Abstract
Purpose
For classical Hodgkin lymphoma (HL), migrant studies could elucidate contributions of environmental factors (including Epstein–Barr virus (EBV)) to the lower rates in non-whites. Given the well-described etiologic complexity of HL, this research requires a large, immigrant population, such as California Hispanics.
Methods
With 1988–2004 California Cancer Registry data (2,595 Hispanic, 8,637 white HL cases) and tumor cell EBV status on a subset (218 Hispanics, 656 whites), we calculated ethnicity- and nativity-specific HL incidence rates simultaneously by age, sex, and histologic subtype, and tumor cell EBV prevalence.
Results
Compared with white rates, Hispanic HL rates were lower overall (70 %) and for nodular sclerosis HL, particularly among young adults (60–65 % for females). However, they were higher among children (200 %) and older adults, and for mixed cellularity HL. Compared with rates in foreign-born Hispanics, rates in US-born Hispanics were higher among young adults (>threefold in females), lower for children and adults over age 70, and consistently intermediate compared with rates in whites. EBV tumor prevalence was 67, 32, and 23 % among foreign-born Hispanics, US-born Hispanics, and whites, respectively, although with variation by age, sex, and histology.
Conclusions
Findings strongly implicate environmental influences, such as nativity-related sociodemographic differences, on HL occurrence. In addition, lower young adult rates and higher EBV prevalence in US-born Hispanics than in whites raise questions about the duration/extent of environmental change for affecting HL rates and also point to ethnic differences in genetic susceptibility. Lesser variation in mixed cellularity HL rates and greater variation in rates for females across groups suggest less modifiable factors interacting with environmental influences.
Keywords: Hodgkin lymphoma, Epstein-Barr virus, Hispanics, Immigration, Nativity, Adolescents and young adults, Cancer epidemiology
Introduction
Hodgkin lymphoma (HL) is an uncommon B-cell malignancy with marked epidemiologic heterogeneity across histologic subtypes and patient age, sex, and race/ethnicity. Studies have long supported socioeconomic influences on disease etiology for children and young adults [1–11]; risk factors include characteristics describing intensity and timing of childhood social exposure, and management of infection [12], notably Epstein–Barr virus (EBV), which is present in monoclonal form in tumor cells of an epidemiologically distinctive proportion of cases [13–16]. Yet, racial/ethnic differences in HL occurrence persist regardless of socioeconomic status (SES) [4, 17], with higher rates [18] and lower tumor EBV prevalence [13, 16] in whites. This variation suggests additional environmental [17, 19] and/or genetic influences [20–23].
These contributions to HL etiology could be explored efficiently through migrant studies, but the paucity of large immigrant cohorts for evaluating this rare and complex disease has limited such research [11, 17, 19–21]. US Hispanics represent an ideal study population: This is a large group with a substantial foreign-born subset, and US- and foreign-born Hispanics likely differ in relevant childhood infectious exposures and other immune system influences [22]. However, migrant studies of HL in US Hispanics have been impeded by methodologic challenges, including cultural and genetic heterogeneity [23–28], which can dilute study findings, and missing cancer registry information on birthplace for a sizeable, non-random segment of cases, which may introduce bias into estimated rates [29–34].
To advance understanding of how environment affects HL incidence variation, we examined the effect of nativity on HL rates in US Hispanics in comparison with non-Hispanic whites, addressing the aforementioned challenges using California Cancer Registry (CCR) data, and nativity-specific numerators and denominators [35, 36] associated with the California Neighborhoods Data System [37]. This approach provided access to a Hispanic population that is large, relatively homogeneous culturally and ethnically (approximately 80 % of Mexican origin in 2001 [38]), and for which missing registry birthplace information for cases was imputed by a validated method [36, 39, 40]. In addition, as the association of tumor cell EBV with HL is strong in Hispanics [13, 41, 42] but not well studied by birthplace, we examined variation in its prevalence across a subset of study cases [16].
Materials and methods
Patient data
We identified all California residents newly diagnosed with primary classical HL (International Classification of Diseases for Oncology, 3rd Edition, morphology codes 9663–9665, 9667 (nodular sclerosis (NS)); 9652 (mixed cellularity (MC)); 9651 (lymphocyte rich); 9653–9655 (lymphocyte depletion), or 9650 (not otherwise specified)) during the period 1988 through 2004 and reported to the CCR. We obtained data (routinely abstracted from the medical record) on patient age, sex, race/ethnicity, social security number (SSN), birthplace, and tumor histology at diagnosis for all 2,754 HLs in Hispanics and 8,971 HLs in non-Hispanic whites (hereafter called whites). Hispanic status, based on patient medical records and death certificates, was enhanced using the North American Association of Central Cancer Registries (NAACCR) Hispanic Identification Algorithm [43]. Because HIV-related HL has unusual epidemiologic profiles and had increased rates in California Hispanics during the study period [44], we excluded 159 Hispanic (5.8 %) and 334 white (3.7 %) HL cases with evidence of HIV or AIDS in registry diagnostic or death certificate data [45]. These exclusions left 2,595 Hispanic and 8,637 white HL cases for analysis.
Nativity
Of the 2,595 Hispanic cases, the CCR listed 1,054 (41 %) as US-born and 849 (33 %) as having a foreign birthplace (Mexico (n = 571), Central America (n = 119), South America (n = 51), Cuba (n = 21), Puerto Rico (n = 14), other North America (n = 6), Europe (n = 8), Asia (n = 4), not US (n = 5), and unspecified (n = 50 with data from the death certificate)). Among the 692 cases (27 %) without a recorded birthplace, we applied a previously validated method using patient SSN (and presumed year of issuance) to impute nativity for 591 cases [36], and, for 101 cases with missing or invalid SSNs, assigned nativity based on its distribution within matched strata of race/ethnicity, sex, and age in the overall CCR Hispanic patient population. Ultimately, we categorized 531 Hispanic HL cases without registry birthplace as US-born, for a total of 1,585 (61 %), and 161 as foreign-born, for a total of 1,010 (39 %).
EBV tumor status
For all incident HL cases diagnosed in 1988–1997 in selected California counties (non-whites only from southern California), we previously linked CCR data to results of tumor cell EBV assays applied to diagnostic specimens [16]. Reflecting regional variation in specimen-releasing practices, specimens were available from 86 % of Greater Bay Area cases and 43 % of southern California cases, and thus for 73 % of whites and 38 % of Hispanics. For these 219 Hispanic cases with EBV results, birthplace had not been recorded for 54 (25 %). For the current study, we determined nativity for 53 of these cases using the methods described above, thus enabling reanalysis in 218 Hispanic cases by nativity compared with 656 whites.
Population data
To compute incidence rates, we obtained California population counts from the 1990 and 2000 Census Summary File 3 by sex, race/ethnicity, nativity, and 5-year age group. To estimate age- and nativity-specific population counts for Hispanics, we used the 5 % Integrated Public Use Microdata Series sample of the Census; for intercensal years, we estimated the percentages of the population that were US-or foreign-born using cohort component methods [36, 46].
Statistical analysis
Incidence rates and rate ratios
For the period 1988–2004, we computed average annual age-adjusted (standardized to the 2000 US standard million population) and age-specific incidence rates per 100,000 population and associated 95 % confidence intervals (CI). To accommodate HL incidence patterns [47], we examined age-specific rates for 5-year age groups (presented only in study figures), for 10-year age groups, and for age ranges 0–14, 15–39, 40–54, and ≥55 years (hereafter called “children,” “adolescents/young adults (AYAs),” “middle-aged adults” and “older adults”). We calculated rates for classical HL overall and, based on sample size, for the two most common histology subtypes (NS and MC). For comparisons of pairs of incidence rates, we calculated incidence rate ratios (IRRs), considering as significant any difference for which the IRR 95 % CI did not include 1.
EBV prevalence by race/ethnicity and nativity
We examined the associations of age group, sex, and histologic subtype (NS, MC, and other) with EBV-positive and EBV-negative HL in foreign-born Hispanics, US-born Hispanics, and whites using descriptive statistics. As EBV prevalence in HL varies markedly by these variables [13], we undertook multivariate analyses to determine whether they were independently associated with EBV-positive HL by calculating prevalence ratios (PRs) and 95 % CIs, controlling for additional cofactors (census block group SES, Ann Arbor stage, and California residential region), as used previously [16].
Analyses were conducted using SEER*Stat software for incidence rates [48] and SAS version 9.3 for EBV prevalence [49]. This study had approval from the institutional review boards of the Cancer Prevention Institute of California and institutions collaborating on the previous study of HL and EBV [16].
Results
Incidence rates
Hispanics
Among California Hispanics, the 1988–2004 age-adjusted HL incidence rate per 100,000 was 2.02 overall (Table 1). Figure 1a and b showed bimodal curves comprising modest modes in AYA males and females but considerably higher rate peaks in adults, particularly males, over age 70–74.
Table 1.
Incidence rates of classical Hodgkin lymphoma (HL) overall and by selected histologic subtype, for Hispanics and whites, and race/ethnicity incidence rate ratios (IRR) and 95 % confidence intervals (CI), by gender and age, 1988–2004, California
