA (BioLegend, San Diego, CA).
To measure cytokine levels in tumor xenografts, tumor tissues were taken, weighed, minced, and homogenized with tissue lysis buffer (1% Triton X-100; 50 mM HEPES, pH 7.4; 150 mM NaCl; 1.5 mM MgCl2; 1 mM ethylene glycol tetraacetic acid (EGTA); 100 mM NaF; 1 mM Na3VO4; 10% glycerol; and 1× protease inhibitor cocktail from Roche Applied Science). The supernatant of the homogenized sample was obtained by centrifugation (12 000 rpm for 10 minutes), and the levels of IFN-γ, IL-2, and TNF-α in the supernatant were assayed by ELISA (BioLegend).
Statistical Analyses
Data are presented as mean ± standard error. The significance of the difference between groups was evaluated with the Student’s t-test; P < .05 was considered significant.
Results
THL Suppresses Tumor Growth in Tumor Cell–Vaccinated Mice
To investigate the antitumor activity of THL in mice, 2 murine CT-26 colon cancer cell lines, CT-26-low and CT-26-high, which have low and high tumorigenic activity, respectively, were established. Subcutaneous injection of 5 × 104 high-tumorigenic CT-26-high cells resulted in 100% tumor formation in syngeneic BALB/c mice. In contrast, in mice subcutaneously injected with 3 × 106 low-tumorigenic CT-26-low cells, only about 50% of mice showed tumor formation (Table 1). We hypothesized that CT-26-low cell may be used as a tumor vaccine to elicit an antitumor immune response in mice. To test this possibility, we compared the tumor-inducing ability of CT-26-high cells in mice previously injected with CT-26-low cells with that in mice not injected with CT-26-low cells. We found that while injection of 5 × 104 CT-26-high cells was enough to induce 100% tumor formation in mice not injected with CT-26-low cells previously, a much higher number (~5 × 106) of CT-26-high cells was required to induce 100% tumor formation in mice previously injected with CT-26-low cells (data not shown). This result suggests that CT-26-low cell injection may elicit antitumor immune responses against CT-26-high cells. Next, we tested whether THL could suppress tumor growth in mice vaccinated with CT-26-low cell. To do this, mice were first injected with 3 × 106 CT-26-low cells and then randomly divided into 2 groups: one orally fed with THL and the other orally fed with water. Then 56 days later, the number of tumor-free mice in the THL-treated group was 12, whereas that in the water-treated group was 5, indicating that administration of THL reduced the frequency of tumor formation. The mice were then injected subcutaneously with 2 × 106 CT-26-high cells as indicated in the experimental protocol shown in Figure 1A. Mice were fed with either THL or water throughout the entire experimental period (200 µL; twice a day). At 42 days after second tumor-cell injection, 4 out of 12 THL-treated mice remained tumor free, whereas only 1 out of 5 water-treated mice was tumor free. The average tumor volume of THL-treated mice was about 48% that of water-treated mice at 42 days after second tumor-cell injection (Figure 1B). These data indicate that THL can suppress tumor growth in tumor cell–vaccinated mice, and this tumor-suppressing activity of THL may be, at least, partly mediated through its ability to promote antitumor immune responses.
To further study the enhancement of antitumor immunity by THL, we used γ-ray-irradiated CT-26-high cells to immunize syngeneic BALB/c mice. Briefly, BALB/c mice were vaccinated twice with 1 × 106 γ-irradiated CT-26-high cells. One week after the second injection of the γ-irradiated cells, the mice were subcutaneously injected with 5 × 106 viable CT-26-high cells, and the growth of tumor xenografts was monitored and measured for 21 days (see Figure 2A for the experimental protocol). As shown in Figure 2B, the tumor growth was slower in THL-treated mice than in water-treated mice. At day 21 after tumor cell implantation, the average tumor volume of the THL-treated mice was 0.55 ± 0.1 cm3, whereas that of the water-treated mice was 1.29 ± 0.2 cm3. In addition, THL did not cause body weight loss (Figure 2C) or other side effects, such as hair loss and lethargy, during the experimental period. These results further demonstrate that THL possesses potent tumor-suppressing activity, and this antitumor activity may, at least partly, be a result of its ability to enhance antitumor immune responses.
