Abstract
Recent evidence suggests that directing attention toward single item-context associations during encoding improves young and older adults’ context memory performance and reduces demands on executive functions during retrieval. In everyday situations, there are many event features competing for our attention, and our ability to successfully recover those details may depend on our ability to ignore others. Failures of selective attention may contribute to older adults’ context memory impairments. In the current electroencephalogram (EEG) study, we assessed the effects of age on processes supporting successful context memory retrieval of selectively attended features as indexed by neural oscillations. During encoding, young and older adults were directed to attend to a picture of an object and its relationship to one of two concurrently presented contextual details: a color or scene. At retrieval, we tested their memory for the object, its attended and unattended context features, and their confidence for both the attended and unattended features. Both groups showed greater memory for attended than unattended contextual features. However, older adults showed evidence of hyper-binding between attended and unattended context features while the young adults did not. EEG results in the theta band suggest that young and older adults recollect similar amounts of information but brain-behavior correlations suggest that this information was supportive of contextual memory performance, particularly for young adults. By contrast, sustained beta desynchronization, indicative of sensory reactivation and episodic reconstruction, was correlated with contextual memory performance for older adults only. We conclude that older adults’ inhibition deficits during encoding reduced the selectivity of their contextual memories, which led to reliance on executive functions like episodic reconstruction to support successful memory retrieval.
Keywords: Context memory, Retrieval, Aging, Theta, Beta
1. Introduction
Healthy aging is commonly associated with declines in episodic memory (for review: Craik & Rose, 2012; Spencer & Raz, 1995). In studies of source or context memory (for review: Mitchell & Johnson, 2009; Spencer & Raz, 1995), where the memory for an item and a property of the item or initial encoding experience (e.g., location, orienting question, item color) are assessed (Johnson, Hashtroudi, & Lindsay, 1993), young adults outperform their older counterparts. This decline is found even when item memory is matched (James, Strunk, Arndt, & Duarte, 2016; Kensinger & Schacter, 2006; Mitchell & Johnson, 2009). Context memory is thought to rely on frontally mediated executive functions to a greater extent than item memory during encoding and retrieval (Mitchell & Johnson, 2009). This is consistent with evidence suggesting that aging is associated with greater declines in cognitive processes reliant on the prefrontal cortex (e.g., the frontal aging hypothesis) (West, 1996).
Emerging evidence suggests that explicitly instructing both younger and older adults to attend to the relationship between an item and its context increases memory for the item-context association in both groups (Dulas & Duarte, 2013, 2014; Glisky & Kong, 2008; Glisky, Rubin, & Davidson, 2001; Hashtroudi, Johnson, Vnek, & Ferguson, 1994; Kuo & Van Petten, 2006; Naveh-Benjamin, Brav, & Levy, 2007). For example, directing attention to an item-color association (e.g., “Is this a likely color for this item?”) increases memory for that association over directing attention to the item alone (e.g., “Is this item smaller than a shoebox?”) (Dulas & Duarte, 2013). One possible mechanism for the memory improvement is that explicit attention strengthens the relationship between the item and its context at encoding (Uncapher, Otten, & Rugg, 2006). Another, non-mutually exclusive possibility is that the strengthened relationship reduces demands on executive functions at retrieval (Cohn, Emrich, & Moscovitch, 2008; Kuo & Van Petten, 2006).
While many experimental tasks assess memory for an item and a single contextual feature, in the real world we are likely to have multiple features competing for our attention. The ability to recover these contextual features at retrieval is likely a product of where we directed our attention at encoding. The ability to selectively attend to relevant features and ignore the irrelevant features is reduced in older adults (Kim, Hasher, & Zacks, 2007). This reduction in selective attention is suggestive of reduced inhibitory control (Hasher & Zacks, 1988). A reduced ability to selectively attend to a specific relationship may lead older adults to hyper-bind (Campbell, Hasher, & Thomas, 2010) and show a conditional dependence (Boywitt, Kuhlmann, & Meiser, 2012; Meiser, Sattler, & Weisser, 2008; Peterson & Naveh-Benjamin, 2016; Starns & Hicks, 2008) between relevant and irrelevant features during memory retrieval. A consequence of hyper-binding, in typical memory tasks, may be an impoverished memory representation for relevant contextual features. This in turn may lead to increased demands on executive functions at retrieval, such as episodic reconstruction and post-retrieval monitoring, in order to make accurate context memory decisions.
In a previous event related potential (ERP) study from our lab (James et al., 2016) we investigated contextual memory in both young and older adults where, at encoding, we directed attention to the relationship between one of two presented contexts: a color and a scene. Participants were required to direct attention to the appropriate (i.e., attended) context and ignore the other (i.e., unattended) context. At retrieval, we tested their memory for both the attended and unattended context features. We found that both groups demonstrated better memory for the attended feature, suggesting they were able to selectively attend to the appropriate context during encoding. Older adults showed conditional dependence between the two contextual features, indicative of hyper-binding, which we concluded was due to a reduced ability to inhibit the unattended context at encoding. The FN400 and parietal old-new effects were found to be similar across age groups. The FN400 has been linked to familiarity-based memory (Duarte, Ranganath, Winward, Hayward, & Knight, 2004; for review: Friedman & Johnson, 2000; Rugg & Curran, 2007) and conceptual priming (Voss, Lucas, & Paller, 2009). The parietal-old new effect is associated with recollection-based memory (Curran, 2000; Friedman & Johnson, 2000; Wilding, 2000), and can be modulated by the amount of information recollected (Vilberg, Moosavi, & Rugg, 2006). The similarity of these effects suggests that both young and older adults had intact recollection and familiarity. We found differences between young and older adults in the late posterior negativity (LPN), in which a reliably larger LPN was found for the older adults, compared to the young adults. The LPN has been linked to episodic reconstruction of the encoding episode through reactivation of context-specifying information (Cycowicz, Friedman, & Snodgrass, 2001; Johansson & Mecklinger, 2003). We concluded that while young and older adults recollected a similar amount of information, more of this information was likely irrelevant with respect to context memory decisions for the older adults. Consequently, older adults relied on episodic reconstructive processes to a greater extent than the young in order to recover relevant contextual information.
The current study uses the data from our previous ERP study to investigate the relationships between aging and context retrieval, with neural oscillations. An advantage of investigating neural oscillations over ERPs, is that they are thought to represent both local and long range communication between cell assemblies and reflect the synchronized inhibitory and excitatory firing rates (Jacobs, Kahana, Ekstrom, & Fried, 2007; Lee, Simpson, Logothetis, & Rainer, 2005). These synchronized fluctuations are thought to be critical for both encoding and retrieval of long-term memory (for review: Axmacher, Mormann, Fernandez, Elger, & Fell, 2006; Duzel, Penny, & Burgess, 2010; Nyhus & Curran, 2010). Another advantage of investigating neural oscillations is that they may contain more information about the underling cognitive processes, as ERPs only reflect a summation of power across all frequencies, and only those that are phase locked (i.e., synchronize at the same time across all individual trials) (Makeig, Debener, Onton, & Delorme, 2004). The current study capitalizes on the additional information provided by neural oscillations to investigate age-related differences in contextual memory retrieval.
Neural oscillations are commonly grouped into specific frequency bands of interest, such as theta (4–7 Hz), alpha (8–12 Hz), and beta (12–30 Hz). Event related synchronization and desynchronization refers to an increase or decrease in power from a resting, or prestimulus, interval (Pfurtscheller & Aranibar, 1977). Both synchronization and desynchronization within these frequency bands have been shown to reflect memory performance during both encoding and retrieval (for review: Hanslmayr & Staudigl, 2014; Klimesch, 1999). Greater theta synchronization, indexed by greater mean power, for correctly identified old items compared to both forgotten items and correctly rejected new items, is consistently found within the first 1000 msec post-stimulus, although the exact latency and topography varies by study. In contrast, for both alpha and beta, greater desynchronization is commonly found ~600 msec post-stimulus and continues through the end of the epoch (for review: Hanslmayr & Staudigl, 2014; Hanslmayr, Staudigl, & Fellner, 2012). Accumulating evidence suggests that these frequency bands reflect separable memory related processes (for review Hanslmayr & Staudigl, 2014). Theta increases at encoding and retrieval for remembered events have been shown to be invariant to various kinds of stimuli and task conditions (for review: Klimesch, 1999; Nyhus & Curran, 2010). It seems likely that theta rhythms reflect domain general operations that contribute to episodic memory (Guderian, Schott, Richardson-Klavehn, & Duzel, 2009). Both human and rodent research suggests that oscillations in the theta frequency band reflect interactions between the hippocampus and cortical areas including the prefrontal cortex, which facilitate encoding and retrieval (for review: Klimesch, 1999; Nyhus & Curran, 2010).
In contrast to the increases in theta synchrony that contributes to successful encoding and retrieval, alpha and beta desynchronization following stimulus onset has been associated with memory success (reviewed in Hanslmayr et al., 2012; Sederberg, Kahana, Howard, Donner, & Madsen, 2003). Furthermore, alpha and beta responses are often correlated, suggesting that they reflect similar cognitive processes. Studies manipulating the strategies with which events were encoded have shown that decreases in alpha/beta power may be particularly related to semantic encoding rather than shallow encoding or other forms of elaborative processing during encoding (Fellner, Bauml, & Hanslmayr, 2013; Hanslmayr, Spitzer, & Bauml, 2009). Intracranial evidence suggests that the left inferior frontal gyrus and hippocampus are at least two generators of these alpha and beta encoding effects (Sederberg et al., 2003). Alpha and beta power decreases have also been linked to successful episodic memory retrieval in several studies (Duzel et al., 2010; Hanslmayr et al., 2012 for reviews). Alpha and beta power decreases as the number of retrieved items increases and varies spatially across the scalp according to the type of perceptual features associated with prior encoded events (Khader & Rosler, 2011; Waldhauser, Braun, & Hanslmayr, 2016; Waldhauser, Johansson, & Hanslmayr, 2012).
Very little work has been done to study the effects of aging on episodic memory with neural oscillations. Some EEG evidence from a visuospatial associative encoding task suggests that age-related reductions in theta synchronization following event onset may contribute to older adults’ memory impairments (Crespo-Garcia, Cantero, & Atienza, 2012). Similarly, magnetoencephalography (MEG) evidence suggests that increased stimulus-related theta power during encoding predicts relational binding success for the young but not the old in a short-term memory task (Rondina et al., 2015). By contrast, alpha and beta desynchronization was greater for the old than the young but did not support memory performance. These results are consistent with findings from short-term memory tasks showing age-related decreases in theta synchronization (Kardos, Toth, Boha, File, & Molnar, 2014; Karrasch, Laine, Rapinoja, & Krause, 2004) and increases in alpha and beta power during memory task performance (Karrasch et al., 2004; Sebastian & Ballesteros, 2012), in both encoding and retrieval. It is important to note that these studies assessed stimulus-induced changes in oscillatory power relative to baseline but did not compare oscillatory power for successful and unsuccessful memory trials (i.e., subsequent memory and old-new effects). Thus, it remains unclear how aging affects neural oscillations that underlie retrieval success.
