Abstract
Quercetin has been reported to act as a senolytic by selectively removing senescent endothelial cells, and thus it would seem quercetin could revolutionize the field of gerontology. However, given quercetin's narrow therapeutic index reported in work done with human umbilical vein endothelial cells (HUVECs), we hypothesized that quercetin is not innocuous for non-senescent adult human vascular endothelial cells at concentrations that have been reported to be safe for proliferating HUVECs. Furthermore, we investigated quercetin 3-D-galactoside (Q3G; hyperoside), an inactive quercetin derivative that needs to be cleaved by beta-galactosidase overexpressed in senescent cells to release quercetin, as a potential safer senolytic. We compared the effectiveness of quercetin and Q3G in primary human coronary artery endothelial cells (HCAEC), which are adult microvascular cells. We found that quercetin caused cell death in non-senescent endothelial cells at a concentration that has been reported to selectively remove senescent cells, and that Q3G was not cytotoxic to either young or senescent cells. Thus, in primary adult human endothelial cells, quercetin and Q3G are not senolytics. Earlier work reporting positive results was done with HUVECs, and given their origin and the disparate findings from the current study, these may not be the best cells for evaluating potential senolytics in clinically relevant endothelial cells.
New and noteworthy
Previously, quercetin has been reported to be a senolytic, a drug that selectively removes senescent cells, in HUVECs. However, we found neither quercetin nor Q3G was effective as a senolytic for adult human endothelial cells.
Introduction
Quercetin is a flavonoid found in significant quantities in our diet with beneficial effects, including anti-thrombotic, anti-inflammatory, and anti-neoplastic properties [1–4]. It is an excellent antioxidant that scavenges many naturally occurring reactive oxygen species, including O2- and ONOO−, and it facilitates zinc trafficking into cells, which in turn functions as an antioxidant [5, 6]. However, quercetin has been reported to induce cell type-specific cytotoxicity in vitro, where quercetin was relatively harmless to murine thymocytes and human lung embryonic fibroblasts at 100 μM, but significantly increased cell death was observed in human umbilical vein endothelial cells (HUVECs) at the same concentration [7, 8]. Despite this, following the finding that clinically relevant concentrations of glutathione completely suppressed quercetin's mutagenicity, and that no significant harm was observed in animals fed quercetin, it was determined to be safe for human consumption [9].
Quercetin 3-D-galactoside (Q3G), also known as hyperoside, is a natural derivative of quercetin produced by Hypericumperforatum L. (St. John's Wort) [10]. Q3G is structurally identical to quercetin, except for a galactoside group attached through an O-glycosidic bond that can be cleaved by beta-galactosidase to liberate quercetin [11]. Like quercetin, Q3G is bioactive with its anti-oxidant properties, even when it is not pre-processed by beta-galactosidase, and has other beneficial functions including inhibiting the growth of several parasites, lowering cholesterol, and fostering cardioprotection after ischemia [10, 12]. In addition, although quercetin-induced cytotoxicity has been reported in non-senescent HUVECs, Q3G is much safer for these cells, yet still confers protective anti-oxidative effect [13]. The major focus of quercetin research has been on its anti-oxidative effects. However, a groundbreaking new role for quercetin has been recently proposed, which may also extend to the quercetin derivative Q3G, based on recently reported findings that quercetin has senolytic properties, or the ability to selectively remove senescent cells. This would be an important development given the contribution of senescent cells to many of the deleterious changes of aging, including increased inflammation [14].
The in vivo development of cellular senescence, where cells halt normal function, irreversibly cease dividing, and secrete damaging inflammatory factors, has been proposed to be one of the major drivers of aging [15]. Cellular senescence is characterized by several prominent biochemical and functional changes, including flattened and enlarged cell morphology, increased lysosomal beta-galactosidase activity, and inflammatory factor secretion [15, 16]. The idea of cellular senescence contributing to the aging process is supported by the finding that senescent cells accumulate in aging organisms and at sites of age-related dysfunction, such as atrophic skin, osteoarthritic lesions, and atherosclerotic plaques [17].
Recent work reporting quercetin's potential as a senolytic used irradiation-induced senescent HUVECs, but HUVECs, which are derived from the umbilical vein of newborns, are far removed from aging adult human arterial vascular endothelial cells (EC). Not surprisingly, important differences have been found between adult EC and HUVEC [18–21]. Furthermore, quercetin’s low therapeutic/toxic ratio in the HUVEC study [14] raised the possibility that quercetin could significantly injure non-senescent cells. It was unclear whether the proliferation of non-senescent cells could be compensating for some of the quercetin-mediated cell death, thus masking its toxicity to the young cells at the lower concentrations found to be selectively cytotoxic to senescent cells. In the current study, we used adult human coronary artery endothelial cells (HCAEC), which are microvascular cells, as a relevant model, and generated two groups of cells from them to better understand the effect of quercetin: EP (early passage; young) and SEN (senescent), as a model of an aging tissue.
Given the known differences between adult EC and HUVECs, we hypothesized that quercetin would exhibit nonspecific cytotoxicity to adult EC. We investigated the effect of quercetin on EP vs. SEN HCAEC, and whether the SEN group was more susceptible to quercetin toxicity, as had been seen in irradiation-induced senescent HUVECs [14]. Furthermore, we tested whether Q3G, an inactive pro-drug that generates quercetin when cleaved by beta-galactosidase overexpressed in senescent cells, would more selectively remove senescent cells, and thus be a safer senolytic.
