Abstract
Weight gain after smoking cessation can lessen the health benefits of, and reduce the incentives for, quitting smoking. Randomized clinical trials of smoking cessation have estimated this weight gain only over short periods of follow-up. We provide an estimate of long-term post-cessation weight gain in the Framingham Heart Study, a prospective observational study. We identified 2001 smokers free of diabetes, cancer, and cardiovascular disease in 1952. Using the parametric g-formula we estimated mean weight in 1972 if all smokers had quit at baseline versus if all had continued smoking. Our estimates were adjusted for demographic, socio-economic, and clinical factors at baseline and during follow-up. The estimated mean weight (95% CI) at 20-years if all smokers had quit smoking was 75.2 kg (73.5, 76.6), compared with 70.2 kg (68.7, 71.8) if they had smoked 20 cigarettes/day and 73.4 kg (71.9, 74.6) if they had smoked 5 cigarettes/day (i.e. an estimated mean weight gain of 5.1 kg (3.1, 6.6) and 1.8 kg (0.8, 2.8), respectively). Smokers who were overweight or obese at baseline had a greater post-cessation weight gain on average. Our estimates suggest that smoking cessation can result in increases in body weight over 20 years. While the benefits of smoking cessation outweigh the risks due to post-cessation weight gain, our results highlight the need for long-term weight management interventions in combination with smoking cessation.
Keywords: parametric g-formula, smoking cessation, weight gain, epidemiologic methods
Introduction
Smoking remains the leading cause of preventable death in the United States [1]. Smoking cessation reduces the risk of cardiovascular disease (CVD) and increases life expectancy [2], but may also cause weight gain. This weight gain is a major reason for smoking recidivism and reduces incentives for smokers to quit [3,4] it may also lessen some of the health benefits of quitting smoking [5-9]. An accurate estimate of the long-term weight gain after smoking cessation is essential both for effectively designing weight-control interventions in those who quit smoking, and for assessing the potential risks associated with this weight gain.
Randomized clinical trials of smoking cessation have only studied short-term weight gain (4 to 5 kg in the first year following smoking cessation) and have been mostly conducted on smokers seeking professional assistance for cessation [10-12]. Thus the results may not be generalizable to the majority of smokers, many of whom quit by themselves [13,14]. Observational studies have studied slightly longer periods ranging from 4 to11 years, and have estimated weight gains of 3 to 6 kg in quitters compared with continuing smokers [15-20]. A recent meta-analysis of population-based studies of weight gain post smoking-cessation found a mean difference between quitters and continuing smokers of 2.6 kg over an average of 5 years of follow-up [21].
The long-term (over a 20-year period) effect of smoking cessation on body weight has not been studied. A valid estimation of this long-term effect requires both measuring time-varying confounders and using appropriate statistical techniques to adjust for them. For example, consider the time-varying confounder “coronary heart disease” (CHD), which leads to both changes in weight and smoking cessation, but it is also affected by prior smoking history. Standard techniques—e.g., including both CHD status and smoking status in a regression model for weight, regardless of whether smoking status is updated after CHD diagnosis—cannot appropriately handle time-varying confounders affected by prior exposure and may lead to bias [22]. Therefore, a method that appropriately adjusts for measured time-varying confounders, like the parametric g-formula, is needed.
Here we provide estimates of the effects of smoking cessation on 20-year weight gain. To do so, we implemented the parametric g-formula to estimate the mean weight in smokers from the Framingham Heart Study after a 20-year smoking cessation strategy.
Methods
Study population
The Framingham Heart Study (FHS) is a longitudinal cohort study that began in Framingham, Massachusetts in 1948. Investigators recruited 5,209 participants between the ages of 30-62 years, who had no history of MI or stroke and no overt symptoms of cardiovascular disease. Participants underwent clinical examinations approximately every two years during which a detailed medical history was collected, as well as information regarding potential CVD risk factors. Blood samples were collected for standard laboratory measures such as lipid and glucose levels. Further details on the design of FHS are available elsewhere [23,24]. We used the FHS limited access data set provided by the U.S. National Heart, Lung and Blood Institute of the National Institutes of Health.
