Abstract
Accumulation of alpha-synuclein (α-syn) in the central nervous system (CNS) is a core feature of Parkinson disease (PD) that leads to activation of the innate immune system, production of inflammatory cytokines and chemokines, and subsequent neurodegeneration. Here, we used heterozygous reporter knock-in mice in which the first exons of the fractalkine receptor (CX3CR1) and of the C-C chemokine receptor type 2 (CCR2) are replaced with fluorescent reporters to study the role of resident microglia (CX3CR1+) and infiltrating peripheral monocytes (CCR2+), respectively, in the CNS. We used an α-syn mouse model induced by viral over-expression of α-syn. We find that in vivo, expression of full-length human α-syn induces robust infiltration of pro-inflammatory CCR2+ peripheral monocytes into the substantia nigra. Genetic deletion of CCR2 prevents α-syn induced monocyte entry, attenuates MHCII expression and blocks the subsequent degeneration of dopaminergic neurons. These results demonstrate that extravasation of pro-inflammatory peripheral monocytes into the CNS plays a key role in neurodegeneration in this model of PD synucleinopathy, and suggest that peripheral monocytes may be a target of neuroprotective therapies for human PD.
Keywords: Monocytes, Microglia, α-syn (alpha-synuclein), Parkinson disease (PD), C-C chemokine receptor type 2 (CCR2), Fractalkine receptor (CX3CR1) Major histocompatibility complex II (MHCII)
Background
Parkinson disease (PD) is the most common neurodegenerative movement disorder and is characterized by a progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) along with the presence of Lewy bodies and Lewy neurites composed of α-syn in the SNpc and other brain regions. Missense mutations or multiplications of the α-syn gene cause aggressive forms of PD (Polymeropoulos 1998; Polymeropoulos, et al. 1997; Ross, et al. 2008) while genome wide association studies (GWAS) show that the locus encoding α-syn is one of the most potent genetic factors in susceptibility to sporadic PD (Houlden and Singleton 2012; Simon-Sanchez, et al. 2009). One of the most common non-coding disease-associated polymorphisms in this region has recently been shown to lead to enhanced α-syn protein expression (Soldner, et al. 2016).
There is growing evidence for a critical role of inflammation in the pathogenesis of PD (Appel 2012; Hirsch and Hunot 2009; Hirsch, et al. 2012; Lim, et al. 2016). In the human PD brain, there is prominent reactive microgliosis (McGeer, et al. 1988), enhanced expression of pro-inflammatory cytokines and chemokines (Blum-Degen, et al. 1995; Mogi, et al. 1994a; Mogi, et al. 1994b), lymphocyte infiltration (Brochard, et al. 2009) and deposition of IgG (Orr, et al. 2005). There is also strong evidence for alterations in the function of the peripheral immune system in PD pathogenesis including alterations in T cell subsets (Saunders, et al. 2012). Many of these features are reproduced in rodent models of PD produced by overexpression or aggregation of α-syn (Allen Reish and Standaert 2015). We have previously shown that the neurotoxicity of α-syn can be attenuated by modulation of key immune mediators, including MHCII (Harms, et al. 2013) and the Fcgamma receptors (Cao, et al. 2012) which mediate interactions between immunoglobins and microglia (Daeron 1997). Genome Wide Association (GWA) studies provide additional support for the importance of immunological mechanisms driving disease, showing that polymorphisms in the HLA-DR (MHCII) locus are associated with sporadic, late-onset PD (Hamza, et al. 2010). Population studies show an association between the long-term use of non-steroidal anti-inflammatory medications and reduced risk of PD (Gao, et al. 2011), providing proof of principal for the use of immunomodulatory strategies.
Monocytes are a subset of myeloid cells that enter tissues, including the brain, during active disease states (Ransohoff 2011; Ransohoff and Cardona 2010). A key regulatory mechanism for tissue entry is the interaction of the chemokine receptor CCR2, found on peripheral myeloid cells, and its ligand, CCL2 (Mahad, et al. 2006). After crossing the blood-brain barrier in response to CCL2 signaling, monocytes can differentiate into macrophage and dendritic cell phenotypes and mediate pro and anti-inflammatory responses (Mahad, et al. 2006; Prinz, et al. 2011; Ransohoff 2011; Ransohoff and Cardona 2010; Saederup, et al. 2010). These peripherally derived myeloid cells are distinct from microglia, the resident innate-immune cells of the brain, and can have critical differential effects on tissue injury and protection (Butovsky, et al. 2014; Butovsky, et al. 2012; Gaupp, et al. 2003; Schmid, et al. 2009; Yamasaki, et al. 2014). In experimental autoimmune encephalomyelitis (EAE), a mouse model for human multiple sclerosis (MS), infiltrating monocytes promote disease progression via continual destruction of myelin (Ajami, et al. 2011), and blocking CCR2-dependent entry of monocytes both reduces inflammation and delays disease onset (Ajami, et al. 2011; Columba-Cabezas, et al. 2002; Fife, et al. 2000; Izikson, et al. 2000).
While relatively little is known about peripheral monocytes in human PD, preliminary studies in patients have shown that classical monocytes expressing CCL2 are enriched in blood isolated from PD patients, and CCL2 expression is elevated in the CSF (Grozdanov, et al. 2014; Reale, et al. 2009), while an additional study showed elevated CCR2 expression on peripheral blood monocytes (Funk, et al. 2013). In addition, peripheral blood monocytes isolated from PD patients showed a hypersensitivity or inflammatory pre-disposition to an inflammatory stimulus (Grozdanov, et al. 2014). Most recently, a study of circulating blood monocytes from young, healthy individuals without PD found that these cells had remarkably high expression of PD-associated genes, including α-syn, LRRK2 and others, and concluded that the inflammatory component of PD susceptibility is strongly driven by myeloid cell types (Raj, et al. 2014).
In this study, we show that in a mouse model of PD that overexpression of α-syn recruits pro-inflammatory CCR2+ peripheral monocytes into the CNS. Blocking this infiltration attenuates α-syn mediated inflammation and subsequent neurodegeneration. Understanding the role of peripherally derived pro-inflammatory monocytes in PD-related inflammation and the mechanisms responsible for their entry into the brain may lead to novel therapeutic intervention strategies for human PD.
Methods
Animals and Treatment
C57BL/6 (catalog # 000664), CX3CR1 reporter knock in (B6.129P-Cx3cr1tm1Litt/J (catalog # 005582), and CCR2 reporter knock in (B6.129(Cg)-Ccr2tm2.1Ifc/J, catalog # 017586) mice maintained on a congenic background were used for these studies and were obtained from Jackson Laboratories (Bar Harbor, Maine). In these strains, a monomeric green fluorescent protein (GFP) (Jung, et al. 2000) and red fluorescent protein (RFP) sequence replaces the coding sequence of the Fractalkine receptor (CX3CR1) and chemokine (C-C motif) receptor 2 (Ccr2) gene (Saederup, et al. 2010), respectively, abolishing gene function.
