Abstract
Stress during consolidation improves recognition memory performance. Generally, this memory benefit is greater for emotionally arousing stimuli than neutral stimuli. The strength of the stressor also plays a role in memory performance, with memory performance improving up to a moderate level of stress and thereafter worsening. As our daily stressors are generally minimal in strength, we chose to induce mild acute stress to determine its effect on memory performance. In the current study, we investigated if mild acute stress during consolidation improves memory performance for emotionally arousing images. To investigate this, we had participants encode highly arousing negative, minimally arousing negative, and neutral images. We induced stress using the Montreal Imaging Stress Task (MIST) in half of the participants and a control task to the other half of the participants directly after encoding (i.e. during consolidation) and tested recognition 48 h later. We found no difference in memory performance between the stress and control group. We found a graded pattern among confidence, with responders in the stress group having the least amount of confidence in their hits and controls having the most. Across groups, we found highly arousing negative images were better remembered than minimally arousing negative or neutral images. Although stress did not affect memory accuracy, responders, as defined by cortisol reactivity, were less confident in their decisions. Our results suggest that the daily stressors humans experience, regardless of their emotional affect, do not have adverse effects on memory.
Keywords: Stress, Cortisol, Arousal, Recognition memory, Emotion
1. Introduction
Stress is something we all experience at some point in our lives or even on a daily basis and can be broadly defined as our body and brain’s response to changing demands. When we feel stress, we experience a set of physiological changes that are collectively known as the stress response. In addition to the physiological changes produced by stress, the stress response also includes changes in the central nervous system (CNS), which have lasting effects on health and cognitive processes (for review, de Kloet, Joels, & Holsboer, 2005). The effects of stress depend on the type of stress experienced. Exposure to chronic stress is generally harmful to health and cognitive processes and can lead to anxiety, depression, high blood pressure and other health issues (for review, de Kloet et al., 2005; McEwen, 2000). While we know that chronic stress is harmful, we know less about the effects of acute stress. Previous studies have resulted in mixed findings on the effects of acute stress on memory, with some finding impairments and some finding improvements (for review, LaBar & Cabeza, 2006; McGaugh, 2000; Shields, Sazma, & Yonelinas, 2016). The direction of the effect appears to depend on multiple factors: the timing of the stressor (for review, Wolf, 2009) the strength of the stressor (Akirav et al., 2004; Diamond, Bennett, Fleshner, & Rose, 1992; Sandi, Loscertales, & Guaza, 1997) and the type of material being encoded (Cahill & Alkire, 2003; Cahill, Gorski, & Le, 2003; Smeets, Otgaar, Candel, & Wolf, 2008). We will discuss each of these factors in turn.
One of the key factors determining whether acute stress will result in improvements or impairments is when the stress is induced (for review, Wolf, 2009). Recognition memory can be separated into three phases: encoding, when information is learned, consolidation, when information is stored and the memory trace is strengthened, and retrieval, when the information is recovered. Stress induced prior to encoding has produced mixed results, with some evidence for memory impairments (Maheu, Collicutt, Kornik, Moszkowski, & Lupien, 2005; Maheu, Joober, Beaulieu, & Lupien, 2004; Preuss & Wolf, 2009; Schwabe & Wolf, 2010) and some evidence for memory improvements (Abercrombie, Kalin, Thurow, Rosenkranz, & Davidson, 2003; Buchanan & Lovallo, 2001; Schwabe, Bohringer, Chatterjee, & Schachinger, 2008). Stress induced prior to retrieval consistently impairs memory (Buchanan, Tranel, & Adolphs, 2006; de Quervain, Aerni, & Roozendaal, 2007; de Quervain, Roozendaal, Nitsch, McGaugh, & Hock, 2000; de Quervain et al., 2003; Kuhlmann, Piel, & Wolf, 2005; Smeets et al., 2008), while stress induced during consolidation consistently enhances memory (Beckner, Tucker, Delville, & Mohr, 2006; Cahill et al., 2003; Preuss & Wolf, 2009; Smeets et al., 2008). One of the first studies in humans to suggest that acute stress during consolidation can be beneficial to memory was a study that administered the cold pressor task (CPT) immediately following learning (Cahill et al., 2003). Subjects were presented with emotionally arousing negative or neutral images and then immediately submerged their hand in ice cold (stress group) or warm (control group) water for 1–3 min. Their recall was tested one week later. Participants who were administered the CPT had higher recall for the emotionally arousing negative images than participants who were administered the control task. Although the mechanism by which stress affects memory consolidation in humans has not been determined, rodent literature suggests that stress results in the immediate release of epinephrine from the adrenal medulla and glucocorticoids (cortisol in humans) from the adrenal cortex. Following its release, epinephrine induces an increase in noradrenergic activity in the basolateral nucleus of the amygdala (BLA). Together this BLA activity and the glucocorticoid activity enhance long-term potentiation (LTP) in the hippocampus and amygdala, subsequently enhancing memory consolidation (for review, Cahill et al., 2003; McGaugh & Roozendaal, 2002; Wolf, 2008).
