Abstract
Hepatic Veno-Occlusive Disease (VOD) is a potentially severe complication of hematopoietic stem cell transplantation (HSCT). Here we report two patients receiving an allogeneic HSCT who developed late onset VOD with atypical clinical features. The two patients presented with only few risk factors, namely, advanced acute leukemia, a myeloablative busulphan-containing regimen and received grafts from an unrelated donor. The first patient did not experience painful hepatomegaly and weight gain and both patients showed only a mild elevation in total serum bilirubin level. Most importantly, the two patients developed clinical signs beyond day 21 post-HSCT. Hepatic transjugular biopsy confirmed the diagnosis of VOD. Intravenous defibrotide was promptly started leading to a marked clinical improvement. Based on our experience, liver biopsy may represent a useful diagnostic tool when the clinical features of VOD are ambiguous. Early therapeutic intervention with defibrotide represents a crucial issue for the successful outcome of patients with VOD.
Keywords: Leukemia, Allogeneic Hematopietic stem cell transplantation, Veno-occlusive disease, VOD
Introduction
Veno-occlusive disease (VOD), also known as sinusoidal obstruction syndrome (SOS), is a potentially life-threatening complication of hematopoietic stem cell transplantation (HSCT).1 The diagnosis of VOD is primarily based on clinical criteria defined almost 20 years ago, including the triad of painful hepatomegaly, jaundice and fluid retention.2–4 This observation could at least partially explain the highly variable incidence of VOD reported in the literature, ranging from 8% to 14%. VOD usually develops within 20–30 days after HSCT. However, few cases of late-onset VOD have been reported.5 According to this observation, the European Group for Bone marrow Transplantation (EBMT) recently endorsed the revised diagnostic criteria for VOD/SOS, which now include either a classical form of VOD and a late-onset variant (Table 1).6
Table 1.
Criteria for definition of Late-Onset VOD (according to “The new classification from the European Society for Blood and Marrow Transplantation”, BMT 2016).6
| Late Onset VOD (> 21 Days after HSCT) |
|---|
Classical VOD/SOS beyond day 21:
|
| OR Histologically proven VOD/SOS |
| OR Two or more of the following criteria:
|
Abbreviations: EBMT =European Society for Blood and Marrow Transplantation; SOS =sinusoidal obstruction syndrome; VOD =veno-occlusive disease. These symptoms/signs should not be attributable to other causes.
Here we describe two HSCT recipients who developed late-onset VOD with atypical clinical features.
Case 1
A 55-year-old male was diagnosed with acute myeloid leukemia in May 2015. He failed to achieve the complete remission (CR) after two induction chemotherapy courses with high dose cytarabine, idarubicin and etoposide and salvage treatment with fludarabine and idarubicin. The presence of a matched unrelated donor in the International Marrow Donor Registries prompted us to proceed with an allogeneic HSCT following a “sequential” conditioning regimen. The patient was initially treated with mitoxantrone (6 mg/sqm/day), etoposide (80 mg/sqm/day) and cytarabine (1 g/sqm/day for four days), followed, 10 days later, by a conditioning which included i.v. Busulphan (3.2 mg/kg/day) and Fludarabine (50 mg/mq/day) for four days and the infusion of mobilized donor peripheral blood stem cells (PBSC). Graft-vs.-Host disease (GVHD) prophylaxis consisted of anti-thymocyte globulin, cyclosporine and a short course of methotrexate. An absolute neutrophil count higher than 0.5 × 109/L and a platelet count higher than 20.000/mcl were achieved on day + 13. On day + 33, the patient suddenly showed abdominal distension with ascites, increase in liver enzymes (AST 391 U/l, ALT 245 U/l) and in total bilirubin (1.2 mg/dL) and signs of liver and renal insufficiency (INR 1.43; aPTT 65.5″; serum creatinine value 3.04 mg/dL). The patient did not present either painful hepatomegaly or weight gain >2%, or signs of intestinal or cutaneous acute GVHD. Viral hepatitis was ruled out by microbiological testing. Ultrasonography showed normal liver parenchyma, regular biliary tract, moderate splenomegaly (15 cm) with ascites and right pleural effusion. Doppler exam ruled out portal vein thrombosis but showed increased portal vein diameter (10 mm) suggestive of portal hypertension. Paracentesis was performed and showed presence of transudate fluid (serum albumin = 3 mg/dL, ascites albumin 1.5 mg/dL, serum-ascites albumin ratio= 2).