We defined the FHS exam 3 (conducted during1952-1956) as our baseline so that data from exams 1 and 2 could be used to adjust for pre-baseline covariates. Participants who had died, had incomplete follow-up (defined as a missing exam), or were diagnosed with diabetes, cancer (except non-melanoma skin cancer), or cardiovascular disease (CVD) at or before exam 3 were excluded. Additionally, participants were excluded at exam 3 if they had missing data on body mass index (BMI), height, cigarettes smoked per day, systolic blood pressure or total cholesterol. Eligible smokers were followed from exam 3 (baseline) until death, censoring due to incomplete follow-up (defined as a single missed examination) or missing time-varying covariates (see below), or exam 13 (conducted during 1972-1976). Our outcome variable is weight in kilograms 20 years after baseline at exam 13, which we calculated using the recorded height and BMI. For simplicity, below we refer to the original FHS exam 3 as exam 0 (our baseline exam), and original FHS exam 13 as exam 10 (end of follow-up).
The average number of cigarettes smoked per day, was self-reported by the participants at each visit. Baseline covariates included age (years), height (meters), sex, occupation before retirement (executive/supervisory, technical, laborer, clerical, sales, housewife), education (≤ 8th grade, some high school, high school graduate, some college, college graduate, post-graduate), and marital status (single, married, widowed/divorced). In our primary analysis, time-varying covariates included weight (kg) at the previous exam; systolic blood pressure (mm/hg), calculated as an average of three resting measures; and total non-fasting cholesterol (mg/dl), measured using the non-enzymatic Abell-Kendall method in either serum or plasma samples [25]. In addition, we used time-varying indicator variables for CVD diagnosis (as defined above) and menopause in women (defined as one year without a menstruation cycle). CVD was defined as fatal or nonfatal myocardial infarction, cerebral vascular disease (cerebral embolism, intracerebral or subarachnoid hemorrhage), or congestive heart failure.
For weight, cigarettes per day, and the time-varying covariates, we carried forward the last observed value for one exam period. Participants were censored at the second exam with missing values. In sensitivity analysis, we included the time-varying covariates: antihypertensive medication use (yes/no) and indicator variables for diabetes and cancer. Diabetes was defined using the FHS definition of abnormal non-fasting blood glucose (≥140 mg/dl), or current use of insulin or oral hypoglycemic agents (this definition also includes pre-diabetes). These variables were not used in primary analysis due to the high number of missing data (>10% in some exams).
Smoking scenarios
We estimated the mean weight if all eligible individuals had followed each of the five following strategies for 20 years after baseline: “quit smoking”, “smoke 5 cigarettes per day”, “smoke 10 cigarettes per day”, “smoke 15 cigarettes per day”, and “smoke 20 cigarettes per day”. We also estimated the mean weight after 20 years following a “do nothing” strategy, i.e., the natural course.
Statistical Analysis: the parametric g-formula
The parametric g-formula is a generalized version of standardization that provides unbiased estimates under the assumptions of no unmeasured confounding, no measurement error, and no model misspecification [26,27]. An outline of the parametric g-formula algorithm is as follows:
Fit parametric regression models for the time-varying covariates at each follow-up time k as a function of treatment (cigarettes per day) and covariate history, among individuals under follow up at k.
Fit a parametric model for weight at 20 years as a function of treatment and covariate history among individuals under follow up at 20 years.
- Use a Monte Carlo simulation to generate a 10,000-individual population under each of the treatment strategies. For each individual:
- The values of baseline covariates at k=0 are randomly sampled with replacement from the individuals in the original study population.
- The values of the time-varying covariates at k>0 are drawn from the parametric distribution estimated in Step A.
- The value of the cigarettes per day is set to the value specified by the strategy, e.g., zero at all times k for the smoking cessation intervention.
- The covariate-specific mean weight at 20 years is estimated from the model in Step B.
Compute the mean weight at 20 years in the population under each strategy, and the mean differences between strategies.