Materials and methods
Cell culture
HCAEC from three different adult human female donors, frozen at passage 3, were purchased [Cell Applications (San Diego, CA, USA) Lot#2228, Cell Applications Lot#2827, Lonza (Mapleton, IL, USA) Lot# 396592]. Donor information for the cells, supplied by the vendors, is as follows: #2228 (21 years old, Caucasian female), #2827 (17 years old, Hispanic female), #396592 (32 years old, Caucasian female). The cause of death and medical history for the donors is personal protected information, and thus unavailable. Common causes of death in younger females are accidents (30–40% of deaths), suicide and homicide (9–18%) [22]. Endothelial cell identity has been confirmed by uptake of acetylated LDL and presence of Factor III. Mycoplasma testing was negative. The cells were cultured in VascuLIFE® VEGF-MV (Lifeline Cell Technology; Frederick, MD, USA), containing 5% FBS, 5 ng/mL FGF, 50 μg/mL ascorbic acid, 10 mM L-Glutamine, 15 ng/mL IGF-1, 5 ng/mL EGF, 5 ng/mL VEGF, and 0.75 U/mL heparin sulfate. Antibiotics and hydrocortisone were not used. Cells were seeded at 3000 cells/cm2 for each passage. Culture medium was changed every two days, and the cells were kept in a 5% CO2 humidified incubator at 37°C.
Establishment of EP and SEN cells
The HCAEC were thawed and allowed to proliferate (Passage 1). After 4 days, they were passaged (Passage 2), and were then cryogenically stored with 2X freezing buffer [40% FBS (GE Life Sciences; Marlborough, MA, USA), 40% VascuLIFE® VEGF-MV (Lifeline Cell Technology), 20% DMSO (Sigma-Aldrich; St. Louis, MO, USA), 100 IU Penicillin-Streptomycin (Thermo Fisher Scientific; Waltham, MA, USA)]. At the time of an experiment, a vial was thawed and cultured for 4 days to allow time for recovery. The subsequent passage (Passage 3) was considered EP, where treatment of quercetin or Q3G began 2 days after this point for 48 hours.
To establish SEN cells, the cells were serially passaged every 4 days and re-plated at the density of 3000 cells/cm2, thereby gradually decreasing the cells' proliferation from the initial rate of 10- to 30-fold increase between passages to less than 2-fold increase. When the proliferation rate had decreased to the point where the cell number failed to double within 4 days, the subsequent passage was considered SEN. Once the passage at which the cells undergo senescence was identified, a different batch of cells was harvested 3- to 4- passages before reaching this point and was cryogenically stored until desired for experiments. We opted to freeze the cells at 3- to 4- passages before reaching senescence, rather than 1 passage prior like the EP cells, because we were concerned the senescent cells may be more prone to injury by the freezing process at this late passage due to their characteristic enlarged cell size. Cellular senescence was confirmed through visual inspection for the characteristic flattened and enlarged morphology, senescence-associated beta-galactosidase (SABG) staining, and decreased expression of Lamin B1 [23]. Thus, cellular senescence and SEN group were operationally, visually, and biochemically defined and established in our study.
Quercetin and Q3G preparation and treatment
Quercetin (CAS# 117-39-5; Cat# 10005169) and Q3G (CAS# 482-36-0; Cat# 18648) were purchased from Cayman Chemical (Ann Arbor, MI, USA). The stock solutions for these chemicals were prepared in DMSO at the concentration of 20 mM, aliquoted into small airtight tubes, and were stored in either liquid nitrogen or -80°C. Quercetin has been reported to degrade once it is in solution [24]. To prevent this, each of the stock aliquots kept in -80°C was used within a month of preparation and discarded after the single thaw for the experiment, as storing at low, freezing temperatures was recommended by other investigators and one supplier (Abcam) [25–27].
For treatment of HCAEC, quercetin and Q3G were first diluted in the culture medium to create working stock solutions, and then these solutions were mixed in different proportions with the culture medium to achieve the concentrations needed for the experiments. The blank culture medium had the same concentration of DMSO as the working stock solutions, so all cells were treated with the same concentration of DMSO. These working solutions were prepared immediately prior to treatment, and were applied to subconfluent cells two days after cell seeding. Based on the preliminary data on cell viability with quercetin/Q3G treatment (data not shown), both EP and SEN cells were treated for 48 hours at the end of their last passages (Fig 1A) at 0.21 mL per cm2 of growth area.
