Abstract
Replicative DNA polymerases cannot insert efficiently nucleotides at sites of base lesions. This function is taken over by specialized translesion DNA synthesis (TLS) polymerases to allow DNA replication completion in the presence of DNA damage. In eukaryotes, Rad6- and Rad18-mediated PCNA ubiquitination at lysine 164 promotes recruitment of TLS polymerases, allowing cells to efficiently cope with DNA damage. However, several studies showed that TLS polymerases can be recruited also in the absence of PCNA ubiquitination. We hypothesized that the stability of the interactions between DNA polymerase δ (Pol δ) subunits and/or between Pol δ and PCNA at the primer/template junction is a crucial factor to determine the requirement of PCNA ubiquitination. To test this hypothesis, we used a structural mutant of Pol δ in which the interaction between Pol3 and Pol31 is inhibited. We found that in yeast, rad18Δ-associated UV hypersensitivity is suppressed by pol3-ct, a mutant allele of the POL3 gene that encodes the catalytic subunit of replicative Pol δ. pol3-ct suppressor effect was specifically dependent on the Rev1 and Pol ζ TLS polymerases. This result strongly suggests that TLS polymerases could rely much less on PCNA ubiquitination when Pol δ interaction with PCNA is partially compromised by mutations. In agreement with this model, we found that the pol3-FI allele suppressed rad18Δ-associated UV sensitivity as observed for pol3-ct. This POL3 allele carries mutations within a putative PCNA Interacting Peptide (PIP) motif. We then provided molecular and genetic evidence that this motif could contribute to Pol δ-PCNA interaction indirectly, although it is not a bona fide PIP. Overall, our results suggest that the primary role of PCNA ubiquitination is to allow TLS polymerases to outcompete Pol δ for PCNA access upon DNA damage.
Author summary
Replicative DNA polymerases have the essential role of replicating genomic DNA during the S phase of each cell cycle. DNA replication occurs smoothly and accurately if the DNA to be replicated is undamaged. Conversely, replicative DNA polymerases stall abruptly when they encounter a damaged base on their template. In this case, alternative specialized DNA polymerases are recruited to insert nucleotides at sites of base lesions. However, these translesion polymerases are not processive and they are poorly accurate. Therefore, they need to be tightly regulated. This is achieved by the covalent binding of the small ubiquitin peptide to the polymerase cofactor PCNA that subsequently triggers the recruitment of translesion polymerases at sites of DNA damage. Yet, recruitment of translesion polymerases independently of PCNA ubiquitination also has been documented, although the underlying mechanism is not known. Moreover, this observation makes more difficult to understand the exact role of PCNA ubiquitination. Here, we present strong genetic evidence in Saccharomyces cerevisiae implying that the replicative DNA polymerase δ (Pol δ) prevents the recruitment of the translesion polymerases Pol ζ and Rev1 following UV irradiation unless PCNA is ubiquitinated. Thus, the primary role of PCNA ubiquitination would be to allow translesion polymerases to outcompete Pol δ upon DNA damage. In addition, our results led us to propose that translesion polymerases could be recruited independently of PCNA ubiquitination when Pol δ association with PCNA is challenged, for instance at difficult-to-replicate loci.
Introduction
Despite the remarkable catalytic activities of replicative DNA polymerases, these enzymes cannot efficiently incorporate nucleotides opposite damaged template DNA. Therefore, in eukaryotes, translesion DNA synthesis (TLS) is carried out by specialized, low stringency damage-tolerant polymerases belonging to the Y-family (Pol η, Pol ι, Pol κ, and Rev1) and the B family (Pol ζ) [1]. The inevitable consequence of the TLS polymerases feature to synthesize across DNA lesions with no associated proofreading activity, is their overall reduced fidelity, even at undamaged templates. This could lead to accumulation of unwanted mutations and therefore, their activity needs to be tightly regulated [2,3].
The main mechanism of damage-induced activation of TLS polymerases involves covalent modifications of the sliding clamp PCNA by ubiquitin or SUMO peptides [4–7]. PCNA mono-ubiquitination at the highly conserved lysine (K) 164 by the ubiquitin-conjugating enzyme (E2) Rad6 and the ubiquitin ligase (E3) Rad18 is a prerequisite for the activation of TLS polymerases [4,8,9]. Subsequently, K164 is poly-ubiquitinated via a K63-linked poly-ubiquitin chain, for which Rad5 and the Mms2-Ubc13 complex are additionally required. PCNA poly-ubiquitination then triggers a template switch mechanism [4,10].
The recruitment of DNA replication and repair proteins to DNA by PCNA is often dependent on the presence of a conserved protein-protein interaction motif, the "PCNA-interacting protein" or PIP-box [11]. The PIP-box consensus sequence, Qxx(M/L/I)xxF(Y/F), is well conserved [12–14]. All Y family DNA polymerases interact with PCNA. PIP domains are found in Pol η, Pol ι and Pol κ [15–21], whereas Rev1 interacts with PCNA through an additional motif [22]. In addition, one or two ubiquitin-binding domains (UBDs) were identified in all eukaryotic members of the Y family [23,24]. They are the prototypes of two distinct classes: the ubiquitin-binding zinc finger (UBZ) and the helical ubiquitin-binding motif (UBM). Mutational inactivation of these motifs abolishes TLS in yeast and prevents damage-induced association of the mutated polymerases with PCNA in mammalian cells [22–29].
In vitro biochemical assays showed that ubiquitinated PCNA activates the replicative Pol δ similarly to unmodified PCNA [30,31]. Pol η takes the place of Pol δ at the 3’ extending end only when DNA synthesis by Pol δ is stalled and PCNA is ubiquitinated [32,33]. These observations lend support to an expanded "tool-belt" model [2]: ubiquitin primarily acts as a supplementary interaction module to which TLS polymerases first bind before the switch between polymerases. Support for this model comes from structural studies on ubiquitinated PCNA, the catalytic core of Pol η, its PIP-box bound to PCNA and its UBZ domain in complex with ubiquitin [34–38]. Particularly, it was observed that ubiquitin attachment to PCNA does not alter its conformation. Moreover, the ubiquitin moiety is positioned on the back side of the PCNA ring, presumably far away from its front side where PIP binding sites are localized and where polymerases position themselves and perform DNA synthesis [37,38].
Although the role of PCNA ubiquitination is firmly established, a number of observations indicate that TLS polymerases can be recruited also in the absence of PCNA modification. In yeast, early genetic studies showed that spontaneous mutagenesis in wild type (WT) and in the rad18Δ mutant, in which PCNA cannot be ubiquitinated, depends on Pol ζ and Rev1 [39–41]. More recently, studies using human cell extracts [42,43], mouse embryo fibroblasts [44–46], mouse pre-B cells [45] or DT40 chicken cells [47,48] reported activation of TLS polymerases with unmodified PCNA in various contexts: bypass of site-specific DNA lesions on plasmids, somatic hypermutation on immunoglobulin genes, and UV radiation- or methyl methanesulfonate-induced DNA damage. These results are puzzling because the conditions that allow the recruitment of TLS polymerases independently of PCNA ubiquitination are currently unknown.
The finding that Pol δ and TLS polymerases share common structural features is another challenging issue to understand the preferential recruitment of Pol δ over TLS polymerases. Pol δ from S. cerevisiae has three subunits: Pol3, Pol31 and Pol32. Pol3 is the catalytic subunit that contains the polymerase and the 3’ to 5’ exonuclease active site domains. In addition, Pol3 carries a C-terminal domain (CTD) with eight conserved cysteine residues that is folded distinctly from the catalytic domain. The first set of four cysteines (CysA) resembles a zinc ribbon motif [49] and is crucial for mediating DNA-dependent interactions between PCNA and Pol δ [50]. The second C-terminal set of cysteines (CysB) is an essential Fe-S cluster [50]. Pol31 is the essential structural B subunit of 55 kDa with which Pol3 CTD interacts. Pol32 is a non-essential subunit of 40 kDa (or C subunit) that is tethered solely via interactions with Pol31 [51]. Moreover, similarly to some TLS polymerases, Pol δ carries PIP motifs. A canonical PIP motif lies in the C-terminal end of Pol32 and has been shown to interact with PCNA by two-hybrid analyses [52]. Non-canonical PIP motifs are present in Pol3 and Pol31 and they contribute to PCNA-stimulated DNA synthesis [53]. Thus, the presence of multiple distant PIP motifs on the different Pol δ subunits could provide a positive advantage for the access to PCNA compared with monomeric TLS polymerases that carry one or several adjacent PIP motifs [20,21].
More surprisingly, Pol δ and Pol ζ share the same B and C subunits (Pol31 and Pol32 in yeast; P50 and P66 in mammals) [54–56]. This raises the possibility that Pol δ preferential access to unmodified PCNA is determined mostly via its catalytic subunit. It has been proposed that in yeast, the Rev3/Rev7 catalytic complex of Pol ζ replaces Pol3 on the Pol31/Pol32 platform to allow the polymerase switch from Pol δ to Pol ζ upon DNA damage [54]. In this scenario, PCNA ubiquitination could trigger the specific poly-ubiquitination of Pol3 by Def1 and its subsequent proteosomal degradation [57].
Given these structural similarities between Pol δ and TLS polymerases, we hypothesized that destabilization of the interaction between Pol δ subunits and/or between Pol δ and PCNA could modify the regulation of replicative and TLS polymerase loading on PCNA. To this aim, we used a mutant allele of POL3 (pol3-ct). First we showed that in budding yeast, this mutant leads to an increase of UV resistance in rad18Δ cells. We then demonstrated that the suppression of the rad18Δ phenotype by pol3-ct occurs via Pol ζ and Rev1, although PCNA is not ubiquitinated. Moreover, mutational inactivation of Pol3 non-canonical PIP motif in the rad18Δ mutant led also to a robust increase in UV resistance. This suggests that a partial loss of the Pol δ-PCNA interaction is responsible for the increased UV resistance, when PCNA is not ubiquitinated. Using isothermal titration calorimetry (ITC), we found that Pol3 non-canonical PIP motif is probably not a bona fide PIP domain and might participate only indirectly in the PCNA-Pol δ interaction. Overall, our results suggest that the stability of the interaction between PCNA and Pol δ a the primer/template junction is a crucial factor to determine the requirement of PCNA ubiquitination.
Results
pol3-ct suppresses UV hypersensitivity associated with RAD18 inactivation
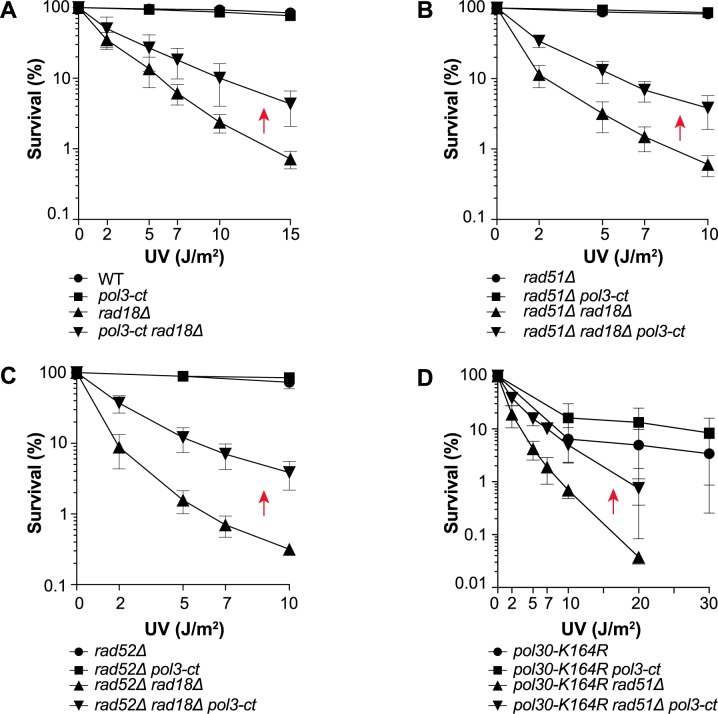
In rad18Δ yeast mutant cells, UV-induced PCNA ubiquitination is abolished and the damage avoidance pathways are inhibited. Indeed, rad18Δ cells displayed UV hypersensitivity compared with WT cells (Fig 1A). Conversely, UV resistance was significantly increased in rad18Δ cells that carried also the pol3-ct allele of POL3 (Fig 1A) in which the substitution of a Leu codon with a stop codon resulted in the loss of the four last C-terminal amino acids (LSKW) of Pol3 [58]. This mutation destabilizes the interaction between Pol3 C-terminal domain and Pol31 [59]. This result suggested that WT Pol δ could contribute to the UV sensitivity associated with RAD18 deletion. To test this hypothesis, we performed a detailed genetic analysis.