Repeat above steps in 500 bootstrap samples in order to obtain 95% confidence intervals (CI).
In models for step A, we included indicator variables for exam, baseline covariates, and time-varying covariates measured at the previous two exams. When a time-varying covariate was not assessed at all exams, only the most recent measurement and a product (“interaction”) term between that measurement and time since measurement was added to the model. In order to predict the joint distribution of time-varying covariates measured at the same exam, k, we modeled each successive covariate as a function of the concurrently measured and previously modeled covariate(s). We chose an arbitrary covariate ordering and explored the robustness of the estimates in sensitivity analyses with alternative orderings. In the model for step B, we included baseline covariates, time-varying covariates from the end of follow-up exam and the previous two exams. Details of the functional form of the models are provided in Online ResourceTable 1.
To assess potential model extrapolation for each strategy we also estimated (1) the cumulative proportion of smokers who would have to change the number of cigarettes they smoked per day at any time during the follow-up to adhere to the strategy, and (2) the average proportion of smokers who would have to change their smoking status at each exam to keep adhering to the strategy.
Our estimates of 20-year weight under each strategy implicitly include an intervention to remove censoring due to loss to follow-up or death [27]. If censoring is non-informative, the observed distribution of outcome and covariates should equal that estimated by the parametric g-formula under no intervention on smoking (i.e., the natural course) when a parametric model for smoking is added to step A. We therefore compared the observed and simulated natural course means for each time-varying covariate and the outcome. Any discrepancies between these values could indicate the presence of informative censoring or misspecification in our models in steps A and B.
We examined effect modification by intensity of smoking at baseline, sex, baseline BMI, and age in sub-group analyses. We conducted sensitivity analyses in which (i) we varied the ordering of the time-varying covariates in our models, and (ii) included three additional time-varying covariates (diabetes, cancer, and use of antihypertensive medication), which were not included in primary analysis due to a large number of missing values. All analyses were conducted using SAS 9.4 software and the GFORMULA macro publically available at http://www.hsph.harvard.edu/causal/software. The SAS code for the GFORMULA macro call for our primary analysis is shown in Online Resource Figure 1.
Results
Our analyses included 2001 eligible smokers (Figure 1). Over the 20-year follow-up period, 239 (12%) died, 776 (39%) were censored due to incomplete follow-up, and 79 (0.04%) were censored due to missing data on time-varying covariates. At baseline the average age was 46.0 years, 55% of participants were male, and the average number of cigarettes smoked per day was 18 (Table 1). Mean weight was 69.0 kg at baseline and 72.4 kg after 20 years (average weight gain of 3.4 kg). Our models closely simulated the observed data (Online Resource Figures 2a-e):the estimated 20-year mean weight (95% CI) under the natural course was 72.7 kg (71.3, 74.0).
Fig. 1.
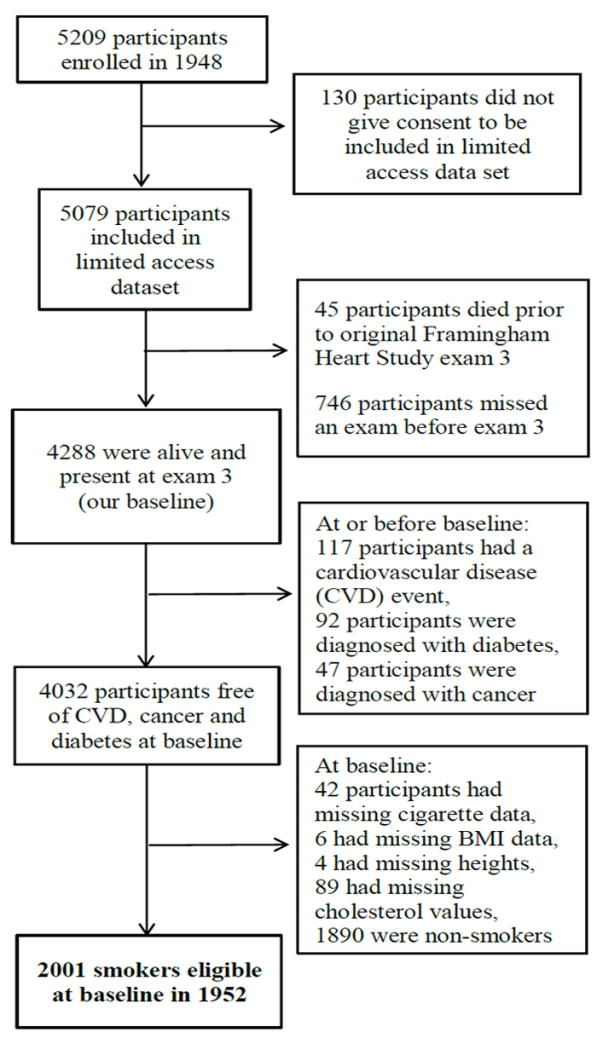
Flowchart for selection of study participants from the Framingham Heart Study
Table 1.