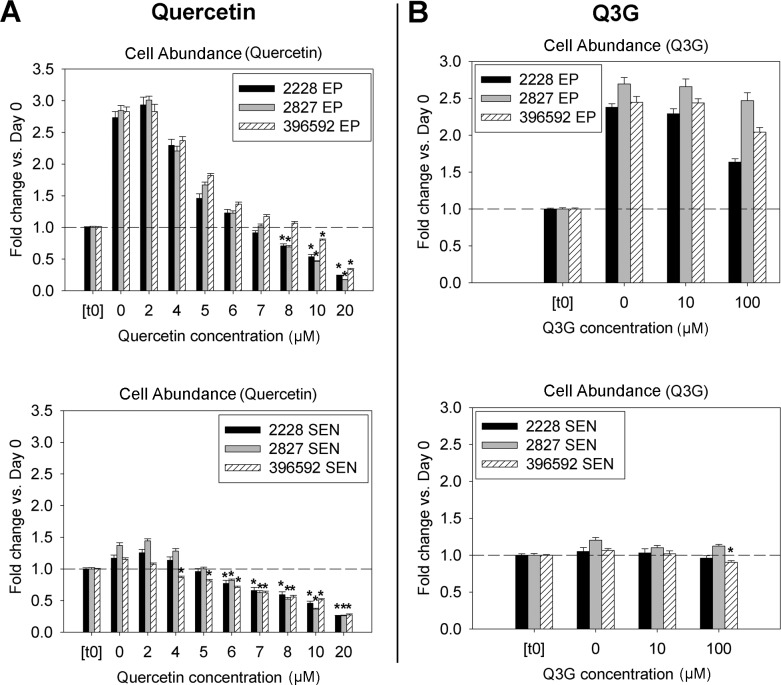
Fig 1.
A) The experimental timeline. Cells were treated with either quercetin or Q3G for 48 hours before assays. B) A representative SABG staining comparing EP and SEN cells. Many of the SEN cells show characteristic flattened morphology, and are stained blue due to increased SABG activity. C) Representative image of the western blot and densitometry data for Lamin B1. *p<0.05 vs. baseline (N = 3 samples/group, per donor), T-test.
Western blot
Whole-cell lysates were prepared by scraping the cultured cells in radio-immunoprecipitation assay (RIPA) buffer containing protease and phosphatase inhibitors (Sigma Aldrich; St. Louis, MO, USA; Cat# P8340, Cat# P0044). The samples were sonicated to facilitate cell lysis, and then centrifuged at 500g for 5 minutes to remove unlysed cells. The samples were separated on a 10% SDS-PAGE gel under reducing and denaturing conditions, and then transferred to nitrocellulose membranes for western blot, as previously described[28]. The following antibodies were used for probing: Lamin B1 (Cell Signaling; Danvers, MA, USA; Cat# 15068), and beta-actin (Sigma Aldrich; St. Louis, MO, USA; Cat# A2228)
Senescence-associated beta-galactosidase staining
SABG staining was carried out according to a previously published protocol [29]. Briefly, EP and SEN cells were fixed with a formaldehyde-glutaraldehyde buffer, and incubated with the X-gal staining solution overnight at 37°C. The cells were then washed with DPBS (Dulbecco's phosphate-buffered saline) and kept in the buffer during light microscopy imaging to prevent desiccation and deformation of cells.
As an alternate assay to confirm the findings for this study, the SABG stained cells were manually counted. To minimize bias, counting of the cells was done by researchers blinded to the cell treatment group. For counts, a well in the culture plate was positioned on the microscope stage without looking into the eyepiece, preventing subconsciously choosing a particular field of view to assay. Then, without re-positioning the plate, both stained and non-stained cells visible in the field of view were counted.
Cell proliferation assay
The DNA content-based fluorescence assay, CyQUANT® Cell Proliferation Assay Kit (Cat# C7026), was purchased from Thermo Fisher Scientific. HCAEC were grown and treated with quercetin/Q3G in black 96-well tissue culture microplates with a clear bottom. Then, t0 (immediately before treatment) and Day 2 (48 hour treatment) plates were washed with DPBS (containing Ca++ and Mg++, to prevent cell detaching) and stored at -80°C. Both plates were treated with the supplied DNA-sensitive fluorescence dye and analyzed at the same time with a microplate reader (Molecular Devices; Sunnyvale, CA, USA), following the manufacturer's protocol.
Live-Dead assay
Cell viability was measured with the LIVE/DEAD® Fixable Green Dead Cell Stain Kit for 488 nm Excitation (Cat# L23101, Thermo Fisher Scientific). EC were grown on six-well tissue culture plates, and the cells were washed twice with DPBS containing Ca++ and Mg++ immediately prior to quercetin/Q3G treatment to remove the small amount of floating dead cells and debris that routinely occur with cell culture. After 48 hours of treatment, the following from each well were collected and centrifuged at 500 rcf for 5 minutes to obtain mixtures of live and dead cells: conditioned culture medium, cells detached with trypsin-EDTA, and DPBS-well washes. Then, the assay was performed according to the manufacturer's protocol for flow cytometry, with formaldehyde fixation. Non-fragmented whole cells were identified and gated with the forward scatter (FSC) parameter (S1A and S1B Fig).
Statistics
Multiple groups were compared using a one-way ANOVA, and Kruskal-Wallis and Mann-Whitney U tests were used for the whole quercetin or Q3G data if data in any group were not normally distributed (Figs 2 and 3). Normally distributed data consisting of only two groups per donor was compared by T-test (Figs 1C and 4). SigmaPlot (Systat Software; San Jose, CA, USA) and SPSS v.23 (IBM; North Castle, NY, USA) were used for all statistical analyses. Data is reported as the mean ± the standard error of the mean (SEM). A p < 0.05 was considered significant.
Fig 2.
Relative cell abundance following quercetin A) and Q3G B) treatment of EP and SEN from three different donors. Cell proliferation was measured 48 hours after treatment, and was compared against the baseline count of cells frozen just prior to beginning treatment (t0). Values significantly lower than the t0 are indicated by asterisks. *p<0.05 vs. baseline (N = 16 wells/group, per donor), Kruskal-Wallis and Mann-Whitney U tests.