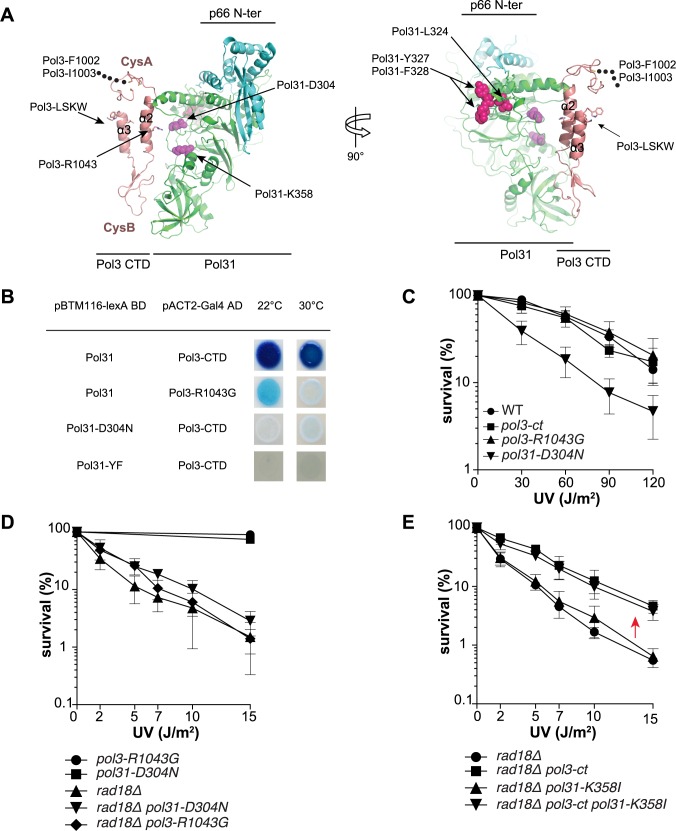
Fig 1. pol3-ct increases UV resistance of yeast cells deficient for PCNA ubiquitination independently of HR.
Survival curves of haploid cells deficient for PCNA ubiquitination after exposure to UV light. Red arrows highlight increased UV resistance in the presence of pol3-ct. (A) Suppression of rad18Δ cells UV hypersensitivity by the pol3-ct allele. (B and C) The increased UV resistance of rad18Δ pol3-ct cells is HR-independent. (D) pol3-ct suppresses UV sensitivity of HR-deficient pol30-K164R cells.
pol3-ct increases UV resistance in PCNA ubiquitination-deficient cells independently of homologous recombination
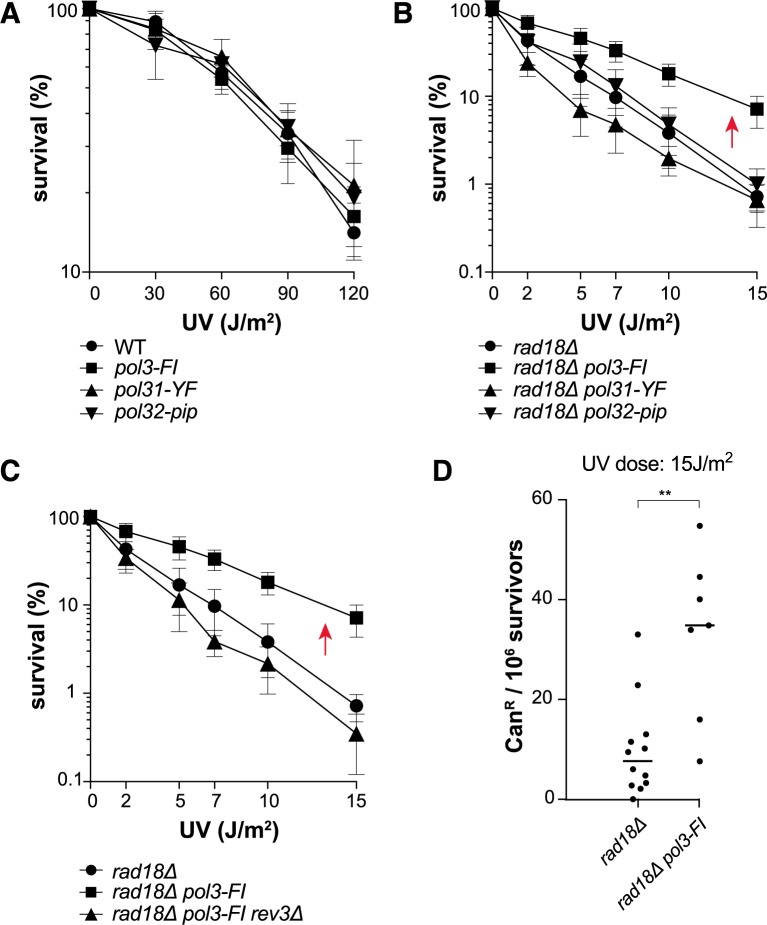
It has been already reported that rad18Δ-associated UV sensitivity is suppressed in the absence of the Srs2 helicase [60] or of the Siz1 SUMO ligase [61]. This suppression requires the homologous recombination (HR) genes RAD51 and RAD52. However, in rad18Δ cells in which RAD51 or RAD52 was also deleted, UV resistance was increased in the presence of pol3-ct (Fig 1B and 1C). Therefore, we concluded that rad18Δ suppression by pol3-ct is mechanistically not related to HR. Moreover, pol3-ct did not suppress UV sensitivity associated with rad51Δ at high UV doses (S1A Fig). These findings suggested that pol3-ct effect could be specific to the DNA damage tolerance (DDT) pathways controlled by PCNA ubiquitination.
To determine whether pol3-ct suppressor effect was related to PCNA ubiquitination, we tested also the impact of pol3-ct in the pol30-K164R mutant. In this mutant, PCNA K164 is mutated, thereby preventing its ubiquitination by Rad6/Rad18 and sumoylation by Siz1. The pol30-K164R mutant was less sensitive to UV than the rad18Δ single mutant (Fig 1D). This phenotype can be explained by the lack of PCNA sumoylation in the pol30-K164R mutant that could lead to inefficient Srs2 recruitment [61,62] and consequently, to frequent channeling of UV-induced DNA lesions towards HR. Moreover, pol30-K164R UV sensitivity was not affected by pol3-ct (Fig 1D). Yet, in the absence of HR, pol3-ct had a clear suppressor effect (Fig 1D), thereby showing that pol3-ct restores UV resistance to both pol30-K164R and rad18Δ cells. Overall, our observations support the hypothesis that pol3-ct relieves partially the requirement of PCNA ubiquitination for UV resistance.
pol3-ct increases UV resistance of mutants on the RAD5- and MMS2-dependent branch of the RAD18 pathway
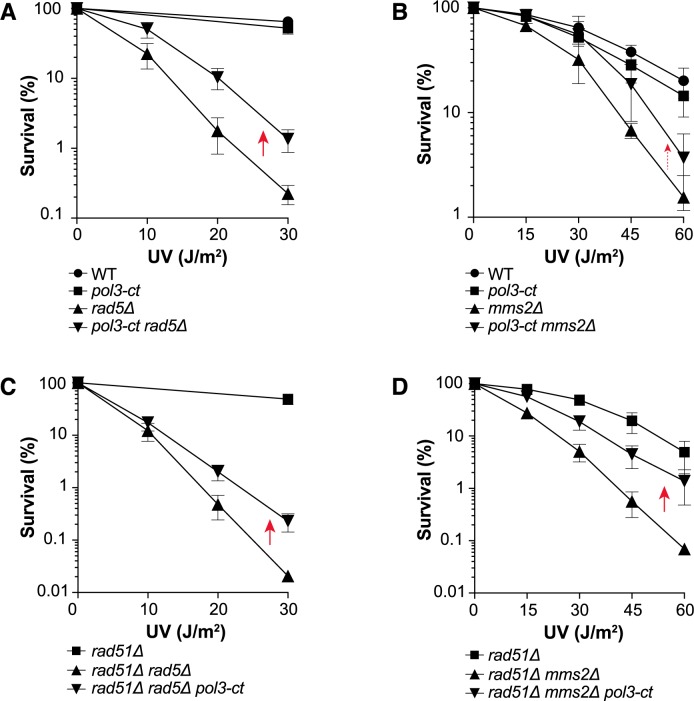
Several DDT pathways are regulated by Rad18 (i.e., TLS activation and template switching). We noticed that pol3-ct suppressed the rad18Δ phenotype only partially and that rad18Δ pol3-ct cells remained sensitive to UV compared with WT cells (Fig 1A). This suggested that pol3-ct suppressor effect could involve mainly one of the Rad18-dependent pathways. To test this hypothesis, we first evaluated pol3-ct effect within the template switching pathway that is triggered by PCNA poly-ubiquitination catalyzed by Rad5 and Mms2-Ubc13. A rad5Δ mutant and, to a lesser extent, an mms2Δ mutant (both defective in PCNA poly-ubiquitination) regained some UV resistance in the presence of pol3-ct (Fig 2A and 2B). Moreover, both rad5Δ and mms2Δ strains showed a negative interaction with rad51Δ upon UV radiation, and the UV sensitivity of the rad5Δ rad51Δ and mms2Δ rad51Δ double mutants was similarly reduced by pol3-ct (Fig 2C and 2D). Thus, some of the UV-induced DNA lesions handled via template switching are bypassed in the rad5Δ and mms2Δ mutants thanks to pol3-ct.
Fig 2. pol3-ct increases UV resistance in the absence of PCNA polyubiquitination.
Survival curves of haploid cells deficient for PCNA poly-ubiquitination after exposure to UV light. Red arrows highlight increased UV resistance in the presence of pol3-ct. (A and C) Suppression of UV hypersensitivity in rad5Δ cells by the pol3-ct allele in the presence or absence of Rad51. (B and D) Suppression of mms2Δ-associated UV sensitivity by the pol3-ct allele in the presence or absence of Rad51. The weaker suppression of mms2Δ UV sensitivity by the pol3-ct allele is shown with a smaller and dotted red arrow.
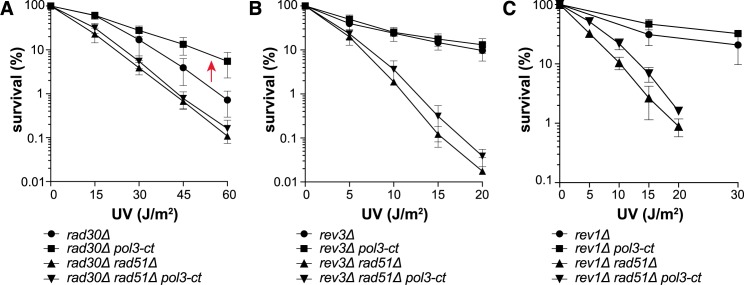
To bypass UV-induced lesions during DNA replication, Rad18 also controls the Pol η-dependent error-free and the Pol ζ- and Rev1-dependent error-prone TLS pathways. Concerning Pol η (encoded by RAD30), pol3-ct partially suppressed the UV sensitivity associated with the rad30Δ allele (Fig 3A). However, this effect was abolished in the absence of Rad51, suggesting a complex relationship between Pol δ-ct and Pol η (S7B Fig). pol3-ct had little effect on UV resistance in the Pol ζ and Rev1 mutants, even at higher UV doses (Fig 3B and 3C; S1B and S1C Fig). We noticed that in the rev3Δ rad51Δ and rev1Δ rad51Δ double mutants, pol3-ct had a minor suppressive effect, suggesting a modest Pol η recruitment (Fig 3B and 3C; S7C Fig). These observations imply that Pol ζ and Rev1 are still required for UV resistance in the pol3-ct mutant. As pol3-ct did not display a pervasive suppressor effect in TLS polymerase-defective mutants, we conclude that Pol δ-ct cannot take the place of TLS polymerases. Conversely, the pol3-ct suppressor effect clearly acts on the RAD18-dependent poly-ubiquitination sub-pathway. To explain these results, we hypothesized that Pol δ-ct reduces the PCNA ubiquitination requirement for TLS polymerase recruitment. In this model, suppression of the rad18Δ phenotype by pol3-ct should require the TLS polymerases.
Fig 3. pol3-ct effects in cells lacking TLS polymerases.
Survival curves of TLS-deficient haploid cells after exposure to UV light. (A) pol3-ct increases the UV resistance of rad30Δ cells (red arrow) only in the presence of Rad51. (B) pol3-ct does not increase UV resistance of rev3Δ cells in the presence of Rad51. A minor pol3-ct effect is observed in the absence of Rad51. (C) pol3-ct does not restore UV resistance of rev1Δ cells in the presence Rad51. A minor pol3-ct effect is observed in the absence of Rad51.
rad18Δ suppression by pol3-ct upon UV radiation depends on Pol ζ and Rev1
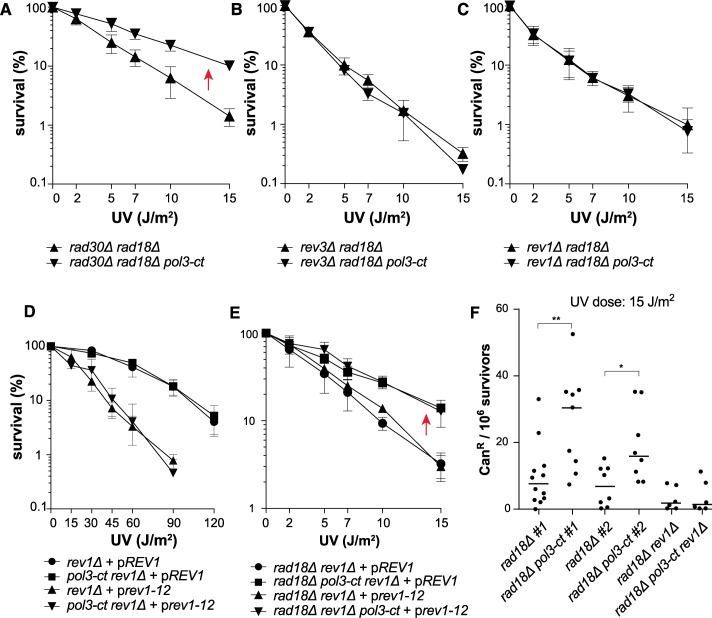
The suppression of rad18Δ UV sensitivity by pol3-ct was still observed in the rad30Δ mutant (Fig 4A), which suggests a minor role for Pol η in the bypass of UV-induced DNA lesions in the pol3-ct rad18Δ double mutant. On the contrary, the rev3Δ rad18Δ pol3-ct and rev1Δ rad18Δ pol3-ct strains were UV hypersensitive, like the rev3Δ rad18Δ and rev1Δ rad18Δ double mutants (Fig 4B and 4C). Thus, suppression of rad18Δ UV sensitivity by pol3-ct occurs only when Pol ζ and Rev1 are present. Interestingly, we observed that suppression of rad5Δ-associated UV sensitivity by pol3-ct was dependent on both Pol ζ and Pol η (S2 Fig), showing that Pol η can play a role in pol3-ct strains when PCNA is ubiquitinated.