The rAAV vectors, rAAV-CBA-EGFP (AAV2-GFP) and rAAV-CBA-Alpha-SYNUCLEIN (AAV2-SYN) were obtained from the University of North Carolina Vector Core via the Michael J. Fox Foundation research tools catalog. (https://www.michaeljfox.org/research/research-tools-catalogue.html). Male red/green and CCR2 knockout mice (8–12 weeks of age) were deeply anesthetized with isoflurane and unilaterally or bilaterally injected with 2 μL of AAV2-SYN or AAV2-GFP (1.5 × 1013 viral genome/mL diluted in sterile PBS) into the right SNpc as previously described (Harms, et al. 2013; Luk, et al. 2012; St Martin, et al. 2007). Co-ordinates were anterior-posterior −3.2 mm from bregma, medio-lateral −1.2 mm from midline, and dorso-ventral −4.6 mm from dura. All research conducted on animals was approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Alabama at Birmingham. The pattern and extent of α-syn expression obtained in vivo after intranigral injection of AAV2-SYN vectors has been reported previously and similar results were obtained in this study (Cao, et al. 2010; Harms, et al. 2013; St Martin, et al. 2007; Theodore, et al. 2008; Thome, et al. 2016; Thome, et al. 2015). These vectors produce localized transduction of neurons in the SNc ipsilateral to the injection site. The extent of transduction of SNpc neurons is typically approximately 50%, but does vary depending on the exact location of the injection site (St Martin, et al. 2007). There is no significant α-syn expression observed in neurons on the contralateral side or in glia on either side. Western blot analysis of nigral tissue shows that these vectors lead to accumulation of human α-syn in both monomeric and high molecular weight forms (Harms et al., 2013).
Immunohistochemistry
At 4 weeks and 6 months post-transduction, animals were deeply anesthetized and transcardially perfused, post-fixed for 24 hours, and cryoprotected as previously described (Harms, et al. 2013). Brains were frozen on dry ice and cryosectioned coronally on a sliding microtome (cut thickness: 40 μm); sections were collected serially throughout the striatum and SNpc, placed into tissue collection solution (50% 0.01 M PBS, 50% glycerol), and stored at −20°C for immunohistochemical analysis.
For fluorescent analysis, free-floating sections were labeled with anti-MHCII (M5/114.15.2, eBiosciences, 1:100) or anti-Tyrosine hydroxylase (TH) (Millipore, 1:2000) antibodies overnight at 4°C. Appropriate Alexa-conjugated secondary antibodies diluted 1:1000 (Life Technologies) were used at room temperature for 2.5 hours. Sections were mounted onto coated glass slides, and cover slips were added using Vectashield Hard Set mounting medium.
For TH neuron quantification using unbiased stereological analysis, free floating sections were stained as previously described (Harms, et al. 2013; Luk, et al. 2012; St Martin, et al. 2007), coded, and analyzed with an Olympus BX51 microscope and MicroBrighfield software (MicroBrightfield Inc., Williston, VT).
Imaging and Quantification
Confocal images were captured using a Leica TCS-SP5 laser scanning confocal microscope. Images were processed using the Leica LASAF software, exported and processed using Adobe Photoshop. For quantification of MHCII and LB509 staining, slides were observed using a Nikon Eclipse E800M fluorescent microscope. Coded slides were scored by using a numerical scale 0 (no staining) to 4 (most intense) by a single observer blind to the treatment paradigm (Harms, et al. 2013; Thome, et al. 2015). TH, MHCII, and LB509 positive cells were manually differentiated under 40x magnification to differentiate cell size, shape, and morphology. Staining within the vicinity of viral transduction (SYN) was considered for scoring while staining immediately surrounding the needle tract was ignored. Scores obtained from 6–8 mice per group were plotted and statistically analyzed using the Mann Whitney test.
Mononuclear Cell Isolation and Flow Cytometry
Mononuclear cells were isolated from bilaterally transduced ventral midbrains using enzymatic digestion with 1 mg/mL Collagenase IV (Sigma) and 20 μg/mL DNAseI (Sigma) diluted in RPMI 1640 with 10% heat inactivated fetal bovine serum and 1% L-glutamine from the pooled ventral midbrains of red/green naïve mice or injected with AAV2-SYN or AAV2-GFP control (n=2–3/group). Mononuclear cells were isolated 2 and 4 weeks post transduction via 30/70% percoll gradient as previously described (Qin, et al. 2012). Isolated cells were first blocked with Fcγ receptor 2.4G2 (1:500), surface stained for MHCII (M5/114.15.2, BioLegend), CD45 (clone 30-F11, BioLegend), Ly6C (clone HK1.4, BioLegend), Ly6G (clone 1A8, BioLegend), CD68 (clone FA-11, BioLegend), CD4 (clone GK1.5, BioLegend) and CD11b (clone M1/70, Biolegend) as previously described, analyzed using the LSR-II flow cytometer (BD biosciences) and data analyzed using FlowJo software. In each experiment, a fixable viability dye (Vibrant Aqua (eBioscience, 1:1000) was used per manufacturer’s instruction.
Each flow cytometry experiment involves 18 to 30 mice. Individual samples were formed by pooling 2–3 ventral midbrains (bilaterally injected), each from different animals. In each experiment, treatment groups (Naïve, AAV2-GFP, AAV2-SYN) consisted of 3–4 of these independent samples. For flow cytometry analysis, the mean +/− SEM of the independent samples in each treatment group were plotted. One-way ANOVA with Tukey’s post hoc test were used to compare the groups.
Results
α-syn expression induces CCR2+ monocyte entry from the periphery
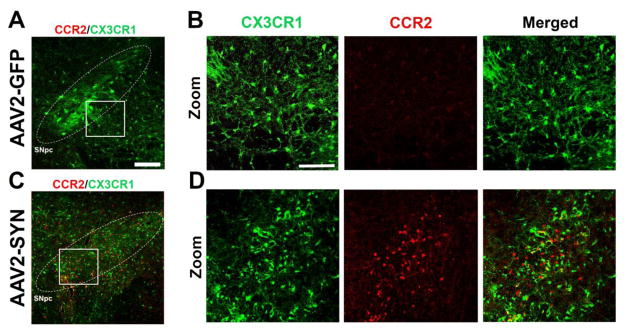
Monocytes are a subset of circulating myeloid cells that can infiltrate into the CNS in disease states. Studying this population of cells in the brain has been challenging due to lack of selective antibodies or reagents and surface marker overlap with resident microglia. To overcome this challenge, we used reporter knock-in mice in which the first exon of the CX3CR1 or CCR2 locus is replaced with a fluorescent reporter (red/green mice) (Jung, et al. 2000; Saederup, et al. 2010). To determine whether monocytes enter into the CNS following α-syn overexpression, 8–12 week old red/green mice received a single, unilateral stereotactic injection of AAV2-SYN or AAV2-GFP control virus (1.5 × 1013 viral genome/mL) into the right SNpc. Four weeks post transduction, CCR2-RFP+ monocytes were observed in response to α-syn expression (Figure 1). The morphology of these RFP+ monocytes were typically small, round cells lacking processes. Further immunohistochemical analysis revealed that these cells co-stained with markers of myeloid lineage including CD11b and CD68 (data not shown). The location of the RFP+ monocytes was closely related to the location of the neuronal somata expressing the AAV2-SYN transgene: they were most frequent within the region of the SNpc and the adjacent SNpr, particularly where there were many virally transduced neurons. Occasionally, we observed RFP+ monocytes clustering around blood vessels near transduced neurons, suggesting that small vessels in the region were a source of infiltration. RFP+ monocytes were quite rare in other parts of the brain; they were generally not observed in the contralateral substantia nigra, nor were they present in the striatum, cortex, or other brain structures outside of the immediate region of the AAV2-SYN injection. RFP+ monocytes were very scarce in the brains of AAV2-GFP control animals (Figures 1A–1D).
Figure 1. α-syn expression induces CCR2+ monocyte entry from the periphery.