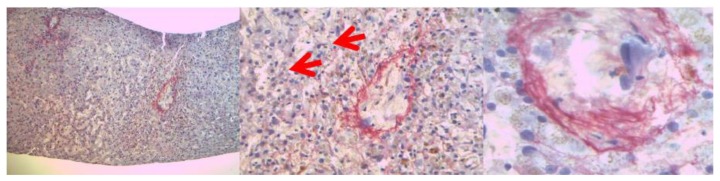
Given that clinical symptoms and laboratory tests did not allow to discriminate between VOD, acute GVHD, toxicity or infective causes, a hepatic transjugular biopsy was performed. Histology studies showed the expansion of the hepatic sinusoid spaces, with gaps in the sinusoidal barrier which were highly suggestive of hepatic VOD in the light of the involvement of zone 3, zone 2 and partially zone 1 of the hepatic acinus (Figure 1).
Figure 1.
Images of hepatic transjugular biopsy: in the middle, the red arrows showed the expansion of hepatic sinusoid spaces, on the right the figure showed a centrilobular vein.
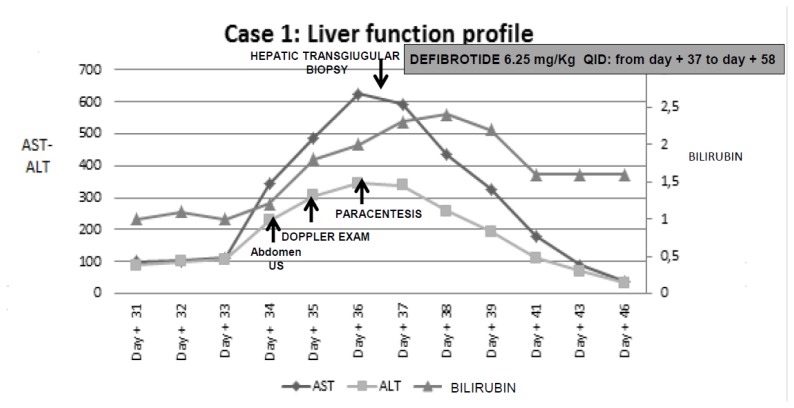
Intravenous defibrotide was started at the dose of 6.25 mg/kg QID on day + 37 for 21 days, along with ancillary therapy including albumin replacement and low dose diuretics; nonsteroids have been administered. Two-three days after the beginning of defibrotide, the patient showed a marked clinical improvement with gradual improvement and normalization of liver and renal function tests (Figure 2). One week after the beginning of defibrotide, the patient developed hemorrhagic cystitis, treated with 2 bladder instillations of hyaluronic acid which led to progressive improvement and complete resolution upon regular discontinuation of defibrotide after 21 days of treatment. Hemorrhagic cystitis did not require an earlier discontinuation of defibrotide. During the treatment course, platelet count remained low between 10.000 to 20.000/mmc even with transfusion support. The patient was discharged on day + 76 in complete remission of his underlying disease on low doses of cyclosporine.
Figure 2.
Diagnostic interventions with liver function profile from clinical onset of VOD until resolution, and treatment of VOD in case 1.
Case 2
A 46-year old male was diagnosed with acute myeloid leukemia - normal karyotype, FLT3, and NPM1 wild-type - in May 2015. The patient was treated with idarubicin plus etoposide and cytarabine with no hematologic response. Complete remission was subsequently obtained with a course of high-dose cytarabine, followed consolidation with 2 additional courses of high-dose cytarabine. An unrelated marrow donor search was started and a partially (one-antigen mismatched) HLA-matched donor was identified. The patient received mobilized donor HSCT after a conditioning regimen with Thiotepa (5 mg/kg/day for 2 days), Busulphan (3,2 mg/kg/day for 3 days) and Fludarabine (50 mg/kg/day for 3 days). GvHD prophylaxis included cyclosporine, short course methotrexate, and anti-thymocyte globulin ATG (2,5 mg/kg/day for 3 days). Neutrophil and platelet engraftment occurred on day +14 and +12 respectively. The patient experienced a transient skin rash suggestive of grade I acute GVHD on day +19, and 3 episodes of CMV reactivation on days +27,+ 43 and + 82 successfully treated with preemptive valganciclovir and immunoglobulins.
