Abstract
Children are especially vulnerable to the health and developmental impacts of environmental hazards and they spend significant portions of their days at school. Yet there are no national-level studies examining school-level environmental inequalities and few have examined disparate exposure to neurological air toxicants, even though chronic exposure to air pollution impacts children’s brain functioning. We paired information about the geographic locations and demographics of each public school in the US with air neurotoxicant exposure estimates pertaining to 24 known neurotoxicants included in the US Environmental Protection Agency’s National Air Toxics Assessment. Using bivariate and multivariate statistics, we tested for environmental injustices in air neurotoxicant exposure at 84,969 US public schools. Metropolitan New York City (EPA Region 2) is the geographic region most burdened by air neurotoxicant exposures at schools since one-third of all schools in that region are in the top 10% for ambient neurotoxicant exposure among all schools nationwide. Students attending “high risk” public schools nationwide are significantly more likely to be eligible for free/reduced price meals, and to be Hispanic, black, or Asian/Pacific Islander (API). They are significantly less likely to be white or of another race. In a multivariate generalized estimating equation controlling for school district effects, schools with greater proportions of Hispanic, black, and API students, schools with higher enrollment, and schools located in more urban (vs. rural) counties face greater risks. Schools serving the youngest students (e.g., pre-kindergarten) have greater levels of risk than schools serving older students. Across all analyses, this study shows that racial/ethnic minority children are bearing the brunt of air neurotoxicant exposures at school, which may be unequally impacting their school performance and future potential.
Keywords: neurological toxics, children, public schools, environmental justice
1. Introduction
Environmental justice (EJ) research focused on children and schools is currently underdeveloped (Strife and Downey 2009). This is despite the fact that children are especially vulnerable to the health and developmental impacts of environmental injustice, due to their unique biological vulnerabilities, age-related patterns of exposure, and lack of control over their own environmental circumstances (Landrigan, Rauh and Galvez 2010). They also spend significant time at school, approximately 6.6 hours per day for 180 days per year for public school students in the US (National Center for Education Statistics 2008).
A handful of previous EJ studies have examined environmental risks for schoolchildren. EJ studies of the Los Angeles Unified School District, CA (USA) (Pastor, Sadd and Morello-Frosch 2002; Pastor, Sadd and Morello-Frosch 2004) and the State of California (Pastor, Morello-Frosch and Sadd 2006) found that racial/ethnic minority students, especially Hispanic students, were overrepresented as attendees of schools with higher outdoor cancer and respiratory air toxics exposures. Using bivariate correlations, another study examined Sacramento (CA) public schools and found that higher levels of PM2.5 emissions from road traffic were correlated with higher percentages of Black, Hispanic and multiethnic students and students eligible for subsidized meals, and a lower percentage of white students (Gaffron and Niemeier 2015). An individual-level study of schoolchildren in Orange County, FL (USA) examined the locations of students’ homes and schools in relation to major point sources, small area emitters, and major roadways, and found that Hispanic and black children were significantly overrepresented (for both school and home locations) relative to non-Hispanic white children in areas proximate to each type of air pollution source (Chakraborty and Zandbergen 2007). Another study focused specifically on the areas surrounding the top 100 point source polluters of developmental and neurological air toxicants in the United States; it found that a significant proportion of the top industrial polluters were located in close proximity to multiple schools and that as the proportion of disadvantaged residents living near the facility went up, so did the number of schools (Legot, London and Shandra 2010). While that particular study provided an important first step in examining air neurotoxicant exposure at US schools, it was limited by restricting the analyses to only the 1–2 miles around each facility and using only correlations.
Thus, while prior EJ studies of schoolchildren suggest particular city- or state-wide patterns of disparate exposure based on race/ethnicity, knowledge of environmental injustices experienced by this vulnerable population remains limited. No national EJ studies of schools have been undertaken, a gap which our proposed study fills. Additionally, previous distributional studies of children and environmental injustice have focused on cancer or respiratory health risks from air pollution.
Rarely have neurological air toxics been examined, even though emerging evidence indicates that chronic exposure to air pollution may negatively impact children’s brain functioning (Brockmeyer and D'Angiulli 2016; Calderón-Garcidueñas et al. 2016). Children are more vulnerable to air pollution than are adults, due to their higher breathing rate to body size ratio, their lungs’ less developed natural barriers, and their propensity to spend more time outside (Brockmeyer and D'Angiulli 2016). Neurological air toxics include ultrafine particulate matter (PM), polychlorinated biphenyls, mercury, manganese and toluene, among many others (Bandyopadhyay 2016; Costa et al. 2014; Grandjean and Landrigan 2014; Loane et al. 2013; World Health Organization 2010). Neurotoxicants are released into the air through combustion engines, industrial activities, power plants, mining, and refuse incineration. While diet has long been considered the primary vehicle of exposure to neurotoxicants, inhalation exposure is a common and important route of exposure (Costa et al. 2014). Inhalation can even be a more toxic route of exposure than ingestion, as is the case with manganese (Environmental Protection Agency 2016b).
When air pollutants are inhaled, they damage the body’s natural barriers (e.g., nasal and lung epitheliums and the blood brain barrier) and gain entry to the body (Calderón-Garcidueñas et al. 2015). Upon arrival, they generate an innate immune response involving small proteins called cytokines, which are present in blood and cerebrospinal fluids. Cytokines cause tissue swelling and immune cell release of cytotoxic species, while signaling the production of additional cytokines. This induces neuroinflammation in the brain and leads to diffuse loss and damage of neural tissues. Cytokines are also associated with the development of white matter hyperintensities (WMH), which are areas of demyelinated neurons in the brain that negatively impact synaptic capacities. WMH are associated with global congitive deficits. Air pollution thus causes cognitive deficits through neuroinflammation and cell loss, and via damange to myelin and neural functioning (Brockmeyer and D'Angiulli 2016). In terms of neurotoxicants specifically, they have additional properties which cause neurological harm. Lead, for example, crosses the blood-brain barrier by substituting as calcium ions and wreaks havoc on the prefrontal cortex, hippocampus, and cerebellum by interfering with the regulartory functioning of calcium (Sanders et al. 2009).
The consequences of inhaling air pollution are far-reaching. Autopsies of otherwise clinically healthy youth, for example, revealed that those from the high pollution megacity of Mexico City had brain structures resembling those with early stage Alzheimer’s disease; similar damage was not present in the brains of youth living in the two low pollution control cities of Veracruz and Tlaxcala (Calderón-Garcidueñas et al. 2008). Given that low income and racial/ethnic minority populations tend to experience the environmental injustice of disproportionately high exposure to pollution (Mohai, Pellow and Roberts 2009; Mohai and Saha 2015; Pais, Crowder and Downey 2014), it is likely that these detrimental effects on brain functioning are not evenly distributed across society. However, this has not yet been investigated.