THL Increases NK Cell and CTL Activities in Tumor Cell–Vaccinated Mice
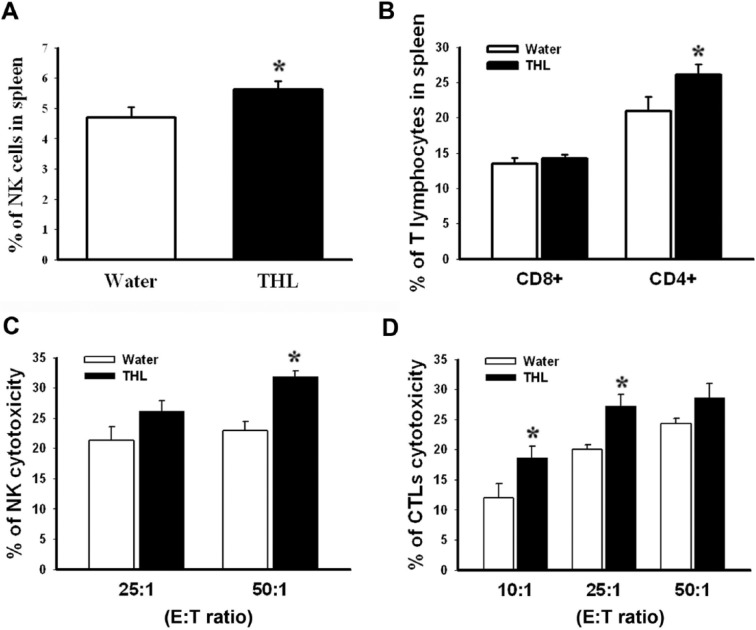
NK cells and CTLs represent 2 major populations of cytotoxic lymphocytes and play a vital role in antitumor defense.9,10 To elucidate the mechanisms for the anticancer efficacy of THL in tumor cell–vaccinated mice, splenocytes were isolated from the mice of the above experiment (see Figure 2), and the populations of NK cells (CD49b+) and T lymphocytes (CD8+ or CD4+) were analyzed by flow cytometry. As shown in Figures 3A and 3B, the populations of NK cells and CD4+ T lymphocytes were higher in the THL-treated mice than in the water-treated mice. To investigate whether the activities of NK cells and CTLs were increased following the THL treatment, ex vivo assays for tumor cell killing by NK cells and CTLs were performed. As shown in Figures 3C and 3D, the tumor-killing activities of NK cells and CTLs of the THL-treated mice were higher than those of the water-treated mice. Together, these results indicate that THL may inhibit tumor growth through the enhancement of the NK cell and CTL activities.
Figure 3.
Tien-Hsien liquid (THL) increased the populations and tumor-killing activities of natural killer (NK) cells and cytotoxic T lymphocytes (CTLs) in mice previously vaccinated with γ-irradiated CT-26-high colon cancer cells. Mice from the experiment in Figure 2 were killed humanely at 21 days after viable CT-26-high cell injection. The splenocytes were then isolated and used to determine the amounts and cytotoxic activities of NK cells and CTLs. The populations of NK cells (A) and T lymphocytes (B) were determined by flow cytometry. Values represent mean ± standard error (SE); n = 9 mice per group. *P < .05 versus the water-treated group. The cytotoxicities of NK cells (C) and CTLs (D) were determined by flow cytometry as described in the Methods section. Values represent mean ± SE; n = 6 mice per group. *P < .05 versus the water-treated group.
