Abstract
Without intervention after spinal cord injury (SCI), paralyzed skeletal muscles undergo myofiber atrophy and slow-to-fast myofiber type transformations. We hypothesized that chronic spasticity-associated neuromuscular activity after SCI would promote recovery from such deleterious changes. We examined segmental tail muscles of chronic spinal rats with longstanding tail spasticity (7 mo after sacral spinal cord transection; older chronic spinals), chronic spinal rats that experienced less spasticity early after injury (young chronic spinals), and rats without spasticity after transection and bilateral deafferentation (spinal isolated). These were compared with tail muscles of age-matched normal rats. Using immunohistochemistry, we observed myofiber distributions of 15.9 ± 3.5% type I, 18.7 ± 10.7% type IIA, 60.8 ± 12.6% type IID(X), and 2.3 ± 1.3% type IIB (means ± SD) in young normals, which were not different in older normals. Young chronic spinals demonstrated transformations toward faster myofiber types with decreased type I and increased type IID(X) paralleled by atrophy of all myofiber types compared with young normals. Spinal isolated rats also demonstrated decreased type I myofiber proportions and increased type II myofiber proportions, and severe myofiber atrophy. After 4 mo of complete spasticity (older chronic spinals), myofiber type transformations were reversed, with no significant differences in type I, IIA, IID(X), or IIB proportions compared with age-matched normals. Moreover, after this prolonged spasticity, type I, IIA, and IIB myofibers recovered from atrophy, and type IID(X) myofibers partially recovered. Our results indicate that early after transection or after long-term spinal isolation, relatively inactive tail myofibers atrophy and transform toward faster myofiber types. However, long-term spasticity apparently produces neuromuscular activity that promotes recovery of myofiber types and myofiber sizes.
INTRODUCTION
After spinal cord injury (SCI, transection or partial injury), reduced neuromuscular activity leads to myofiber atrophy in muscles innervated below the level of the lesion (Dupont-Versteegden et al. 1998; Lotta et al. 1991) especially when muscle activity is further reduced by spinal cord transections combined with bilateral deafferentation (i.e., spinal isolation) (Roy et al. 2000). These muscles typically also undergo transformations in myofiber types and isoforms of the associated myosin heavy chain (MyHC) proteins from slower, fatigue-resistant myofibers to faster, more fatigable myofibers (Du-pont-Versteegden et al. 1998; Hartkopp et al. 2003; Lieber et al. 1986a; Roy et al. 2000). Consequently, faster, weaker, and more fatigable muscle contractile properties often result (Cope et al. 1986; Hartkopp et al. 2003; Lieber et al. 1986b; Roy et al. 1999, 2002b).
Interestingly, exercise or muscle activity induced by electrical stimulation attenuates or reverses such detrimental changes in muscle properties after SCI (Dupont-Versteegden et al. 1998; Hartkopp et al. 2003; Kern et al. 2004; Murphy et al. 1999; Roy et al. 1992, 1999, 2002a; Shields and Dudley-Javoroski 2006). That is, generating muscle activity after SCI interrupts the slow-to-fast myofiber transformations and atrophy, and ultimately assists in preserving the normal muscle contractile properties.
Importantly, the classical atrophy and slow-to-fast myofiber transformations associated with SCI (described in the preceding text) are usually seen in muscles that are rendered relatively inactive (i.e., flaccid paralysis) by the injury (Cope et al. 1986; Dupont-Versteegden et al. 1998; Hartkopp et al. 2003; Lieber et al. 1986a). However, in humans and in some animal models, considerable neuromuscular activity sometimes develops after SCI in the form of spasticity, a syndrome that includes hyper-reflexia, hypertonus, and long-lasting spasms (Bennett et al. 2004; Fujimori et al. 1968; Heckman 1994; Kuhn and Macht 1948; Lance and Burke 1974; Ritz et al. 1992; Taylor et al. 1997). Furthermore, preservation of slow (Hidler et al. 2002; Thomas and Ross 1997; Zijdewind and Thomas 2003) and fatigue-resistant (Hartkopp et al. 2003) muscle contractile properties has been observed in conjunction with spasticity after SCI in humans. Thus in principle, this spastic muscle activity that develops after SCI may, like exercise or electrical stimulation (discussed in the preceding text), act to preserve the normal muscle properties by interrupting the slow-to-fast myofiber transformation and atrophy. The purpose of the current study was to test this idea in a spinal cord transected rat with spasticity, by examining whether the classical slow-to-fast myofiber transformation and atrophy occur when spasticity is not present (early after transection), whether this myofiber transformation and atrophy are reversed to normal as spasticity subsequently develops (long-term transection), and whether the classical slow-to-fast myofiber transformation and atrophy persist and become worse when spasticity is eliminated by a combination of spinal cord transection and bilateral deafferentation below the transection (spinal isolation).
Recently, it has been shown that after sacral spinal cord transection, rats develop a pronounced spastic syndrome in the segmental muscles of the tail over a period of months (chronic spinal rats) (Bennett et al. 1999, 2001, 2004). This spasticity is associated with large muscle spasms lasting many seconds that are evoked by brief, normally innocuous sensory inputs to the tail, such as brushing of the tail on the cage bedding during walking; further, there is ongoing spontaneous EMG activity in the muscles lasting for hours at a time (Bennett et al. 2001). In contrast, after spinal cord transections combined with bilateral deafferentation (spinal isolation), the affected muscles exhibit almost no activity (Pierotti et al. 1991). Thus in the current study we used rat models of spinal cord transection and spinal isolation to evaluate changes in muscle properties with and without spasticity after injury.
In sacral spinal rats, the tail muscles are flaccid for the first 2 wk; then spasticity slowly develops, and the muscles become completely spastic 2–3 mo postinjury (Bennett et al. 2004). In response to reduced muscle activity after spinal cord transection in other preparations without spasticity, transformations in rat myofiber types generally occur as early as 1 wk after SCI (Dupont-Versteegden et al. 1998) but require months to reach a new steady state (Talmadge et al. 1999). Thus if spasticity has the effects hypothesized in the preceding text, after sacral spinal cord transection in the rat, the largest effects of the initial period of reduced activity (i.e., paralysis) should be seen a few months after injury but not too long after activity (i.e., spasticity) begins to develop. Therefore as a compromise, we selected a time point 3 mo postinjury to study the effects of relative muscle inactivity on muscle properties (young chronic spinal rats). To study the full effects of spasticity in sacral spinal rats, we evaluated the properties of muscles that had been spastic for many months (completely spastic for 4 mo at 7 mo postinjury; older chronic spinal rats). Finally, to compare the effects of spasticity with the effects of long-term elimination of muscle activity, we assessed tail muscle properties in rats that had experienced many months of almost complete neuromuscular silence (7 mo of spinal isolation; spinal isolated rats).
We specifically chose to evaluate the SCI-induced changes in the ventrolateral segmental muscles of the tail because motor-unit recordings in these muscles have shown that they are very active in the spastic rat with spontaneous motor unit firing lasting hours at a time, in association with hypertonus, and bursts of even more intense firing associated with spasms (Bennett et al. 2001). In these segmental tail muscles, we evaluated changes in the distributions of myofiber types and sizes and the associated MyHC protein isoforms, using immunohistochemistry and gel electrophoresis. Importantly, rat segmental tail muscles have both slow and fast motor units (Harris et al. 2006; Steg 1964), which allowed us to study the effects of SCI and spasticity on slow and fast myofiber types and ultimately to evaluate transformations between these types. Parts of this work have previously been reported in abstract form (Harris et al. 2005).
METHODS
Animal procedures
Adult, female, Sprague-Dawley rats were used in this study with the approval of the University of Alberta Animal Welfare and Policy Committee and in accordance with the guidelines of the Canadian Council for Animal Care. Respectively, two experimental groups of young (n = 5) and older (n = 9) chronic sacral spinal adult rats were killed at 3 and 7 mo after spinal cord transection (spinal cord transection at 2 mo of age; see introduction for rationale). One experimental group of spinal isolated adult rats (n = 7) was killed at 7 mo after spinal isolation (isolation at 2 mo of age; age-matched to older chronic spinal rats). Two control groups of young (n = 5, killed at 5 mo of age) and older (n = 9, killed at 9 mo of age) normal adult rats were age-matched to the injured rats. Some of the chronic sacral spinal rats were the same as those used in earlier studies (Bennett et al. 2004) and within 2–3 mo, as previously documented (Bennett et al. 2001, 2004) according to muscle tone, reflexes, and clonus, spasticity developed in the tail muscles and continued indefinitely. In long-term sacral spinal animals the spasticity rating was always 4/5 or 5/5 (see Bennett et al. 1999, 2004 for further details of the spasticity assessment). In contrast, the tail muscles of the spinal isolated rats were completely areflexive, and unresponsive to any external stimuli (spasticity rating = 0/5).
Spinal cord injury
In the chronic spinal rats, spinal cord transection was performed at the S2 spinal level under sodium pentobarbital anesthesia (58 mg/kg body mass) as previously described (Bennett et al. 1999) in adult rats (at 2 mo of age). Transection at this S2 level does not impair hindlimb, bladder, or bowel functions; it only affects tail function.