To begin to address this important knowledge gap, this study tests for environmental injustices in exposures to air neurotoxicants in an analysis that includes all US public schools. The first research question is: which US EPA regions house disproportionate numbers of “high risk” schools? “High risk” schools are those in the top 10% of all schools for neurotoxicant risk. The second research question is: are racial/ethnic minority and economically-deprived students disproportionately at “high risk” to ambient neurotoxicants as compared to all US public school students? Finally, the third research question is: accounting for geographic clustering and other factors known to influence exposure to toxics, how do the racial/ethnic and economic composition of the schools and the grade level of student served relate to air neurotoxicant risk?
2. Materials and Methods
2.1 Unit of Analysis: United States Public Schools
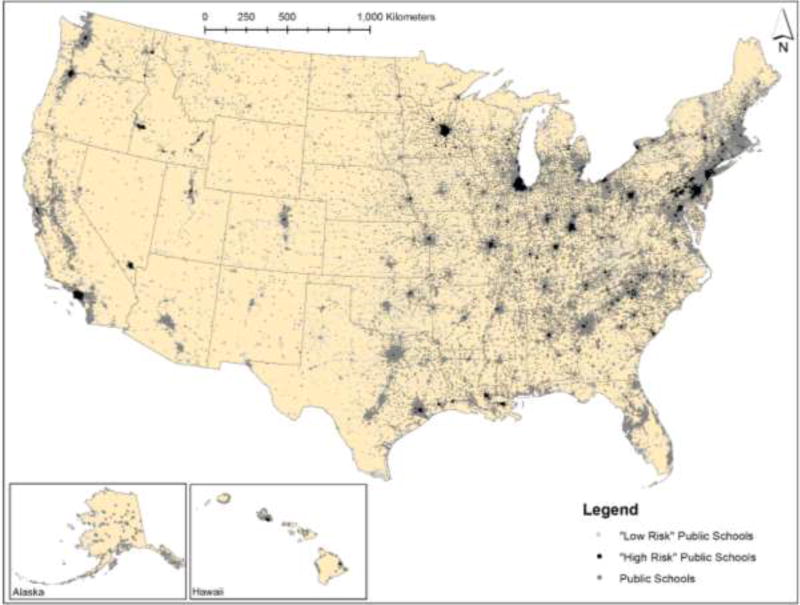
We downloaded the geographic locations and demographics of each public school in the US for 2011 from the National Center for Education Statistics using the ElSi Table Generator tool (National Center for Education Statistics 2017). We selected 2011 since it aligns with the most recent National Air Toxics Assessment. This ElSi tool provides users with access to the Common Core of Data (CCD), which is the US Department of Education's primary database on public elementary and secondary schools in the United States. The CCD surveys are conducted annually at all public elementary and secondary schools throughout the United States. CCD contains general descriptive information on schools and on their students and staff. Of relevance to this project, we downloaded the latitude and longitude of each school, information about the racial/ethnic make-up of the student body, eligibility for free and reduced price meals, and the lowest and highest grade offered at the school. Using ArcGIS 10, we plotted all schools based on their latitude and longitude (Figure 1). We removed schools with fewer than 100 children in 2011 from the analysis (n=8,876) to create more stable proportion variables.
Figure 1.
U.S. public schools (2011), including those at “high” and “low” risk from ambient neurotoxicants
Note: “High Risk” schools are in the top 10% for neurotoxicant exposure nationwide, based on our metric. “Low Risk” schools are in the bottom 10%.
Mapping the schools allowed us to create other variables that were needed for the analysis. Using the spatial join tool in ArcGIS 10, we were able to determine which EPA region and school district each school fell within. To do this, we obtained spatial data for US school districts in 2011 from the National Historical Geographic Information System (NHGIS) portal (IPUMS 2016) and located each school within its school district (n=11,845).
2.2 Analysis Variables
2.2.1 Dependent Variables
The neurotoxicant measures come from the US Environmental Protection Agency’s National Air Toxics Assessment (NATA). The NATA includes all hazardous air pollutants (HAPs) regulated by the US Clean Air Act (except criteria pollutants) that are known or suspected to cause cancer or neurological, developmental, respiratory, immunological, and reproductive damage (Environmental Protection Agency 2016a). The EPA models the exposure risks of different pollutants additively to estimate an aggregate risk score for each census tract (Environmental Protection Agency 2016a). Exposure risk scores are based on the concentration of each chemical to which people are exposed and each chemical’s reference concentration (i.e., the amount of toxicity below which long-term exposure to the general population is not expected to result in adverse effects). The NATA is currently the best available secondary data source for spatially explicit characterization of toxic air pollution exposure risk in the US and has been used to measure census tract-level pollution in high-tier EJ and environmental health studies (Linder, Marko and Sexton 2008; Roberts et al. 2013).
Air neurotoxicant exposure estimates for the schools were created from the EPA’s NATA census tract-level databases from 2011 using ArcGIS 10. The 24 known neurotoxicants included in the NATA [see Appendix H in Environmental Protection Agency (2015)] are: 1,1,1-Trichloroethane, 2,4-Dinitrotoluene, Acrylamide, Allyl Chloride, Benzidine, Calcium Cyanamide, Carbon Disulfide, Carbonyl Sulfide, Cresol/Cresylic Acid, Cyanide Compounds, Dichlorvos, Ethylene Oxide, Hexachloroethane, Hexane, Lead Compounds, Manganese Compounds, Mercury Compounds, Methyl Chloride (Chloromethane), Selenium Compounds, Styrene, Tetrachloroethylene, Toluene, Trichloroethylene, and Xylenes. The EPA recommends summing the exposure risk estimates for each chemical known to effect the same target organ (e.g., nervous system in this case) to create a cumulative exposure risk score, given the high degree of uncertainty regarding non-additive interactions between chemicals (Environmental Protection Agency 2015).
We created three variables using these NATA data. First, to create a summative score, we examined the distribution of each of the 24 HAPs by quartile and excluded HAPs with no variability across the 25th, 50th and 75th percentiles. For the remaining 15 HAPs with sufficient variability, we recoded each HAP into quartiles and summed across the 15 HAPs (Talbott et al. 2015; Windham et al. 2006). Using that variable, we created a “high risk” variable by taking the top 10% of all schools in terms of the summed measure, and a “low risk” variable, which is the lowest 10% of all schools on the summed measure; those schools are depicted in Figure 1.
2.2.2 Independent Variables
Total Number of Students
We use the number of students attending each school in the analyses for all three research questions.