In the current study, we assessed effects of age on neural oscillations during context memory retrieval with the data from our previously published ERP study (James et al., 2016). As discussed above, participants were directed to attend to a gray scale object and one of two concurrent contextual details (i.e., color and scene) at encoding. At retrieval, we tested their memory for the object, its attended and unattended features, and their confidence in both context judgments. Given that we found ERP evidence that older adults relied on episodic reconstructive processes at retrieval to support memory performance to a greater extent than the young, we expected to find age differences within frequency bands that reflect episodic reconstruction. A likely frequency band in which reconstructive processes might be reflected is the alpha/beta band, given its association with sensory reactivation during retrieval (Khader & Rosler, 2011; Waldhauser et al., 2012). Additionally, neural oscillations allow for investigation of non-phased locked activity, and the separation of frequency bands may reveal age-related effects in the time series that are masked by the ERP analysis.
2. Method
2.1. Participants
The current study included the same participants from our previously published ERP study (James et al., 2016). This included 22 young (18–35) and 21 older (60–80) healthy, right-handed adults. All participants were native English speakers and had normal or corrected vision, were compensated with course credit or $10 per hour, and were recruited from the Georgia Institute of Technology and surrounding community. None of the participants reported neurological or psychiatric disorders, vascular disease, or use of any medications affecting the central nervous system. Participants completed a standardized neurological battery and were only included if their scores fell within two standard deviations of the group mean. All participants signed consent forms approved by the Georgia Institute of Technology Institutional Review Board.
2.2. Materials
All 432 grayscale object images were collected from the Hemera Technologies Photo-Object DVDs and Google images. At study, 288 object images were encoded (144 in each attended to group), and 144 object images were used as new items during test. Study and test items were counter balanced across subjects. Each object was presented center screen on a white background, flanked by both a colored square and a scene. The color and scene images served to provide a context to which participants were instructed to attend. The possible scenes included a studio apartment, cityscape, or island. The possible colored squares included green, brown, or red. Each context and object image spanned a maximum vertical and horizontal visual angle of approximately 3°.
2.3. Procedure
A sketch of both the study and test trials is depicted in Fig. 1. During study, the participant made a subjective yes/no judgment about the relationship between the object and either the colored square (i.e., Is this color likely for this object?) or the scene (i.e., Is this object likely to appear in this scene?). Both written and verbal instructions clearly stated that on any particular trial the participant should attend to one context and ignore the other context. Each study block was divided into 4 mini-blocks containing 18 trials each. Within each mini-block only one type of context question was asked, and this was indicated both at the beginning of the mini-block and underneath the images during each trial. The order of the context questions was counterbalanced across subjects. The location of the color and scene context images was switched between blocks, so that for each participant the image and scene contexts appeared on the left for two blocks and on the right for two blocks.
Fig. 1.
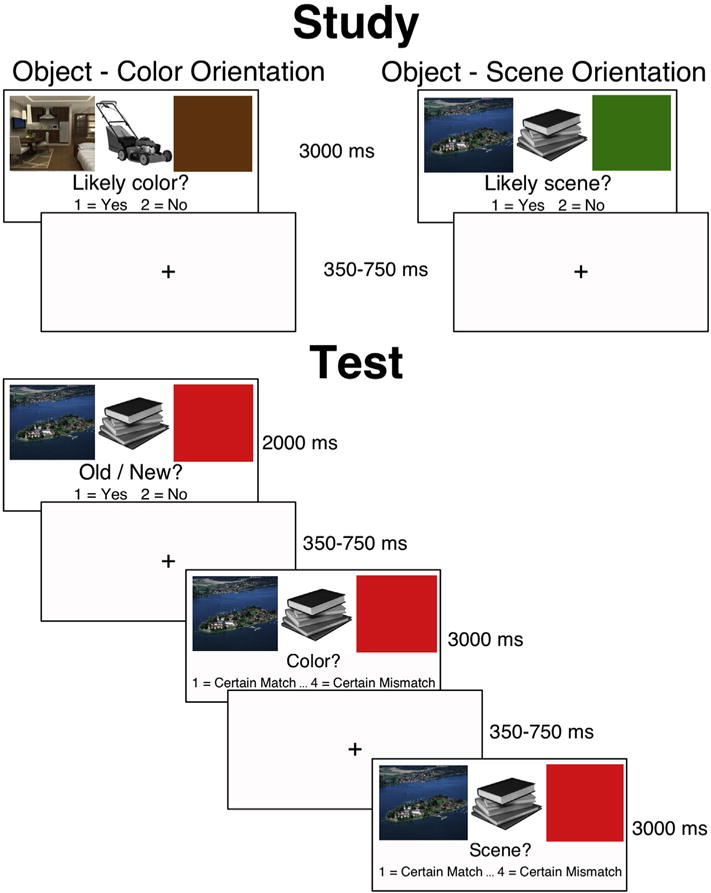
Study and test task design.
During test, all the 288 old objects and 144 new object were used. Each object was flanked by both a scene and a colored square, with the same positioning as presented during study. For each object, the participant decided whether it was an old or a new image. If the participant responded that it was a new image the next trial began after 2000 msec. If they responded that it was an old image, they were asked to make two context judgments: one about the colored square and another about the scene. Which context question was asked first was counterbalanced across participants. For old items, the pairing was arranged so that an equal number of old objects were presented with: (1) the original context images, (2) only the scene switched, (3) only the color switched, and (4) both context images switched. Responses to the context questions were made on a scale from 1 (certain match) to 4 (certain mismatch). For those items correctly identified as ‘Old’, we classified a ‘Context-Hit’ as correctly identifying whether the attended context (scene or color) was the same as or different from encoding, regardless of memory for the unattended context. An incorrect response was classified as a ‘Context-Miss’.
In total, there were four study and four test blocks. Young adults completed all four study blocks before the four test blocks. For older adults, in order to better equate item memory performance with young adults, the memory load was halved so that they completed a two-block study-test cycle twice (two study, two test, two study, two test). Both younger and older adults completed a short practice of both the study and test blocks before starting the first study block. Thus, both younger and older adults knew of the upcoming memory test.
2.4. EEG recording
Continuous scalp-recorded EEG data were collected from 32 Ag-AgCl electrodes using an ActiveTwo amplifier system (BioSemi, Amsterdam, Netherlands). Electrode position follows the extended 10–20 system (Nuwer et al., 1998). Electrode positions included: AF3, AF4, FC1, FC2, FC5, FC6, FP1, FP2, F7, F3, Fz, F4, F8, C3, Cz, C4, CP1, CP2, CP5, CP6, P7, PO3, PO4, P3, Pz, P4, P8, T7, T8, O1, Oz, and O2. External left and right mastoid electrodes were used for referencing offline. Two electrodes placed superior and inferior to the right eye recorded vertical electrooculogram (VEOG) and two additional electrodes recorded horizontal electrooculogram (HEOG) at the lateral canthi of the left and right eyes. The ActiveTwo system replaces the traditional reference with a Common Mode Sense (CMS) active electrode and the ground with a Driven Right Leg (DRL) passive electrode. EEG was sampled at 1024 Hz with 24-bit resolution without high or low pass filtering.
2.5. EEG preprocessing
Offline analysis of the EEG data was done in MATLAB 2015b with the EEGLAB (Delorme & Makeig, 2004), ERPLAB (Lopez-Calderon & Luck, 2014), and FIELDTRIP (Oostenveld, Fries, Maris, & Schoffelen, 2011) toolboxes. The continuous data were down sampled to 256 Hz, referenced to the average of the left and right mastoid electrodes, and band pass filter between .5 Hz and 125 Hz. The data were then epoched from −1000 msec to 3000 msec from stimulus presentation of the first retrieval question (old/new). The time range of interest was set to −300 msec to 2000 msec, but a longer epoch is needed to account for signal loss at both ends of the epoch during wavelet transformation. Each epoch was baseline corrected to the average of the whole epoch, and an automatic rejection process removed epochs with extreme voltage shifts that spanned across two or more electrodes, or epochs in which a blink occurred during stimulus onset. The automated rejection processes identified epochs with the following parameters in the raw data: 1) The voltage range was greater than 99th percentile of all epoch voltage ranges within a 400 msec window (sliding in 100 msec intervals across each epoch). 2) The linear trend slope exceeded the 95th percentile of all epoch ranges with a min R2 value of .3.3) The voltage range was greater than 95th percentile of all epoch voltage ranges within a 100 msec window (sliding in 25 msec intervals across each epoch), between −150 and 150 msec from stimulus onset for frontal and eye electrodes only. This process was iterated twice. Then an independent component analysis (ICA) was run on all head electrodes in order to identify additional artifacts highlighted by the components. The following parameters were used on the components to reject epochs: 1) The voltage range was greater than 99th percentile of all epoch voltage ranges within a 400 msec window (sliding in 100 msec intervals across each epoch). 2) The kurtosis or joint probability exceeded 15 standard deviations within the component or 23 standard deviations of all components for the epoch. In order to identify activity related to ocular artifacts (i.e., blinks and horizontal eye movements), ICA was run on the first 20 principle components of the head electrodes for the accepted epochs. Components related to ocular artifacts were removed from the data by visually inspecting the topographic component maps and component time course with the ocular electrodes (Bell & Sejnowski, 1995; Delorme, Sejnowski, & Makeig, 2007; Hoffmann & Falkenstein, 2008). Finally, each epoch was re-baselined to the −300 to −100 msec time period and manually inspected for additional artifacts (e.g., amplifier saturation, spiking, extreme values, uncorrected ocular activity). If a dataset contained a noisy electrode (e.g., greater than 30% of the data needed to be rejected), it was removed from the processing stream and interpolated before running the time-frequency process. On average, after all processing steps 14% (SD = 9%) of the epochs were removed.
2.6. Frequency decomposition
Each epoch was transformed into a time frequency representation using Morlet wavelets (Percival & Walden, 1993) with 40 linearly spaced frequencies between 3 and 30 Hz, at 5 cycles. During the wavelet transformation, each epoch was reduced to the time range of interest and down sampled to 50.25 Hz (Cohen, 2014). Then, condition averages with a 10% trimmed mean (Wilcox & Keselman, 2003) were made for each subject. Frequencies of interest were set to: Theta (4–7 Hz), Alpha (8–12 Hz), and Beta (14–24 Hz). Each condition was baseline corrected to the decibel change from the −300 to −100 msec time range. Trial numbers for the conditions of interest are as follows: Context-Hits (young mean = 123, SD = 47, minimum = 53; old mean = 92, SD = 31, minimum = 33), Correct Rejections (young mean = 107, SD = 17, minimum = 67; old mean = 102, SD = 20, minimum = 56). The within subject condition averages were used for all significance tests.