Baseline characteristics of 2001 eligible smokers, Framingham Heart Study, year 1952a
| Characteristics | |
|---|---|
| Age (years) mean ± std | 46 ± 8 |
| Male | 1105 (55) |
| Weight (kg) mean ± std | 69.0 ± 12.9 |
| Body Mass Index (BMI) mean ± std | 25.0 ± 3.8 |
| Normal weight (BMI < 25 kg/m2) | 1054 (53) |
| Overweight (25 kg/m2 ≤ BMI <30 kg/m2) | 769 (38) |
| Obese (BMI ≥ 30 kg/m2) | 178 (9) |
| Height (m) mean ± std | 1.7 ± 0.1 |
| Cigarettes smoked per day, mean ± std | 18 ± 11 |
| Marital Status | |
| Married | 1783 (89) |
| Single | 126 (6) |
| Widowed/Divorced | 92 (5) |
| Education b | |
| 8th grade or less | 441 (22) |
| Some high school | 312 (16) |
| High school graduate | 662 (33) |
| Some College | 157 (8) |
| College graduate | 160 (8) |
| Post graduate | 231 (12) |
| Employment before retirement c | |
| Executive/Supervisory/Technical | 393 (20) |
| Technical | 117 (6) |
| Laborer | 502 (25) |
| Clerical | 113 (6) |
| Sales | 87 (4) |
| Housewife | 542 (27) |
| Total cholesterol (mg/dl) mean ± std | 228 ± 50 |
| Systolic blood pressure (mmHg) mean ± std | 127 ± 19 |
| Menopause, women only | 421 (47) |
Values are N (%) of baseline population unless otherwise specified. STD is standard deviation.
Missing in 38 (2%) participants
Missing in 249 (12%) participants
The estimated 20-year mean weight was 75.2 kg (73.5, 76.6) under the smoking cessation strategy, 73.4 kg (71.9, 74.6) if smoking 5 cigarettes per day, 71.9 kg (70.3, 73.4) if smoking 10 cigarettes per day, 70.8 kg (69.3, 72.4) if smoking 15 cigarettes per day, and 70.2 kg (68.7, 71.8) if smoking 20 cigarettes per day (Table 2). That is, enforcing smoking cessation strategy resulted in an estimated weight gain of 1.8 kg compared with enforcing smoking 5 cigarettes per day and 5.1 kg (3.1, 6.6) compared with enforcing smoking 20 cigarettes per day (Table 2). We estimated that maintaining adherence to the smoking cessation strategy in this particular population would require changing the smoking status of an average of 18% of participants at each exam period. Sustaining the smoking strategies in this population would require changing the smoking status of an average of 80% of participants at each exam period (Table 2).
Table 2.