THL Increases the Cytokine Secretion in Tumor Cell–Vaccinated Mice
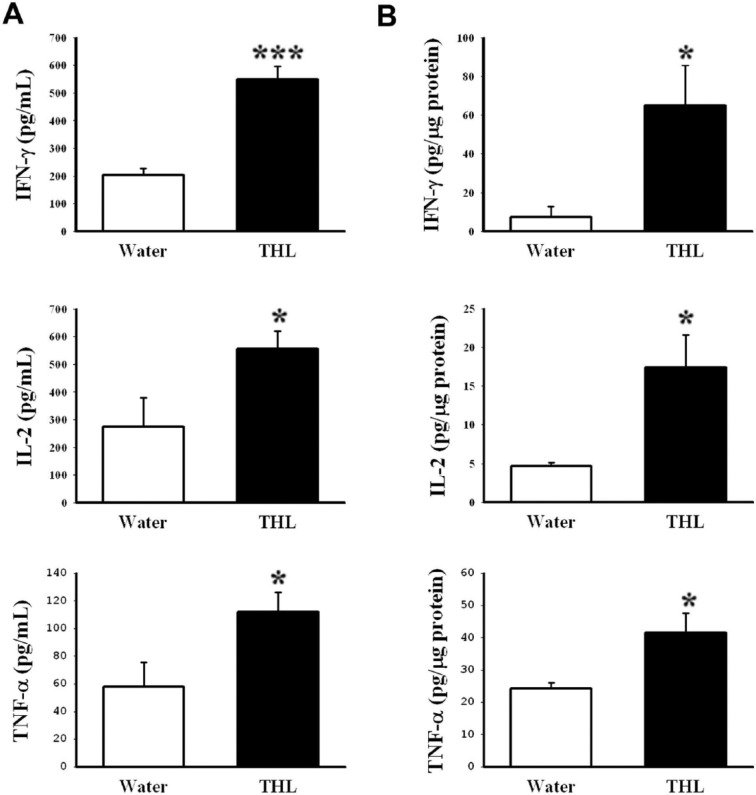
Cytokines, such as IFN-γ, IL-2, and TNF-α, play an essential role in NK cell and CTL activation. We thus investigated the effect of THL on the production of cytokines IFN-γ, IL-2, and TNF-α from Con A–stimulated splenocytes isolated from mice vaccinated with γ-irradiated CT-26-high cells by ELISA. As shown in Figure 4A, splenocytes isolated from the THL-treated mice produced higher levels of IFN-γ, IL-2, and TNF-α than splenocytes isolated from the water-fed mice. In addition, the infiltrating cytokines in tumor xenografts were also examined by ELISA analyses. Similarly, the levels of IFN-γ, IL-2, and TNF-α were higher in THL-treated tumors than in water-treated tumors (Figure 4B). Together, these data indicate that THL increases the production of IFN-γ, IL-2, and TNF-α in tumor cell–vaccinated mice and suggest that THL may promote the activities of NK cells and CTLs by inducing cytokine production.
Figure 4.
Tien-Hsien liquid (THL) increased the level of cytokines in mice previously vaccinated with γ-irradiated CT-26-high colon cancer cells. Mice from the experiment in Figure 2 were killed humanely at 21 days after viable CT-26-high cell injection. The production of interferon (IFN)-γ, interleukin (IL)-2, and tumor necrosis factor (TNF)-α from splenocytes isolated from mice (A) and in tumor xenografts (B) was determined. (A) Splenocytes were cultured with 5 µg/mL concanavalin A for 24 hours, and the levels of IFN-γ, IL-2, and TNF-α in the supernatant were measured by ELISA. Values represent mean ± standard error (SE); n = 6 mice per group. *P < .05; ***P < .001 versus the water-treated group. (B) The levels of IFN-γ, IL-2, and TNF-α in tumor xenografts were measured by ELISA. Values represent mean ± SE; n = 4 mice per group. *P < .05 versus the water-treated group.
Discussion
THL is a Chinese herbal mixture that has been used as an anticancer dietary supplement for many years. It has been suggested that the antitumor activity of traditional Chinese herbs is mediated through the augmentation of the immune responses.27 It also has been reported that THL can modulate immune responses.22,23,28 However, it is still not clear whether THL could suppress tumor growth through enhancing antitumor immunity. In this study, we investigated the immune-enhancing activity of THL. We found that THL can efficiently suppress the growth of CT-26-high tumors in syngeneic BALB/c mice previously vaccinated with either low tumorigenic CT-26-low cells or γ-irradiated CT-26-high cells. We also found that the tumor-killing activities of NK cells and CTLs in the THL-treated mice were significantly higher than those in the water-treated mice, which was correlated with the increased production of cytokines, including IFN-γ, IL-2, and TNF-α, in THL-treated mice. Together, these results indicate that THL can enhance the antitumor immune responses.