Fig 4. Suppression of rad18Δ UV phenotypes by pol3-ct requires REV3 and REV1.
Survival curves of UV-irradiated haploid cells with defective PCNA ubiquitination and TLS. (A) Suppression of rad18Δ UV sensitivity by pol3-ct (red arrow) does not require RAD30. (B) Suppression of rad18Δ UV sensitivity by pol3-ct requires REV3. (C) Suppression of rad18Δ UV sensitivity by pol3-ct requires REV1. (D) pol3-ct does not suppress the effect of the rev1-12 allele. rev1Δ cells are complemented with a plasmid containing either the WT REV1 gene (pREV1) or the rev1-12 allele (prev1-12). (E) Suppression of rad18Δ UV sensitivity by pol3-ct (red arrow) does not require a functional UBM domain in Rev1. rad18Δ rev1Δ cells are complemented with a plasmid containing either the WT REV1 gene (pREV1) or the rev1-12 allele (prev1-12). (F) Increase of UV-induced mutagenesis in rad18Δ cells carrying pol3-ct. Each dot represents the canR frequency measured in one experiment. The median value for each strain is represented by the horizontal bar. For this experiment, the rad18Δ and rad18Δ pol3-ct strains were isolated from two independent progenies (#1 and #2), and in both cases the canR frequency between rad18Δ and rad18Δ pol3-ct was significantly different (Mann-Whitney test): **, P = 0.0043 (#1) and *, P = 0.0353 (#2).
Rev1 carries a conserved UBM motif in its C-terminus. As pol3-ct relieves partially the requirement for PCNA ubiquitination, the Rev1 UBM should be dispensable in the pol3-ct mutant. Point mutations within this motif (L821A, P822A, I825A) abolish its functional interaction with ubiquitinated PCNA in vitro and strongly lower cell resistance to UV radiation in vivo [29]. In the pol3-ct background, UV sensitivity was not reduced in the rev1-12 mutant that carries the L821A, P822A, I825A mutations, (Fig 4D). However, the rad18Δ phenotype was suppressed by pol3-ct in the rev1-12 mutant (Fig 4E). Thus, we conclude that in the pol3-ct mutant, Pol ζ and Rev1 bypass UV-induced DNA lesions in the absence of PCNA ubiquitination independently of Rev1 UBM.
UV-induced mutagenesis mostly depends on Pol ζ and Rev1 and on PCNA ubiquitination [8,63–65]. In the pol3-ct mutant, the role of PCNA ubiquitination in UV-induced mutagenesis could be less important. To test this hypothesis, we monitored UV-induced mutagenesis with the CAN1 forward mutation assay after exposure to a UV dose of 15 J/m2. At this dose, only about 1% of rad18Δ cells survived and pol3-ct strongly suppressed rad18Δ-associated UV sensitivity (Fig 1A). Interestingly, we observed that the frequency of UV-induced canavanine resistant (CanR) cells was higher in the rad18Δ pol3-ct double mutant compared with rad18Δ cells (Fig 4F). This result was obtained with strains coming from two different progenies and is no longer observed in absence of Rev1 (Fig 4F). Thus, these observations further support the hypothesis that upon UV irradiation, Pol ζ and Rev1 are recruited independently of PCNA ubiquitination in the pol3-ct mutant.
Pol3 and Pol31 mutations that affect the Pol3-CTD/Pol31 complex like Pol3-ct
Pol3-ct interacts weakly with Pol31 [59]. To determine whether this interaction plays a role in PCNA ubiquitination requirement, we wanted to identify mutations that affect this interaction and then test them genetically in the rad18Δ mutant. Based on our previous three-dimensional model of the Pol3-CTD and Pol31 complex from the published structures of human p50 and of Pol α [59,66,67] (Fig 5A), we focused our analysis on Arg1043 of Pol3 and Asp304 of Pol31, which is opposite to Pol3 Arg1043 (Fig 5A). Two-hybrid analyses [59] indicated that co-transformants carrying pBTM116-Pol31 and pACT2-Pol3-R1043G displayed temperature-sensitive β-galactosidase activity after incubation for two days. Compared with the activity of pBTM116-Pol31 and pACT2-Pol3-CTD co-transformants, their activity was low at 22°C and even lower at 30°C, showing that Pol3-R1043G gives similar results than Pol3-ct (Fig 5B; [59]). Co-transformants carrying pBTM116-Pol31-D304N and pACT2-Pol3-CTD did not show any measurable β-galactosidase activity at either temperature (Fig 5B).
Fig 5. Functional analysis of the interaction between Pol3-CTD and Pol31 in cell survival after UV irradiation.
(A) Structural model of the Pol3-CTD/Pol31/P66 complex. Pol3 CTD contains two conserved cysteine-rich metal-binding motifs (CysA and CysB) that are separated by two α-helices. The Arg1043 residue of Pol3 lies within helix α2 between the Pol3 LSKW residues and Pol31. In addition, Pol31 Asp304 is very close to Pol31 interacting surface opposite to Pol3 Arg1043. The position of the LSKW motif at the C-terminal end of Pol 3 CTD is indicated by Pol3-LSKW. The conserved L1094 and W1097 residues of this motif are indicated by sticks. This motif is deleted in the pol3-ct mutant. The Pol3 residue R1043 within helix α2 is also indicated by sticks. The Pol3 residues F1002 and I1003 are located five amino-acids upstream of C1009 (the first amino-acid included in the structural model). These five amino-acids are indicated by black dots to show the proximity of Pol3-F1002 and -I1003 with Pol3-C1009. Pol31-D304 and -K358 residues are highlighted as magenta spheres. Pol31-L324, -Y327 and -F328 residues are shown as red spheres. Note that Pol31-L324 is buried in our model and not available for intermolecular interactions. (B) Yeast two-hybrid assays were performed using pBTM116 plasmids carrying WT and mutated Pol31 fused to the lexA binding domain (BD) and pACT2 plasmids carrying WT or mutated Pol3 C-terminal amino acids 1032–1097 fused to the Gal4 activating domain (AD). Each spots illustrates an individual co-transformant obtained following transformation of CTY10-5d. β-galactosidase activity was tested using an overlay plate assay at 22°C and 30°C. (C) Survival curves obtained after UV-irradiation of Pol δ mutant strains in which the interaction between Pol3 CTD and Pol31 is impaired. (D and E) Survival curves obtained after UV-irradiation of PCNA ubiquitination-deficient Pol δ mutant strains. (D) The pol3-R1043G and pol31-D304N alleles do not suppress rad18Δ-associated UV sensitivity. (E) The pol31K358I allele does not affect the suppression of rad18Δ-associated UV sensitivity by pol3-ct (red arrow).
pol3-R1043G did not confer sensitivity to the genotoxic agent hydroxyurea (HU) differently from pol3-ct (S3A Fig). Yet, we observed synthetic lethality between pol3-R1043G and pol32Δ, a phenotype shared with pol3-ct (Material & Methods; S4 Fig). The pol31-D304N allele (originally named hys2-2; [68]) conferred temperature and HU sensitivities (S3A Fig) and was synthetic lethal with pol32Δ (S4 Fig). In summary, the Pol3-R1043G mutation affected the structure of the Pol δ holoenzyme although to a lesser extent than the Pol3-ct and the Pol31-D304N mutations.
Partial loss of interaction between Pol3 CTD and Pol31 does not promote Rad18-independent TLS
To determine the role of the interaction between Pol3 CTD and Pol31 in bypassing PCNA ubiquitination, we first evaluated UV sensitivity in the pol3-ct, pol3-R1043G and pol31-D304N strains. The pol3-ct and pol3-R1043G mutants were not UV sensitive and showed UV-induced mutagenesis frequencies similar to those of WT cells (Fig 5C; S5 Fig). Conversely, the pol31-D304N allele increased UV sensitivity (Fig 5C). As Pol31 is a member of the Pol ζ complex, we hypothesized that this phenotype could be caused by a partial impairment of the interaction between Pol31 and Rev3 CTD. Interestingly, UV-induced mutagenesis in the pol31-D304N mutant was significantly decreased, but not abolished (S5 Fig).
Based on the UV phenotypes of Pol δ mutants, we hypothesized that rad18Δ UV hypersensitivity could be suppressed by pol3-R1043G, but not by pol31-D304N due to its possible defect in the Pol ζ-dependent pathway. However, neither pol3-R1043G nor pol31-D304N suppressed the rad18Δ phenotype (Fig 5D). Thus, in disagreement with our initial prediction, suppression of rad18Δ UV sensitivity by pol3-ct might not be related to a defective interaction between Pol3 CTD and Pol31. To test genetically this hypothesis, we used the pol31-K358I allele that suppresses pol3-ct effect [59]. K358 is accessible for interaction with Pol3 CTD residues (Fig 5A). The pol31-K358I allele did not modify the suppression of rad18Δ UV hypersensitivity by pol3-ct, reinforcing the conclusion that pol3-ct effect on rad18Δ is not related to the interaction between Pol3 CTD and Pol31 (Fig 5E).
Among the PIP motifs in the three subunits of Pol δ, only Pol32 PIP motif interacts with PCNA
As an alternative hypothesis, TLS polymerase recruitment could be facilitated by Pol δ mutations that weaken its interaction with PCNA. Our strategy was again to find these mutations and to test them subsequently in rad18Δ strains. However, this search was hampered by the lack of a ternary structure of PCNA complexed with Pol δ on DNA. Hence, we focused on Pol δ sites that were described as PIP motifs [52,53]. Pol32 PIP motif (QxxLxxFF) contains the PIP consensus sequence, is highly conserved in eukaryotes [52] (S6C Fig) and has been implicated in PCNA interaction by two-hybrid screens [52]. The Pol31 motif LxxYF lacks the conserved Gln residue, is not conserved and is located in the Pol31 PDE domain [53,67] (S6B Fig). In our Pol31 model, the aromatic dyad is exposed and the preceding L324 could be buried (Fig 5A). The Pol3 motif QxxxLxxFI differs from the consensus PIP motif by the presence of an extra residue between the conserved Gln and Leu residues and by the lack of the second aromatic residue at the end of the motif. Moreover, this motif is located just upstream of the highly conserved CysA cysteine module (Fig 5A), and only the Leu and Phe residues are conserved in eukaryotes (S6A Fig).
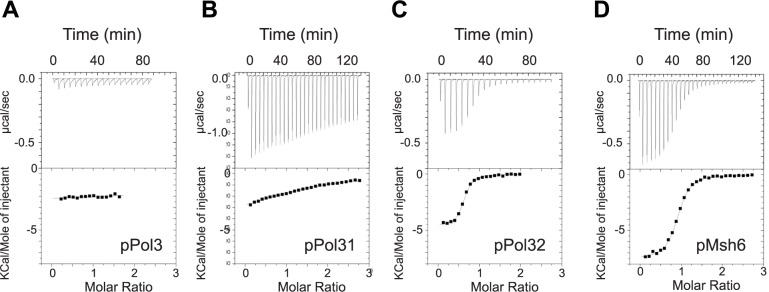
To test the functionality of these three motifs, we characterized by microcalorimetry the affinity and stoichiometry of the interaction between purified PCNA and synthetic peptides that contain the putative PIP-boxes found in Pol δ. The Pol3 and Pol31 peptides were of equal length (21 amino acids), whereas the Pol32 PIP motif, which is located in the extreme C-terminus of Pol32, was three amino acids shorter. In parallel, we tested the canonical PIP motif of Msh6 that mediates the interaction of MutSα with PCNA [69]. The binding reactions between Msh6 and Pol32 peptides with PCNA gave large exothermic signals and the interactions could be fitted with a one-site binding model after integration. The dissociation constant (Kd) of the Msh6 and Pol32 PIP motifs were 0.31 μM and 0.46 μM at 30°C, respectively (Table 1, Fig 6C and 6D). Both interactions presented favorable enthalpy and entropy, as previously observed for other canonical PIP motifs [14,70,71]. In the same experimental condition, the non-canonical Pol3 and Pol31 PIP motifs showed no interaction or very weak interaction, respectively (Fig 6A and 6B).