2.7. Frequency significance testing
To assess significance in the oscillatory data, Monte Carlo permutation tests were performed with the FIELDTRIP toolbox (Blair & Karniski, 1993; Maris & Oostenveld, 2007). Briefly, this method tests the two-tailed t-statistic between two conditions of interest against an expected null distribution of a two-tailed t-statistics created by randomizing the data between the two conditions of interest (e.g., across subject mean power for context-hits and correct rejections). The randomization procedure to create the null distribution of t-statistics was repeated 2000 times. The test statistic was considered significant if, at an alpha level of .05 for a two-tailed test, the value was greater than 97.5%, or less than 2.5% of the null distribution. The permutation processes is the same for both within and between analysis, with the exception that a paired sample t-test was used for all within subject analyses and an independent sample t-test was used for across group analyses.
2.8 Identification of time regions
For each group and frequency band, within the contrast of interest, consecutive 100 msec time intervals from stimulus onset to 2000 msec post stimulus were used to identify time regions of interest. Each electrode within each time window and frequency band was subjected to an across-subject permutation test to identify clusters of electrodes showing significant effects. Assuming independence between all 32 electrodes and an alpha rate of .05, the expected Type 1 error rate would lead to 1.6 electrodes (32*.05) per time window. Thus, we only identified significant time windows if two or more neighboring electrodes were significant and they extended across two or more interval time windows. After identifying significant extended time windows, we averaged across them and ran an additional permutation tests on the extended windows. Only results from the extended time windows are reported. This approach is similar to that used in previous retrieval studies (Addante, Watrous, Yonelinas, Ekstrom, & Ranganath, 2011; Gruber, Watrous, Ekstrom, Ranganath, & Otten, 2013).
In the second step, after establishing times ranges of interest for the planned contrast, we tested for significant differences between the young and old adults within the contrast of interest. Because we identified time ranges of interest separately for both young and older adults, it is unsurprising that we found similar effects with slightly different time windows for each age group. In order to avoid biasing our results, when time ranges overlapped (e.g., young: 1000–2000 msec; old: 1500–2000 msec), we tested for group differences for a particular effect (e.g., beta desynchronization) for the overlapping periods and the non-overlapping periods (i.e., 1000–1500 msec and 1500–2000 msec).
2.9. Correlations
In order to establish whether EEG effects showed a relationship with context memory performance (proportion of context-hits), an independent samples regression t-test was run within each age group, during each time region of interest, on each electrode. Significance was based on permutation testing as discussed above, with a minimum threshold of two significant contiguous electrodes. Those electrodes with a significant relationship between behavior and power were averaged together in-order to quantify and report the relationship, and the Pearson correlation between average power and context memory performance is reported. It should be noted that electrodes that show a significant linear relationship with performance might not be the same as those that show mean level differences.
3. Results
3.1. Behavior
Results from the neuropsychological battery can be found in Table 1, and full behavioral results and analysis can be found in James et al. (2016). In consideration of our current results mean proportion of item hits, false alarms, and item hit associated with correct context judgments for attended and unattended features can be found in Table 2. Item memory for object stimuli was assessed with Pr: p(hits) − p(false alarms (Snodgrass and Corwin, 1988). Pr for young adults was .67; (SD = .15), and for older adults was .61 (SD = .15). No group differences were found for item Pr [t(41) = .78, p = .44, d = .24] Participants judged contexts as either matching or mismatching the context that was presented during study for each object. Thus, chance performance is .5. As can be seen in Table 2, context accuracy for unattended features was near chance for both age groups. Unattended context accuracy exceeded chance for young [t(21) = 3.93, p = .001, d = 1.72] but not older adults [t(20) = 1.72, p = .10, d = .77]. Accuracy for attended features was well above chance for both the young [t(21) = 14.19, p < .001, d = 3.03] and old [t(20) = 8.42, p < .001 d = 1.84]. Finally, young adults outperformed the older adults in the ability to correctly identify attended features [t(41) = 5.18, p < .001, d = 1.62].
Table 1.
Group characteristics and neuropsychological scores.
| Measure | Young (n = 22) |
Old (n = 21) |
|---|---|---|
| Age | 21.33 (19.41, 23.25) | 67.86 (66.06, 69.66) |
| Gender (F/M) | 9/13 | 14/7 |
| Education | 14.21 (13.51, 14.91) | 15.21 (14.22, 16.20) |
| MOCA (older adults only) | – | 27.06 (26.02, 28.10)) |
| Trails A (in seconds) | 23.89 (20.62, 27.16) | 36.48 (25.94, 47.03)** |
| Trails B (in seconds) | 47.45 (41.08, 53.83) | 84.81 (67.30, 102.31)** |
| Visual recognition | 18.17 (17.26, 19.07) | 16.68 (15.57, 17.80)** |
| Delayed visual recognition | 19.11 (18.41, 19.81) | 16.47 (15.20, 17.74)** |
| Visual reproduction | 8.89 (8.33, 9.45) | 5.58 (4.43, 6.73)** |
| Letter fluency | 46.39 (40.30, 52.48) | 50.61 (40.93, 60.29) |
| List recall (Immediate) | 10.28 (9.43, 11.13) | 9.16 (7.74, 10.58) |
| List recall (Immediate, Cued) | 10.28 (9.56, 11.00) | 10.26 (9.37, 11.16) |
| List recall (Delayed) | 11.28 (10.64, 11.91) | 10.05 (8.45, 11.66) |
| List recall (Delayed, Cued) | 11.17 (10.44, 11.90) | 10.79 (9.99, 11.59) |
| List recognition | 12.00 (12.00, 12.00) | 11.61 (11.31, 11.91) |
| MAS digit span forward | 7.61 (6.95, 8.27) | 7.0 (6.38, 7.62) |
| MAS digit span backward | 5.50 (4.77, 6.23) | 4.78 (4.04, 5.51) |
Note: Reported test scores are the raw values and the 95% confidence interval for the mean is in parentheses.
Significant group difference (p < .05).
Table 2.
Mean proportion of hits, false alarms to new items, and correct context judgments for both the attended and unattended context.
| Young | Old | |
|---|---|---|
| Hits | .73 (.67, .80) | .70 (.64, .76) |
| False alarms | .06 (.04, .08) | .10 (.07, .12) |
| Attended context accuracy | .74 (.71, .78) | .62 (.59, .65) |
| Unattended context accuracy | .53 (.51, .54) | .52 (.50, .54) |
Note: Context accuracy represents the percentage of trials on which participants correctly identified the item (hits) and judged context (attended or unattended) accurately. The 95% confidence interval for the mean is presented in parentheses and all values have been rounded to the nearest hundredth.
Older adults showed greater conditional dependence between attended and unattended context accuracy. A full analysis of these probabilities is reported in James et al. (2016). Specifically, the probability of correctly endorsing the attended context if the unattended context was correct was .74 in young adults and .64 for older adults. The probability of correctly endorsing the attended context if the unattended context was incorrect was .74 for young adults and .60 for older adults. Context accuracy was greater for the attended feature if accuracy for the unattended feature was also correct as opposed to incorrect for older adults [F(1,20) = 4.84, p = .04, η2 partial = .20] but not young adults [F(1,21) < 1, η2 partial = 0.002]. To ensure that the conditional dependence found in older adults was not a result of experience from the first study-test cycle, we tested for differences in conditional dependence between the two cycles. The first and second study-test cycle in older adults did not differ in conditional dependence [t(20) = −.083, p = .935]. These results are consistent with the idea that reduced selective attention leads to hyper-binding of target and distractor context features in older adults.
3.2. EEG results
Time frequency analysis was restricted to the comparison between context-hits and correct rejections. Context-hits refer to the correctly recognized items for which the attended context was correctly identified during retrieval, regardless of whether the unattended context was or was not identified. This comparison between context-miss (i.e., item-only) and context-hits trials would be the most sensitive index of context memory accuracy. Unfortunately, there were insufficient numbers of context-misses to obtain robust EEG signals for all participants. In order to maximize power to find potential correlates of contextual retrieval, we restricted our analysis to the comparison between context-hits and correct rejections, as has been done in previous studies (Duverne, Motamedinia, & Rugg, 2009; Morcom, Li, & Rugg, 2007; Newsome, Dulas, & Duarte, 2012). Although robust, this contrast likely reflects both item and context recognition. As discussed in the methods, we identified significant time regions within each frequency band for young and older adults separately.
Time-series plots and topographic maps for significant effects for both young and older adults are shown in Fig. 2. Topographic maps and bar graphs showing significant group differences are shown in Fig. 3. We found that both groups showed greater theta synchronization for context-hits than correct rejections during an early time range, followed by greater theta power for correct rejections. Widespread alpha and beta desynchronization effects were evident over the late part of the epoch for both age groups. While theta synchronization effects were supportive of context memory accuracy in young adults, beta desynchronization effects supported performance for older adults.
Fig. 2.
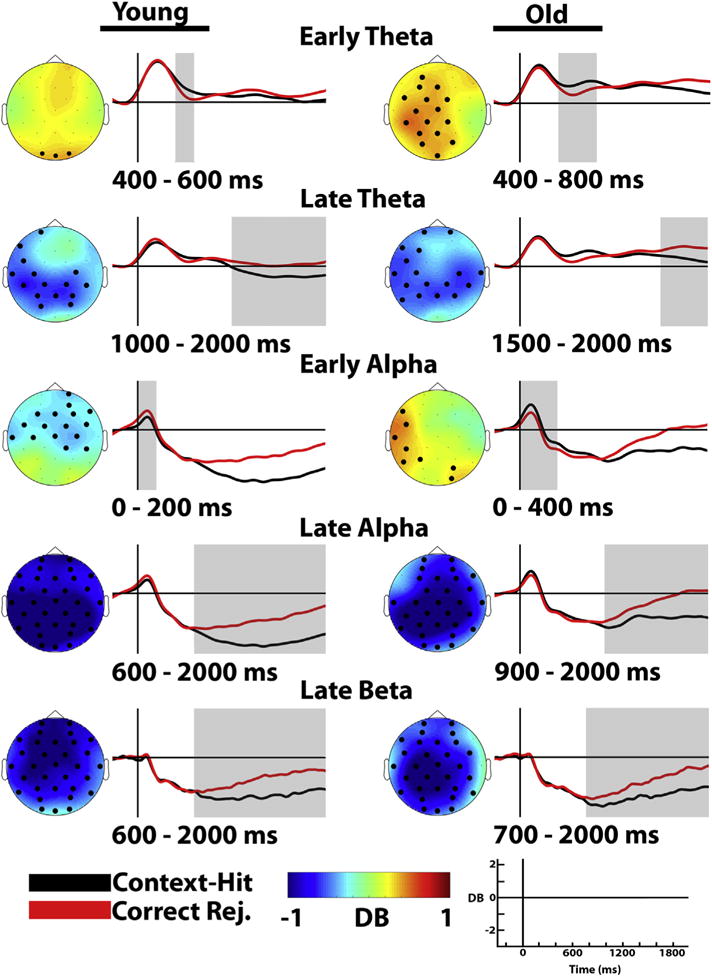
Topographic maps and time course for context-hits and correct rejections for both young and older adults. Topographic maps represent the contrast of context-hits minus correct rejections for the identified time range, as indicated in the label and gray area of the time course plots. Significant electrodes are shown in bold. The time course represents the decibel signal change for context-hits (black lines) and correct rejections (red lines) averaged across the significant electrodes from the prestimulus baseline (−300 to −100 msec).