To investigate the ability of THL to induce antitumor immunity, we used viable low-tumorigenic CT-26-low cells or γ-irradiated CT-26-high cells as tumor vaccines to immunize syngeneic BALB/c mice. Both viable CT-26-low cells and γ-irradiated CT-26-high cells were capable of inducing antitumor immune responses because a much higher number of CT-26-high cells was required to induce tumor formation in mice previously injected with viable CT-26-low cells or γ-irradiated CT-26-high cells compared with mice not previously injected with these cells (data not shown). The observation that γ-irradiated apoptotic tumor cells can induce a potent antitumor immune response in vivo has been reported before.20,21 We found that THL can effectively suppress the growth of CT-26-high tumor in mice previously vaccinated with CT-26-low cells or γ-irradiated CT-26-high cells (Figures 1 and 2). This tumor-suppressing effect of THL is most likely mediated by its ability to enhance antitumor immunity in tumor cell–vaccinated mice, based on the following reasons. First, THL significantly increased the tumor-killing activities of NK cells and CTLs in tumor cell–vaccinated mice (Figure 3). Second, THL significantly increased the secretion of IFN-γ, IL-2, and TNF-α in tumor cell–vaccinated mice (Figure 4). Third, our preliminary data indicated that THL could only slightly inhibit the growth of CT-26-high tumor in nonvaccinated mice (statistically not significant). This result suggests that THL’s tumor-suppressing activity is largely mediated by its ability to enhance antitumor immunity rather than by its ability to kill CT-26-high tumor cells directly.
Our previous studies have shown that THL has immune-modulating activity and can modulate the antigen-stimulated cytokine production by T-cells.22,23 More recently, Kuo et al26 reported that in a phase IIa trial, THL showed immune-modulating effects in patients with refractory metastatic breast cancer. The levels of T lymphocytes (CD3+, CD4+, and CD8+), B lymphocytes (CD19+), and mature NK cells (CD16+/CD56+) in peripheral blood of THL-treated patients were elevated.26 In the present study, we further showed that THL could increase the tumor-killing activities of CTL and NK cells in tumor cell–vaccinated mice (Figure 3). This effect of THL may be derived from its ability to stimulate the production of IFN-γ, IL-2, and TNF-α in tumor cell–vaccinated mice (Figure 4). IFN-γ is known to play a central role in the induction of host defenses against tumors.29,30 Its antitumor effects can be mediated directly through inhibition of tumor cell growth and/or indirectly by activation of CTLs, NK cells, and macrophages, which are involved in innate as well as adaptive antitumor immune responses.31-33 IFN-γ also facilitates antigen processing and presentation by both the MHC class I and II pathways in tumor cells and antigen-presenting cells, leading to tumor recognition and subsequent destruction by CTLs.32-37 IL-2 has long been considered as a T-cell growth factor and thought to play an important role in mediating clonal expansion of T-cells38-41 and in driving effector differentiation to CTLs.42,43 It can promote tumor-reactive lymphocyte proliferation and cytotoxicity.38,44 TNF-α is an important effector molecule in CTL and NK cell killing of immunogenic tumor cells.45-47 When expressed locally by immune cells, TNF-α can destroy tumor blood vessels and induce the apoptosis and necrosis of tumor cells.9,47 TNF-α has also been shown to be able to inhibit the growth of tumors through the recruitment of macrophages and NK cells.48 Together, these studies suggest that the above cytokines may exert their antitumor activities directly through inhibiting tumor growth or indirectly through modulating innate as well as adaptive immune responses against tumors. Our finding that THL can suppress tumor growth and promote the tumor-killing activities of NK cells and CTLs may be related to its ability to stimulate the secretion of IFN-γ, IL-2, and TNF-α.
In addition to the immune-enhancing and tumor-suppressing activities, depending on the tumor microenvironment, cytokines can also promote the initiation, progression, invasion, and metastasis of cancer through various mechanisms, including modulation of the immune system.49,50 For example, TNF-α has been shown to participate in the initiation, progression, and metastasis of cancer.47,49 A number of studies also showed that IFN-γ may be intimately involved in immunosuppressive mechanisms.51 In this study, we cannot exclude the possibility that cytokines stimulated by THL also display tumor-promoting activities. Further investigation is needed to clarify the exact roles of these cytokines in our tumor model.