Another factor that determines whether acute stress will result in improvements or impairments in recognition memory is the strength of the stressor. Animal research has suggested a curvilinear relationship between stress level and performance, in which moderate levels of stress improves performance while mild and severe levels of stress may not (Akirav et al., 2004; Diamond et al., 1992; Sandi et al., 1997). Yerkes and Dodson were the first to observe this relationship (Yerkes & Dodson, 1908). In their study, rats were put in front of two rooms, one black and one white, and had to choose which room to enter. If they chose the black room they were given either a mild, moderate or strong electrical shock. As voltage increased, so did learning, but once the voltage passed the moderate level, rats’ performance significantly decreased. Animal research suggests that moderate acute stress leads to elevated levels of glucocorticoids that effectively induce LTP and lead to enhanced memory performance. Whereas, severe acute stress leads to abnormal levels of glucocorticoids that impair LTP and subsequently impair memory performance (for review, de Kloet et al., 2005). A similar curvilinear relationship between stress level and performance has been found in humans. Andreano and Cahill (2006) administered CPT to male and female participants immediately after having them read a neutral story and tested their retention of this story 1 week later. Male participants with a moderate increase in cortisol had the highest subsequent memory performance relative to participants with low or high increases in cortisol. Although moderate levels of stress have been suggested to be optimal with regard to memory performance, it is important to understand the effects of mild acute stress since it is more consistent with what humans encounter on a daily basis (i.e. difficult homework, an argument). Understanding the relationship between mild levels of stress and memory performance is one of the goals of the present study.
Another factor that has not been thoroughly assessed in stress studies in humans, but is a key factor in determining how well an event will be remembered, is the type of material being encoded. Long-term memory for emotional events is superior to memory for neutral events (for review, Talmi et al., 2013). Emotion can be defined in terms of valence and arousal. Valence refers to how positive or negative an event is, while arousal indicates the intensity of the emotion (Lang, Greenwald, Bradley, & Hamm, 1993; Russell, 1980). The degree of arousal, rather than valence, is thought to be the primary factor underlying emotion-related memory benefits (Cahill & Alkire, 2003; Dolcos, LaBar, & Cabeza, 2004b; Kensinger & Schacter, 2005; Nielson & Powless, 2007; Smeets et al., 2008). The “modulation hypothesis of emotional memory” states that arousing materials exert their beneficial effect on memory by enhancing activity in both the amygdala and the medial temporal lobe (MTL) memory system, thereby modulating memory consolidation (Dolcos et al., 2004b). Because emotional arousal and stress both independently enhance activity in the amygdala, in combination they are thought to selectively modulate memory consolidation, such that highly arousing negative stimuli will be better remembered relative to minimally arousing negative and neutral stimuli (Cahill & Alkire, 2003; Cahill et al., 2003; Smeets et al., 2008).
Typically, stress studies have used either the Trier Social Stress Task (Kirschbaum, Pirke, & Hellhammer, 1993), which has participants give a speech and do mental math in front of an audience (Abercrombie, Speck, & Monticelli, 2006; Preuss & Wolf, 2009), or the CPT (Cahill et al., 2003; Smeets et al., 2008). An issue with these stressors is that they cannot readily be used in fMRI studies. Having a stress task that can be incorporated into fMRI studies is very important as researchers attempt to understand the neural underpinnings of stress-related memory enhancements. We chose to use the Montreal Imaging Stress Task (MIST), a stress task designed to be used in an fMRI scanner, as our stressor in the current study. The MIST is used to induce mild psychological stress in the laboratory (Dedovic et al., 2005). In the stress condition, the MIST requires participants to complete mental math problems faster than they are able to solve and input their answers. Stress participants also receive negative feedback from the experimenter in between runs. As levels of stress in humans have not been specifically defined, in terms of cortisol response or by the type of stressor, we are defining the MIST as a mild stressor because it usually results in small increases in cortisol relative to other stressors. The MIST typically increases cortisol 50–100% above baseline, whereas the TSST consistently produces increases in cortisol about 200–400% above baseline (Dedovic et al., 2005; Kirschbaum et al., 1993). In order to assess the feasibility of the MIST, we chose to first assess it behaviorally before incorporating it into any of our fMRI studies.