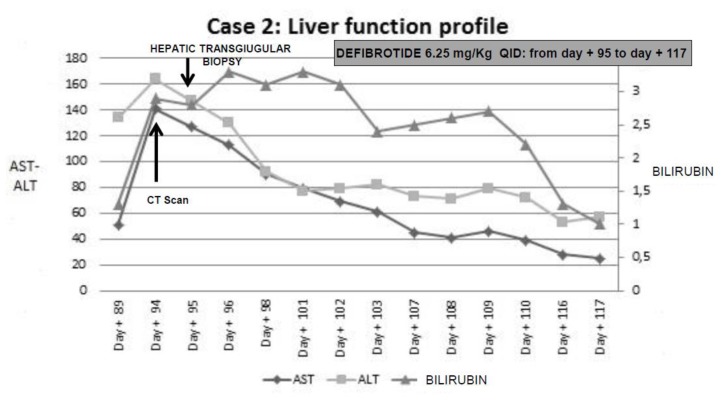
The patient was readmitted because of severe anemia and thrombocytopenia (Hb 5,8 gr/dl; platelet 5000/ul) and complaints of right abdominal pain with melena on day +89. Significant weight gain (+7 kg) along with abdominal distension and anasarca were observed on day +91. Laboratory exams showed total bilirubin 3,30 mg/dl, AST/ALT 140/164 UI, GGT 725 Ul, INR 1,7; aPTT 41,3″, serum creatinine 2,0 mg/dl; platelet count was 20.000/mmc. An abdominal CT scan revealed ascites and hepatic veins compression. Transjugular measurement of the hepatic venous pressure gradient showed severe sinusoidal portal hypertension with a significant transhepatic/caval gradient diagnostic for severe VOD. Transjugular liver biopsy showed sinusoidal dilation and bleeding with erythrocytes in the Disse space, and significant iron overload. Histology studies were consistent with severe VOD.
Intravenous defibrotide was promptly started at the dose of 6.25 mg/kg QID for 22 days. Ancillary therapy included plasma and red blood cells transfusions, no steroid have been administered. A complete and sustained response was achieved. The patient was discharged on day +121. The patient is currently alive, 188 days after transplant, with normal liver function, no evidence of GvHD, or any other relevant clinical complication. A bone marrow aspirate showed complete remission of his underlying disease.
Discussion
Recognition of potential risk factors for VOD is a key point for early diagnosis and prompt therapeutic intervention. Recently, the EBMT group has categorized these risk factors as transplant-, hepatic-, patient- and disease-related.7–9 Interestingly, our patients presented with only a few risk factors, namely, advanced acute leukemia, a myeloablative busulphan-containing conditioning and an unrelated donor. Moreover, both patients did not show the typical clinical VOD features described by the Seattle2–3 and Baltimore4 criteria. In particular, the first patient did not experience either painful hepatomegaly or weight gain, and only a mild elevation in total serum bilirubin level was observed after the development of ascites, while the second patient showed only mild hyper-bilirubinemia concurrent with painful hepatomegaly and significant weight gain. Most importantly, both patients developed clinical signs beyond day 21 post-HSCT (on days + 33 and + 89 respectively). In this respect, it should be emphasized that the EBMT consensus6 has now recognized the existence of a “late onset” VOD, defined with less stringent diagnostic criteria and where hyper-bilirubinemia should no longer be mandatory for diagnosis. Overall, in these two patients, the short time between the onset of clinical symptoms and the final diagnosis, and the higher than 5 fold increase in transaminases combine to diagnose a severe form of VOD by the new EBMT criteria (Table 1)
Both the British guidelines10 and the EBMT recommendations indicate that liver biopsy should be reserved for those patients in whom the diagnosis of VOD is unclear, and there is an urgent need to rule out other possible causes of liver dysfunction. In our experience, a transjugular liver biopsy was safe despite severe thrombocytopenia and, most importantly, was conclusive for the diagnosis of VOD ruling out drug toxicities, viral infections, sepsis or GVHD.11–13 In keeping with our findings, Kis et al. reported only 1.8% of major complications during 166 transjugular liver biopsies.14
Defibrotide is the only agent approved for the treatment of VOD in Europe. Defibrotide has been shown to have antithrombotic and anti-inflammatory properties and may promote revascularization inducing endothelial cell proliferation and angiogenesis.15 In our patients, the combination of clinical features and histology studies prompted us to start defibrotide only a few days after the onset of symptoms. Our experience strengthens the observation reported by Richardson et al.,16 that the timely administration of defibrotide may represent a crucial issue for the successful outcome of patients with VOD. Sixty % of patients were alive when defibrotide was started within 2 days from the onset of symptoms as compared with 14% when treatment was delayed and started after 7 days. Similar results were reported by Corbacioglu et al.17 Our patients began defibrotide treatment within 7 days from the onset of symptoms but within 1 day from the histological diagnosis. They did not fall exactly in the early treatment category. Nevertheless, defibrotide has been initiated within 7 days, representing the crucial threshold to achieve a good outcome. Phase 2 and 3 studies18–22 demonstrated that defibrotide was generally well tolerated with manageable toxicity. Hemorrhagic complications were reported as the most frequent adverse event. Hemorrhagic cystitis, which occurred in one of our patients, is a common complication in HSCT recipients. Though other causes may have been involved, we could not rule out that it may have been related to defibrotide treatment given its prompt resolution upon drug discontinuation.22
Conclusions
VOD should be considered in the differential diagnosis of HSCT recipients who present with unexplained liver injuries, ascites and/or MOF. Liver biopsy may represent a useful diagnostic tool when the clinical criteria for VOD are not entirely fulfilled. Early therapeutic intervention with defibrotide may improve the clinical outcomes of these patients.