Fig. 3.
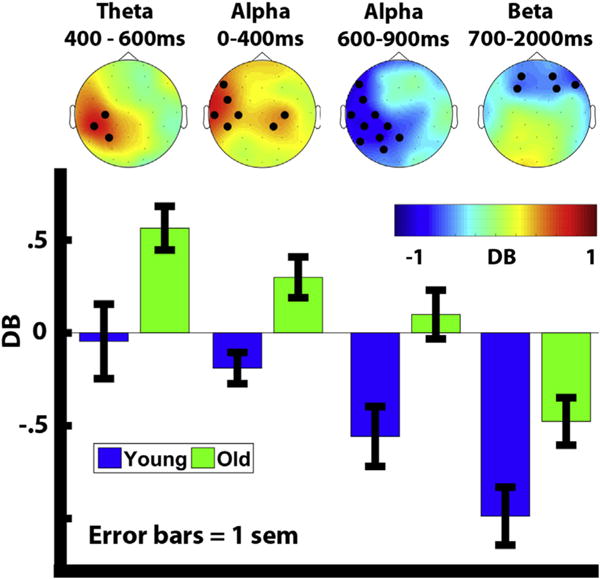
Group differences in decibel power between context-hits minus correct rejections. Significant electrodes are shown in bold. Average power for young (blue) and old (green) adults is plotted under each topographic map for significantly different electrodes. Error bars depict standard error of the mean.
3.2.1. Theta power
3.2.1.1. Young adults
For young adults, theta power was greater for context-hits than correct rejections, between 400 and 600 msec over occipital electrodes (O2, Oz, O1). Between 1000 and 2000 msec we found significantly less theta power for context-hits compared to correct rejections, maximal across posterior electrodes (Fp1, F7, FC5, T7, C3, CP1, CP5, P3, Pz, PO3, PO4, P4, CP6, CP2, C4). The magnitude of the early (400–600 msec) theta synchronization effect (context-hits – correct rejections) was positively related to the proportion of context-hits in a cluster of left posterior electrodes (CP1, P7, P3, Pz, PO3) [r(20) = .535, p = .01], as shown in Fig. 4. The correlation was also reliable in the later part of the epoch between 1000 and 2000 msec (CP1, P3, Pz, PO3, CP2) [r(20) = .471, p = .027]. Neither the early [r(20) = .219, p = .327] nor the late [r(20) = .416, p = .054] effect significantly correlated with item memory (Pr). This suggests that the theta synchronization effect supported better context memory accuracy for young adults.
Fig. 4.
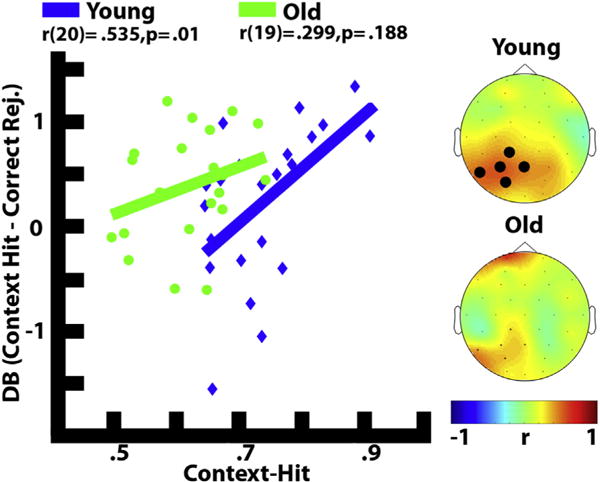
Correlation between context-hits and early theta for both the young (blue) (400–600 msec) and old (green) (400–800 msec). Significant electrodes are shown in bold. Early theta power corresponds to the decibel signal difference of context-hits minus correct rejections. Topographic maps represent the Pearson correlation coefficient for each electrode. Only correlations with two or more contiguous electrodes were considered significant, and the average power over all significant electrodes was used to represent the brain-behavior relationship.
3.2.1.2. Old adults
For older adults, theta power was greater for context-hits than correct rejections in the 400–800 msec time range over left central electrodes (AF3, F3, FC1, FC5, C3, CP1, CP5, P3, Pz, PO3, Oz, PO4, CP2, FC2, Fz, Cz). In the 1500–2000 msec time range, we found significantly less theta power for context-hits compared to correct rejections, which was maximal across the posterior electrodes (Fp1, F7, F3, FC5, T7, C3, CP1, CP5, P7, P3, Pz, P4, CP6, CP2, C4, T8, Fp2). No significant correlations between theta memory effects and the proportion of context-hits were observed for older adults.
3.2.1.3. Between-group analysis
For the 600–800 msec time-range, we found significantly greater theta synchronization effects (context-hits-correct rejections) for older adults compared to young adults over three contiguous left posterior electrodes (C3, CP5, and P3). In order to identify if the significant group differences can be attributed to age group or differences in context memory performance we ran a oneway ANCOVA between age group (Young, Old) on average cluster power controlling for context-hit performance. After controlling for context-hit performance age group was still significant [F(1,40) = 11.699, p = .001].
3.2.2. Alpha power
3.2.2.1. Young adults
Between 0 and 200 msec, we found greater alpha desynchronization for correct rejections compared to context-hits over frontocentral electrodes (F3, FC1, FC5, T7, C3, CP6, CP2, C4, FC6, FC2, F4, F8, AF4, Fp2, Fz, Cz). This was followed in the 600–2000 msec time-range by a large and widespread desynchronization for context-hits compared to correct rejections (Fp1, AF3, F7, F3, FC1, FC5, T7, C3, CP1, CP5, P7, P3, Pz, PO3, O1, Oz, O2, PO4, P4, P8, CP6, CP2, C4, T8, FC6, FC2, F4, F8, AF4, Fp2, Fz, Cz).
3.2.2.2. Old adults
Between 0 and 400 msec, we found greater alpha synchronization for context-hits compared to correct rejections over left lateralized electrodes (F7, FC5, T7, CP5, P7, P3, O2, PO4). In the 900–2000 msec time range, we found a large widespread desynchronization for context-hits compared to correct rejections (Fp1, AF3, F3, FC1, T7, C3, CP1, CP5, P7, P3, Pz, PO3, O1, Oz, O2, PO4, P4, P8, CP6, CP2, C4, T8, FC6, FC2, F4, F8, AF4, Fp2, Fz, Cz).
3.2.2.3. Between-group analysis
For the 0–400 msec time range, we found significant crossover effects for alpha, with greater power for context-hits than correct rejections for older adults, and the opposite pattern for the young over frontocentral electrodes (F7, FC5, T7, C3, CP5, CP2, C4). Young adults showed greater alpha desynchronization effects between 600 and 900 msec than older adults over left lateralized electrodes (F7, FC5, T7, C3, CP1, CP5, P7, P3, Pz, PO3). These group differences disappeared in the 900–2000 msec time range. This latter result suggests that alpha desynchronization started earlier for young adults compared to the old. A one-way ANCOVA between age group (Young, Old) on average cluster power controlling for context-hit performance revealed a significant effect of age on both early [F(1,40) = 6.338, p = .016] and late [F(1,40) = 5.260, p = .027] alpha power.
3.2.3. Beta power
3.2.3.1. Young adults
Widespread beta desynchronization effects were evident between 600 and 2000 msec for context-hits compared to correct rejections (Fp1, AF3, F7, F3, FC1, FC5, T7, C3, CP1, CP5, P7, P3, Pz, PO3, O1, Oz, O2, PO4, P4, P8, CP6, CP2, C4, T8, FC6, FC2, F4, F8, AF4, Fp2, Fz, Cz).
3.2.3.2. Old adults
Widespread beta desynchronization effects were also evident for older adults between 700 and 2000 msec (Fp1, AF3, F7, F3, FC1, FC5, T7, C3, CP1, CP5, P7, P3, Pz, PO3, O1, Oz, O2, PO4, P4, P8, CP6, CP2, C4, FC6, FC2, F4, AF4, Fp2, Fz, Cz). Interestingly, this effect correlated negatively with the proportion of context-hits across mostly left lateralized electrodes (FC1, T7, C3, CP1, P3, PO3, Fp2) for older adults only [r(19) = −.516, p = .017], as can be seen in Fig. 5. In addition, we also found a correlation between item memory (Pr) and this electrode cluster [r(19) = −.442, p = .045]. This suggests that the beta desynchronization effect supported both item and context memory accuracy for older adults.
Fig. 5.
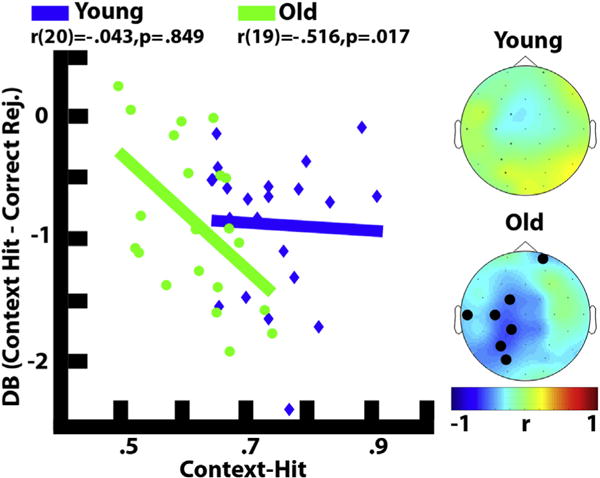
Correlation between context-hits and late beta for both the young (blue) (600–2000 msec) and old (green) (700–2000 msec). Significant electrodes are shown in bold. Late beta power corresponds to the decibel signal difference of context-hits minus correct rejections. Topographic maps represent the Pearson correlation coefficient for each electrode. Only correlations with two or more contiguous electrodes were considered significant, and the average power over all significant electrodes was used to represent the brain-behavior relationship.
3.2.3.3. Between-group analysis
For the 700–2000 msec time range, beta desynchronization was greater for the young compared to the old adults over bilateral frontal electrodes (AF3, F3, AF4, F4, F8). A one-way ANCOVA between age group (Young, Old) on average cluster power controlling for context-hit performance revealed that age group was not significant after controlling for context-hit performance [F(1,40) = 1.763, p = .192], suggesting that increased negativity across the frontal electrodes reflects differences in performance rather than age.