(A) No detectable CCR2-RFP+ cells (CCR2, red) are observed in the SNpc (white circle, inset) of AAV2-GFP injected controls animals 4 weeks post-transduction in red/green mice. CX3CR1-GFP+ resident microglia (CX3CR1, green). Scale bar is 50 microns. (B) Zoom inset of AAV2-GFP injected controls. Single channels and merged images. Scale bar is 75 microns. (C) CCR2-RFP+ cells (CCR2, red) infiltrate from the periphery in the SNpc (white circle, inset) of AAV2-SYN injected animals 4 weeks post-transduction in red/green mice. (D) Zoom inset of AAV2-SYN injected animals. Single channel and merged images.
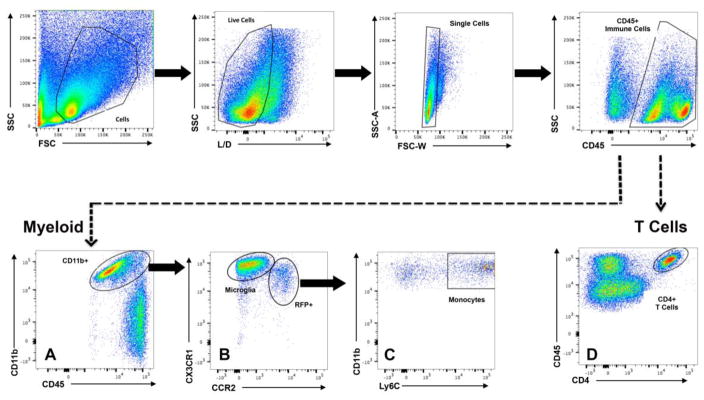
Flow cytometric analysis of ventral midbrain mononuclear cell isolates from Naïve, AAV2-GFP or AAV2-SYN treated red/green mice was used to further characterize the infiltrating cells. Flow data were gated sequentially, first on a forward (FSC) and side scatter (SSC), and then on live cells (Live/Dead), and CD45+ expression (Figure 2, top). This gating strategy allowed us to select all immune cells (resident and peripheral) and eliminate doublets from analysis (Figure 2, single cells). Further gating analysis revealed two distinct populations of cells: a CD45+ CD11b expressing population, characteristic of myeloid cells (Figure 2A), and a CD45+, CD4 expressing population corresponding to T cells (Figure 2D). All of the CX3CR1-GFP+ expressing resident microglia and CCR2-RFP+ expressing infiltrating monocytes (Figure 2B) were found in the CD11b+, Ly6C+ population (Figure 2C). To ensure specificity of Ly6C to monocytes, cells that were positive for Ly6G+ were excluded from analysis. In the cell populations that were CD45+, CD11b− (Figure 2A, ungated population) we performed flow cytometric analysis at 4 weeks post transduction with antibodies for B220 (B cells), NK1.1 (natural killer cells), and CD4 and CD8 (T cells). We we did not observe any difference in the percent of these cell populations expressing CCR2 (data not shown). Additionally, with α-syn overexpression, we found no significant changes in the number of these cell types over Naïve and AAV2-GFP control (data not shown) with the exception of CD4 T cells (Supplemental Figure 1).
Figure 2. Flow cytometry gating strategy to differentiate between T cells, resident microglia, and infiltrating monocytes.
Flow cytometric analysis of ventral midbrain isolates and gating scheme for isolating mononuclear cells from Naïve, AAV2-GFP, and AAV2-SYN transduced red/green mice. Mononuclear cells were gated on forward (FSC) and side scatter (SSC), live cells (Live/Dead), and CD45+ expression. Gating was designed to distinguish two distinct populations of cells: (Myeloid) a CD45+ CD11b expressing population which are myeloid, and (D) a CD45+, CD4 expressing population which are T cells. (A) Isolation of the CD45+, CD11b+ myeloid population reveals (B) two distinct gates of resident microglia which are CX3CR1-GFPhi and infiltrating monocytes which are CCR2-RFP+. (C) Further gating of CCR2-RFP+ monocytes indicate that the monocytes are Ly6C+. Cells that were Ly6G+ were excluded from analysis (not shown).
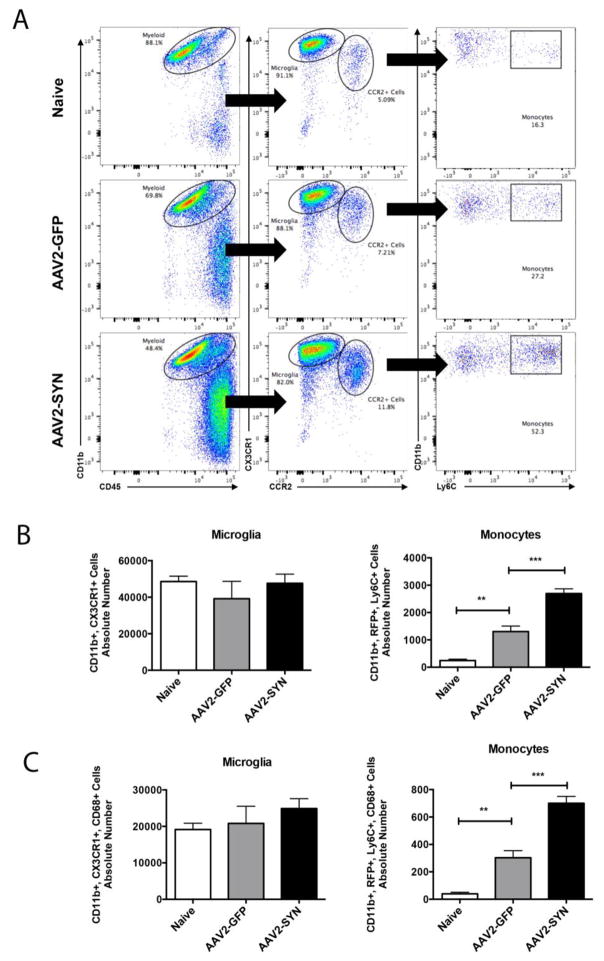
Quantitative analysis of the effects of AAV2-SYN expression on peripheral monocyte entry into the CNS was performed by gating for CD45+, CD11b+ myeloid cells, and comparing CX3CR1-GFP+hi CCR2-RFP- (resident microglia) and CX3CR1-GFP+lo, CCR2-RFP+, Ly6C+ (infiltrating monocyte) populations in Naïve, AAV2-SYN and AAV2-GFP treated red/green animals (Figure 3A). Analysis of the absolute number of cells demonstrated a marked increase in CX3CR1-GFP+lo, CCR2-RFP+, Ly6C+, and CD68+ monocytes after AAV2-SYN (Figures 3A–C), but no significant difference in the absolute number of CX3CR1-GFPhi, CCR2-RFP−, and CD68+ resident microglia in the ventral midbrain (Figures 3B, C) four weeks post transduction.
Figure 3. AAV2-SYN expression induces CCR2+, LY6C+ monocyte entry from the periphery.
(A) CD45+, CD11b+, myeloid cell populations were gated into two distinct populations: CX3CR1-GFPhi, CCR2-RFP− resident microglia and CX3CR1-GFPlo, CCR2-RFP+ infiltrating myeloid cells. Further gating on Ly6C showed and increase in the percent of CCR2-RFP+ infiltrating monocytes in AAV2-SYN injected red/green mice. (B) Quantification of the absolute number of microglia and monocytes in the ventral midbrain of Naïve, AAV2-GFP, and AAV2-SYN transduced red/green mice. This experiment involves 28 mice. Individual samples were formed by pooling 2–3 ventral midbrains (bilaterally injected), each from different animals. In each experiment, treatment groups (Naïve, AAV2-GFP, AAV2-SYN) consisted of 4 of these independent samples. For flow cytometry analysis, the mean +/− SEM of the independent samples in each treatment group were plotted. One-way ANOVA with Tukey’s post hoc test, **p<0.01, ***p<0.001. (C) Quantification of the absolute number of CD68+ microglia and monocytes in the ventral midbrain of Naïve, AAV2-GFP, and AAV2-SYN transduced red/green mice. This experiment involves 28 mice. Individual samples were formed by pooling 2 ventral midbrains, each from different animals (bilaterally injected). In each experiment, treatment groups (Naïve, AAV2-GFP, AAV2-SYN) consisted of 4 of these independent samples. For flow cytometry analysis, the mean +/− SEM of the independent samples in each treatment group were plotted. One-way ANOVA with Tukey’s post hoc test, **p<0.01, ***p<0.001. Infiltrating monocytes as well as resident microglia express the myeloid marker, CD68.