| All HL | Nodular sclerosis HL | Mixed cellularity HL | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hispanics | Whites | Hispanic vs. White |
Hispanics | Whites | Hispanic vs. White |
Hispanics | Whites | Hispanic vs. White |
||||||||||||||||
| N | Rate* | 95% CI | N | Rate* | 95% CI | IRR | 95% CI | N | Rate* | 95% CI | N | Rate* | 95% CI | IRR | 95% CI | N | Rate* | 95% CI | N | Rate* | 95% CI | IRR | 95% CI | |
| TOTAL | ||||||||||||||||||||||||
| Age (10-year groups) | ||||||||||||||||||||||||
| 00–09 | 134 | 0.38 | 0.32–0.45 | 56 | 0.17 | 0.13–0.22 | 2.24 | 1.64–3.05 | 77 | 0.22 | 0.17–0.27 | 32 | 0.10 | 0.07–0.14 | 2.20 | 1.46–3.32 | 44 | 0.13 | 0.09–0.17 | 13 | 0.04 | 0.02–0.07 | 3.25 | 1.75–6.03 |
| 10–19 | 465 | 1.54 | 1.40–1.69 | 778 | 2.43 | 2.26–2.61 | 0.63 | 0.56–0.71 | 360 | 1.19 | 1.07–1.32 | 632 | 1.97 | 1.82–2.13 | 0.60 | 0.53–0.69 | 54 | 0.18 | 0.13–0.23 | 67 | 0.21 | 0.16–0.27 | 0.86 | 0.60–1.23 |
| 20–29 | 648 | 1.98 | 1.83–2.14 | 2,071 | 5.65 | 5.41–5.90 | 0.35 | 0.32–0.38 | 474 | 1.45 | 1.32–1.59 | 1,650 | 4.51 | 4.29–4.73 | 0.32 | 0.29–0.36 | 93 | 0.29 | 0.23–0.35 | 192 | 0.52 | 0.45–0.60 | 0.56 | 0.44–0.71 |
| 30–39 | 415 | 1.44 | 1.31–1.59 | 1,842 | 3.89 | 3.72–4.07 | 0.37 | 0.33–0.41 | 278 | 0.96 | 0.85–1.08 | 1,429 | 3.01 | 2.86–3.17 | 0.32 | 0.28–0.36 | 79 | 0.28 | 0.22–0.35 | 203 | 0.43 | 0.38–0.50 | 0.65 | 0.50–0.84 |
| 40–49 | 254 | 1.45 | 1.28–1.64 | 1,133 | 2.56 | 2.42–2.72 | 0.57 | 0.49–0.65 | 127 | 0.72 | 0.60–0.86 | 769 | 1.74 | 1.62–1.87 | 0.41 | 0.34–0.50 | 69 | 0.40 | 0.31–0.51 | 176 | 0.40 | 0.34–0.46 | 1.00 | 0.76–1.32 |
| 50–59 | 222 | 2.33 | 2.03–2.66 | 759 | 2.34 | 2.18–2.52 | 1.00 | 0.86–1.16 | 86 | 0.90 | 0.72–1.11 | 415 | 1.28 | 1.16–1.41 | 0.70 | 0.56–0.89 | 72 | 0.75 | 0.59–0.95 | 188 | 0.58 | 0.50–0.67 | 1.29 | 0.99–1.70 |
| 60–69 | 207 | 3.65 | 3.17–4.18 | 792 | 3.23 | 3.01–3.46 | 1.13 | 0.97–1.32 | 62 | 1.09 | 0.84–1.40 | 334 | 1.36 | 1.22–1.52 | 0.80 | 0.61–1.05 | 85 | 1.50 | 1.20–1.86 | 226 | 0.92 | 0.80–1.05 | 1.63 | 1.27–2.09 |
| 70–79 | 171 | 5.46 | 4.67–6.35 | 774 | 3.94 | 3.66–4.22 | 1.39 | 1.17–1.64 | 54 | 1.74 | 1.30–2.27 | 270 | 1.37 | 1.21–1.55 | 1.27 | 0.95–1.70 | 56 | 1.75 | 1.32–2.28 | 246 | 1.25 | 1.10–1.42 | 1.40 | 1.05–1.87 |
| 80+ | 79 | 5.76 | 4.56–7.19 | 432 | 3.84 | 3.48–4.22 | 1.50 | 1.18–1.91 | 30 | 2.20 | 1.48–3.14 | 156 | 1.39 | 1.18–1.62 | 1.58 | 1.07–2.34 | 29 | 2.10 | 1.40–3.01 | 120 | 1.06 | 0.88–1.27 | 1.98 | 1.32–2.97 |
| Age groups | ||||||||||||||||||||||||
| 00–14 | 298 | 0.61 | 0.54–0.69 | 268 | 0.54 | 0.48–0.61 | 1.13 | 0.96–1.33 | 195 | 0.40 | 0.35–0.46 | 202 | 0.41 | 0.36–0.47 | 0.98 | 0.80–1.19 | 75 | 0.15 | 0.12–0.19 | 34 | 0.07 | 0.05–0.10 | 2.14 | 1.43–3.21 |
| 15–39 | 1,364 | 1.76 | 1.67–1.86 | 4,479 | 4.48 | 4.35–4.62 | 0.39 | 0.37–0.42 | 994 | 1.28 | 1.20–1.36 | 3,541 | 3.55 | 3.43–3.67 | 0.36 | 0.34–0.39 | 195 | 0.26 | 0.22–0.29 | 441 | 0.44 | 0.40–0.48 | 0.59 | 0.50–0.70 |
| 40–54 | 360 | 1.59 | 1.43–1.76 | 1,531 | 2.47 | 2.35–2.60 | 0.64 | 0.57–0.72 | 170 | 0.74 | 0.63–0.86 | 990 | 1.60 | 1.50–1.70 | 0.46 | 0.39–0.54 | 105 | 0.48 | 0.39–0.58 | 272 | 0.44 | 0.39–0.49 | 1.09 | 0.87–1.37 |
| 55+ | 573 | 4.30 | 3.94–4.68 | 2,359 | 3.35 | 3.21–3.49 | 1.28 | 1.17–1.41 | 189 | 1.43 | 1.23–1.66 | 954 | 1.36 | 1.28–1.45 | 1.05 | 0.90–1.23 | 206 | 1.52 | 1.32–1.76 | 684 | 0.97 | 0.90–1.04 | 1.57 | 1.34–1.83 |
| TOTAL | 2,595 | 2.02 | 1.93–2.11 | 8,637 | 2.96 | 2.90–3.02 | 0.68 | 0.65–0.71 | 1,548 | 1.01 | 0.95–1.07 | 5,687 | 1.99 | 1.93–2.04 | 0.51 | 0.48–0.54 | 581 | 0.55 | 0.50–0.61 | 1,431 | 0.47 | 0.45–0.50 | 1.17 | 1.06–1.29 |
| MALES | ||||||||||||||||||||||||
| Age (10-year groups) | ||||||||||||||||||||||||
| 00–09 | 100 | 0.56 | 0.45–0.68 | 38 | 0.22 | 0.16–0.30 | 2.55 | 1.75–3.70 | 57 | 0.32 | 0.24–0.41 | 18 | 0.10 | 0.06–0.17 | 3.20 | 1.88–5.44 | 36 | 0.20 | 0.14–0.28 | 10 | 0.06 | 0.03–0.11 | 3.33 | 1.65–6.72 |
| 10–19 | 243 | 1.54 | 1.35–1.75 | 376 | 2.27 | 2.05–2.52 | 0.68 | 0.58–0.80 | 175 | 1.11 | 0.95–1.29 | 282 | 1.71 | 1.51–1.92 | 0.65 | 0.54–0.78 | 37 | 0.23 | 0.17–0.32 | 46 | 0.28 | 0.20–0.37 | 0.82 | 0.53–1.27 |
| 20–29 | 343 | 1.92 | 1.72–2.13 | 1,045 | 5.52 | 5.19–5.87 | 0.35 | 0.31–0.39 | 217 | 1.22 | 1.06–1.39 | 779 | 4.13 | 3.85–4.43 | 0.30 | 0.25–0.34 | 75 | 0.42 | 0.33–0.53 | 129 | 0.68 | 0.57–0.81 | 0.62 | 0.46–0.82 |
| 30–39 | 219 | 1.45 | 1.26–1.65 | 982 | 4.07 | 3.82–4.33 | 0.36 | 0.31–0.41 | 131 | 0.86 | 0.72–1.03 | 696 | 2.88 | 2.67–3.10 | 0.30 | 0.25–0.36 | 51 | 0.33 | 0.25–0.44 | 149 | 0.62 | 0.53–0.73 | 0.53 | 0.39–0.73 |
| 40–49 | 151 | 1.72 | 1.45–2.02 | 663 | 2.97 | 2.75–3.20 | 0.58 | 0.49–0.69 | 69 | 0.78 | 0.61–0.99 | 398 | 1.78 | 1.61–1.97 | 0.44 | 0.34–0.57 | 48 | 0.56 | 0.41–0.75 | 128 | 0.57 | 0.48–0.68 | 0.98 | 0.71–1.37 |
| 50–59 | 130 | 2.84 | 2.37–3.37 | 477 | 2.97 | 2.71–3.25 | 0.96 | 0.79–1.16 | 43 | 0.94 | 0.68–1.26 | 236 | 1.47 | 1.29–1.67 | 0.64 | 0.46–0.89 | 38 | 0.83 | 0.59–1.14 | 128 | 0.80 | 0.67–0.95 | 1.04 | 0.72–1.49 |
| 60–69 | 123 | 4.75 | 3.94–5.67 | 464 | 4.01 | 3.66–4.39 | 1.18 | 0.97–1.45 | 39 | 1.50 | 1.06–2.05 | 192 | 1.66 | 1.43–1.91 | 0.90 | 0.64–1.27 | 44 | 1.72 | 1.25–2.30 | 138 | 1.19 | 1.00–1.41 | 1.45 | 1.03–2.03 |
| 70–79 | 96 | 7.33 | 5.93–8.97 | 387 | 4.54 | 4.10–5.02 | 1.61 | 1.29–2.02 | 27 | 2.06 | 1.35–3.01 | 134 | 1.58 | 1.32–1.87 | 1.30 | 0.86–1.97 | 34 | 2.55 | 1.76–3.57 | 120 | 1.41 | 1.17–1.68 | 1.81 | 1.24–2.65 |
| 80+ | 40 | 8.01 | 5.71–10.93 | 198 | 5.13 | 4.43–5.90 | 1.56 | 1.11–2.19 | 15 | 3.07 | 1.71–5.07 | 65 | 1.68 | 1.29–2.15 | 1.83 | 1.04–3.20 | 17 | 3.30 | 1.91–5.30 | 61 | 1.57 | 1.19–2.02 | 2.10 | 1.23–3.60 |
| Age groups | ||||||||||||||||||||||||
| 00–14 | 195 | 0.78 | 0.67–0.89 | 150 | 0.59 | 0.50–0.69 | 1.32 | 1.07–1.64 | 120 | 0.48 | 0.40–0.57 | 96 | 0.38 | 0.31–0.46 | 1.26 | 0.97–1.65 | 57 | 0.22 | 0.17–0.29 | 28 | 0.11 | 0.07–0.16 | 2.00 | 1.27–3.14 |
| 15–39 | 710 | 1.71 | 1.58–1.84 | 2,291 | 4.44 | 4.26–4.63 | 0.39 | 0.35–0.42 | 460 | 1.11 | 1.01–1.22 | 1,679 | 3.27 | 3.11–3.43 | 0.34 | 0.31–0.38 | 142 | 0.34 | 0.28–0.40 | 306 | 0.59 | 0.52–0.66 | 0.58 | 0.47–0.70 |
| 40–54 | 213 | 1.89 | 1.64–2.16 | 913 | 2.93 | 2.74–3.12 | 0.65 | 0.56–0.75 | 89 | 0.77 | 0.62–0.95 | 518 | 1.66 | 1.52–1.81 | 0.46 | 0.37–0.58 | 67 | 0.60 | 0.47–0.77 | 197 | 0.63 | 0.55–0.73 | 0.95 | 0.72–1.26 |
| 55+ | 327 | 5.69 | 5.05–6.39 | 1,276 | 4.14 | 3.91–4.37 | 1.37 | 1.22–1.55 | 104 | 1.83 | 1.47–2.25 | 507 | 1.63 | 1.49–1.78 | 1.12 | 0.91–1.39 | 114 | 2.02 | 1.65–2.46 | 378 | 1.23 | 1.10–1.36 | 1.64 | 1.33–2.02 |
| TOTAL | 1,445 | 2.40 | 2.24–2.56 | 4,630 | 3.22 | 3.13–3.32 | 0.75 | 0.70–0.79 | 773 | 1.05 | 0.96–1.16 | 2,800 | 1.95 | 1.88–2.02 | 0.54 | 0.50–0.58 | 380 | 0.73 | 0.64–0.83 | 909 | 0.63 | 0.59–0.67 | 1.16 | 1.03–1.31 |
| FEMALES | ||||||||||||||||||||||||
| Age (10-year groups) | ||||||||||||||||||||||||
| 00–09 | 34 | 0.20 | 0.14–0.28 | 18 | 0.11 | 0.07–0.18 | 1.82 | 1.03–3.22 | 20 | 0.12 | 0.07–0.18 | 14 | 0.09 | 0.05–0.14 | 1.33 | 0.67–2.64 | 8 | 0.05 | 0.02–0.09 | <5 | 0.02 | 0.00–0.05 | 2.50 | 0.66–9.42 |
| 10–19 | 222 | 1.54 | 1.35–1.76 | 402 | 2.60 | 2.35–2.86 | 0.59 | 0.50–0.70 | 185 | 1.29 | 1.11–1.49 | 350 | 2.26 | 2.03–2.51 | 0.57 | 0.48–0.68 | 17 | 0.12 | 0.07–0.19 | 21 | 0.14 | 0.08–0.21 | 0.86 | 0.45–1.62 |
| 20–29 | 305 | 2.06 | 1.83–2.30 | 1,026 | 5.78 | 5.43–6.15 | 0.36 | 0.31–0.40 | 257 | 1.74 | 1.53–1.96 | 871 | 4.91 | 4.59–5.25 | 0.35 | 0.31–0.41 | 18 | 0.12 | 0.07–0.19 | 63 | 0.36 | 0.27–0.46 | 0.33 | 0.20–0.56 |
| 30–39 | 196 | 1.44 | 1.24–1.66 | 860 | 3.71 | 3.46–3.96 | 0.39 | 0.33–0.45 | 147 | 1.07 | 0.90–1.26 | 733 | 3.16 | 2.93–3.39 | 0.34 | 0.28–0.40 | 28 | 0.22 | 0.14–0.31 | 54 | 0.23 | 0.18–0.31 | 0.96 | 0.61–1.51 |
| 40–49 | 103 | 1.18 | 0.96–1.44 | 470 | 2.15 | 1.96–2.35 | 0.55 | 0.44–0.68 | 58 | 0.66 | 0.50–0.85 | 371 | 1.70 | 1.53–1.88 | 0.39 | 0.29–0.51 | 21 | 0.25 | 0.15–0.38 | 48 | 0.22 | 0.16–0.29 | 1.14 | 0.68–1.90 |
| 50–59 | 92 | 1.86 | 1.50–2.28 | 282 | 1.73 | 1.53–1.94 | 1.08 | 0.85–1.36 | 43 | 0.87 | 0.63–1.17 | 179 | 1.10 | 0.95–1.28 | 0.79 | 0.57–1.10 | 34 | 0.69 | 0.48–0.96 | 60 | 0.37 | 0.28–0.47 | 1.86 | 1.22–2.84 |
| 60–69 | 84 | 2.73 | 2.18–3.38 | 328 | 2.52 | 2.25–2.81 | 1.08 | 0.85–1.38 | 23 | 0.75 | 0.47–1.12 | 142 | 1.10 | 0.93–1.30 | 0.68 | 0.44–1.06 | 41 | 1.33 | 0.95–1.80 | 88 | 0.67 | 0.54–0.83 | 1.99 | 1.37–2.88 |
| 70–79 | 75 | 4.12 | 3.24–5.17 | 387 | 3.46 | 3.13–3.83 | 1.19 | 0.93–1.52 | 27 | 1.50 | 0.99–2.19 | 136 | 1.22 | 1.02–1.44 | 1.23 | 0.81–1.86 | 22 | 1.18 | 0.74–1.79 | 126 | 1.13 | 0.94–1.34 | 1.04 | 0.66–1.64 |
| 80+ | 39 | 4.48 | 3.19–6.13 | 234 | 3.20 | 2.80–3.64 | 1.40 | 1.00–1.96 | 15 | 1.72 | 0.96–2.84 | 91 | 1.24 | 1.00–1.52 | 1.39 | 0.80–2.40 | 12 | 1.38 | 0.71–2.41 | 59 | 0.81 | 0.62–1.04 | 1.70 | 0.92–3.17 |
| Age groups | ||||||||||||||||||||||||
| 00–14 | 103 | 0.44 | 0.36–0.53 | 118 | 0.49 | 0.41–0.59 | 0.90 | 0.69–1.17 | 75 | 0.32 | 0.25–0.40 | 106 | 0.44 | 0.36–0.54 | 0.73 | 0.54–0.98 | 18 | 0.08 | 0.05–0.12 | 6 | 0.02 | 0.01–0.05 | 4.00 | 1.59–10.08 |
| 15–39 | 654 | 1.82 | 1.68–1.97 | 2,188 | 4.53 | 4.34–4.72 | 0.40 | 0.37–0.44 | 534 | 1.47 | 1.35–1.61 | 1,862 | 3.85 | 3.68–4.03 | 0.38 | 0.35–0.42 | 53 | 0.16 | 0.12–0.21 | 135 | 0.28 | 0.23–0.33 | 0.57 | 0.42–0.79 |
| 40–54 | 147 | 1.29 | 1.09–1.52 | 618 | 2.01 | 1.85–2.17 | 0.64 | 0.54–0.77 | 81 | 0.70 | 0.56–0.88 | 472 | 1.53 | 1.40–1.68 | 0.46 | 0.36–0.58 | 38 | 0.35 | 0.25–0.48 | 75 | 0.24 | 0.19–0.31 | 1.46 | 0.99–2.15 |
| 55+ | 246 | 3.28 | 2.87–3.72 | 1,083 | 2.73 | 2.57–2.90 | 1.20 | 1.05–1.38 | 85 | 1.15 | 0.91–1.43 | 447 | 1.14 | 1.04–1.26 | 1.01 | 0.80–1.27 | 92 | 1.18 | 0.94–1.45 | 306 | 0.77 | 0.68–0.86 | 1.53 | 1.21–1.93 |
| TOTAL | 1,150 | 1.72 | 1.61–1.84 | 4,007 | 2.73 | 2.65–2.82 | 0.63 | 0.59–0.67 | 775 | 0.99 | 0.91–1.07 | 2,887 | 2.04 | 1.96–2.12 | 0.49 | 0.45–0.53 | 201 | 0.40 | 0.34–0.46 | 522 | 0.32 | 0.29–0.35 | 1.25 | 1.06–1.47 |
Rates are per 100,000 and age adjusted to the 2000 US Standard Population (19 age groups–Census P25–1130); confidence intervals (Tiwari modification) are 95 % for rates
Bolded IRR and 95 % CI are statistically significant
To comply with California Cancer Registry confidentiality regulations, case counts fewer than 5 are not presented
Fig. 1.