Racial/Ethnic Composition
For research question 2, we began with the numbers of students who were non-Hispanic white, Hispanic and non-Hispanic black. Asian and Pacific Islander/Native Hawaiian were combined into an API (Asian/Pacific Islander) category, due to small counts; multiracial and Native American were also collapsed into proportion other race. For research question 3, we created variables that represent the proportion of each school’s students who are in each of the five racial/ethnic categories using the total number of students as the denominator. Proportion white was excluded as the reference group.
Economic Deprivation
We use student eligibility for free or reduced price meals as a proxy for economic deprivation, since a student’s family must be of low income to qualify for this assistance. Specifically, children qualify if their family is at or below 185% of the federal poverty line. Eligibility is determined based on the number of people sharing a home with the child and the household income. For research question 2, we use the number of eligible children. For question 3, we use the proportion of students in the school that are eligible and a squared free/reduced price meals term because the association between economic deprivation and air neurotoxicant exposure may be non-linear. This is because economically deprived areas tend to have fewer economic activities and hence fewer sources of pollution, while economically advantaged areas typically have the political power to resist pollution sources; thus, more air pollution risks are expected in areas that are neither economically deprived or advantaged (Pastor, Morello-Frosch and Sadd 2005).
Grade Level of Students Served
For research question 3, we created a five-category variable to capture the grade level of students served in each school using the variables “highest” and “lowest grade offered at the school”. The five categories, which map to natural breaks in the data and the organization of schools in the US, are: schools which offer all grades or are ungraded (“ungraded/all grades”); schools with the highest grade offered being pre-kindergarten, kindergarten or first grade (“early childhood centers”); schools with the highest grade being second through sixth (“elementary school”), schools with the highest grade being seventh through ninth (“junior high”), and schools with the highest grade being tenth-twelfth (“high school”). We separated out schools that serve the youngest children from elementary school-aged children, because young children are especially vulnerable to the health and developmental impacts of environmental injustice (Landrigan, Rauh and Galvez 2010). We use “elementary school” as the reference category, since it includes the most schools.
Urban Location
Based on the 2010 US Census, we used the proportion of the 2010 population in the county containing each school living in an “urban area” for research question 3. Urban areas are densely settled groups of census tracts that meet minimum population density requirements and have at least 50,000 or more people. This is an important control variable since there are more pollution generating activities in urban areas (Chakraborty et al. 2014; Collins, Grineski and Morales 2017; Gould 1986 ; Lievanos 2015).
2.3 Analysis Approach
2.3.1 Descriptive Analysis
To answer the first research question, we determined the total number of schools and the number of those schools at “high risk” (i.e., in the top 10% for summed air neurotoxicant risk nationally) in each EPA region. Then, we calculated the proportion of schools in each region that were “high risk” by dividing the number of high risk schools by the total number of schools in each region.
2.3.2 Bivariate Statistics
To answer the second research question, we calculated the proportion of students attending public schools in the US who qualify for free/reduced price meals, and belong to each racial/ethnic group. Then, we calculated the proportion of students attending public schools in the US that attended “high risk” schools. Finally, we calculated the proportion of students in the US attending “high risk” schools that qualify for free/reduced price meals and belong to each racial/ethnic group. We then used a z-test for difference in proportions to determine if low income or racial/ethnic minority students are disproportionately at “high risk” as compared to students on average in the US. Unlike the school-level analyses to be conducted as part of research question 3, this is an analysis of children in schools (child-level).
2.3.3 Multivariate Statistics
We used a generalized estimating equation (GEE) to answer the third research question. This involved predicting the summed air neurotoxicant score using the four racial/ethnic composition variables, the economic deprivation variable and its squared term, and the level of student served and urban location variables, while accounting for clustering at the level of the school district. This clustering definition led to the assignment of between 1 and 1,646 schools to each cluster.
GEEs expand the generalized liner model to accommodate clustered data (Liang and Zeger 1986; Nelder and Wedderburn 1972). In this case, the schools are clustered within school districts. GEEs require the specification of an intracluster dependency correlation matrix (Liang and Zeger 1986; Zeger and Liang 1986). Three correlation structure specifications were substantive candidates (Garson 2012): (1) independent, which assumes the nonexistence of dependency, so that all off-diagonal elements of the working correlation matrix are zero; (2) exchangeable, which assumes constant intracluster dependency, so that all the off-diagonal elements of the correlation matrix are equal; and (3) unstructured, which assumes a completely general correlation matrix, which is estimated without constraints. We modeled the GEEs with the three matrices, using goodness-of-fit coefficients to determine the best specification (Garson 2012). The exchangeable correlation structure specification was selected for the results reported here, as it was better fitting than the independent and unstructured specifications.
To select the best fitting models, we estimated a series of GEEs by varying the model specifications. We tested normal, gamma and inverse Gaussian distributions with logarithmic and identity link functions. Visual inspection of a histogram of our dependent variable suggested that these were the most appropriate options, given that the dependent variable was not normally distributed. The inverse Gaussian distribution with a logarithmic link function was the best fitting. Finally, we examined possible multicollinearity among the analysis variables; based on variance inflation factor, tolerance, and condition index criteria, inferences from the GEEs are not affected by multicollinearity. All continuous independent variables were standardized before inclusion in the GEE. We define statistical significance as p<0.001, due to the large sample.
3. Results
3.1 Research Question 1: EPA Region
Table 2 presents the number and percent of “high risk” schools by region. In Region 2, which includes all schools in New York and New Jersey, one-third of schools are at “high risk” for air neurotoxicant exposures. Schools in Regions 3 and 5 have the second and third highest levels of air neurotoxicants, with 16–17% of schools being “high risk”. On the other end, Region 8 has a very low percentage of schools at “high risk”.
Table 2.
Number and Percent of Schools in Top 10% for Neurotoxicant Exposure in each EPA region
| EPA Region |
US States in the Region | Number of High Risk Schools |
Number of Schools |
% High Risk Schools per Region |
|---|---|---|---|---|
| 2 | NY, NJ | 2,318 | 6,958 | 33.3 |
| 3 | DE, MD, PA, VA, WV, District of Columbia | 1,272 | 7,333 | 17.3 |
| 5 | IL, IN, MI, MN, OH, WI | 2,560 | 15,774 | 16.2 |
| 9 | AZ, CA, HI, NV | 1,167 | 11,223 | 10.4 |
| 10 | AK, ID, OR, WA | 400 | 3,955 | 10.1 |
| 1 | CT, ME, MA, NH, RI, VT | 146 | 4,203 | 3.5 |
| 7 | IA, MO, KS, NE | 160 | 5,216 | 3.1 |
| 4 | AL, GA, KY, MS, NC, TN, FL, SC | 388 | 14,285 | 2.7 |
| 6 | AR, LA, OK, NM, TX | 289 | 12,330 | 2.3 |
| 8 | CO, MT, ND, SD, UT, WY | 18 | 3,692 | 0.5 |
3.2 Research Question 2: Disproportionate Risk for Minority and Economically-Deprived Students
Table 3 shows that students attending “high risk” public schools nationwide are significantly more likely to eligible for free/reduced price meals, and to be Hispanic, black, or API. They are significantly less likely to be white or other race. As an example of how to interpret the table, the second column in Table 3 shows the proportion of all children in that group. For example, 45.4% of all public school children are eligible for free/reduced price meals. The third column shows the proportion of children at “high risk” who are in that group. In this case, 56.5% of children at “high risk” are eligible for free/reduced price meals. The difference is 11.1% and it is statistically significant.