Fig 3.
Live-Dead assay for quercetin (A) and Q3G (B) treatment of EP and SEN cells from the same three different donors. Data reflect the percentage of dead cells in response to different concentrations of quercetin or Q3G. N ≥ 6 wells/group, per donor. Approximately 1000 non-fragmented cells were scored for each N. *p<0.05 vs. baseline value. One-way ANOVA was used for Q3G data, which are normally distributed. Kruskal-Wallis and Mann-Whitney U tests were used for quercetin treatment to account for non-normality.
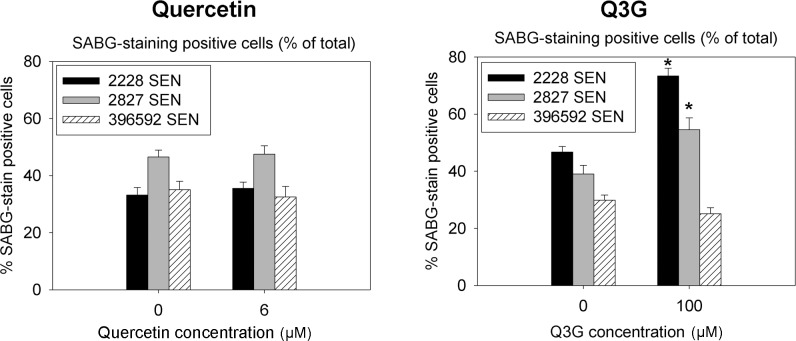
Fig 4. Manual quantification of SABG stain-positive cells.
SEN cells from the same three donors were treated with 6 μM quercetin and 100 μM Q3G followed by SABG staining. Manual counting was done to assess percent of SABG positive cells. *p<0.05 vs. baseline value (N = 12 wells/group, per donor, where each N ranged from 40 to 160 cells). T-test was used to determine statistical significance between control and test groups for each donor.
Results
For all experiments, HCAEC isolated from three different female donors were used to account for individual genetic variations, and only the data points that were positive for all three donors were considered relevant. For example, if only two of the three donors showed toxicity at a certain concentration of quercetin or Q3G, then this concentration was not considered damaging.
Establishment of EP and SEN cells
Similar numbers of passages (10 to 13) were required to reach senescence with characteristic morphologic changes and expression of SABG (Fig 1B), as well as decreased expression of Lamin B1 (Fig 1C), for the three donors. For each donor, the passage number for onset of senescence was highly reproducible. The experimental time line is summarized in Fig 1A. A small subset of cells was still proliferating at this stage, as indicated by the cell proliferation assay, discussed below (Fig 2), but the overwhelming majority of the cells showed the characteristic morphological changes associated with cellular senescence, including the enlargement and flattening of the cells.
Anti-proliferative effect of quercetin, but not Q3G, in EP and SEN cells
The proliferation of ECs was assessed following treatment with either quercetin or Q3G (Fig 2). The cell counts for both EP and SEN cells were increased in the respective 0 μM (vehicle only) groups compared to baseline values at Day 0 ("t0"), reflecting their natural proliferation over 48 hours (Fig 2A). Cell counts decreased with the increasing concentration of quercetin, consistent with a dose-dependent anti-proliferative effect, which has been previously reported in various cell types [2]. However, when the cell counts dropped below the initial baseline value, this suggested that cell death had occurred. At 6 μM quercetin, SEN cell numbers for all three donors were significantly lower than their respective baseline values (p ≤ 0.004). EP cells did not manifest quercetin toxicity until 10 μM, consistent with SEN cells' increased sensitivity to quercetin's cytotoxicity. In contrast, Q3G did not reduce the cell proliferation even at 100 μM, except for SEN cells from one donor, where a small, but significant dip in cell counts occurred (Fig 2B).
Quercetin toxicity to EP and SEN EC
The Live-Dead assay was performed with flow cytometry to directly assess cell death with quercetin/Q3G treatment (S1A and S1B Fig; Fig 3). This assay revealed quercetin-mediated toxicity in EP cells at a lower concentration than our previously determined threshold of 10 μM (Fig 2A): EP cells from all three donors had a significant increase in cell death when treated with 6 or more μM of quercetin (Fig 3A). Thus, the EP cells' apparent resistance to quercetin-induced cell death exhibited in the cell proliferation assay (Fig 2) was likely due the lost cells being replaced by proliferation, thereby masking quercetin's toxicity. As for SEN cells, results similar to EP cells were observed (Fig 3), where all three donors also had significantly elevated cell death beginning at 6 μM, suggesting non-specific toxicity of quercetin. Q3G did not affect cell death at any concentration in EP or SEN cells (Fig 3B). Thus, the Live-Dead assay clearly demonstrates an increase in cell death in EP cells, as well as SEN cells, rather than selective culling of SEN cells.
Quercetin and Q3G treatment on the prevalence of senescent cells
To directly assess whether senescent cells treated with quercetin or Q3G decreased in number, we used direct counting of SABG stain-positive cells by researchers blinded to treatment groups (Fig 4). SEN cells were treated with 6 μM quercetin based on the cell proliferation data, which had indicated that this concentration was nontoxic for EP cells, but reduced the number of SEN cells (Fig 2). For Q3G, 100 μM was used to determine if it was capable of reducing the number of senescent cells. The SEN cells treated with 6 μM quercetin showed no reduction in SABG stain-positive, senescent cells (Fig 4). Paradoxically, we found an increase in the population of senescent cells for two of the three donors treated with 100 μM Q3G, but as not all donors showed the same response, we deemed this inconclusive. Thus, neither quercetin nor Q3G reduced the prevalence of senescent cells.