Chemotherapy and radiotherapy are the major therapeutic modalities commonly used for treatment of a variety of cancer patients. However, in many cases, chemotherapy or radiotherapy alone cannot achieve a satisfactory therapeutic outcome because of severe adverse effects induced at therapeutic doses.52 Manipulation of the immune system has been considered an alternative strategy for the prevention and cure of cancers and as a promising means of suppressing tumor growth without harming the host.7,8 Here, we show that THL can enhance the antitumor immunity in mice previously injected with γ-irradiated tumor cells. Both chemotherapy and radiotherapy can induce tumor cell apoptosis. The tumor antigens in the apoptotic cells may be processed and presented by professional antigen-presenting cells and induce antigen-specific immune response in the presence of immune-enhancing agents such as THL. The observation that herbal mixtures can enhance the therapeutic efficacy of ionizing radiation and augment antitumor immune response in tumor-bearing mice has been reported before.53 THL has been shown to be a safe adjuvant regimen for patients with refractory metastatic breast cancer in a phase IIa trial and can effectively palliate cancer-related syndromes and improve quality of life.26 It is possible that THL can be used as a complementary medicine to enhance the therapeutic efficacy and lower therapeutic doses of chemotherapy or radiotherapy in cancer patients. Immunotherapy using γ-irradiated tumor cell vaccines is an alternative strategy for cancer treatment.15,16,20,21 Also, it is possible that THL may enhance the therapeutic efficacy of γ-irradiated tumor cell vaccines via augmenting antitumor immunity.
Conclusions
THL, a Chinese herbal mixture, has been shown to have various anticancer activities. In this study, we further show that THL can suppress tumor growth in mice previously vaccinated with γ-irradiated tumor cells. This function of THL may be related to its ability to enhance the tumor-killing activity of NK cells and CTLs and increase the secretion of IFN-γ, IL-2, and TNF-α in mice vaccinated with γ-irradiated tumor cells. Together, these results suggest that THL may be used as a complementary medicine for cancer patients previously treated with γ-irradiated apoptotic tumor cell vaccines, radiotherapy, or chemotherapy.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant from Ching-Hsing Medical Foundation, Taipei, Taiwan.
References
- 1. Chia JS, Du JL, Hsu WB, Sun A, Chiang CP, Wang WB. Inhibition of metastasis, angiogenesis, and tumor growth by Chinese herbal cocktail Tien-Hsien liquid. BMC Cancer. 2010;10:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sun A, Chia JS, Chiang CP, et al. The Chinese herbal medicine Tien-Hsien liquid inhibits cell growth and induces apoptosis in a wide variety of human cancer cells. J Altern Complement Med. 2005;11:245-256. [DOI] [PubMed] [Google Scholar]
- 3. Yao CJ, Yang CM, Chuang SE, et al. Targeting PML-RARα and oncogenic signaling pathways by Chinese herbal mixture Tien-Hsien liquid in acute promyelocytic leukemia NB4 cells. Evid Based Complement Alternat Med. 2011;2011:984154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sze SC, Wong KL, Liu WK, et al. Regulation of p21, MMP-1, and MDR-1 expression in human colon carcinoma HT29 cells by Tian Xian liquid, a Chinese medicinal formula, in vitro and in vivo. Integr Cancer Ther. 2011;10:58-69. [DOI] [PubMed] [Google Scholar]
- 5. Chu ES, Sze SC, Cheung HP, et al. Differential effects of anti-metastatic mechanism of Tian-Xian liquid (TXL) and its bioactive fractions on human colorectal cancer models. J Ethnopharmacol. 2011;137:403-413. [DOI] [PubMed] [Google Scholar]
- 6. Yao CJ, Yeh CT, Lee LM, et al. Elimination of cancer stem-like “side population” cells in hepatoma cell lines by Chinese herbal mixture “Tien-Hsien liquid”. Evid Based Complement Alternat Med. 2012;2012:617085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mitchell MS. Immunotherapy as part of combinations for the treatment of cancer. Int Immunopharmacol. 2003;3:1051-1059. [DOI] [PubMed] [Google Scholar]