Figure 3.
Diagnostic interventions with liver function profile from clinical onset of VOD until resolution, and treatment of VOD in case 2.
Footnotes
Competing interests: The authors have declared that no competing interests exist.
References
- 1.Carreras E, et al. Veno-occlusive disease of the liver after hemopoietic cell transplantation. Eur J Haematol. 2000;64:281–291. doi: 10.1034/j.1600-0609.2000.9r200.x. https://doi.org/10.1034/j.1600-0609.2000.9r200.x. [DOI] [PubMed] [Google Scholar]
- 2.McDonald GB, Hinds MS, Fisher LD, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Annals of Internal Medicine. 1993;118:255–267. doi: 10.7326/0003-4819-118-4-199302150-00003. https://doi.org/10.7326/0003-4819-118-4-199302150-00003. [DOI] [PubMed] [Google Scholar]
- 3.Jones RJ, Lee KS, Beschorner WE, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44:778–783. doi: 10.1097/00007890-198712000-00011. https://doi.org/10.1097/00007890-198712000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Lee JL, Gooley T, Bensinger W, et al. Veno-occlusive disease of the liver after busulfan, melphalan, and thiotepa conditioning therapy: incidence, risk factors, and outcome. Biology of Blood and Marrow Transplantation. 1999;5:306–315. doi: 10.1016/s1083-8791(99)70006-6. https://doi.org/10.1016/S1083-8791(99)70006-6. [DOI] [PubMed] [Google Scholar]
- 5.Coppell JA, Richardson PG, Soiffer R, Martin PL, Kernan NA, Chen A, et al. Hepatic veno-occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biol Blood Marrow Transplant. 2010;16:157–168. doi: 10.1016/j.bbmt.2009.08.024. https://doi.org/10.1016/j.bbmt.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohty M, Malard F, Abecassis M, et al. Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a new classification from the European Society for Blood and Marrow Transplantation. BMT. 2016:1–7. doi: 10.1038/bmt.2016.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bearman SI, et al. The syndrome of hepatic veno-occlusive disease after marrow transplantation. Blood. 1995;85:3005–20. [PubMed] [Google Scholar]
- 8.Carreras E, Bertz H, Arcese W, et al. Incidence and outcome of hepatic veno-occlusive disease after blood or marrow transplantation: A prospective cohort study of the European Group for Blood and Marrow Transplantation: European Group for Blood and Marrow Transplantation Chronic Leukemia Working Party. Blood. 1998;92:3599–604. [PubMed] [Google Scholar]
- 9.Cesaro S, Pillon M, Talenti E, et al. A prospective survey on incidence, risk factors and therapy of hepatic veno-occlusive disease in children after hematopoietic stem cell transplantation. Haematologica. 2005;90:1396–1404. [PubMed] [Google Scholar]
- 10.Dignan FL, Wynn RF, Hadzic N, et al. BCSH/BSBMT guideline: diagnosis and management of veno-occlusive disease (sinusoidal obstruction syndrome) following haematopoietic stem cell transplantation. BJH. 2013;163:444–457. doi: 10.1111/bjh.12558. https://doi.org/10.1111/bjh.12558. [DOI] [PubMed] [Google Scholar]
- 11.DeLeve LD, Shulman HM, McDonald GB. Toxic injury to hepatic sinusoids: sinusoidal obstruction syndrome (veno-occlusive disease) Seminars in Liver Disease. 2002;22:27. doi: 10.1055/s-2002-23204. https://doi.org/10.1055/s-2002-23204. [DOI] [PubMed] [Google Scholar]