The goal of the current study was to determine the effect of mild acute stress during consolidation for emotional events. To be consistent with previous studies, we measured the stress response by assaying salivary cortisol levels (Andreano & Cahill, 2006; Cahill et al., 2003; Dedovic et al., 2009; McCullough, Ritchey, Ranganath, & Yonelinas, 2015; for review, Shields, Sazma, McCullough, & Yonelinas, 2017). We chose to only include male participants because of known sex differences in HPA axis functioning (Kajantie & Phillips, 2006; Kudielka & Kirschbaum, 2005). Specifically, a woman’s menstrual phase and the use of oral contraceptives can affect her cortisol response to stressors (Kajantie & Phillips, 2006). Excluding female participants reduces the variability in the cortisol data and is consistent with previous stress studies that have only used male participants (Abercrombie et al., 2003; de Quervain et al., 2003; Khalili-Mahani, Dedovic, Engert, Pruessner, & Pruessner, 2010; Maheu et al., 2004, 2005; Oei et al., 2007; Pruessner et al., 2008; Schwabe, Romer, et al., 2009). While moderate acute stress during consolidation appears to be optimal for later memory performance, the effects of mild acute stress during consolidation in humans is unknown. We hypothesize that memory for highly arousing negative material has the greatest likelihood of being enhanced by mild acute stress during consolidation. To investigate this, we had participants encode highly arousing negative images, minimally arousing negative images and neutral images. We administered a mild acute stressor to half the participants and a control task to the other half of participants immediately after encoding and assessed recognition memory performance 48 h later. The purpose of this delay period was to maximize the likelihood that the stressor affected consolidation mechanisms rather than retrieval. Previous emotional memory studies have implemented similar delays (Dolcos et al., 2004b; LaBar & Phelps, 1998; Sharot & Phelps, 2004). In addition to recognition, we also collected confidence ratings at retrieval in order to examine the quality of the retrieved memories. Based on previous research, we made the following predictions: If a mild acute stressor improves memory performance: (1) The stress group would have higher memory performance than the control group, especially for the highly arousing negative images. (2) Increases in cortisol following stress would be correlated with memory performance, with greater increases in cortisol corresponding to better memory performance. Alternatively, if a mild acute stressor does not improve memory performance then there will be no difference between the two groups or impaired memory performance for the stress group.
2. Method
2.1. Participants
The participants for this study were 78 young adult males (mean age = 20.31, SD = 2.43, range = 18–29). There were 39 participants in the stress group and 39 participants in the control group. Participants received either $10 per hour plus $5 per day for travel expenses or course credit as compensation. All participants received a $5 bonus for showing up for the second session. All participants signed consent forms approved by the Georgia Institute of Technology Institutional Review Board.
Participants completed a health questionnaire before their first session to ensure that they did not have any medical conditions that could affect either the results of the study or the individual’s ability to participate in the study. Potential participants who reported any of the following conditions were excluded from the study: Epilepsy, Parkinson’s disease, a history of stroke or seizure, untreated depression, untreated anxiety, Attention Deficit Disorder, Multiple Sclerosis, uncontrolled hyper- or hypo-tension, untreated Diabetes, Sickle Cell Anemia, smoking or other regular use of nicotine, use of beta blockers, alcoholism, and regular use of illegal drugs. Participants were asked when they usually wake up in the morning so that they would not be scheduled to participate within two hours of waking. Endogenous cortisol levels are highest at waking and then decline during the day (Kirschbaum et al., 1993; Rimmele, Meier, Lange, & Born, 2010; Wilhelm, Born, Kudielka, Schlotz, & Wust, 2007). This decline is rapid in the morning and slower in the afternoon (Het, Ramlow, & Wolf, 2005; Maheu et al., 2005). Because we expected an acute increase in cortisol as a result of the stress manipulation, we wanted endogenous cortisol levels to be relatively low at the start of the experiment. For this reason, we ran all of the participants in the afternoon (1 pm to 6 pm).
At the beginning of each experimental session, participants were asked when they woke up that day, if they did any physical activity, and if they had any caffeine or nicotine. Participants who said they did some physical activity were asked what time they did that activity and for how long they did that activity. Participants who said they had caffeine or nicotine were asked when they had the caffeine or nicotine. Participants were informed that they were to refrain from physical exercise, caffeine, and nicotine within two hours of an experimental session when they were scheduled. Participants who did not comply with these instructions were not allowed to complete the study.
2.2. Materials
Stimuli consisted of 450 color photographs from the Nencki Affective Picture System (NAPS) and contained 150 highly arousing negative images, 150 minimally arousing negative images, and 150 neutral images (Marchewka, Zurawski, Jednorog, & Grabowska, 2013). Stimuli were chosen based on their provided normative ratings for emotional arousal and valence assessed with a computerized version of the Self-Assessment Manikin (SAM) Scale, each ranging from 1 to 9 (1 = very negative, 9 = very positive; 1 = relaxed, 9 = aroused). The NAPS images consisted of photographs grouped into 5 categories based on their content: animals, faces, landscapes, objects, and people. The average valence and arousal ratings for these images can be found in Table 1. Neutral images had higher valence ratings than the minimally arousing negative images [t(298) = 55.559, p < 0.001] and the highly arousing negative images [t(298) = 31.977, p < 0.001], the latter of which did not differ [t(298) = 0.580, p = 0.563]. The highly arousing negative images had higher arousal ratings than both minimally arousing negative images [t(298) = 25.073, p < 0.001] and neutral images [t(298) = 29.369, p < 0.001]. The minimally arousing negative images had higher arousal ratings than the neutral images [t(298) = 12.483, p < 0.001].