The spinal isolation procedure was adapted from the previously described sacral spinal cord transection method (Bennett et al. 1999) and modified from models of lumbar spinal isolation in rats and cats (Eldridge 1984; Pierotti et al. 1991; Tower 1937). Specifically, at 2 mo of age, in addition to the S2 spinal cord transection, all the dorsal roots caudal to the transection were cut bilaterally. To this end, a more caudal laminectomy was performed to expose the sacral (S) and caudal (Ca) dorsal roots at their entry points to the spinal cord, and the dura mater was incised above these roots. The sacral S2–S4 and all caudal dorsal roots were cut bilaterally and intradurally, taking great care not to damage the sacral spinal cord, 1–2 mm from their points of entry to the cord. Unlike with lumbar spinal isolation a second, more caudal transection is not required with sacrocaudal spinal isolation because the caudal cord is the end of the spinal cord.
EMG recordings
Recordings were performed in acutely spinalized animals (2 days postinjury at 2 mo of age, n = 5), in older chronic spinal (n = 5) and spinal isolated (n = 7) rats, as well as in older normal animals (age-matched to chronic spinal and spinal isolated rats; n = 5). Subcutaneous 24-h EMG electrodes were made using ultra miniature stainless steel medical wire (fluorocarbon-insulated, total diameter: 78 µm; Cooner Wire, Chatsworth CA, No. AS-632), which was threaded through 16-cm-long, 22-gauge needles with blunted and smoothed tips. Each 1-mm end of the wire was deinsulated, and the wires and needles were then sterilized.
Electrodes were inserted while animals were under continuous inhalation anesthesia with halothane (2% in oxygen). The left lateral surface of the tail was marked at the rostral and caudal borders of the 14th segment of the tail, at which segment the 14th ventrolateral intersegmental tail muscle is located. The skin over the lumbar vertebrae was shaved and disinfected, and a 1.5-cm skin incision was made perpendicular to the vertebral column. A blunt sterile needle was inserted at the incision and threaded under the skin until the tip of the needle could be seen subcutaneously 0.5 mm caudal to the 14th tail segment, on the left side. Then the tail was clamped firmly with thumb and forefinger at this level to maintain the electrode wire tip at this position while the needle was carefully removed. Using this method a second subcutaneous electrode was inserted 0.5 mm rostral to the 14th tail segment on the left side to permit bipolar recording. A third subcutaneous electrode was inserted above the third tail segment on the right (opposite) side, and this electrode served as the ground. Each wire (electrode) was labeled immediately after insertion.
The three electrodes were wound once in a 1-cm-diam loop, which was sutured subcutaneously to the fascia at the level of the incision. This loop served as slack, to ensure that the electrodes would not move in the event that tension was accidentally exerted on the external portion of the wire. The incision was sutured, with the free end of the electrodes exiting the skin at one end of the skin closure. The external ends of the electrodes were soldered into machine pins connected to 1-m-long insulated and electrically shielded multiconductor wires (Cooner Wire, No. NMUF 1/30-4046 SJ) that led to a digital data-acquisition system. These 1-m-long leads were hung from a spring-loaded pulley system, which ensured that no tension was exerted on the wires, and thus the animal experienced no discomfort.
On cessation of anesthesia, the animal was placed in an open-topped cage with standard base dimensions and 75-cm-high walls. Standard bedding was provided, and food and water were provided ad libitum. The animal was monitored continuously by the experimenters throughout the 24-h recording session. The EMG signal was high-pass filtered at 100 Hz with a first-order filter to remove movement artifact and 60-Hz noise. Then it was rectified and low-pass filtered at 10 Hz to determine the envelope of the EMG activity. Finally, it was sampled at 20 Hz by a data-acquisition system (Axoscope, Axon Instruments, Union City, CA). Lighting was adjusted according to the animal’s regular 12-h light/dark cycle. When the recording session was complete, the animal was either anesthetized for tail muscle extraction (see following text) or killed immediately.
Data were analyzed in consecutive 0.5-h-long bins. For each animal, Clampfit (Axon Instruments) was used to measure the baseline-adjusted absolute mean rectified EMG amplitude in each 0.5-h-long bin. For each animal these 42 measured means were gain-corrected and averaged to calculate the mean 24-h EMG amplitude.
Muscle preparation and sectioning
At 3 mo after injury (young chronic spinal rats) and at 7 mo after injury (older chronic spinal rats and spinal isolated rats) in experimental animals or at corresponding ages in age-matched normals (5 mo of age and 9 mo of age), animals were anesthetized with sodium pentobarbital (58 mg/kg body mass) and tail muscles were removed. Specifically, a 2.5-cm-long incision on the left lateral side of the tail exposed the 14th ventrolateral segmental tail muscle. This muscle was completely freed from the underlying bone and from surrounding connective tissue along its entire length and removed. The muscle was coated in Tissue Tek Embedding Medium (Sakura Finetek, Torrance CA) in a cryomold, frozen in liquid nitrogen-cooled, melting isopentane, and immediately stored at −80°C until sectioning. Finally, animals were killed with an overdose of sodium pentobarbital. Transverse sections (10 µm thick) were cut from the belly of frozen muscles in a −24°C cryostat after 30 min of equilibration at this temperature. Sections were mounted on precleaned Colorfrost Plus slides (Fisher Scientific, Pittsburgh, PA) and stored at −80°C until used for immunohistochemical staining.
Immunohistochemical staining of myofibers with antibodies against myosin heavy chain isoforms
Myofibers were assessed by immunohistochemical staining (Putman et al. 2000, 2003). Primary monoclonal antibodies directed against adult rodent myosin heavy chain (MyHC) isoforms were used to identify individual myofiber types in serial sections (Fig. 1,A–Q): slow type I (clone BA-D5, IgG), fast type IIA (clone SC-71, IgG), and fast type IIB (clone BF-F3, IgM). Primary antibodies against cardiac type Iα myofibers (clone F88-12F8.1, IgG) and embryonic myofibers (clone BF-45, IgG) were used for preliminary staining that revealed no positive reactions in tail muscles from control or experimental animals and thus were not used further. Immunoreactivity was subsequently localized with biotinylated secondary antibodies (Vector Laboratories, Burlingame, CA): goat anti-mouse IgM directed against clone BF-F3, or horse anti-mouse IgG (rat absorbed, affinity purified) directed against all other primary antibodies. Immunohistochemical staining always included negative control tissue sections to which these biotinylated secondary antibodies were applied without prior application of primary antibodies directed against MyHC isoforms. Color development of sections (Putman et al. 1999) was achieved by incubation in a biotin-avidin horseradish peroxidase complex followed by a buffered mixture of diaminobenzidine (DAB) and hydrogen peroxide (Vector Laboratories, Burlingame, CA). Segmental tail muscle sections were accompanied on every slide by a positive control section from mixed extensor digitorum longus (EDL) hind-limb muscles from normal rats in this study (Fig. 1,R–T).
FIG. 1.
Representative photomicrographs show immunohistochemical staining of rat segmental tail muscles in serial cross sections. A–C: young normal rat. D–F: young chronic spinal rat. G, H, and J: older normal rat. K–M: older chronic spinal rat. N, P, and Q: older spinal isolated rat. R–T: for reference, sections are shown from an un-paralyzed control hindlimb extensor digitorum longus (EDL) muscle from a young normal rat. Sample myofibers marked “I” (type I), “IIA” (type IIA), and “IIB” (type IIB) were identified according to their positive staining reaction with the corresponding myosin heavy chain (MyHC) antibody, as indicated (left: type I, clone BA-D5; middle: type IIA, clone SC-71; right: type IIB, clone BF-F3). Sample myofibers marked “IID(X)” (type IID(X)) were identified by the absence of staining reactions with these antibodies (see methods); this “subtraction” method of staining consistently identified type IID(X) myofibers, as determined by direct comparison to staining patterns observed with clone BF-35 against all MyHCs except MyHC IId(x). Bar is 50 µm.
Stained sections were photomicrographed at 160 times in grayscale. Myofibers were assessed in photomicrographs for reactions with antibodies and for cross-sectional area using custom designed image analysis software (Putman et al. 2000). An average of 284 ± 147 myofibers per section was analyzed, ensuring adequate myofiber numbers for statistical analyses of myofiber proportions and sizes (McGuigan et al. 2002). Type I, type IIA, and type IIB myofibers were identified by positive staining reactions with the corresponding primary monoclonal antibodies (see preceding text). The absence of staining reactions with this series of antibodies was used to identify type IID(X) (also called type IID, type IID/X, or type IIX) myofibers (Putman et al. 2003). Although this “subtraction” method for the identification of type IID(X) myofibers previously has been reliably applied in rodent hindlimb skeletal muscle using the same antibodies (Rosenblatt and Parry 1992), we wanted to validate the reliability of this approach for the characterization of rat tail muscles. Thus in 10 animals we also used limited quantities of a primary antibody that stains all myofibers except type IID(X) myofibers (clone BF-35, IgG). Then, in each animal, we compared the total number of myofibers that could be identified as type IID(X) according to both classifications. When the values obtained from each method were assessed with a paired Student’s t-test across all 10 animals, they were not significantly different (P > 0.05); thus we employed the subtraction method for all subsequent analyses.
For each muscle, the number of myofibers expressing a given MyHC isoform was reported as a proportion of the total number of myofibers in that muscle. The cross-sectional area of individual myofibers was calculated by custom designed image analysis software (Putman et al. 2000) using a conversion factor determined from a 1-µm graticule photomicrographed at 160 times. The area density of each muscle was also determined; that is, the total myofibrillar cross-sectional area that expressed each isoform (i.e., the sum of the cross-sectional areas of all myofibers of that type) was reported as a proportion of the total cross-sectional area of all the myofibers in that muscle (i.e., the sum of the cross-sectional areas of all the myofibers regardless of type).