Table 1. Thermodynamic parameters of the interactions between PCNA and the proposed Pol32, Pol31 and Pol3 PIP motifs measured by calorimetry.
| Peptide | Sequence | Length (aa) |
Kd (μM) |
ΔH° (kcal/mol) |
-TΔS° (kcal/mol) |
ΔG° (kcal/mol) |
|---|---|---|---|---|---|---|
| pPol3 992–1012 |
TGSQKGGLMSFIKKVEACKSC | 21 | NI | NI | NI | NI |
| pPol31 317–337 |
KSLFDKSLESYFNGSNKEILN | 21 | >50 | nd | nd | nd |
| pPol32 333–350 |
KRLKKQGTLESFFKRKAKa | 18 | 0.46 ± 0.15 | -4.45 ± 0.8 | -4.65 ± 1.0 | -9.05 ± 0.2 |
| pMsh6 22–43 |
QKKMKQSSLLSFFSKQVPSGTP | 26 | 0.31 ± 0.1 | -6.40 ± 1.0 | -2.39 ± 1.06 | -8.8 ± 0.2 |
All ITC experiments were done at 30°C. Data are shown as the mean value ± standard deviation of at least two measurements. The amino acids of the proposed PIP motifs are in bold underlined characters. The numbers under the peptides’ names indicate to the amino acid position in the sequence of the corresponding protein. The Kd for Pol31 corresponds to a lower value estimated from the weak signal observed with this peptide. The thermograms and isotherms are reported in Fig 6. NI: no interaction; nd: not determined.
aK is the last residue of Pol32.
Fig 6. Physical interactions between PCNA and the proposed PIP-boxes of S. cerevisiae Pol32, Pol31 and Pol3.
The thermograms (upper panels) and binding isotherms (lower panels) of the calorimetric titrations of PCNA by the assayed peptides at 303 K are presented. The corresponding thermodynamic parameters are reported in Table 1. (A, B, C, D) Interaction between PCNA and the peptides containing the (putative and canonical) Pol3, Pol31, Pol32 and Msh6 PIP motifs, respectively. The thermodynamic parameters ΔH, N, and Ka were obtained by non-linear least-squares fitting (represented in line mode) of the experimental data with a single site model.
In summary, while the Pol32 PIP motif has the characteristics of a functional PIP motif, the proposed Pol3 and Pol31 non-canonical motifs may have a role in Pol δ function or in Pol δ interaction with PCNA, but possibly not as bona fide PIP domains.
The viability of the triple pol3-FI pol31-YF pol32-pip mutant implies a functional PCNA-Pol δ interaction in this mutant
Although they do not interact with PCNA, the Pol3 and Pol31 non-canonical PIP motifs contribute to PCNA-stimulated DNA synthesis in vitro [53]. To study their role in vivo, we mutated the Pol3 QxxxLxxFI and Pol31 LxxYF motifs and generated strains that carry either the pol3-FI1002-1003AA allele (thereafter, named pol3-FI) or the pol31-YF327-328AA allele (thereafter, named pol31-YF). We also produced the pol32-FF344-345LL allele (thereafter, named pol32-pip) by directed mutagenesis of the Pol32 PIP motif. Mutants carrying these alleles did show neither slow growth, nor temperature or HU sensitivity (S3 Fig). In addition, all double mutants were viable and thermo-resistant. Only the pol3-FI pol32-pip double mutant was sensitive to HU (S3B Fig). Importantly, the pol3-FI pol31-YF pol32-pip triple mutant was viable, strongly suggesting that in this mutant, PCNA is still a processivity factor for Pol δ (S3B Fig). Moreover, the triple mutant was more sensitive to HU than the pol3-FI pol32-pip double mutant. This suggests that pol31-YF has some effect on Pol δ structure stability. Accordingly, pol31-YF showed synthetic lethality with pol32Δ (S4 Fig) and an additive effect with pol3-ct upon HU treatment (S3A Fig). Finally, the Pol31 LxxYF motif is close to the Pol31-D304 residue involved in the interaction with Pol3 CTD (Fig 5A). By two-hybrid assay, we found that the Pol31 YF residues were as important for this interaction as the D304 residue (Fig 5B). This also suggests that these residues contribute to the overall stability of the Pol δ holoenzyme.
rad18Δ-associated UV hypersensitivity is partially suppressed by mutational inactivation of the Pol3 F1002-I1003 residues upstream of the Pol3 CysA module
UV resistance (Fig 7A) and UV-induced mutagenesis (S5 Fig) were comparable in the three pol3-FI, pol31-YF and pol32-pip single Pol δ mutants and in WT cells. Thus, these mutants carry a functional Pol ζ. Therefore, we could test the role of the Pol δ mutated motifs in the absence of PCNA ubiquitination. UV sensitivity was comparable in the pol32-pip rad18Δ double mutant and in the rad18Δ single mutant (Fig 7B). Thus, the Pol32-pip mutation did not affect the competition for PCNA access between Pol δ and Pol ζ. We obtained similar results with the pol31-YF rad18Δ mutant (Fig 7B). Conversely, pol3-FI suppressed rad18Δ-associated UV hypersensitivity and this effect was REV3 dependent (Fig 7C). In agreement with these observations, UV-induced mutagenesis was significantly increased in rad18Δ pol3-FI cells compared with rad18Δ cells (Fig 7D). Thus, only pol3-FI recapitulates the phenotypes of the pol3-ct allele in the rad18Δ background. The Pol3 FI residues (mutated in the pol3-FI strain) and Pol3 LSKW residues (lost in the pol3-ct strain) are close to the CysA module of Pol3-CTD in our structural model (Fig 5A). Therefore, the phenotypes shared by pol3-ct and pol3-FI could be due to destabilization of the CysA module that has been proposed to be essential for the Pol δ-PCNA interaction ([50], Discussion).
Fig 7. Suppression of rad18Δ UV phenotypes by pol3-FI.
(A) Survival curves of UV-irradiated mutant strains carrying the pol3-FI, pol31-YF or pol32-pip allele. (B) pol3-FI suppresses rad18Δ-associated UV sensitivity (red arrow), while pol31-YF and pol32-pip do not. (C) Suppression of rad18Δ-associated UV sensitivity by pol3-FI (red arrow) depends on REV3. (D) Increase of UV-induced mutagenesis in rad18Δ cells carrying pol3-FI. Each dot represents the canR frequency measured in one experiment. The median for each strain is represented by a horizontal bar: **, P = 0.0026 (Mann-Whitney test).
Discussion
In the present study, we showed that Pol ζ and Rev1 can be activated in vivo independently of PCNA ubiquitination in the pol3-ct and pol3-FI mutants. From this genetic observation, we infer that the primary role of PCNA ubiquitination is to promote the recruitment of TLS polymerases that would otherwise be outcompeted by Pol δ for access to the front side of PCNA and to the 3’ primer ends, even in the presence of UV-induced DNA lesions leading to fork stalling. In the next paragraphs, we will emphasize that the suppression of rad18Δ cells UV sensitivity by these Pol δ mutants is novel and robust. Then, we will discuss the possible mechanism by which this suppression occurs. We will argue that it is not mediated through the easier replacement of Pol3 by Rev3/Rev7 on a putative Pol31/Pol32 platform, but through the partial loss of interaction between Pol3 and PCNA. Finally, we will present a working model that takes into account the finding that rad18Δ suppression by pol3-ct and pol3-FI occurs mostly through Pol ζ and Rev1 and not through Pol η.
pol3-ct and pol3-FI are novel suppressors of rad18Δ
The pol3-ct and pol3-FI alleles do not confer UV sensitivity to WT cells or to cells with defects in one or several UV-induced DNA lesion tolerance pathways ([59], Figs 5C and 7A, S1 Fig). Moreover, these alleles are not associated with a defect in UV-induced mutagenesis (S5 Fig). These key features allowed us to detect the partial rescue of the rad18Δ mutant by pol3-ct and pol3-FI following UV radiation. Although rad18Δ still confers high UV sensitivity in the presence of the POL3 alleles (Figs 1A and 7B), we considered the phenotypic suppression robust for the following reasons. At higher UV doses, rad18Δ cell survival is increased at least ten-fold in the presence of the pol3-ct and pol3-FI alleles. pol3-ct suppresses the rad18Δ phenotype in the W303 and the FF genetic backgrounds and in several genetic contexts (pol30-K164R, rad5Δ and mms2Δ). Finally, this suppression seems to occur only through the error-prone repair pathway, which explains why rad18Δ cells still retain high UV sensitivity. In addition, pol3-ct suppression of rad18Δ UV sensitivity is novel. It does not require functional HR, differently from the srs2Δ- and siz1Δ-dependent suppressions [60,61]. Moreover, studies based on the inactivation of the Pol δ proofreading domain in yeast and DT40 cells indicated that Pol δ contributes to TLS in vivo [72,73]. This propensity of the exonuclease-dead Pol δ to perform TLS in yeast is independent of Pol ζ and remarkably suppresses also rad18Δ-associated hypersensitivity to DNA damaging agents [72]. On the other hand, rad18Δ suppression by Pol3-ct and pol3-FI depends on Pol ζ and Rev1. Our results allow us to revisit the role of PCNA ubiquitination and of polymerase switch upon UV radiation.
Pol ζ recruitment independently of PCNA ubiquitination might not occur through a switch between Pol3 and Rev3/Rev7 on a Pol31/Pol32 platform
How to explain that Pol δ-ct and Pol δ-FI allow the recruitment of Rev1 and Pol ζ independently of PCNA ubiquitination? The finding that Pol ζ may function as a four-subunit enzyme (Pol ζ4) has led to the suggestion that the polymerase switch involves dissociation of the Pol δ catalytic subunit (Pol3 in yeast) from its structural subunits (Pol31 and Pol32 in yeast), which will become part of Pol ζ [54–56]. In addition, DNA damage and PCNA ubiquitination trigger Def1-dependent degradation of the Pol3 catalytic subunit of yeast Pol δ [57]. Def1-dependent Pol3 degradation could be the initial event leading to polymerase switching on the Pol31/Pol32 platform [57]. The genetic suppression of rad18Δ by pol3-ct and pol3-FI described here depends on Pol ζ. In addition, pol3-ct partially impairs the interaction between Pol3 and Pol31 [59]. These observations fit well with a model in which in our pol3 mutants, the switch between Pol3 and Rev3/Rev7 on the Pol31/Pol32 platform occurs spontaneously without the need of PCNA ubiquitination and the subsequent Pol3 poly-ubiquitination by Def1.
Yet, and rather unexpectedly, the results of our genetic analyses imply that the impaired interaction between Pol3 and Pol31 is not involved in the suppression of rad18Δ. The Pol3-R1043G mutant protein displays the same defect as the pol3-ct mutant in the two-hybrid interaction with Pol31, and both pol3-R1043G and pol3-ct are synthetic lethal with pol32Δ. However, pol3-R1043G does not suppress rad18Δ (Fig 5D). Similarly, the simultaneous mutational inactivation of Pol31 Y327 and F328 impairs the interaction between Pol31 and Pol3-CTD (Fig 5B) and leads to synthetic lethality with pol32Δ (S4 Fig). However, the pol31-YF mutant allele does not suppress rad18Δ (Fig 7B). The Pol31-K358I mutation restores stable Pol3-Pol31 interactions in the pol3-ct mutant and pol31-K358I suppresses pol3-ct-associated HU sensitivity [59]. On the contrary, pol31-K358I does not affect rad18Δ suppression by pol3-ct (Fig 5E). Finally, the pol3-FI allele suppresses rad18Δ, although the Pol3 F1002 and I1003 residues are upstream of the C-terminal Pol3 domain that is sufficient for interaction with Pol31 [74] (Fig 7C). Thus, the model implying a switch between Pol3 and Rev3/Rev7 on a Pol31/Pol32 platform does not fully account for the bypass of PCNA ubiquitination observed in the pol3 mutants.
A partial loss of interaction between Pol3 CTD and PCNA might allow the suppression of rad18Δ-associated UV hypersensitivity
These observations suggest that the effect of the Pol3-ct mutation on Pol δ structure does not impair only the interaction with Pol31. Therefore, we hypothesized that pol3-ct destabilizes the interaction between Pol δ and PCNA. Hence, we expected to find Pol δ mutations that affect this interaction and that would share the pol3-ct phenotypes. However, the lack of a ternary structure that includes Pol δ bound to PCNA at primer ends made our search uncertain. Moreover, Pol δ interacts with PCNA through multiple sites and a possible redundancy in binding interactions could allow Pol δ to adopt flexible configurations with PCNA [75,76]. Our understanding of PCNA-Pol δ interactions is even more challenged by our finding that among the three proposed PIP motifs of Pol δ, only the Pol32 motif is a bona fide PIP motif (Table 1, Fig 6A)[53]. The observation that the pol3-FI pol31-YF pol32-pip triple mutant is viable in our strain backgrounds (W303 and FF18733; S4 Fig and S1 Table) clearly indicates that Pol δ interacts with PCNA in this triple mutant.