Table 1.
Normed valence and arousal ratings for stimuli.
| Valence | Arousal | |
|---|---|---|
| Neutral | 6.489 (0.273) | 4.547 (0.745) |
| Minimally arousing negative | 3.801 (0.526)a | 5.416 (0.414)a |
| Highly arousing negative | 3.747 (1.014)a | 6.503 (0.333)a,b |
Note: Standard deviations in parentheses.
Neutral > Negative.
Highly Arousing Negative > Minimally Arousing Negative.
Salivary cortisol levels were assessed using the Salimetrics Oral Swab (SOS) and were sent to Salimetrics for immunoassay. The collection and storage of saliva samples were done in accordance with the requirements for safe handling of biological materials from the Georgia Institute of Technology Environmental Health and Safety Office. Salimetrics provided this information about the immunoassay procedure and reliability: Saliva samples were assayed in duplicate to determine cortisol levels using a highly sensitive enzyme immunoassay (Salimetrics, State College, PA). The test used 25 μL of saliva per determination, has a lower limit of sensitivity of 0.007 μg/dL, standard curve range from 0.012 μg/dL to 3.0 μg/dL, an average intra-assay coefficient of variation of 4.6% and an average inter-assay coefficient of variation of 5.9%. Method accuracy determined by spike and recovery averaged 105.3% and linearity determined by serial dilution averaged 105.3%. Values from matched serum and saliva samples show the expected strong linear relationship, r(47) = 0.91, p < 0.0001.
2.3. Procedure
There were 2 lab sessions 48 h apart. The use of this memory delay is consistent with the literature and ensured that the stress only occurs during consolidation and did not carry over into retrieval (for review, Wolf, 2008). Participants were asked to refrain from caffeine and nicotine for the 2 h prior to each session. All sessions took place in the afternoon (between 1 pm and 6 pm) so that basal cortisol levels were relatively low and stable (Het et al., 2005; Kirschbaum et al., 1993; Maheu et al., 2005). Fig. 1a shows the procedure for the experiment.
Fig. 1.
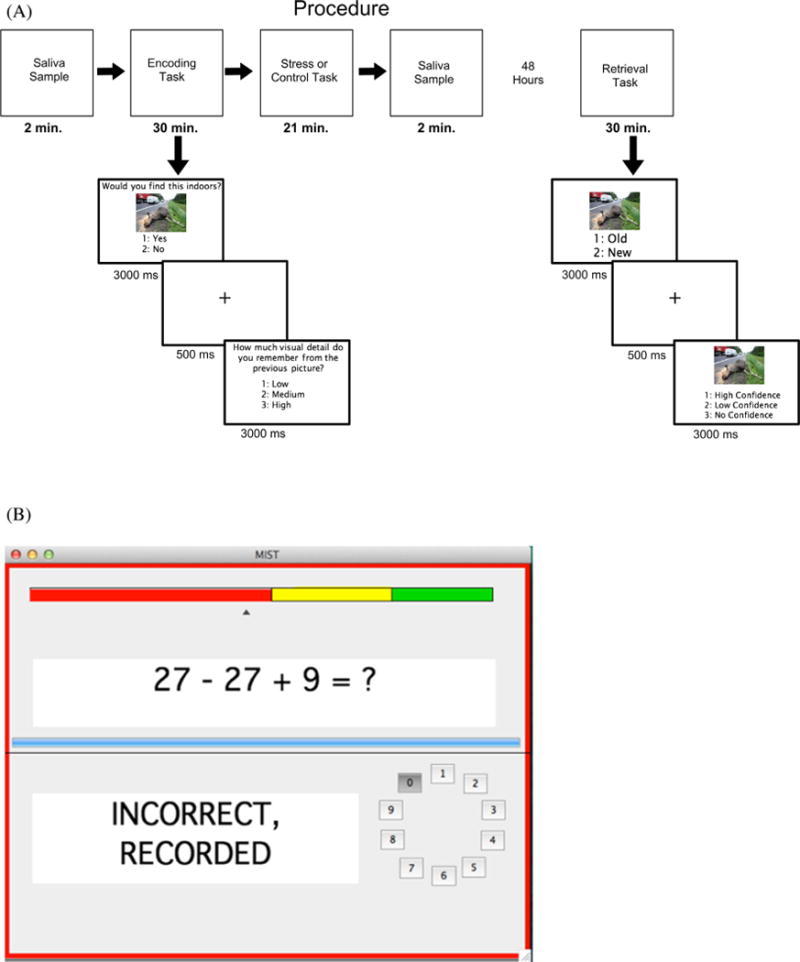
(A) Task design for both encoding and retrieval. Time values represent the mean latency of each task. (B) The participants’ view of the MIST during the stress condition.