Electrophoresis of myosin heavy chain protein isoforms
The relative contents of MyHC protein isoforms MyHC I, MyHC IIa, MyHC IId(x), and MyHC IIb were determined electrophoretically for each tail muscle (Fig. 2) as previously described (Putman et al. 2003). Briefly, MyHC protein was extracted from frozen muscle tissue homogenates and separated by SDS-PAGE for 24 h at 275 V. Gels were silver stained (Oakley et al. 1980). Integrated densitometry (ChemiGenius, GeneSnap, and GeneTools, Syngene, Frederick MD) was used to determine individual isoform amounts expressed as relative proportions of the total MyHC protein present in each muscle. A hindlimb medial gastrocnemius muscle sampled from an age-matched control rat in this study was included on each gel as a control for the position of each MyHC protein band (Fig. 2).
FIG. 2.
The relative muscle contents of MyHC protein isoforms MyHC I, MyHC IIa, MyHC IId(x), and MyHC IIb in protein extracts from whole muscle homogenates were assessed by integrated densitometry after electrophoretic separation of these isoforms on a polyacrylamide gel. Elec-trophoresis was used to separate MyHC isoforms in seg-mental tail muscle homogenates, as labeled for representative samples from young normal, young chronic spinal, older normal, older chronic spinal, and older spinal isolated rats. Also, on each gel, a sample extract from a normal rat hindlimb medial gastrocnemius (MG) was used as a positive control to confirm the position of each MyHC band.
Statistical analyses
All data are reported as means ± SD. Unpaired Student’s t-tests were used to determine statistical significance between means (SigmaPlot, SPSS, Chicago IL). Significance was accepted at P < 0.05. As required for the t-test, normality of the data were verified using a Kolmogorov-Smirnov test (P < 0.05).
RESULTS
Spinal isolation eliminates spasticity
To compare muscle activity in chronic spinal, spinal isolated, and age-matched normal animals, we recorded segmental tail muscle EMG over a 24-h period in each of these groups of rats (Fig. 3). In normal rats, daily tail muscle EMG activity was characterized by bursts in association with voluntary movements such as during locomotion (older normal rats, n = 5). These bursts were usually followed by long periods of sustained low-amplitude firing that lasted a few to several minutes in association with maintained postural control of the tail during tasks such as feeding and grooming (Fig. 3A). In acutely spinalized animals, the tail muscles were virtually silent, exhibiting only rare, low-amplitude spontaneous firing that was never sustained (2 days after transection, n = 5; Fig. 3B), consistent with the flaccid paralysis exhibited by these muscles during the first 2 wk (Bennett et al. 2004). Even skin stimulation, such as pinch (not shown), could not evoke prolonged spasms. Previously, we have quantified the gradual increase in EMG and reflex evoked muscles spasms that emerge over the first few months after injury (Bennett et al. 2004), and thus this was not repeated here. Relevant to the current study is that muscle properties depend on the activity over the past few months (see introduction), and thus muscle properties of young chronic spinal rats suffered the deleterious effects of inactivity early after injury.
FIG. 3.
A–D: representative tail muscle EMG records taken from 24-h electromyographic (EMG) recording sessions. Left: 10-min-long records of rectified tail muscle EMG from old normal (n = 5; A), acute spinal (n = 5; B), old chronic spinal (n = 5; C), and old spinal isolated (n = 7; D) animals. Note that there is ongoing large-amplitude activity in the normal and chronic spinal animals, whereas no large-amplitude or continuous activity is observed in acute spinal or spinal isolated animals. Right: records on an expanded scale show 30-s-long detail of the activity associated with normal behavior in normal animals (A), associated with spasticity in chronic spinal animals (C), and associated with the nominal bursts occasionally observed in acutely spinalized (B) and spinal isolated (D) animals. ↑ , t = 0 in the right-hand column. E: mean 24-h EMG amplitude is shown for each group (see methods for calculation). *, significant difference compared with normal. Significant differences were accepted at P < 0.05.
Compared with in acute spinal rats, the tail muscles in older chronic spinal animals exhibited a large and significant increase in 24-h EMG activity (n = 5). There was continuous low-level EMG activity (lasting hours at a time), interspersed with intense EMG activity lasting for several seconds (e.g., at arrow in Fig. 3C) and associated with tail spasms (i.e., coiling) (also see Bennett et al. 1999). This EMG activity and these spasms were evoked by sensory inputs, such as dragging of the tail on the ground during walking and bending/pressing on the tail when the animal sat or slept on it. The EMG activity observed in chronic spinal animals (n = 5) tended to be larger in magnitude than that observed in age-matched normal animals (n = 5), but there was no significant difference between these groups in the tail EMG amplitude averaged over 24-hours (Fig. 3E). This indicated that muscle activity in chronic spinals was close to that in normals, though more spasm-like in nature. In the spinal isolated animals, daily EMG activity was similar to that in acutely transected animals with little or no activity, except for the occasional, brief low-amplitude spontaneous bursts (n = 7; Fig. 3D). These brief EMG bursts were completely centrally generated, because they could not be evoked by any kind of direct tail stimulation (e.g., pinching, not shown). In summary, acute spinal rats (n = 5) and older spinal isolated rats exhibited no spasticity, and their mean 24-h EMG amplitudes were significantly lower than both normal and chronic spinal rats (Fig. 3E).
Myofiber composition is not affected by differences in the ages of the rats studied
The segmental tail muscles of normal rats were of mixed myofiber distribution (Fig. 1,A–C,G–J). In young normal rats (5 mo of age, n = 5), we observed myofiber proportions of 15.9 ± 3.5% slow type I, 18.7 ± 10.7% fast type IIA, 60.8 ± 12.6% fast type IID(X), and very little fast type IIB (2.3 ± 1.3%; Fig. 4,A–D). Hybrid type I/IIA myofibers were negligible (1.5 ± 1.7%, not significantly different from 0). In older normals (9 mo of age, n = 9), the distribution of myofibers was not significantly different from that in young normals (Fig. 4,A–D), and thus there was no age effect observed between our two normal groups, which were age-matched to our young and older chronic spinal rats. As a positive control, we assessed the mixed fast twitch hindlimb extensor digitorum longus muscle (Fig. 1,R–T), which displayed a myofiber distribution (not shown, n = 5) of 10.1 ± 3.8% type I, 25.1 ± 1.9% type IIA, 28.5 ± 4.1% type IID(X), 28.3 ± 2.6% type IIB, and 8.0 ± 2.0% hybrid type I/IIA. These values were similar to previous descriptions of extensor digitorum longus myofiber proportions (Bamford et al. 2003; Dupont-Versteegden et al. 1998; Putman et al. 2000).
FIG. 4.
A–D: mean proportion of each myofiber type is shown for young normals (n = 5) and young chronic spinal rats (n = 5; □), older normals (n= 9) and older chronic spinal rats (n = 9; ■), and older spinal isolated rats (n= 7;
 ), as determined from immunohistochemical staining of frozen muscle sections (cf. Fig. 1; values of n supplied here apply in all subsequent figures). E–H: also shown are percentage changes (see results for calculation) in myofiber type proportions in young chronic spinal rats relative to age-matched young normals (YC, □), in older chronic spinal rats relative to age-matched older normals (OC, ■), and in older spinal isolated rats (SI,
), as determined from immunohistochemical staining of frozen muscle sections (cf. Fig. 1; values of n supplied here apply in all subsequent figures). E–H: also shown are percentage changes (see results for calculation) in myofiber type proportions in young chronic spinal rats relative to age-matched young normals (YC, □), in older chronic spinal rats relative to age-matched older normals (OC, ■), and in older spinal isolated rats (SI,
 ) relative to age-matched normals. A and E: slow type I. B and F: fast type IIA. C and G: fast type IID(X). D and H: fast type IIB. Please note that in A, B, and D, the y axis extends to 60%, whereas in C, the y axis extends to 100%. Note: the following statisical symbols apply here and in the subsequent Figs. 5 and 7. *, significant difference between each chronic experimental group and its age-matched normal group (A–D) or a significant difference from 0 in the percentage change of an injured experimental group relative to its age-matched normal group (E–H). ‡, significant difference between the old spinal isolated group and the old chronic spinal group (A–D) and between the percent changes in these groups relative to their age-matched old normal group (SI vs. OC in E–H). Significant differences were accepted at P < 0.05.
) relative to age-matched normals. A and E: slow type I. B and F: fast type IIA. C and G: fast type IID(X). D and H: fast type IIB. Please note that in A, B, and D, the y axis extends to 60%, whereas in C, the y axis extends to 100%. Note: the following statisical symbols apply here and in the subsequent Figs. 5 and 7. *, significant difference between each chronic experimental group and its age-matched normal group (A–D) or a significant difference from 0 in the percentage change of an injured experimental group relative to its age-matched normal group (E–H). ‡, significant difference between the old spinal isolated group and the old chronic spinal group (A–D) and between the percent changes in these groups relative to their age-matched old normal group (SI vs. OC in E–H). Significant differences were accepted at P < 0.05.