Estimated mean weight and weight gain after 20 years of following five cigarette smoking strategies, 2001 smokers from the Framingham Heart Study a
| Strategy | Mean 20-year weight, kg (95% CI) | Mean 20-year weight gain, kg (95% CI)b | Cumulative % intervened on | Average % intervened on |
|---|---|---|---|---|
| Smoking cessation | 75.2 (73.5, 76.6) | - | 100.0 | 17.6 |
| Smoke 5 cigarettes per day | 73.4 (71.9, 74.6) | 1.8 (0.8, 2.8) | 100.0 | 80.3 |
| Smoke 10 cigarettes per day | 71.9 (70.3, 73.4) | 3.3 (1.7, 4.6) | 100.0 | 80.9 |
| Smoke 15 cigarettes per day | 70.8 (69.3, 72.4) | 4.4 (2.5, 5.8) | 100.0 | 80.7 |
| Smoke 20 cigarettes per day | 70.2 (68.7, 71.8) | 5.1 (3.1, 6.6) | 100.0 | 78.6 |
Estimated using the parametric g-formula with fixed covariates: age, height, occupation, education, and marital status; and time-varying covariates: lagged weight, cigarettes smoked per day, systolic blood pressure, total cholesterol, and cardiovascular disease diagnosis.
20-year weight gain post smoking cessation, with the cigarette smoking strategy as reference.
The estimated weight gain comparing smoking cessation with smoking 20 cigarettes per day was greater in participants who were overweight/obese at baseline; they had a post-cessation weight gain of 6.7 kg (4.1, 9.3) compared to 3.3 kg (1.5, 5.2) for participants with a normal BMI (p=0.04) (Table 3). Those younger, and who smoked more than 20 cigarettes (one pack) per day at baseline also gained more weight post smoking cessation, however, the 95% confidence intervals for these differences were wide.
Table 3.
Estimated mean weight and weight gain at 20 years of follow-up comparing smoking cessation strategy to continued smoking by subgroups defined at baseline, 2001 smokers from the Framingham Heart Studya
| Subgroup | Mean Weight, kg (95% CI) | Mean Weight Gain, kg (95% CI) | p-value for Heterogeneity | |
|---|---|---|---|---|
|
| ||||
| Smoke 20 cigarettes/day | Quit smoking | |||
| Smoked < 20 cigs/day | 68.7 (62.5, 71.7) | 70.7 (67.4, 73.5) | 2.0 (-1.0, 5.7) | 0.24 |
| Smoked ≥ 20 cigs/day | 73.7 (71.5, 75.9) | 78.2 (75.9, 80.2) | 4.5 (2.6, 6.5) | |
|
| ||||
| Maleb | 76.3 (74.5, 77.9) | 80.9 (79.5, 82.4) | 4.6 (2.8, 6.7) | 0.70 |
| Femaleb | 64.1 (62.8, 66.1) | 68.0 (66.0, 70.4) | 3.9 (0.9, 6.7) | |
|
| ||||
| BMI < 25 kg/m2 | 64.6 (63.3, 66.0) | 67.9 (66.6, 69.2) | 3.3 (1.5, 5.2) | 0.04 |
| BMI ≥ 25 kg/m2 | 77.6 (75.6, 79.5) | 84.3 (82.2, 86.5) | 6.7 (4.1, 9.3) | |
|
| ||||
| Age < 46 yearsc | 72.5 (71.2, 74.4) | 77.8 (75.8, 79.8) | 5.3 (2.8, 7.2) | 0.14 |
| Age ≥ 46 yearsc | 65.3 (62.6, 70.6) | 67.9 (65.6, 72.4) | 2.6 (-0.5, 5.6) | |
Estimated using the parametric g-formula with fixed covariates: age, height, occupation, education, and marital status; and time-varying covariates: lagged weight, cigarettes smoked per day, systolic blood pressure, total cholesterol, and cardiovascular disease diagnosis.
Sex not included in models; occupation, marital status removed as a fixed covariate and menopause added as a time-varying covariate in analysis for the female subgroup
46 years is the mean age at baseline.
Our results did not change materially in any of the sensitivity analyses conducted (Online Resource Tables 2, 3).