CCR2-RFP+ infiltrating monocytes are pro-inflammatory
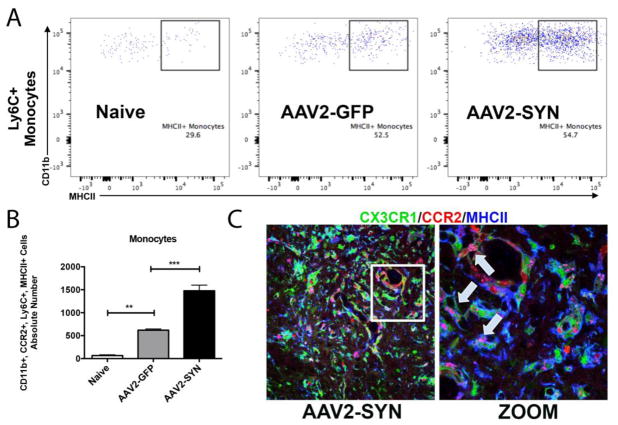
Previous studies have shown that once in the CNS, monocytes can differentiate along a spectrum of inflammatory states (Butovsky, et al. 2014; Butovsky, et al. 2012; Gaupp, et al. 2003; Ransohoff 2011; Ransohoff and Cardona 2010; Schmid, et al. 2009). These can be described broadly as pro-inflammatory (M1) and anti-inflammatory (M2) phenotypes, although it is clear that there are also intermediate and overlapping states of activation in some model systems (Butovsky, et al. 2014; Butovsky, et al. 2012; Gaupp, et al. 2003; Moehle and West 2015; Schmid, et al. 2009). To determine the characteristic of the infiltrating monocytes in the AAV2-SYN model of PD, we studied expression of an M1 marker, MHCII, and an M2 marker, CD206 (mannose receptor), 4 weeks after injection of AAV2-GFP or AAV2-SYN in the right ventral midbrain. (Figure 4). Histopathological analysis of SNpc sections from AAV2-SYN injected red/green mice revealed that many of the CCR2-RFP+ monocytes exhibited strong MHCII expression (Figure 4C). MHCII expression was also seen in CX3CR1-GFP+ microglia (Figure 4C). The distribution of MHCII expression was examined further by mononuclear cell isolation and flow cytometry. Mononuclear cell isolates from Naïve, AAV2-GFP, and AAV2-SYN treated red/green mice were gated to identify CD11b+ CX3CR1-GFP+lo, CCR2-RFP+, Ly6C+ cells (infiltrating monocytes) (Figure 2, 4A). We found significantly more MHCII+ infiltrating monocytes in response to α-syn expression when compared to naïve and AAV2-GFP controls (Figure 4B, D). We observed no significant difference in the number of CD206 expressing cells (Data not shown). In addition, as previously reported by our group and others, while the number of resident microglia did not change (Figure 3B, C), there was a significant increase in the percent of resident microglia expressing MHCII in response to α-syn expression when compared to AAV2-GFP control (GFP: 4.76 + 0.71, SYN: 17.43+ 2.027 **p<0.0011, n=4/group). Together, these observations show that AAV2-SYN expression leads to activation of resident microglia, significant infiltration of pro-inflammatory monocytes.
Figure 4. CCR2-RFP+ peripheral monocytes are pro-inflammatory via MHCII expression.
(A) CD45+, CD11b+, myeloid cell populations were gated to isolate CX3CR1-GFPlo, CCR2-RFP+ infiltrating myeloid cells. Further gating on Ly6C and M1 marker, MHCII showed and increase in the absolute number of CCR2-RFP+, Ly6C+, MHCII+ infiltrating monocytes in AAV2-SYN injected red/green mice when compared to Naïve and AAV2-GFP controls (B). Quantification of the absolute number of MHCII+, Ly6C+ monocytes in the ventral midbrain of Naïve, AAV2-GFP, and AAV2-SYN transduced red/green mice. This experiment involves 28 mice. Individual samples were formed by pooling 2 ventral midbrains, each from different animals (bilaterally injected). In each experiment, treatment groups (Naïve, AAV2-GFP, AAV2-SYN) consisted of 4 of these independent samples. For flow cytometry analysis, the mean +/− SEM of the independent samples in each treatment group were plotted. One-way ANOVA with Tukey’s post hoc test, **p<0.01, ***p<0.001. (C) CCR2-RFP+ infiltrating cells (red) co-localize with MHCII I-A/I-E proteins (blue, arrow inset) in the AAV2-SYN transduced SNpc of red/green mice at 4 weeks. GFP is CX3CR1.
Blocking peripheral monocyte entry is neuroprotective in an α-syn model of PD
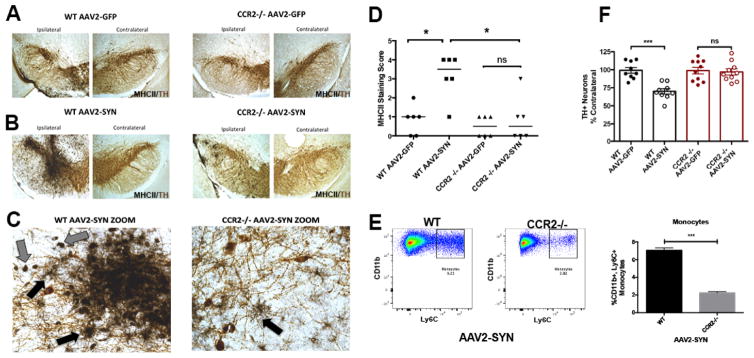
Previous studies have shown that in an experimental autoimmune encephalomyelitis (EAE) mouse model of Multiple Sclerosis (MS), infiltrating monocytes are responsible for disease progression and blocking CCR2-dependent monocyte entry reduces inflammation and delays disease onset (Ajami, et al. 2011; Gaupp, et al. 2003; Izikson, et al. 2000). To determine if peripheral monocyte entry is required for α-syn-induced inflammation and neurodegeneration, we used WT (C57BL/6) and CCR2 KO mice on a congenic background, and performed unilateral stereotactic injections of AAV2-SYN or AAV2-GFP virus into the right SNpc. Four weeks post transduction, we observed prominent expression of MHCII+ via via IHC on microglia (Figure 5C, black arrows) and monocytes (Figure 5C, gray arrows) in AAV-SYN injected WT animals (Figure 5B, C), but not in those injected with AAV-GFP (Figure 5A). Knockout of CCR2 markedly attenuated α-syn-induced MHCII expression in the ipsilateral SNpc (Figure 5A–C) via immunohistochemistry and Ly6C+ monocyte infiltration via flow cytometry (Figure 5E). Rating of the intensity of the MHCII staining, performed by an observed blinded to the treatment conditions, confirmed the prominent induction of MHCII expression by AAV2-SYN in WT animals, and near complete blockade of this response in the CCR2 KO animals (Fig 5D). Six months post transduction, tyrosine hydroxylase (TH) positive cell number was evaluated using unbiased stereology We found that genetic knockout of CCR2 completely blocked the 25–30% loss of TH immunopositive cells at 6 months observed in the WT controls after injection of AAV-SYN (Figure 5F). We also examined infiltration of CD4+ T cells in these models; while AAV2-SYN did induce infiltration of CD4+ cells, the numbers were not reduced in the CCR2 KO animals (Supplemental Figure 1). Additionally, AAV2-SYN induced protein expression, assessed by immunohistochemistry using a human synuclein specific antibody, was not altered in the CCR2 knockout animals (Supplemental Figure 2).