Transformation toward a faster myofiber composition early after spinal cord transection is reversed by prolonged spasticity
After sacral spinal cord transection in adult rats, segmental tail muscles demonstrated flaccid paralysis for 2 wk, and then over the subsequent 2–3 mo these muscles only gradually developed complete spasticity. Thus young chronic spinal rats (3 mo postinjury) had experienced a 3-mo period of relatively reduced tail muscle activity, whereas older chronic spinal rats (7 mo postinjury) had experienced a long-term augmentation of tail muscle activity due to 4 mo of complete spasticity. A transformation toward a faster myofiber type distribution was associated with this period of relative inactivity in young chronic spinal rats. Specifically, in young chronic spinal rats (n = 5), the proportion of slow type I tail myofibers was significantly lower than in age-matched young normals (n = 5; Fig. 4A), whereas the proportion of fast type IID(X) myofibers (the dominant myofiber type) was significantly higher than in young normals (Fig. 4C). The mean proportions of type IIA and type IIB myofibers did not change significantly. A recovery from this type I to type IID(X) myofiber transformation was associated with the 4 mo of complete spasticity experienced by the older chronic spinal rats. Specifically, in older chronic spinal rats (n = 9), proportions of both slow type I myofibers (Fig. 4A) and fast type IID(X) myofibers (Fig. 4C) were not significantly different from those in age-matched older normals (n = 9). In summary, in young chronic spinal rats, a transformation toward faster myofiber types was associated with reduced activity and was apparent in tail muscles after 3 mo of injury, whereas older chronic spinal rats that were injured for 7 mo and completely spastic for 4 mo exhibited a recovery from reduced activity-associated alterations in myofiber types. Hybrid myofibers did not emerge after 3 or 7 mo of spinal cord transection.
A slow-to-fast myofiber type transformation was associated with the 7 mo of near-elimination of muscle activity after spinal isolation. Specifically, in spinal isolated rats (n = 7), there were a significant decrease in the proportion of type I myofibers (Fig. 4A) and significant increases in the proportions of both type IIA and type IIB myofibers (Fig. 4,B,and D). Interestingly, there was also a significant reduction in the proportion of the normally dominant type IID(X) myofibers (Fig. 4C), which apparently transformed to type IIA myofibers (Fig. 4B) for unknown reasons. Also in spinal isolated rats, a small proportion of hybrid type I/IIA myofibers emerged (co-expressing both MyHC I and MyHC IIa isoforms, not shown; 4.5 ± 3.4%, significantly different from 0).
Mean myofiber proportions were not significantly different in young normals compared with in older normals, consistent with previous evidence that there should be no differences in myofiber proportions among adult animals of these ages (Ansved and Larsson 1989). However, there tended to be a discrepancy between these two groups for type IIA and type IID(X) myofibers (Fig. 4,B,and C). Thus despite the absence of a significant age effect between these normal groups, we wanted to account for the potentially confounding effects of age on altered myofiber proportions. To this end, the percent change in myofiber proportion (Y) for each young chronic spinal rat was computed relative to the mean myofiber proportion (Z) in age-matched young normal rats (see Fig. 4E–H, □); that is, the percent change = (Y – Z)/Z × 100%. Likewise, myofiber proportions from older chronic spinal rats (Fig. 4E–H, ■) and from spinal isolated rats (Fig. 4E–H
 ) were expressed as percent changes with respect to age-matched old normals. In young chronic spinal rats, the mean percent changes in myofiber proportions relative to in young normal rats were large and significantly different from zero, with a −60.0 ± 19.1% decrease in type I, a −49.7 ± 22.3% decrease in type IIA, and a +30.5 ± 10.3% increase in type IID(X) myofiber proportions with injury (Fig. 4E, F,and G). In older chronic spinal rats with spasticity, these percent changes in myofiber proportions tended to be much smaller in magnitude compared with in young chronic spinal rats; specifically, the percent change in type IIA myofibers was reduced so much that it was no longer significantly different from zero (Fig. 4F), indicating a substantial recovery of type IIA proportions in spastic tail muscles. Furthermore, in older chronic spinal rats with spasticity the percent change in type IID(X) was only +9.7 ± 8.6%, which was significantly smaller than the percent change in type IID(X) for young chronic spinal rats (+30.5 ± 10.3%, Fig. 4G). In contrast, in spinal isolated rats there was a +376.3 ± 124.9% increase in the type IIA myofiber proportion relative to normal, and there was a −47.5 ± 2.3% decrease in the type IID(X) myofiber proportion, and these values were both significantly different from zero and significantly different from the changes observed for old chronic spinal rats. This demonstrates a much different effect associated the with long-term increased muscle activity due to spasticity after chronic spinal cord transection, compared with the long-term near-elimination of activity after chronic spinal isolation. In all injured groups, the large variability in the percent changes in type IIB myofiber proportions (Fig. 4H) occurred because these myofibers were so infrequently observed (Fig. 4D).
) were expressed as percent changes with respect to age-matched old normals. In young chronic spinal rats, the mean percent changes in myofiber proportions relative to in young normal rats were large and significantly different from zero, with a −60.0 ± 19.1% decrease in type I, a −49.7 ± 22.3% decrease in type IIA, and a +30.5 ± 10.3% increase in type IID(X) myofiber proportions with injury (Fig. 4E, F,and G). In older chronic spinal rats with spasticity, these percent changes in myofiber proportions tended to be much smaller in magnitude compared with in young chronic spinal rats; specifically, the percent change in type IIA myofibers was reduced so much that it was no longer significantly different from zero (Fig. 4F), indicating a substantial recovery of type IIA proportions in spastic tail muscles. Furthermore, in older chronic spinal rats with spasticity the percent change in type IID(X) was only +9.7 ± 8.6%, which was significantly smaller than the percent change in type IID(X) for young chronic spinal rats (+30.5 ± 10.3%, Fig. 4G). In contrast, in spinal isolated rats there was a +376.3 ± 124.9% increase in the type IIA myofiber proportion relative to normal, and there was a −47.5 ± 2.3% decrease in the type IID(X) myofiber proportion, and these values were both significantly different from zero and significantly different from the changes observed for old chronic spinal rats. This demonstrates a much different effect associated the with long-term increased muscle activity due to spasticity after chronic spinal cord transection, compared with the long-term near-elimination of activity after chronic spinal isolation. In all injured groups, the large variability in the percent changes in type IIB myofiber proportions (Fig. 4H) occurred because these myofibers were so infrequently observed (Fig. 4D).
Overall, the observed myofiber distributions and changes due to SCI suggest that muscles initially exhibiting flaccid paralysis (young chronic spinal rats) demonstrated a modest transformation toward faster myofibers, and muscles experiencing a long-term near-elimination of activity (older spinal isolated rats) demonstrated a persistence of this slow-to-fast myofiber type transformation. However, 4 mo of complete spasticity (older chronic spinal rats) resulted in a partial re-establishment of the normal distribution of myofiber types.
Tail myofiber size is not affected by differences in the ages of the rats studied
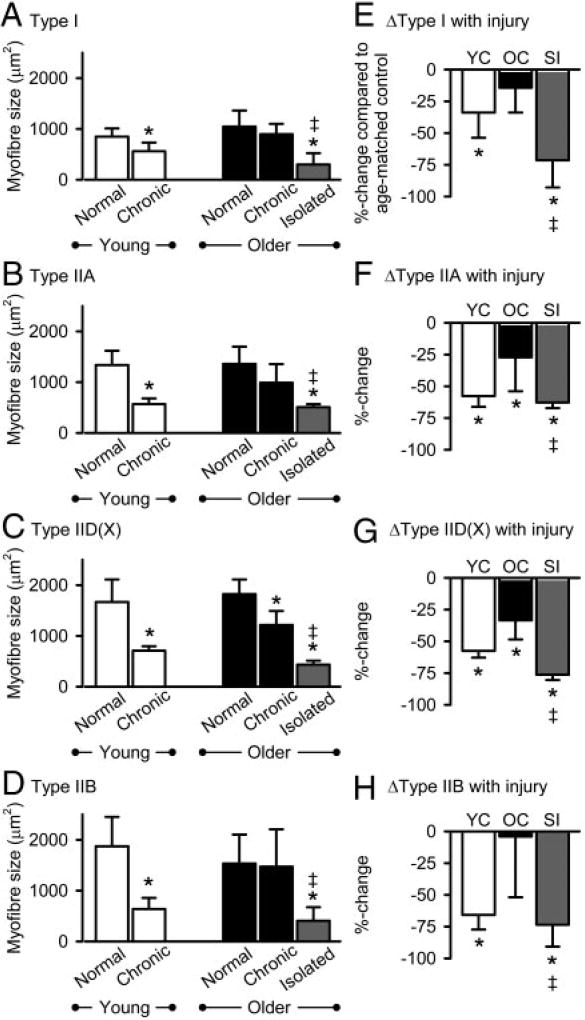
The mean cross-sectional area of all the myofibers that were assessed in segmental tail muscles from young normals (n = 5) was 1,474 ± 714 µm2 (minimum = 270 µm2, maximum = 5,604 µm2). Myofibers from older normals (n = 9) were of similar size (mean = 1,615 ± 797 pum2, minimum = 135 µm2, maximum = 5,049 µm2), and the mean sizes of individual myofiber types were not significantly different from young normals (Fig. 5A–D); thus there was no effect of age on tail myofiber size in normal adult rats. In normal hindlimb extensor digitorum longus muscles from these same animals, the mean cross-sectional area of myofibers was 2,096 ± 972 µm2 (minimum = 463 µm2, maximum = 7,407 µm2), similar to that reported previously (Dupont-Versteegden et al. 1998; Lieber et al. 1986a). Consistent with previous assessments of myofibers from a wide selection of rat skeletal muscles of various locations and serving a variety of functions (Bamford et al. 2003; Delp and Duan 1996), both tail muscles and hindlimb extensor digitorum longus muscles demonstrated mean myofiber cross-sectional areas ranked type I < type IIA < type IID(X) < type IIB.
FIG. 5.
