Abstract
Background
Yaws is a neglected tropical disease, caused by Treponema pallidum subsp. pertenue. The disease causes chronic lesions, primarily in young children living in remote villages in tropical climates. As part of a global yaws eradication campaign initiated by the World Health Organization, we sought to develop and evaluate a molecular typing method to distinguish different strains of T. pallidum subsp. pertenue for disease control and epidemiological purposes.
Methods and principal findings
Published genome sequences of strains of T. pallidum subsp. pertenue and pallidum were compared to identify polymorphic genetic loci among the strains. DNA from a number of existing historical Treponema isolates, as well as a subset of samples from yaws patients collected in Lihir Island, Papua New Guinea, were analyzed using these targets. From these data, three genes (tp0548, tp0136 and tp0326) were ultimately selected to give a high discriminating capability among the T. pallidum subsp. pertenue samples tested. Intragenic regions of these three target genes were then selected to enhance the discriminating capability of the typing scheme using short readily amplifiable loci. This 3-gene multilocus sequence typing (MLST) method was applied to existing historical human yaws strains, the Fribourg-Blanc simian isolate, and DNA from 194 lesion swabs from yaws patients on Lihir Island, Papua New Guinea. Among all samples tested, fourteen molecular types were identified, seven of which were found in patient samples and seven among historical isolates or DNA. Three types (JG8, TD6, and SE7) were predominant on Lihir Island.
Conclusions
This MLST approach allows molecular typing and differentiation of yaws strains. This method could be a useful tool to complement epidemiological studies in regions where T. pallidum subsp. pertenue is prevalent with the overall goals of improving our understanding of yaws transmission dynamics and helping the yaws eradication campaign to succeed.
Author summary
Yaws is a neglected treponemal infection that is often transmitted among children in developing countries. Eradication programs in the 1940–50’s significantly reduced the incidence of yaws, but the disease has resurged. The World Health Organization has proposed to eliminate yaws by 2020, and mass treatment trials are underway in a number of countries. To assist in investigating the molecular epidemiology of yaws, we propose a new method for differentiating strains of the causative agent, Treponema pallidum subsp. pertenue. Using this typing method, we identified seven molecular types in yaws patients from a small island in Papua New Guinea. This method may prove useful in clarifying reinfection vs. relapse, in detecting cases newly imported into a village, in tracking the development of macrolide resistant strains, and in helping to define the transmission of yaws strains within a region.
Introduction
Yaws is a highly contagious treponemal infection caused by the bacterium Treponema pallidum subsp. pertenue (T.p. pertenue). It is transmitted by direct skin contact and is symptomatic predominantly in children <15 years of age, usually manifesting as chronic ulcers on the extremities. Latent, or inapparent, infection can persist for decades, often re-emerging as skin lesions or causing painful bone and joint damage [1,2]. Yaws continues to be endemic in a number of tropical countries, particularly in rural regions with lack of public health surveillance. In 2012, the World Health Organization (WHO) proposed a program to eradicate yaws by 2020 [3] using mass drug administration (MDA) with single dose azithromycin. To aid in post-MDA surveillance, a molecular typing scheme is needed to discriminate among T.p. pertenue strains, thus permitting investigators to track the movement of genetically distinct strains in populations and to identify strains newly introduced to already-treated populations. Careful molecular epidemiological studies using typing can assist in understanding the dynamics of disease transmission to improve control of future outbreaks.
T.p. pertenue is closely related to T. pallidum subsp. pallidum, the causative agent of venereal syphilis, which differs from pertenue by less than 0.2% of their genome sequences [4]. These subspecies are indistinguishable serologically and morphologically [1,2], but can be differentiated on the basis of molecular signatures [4–9]. For a number of years, molecular typing has been used worldwide for typing treponemes from syphilis patients. This method is based upon 1) the number of 60-base pair repeats in the acidic repeat gene (arp) gene (tp0433); 2) the restriction fragment length pattern of the Subfamily II Treponema pallidum repeat (tpr) E, G, and J genes (tp0313, tp0317, and tp0621, respectively) [10], and 3) is enhanced by inclusion of the sequence of a polymorphic 300 bp region of the tp0548 gene [11]. This typing scheme has been adopted globally in recent years to create a molecular epidemiology database for syphilis, and also to analyze linkage of specific T.p. pallidum molecular types to specific disease manifestations [11,12]. Nonetheless, the 1) well-recognized difficulty in amplifying the arp and tprE/G/J loci in samples where treponemal DNA is not abundant, 2) the concerns that amplification of the arp target might yield inconsistent results [13,14], and 3) the difficulty that sometimes arises in identifying unambiguously the tprE/G/J restriction patterns have prompted investigators to propose modifications to the typing approach. These include multilocus sequence typing (MLST) approaches with the capability of discriminating genetic differences among syphilis strains without the risk of ambiguous results. New target loci have included tp0136 [5,8,15–17] and tp0279 [18]. Compared to typing methods that rely on restriction fragment length polymorphisms or analysis of tandem repeats, a MLST of proven efficacy would also be more likely to be routinely adopted in research and clinical laboratories.
To provide a better understanding of the current yaws status and to guide control efforts, development of a molecular typing method for T.p. pertenue is highly desirable. Therefore, we sought a sequenced-based typing method using small gene regions that can readily be amplified even from clinical samples with low concentrations of T. pallidum DNA and whose analysis could unambiguously identify yaws isolates carrying different genetic signatures in these loci.
We propose a MLST method for differentiating T.p. pertenue strains using defined regions of tp0548, tp0136 and tp0326. Each of these genes codes for putative (tp0548) or bona fide (tp0136 and tp0326) treponemal surface-exposed proteins shown to be implicated in maintaining the homeostasis of the bacterial cell envelope (tp0326) [19,20], in mediating adhesion to host components (tp0136) [8,21], or hypothesized to mediate nutrient acquisition (tp0548). These typing targets yield a highly discriminating molecular method for distinguishing T.p. pertenue strains.
Methods
Sources of T. pallidum subsp. pertenue strains
Historical T.p. pertenue isolates (Table 1) were propagated in New Zealand white rabbits by intratesticular inoculation as previously described [22]. DNA was extracted for PCR amplification using the QIAamp DNA Mini Kit (Qiagen, Valencia CA) following the manufacturer’s instructions, but adding 50 μl of proteinase K (100 mg/ml stock solution) instead of 20 μl and incubating the sample for 2 hours at 56°C. Samples were eluted in 200 μl of H2O and stored at -20C until used for PCR.
Table 1. Treponema pallidum subsp. pertenue strains used in this study.
| Strain name | Source | Location | Year of isolation | Ref. | |
|---|---|---|---|---|---|
| Human isolates of T.p. pertenue |
Gauthier a | Skin lesion | Nigeria | 1963 | [23] |
| CDC1b | Skin lesion | Densuso, Ghana | 1980 | [24] | |
| CDC2b | Skin lesion | Akorabo, Ghana | 1980 | [24] | |
| Samoa D c | Skin | Western Samoa | 1953 | [25] | |
| Samoa Fc | Skin | Western Samoa | 1953 | [25] | |
| Ghana051d | Unknown | Ghana | 1988 | [26] | |
| Simian isolate | Fribourg-Blanc c | Lymph node | Guinea | 1966 | [27] |
a Provided by Peter Perine, Centers for Diseases Control and Prevention, Atlanta, GA.
b Provided by Robert George and Victoria Pope, Centers for Disease Control and Prevention, Atlanta, GA.
c Provided by Paul Hardy and Ellen Nell, Johns Hopkins University, Baltimore, MD.
d Provided by Leo Schouls, National Institute for Public Health and Environment, Bilthoven, and Gerda Noordhoek, Public Health Laboratory, Friesland, The Netherlands. DNA only, no known isolate in existence.
Patient samples
Swab samples containing T.p. pertenue were collected from study participants with exudative skin ulcers in Lihir Island, Papua New Guinea (PNG), during a yaws elimination campaign, between May 2013 and October 2016. Following baseline examination and sample collection, mass treatment with single dose azithromycin was administered. Treatment coverage was 84%. The population was re-examined at 6 month intervals for 42 months. At re-examination, swabs were collected from individuals with yaws-like ulcers, and targeted azithromycin treatment was provided to these persons and their family/childhood contacts. Details of the study have been published elsewhere [28,29]. Immediately after collection, the swabs were placed in 1 ml of 1x lysis buffer (10mM Tris-HCl, 0.1mM EDTA, 0.5% SDS), frozen, and transported to the University of Washington. DNA was extracted using the QIAamp DNA Mini Kit (Qiagen) according to the manufacturer’s instructions. Presence of T. pallidum DNA was assessed initially by PCR of the Tp47 gene (tp0574), which is conserved in all Treponema subspecies, including pertenue. Samples positive for T. pallidum DNA underwent amplification of the tp1031 (tprL) gene as previously described [30] and the amplicon size was used to determine the pertenue (vs. pallidum) subspecies. All T. pallidum-positive samples from Lihir Island (n = 232 with duplicates removed), corresponding to 30.7% of all lesion samples analyzed) were confirmed as T.p. pertenue and were used for molecular typing studies. Of these samples, 83.6% (n = 194) were fully typeable with our approach.