4. Discussion
The current study is the first, to our knowledge, to investigate age-related differences in context memory retrieval with neural oscillations. Participants selectively attended to one of two contextual features presented concurrently during encoding, allowing us to examine the effect of selective attention during encoding on the processes supporting context memory retrieval. Both groups showed greater memory for attended than unattended contextual features. Older adults showed evidence of hyper-binding between attended and unattended context features. Time frequency results in the theta band suggest that young and older adults recollected similar amounts of information. However, brain-behavior correlations suggest that for young adults only, the amount of information recollected was supportive of contextual memory performance. By contrast, for older adults only, beta desynchronization was correlated with contextual memory accuracy, suggesting that their performance was supported by late on setting executive functions, such as episodic reconstruction. These results and their implications are discussed below.
4.1. Behavior
The goal of the current study was to investigate neural oscillations during contextual memory retrieval. Since a full behavioral discussion has been previously published, we direct interested readers to James et al. (2016). Thus, we will only briefly recap the relevant results and interpretations that we used to interpret the neural oscillations. In our study, young and older adults showed equivalent item-memory performance. This allowed us to investigate age-related differences in contextual memory, unconfounded by age-related differences in item memory. Older adults completed two study-test cycles to ensure equivalent item memory between young and older adults, which effectively halved the memory load for older adults. Large differences in task performance are potential confounds in aging research, and care must be taken to ensure that the effects of age are not completely accounted for by these differences (Rugg & Morcom, 2005). Memory for contextual features was greater for the attended, compared to the unattended features, in both groups. Both groups were near chance for the unattended features. This suggests that both young and older adults were successful at selectively attending to the appropriate context during encoding.
Even though we found that both young and older adults were able to successfully selective attend during encoding, we found evidence that older adults were less able to ignore the unattended contextual feature. Memory for attended contextual features was lower for older adults, compared to younger adults. A conditional probability analysis revealed that older adults’ ability to remember attended contextual features was conditional on their ability to remember the unattended contextual features, and vice versa. In contrast, young adults did not show a conditional probability between attended and unattended contextual memory performance. Thus, older adults were more likely to encode the attended with the unattended context, and this reduced their ability to selectively retrieve the appropriate contextual details. This is consistent with hyper-binding, where older adults are more likely to encode target and distractor items appearing simultaneously (Campbell et al., 2010) or occurring close in temporal proximity (Campbell, Trelle, & Hasher, 2014). Importantly, the conditional dependence found in older adults did not differ between the two study-test cycles, indicating that this result was not a function of task experience during the first study-test cycle. We suggest that inhibitory deficits in the older adults likely led to a reduced ability to encode the appropriate item-context relationship, and this led to age-related declines in contextual memory performance.
Research directly investigating conditional dependence between multiple context details suggests that young adults are more likely to bind multiple context features in memory, compared to older adults (Boywitt et al., 2012; Peterson & Naveh-Benjamin, 2016). In the current study, we found the opposite: older adults conditionally bound context details while younger adults did not. Given our hypothesis that younger adults are more effective at selectively attending to the appropriate context than older adults, this would be the expected outcome. It is worth noting that the mechanisms underlying hyper-binding and conditional dependence in general are still under investigation, and previous studies suggest that context information may be bound to the item and not to each other (Hicks & Starns, 2016; Starns & Hicks, 2008; Vogt & Broder, 2007). The present results are consistent with the idea that irrelevant contextual features affected the manner in which events were encoded by older adults, although the exact manner remains to be determined. Future studies directly manipulating conditional dependence in aging between perceptual and semantic encoding would valuable in determining the boundaries of contextual binding.
4.2. EEG results
4.2.1. Theta synchronization and desynchronization
Consistent with previous research investigating old-new and contextual memory effects in young adults, we found greater theta synchronization for correctly identified context-hits, compared to correctly rejected new items (Addante et al., 2011; Burgess & Gruzelier, 1997; Gruber, Tsivilis, Giabbiconi, & Muller, 2008; Guderian & Düzel, 2005; Klimesch, Doppelmayr, Schwaiger, Winkler, & Gruber, 2000; Klimesch et al., 2006). Previous studies have shown a similar theta synchronization effect for context-hits, compared to context misses, within a similar time range (400–800 msec) (Gruber et al., 2008; Guderian & Diizel, 2005). In addition, the correlation between theta power and item memory was not significant. Thus, we believe the theta effect observed here reflects successful recollection, rather than item recognition. For young adults, we found that theta power was correlated with context memory accuracy over left parietal electrodes, similar to previously published research (Addante et al., 2011). Theta power has been shown to vary with amount of information retrieved (Khader & Rosler, 2011) and source memory accuracy (Addante et al., 2011), but not the nature of the stimulus materials (Khader & Rosler, 2011), consistent with the idea that theta power reflects domain general memory operations (Hanslmayr, Staudigl, Aslan, & Bauml, 2010; Staudigl, Hanslmayr, & Bauml, 2010). We suggest that younger adults were successful at selectively attending during encoding, and the amount of retrieved contextual information was directly supportive of contextual memory performance. In older adults, we did not find a significant relationship between theta and context memory performance. A similar lack of relationship between theta and associative memory accuracy in a short-term memory task has been previously shown for older adults (Rondina et al., 2015). Instead, we found a longer lasting theta synchronization effect over left parietal electrodes for the old, compared to the young, which remained significant after controlling for age differences in context memory.
Research in young adults has found that the parietal old-new ERP is related to the old-new theta effects but is functionally distinct (Jacobs, Hwang, Curran, & Kahana, 2006; Klimesch et al., 2000). For example, they are both related to retrieval success and show similar time course, but filtering out the theta frequency range does not remove the parietal old-new ERP. Instead, filtering out sub-delta frequency ranges has been found to remove the parietal old-new ERP (Klimesch et al., 2000). While both effects are sensitive to manipulations at retrieval, the ERP is a combination of frequencies and phase angles, which likely reflects multiple cognitive processes activating in concert, while theta activity may only represent a single or subset of these process related to the retrieval process itself (Jacobs et al., 2006).
Considering the similarities in the average theta response between young and old adults, we suggest that both groups were able to retrieve criterial episodic information. This result is consistent with our ERP results showing age invariance of the parietal old-new effect (James et al., 2016), which has been linked to recollection in numerous previous studies (Curran, 2000; Friedman & Johnson, 2000; Rugg & Curran, 2007; Wilding, 2000). Previous research suggests that both young and older adults may recollect a similar amount of information, although they differ on the retrieved content (Leshikar, Dulas, & Duarte, 2015). Specifically, older adults have been shown to recollect more ‘non-criterial’ details, such as thoughts and feelings from encoding (Mollison & Curran, 2012; Yonelinas & Jacoby, 1996). Interestingly, for older adults, the sustained theta response and lack of correlation between theta and context memory performance suggests that they may be retrieving additional irrelevant contextual details that do not support performance. It is also possible that older adults recollected lower quality perceptual details that contributed to their reduced scene and color context accuracy. In line with previous studies, we suggest that while older adults are recollecting a similar amount or more information as the young, it was less robust and/or less relevant for contextual memory performance (Duarte, Henson, & Graham, 2008; Duarte, Ranganath, Trujillo, & Knight, 2006). It remains an open question as to how aging affects retrieval processes reflected by increased theta synchronization. For example, the increase in theta could reflect the additional retrieval of non-criterial elements or the unattended context feature from encoding. These differences may also reflect general age differences in cognition, such as age related slowing (Salthouse, 1996), and the increase in theta is reflecting a longer retrieval time and not necessarily a difference in quantity.
Interestingly, we also found a reversal of theta power (i.e., correct rejections > context-hits) in the later part of the epoch, between 1000 and 2000 msec, that did not differ between the groups. Task related theta power reductions in this later time range are not reported in the literature as much as increases in theta power. It is worth noting that this reduced theta power for context-hits was much smaller than the desynchronization effects found for alpha and beta, as discussed below. At least one study has found that increasing memory load in a working memory task modulated widespread negative theta following the probe, suggesting that negative theta is related to maintaining the study items (Jacobs et al., 2006). In our design, participants answered a series of questions if they responded with an ‘old’ response during the initial old/new question. For the context-hits, the participants were required to make additional judgments for the contextual details, while for correct rejections they did not need to make any additional judgments. Thus, the negative theta may reflect maintenance of retrieved episodic content used to support context memory decisions. It should be noted that the relationship between this late theta effect and context memory accuracy was positive, meaning that young adults who performed best had greater theta power for context-hits than correct rejections. Collectively, we believe these results suggest that greater theta power reflects successful context memory retrieval. By contrast, lower theta power reflects sustained maintenance of retrieved contents, which is particularly evident when contextual features are not readily recovered.
Low frequency bands, such as theta, are thought to allow for long distance communication between separate neural regions (for review: Fries, 2005). Theta is the dominant frequency in the hippocampus and is thought to facilitate communication between the hippocampus and neocortex (for review: Bastiaansen & Hagoort, 2003; Nyhus & Curran, 2010). While it is unlikely that we are able to record theta activity directly from the hippocampus through scalp electrodes, we are likely able to detect communication between the hippocampus and prefrontal cortex (for review: Hsieh & Ranganath, 2014; Jensen, 2005). We tentatively suggest that this prefrontal-hippocampal communication may be age-invariant during context retrieval. This would be consistent with previous neuroimaging findings showing age invariance for medial temporal lobe contributions during episodic retrieval (Dulas & Duarte, 2014; Duverne et al., 2009; Morcom et al., 2007). Nonetheless, the diagnostic quality of the episodic information, with respect to the contextual decision, reflected in this communication likely differs between young and older adults.
4.2.2. Alpha synchronization, alpha and beta desynchronization
We found an age-related crossover for early alpha effects, with young adults showing greater alpha power for correct rejections than context-hits and older adults showing the reverse pattern. While these early alpha effects were not necessarily predicted, the latency of the effects, which appear to begin slightly prior to probe onset, suggest that they reflect processes that contribute to retrieval rather than operate on the products of retrieval. The different distributions of these effects, bilateral frontal for young and left hemisphere for old, further suggest that they reflect different functional processes for each age group. The reduction in alpha power for young adults may reflect anticipatory attention, which has been linked to alpha desynchronization in many previous studies (for review: Klimesch, 2012). By contrast, the increase in alpha power for older adults may reflect attempts to inhibit task irrelevant brain regions/processes. Alpha synchronization has been tied to neural and cognitive inhibition in numerous studies (for review: Hanslmayr et al., 2012; Klimesch, 1999; Klimesch, 2012; Klimesch, Sauseng, & Hanslmayr, 2007). For example, when participants are asked to retrieve a target color previously paired with a shape that was also paired with a competing color, early alpha power (~90–430 msec) increases over the hemisphere in which the distractor color is stored (Waldhauser et al., 2012). The early latency of this effect suggests that inhibition can be rapidly engaged to suppress competing memories during selective retrieval. We have argued that older adults show a reduced ability to ignore distractor context features during encoding, compared to the young. One potential explanation for the early alpha synchronization effect is that more competing memories were reactivated during retrieval for older adults leading to attempts to suppress memories of these features in order to make item and context retrieval decisions. It should be noted that neither the early alpha desynchronization effect for the young nor the synchronization effect for the old correlated with context memory performance. Thus, it is not clear that these early alpha effects contribute substantially to context memory accuracy. Future studies that directly interrogate inhibition and anticipatory attention mechanisms will be necessary to fully interpret the significance of these early alpha effects.