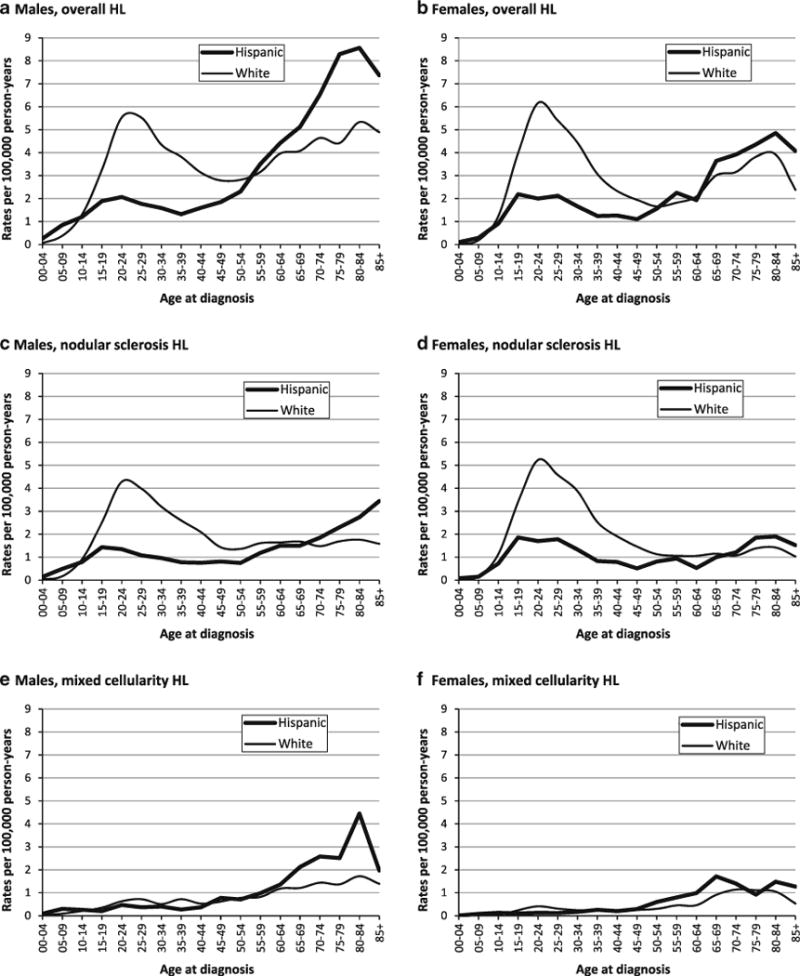
Age- and sex-specific incidence rates of classical Hodgkin lymphoma (HL) overall and for selected histologic subtypes in Hispanics and whites, 1988–2004, California
Hispanics versus whites
Compared with whites, Hispanics had overall HL rates that were approximately 30 % lower and showed a larger male excess (male–female (M–F) IRRs with 95 % CI: Hispanics, 1.39 (1.27–1.53); whites, 1.18 (1.13–1.23)), reflecting relatively lower female than male rates (Table 1). For NS, rates in Hispanics were half those in whites; for MC, they were slightly higher than in whites. Age-specific data (Fig. 1a–f) show that AYA incidence peaks were markedly lower in Hispanics than in whites for HL overall; for NS, peak rates (at ages 20–29) were higher in females than in males for both Hispanics (M–F IRR: 0.70 (0.58–0.84)) and whites (M–F IRR: 0.84 (0.76–0.93)). For children and older adults, HL rates were higher for Hispanics than for whites, notably in males. For NS, similar patterns occurred, with higher rates in Hispanic than in white males, but not in females, under age 10 (M–F IRRs: Hispanics, 2.77 (1.64–4.87); whites, 1.21 (0.57–2.62)) and at ages 80 and over. For MC, Hispanics had higher rates than whites in children and older adults of both genders.
Hispanics by nativity
HL incidence rates were higher in US- than in foreign-born Hispanics, 55 % for females and 22 % for males (Table 2). AYA rates were substantially higher in the US-born than in the foreign-born (Fig. 2a, b), approximately two times among males and more than three times among females. For NS (Fig. 2c, d), rates across age groups 10–19 through 50–59 were up to 2.7 times higher in the US- than in the foreign-born for males and three to nearly five times higher for females, due to a female rate excess among the US-born. For MC (Fig. 2e, f), AYA rates were substantially higher in US- than in foreign-born Hispanics (fourfold for females at ages 20–29), although higher in males than in females in both the US-born (M–F IRR: 1.84 (1.21–2.87)) and foreign-born (M–F IRR: 2.94 (1.65–5.56)). Among children ages 5–9, rates per 100,000 were lower in US-than in foreign-born Hispanics overall and for MC (0.14 and 0.58, respectively), while for NS, they were higher for US- than for foreign-born boys (0.52 and 0.26) and similar for girls (0.15 and 0.19).
Table 2.
Incidence rates of classical Hodgkin lymphoma (HL) overall and by selected histologic subtype, for US- and foreign-born Hispanics, and nativity incidence rate ratios (IRR) and 95 % confidence intervals (CI), by gender and age, 1988–2004, California
| All HL | Nodular sclerosis HL | Mixed cellularity HL | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| US-born Hispanics | Foreign-born Hispanics | US-born vs. Foreign born |
US-born Hispanics | Foreign-born Hispanics | US-born vs. Foreign-born |
US-born Hispanics | Foreign-born Hispanics |
US-born vs. Foreign-born |
||||||||||||||||
| N | Rate* | 95% CI | N | Rate* | 95% CI | IRR | 95% CI | N | Rate* | 95% CI | N | Rate* | 95% CI | IRR | 95% CI | N | Rate* | 95% CI | N | Rate* | 95% CI | IRR | 95% CI | |
| TOTALS | ||||||||||||||||||||||||
| Age (10-year groups) | ||||||||||||||||||||||||
| 00–09 | 105 | 0.34 | 0.28–0.41 | 29 | 0.71 | 0.48–1.04 | 0.47 | 0.31–0.74 | 67 | 0.22 | 0.17–0.28 | 10 | 0.27 | 0.13–0.51 | 0.81 | 0.40–1.77 | 30 | 0.10 | 0.06–0.14 | 14 | 0.33 | 0.18–0.57 | 0.29 | 0.15–0.59 |
| 10–19 | 356 | 1.73 | 1.55–1.92 | 109 | 1.26 | 1.03–1.53 | 1.37 | 1.10–1.72 | 280 | 1.37 | 1.21–1.54 | 80 | 0.92 | 0.73–1.15 | 1.48 | 1.15–1.94 | 41 | 0.19 | 0.14–0.26 | 13 | 0.17 | 0.09–0.29 | 1.12 | 0.59–2.32 |
| 20–29 | 439 | 3.16 | 2.87–3.47 | 209 | 1.12 | 0.97–1.28 | 2.83 | 2.40–3.36 | 326 | 2.35 | 2.10–2.62 | 148 | 0.79 | 0.67–0.93 | 2.96 | 2.43–3.62 | 56 | 0.40 | 0.30–0.51 | 37 | 0.19 | 0.14–0.27 | 2.04 | 1.32–3.19 |
| 30–39 | 250 | 2.57 | 2.26–2.92 | 165 | 0.87 | 0.74–1.01 | 2.96 | 2.41–3.63 | 177 | 1.81 | 1.55–2.10 | 101 | 0.53 | 0.43–0.64 | 3.43 | 2.67–4.45 | 43 | 0.44 | 0.32–0.60 | 36 | 0.19 | 0.14–0.27 | 2.27 | 1.41–3.66 |
| 40–49 | 140 | 2.29 | 1.93–2.70 | 114 | 1.00 | 0.83–1.21 | 2.28 | 1.77–2.95 | 76 | 1.24 | 0.97–1.55 | 51 | 0.44 | 0.33–0.58 | 2.79 | 1.93–4.06 | 34 | 0.57 | 0.39–0.80 | 35 | 0.31 | 0.22–0.44 | 1.81 | 1.10–3.00 |
| 50–59 | 95 | 2.60 | 2.10–3.18 | 127 | 2.17 | 1.81–2.58 | 1.20 | 0.91–1.58 | 43 | 1.18 | 0.85–1.59 | 43 | 0.73 | 0.53–0.99 | 1.60 | 1.03–2.51 | 25 | 0.68 | 0.44–1.01 | 47 | 0.80 | 0.59–1.06 | 0.85 | 0.50–1.42 |
| 60–69 | 104 | 3.98 | 3.25–4.83 | 103 | 3.38 | 2.76–4.10 | 1.18 | 0.89–1.56 | 26 | 1.00 | 0.65–1.46 | 36 | 1.17 | 0.82–1.63 | 0.85 | 0.49–1.45 | 42 | 1.61 | 1.16–2.18 | 43 | 1.42 | 1.03–1.92 | 1.13 | 0.72–1.78 |
| 70–79 | 72 | 4.67 | 3.65–5.90 | 99 | 6.17 | 5.01–7.52 | 0.76 | 0.55–1.04 | 25 | 1.64 | 1.06–2.43 | 29 | 1.82 | 1.22–2.62 | 0.90 | 0.50–1.60 | 23 | 1.42 | 0.90–2.14 | 33 | 2.05 | 1.41–2.88 | 0.69 | 0.39–1.22 |
| 80+ | 24 | 4.22 | 2.69–6.32 | 55 | 6.65 | 5.01–8.65 | 0.64 | 0.37–1.05 | 10 | 1.75 | 0.83–3.27 | 20 | 2.41 | 1.47–3.73 | 0.73 | 0.30–1.65 | 10 | 1.75 | 0.83–3.27 | 19 | 2.30 | 1.38–3.59 | 0.76 | 0.31–1.75 |
| Age groups | ||||||||||||||||||||||||
| 00–14 | 227 | 0.56 | 0.49–0.64 | 71 | 0.90 | 0.70–1.15 | 0.62 | 0.47–0.83 | 155 | 0.39 | 0.33–0.45 | 40 | 0.49 | 0.34–0.68 | 0.80 | 0.55–1.18 | 53 | 0.13 | 0.10–0.17 | 22 | 0.30 | 0.19–0.46 | 0.43 | 0.25–0.74 |
| 15–39 | 923 | 2.77 | 2.58–2.96 | 441 | 1.04 | 0.94–1.15 | 2.66 | 2.36–3.01 | 695 | 2.05 | 1.90–2.22 | 299 | 0.71 | 0.63–0.80 | 2.89 | 2.51–3.35 | 117 | 0.37 | 0.31–0.45 | 78 | 0.17 | 0.14–0.22 | 2.15 | 1.58–2.93 |
| 40–54 | 189 | 2.32 | 2.00–2.68 | 171 | 1.19 | 1.02–1.38 | 1.96 | 1.58–2.42 | 99 | 1.21 | 0.98–1.47 | 71 | 0.48 | 0.38–0.61 | 2.50 | 1.82–3.46 | 47 | 0.59 | 0.43–0.79 | 58 | 0.42 | 0.31–0.54 | 1.42 | 0.94–2.13 |
| 55+ | 246 | 3.95 | 3.46–4.50 | 327 | 4.54 | 4.04–5.08 | 0.87 | 0.73–1.04 | 81 | 1.35 | 1.06–1.69 | 108 | 1.49 | 1.21–1.82 | 0.90 | 0.66–1.23 | 87 | 1.38 | 1.10–1.72 | 119 | 1.63 | 1.34–1.97 | 0.85 | 0.63–1.14 |
| TOTAL | #### | 2.45 | 2.31–2.60 | #### | 1.79 | 1.66–1.92 | 1.37 | 1.25–1.51 | 1,030 | 1.36 | 1.26–1.47 | 518 | 0.78 | 0.70–0.86 | 1.75 | 1.53–1.99 | 304 | 0.58 | 0.51–0.67 | 277 | 0.56 | 0.49–0.65 | 1.03 | 0.85–1.25 |
| MALES | ||||||||||||||||||||||||
| Age (10-year groups) | ||||||||||||||||||||||||
| 00–09 | 81 | 0.51 | 0.41–0.64 | 19 | 0.91 | 0.54–1.44 | 0.57 | 0.34–0.99 | 51 | 0.33 | 0.24–0.43 | 6 | 0.31 | 0.11–0.69 | 1.05 | 0.44–3.03 | 25 | 0.16 | 0.10–0.23 | 11 | 0.51 | 0.25–0.93 | 0.31 | 0.14–0.70 |
| 10–19 | 184 | 1.73 | 1.48–1.99 | 59 | 1.29 | 0.97–1.68 | 1.34 | 0.98–1.85 | 136 | 1.28 | 1.07–1.52 | 39 | 0.83 | 0.58–1.15 | 1.55 | 1.06–2.30 | 26 | 0.24 | 0.15–0.35 | 11 | 0.27 | 0.13–0.49 | 0.87 | 0.41–1.99 |
| 20–29 | 210 | 2.90 | 2.52–3.33 | 133 | 1.24 | 1.04–1.47 | 2.34 | 1.87–2.93 | 135 | 1.87 | 1.56–2.21 | 82 | 0.77 | 0.61–0.96 | 2.41 | 1.82–3.22 | 42 | 0.57 | 0.41–0.77 | 33 | 0.30 | 0.21–0.43 | 1.88 | 1.16–3.07 |