Ethics statement
Participants with suspected yaws or, for young children, their parents or guardians provided written consent for inclusion in clinical surveys and etiological studies, including collection of swabs used in this study. The study was approved by the National Medical Research Advisory Committee of the Papua New Guinea Ministry of Health (MRAC no. 12.36). Only coded samples were sent to the University of Washington for testing.
Evaluation of typing targets, amplification, sequencing, and sequence analysis
Based upon published T.p. pallidum and pertenue genome sequences, we evaluated a number of genes that are polymorphic among strains; these included tp0136, tp0548, bamA (tp0326), tprC (tp0117), tprD (tp0131), and tp0619. Published full length sequences of these genes were initially examined from three human T.p. pertenue strains (Gauthier, CDC2, Samoa D; Table 1) and the Fribourg-Blanc simian isolate. The members of the tpr gene family (tprC and tprD) were not examined further due to the high homology between these two genes and other members of the tpr family, making specific amplification problematic. With the exception of tp0548, which has an already-identified region that is used for T. pallidum subsp. pallidum typing, we identified, within each of the remaining targets (tp0136, tp0326, and tp0619), regions containing polymorphisms potentially suitable to differentiate the strains. Primers were designed to amplify large regions of these genes for preliminary sequence analysis (Table 2). From 95 T.p. pertenue-positive PNG samples collected from baseline through 18 months of the study, we were able to successfully amplify and obtain good sequences from 66 (69%) samples for tp0619, 87 (92%) for tp0136, and 42 (44%) for tp0326. PCR amplifications for these targets were performed using genomic DNA in a 50-μl final volume containing 200 μM deoxynucleoside triphosphates, 1.5 mM MgCl2, 0.8 μM primers (Table 2) and 2.5 U of GoTaq DNA polymerase (Promega, Madison, WI). Cycling conditions for the tp0136 PCR were 95°C for 3 mins, then 45 cycles of 95°C for 1 min, 60°C for 2 min, 72°for 1 min; followed by 72°C for 10 mins. The conditions for tp0326 were 95°C for 3 mins, then 45 cycles of 95°C for 1 min, 56°C for 1 min, 72°for 1 min; followed by 72°C for 10 mins. For tp0619, cycling conditions were 95°C for 5 mins, then 45 cycles of 95°C for 1 min, 55°C for 1 min, 72°for 1 min; followed by 72°C for 10 mins.
Table 2. Primers used for the preliminary analysis of a subset of PNG samples.
| Gene | Primer | Sequence 5’-3’ | Product Size (nt) | ORF Region (nt) |
|---|---|---|---|---|
| tp0619 | Sense | 5’-TACAAGCTCCCACAATGCCA-3’ | ||
| Antisense | 5’-TTACCCAGACATTTTCTCCACATA-3’ | 6531 | 224–798 | |
| Sequencing | 5’-TACAAGCTCCCACAATGCCA-3’ | |||
| tp0136 | Sense | 5’-GAAGAGGGCGTTTTGTG-3’ | ||
| Antisense2 | 5’-CTCCCAGCTCAGCCGAATCTC-3’ | 16702 | 1–1485 | |
| Sequencing | 5’-GAAGAGGGCGTTTTGTG-3’ | |||
| 5’-CTCCCAGCTCAGCCGAATCTC-3’ | ||||
| 5’-CCATCCAGTCGGAAGTGC-3’ | ||||
| 5’-AACTACGTAGATTTTCTGCAC-3’ | ||||
| tp0326 | Sense | 5’-CTGACGGTGGGCTTTGAC-3’ | ||
| Antisense3 | 5’-GCATCTATGACGGCAAAGCG-3’ | 11693 | 1538–2559 | |
| Sequencing | 5’-AGCACGCCGTCTATTACCAG-3’ | |||
| 5’-AAGAGCATTCGTTTCGCTCC-3’ | ||||
| 5’-CTGACGGTGGGCTTTGAC-3’ |
1 Amplicon also contains tp0618-tp0619 intergenic region (58 nt) and 20 nt of tp0618
2 Amplicon also contains tp0135-tp0136 intergenic region (20 nt at 5’-end of tp0136) and 165 nt of tp0137
3 Amplicon also contains 148 nt of tp0327
Based on the alignments from the historical strains and 95 initial PNG samples, tp0619 proved not to be a suitable typing target: all historical strains had the same tp0619 sequence and there were 5 types identified in the amplified PNG samples (S1 Fig). In comparison, even from the low number of tp0326 sequences that we obtained with these initial primers, we were able to identify 8 tp0326 types. Thus, the three targets selected for further investigation were tp0548, tp0136, and tp0326. Based upon analysis of these large amplicons, we identified relatively short regions containing polymorphisms yielding the maximum number of unique “types” among the samples tested, and selected those as typing targets for our MLST protocol. Primers, amplicon size, and region identifications are shown in Table 3. Amplifications of these targets (tp0548, tp0136, and tp0326 gene fragments) were performed using genomic DNA in a 50-μl final volume containing 200 μM deoxynucleoside triphosphates, 1.5 mM MgCl2, 0.8 μM primers (Table 3) and 2.5 U of GoTaq DNA polymerase (Promega, Madison, WI). Thermocycling conditions for tp0548 have previously been described [11]. Conditions for the tp0136 PCR were 95°C for 3 mins, then 45 cycles of 95°C for 1 min, 59°C for 2 min, 72°for 1 min; followed by 72°C for 10 mins. The conditions for tp0326 were 95°C for 5 mins, then 45 cycles of 95°C for 1 min, 58°C for 1 min, 72°for 1 min; followed by 72°C for 10 mins. All amplified products were treated with ExoSAP-IT PCR Product Cleanup Reagent (Affymetrix, Santa Clara CA) for dye deoxy terminator sequencing in one direction. If ambiguities in base-calling were seen in the electropherograms, we repeated the sequencing in both directions, repeating the PCR when necessary. Further, all gene alleles described in our study were found in more than one clinical sample, thus providing confidence that the typing sequences are correct.
Table 3. Primers used for the MLST scheme.
| Gene | Primer | Sequence 5’-3’ | Product Size (nt) | ORF Region (nt) |
|---|---|---|---|---|
| tp0548 | Sense1 | 5’-GGTCCCTATGATATCGTGTTCG-3’ | 300 | 130–212 |
| Antisense1 | 5’-CGTTTCGGTGTGTGAGTCAT-3’ | |||
| Sequencing | 5’-GTCATGGATCTGCGAGTGG-3’ | |||
| tp0136 | Sense | 5’-CCATCCAGTCGGAAGTGC-3’ | 223–675 | |
| Antisense2 | 5’-CATATCGAGAAACTGTTCGCC-3’ | 563 | ||
| Antisense3 | 5`-CGTGCAGGCAGAACTCATT-3’ | 464 | ||
| Sequencing | 5’-CCATCCAGTCGGAAGTGC-3’ | |||
| tp0326 | Sense | 5’-AAGAGCATTCGTTTCGCTCC-3’ | 441 | 2031–2342 |
| Antisense | 5’-CCGGACCGTAGCTCATTTTG-3’ | |||
| Sequencing | 5’-GACACCAAGGCCGAGTTCTA-3’ |
1 Published tp0548 primers [11]
2 Antisense primer for tp0136 subtypes A-F
3 Antisense primer for tp0136 subtype G
Sequence analysis and alignments of the six human yaws strains of T. pallidum subsp. pertenue (Gauthier, Ghana051, CDC1, CDC2, Samoa D, Samoa F), the Fribourg-Blanc strain, and the PNG samples were performed using Bioedit [31] (http://www.mbio.ncsu.edu/BioEdit/bioedit.html) and Clustal W (http://www.ebi.ac.uk/Tools/msa/clustalw2/). Phylogenetic analysis was conducted by first constructing multiple alignments using the Muscle algorithm implemented in Molecular Evolutionary Genetics Analysis (MEGA) version 7.0 software (http://www.megasoftware.net/) [32], and drawing phylogenetic trees using the Neighbor-Joining method and the number of differences model, with pairwise deletion of gaps and 1000 bootstrap repetitions.
GenBank accession numbers for the new tp0548 types are as follows: R, MF425823; S, MF425824; T, MF425825; U, HM585227; V, HM243495; W HM245777; X, CP020365. The authors that described tp0548 types M, N, P, and Q did not submit the sequences to GenBank, so no accession numbers are available The tp0136 types A-G are MF425826-MF425831 and MF425833. The tp0326 types 1–8 are MF425834-MF425836 and MF425838-MF425842.
