Abstract
Background
The accuracy of sentinel lymph node dissection (SLND) in clinically node-positive patients who receive neoadjuvant chemotherapy has been investigated in clinical trials. This survey was designed to assess familiarity and impact of these trial findings into practice.
Methods
American Society of Breast Surgeons members were invited by e-mail to complete an anonymous online survey. 642 members responded (21% of 3090 eligible members). Results were summarized as proportions based on the number of responses to each question.
Results
Respondents indicated knowledge of the Z1071 (86%), SENTINA (57%), and SN-FNAC (39%) trials. The published false negative rates (FNR) of the trials were correctly reported by 53% (336/638) of respondents. Before the trials, 45% (285/636) offered SLND compared to 85% (543/638) after the trials. In the 556 respondents who reported knowledge of at least one trial, 310 (56%) currently offer SLND to >50% of patients, 175 (31%) offer to <50%, and 70 (13%) routinely perform axillary lymph node dissection. Respondents who reported knowledge of the trials but did not change their practice to incorporate SLND (n=67) cited concerns over lack of outcome data (64%), worries about FNR (42%), lack of resources (34%), or objections from radiation oncologists (25%), medical oncologists (18%), or other surgeons (8%).
Conclusions
The publication of trials evaluating SLND in clinically node positive patients has resulted in changes in practice. Concerns over the FNR and lack of outcome data limit incorporation of SLND into practice by some surgeons.
The presence of nodal metastases often guides treatment decisions in breast cancer. Many patients with clinically node-positive breast cancer receive neoadjuvant chemotherapy which can eradicate nodal disease in 40–75% of patients.1–5 Despite high rates of nodal pathologic complete response, standard practice has been to perform axillary lymph node dissection (ALND) upon completion of chemotherapy. While it is unlikely that performing extensive axillary surgery in patients without residual disease confers oncologic benefit, identifying patients who may not require ALND has been challenging. Initial reports addressing the use of sentinel lymph node dissection (SLND) after chemotherapy reported false negative rates (FNR) ranging from 5–30%. However, these were largely retrospective studies without standardized surgical techniques, therefore difficult to interpret.6–11
Prospective trials evaluating the accuracy of SLND after neoadjuvant chemotherapy in clinically node-positive patients have been completed. The American College of Surgeons Oncology Group (ACOSOG) Z1071 trial reported a FNR of 12.6% for SLND in patients with cN1 disease who had at least 2 sentinel lymph nodes (SLN) removed.12 While the trial did not meet its prespecified success threshold of 10%, subgroup analyses revealed that technical aspects of SLND could lower the FNR.12–14 The European SENTinel NeoAdjuvant (SENTINA) trial and the Canadian Sentinel Node Biopsy Following Neoadjuvant Chemotherapy (SN FNAC) trial, corroborated these findings.15,16 Techniques such as use of dual tracers (blue dye and radioisotope) and removal of >2 SLNs were shown to lower the FNR in all three trials. Additional factors were assessed in some of the trials including the use of ultrasound to assess nodal response, placing clips to mark nodes with biopsy-confirmed disease prior to chemotherapy and ensuring removal and evaluation after chemotherapy, as well as use of immunohistochemistry for pathologic evaluation.13–15,17
Since publication of these trials, some surgeons are offering SLND with the intent of omitting ALND if the pathology result is negative while others continue to routinely perform ALND. Amongst surgeons who have incorporated SLND into their practice, there is significant variation in techniques employed to minimize the FNR.18–21 The goal of this study was to determine surgeon familiarity with the trials and to determine if and how they have incorporated the results into their practice. We also sought to identify barriers and issues that have contributed to lack of acceptance of the trial results, which might elucidate opportunities for future studies.
Methods
The study involved a survey of members of the American Society of Breast Surgeons (ASBrS) conducted over a 4 week period. ASBrS members were sent an email invitation with a web-based link to the 11-question, anonymous survey (Figure 1). Questions assessed practice environment, years in practice, and knowledge of trial data. The remaining questions focused on surgeon practice before and after publication of the trials, relative importance of patient and technical aspects, and barriers to implementation.
Figure 1.
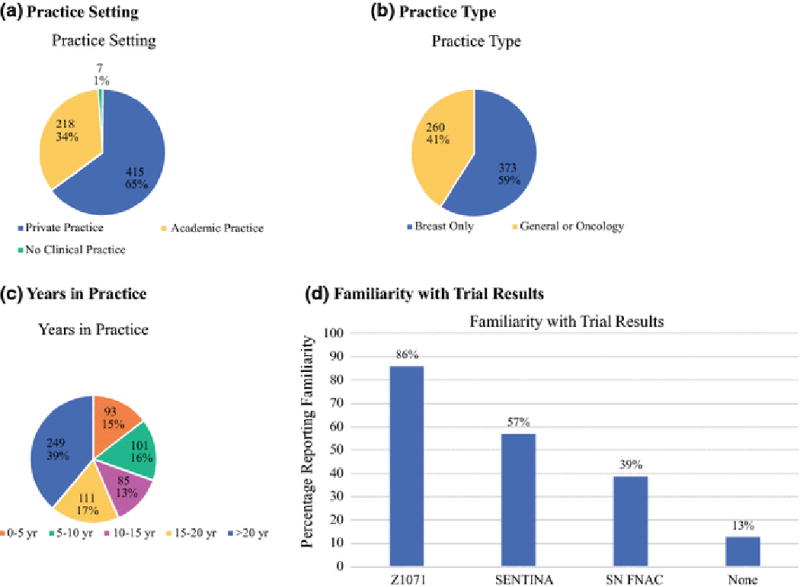
Characteristics of Survey Respondents
(a) Practice Setting
(b) Practice Type
(c) Years ibn Practice
(d) Practice Type
(e) Familiarity with Trial Results
Responses were received from 642 (21%) of 3090 eligible members. Results were summarized as proportions based on the number of responses to each specific question. Statistical comparison was performed using chi-square tests with a significance level of 0.05. (SAS Enterprise Guide 5.1, Cary, NC). The study was approved by the ASBrS Research Committee and Board of Directors, and the MD Anderson Institutional Review Board.