| 30–39 | 115 | 2.35 | 1.94–2.83 | 104 | 1.02 | 0.83–1.24 | 2.31 | 1.75–3.06 | 70 | 1.43 | 1.11–1.82 | 61 | 0.60 | 0.45–0.77 | 2.40 | 1.67–3.46 | 26 | 0.51 | 0.33–0.76 | 25 | 0.25 | 0.16–0.36 | 2.09 | 1.15–3.81 |
| 40–49 | 80 | 2.67 | 2.12–3.33 | 71 | 1.23 | 0.96–1.55 | 2.17 | 1.55–3.03 | 40 | 1.33 | 0.95–1.81 | 29 | 0.50 | 0.34–0.72 | 2.65 | 1.60–4.44 | 24 | 0.82 | 0.53–1.22 | 24 | 0.43 | 0.27–0.64 | 1.92 | 1.04–3.53 |
| 50–59 | 56 | 3.17 | 2.40–4.12 | 74 | 2.64 | 2.07–3.32 | 1.20 | 0.83–1.72 | 23 | 1.30 | 0.82–1.95 | 20 | 0.71 | 0.43–1.10 | 1.83 | 0.96–3.52 | 15 | 0.85 | 0.48–1.40 | 23 | 0.82 | 0.52–1.23 | 1.04 | 0.50–2.07 |
| 60–69 | 64 | 5.24 | 4.04–6.70 | 59 | 4.32 | 3.28–5.58 | 1.21 | 0.84–1.76 | 17 | 1.39 | 0.81–2.22 | 22 | 1.60 | 1.00–2.44 | 0.87 | 0.43–1.71 | 23 | 1.89 | 1.20–2.84 | 21 | 1.56 | 0.96–2.38 | 1.22 | 0.64–2.32 |
| 70–79 | 44 | 6.58 | 4.76–8.88 | 52 | 8.03 | 5.98–10.55 | 0.82 | 0.53–1.26 | 13 | 1.96 | 1.03–3.38 | 14 | 2.15 | 1.17–3.62 | 0.91 | 0.39–2.11 | 16 | 2.25 | 1.28–3.69 | 18 | 2.80 | 1.65–4.44 | 0.80 | 0.38–1.69 |
| 80+ | 9 | 4.37 | 1.96–8.51 | 31 | 10.22 | 6.94–14.51 | 0.43 | 0.18–0.94 | 5 | 2.65 | 0.83–6.33 | 10 | 3.32 | 1.59–6.10 | 0.80 | 0.21–2.62 | <5 | 1.29 | 0.27–4.08 | 14 | 4.57 | 2.50–7.67 | 0.28 | 0.05–1.09 |
| Age groups | ||||||||||||||||||||||||
| 00–14 | 152 | 0.73 | 0.62–0.85 | 43 | 1.07 | 0.77–1.47 | 0.68 | 0.47–0.99 | 100 | 0.48 | 0.39–0.59 | 20 | 0.48 | 0.29–0.77 | 1.00 | 0.60–1.75 | 39 | 0.18 | 0.13–0.25 | 18 | 0.47 | 0.28–0.77 | 0.38 | 0.21–0.72 |
| 15–39 | 438 | 2.55 | 2.30–2.82 | 272 | 1.13 | 1.00–1.28 | 2.25 | 1.91–2.64 | 292 | 1.66 | 1.47–1.88 | 168 | 0.71 | 0.60–0.83 | 2.34 | 1.91–2.87 | 80 | 0.48 | 0.38–0.60 | 62 | 0.24 | 0.19–0.32 | 1.96 | 1.37–2.82 |
| 40–54 | 109 | 2.75 | 2.25–3.32 | 104 | 1.44 | 1.17–1.75 | 1.91 | 1.44–2.53 | 50 | 1.24 | 0.92–1.64 | 39 | 0.53 | 0.37–0.72 | 2.35 | 1.51–3.69 | 33 | 0.85 | 0.58–1.19 | 34 | 0.47 | 0.33–0.67 | 1.78 | 1.07–2.98 |
| 55+ | 144 | 5.08 | 4.23–6.05 | 183 | 6.09 | 5.18–7.10 | 0.83 | 0.66–1.06 | 48 | 1.81 | 1.29–2.48 | 56 | 1.85 | 1.37–2.45 | 0.98 | 0.62–1.52 | 48 | 1.64 | 1.19–2.22 | 66 | 2.27 | 1.73–2.93 | 0.72 | 0.48–1.10 |
| TOTAL | 843 | 2.74 | 2.51–2.99 | 602 | 2.24 | 2.02–2.48 | 1.22 | 1.07–1.40 | 490 | 1.35 | 1.19–1.52 | 283 | 0.87 | 0.74–1.01 | 1.56 | 1.28–1.90 | 200 | 0.74 | 0.62–0.88 | 180 | 0.78 | 0.64–0.93 | 0.96 | 0.74–1.24 |
| FEMALES | ||||||||||||||||||||||||
| Age (10-year groups) | ||||||||||||||||||||||||
| 00–09 | 24 | 0.16 | 0.10–0.23 | 10 | 0.51 | 0.24–0.97 | 0.31 | 0.14–0.73 | 16 | 0.10 | 0.06–0.17 | <5 | 0.23 | 0.06–0.60 | 0.46 | 0.14–1.94 | 5 | 0.03 | 0.01–0.08 | <5 | 0.14 | 0.03–0.46 | 0.23 | 0.04–1.48 |
| 10–19 | 172 | 1.73 | 1.48–2.01 | 50 | 1.24 | 0.91–1.64 | 1.39 | 1.01–1.96 | 144 | 1.46 | 1.23–1.72 | 41 | 1.03 | 0.73–1.41 | 1.41 | 0.99–2.07 | 15 | 0.14 | 0.08–0.23 | <5 | 0.05 | 0.01–0.19 | 2.66 | 0.61–25.61 |
| 20–29 | 229 | 3.43 | 3.00–3.91 | 76 | 0.95 | 0.74–1.19 | 3.63 | 2.78–4.78 | 191 | 2.86 | 2.47–3.30 | 66 | 0.82 | 0.63–1.05 | 3.49 | 2.62–4.70 | 14 | 0.21 | 0.11–0.35 | <5 | 0.05 | 0.01–0.12 | 4.41 | 1.36–18.67 |
| 30–39 | 135 | 2.79 | 2.34–3.31 | 61 | 0.70 | 0.53–0.90 | 4.02 | 2.94–5.55 | 107 | 2.19 | 1.79–2.65 | 40 | 0.45 | 0.32–0.61 | 4.90 | 3.36–7.27 | 17 | 0.37 | 0.21–0.59 | 11 | 0.13 | 0.07–0.24 | 2.74 | 1.21–6.53 |
| 40–49 | 60 | 1.92 | 1.47–2.48 | 43 | 0.77 | 0.56–1.04 | 2.50 | 1.66–3.80 | 36 | 1.15 | 0.80–1.59 | 22 | 0.39 | 0.24–0.59 | 2.98 | 1.70–5.32 | 10 | 0.33 | 0.16–0.61 | 11 | 0.20 | 0.10–0.36 | 1.66 | 0.63–4.31 |
| 50–59 | 39 | 2.07 | 1.47–2.82 | 53 | 1.74 | 1.30–2.28 | 1.19 | 0.76–1.83 | 20 | 1.06 | 0.65–1.64 | 23 | 0.76 | 0.48–1.13 | 1.41 | 0.73–2.68 | 10 | 0.53 | 0.25–0.97 | 24 | 0.78 | 0.50–1.17 | 0.67 | 0.29–1.46 |
| 60–69 | 40 | 2.87 | 2.05–3.91 | 44 | 2.62 | 1.90–3.51 | 1.10 | 0.70–1.73 | 9 | 0.65 | 0.30–1.23 | 14 | 0.83 | 0.45–1.39 | 0.78 | 0.30–1.94 | 19 | 1.36 | 0.82–2.13 | 22 | 1.31 | 0.82–1.99 | 1.04 | 0.53–2.01 |
| 70–79 | 28 | 3.21 | 2.13–4.65 | 47 | 4.91 | 3.61–6.54 | 0.65 | 0.39–1.07 | 12 | 1.39 | 0.72–2.44 | 15 | 1.60 | 0.89–2.63 | 0.87 | 0.37–2.01 | 7 | 0.77 | 0.31–1.60 | 15 | 1.55 | 0.86–2.55 | 0.50 | 0.17–1.31 |
| 80+ | 15 | 4.16 | 2.32–6.91 | 24 | 4.56 | 2.92–6.78 | 0.91 | 0.44–1.82 | 5 | 1.32 | 0.43–3.15 | 10 | 1.89 | 0.91–3.48 | 0.70 | 0.19–2.29 | 7 | 1.98 | 0.79–4.12 | 5 | 0.95 | 0.31–2.21 | 2.09 | 0.57–8.44 |
| Age groups | ||||||||||||||||||||||||
| 00–14 | 75 | 0.39 | 0.31–0.49 | 28 | 0.72 | 0.47–1.07 | 0.54 | 0.34–0.88 | 55 | 0.29 | 0.22–0.38 | 20 | 0.49 | 0.29–0.79 | 0.59 | 0.33–1.06 | 14 | 0.07 | 0.04–0.12 | <5 | 0.12 | 0.03–0.32 | 0.63 | 0.18–2.66 |
| 15–39 | 485 | 2.99 | 2.72–3.29 | 169 | 0.92 | 0.78–1.08 | 3.25 | 2.70–3.93 | 403 | 2.46 | 2.21–2.72 | 131 | 0.71 | 0.59–0.85 | 3.47 | 2.82–4.30 | 37 | 0.26 | 0.18–0.36 | 16 | 0.08 | 0.05–0.14 | 3.13 | 1.66–6.13 |
| 40–54 | 80 | 1.92 | 1.52–2.39 | 67 | 0.94 | 0.72–1.19 | 2.05 | 1.46–2.89 | 49 | 1.18 | 0.87–1.56 | 32 | 0.44 | 0.30–0.62 | 2.69 | 1.68–4.36 | 14 | 0.34 | 0.19–0.58 | 24 | 0.35 | 0.23–0.53 | 0.98 | 0.47–1.97 |
| 55+ | 102 | 3.03 | 2.46–3.70 | 1.44 | 3.47 | 2.91–4.10 | 0.87 | 0.67–1.14 | 33 | 1.00 | 0.68–1.42 | 52 | 1.25 | 0.92–1.64 | 0.80 | 0.50–1.28 | 39 | 1.15 | 0.81–1.59 | 53 | 1.21 | 0.90–1.60 | 0.95 | 0.60–1.48 |
| TOTAL | 742 | 2.21 | 2.03–2.41 | 408 | 1.43 | 1.28–1.59 | 1.55 | 1.35–1.79 | 540 | 1.40 | 1.27–1.55 | 235 | 0.72 | 0.62–0.83 | 1.95 | 1.63–2.34 | 104 | 0.43 | 0.34–0.53 | 97 | 0.39 | 0.31–0.48 | 1.10 | 0.80–1.50 |
Rates are per 100,000 and age adjusted to the 2000 US Standard Population (19 age groups–Census P25–1130); confidence intervals (Tiwari modification) are 95 % for rates
Bolded IRR and 95 % CI are statistically significant
To comply with California Cancer Registry confidentiality regulations, case counts fewer than 5 are not presented
Fig. 2.
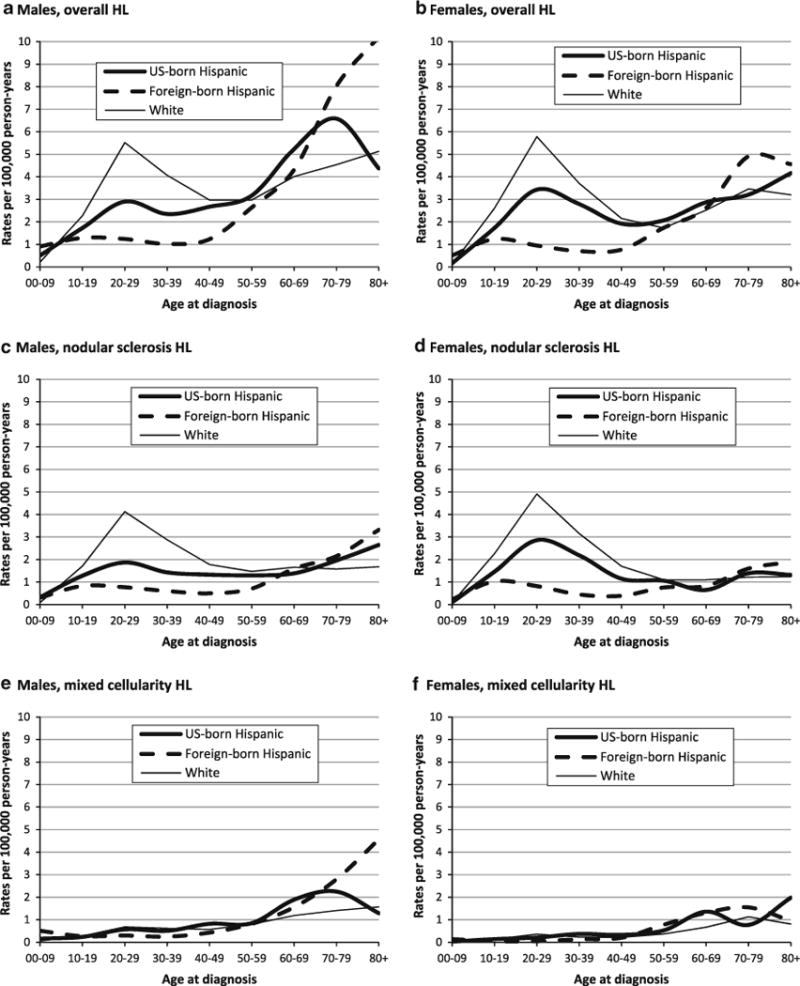
Age- and sex-specific incidence rates of classical Hodgkin lymphoma (HL) overall and for selected histologic subtypes in US-born and foreign-born Hispanics and whites, 1988–2004, California
Whites, US-born and Foreign-born Hispanics
Overall HL rates were lower among both US-born and foreign-born Hispanics than whites (Table 3), although less so for the US-born, notably females, for whom rates in US-and foreign-born Hispanics were approximately 80 and 50 % those of whites. In contrast, for children, HL rates were twofold higher among US-born Hispanics and fourfold higher among foreign-born Hispanics than whites; similar, but smaller, differences occurred for adults over age 70. For MC, rates were higher in Hispanics, irrespective of nativity, than in whites overall, although these differences were smaller for US-born than for foreign-born Hispanics. Among children, MC rates in Hispanics were eightfold higher in the foreign-born and about 2.5-fold higher in the US-born than in whites.
Table 3.