Table 3.
Results of a Z-test for differences in proportions comparing students based on economic deprivation and race/ethnicity to all US public school students
| Proportion of all children |
Proportion of children at high risk |
Difference | Z | p | |
|---|---|---|---|---|---|
| Free/Reduced Meals | 0.454 | 0.565 | −0.111 | −485.895 | <0.0001 |
| White | 0.516 | 0.284 | 0.232 | 1019.9 | <0.0001 |
| Hispanic | 0.238 | 0.344 | −0.106 | −540.505 | <0.0001 |
| Black | 0.158 | 0.268 | −0.11 | −644.378 | <0.0001 |
| API | 0.051 | 0.08 | −0.029 | −277.785 | <0.0001 |
| Other Race | 0.036 | 0.024 | 0.012 | 149.879 | <0.0001 |
3.3 Research Question 3: Predicting Summed Neurotoxicant Risk
Table 4 reports results of a model predicting the summed neurotoxicant risk variable. Accounting for school district as a nuisance parameter, we find that an increase in the number of students is associated with higher air neurotoxicant risks. The relationship with economic deprivation (proportion free/reduced price meals) was not significant. The racial/ethnic minority group variables are significant in the direction of more minority students, more risk, with the exception of other race. In terms of the level of student served, schools serving children in the earliest grades face increased risk relative to elementary schools. Students attending junior high schools, high schools, and ungraded schools have less risk than those attending elementary schools. Schools located in increasingly urban counties have significantly greater air neurotoxicant risks. Proportion urban was the most important predictor of summed neurotoxicant risk, followed by ungraded/all grades and proportion black.
Table 4.
Results of a GEE predicting summed neurotoxicant risk at US public school
| Parameter | Parameter Estimate |
Standard Error |
Lower 95% Confidence Interval |
Upper 95% Confidence Interval |
p |
|---|---|---|---|---|---|
| Intercept | 3.582 | 0.003 | 3.576 | 3.588 | <0.001 |
| Total Students | 0.011 | 0.001 | 0.009 | 0.014 | <0.001 |
| Prop. Free/Reduced Meals | 0.001 | 0.003 | −0.006 | 0.006 | 0.964 |
| Prop. Free/Reduced Meals Squared | 0.001 | 0.003 | −0.005 | 0.008 | 0.732 |
| Prop. Hispanic | 0.017 | 0.003 | 0.012 | 0.022 | <0.001 |
| Prop. Black | 0.031 | 0.003 | 0.026 | 0.037 | <0.001 |
| Prop. API | 0.012 | 0.002 | 0.008 | 0.015 | <0.001 |
| Prop. Other | −0.015 | 0.002 | −0.019 | −0.012 | <0.001 |
| Early Education1 | 0.031 | 0.003 | 0.026 | 0.037 | <0.001 |
| Junior High1 | −0.004 | 0.001 | −0.006 | −0.002 | <0.001 |
| High School1 | −0.011 | 0.002 | −0.015 | −0.008 | <0.001 |
| Ungraded/All Grades1 | −0.037 | 0.005 | −0.045 | −0.028 | <0.001 |
| Prop. Urban (in county) | 0.170 | 0.004 | 0.163 | 0.177 | <0.001 |
| Scale | 0.002 |
Notes: All continuous predictors were standardized. Model uses an exchangeable correlation matrix, inverse Gaussian with logarithmic link, and adjusts for clustering at the school district level.
Reference: Elementary School
While the parameter estimate values for the proportion variables shown in Table 4 are interpretable as the unit change in the neurotoxicant sum per one standard deviation increase in the variable, a 10% increase may be more meaningful to some readers. When interpreting the results that way, we find that a 10% increase in the percent of Hispanic children in the school is associated with a 0.006 unit increase in the neurotoxicant risk score. Ten percent increases in percent black and API, respectively, are associated with 0.013 and 0.014 unit increases in the risk score. As the percent of other race students increases by 10%, the summed neurotoxicant risk score drops by 0.019 unit. As the urban population in the county housing the school increases by 10%, the neurotoxicant risk measure increases by 0.042 unit. For every 100 additional students attending a school, the neurotoxicant risk score increases by 0.003 unit.
4. Discussion
Findings reveal geographically and socially disparate patterns of risk to air neurotoxicants, which likely have serious implications for the health of US children. Regionally, the New York City metro area (EPA Region 2) emerged as a location of concern, since one third of schools in that region are in the top 10% for air neurotoxicant exposure nationwide (Table 2). While EPA Region 2 also includes Puerto Rico and the U.S. Virgin Islands, schools from those places are not part of the study. Results suggest that ambient neurotoxicants must be centered on the radar screen in EPA Region 2. Currently, the key issues posted on the EPA Region 2 website include climate change and projects to address water contamination (U.S. Environmental Protection Agency 2017). While those issues are locally relevant and important, action is needed to reduce air emissions containing neurotoxicants from multiple sources (e.g., point and transportation), especially near public schools. A national regulatory structure could help to enable that. Local EJ groups in the area, like the New York City Environmental Justice Alliance and WE ACT for Environmental Justice, could also play a role in advocacy surrounding this issue as part of their ongoing air quality actions.
Results reveal a strong pattern of disparate risk based on race, especially for black, API and Hispanic children across the analyses, while white children seem to be protected. The proportion black and Hispanic students attending “high risk” schools nationwide deviated the most from the national averages in the positive direction, suggesting the greatest risk disparities (Table 3). In the multivariate model (Table 4), the strongest relationship with air neurotoxicant risk in terms of race/ethnicity was for proportion black students, this was followed by Hispanic and API. These findings point toward substantial risk for black, API and Hispanic students nationwide.