Figure 5. Blocking peripheral monocyte entry is neuroprotective in an α-syn model of PD.
(A–B) Four weeks post transduction, MHCII expression (Ni-DAB, black) in the SNpc (TH, brown), indicates that blocking myeloid cell infiltration using a CCR2 knockout animal inhibits α-syn-induced inflammation. (C) 40x images of the ipsilateral SNpc of AAV2-SYN injected WT and CCR2−/− mice. Gray arrows indicate indicate MHCII+ (NiDAB-black) monocytes. Black arrows indicate MHCII+ (NiDAB-black) microglia. (D) Quantification of MHCII staining in the SNpc of AAV2-GFP (control) and AAV2-SYN injected WT and CCR2 −/− mice at 4 weeks post-transduction. Data represents the median (n=6/treatment) *p<0.05, Kruskal Wallis test. (E) Representative dot plots of Ly6C+ monocyte entry observed in WT and CCR2−/− mice mice in response to α-syn overexpression. Quantification of the percent of Ly6C+ monocytes in the ventral midbrain of WT and CCR2−/− AAV2-SYN injected mice. This experiment involves 18 mice. Individual samples were formed by pooling 3 ventral midbrains (bilaterally injected), each from different animals. In this experiment each genotype (WT, CCR2−/−) consisted of 3 of these independent samples. For flow cytometry analysis, the mean +/− SEM of the independent samples in each treatment group were plotted. Unpaired t test. ***p<0.0001. No significant Ly6C+ monocyte entry was observed in CCR2−/− AAV2-SYN transduced mice when compared to Naïve (uninjected) control. p=0.549. (F) Six months post transduction, genetic knockout of CCR2 attenuates AAV2-SYN-inuduced TH positive neuron loss in the SNpc. Neuron loss is reported via unbiased stereology and as a percentage of contralateral side. n=8–10/group. Two-way ANOVA with Conferring selected comparison post hoc test. ***p<0.001.
Discussion
In this study, we show that a key component of the brain’s response to α-syn is recruitment of pro-inflammatory CCR2+ peripheral monocytes into the CNS. We observed robust infiltration of CCR2+, Ly6C+ peripheral monocytes in to the SNpc of AAV2-SYN transduced mice. At four weeks after AAV2-SYN, these infiltrating cells have a pro-inflammatory phenotype with marked expression of MHCII expression. We also found that genetic knockout of CCR2 prevents α-syn induced monocyte infiltration in response to AAV2-SYN. Remarkably, genetic deletion of CCR2 also blocks the induction of the pro-inflammatory MHCII response in the SNpc and prevents α-syn-induced degeneration of dopaminergic neurons. These results indicate a critical role for peripheral derived cells in α-syn-induced inflammation and neurodegeneration.
An important insight from the present study is that the relationship between microglial activation, monocyte entry, and degeneration in PD models is more complex that previous investigations suggested. Microglial activation and pro-inflammatory cytokine/chemokine expression has been described as a key mediator of injury in both α-syn and neurotoxin animal models of PD (Cao, et al. 2010; Harms, et al. 2013; Langston, et al. 1999; M.B. Watson 2011; Mount, et al. 2007; Sanchez-Guajardo, et al. 2010; Theodore, et al. 2008; Watson, et al. 2012). In 6-hydroxydopamine and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) models, activation of microglia has been described, and therapeutic strategies targeting pro-inflammatory cytokines and chemokines have proven to reduce dopaminergic neurodegeneration (Appel 2012; Gao, et al. 2008; Hirsch and Hunot 2009; Moehle and West 2015; Saijo and Glass 2011; Tansey and Goldberg 2010; Whitton 2007). In α-syn models, including α-syn transgenic mice, reactive microgliosis and inflammation have been reported to occur prior to dopamine cell loss (Watson, et al. 2012). Our findings require that these prior reports be viewed in a new light. The methods employed in these prior studies of “microgliosis” in fact cannot distinguish between activation of intrinsic microglia and entry of peripheral monocytes. Our studies suggest that many of the neuroinflammatory effects in PD models previously attributed to microglia could be a result of entry and differentiation of peripheral monocytes. Although the resident brain microglia do participate in the inflammatory response to brain α-syn, activation of the resident cells seems to be largely dependent on the entry of the peripheral monocytes.
In this study, we have shown that overexpression of α-syn results in robust infiltration of CCR2-RFP+ monocytes (Figure 1, 3) at 4 weeks post-transduction. This time point coincides with activation of a broad range of inflammatory markers (cytokines and chemokines) observed in the AAV2-SYN model of PD, providing further support for the idea that they are a critical part of the pro-inflammatory process in α-syn-mediated neuroinflammation (Allen Reish and Standaert 2015; Cao, et al. 2010; Harms, et al. 2013; Theodore, et al. 2008; Thome, et al. 2016; Thome, et al. 2015). In this study, we selected the AAV2-SYN model of PD. Utilizing this model, we were able to overexpress human full-length α-syn directly in dopaminergic neurons of the SNpc of red/green mice, successfully re-creating the neuroinflammatory and neurodegenerative phenotype as previously described (Harms, et al. 2013; Thome, et al. 2016; Thome, et al. 2015). While there are caveats of this particular model, including the high expression level of α-syn and brief disruption of the blood-brain-barrier during the stereotaxic surgery, the inflammation associated with α-syn has been extensively characterized and is reproducible (Cao, et al. 2010; Harms, et al. 2013; Theodore, et al. 2008; Thome, et al. 2016; Thome, et al. 2015; Volpicelli-Daley, et al. 2016). In order to control for these challenges, we incorporated flow cytometric analysis on naïve and AAV2-GFP injected animals. We did see modest neuroinflammatory effects in AAV2-GFP injected animals, detectable by comparison with either the contralateral (uninjected side) of the brain or with tissue from naïve (uninjected animals) (Figure 3). We believe these AAV2-GFP induced changes may result from either the surgery or non-specific effects of the vector as no neurodegeneration was observed in AAV2-GFP injected animals. In either case these effects were always significantly less than the inflammatory response produced by expression of wild type human α-syn with AAV2-SYN vector, which is identical to AAV2-GFP except for the protein encoded. Future studies utilizing other α-syn models are warranted to further evaluate the role of peripheral monocytes in PD-related neurodegenerative processes.