Incidence rate* ratios (IRR) and 95 % confidence intervals (CI) for classical HL overall and by histologic subtype for US-born Hispanics versus whites and foreign-born Hispanics versus whites, by age and gender, 1988–2004, California
| All HL
|
Nodular sclerosis HL
|
Mixed cellularity HL
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| US-born Hispanics versus Whites
|
Foreign-born Hispanics versus Whites
|
US-born Hispanics versus Whites
|
Foreign-born Hispanics versus Whites
|
US-born Hispanics versus Whites
|
Foreign-born Hispanics versus Whites
|
|||||||
| IRR | 95 % CI | IRR | 95 % CI | IRR | 95 % CI | IRR | 95 % CI | IRR | 95 % CI | IRR | 95 % CI | |
| Total | ||||||||||||
| Age (10-year groups) | ||||||||||||
| 00–09 | 2.00 | 1.45–2.77 | 4.18 | 2.67–6.54 | 2.20 | 1.44–3.35 | 2.70 | 1.33–5.49 | 2.50 | 1.30–4.79 | 8.25 | 3.88–17.55 |
| 10–19 | 0.71 | 0.63–0.81 | 0.52 | 0.42–0.63 | 0.70 | 0.60–0.80 | 0.47 | 0.37–0.59 | 0.90 | 0.61–1.33 | 0.81 | 0.45–1.47 |
| 20–29 | 0.56 | 0.50–0.62 | 0.20 | 0.17–0.23 | 0.52 | 0.46–0.59 | 0.18 | 0.15–0.21 | 0.77 | 0.57–1.04 | 0.37 | 0.26–0.52 |
| 30–39 | 0.66 | 0.58–0.75 | 0.22 | 0.19–0.26 | 0.60 | 0.51–0.70 | 0.18 | 0.14–0.22 | 1.02 | 0.74–1.42 | 0.44 | 0.31–0.63 |
| 40–49 | 0.89 | 0.75–1.07 | 0.39 | 0.32–0.47 | 0.71 | 0.56–0.90 | 0.25 | 0.19–0.34 | 1.43 | 0.99–2.06 | 0.78 | 0.54–1.11 |
| 50–59 | 1.11 | 0.90–1.38 | 0.93 | 0.77–1.12 | 0.92 | 0.67–1.26 | 0.57 | 0.42–0.78 | 1.17 | 0.77–1.78 | 1.38 | 1.00–1.90 |
| 60–69 | 1.23 | 1.00–1.51 | 1.05 | 0.85–1.28 | 0.74 | 0.49–1.10 | 0.86 | 0.61–1.21 | 1.75 | 1.26–2.43 | 1.54 | 1.11–2.14 |
| 70–79 | 1.19 | 0.93–1.51 | 1.57 | 1.27–1.93 | 1.20 | 0.79–1.80 | 1.33 | 0.91–1.95 | 1.14 | 0.74–1.74 | 1.64 | 1.14–2.36 |
| 80+ | 1.10 | 0.73–1.66 | 1.73 | 1.31–2.29 | 1.26 | 0.66–2.39 | 1.73 | 1.09–2.76 | 1.65 | 0.87–3.15 | 2.17 | 1.34–3.52 |
| Age groups | ||||||||||||
| 00–14 | 1.04 | 0.87–1.24 | 1.67 | 1.28–2.17 | 0.95 | 0.77–1.17 | 1.20 | 0.85–1.68 | 1.86 | 1.21–2.86 | 4.29 | 2.51–7.33 |
| 15–39 | 0.62 | 0.58–0.66 | 0.23 | 0.21–0.26 | 0.58 | 0.53–0.63 | 0.20 | 0.18–0.23 | 0.84 | 0.69–1.03 | 0.39 | 0.30–0.49 |
| 40–54 | 0.94 | 0.81–1.09 | 0.48 | 0.41–0.56 | 0.76 | 0.62–0.93 | 0.30 | 0.24–0.38 | 1.34 | 0.98–1.83 | 0.95 | 0.72–1.27 |
| 55+ | 1.18 | 1.03–1.34 | 1.36 | 1.21–1.52 | 0.99 | 0.79–1.25 | 1.10 | 0.90–1.34 | 1.42 | 1.14–1.78 | 1.68 | 1.38–2.04 |
| Total | 0.83 | 0.78–0.87 | 0.60 | 0.57–0.65 | 0.68 | 0.64–0.73 | 0.39 | 0.36–0.43 | 1.23 | 1.09–1.40 | 1.19 | 1.05–1.36 |
| Males | ||||||||||||
| Age (10-year groups) | ||||||||||||
| 00–09 | 2.32 | 1.58–3.41 | 4.14 | 2.38–7.17 | 3.30 | 1.93–5.65 | 3.10 | 1.23–7.81 | 2.67 | 1.28–5.55 | 8.50 | 3.61–20.01 |
| 10–19 | 0.76 | 0.64–0.91 | 0.57 | 0.43–0.75 | 0.75 | 0.61–0.92 | 0.49 | 0.35–0.68 | 0.86 | 0.53–1.39 | 0.96 | 0.50–1.86 |
| 20–29 | 0.53 | 0.45–0.61 | 0.22 | 0.19–0.27 | 0.45 | 0.38–0.54 | 0.19 | 0.15–0.23 | 0.84 | 0.59–1.19 | 0.44 | 0.30–0.65 |
| 30–39 | 0.58 | 0.48–0.70 | 0.25 | 0.20–0.31 | 0.50 | 0.39–0.63 | 0.21 | 0.16–0.27 | 0.82 | 0.54–1.25 | 0.40 | 0.26–0.62 |
| 40–49 | 0.90 | 0.71–1.13 | 0.41 | 0.32–0.53 | 0.75 | 0.54–1.03 | 0.28 | 0.19–0.41 | 1.44 | 0.93–2.22 | 0.75 | 0.49–1.17 |
| 50–59 | 1.07 | 0.81–1.41 | 0.89 | 0.70–1.14 | 0.88 | 0.58–1.36 | 0.48 | 0.31–0.76 | 1.06 | 0.62–1.81 | 1.03 | 0.66–1.60 |
| 60–69 | 1.31 | 1.01–1.70 | 1.08 | 0.82–1.41 | 0.84 | 0.51–1.37 | 0.96 | 0.62–1.50 | 1.59 | 1.02–2.47 | 1.31 | 0.83–2.07 |
| 70–79 | 1.45 | 1.06–1.98 | 1.77 | 1.32–2.36 | 1.24 | 0.70–2.19 | 1.36 | 0.78–2.36 | 1.60 | 0.95–2.69 | 1.99 | 1.21–3.26 |
| 80+ | 0.85 | 0.44–1.66 | 1.99 | 1.36–2.91 | 1.58 | 0.64–3.92 | 1.98 | 1.02–3.85 | 0.82 | 0.26–2.62 | 2.91 | 1.63–5.20 |
| Age groups | ||||||||||||
| 00–14 | 1.24 | 0.99–1.55 | 1.81 | 1.29–2.55 | 1.26 | 0.95–1.67 | 1.26 | 0.78–2.04 | 1.64 | 1.01–2.66 | 4.27 | 2.36–7.72 |
| 15–39 | 0.57 | 0.52–0.64 | 0.25 | 0.22–0.29 | 0.51 | 0.45–0.57 | 0.22 | 0.19–0.25 | 0.81 | 0.64–1.04 | 0.41 | 0.31–0.53 |
| 40–54 | 0.94 | 0.77–1.14 | 0.49 | 0.40–0.60 | 0.75 | 0.56–1.00 | 0.32 | 0.23–0.44 | 1.35 | 0.93–1.95 | 0.75 | 0.52–1.07 |
| 55+ | 1.23 | 1.03–1.46 | 1.47 | 1.26–1.72 | 1.11 | 0.83–1.49 | 1.13 | 0.86–1.50 | 1.33 | 0.99–1.80 | 1.85 | 1.42–2.40 |
| Total | 0.85 | 0.79–0.92 | 0.70 | 0.64–0.76 | 0.69 | 0.63–0.76 | 0.45 | 0.39–0.50 | 1.17 | 1.01–1.37 | 1.24 | 1.06–1.45 |
| Females | ||||||||||||
| Age (10-year groups) | ||||||||||||
| 00–09 | 1.45 | 0.79–2.68 | 4.64 | 2.14–10.04 | 1.11 | 0.54–2.28 | 2.56 | 0.84–7.76 | 1.50 | 0.36–6.28 | 7.00 | 1.41–34.68 |
| 10–19 | 0.67 | 0.56–0.80 | 0.48 | 0.36–0.64 | 0.65 | 0.53–0.78 | 0.46 | 0.33–0.63 | 1.00 | 0.52–1.94 | 0.36 | 0.08–1.52 |
| 20–29 | 0.59 | 0.51–0.68 | 0.16 | 0.13–0.21 | 0.58 | 0.50–0.68 | 0.17 | 0.13–0.21 | 0.58 | 0.33–1.04 | 0.14 | 0.05–0.38 |
| 30–39 | 0.75 | 0.63–0.90 | 0.19 | 0.15–0.24 | 0.69 | 0.57–0.85 | 0.14 | 0.10–0.20 | 1.61 | 0.93–2.77 | 0.57 | 0.30–1.08 |
| 40–49 | 0.89 | 0.68–1.17 | 0.36 | 0.26–0.49 | 0.68 | 0.48–0.95 | 0.23 | 0.15–0.35 | 1.50 | 0.76–2.96 | 0.91 | 0.47–1.75 |
| 50–59 | 1.20 | 0.86–1.67 | 1.01 | 0.75–1.35 | 0.96 | 0.61–1.53 | 0.69 | 0.45–1.07 | 1.43 | 0.73–2.80 | 2.11 | 1.31–3.38 |
| 60–69 | 1.14 | 0.82–1.58 | 1.04 | 0.76–1.42 | 0.59 | 0.30–1.16 | 0.75 | 0.44–1.31 | 2.03 | 1.24–3.33 | 1.96 | 1.23–3.12 |
| 70–79 | 0.93 | 0.63–1.36 | 1.42 | 1.05–1.92 | 1.14 | 0.63–2.06 | 1.31 | 0.77–2.24 | 0.68 | 0.32–1.46 | 1.37 | 0.80–2.34 |
| 80+ | 1.30 | 0.77–2.19 | 1.43 | 0.94–2.17 | 1.06 | 0.43–2.62 | 1.52 | 0.79–2.93 | 2.44 | 1.12–5.35 | 1.17 | 0.47–2.92 |
| Age groups | ||||||||||||
| 00–14 | 0.80 | 0.60–1.06 | 1.47 | 0.97–2.22 | 0.66 | 0.48–0.91 | 1.11 | 0.69–1.80 | 3.50 | 1.35–9.11 | 6.00 | 1.69–21.26 |
| 15–39 | 0.66 | 0.60–0.73 | 0.20 | 0.17–0.24 | 0.64 | 0.57–0.71 | 0.18 | 0.15–0.22 | 0.93 | 0.65–1.34 | 0.29 | 0.17–0.48 |
| 40–54 | 0.96 | 0.76–1.21 | 0.47 | 0.36–0.60 | 0.77 | 0.57–1.04 | 0.29 | 0.20–0.41 | 1.42 | 0.80–2.51 | 1.46 | 0.92–2.31 |
| 55+ | 1.11 | 0.91–1.36 | 1.27 | 1.07–1.51 | 0.88 | 0.62–1.25 | 1.10 | 0.82–1.46 | 1.49 | 1.07–2.08 | 1.57 | 1.17–2.10 |
| Total | 0.81 | 0.75–0.88 | 0.52 | 0.47–0.58 | 0.69 | 0.63–0.75 | 0.35 | 0.31–0.40 | 1.34 | 1.09–1.66 | 1.22 | 0.98–1.51 |
Rates are per 100,000 and age-adjusted to the 2000 US Standard Population (19 age groups–Census P25–1130)
Bolded IRR and 95 % CI are statistically significant
EBV associations
Tumor cell EBV prevalences were highest in foreign-born Hispanics, followed by US-born Hispanics and whites, in almost every age, sex, and histology subtype group (Table 4). For children and older adults, EBV prevalences were relatively similar for foreign- and US-born Hispanics (albeit higher in the former), but much lower for whites. For AYAs, EBV prevalences were higher for foreign-born than for US-born Hispanics but similar for US-born Hispanics and whites; however, this latter pattern was limited to females (EBV prevalences for males and females, respectively: 48 and 27 % in foreign-born Hispanics (n = 25 male and 11 female HL cases), 35 and 11 % in US-born Hispanics (n = 37 and 38), and 26 and 13 % in whites (n = 174 and 220)). Adjusted EBV PRs were increased for older adults versus AYAs and for MC versus NS in all three Hispanic/white groups, and for children versus AYAs in US- and foreign-born Hispanics. Adjusted EBV PRs for males versus females were higher for whites than for foreign-born and US-born Hispanics, reflecting the relatively low EBV prevalence in white women.
Table 4.
Number of classical Hodgkin lymphoma cases, percentages with EBV-positive tumors, and adjusted EBV prevalence ratios (PR) and 95 % confidence intervals (CI), in patient demographic and tumor subgroups, for foreign-born and US-born Hispanics and whites, 1988–1997, California regions
| Characteristic | Foreign-born Hispanics
|
US-born Hispanics
|
Whites
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % EBV+ | PR* | 95 % CI | N | % EBV+ | PR† | 95 % CI | N | % EBV+ | PR‡ | 95 % CI | |
| Age groups | ||||||||||||
| 0–14 | 7 | 85.7 | 1.7 | 1.03–2.7 | 22 | 72.7 | 2.8 | 1.7–4.5 | 21 | 28.6 | 1.4 | 0.8–2.6 |
| 15–39 | 36 | 41.7 | 1.0 | 75 | 22.7 | 1.0 | 394 | 18.5 | 1.0 | |||
| 40–54 | 14 | 64.3 | 1.4 | 0.7–2.6 | 27 | 29.6 | 1.5 | 0.8–3.2 | 104 | 11.5 | 0.6 | 0.3–0.97 |
| 55+ | 24 | 83.3 | 1.6 | 1.03–2.6 | 13 | 76.9 | 3.4 | 2.0–5.7 | 137 | 43.8 | 1.6 | 1.2–2.2 |
| p** | 0.005 | <.0001 | <.0001 | |||||||||
| Sex | ||||||||||||
| Male | 48 | 66.7 | 1.1 | 0.7–1.6 | 70 | 44.3 | 1.3 | 0.9–2.1 | 304 | 29.9 | 1.6 | 1.2–2.0 |
| Female | 33 | 54.6 | 1.0 | 67 | 29.9 | 1.0 | 352 | 17.1 | 1.0 | |||
| p** | 0.36 | 0.07 | <.0001 | |||||||||
| Histologic subtype | ||||||||||||
| NS | 40 | 42.5 | 1.0 | 95 | 30.5 | 1.0 | 493 | 15.2 | 1.0 | |||
| MC | 28 | 85.7 | 1.7 | 1.1–2.5 | 23 | 60.8 | 1.9 | 1.2–2.9 | 91 | 59.3 | 3.0 | 2.2–4.0 |
| Other | 13 | 69.3 | 1.2 | 0.7–2.0 | 19 | 42.1 | 1.1 | 0.6–1.8 | 72 | 30.6 | 1.4 | 0.9–2.1 |
| p** | 0.001 | 0.02 | <.0001 | |||||||||
| Census block group SES [16] | ||||||||||||
| Low | 50 | 60.0 | 0.9 | 0.6–1.4 | 59 | 50.9 | 1.5 | 0.97–2.4 | 52 | 26.9 | 1.0 | 0.7–1.6 |
| Mid-high | 31 | 64.5 | 1.0 | 78 | 26.9 | 1.0 | 604 | 22.7 | 1.0 | |||
| p** | 0.68 | 0.004 | 0.49 | |||||||||
| Ann Arbor stage [16] | ||||||||||||
| I/II | 45 | 51.1 | 1.0 | 72 | 40.3 | 1.0 | 402 | 20.7 | 1.0 | |||
| III/IV | 31 | 71.0 | 1.3 | 0.9–1.9 | 59 | 33.9 | 0.8 | 0.5–1.2 | 231 | 26.8 | 1.1 | 0.9–1.5 |
| Unknown | 5 | 100.0 | 1.5 | 1.02–2.3 | 6 | 33.3 | 0.5 | 0.2–1.1 | 23 | 26.1 | 1.1 | 0.6–1.8 |
| p** | 0.03 | 0.75 | 0.18 | |||||||||
| California region [16] | ||||||||||||
| Greater Bay area | 36 | 58.3 | 1.0 | 79 | 30.4 | 656 | 23.0 | |||||
| Southern CA | 45 | 64.4 | 1.1 | 0.7–1.5 | 58 | 46.6 | 1.2 | 0.8–1.8 | – | |||
| p** | 0.57 | 0.05 | ||||||||||
| Total | 81 | 61.7 | 137 | 37.2 | 656 | 23.0 | ||||||
Adjusted for age (4 groups), gender, histologic group (3 groups), Ann Arbor stage (3 groups), SES (2 groups), California region
Adjusted for age (4 groups), gender, histologic group (3 groups), Ann Arbor stage (3 groups), SES (2 groups), California region
Adjusted for age (4 groups), gender, histologic group (3 groups), Ann Arbor stage (3 groups), SES (2 groups)
p value for Chi-square statistic
Discussion
This study is the first to examine HL incidence rates using a case series of US Hispanics whose large size, relative ethnic homogeneity, fully characterized birthplace, and tumor cell EBV status for a subset, permitted detailed insights into immigration-related differences in occurrence. Overall, the lower HL rates in Hispanics than in whites among AYAs, but higher rates in children and older adults (notably males), varied by nativity. Ethnic differences were quite marked in foreign-born Hispanics, particularly younger children, but attenuated for US-born Hispanics, especially for women and for the NS subtype. EBV prevalence in HL tumors declined from foreign-born Hispanics to US-born Hispanics to whites, more dramatically in AYA women than in other age–sex groups. Together, these patterns extend evidence for strong socioeconomically associated environmental influences on HL incidence and on its EBV-defined subtypes. They also support the hypothesis that HL results from age-differing challenges to immune function [12], with higher risk in children exposed to intense, early infection, consistent with their larger family sizes [50]; higher risk in AYAs with delayed exposures to infection related to fewer childhood social contacts (e.g., fewer older siblings [51], less nursery school attendance [52]); and higher risk in older adults linked with more social contacts, likely because control of infection, especially with chronic herpesviruses, diminishes with immunosenescence. Beyond that, HL rate differences between US-born Hispanics and whites raise questions about the timing, duration, and/or extent of environmental change required to reduce ethnic variation in rates. Differences in nativity-specific variation for NS and MC underscore distinct etiologies. The markedly larger nativity effect on NS rates in AYA females than in males, but the lesser effect on rates for girls than for boys, illustrates the strong, age-varying influence of gender on NS HL occurrence.