While readers may have expected the risk for black and Hispanic students, they may be surprised by the API findings. Risks for black and Hispanic children have been highlighted in other case studies looking at school-based exposure to toxics in Florida (Chakraborty and Zandbergen 2007) and California (Gaffron and Niemeier 2015; Green et al. 2004), although this is the first study to show that these disparate school-based risks exist nationwide. Black and Hispanic children disproportionately face other environmental risks outside of school. They are more likely than white children to live in substandard housing, which includes poor physical conditions (e.g., noise), chemical exposures (e.g., carbon monoxide), biological exposures (e.g., cockroaches), and unsafe building conditions (e.g., posing accident risks) (Jacobs 2011). Black children under age six have higher blood lead levels than their peers of other racial/ethnic groups (White, Bonilha and Ellis 2016) and high rates of asthma (22%); 14% of Hispanic children and 12% of white children also have asthma (Bloom, Jones and Freeman 2013). Compared to other racial/ethnic groups, black families in the US experience the highest levels of residential segregation (Massey and Denton 1993), and neighborhoods with high concentrations of black residents tend to have a host of health-compromising characteristics (e.g. high poverty levels, greater numbers of unemployed adults, and institutional disinvestment) (Bécares et al. 2012); this pattern could extend to schools with high proportions of black students.
Unlike health risks associated with black ethnic density, living in ethnically dense Hispanic neighborhoods is associated with better health, hypothetically due to social support, reduced exposure to racism, decreased low status stigma, recent chain migration, and less hyper segregation (as compared to black neighborhoods) (Bécares et al. 2012). It could be that proportion Hispanic students (at the school level) is protective against some of the negative effects of neurotoxicant exposures, but this has not been investigated. Potential protective effects align with the Hispanic health paradox, which refers to the observation that Hispanics (and Mexican immigrants in particular) have better health outcomes than expected, given their low socioeconomic status (Morales et al. 2002). Disparate air neurotoxicant exposures at school, taken together with the heightened risks summarized above, outline a situation of multiple jeopardy for black American children. This could also be the case for Hispanic American children, but the ethnic density and Hispanic health paradox literatures suggest that the relationship could be more complex. Our findings coupled with the literature point toward interacting exposures that demand further investigation.
The disparate air neurotoxicant risks for API students may come as more of a surprise since API populations are included less frequently than blacks and Hispanics in EJ studies, based on the assumption that they have similar risk profiles to whites. When API Americans are actually examined in EJ research, results typically indicate that they face higher risks from environmental hazards than whites (Clark, Millet and Marshall 2014; Cushing et al. 2015; Grineski, Collins and Morales 2017; Lievanos 2015; McKelvey et al. 2007; Morello-Frosch and Jesdale 2006). However, results indicative of disproportionate risk for API Americans have been de-emphasized in many studies. Our findings related to air neurotoxicant exposures of API children at US public schools contribute to a growing body of work that is raising awareness of the environmental risks that API Americans face in the US (Grineski, Collins and Morales 2017; Sze 2004). This work is important since it counters the “model minority” stereotype, which has been applied to Asian Americans to suggest that they do not experience discrimination or racism in the US. While Asian Americans have high incomes and levels of education, these statistics bely the racialized experiences of API individuals. Asians in the US are held up as the successful and high achieving model minority group while they are simultaneously marginalized as outsiders (Xu and Lee 2013), and, as this study shows, overrepresented as students in public schools that are at high risk to air neurotoxicants.
In addition to racial/ethnic risk disparities, multivariate results also showed that younger children (i.e., those in earlier grade levels) are exposed to greater risks than older children while attending public schools in the US. Schools serving the youngest children (e.g., pre-kindergarten and kindergarten) had greater risk than those attending elementary schools, which had greater risk than junior high and high schools. The disproportionate presence of air neurotoxicants at schools serving the youngest Americans is cause for serious concern. Children are highly susceptible to the effects of chemical hazards because of heavy exposures (i.e., they consume more air and food per unit of body weight than do adults), biologic sensitivity associated with growth and development, and their long future lifetimes as early insults can manifest in adult diseases (Perera 2008).
This analysis suffers from several limitations. The NATA considers only neurotoxicants emitted into the air from ambient sources and not those from other sources, such as through food or household products even though those are additional pathways of exposure. Our comprehensive measure of air neurotoxicant exposure included 24 toxicants, which are the chemicals that affect the neurological system, as designated by the EPA [see Appendix H in Environmental Protection Agency (2015)] and regulated by the Clean Air Act; chemicals not listed or regulated are not included. We also examined only exposure at school and not at home, which means we do not capture a full picture of children’s exposure. Therefore, children’s exposure to neurotoxicants may be underestimated. Ultrafine particulate matter is a critical neurotoxicant of concern (Loane et al. 2013), which is missing from this study, due to data limitations. The study focused on the year 2011, due to limited availability of air neurotoxicant estimates available from the National Air Toxics Assessment, even though more recent data on school demographics are available. While the Common Core school-level data provided many relevant variables, our selections were limited by what was available; the median education level of children’s parents, for example, was not an option. While it made sense to focus our analysis the neurotoxicants listed as such by the EPA as part of the NATA documentation, this is an incomplete list and there are other known and potential neurotoxicants that could be considered in future research. Finally, the study was cross-sectional, capturing associations at one point in time, and cannot speak to how the observed pattern developed or changed over time.
5. Conclusion
The unmeasured losses in societal-level human capital due to these documented air neurotoxicant exposures among US schoolchildren could be expansive. For example, it has been suggested that people exposed to lead in countries that used leaded gasoline suffered widespread low-grade lead poisoning that lowered the average IQ of the population by about five points (Lanphear et al. 2000). Such widespread cognitive impairment can reduce the intelligence and lifelong economic productivity of entire generations, thus undermining the developmental trajectory of whole societies (Landrigan and Fuller 2014; Suk et al. 2016). These neurotoxicant exposures may be decreasing societal capacities for innovation and causing economic losses associated with direct medical expenses, diminished productivity, and missed opportunities (Colburn, Dumanoski and Myers 1997; Suk et al. 2016).
For the children in this study, the exposures may be linked to their abilities to do well in school. Children attending schools in more polluted areas have lower aggregated standardized test scores than those in less polluted areas, even accounting for other factors known to influence academic achievement (Mohai et al. 2011; Pastor, Morello-Frosch and Sadd 2006; Pastor, Sadd and Morello-Frosch 2004; Scharber et al. 2013); they also have lower GPAs (Grineski, Clark-Reyna and Collins 2015). Subpar academic performance at a young age can have lifelong impacts on a child’s life chances, including lower economic and educational attainment in adulthood (Case, Fertig and Paxson 2005). This study shows that racial/ethnic minority children are bearing the brunt of neurotoxicant exposures at school, which may be unequally impacting their school performance and future prospects.