We used two primary markers to distinguish monocytes from resident microglia: CCR2 and CX3CR1. Expression profiling studies have shown CNS resident microglia express high amounts of CX3CR1 and very low CCR2, while peripheral monocytes express low levels of CX3CR1 and high CCR2 (Butovsky, et al. 2014; Mahad, et al. 2006; Mizutani, et al. 2012; Prinz, et al. 2011; Ransohoff 2011; Ransohoff and Cardona 2010; Saederup, et al. 2010). In our study, we utilized a novel red/green mouse in which resident microglia are CX3CR1-GFP+hi, CCR2-RFP− and infiltrating monocytes are CX3CR1-GFP+lo CCR2-RFP + (Figures 1–4). Since the mice retain a normal allele of both CCR2 and CX3CR1, they have normal immunological function (Saederup, et al. 2010). We have confirmed that although they are haploinsufficient for these alleles, they still display the expected neuroinflammatory phenotype in the AAV-SYN model, with a 30% TH positive cell loss in response to α-syn overexpression at six months after injection. We verified the monocytic nature of the CCR2-RFP+ infiltrating cells and examined their inflammatory status via flow cytometry using Ly6C expression as a marker (Figures 3, 4). CD11b+, Ly6Chi expression is a marker of circulating peripheral blood monocytes. It is important to note that CCR2 can be expressed by other cell types including natural killer cells, neutrophils, B cells, and T cells. We performed flow cytometric analysis at 4 weeks post transduction with antibodies for Ly6G (neutrophils), B220 (B cells), NK1.1 (natural killer cells), and CD4 and CD8 (T cells). We did not observe any difference in the percent of these cell populations expressing CCR2, particularly CD4 T cells. Additionally, with α-syn overexpression, we found no significant changes in the number of these cell types with the exception of CD4 T cells, which were increased in both WT and CCR2 KO (Supplemental Figure 1). It is possible that T cells do also contribute to neurodegeneration in this model. Since their numbers were not altered by CCR2 deletion, and the expression of CCR2 is not required for their entry into the CNS, the magnitude of this contribution cannot be directly determined with this approach and will need to be studied with other approaches that either assess the functional properties of T cells or modulate T cell entry. Recently, expression-profiling studies have identified several microglia-enriched proteins including transmembrane protein 119 (TMEM119), purinergic receptor P2ry12 (P2ry12) (Bennett, et al. 2016; Butovsky, et al. 2014) which may allow similar studies of microglia and macrophages in non-transgenic systems.
To determine the role of these pro-inflammatory monocytes of peripheral origin on α-syn-induced inflammation and neurodegeneration, we performed studies in animals with complete deletion of CCR2. Previous studies have shown that CCR2 expression is required for monocyte entry into the CNS (Mahad, et al. 2006) although other cell types, including T cells, can still enter. In the AAV2-SYN model, we found that CCR2 knockout attenuated α-syn-induced monocyte infiltration in the ipsilateral SNpc (Figure 5). It also greatly reduced induction of MHCII expression, not only within infiltrating cells, but also in the resident microglia via immunohistochemistry (Figures 5A–D). Absence of CCR2 expression and monocyte entry also blocked AAV2-SYN neurodegeneration (Figure 5F), indicating a critical role for peripheral monocytes in α-syn mediated neurodegeneration.
Our data suggests that CCL2, the ligand for CCR2, is important for monocyte infiltration. It is possible that this chemokine arises from the resident microglia; since activation of these cells is a spectrum of response and not a binary state, they might secrete chemokines with or without classical M1 activation. Alternatively, the chemokine may arise from monocyte pools engaged in surveillance. These results are consistent with findings using immunohistochemistry and confocal microscopy (Figure 4C) as well as our previously published results where we described the importance of MHCII in α-syn-induced neuroinflammation and neurodegeneration (Harms, et al. 2013; Thome, et al. 2016). Knowing whether they are actively contributing to disease pathogenesis by expressing M1 markers, or resolving α-syn-induced neuroinflammation by infiltrating with an M2 phenotype will have a great influence on potential therapeutic strategies in the future.
In human PD, microgliosis has been described as a feature of the disease and activated microglia are prominent in postmortem studies of idiopathic PD (McGeer, et al. 1988). Interestingly, microgliosis has also been observed in human brains many years after intoxication with MPTP, demonstrating that neurotoxin-induced PD also leads to long-lasting inflammatory effects in human brain (Langston, et al. 1999). A critical question is whether monocyte entry of the kind we have observed in animal models of PD also occurs in human disease. Is part or all of the “microgliosis” described in human PD in fact the result of infiltrating monocytes? At present there is no satisfactory method to directly address this important question, since the morphology of microglia and differentiated monocytes are indistinguishable and reliable molecular markers for human studies are lacking. There is, however, substantial evidence for entry of other immune cell types into the brain in human PD: CD4+ and CD8+ T cells are found concentrated in areas of dopaminergic cell degeneration including the SNpc (Brochard, et al. 2009), and these are associated with alterations in circulating T cell subsets in human PD patients (Saunders, et al. 2012). It will be important to develop markers that can assess monocyte entry into human brain, and determine the roles that these cells may play in the human disorder.
We propose that the entry of monocytes into the brain is a necessary component of the inflammatory cascade by which α-syn can induce neurodegeneration in PD. This appears to require CCR2 signaling, which leads to the question of the nature of the upstream mechanisms responsible for secretion of this chemokine. There is abundant evidence for the presence of abnormal forms of α-syn in PD, which include misfolded, aggregated, and modified (phosphorylated and nitrated) forms of the protein (Luk, et al. 2012; Reynolds, et al. 2008; Reynolds, et al. 2009; Volpicelli-Daley, et al. 2011). We have previously shown that a key requirement for toxicity in the setting of α-syn overexpression is MHCII-mediated antigen presentation mediated by resident microglia (Harms, et al. 2013). While the exact nature of the triggering antigen is unclear, it is likely that the consequence of this antigen presentation is interaction with T cells, and production of chemokines including CCL2. The data here suggest that the downstream consequence of the interaction of resident microglia and T cells is the migration of monocytes into the brain follow by differentiation to a strong pro-inflammatory phenotype. Once in the CNS, these peripheral cells drive further α-syn-dependent neuroinflammation and neurodegeneration.
Conclusions
Our data point to an essential role of peripheral monocyte infiltration early in the PD neuroinflammatory process. These cells appear to be essential to drive both local brain inflammatory responses as well as the engagement of peripheral immune mechanisms. These myeloid cells may be a key target for the development of potentially neuroprotective immunotherapies in PD.
Supplementary Material
(A) AAV2-SYN increases the number of CD45+, CD4+ T cells in the ventral midbrain of red/green mice. (B) Quantification of the absolute number of CD45+, CD4+ T cells in Naïve, AAV2-GFP and AAV2-SYN injected red/green mice. This experiment involves 28 mice. Individual samples were formed by pooling 2 ventral midbrains (bilaterally injected), each from different animals. In each experiment, treatment groups (Naïve, AAV2-GFP, AAV2-SYN) consisted of 3–4 of these independent samples. For flow cytometry analysis, the mean +/− SEM of the independent samples in each treatment group were plotted. One-way ANOVA with Tukey’s post hoc test. *p<0.05. (C) Quantification of the absolute number of CD4+ T cells in WT and CCR2−/− mice. This experiment involves 28 mice. Individual samples were formed by pooling 2–3 ventral midbrains (bilaterally injected), each from different animals. In each experiment, treatment group (AAV2-SYN) consisted of 4 of these independent samples. For flow cytometry analysis, the mean +/− SEM of the independent samples in each treatment group were plotted.
(A) human-specific α-syn immunostaining (LB509) in the ipsilateral SNpc of WT and CCR2−/− mice 6 months post-transduction (Ni-DAB, black). 10x brightfield images. (B) human-specific α-syn immunostaining (LB509) in the contralateral SNpc of WT and CCR2−/− mice 6 months post-transduction (Ni-DAB, black). 10x brightfield images. (C) Quantification of LB509 immunostaining in the SNpc of AAV2-SYN injected WT and CCR2 −/− mice at 6 months post-transduction. Data represents the median (n=6/treatment) Mann Whitney test. No significant difference was observed.