Previous studies reported lower overall HL rates in US Hispanics, especially in AYAs, but higher rates for children and older adults, although most studies did not have adequate sample sizes to calculate rates simultaneously by age, sex, and histologic subtype [3, 4, 11, 17, 19, 53–55]. The three prior studies evaluating the effect of nativity on HL in Hispanics [11, 17, 19] lacked either the numbers of cases to stratify data by age, sex, and histologic subtype [17, 19], or nativity for all cases [11]; none included EBV information. In our study, nativity results generally resembled the prior findings. However, whereas Cozen et al. [17] showed higher NS rates in foreign- than in US-born Latinos under age 14 in Los Angeles County in 1972–1985, we observed no significant nativity differences at these ages, suggesting possible temporal or cohort changes in NS incidence. In SEER-wide data (2,067 Hispanic cases from 1992–2007), Evens et al. [11] reported lower proportions of MC in the US- than in the foreign-born; in our study, the lack of difference in MC rates by nativity, likely reflecting adjustment for age differences between groups, indicates little impact of birthplace on overall incidence of this histologic subtype. Numerous studies of EBV-related HL have described higher tumor EBV prevalence in Hispanics than in whites [16, 41, 42, 56–59]. Although none considered nativity, prevalences in our foreign-born cases were similar to those reported from Mexico and Costa Rica [42, 58].
As HL rates vary with economic conditions [1, 2, 4, 5, 7, 10, 17], some of the ethnic and nativity variation in rates reported here likely reflects socioeconomic differences. In 2001, California Hispanics differed from whites in having less education (74 vs. 31 % completing high school or less), more poverty (35 vs. 6 % living at or below the federal poverty level), and larger households (86 vs. 56 % with 3 or more persons) [60]—all characteristics associated with lower HL risk [1–11]. Similarly, foreign-born Hispanics differed from US-born Hispanics in reporting less education (84 vs. 57 % completing high school or less) and more poverty (42 vs. 28 % living at or below the federal poverty level) [60]. These socioeconomic conditions likely reflect aspects of the timing and intensity of exposure to infection linked to HL risk [12]. In several studies, Mexican-Americans had higher seroprevalence and overall burdens of common, latent infections even after control for social class [61, 62], and earlier age at infection [63, 64] than whites, and foreign birthplace was an independent predictor of these associations [61–66]. For children, these findings may describe the early and intense infections assumed to predict higher HL risk and may help explain the higher HL rates in foreign-born Hispanic children. For AYAs, they may speak to a well-primed childhood immune system, particularly in the foreign-born, consistent with their much lower young adult HL rates [67]. For older adults, greater infectious disease burdens among Mexican-Americans, particularly the foreign-born [68], may relate to the increased HL rates in Hispanics compared with whites; specifically, higher antibody titers against latent herpesviruses in Hispanics [69] may reflect diminished immune function and poorer control of latent infection due to stress [68, 70–72], lower SES [61, 69, 72, 73], or related aspects of immigration [74, 75] that could be related to HL development.
EBV-positive HL tumors appear to result from an aberrant immune response to EBV infection due to abnormal immune function [25, 76, 77]. The higher EBV prevalence in tumors of foreign-born than US-born Hispanics than whites in our study, which recalls the higher EBV prevalence in HL in populations from developing than developed economies [13, 42, 57, 78–80], and in some but not other European populations [13], suggests that foreign-born Hispanics have poorer immune control of EBV than US-born Hispanics and whites, particularly among children and older adults. For Hispanics, some data indirectly support this conjecture. In children and teenagers, adjusted PRs for EBV seroprevalence [81] and absolute antibody titers [81, 82] were higher in Mexican-Americans than whites, foreign- than US-born individuals, girls than boys, and children from lower versus the highest quartiles of household income [81]. In adults ages 24–32, EBV antibody titers were increased for Hispanics, females, and having lower SES during adolescence, suggesting long-lasting impacts of early environment on immune control of EBV [71]. EBV seroprevalence and antibody titers against lytic and latent antigens were higher in Mexicans than in Italians, Dutch, Israelis, Columbians, or Papua New Guineans [83], although a Texas community study found lower antibody titers against EBV lytic antigens in Hispanics than in whites [72]. Our finding of a lower EBV prevalence in HL tumors among US-than foreign-born Hispanics thus may reflect improved immune function as related to EBV control. However, given their higher HL rates, it also may reflect increased exposure to, or greater prevalence of, other environmental causes.
The deficit of AYA HL rates even in US-born Hispanics compared with whites in these data may diminish with increasing acculturation. In 2001, the California Hispanic population was still relatively recently immigrated (46 % foreign-born, 30 % residing in the US for under 10 years [60]). Nevertheless, to date, no study of HL incidence has observed an equivalence of rates in immigrants to those in the host country or of rates in young adult non-whites with those in whites over time [10, 11, 20, 21, 84]. For His-panics, young adult HL rates did not change over the 1990s and 2000s in either the US- or foreign-born [11]. Thus, persistent differences in HL rates between US-born Hispanics and whites may in part also reflect less modifiable aspects of HL etiology, such as HLA genotype [85–88], which manifests racial/ethnic variation in its association with HL overall [89] and by tumor cell EBV status [87]. Prevalence of the HLA-A2 allele, protective against EBV-positive HL [87, 90], varies across racial/ethnic groups, including Hispanics [87, 91, 92], and Mexican-Americans have specific HLA region determinants of immune response to EBV infection, including SNPs associated with HL [93].
The greater rate stability with immigration for the MC than for the NS subtypes in our data and the stronger association of EBV with MC irrespective of ethnicity and nativity support separate histology-specific etiologies [94] and, for MC, a larger component of invariable etiologic pathways across populations. Nevertheless, the nativity variation in MC rates also reveals environmental sensitivity in MC etiology, possibly through changes in immune function: Barros et al. [59] hypothesized that, as in HIV-related HL, EBV infection in children with poorer immune responses due to challenging environments may promote development of MC, while those with stronger immune function associated with healthier environments would develop NS. For NS, the higher rates in US- than in foreign-born AYAs, together with the lower EBV prevalences, confirm the view that EBV is not a dominant etiologic factor for this HL subtype [47]. However, as infectious mononucleosis, a strong risk factor for young adult EBV-associated HL [95, 96], seems less likely in US-born Hispanic AYAs than in whites in California, given their socioeconomic profiles [60], other pathways may lead to the observed higher EBV prevalences in HL in AYA US-born Hispanic males.
The larger immigration-related variation in HL rates in females than in males may reflect acculturation-related exposures together with biologic differences. If hormonal exposures related to reproduction interact with exposures to children to affect HL risk in women [97], the greater HL rate variation by ethnicity and nativity among AYA females than males may reflect protection to Hispanic women from earlier and more intense household exposure to infection. Among California women ages 15–39 in 2001, 41 % of foreign-born Hispanics, 17 % of US-born Hispanics, and 12 % of whites had had three or more children [60]. Among older men, the high HL rates in foreign-born than in US-born Hispanics might result in part from chronic inflammation from higher lifetime infection burdens [75].
Our findings are subject to some limitations. First, our inability to account for duration of US residence among foreign-born Hispanics, or degree of acculturation among US-born Hispanics, limits the precision of estimated rates and may bias our nativity-specific findings [17]. Second, although SES is a predictor of racial/ethnic differences in HL incidence [4, 13, 16], we lacked the denominators to calculate nativity- and neighborhood-level SES-specific rates. Third, tumor cell EBV status was available only on a small proportion of cases, limiting statistical power. HL tumors not retrievable for EBV testing were more likely to be from cases that were non-white, male, and from southern California [16], potentially resulting in selection bias. However, for 704 Hispanic cases in that study, birthplace distributions (US, foreign, unknown) did not differ significantly between those with and without available specimens (data not shown). Fourth, estimated nativity via imputation could underestimate rates for the foreign-born to the extent that they were undocumented, and thus be counted in the cancer registry but not in the census. Fifth, registry-recorded Hispanic ethnicity is subject to some misclassification [32] and may undercount Hispanics, enhancing ethnic rate differentials [24].
Our study also has several notable strengths. First, the case series is both large enough for evaluating HL rates simultaneously by age group, sex, and histologic subtype and for allowing evaluation of the joint effects of race and nativity on HL by tumor cell EBV status [16]. Second, as cancer registry cases without recorded birthplace are younger, better educated, and more likely to be US-born [29–34], our imputation of nativity where missing, rather than exclusion or proportional assignment of such cases, eliminates underestimation of rates in US-born Hispanics and subsequent attenuation of nativity comparisons [11]. Third, use of population-based cancer registry and census data for a relatively ethnically homogeneous population of Hispanics assures more precise study findings, given genetic admixture among Hispanics [27, 28] and enhances representativeness and generalizability of study findings. Fourth, by calculating rates with annual nativity-specific denominators based on statistical modeling, we avoided the likely biases associated with applying single-year census estimates to a longer time period.
Summary
In the large California Hispanic and white populations, this study was able to detect complex variation in HL incidence rates and EBV associations consistent with strong but age-varying effects of socioeconomic and related factors on HL incidence. However, persistent rate deficits in AYA US-born Hispanics versus whites and higher tumor EBV prevalence for US-born Hispanic than for white children and older adults suggest less modifiable aspects of HL etiology. The lesser ethnic and nativity variability, and stronger and persistent association of EBV, for MC than for NS point to differing etiologies, with a larger component of invariable biological influences for the former. The much stronger impact of nativity on HL rates for females than for males, particularly in AYAs, highlights underexplored age-varying influences of gender on HL occurrence. Together, these findings not only underscore the substantial etiologic heterogeneity of HL, but also point to the need for further investigation of genetic and hormonal factors that interact with environmental influences associated with HL occurrence.
Acknowledgments
This study was supported in part by NCI funds R01CA65661, R03CA63245, and N01-PC-65107 (SEER Rapid Response Surveillance Study mechanism) to Dr. Glaser; and P50CA096888 to Dr. Richard Ambinder. The authors thank the late Sarah Shema, Daphne Lichtensztajn, Rita Leung, and Kristine Winters for their contributions. The collection of cancer incidence data used in this study was supported by the California Department of Health Services as part of the statewide cancer-reporting program mandated by California Health and Safety Code, Section 103885; by the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program under contracts N01-PC-35136 awarded to the Cancer Prevention Institute of California, N02-PC-15105 awarded to the Public Health Institute, HHSN261201000140C awarded to the Cancer Prevention Institute of California, HHSN261201000035C awarded to the University of Southern California, and HHSN261201000034C awarded to the Public Health Institute; and by the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreements U55/CCR921930-02 awarded to the Public Health Institute and U58DP003862-01 awarded to the California Department of Public Health. The ideas and opinions expressed herein are those of the author(s), and endorsement by the State of California Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be inferred.
Contributor Information
S. L. Glaser, Cancer Prevention Institute of California, 2201 Walnut Avenue, Suite 300, Fremont, CA 94538, USA Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA 94306, USA.
C. A. Clarke, Cancer Prevention Institute of California, 2201 Walnut Avenue, Suite 300, Fremont, CA 94538, USA Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA 94306, USA.
E. T. Chang, Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA 94306, USA Health Sciences Practice, Exponent, Inc., 149 Commonwealth Drive, Menlo Park, CA 94025, USA.
J. Yang, Cancer Prevention Institute of California, 2201 Walnut Avenue, Suite 300, Fremont, CA 94538, USA
S. L. Gomez, Cancer Prevention Institute of California, 2201 Walnut Avenue, Suite 300, Fremont, CA 94538, USA
T. H. Keegan, Cancer Prevention Institute of California, 2201 Walnut Avenue, Suite 300, Fremont, CA 94538, USA Department of Health Research and Policy, Stanford University School of Medicine, Stanford, CA 94306, USA.