A–D: mean cross-sectional area of each myofiber type for young normals and young chronic spinal rats (□), older normals and older chronic spinal rats (■), and older spinal isolated rats (
 ) is shown in units of cross-sectional area (µm2), as determined from immunohistochemical staining of frozen muscle sections (cf. Fig. 1). E–H: also shown are percentage changes (see results for calculation) in myofiber cross-sectional area in young chronic spinal rats relative to in age-matched young normal rats (YC, □), in older chronic spinal rats relative to in age-matched older normals (OC, ■), and in older spinal isolated rats relative to in age-matched normals (SI,
) is shown in units of cross-sectional area (µm2), as determined from immunohistochemical staining of frozen muscle sections (cf. Fig. 1). E–H: also shown are percentage changes (see results for calculation) in myofiber cross-sectional area in young chronic spinal rats relative to in age-matched young normal rats (YC, □), in older chronic spinal rats relative to in age-matched older normals (OC, ■), and in older spinal isolated rats relative to in age-matched normals (SI,
 ). Format, statistical symols, and values of n are the same as those in the previous Fig. 4. Significant differences were accepted at P < 0.05.
). Format, statistical symols, and values of n are the same as those in the previous Fig. 4. Significant differences were accepted at P < 0.05.
Myofibers atrophy early after spinal cord transection but recover with prolonged spasticity
Tail muscles exhibited significant myofiber atrophy early after spinal cord transection (young chronic spinal rats, n = 5; for all myofibers: mean = 683 ± 250 µm2, minimum =118 µm2, maximum = 1,805 µm2). Specifically, slow type I, fast type IIA, fast type IID(X), and fast type IIB tail myofibers in young chronic spinal rats were all significantly atrophied compared with in young normals (Fig. 5,A–D). However, 4 mo of complete tail muscle spasticity was associated with a substantial recovery from myofiber atrophy (older chronic spinal rats, n = 9; for all myofibers: mean = 1,054 ± 514 µm2, minimum = 101 µm2, maximum = 3,710 µm2). Specifically, the type I, type IIA, and type IIB myofibers in older chronic spinal rats recovered from atrophy in that they were significantly larger than in young chronic spinal rats and were not significantly smaller than in older normals (Fig. 5,A, B, andD). Type IID(X) myofibers also recovered in older chronic spinal rats in that they were significantly larger than in young chronic spinal rats, although they remained significantly smaller than in older normals (Fig. 5C). Interestingly, the type IID(X) myofibers are the dominant myofiber type in this muscle and, with the exception of the infrequently observed type IIB myofibers, are likely to be in the highest threshold motor units and thus least active during spasticity. This partial recovery from atrophy of type IID(X) myofibers is also consistent with previous observations that following periods of reduced muscle activity, the predominant myofiber type tends to exhibit the greatest atrophy (Ohira et al. 2002).
In contrast to the recovery of myofiber size observed with spasticity, there was no recovery from atrophy with long-term near-elimination of muscle activity after spinal isolation (spinal isolated rats, n = 7; for all myofibers: mean = 508 ± 328 µm2, minimum = 53 µm2, maximum = 2,302 µm2). Specifically, myofibers of all types in spinal isolated rats were atrophied compared with in age-matched old normal and old chronic spinal rats (Fig. 5,A-D). These data indicate that reduced muscle activity is associated with tail myofiber atrophy 3 mo after spinal cord transection, and this association of reduced muscle activity with myofiber atrophy is also apparent 7 mo after spinal isolation. In contrast, prolonged spasticity after spinal cord transection is apparently associated with a persistent increase in muscle activity that promotes recovery of myofiber size via compensatory hypertrophy.
We wanted to account for the potentially confounding effects of age on altered myofiber cross-sectional areas; to this end, the percent changes in myofiber sizes in injured rats were computed relative in age matched normal rats, as also done for myofiber proportions (see preceding text). In young chronic spinal rats, these percent changes in myofiber size were large and significantly different from zero, with decreases of −33.9 ± 19.9% for type I, −57.7 ± 8.5% for type IIA, − 57.6 ± 5.3% for type IID(X), and −65.8 ± 11.5% for type IIB myofibers (Fig. 5,E–H), again suggesting considerable atrophy compared with in young normal rats. Myofibers recovered from this atrophy in older chronic spinal rats with spasticity; specifically, the percent changes in the sizes of type I and of type IIB myofibers were no longer significantly different from zero (Fig. 5,E, and H). Moreover, the percent changes in the sizes of type IIA and type IID(X) myofibers from older chronic spinal rats relative to normal were reduced to −27.1 ± 26.8 and −33.6 ± 15.1%, respectively (Fig. 5,F, and G), significantly less than the percent changes for atrophied type IIA and type IID(X) myofibers in young chronic spinal rats relative to normal (−57.7 ± 8.5 and −57.6 ± 5.3%, respectively). In contrast, in spinal isolated rats the percent changes (decreases) in cross-sectional area for all myofiber types were both significantly different from zero and significantly larger than the relatively small percent decreases observed in old chronic spinal rats relative to normal (Fig. 5,E–H).
Myofiber cross-sectional areas were further analyzed for normal and injured rats by separating myofibers into bins 500 µm2 in size, as shown in Fig. 6 (i.e., 1–500 µm2, 501–1,000 µm2, and so on up to 3,501–4,000 µm2; the top of each range is specified on the x-axis throughout Fig. 6, and all bins are listed in the Fig. 6 legend). Because myofibers with cross-sectional areas >4,000 µm2 were observed in very low proportions, axes extend only to 4,000 µm2 (Fig. 6).
FIG. 6.
The mean myofiber cross-sectional areas shown in Fig. 5 are detailed according to size distributions in the following bins of 500 µm2 each: 1–500 µm2, 501–1,000 µm2, 1,001–1,500 µm2, 1,501–2,000 µm2, 2,001–2,500 µm2, 2,501–3,000 µm2, 3,001–3,500 µm2, and 3,501–4,000 µm2A: young normals, □. B: older normals, ■. C: young chronic spinal rats, □. D: older chronic spinal rats that had complete spasticity for 4 mo, ■. E: older spinal isolated rats that had near-elimination of muscle activity for 7 mo,
 s. See Fig. 4 caption for values of n. Inset: minimum, maximum, and mean myofiber size for each group (SDs are found in the text of the results).
s. See Fig. 4 caption for values of n. Inset: minimum, maximum, and mean myofiber size for each group (SDs are found in the text of the results).
Tail myofiber size distributions were broadly right-skewed with peaks at 1,001–1,500 µm2 in both young and older normals (Fig. 6,A, and B). Overall in these normal rats, the majority of myofibers were <2,000 µm2, although very few myofibers were observed in the smallest 1–500 µm2 bin. No large differences due to age were apparent in older normals (Fig. 6B) compared with in young normals (Fig. 6,A).
In young chronic spinal rats compared with in young normals, the distribution of myofiber sizes narrowed substantially, becoming more bell-shaped and shifting leftward, with the peak at 501–1,000 µm2 and the majority of myofibers < 1,000 µm2 (Fig. 4C). In older chronic spinal rats, although the peak of the distribution remained at 501–1,000 µm2, the proportion of myofibers in this bin was much smaller than in young chronic spinal rats, and the distribution was recovered closer to normal (peak shifted back toward the right) with increased numbers of larger myofibers; specifically, the proportion of myofibers that were 1,001–1,500 µm2 more than quadrupled in older chronic spinals compared with in young chronic spinals (Fig. 6,C, and D). In contrast, the myofiber size distribution for spinal isolated rats was sharply right-skewed compared with chronic spinal rats, and the majority of myofibers were <500 µm2 (Fig. 6E).
Interestingly, a relatively large population of very small myofibers appeared early after injury in young chronic spinal rats (~25% of myofibers 1–500 µm2; Fig. 6C). Yet associated with 4 mo of complete spasticity in older chronic spinal rats, hypertrophy resulted in a substantial decrease in the number of these very small myofibers (~10% of myofibers 1–500 µm2; Fig. 6D). However, in spinal isolated rats, these small myofibers accounted for −65% of all myofibers (Fig. 6E). In all injured rats, these small myofibers appeared in every myofiber type but did not express embryonic MyHC and had normal morphological appearance, indicating that they were neither newly formed nor necrotic.
Electrophoretically determined MyHC isoform content and computed area density are stable with long-term spinal cord transection and spasticity
The proportions of MyHC isoforms were assessed by gel electrophoresis (Fig. 2). When young normals (n = 5) were compared with older normals (n = 9), there were no significant age-related differences in the relative muscle contents of MyHC I, MyHC IIa, MyHC IId(x), or MyHC IIb as assessed by integrated densitometry after gel electrophoresis (Fig. 7A–D). Likewise, comparing young chronic spinal rats (n = 5) to young age-matched normals there were no significant differences early after injury in the relative contents of MyHC I, MyHC IId(x), or MyHC IIb proteins (Fig. 7,A, C, and D), but there was a significant decrease in the relative content of MyHC IIa protein (Fig. 7B). Comparing older chronic spinal rats (n = 9) to age-matched older normals, there were no significant differences after prolonged spasticity in the relative contents of MyHC I, MyHC IIa, MyHC IId(x), or MyHC IIb proteins (Fig. 7,A-D). Comparing spinal isolated rats (n = 7) to both age-matched old normals and old chronic spinal rats, there was a significant increase in the relative content of MyHC IIa (Fig. 7B) and a significant decrease in the relative content of MyHC IId(x) (Fig. 7C) as similarly observed for the proportion of myofibers expressing these isoforms (Fig. 4), but there were no changes in the relative contents of either MyHC I or MyHC IIb (Fig. 7A, and D).