Results
Definition of the MLST typing targets
Based upon our analyses of historical strains and a subset of PNG clinical samples, we chose tp0136, tp0548, and tp0326 as the most promising targets for use as a T.p. pertenue typing system. Primers were designed and tested for amplification of these regions and those with robust amplification were selected for the MLST scheme (Table 3) The targets, all of which are putative or bona fide outer membrane proteins, each contain small (300–600 nt) readily amplifiable regions with sequence heterogeneity among strains. While the selection of additional, or longer, targets could have increased discrimination, we weighed the resulting requirement for increased sample volume, cost, and time of adding more targets with the risk of losing the ability to fully type some samples. The proposed nomenclature for different T.p. pertenue strain types is expressed as two letters, representing tp0548 and tp0136 types, followed by a number, representing tp0326 types, e.g. JG8.
Using these new MLST primers, we attempted to amplify and sequence typing products from 232 T. pallidum–positive swab samples, collected over 42 months, from persons with chronic ulcers on Lihir Island; 194 (83.6%) samples could be completely typed.
Sequence diversity of the selected polymorphic markers
tp0548
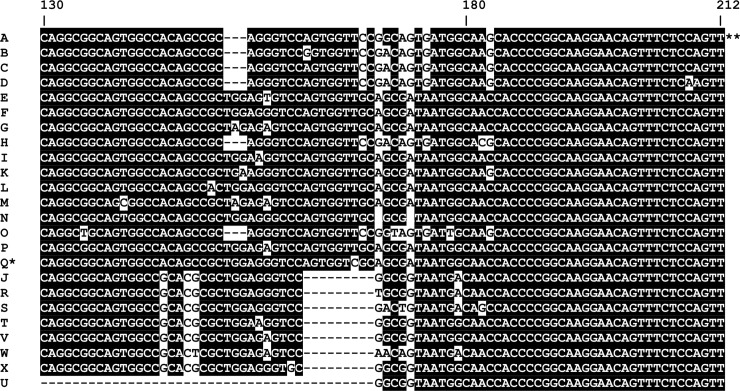
Sequence analysis of nucleotides 130–212 (cognate to Nichols strain genome [AE 000520.1]) of tp0548 from the published genomes and newly sequenced tp0548 loci of historical yaws strains resulted in the identification of four tp0548 genotypes types (designated here as types R, V, W, and X. Type O had been defined by Knauf et al.[33]. While this manuscript was under review, two new tp0548 types were published: one was defined as P by Mikalova et al. [34] and the other was incorrectly defined as type O by Li et al et al. [35] (here re-defined as Q). Analysis of the PNG samples resulted in the identification of two tp0548 genotypes (sequences designated S and T) that had not previously been identified in the literature. The Fribourg-Blanc isolate tp0548 sequence has a 47 bp deletion from coordinates 122–168 (cognate to the Nichols strain) [7] and was assigned type U (Fig 1).
Fig 1. Sequence alignment of Tp0548 types A through X.
Sequence alignment is for different strains of T. pallidum subsp. pallidum and T. pallidum subsp. pertenue. The coordinates of nucleotides 130–212 shown above the alignment are based on the Nichols strain genome (AE 000520.1) as indicated by **. Published reference sequences for each tp0548 type are as follows: Types A-I:[11]; Type J: [36]; Type K: [17]; Type L: [37]; Type M-N:[38]; Type O:[33]; Type P: [34] Type Q: [35]; Type R,V,W: [5]; Type S-T and X: this work; Type U: [7] Type Q (indicated by *) was originally incorrectly published as Type O; it was renamed in this manuscript.
In our analysis of the PNG samples, we found a large number (n = 160) of samples with the previously described type “J” tp0548 sequence, first identified in Paris by Grange et al. in a genital ulcer of a man with recent sexual exposure in Pakistan [36]. In developing the nomenclature for the T.p. pertenue typing system, we debated whether to continue adding letters to the already extensive list of tp0548 type sequences (Fig 1) but, when the Paris sample (subsequently determined to be T. pallidum subsp. endemicum) was used to define type “J” in that list, we elected to continue adding the new PNG sequences to the existing list.
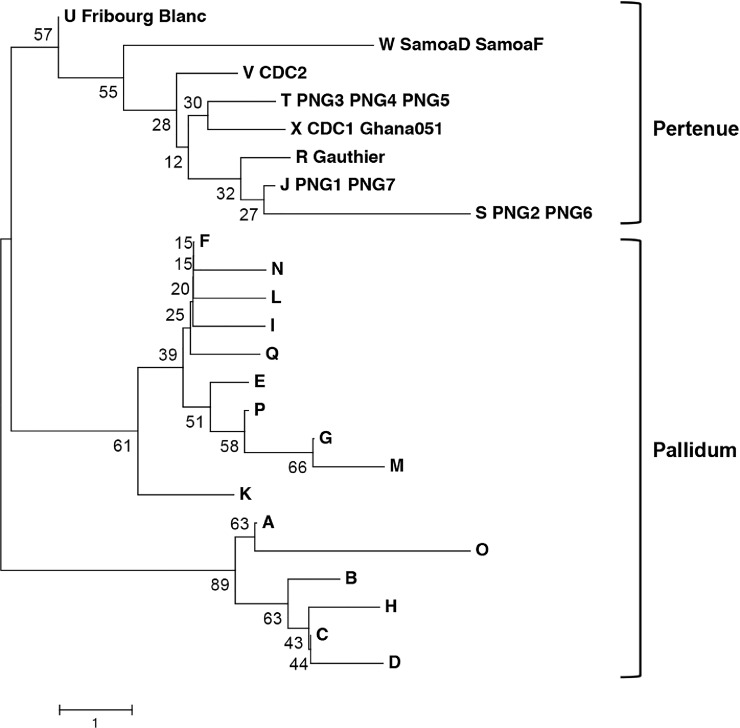
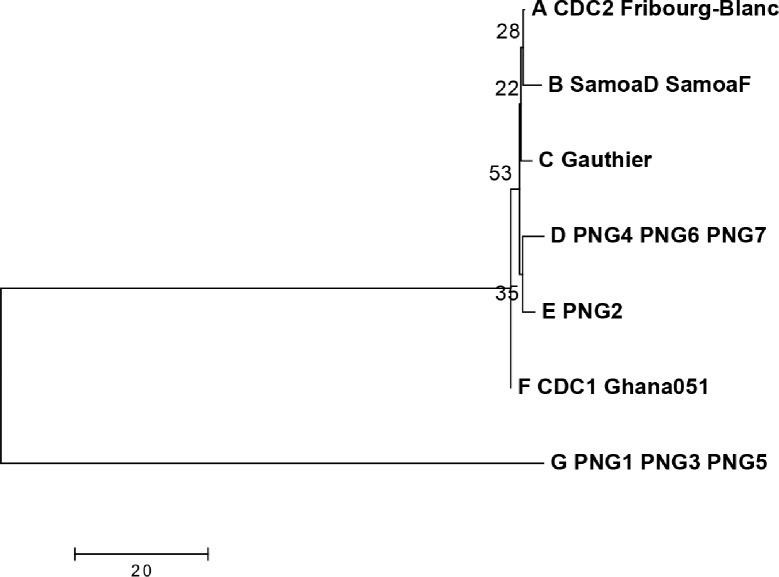
Phylogenetic analysis of the tp0548 typing sequences divides the Treponema into three clades, two containing subspecies pallidum strains and one containing the pertenue strains (Fig 2). Based on tp0548 alone, there was limited bootstrap support to divide the pertenue types, with type W being the most distinct, albeit with a bootstrap value of only 51.