2.3.1. Session 1
The first session lasted approximately 1.5 h. Participants first completed a practice encoding task with 10 trials. The first saliva sample was collected immediately following the practice encoding task. The encoding task contained 75 minimally arousing negative images, 75 highly arousing negative images, and 75 neutral images. There were 5 blocks, each with 15 minimally arousing negative images, 15 highly arousing negative images, and 15 neutral images. First, participants saw each image for 3000 ms in the center of the screen with the question “Would you find this indoors?” written above the image. Participants responded with “1” on the number pad for yes and “2” on the number pad for no. These response choices were displayed on the screen below the image. After the image disappeared, participants were asked how much visual detail they remembered from the image. Participants responded with “1” for low visual detail, “2” for medium visual detail, and “3” for high visual detail. These response choices were displayed in the center of the screen for 3000 ms. Participants were not informed that their memory for these images would be tested.
We asked participants to report how much visual detail they remembered during encoding to determine if the level of visual detail of the NAPS stimuli were different between arousal/valence categories. In the International Affective Picture System images (IAPS), neutral stimuli generally consist of objects with minimal detail, while emotional stimuli generally consist of scenes and faces with vivid detail (Lang, Bradley, & Cuthbert, 1995). Previous research has generally used the IAPS photos as stimuli and found that emotional stimuli are better remembered than neutral stimuli. Thus, the memory benefit for emotional stimuli could be due to the visual complexity of the photos rather than the emotional properties of the photos.
We used the Montreal Imaging Stress Task (MIST) to induce acute psychological stress immediately following encoding (Dedovic et al., 2005). The user interface of the MIST is shown in Fig. 1b. Consistent with the administration of the MIST by Dedovic et al. (2009), participants in the stress condition were asked to solve mental arithmetic problems under a restrictive time limit (≤5000 ms). The participant was told that they were being evaluated by the experimenters and that their performance should be in line with the average user. The participant received feedback on their responses (“correct”, “incorrect”, “timeout”) and was able to see how their performance compared to the average user throughout the task on a color bar, with their performance usually in the “red zone” and the average user in the “green zone.” In addition, the participant received verbal negative feedback about their performance from the researcher after each run, in which they were told they were performing poorly and they should be performing at the level of the average user. The program also adjusted to the participant’s performance in order to keep their response rate at 45–55% correct, by either increasing task difficulty or decreasing their time limit. Participants in the control condition were asked to solve the same mental arithmetic problems but without a restrictive time limit (<10,000 ms). The participants did not receive any negative feedback nor were told they were being evaluated or that they had to perform at a certain level. All participants completed three seven-minute runs of either the control condition or the experimental condition of the MIST. The second saliva sample was collected immediately following the completion of the MIST.
2.3.2. Session 2
The second session lasted approximately one hour. Participants completed a practice retrieval task with 10 trials. The retrieval task included the 225 images from the encoding task as well as 225 new images (75 minimally arousing negative, 75 highly arousing negative, and 75 neutral). Retrieval was split into 10 blocks to avoid fatigue. Participants responded to 2 questions for each stimulus. First, participants viewed the image for 3000 ms and indicated if the image was old or new. They responded with “1” for old and “2” for new on the number pad. Participants were instructed to respond “old” if they remembered seeing the image during the first session and “new” if they did not remember seeing the image during the first session. Second, participants viewed the image for 3000 ms and said how confident they were that the image was old or new. Participants responded with “1” for high confidence, “2” for low confidence, and “3” for no confidence. Participants were instructed to respond “high confidence” if they were completely sure of their response, “low confidence” if they were somewhat sure, but not completely sure of their response, and “no confidence” if they were just guessing. A fixation cross was displayed in the center of the screen for 500 ms between each of these questions. For all responses, stimulus duration was fixed such that if the participant did not respond or responded more than once, their response was not included in the analyses. On average this amounted to less than 1% of the trials.
For all statistical tests, the Huynh-Feldt correction was applied where appropriate and is reflected in the p values and the error terms.
3. Results
3.1. Encoding
We analyzed the visual detail ratings from encoding to determine how much visual detail participants reported remembering for each stimulus category. We created a visual detail score by giving a 3 to high visual detail ratings, a 2 to medium visual detail ratings, and a 1 to low visual detail ratings. We averaged this score from each participant for each stimulus category. These average visual detail ratings are shown in Fig. 2.
Fig. 2.
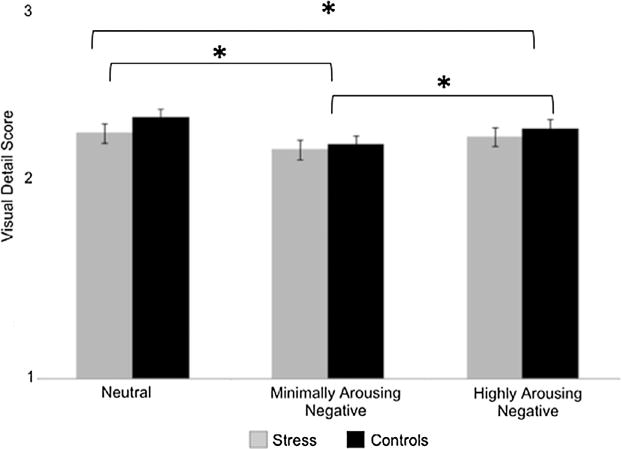
Average visual detail rating for each group at encoding for each stimulus category. Error bars represent the standard error of the mean. Across groups, neutral images were remembered with the most visual detail followed by highly arousing negative images and then minimally arousing negative images.