- 12.Shulman HM, Gown AM, Nugent DJ. Hepatic veno-occlusive disease after bone marrow transplantation. Immunohistochemical identification of the material within occluded central venules. The American Journal of Pathology. 1987;127:549–558. [PMC free article] [PubMed] [Google Scholar]
- 13.Shulman HM, Fisher LB, Schoch HG, Henne KW, McDonald GB. Veno-occlusive disease of the liver after marrow transplantation: histological correlates of clinical signs and symptoms. Hepatology. 1994;19:1171–1181. https://doi.org/10.1002/hep.1840190515. [PubMed] [Google Scholar]
- 14.Kis b, Pamarthi V, Fan C-M, et al. Safety and Utility of Transjugular Liver Biopsy in Hematopoietic Stem Cell Transplant Recipients. J Vasc Interv Radiol. 2013;24:85–89. doi: 10.1016/j.jvir.2012.09.011. https://doi.org/10.1016/j.jvir.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Richardson PG, Elias AD, Krishnan A, et al. Treatment of severe veno-occlusive disease with defibrotide: compassionate use results in response without significant toxicity in a high-risk population. Blood. 1998;92:737–744. [PubMed] [Google Scholar]
- 16.Richardson PG, Smith AR, Triplett BM, et al. Early Initiation of Defibrotide in Patients with Hepatic Veno-Occlusive Disease/Sinusoidal Obstruction Syndrome Following Hematopoietic Stem Cell Transplantation Improves Day +100 Survival. ASH. 2015 Poster Abs. [Google Scholar]
- 17.Corbacioglu S, Greil J, Peters C, et al. Defibrotide in the treatment of children with veno-occlusive disease (VOD): a retrospective multicentre study demonstrates therapeutic efficacy upon early intervention. BMT. 2004 Jan;33(2):189–95. doi: 10.1038/sj.bmt.1704329. https://doi.org/10.1038/sj.bmt.1704329. [DOI] [PubMed] [Google Scholar]
- 18.Bearman SI, Anderson GL, Mori M, Hinds MS, Shulman HM, McDonald GB. Venoocclusive disease of the liver: development of a model for predicting fatal outcome after marrow transplantation. Journal of Clinical Oncology. 1993;11:1729–1736. doi: 10.1200/JCO.1993.11.9.1729. https://doi.org/10.1200/JCO.1993.11.9.1729. [DOI] [PubMed] [Google Scholar]
- 19.Chopra R, Eaton JD, Grassi A, Potter M, Shaw B, Salat C, et al. Defibrotide for the treatment of hepatic veno-occlusive disease: Results of the European compassionate-use study. Br J Haematol. 2000;111:1122–9. doi: 10.1046/j.1365-2141.2000.02475.x. https://doi.org/10.1046/j.1365-2141.2000.02475.x. [DOI] [PubMed] [Google Scholar]
- 20.Richardson PG, Murakami C, Jin Z, Warren D, Momtaz P, Hoppensteadt D, et al. Multi-institutional use of defibrotide in 88 patients after stem cell transplantation with severe veno-occlusive disease and multisystem organ failure: response without significant toxicity in a high-risk population and factors predictive of outcome. Blood. 2002;100:4337–43. doi: 10.1182/blood-2002-04-1216. https://doi.org/10.1182/blood-2002-04-1216. [DOI] [PubMed] [Google Scholar]
- 21.Richardson PG, Soiffer R, Antin J, Jin Z, Kurtzberg J, Martin P, et al. Defibrotide (DF) for the treatment of severe veno-occlusive disease (sVOD) and multi-organ failure (MOF) post SCT: Final results of a multi-center, randomized, dose-finding trial. Blood. 2006;108:178. [Google Scholar]
- 22.Richardson PG, Riches ML, Kernan NA, et al. Phase 3 trial of defibrotide for the treatment of severe veno-occlusive disease and multi-organ failure. Blood. 2016;127(13):1656–65. doi: 10.1182/blood-2015-10-676924. https://doi.org/10.1182/blood-2015-10-676924. [DOI] [PMC free article] [PubMed] [Google Scholar]