Despite the uncertainties surrounding PCNA-Pol δ interaction, the Pol3 F1002 and I1003 residues are separated by only five amino-acids from Pol3 C1009, the first cysteine of the CysA zinc-binding segment of Pol3 CTD (Fig 5A). This CysA motif is likely to be crucial for the interaction with PCNA [50]. Thus, Pol3 F1002 and I1003, while not directly required for the interaction with PCNA, might contribute to the optimum structural conformation of Pol3 CysA. Destabilization of this CysA module could affect the interaction between Pol δ and PCNA and consequently facilitate the recruitment of TLS polymerases to PCNA. To substantiate this hypothesis, we wanted to mutate the CysA cysteines (C1009, C1012 or C1024), but failed. This suggests that these cysteines are essential and that the CysA module is crucial for PCNA-Pol δ complex stability [50,77]. The last Pol3 C-terminal W1097 residue is close to the CysA module and therefore, could play a role in its stabilization as well (Fig 5A). Thus, the loss of the last four LSKW residues in the pol3-ct mutant might affect the interaction with Pol31 and also with PCNA. Our genetic observations support a role for Pol3 F1002 and I1003 and for Pol3 W1097 in promoting stable interactions between Pol3 and PCNA. Only pol3-ct and pol3-FI suppress the UV hypersensitivity associated with rad18Δ and remarkably, only these two POL3 alleles display an additive negative effect with the pol32-pip allele upon HU treatment (S3 Fig). Similarly, when both Pol3 CysA and Pol32 PIP motifs are mutated, the PCNA-dependent replication activity of Pol δ is almost abolished, showing an additive negative effect between these sites in vitro [50]. Therefore, we propose that the impaired interaction between a Pol3 CTD mutant and PCNA is the origin of the suppression of rad18Δ-dependent UV sensitivity.
Model for PCNA ubiquitination-dependent polymerase switch at UV-induced DNA lesions
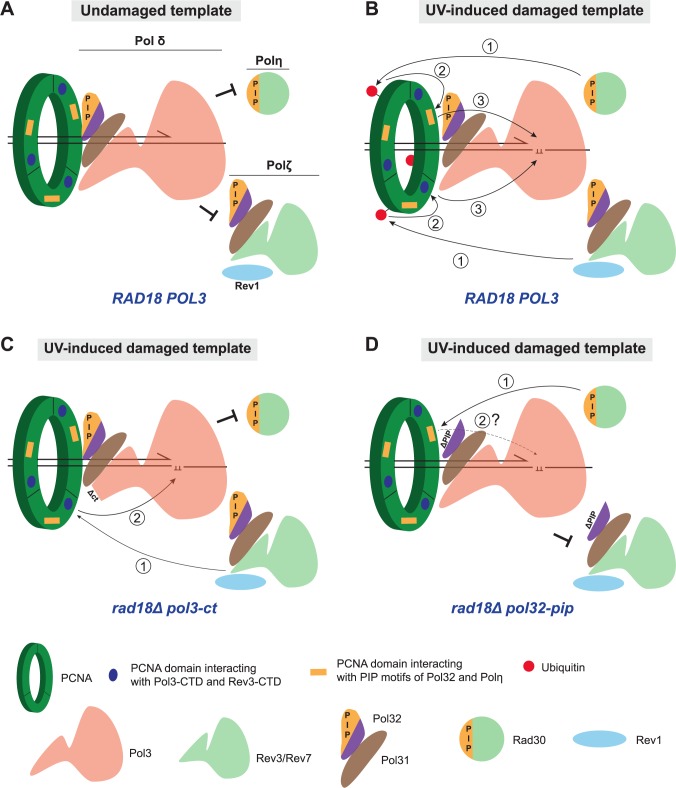
The observation that pol3-ct and pol3-FI suppress rad18Δ-associated UV hypersensitivity leads to the conclusion that WT Pol δ is in part responsible for this sensitivity. In addition, our data suggest that Pol δ competes with TLS polymerases for the access to primer/template junctions in the front side of PCNA (Fig 8A). Therefore, PCNA ubiquitination might provide a docking site on the back side of PCNA for recruiting TLS polymerases, thereby increasing their local accumulation near a stalled 3’ end (Fig 8B). Spontaneous or active Pol δ displacement from its interaction domains with PCNA at sites of DNA damage would be accompanied by the concomitant binding of a TLS polymerase to the front side of PCNA and its access to the primer end. In the absence of PCNA ubiquitination, preferential binding of Pol δ to the front side of PCNA due to stronger affinity or mass action would inhibit the polymerase switch at blocking DNA lesions.
Fig 8. Polymerase switching in Pol δ structural mutants: A working model.
(A) DNA synthesis on an undamaged DNA template is catalyzed by Pol δ. Thanks to PCNA trimeric structure, simultaneous binding of two or even three Pol δ molecules could be envisaged. This possibility is not depicted here for the sake of simplicity. Pol δ outcompetes TLS polymerases for the access to the 3’ DNA primer terminus by stronger affinity or mass action. Pol δ subunits interact with several PCNA domains. The PCNA domain interacting with PIP motifs is represented by an orange rectangle on PCNA, whereas the one interacting with the CysA module of Pol3-CTD and possibly with the CysA module of Rev3-CTD is represented by a blue filled circle (note that this PCNA domain has not been identified yet). (B) Pol δ stalls when the 3’ end of the newly synthesized strand encounters a UV-induced DNA lesion such as a pyrimidine dimer. Rad6/Rad18 activation triggers PCNA ubiquitination at K164 (red filled circle). TLS polymerases access to the 3’ end is envisaged in three steps: 1) the TLS polymerase-ubiquitin interaction brings these polymerases closer to their PCNA interacting domain and therefore increases their local concentration near the 3’ end; 2) thanks to this proximity, TLS polymerases can compete with Pol δ for binding to their specific PCNA interaction domain: PIP interacting domain (orange rectangle) for Pol η and a not-yet-identified PCNA domain (blue filled circle) for Pol ζ via its CysA module. Following its displacement, Pol δ disengages from binding to the primer terminus; 3) tight binding of TLS polymerases on the front face of PCNA allows the insertion of the 3’ extending end and the template UV-induced DNA lesion into the catalytic site of TLS polymerases for translesion synthesis. (C) Pol δ-ct (and Pol3-FI) leads locally to the destabilization of the Pol3-CTD-PCNA interaction. With the help of Rev1, this could facilitate Pol ζ competition for the common PCNA interacting domain. Thus, the initial binding step to ubiquitinated PCNA is bypassed and Pol ζ has access to the 3’ end in two steps only: 1) Pol ζ binds to its PCNA interacting domain (blue filled circle); and then 2) Translesion synthesis takes place. Conversely, Pol η does not have access to its PCNA interacting domain in pol3-ct. (D) When Pol δ carries the mutant Pol32-ΔPIP subunit, Pol η PIP domain should readily interact with PCNA PIP interacting domain (orange rectangle, step 1) and the requirement of PCNA ubiquitination should be bypassed. This hypothesis was proved to be false because the pol32-pip allele does not suppress UV sensitivity associated with rad18Δ. Thus, Pol η cannot take over DNA synthesis at UV-induced DNA lesions (step 2 indicated by a dashed arrowed line and a question mark). Pol η might require additional PCNA interactions to accommodate pyrimidine dimers on its catalytic site.
Our model is based on the activation of Pol ζ and Rev1 independently of PCNA ubiquitination observed in the pol3-ct and pol3-FI mutants. A noticeable issue in our model is the absence of a similar activation of Pol η in rad18Δ cells. From this unexpected result, we hypothesized that the key factor of rad18Δ suppression might be the specific interaction between Pol η or Pol ζ with PCNA (Fig 8B). Given the structurally conserved organization of the catalytic Pol3 and Rev3 subunits [50], the CysA metal-binding motif in Rev3 CTD and the CysA motif of Pol3 might interact with the same PCNA region. Thus, upon destabilization of Pol3 CTD, PCNA ubiquitination could be bypassed only through the binding of Rev1 and Rev3 CTD to PCNA (Fig 8C). Differently from Pol ζ, Pol η relies on a PIP motif for its interaction with PCNA and does not carry a metal-binding motif in its CTD. If the PCNA domain for interaction with CysA modules were different from the domain that interacts with the PIP motifs, Pol η would not be able to compete with Pol δ even when Pol δ harbors a mutated CTD (Fig 8C). According to this hypothesis, pol32-pip should suppress rad18Δ-associated UV sensitivity in a Pol η-dependent manner (Fig 8D). However, this prediction is not supported by our results suggesting that Pol η requires additional PCNA-dependent engagement at the primer terminus to incorporate distorting DNA lesions, such as pyrimidine dimers, into its catalytic site. To test this hypothesis, it will be important to investigate the suppression of rad18Δ-associated phenotypes by new Pol δ structural mutants or at DNA lesions different from those induced by UV exposure.
A regulatory role for Pol δ in DNA damage tolerance
Our results also bring some insights into Pol δ regulatory role at stalled replication forks with ubiquitinated PCNA (S7 Fig). The pol3-ct allele does not increase UV-induced mutagenesis and is not associated with UV sensitivity. Thus, the pol3-ct effect on the choice of DDT pathway might be too subtle to be detected in the presence of PCNA ubiquitination (S7A Fig). However, the rad30Δ strain carrying pol3-ct showed higher UV resistance, and this effect was abolished in the rad30Δ rad51Δ pol3-ct mutant (Fig 3A). This observation suggests that the Pol δ-ct defect might facilitate the channeling of UV-induced DNA lesions processed by Pol η towards the HR pathway (S7B Fig). We observed a minor pol3-ct-dependent suppression of UV sensitivity with the rev3Δ strains (S1B Fig) and with the rev3Δ rad51Δ and the rev1Δ rad51Δ strains (Fig 3B and 3C). These last observations suggest an easier Pol η recruitment in competition with Pol δ-ct (S7C Fig). This hypothesis is supported by the clear suppression of the rad5Δ- and mms2Δ-associated UV sensitivity by pol3-ct (Fig 2) that depends on Pol ζ but also on Pol η (S2 Fig; S7D Fig). Pol η would be more suitable to displace the mutant Pol δ-ct in the rad5Δ and mms2Δ mutants than in the rad18Δ mutant thanks to its initial binding to ubiquitin on PCNA K164. It remains to be determined whether Pol η always requires PCNA mono-ubiquitination to efficiently outcompete Pol δ-ct or whether this requirement is less stringent for DNA lesions not induced by UV. Nevertheless, although PCNA undergoes mono-ubiquitination following UV radiation in the rad5Δ and mms2Δ mutants, WT Pol δ still competes with TLS polymerases. Therefore, the competition between Pol δ and the TLS polymerases could play a role in the choice leading to the template switching pathway. For instance, Pol δ preferential binding to PCNA in suitable genomic contexts could prevent TLS and allow PCNA poly-ubiquitination to occur more frequently. Conversely, genomic sequences that challenge the replication fork stability and the stable interaction between Pol δ and PCNA could favor the recruitment of TLS polymerases. Interestingly, no increase in UV resistance in absence of Rad51 is observed with Pol δ-ct (S1 and S7E Figs). This suggests that Pol δ regulator role in the presence of ubiquitinated PCNA might take place preferentially at stalled replication forks rather than at single-strand gaps behind the forks that could be processed by HR.
Finally, such a regulatory role for Pol δ in DNA damage tolerance could be also a key feature in TLS that occurs independently of PCNA ubiquitination (see Introduction). In agreement with this hypothesis, some genomic regions, such as repetitive sequence elements, hinder elongation by Pol δ and lead to its dissociation from the replication forks [78]. On the other hand, TLS polymerases are emerging as major actors in DNA synthesis at repetitive DNA sequences and their resulting non-B DNA structures [79]. Thus, a simple prediction would be that TLS polymerases sparsely rely on PCNA ubiquitination for their recruitment at intrinsically difficult to replicate loci. Other post-translational modifications might be at work in these situations, as exemplified by human Pol η recruitment to replication forks thanks to its sumoylation, but independently of PCNA ubiquitination [80].
Materials and methods
Yeast strains, plasmids and media
The S. cerevisiae strains used in the present study are isogenic derivatives of W303-1A [81] or FF18733 (his7-2, leu2-3,112, lys1-1, trp1-289, ura3-52) and are listed in S1 Table. Gene deletions were performed by using a PCR-mediated one-step replacement technique [82,83]. All deletions were confirmed by PCR amplification of genomic DNA. Meiotic segregation of the pol30-K164R allele was followed by PCR amplification with the primers P96 and P97. To study the effect of the rev1-12 allele, rev1Δ strains were transformed with centromeric pBL820 plasmids carrying either REV1 or rev1-12 (a generous gift by Dr Peter Burgers) [29]. The genomic pol3-R1043G mutation was generated by using the plasmid pL11. pL11 is a derivative of pL6 obtained by cloning the C-terminal end of POL3 (from +2503 of the ORF to 523 bp downstream the stop codon) in pRS306 [58]. The pol3-R1043G mutation was inserted in pL11 after site-directed mutagenesis performed in pL6. For targeting the mutation by the two-step transplacement procedure [84], pL11 was digested with HindIII (site located 425 bp downstream of the pol3-R1043G mutation). The pol3-FI1002-1003AA and pol31-YF327-328AA mutations were generated by using the pPOL519 and pPOL545 plasmids (kindly provided by Dr Louise Prakash), respectively [53]. For targeting the mutations, pPOL519 and pPOL545 were digested with KpnI and MfeI, respectively. The pol32-FF344-345LL mutations were generated using plasmid pL19 that was obtained by cloning the C-terminal end of POL32 (from +273 of the ORF to 500 bp downstream the stop codon) in pRS306 of. Prior to transformation, pL19 was digested with XbaI.