FIG. 7.
A–D: relative muscle content of each MyHC isoform is shown for young normals and young chronic spinal rats (□), older normals and older chronic spinal rats (■), and older spinal isolated rats (
 ) as determined by integrated densitometry after electrophoretic separation of these MyHC iso-forms on a polyacrylamide gel (cf. Fig. 2). E–H: area densities of the corresponding MyHC isoforms are shown as computed from immunohisto-chemical staining of frozen muscle sections (see results for calculation). A and E: MyHC I. B and F: MyHC IIa. C and G: MyHC IId(x). D and H: MyHC IIb. The values of n and formats for A–D and for E–H are the same as those for Fig. 4A–D, except that in Fig. 7B, and F, the vertical scale extends to 75%. Significant differences were accepted at P < 0.05.
) as determined by integrated densitometry after electrophoretic separation of these MyHC iso-forms on a polyacrylamide gel (cf. Fig. 2). E–H: area densities of the corresponding MyHC isoforms are shown as computed from immunohisto-chemical staining of frozen muscle sections (see results for calculation). A and E: MyHC I. B and F: MyHC IIa. C and G: MyHC IId(x). D and H: MyHC IIb. The values of n and formats for A–D and for E–H are the same as those for Fig. 4A–D, except that in Fig. 7B, and F, the vertical scale extends to 75%. Significant differences were accepted at P < 0.05.
The MyHC isoform contents determined from gel electrophoresis correspond to the combined influence of both myofiber proportion and myofiber cross-sectional area. Specifically, MyHC isoform contents correspond to the area density, which is defined as the total myofibrillar cross-sectional area expressing each isoform in a given muscle, reported as a proportion of the total cross-sectional area of all the myofibers in that muscle (Fry et al. 1994; Hansen et al. 2004). Mathematically, the area density of a given MyHC isoform (or myofiber type) is equivalent to the product of the myofiber proportion of that type (Fig. 4A-D) and the mean cross-sectional area of that myofiber type (Fig. 5A–D), normalized to the mean cross-sectional area of all myofibers. As with electrophoresis, immuno-histochemical stains showed no significant changes in these computed area densities for MyHC I or MyHC IIb with age or injury (Fig. 7E, and H); however, as also observed with electrophoresis, in young chronic spinal rats (n = 5) compared with in young normals (n = 5), the MyHC IIa area density was decreased (Fig. 7,F), and in spinal isolated rats (n = 7) compared with in both age-matched old normals (n = 9) and old chronic spinal rats (n = 9), the MyHC IIa area density was increased and the MyHC IId(x) area density was decreased (Fig. 7,F, and G).
DISCUSSION
Spasticity promotes recovery from myofiber atrophy after chronic spinal cord transection
Our results from adult rats with sacral spinal cord transection demonstrate that early after spinal cord transection, tail myofibers are atrophied relative to age-matched normals, likely due to flaccid paralysis during the early period after spinal cord transection. After long-term spasticity in older chronic spinal rats, tail myofibers recover from this atrophy. This myofiber size recovery is nearly complete for all myofiber types with the exception of the type IID(X) myofibers, which, interestingly, should represent the highest threshold motor units and thus be the least active during spasticity. This recovery is due to spastic muscle activity because when spasticity is eliminated by cutting the dorsal roots bilaterally in addition to transecting the cord (spinal isolation), atrophy then only further increases with time after injury. These results are consistent with previous findings indicating that after long-term SCI recovery from myofiber atrophy occurs with exercise-induced muscle activity (Crameri et al. 2004; Dupont-Versteegden et al. 1998; Roy et al. 1998, 1999). Previously, we have shown that spastic tail muscle activity results from a spontaneous recovery of motoneuron excitability after injury combined with exaggerated reflex-evoked synaptic inputs to motoneurons (Bennett et al. 2004), and thus ultimately, muscle properties are indirectly linked to changes in motoneuron properties.
Spasticity opposes transformation of myofiber types after chronic spinal cord transection
Our results also demonstrate that early after spinal cord transection, tail muscles in young chronic spinal rats undergo a modest transformation in their myofiber types toward faster MyHC isoforms compared with in young normal rats, with smaller proportions of slow type I myofibers and larger proportions of fast type IID(X) myofibers, consistent with the classic effects of reduced muscle activity (Roy et al. 2000; Talmadge et al. 1999). Again, after 4 mo of complete spasticity in older chronic spinal rats, these proportions recover to no different from in age-matched older normal rats. The magnitudes of transformation in young chronic spinal rats and of recovery in older chronic spinal rats are small due to the already high proportion of fast type IID(X) myofibers in normal rats. However, the relative changes in type I and type IIA myofiber proportions early after injury are very large (reduced to approximately half of young normal). Again this recovery is due to spastic muscle activity because eliminating spasticity in the spinal isolated animals eliminates the recovery and only further augments the slow-to-fast myofiber type transformation.
Unexpectedly after spinal isolation, within the fast type II myofiber population, there is a large decrease in the proportion of type IID(X) myofibers and a large increase in the proportion of type IIA myofibers, which is not consistent with observations of other muscles after spinal isolation (Roy et al. 2000) but which may be associated with altered muscle loading (see following text). Associated with this there is an emergence of type IIB and hybrid type I/IIA myofibers that were very low in number or absent in all other conditions. There is also an emergence of many more very small myofibers, which are neither regenerating (no embryonic MyHC) nor necrotic (normal morphology). Thus this seems to be a stable tail muscle phenotype after long-term sacral spinal isolation in the adult rat.
Recovery of myofiber types and myofiber morphology resembles the effects of exercise
It is well documented that in the absence of muscle activity, myofibers often transform robustly toward faster, more fatigable types and undergo considerable atrophy (Roy et al. 2002a). Early after spinal cord transection and after long-term spinal isolation in our rats, reduced muscle activity results in a similar atrophy and decrease in type I myofiber type proportions as seen in our results.
It is also well documented that after SCI (in animals and humans), cauda equina injuries (in humans), and spinal isolation (in animals), reflex-generated exercise or direct electrical stimulation of muscle nerves induces recovery from myofiber atrophy in both primarily slow and primarily fast muscles and also opposes transformations in myofiber types toward faster isoforms (Dupont-Versteegden et al. 1998; Hartkopp et al. 2003; Kern et al. 2004; Murphy et al. 1999; Roy et al. 1999, 2002a; Shields and Dudley-Javoroski 2006). Thus the recovery of myofiber sizes and proportions in chronic spinal rats with long-term spasticity resembles the effects of training interventions that provide muscle activity after CNS lesions in animals and humans. Consistent with previous evidence, the recovery we observe in both myofiber types and myofiber size is not associated with changes in myofibers due to age (Ansved and Larsson 1989; Putman et al. 2001), as described in results, and is associated with spasticity because the recovery is eliminated when spasticity is eliminated by sectioning the dorsal roots in addition to transecting the spinal cord (spinal isolation). Taken together, our histochemical and 24-h EMG results from older chronic spinal rats and their age-matched older spinal isolated rats demonstrate that spasticity provides sufficient muscle activity to promote the recovery from reduced activity-dependent changes early after injury.
The mechanical consequences of the histological changes we observed in myofibers should be that early after injury, the muscles are weaker, faster, and more fatigable, as seen in previous investigations (Cope et al. 1986; Hartkopp et al. 2003; Lieber et al. 1986b; Roy et al. 1999). Furthermore, with long-term injury and spasticity, compared with the earlier deleterious adaptations, muscles should recover their force-generating capability, become slower, and become less fatigable, consistent with previous investigations in humans that indicate a role for spasticity in preserving slower muscle contractile properties (Hartkopp et al. 1999; Hidler et al. 2002; Thomas 1997; Zijdewind and Thomas 2003). The actual changes in tail muscle contractile properties in chronic spinal rats with long-term spasticity, which include a slowing of muscle contractile speed and an increase in muscle twitch force, but losses of fatigue-resistance and of tetanic force, are explored in a companion study (Harris et al. 2006).
Rat segmental tail muscles can be reliably assessed with routine immunohistochemical staining and gel electrophoresis
Our study is the first to report myofiber type composition, MyHC isoform distributions, and myofiber size in the segmental tail muscles of the rat. The prominent features of these muscles are: small myofibers, a high proportion of type IID(X) myofibers, and a conspicuous absence of both type IIB and hybrid myofibers (~15% type I, 15% type IIA, 67.5% type IID(X), 2.5% type IIB, and no hybrids). In contrast, hindlimb muscles such as the extensor digitorum longus (EDL, stained as positive control in our study) often have: larger myofibers, fewer type IID(X) myofibers, and numerous type IIB and hybrid myofibers (~10% type I, 25% type IIA, 30% type IID(X), 30% type IIB, and 5% hybrids for EDL in this study).
Importantly, the close agreement of our immunohistochemical and electrophoretic methods shows that tail myofibers can be reliably assessed by immunohistochemistry, and this is especially important for type IID(X) myofibers, which we quantified indirectly by counting myofibers that are not immunostained (subtraction method). Further validation of our methodology for assessing rat segmental tail muscles is derived from the close agreement between the results of our subtraction method and those of our immunostaining with clone BF-35 (a monoclonal primary antibody that labels all MyHC isoforms except MyHC IId(x); see methods) in the identification of type IID(X) myofibers as well as from the close agreement of our histochemical evaluation of the positive control hindlimb EDL muscle with that performed in previous studies (Bamford et al. 2003; Delp and Duan 1996; Dupont-Versteegden et al. 1998; Lieber et al. 1986a; Putman et al. 2000).