Highlights.
α-syn expression induces infiltration of pro-inflammatory CCR2+ peripheral monocytes into the substantia nigra
Genetic deletion of CCR2 prevents α-syn induced monocyte entry, attenuates MHCII expression and blocks the subsequent degeneration of dopaminergic neurons.
Acknowledgments
Funding
The work from these studies is generously supported by the RJG Foundation, NIH 2P30 NS047466, and NIH/NINDS P20 NS092530.
We would like to thank Aster Jurkuvenaite, Hao Yu, Sara Gibson, and the UAB Rheumatic Diseases Core Center Comprehensive Flow Cytometry Core (NIH P30 AR48311) for assistance with flow cytometry and the UAB Molecular Detection and Stereology Core.
Abbreviations
- α-syn
alpha-synuclein
- CNS
central nervous system
- PD
Parkinson disease
- CX3CR1
fractalkine receptor
- CCR2
C-C chemokine receptor type 2
- CCL2
C-C chemokine ligand 2
- SNpc
substantia nigra pars compacta
- GWAS
genome wide association studies
- MHCII
major histocompatibility complex II
- GFP
green fluorescent protein
- RFP
red fluorescent protein
- AAV2
adeno-associated virus serotype 2
- TH
tyrosine hydroxylase
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
Footnotes
Competing Interests
The authors in this manuscript declare no competing financial interests.
Author Contributions
ASH designed all studies, carried out, performed data analysis, and drafted the manuscript. ADT designed, carried out stereotaxic surgeries, performed data analysis for flow cytometry and stereology for dopamine neurons. ZY designed, carried out, performed data analysis for flow cytometry. AMS carried out and performed data analysis for flow cytometry experiments. GPW carried out and performed data analysis for flow cytometry experiments. XL performed the stereotaxic surgeries and tissue processing for immunohistochemistry studies. YL participated in the design and execution of flow cytometry experiments. HQ participated in the design of flow cytometry studies and edited the manuscript. ENB participated in the design of flow cytometry studies and edited the manuscript. DGS participated in the design of the study and edited the manuscript. All authors read and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ajami B, et al. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14(9):1142–9. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- Allen Reish HE, Standaert DG. Role of alpha-synuclein in inducing innate and adaptive immunity in Parkinson disease. J Parkinsons Dis. 2015;5(1):1–19. doi: 10.3233/JPD-140491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel SH. Inflammation in Parkinson’s disease: cause or consequence? Mov Disord. 2012;27(9):1075–7. doi: 10.1002/mds.25111. [DOI] [PubMed] [Google Scholar]
- Bennett ML, et al. New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci U S A. 2016;113(12):E1738–46. doi: 10.1073/pnas.1525528113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum-Degen D, et al. Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson’s disease patients. Neuroscience letters. 1995;202(1–2):17–20. doi: 10.1016/0304-3940(95)12192-7. [DOI] [PubMed] [Google Scholar]
- Brochard V, et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. The Journal of clinical investigation. 2009;119(1):182–92. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, et al. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat Neurosci. 2014;17(1):131–43. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, et al. Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. The Journal of clinical investigation. 2012;122(9):3063–87. doi: 10.1172/JCI62636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S, Standaert DG, Harms AS. The gamma chain subunit of Fc receptors is required for alpha-synuclein-induced pro-inflammatory signaling in microglia. J Neuroinflammation. 2012;9(1):259. doi: 10.1186/1742-2094-9-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S, Theodore S, Standaert DG. Fcgamma receptors are required for NF-kappaB signaling, microglial activation and dopaminergic neurodegeneration in an AAV-synuclein mouse model of Parkinson’s disease. Molecular neurodegeneration. 2010;5:42. doi: 10.1186/1750-1326-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Columba-Cabezas S, et al. Induction of macrophage-derived chemokine/CCL22 expression in experimental autoimmune encephalomyelitis and cultured microglia: implications for disease regulation. Journal of neuroimmunology. 2002;130(1–2):10–21. doi: 10.1016/s0165-5728(02)00170-4. [DOI] [PubMed] [Google Scholar]
- Daeron M. Fc receptor biology. Annual review of immunology. 1997;15:203–34. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- Fife BT, et al. CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192(6):899–905. doi: 10.1084/jem.192.6.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk N, et al. Characterization of peripheral hematopoietic stem cells and monocytes in Parkinson’s disease. Mov Disord. 2013;28(3):392–5. doi: 10.1002/mds.25300. [DOI] [PubMed] [Google Scholar]
- Gao HM, et al. Neuroinflammation and oxidation/nitration of alpha-synuclein linked to dopaminergic neurodegeneration. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28(30):7687–98. doi: 10.1523/JNEUROSCI.0143-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, et al. Use of ibuprofen and risk of Parkinson disease. Neurology. 2011;76(10):863–9. doi: 10.1212/WNL.0b013e31820f2d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaupp S, et al. Experimental autoimmune encephalomyelitis (EAE) in CCR2(−/−) mice: susceptibility in multiple strains. Am J Pathol. 2003;162(1):139–50. doi: 10.1016/S0002-9440(10)63805-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozdanov V, et al. Inflammatory dysregulation of blood monocytes in Parkinson’s disease patients. Acta Neuropathol. 2014;128(5):651–63. doi: 10.1007/s00401-014-1345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza TH, et al. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nature genetics. 2010;42(9):781–5. doi: 10.1038/ng.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms AS, et al. MHCII is required for alpha-synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33(23):9592–600. doi: 10.1523/JNEUROSCI.5610-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol. 2009;8(4):382–97. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Vyas S, Hunot S. Neuroinflammation in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18(Suppl 1):S210–2. doi: 10.1016/S1353-8020(11)70065-7. [DOI] [PubMed] [Google Scholar]
- Houlden H, Singleton AB. The genetics and neuropathology of Parkinson’s disease. Acta Neuropathol. 2012;124(3):325–38. doi: 10.1007/s00401-012-1013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izikson L, et al. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2. J Exp Med. 2000;192(7):1075–80. doi: 10.1084/jem.192.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20(11):4106–14. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston JW, et al. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Annals of neurology. 1999;46(4):598–605. doi: 10.1002/1531-8249(199910)46:4<598::aid-ana7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Lim S, et al. Neuroinflammation in Synucleinopathies. Brain Pathol. 2016;26(3):404–9. doi: 10.1111/bpa.12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, et al. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338(6109):949–53. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson MB, Lee SK, Richter F, Masliah E, Chesselet M-F. 2011 Regionally specific microglial activation precedes neuropathology and peripheral immune response in mice over-expressing wildtype alpha synuclein. Washington, D.C: Society for Neuroscience; 2011. [Google Scholar]
- Mahad D, et al. Modulating CCR2 and CCL2 at the blood-brain barrier: relevance for multiple sclerosis pathogenesis. Brain: a journal of neurology. 2006;129(Pt 1):212–23. doi: 10.1093/brain/awh655. [DOI] [PubMed] [Google Scholar]
- McGeer PL, et al. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38(8):1285–91. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- Mizutani M, et al. The fractalkine receptor but not CCR2 is present on microglia from embryonic development throughout adulthood. Journal of immunology. 2012;188(1):29–36. doi: 10.4049/jimmunol.1100421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehle MS, West AB. M1 and M2 immune activation in Parkinson’s Disease: Foe and ally? Neuroscience. 2015;302:59–73. doi: 10.1016/j.neuroscience.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi M, et al. Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neuroscience letters. 1994a;180(2):147–50. doi: 10.1016/0304-3940(94)90508-8. [DOI] [PubMed] [Google Scholar]