Fig 2. Phylogenetic relationships of the tp0548 types.
tp0548 types are shown for T.p. pallidum, T.p. pertenue, and Fribourg Blanc isolates/strains and for PNG samples, as shown in Fig 1. Sequences were first aligned using the Muscle algorithm, using default parameters. The evolutionary history was inferred using the Neighbor-Joining method. The optimal tree is shown, with branch lengths equivalent to the evolutionary distance as indicated by the scale. Evolutionary distance was measured using the number of differences per sequence, with pairwise deletion of gaps. The percentage of replicate trees in which the associated molecular types clustered together in the bootstrap test (1000 replicates) is shown next to the branches. Analyses were conducted in MEGA version 7.0 [32].
tp0136
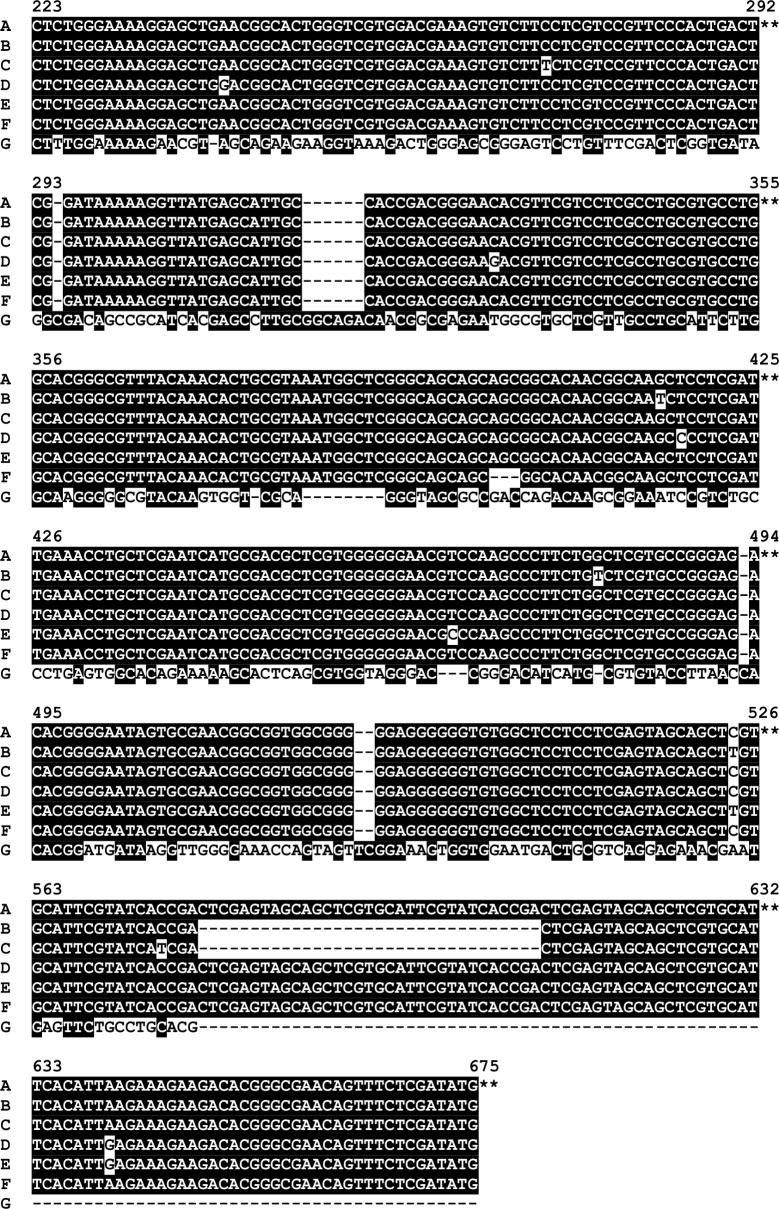
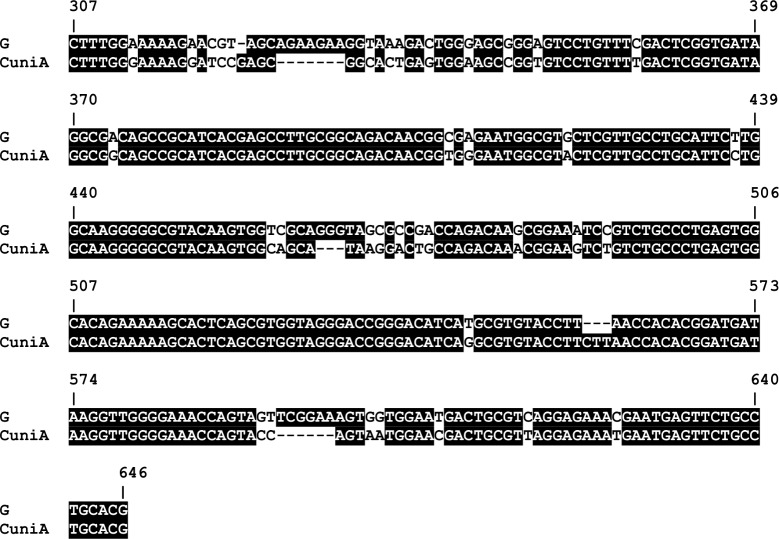
Sequence diversity in tp0136 has been described by others [8,21]. In addition, Flasarova et al. used the Tp0136 gene as an adjunct to the CDC typing method for T.p. pallidum strains and have found sequence variation among nucleotides 303–1452 [15,16]. Because we wanted to focus on smaller amplicons for greater sensitivity in typing clinical samples, we designed primers that amplified the region between nucleotides 223–675 (cognate to CDC2 sequence). These primers successfully amplified the historical/reference strains (Table 1) and a subset of the PNG clinical samples, resulting in the identification of four types (A-D) in the historical yaws strains and two additional tp0136 types (E-F) in the PNG samples (Fig 3). A large subset (n = 164, 70.7%) of the PNG samples could not be amplified by these primers and we therefore designed another antisense primer (Antisense 2, Table 3) which successfully amplified the tp0136 typing region from this latter group of samples. Sequence analysis showed that these samples contained a tp0136 sequence with relatively high divergence compared to the other sequences, and this was designated type G (Fig 3). Interestingly, BLAST analysis indicated that this sequence was similar to the tp0136 sequence from Treponema paraluiscuniculi A [39] as shown in Fig 4.
Fig 3. Sequence alignment of Tp0136 types A through G from T. pallidum subsp. pertenue isolates and Papua New Guinea samples.
The coordinates in the alignment between nucleotides 223 and 675 in T. pallidum subsp. pertenue strains and Papua Guinea samples are in reference to strain CDC2 in GenBank (Accession No. CP002375.1) as indicated by **. A: Fribourg-Blanc, CDC2; B: Samoa D, Samoa F; C: Gauthier, F: CDC1, Ghana051; D, E, and G: Papua New Guinea samples.
Fig 4. Sequence alignment of tp0136 type G with Treponema paraluiscuniculi A.
The very unusual sequence (Type G) found in tp0136 from the majority of PNG samples was more closely aligned with the sequence from T. paraluiscuniculi than with the other T.p. pertenue strains (types A-F in Fig 3). The coordinates in the alignment are in reference to T. paraluiscuniculi A strain (Accession No. CP002103) as indicated by **.
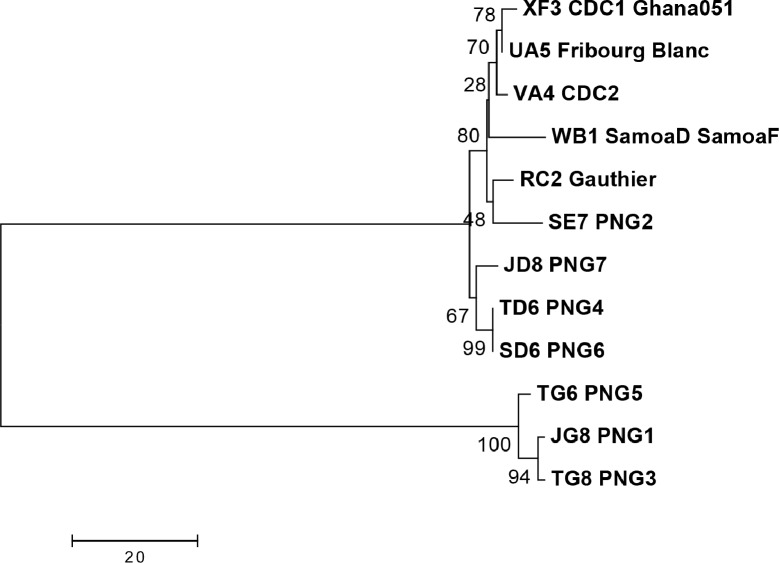
In the phylogenetic analysis (Fig 5), the tp0136 marker divides the PNG strains into two major clusters, with one containing all of the historical strains and the four very closely related PNG2, 4, 6, and 7 types, while type G, found in three groups (PNG1, 3, 5), showed high divergence from all other types as indicated by the multiple alignments.
Fig 5. Phylogenetic relationships of the tp0136 typing region.
tp0136 types are shown for T.p. pertenue and Fribourg Blanc isolates and PNG samples; typing designations are as described in Fig 3. Sequences were first aligned using the Muscle algorithm, using default parameters. The evolutionary history was inferred using the Neighbor-Joining method. The optimal tree is shown, with branch lengths equivalent to the evolutionary distance as indicated by the scale. Evolutionary distance was measured using the number of differences per sequence, with pairwise deletion of gaps. The percentage of replicate trees in which the associated molecular types clustered together in the bootstrap test (1000 replicates) is shown next to the branches. Analyses were conducted in MEGA version 7.0 [32].
tp0326
Sequence diversity in the third typing target, tp0326 (originally called Tp92), was initially demonstrated by Cameron et al. [19]. This gene encodes an orthologue of BamA, which is part of the outer membrane protein assembly machinery [20]. The tp0326 typing region (nucleotides 2031–2345, cognate to CDC2) defined five genotypes (designated 1–5) among the historical strains and three new genotypes (designated 6–8) in the PNG samples (Fig 6).