- 8. Yuan H, Song J, Li X, Li N, Dai J. Immunomodulation and antitumor activity of kappa-carrageenan oligosaccharides. Cancer Lett. 2006;243:228-234. [DOI] [PubMed] [Google Scholar]
- 9. Medzhitov R, Janeway CA., Jr. Innate immunity: impact on the adaptive immune response. Curr Opin Immunol. 1997;9:4-9. [DOI] [PubMed] [Google Scholar]
- 10. Moretta L, Bottino C, Cantoni C, Mingari MC, Moretta A. Human natural killer cell function and receptors. Curr Opin Pharmacol. 2001;1:387-391. [DOI] [PubMed] [Google Scholar]
- 11. Herberman RB. Cancer immunotherapy with natural killer cells. Semin Oncol. 2002;29:27-30. [DOI] [PubMed] [Google Scholar]
- 12. Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503-510. [DOI] [PubMed] [Google Scholar]
- 14. Zhang J, Sun R, Wei H, Tian Z. Antitumor effects of recombinant human prolactin in human adenocarcinoma-bearing SCID mice with human NK cell xenograft. Int Immunopharmacol. 2005;5:417-425. [DOI] [PubMed] [Google Scholar]
- 15. Anderson KS. Tumor vaccines for breast cancer. Cancer Invest. 2009;27:361-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ellebaek E, Andersen MH, Svane IM, Straten PT. Immunotherapy for metastatic colorectal cancer: present status and new options. Scand J Gastroenterol. 2012;47:315-324. [DOI] [PubMed] [Google Scholar]
- 17. Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993;90:3539-3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jaffee EM, Hruban RH, Biedrzycki B, et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol. 2001;19:145-156. [DOI] [PubMed] [Google Scholar]
- 19. Simon RM, Steinberg SM, Hamilton M, et al. Clinical trial designs for the early clinical development of therapeutic cancer vaccines. J Clin Oncol. 2001;19:1848-1854. [DOI] [PubMed] [Google Scholar]
- 20. Henry F, Boisteau O, Bretaudeau L, Lieubeau B, Meflah K, Gregoire M. Antigen-presenting cells that phagocytose apoptotic tumor-derived cells are potent tumor vaccines. Cancer Res. 1999;59:3329-3332. [PubMed] [Google Scholar]
- 21. Scheffer SR, Nave H, Korangy F, et al. Apoptotic, but not necrotic, tumor cell vaccines induce a potent immune response in vivo. Int J Cancer. 2003;103:205-211. [DOI] [PubMed] [Google Scholar]
- 22. Sun A, Chia JS, Wang WB, Chiang CP. Immunomodulating effects of “Tien-Hsien liquid” on peripheral blood mononuclear cells and T-lymphocytes from patients with recurrent aphthous ulcerations. Am J Chin Med. 2004;32:221-234. [DOI] [PubMed] [Google Scholar]
- 23. Sun A, Chia JS, Wang WB, Chiang CP. “Tien-Hsien liquid” can modulate antigen-stimulated cytokine production by T-cells isolated from patients with recurrent aphthous ulcerations. Am J Chin Med. 2005;33:559-571. [DOI] [PubMed] [Google Scholar]
- 24. Hu HM. Chinese Herbs. Shanghai, China: Shanghai Science and Technology; 1996:164-169, 182,-187, 517,-525, 814,-835, 866,-884, 1217,-1225, 1269,-1294, 1464,-1470, 1530,-1533, 1594,-1597, 1893,-1902, 2330,-2337, 2367-2372. [Google Scholar]
- 25. Luoh HS. Immunopharmacology of Chinese Medicinal Herbs. Beijing, China: Union Publishing Company of Beijing Medical University and Peking Union Medical College; 1999:26-31, 40,-53, 103,-106, 130,-133, 147,-152, 218,-222, 295,-297, 404-405. [Google Scholar]
- 26. Kuo WH, Yao CA, Lin CH, Chang KJ. Safety and efficacy of Tien-Hsien liquid practical in patients with refractory metastatic breast cancer: a randomized, double-blind, placebo-controlled, parallel-group, phase IIA trial. Evid Based Complement Alternat Med. 2012;2012:803239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ruan WJ, Lai MD, Zhou JG. Anticancer effects of Chinese herbal medicine, science or myth? J Zhejiang Univ Sci B. 2006;7:1006-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sun A, Chia JS, Wang WB, Chiang CP. “Tien-Hsien” liquid modulates antigen-stimulated cytokine production by T-cells from patients with erosive oral lichen planus. J Dent Sci. 2008;3:159-166. [Google Scholar]