Consistent with previous research, we found greater alpha and beta desynchronization for correctly identified contexthits, compared to correctly rejected new items (for review: Hanslmayr et al., 2012; Spitzer, Hanslmayr, Opitz, Mecklinger, & Bauml, 2009; Zion-Golumbic, Kutas, & Bentin, 2010). Alpha and beta memory effects, both during encoding and retrieval, are often correlated, suggesting that they reflect similar cognitive processes (for review: Klimesch, 2012). Previous studies have shown that the topography of the alpha/beta desynchronization effects vary according to the nature of the sought after information during retrieval (e.g., words, faces, spatial location) (Burgess & Gruzelier, 2000; Khader & Rosler, 2011; Waldhauser et al., 2012). These results have been taken to suggest that alpha/beta power decreases reflect the reactivation of target-related sensory information from the encoded episode (for review: Klimesch, 2012; Waldhauser et al., 2012, 2016). In the current study, these effects were widespread, which might be expected given the multiple kinds of stimuli presented during encoding and retrieval (i.e., verbalizable objects, scenes, colors).
Some recent evidence shows that alpha/beta desynchronization effects implicated in sensory reactivation during episodic retrieval occur very early, roughly 100 msec following retrieval probe onset (Waldhauser et al., 2016). Furthermore, transcranial magnetic stimulation (TMS) induced in this early time period revealed that this reactivation is necessary for successful retrieval, which begins a few hundred milliseconds later (Rugg & Curran, 2007). These data were taken to support the hypothesis that recollection is dependent upon reinstatement of sensory features from prior encoding episodes (Tulving, 1983). In the current study, alpha/beta power reductions onset at ~600 msec in young adults, somewhat later for older adults, and were sustained across the scalp through the end of the epoch. The onset and time course of these effects are consistent with the idea that they reflect processes that operate after initial retrieval. In our ERP results from these data, we found a LPN component between 1000 and 1600 msec, which followed the parietal old-new effect and preceded participants’ responses by ~100–500 msec (James et al., 2016). We argued that the LPN reflected processes that act to reconstruct encoding episodes when context-specifying information was not readily recollected, which in turn contributed to context memory decisions. We believe that a similar argument can be made for the alpha/beta desynchronization effects observed here. Furthermore, the extended time course and widespread distribution of these effects may suggest they reflect not only reconstruction processes but also the evaluation and maintenance of the reconstructed episode that may continue following response (Herron, 2007).
Desynchronization effects were largely similar in time course and topography for alpha and beta bands, consistent with many previous studies (for review: Hanslmayr et al., 2012; Klimesch, 2012). There are a few results, however, that point to a potential dissociation between these effects. First, age-related reductions in these effects were observed over different time ranges and electrodes, with relatively minimal group differences for alpha. Second, the magnitude of the desynchronization effect predicted better item and context memory accuracy for the beta band only in older adults. Although this study was not designed to disambiguate retrieval effects for alpha and beta bands, some previous findings also suggest partially distinct roles for these frequencies in episodic memory. For example, one previous study found that while sustained frontal beta desynchronization predicted successful encoding for elaborately encoded events only, alpha desynchronization also predicted successful encoding for shallowly encoded events (Hanslmayr et al., 2009). These authors concluded that beta desynchronization is particularly related to semantic processes that support memory. In another study investigating episodic retrieval, the topographies of alpha and beta power reductions were sensitive to the kind of information being retrieved (i.e., objects, spatial locations), but the particular spatial distributions differed between frequencies (Khader & Rosler, 2011). We tentatively suggest that both alpha and beta desynchronization reflect reactivation of context-specifying information during attempts to reconstruct prior encoding episodes. Beta desynchronization, particularly over frontal channels, may additionally reflect post-retrieval evaluation processes that likely involve semantic elaboration.
Finally, we believe that the age group differences in beta desynchronization may be another consequence of older adults’ inhibitory deficits during encoding. First, the significant correlation between beta desynchronization over centroposterior channels and memory performance for only the older adults may suggest that they rely on episodic reconstruction processes to support successful item and context memory performance. As we have argued (James et al., 2016), if the quality of the recollected information was poorer or noncriterial for older adults due to suppression deficits during encoding, reconstruction may have been needed to support context memory decisions. Second, although older adults showed reduced beta effects compared to the young over frontal electrodes from 700 msec through the end of the epoch, this did not remain significant after controlling for context memory performance. Since our effect of age on average beta desynchronization was no longer significant after controlling for context memory performance, we suggest that successful reactivation and maintenance of the original encoding details is important for successful memory performance across age groups. Given that beta desynchronization correlated with both item and context memory in the older adults and not the young adults, we cautiously suggest that older adults were less able to engage this operation, potentially contributing to age related context memory impairments. Future studies that modulate demands on suppression during encoding and reactivation during retrieval will be needed to determine whether these age-related changes in retrieval oscillations can be reduced.
A limitation of the current study was that two context images were presented during retrieval in both match, color only match, scene only match, and neither match configurations. Thus, we were unable to look at stimulus specific reactivation due to the mixture of stimulus processing and retrieval, the attempted retrieval of both context details, and the combined inhibition and reactivation of context cues across the retrieval configurations. Future studies with explicit recall of context features would be beneficial in delineating stimulus specific retrieval effects.
5. Conclusion
This study provides the first evidence that age-related changes in oscillatory EEG signals during retrieval contribute to older adults’ contextual memory impairments. Using a selective encoding design, we show that young and older adults can selectively attend to and encode target contextual features. Consistent with the inhibition deficit hypothesis of aging, older adults are less able to suppress co-occurring distractor features, which in turn leads to less selective and less confident contextual memory performance. Oscillatory EEG signals suggest that older adults may recollect a similar or greater amount of information, compared to the young, but this information is more directly supportive of contextual memory performance for young adults. Suppression deficits in older adults may have led to less context-relevant recollection and a greater reliance on processes that act to reconstruct prior episodes in order to support context memory decisions. These results have implications for context memory abilities in real world situations in which older adults may be especially likely to experience difficulties.
Acknowledgments
This study was supported by National Science Foundation grant # 1125683 awarded to Audrey Duarte. This research was also supported in part by a NIA Ruth L. Kirschstein National Research Service Award (NRSA) Institutional Research Training Grant, Grant # 5T32AG000175. We thank all of our research participants.
References
- Addante RJ, Watrous AJ, Yonelinas AP, Ekstrom AD, Ranganath C. Prestimulus theta activity predicts correct source memory retrieval. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(26):10702–10707. doi: 10.1073/pnas.1014528108. http://dx.doi.org/10.1073/pnas.1014528108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axmacher N, Mormann F, Fernandez G, Elger CE, Fell J. Memory formation by neuronal synchronization. Brain Research Reviews. 2006;52(1):170–182. doi: 10.1016/j.brainresrev.2006.01.007. http://dx.doi.org/10.1016/j.brainresrev.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Bastiaansen M, Hagoort P. Event-induced theta responses as a window on the dynamics of memory. Cortex. 2003;39(4):967–992. doi: 10.1016/s0010-9452(08)70873-6. [DOI] [PubMed] [Google Scholar]
- Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Computation. 1995;7(6):1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- Blair RC, Karniski W. An alternative method for significance testing of waveform difference potentials. Psychophysiology. 1993;30(5):518–524. doi: 10.1111/j.1469-8986.1993.tb02075.x. [DOI] [PubMed] [Google Scholar]
- Boywitt CD, Kuhlmann BG, Meiser T. The role of source memory in older adults’ recollective experience. Psychology and Aging. 2012;27(2):484–497. doi: 10.1037/a0024729. http://dx.doi.org/10.1037/a0024729. [DOI] [PubMed] [Google Scholar]
- Burgess AP, Gruzelier JH. Short duration synchronization of human theta rhythm during recognition memory. NeuroReport. 1997;8(4):1039–1042. doi: 10.1097/00001756-199703030-00044. [DOI] [PubMed] [Google Scholar]
- Burgess A, Gruzelier J. Short duration power changes in the EEG during recognition memory for words and faces. Psychophysiology. 2000;37(5):596–606. [PubMed] [Google Scholar]
- Campbell KL, Hasher L, Thomas RC. Hyper-Binding a unique age effect. Psychological Science. 2010;21(3):399–405. doi: 10.1177/0956797609359910. http://dx.doi.org/10.1177/0956797609359910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KL, Trelle A, Hasher L. Hyper-binding across time: Age differences in the effect of temporal proximity on paired-associate learning. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2014;40(1):293. doi: 10.1037/a0034109. [DOI] [PubMed] [Google Scholar]
- Cohen MX. Analyzing neural time series data: Theory and practice Cambridge. Massachusetts: The MIT Press; 2014. [Google Scholar]
- Cohn M, Emrich SM, Moscovitch M. Age-related deficits in associative memory: The influence of impaired strategic retrieval. Psychology and Aging. 2008;23(1):93. doi: 10.1037/0882-7974.23.1.93. [DOI] [PubMed] [Google Scholar]