All media were prepared as previously described [85]. Mutants were selected on YPD medium containing 300 mg/L of geneticin (Sigma) or nourseothricine (cloNAT; Werner BioAgents).
UV irradiation and UV-induced mutagenesis
Cells in stationary phase were plated at appropriate dilutions on YPD and synthetic plates containing canavanine prior to UV irradiation. UV irradiation was performed using a 264nm source. Survival was determined as the number of cells forming colonies on YPD plates following exposure to a given UV dose divided by the number of cells forming colonies on YPD plates in absence of irradiation. Data used to draw graphs represent the mean ± SEM of at least 3 independent experiments. UV-induced mutagenesis was assessed with the CAN1 forward-mutation assay. UV-induced mutagenesis frequencies were obtained by dividing the number of colonies growing on selective medium containing canavanine (i.e., canavanine-resistant, canR, cells) by the number of cells that survived irradiation. The number of canR colonies obtained after irradiation was corrected by subtracting the number of canR colonies present on the non-irradiated plates and corresponding to spontaneous mutation events. UV-induced mutagenesis in the rad18Δ background has been measured at 15 J/m2.
Thermo- and HU-sensitivity
Yeast cells were directly picked from fresh YPD plates, suspended in sterile water, serially diluted and spotted onto plates. 5 μl of the various dilutions containing 625, 125, 25 and 5 cells were deposited for each sample. Replicates were made on YPD plates and YPD plates containing increasing concentrations of HU. For each experiment, one YPD plate without HU was incubated at 37°C. Plates were incubated for three days at 30°C.
Synthetic lethality
Synthetic lethality between Pol δ mutant alleles was tested by using crosses, sporulation, tetrad dissection as well as genetic and PCR analyses. Meiotic segregation of a given allele was followed within tetrads by PCR analysis. Primers for pol3-ct: P13 (GCAGGAGAAAGTAGAACAATT) and P4187 (TACGCCTTCTTATGTAGCGC); Primers for pol3-R1043G: P217 (CATAAAGGCATTATACGATGTCG) and P4187; Primers for pol31-D304N: P180 (GATATTATGCCCGGAACCAATA) and P181 (TCAATCTTGACCGTCTCTGC); Primers for pol3-FI1002-1003AA: P182 (GGAGGCTTGATGAGCTTTATT) and P184 (CGGTCTCATTAGAATTGCAGC); Primers for pol31-YF327-328AA: P158 (AGTCCCTAGAATCAGCCGC) and P159 (GTCAATCTTGACCGTCTCTGC); Primers for pol32-FF344-345LL: P221 (GGAACATTGGAAAGCTTGTTG) and P222 (TGAGGGACAGAGAAGATTGG).
Yeast two-hybrid assays
The strain CTY10-5d (MATa, ade2-101, his3Δ200, leu2Δ1, trp1Δ901, gal4, gal80, URA3::lexAop-lacZ) was used for two-hybrid analysis. Pol31 was fused in frame with the lexA binding domain in plasmid pBTM116, while the Pol3 C-terminal amino-acids 1032–1097 were fused with the Gal4 activating domain of pACT2 to generate the plasmid pACT2-Pol3-ZnF2 [74]. The Pol3-R1043G, Pol31-YF327-328AA and Pol31-D304N mutations were introduced by site-directed mutagenesis and were confirmed by DNA sequencing. The resulting plasmids were called pACT2-Pol3-R1043G, pBTM116-Pol31-YF and pBTM116-Pol31-D304N, respectively. CTY10-5d was co-transformed with the pACT2 and pBTM116 plasmids that carry WT or mutated Pol3 CTD or Pol31 (Fig 5B). Cells were plated in -Leu -Trp selective medium supplemented with methionine at 22°C or 30°C for 3 to 4 days, and tested for β-galactosidase production in an overlay plate assay [59].
Isothermal Titration Calorimetry (ITC)
Recombinant S. cerevisiae PCNA with an His-Tag was produced in E coli using the pET28c bacterial expression vector (Novagen) as described in [4] and purified on a 5 ml HisTrap column (GE Healthcare). The synthetic peptides containing the PIP-box motifs of S. cerevisiae Pol3, Pol31, Pol32 and Msh6 were purchased from Genecust at 95% purity, and the concentrations of the stock peptide solutions were determined by amino acid composition.The interactions between PCNA and the different PIP-box peptides were determined by isothermal titration calorimetry (ITC) using a VP-ITC calorimeter (Microcal). Prior to measurements, all solutions were degassed under vacuum. The ITC reaction cell (vol 1.8 mL) was loaded with 20–25 μM of PCNA solution. The syringe (500 μL) was filled with the different PIP-box peptides at a concentration of 270 μM. Proteins were extensively dialyzed against buffer T (20 mM Tris [pH 7.5], 50 mM NaCl, and 20 mM β-mercaptoethanol) before ITC. The thermodynamic parameters ΔH, N, and Ka were obtained by non-linear least-squares fitting of the experimental data using the single set of independent binding sites model of the Origin software provided with the instrument. The free energy of binding (ΔG) and the entropy (ΔS) were determined using the classical thermodynamic formula, ΔG = -RT ln(Ka) and ΔG = ΔH -TΔS. All binding experiments were performed in duplicate or triplicate at 30°C.
Supporting information
Survival curves of haploid cells after exposure to UV light. (A) pol3-ct does not affect the UV sensitivity of rad51Δ cells. (B and C) pol3-ct marginally affects rev3Δ and rev1Δ cells UV sensitivity.
(EPS)
Survival curves of haploid cells after exposure to UV light. (A) pol3-ct partially suppresses UV sensitivity associated with rad5Δ. (B) pol3-ct does not suppress UV sensitivity of the rad5Δ rad30Δ double mutant. (C) pol3-ct does not suppress UV sensitivity of the rad5Δ rev3Δ double mutant.
(EPS)
(A) Single mutants and double mutants with pol3-ct. (B) Different genetic combinations between the pol3-FI, pol31YF and pol32-pip alleles. Note that the triple mutant in the W303 background is viable and not thermosensitive.
(EPS)
(A) Following sporulation of diploid cells, four-spore asci were dissected on YPD plates. Spores individually separated under the microscope formed colonies after incubation at 30°C for three days. Images of 12 dissected asci are shown. The relevant genotype of the haploid parental strains for each cross (symbolized by x) is indicated underneath the image of the exemplified dissection plate. Genetic and PCR analyses allowed following the meiotic segregation of each allele of interest in each tetrad (see Materials and Methods). Circles highlight spores that could not form a colony because they carry the two tested mutant alleles, thereby demonstrating the synthetic lethality between these alleles. The two alleles showed as examples are pol3-R1043G and pol32Δ. (B) Summary of the synthetic lethality found between mutant alleles of the genes that encode the three Pol δ subunits Pol3, Pol31 and Pol32. POL3 mutant alleles are shown in blue. POL31 mutant alleles are in red. POL32 mutant alleles are in green. SL: synthetic lethality between the tested alleles; Viable: spores carrying the two mutant alleles can grow. Note that pol3-ct and pol3-FI display similar phenotypes. Moreover, while pol32Δ shows synthetic lethality with all tested alleles, pol32-pip is viable. Finally, spores carrying pol3-FI, pol31-YF and pol32-pip simultaneously can form colonies.
(EPS)
Each dot represents the canR frequency obtained for one experiment for each strain. The median value for each strain is represented by a horizontal bar: ***, P = 0.0001 (Mann-Whitney test between WT and pol31-D304N).
(EPS)
Multiple sequence alignments of the potential motifs found in Pol3, Pol31 and Pol32 show the conservation of the Pol32 PIP residues and the lack of conservation for the sequences of the Pol3 and Pol31 motifs. The potential position of the canonical PIP-box residues is indicated by a star. Conservation of the residue at the PIP position is underlined in yellow. Color code: green for hydrophobic residues (V,L,I,M); light green for tryptophan (W); cyan for aromatic (F,Y); blue for basic residues (R,K); red for acidic residues (D,E); grey blue for polar (S,T,Q,N); brown for cysteine (C); orange for glycine (G); light blue for Alanine (A); light brown for proline (P); magenta for histidine (H).
(EPS)
(A) Schematic shows a stalled 3’end primer terminus at a pyrimidine dimer with PCNA ubiquitinatination (red circles) as a consequence of the stalling. Pol δ-ct residence time at the junction and on PCNA can be shorter than that of WT Pol δ. (B) The rad30Δ mutant is UV sensitive and pol3-ct partially suppresses this sensitivity. This suppression is no longer observed in the rad51Δ mutant, thereby suggesting that in this case, UV-induced DNA lesions can be channeled towards the HR pathway more frequently thanks to Pol3-ct (red dashed arrow). (C) In the rev3Δ and rev1Δ mutants, the error-prone pathway is abolished and the mutants display UV sensitivity. pol3-ct has a minor effect in these mutants and might weakly favor Pol η recruitment (dashed red arrow). (D) In the rad5Δ and mms2Δ mutants, the template switching (TS) pathway is defective. pol3-ct suppresses UV sensitivity associated with rad5Δ and mms2Δ, and this effect depends on Pol η and Pol ζ. Thus, UV DNA lesions are channeled to the TLS pathways in the Pol δ-ct mutant (red arrows). (E) In the rad51Δ mutant, pol3-ct has no effect upon UV radiation, thereby indicating no additional channeling of UV-induced DNA lesions from the HR pathway to the other DDT pathways.