These data were submitted to a 2 Group (stress, control) ×3 Category (neutral, minimally arousing negative, highly arousing negative) ANOVA. There was a main effect of Category [F(2,152) = 28.315, p < 0.001, η2 = 0.271], no main effect of Group [F(1,76) < 1, η2 = 0.008] and no Category × Group interaction [F(2,152) = 1.691, p = 0.188, η2 = 0.022]. As these detail ratings were made prior to stress induction, we did not expect there to be any group differences in these ratings. Follow-up t-tests indicated that participants reported remembering more visual details from neutral images than from minimally arousing negative images [t(77) = 7.659, p < 0.001] and highly arousing negative images [t(77) = 2.437, p = 0.017]. Participants also reported remembering more visual details from highly arousing negative images than from minimally arousing negative images [t(77) = 5.137, p < 0.001].
3.2. Cortisol
In order to verify that the MIST induced a stress response, we submitted the cortisol levels to a 2 Time Point (Pre-MIST, Post-MIST) × 2 Group (stress, control) ANOVA. We found a main effect of Time Point [F(1,75) = 13.501, p < 0.001, η2 = 0.153], no main effect of Group [F(1,75) = 1.866, p = 0.176, η2 = 0.024] and no Time Point × Group Interaction [F(1,75) < 1, η2 = 0.001]. Follow-up t-tests showed that, across groups, Pre-MIST cortisol levels were higher than Post-MIST cortisol levels [t(77) = 3.865, p < 0.001]. This overall decrease is consistent with the circadian rhythm of endogenous cortisol, which decreases throughout the day.
Consistent with previous stress studies, we separated the stress group into responders and non-responders (Buchanan et al., 2006; Dedovic et al., 2009; Khalili-Mahani et al., 2010; Pruessner et al., 2008). We separated our participants using a tertile split based upon their change in cortisol level following the MIST (Post-MIST Pre - MIST). Participants who had a positive change in cortisol in the top third of the stress group were classified as responders, and participants who had a change in cortisol in the bottom third of the stress group were classified as non-responders. Participants whose change in cortisol was in the middle third were removed from the analyses. There were 13 responders and 13 non-responders. The average change in cortisol for responders, non-responders, and the controls is displayed in Fig. 3. As can be seen in the figure, the change in cortisol in the responders was significantly greater than zero [t(12) = 4.851, p < 0.001], while the change in cortisol in the controls [t(38) = 3.125, p = 0.003] and non-responders [t(12) = 4.443, p = 0.001] were significantly less than zero. As a parallel to the analysis above, we ran a 2 Time Point (Pre-MIST, Post-MIST) × 3 Group (responders, non-responders, controls) ANOVA on the cortisol levels. Unsurprisingly, we observed a Time Point × Group interaction [F(1,62) = 14.172, p < 0.001, η2 = 0.314]. Responders had higher Post-MIST than Pre-MIST cortisol levels [t(12) = 4.851, p < 0.001], while non-responders [t(12) = 4.443, p = 0.001] and controls [t(38) = 3.125, p = 0.003] had higher Pre-MIST than Post-MIST cortisol levels. An independent t-test confirmed that the reduction in cortisol following the MIST was greater for non-responders than controls [t(50) = 2.994, p = 0.004].
Fig. 3.
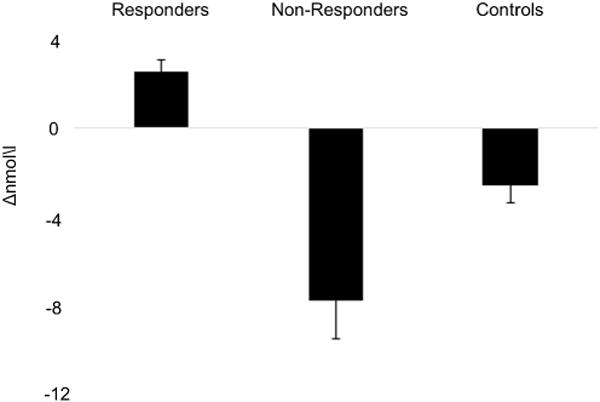
Average change (Post-MIST – Pre-MIST) in cortisol following the MIST. Error bars represent the standard error of the mean.