Urgent action is needed to protect children from these harmful exposures. Enforcement strategies at the federal and state level are needed to improve ambient air quality, minimize children’s exposure, and encourage the redesign of engine and fuel technologies to produce fewer neurotoxic HAPs (Genc et al. 2012). Specific policies to prohibit schools from being located in polluted areas, as well as to prohibit highly polluting land uses near existing schools, are needed. There is no overarching federal agency nor specific policies in place to protect children from attending schools in polluted or toxic locations in the US (Sampson 2012). The World Health Organization and the US Environmental Protection Agency have put forth school siting guidelines (U.S. Environmental Protection Agency 2014; Wargo 2004), but these are to inform voluntary decision making only, since they are not legally enforceable. In the US, voluntary guidelines are not being widely adopted as only 10 US states prohibit school siting near environmental hazards (Gaffron and Niemeier 2015). Given that these voluntary guidelines have been adopted in a limited way, federal regulation is required to ensure that children are protected. All US states have compulsory education laws that require children to attend public (or accredited private schools) for a specific period of time, usually between the ages of 6 and at least 16, for 180 days per year (FindLaw 2013). While attending public school, the health and wellbeing of these children is the responsibility of the government, which needs to adequately protect them from risks.
In addition to policies aimed at reducing exposure, other strategies should be implemented to better document the risks and to protect children from them. These include augmenting current air quality monitoring efforts to ensure that neurotoxicants, such as heavy metals and ultrafine particulate matter, are measured and their levels reported publically, as is done with criteria pollutants (Mandal and Bandyopadhyay 2013). These data could then be paired with neurological health registries, which could be initiated at the clinic/hospital, municipal, or state level, to enable researchers to more accurately trace the impact of ambient neurotoxicants on neurological health conditions and report these findings to the general public (Bandyopadhyay 2016). An awareness program, which highlights existing and new research, should be undertaken by regulators and academics to better publicize the myriad ways in which pollution affects children’s health, including their neurodevelopment (Bandyopadhyay 2016).
Table 1.
Descriptive Statistics for Analysis Variables
| Variables | N | Min. | Max. | Mean | Std. Dev. |
|
| |||||
| Total Number of Students | 84969 | 100 | 11640 | 574.51 | 431.016 |
| Prop. Free/Reduced Meals | 84969 | 0 | 0.999 | 0.485 | 0.286 |
| Prop. White | 84969 | 0 | 1 | 0.548 | 0.336 |
| Prop. Hispanic | 84969 | 0 | 1 | 0.211 | 0.262 |
| Prop. Black | 84969 | 0 | 1 | 0.159 | 0.246 |
| Prop. API | 84969 | 0 | 1 | 0.041 | 0.086 |
| Prop. Other | 84969 | 0 | 1 | 0.041 | 0.079 |
| Prop. Urban (county) | 84969 | 0 | 100 | 0.634 | 0.403 |
| Summed Air Neurotoxicant Risk | 84969 | 15 | 59 | 37.676 | 10.047 |
|
| |||||
| N | Min. | Max. | Variable=1 | Variable=0 | |
|
| |||||
| “ High Risk” School | 84969 | 0 | 1 | 8718 | 76251 |
| “ Low Risk” School | 84969 | 0 | 1 | 8864 | 76105 |
| Level of Student Served | |||||
| Early Childhood | 84969 | 0 | 1 | 1404 | 83565 |
| Elementary | 84969 | 0 | 1 | 44082 | 40887 |
| Junior High | 84969 | 0 | 1 | 20282 | 64687 |
| High School | 84969 | 0 | 1 | 18427 | 66542 |
| Ungraded/All | 84969 | 0 | 1 | 774 | 84195 |
Highlights.
Ambient neurotoxicants pose serious risks at US public schools.
There are social and spatial disparities in ambient neurotoxicant risks.
One-third of all schools in EPA Region 2 are at “high risk” for neurotoxicant exposure.
Schools with higher % of Hispanic, black, and Asian students face disparate risks.
Schools serving the youngest (vs. older) students face disparate risks.
Acknowledgments
Statement of Funding
Research reported in this paper was supported by the National Institute of General Medical Sciences of the National Institutes of Health under linked Award Numbers RL5GM118969, TL4GM118971, and UL1GM118970. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bandyopadhyay A. Neurological Disorders from Ambient (Urban) Air Pollution Emphasizing UFPM and PM2.5. Current Pollution Reports. 2016;2(3):203–11. [Google Scholar]
- Bécares L, Shaw R, Nazroo J, Stafford M, Albor C, Atkin K, Kiernan K, Wilkinson R, Pickett K. Ethnic density effects on physical morbidity, mortality, and health behaviors: a systematic review of the literature. American Journal of Public Health. 2012;12:e33–66. doi: 10.2105/AJPH.2012.300832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom Barbara, Jones Ll, Freeman Gulnur. Summary Health Statistics for U.S. Children: National Health Interview Survey, 2012. Vital and Health Statistics. 2013;10(258):1–81. [PubMed] [Google Scholar]
- Brockmeyer S, D'Angiulli A. How air pollution alters brain development: The role of neuroinflammation. Translational Neuroscience. 2016;7(1):24–30. doi: 10.1515/tnsci-2016-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Leray E, Heydarpour P, Torres-Jardon R, Reis J. Air pollution, a rising environmental risk factor for cognition, neuroinflammation and neurodegeneration: The clinical impact on children and beyond. Revue Neurologique. 2016;172(1):69–80. doi: 10.1016/j.neurol.2015.10.008. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Solt AC, Henriquez-Roldan C, Torres-Jardon R, Nuse B, Herritt L, Villarreal-Calderón R, Osnaya N, Stone I, García R, Brooks DM, González-Maciel A, Reynoso-Robles R, Delgado-Chávez R, Reed W. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicologic Pathology. 2008;36(2):289–310. doi: 10.1177/0192623307313011. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Vojdani A, Blaurock-Busch E, Busch Y, Friedle A, Franco-Lira M, Sarathi-Mukherjee P, Martínez-Aguirre X, Park SB, Torres-Jardón R, D'Angiulli A. Air pollution and children: neural and tight junction antibodies and combustion metals, the role of barrier breakdown and brain immunity in neurodegeneration. Journal of Alzheimer's Disease. 2015;43(3):1039–58. doi: 10.3233/JAD-141365. [DOI] [PubMed] [Google Scholar]
- Case A, Fertig A, Paxson C. The lasting impact of childhood health and circumstance. Journal of Health Economics. 2005;24:365–89. doi: 10.1016/j.jhealeco.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Chakraborty J, Collins Timothy W, Montgomery Marilyn C, Grineski SE, Hernandez Maricarmen. Comparing Disproportionate Exposure to Acute and Chronic Pollution Risks: A Case Study in Houston, Texas. Risk Analysis. 2014;34(11):2005–20. doi: 10.1111/risa.12224. [DOI] [PubMed] [Google Scholar]