References
- 1.Correa P, O’Conor GT. Epidemiologic patterns of Hodgkin’s disease. Int J Cancer. 1971;8:192–201. doi: 10.1002/ijc.2910080203. [DOI] [PubMed] [Google Scholar]
- 2.Macfarlane G, Evstifeeva T, Boyle P, Grufferman S. International patterns in the occurrence of Hodgkin’s disease in children and young adult males. Int J Cancer. 1995;61:165–169. doi: 10.1002/ijc.2910610204. [DOI] [PubMed] [Google Scholar]
- 3.Henderson BE, Dworsky R, Menck H, Alena B, Henle W, Henle G, Terasaki P, Newell GR, Rawlings W, Kinnear BK. Case–control study of Hodgkin’s disease. II. Herpesvirus group antibody titers and HL-A type. J Natl Cancer Inst. 1973;51:1443–1447. [PubMed] [Google Scholar]
- 4.Clarke CA, Glaser SL, Keegan THM, Stroup A. Neighborhood socioeconomic status and Hodgkin lymphoma incidence in California. Cancer Epidemiol Biomarkers Prev. 2005;14:1441–1447. doi: 10.1158/1055-9965.EPI-04-0567. [DOI] [PubMed] [Google Scholar]
- 5.Glaser SL. Recent incidence and secular trends in Hodgkin’s disease and its histologic subtypes. J Chronic Dis. 1986;39:789–798. doi: 10.1016/0021-9681(86)90081-0. [DOI] [PubMed] [Google Scholar]
- 6.Medeiros LJ, Greiner TC. Hodgkin’s disease. Cancer. 1995;75:357–369. doi: 10.1002/1097-0142(19950101)75:1+<357::aid-cncr2820751318>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 7.Chen YT, Zheng T, Mei-Chu C, Boyle P, Holford TR. The increase in Hodgkin’s disease incidence among young adults. Experience in Connecticut, 1935–1992. Cancer. 1997;79:2209–2218. [PubMed] [Google Scholar]
- 8.Liu S, Semenciw R, Waters C, Shi WuW, Mao Y. Time trends and sex patterns in Hodgkin’s disease incidence in Canada, 1970–1995. Can J Public Health. 2000;91:188–192. doi: 10.1007/BF03404269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adamson P, Bray F, Costantini AS, Tao M-H, Weiderpass E, Roman E. Time trends in the registration of Hodgkin and non-Hodgkin lymphomas in Europe. Eur J Cancer. 2007;43:391–401. doi: 10.1016/j.ejca.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Hjalgrim H, Seow A, Rostgaard K, Friborg J. Changing patterns of Hodgkin lymphoma incidence in Singapore. Int J Cancer. 2008;123:716–719. doi: 10.1002/ijc.23504. [DOI] [PubMed] [Google Scholar]
- 11.Evens AM, Antillón M, Aschebrook-Kilfoy B, Chiu BC-H. Racial disparities in Hodgkin’s lymphoma: a comprehensive population-based analysis. Ann Oncol. 2012;23:2128–2137. doi: 10.1093/annonc/mdr578. [DOI] [PubMed] [Google Scholar]
- 12.Mueller NE, Grufferman S, Chang ET. The epidemiology of Hodgkin Lymphoma. In: Hoppe R, Mauch P, Armitage J, Diehl V, Weiss L, editors. Hodgkin’s disease. 2nd. Lippincott-Raven; New York: 2007. pp. 7–24. [Google Scholar]
- 13.Glaser SL, Lin RJ, Stewart SL, Ambinder RF, Jarrett RF, Brousset P, Pallesen G, Gulley ML, Khan G, O’Grady J, Hummel M, Preciado MV, Knecht H, Chan JK, Claviez A. Epstein-Barr virus-associated Hodgkin’s disease: epidemiologic characteristics in international data. Int J Cancer. 1997;70:375–382. doi: 10.1002/(sici)1097-0215(19970207)70:4<375::aid-ijc1>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 14.Farrell K, Jarrett RF. The molecular pathogenesis of Hodgkin lymphoma. Histopathology. 2011;58:15–25. doi: 10.1111/j.1365-2559.2010.03705.x. [DOI] [PubMed] [Google Scholar]
- 15.Jarrett RF, Krajewski AS, Angus B, Freeland J, Taylor PR, Taylor GM, Alexander FE. The Scotland and Newcastle epidemiological study of Hodgkin’s disease: impact of histopathological review and EBV status on incidence estimates. J Clin Pathol. 2003;56:811–816. doi: 10.1136/jcp.56.11.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glaser SL, Gulley ML, Clarke CA, Keegan TH, Chang ET, Shema SJ, Craig FE, DiGiuseppe JA, Dorfman RF, Mann RB, Anton-Culver H, Ambinder RF. Racial/ethnic variation in EBV-positive classical Hodgkin lymphoma in California populations. Int J Cancer. 2008;123:1499–1507. doi: 10.1002/ijc.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cozen W, Katz J, Mack TM. Risk patterns of Hodgkin’s disease in Los Angeles vary by cell type. Cancer Epidemiol Biomarkers Prev. 1992;1:261–268. [PubMed] [Google Scholar]
- 18.Shenoy P, Maggioncalda A, Malik N, Flowers CR. Incidence patterns and outcomes for Hodgkin lymphoma patients in the United States. Adv Hematol. 2011;2011:725219. doi: 10.1155/2011/725219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menck HR, Henderson BE, Pike MC, Mack T, Martin SP, Soo-Hoo J. Cancer incidence in the Mexican-American. J Natl Cancer Inst. 1975;55:531–536. doi: 10.1093/jnci/55.3.531. [DOI] [PubMed] [Google Scholar]
- 20.Au WY, Gascoyne RD, Gallagher RE, Le N, Klasa RD, Liang RHS, Choy C, Foo W, Connors JM. Hodgkin’s lymphoma in Chinese migrants to British Columbia: a 25-year survey. Ann Oncol. 2004;15:626–630. doi: 10.1093/annonc/mdh132. [DOI] [PubMed] [Google Scholar]
- 21.Clarke CA, Glaser SL, Gomez SL, Wang SS, Keegan TH, Yang J, Chang ET. Lymphoid malignancies in U.S. Asians: incidence rate differences by birthplace and acculturation. Cancer Epidemiol Biomarkers Prev. 2011;20:1064–1077. doi: 10.1158/1055-9965.EPI-11-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez Burchard E, Borrell LN, Choudhry S, Naqvi M, Tsai HJ, Rodriguez-Santana JR, Chapela R, Rogers SD, Mei R, Rodriguez-Cintron W, Arena JF, Kittles R, Perez-Stable EJ, Ziv E, Risch N. Latino populations: a unique opportunity for the study of race, genetics, and social environment in epidemiological research. Am J Public Health. 2005;95:2161–2168. doi: 10.2105/AJPH.2005.068668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinheiro PS, Sherman RL, Trapido EJ, Fleming LE, Huang Y, Gomez-Marin O, Lee D. Cancer incidence in first generation U.S. Hispanics: Cubans, Mexicans, Puerto Ricans, and new Latinos. Cancer Epidemiol Biomarkers Prev. 2009;18:2162–2169. doi: 10.1158/1055-9965.EPI-09-0329. [DOI] [PubMed] [Google Scholar]
- 24.Ho GY, Figueroa-Valles NR, De La Torre-Feliciano T, Tucker KL, Tortolero-Luna G, Rivera WT, Jimenez-Velazquez IZ, Ortiz-Martinez AP, Rohan TE. Cancer disparities between mainland and island Puerto Ricans. Rev Panam Salud Publica. 2009;25:394–400. doi: 10.1590/s1020-49892009000500003. [DOI] [PubMed] [Google Scholar]
- 25.Haile RW, John EM, Levine AJ, Cortessis VK, Unger JB, Gonzales M, Ziv E, Thompson P, Spruijt-Metz D, Tucker KL, Bernstein JL, Rohan TE, Ho GYF, Bondy ML, Martinez ME, Cook L, Stern MC, Correa MC, Wright J, Schwartz SJ, Baezconde-Garbanati L, Blinder V, Miranda P, Hayes R, Friedman-Jiménez G, Monroe KR, Haiman CA, Henderson BE, Thomas DC, Boffetta P. A review of cancer in U.S. Hispanic populations. Cancer Prev Res. 2012;5:150–163. doi: 10.1158/1940-6207.CAPR-11-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spitz MR, Sider JG, Johnson CC, Butler JJ, Pollack ES, Newell GR. Ethnic patterns of Hodgkin’s disease incidence among children and adolescents in the United States, 1973–82. J Natl Cancer Inst. 1986;76:235–239. [PubMed] [Google Scholar]
- 27.Hanis CL, Hewett-Emmett D, Bertin TK, Schull WJ. Origins of U.S. Hispanics. Implications for diabetes. Diabetes Care. 1991;14:618–627. doi: 10.2337/diacare.14.7.618. [DOI] [PubMed] [Google Scholar]
- 28.Klitz W, Gragert L, Maiers M, Tu B, Lazaro A, Yang R, Xu Q, Masaberg C, Ng J, Hurley CK. Four-locus high-resolution HLA typing in a sample of Mexican Americans. Tissue Antigens. 2009;74:508–513. doi: 10.1111/j.1399-0039.2009.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomez SL, Glaser SL. Quality of birthplace information obtained from death certificates for Hispanics, Asians, and Pacific Islanders. Ethn Dis. 2004;14:292–295. [PubMed] [Google Scholar]
- 30.Gomez SL, Glaser SL, Kelsey JL, Lee MM. Bias in completeness of birthplace data for Asian groups in a population-based cancer registry (United States) Cancer Causes Control. 2004;15:243–253. doi: 10.1023/B:CACO.0000024244.91775.64. [DOI] [PubMed] [Google Scholar]
- 31.Gomez SL, Glaser SL. Quality of cancer registry birthplace data for Hispanics living in the United States. Cancer Causes Control. 2005;16:713–723. doi: 10.1007/s10552-005-0694-7. [DOI] [PubMed] [Google Scholar]
- 32.Gomez SL, Glaser SL. Misclassification of race/ethnicity in a population-based cancer registry (United States) Cancer Causes Control. 2006;17:771–781. doi: 10.1007/s10552-006-0013-y. [DOI] [PubMed] [Google Scholar]
- 33.Lin SS, Glaser SL, Stewart SL. Reliability of self-reported reproductive factors and childhood social-class indicators in a case–control study of women. Ann Epidemiol. 2002;12:242–247. doi: 10.1016/s1047-2797(01)00262-9. [DOI] [PubMed] [Google Scholar]
- 34.Lin SS, O’Malley CD, Lui SW. Factors associated with missing birthplace information in a population-based cancer registry. Ethn Dis. 2001;11:598–605. [PubMed] [Google Scholar]
- 35.Chang ET, Yang J, Alfaro-Velcamp T, So SK, Glaser SL, Gomez SL. Disparities in liver cancer incidence by nativity, acculturation, and socioeconomic status in California Hispanics and Asians. Cancer Epidemiol Biomarkers Prev. 2010;19:3106–3118. doi: 10.1158/1055-9965.EPI-10-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang ET, Gomez SL, Fish K, Schupp CW, Parsonnet J, DeRouen MC, Keegan THM, Clarke CA, Glaser SL. Gastric cancer incidence among Hispanics in California: patterns by time, nativity, and neighborhood characteristics. Cancer Epidemiol Biomark Prev. 2012;21:709–719. doi: 10.1158/1055-9965.EPI-11-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gomez SL, Glaser SL, McClure LA, Shema SJ, Kealey M, Keegan TH, Satariano WA. The California Neighborhoods Data System: a new resource for examining the impact of neighborhood characteristics on cancer incidence and outcomes in populations. Cancer Causes Control. 2011;22:631–647. doi: 10.1007/s10552-011-9736-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.The Hispanic Population: Census 2000 Brief. U.S. Census Bureau; Washington, DC: 2001. [Google Scholar]
- 39.Raz DJ, Gomez SL, Chang ET, Kim JY, Keegan THM, Pham J, Kukreja J, Hiatt RA, Jablons DM. Epidemiology of non-small cell lung cancer in Asian Americans: incidence patterns among six subgroups by nativity. J Thorac Oncol. 2008;3:1391–1397. doi: 10.097/JTO.0b013e31818ddff7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horn-Ross PL, Chang ET, Clarke CA, Keegan THM, Rull RP, Quach T, Gomez SL. Nativity and papillary thyroid cancer incidence rates among Hispanic women in California. Cancer. 2012;118:216–222. doi: 10.1002/cncr.26223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ambinder RF, Browning PJ, Lorenzana I, Leventhal BG, Cosenza H, Mann RB, MacMahon EM, Medina R, Cardona V, Grufferman S. Epstein-Barr virus and childhood Hodgkin’s disease in Honduras and the United States. Blood. 1993;81:462–467. [PubMed] [Google Scholar]
- 42.Gulley ML, Eagan PA, Quintanilla-Martinez L, Picado AL, Smir BN, Childs C, Craig FE, Williams JW, Banks PM. Epstein-Barr virus DNA is abundant and monoclonal in the Reed-Sternberg cells of Hodgkin’s disease: association with mixed cellularity subtype and Hispanic American ethnicity. Blood. 1994;83:1595–1602. [PubMed] [Google Scholar]
- 43.Group NLRW. NAACCR guideline for enhancing Hispanic-Latino identification: revised NAACCR Hispanic/Latino identification algorithm [NHIA v2] North American Association of Central Cancer Registries, Springfield; 2005. [Google Scholar]
- 44.Glaser SL, Clarke CA, Gulley ML, Craig FD, DiGiuseppe JA, Dorfman RF, Mann R, Ambinder RF. Population-based patterns of human immunodeficiency virus-related Hodgkin lymphoma in the Greater San Francisco Bay Area, 1988–1998. Cancer. 2003;98:300–309. doi: 10.1002/cncr.11459. [DOI] [PubMed] [Google Scholar]
- 45.Clarke C, Glaser SL. Population-based surveillance of HIV-associated cancers: utility of cancer registry data. J Acquir Immune Defic Syndr. 2004;36:1083–1091. doi: 10.1097/00126334-200408150-00012. [DOI] [PubMed] [Google Scholar]
- 46.Keegan TH, Quach T, Shema S, Glaser SL, Gomez SL. The influence of nativity and neighborhoods on breast cancer stage at diagnosis and survival among California Hispanic women. BMC Cancer. 2010;10:603. doi: 10.1186/1471-2407-10-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hjalgrim H, Engels EA. Infectious aetiology of Hodgkin and non-Hodgkin lymphomas: a review of the epidemiological evidence. J Int Med. 2008;264:537–548. doi: 10.1111/j.1365-2796.2008.02031.x. [DOI] [PubMed] [Google Scholar]
- 48.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov http://www.seer.cancer.gov) SEER*Stat database: incidence–SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2012 Sub (2000–2010) < Katrina/Rita Population Adjustment > –Linked To County Attributes–Total U.S., 1969–2011 Counties, National Cancer Institute DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2013, based on the November 2012 submission
- 49.(2009) Surveillance, Epidemiology, and End Results Website. SEER*Stat Software version 8.0.1
- 50.Gutensohn NM, Shapiro DS. Social class risk factors among children with Hodgkin’s disease. Int J Cancer. 1982;30:433–435. doi: 10.1002/ijc.2910300409. [DOI] [PubMed] [Google Scholar]
- 51.Chang ET, Montgomery SM, Richiardi L, Ehlin A, Ekbom A, Lambe M. Number of siblings and risk of Hodgkin’s lymphoma. Cancer Epidemiol Biomarkers Prev. 2004;13:1236–1243. [PubMed] [Google Scholar]
- 52.Chang ET, Zheng T, Weir EG, Borowitz M, Mann RB, Spiegelman D, Mueller NE. Childhood social environment and Hodgkin’s lymphoma: new findings from a population-based case–control study. Cancer Epidemiol Biomarkers Prev. 2004;13:1361–1370. [PubMed] [Google Scholar]