Discussion
Previously, quercetin was reported to be a senolytic in irradiation-induced senescent HUVECs [14]. HUVECs are derived from the umbilical cord of newborn babies, and for a long time were the only model of primary human EC; however, these cells are not the best model of diseases associated with human arterial aging. HUVECs have been shown to differ substantially from primary endothelial cells derived from adult human vasculature [18–21]. In the current study, we investigated whether quercetin is a senolytic in adult EC, and evaluated whether Q3G would be a more selective senolytic. Our key findings are that quercetin at a concentration that reduced SEN EC also caused significant EP EC cell death (Fig 3), and that there was no evidence of senescent cell-specific cell death mediated by quercetin (Fig 4). Thus, quercetin is not a selective senolytic in adult human arterial endothelial cells, where both EP and SEN cells responded similarly to quercetin's toxicity. In contrast, Q3G had no significant cytotoxicity at all (Figs 2B, 3B and 4).
In this study, we performed three different assays to evaluate quercetin and Q3G. Our SEN cells are a mixture of senescent cells positive for SABG staining (25 to 45%) and near-senescent cells, which are beginning to show the characteristic morphological changes related to cellular senescence, but are SABG stain-negative and slowly proliferate (Figs 1, 2 and 4). As cellular senescence induced by replication, which is a physiologically relevant stress, and ionizing radiation, have similar, but nevertheless distinct phenotypes [30], we think this is a more representative model of aging tissue.
SEN cells were more sensitive to quercetin treatment than EP ECs. EP cells did not begin to show cytotoxicity until 10 μM quercetin, which differed substantially from the previous work with HUVECs, where proliferating cells tolerated up to 20 μM quercetin [14]. HCAECs' increased sensitivity to quercetin's cytotoxicity compared to HUVECs is consistent with previous studies that reported HUVECs' disparate responses compared to adult EC [18, 20].
To determine if quercetin's toxicity in EP cells was masked by their proliferation, we directly examined the number of dead cells. Quercetin has been reported to be capable of inducing both necrosis and apoptosis. [31] The Live-Dead assay, which captures both types of cell death, showed that EP cell death was increased (Fig 3) at a concentration that appeared to be safe based on the cell proliferation data (Fig 2). Importantly, both EP and SEN cells exhibited signs of increased cell death at the same quercetin concentration. Thus, quercetin toxicity was not selective.
In the Live-Dead assay, we evaluated only non-fragmented whole cells by flow cytometry and thus may have undercounted cell death. Therefore, we manually counted senescent cells treated with quercetin or Q3G (Fig 4) to determine if a selective decrease of senescent cells occurred in the SEN cell mixed population. This manual count showed that neither quercetin nor Q3G selectively decreased senescent cells.
Quercetin and the heat shock response
An important detrimental property of quercetin is inhibition of the heat shock response (HSR) [32]. The HSR is an important cytoprotective cellular response conferred by the induction of heat shock proteins (HSPs). The primary role of HSR is to mitigate proteotoxic damage stemming from a wide range of stresses, including heat, radiation, inflammation, ischemia/reperfusion injury, stretch, and reactive oxygen species [33–36]. However, the HSR is blunted with aging, as lower activity of HSF1, the primary transcription factor for the HSR and HSP expression, and HSF1-mediated HSR has been observed in senescent cells [37, 38], in the aging heart [28] and in aging humans [39, 40]. Given quercetin's direct effect on HSF1, which downregulates both its level and activation [32], treating older individuals, who would already have an impaired HSR, with quercetin either through high dose dietary supplements or intravenous administration as an anti-senescence treatment may have a significant downside, including accumulation of misfolded proteins [41].
Senolytics and aging
The first study demonstrating the physiological benefits of selective removal of senescent cells utilized transgenic progeroid mice with a novel, inducible transgene, which would eliminate p16Ink4a-positive, senescent cells [42]. This selective removal of senescent cells was able to prevent key age-related dysfunctions in the mice, including lordokyphosis, muscle atrophy, and cataract development [42].
The first clinically relevant senolytics for humans were discussed in a study published in 2015: quercetin and dasatinib [14]. Since then, several additional senolytic agents have been reported [43–45], and the development of this class of drugs is ongoing. Recently, geldanamycin and tanespimycin, the inhibitors of heat shock protein 90 (HSP90), were identified as senolytics [45]. Inhibiting HSP90 leads to the activation of HSF1 and upregulation of HSPs [46–48], which is the opposite effect of quercetin. However, it is unclear if activation of HSF1 is necessary for the senolytic activity of geldanamycin and tanespimycin.
Q3G as a selective alternative to quercetin
To circumvent quercetin's toxicity on healthy, non-senescent cells, we investigated Q3G, a derivative of quercetin with limited toxicity to endothelial cells, which is processed by SABG enriched in senescent cells to release quercetin in situ [10–13]. Q3G could act as a selective prodrug in senescent cells. However, Q3G had no significant toxicity to either EP or SEN EC. The ability of Q3G to cross the cell membrane despite its large polar structure has been demonstrated by its in vitro bioactivity in treated cells [49], and Q3G has been found to be able to release quercetin through the activity of exogenous beta-galactosidase [11]. The lack of Q3G's toxicity in the current study may be due to Q3G being unable to enter the beta-galactosidase-rich lysosomes [16], or alternatively, Q3G being able to translocate to the lysosomes to release quercetin, which is further processed into an inert compound.