Fig 6. Sequence alignment of tp0326 typing region.
Sequence alignment of tp0326 types from T. pallidum subsp. pertenue isolates and Papua New Guinea samples. Nucleotide numbering between 2031and 2342 in the alignment refers to coordinates in strain CDC2 in GenBank (Accession No. CP002375.1) as indicated by **. The sequences between coordinates 2117 and 2297 are conserved in all strains examined. 1: Samoa D, Samoa F; 2: Gauthier; 3: Ghana051, CDC1; 4: CDC2; 5: Fribourg-Blanc; 6–8: Papua New Guinea samples.
Phylogenetic analysis of tp0326 showed the relatively low diversity of this marker, however the polymorphisms present divided the eight groups into distinct clusters (Fig 7). Interestingly, PNG groups 2, 4, 5, and 6, clustered with the Gauthier strain, which was isolated in Africa, while PNG groups 1, 3, and 7 clustered with Samoa D and F strains, which were isolated in the South Pacific, near Papua New Guinea.
Fig 7. Phylogenetic relationships of the tp0326 types.
tp0326 types are shown for T.p. pertenue and Fribourg Blanc isolates and PNG samples; typing designations are as described in Fig 6. Sequences were first aligned using the Muscle algorithm, using default parameters. The evolutionary history was inferred using the Neighbor-Joining method. The optimal tree is shown, with branch lengths equivalent to the evolutionary distance as indicated by the scale. Evolutionary distance was measured using the number of differences per sequence, with pairwise deletion of gaps. The percentage of replicate trees in which the associated molecular types clustered together in the bootstrap test (1000 replicates) is shown next to the branches. Analyses were conducted in MEGA version 7.0 [32].
MLST typing of T.p. pertenue from a yaws-endemic area of Papua New Guinea
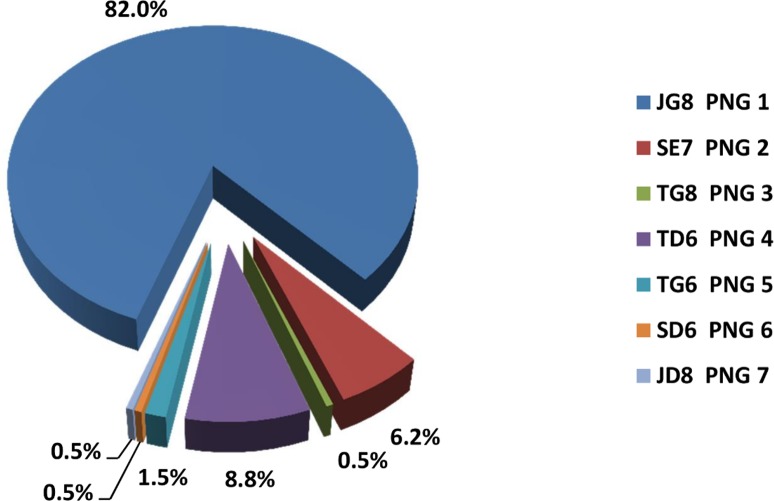
The MLST typing approach based on the three chosen markers was then applied to T.p. pertenue isolates from PNG. Of the 232 total PNG T.p. pertenue-containing samples, 194 (83.6%) were successfully typed at all three loci; 22 (9%) could be partially typed; and 16 (7%) could not be typed at all. Only fully typed samples were further analyzed. During the 3.5 years of subsequent surveys and sampling, a total of seven types (dividing the samples into groups PNG 1 to PNG 7) were observed, with type JG8 (PNG 1) being predominant throughout that period (82%, Fig 8). The distribution of the molecular types during the course of the survey is discussed elsewhere (manuscript submitted).
Fig 8. T. pallidum subsp. pertenue types collected on Lihir Island, Papua New Guinea, from May 2013 and October 2016.
Data for the 194 fully typeable samples are included here, and proportions for each type are shown.
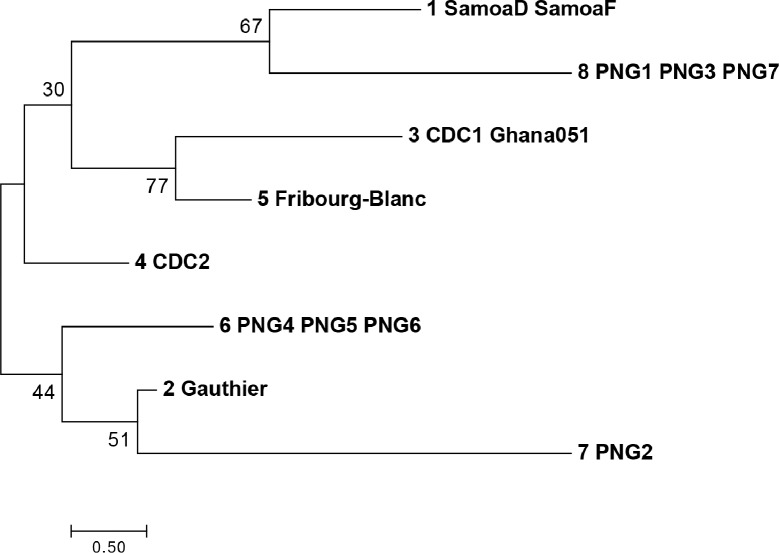
A phylogenetic analysis of the final tripartite MLST system for T.p. pertenue, based upon the haplotypes (e.g. concatenated tp0548, tp0136, and tp0326 genotypes), is shown in Fig 9. This divided the haplotypes into two major clusters with high bootstrap values, with one containing the three haplotypes with the divergent tp0136 G genotype (PNG 1,3,5), and the other cluster comprising two minor clusters, one containing all historical isolates and the PNG 2 haplotype (SE7), and the other containing the haplotypes with the tp0136 D genotype (PNG 4,6,7).
Fig 9. Phylogenetic analysis of Tp multilocus sequence types.
Multilocus Sequence Types (MLSTs) were defined by sequencing regions of three genes: tp0548, tp0136 and tp0326. Concatenated sequences were first aligned using the Muscle algorithm, using default parameters. The evolutionary history of the MLSTs was inferred using the Neighbor-Joining method. The optimal tree is shown, with branch lengths equivalent to the evolutionary distance as indicated by the scale. Evolutionary distance was measured using the number of differences per sequence, with pairwise deletion of gaps. The percentage of replicate trees in which the associated molecular types clustered together in the bootstrap test (1000 replicates) is shown next to the branches. Analyses were conducted in MEGA version 7.0 [32].
Discussion
Whole genome sequencing of the Samoa D, Gauthier, and CDC2 T.p. pertenue strains provided an excellent resource for beginning to develop a genotyping tool for yaws clinical samples [4]. For several years, a molecular typing method originally developed at the Centers for Disease Control [10] has been used to identify circulating strains of T. pallidum subsp. pallidum for epidemiological studies [10,11,16,18,40,41]. The enhanced typing method developed by Marra et al. built upon the earlier method, proved to provide greater discrimination, and has been widely adopted for typing syphilis strains [11,13,16,41]. Similarly, a typing scheme for yaws organisms could help to inform WHO’s yaws eradication program by permitting an examination of the diversity, stability, and movement of strains throughout a geographical area, and the importation of strains by travelers. The typing system will provide a tool to help to identify the resilience of a bacterial population (e.g. the emergence or importation of strains with enhanced virulence or drug resistance, or the occurrence of an outbreak). Also, the new strain-typing technique will help to improve the understanding of yaws transmission pathways, which will inform the development of improved management and preventative interventions. For example, this tool will help to determine the degree to which yaws cases are clustered within villages and districts; identifying the mechanisms for that clustering could contribute to determination of optimal implementation units for interventions. If inter-village yaws transmission were to be identified, public health officials might want to consider establishing larger implementation units. For evaluating clinical episodes, molecular typing may clarify whether repeated episodes of yaws are due to reinfection rather than relapse in patients in whom genotypically different strains of T.p. pertenue were detected from lesions during each of the separate episodes of ulcer.
Because of the significant difficulty inherent to the syphilis typing method, which relies heavily on analysis of restriction fragment length polymorphisms and of variable numbers of repeats, we sought to develop a multilocus sequence typing (MLST) approach for yaws samples that would be more straightforward and reliable to execute and would provide greater resolution while limiting ambiguous results. Based upon our analysis of sequenced yaws strain genomes and a subset of PNG samples, we chose fragments of the tp0136, tp0548, and tp0326 genes as the most promising targets for a T.p. pertenue typing system. Our selection was based primarily upon the level of strain discrimination afforded by the genes and the robustness of the PCR assay in samples containing low concentration of treponemal DNA. We weighed the increased cost and time of adding more targets with the risk of losing the ability to fully type some samples. We fully recognize that, by limiting the size of the gene fragments used in the typing system, we risk losing some discriminating capability. Our experience with typing clinical samples, often from distant locations where optimal handling of DNA is not practical, has convinced us however that the ability to derive a complete molecular typing designation from a high proportion of samples is preferable to a more discriminating system in which a lower percentage of samples can be fully typed. We do not exclude, however, that in the future additional targets might be added to our MLST. Preliminary evidence suggests, for example, that tp0488 might be a suitable typing targets for T. pallidum subsp. pertenue, and its use should be further evaluated.