At the beginning of session 1, participants were asked what time they woke up that morning. We calculated how long the participants had been awake by subtracting the time they woke up from the time session 1 began. On average, responders were awake for 5.86 h (SD = 1.89), non-responders were awake for 5.90 h (SD = 2.39) and the controls were awake for 6.48 h (SD = 1.86). We ran a one-way ANOVA on the responders, the non-responders, and the control participants to determine if there were any differences in how long they had been awake when they came in for the first session. There was no main effect of Group [F(2,62) < 1] suggesting that group differences in cortisol or behavioral measures, discussed below, are not confounded by differences in time awake.
3.3. Recognition memory
Proportions of hits, misses, and false alarms for each of the stimulus categories and groups are shown in Table 2.
Table 2.
Mean proportion of hits, misses, and false alarms for the responders, non-responders, and controls for each stimulus category.
| Responders | Non-responders | Controls | |
|---|---|---|---|
| Highly arousing negative | |||
| Hits | 0.73 (0.05) | 0.73 (0.04) | 0.79 (0.02) |
| Misses | 0.27 (0.05) | 0.27 (0.04) | 0.21 (0.03) |
| FAs | 0.22 (0.03) | 0.18 (0.04) | 0.22 (0.02) |
| Minimally arousing negative | |||
| Hits | 0.66 (0.05) | 0.67 (0.04) | 0.75 (0.03) |
| Misses | 0.34 (0.06) | 0.33 (0.04) | 0.25 (0.03) |
| FAs | 0.20 (0.03) | 0.16 (0.04) | 0.20 (0.02) |
| Neutral | |||
| Hits | 0.67 (0.07) | 0.67 (0.05) | 0.72 (0.03) |
| Misses | 0.34 (0.07) | 0.33 (0.05) | 0.28 (0.03) |
| FAs | 0.19 (0.02) | 0.13 (0.03) | 0.19 (0.02) |
Note: FAs = false alarms; standard error of the means in parentheses.
We first analyzed item memory performance using the Pr discrimination index (hit rate – false alarm rate). The Pr data were submitted to a 3 Group (responders, non-responders, controls) × 3 Category (neutral, minimally arousing negative, highly arousing negative) ANOVA. These estimates are shown in Fig. 4. There was a marginal main effect of Category [F(2,124) = 2.895, p = 0.059, η2 = 0.045]. There was no main effect of Group [F(1,62) < 1, η2 = 0.025] and no interaction [F(4,124) < 1, η2 = 0.018]. Follow-up t-tests indicated that participants had better memory for the highly arousing negative images than both the neutral images [t (64) = 2.216, p = 0.030] and the minimally arousing negative images [t(64) = 2.047, p = 0.045]. There was no difference in memory between the neutral images and the minimally arousing negative images [t(64) < 1].
Fig. 4.
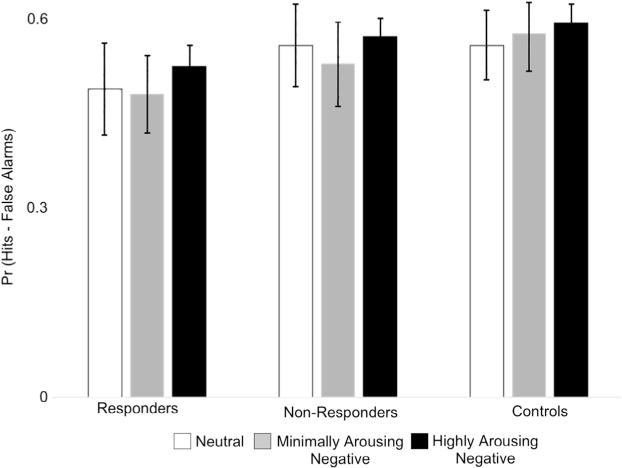
Memory accuracy (Pr) for each group for each stimulus category. Error bars represent the standard error of the mean. Across groups, highly arousing negative images were better remembered than minimally arousing negative images and neutral images.
We then analyzed participants’ confidence ratings for hits. Mean proportions of their high confidence, low confidence and no confidence hits for each stimulus category and group are shown in Table 3.
Table 3.
Mean proportion of high confidence, low confidence and no confidence hits for the responders, non-responders, and controls for each stimulus category.
| Responders | Non-responders | Controls | ||
|---|---|---|---|---|
| Highly arousing negative | High confidence | 0.76 (0.18) | 0.83 (0.12) | 0.86 (0.09) |
| Low confidence | 0.19 (0.15) | 0.14 (0.11) | 0.12 (0.07) | |
| No confidence | 0.05 (0.05) | 0.03 (0.03) | 0.02 (0.03) | |
| Minimally arousing negative | High confidence | 0.73 (0.16) | 0.81 (0.14) | 0.85 (0.11) |
| Low confidence | 0.22 (0.11) | 0.15 (0.12) | 0.14 (0.09) | |
| No confidence | 0.05 (0.06) | 0.04 (0.06) | 0.01 (0.02) | |
| Neutral | High confidence | 0.73 (0.17) | 0.83 (0.15) | 0.85 (0.10) |
| Low confidence | 0.23 (0.15) | 0.14 (0.13) | 0.14 (0.09) | |
| No confidence | 0.04 (0.07) | 0.03 (0.04) | 0.01 (0.02) |
Note: Standard deviation in parentheses.