(EPS)
(XLS)
Acknowledgments
We thank Dr Peter Burgers for his generous gift of plasmids carrying either REV1 or its rev1-12 mutant allele. We thank Dr. Louise Prakash for her generous gift of plasmids to allow the genomic integration of the pol3 mutations FI-1002-1003AA and pol31 mutations YF327-328AA. We are grateful to E. Despras, S. Marcand, T. Guerin and P. Radicella for critical and careful reading of the manuscript. We also appreciate the help of Elisabetta Andermarcher with the English editing.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The research was supported by the Agence Nationale de la Recherche (ANR-09-PIRI-0015-03 and ANR-10-INBS-05), by the Centre national de la recherche scientifique (recurrent funding) and the Commissariat aux Energies Atomiques et Alternatives (recurrent funding). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Waters LS, Minesinger BK, Wiltrout ME, D’Souza S, Woodruff R V, Walker GC. Eukaryotic translesion polymerases and their roles and regulation in DNA damage tolerance. Microbiol Mol Biol Rev. 2009;73: 134–154. doi: 10.1128/MMBR.00034-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pagès V, Fuchs RPP. How DNA lesions are turned into mutations within cells? Oncogene. 2002;21: 8957–8966. doi: 10.1038/sj.onc.1206006 [DOI] [PubMed] [Google Scholar]
- 3.Lehmann AR, Niimi A, Ogi T, Brown S, Sabbioneda S, Wing JF, et al. Translesion synthesis: Y-family polymerases and the polymerase switch. DNA Repair (Amst). 2007;6: 891–899. doi: 10.1016/j.dnarep.2007.02.003 [DOI] [PubMed] [Google Scholar]
- 4.Hoege C, Pfander B, Moldovan G-L, Pyrowolakis G, Jentsch S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature. 2002;419: 135–141. doi: 10.1038/nature00991 [DOI] [PubMed] [Google Scholar]
- 5.Bergink S, Jentsch S. Principles of ubiquitin and SUMO modifications in DNA repair. Nature. 2009;458: 461–467. doi: 10.1038/nature07963 [DOI] [PubMed] [Google Scholar]
- 6.Ulrich HD. Regulating post-translational modifications of the eukaryotic replication clamp PCNA. DNA Repair (Amst). 2009;8: 461–469. doi: 10.1016/j.dnarep.2009.01.006 [DOI] [PubMed] [Google Scholar]
- 7.Ulrich HD, Walden H. Ubiquitin signalling in DNA replication and repair. Nat Rev Mol Cell Biol. 2010;11: 479–489. doi: 10.1038/nrm2921 [DOI] [PubMed] [Google Scholar]
- 8.Stelter P, Ulrich HD. Control of spontaneous and damage-induced mutagenesis by SUMO and ubiquitin conjugation. Nature. 2003;425: 188–191. doi: 10.1038/nature01965 [DOI] [PubMed] [Google Scholar]
- 9.Yoon J-H, Prakash S, Prakash L. Requirement of Rad18 protein for replication through DNA lesions in mouse and human cells. Proc Natl Acad Sci. 2012;109: 7799–7804. doi: 10.1073/pnas.1204105109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torres-Ramos CA, Prakash S, Prakash L. Requirement of RAD5 and MMS2 for Postreplication Repair of UV-Damaged DNA in Saccharomyces cerevisiae. Mol Cell Biol. 2002;22: 2419–2426. doi: 10.1128/MCB.22.7.2419-2426.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warbrick E. PCNA binding through a conserved motif. BioEssays. 1998;20: 195–199. doi: 10.1002/(SICI)1521-1878(199803)20:3<195::AID-BIES2>3.0.CO;2-R [DOI] [PubMed] [Google Scholar]
- 12.Hingorani MM, O’Donnell M. Sliding clamps: A (tail)ored fit. Curr Biol. 2000;10: 25–29. doi: 10.1016/S0960-9822(99)00252-3 [DOI] [PubMed] [Google Scholar]
- 13.Klemperer N, Zhang D, Skangalis M, O’Donnell M. Cross-utilization of the β sliding clamp by replicative polymerases of evolutionary divergent organisms. J Biol Chem. 2000;275: 26136–26143. doi: 10.1074/jbc.M002566200 [DOI] [PubMed] [Google Scholar]
- 14.Bruning JB, Shamoo Y. Structural and thermodynamic analysis of human PCNA with peptides derived from DNA polymerase-delta p66 subunit and flap endonuclease-1. Structure. 2004;12: 2209–19. doi: 10.1016/j.str.2004.09.018 [DOI] [PubMed] [Google Scholar]
- 15.Haracska L, Kondratick CM, Unk I, Prakash S, Prakash L. Interaction with PCNA Is Essential for Yeast DNA Polymerase η Function. Mol Cell. 2001;8: 407–415. doi: 10.1016/S1097-2765(01)00319-7 [DOI] [PubMed] [Google Scholar]
- 16.Haracska L, Johnson RE, Unk I, Phillips B, Hurwitz J, Prakash L, et al. Physical and functional interactions of human DNA polymerase eta with PCNA. Mol Cell Biol. 2001;21: 7199–206. doi: 10.1128/MCB.21.21.7199-7206.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haracska L, Johnson RE, Unk I, Phillips BB, Hurwitz J, Prakash L, et al. Targeting of human DNA polymerase iota to the replication machinery via interaction with PCNA. Proc Natl Acad Sci U S A. 2001;98: 14256–61. doi: 10.1073/pnas.261560798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vidal AE, Kannouche P, Podust VN, Yang W, Lehmann AR, Woodgate R. Proliferating cell nuclear antigen-dependent coordination of the biological functions of human DNA polymerase iota. J Biol Chem. 2004;279: 48360–8. doi: 10.1074/jbc.M406511200 [DOI] [PubMed] [Google Scholar]
- 19.Ogi T, Kannouche P, Lehmann AR. Localisation of human Y-family DNA polymerase kappa: relationship to PCNA foci. J Cell Sci. 2005;118: 129–136. doi: 10.1242/jcs.01603 [DOI] [PubMed] [Google Scholar]
- 20.Yoon J-H, Acharya N, Park J, Basu D, Prakash S, Prakash L. Identification of two functional PCNA-binding domains in human DNA polymerase κ. Genes Cells. 2014;19: 594–601. doi: 10.1111/gtc.12156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masuda Y, Kanao R, Kaji K, Ohmori H, Hanaoka F, Masutani C. Different types of interaction between PCNA and PIP boxes contribute to distinct cellular functions of Y-family DNA polymerases. Nucleic Acids Res. 2015;43: 7898–7910. doi: 10.1093/nar/gkv712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo C, Sonoda E, Tang TS, Parker JL, Bielen AB, Takeda S, et al. REV1 protein interacts with PCNA: significance of the REV1 BRCT domain in vitro and in vivo. Mol Cell. 2006;23: 265–271. doi: 10.1016/j.molcel.2006.05.038 [DOI] [PubMed] [Google Scholar]
- 23.Bienko M, Green CM, Crosetto N, Rudolf F, Zapart G, Coull B, et al. Ubiquitin-Binding Domains in Y-Family Polymerases Regulate Translesion Synthesis. Science (80-). 2005;310: 1821–1824. doi: 10.1126/science.1120615 [DOI] [PubMed] [Google Scholar]
- 24.Plosky BS, Vidal AE, Fernández de Henestrosa AR, McLenigan MP, McDonald JP, Mead S, et al. Controlling the subcellular localization of DNA polymerases iota and eta via interactions with ubiquitin. EMBO J. 2006;25: 2847–2855. doi: 10.1038/sj.emboj.7601178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kannouche PL, Wing J, Lehmann AR. Interaction of human DNA polymerase eta with monoubiquitinated PCNA: a possible mechanism for the polymerase switch in response to DNA damage. Mol Cell. 2004;14: 491–500. doi:S109727650400259X [pii] [DOI] [PubMed] [Google Scholar]
- 26.Bomar MG, D’Souza S, Bienko M, Dikic I, Walker GC, Zhou P. Unconventional Ubiquitin Recognition by the Ubiquitin-Binding Motif within the Y Family DNA Polymerases iota and Rev1. Mol Cell. 2010;37: 408–417. doi: 10.1016/j.molcel.2009.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo C, Tang T-S, Bienko M, Dikic I, Friedberg EC. Requirements for the interaction of mouse Polkappa with ubiquitin and its biological significance. J Biol Chem. 2008;283: 4658–64. doi: 10.1074/jbc.M709275200 [DOI] [PubMed] [Google Scholar]
- 28.Parker JL, Bielen AB, Dikic I, Ulrich HD. Contributions of ubiquitin- and PCNA-binding domains to the activity of Polymerase η in Saccharomyces cerevisiae. Nucleic Acids Res. 2007;35: 881–889. doi: 10.1093/nar/gkl1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wood A, Garg P, Burgers PMJ. A ubiquitin-binding motif in the translesion DNA polymerase Rev1 mediates its essential functional interaction with ubiquitinated proliferating cell nuclear antigen in response to DNA damage. J Biol Chem. 2007;282: 20256–63. doi: 10.1074/jbc.M702366200 [DOI] [PubMed] [Google Scholar]
- 30.Garg P, Burgers PM. Ubiquitinated proliferating cell nuclear antigen activates translesion DNA polymerases eta and REV1. Proc Natl Acad Sci U S A. 2005;102: 18361–18366. doi: 10.1073/pnas.0505949102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hedglin M, Pandey B, Benkovic SJ. Stability of the human polymerase δ holoenzyme and its implications in lagging strand DNA synthesis. Proc Natl Acad Sci. 2016; 201523653. doi: 10.1073/pnas.1523653113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhuang Z, Johnson RE, Haracska L, Prakash L, Prakash S, Benkovic SJ. Regulation of polymerase exchange between Pol eta and Pol delta by monoubiquitination of PCNA and the movement of DNA polymerase holoenzyme. Proc Natl Acad Sci U S A. 2008;105: 5361–5366. doi: 10.1073/pnas.0801310105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masuda Y, Piao J, Kamiya K. DNA Replication-Coupled PCNA Mono-Ubiquitination and Polymerase Switching in a Human In Vitro System. J Mol Biol. 2010;396: 487–500. doi: 10.1016/j.jmb.2010.01.003 [DOI] [PubMed] [Google Scholar]
- 34.Trincao J, Johnson RE, Escalante CR, Prakash S, Prakash L, Aggarwal AK. Structure of the Catalytic Core of S. cerevisiae DNA Polymerase η: Implications for Translesion DNA Synthesis. Mol Cell. 2001;8: 417–426. doi: 10.1016/S1097-2765(01)00306-9 [DOI] [PubMed] [Google Scholar]
- 35.Bomar MG, Pai M-T, Tzeng S-R, Li SS-C, Zhou P. Structure of the ubiquitin-binding zinc finger domain of human DNA Y-polymerase eta. EMBO Rep. 2007;8: 247–251. doi: 10.1038/sj.embor.7400901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hishiki A, Hashimoto H, Hanafusa T, Kamei K, Ohashi E, Shimizu T, et al. Structural basis for novel interactions between human translesion synthesis polymerases and proliferating cell nuclear antigen. J Biol Chem. 2009;284: 10552–10560. doi: 10.1074/jbc.M809745200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freudenthal BD, Gakhar L, Ramaswamy S, Washington MT. Structure of monoubiquitinated PCNA and implications for translesion synthesis and DNA polymerase exchange. Nat Struct Mol Biol. 2010;17: 479–484. doi: 10.1038/nsmb.1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Z, Zhang S, Lin SHS, Wang X, Wu L, Lee EYC, et al. Structure of monoubiquitinated PCNA: implications for DNA polymerase switching and Okazaki fragment maturation. Cell Cycle. 2012;11: 2128–36. doi: 10.4161/cc.20595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roche H, Gietz RD, Kunz BA. Specificities of the Saccharomyces cerevisiae rad6, rad18, and rad52 mutators exhibit different degrees of dependence on the REV3 gene product, a putative nonessential DNA polymerase. Genetics. 1995;140: 443–456. Available: http://www.ncbi.nlm.nih.gov/pubmed/7498727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liefshitz B, Steinlauf R, Friedl A, Eckardt-Schupp F, Kupiec M. Genetic interactions between mutants of the “error-prone” repair group of Saccharomyces cerevisiae and their effect on recombination and mutagenesis. Mutat Res. 1998;407: 135–145. doi: 10.1016/S0921-8777(97)00070-0 [DOI] [PubMed] [Google Scholar]
- 41.Minesinger BK, Jinks-Robertson S. Roles of RAD6 epistasis group members in spontaneous polzeta-dependent translesion synthesis in Saccharomyces cerevisiae. Genetics. 2005;169: 1939–55. doi: 10.1534/genetics.104.033894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nikolaishvili-Feinberg N, Jenkins GS, Nevis KR, Staus DP, Scarlett CO, Unsal-Kaçmaz K, et al. Ubiquitylation of proliferating cell nuclear antigen and recruitment of human DNA polymerase eta. Biochemistry. 2008;47: 4141–50. doi: 10.1021/bi702329h [DOI] [PubMed] [Google Scholar]
- 43.Schmutz V, Janel-Bintz R, Wagner J, Biard D, Shiomi N, Fuchs RP, et al. Role of the ubiquitin-binding domain of Polη in Rad18-independent translesion DNA synthesis in human cell extracts. Nucleic Acids Research. 2010. pp. 6456–6465. doi: 10.1093/nar/gkq403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hendel A, Krijger PHL, Diamant N, Goren Z, Langerak P, Kim J, et al. PCNA ubiquitination is important, but not essential for translesion DNA synthesis in mammalian cells. PLoS Genet. 2011;7: 1–13. doi: 10.1371/journal.pgen.1002262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krijger PHL, Van Den Berk PCM, Wit N, Langerak P, Jansen JG, Reynaud CA, et al. PCNA ubiquitination-independent activation of polymerase η during somatic hypermutation and DNA damage tolerance. DNA Repair (Amst). 2011;10: 1051–1059. doi: 10.1016/j.dnarep.2011.08.005 [DOI] [PubMed] [Google Scholar]
- 46.Wit N, Buoninfante OA, van den Berk PCM, Jansen JG, Hogenbirk MA, de Wind N, et al. Roles of PCNA ubiquitination and TLS polymerases κ and η in the bypass of methyl methanesulfonate-induced DNA damage. Nucleic Acids Res. 2015;43: 282–94. doi: 10.1093/nar/gku1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okada T, Sonoda E, Yamashita YM, Koyoshi S, Tateishi S, Yamaizumi M, et al. Involvement of vertebrate polkappa in Rad18-independent postreplication repair of UV damage. J Biol Chem. 2002;277: 48690–5. doi: 10.1074/jbc.M207957200 [DOI] [PubMed] [Google Scholar]
- 48.Edmunds CE, Simpson LJ, Sale JE. PCNA Ubiquitination and REV1 Define Temporally Distinct Mechanisms for Controlling Translesion Synthesis in the Avian Cell Line DT40. Mol Cell. 2008;30: 519–529. doi: 10.1016/j.molcel.2008.03.024 [DOI] [PubMed] [Google Scholar]
- 49.Krishna SS, Majumdar I, Grishin N V. Structural classification of zinc fingers. Nucleic Acids Res. 2003;31: 532–550. doi: 10.1093/nar/gkg161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Netz DJA, Stith CM, Stümpfig M, Köpf G, Vogel D, Genau HM, et al. Eukaryotic DNA polymerases require an iron-sulfur cluster for the formation of active complexes. Nat Chem Biol. 2011;8: 125–132. doi: 10.1038/nchembio.721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gerik KJ, Li X, Pautz A, Burgers PMJ. Characterization of the Two Small Subunits of Saccharomyces cerevisiae DNA Polymerase δ. J Biol Chem. 1998;273: 19747–19755. doi: 10.1074/jbc.273.31.19747 [DOI] [PubMed] [Google Scholar]