Results
Overall Findings
Survey results for the 642 respondents are summarized in table 1. Practice type and duration as well as trial familiarity are summarized in figure 1. Respondents reported familiarity with ACOSOG Z1071 (85.5%, n=549), SENTINA (57.0%, n=366), and SN-FNAC (39.4%, n=253). Thirteen percent (n=86) reported that they were not familiar with any of the trials, 27.6% (n=177) were familiar with one trial, 22.7% (n=146) were familiar with two, and 36.3% (n=233) were familiar with all three trials. The FNR of SLND reported in the trials was correctly identified by 52.7% (n=336/638) of respondents. Before the trials were published, 17.3% (110/636) offered SLND with possible omission of ALND in most patients and 27.5% (175/636) offered it in selected patients. After the trials, 53.6% (342/638) offered it to > 50% of their patients and 31.5% (201/638) offered it to selected patients. When considering whether to offer SLND, over half of respondents considered the number of abnormal nodes seen on initial ultrasound (62.3%, 349/560), nodal response seen on ultrasound (61.8%, 346/560), and whether the patient would receive adjuvant radiotherapy (63.2%, 354/560).
Table 1.
Survey results in all respondents
| Total Respondents | N=642 | ||
|---|---|---|---|
|
| |||
| The FNR of SLND is: | 638 Responses | ||
| <5% | 42 (7%) | ||
| 5–9% | 155 (24%) | ||
| 10–15% | 336 (53%) | ||
| >15% | 25 (4%) | ||
| Not familiar with the trial results | 80 (13%) | ||
|
| |||
| Use of SLND alone before publication of trials: | 636 Responses | ||
| Most of the time | 110 (17%) | ||
| In select patients | 175 (28%) | ||
| No | 351 (55%) | ||
|
| |||
| Currently offer SLND with possible omission of ALND: | 638 Responses | ||
| In the majority (>50%) | 342 (54%) | ||
| In select patients (<50%) | 201 (32%) | ||
| Perform ALND in all patients | 95 (15%) | ||
|
| |||
| Features considered in determining if eligible for SLND: | 560 Responses | ||
| Tumor size | 116 (21%) | ||
| Number of abnormal axillary nodes on ultrasound at diagnosis | 349 (62%) | ||
| Status of nodes on ultrasound after completing chemotherapy | 346 (62%) | ||
| Tumor subtype | 200 (36%) | ||
| Patient age | 235 (42%) | ||
| Planned postoperative radiation | 354 (63%) | ||
| Do not consider any of these | 43 (8%) | ||
|
| |||
| Technical aspects considered critical for SLND: | Yes | No | No Response |
| Dual tracer | 481 (86%) | 77 (14%) | 84 |
| Removal of ≥ 2 SLN | 332 (70%) | 139 (30%) | 171 |
| Removal of ≥3 SLN | 292 (61%) | 184 (39%) | 166 |
| Placing clip to mark biopsied node and ensured removal | 435 (82%) | 97 (18%) | 110 |
| Immunohistochemistry | 247 (50%) | 247 (50%) | 148 |
| Normalization of nodes on ultrasound | 247 (49%) | 258 (51%) | 137 |
|
| |||
| Routinely place clips to mark biopsied nodes: | 559 Responses | ||
| Yes | 373 (67%) | ||
| No | 186 (33%) | ||
|
| |||
| Intra-operative handling when clips are placed: | 386 Responses | ||
| Wire localization | 202 (52%) | ||
| Seed localization | 36 (9%) | ||
| Other localization technique | 38 (10%) | ||
| X-ray to confirm removal but no localization | 90 (23%) | ||
| No assessment for clipped node | 20 (5%) | ||
|
| |||
| Reasons SLND is not used: | 212 Responses | ||
| Concerns about false negative rate | 44 (21%) | ||
| Lack of oncologic outcome data | 112 (53%) | ||
| Do not have the institutional resources required | 64 (30%) | ||
| Resistance from medical oncologists | 56 (26%) | ||
| Resistance from radiation oncologists | 50 (24%) | ||
| Resistance from other surgeons | 32 (15%) | ||
| Institution is collecting data | 15 (7%) | ||
Surgeons identified multiple technical features they considered critical to performance (table 1). Two-thirds (373/559) of respondents routinely place clips in lymph nodes with biopsy-proven metastases prior to the initiation of chemotherapy. A total of 386 participants described their practice of assessment of the clipped node intra-operatively. The clip is localized for removal by 71.5% (276/386) using a wire (73.2%, 202/276), radioactive seed (13.0%, 36/276), or other localization method (13.8%, 38/276). The remaining respondents stated that either they do not localize the clip but use x-ray to confirm removal (81.8%, 90/110) or use no clip assessment (18.2%, 20/110). (Figure 2)
Figure 2.