- 29. Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998;188:2357-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fallarino F, Gajewski TF. Cutting edge: differentiation of antitumor CTL in vivo requires host expression of Stat1. J Immunol. 1999;163:4109-4113. [PubMed] [Google Scholar]
- 31. Krasagakis K, Garbe C, Zouboulis CC, Orfanos CE. Growth control of melanoma cells and melanocytes by cytokines. Recent Results Cancer Res. 1995;139:169-182. [DOI] [PubMed] [Google Scholar]
- 32. Bohm W, Thoma S, Leithauser F, Moller P, Schirmbeck R, Reimann J. T cell-mediated, IFN-gamma-facilitated rejection of murine B16 melanomas. J Immunol. 1998;161:897-908. [PubMed] [Google Scholar]
- 33. Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749-795. [DOI] [PubMed] [Google Scholar]
- 34. Kaplan DH, Shankaran V, Dighe AS, et al. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci U S A. 1998;95:7556-7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ogasawara M, Rosenberg SA. Enhanced expression of HLA molecules and stimulation of autologous human tumor infiltrating lymphocytes following transduction of melanoma cells with gamma-interferon genes. Cancer Res. 1993;53:3561-3568. [PubMed] [Google Scholar]
- 36. Weber JS, Jay G, Tanaka K, Rosenberg SA. Immunotherapy of a murine tumor with interleukin 2: increased sensitivity after MHC class I gene transfection. J Exp Med. 1987;166:1716-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Restifo NP, Esquivel F, Asher AL, et al. Defective presentation of endogenous antigens by a murine sarcoma: implications for the failure of an anti-tumor immune response. J Immunol. 1991;147:1453-1459. [PMC free article] [PubMed] [Google Scholar]
- 38. Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192:5451-5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cantrell DA, Smith KA. The interleukin-2 T-cell system: a new cell growth model. Science. 1984;224:1312-1316. [DOI] [PubMed] [Google Scholar]
- 40. Gillis S, Smith KA. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977;268:154-156. [DOI] [PubMed] [Google Scholar]
- 41. Morgan DA, Ruscetti FW, Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976;193:1007-1008. [DOI] [PubMed] [Google Scholar]
- 42. Yu A, Zhou J, Marten N, et al. Efficient induction of primary and secondary T cell-dependent immune responses in vivo in the absence of functional IL-2 and IL-15 receptors. J Immunol. 2003;170:236-242. [DOI] [PubMed] [Google Scholar]
- 43. D’Souza WN, Lefrancois L. IL-2 is not required for the initiation of CD8 T cell cycling but sustains expansion. J Immunol. 2003;171:5727-5735. [DOI] [PubMed] [Google Scholar]
- 44. Muul LM, Spiess PJ, Director EP, Rosenberg SA. Identification of specific cytolytic immune responses against autologous tumor in humans bearing malignant melanoma. J Immunol. 1987;138:989-995. [PubMed] [Google Scholar]
- 45. Kashii Y, Giorda R, Herberman RB, Whiteside TL, Vujanovic NL. Constitutive expression and role of the TNF family ligands in apoptotic killing of tumor cells by human NK cells. J Immunol. 1999;163:5358-5366. [PubMed] [Google Scholar]
- 46. Prevost-Blondel A, Roth E, Rosenthal FM, Pircher H. Crucial role of TNF-alpha in CD8 T cell-mediated elimination of 3LL-A9 Lewis lung carcinoma cells in vivo. J Immunol. 2000;164:3645-3651. [DOI] [PubMed] [Google Scholar]
- 47. Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361-371. [DOI] [PubMed] [Google Scholar]
- 48. Blankenstein T, Qin ZH, Uberla K, et al. Tumor suppression after tumor cell-targeted tumor necrosis factor alpha gene transfer. J Exp Med. 1991;173:1047-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Charles KA, Kulbe H, Soper R, et al. The tumor-promoting actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J Clin Invest. 2009;119:3011-3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zaidi MR, Merlino G. The two faces of interferon-gamma in cancer. Clin Cancer Res. 2011;17:6118-6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153-164. [DOI] [PubMed] [Google Scholar]
- 53. Park HR, Ju EJ, Jo SK, Jung U, Kim SH. HemoHIM enhances the therapeutic efficacy of ionizing radiation treatment in tumor-bearing mice. J Med Food. 2010;13:47-53. [DOI] [PubMed] [Google Scholar]