- 53.Glaser SL, Jarrett RF. The epidemiology of Hodgkin’s disease. Bailliere’s Clin Haematol. 1996;9:401–416. doi: 10.1016/s0950-3536(96)80018-7. [DOI] [PubMed] [Google Scholar]
- 54.Canto MT, Chu KC. Annual cancer incidence rates for Hispanics in the United States: surveillance, epidemiology, and end results, 1992–1996. Cancer. 2000;88:2642–2652. doi: 10.1002/1097-0142(20000601)88:11<2642::aid-cncr29>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 55.Henderson BE, Dworsky R, Pike MC, Baptista J, Menck H, Preston-Martin S, Mack T. Risk factors for nodular sclerosis and other types of Hodgkin’s disease. Cancer Res. 1979;39:4507–4511. [PubMed] [Google Scholar]
- 56.Dirnhofer S, Angeles-Angeles A, Ortiz-Hidalgo C, Reyes E, Gredler E, Krugmann J, Fend F, Quintanilla-Martinez L. High prevalence of a 30-base pair deletion in the Epstein-Barr virus (EBV) latent membrane protein 1 gene and of strain type B EBV in Mexican classical Hodgkin’s disease and reactive lymphoid tissue. Hum Pathol. 1999;30:781–787. doi: 10.1016/s0046-8177(99)90138-7. [DOI] [PubMed] [Google Scholar]
- 57.Zarate-Osorno A, Roman LN, Kingma DW, Meneses-Garcia A, Jaffe ES. Hodgkin’s disease in Mexico. Prevalence of Epstein-Barr virus sequences and correlations with histologic subtype. Cancer. 1995;75:1360–1366. doi: 10.1002/1097-0142(19950315)75:6<1360::aid-cncr2820750619>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 58.Palma I, Sánchez AE, Jiménez-Hernández E, Alvarez-Rodríguez F, Nava-Frias M, Valencia-Mayoral P, Salinas-Lara C, Velazquez-Guadarrama N, Portilla-Aguilar J, Pena RY, Ramos-Salazar P, Contreras A, Alfaro A, Espinosa AM, Nájera N, Gutierrez G, Mejia-Arangure JM, Arellano-Galindo J. Detection of Epstein-Barr virus and genotyping based on EBNA2 protein in Mexican patients With Hodgkin lymphoma: a comparative study in children and adults. Clin Lymphoma Myeloma Leuk. 2013;13:266–272. doi: 10.1016/j.clml.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 59.Barros MH, Hassan R, Niedobitek G. Disease patterns in pediatric classical Hodgkin lymphoma: a report from a developing area in Brazil. Hematol Oncol. 2011;29:190–195. doi: 10.1002/hon.984. [DOI] [PubMed] [Google Scholar]
- 60.California Health Interview Survey (CHIS) UCLA Center for Health Policy Research; Los Angeles, CA: 2001. [Google Scholar]
- 61.Zajacova A, Dowd JB, Aiello AE. Socioeconomic and race/ethnic patterns in persistent infection burden among U.S. adults. J Gerontol A Biol Sci Med Sci. 2009;64A:272–279. doi: 10.1093/gerona/gln012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McQuillan GM, Kruszon-Moran D, Kottiri BJ, Curtin LR, Lucas JW, Kington RS. Racial and ethnic differences in the seroprevalence of 6 infectious diseases in the United States: data from NHANES III, 1988–1994. Am J Public Health. 2004;94:1952–1958. doi: 10.2105/ajph.94.11.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Staras SAS, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin Infect Dis. 2006;43:1143–1151. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- 64.Schillinger JA, Xu F, Sternberg MR, Armstrong GL, Lee FK, Nahmias AJ, McQuillan GM, Louis ME, Markowitz LE. National seroprevalence and trends in Herpes Simplex Virus Type 1 in the United States, 1976–1994. Sex Transm Dis. 2004;31:753–760. doi: 10.1097/01.olq.0000145852.43262.c3. [DOI] [PubMed] [Google Scholar]
- 65.Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the National Health and Nutrition Examination Surveys, 1988–2004. Clin Infect Dis. 2010;50:1439–1447. doi: 10.1086/652438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kruszon-Moran D, McQuillan G. Advance data from Vital & health statistics of the. National Center for Health Statistics; 2005. Seroprevalence of six infectious diseases among adults in the United States by race/ethnicity: data from the third National Health and Nutrition Examination Survey, 1988–94; pp. 1–9. [PubMed] [Google Scholar]
- 67.Cozen W, Hamilton AS, Zhao P, Salam MT, Deapen DM, Nathwani BN, Weiss LM, Mack TM. A protective role for early oral exposures in the etiology of young adult Hodgkin lymphoma. Blood. 2009;114:4014–4020. doi: 10.1182/blood-2009-03-209601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dowd JB, Zajacova A, Aiello A. Early origins of health disparities: burden of infection, health, and socioeconomic status in U.S. children. Soc Sci Med. 2009;68:699–707. doi: 10.1016/j.socscimed.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dowd JB, Haan MN, Blythe L, Moore K, Aiello AE. Socioeconomic gradients in immune response to latent infection. Am J Epidemiol. 2008;167:112–120. doi: 10.1093/aje/kwm247. [DOI] [PubMed] [Google Scholar]
- 70.Godbout JP, Glaser R. Stress-induced immune dysregulation: implications for wound healing, infectious disease and cancer. Jrnl Neuroimmune Pharm. 2006;1:421–427. doi: 10.1007/s11481-006-9036-0. [DOI] [PubMed] [Google Scholar]
- 71.Slopen N, McLaughlin KA, Dunn EC, Koenen KC. Childhood adversity and cell-mediated immunity in young adulthood: does type and timing matter? Brain Behav Immun. 2013;28:63–71. doi: 10.1016/j.bbi.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stowe RP, Peek MK, Perez NA, Yetman DL, Cutchin MP, Goodwin JS. Herpesvirus reactivation and socioeconomic position: a community-based study. J Epidemiol Community Health. 2010;64:666–671. doi: 10.1136/jech.2008.078808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stowe RP, Kozlova EV, Yetman DL, Walling DM, Goodwin JS, Glaser R. Chronic herpesvirus reactivation occurs in aging. Exp Gerontol. 2007;42:563–570. doi: 10.1016/j.exger.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pérez DJ, Fortuna L, Alegría M. Prevalence and correlates of everyday discrimination among U.S. Latinos. J Community Psychol. 2008;36:421–433. doi: 10.1002/jcop.20221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McClure HH, Martinez CR, Snodgrass J, Eddy J, Jimenez RA, Isiordia LE, McDade TW. Discrimination-related stress, blood pressure and Epstein-Barr virus antibodies among Latin American immigrants in Oregon, US. J Biosoc Sci. 2010;42:433–461. doi: 10.1017/S0021932010000039. [DOI] [PubMed] [Google Scholar]
- 76.Chang ET, Zheng T, Lennette ET, Weir EG, Borowitz M, Mann RB, Spiegelman D, Mueller NE. Heterogeneity of risk factors and antibody profiles in Epstein-barr virus genome-positive and -negative Hodgkin lymphoma. J Infect Dis. 2004;189:2271–2281. doi: 10.1086/420886. [DOI] [PubMed] [Google Scholar]
- 77.Levin LI, Chang ET, Ambinder RF, Lennette ET, Rubertone MV, Mann RB, Borowitz M, Weir EG, Abbondanzo SL, Mueller NE. Atypical prediagnosis Epstein-Barr virus serology restricted to EBV-positive Hodgkin lymphoma. Blood. 2012;120:3750–3755. doi: 10.1182/blood-2011-12-390823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fatima S, Ahmed R, Ahmed A. Hodgkin lymphoma in Pakistan: an analysis of subtypes and their correlation with Epstein Barr virus. Asian Pac J Cancer Prev. 2011;12:1385–1388. [PubMed] [Google Scholar]
- 79.Huang X, Nolte I, Gao Z, Vos H, Hepkema B, Poppema S, van den Berg A, Diepstra A. Epidemiology of classical Hodgkin lymphoma and its association with Epstein Barr virus in Northern China. PLoS ONE. 2011;6:e21152. doi: 10.1371/journal.pone.0021152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Koh YW, Kang HJ, Yoon DH, Suh C, Kim JE, Kim CW, Huh J. Changing trend of Epstein-Barr virus association in Hodgkin lymphoma in the Republic of Korea. Ann Hematol. 2013;92:1653–1660. doi: 10.1007/s00277-013-1837-7. [DOI] [PubMed] [Google Scholar]
- 81.Dowd JB, Palermo T, Brite J, McDade TW, Aiello A. Seroprevalence of Epstein-Barr virus infection in U.S. children ages 6–19, 2003–2010. PLoS ONE. 2013;8:e64921. doi: 10.1371/journal.pone.0064921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ford JL, Stowe RP. Racial–ethnic differences in Epstein-Barr virus antibody titers among U.S. children and adolescents. Ann Epidemiol. 2013;23:275–280. doi: 10.1016/j.annepidem.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 83.Shapira Y, PoratKatz B-S, Gilburd B, Barzilai O, Ram M, Blank M, Lindeberg S, Frostegård J, Anaya J-M, Bizzaro N, Jara L, Damoiseaux J, Shoenfeld Y, Agmon Levin N. Geographical differences in autoantibodies and anti-infectious agents antibodies among healthy adults. Clinic Rev Allerg Immunol. 2012;42:154–163. doi: 10.1007/s12016-010-8241-z. [DOI] [PubMed] [Google Scholar]
- 84.Glaser SL, Hsu JL. Hodgkin’s disease in Asians: incidence patterns and risk factors in population-based data. Leuk Res. 2002;26:261–269. doi: 10.1016/s0145-2126(01)00126-6. [DOI] [PubMed] [Google Scholar]
- 85.Enciso-Mora V, Broderick P, Ma Y, Jarrett RF, Hjalgrim H, Hemminki K, van den Berg A, Olver B, Lloyd A, Dobbins SE, Lightfoot T, van Leeuwen FE, Forsti A, Diepstra A, Broeks A, Vijayakrishnan J, Shield L, Lake A, Montgomery D, Roman E, Engert A, von Strandmann EP, Reiners KS, Nolte IM, Smedby KE, Adami HO, Russell NS, Glimelius B, Hamilton-Dutoit S, de Bruin M, Ryder LP, Molin D, Sorensen KM, Chang ET, Taylor M, Cooke R, Hofstra R, Westers H, van Wezel T, van Eijk R, Ashworth A, Rostgaard K, Melbye M, Swerdlow AJ, Houlston RS. A genome-wide association study of Hodgkin’s lymphoma identifies new susceptibility loci at 2p16.1 (REL), 8q24.21 and 10p14 (GATA3) Nat Genet. 2010;42:1126–1130. doi: 10.1038/ng.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chang ET, Cronin-Fenton DP, Friis S, Hjalgrim H, Sørensen HT, Pedersen L. Aspirin and other nonsteroidal anti-inflammatory drugs in relation to Hodgkin lymphoma risk in northern Denmark. Cancer Epidemiol Biomarkers Prev. 2010;19:59–64. doi: 10.1158/1055-9965.EPI-09-0909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang X, Hepkema B, Nolte I, Kushekhar K, Jongsma T, Veenstra R, Poppema S, Gao Z, Visser L, Diepstra A, van den Berg A. HLA-A*02:07 is a protective allele for EBV negative and a susceptibility allele for EBV positive classical Hodgkin lymphoma in China. PLoS ONE. 2012;7:e31865. doi: 10.1371/journal.pone.0031865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Urayama KY, Jarrett RF, Hjalgrim H, Diepstra A, Kamatani Y, Chabrier A, Gaborieau V, Boland A, Nieters A, Becker N, Foretova L, Benavente Y, Maynadie M, Staines A, Shield L, Lake A, Montgomery D, Taylor M, Smedby KE, Amini RM, Adami HO, Glimelius B, Feenstra B, Nolte IM, Visser L, van Imhoff GW, Lightfoot T, Cocco P, Kiemeney L, Vermeulen SH, Holcatova I, Vatten L, Macfarlane GJ, Thomson P, Conway DI, Benhamou S, Agudo A, Healy CM, Overvad K, Tjonneland A, Melin B, Canzian F, Khaw KT, Travis RC, Peeters PH, Gonzalez CA, Quiros JR, Sanchez MJ, Huerta JM, Ardanaz E, Dorronsoro M, Clavel-Chapelon F, Bueno-de-Mesquita HB, Riboli E, Roman E, Boffetta P, de Sanjose S, Zelenika D, Melbye M, van den Berg A, Lathrop M, Brennan P, McKay JD. Genome-wide association study of classical Hodgkin lymphoma and Epstein-Barr virus status-defined subgroups. J Natl Cancer Inst. 2012;104:240–253. doi: 10.1093/jnci/djr516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oza AM, Tonks S, Lim J, Fleetwood MA, Lister TA, Bodmer JG. A clinical and epidemiological study of human leukocyte antigen-DPB alleles in Hodgkin’s disease. Cancer Res. 1994;54:5101–5105. [PubMed] [Google Scholar]
- 90.Niens M, Jarrett RF, Hepkema B, Nolte IM, Diepstra A, Platteel M, Kouprie N, Delury CP, Gallagher A, Visser L, Poppema S, Te Meerman GJ, van den Berg A. HLA-A*02 is associated with a reduced risk and HLA-A*01 with an increased risk of developing EBV-positive Hodgkin lymphoma. Blood. 2007;110:3310–3315. doi: 10.1182/blood-2007-05-086934. [DOI] [PubMed] [Google Scholar]
- 91.Ellis JM, Henson V, Slack R, Ng J, Hartzman RJ, Katovich Hurley C. Frequencies of HLA-A2 alleles in five U.S. population groups. Predominance of A*02011 and identification of HLA-A*0231. Hum Immunol. 2000;61:334–340. doi: 10.1016/s0198-8859(99)00155-x. [DOI] [PubMed] [Google Scholar]
- 92.Krausa P, Brywka M, Savage D, Hui KM, Bunce M, Ngai JLF, Teo DLT, Ong YW, Barouch D, Allsop CEM, Hill AVS, McMichael AJ, Bodmer JG, Browning MJ. Genetic polymorphism within HLA-A*02: significant allelic variation revealed in different populations. Tissue Antigens. 1995;45:223–231. doi: 10.1111/j.1399-0039.1995.tb02444.x. [DOI] [PubMed] [Google Scholar]
- 93.Rubicz R, Yolken R, Drigalenko E, Carless MA, Dyer TD, Bauman L, Melton PE, Kent JW, Jr, Harley JB, Curran JE, Johnson MP, Cole SA, Almasy L, Moses EK, Dhurandhar NV, Kraig E, Blangero J, Leach CT, Goring HH. A genome-wide integrative genomic study localizes genetic factors influencing antibodies against Epstein-Barr virus nuclear antigen 1 (EBNA-1) PLoS Genet. 2013;9:e1003147. doi: 10.1371/journal.pgen.1003147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hjalgrim H, Smedby KE, Rostgaard K, Molin D, Hamilton-Dutoit S, Chang ET, Ralfkiaer E, Sundstrom C, Adami HO, Glimelius B, Melbye M. Infectious mononucleosis, childhood social environment, and risk of Hodgkin lymphoma. Cancer Res. 2007;67:2382–2388. doi: 10.1158/0008-5472.CAN-06-3566. [DOI] [PubMed] [Google Scholar]
- 95.Jarrett RF. Viruses and Hodgkin’s lymphoma. Ann Oncol. 2002;13(Suppl 1):23–29. doi: 10.1093/annonc/13.s1.23. [DOI] [PubMed] [Google Scholar]
- 96.Jarrett RF. Viruses and lymphoma/leukaemia. J Pathol. 2006;208:176–186. doi: 10.1002/path.1905. [DOI] [PubMed] [Google Scholar]
- 97.Glaser SL, Clarke CA, Nugent RA, Stearns CB, Dorfman RF. Reproductive risk factors in Hodgkin’s disease in women. Am J Epidemiol. 2003;158:553–563. doi: 10.1093/aje/kwg198. [DOI] [PubMed] [Google Scholar]