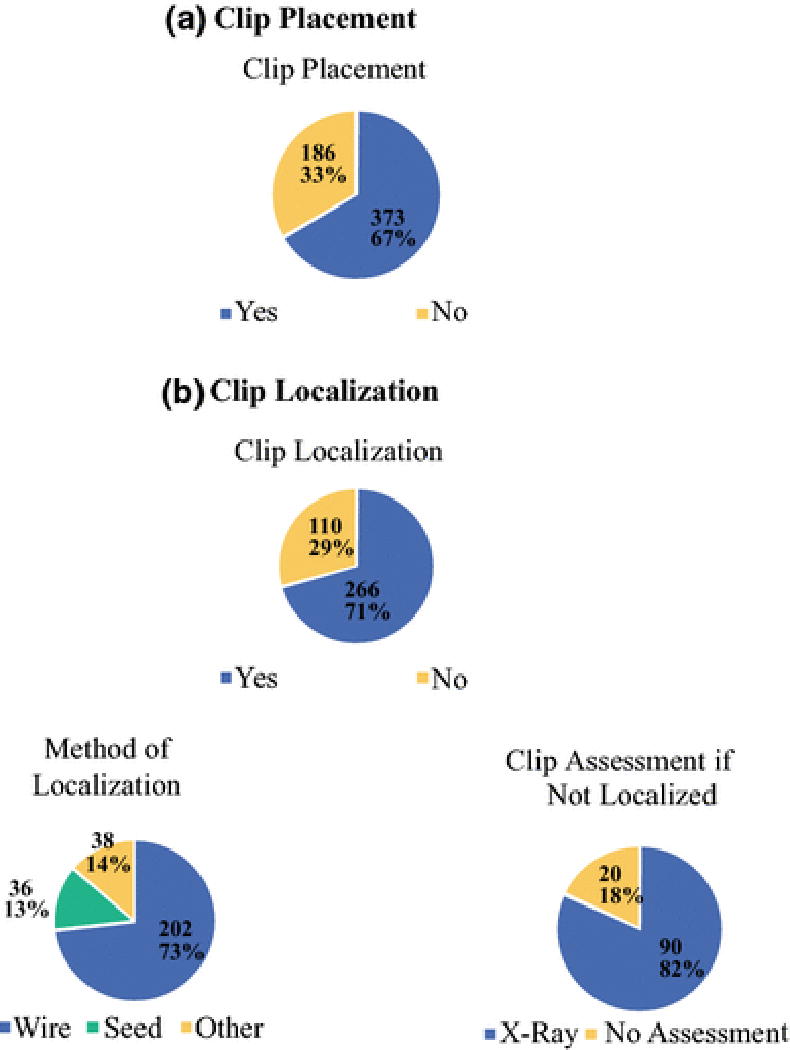
Use of Clips in Biopsied Lymph Nodes
(a) Clip Placement
(b) Clip Localization
There were 212 responses to the question assessing barriers to incorporation of SLND into practice. The primary concerns were lack of outcome data (52.8%, n=112), lack of resources (30.2%, n=64), resistance from medical oncology (26.4%, n=56) or radiation oncology (23.6%, n=50), and concerns about the FNR (20.8%, n=44).
Current Practice of Surgeons with Familiarity with Trials
Surgeons who reported knowledge of the trials were more likely to perform SLND currently (87.4%, 485/555) than those that did not know about the trials (69.2%, 54/78) (p<.0001). In order to assess how surgeons who have knowledge of the trials have decided to incorporate the data into practice, an analysis was performed limited to surgeons who reported familiarity with at least one trial categorized by their current practice (Table 2). Of 555 respondents familiar with a trial, 55.9% (n=310) offer SLND to > 50% of their patients with planned omission of ALND if SLNs are negative, 31.5% (n=175) to <50% of patients, and 12.6% (n=70) do not offer SLND to their patients with initial node-positive disease. Surgeons who reported knowledge of the trials but have not incorporated SLND into practice were more likely to identify the correct FNR of the trials (69.6%, 48/69) than those that offer SLND (57.4%, 278/484) although this did not reach statistical significance (p=0.07). A proportion of surgeons now incorporating SLND also offered SLND before publication of the trials. In those now offering SLND in > 50% of patients, 24.4% (75/308) offered it to most patients before the trials and 27.6% (85/308) offered it in selected patients. Amongst surgeons currently using SLND in < 50% of cases, 5.2% (9/174) offered it to most patients before the trials and 38.5% (67/174) in selected patients (p<0.0001).
Table 2.