- Craik FI, Rose NS. Memory encoding and aging: A neurocognitive perspective. Neuroscience and Biobehavioral Reviews. 2012;36(7):1729–1739. doi: 10.1016/j.neubiorev.2011.11.007. http://dx.doi.org/10.1016/j.neubiorev.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Crespo-Garcia M, Cantero JL, Atienza M. Effects of semantic relatedness on age-related associative memory deficits: The role of theta oscillations. NeuroImage. 2012;61(4):1235–1248. doi: 10.1016/j.neuroimage.2012.03.034. http://dx.doi.org/10.1016/j.neuroimage.2012.03.034. [DOI] [PubMed] [Google Scholar]
- Curran T. Brain potentials of recollection and familiarity. Memory & Cognition. 2000;28(6):923–938. doi: 10.3758/bf03209340. [DOI] [PubMed] [Google Scholar]
- Cycowicz YM, Friedman D, Snodgrass JG. Remembering the color of objects: An ERP investigation of source memory. Cerebral Cortex. 2001;11(4):322–334. doi: 10.1093/cercor/11.4.322. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. http://dx.doi.org/10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Delorme A, Sejnowski T, Makeig S. Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. NeuroImage. 2007;34(4):1443–1449. doi: 10.1016/j.neuroimage.2006.11.004. http://dx.doi.org/10.1016/j.neuroimage.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Henson RN, Graham KS. The effects of aging on the neural correlates of subjective and objective recollection. Cerebral Cortex. 2008;18(9):2169–2180. doi: 10.1093/cercor/bhm243. http://dx.doi.org/10.1093/cercor/bhm243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Ranganath C, Trujillo C, Knight RT. Intact recollection memory in high-performing older adults: ERP and behavioral evidence. Journal of Cognitive Neurosciences. 2006;18(1):33–47. doi: 10.1162/089892906775249988. http://dx.doi.org/10.1162/089892906775249988. [DOI] [PubMed] [Google Scholar]
- Duarte A, Ranganath C, Winward L, Hayward D, Knight RT. Dissociable neural correlates for familiarity and recollection during the encoding and retrieval of pictures. Brain Research Cognitive Brain Research. 2004;18(3):255–272. doi: 10.1016/j.cogbrainres.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Dulas MR, Duarte A. The influence of directed attention at encoding on source memory retrieval in the young and old: An ERP study. Brain Research. 2013;1500:55–71. doi: 10.1016/j.brainres.2013.01.018. http://dx.doi.org/10.1016/j.brainres.2013.01.018. [DOI] [PubMed] [Google Scholar]
- Dulas MR, Duarte A. Aging affects the interaction between attentional control and source memory: An fMRI study. Journal of Cognitive Neurosciences. 2014;26(12):2653–2669. doi: 10.1162/jocn_a_00663. http://dx.doi.org/10.1162/jocn_a_00663. [DOI] [PubMed] [Google Scholar]
- Duverne S, Motamedinia S, Rugg MD. The relationship between aging, performance, and the neural correlates of successful memory encoding. Cerebral Cortex. 2009;19(3):733–744. doi: 10.1093/cercor/bhn122. http://dx.doi.org/10.1093/cercor/bhn122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duzel E, Penny WD, Burgess N. Brain oscillations and memory. Current Opinion in Neurobiology. 2010;20(2):143–149. doi: 10.1016/j.conb.2010.01.004. http://dx.doi.org/10.1016/j.conb.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Fellner MC, Bauml KH, Hanslmayr S. Brain oscillatory subsequent memory effects differ in power and long-range synchronization between semantic and survival processing. NeuroImage. 2013;79:361–370. doi: 10.1016/j.neuroimage.2013.04.121. http://dx.doi.org/10.1016/j.neuroimage.2013.04.121. [DOI] [PubMed] [Google Scholar]
- Friedman D, Johnson R., Jr Event-related potential (ERP) studies of memory encoding and retrieval: A selective review. Microscopy Research and Technique. 2000;51(1):6–28. doi: 10.1002/1097-0029(20001001)51:1<6::AID-JEMT2>3.0.CO;2-R. http://dx.doi.org/10.1002/1097-0029(20001001)51:1<6::AID-JEMT2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: Neuronal communication through neuronal coherence. Trends in Cognitive Sciences. 2005;9(10):474–480. doi: 10.1016/j.tics.2005.08.011. http://dx.doi.org/10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Glisky EL, Kong LL. Do young and older adults rely on different processes in source memory tasks? A neuropsychological study. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2008;34(4):809. doi: 10.1037/0278-7393.34.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisky EL, Rubin SR, Davidson PS. Source memory in older adults: An encoding or retrieval problem? Journal of Experimental Psychology: Learning, Memory, and Cognition. 2001;27(5):1131. doi: 10.1037//0278-7393.27.5.1131. [DOI] [PubMed] [Google Scholar]
- Gruber T, Tsivilis D, Giabbiconi CM, Muller MM. Induced electroencephalogram oscillations during source memory: Familiarity is reflected in the gamma band, recollection in the theta band. Journal of Cognitive Neurosciences. 2008;20(6):1043–1053. doi: 10.1162/jocn.2008.20068. http://dx.doi.org/10.1162/jocn.2008.20068. [DOI] [PubMed] [Google Scholar]
- Gruber MJ, Watrous AJ, Ekstrom AD, Ranganath C, Otten LJ. Expected reward modulates encoding-related theta activity before an event. NeuroImage. 2013;64:68–74. doi: 10.1016/j.neuroimage.2012.07.064. http://dx.doi.org/10.1016/j.neuroimage.2012.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guderian S, Düzel E. Induced theta oscillations mediate large-scale synchrony with mediotemporal areas during recollection in humans. Hippocampus. 2005;15(7):901–912. doi: 10.1002/hipo.20125. [DOI] [PubMed] [Google Scholar]
- Guderian S, Schott BH, Richardson-Klavehn A, Duzel E. Medial temporal theta state before an event predicts episodic encoding success in humans. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(13):5365–5370. doi: 10.1073/pnas.0900289106. http://dx.doi.org/10.1073/pnas.0900289106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr S, Spitzer B, Bauml KH. Brain oscillations dissociate between semantic and nonsemantic encoding of episodic memories. Cerebral Cortex. 2009;19(7):1631–1640. doi: 10.1093/cercor/bhn197. http://dx.doi.org/10.1093/cercor/bhn197. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Staudigl T. How brain oscillations form memories–a processing based perspective on oscillatory subsequent memory effects. NeuroImage. 2014;85(Pt 2):648–655. doi: 10.1016/j.neuroimage.2013.05.121. http://dx.doi.org/10.1016/j.neuroimage.2013.05.121. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Staudigl T, Aslan A, Bauml KH. Theta oscillations predict the detrimental effects of memory retrieval. Cognitive, Affective & Behavioral Neuroscience. 2010;10(3):329–338. doi: 10.3758/CABN.10.3.329. http://dx.doi.org/10.3758/CABN.10.3.329. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Staudigl T, Fellner MC. Oscillatory power decreases and long-term memory: The information via desynchronization hypothesis. Frontiers in Human Neuroscience. 2012;6:74. doi: 10.3389/fnhum.2012.00074. http://dx.doi.org/10.3389/fnhum.2012.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension, and aging: A review and new view. In: Bower GH, editor. The psychology of learning and motivation. Vol. 22. San Diego, CA: Academic Press; 1988. pp. 193–225. [Google Scholar]
- Hashtroudi S, Johnson MK, Vnek N, Ferguson SA. Aging and the effects of affective and factual focus on source monitoring and recall. Psychology and Aging. 1994;9(1):160. doi: 10.1037//0882-7974.9.1.160. [DOI] [PubMed] [Google Scholar]
- Herron JE. Decomposition of the ERP late posterior negativity: Effects of retrieval and response fluency. Psychophysiology. 2007;44(2):233–244. doi: 10.1111/j.1469-8986.2006.00489.x. [DOI] [PubMed] [Google Scholar]
- Hicks JL, Starns JJ. Successful cuing of gender source memory does not improve location source memory. Memory & Cognition. 2016;44(4):650–659. doi: 10.3758/s13421-016-0586-y. http://dx.doi.org/10.3758/s13421-016-0586-y. [DOI] [PubMed] [Google Scholar]
- Hoffmann S, Falkenstein M. The correction of eye blink artefacts in the EEG: A comparison of two prominent methods. PLoS One. 2008;3(8):e3004. doi: 10.1371/journal.pone.0003004. http://dx.doi.org/10.1371/journal.pone.0003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh LT, Ranganath C. Frontal midline theta oscillations during working memory maintenance and episodic encoding and retrieval. NeuroImage. 2014;85:721–729. doi: 10.1016/j.neuroimage.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Hwang G, Curran T, Kahana MJ. EEG oscillations and recognition memory: Theta correlates of memory retrieval and decision making. NeuroImage. 2006;32(2):978–987. doi: 10.1016/j.neuroimage.2006.02.018. http://dx.doi.org/10.1016/j.neuroimage.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Kahana MJ, Ekstrom AD, Fried I. Brain oscillations control timing of single-neuron activity in humans. The Journal of Neuroscience. 2007;27(14):3839–3844. doi: 10.1523/JNEUROSCI.4636-06.2007. http://dx.doi.org/10.1523/JNEUROSCI.4636-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James T, Strunk J, Arndt J, Duarte A. Age-related deficits in selective attention during encoding increase demands on episodic reconstruction during context retrieval: An ERP study. Neuropsychologia. 2016;86:66–79. doi: 10.1016/j.neuropsychologia.2016.04.009. http://dx.doi.org/10.1016/j.neuropsychologia.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O. Reading the hippocampal code by theta phase-locking. Trends in Cognitive Sciences. 2005;9(12):551–553. doi: 10.1016/j.tics.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Johansson M, Mecklinger A. The late posterior negativity in ERP studies of episodic memory: Action monitoring and retrieval of attribute conjunctions. Biological Psychology. 2003;64(1):91–117. doi: 10.1016/s0301-0511(03)00104-2. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Hashtroudi S, Lindsay DS. Source monitoring. Psychological Bulletin. 1993;114(1):3–28. doi: 10.1037/0033-2909.114.1.3. [DOI] [PubMed] [Google Scholar]
- Kardos Z, Toth B, Boha R, File B, Molnar M. Age-related changes of frontal-midline theta is predictive of efficient memory maintenance. Neuroscience. 2014;273:152–162. doi: 10.1016/j.neuroscience.2014.04.071. http://dx.doi.org/10.1016/j.neuroscience.2014.04.071. [DOI] [PubMed] [Google Scholar]
- Karrasch M, Laine M, Rapinoja P, Krause CM. Effects of normal aging on event-related desynchronization/synchronization during a memory task in humans. Neuroscience Letters. 2004;366(1):18–23. doi: 10.1016/j.neulet.2004.05.010. http://dx.doi.org/10.1016/j.neulet.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Amygdala activity is associated with the successful encoding of item, but not source, information for positive and negative stimuli. The Journal of Neuroscience. 2006;26(9):2564–2570. doi: 10.1523/JNEUROSCI.5241-05.2006. http://dx.doi.org/10.1523/JNEUROSCI.5241-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khader PH, Rosler F. EEG power changes reflect distinct mechanisms during long-term memory retrieval. Psychophysiology. 2011;48(3):362–369. doi: 10.1111/j.1469-8986.2010.01063.x. http://dx.doi.org/10.1111/j.1469-8986.2010.01063.x. [DOI] [PubMed] [Google Scholar]