- Mogi M, et al. Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neuroscience letters. 1994b;165(1–2):208–10. doi: 10.1016/0304-3940(94)90746-3. [DOI] [PubMed] [Google Scholar]
- Mount MP, et al. Involvement of interferon-gamma in microglial-mediated loss of dopaminergic neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27(12):3328–37. doi: 10.1523/JNEUROSCI.5321-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr CF, et al. A possible role for humoral immunity in the pathogenesis of Parkinson’s disease. Brain: a journal of neurology. 2005;128(Pt 11):2665–74. doi: 10.1093/brain/awh625. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH. Autosomal dominant Parkinson’s disease and alpha-synuclein. Ann Neurol. 1998;44(3 Suppl 1):S63–4. doi: 10.1002/ana.410440710. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276(5321):2045–7. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Prinz M, et al. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat Neurosci. 2011;14(10):1227–35. doi: 10.1038/nn.2923. [DOI] [PubMed] [Google Scholar]
- Qin H, et al. Signal transducer and activator of transcription-3/suppressor of cytokine signaling-3 (STAT3/SOCS3) axis in myeloid cells regulates neuroinflammation. Proc Natl Acad Sci U S A. 2012;109(13):5004–9. doi: 10.1073/pnas.1117218109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj T, et al. Polarization of the effects of autoimmune and neurodegenerative risk alleles in leukocytes. Science. 2014;344(6183):519–23. doi: 10.1126/science.1249547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM. Microglia and monocytes: ‘tis plain the twain meet in the brain. Nat Neurosci. 2011;14(9):1098–100. doi: 10.1038/nn.2917. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature. 2010;468(7321):253–62. doi: 10.1038/nature09615. [DOI] [PubMed] [Google Scholar]
- Reale M, et al. Peripheral cytokines profile in Parkinson’s disease. Brain Behav Immun. 2009;23(1):55–63. doi: 10.1016/j.bbi.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Reynolds AD, et al. Nitrated alpha-synuclein-activated microglial profiling for Parkinson’s disease. Journal of neurochemistry. 2008;104(6):1504–25. doi: 10.1111/j.1471-4159.2007.05087.x. [DOI] [PubMed] [Google Scholar]
- Reynolds AD, et al. Nitrated {alpha}-synuclein-induced alterations in microglial immunity are regulated by CD4+ T cell subsets. Journal of immunology. 2009;182(7):4137–49. doi: 10.4049/jimmunol.0803982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross OA, et al. Genomic investigation of alpha-synuclein multiplication and parkinsonism. Annals of neurology. 2008;63(6):743–50. doi: 10.1002/ana.21380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saederup N, et al. Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. PLoS One. 2010;5(10):e13693. doi: 10.1371/journal.pone.0013693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol. 2011;11(11):775–87. doi: 10.1038/nri3086. [DOI] [PubMed] [Google Scholar]
- Sanchez-Guajardo V, et al. Microglia acquire distinct activation profiles depending on the degree of alpha-synuclein neuropathology in a rAAV based model of Parkinson’s disease. PLoS One. 2010;5(1):e8784. doi: 10.1371/journal.pone.0008784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JA, et al. CD4+ regulatory and effector/memory T cell subsets profile motor dysfunction in Parkinson’s disease. J Neuroimmune Pharmacol. 2012;7(4):927–38. doi: 10.1007/s11481-012-9402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CD, et al. Differential gene expression in LPS/IFNgamma activated microglia and macrophages: in vitro versus in vivo. Journal of neurochemistry. 2009;109(Suppl 1):117–25. doi: 10.1111/j.1471-4159.2009.05984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon-Sanchez J, et al. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet. 2009;41(12):1308–12. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldner F, et al. Parkinson-associated risk variant in distal enhancer of alpha-synuclein modulates target gene expression. Nature. 2016;533(7601):95–9. doi: 10.1038/nature17939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Martin JL, et al. Dopaminergic neuron loss and up-regulation of chaperone protein mRNA induced by targeted over-expression of alpha-synuclein in mouse substantia nigra. Journal of neurochemistry. 2007;100(6):1449–57. doi: 10.1111/j.1471-4159.2006.04310.x. [DOI] [PubMed] [Google Scholar]
- Tansey MG, Goldberg MS. Neuroinflammation in Parkinson’s disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis. 2010;37(3):510–8. doi: 10.1016/j.nbd.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodore S, et al. Targeted overexpression of human alpha-synuclein triggers microglial activation and an adaptive immune response in a mouse model of Parkinson disease. Journal of neuropathology and experimental neurology. 2008;67(12):1149–58. doi: 10.1097/NEN.0b013e31818e5e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome AD, et al. microRNA-155 Regulates Alpha-Synuclein-Induced Inflammatory Responses in Models of Parkinson Disease. J Neurosci. 2016;36(8):2383–90. doi: 10.1523/JNEUROSCI.3900-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome AD, Standaert DG, Harms AS. Fractalkine Signaling Regulates the Inflammatory Response in an alpha-Synuclein Model of Parkinson Disease. PLoS One. 2015;10(10):e0140566. doi: 10.1371/journal.pone.0140566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, et al. How can rAAV-alpha-synuclein and the fibril alpha-synuclein models advance our understanding of Parkinson disease? J Neurochem. 2016 doi: 10.1111/jnc.13627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, et al. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72(1):57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson MB, et al. Regionally-specific microglial activation in young mice over-expressing human wildtype alpha-synuclein. Exp Neurol. 2012;237(2):318–34. doi: 10.1016/j.expneurol.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton PS. Inflammation as a causative factor in the aetiology of Parkinson’s disease. Br J Pharmacol. 2007;150(8):963–76. doi: 10.1038/sj.bjp.0707167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki R, et al. Differential roles of microglia and monocytes in the inflamed central nervous system. J Exp Med. 2014;211(8):1533–49. doi: 10.1084/jem.20132477. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) AAV2-SYN increases the number of CD45+, CD4+ T cells in the ventral midbrain of red/green mice. (B) Quantification of the absolute number of CD45+, CD4+ T cells in Naïve, AAV2-GFP and AAV2-SYN injected red/green mice. This experiment involves 28 mice. Individual samples were formed by pooling 2 ventral midbrains (bilaterally injected), each from different animals. In each experiment, treatment groups (Naïve, AAV2-GFP, AAV2-SYN) consisted of 3–4 of these independent samples. For flow cytometry analysis, the mean +/− SEM of the independent samples in each treatment group were plotted. One-way ANOVA with Tukey’s post hoc test. *p<0.05. (C) Quantification of the absolute number of CD4+ T cells in WT and CCR2−/− mice. This experiment involves 28 mice. Individual samples were formed by pooling 2–3 ventral midbrains (bilaterally injected), each from different animals. In each experiment, treatment group (AAV2-SYN) consisted of 4 of these independent samples. For flow cytometry analysis, the mean +/− SEM of the independent samples in each treatment group were plotted.
(A) human-specific α-syn immunostaining (LB509) in the ipsilateral SNpc of WT and CCR2−/− mice 6 months post-transduction (Ni-DAB, black). 10x brightfield images. (B) human-specific α-syn immunostaining (LB509) in the contralateral SNpc of WT and CCR2−/− mice 6 months post-transduction (Ni-DAB, black). 10x brightfield images. (C) Quantification of LB509 immunostaining in the SNpc of AAV2-SYN injected WT and CCR2 −/− mice at 6 months post-transduction. Data represents the median (n=6/treatment) Mann Whitney test. No significant difference was observed.