Surgeons who reported knowledge of at least one trial characterized by their current practice
| Offer SLND in >50% of Patients N=310 |
Offer SLND in <50% of Patients N=175 |
Still Do ALND N= 70 |
P Value | |
|---|---|---|---|---|
|
| ||||
| Respondents familiar with: | ||||
| Z1071 | 306 (99%) | 174 (99%) | 69 (99%) | 0.7 |
| SENTINA | 207 (67%) | 108 (62%) | 50 (71%) | 0.3 |
| SN FNAC | 148 (48%) | 74 (42%) | 31 (44%) | 0.5 |
|
| ||||
| The FNR of SLND is: | 309 Responses | 175 Responses | 69 Responses | |
| <5% | 28 (9%) | 7 (4%) | 1 (1%) | 0.03 |
| 5–9% | 91 (29%) | 43 (25%) | 13 (19%) | |
| 10–15% | 171 (55%) | 107 (61%) | 48 (70%) | |
| >15% | 9 (3%) | 11 (6%) | 5 (7%) | |
| Not familiar with this data | 10 (3%) | 7 (4%) | 2 (3%) | |
|
| ||||
| Use of SLND alone before trials: | 308 Responses | 174 Responses | 70 Responses | |
| Most of the time | 75 (24%) | 9 (5%) | 0 | <0.000 |
| In select patients | 85 (28%) | 67 (39%) | 1 (1%) | 1 |
| No | 148 (48%) | 98 (56%) | 69 (99%) | |
|
| ||||
| Features considered in determining if eligible for SLND: | 310 Responses | 175 Responses | 10 Responses | |
| Tumor size | 54 (17%) | 46 (26%) | 2 (20%) | 0.07 |
| Number of abnormal nodes on US | 165 (53%) | 139 (79%) | 7 (70%) | <0.0001 |
| Ultrasound after completing NAC | 197 (64%) | 114 (65%) | 4 (40%) | 0.3 |
| Tumor subtype | 90 (29%) | 83 (47%) | 2 (20%) | 0.0001 |
| Patient age | 105 (34%) | 97 (55%) | 4 (40%) | <0.0001 |
| Plans for adjuvant radiation | 184 (59%) | 123 (70%) | 6 (60%) | 0.06 |
| Do not consider any of these | 31 (10%) | 3 (2%) | 2 (20%) | 0.001 |
|
| ||||
| Technical aspects considered critical: | 310 Responses | 175 Responses | 11 Responses | |
| Dual tracer | 367/307 (87%) | 156/174 (90%) | 8/11 (73%) | 0.2 |
| Removal of ≥2 SLNs | 186/257 (72%) | 103/147 (70%) | 5/9 (56%) | 0.5 |
| Removal of ≥3 SLNs | 166/265 (63%) | 97/147 (66%) | 7/11 (64%) | 0.8 |
| Placing clip and ensuring removal | 244/296 (82%) | 139/165 (84%) | 10/11 (91%) | 0.7 |
| Immunohistochemistry | 126/268 (47%) | 85/157 (54%) | 8/10 (80%) | 0.06 |
| Normalization of nodes on ultrasound | 135/279 (48%) | 90/159 (57%) | 3/11 (27%) | 0.07 |
|
| ||||
| Reasons SLND is not used: | 25 Responses | 80 Responses | 68 Responses | |
| Concerns about false negative rate | 1 (4%) | 10 (13%) | 29 (43%) | <0.001 |
| Lack of oncologic outcome data | 8 (32%) | 41 (51%) | 44 (65%) | 0.02 |
| Do not have institutional resources | 4 (16%) | 24 (30%) | 23 (34%) | 0.2 |
| Resistance from medical oncologists | 8 (32%) | 20 (25%) | 12 (18%) | 0.3 |
| Resistance from radiation oncologists | 9 (36%) | 16 (20%) | 17 (25%) | 0.3 |
| Resistance from other surgeons | 7 (28%) | 15 (19%) | 5 (7%) | 0.03 |
| Institution is collecting data | 1 (4%) | 9 (11%) | 5 (7%) | 0.5 |
There were significant differences noted between the groups in regards to patient selection factors for SLND eligibility with surgeons offering SLND to <50% of patients reporting many factors utilized in their decision to offer SLND. Surgeons performing SLND in <50% of patients were more likely to consider number of abnormal nodes on ultrasound (79.4% vs 53.2%,p<0.0001), tumor subtype (47.4% vs. 29.0%, p<0.0001), patient age (55.4% vs. 33.9%, p<0.0001), plans for adjuvant radiotherapy (70.3% vs. 59.4%, p=0.02) and tumor size (26.3% vs. 17.4%, p=0.02) than surgeons offering SLND to the majority of their patients. Surgeons who reported knowledge of the trials but did not incorporate SLND (n=68) cited concerns over lack of outcome data (64.7%, n=44), worries about FNR (42.6%, n=29), lack of resources (33.8%, n=23), or objections from radiation oncologists (25%, n=17), medical oncologists (17.6%, n=12), or other surgeons (7.4%, n=5) (Table 3).
Table 3.