Evidence for the utility of our novel T.p. pertenue typing system can be found by examining the strain types of the six historical yaws treponemes, which were collected from disparate geographical regions over nearly 3 decades, and could be divided into four molecular types based on our typing system. It was not unexpected to see that Samoa D and Samoa F, which were both isolated from children in Apia, Western Samoa, in January,1953 [25], had the identical molecular type, WB1. Typing and careful literature research can also lead to questioning of the origins of some DNA samples. We initially conducted typing analysis on DNA from two strains (called CDC2571 and Brazzaville) obtained from a laboratory in the Netherlands, and for which no known isolated strains exist. In carefully researching the origin of this DNA, we were unable to find published references describing the isolation of either strain by those names. In our typing analysis, we found that the Brazzaville strain had identical type sequences to the Gauthier strain (S2 Fig). The 1963 publication describing the isolation of the Gauthier strain [23] describes the collection of a sample from Nigeria in 1960 by a physician in Brazzaville; this publication names the sample “Gauthier, Eastern Nigeria”. We therefore suspect that the “Brazzaville strain” is actually the same as the Gauthier isolate.
Similarly, CDC2571 had the same type sequences as CDC1 and Ghana051 (S3 Fig). There is no known description of the isolation of CDC2575 which was provided to the Netherlands lab by Dr. Peter Perine [42]. The cited reference [24] for CDC2575 describes the isolation in hamsters of treponemes from three children with yaws; all hamster inoculations were conducted on the same date, and the children were residents of two towns in Ghana. Only two of the three strains were successfully transferred and propagated in subsequent animals, and these two are named CDC1 and CDC2; the third un-named strain was apparently lost. We therefore suspect that CDC2575 is actually strain CDC1.
The reference that is typically cited for strain Ghana051 [26] describes the 1988 isolation of the organism from a child who had recently emigrated from Ghana, although this publication does not name the strain. While this manuscript was under review, a publication from Strouhal et al. [43] described the genome sequences of CDC2575 and Ghana051, which were virtually identical. The existence of a description of the isolation of the Ghana051 strain and the clear difference in years of reported isolation suggests that Ghana051 (1988) is actually a different strain from CDC1 (1980) and CDC2575 (no description of isolation). The lack of published strain nomenclature for the 1988 isolate leaves the question open, however, as to whether strains were confused or mislabeled during passage or handling over the years. Even whole genome sequencing cannot always determine whether strain mislabeling has occurred.
The utility of strain typing is also apparent in the saga of the Paris case report by Grange et al. [36]. The penile lesion was initially thought to be caused by T.p. pallidum acquired by sexual contact in Pakistan, but the tp0548 sequence, named type J, suggested that it was T.p. pertenue. It was the astute observation of the unusual sequence, called type J, by Mikalova et al. [44] that suggested that the agent was not a pallidum subspecies. Subsequent more extensive analyses suggest that the treponeme present in this ulcer is actually most closely related to T. pallidum subsp. endemicum, the cause of bejel or endemic syphilis. It has been proposed by Mikalova et al. that the tp0548 sequence from this patient is the result of recombination between pertenue and endemicum subspecies [45]. Notably, tp0548 type J is the most prevalent type in the PNG samples that we examined, demonstrating that the tp0548 type J sequence is seen in modern T.p. pertenue strains, as well as in the putative hybrid T.p. endemicum strain that was presumably sexually acquired in Pakistan. The “Paris” sample also provides evidence that the oft-stated belief that only T.p. pallidum is sexually transmitted is not true. With more molecular analyses being conducted on pathogenic Treponema, we increasingly realize that the strict “distinctions” concerning the modes of transmission and, potentially, the clinical manifestations of the T.p. subspecies are becoming significantly blurred [2].
The overlap among subspecies in transmission and clinical manifestations is further suggested by the finding that the agent causing genital ulcerations (typically ascribed to the pallidum subspecies) in wild baboons [46] is most closely related to the yaws-causing pertenue subspecies. Subsequent analyses of the material from these animals revealed a pertenue-like lineage that was nonetheless distinct compared to the historical human yaws strains [47]. It is striking that analysis of DNA from flies associated with baboon lesions [33] revealed that some flies contained tp0548 sequences that clustered with the pertenue subspecies, while others contained Type J tp0548 sequences, discussed above as having been first identified in a T. pallidum subsp. endemicum human genital ulcer swab [36,44,45] and later found by us in the majority of samples from children with yaws (molecularly defined as pertenue) in Papua New Guinea. Molecular typing and gene sequencing has revealed the intersection of the subspecies [30,45,48].
This picture is further complicated by our finding that a majority of the PNG samples described in this study have a tp0136 allele that has previously been described only in Treponema paraluiscuniculi, which causes a venereal infection in wild rabbits and is thought not to be infectious for humans [49]. In other cases in which alleles thought to belong to one subspecies are found in another subspecies, it has been proposed that inter-subspecies recombination has occurred [45,48]. Might our finding represent an example of possible recombination between two treponemal species?
Aside from triggering deeper evaluations of the nature of T. pallidum subspecies discussed above, the establishment of a typing system for a pathogen might assist in assessing the association of a particular molecular type with a disease manifestation. If clear associations can be determined through careful epidemiological studies, typing could have a predictive value for regional clinicians and public health officials. For example, if a T.p. pertenue type strain associated with severe joint inflammation were found to be circulating in a community, local health workers could be on heightened alert for identifying and treating such cases. If associations are strong enough, it might justify the adoption of a typing system in routine surveillance programs or in clinical laboratories. Identification of links between genotype and clinical manifestations in yaws is speculative at this time and awaits further study, but a few studies have found associations of specific T. p. pallidum strain types and syphilis manifestations. For example, the 14D/f strain type of T.p. pallidum was significantly associated with neurosyphilis in a large prospective study [11]. In more recent studies, a cluster of T.p. pallidum type 8D/g strains was seen in cases of ocular syphilis in Seattle [12], and infection with the 14I/a type was found to be a significant predictor of serofast status among syphilis-infected patients [50].
With regard to yaws, infection is commonly believed not to affect the cardiovascular and central nervous systems, and not to be transmitted to the fetus during pregnancy. This oft-repeated “maxim” may reflect lack of extensive knowledge on the pathogenesis of yaws. Alternatively, there may be differences in strain invasiveness. Studies conducted by Edington identified syphilis-like aortitis as a major cause of death in people from Ghana where yaws is endemic [51], while Roman and Roman suggested that there is evidence in the literature to support not only neurological and cardiovascular involvement in yaws patients, but also vertical transmission of the pathogen [52]. In the future, discordant observations and conclusions concerning yaws pathogenesis and manifestations may be explained by genetic differences among strains, and with sufficient clinical data, our typing system might assist in linking genotype and phenotype in T.p. pertenue.
In summary, we have described a new sequence-based typing system for T. pallidum subsp. pertenue, based upon tp0548, tp0136, and tp0326 genes. The proposed method was developed to maximize the discriminating capability of the sequence target regions, balanced by the robustness of the PCR to amplify samples with limiting amounts of treponemal DNA. In this study, we limited our analysis to the aggregated typing results from clinical samples obtained during the 3.5 years of examinations of the population of Lihir Island. An analysis of the geographical clustering of the strain types across the island and the correlation of strain type with population migration or travel will provide critical information for developing protocols and monitoring progress of yaws eradication activities in the future. Those analyses are ongoing.
While this new typing system has been quite useful in examining strains circulating on Lihir Island, it is very important to assess its applicability to samples from yaws lesions from other geographical regions. It is fully expected that more strain types will be identified as the typing method is applied to more yaws-affected populations, and that modifications to the primer sets may be needed. It should also be remembered that no typing system will be universally sensitive, particularly for samples that cannot be collected, stored, or transported under optimal conditions. The discriminating ability of the typing system described here for historical T.p. pertenue isolates from Pacific Islands and Africa, as well as clinical samples, suggests however that it is a good prototype that will be readily applicable to the current WHO campaign to eliminate yaws.
Supporting information
Alignment of the tp0619 sequences from historical strains and the five molecular types identified from 95 PNG samples. The Samoa D tp0619 sequence is identical to that of all other historical pertenue strains analyzed (Samoa F, Gauthier, Brazzaville, CDC 1, CDC 2, CDC2575, and Ghana051).