Although hybrid myofibers are often present in muscles transforming both to slower and to faster distributions of myosin heavy chain (MyHC) isoforms and myofibers (Putman et al. 2001; Talmadge et al. 1999), there are no hybrid type I/IIA or hybrid type IIA/B myofibers in normal rat tail muscles or after spinal cord transection alone. The lack of hybrid myofibers in tail muscles in this study is not a result of limitations in our methodology because we observe the expected number of hybrid myofibers in the normal hindlimb EDL muscle, which is used as a positive control. Moreover, a small but significant proportion of hybrid type I/IIA myofibers does emerge in tail muscles after spinal isolation. This is consistent with the idea that the sacrocaudal spinal isolation (sacral spinal cord transection combined with bilateral sacrocaudal deafferentation) results in a drastic long-term reduction of neuromuscular activity among tail motor units, just as a more rostral lumbar spinal isolation (bilateral lumbar deafferentation between transections at low thoracic and high sacral levels) dramatically reduces muscle activity in rats and cats (Pierotti et al. 1991; Roy et al. 2000). No antibody specific for MyHC IId(x) was available; thus it is not known if there are hybrid tail myofibers coexpressing the MyHC IId(x) isoform (e.g., type IIA/D(X) or type IID(X)/B myofibers).
Altered muscle loading may influence tail myofiber type proportions after spinal isolation
The ventrolateral segmental tail muscles are small intervertebral flexor muscles with only ~12 motor units each, and, in the normal animal, these muscles primarily provide intervertebral postural stability during activation of the tail by much larger muscles located at the base of the tail (Brink and Pfaff 1980; Steg 1964). Thus it is likely that the unique functional demands of the segmental tail muscles lead to their myofiber composition, including the high proportion of type IID(X) myofibers.
In older chronic spinal rats compared with in age-matched older normals, there is no change in the proportion of type IID(X) myofibers (or in the proportions of any other myofiber types) in the long-term spastic muscles. This suggests that the daily intermittent load these muscles must bear during spasms (i.e., ventroflexion) (see also Bennett et al. 1999, 2004) is similar to the daily load experienced by normal, unparalyzed segmental tail muscles, and this is supported by the fact that tail muscles from these two groups of animals experience similar amounts of average daily EMG activity. On the other hand, tail muscles experience little daily EMG activity following spinal isolation (rare and low-amplitude spontaneous bursts), and thus the daily contractile activity of tail muscles in spinal isolated animals is likely negligible.
After spinal isolation, paralysis of the tail muscles may result in a transfer of the active contractile role of the type I myofibers during posture to a passive postural role of all myofiber types in bearing the mass of the tail. Thus paradoxically, both due to and in spite of the dramatic reduction in neuromuscular activity after spinal isolation, it is possible that the unexpected type IID(X) to type IIA myofiber transformation observed in the tail muscles of spinal isolated animals in our study is the result of passive stretch experienced by these muscles. This is supported by the observation that in the rat hindlimb gastrocnemius, which like segmental tail muscles is a mixed type muscle dominated by fast myosin heavy chain isoforms, passive stretch robustly upregulates the MyHC IIa gene and downregulates the MyHC IIb gene (Loughna et al. 1990).
Summary
Early after sacral spinal cord transection, there is little tail muscle activity, a condition that is associated with considerable myofiber atrophy and a transformation toward a faster myofiber distribution in young chronic spinal rats. After long-term spinal isolation (spinal cord transection combined with bilateral deafferentation), there is a permanent near-elimination of muscle activity, which is associated with further myofiber atrophy and a transformation toward a faster myofiber distribution in spinal isolated rats. However, many months after spasticity develops in older chronic spinal rats, tail muscles recover from these deleterious changes with a partial re-establishment of normal myofiber types and morphology.
Acknowledgments
The authors thank Dr. M. A. Gorassini for generously donating laboratory space to perform the 24-h EMG recordings. The authors also thank N. Tireman and I. McLean for expert technical assistance.
GRANTS
Funding was provided by the Natural Sciences and Engineering Research Council of Canada, by the Canadian Foundation for Innovation, by the Canadian Institutes of Health Research, and by the Alberta Heritage Foundation for Medical Research (AHFMR). D. J. Bennett is an AHFMR Senior Scholar and C. T. Putman is an AHFMR Medical Scholar.
References
- Ansved T, Larsson L. Effects of ageing on enzyme-histochemical, morpho-metrical and contractile properties of the soleus muscle in the rat. J Neurol Sci. 1989;93:105–124. doi: 10.1016/0022-510x(89)90165-2. [DOI] [PubMed] [Google Scholar]
- Bamford JA, Lopaschuk GD, MacLean IM, Reinhart ML, Dixon WT, Putman CT. Effects of chronic AICAR administration on the metabolic and contractile phenotypes of rat slow- and fast-twitch skeletal muscles. Can J Physiol Pharmacol. 2003;81:1072–1082. doi: 10.1139/y03-110. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Gorassini M, Fouad K, Sanelli L, Han Y, Cheng J. Spasticity in rats with sacral spinal cord injury. J Neurotrauma. 1999;16:69–84. doi: 10.1089/neu.1999.16.69. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Harvey PJ, Gorassini M. Evidence for plateau potentials in tail motoneurons of awake chronic spinal rats with spasticity. J Neuro-physiol. 2001;86:1972–1982. doi: 10.1152/jn.2001.86.4.1972. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Sanelli L, Cooke CL, Harvey PJ, Gorassini MA. Spastic long-lasting reflexes in the awake rat after sacral spinal cord injury. J Neu-rophysiol. 2004;91:2247–2258. doi: 10.1152/jn.00946.2003. [DOI] [PubMed] [Google Scholar]
- Brink EE, Pfaff DW. Vertebral muscles of the back and tail of the albino rat (Rattus norvegicus albinus) Brain Behav Evol. 1980;17:1–47. doi: 10.1159/000121788. [DOI] [PubMed] [Google Scholar]
- Cope TC, Bodine SC, Fournier M, Edgerton VR. Soleus motor units in chronic spinal transected cats: physiological and morphological alterations. J Neurophysiol. 1986;55:1202–1220. doi: 10.1152/jn.1986.55.6.1202. [DOI] [PubMed] [Google Scholar]
- Crameri RM, Cooper P, Sinclair PJ, Bryant G, Weston A. Effect of load during electrical stimulation training in spinal cord injury. Muscle Nerve. 2004;29:104–111. doi: 10.1002/mus.10522. [DOI] [PubMed] [Google Scholar]
- Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol. 1996;80:261–270. doi: 10.1152/jappl.1996.80.1.261. [DOI] [PubMed] [Google Scholar]
- Dupont-Versteegden EE, Houle JD, Gurley CM, Peterson CA. Early changes in muscle fiber size and gene expression in response to spinal cord transection and exercise. Am J Physiol Cell Physiol. 1998;275:C1124–1133. doi: 10.1152/ajpcell.1998.275.4.C1124. [DOI] [PubMed] [Google Scholar]
- Eldridge L. Lumbosacral spinal isolation in cat: surgical preparation and health maintenance. Exp Neurol. 1984;83:318–327. doi: 10.1016/S0014-4886(84)90101-8. [DOI] [PubMed] [Google Scholar]
- Fry AC, Allemeier CA, Staron RS. Correlation between percentage fiber type area and myosin heavy chain content in human skeletal muscle. Eur J Appl Physiol Occup Physiol. 1994;68:246–251. doi: 10.1007/BF00376773. [DOI] [PubMed] [Google Scholar]
- Fujimori B, Shimamura M, Kato M, Yamauchi T, Aoki M, Tanji J. Studies on the mechanisms of spasticity and rigidity following spinal lesions and ischaemia. Proc Aust Assoc Neurol. 1968;5:35–39. [PubMed] [Google Scholar]
- Hansen G, Martinuk KJ, Bell GJ, MacLean IM, Martin TP, Putman CT. Effects of spaceflight on myosin heavy-chain content, fibre morphology and succinate dehydrogenase activity in rat diaphragm. Pfluegers. 2004;448:239–247. doi: 10.1007/s00424-003-1230-9. [DOI] [PubMed] [Google Scholar]
- Harris RLW, Bobet J, Sanelli L, Bennett DJ. Tail muscles become slow but fatigable in chronic sacral spinal rats with spasticity. J Neurophysiol. 2006;95:1124–1133. doi: 10.1152/jn.00456.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RLW, Sanelli L, Putman CT, Bennett DJ. XXXV International Congress of the Physiological Sciences. San Diego, CA: 2005. Spasticity reverses atrophy and transformation of skeletal muscle fibers after spinal cord injury. [Google Scholar]