In conclusion, our results demonstrate that neither quercetin nor Q3G is effective as a senolytic for adult EC in vitro. The difference in the findings between our work and the results of Zhu et al. [14] may reflect the important differences between HUVECs and mature adult EC, as well as different models of senescence–radiation vs. replicative senescence, which is a more physiological model of aging. Given the disparity in the sources and phenotypes of the cells, HCAECs may be a more relevant model for studying senolytics than HUVECs.
Supporting information
–A) Representative scatter plots for flow cytometry with different concentrations of quercetin treatment. B) Representative scatter plots for flow cytometry with different concentrations of Q3G treatment. For both panels, the number at the lower left in the plot indicates the percentage of events within the gate compared to the entire events shown in the plot.
(TIF)
(XLS)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by a Merit Award (5101BX000839) from the U.S. Department of Veterans’ Affairs, Office of Research and Development, Biomedical Laboratory Research Program (AAK), https://www.research.va.gov/. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Disclaimer – The contents reported do not represent the views of the Department of Veterans Affairs or the United States Government.
References
- 1.D'Andrea G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia. 2015;106:256–71. 10.1016/j.fitote.2015.09.018. 10.1016/j.fitote.2015.09.018 [DOI] [PubMed] [Google Scholar]
- 2.Scambia G, Ranelletti F, Panici PB, Piantelli M, Bonanno G, De Vincenzo R, et al. Inhibitory effect of quercetin on OVCA 433 cells and presence of type II oestrogen binding sites in primary ovarian tumours and cultured cells. British journal of cancer. 1990;62(6):942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wright B, Moraes LA, Kemp CF, Mullen W, Crozier A, Lovegrove JA, et al. A structural basis for the inhibition of collagen-stimulated platelet function by quercetin and structurally related flavonoids. British journal of pharmacology. 2010;159(6):1312–25. Epub 2010/02/13. 10.1111/j.1476-5381.2009.00632.x ; PubMed Central PMCID: PMCPmc2848935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boesch-Saadatmandi C, Loboda A, Wagner AE, Stachurska A, Jozkowicz A, Dulak J, et al. Effect of quercetin and its metabolites isorhamnetin and quercetin-3-glucuronide on inflammatory gene expression: role of miR-155. J Nutr Biochem. 2011;22(3):293–9. Epub 2010/06/29. 10.1016/j.jnutbio.2010.02.008 . [DOI] [PubMed] [Google Scholar]
- 5.Boots AW, Haenen GRMM, Bast A. Health effects of quercetin: From antioxidant to nutraceutical. European Journal of Pharmacology. 2008;585(2–3):325–37. 10.1016/j.ejphar.2008.03.008. 10.1016/j.ejphar.2008.03.008 [DOI] [PubMed] [Google Scholar]
- 6.Dabbagh-Bazarbachi H, Clergeaud G, Quesada IM, Ortiz M, O’Sullivan CK, Fernández-Larrea JB. Zinc ionophore activity of quercetin and epigallocatechin-gallate: from Hepa 1–6 cells to a liposome model. Journal of Agricultural and Food Chemistry. 2014;62(32):8085–93. 10.1021/jf5014633 [DOI] [PubMed] [Google Scholar]
- 7.Matsuo M, Sasaki N, Saga K, Kaneko T. Cytotoxicity of flavonoids toward cultured normal human cells. Biological & pharmaceutical bulletin. 2005;28(2):253–9. Epub 2005/02/03. . [DOI] [PubMed] [Google Scholar]
- 8.Lee J-C, Kim J, Park J-K, Chung G-H, Jang Y-S. The antioxidant, rather than prooxidant, activities of quercetin on normal cells:: quercetin protects mouse thymocytes from glucose oxidase-mediated apoptosis. Experimental Cell Research. 2003;291(2):386–97. 10.1016/S0014-4827(03)00410-5. [DOI] [PubMed] [Google Scholar]
- 9.Harwood M, Danielewska-Nikiel B, Borzelleca JF, Flamm GW, Williams GM, Lines TC. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food and Chemical Toxicology. 2007;45(11):2179–205. 10.1016/j.fct.2007.05.015. 10.1016/j.fct.2007.05.015 [DOI] [PubMed] [Google Scholar]
- 10.Liu Z, Tao X, Zhang C, Lu Y, Wei D. Protective effects of hyperoside (quercetin-3-o-galactoside) to PC12 cells against cytotoxicity induced by hydrogen peroxide and tert-butyl hydroperoxide. Biomedicine & Pharmacotherapy. 2005;59(9):481–90. 10.1016/j.biopha.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Boukes GJ, van de Venter M. The apoptotic and autophagic properties of two natural occurring prodrugs, hyperoside and hypoxoside, against pancreatic cancer cell lines. Biomedicine & Pharmacotherapy. 2016;83:617–26. 10.1016/j.biopha.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 12.Bernatoniene J, Masteikova R, Majiene D, Savickas A, Kevelaitis E, Bernatoniene R, et al. Free radical-scavenging activities of Crataegus monogyna extracts. Medicina (Kaunas, Lithuania). 2008;44(9):706–12. Epub 2008/10/31. . [PubMed] [Google Scholar]