Analysis limited to surgeons who did not perform SLND before the trials and reported knowledge of the trials. This is a comparison of those that changed their practice to incorporate SLND after the trials to those that did not change their practice (and still routinely do ALND)
| Changed Practice to Use SLND N=246 |
Did Not Change Practice (Still do ALND) N=69 |
P Value | |
|---|---|---|---|
|
| |||
| Familiar With Trials: | |||
| Z1071 | 245 (100%) | 68 (99%) | 1.0 |
| SENTINA | 175 (71%) | 49 (71%) | 1.0 |
| SN FNAC | 120 (49%) | 30 (44%) | 0.4 |
|
| |||
| The FNR of SLND is: | 245 Responses | 68 Responses | |
| <5% | 11 (5%) | 1 (2%) | 0.3 |
| 5–9% | 63 (26%) | 13 (19%) | |
| 10–15% | 159 (65%) | 47 (69%) | |
| >15% | 8 (3%) | 5 (7%) | |
| Not familiar with data | 4 (2%) | 2 (3%) | |
|
| |||
| Reasons SLND is not used: | 59 Responses | 67 Responses | |
| Concerns about false negative rate | 8 (14%) | 28 (42%) | <0.001 |
| Lack of oncologic outcome data | 29 (49%) | 43 (64%) | 0.1 |
| Do not have institutional resources | 14 (24%) | 23 (34%) | 0.2 |
| Resistance from medical oncologists | 12 (20%) | 12 (18%) | 0.7 |
| Resistance from radiation oncologists | 13 (22%) | 17 (25%) | 0.7 |
| Resistance from other surgeons | 11 (19%) | 5 (8%) | 0.1 |
| Institution is collecting data | 4 (7%) | 4 (6%) | 0.9 |
Impact of Trials on Surgeon Practice
Lastly, we evaluated surgeons who did not perform SLND before publication of the trials and reported knowledge of the trials to assess differences between those that decided to change their practice to incorporate SLND (78.1%, 246/315) compared to those that did not change their practice and continue to routinely perform ALND (21.9%, 69/315) (Table 3). There were no differences in regards to knowledge of specific trials or knowledge of the reported FNRs. Those that did not change their practice (and still routinely perform ALND) were more likely to show concern over the FNR (41.8%, 28/67) than those that now use SLND (13.6%, 8/59) (p=<.001). There were no significant differences regarding barriers to implementation.
Discussion
The publication of trials evaluating SLND in clinically node-positive patients who receive neoadjuvant chemotherapy has resulted in practice changes in a majority of surgeons who responded to the survey. While 45% of surgeons reported offering SLND with possible omission of ALND before the trials, 85% now offer it to at least some of their patients after chemotherapy. However, there is variation in patient selection and technical features thought to be critical for accuracy. There are also barriers to incorporation; primarily concerns over the lack of outcome data and reservations about the FNR.
One challenge of clinical trials is disseminating the data so that clinicians can determine if practice change is warranted. While 86% of respondents reported knowledge of ACOSOG Z1071, there was less awareness of the related SENTINA (57%) and SN FNAC (39%). However, even in respondents reporting knowledge of the trials, only 59% (326/553) accurately identified the FNR of SLND, the primary endpoint of the trials. Interestingly, those who knew of the trials but had decided not to change their practice to incorporate SLND were more likely to correctly identify the FNR. The majority (87%) of surgeons who knew about the trials now perform SLND in at least some patients. However, 69% of the surgeons who were not familiar with the trials are also performing SLND pointing to routes of practice change other than direct knowledge of clinical trials.
Important lessons learned from these trials were that surgical technique impacts the accuracy of SLND. For instance, the use of dual tracers in ACOSOG Z1071 decreased the FNR from 20.3% to 10.8%.12 Similar results were seen in SENTINA (16% to 8.6%)16 and SN FNAC (16% to 5.2%).15 In addition, retrieval of at least 3 SLNs lowered the FNR to 9% from 31% if one SLN was removed in ACOSOG Z1071.12 In the SENTINA trial, the removal of one SLN resulted in a FNR of 24% which was reduced to 5% if 3 or more nodes were removed.16 The SN FNAC trial corroborated this finding with a FNR of 18% with one SLN removed compared to 5% if at least 2 were removed.15 More intense pathologic evaluation with immunohistochemistry (IHC) reduced the FNR from 12.6% to 8.7% in ACOSOG Z1071 and from 13.3% to 8.4% in the SN FNAC trial.14,15 The investigators of the Z1071 trial have also recommended the use of axillary ultrasound after chemotherapy, noting that the FNR could have potentially been reduced to 9.8% if SLND had only been performed on patients with a normal axillary ultrasound at the completion of chemotherapy.17 However, the SENTINA investigators concluded that the combination of palpation and axillary ultrasound after chemotherapy did not reliably assess response.22 Our survey shows that surgeons recognize the limitations of the available ultrasound data as only half of respondents felt that ultrasound was critical. In contrast, surgeons recognize the importance of technical aspects such as use of dual tracer technique (86%) and removal of at least 2 SLNs (70%).