(TIF)
(TIF)
(TIF)
Acknowledgments
We thank the people of Lihir Island for their willingness to participate in the study; the field teams for their hard work in conducting the study; and the Papua New Guinea National Department of Health for oversight of the trial and continued cooperation.
Data Availability
All relevant data are within the paper. GenBank accession numbers for the new tp0548 types are as follows: R, MF425823; S, MF425824; T, MF425825; U, HM585227; V, HM243495; W HM245777; X, CP020365. The authors that described tp0548 types M, N, P, and Q did not submit the sequences to GenBank, so no accession numbers are available. References for all tp0548 types, including M, N, P, and Q sequences are shown in the legend of Fig 1 in the Results section. The tp0136 types A-G are MF425826-MF425831 and MF425833. The tp0326 types 1-8 are MF425834-MF425836 and MF425838-MF425842.
Funding Statement
Research reported in this publication was supported by the National Institute of Allergy & Infectious Diseases of the National Institutes of Health under award number R01AI42143 (SAL, LG) and by ISDIN Laboratories (OM). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Antal GM, Lukehart SA, Meheus AZ. The Endemic Treponematoses. Microbes Infect. 2002;4: 83–94. [DOI] [PubMed] [Google Scholar]
- 2.Giacani L, Lukehart SA. The Endemic Treponematoses. Clin Microbiol Rev. 2014;27: 89–115. doi: 10.1128/CMR.00070-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asiedu K, Fitzpatrick C, Jannin J. Eradication of Yaws: Historical Efforts and Achieving WHO’s 2020 Target. PLoS Negl Trop Dis. 2014;8: e3016 doi: 10.1371/journal.pntd.0003016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Čejková D, Zobaníková M, Chen L, Pospíšilová P, Strouhal M, Qin X, et al. Whole Genome Sequences of Three Treponema pallidum ssp. pertenue Strains: Yaws and Syphilis Treponemes Differ in Less than 0.2% of the Genome Sequence. PLoS Negl Trop Dis. 2012;6: e1471 doi: 10.1371/journal.pntd.0001471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mikalová L, Strouhal M, Čejková D, Zobaníková M, Pospíšilová P, Norris SJ, et al. Genome Analysis of Treponema pallidum subsp. pallidum and subsp. pertenue Strains: Most of the Genetic Differences Are Localized in Six Regions. PLoS ONE. 2010;5: e15713 doi: 10.1371/journal.pone.0015713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centurion-Lara A, Molini BJ, Godornes C, Sun E, Hevner K, Van Voorhis WC, et al. Molecular Differentiation of Treponema pallidum subspecies. J Clin Microbiol. 2006;44: 3377–80. doi: 10.1128/JCM.00784-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zobaníková M, Strouhal M, Mikalová L, Čejková D, Ambrožová L, Pospíšilová P, et al. Whole Genome Sequence of the Treponema Fribourg-Blanc: Unspecified Simian Isolate Is Highly Similar to the Yaws Subspecies. PLoS Negl Trop Dis. 2013;7: e2172 doi: 10.1371/journal.pntd.0002172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brinkman MB, McGill MA, Pettersson J, Rogers A, Matějková P, Šmajs D, et al. A Novel Treponema pallidum Antigen, TP0136, Is an Outer Membrane Protein That Binds Human Fibronectin. Infect Immun. 2008;76: 1848–1857. doi: 10.1128/IAI.01424-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centurion-Lara A, Castro C, Castillo R, Shaffer JM, Van Voorhis WC, Lukehart SA. The Flanking Region Sequences of the 15-kDa Lipoprotein Gene Differentiate Pathogenic Treponemes. J Infect Dis. 1998;177: 1036–40. [DOI] [PubMed] [Google Scholar]
- 10.Pillay A, Liu H, Chen CY, Holloway B, Sturm AW, Steiner B, et al. Molecular subtyping of Treponema pallidum subspecies pallidum. Sex Transm Dis. 1998;25: 408–14. [DOI] [PubMed] [Google Scholar]
- 11.Marra CM, Sahi SK, Tantalo LC, Godornes C, Reid T, Behets F, et al. Enhanced Molecular Typing of Treponema pallidum: Geographical Distribution of Strain Types and Association with Neurosyphilis. J Infect Dis. 2010;202: 1380–1388. doi: 10.1086/656533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oliver S, Sahi SK, Tantalo LC, Godornes C, Neblett Fanfair R, Markowitz LE, et al. Molecular Typing of Treponema pallidum in Ocular Syphilis. Sex Transm Dis. 2016;43: 524–527. doi: 10.1097/OLQ.0000000000000478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mikalová L, Pospíšilová P, Woznicová V, Kuklová I, Zákoucká H, Šmajs D. Comparison of CDC and Sequence-based Molecular Typing of Syphilis Treponemes: tpr and arp loci are Variable in Multiple Samples From the Same Patient. BMC Microbiol. 2013;13: 178–178. doi: 10.1186/1471-2180-13-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Florindo C, Reigado V, Gomes JP, Azevedo J, Santo I, Borrego MJ. Molecular typing of Treponema pallidum clinical strains from Lisbon, Portugal. J Clin Microbiol. 2008;46: 3802–3. doi: 10.1128/JCM.00128-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flasarová M, Pospíšilová P, Mikalová L, Vališová Z, Dastychová E, Strnadel R, et al. Sequencing-based Molecular Typing of Treponema pallidum Strains in the Czech Republic: All Identified Genotypes Are Related to the Sequence of the SS14 strain. Acta Derm Venereol. 2012;92: 669–674. doi: 10.2340/00015555-1335 [DOI] [PubMed] [Google Scholar]
- 16.Flasarová M, Smajs D, Matejková P, Woznicová V, Heroldová-Dvoráková M, Votava M. [Molecular Detection and Subtyping of Treponema pallidum subsp. pallidum In Clinical specimens]. Epidemiol Mikrobiol Imunol Cas Spolecnosti Epidemiol Mikrobiol Ceské Lékarské Spolecnosti JE Purkyne. 2006;55: 105–111. [PubMed] [Google Scholar]
- 17.Grillová L, Pĕtrošová H, Mikalová L, Strnadel R, Dastychová E, Kuklová I, et al. Molecular Typing of Treponema pallidum in the Czech Republic During 2011 to 2013: Increased Prevalence of Identified Genotypes and of Isolates with Macrolide Resistance. Munson E, editor. J Clin Microbiol. 2014;52: 3693–3700. doi: 10.1128/JCM.01292-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katz KA, Pillay A, Ahrens K, Kohn RP, Hermanstyne K, Bernstein KT, et al. Molecular Epidemiology of Syphilis—San Francisco, 2004–2007: Sex Transm Dis. 2010; 1. [DOI] [PubMed] [Google Scholar]
- 19.Cameron CE, Lukehart SA, Castro C, Molini B, Godornes C, Van Voorhis WC. Opsonic Potential, Protective Capacity, and Sequence Conservation of the Treponema pallidum subspecies pallidum Tp92. J Infect Dis. 2000;181: 1401–13. doi: 10.1086/315399 [DOI] [PubMed] [Google Scholar]
- 20.Desrosiers DC, Anand A, Luthra A, Dunham-Ems SM, LeDoyt M, Cummings MA, et al. TP0326, a Treponema pallidum Beta-Barrel Assembly Machinery A (BamA) Orthologue and Rare Outer Membrane Protein. Mol Microbiol. 2011;80: 1496–515. doi: 10.1111/j.1365-2958.2011.07662.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ke W, Molini BJ, Lukehart SA, Giacani L. Treponema pallidum subsp. pallidum TP0136 Protein Is Heterogeneous Among Isolates and Binds Cellular and Plasma Fibronectin via its NH(2)-Terminal End. PLoS Negl Trop Dis. 2015;9: e0003662 doi: 10.1371/journal.pntd.0003662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lukehart SA, Marra CM. Isolation and laboratory maintenance of Treponema pallidum. Curr Protoc Microbiol. 2007;Chapter 12: Unit 12A 1. [DOI] [PubMed] [Google Scholar]
- 23.Gastinel P, Vaisman A, Hamelin A, Dunoyer F. [Study of a recently isolated strain of Treponema pertenue]. Ann Dermatol Syphiligr Paris. 1963;90: 155–61. [PubMed] [Google Scholar]
- 24.Liska SL, Perine PL, Hunter EF, Crawford JA, Feeley JC. Isolation and Transportation of Treponema pertenue in Golden Hamsters. Curr Microbiol. 1982;7: 41–3. [Google Scholar]
- 25.Turner TB, Hollander DH. Biology of the Treponematoses. Geneva: World Health Organization; 1957. [PubMed] [Google Scholar]
- 26.Engelkens HJ, Oranje AP, Stolz E. Early Yaws, Imported in The Netherlands. Genitourin Med. 1989;65: 316–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fribourg-Blanc A, Mollaret HH, Niel G. [Serologic and microscopic confirmation of treponemosis in Guinea baboons]. Bull Soc Pathol Exot Fil. 1966;59: 54–9. [PubMed] [Google Scholar]
- 28.Mitjà O, Houinei W, Moses P, Kapa A, Paru R, Hays R, et al. Mass Treatment with Single-Dose Azithromycin for Yaws. N Engl J Med. 2015;372: 703–710. doi: 10.1056/NEJMoa1408586 [DOI] [PubMed] [Google Scholar]
- 29.Mitjà O, Lukehart SA, Pokowas G, Moses P, Kapa A, Godornes C, et al. Haemophilus ducreyi as a Cause of Skin Ulcers in Children from a Yaws-Endemic Area of Papua New Guinea: A Prospective Cohort Study. Lancet Glob Health. 2014;2: e235–e241. doi: 10.1016/S2214-109X(14)70019-1 [DOI] [PubMed] [Google Scholar]
- 30.Centurion-Lara A, Giacani L, Godornes C, Molini BJ, Brinck Reid T, Lukehart SA. Fine Analysis of Genetic Diversity of the tpr Gene Family among Treponemal Species, Subspecies and Strains. PLoS Negl Trop Dis. 2013;7: e2222 doi: 10.1371/journal.pntd.0002222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Ser. 41:95–98. Nucl Acids Symp. 1999;Ser. 41: 95–98.