- Kim S, Hasher L, Zacks RT. Aging and a benefit of distractibility. Psychonomic Bulletin & Review. 2007;14(2):301–305. doi: 10.3758/bf03194068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Research Brain Research Reviews. 1999;29(2–3):169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W. Alpha-band oscillations, attention, and controlled access to stored information. Trends in Cognitive Sciences. 2012;16(12):606–617. doi: 10.1016/j.tics.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Schwaiger J, Winkler T, Gruber W. Theta oscillations and the ERP old/new effect: Independent phenomena? Clinical Neurophysiology. 2000;111(5):781–793. doi: 10.1016/s1388-2457(00)00254-6. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Hanslmayr S, Sauseng P, Gruber W, Brozinsky CJ, Kroll NEA, Doppelmayr M. Oscillatory EEG correlates of episodic trace decay. Cerebral Cortex. 2006;16(2):280–290. doi: 10.1093/cercor/bhi107. http://dx.doi.org/10.1093/cercor/bhi107. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: The inhibition-timing hypothesis. Brain Research Reviews. 2007;53(1):63–88. doi: 10.1016/j.brainresrev.2006.06.003. http://dx.doi.org/10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Kuo TY, Van Petten C. Prefrontal engagement during source memory retrieval depends on the prior encoding task. Journal of Cognitive Neurosciences. 2006;18(7):1133–1146. doi: 10.1162/jocn.2006.18.7.1133. http://dx.doi.org/10.1162/jocn.2006.18.7.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Simpson GV, Logothetis NK, Rainer G. Phase locking of single neuron activity to theta oscillations during working memory in monkey extrastriate visual cortex. Neuron. 2005;45(1):147–156. doi: 10.1016/j.neuron.2004.12.025. http://dx.doi.org/10.1016/j.neuron.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Leshikar ED, Dulas MR, Duarte A. Self-referencing enhances recollection in both young and older adults. Aging, Neuropsychology, and Cognition. 2015;22(4):388–412. doi: 10.1080/13825585.2014.957150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Calderon J, Luck SJ. ERPLAB: An open-source toolbox for the analysis of event-related potentials. Frontiers in Human Neuroscience. 2014;8 doi: 10.3389/fnhum.2014.00213. http://dx.doi.org/10.3389/fnhum.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeig S, Debener S, Onton J, Delorme A. Mining event-related brain dynamics. Trends in Cognitive Sciences. 2004;8(5):204–210. doi: 10.1016/j.tics.2004.03.008. http://dx.doi.org/10.1016/j.tics.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. Journal of Neuroscience Methods. 2007;164(1):177–190. doi: 10.1016/j.jneumeth.2007.03.024. http://dx.doi.org/10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Meiser T, Sattler C, Weisser K. Binding of multidimensional context information as a distinctive characteristic of remember judgments. Journal of Experimental Psychology Learning, Memory, and Cognition. 2008;34(1):32–49. doi: 10.1037/0278-7393.34.1.32. http://dx.doi.org/10.1037/0278-7393.34.1.32. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK. Source monitoring 15 years later: What have we learned from fMRI about the neural mechanisms of source memory? Psychological Bulletin. 2009;135(4):638–677. doi: 10.1037/a0015849. http://dx.doi.org/10.1037/A0015849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollison MV, Curran T. Familiarity in source memory. Neuropsychologia. 2012;50(11):2546–2565. doi: 10.1016/j.neuropsychologia.2012.06.027. http://dx.doi.org/10.1016/j.neuropsychologia.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcom AM, Li J, Rugg MD. Age effects on the neural correlates of episodic retrieval: Increased cortical recruitment with matched performance. Cerebral Cortex. 2007;17(11):2491–2506. doi: 10.1093/cercor/bhl155. http://dx.doi.org/10.1093/cercor/bhl155. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Brav TK, Levy O. The associative memory deficit of older adults: The role of strategy utilization. Psychology and Aging. 2007;22(1):202–208. doi: 10.1037/0882-7974.22.1.202. http://dx.doi.org/10.1037/0882-7974.22.1.202. [DOI] [PubMed] [Google Scholar]
- Newsome RN, Dulas MR, Duarte A. The effects of aging on emotion-induced modulations of source retrieval ERPs: Evidence for valence biases. Neuropsychologia. 2012;50(14):3370–3384. doi: 10.1016/j.neuropsychologia.2012.09.024. http://dx.doi.org/10.1016/j.neuropsychologia.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuwer MR, Comi G, Emerson R, Fuglsang-Frederiksen A, Guerit JM, Hinrichs H, Rappelsburger P. IFCN standards for digital recording of clinical EEG. International Federation of Clinical Neurophysiology. Electroencephalography and Clinical Neurophysiology. 1998;106(3):259–261. doi: 10.1016/s0013-4694(97)00106-5. [DOI] [PubMed] [Google Scholar]
- Nyhus E, Curran T. Functional role of gamma and theta oscillations in episodic memory. Neuroscience and Biobehavioral Reviews. 2010;34(7):1023–1035. doi: 10.1016/j.neubiorev.2009.12.014. http://dx.doi.org/10.1016/j.neubiorev.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational Intelligence and Neuroscience. 2011;2011:156869. doi: 10.1155/2011/156869. http://dx.doi.org/10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival DB, Walden AT. Spectral analysis for physical applications: Multitaper and conventional univariate techniques. Cambridge; New York, NY, USA: Cambridge University Press; 1993. [Google Scholar]
- Peterson DJ, Naveh-Benjamin M. The role of aging in intra-item and item-context binding processes in visual working memory. Journal of Experimental Psychology Learning, Memory, and Cognition. 2016;42(11):1713–1730. doi: 10.1037/xlm0000275. http://dx.doi.org/10.1037/xlm0000275. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Aranibar A. Event-related cortical desynchronization detected by power measurements of scalp EEG. Electroencephalography and Clinical Neurophysiology. 1977;42(6):817–826. doi: 10.1016/0013-4694(77)90235-8. [DOI] [PubMed] [Google Scholar]
- Rondina R, 2nd, Olsen RK, McQuiggan DA, Fatima Z, Li L, Oziel E, Ryan JD. Age-related changes to oscillatory dynamics in hippocampal and neocortical networks. Neurobiology of Learning and Memory. 2015 doi: 10.1016/j.nlm.2015.11.017. http://dx.doi.org/10.1016/j.nlm.2015.11.017. [DOI] [PubMed]
- Rugg MD, Curran T. Event-related potentials and recognition memory. Trends in Cognitive Sciences. 2007;11(6):251–257. doi: 10.1016/j.tics.2007.04.004. http://dx.doi.org/10.1016/j.tics.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Morcom AM. The relationship between brain activity, cognitive performance, and aging. Cognitive neuroscience of aging: Linking cognitive and cerebral aging. 2005:132–154. [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103(3):403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Sebastian M, Ballesteros S. Effects of normal aging on event-related potentials and oscillatory brain activity during a haptic repetition priming task. NeuroImage. 2012;60(1):7–20. doi: 10.1016/j.neuroimage.2011.11.060. http://dx.doi.org/10.1016/j.neuroimage.2011.11.060. [DOI] [PubMed] [Google Scholar]
- Sederberg PB, Kahana MJ, Howard MW, Donner EJ, Madsen JR. Theta and gamma oscillations during encoding predict subsequent recall. The Journal of Neuroscience. 2003;23(34):10809–10814. doi: 10.1523/JNEUROSCI.23-34-10809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer WD, Raz N. Differential effects of aging on memory for content and context: A meta-analysis. Psychology and Aging. 1995;10(4):527–539. doi: 10.1037//0882-7974.10.4.527. http://dx.doi.org/10.1037/0882-7974.10.4.527. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: Applications to dementia and amnesia. Journal of Experimental Psychology: General. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Spitzer B, Hanslmayr S, Opitz B, Mecklinger A, Bauml KH. Oscillatory correlates of retrieval-induced forgetting in recognition memory. Journal of Cognitive Neurosciences. 2009;21(5):976–990. doi: 10.1162/jocn.2009.21072. http://dx.doi.org/10.1162/jocn.2009.21072. [DOI] [PubMed] [Google Scholar]
- Starns JJ, Hicks JL. Context attributes in memory are bound to item information, but not to one another. Psychonomic Bulletin & Review. 2008;15(2):309–314. doi: 10.3758/pbr.15.2.309. [DOI] [PubMed] [Google Scholar]
- Staudigl T, Hanslmayr S, Bauml KH. Theta oscillations reflect the dynamics of interference in episodic memory retrieval. The Journal of Neuroscience. 2010;30(34):11356–11362. doi: 10.1523/JNEUROSCI.0637-10.2010. http://dx.doi.org/10.1523/JNEUROSCI.0637-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Elements of episodic memory. Oxford Oxfordshire New York: Clarendon Press; Oxford University Press; 1983. [Google Scholar]
- Uncapher MR, Otten LJ, Rugg MD. Episodic encoding is more than the sum of its parts: An fMRI investigation of multifeatural contextual encoding. Neuron. 2006;52(3):547–556. doi: 10.1016/j.neuron.2006.08.011. http://dx.doi.org/10.1016/j.neuron.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Moosavi RF, Rugg MD. The relationship between electrophysiological correlates of recollection and amount of information retrieved. Brain Research. 2006;1122(1):161–170. doi: 10.1016/j.brainres.2006.09.023. http://dx.doi.org/10.1016/j.brainres.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V, Broder A. Independent retrieval of source dimensions: An extension of results by Starns and Hicks (2005) and a comment on the ACSIM measure. Journal of Experimental Psychology Learning, Memory, and Cognition. 2007;33(2):443–450. doi: 10.1037/0278-7393.33.2.443. http://dx.doi.org/10.1037/0278-7393.33.2.443. [DOI] [PubMed] [Google Scholar]
- Voss JL, Lucas HD, Paller KA. Conceptual Priming and Familiarity: Different Expressions of Memory during Recognition Testing with Distinct Neurophysiological Correlates. J Cogn Neurosci. 2009;22(11):2638–2651. doi: 10.1162/jocn.2009.21341. http://dx.doi.org/10.1162/jocn.2009.21341. [DOI] [PubMed] [Google Scholar]
- Waldhauser GT, Braun V, Hanslmayr S. Episodic memory retrieval functionally relies on very rapid reactivation of sensory information. The Journal of Neuroscience. 2016;36(1):251–260. doi: 10.1523/JNEUROSCI.2101-15.2016. http://dx.doi.org/10.1523/JNEUROSCI.2101-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldhauser GT, Johansson M, Hanslmayr S. Alpha/beta oscillations indicate inhibition of interfering visual memories. The Journal of Neuroscience. 2012;32(6):1953–1961. doi: 10.1523/JNEUROSCI.4201-11.2012. http://dx.doi.org/10.1523/JNEUROSCI.4201-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin. 1996;120(2):272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Wilcox RR, Keselman H. Modern robust data analysis methods: Measures of central tendency. Psychological Methods. 2003;8(3):254. doi: 10.1037/1082-989X.8.3.254. [DOI] [PubMed] [Google Scholar]
- Wilding EL. In what way does the parietal ERP old/new effect index recollection? International Journal of Psychophysiology. 2000;35(1):81–87. doi: 10.1016/s0167-8760(99)00095-1. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Jacoby LL. Noncriterial recollection: Familiarity as automatic, irrelevant recollection. Consciousness and Cognition. 1996;5(1–2):131–141. doi: 10.1006/ccog.1996.0008. http://dx.doi.org/10.1006/ccog.1996.0008. [DOI] [PubMed] [Google Scholar]
- Zion-Golumbic E, Kutas M, Bentin S. Neural dynamics associated with semantic and episodic memory for faces: Evidence from multiple frequency bands. Journal of Cognitive Neurosciences. 2010;22(2):263–277. doi: 10.1162/jocn.2009.21251. http://dx.doi.org/10.1162/jocn.2009.21251. [DOI] [PMC free article] [PubMed] [Google Scholar]