Additionally, data reported from the ACOSOG Z1071 trial demonstrated that placement of a clip in nodes with biopsy-proven metastases may be useful. In that study, 170 patients had a clip placed in the lymph node containing metastases at the time of initial biopsy. In the 107 patients where the clipped node was retrieved as a SLN, the FNR was 6.8% (95% CI 1.9–16.5%).13 A recent study from our institution also showed utility in placing clips in lymph nodes with biopsy-proven metastases and ensuring removal of these nodes for evaluation. In our study, the FNR for SLND alone was 10.1% (95% CI 4.2–19.8). Evaluation of the clipped node alone had a FNR of 4.2% (95% CI 1.4–9.5).23 When we assured removal of all SLNs and the clipped node, the FNR was reduced to 1.4% (95% CI 0.03–7.3). In 23% of patients, the clipped node was not a SLN. We have therefore proposed targeted axillary dissection, which entails the removal of SLNs as well as selective localization and removal of clipped nodes.23,24 In this survey, 82% of surgeons stated that placing clips and ensuring removal was critical for the accuracy of SLND. The survey showed that 67% now routinely place clips in biopsied nodes and many selectively remove it with localization.
This survey also confirmed barriers to acceptance of the trial data and incorporation of SLND in these patients in clinical practice. The primary concerns were hesitation about the FNR and lack of outcomes data. With a FNR of 12.6%, the ACOSOG Z1017 trial did not meet its threshold of 10%.12 This has prompted many to question whether SLND should be incorporated into practice based on a negative trial. While the techniques mentioned above decreased the FNR, conclusions must be based on subgroup analyses which have insufficient power to be definitive. There is currently no data to know the true FNR when all of the optimal technical factors are used. In addition, there is no outcome data available to assess the oncologic safety of omission of ALND after nodal conversion. Finally other than limiting to patients with clinical N1 disease, there is limited data to guide surgeons in selecting which patients would be appropriate candidates. Surgeons with knowledge of the trials that currently offer SLND selectively were more likely to consider tumor subtype, patient age, and the number of abnormal nodes in their decision to offer SLND then those that offer SLND routinely.
The primary limitations to this study are related to sampling. Only ASBrS members were invited to complete this survey. Given membership in this breast-specific organization, members are more likely to be familiar with breast cancer trials. Because it was a voluntary study with a 21% response rate, there is selection bias for the surgeons that chose to participate. It is likely that a survey of national practice patterns might show a different level of incorporation into practice. However, the surgical management of breast cancer patients is increasingly ascribed to surgeons with a breast focus, surgeons likely to be members of ASBrS. One publication based on the American Board of Surgery recertification data estimated that 90% of breast cancer surgery was performed by 25% of practicing surgeons.25 As with any survey, answers had to be categorized and may not accurately assess nuances associated with practice decisions. Lastly, the goal of this survey was to assess practice patterns, but the scope does not allow for recommendations on the use of SLND or the optimal technique if performed.
In conclusion, the majority of surgeons responding to the survey report familiarity with the recently published trials evaluating the use of SLND for axillary staging in clinically node-positive breast cancer patients who receive neoadjuvant chemotherapy. These trials have resulted in practice changes, although many surgeons have chosen to incorporate the results in a selective manner. There remain concerns over the FNR and of the lack of oncologic outcome data which could present opportunity for future study.
Synopsis.
Trials have evaluated the accuracy of SLND in clinically node-positive patients after chemotherapy. This survey of American Society of Breast Surgeons members reports that 45% (285/636) offered SLND before the trials compared to 85% (543/638) after the trials.
Acknowledgments
Laura Randel and the American Society of Breast Surgeons provided the administrative support for the survey. Grant Support was provided by a Cancer Center Support Grant from the NIH (CA16672).
Appendix 1 – Survey sent to American Society of Breast Surgeons membership
- Which best describes your clinical practice?
- Private practice general or oncologic surgeon who performs breast surgeries
- Private practice breast-only surgeon
- Academic general or oncologic surgeon who performs breast surgeries
- Academic breast-only surgeon
- I do not provide clinical care
- How long have you been in clinical practice?
- 0 to 5 years
- 5 to 10 years
- 10 to 15 years
- 15 to 20 years
- Greater than 20 years
- Are you familiar with these recent multi-institutional trials evaluating the accuracy of sentinel lymph node biopsy in clinically node positive breast cancer patients who receive neoadjuvant therapy? Please check all trials with which you are familiar:
- Yes, I am familiar with the ACOSOG Z1071 trial results
- Yes, I am familiar with the SENTINA trial results
- Yes, I am familiar with the SN FNAC trial results
- No, I am not familiar with any of these trial result
- The ACOSOG Z1071, SENTINA and SN FNAC trials all enrolled patients with clinically node positive breast cancer who received neoadjuvant chemotherapy then went on to SLND with planned completion ALND in order to determine the false negative rate of SLND. For patients who presented with clinical N1 (cN1) disease and had their SLN(s) examined by hematoxylin and eosin staining, the false negative rates in all of these studies was:
- Less than 5%
- 5% – 9%
- 10%–15%
- >15%
- I am not familiar with these trial results
- Before publication of these trial results, did you perform SLND on clinically node positive patients after neoadjuvant chemotherapy with the intent to omit axillary lymph node dissection (ALND) if no residual disease was identified in the SLN(s)?
- Most of the time
- In select patients
- No, my standard practice was to perform ALND
- What is your current practice in regards to surgical management of clinically node positive patients (cN1) who receive neoadjuvant chemotherapy?