Discussion
Our findings from the Framingham Heart Study suggest that smoking cessation leads to detectable weight gain even after 20 years of follow-up. The estimated weight gained 20-years after cessation ranged from 1.8 kg when compared with smoking 5 cigarettes per day, to 5.1 kg when compared with smoking 20 cigarettes per day.
Compared with previous observational studies, our results extend the period during which post-cessation weight gain can be detected by about 10 years. Some of those observational studies had suggested greater post-cessation weight gain in population sub-groups defined by higher intensity of smoking at baseline [19,28], younger age [19,29], lower education [28,29], and a higher baseline BMI [17,28][17,28][17,28][17,28][17,28]. We found greater post-cessation weight gain in those with BMI greater or equal to 25 at baseline. We also saw increased weight gain in those who smoked 20 or more cigarettes per day and those above the average age in the study (46 years), but these estimates were too imprecise for firm conclusions. Identifying populations at the highest risk of weight gain may help tailor weight management interventions post smoking cessation.
For our results to be applicable to current populations in which smoking cessation is being considered, the target population should have the same distribution of effect modifiers and interference patterns as our study population [30]. Our study population from 1950-1970 may not be representative of contemporary populations even in the US, for example, racial minorities were not enrolled in the FHS original cohort. Additionally, smoking cessation can be achieved through different mechanisms, such as the use of nicotine replacement products or quitting through a counselling program [30].Our analysis implicitly assumes that all these versions of the treatment “smoking cessation” are have the same impact on weight gain [28,31,32].
Like for any observational estimates, the validity of our estimates relies on the assumptions of no unmeasured confounding, no model misspecification, and no measurement error. We included all important fixed and time-varying confounders that were available to us, but it is possible that additional health conditions, and lifestyle characteristics, such as diet and exercise, might also be necessary to fully adjust for confounding. However, the inclusion of diabetes, cancer, and use of anti-hypertensive medication in sensitivity analyses did not change the results of our primary analysis. The agreement of the mean estimated values of all variables under the natural course with their observed values supports no model misspecification under the natural course. Finally, our analysis is based on clinically measured (as opposed to self-reported) weight, laboratory values, and disease diagnoses at each exam.
Smoking cessation remains an important public health goal, regardless of body weight changes. Despite being heavier than continuing smokers, those who quit smoking have significantly reduced mortality due to all-causes and reduced risks of cardiovascular disease and cancers [33]. Furthermore, the economic benefits to society for smoking cessation far outweigh the costs [34]. However, weight gain caused by smoking cessation remains a significant barrier to quitting smoking [35]. Our work adds important evidence that post-cessation weight gain may persist over long periods, further highlighting the problem of inadequate post-cessation weight management interventions [32]. The prevention of this weight gain needs to be addressed through interdisciplinary measures involving long-term weight management in combination with tobacco control programs.
Supplementary Material
Footnotes
Conflict of interest
The authors declare they have no conflict of interest.
References