- 52.Johansson E, Garg P, Burgers PMJ. The Pol32 Subunit of DNA Polymerase δ Contains Separable Domains for Processive Replication and Proliferating Cell Nuclear Antigen (PCNA) Binding. J Biol Chem. 2004;279: 1907–1915. doi: 10.1074/jbc.M310362200 [DOI] [PubMed] [Google Scholar]
- 53.Acharya N, Klassen R, Johnson RE, Prakash L, Prakash S. PCNA binding domains in all three subunits of yeast DNA polymerase δ modulate its function in DNA replication. Proc Natl Acad Sci. 2011;108: 17927–17932. doi: 10.1073/pnas.1109981108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baranovskiy AG, Lada AG, Siebler HM, Zhang Y, Pavlov YI, Tahirov TH. DNA polymerase δ and ζ switch by sharing accessory subunits of DNA polymerase δ. J Biol Chem. 2012;287: 17281–17287. doi: 10.1074/jbc.M112.351122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnson RE, Prakash L, Prakash S. Pol31 and Pol32 subunits of yeast DNA polymerase δ are also essential subunits of DNA polymerase ζ. Proc Natl Acad Sci. 2012;109: 12455–12460. doi: 10.1073/pnas.1206052109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Makarova A V, Stodola JL, Burgers PM. A four-subunit DNA polymerase ζ complex containing Pol δ accessory subunits is essential for PCNA-mediated mutagenesis. Nucleic Acids Res. 2012;40: 11618–11626. doi: 10.1093/nar/gks948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daraba A, Gali VK, Halmai M, Haracska L, Unk I. Def1 Promotes the Degradation of Pol3 for Polymerase Exchange to Occur During DNA-Damage-Induced Mutagenesis in Saccharomyces cerevisiae. PLoS Biol. 2014;12 doi: 10.1371/journal.pbio.1001771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maloisel L, Bhargava J, Roeder GS. A role for DNA polymerase delta in gene conversion and crossing over during meiosis in Saccharomyces cerevisiae. Genetics. 2004;167: 1133–42. doi: 10.1534/genetics.104.026260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brocas C, Charbonnier J-B, Dhérin C, Gangloff S, Maloisel L. Stable interactions between DNA polymerase δ catalytic and structural subunits are essential for efficient DNA repair. DNA Repair (Amst). 2010;9: 1098–1111. doi: 10.1016/j.dnarep.2010.07.013 [DOI] [PubMed] [Google Scholar]
- 60.Schiestl RH, Prakash S, Prakash L. The SRS2 suppressor of rad6 mutations of Saccharomyces cerevisiae acts by channeling DNA lesions into the RAD52 DNA repair pathway. Genetics. 1990;124: 817–31. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1203974&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Papouli E, Chen SH, Davies AA, Huttner D, Krejci L, Sung P, et al. Crosstalk between SUMO and ubiquitin on PCNA is mediated by recruitment of the helicase Srs2p. Mol Cell. 2005;19: 123–133. doi: 10.1016/j.molcel.2005.06.001 [DOI] [PubMed] [Google Scholar]
- 62.Pfander B, Moldovan GL, Sacher M, Hoege C, Jentsch S. SUMO-modified PCNA recruits Srs2 to prevent recombination during S phase. Nature. 2005;436: 428–433. doi: 10.1038/nature03665 [DOI] [PubMed] [Google Scholar]
- 63.Lawrence CW, Maher VM. Mutagenesis in eukaryotes dependent on DNA polymerase zeta and Rev1p. Philos Trans R Soc Lond B Biol Sci. 2001;356: 41–6. doi: 10.1098/rstb.2000.0746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cassier-Chauvat C, Fabre F. A similar defect in UV-induced mutagenesis conferred by the rad6 and rad18 mutations of Saccharomyces cerevisiae. Mutat Res Repair. 1991;254: 247–253. doi: 10.1016/0921-8777(91)90063-U [DOI] [PubMed] [Google Scholar]
- 65.Armstrong JD, Chadee DN, Kunz BA. Roles for the yeast RAD18 and RAD52 DNA repair genes in UV mutagenesis. Mutat Res Repair. 1994;315: 281–293. doi: 10.1016/0921-8777(94)90039-6 [DOI] [PubMed] [Google Scholar]
- 66.Klinge S, Núñez-Ramírez R, Llorca O, Pellegrini L. 3D architecture of DNA Pol alpha reveals the functional core of multi-subunit replicative polymerases. EMBO J. 2009;28: 1978–1987. doi: 10.1038/emboj.2009.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baranovskiy AG, Babayeva ND, Liston VG, Rogozin IB, Koonin E V, Pavlov YI, et al. X-ray structure of the complex of regulatory subunits of human DNA polymerase delta. Cell cycle. 2008;7: 3026–3036. doi:6720 [pii] doi: 10.4161/cc.7.19.6720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hashimoto K, Nakashima N, Ohara T, Maki S, Sugino A. The second subunit of DNA polymerase III (delta) is encoded by the HYS2 gene in Saccharomyces cerevisiae. Nucl Acids Res. 1998;26: 477–485. doi: 10.1093/nar/26.2.477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boehm EM, Powers KT, Kondratick CM, Spies M, Houtman JCD, Washington MT. The proliferating cell nuclear antigen (PCNA)-interacting Protein (PIP) motif of DNA polymerase η Mediates Its interaction with the C-terminal domain of Rev1. J Biol Chem. 2016;291: 8735–8744. doi: 10.1074/jbc.M115.697938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liberti SE, Andersen SD, Wang J, May A, Miron S, Perderiset M, et al. Bi-directional routing of DNA mismatch repair protein human exonuclease 1 to replication foci and DNA double strand breaks. DNA Repair (Amst). 2011;10: 73–86. doi: 10.1016/j.dnarep.2010.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bacquin A, Pouvelle C, Siaud N, Perderiset M, Salomé-Desnoulez S, Tellier-Lebegue C, et al. The helicase FBH1 is tightly regulated by PCNA via CRL4(Cdt2)-mediated proteolysis in human cells. Nucleic Acids Res. 2013;41: 6501–6513. doi: 10.1093/nar/gkt397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lis ET, O'Neill BM, Gil-Lamaignere C, Chin JK, Romesberg FE. Identification of pathways controlling DNA damage induced mutation in Saccharomyces cerevisiae. DNA Repair (Amst). 2008;7: 801–810. doi: 10.1016/j.dnarep.2008.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hirota K, Tsuda M, Mohiuddin, Tsurimoto T, Cohen IS, Livneh Z, et al. In vivo evidence for translesion synthesis by the replicative DNA polymerase δ Nucleic Acids Res. 2016;44: 7242–7250. doi: 10.1093/nar/gkw439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Garcia JS, Ciufo LF, Yang X, Kearsey SE, MacNeill S a. The C-terminal zinc finger of the catalytic subunit of DNA polymerase δ is responsible for direct interaction with the B-subunit. Nucleic Acids Res. 2004;32: 3005–3016. doi: 10.1093/nar/gkh623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li H, Xie B, Zhou Y, Rahmeh A, Trusa S, Zhang S, et al. Functional roles of p12, the fourth subunit of human DNA polymerase delta. J Biol Chem. 2006;281: 14748–14755. doi: 10.1074/jbc.M600322200 [DOI] [PubMed] [Google Scholar]
- 76.Wang Y, Zhang Q, Chen H, Li X, Mai W, Chen K, et al. P50, the small subunit of DNA polymerase delta, is required for mediation of the interaction of polymerase delta subassemblies with PCNA. PLoS One. 2011;6 doi: 10.1371/journal.pone.0027092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Giot L, Chanet R, Simon M, Facca C, Faye G. Involvement of the yeast DNA polymerase delta in DNA repair in vivo. Genetics. 1997;146: 1239–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Walsh E, Wang X, Lee MY, Eckert KA. Mechanism of replicative DNA polymerase delta pausing and a potential role for DNA polymerase kappa in common fragile site replication. J Mol Biol. 2013;425: 232–243. doi: 10.1016/j.jmb.2012.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boyer AS, Grgurevic S, Cazaux C, Hoffmann JS. The human specialized DNA polymerases and non-B DNA: Vital relationships to preserve genome integrity. Journal of Molecular Biology. 2013. pp. 4767–4781. doi: 10.1016/j.jmb.2013.09.022 [DOI] [PubMed] [Google Scholar]
- 80.Despras E, Sittewelle M, Pouvelle C, Delrieu N, Cordonnier AM, Kannouche PL. Rad18-dependent SUMOylation of human specialized DNA polymerase eta is required to prevent under-replicated DNA. Nat Commun. 2016;7: 13326 doi: 10.1038/ncomms13326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thomas BJ, Rothstein R. The genetic control of direct-repeat recombination in Saccharomyces: the effect of rad52 and rad1 on mitotic recombination at GAL10, a transcriptionally regulated gene. Genetics. 1989;123: 725–38. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1203884&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baudin a, Ozier-Kalogeropoulos O, Denouel a, Lacroute F, Cullin C. A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae. Nucleic Acids Res. 1993;21: 3329–3330. doi: 10.1093/nar/21.14.3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Longtine MS, McKenzie A, Demarini DJ, Shah NG, Wach A, Brachat A, et al. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14: 953–61. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U [DOI] [PubMed] [Google Scholar]
- 84.Rothstein R. Targeting, disruption, replacement, and allele rescue: Integrative DNA transformation in yeast. Methods Enzymol. 1991;194: 281–301. doi: 10.1016/0076-6879(91)94022-5 [DOI] [PubMed] [Google Scholar]
- 85.Sherman F. Getting started with yeast. Methods Enzymol. 2002;350: 3–41. doi: 10.1016/S0076-6879(02)50954-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Survival curves of haploid cells after exposure to UV light. (A) pol3-ct does not affect the UV sensitivity of rad51Δ cells. (B and C) pol3-ct marginally affects rev3Δ and rev1Δ cells UV sensitivity.
(EPS)
Survival curves of haploid cells after exposure to UV light. (A) pol3-ct partially suppresses UV sensitivity associated with rad5Δ. (B) pol3-ct does not suppress UV sensitivity of the rad5Δ rad30Δ double mutant. (C) pol3-ct does not suppress UV sensitivity of the rad5Δ rev3Δ double mutant.
(EPS)
(A) Single mutants and double mutants with pol3-ct. (B) Different genetic combinations between the pol3-FI, pol31YF and pol32-pip alleles. Note that the triple mutant in the W303 background is viable and not thermosensitive.
(EPS)
(A) Following sporulation of diploid cells, four-spore asci were dissected on YPD plates. Spores individually separated under the microscope formed colonies after incubation at 30°C for three days. Images of 12 dissected asci are shown. The relevant genotype of the haploid parental strains for each cross (symbolized by x) is indicated underneath the image of the exemplified dissection plate. Genetic and PCR analyses allowed following the meiotic segregation of each allele of interest in each tetrad (see Materials and Methods). Circles highlight spores that could not form a colony because they carry the two tested mutant alleles, thereby demonstrating the synthetic lethality between these alleles. The two alleles showed as examples are pol3-R1043G and pol32Δ. (B) Summary of the synthetic lethality found between mutant alleles of the genes that encode the three Pol δ subunits Pol3, Pol31 and Pol32. POL3 mutant alleles are shown in blue. POL31 mutant alleles are in red. POL32 mutant alleles are in green. SL: synthetic lethality between the tested alleles; Viable: spores carrying the two mutant alleles can grow. Note that pol3-ct and pol3-FI display similar phenotypes. Moreover, while pol32Δ shows synthetic lethality with all tested alleles, pol32-pip is viable. Finally, spores carrying pol3-FI, pol31-YF and pol32-pip simultaneously can form colonies.
(EPS)
Each dot represents the canR frequency obtained for one experiment for each strain. The median value for each strain is represented by a horizontal bar: ***, P = 0.0001 (Mann-Whitney test between WT and pol31-D304N).
(EPS)
Multiple sequence alignments of the potential motifs found in Pol3, Pol31 and Pol32 show the conservation of the Pol32 PIP residues and the lack of conservation for the sequences of the Pol3 and Pol31 motifs. The potential position of the canonical PIP-box residues is indicated by a star. Conservation of the residue at the PIP position is underlined in yellow. Color code: green for hydrophobic residues (V,L,I,M); light green for tryptophan (W); cyan for aromatic (F,Y); blue for basic residues (R,K); red for acidic residues (D,E); grey blue for polar (S,T,Q,N); brown for cysteine (C); orange for glycine (G); light blue for Alanine (A); light brown for proline (P); magenta for histidine (H).
(EPS)
(A) Schematic shows a stalled 3’end primer terminus at a pyrimidine dimer with PCNA ubiquitinatination (red circles) as a consequence of the stalling. Pol δ-ct residence time at the junction and on PCNA can be shorter than that of WT Pol δ. (B) The rad30Δ mutant is UV sensitive and pol3-ct partially suppresses this sensitivity. This suppression is no longer observed in the rad51Δ mutant, thereby suggesting that in this case, UV-induced DNA lesions can be channeled towards the HR pathway more frequently thanks to Pol3-ct (red dashed arrow). (C) In the rev3Δ and rev1Δ mutants, the error-prone pathway is abolished and the mutants display UV sensitivity. pol3-ct has a minor effect in these mutants and might weakly favor Pol η recruitment (dashed red arrow). (D) In the rad5Δ and mms2Δ mutants, the template switching (TS) pathway is defective. pol3-ct suppresses UV sensitivity associated with rad5Δ and mms2Δ, and this effect depends on Pol η and Pol ζ. Thus, UV DNA lesions are channeled to the TLS pathways in the Pol δ-ct mutant (red arrows). (E) In the rad51Δ mutant, pol3-ct has no effect upon UV radiation, thereby indicating no additional channeling of UV-induced DNA lesions from the HR pathway to the other DDT pathways.
(EPS)
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.