- In the majority of patients (>50%), I perform SLND with the intent of omitting ALND if no disease is identified in the SLN(s)
- In a select group of patients (<50%), I perform SLND with the intent of omitting ALND if no residual disease is identified in the SLN(s)
- My standard practice is to perform ALND in all patients (Skip to question 11)
- The aim of the following question is to determine which clinicopathologic features impact your pre-operative decision in determining whether a patient is appropriate for SLND and consideration of omission of ALND if no metastases are seen in the SLN(s) after neoadjuvant therapy. Please check all variables that you consider when determining eligibility for SLND in these patients: (Please check all that apply)
- Primary tumor size
- Number of abnormal axillary lymph nodes seen on US performed at the time of diagnosis before initiation of neoadjuvant chemotherapy
- Status of axillary lymph nodes seen on US performed preoperatively after completion of neoadjuvant chemotherapy
- Tumor subtype (Hormone receptor positive, HER2 positive, triple negative)
- Patient age
- Planned postoperative radiation
- I do not consider any of these variables in my decision
- The following question is to understand technical aspects that you consider crucial to the accuracy of SLND in clinically node positive patients who receive neoadjuvant chemotherapy. I believe that the following components must be in place for SLND results to be accurate:
Dual tracer technique (i.e. blue dye and radioisotope) Yes No Removal of ≥ 2 SLNs Yes No Removal of ≥ 3 SLNs Yes No The biopsied node has a clip placed at the time of diagnosis and removal of the clipped node at surgery is confirmed Yes No Immunohistochemistry is performed to confirm no residual metastasis Yes No Preoperative ultrasonography following neoadjuvant chemotherapy showing normalization of nodes Yes No Do you routinely have a clip placed in axillary nodes with biopsy-proven metastases? (If your answer is no, please skip to question #11.) Yes No - If a clip is placed in the biopsied node, how is this clipped node handled intra-operatively?
- We place clips but do not assess for their removal at surgery
- I do not selectively remove the clipped node, but I perform an x-ray of the nodes to confirm clip removal
- I localize clipped nodes with wire/needle localization
- I localize clipped nodes with I125 seeds
- I localize clipped nodes with a method other than wire or seed localization
- We do not place clips in nodes
- 11. If you do not rely on SLND to stage clinically node positive patients after neoadjuvant chemotherapy, what has limited your use of this technique? (Please check all that apply)
- I do not feel the reported false negative rates for SLND are low enough to accurately assess axillary nodes after neoadjuvant chemotherapy
- I do not feel that we have adequate data regarding the long-term, oncologic outcomes when ALND is omitted in these patients
- I feel that SLND may be appropriate in some patients, but my institution does not have the resources that I feel are essential to accuracy of the technique (such as clip placement in biopsied nodes, or inability to localize clipped nodes)
- I feel that SLND may be appropriate in some patients, but medical oncologists in my institution do not feel this is appropriate
- I feel that SLND may be appropriate in some patients, but radiation oncologists in my institution do not feel this is appropriate
- I feel that SLND may be appropriate in some patients, but other surgeons in my practice do not feel this is appropriate
- My institution is currently collecting internal data to determine the FNR of SLND in our institution
- I use SLND in this population with omission of ALND when no metastases are identified
Footnotes
The authors have no financial disclosures
References
- 1.Boughey J, McCall L, Ballman K, et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial. Ann Surg. 2014 Oct;260(4):608–614. doi: 10.1097/SLA.0000000000000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuerer H, Sahin A, Hunt K, et al. Incidence and impact of documented eradication of breast cancer axillary lymph node metastases before surgery in patients treated with neoadjuvant chemotherapy. Ann Surg. 1999 Jul;230(1):72–78. doi: 10.1097/00000658-199907000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buzdar A, Ibrahim N, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005 Jun 1;23(16):3676–3684. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 4.Dominici L, Mittendorf E, Wang X, et al. Implications of constructed biologic subtype and its relationship to locoregional recurrence following mastectomy. Breast Cancer Res. 2012 May 23;14(3):R82. doi: 10.1186/bcr3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hennessy B, Hortobagyi G, Rouzier R, et al. Outcome after pathologic complete eradication of cytologically proven breast cancer axillary node metastases following primary chemotherapy. J Clin Oncol. 2005 Dec 20;23(36):9304–9311. doi: 10.1200/JCO.2005.02.5023. [DOI] [PubMed] [Google Scholar]
- 6.Alvarado R, Yi M, Le-Petross H, et al. The Role for Sentinel Lymph Node Dissection after Neoadjuvant Chemotherapy in Patients who Present with Node-Positive Breast Cancer. Ann Surg Oncol. 2012 Oct;19(10):3177–3183. doi: 10.1245/s10434-012-2484-2. [DOI] [PubMed] [Google Scholar]