- 13.Li Z-l, Liu J-c, Hu J, Li X-q, Wang S-w, Yi D-h, et al. Protective effects of hyperoside against human umbilical vein endothelial cell damage induced by hydrogen peroxide. Journal of Ethnopharmacology. 2012;139(2):388–94. 10.1016/j.jep.2011.11.020. 10.1016/j.jep.2011.11.020 [DOI] [PubMed] [Google Scholar]
- 14.Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging cell. 2015;14(4):644–58. 10.1111/acel.12344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campisi J. Aging, cellular senescence, and cancer. Annual review of physiology. 2013;75:685–705. Epub 2012/11/13. 10.1146/annurev-physiol-030212-183653 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee BY, Han JA, Im JS, Morrone A, Johung K, Goodwin EC, et al. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell. 2006;5(2):187–95. Epub 2006/04/22. 10.1111/j.1474-9726.2006.00199.x . [DOI] [PubMed] [Google Scholar]
- 17.Jeyapalan JC, Sedivy JM. Cellular senescence and organismal aging. Mech Ageing Dev. 2008;129(7–8):467–74. Epub 2008/05/27. 10.1016/j.mad.2008.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan PH, Chan C, Xue SA, Dong R, Ananthesayanan B, Manunta M, et al. Phenotypic and functional differences between human saphenous vein (HSVEC) and umbilical vein (HUVEC) endothelial cells. Atherosclerosis. 2004;173(2):171–83. 10.1016/j.atherosclerosis.2003.12.011. 10.1016/j.atherosclerosis.2003.12.011 [DOI] [PubMed] [Google Scholar]
- 19.McCall MN, Kent OA, Yu J, Fox-Talbot K, Zaiman AL, Halushka MK. MicroRNA profiling of diverse endothelial cell types. BMC medical genomics. 2011;4(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajavashisth T, Andalibi A, Territo M, Berliner J, Navab M. Induction of endothelial cell expression of granulocyte and macrophage colony-stimulating factors by modified low-density lipoproteins. Nature. 1990;344(6263):254 10.1038/344254a0 [DOI] [PubMed] [Google Scholar]
- 21.Malavolta M, Costarelli L, Giacconi R, Basso A, Piacenza F, Pierpaoli E, et al. Changes in Zn homeostasis during long term culture of primary endothelial cells and effects of Zn on endothelial cell senescence. Experimental Gerontology. 2017;99(Supplement C):35–45. 10.1016/j.exger.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Heron M. Deaths: leading causes for 2014. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2016;65(5):1–96. Epub 2016/07/06. . [PubMed] [Google Scholar]
- 23.Freund A, Laberge R-M, Demaria M, Campisi J. Lamin B1 loss is a senescence-associated biomarker. Molecular biology of the cell. 2012;23(11):2066–75. 10.1091/mbc.E11-10-0884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bancirova M. Changes of the quercetin absorption spectra in dependence on solvent. Chemistry Journal. 2015;1:31–4. [Google Scholar]
- 25.Abcam. Abcam; [cited 2017 August 4]. Available from: http://www.abcam.com/quercetin-ab120247.html.
- 26.Samuel T, Fadlalla K, Turner T, Yehualaeshet TE. The flavonoid quercetin transiently inhibits the activity of taxol and nocodazole through interference with the cell cycle. Nutrition and cancer. 2010;62(8):1025–35. 10.1080/01635581.2010.492087. PMC3021775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duo J, Ying GG, Wang GW, Zhang L. Quercetin inhibits human breast cancer cell proliferation and induces apoptosis via Bcl-2 and Bax regulation. Molecular medicine reports. 2012;5(6):1453–6. Epub 2012/03/27. 10.3892/mmr.2012.845 . [DOI] [PubMed] [Google Scholar]
- 28.Stice JP, Chen L, Kim SC, Jung JS, Tran AL, Liu TT, et al. 17beta-estradiol, aging, inflammation, and the stress response in the female heart. Endocrinology. 2011;152(4):1589–98. Epub 2011/02/10. 10.1210/en.2010-0627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Debacq-Chainiaux F, Erusalimsky JD, Campisi J, Toussaint O. Protocols to detect senescence-associated beta-galactosidase (SA-[beta]gal) activity, a biomarker of senescent cells in culture and in vivo. Nat Protocols. 2009;4(12):1798–806. 10.1038/nprot.2009.191 [DOI] [PubMed] [Google Scholar]
- 30.Webley K, Bond JA, Jones CJ, Blaydes JP, Craig A, Hupp T, et al. Posttranslational modifications of p53 in replicative senescence overlapping but distinct from those induced by DNA damage. Molecular and cellular biology. 2000;20(8):2803–8. PMC85496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Avci CB, Yilmaz S, Dogan ZO, Saydam G, Dodurga Y, Ekiz HA, et al. Quercetin-induced apoptosis involves increased hTERT enzyme activity of leukemic cells. Hematology. 2011;16(5):303–7. 10.1179/102453311X13085644680104 [DOI] [PubMed] [Google Scholar]
- 32.Nagai N, Nakai A, Nagata K. Quercetin suppresses heat shock response by down-regulation of HSF1. Biochemical and Biophysical Research Communications. 1995;208(3):1099–105. 10.1006/bbrc.1995.1447. 10.1006/bbrc.1995.1447 [DOI] [PubMed] [Google Scholar]