- Chakraborty Jayajit, Zandbergen Paul A. Children at risk: measuring racial/ethnic disparities in potential exposure to air pollution at school and home. Journal of Epidemiology and Community Health. 2007;61(12):1074–79. doi: 10.1136/jech.2006.054130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LP, Millet DB, Marshall JD. National Patterns in Environmental Injustice and Inequality: Outdoor NO2 Air Pollution in the United States. Plos One. 2014;9(4) doi: 10.1371/journal.pone.0094431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colburn T, Dumanoski D, Myers JP. Our Stolen Future: Are We Threatening Our Fertility, Intelligence, and Survival? A Scientific Detective Story. New York, NY: Plume Books; 1997. [Google Scholar]
- Collins Timothy, Grineski SE, Morales Danielle X. Sexual Orientation, Gender, and Environmental Injustice: Unequal Carcinogenic Air Pollution Risks in Greater Houston. Annals of the Association of American Geographers. 2017;107(1):72–92. doi: 10.1080/24694452.2016.1218270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa Lucio G, Cole Toby B, Coburn Jacki, Chang Yu-Chi, Dao Khoi, Roque Pamela. Neurotoxicants Are in the Air: Convergence of Human, Animal, and In Vitro Studies on the Effects of Air Pollution on the Brain. BioMed Research International. 2014:8. doi: 10.1155/2014/736385. Article ID 736385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing L, Faust J, August LM, Cendak R, Wieland W, Alexeeff G. Racial/Ethnic Disparities in Cumulative Environmental Health Impacts in California: Evidence From a Statewide Environmental Justice Screening Tool (CalEnviroScreen 1.1) American Journal of Public Health. 2015;105(11):2341–48. doi: 10.2105/AJPH.2015.302643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Environmental Protection Agency. Technical Support Document: EPA’s 2011 National-scale Air Toxics Assessment. Research Triangle Park, North Carolina: Office of Air Quality, Planning, and Standards; 2015. p. 361. [Google Scholar]
- Environmental Protection Agency. 2011 NATA: Assessment Results. Washington DC: 2016a. [Google Scholar]
- Environmental Protection Agency. TEACH: Toxicity and Exposure Assessment for Children’s Health. Washington, D.C: 2016b. Manganese. [Google Scholar]
- FindLaw. Compulsory Education Laws: Background. Thompson Reuters: 2013. [Google Scholar]
- Gaffron P, Niemeier D. School Locations and Traffic Emissions - Environmental (In)Justice Findings Using a New Screening Method. International Journal of Environmental Research and Public Health. 2015;12(2):2009–25. doi: 10.3390/ijerph120202009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garson G. Generalized linear models and generalized estimating equations. Asheboro, NC: Statistical Associates Publishing; 2012. [Google Scholar]
- Genc Sermin, Zadeoglulari Zeynep, Fuss Stefan H, Genc Kursad. The adverse effects of air pollution on the nervous system. Journal of Toxicology. 2012:23. doi: 10.1155/2012/782462. Article ID 782462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould Jay. Quality of Life in American Neighborhoods. Boulder, Colorado: Westview Press; 1986. [Google Scholar]
- Grandjean Philippe, Landrigan Philip J. Neurobehavioural effects of developmental toxicity. Lancet Neurology. 2014;13(3):330–38. doi: 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RS, Smorodinsky S, Kim JJ, McLaughlin R, Ostro B. Proximity of California public schools to busy roads. Environmental Health Perspectives. 2004;112:61–66. doi: 10.1289/ehp.6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grineski Sara E, Clark-Reyna Stephanie E, Collins Timothy. School-based Exposure to Hazardous Air Pollutants and Grade Point Average: A multi-level study. Environmental Research. 2015;147:164–71. doi: 10.1016/j.envres.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grineski Sara E, Collins Timothy W, Morales Danielle X. Asian Americans and disproportionate exposure to carcinogenic hazardous air pollutants: A national study. Social Science & Medicine. 2017;185:71–80. doi: 10.1016/j.socscimed.2017.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPUMS. U.S. Geographic Summary Data and Boundary Files. Minnesota Population Center; St. Paul, MN: 2016. [Google Scholar]
- Jacobs DE. Environmental Health Disparities in Housing. American Journal of Public Health. 2011;101:S115–S22. doi: 10.2105/AJPH.2010.300058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan PJ, Rauh VA, Galvez MP. Environmental Justice and the Health of Children. Mount Sinai Journal of Medicine. 2010;77(2):178–87. doi: 10.1002/msj.20173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan PJ, Fuller R. Environmental pollution: an enormous and invisible burden on health systems in low- and middle-income counties. World Hospitals and Health Services. 2014;50(4):35–40. [PubMed] [Google Scholar]
- Lanphear BP, Dietrich K, Auinger P, Cox C. Cognitive deficits associated with blood lead concentrations <10 microg/dL in U.S. children and adolescents. Public Health Reports. 2000;115(6):521–29. doi: 10.1093/phr/115.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legot C, London B, Shandra J. Environmental Ascription: High-Volume Polluters, Schools, and Human Capital. Organization & Environment. 2010;23(3):271–90. [Google Scholar]
- Liang K, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Lievanos RS. Race, deprivation, and immigrant isolation: The spatial demography of air-toxic clusters in the continental United States. Social Science Research. 2015;54:50–67. doi: 10.1016/j.ssresearch.2015.06.014. [DOI] [PubMed] [Google Scholar]
- Linder Stephen H, Marko Dritana, Sexton Ken. Cumulative Cancer Risk from Air Pollution in Houston: Disparities in Risk Burden and Social Disadvantage. Environmental Science & Technology. 2008;42(12):4312–22. doi: 10.1021/es072042u. [DOI] [PubMed] [Google Scholar]
- Loane C, Pilinis C, Lekkas TD, Politis M. Ambient particulate matter and its potential neurological consequences. Reviews in Neuroscience. 2013;24(3):323–25. doi: 10.1515/revneuro-2013-0001. [DOI] [PubMed] [Google Scholar]
- Mandal PK, Bandyopadhyay A. Ambient air quality in the Kolkata Municipal Corporation Area: assessment of practices and policy from 2003 to 2010 with policy recommendations for air quality improvement. Environmental Quality and Management. 2013;23(1):83–106. [Google Scholar]
- Massey Douglas S, Denton NA. American apartheid: segregation and the making of the underclass. Cambridge, MA: Harvard University Press; 1993. [Google Scholar]