- 1.Danaei G, Ding EL, Mozaffarian D, et al. The Preventable Causes of Death in the United States: Comparative Risk Assessment of Dietary, Lifestyle, and Metabolic Risk Factors. In: Hales S, editor. PLoS Med. 4. Vol. 6. 2009. p. e1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jha P, Ramasundarahettige C, Landsman V, et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368(4):341–350. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 3.Pisinger C, Jorgensen T. Weight concerns and smoking in a general population: the Inter99 study. Prev Med (Baltim) 2007;44(4):283–289. doi: 10.1016/j.ypmed.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Shang C, Chaloupka FJ, Fong GT, Thompson M, Siahpush M, Ridgeway W. Weight control belief and its impact on the effectiveness of tobacco control policies on quit attempts: findings from the ITC 4 Country Project. Tob Control. 2015 doi: 10.1136/tobaccocontrol-2014-051886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stadler M, Tomann L, Storka A, et al. Effects of smoking cessation on β-cell function, insulin sensitivity, body weight, and appetite. Eur J Endocrinol. 2014;170(2):219–7. doi: 10.1530/EJE-13-0590. [DOI] [PubMed] [Google Scholar]
- 6.Stein JH, Asthana A, Smith SS, et al. Smoking cessation and the risk of diabetes mellitus and impaired fasting glucose: three-year outcomes after a quit attempt. PLoS One. 2014;9(6):e98278. doi: 10.1371/journal.pone.0098278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chinn S, Jarvis D, Melotti R, et al. Smoking cessation, lung function, and weight gain: a follow-up study. Lancet (London, England) 365(9471):1629–1635. doi: 10.1016/S0140-6736(05)66511-7. discussion 1600-1601. [DOI] [PubMed] [Google Scholar]
- 8.Komiyama M, Wada H, Ura S, et al. The effects of weight gain after smoking cessation on atherogenic α1-antitrypsin-low-density lipoprotein. Heart Vessels. 2014 doi: 10.1007/s00380-014-0549-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeh H-C, Duncan BB, Schmidt MI, Wang N-Y, Brancati FL. Smoking, smoking cessation, and risk for type 2 diabetes mellitus: a cohort study. Ann Intern Med. 2010;152(1):10–17. doi: 10.7326/0003-4819-152-1-201001050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aubin H-J, Farley A, Lycett D, Lahmek P, Aveyard P. Weight gain in smokers after quitting cigarettes: meta-analysis. BMJ. 2012;345:e4439. doi: 10.1136/bmj.e4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Locatelli I, Collet T-H, Clair C, Rodondi N, Cornuz J. The joint influence of gender and amount of smoking on weight gain one year after smoking cessation. Int J Environ Res Public Health. 2014;11(8):8443–8455. doi: 10.3390/ijerph110808443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gennuso KP, Thraen-Borowski KM, Schlam TR, et al. Smokers’ physical activity and weight gain one year after a successful versus unsuccessful quit attempt. Prev Med (Baltim) 2014;67:189–192. doi: 10.1016/j.ypmed.2014.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernández E, Chapman S. Quitting smoking and gaining weight: the odd couple. BMJ. 2012;345(jul10_2):e4544. doi: 10.1136/bmj.e4544. [DOI] [PubMed] [Google Scholar]
- 14.Chapman S, MacKenzie R. The global research neglect of unassisted smoking cessation: causes and consequences. PLoS Med. 2010;7(2):e1000216. doi: 10.1371/journal.pmed.1000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clair C, Rigotti NA, Porneala B, et al. Association of smoking cessation and weight change with cardiovascular disease among adults with and without diabetes. JAMA. 2013;309(10):1014–1021. doi: 10.1001/jama.2013.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janzon E, Hedblad B, Berglund G, Engström G. Changes in blood pressure and body weight following smoking cessation in women. J Intern Med. 2004;255(2):266–272. doi: 10.1046/j.1365-2796.2003.01293.x. [DOI] [PubMed] [Google Scholar]
- 17.Lycett D, Munafò M, Johnstone E, Murphy M, Aveyard P. Associations between weight change over 8 years and baseline body mass index in a cohort of continuing and quitting smokers. Addiction. 2011;106(1):188–196. doi: 10.1111/j.1360-0443.2010.03136.x. [DOI] [PubMed] [Google Scholar]
- 18.Tamura U, Tanaka T, Okamura T, et al. Changes in Weight, cardiovascular risk factors and estimated risk of coronary heart disease following smoking cessation in Japanese male workers: HIPOP-OHP study. J Atheroscler Thromb. 2010;17(1):12–20. doi: 10.5551/jat.1800. [DOI] [PubMed] [Google Scholar]
- 19.Williamson DF, Madans J, Anda RF, Kleinman JC, Giovino GA, Byers T. Smoking cessation and severity of weight gain in a national cohort. N Engl J Med. 1991;324(11):739–745. doi: 10.1056/NEJM199103143241106. [DOI] [PubMed] [Google Scholar]