- 33.Dai C, Whitesell L, Rogers AB, Lindquist S. Heat Shock Factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell. 2007;130(6):1005–18. Epub 2007/09/25. 10.1016/j.cell.2007.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishizawa J, Nakai A, Matsuda K, Komeda M, Ban T, Nagata K. Reactive oxygen species play an important role in the activation of Heat Shock Factor 1 in ischemic-reperfused heart. Circulation. 1999;99(7):934–41. 10.1161/01.cir.99.7.934 [DOI] [PubMed] [Google Scholar]
- 35.Wang J, He H, Yu L, Xia HH-x, Lin MC, Gu Q, et al. HSF1 down-regulates XAF1 through transcriptional regulation. Journal of Biological Chemistry. 2006;281(5):2451–9. 10.1074/jbc.M505890200 [DOI] [PubMed] [Google Scholar]
- 36.Fulda S, Gorman AM, Hori O, Samali A. Cellular stress responses: cell survival and cell death. International Journal of Cell Biology. 2010;2010:23 10.1155/2010/214074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim G, Meriin AB, Gabai VL, Christians E, Benjamin I, Wilson A, et al. The heat shock transcription factor Hsf1 is downregulated in DNA damage–associated senescence, contributing to the maintenance of senescence phenotype. Aging cell. 2012;11(4):617–27. 10.1111/j.1474-9726.2012.00827.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luce MC, Cristofalo VJ. Reduction in heat shock gene expression correlates with increased thermosensitivity in senescent human fibroblasts. Experimental Cell Research. 1992;202(1):9–16. 10.1016/0014-4827(92)90398-R. [DOI] [PubMed] [Google Scholar]
- 39.Gutsmann-Conrad A, Heydari AR, You S, Richardson A. The expression of Heat Shock Protein 70 decreases with cellular senescence in vitro and in cells derived from young and old human subjects. Experimental Cell Research. 1998;241(2):404–13. 10.1006/excr.1998.4069. 10.1006/excr.1998.4069 [DOI] [PubMed] [Google Scholar]
- 40.Anckar J, Sistonen L. Regulation of HSF1 function in the heat stress response: implications in aging and disease. Annual review of biochemistry. 2011;80:1089–115. Epub 2011/03/23. 10.1146/annurev-biochem-060809-095203 . [DOI] [PubMed] [Google Scholar]
- 41.Shringarpure R, Davies KJA. Protein turnover by the proteasome in aging and disease. Free Radical Biology and Medicine. 2002;32(11):1084–9. 10.1016/S0891-5849(02)00824-9. [DOI] [PubMed] [Google Scholar]
- 42.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479(7372):232–6. http://www.nature.com/nature/journal/v479/n7372/abs/nature10600.html#supplementary-information. 10.1038/nature10600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu Y, Tchkonia T, Fuhrmann‐Stroissnigg H, Dai HM, Ling YY, Stout MB, et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl‐2 family of anti‐apoptotic factors. Aging Cell. 2016;15(3):428–35. 10.1111/acel.12445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Chang J, Liu X, Zhang X, Zhang S, Zhang X, et al. Discovery of piperlongumine as a potential novel lead for the development of senolytic agents. Aging (Albany NY). 2016;8(11):2915–26. 10.18632/aging.101100. PMC5191878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fuhrmann-Stroissnigg H, Ling YY, Zhao J, McGowan SJ, Zhu Y, Brooks RW, et al. Identification of HSP90 inhibitors as a novel class of senolytics. Nature Communications. 2017;8:422 10.1038/s41467-017-00314-z. PMC5583353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Powers MV, Valenti M, Miranda S, Maloney A, Eccles SA, Thomas G, et al. Mode of cell death induced by the HSP90 inhibitor 17-AAG (tanespimycin) is dependent on the expression of pro-apoptotic BAX. Oncotarget. 2013;4(11):1963–75. PMC3875762. 10.18632/oncotarget.1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim HR, Kang HS, Kim HD. Geldanamycin induces heat shock protein expression through activation of HSF1 in K562 erythroleukemic cells. IUBMB Life. 1999;48(4):429–33. 10.1080/713803536 [DOI] [PubMed] [Google Scholar]
- 48.Conde R, Belak ZR, Nair M, O’Carroll RF, Ovsenek N. Modulation of Hsf1 activity by novobiocin and geldanamycin. Biochemistry and Cell Biology. 2009;87(6):845–51. 10.1139/o09-049 [DOI] [PubMed] [Google Scholar]
- 49.Wang L, Yue Z, Guo M, Fang L, Bai L, Li X, et al. Dietary flavonoid hyperoside induces apoptosis of activated human LX-2 hepatic stellate cell by suppressing canonical NF-κB signaling. BioMed Research International. 2016;2016:1068528 10.1155/2016/1068528. PMC4826685. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
–A) Representative scatter plots for flow cytometry with different concentrations of quercetin treatment. B) Representative scatter plots for flow cytometry with different concentrations of Q3G treatment. For both panels, the number at the lower left in the plot indicates the percentage of events within the gate compared to the entire events shown in the plot.
(TIF)
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.