- McKelvey Wendy R, Gwynn Charon, Jeffery Nancy, Kass Daniel, Thorpe Lorna E, Garg Renu K, Palmer Christopher D, Parsons Patrick J. A biomonitoring study of lead, cadmium, and mercury in the blood of New York city adults. Environmental Health Perspectives. 2007;115(10):1435–41. doi: 10.1289/ehp.10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohai P, Pellow DN, Roberts J Timmons. Environmental Justice. Annual Review of Environment and Resources. 2009;34:405–30. [Google Scholar]
- Mohai P, Saha R. Which came first, people or pollution? Assessing the disparate siting and post-siting demographic change hypotheses of environmental injustice. Environmental Research Letters. 2015;10(11) [Google Scholar]
- Mohai Paul, Kweon Byoung-Suk, Lee Sangyun, Ard Kerry. Air Pollution Around Schools Is Linked To Poorer Student Health And Academic Performance. Health Affairs. 2011;30(5):852–62. doi: 10.1377/hlthaff.2011.0077. [DOI] [PubMed] [Google Scholar]
- Morales LS, Lara M, Kington RS, Valdez RO, Escarce JJ. Socioeconomic, Cultural and Behavioral Factors Affecting Hispanic Health Outcomes. Journal of Health Care for the Poor & Underserved. 2002;13(4):477–503. doi: 10.1177/104920802237532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello-Frosch R, Jesdale BM. Separate and unequal: Residential segregation and estimated cancer risks associated with ambient air toxics in US metropolitan areas. Environmental Health Perspectives. 2006;114(3):386–93. doi: 10.1289/ehp.8500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Education Statistics. Average number of hours in the school day and average number of days in the school year for public schools, by state: 2007–08. U.S. Department of Education; Washington D.C: 2008. [Google Scholar]
- National Center for Education Statistics. ElSi tableGenerator. U.S. Department of Education; Washington D.C: 2017. [Google Scholar]
- Nelder J, Wedderburn R. Generalized linear models. Journal of the Royal Statistical Society, Series A. 1972;135:370–84. [Google Scholar]
- Pais J, Crowder K, Downey L. Unequal Trajectories: Racial and Class Differences in Residential Exposure to Industrial Hazard. Social Forces. 2014;92(3):1189–215. doi: 10.1093/sf/sot099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor M, Morello-Frosch R, Sadd JL. Breathless: Schools, air toxics, and environmental justice in California. Policy Studies Journal. 2006;34(3):337–62. [Google Scholar]
- Pastor M, Sadd JL, Morello-Frosch R. Who's minding the kids? Pollution, public schools, and environmental justice in Los Angeles. Social Science Quarterly. 2002;83(1):263–80. [Google Scholar]
- Pastor Manuel, Morello-Frosch Rachel, Sadd James L. The air is always cleaner on the other side: Race, space, and ambient air toxics exposures in California. Journal of Urban Affairs. 2005;27(2):127–48. [Google Scholar]
- Pastor Manuel, Sadd James L, Morello-Frosch Rachel. Reading, writing, and toxics: children's health, academic performance, and environmental justice in Los Angeles. Environment and Planning C. 2004;22:271–90. [Google Scholar]
- Perera F. Children are likely to suffer most from our fossil fuel addiction. Environmental Health Perspectives, 116: 987–990. 2008;116:987–90. doi: 10.1289/ehp.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AL, Lyall K, Hart JE, Laden F, Just AC, Bobb JF, Koenen KC, Ascherio A, Weisskopf MG. Perinatal Air Pollutant Exposures and Autism Spectrum Disorder in the Children of Nurses' Health Study II Participants. Environmental Health Perspectives. 2013;121(8):978–84. doi: 10.1289/ehp.1206187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson N. Environmental Justice at School: Understanding Research, Policy, and Practice to Improve Our Children's Health. Journal of School Health. 2012;82(5):246–52. doi: 10.1111/j.1746-1561.2012.00694.x. [DOI] [PubMed] [Google Scholar]
- Sanders T, Liu Y, Buchner V, Tchounwou PB. Neurotoxic Effects and Biomarkers of Lead Exposure: A Review. Reviews on Environmental Health. 2009;24(1):15–45. doi: 10.1515/reveh.2009.24.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharber H, Lucier C, London B, Rosofsky A, Shandra J. The consequences of exposure to, neurological, and respiratory toxins for school performance: a closer look at environmental ascription in East Baton Rouge, Louisiana. Population and Environment. 2013;35:205–24. [Google Scholar]
- Strife S, Downey L. Childhood development and access to nature a new direction for environmental inequality research. Organization & Environment. 2009;22(1):99–122. doi: 10.1177/1086026609333340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suk WA, Ahanchian H, Asante KA, Carpenter DO, Diaz-Barriga F, Ha EH, Huo X, King M, Ruchirawat M, da Silva ER, Sly L, Sly PD, Stein RT, van den Berg M, Zar H, Landrigan PJ. Environmental Pollution: An Under-recognized Threat to Children's Health, Especially in Low- and Middle-Income Countries. Environmental Health Perspectives. 2016;124(3):A41–A45. doi: 10.1289/ehp.1510517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze Julie. Asian American Activism for Environmental Justice. Peace Review. 2004;16(2):149–56. [Google Scholar]
- Talbott EO, Marshall LP, Rager JR, Arena VC, Sharma RK, Stacy SL. Air toxics and the risk of autism spectrum disorder: the results of a population based case-control study in southwestern Pennsylvania. Environmental Health. 2015;14 doi: 10.1186/s12940-015-0064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. School Siting Guidelines. Washington, D.C: 2014. [Google Scholar]
- U.S. Environmental Protection Agency. EPA Region 2. 2017 [PubMed] [Google Scholar]
- Wargo John. The Physical School Environment: An Essential Component of a Health-Promoting School. Geneva: World Health Organization; 2004. [Google Scholar]
- White BM, Bonilha HS, Ellis C. Racial/Ethnic Differences in Childhood Blood Lead Levels Among Children < 72 Months of Age in the United States: a Systematic Review of the Literature. Journal of Racial and Ethnic Health Disparities. 2016;3(1):145–53. doi: 10.1007/s40615-015-0124-9. [DOI] [PubMed] [Google Scholar]
- Windham GC, Zhang L, Gunier R, Croen LA, Grether JK. Autism Spectrum Disorders in Relation to Distribution of Hazardous Air Pollutants in the San Francisco Bay Area. Environmental Health Perspectives. 2006;114(9):1438–44. doi: 10.1289/ehp.9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Children's expsosure to mercury compounds. Geneva, Switzerland: 2010. [Google Scholar]
- Xu J, Lee JC. "The marginalized" model" minority: An empirical examination of the racial triangulation of Asian Americans. Social Problems. 2013;91(4):1363–97. [Google Scholar]
- Zeger S, Liang K. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]