- Hartkopp A, Andersen JL, Harridge SD, Crone C, Gruschy-Knudsen T, Kjaer M, Masao M, Ratkevicius A, Quistorff B, Zhou S, Biering-Sorensen F. High expression of MHC I in the tibialis anterior muscle of a paraplegic patient. Muscle Nerve. 1999;22:1731–1737. doi: 10.1002/(sici)1097-4598(199912)22:12<1731::aid-mus20>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Hartkopp A, Harridge SD, Mizuno M, Ratkevicius A, Quistorff B, Kjaer M, Biering-Sorensen F. Effect of training on contractile and metabolic properties of wrist extensors in spinal cord-injured individuals. Muscle Nerve. 2003;27:72–80. doi: 10.1002/mus.10290. [DOI] [PubMed] [Google Scholar]
- Heckman CJ. Alterations in synaptic input to motoneurons during partial spinal cord injury. Med Sci Sports Exerc. 1994;26:1480–1490. [PubMed] [Google Scholar]
- Hidler JM, Harvey RL, Rymer WZ. Frequency response characteristics of ankle plantar flexors in humans following spinal cord injury: relation to degree of spasticity. Ann Biomed Eng. 2002;30:969–981. doi: 10.1114/1.1500409. [DOI] [PubMed] [Google Scholar]
- Kern H, Boncompagni S, Rossini K, Mayr W, Fano G, Zanin ME, Podhorska-Okolow M, Protasi F, Carraro U. Long-term denervation in humans causes degeneration of both contractile and excitation-contraction coupling apparatus, which is reversible by functional electrical stimulation (FES): a role for myofiber regeneration? J Neuropathol Exp Neurol. 2004;63:919–931. doi: 10.1093/jnen/63.9.919. [DOI] [PubMed] [Google Scholar]
- Kuhn RA, Macht MB. Some manifestations of reflex activity in spinal man with particular reference to the occurrence of extensor spasm. Bull Johns Hopkins Hosp. 1948;84:43–75. [PubMed] [Google Scholar]
- Lance JW, Burke D. Mechanisms of spasticity. Arch Phys Med Rehabil. 1974;55:332–337. [PubMed] [Google Scholar]
- Lieber RL, Friden JO, Hargens AR, Feringa ER. Long-term effects of spinal cord transection on fast, slow rat skeletal muscle II Morphomet-ric properties. Exp Neurol. 1986a;91:435–448. doi: 10.1016/0014-4886(86)90042-7. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Johansson CB, Vahlsing HL, Hargens AR, Feringa ER. Long-term effects of spinal cord transection on fast, slow rat skeletal muscle I Contractile properties. Exp Neurol. 1986b;91:423–434. doi: 10.1016/0014-4886(86)90041-5. [DOI] [PubMed] [Google Scholar]
- Lotta S, Scelsi R, Alfonsi E, Saitta A, Nicolotti D, Epifani P, Carraro U. Morphometric and neurophysiological analysis of skeletal muscle in paraplegic patients with traumatic cord lesion. Paraplegia. 1991;29:247–252. doi: 10.1038/sc.1991.35. [DOI] [PubMed] [Google Scholar]
- Loughna PT, Izumo S, Goldspink G, Nadal-Ginard B. Disuse and passive stretch cause rapid alterations in expression of developmental and adult contractile protein genes in skeletal muscle. Development. 1990;109:217–223. doi: 10.1242/dev.109.1.217. [DOI] [PubMed] [Google Scholar]
- McGuigan MR, Kraemer WJ, Deschenes MR, Gordon SE, Kitaura T, Scheett TP, Sharman MJ, Staron RS. Statistical analysis of fiber area in human skeletal muscle. Can J Appl Physiol. 2002;27:415–422. doi: 10.1139/h02-022. [DOI] [PubMed] [Google Scholar]
- Murphy RJ, Hartkopp A, Gardiner PF, Kjaer M, Beliveau L. Salbutamol effect in spinal cord injured individuals undergoing functional electrical stimulation training. Arch Phys Med Rehabil. 1999;80:1264–1267. doi: 10.1016/s0003-9993(99)90027-8. [DOI] [PubMed] [Google Scholar]
- Oakley BR, Kirsch DR, Morris NR. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980;105:361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Ohira Y, Yoshinaga T, Nomura T, Kawano F, Ishihara A, Nonaka I, Roy RR, Edgerton VR. Gravitational unloading effects on muscle fiber size, phenotype and myonuclear number. Adv Space Res. 2002;30:777–781. doi: 10.1016/s0273-1177(02)00395-2. [DOI] [PubMed] [Google Scholar]
- Pierotti DJ, Roy RR, Bodine-Fowler SC, Hodgson JA, Edgerton VR. Mechanical and morphological properties of chronically inactive cat tibialis anterior motor units. J Physiol. 1991;444:175–192. doi: 10.1113/jphysiol.1991.sp018872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putman CT, Conjard A, Peuker H, Pette D. Alpha-cardiac-like myosin heavy chain MHCI alpha is not upregulated in transforming rat muscle. J Muscle Res Cell Motil. 1999;20:155–162. doi: 10.1023/a:1005430115402. [DOI] [PubMed] [Google Scholar]
- Putman CT, Dusterhoft S, Pette D. Satellite cell proliferation in low frequency-stimulated fast muscle of hypothyroid rat. Am J Physiol Cell Physiol. 2000;279:C682–690. doi: 10.1152/ajpcell.2000.279.3.C682. [DOI] [PubMed] [Google Scholar]
- Putman CT, Kiricsi M, Pearcey J, MacLean IM, Bamford JA, Murdoch GK, Dixon WT, Pette D. AMPK activation increases uncoupling protein-3 expression and mitochondrial enzyme activities in rat muscle without fibre type transitions. J Physiol. 2003;551:169–178. doi: 10.1113/jphysiol.2003.040691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putman CT, Sultan KR, Wassmer T, Bamford JA, Skorjanc D, Pette D. Fiber-type transitions and satellite cell activation in low-frequency-stimulated muscles of young and aging rats. J Gerontol A Biol Sci Med Sci. 2001;56:B510–519. doi: 10.1093/gerona/56.12.b510. [DOI] [PubMed] [Google Scholar]
- Ritz LA, Friedman RM, Rhoton EL, Sparkes ML, Vierck CJ., Jr Lesions of cat sacrocaudal spinal cord: a minimally disruptive model of injury. J Neurotrauma. 1992;9:219–230. doi: 10.1089/neu.1992.9.219. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JD, Parry DJ. Gamma irradiation prevents compensatory hypertrophy of overloaded mouse extensor digitorum longus muscle. J Appl Physiol. 1992;73:2538–2543. doi: 10.1152/jappl.1992.73.6.2538. [DOI] [PubMed] [Google Scholar]
- Roy RR, Kim JA, Grossman EJ, Bekmezian A, Talmadge RJ, Zhong H, Edgerton VR. Persistence of myosin heavy chain-based fiber types in innervated but silenced rat fast muscle. Muscle Nerve. 2000;23:735–747. doi: 10.1002/(sici)1097-4598(200005)23:5<735::aid-mus11>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Roy RR, Pierotti DJ, Flores V, Rudolph W, Edgerton VR. Fibre size and type adaptations to spinal isolation and cyclical passive stretch in cat hindlimb. J Anat. 1992;180:491–499. [PMC free article] [PubMed] [Google Scholar]
- Roy RR, Talmadge RJ, Hodgson JA, Oishi Y, Baldwin KM, Edgerton VR. Differential response of fast hindlimb extensor and flexor muscles to exercise in adult spinalized cats. Muscle Nerve. 1999;22:230–241. doi: 10.1002/(sici)1097-4598(199902)22:2<230::aid-mus11>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Roy RR, Talmadge RJ, Hodgson JA, Zhong H, Baldwin KM, Edgerton VR. Training effects on soleus of cats spinal cord transected (T12–13) as adults. Muscle Nerve. 1998;21:63–71. doi: 10.1002/(sici)1097-4598(199801)21:1<63::aid-mus9>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Roy RR, Zhong H, Hodgson JA, Grossman EJ, Siengthai B, Talmadge RJ, Edgerton VR. Influences of electromechanical events in defining skeletal muscle properties. Muscle Nerve. 2002a;26:238–251. doi: 10.1002/mus.10189. [DOI] [PubMed] [Google Scholar]
- Roy RR, Zhong H, Monti RJ, Vallance KA, Edgerton VR. Mechanical properties of the electrically silent adult rat soleus muscle. Muscle Nerve. 2002b;26:404–412. doi: 10.1002/mus.10219. [DOI] [PubMed] [Google Scholar]
- Shields RK, Dudley-Javoroski S. Musculoskeletal plasticity after acute spinal cord injury: effects of long-term neuromuscular electrical stimulation training. J Neurophysiol. 2006;95:2380–2390. doi: 10.1152/jn.01181.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steg G. Efferent muscle innervation and rigidity. Acta Physiol Scand 61 Suppl. 1964;225:1–53. [PubMed] [Google Scholar]
- Talmadge RJ, Roy RR, Edgerton VR. Persistence of hybrid fibers in rat soleus after spinal cord transection. Anat Rec. 1999;255:188–201. doi: 10.1002/(SICI)1097-0185(19990601)255:2<188::AID-AR9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Taylor JS, Friedman RF, Munson JB, Vierck CJ., Jr Stretch hyperreflexia of triceps surae muscles in the conscious cat after dorsolateral spinal lesions. J Neurosci. 1997;17:5004–5015. doi: 10.1523/JNEUROSCI.17-13-05004.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CK. Contractile properties of human thenar muscles paralyzed by spinal cord injury. Muscle Nerve. 1997;20:788–799. doi: 10.1002/(sici)1097-4598(199707)20:7<788::aid-mus2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Ross BH. Distinct patterns of motor unit behavior during muscle spasms in spinal cord injured subjects. J Neurophysiol. 1997;77:2847–2850. doi: 10.1152/jn.1997.77.5.2847. [DOI] [PubMed] [Google Scholar]
- Tower SS. Function and structure in the chronically isolated lumbo-sacral spinal cord of the dog. J Comp Neurol. 1937;67:109–131. [Google Scholar]
- Zijdewind I, Thomas CK. Motor unit firing during and after voluntary contractions of human thenar muscles weakened by spinal cord injury. J Neurophysiol. 2003;89:2065–2071. doi: 10.1152/jn.00492.2002. [DOI] [PubMed] [Google Scholar]