- 7.Classe J, Bordes V, Campion L, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy for advanced breast cancer: results of Ganglion Sentinelle et Chimiotherapie Neoadjuvante, a French prospective multicentric study. J Clin Oncol. 2009 Feb 10;27(5):726–732. doi: 10.1200/JCO.2008.18.3228. [DOI] [PubMed] [Google Scholar]
- 8.Newman E, Sabel M, Nees A, et al. Sentinel lymph node biopsy performed after neoadjuvant chemotherapy is accurate in patients with documented node-positive breast cancer at presentation. Ann Sug Oncol. 2007;14(10):2946–2952. doi: 10.1245/s10434-007-9403-y. [DOI] [PubMed] [Google Scholar]
- 9.Mamounas E, Brown A, Anderson S, et al. Sentinel node biopsy after neoadjuvant chemotherapy in breast cancer: results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2005;23(12):2694–2702. doi: 10.1200/JCO.2005.05.188. [DOI] [PubMed] [Google Scholar]
- 10.Balch G, Mithani S, Richards K, Beauchamp R, Kelley M. Lymphatic mapping and sentinel lymphadenectomy after preoperative therapy for stage II and III breast cancer. Ann Surg Oncol. 2003 Jul;10(6):616–621. doi: 10.1245/aso.2003.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Vriens B, Keymeulen K, Kroep J, et al. Axillary staging in breast cancer patients treated with neoadjuvant chemotherapy in two Dutch phase III studies. Oncotarget. 2017 doi: 10.18632/oncotarget.15101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boughey J, Suman V, Mittendorf E, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013 Oct 9;310(14):1455–1461. doi: 10.1001/jama.2013.278932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boughey J, Ballman K, Le-Petross H, et al. Identification and Resection of Clipped Node Decreases the False-negative Rate of Sentinel Lymph Node Surgery in Patients Presenting With Node-positive Breast Cancer (T0-T4, N1-N2) Who Receive Neoadjuvant Chemotherapy: Results From ACOSOG Z1071 (Alliance) Ann Surg. 2016 Apr;263(4):802–807. doi: 10.1097/SLA.0000000000001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boughey J, Ballman K, Symmans W, et al. Methods impacting the false negative rate of sentinel lymph node surgery in patients presenting with node positive breast cancer (T0-T4,N1-2) who receive neoadjuvant chemotherapy – Results from a prospective trial – ACOSOG Z1071 (Alliance) San Antonio Breast Cancer Symposium 2014. 2014 Poster Presentation. Available at: http://eposter.abstractsonline.com/sabcs. Accessed 1/31/2015.
- 15.Boileau JF, Poirier B, Basik M, et al. Sentinel Node Biopsy After Neoadjuvant Chemotherapy in Biopsy-Proven Node-Positive Breast Cancer: The SN FNAC Study. J Clin Oncol. 2015 Jan 20;33(3):258–264. doi: 10.1200/JCO.2014.55.7827. [DOI] [PubMed] [Google Scholar]
- 16.Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013 Jun;14(7):609–618. doi: 10.1016/S1470-2045(13)70166-9. [DOI] [PubMed] [Google Scholar]
- 17.Boughey J, Ballman K, Hunt K, et al. Axillary Ultrasound After Neoadjuvant Chemotherapy and Its Impact on Sentinel Lymph Node Surgery: Results From the American College of Surgeons Oncology Group Z1071 Trial (Alliance) J Clin Oncol. 2015 Oct 20;33(30):3368–3393. doi: 10.1200/JCO.2014.57.8401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilewskie M, Morrow M. Axillary Nodal Management Following Neoadjuvant Chemotherapy: A Review. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2016.4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mittendorf E, Caudle A, Yang W, et al. Implementation of the american college of surgeons oncology group z1071 trial data in clinical practice: is there a way forward for sentinel lymph node dissection in clinically node-positive breast cancer patients treated with neoadjuvant chemotherapy? Ann Surg Oncol. 2014 Aug;21(8):2468–2473. doi: 10.1245/s10434-014-3775-6. [DOI] [PubMed] [Google Scholar]
- 20.Mamtani A, Barrio A, Van Zee K, et al. How Often Does Neoadjuvant Chemotherapy Avoid Axillary Dissection in Patients With Histologically Confirmed Nodal Metastases? Results of a Prospective Study. Ann Surg Oncol. 2016 Oct;23(11):3467–3474. doi: 10.1245/s10434-016-5246-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jatoi I, Benson J, Toi M. De-escalation of axillary surgery in early breast cancer. Lancet Oncol. 2016 Oct;17(10):e430–441. doi: 10.1016/S1470-2045(16)30311-4. [DOI] [PubMed] [Google Scholar]
- 22.Schwentner L, Helms G, Neklijudova V, et al. Using ultrasound and palpation for predicting axillary lymph node status following neoadjuvant chemotherapy - Results from the multi-center SENTINA trial. Breast. 2017 Feb;31:202–207. doi: 10.1016/j.breast.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 23.Caudle A, Yang W, Krishnamurthy S, et al. Improved Axillary Evaluation Following Neoadjuvant Therapy for Patients With Node-Positive Breast Cancer Using Selective Evaluation of Clipped Nodes: Implementation of Targeted Axillary Dissection. J Clin Oncol. 2016 Apr 1;34(10):1072–1078. doi: 10.1200/JCO.2015.64.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caudle A, Yang W, Mittendorf E, et al. Selective Surgical Localization of Axillary Lymph Nodes Containing Metastases in Patients With Breast Cancer: A Prospective Feasibility Trial. JAMA Surg. 2014 Apr 1;34(10):1072–1078. doi: 10.1001/jamasurg.2014.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pass H, Klimberg S, Copeland E. Are “Breast-Focused” Surgeons More Competent? Ann Surg Oncol. 2008;15(4):953–955. doi: 10.1245/s10434-008-9835-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