- 32.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol Biol Evol. 2016;33: 1870–1874. doi: 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knauf S, Raphael J, Mitjà O, Lejora IAV, Chuma IS, Batamuzi EK, et al. Isolation of Treponema DNA from Necrophagous Flies in a Natural Ecosystem. EBioMedicine. 2016;11: 85–90. doi: 10.1016/j.ebiom.2016.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mikalová L, Grillová L, Osbak K, Strouhal M, Kenyon C, Crucitti T, et al. Molecular Typing of Syphilis-causing Strains Among Human Immunodeficiency Virus-positive Patients in Antwerp, Belgium. Sex Transm Dis. 2017;44: 376–379. doi: 10.1097/OLQ.0000000000000600 [DOI] [PubMed] [Google Scholar]
- 35.Li Z, Wang C, Xiao H, Zhao W, Li Z, Zheng R, et al. Enhanced Molecular Typing of Treponema pallidum Identified a New Tp0548 Gene type in Shandong, China. APMIS. 2017;125: 937–939. doi: 10.1111/apm.12724 [DOI] [PubMed] [Google Scholar]
- 36.Grange PA, Allix-Beguec C, Chanal J, Benhaddou N, Gerhardt P, Morini J-P, et al. Molecular Subtyping of Treponema pallidum in Paris, France. Sex Transm Dis. 2013;40. [DOI] [PubMed] [Google Scholar]
- 37.Tian H, Li Z, Li Z, Hou J, Zheng R, Li F, et al. Molecular Typing of Treponema pallidum: Identification of a New Sequence of tp0548 Gene in Shandong, China. Sex Transm Dis. 2014;41. [DOI] [PubMed] [Google Scholar]
- 38.Read P, Tagg KA, Jeoffreys N, Guy RJ, Gilbert GL, Donovan B. Treponema pallidum Strain-Types and Association with Macrolide Resistance in Sydney, Australia: New tp0548 Types Identified. J Clin Microbiol. 2016; 54:2172–2174. doi: 10.1128/JCM.00959-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Šmajs D, Zobaníková M, Strouhal M, Čejková D, Dugan-Rocha S, Pospíšilová P, et al. Complete Genome Sequence of Treponema paraluiscuniculi, Strain Cuniculi A: The Loss of Infectivity to Humans Is Associated with Genome Decay. PLoS ONE. 2011;6: e20415 doi: 10.1371/journal.pone.0020415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castro R, Prieto E, A M.J., NManata MJ, Botas J, Pereira FM. Molecular subtyping of Treponema pallidum subsp.pallidum in Lisbon, Portugal. J Clin Microbiol. 2009;47: 2510–2512. doi: 10.1128/JCM.00287-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salado-Rasmussen K, Cowan S, Gerstoft J, Kiellberg Larsen H, Hoffmann S, Bygum Knudsen T, et al. Molecular Typing of Treponema pallidum in Denmark: A Nationwide Study of Syphilis. Acta Derm Venereol. 2014; 96:202–206 [DOI] [PubMed] [Google Scholar]
- 42.Noordhoek GT, Hermans PW, Paul AN, Schouls LM, van der Sluis JJ, van Embden JD. Treponema pallidum subspecies pallidum (Nichols) and Treponema pallidum subspecies pertenue (CDC 2575) differ in at least one nucleotide: comparison of two homologous antigens. Microb Pathog. 1989;6: 29–42. [DOI] [PubMed] [Google Scholar]
- 43.Strouhal M, Mikalová L, Havlíčková P, Tenti P, Čejková D, Rychlík I, et al. Complete Genome Sequences of Two Strains of Treponema pallidum subsp. pertenue from Ghana, Africa: Identical Genome Sequences in Samples Isolated More Than 7 Years Apart. PLoS Negl Trop Dis. 2017;11: e0005894 doi: 10.1371/journal.pntd.0005894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mikalová L, Strouhal M, Grillová L, Šmajs D. The Molecular Typing Data of Recently Identified Subtype 11q/j of Treponema pallidum subsp. pallidum Suggest Imported Case of Yaws. Sex Transm Dis. 2014;41. [DOI] [PubMed] [Google Scholar]
- 45.Mikalová L, Strouhal M, Oppelt J, Grange PA, Janier M, Benhaddou N, et al. Human Treponema pallidum 11q/j Isolate Belongs to subsp. endemicum But Contains Two Loci with a Sequence in TP0548 and TP0488 similar to subsp. pertenue and subsp. pallidum, Respectively. PLoS Negl Trop Dis. 2017;11: e0005434 doi: 10.1371/journal.pntd.0005434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knauf S, Batamuzi EK, Mlengeya T, Kilewo M, Lejora IA, Nordhoff M, et al. Treponema Infection associated with Genital Ulceration in Wild Baboons. Vet Pathol. 2012;49: 292–303. doi: 10.1177/0300985811402839 [DOI] [PubMed] [Google Scholar]
- 47.Harper KN, Fyumagwa RD, Hoare R, Wambura PN, Coppenhaver DH, Sapolsky RM, et al. Treponema pallidum Infection in the Wild Baboons of East Africa: Distribution and Genetic Characterization of the Strains Responsible. PLOS ONE. 2012;7: e50882 doi: 10.1371/journal.pone.0050882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pětrošová H, Zobaníková M, Čejková D, Mikalová L, Pospíšilová P, Strouhal M, et al. Whole Genome Sequence of Treponema pallidum ssp. pallidum, Strain Mexico A, Suggests Recombination between Yaws and Syphilis Strains. PLoS Negl Trop Dis. 2012;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Graves S, Downes J. Experimental infection of man with rabbit-virulent Treponema paraluis- cuniculi. Br J Vener Dis. 1981;57: 7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang R-L, Wang Q-Q, Zhang J-P, Yang L-J. Molecular Subtyping of Treponema pallidum and Associated Factors of Serofast Status in Early Syphilis Patients: Identified Novel Genotype and Cytokine Marker. PloS One. 2017;12: e0175477 doi: 10.1371/journal.pone.0175477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Edington GM. Cardiovascular Disease as a Cause of Death in the Gold Coast African. Trans R Soc Trop Med Hyg. 1954;48: 419–425. [DOI] [PubMed] [Google Scholar]
- 52.Roman GC, Roman LN. Occurrence of Congenital, Cardiovascular, Visceral, Neurologic, and Neuro-Ophthalmologic Complications in Late Yaws: A Theme For Future Research. Rev Infect Dis. 1986;8: 760–70. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Alignment of the tp0619 sequences from historical strains and the five molecular types identified from 95 PNG samples. The Samoa D tp0619 sequence is identical to that of all other historical pertenue strains analyzed (Samoa F, Gauthier, Brazzaville, CDC 1, CDC 2, CDC2575, and Ghana051).
(TIF)
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the paper. GenBank accession numbers for the new tp0548 types are as follows: R, MF425823; S, MF425824; T, MF425825; U, HM585227; V, HM243495; W HM245777; X, CP020365. The authors that described tp0548 types M, N, P, and Q did not submit the sequences to GenBank, so no accession numbers are available. References for all tp0548 types, including M, N, P, and Q sequences are shown in the legend of Fig 1 in the Results section. The tp0136 types A-G are MF425826-MF425831 and MF425833. The tp0326 types 1-8 are MF425834-MF425836 and MF425838-MF425842.