- 20.Veldheer S, Yingst J, Zhu J, Foulds J. Ten-year weight gain in smokers who quit, smokers who continued smoking and never smokers in the United States, NHANES 2003-2012. Int J Obes (Lond) 2015;39(12):1727–1732. doi: 10.1038/ijo.2015.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian J, Venn A, Otahal P, Gall S. The association between quitting smoking and weight gain: a systemic review and meta-analysis of prospective cohort studies. Obes Rev. 2015;16(10):883–901. doi: 10.1111/obr.12304. [DOI] [PubMed] [Google Scholar]
- 22.Taubman SL, Robins JM, Mittleman MA, Hernán MA. Intervening on risk factors for coronary heart disease: an application of the parametric g-formula. Int J Epidemiol. 2009;38(6):1599–1611. doi: 10.1093/ije/dyp192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kannel WB, Dawber TR, Kagan A, Revotskie N, Stokes J. Factors of risk in the development of coronary heart disease--six year follow-up experience. The Framingham Study. Ann Intern Med. 1961;55:33–50. doi: 10.7326/0003-4819-55-1-33. [DOI] [PubMed] [Google Scholar]
- 24.Dawber TR, Meadors GF, Moore FE. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41(3):279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson PW, Hoeg JM, D’Agostino RB, et al. Cumulative effects of high cholesterol levels, high blood pressure, and cigarette smoking on carotid stenosis. N Engl J Med. 1997;337(8):516–522. doi: 10.1056/NEJM199708213370802. [DOI] [PubMed] [Google Scholar]
- 26.Robins J. A graphical approach to the identification and estimation of causal parameters in mortality studies with sustained exposure periods. [September 4, 2014];J Chronic Dis. 1987 40(Suppl 2):139S–161S. doi: 10.1016/s0021-9681(87)80018-8. http://www.ncbi.nlm.nih.gov/pubmed/3667861. [DOI] [PubMed] [Google Scholar]
- 27.Young JG, Cain LE, Robins JM, O’Reilly EJ, Hernán MA. Comparative effectiveness of dynamic treatment regimes: an application of the parametric g-formula. Stat Biosci. 2011;3(1):119–143. doi: 10.1007/s12561-011-9040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scherr A, Seifert B, Kuster M, et al. Predictors of marked weight gain in a population of health care and industrial workers following smoking cessation. BMC Public Health. 2015;15:520. doi: 10.1186/s12889-015-1854-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swan GE, Carmelli D. Characteristics associated with excessive weight gain after smoking cessation in men. [June 15, 2015];Am J Public Health. 1995 85(1):73–77. doi: 10.2105/ajph.85.1.73. /pmc/articles/PMC1615288/?report=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hernán MA, VanderWeele TJ. Compound treatments and transportability of causal inference. Epidemiology. 2011;22(3):368–377. doi: 10.1097/EDE.0b013e3182109296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allen AM, Kleppinger A, Lando H, Oncken C. Effect of nicotine patch on energy intake and weight gain in postmenopausal women during smoking cessation. Eat Behav. 2013;14(4):420–423. doi: 10.1016/j.eatbeh.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farley AC, Hajek P, Lycett D, Aveyard P. Interventions for preventing weight gain after smoking cessation. Cochrane database Syst Rev. 2012;1 doi: 10.1002/14651858.CD006219.pub3. CD006219. [DOI] [PubMed] [Google Scholar]
- 33.Siahpush M, Singh GK, Tibbits M, Pinard CA, Shaikh RA, Yaroch A. It is better to be a fat ex-smoker than a thin smoker: findings from the 1997-2004 National Health Interview Survey-National Death Index linkage study. Tob Control. 2014;23(5):395–402. doi: 10.1136/tobaccocontrol-2012-050912. [DOI] [PubMed] [Google Scholar]
- 34.Pieroni L, Minelli L, Salmasi L. Economic Evaluation of the effect of Quitting Smoking on Weight Gains: Evidence from the United Kingdom. Value Health. 2015;18(6):791–799. doi: 10.1016/j.jval.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 35.White MA, McKee SA, O’malley SS. Smoke and mirrors: magnified beliefs that cigarette smoking suppresses weight. Addict Behav. 2007;32(10):2200–2210. doi: 10.1016/j.addbeh.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