The data was submitted to a 3 Group (responders, non-responders, controls) × 3 Category (neutral, minimally arousing negative, highly arousing negative) × 3 Confidence (high confidence, low confidence, no confidence) ANOVA. There was a main effect of Confidence [F(2,124) = 710.780, p < 0.001, η2 = 0.920], a Confidence × Group Interaction [F(4,124) = 4.013, p = 0.018, η2 = 0.115], but no other effects [F’s < 2.137, p’s > 0.1, η2’s > 0.02]. Follow-up paired t-tests on the main effect of Confidence revealed that across groups and categories, participants reported more high confidence hits than low [t(64) = 24.489, p < 0.001] or no confidence hits [t(64) = 43.625, p < 0.001] and more low than no confidence hits [t(64) = 11.099, p < 0.001]. Independent t-tests revealed that controls had more high confidence hits than did responders [t(50) = 3.121, p = 0.003], while responders had more low [t(50) = 2.577, p = 0.013] and no confidence [t(50) = 3.332, p = 0.002] hits than controls and that non-responders had more no confidence hits than did controls [t(50) = 2.064, p = 0.044], there were no other significant differences [t’s < 1.426, p’s < 0.657]. Given that there was no effect of category, we collapsed across category for visualization purposes in Fig. 5.
Fig. 5.
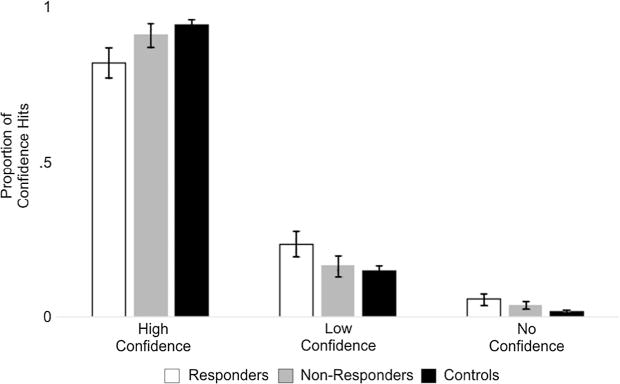
The mean proportion of high confidence, low confidence and no confidence hits for each group. There was a graded pattern among confidence with responders having the highest amount of low and no confidence hits and controls having the highest amount of high confidence hits.
Third, we analyzed whether the visual detail ratings given at encoding affected subsequent memory performance in the responders, non-responders, and controls. We used the hit rate, rather than Pr, for this analysis because we only have the visual detail ratings for items that were presented at encoding. The hit rate data were submitted to a 3 Group (responders, non-responders, controls) × 3 Category (neutral, minimally arousing negative, highly arousing negative) × 3 Visual Detail (high, medium, low) ANOVA. There was a main effect of Visual Detail [F(2,124) = 22.904, p < 0.001, η2 = 0.270]. None of the other effects were significant [all F’s < 1.772, all p’s > 0.318, all η2’s < 0.023]. Follow-up t-tests indicated that participants were more likely to remember items for which they reported high visual detail at encoding than items with medium visual detail [t(64) = 6.149, p < 0.001] or low visual detail [t(64) = 6.170, p < 0.001]. Participants were also more likely to remember medium visual detail items than low visual detail items [t(64) = 4.129, p < 0.001].
4. Discussion
The current study investigated whether mild acute psychological stress during consolidation improves memory performance for emotionally arousing materials. We predicted that stress and arousal would interact such that memory would be greater for highly arousing negative images relative to neutral images. We additionally predicted that this pattern would be greater for stress than control participants. Highly arousing negative images were better remembered relative to minimally arousing negative and neutral images. Contrary to our hypothesis, this pattern was similar across groups. The possible interpretations of this finding and its implications are discussed below.
One important difference between our study and other studies of emotion is that we used a different stimulus database, the Nencki Affective Picture System (NAPS). The International Affective Picture System (IAPS) images, which are commonly used as emotional stimuli, differ in visual complexity across category. Specifically, the emotional images are often visually complex scenes, while the neutral images are often objects (Bradley & Lang, 2007). This difference in visual complexity may contribute to the negative IAPS images being better remembered and with greater detail than neutral IAPS images (Cahill et al., 2003; for review, Kensinger, 2009; Kensinger & Schacter, 2008). This could be a potential confound to any memory results as the visual complexity of the emotional IAPS images may lead to their advantage in later memory performance, rather than the emotional properties of the images, per se. Given that we were using stimuli that are not typically used, we had participants rate the visual detail of the stimuli during encoding to ensure that memory differences between the categories were not confounded by the difference in visual details between them. We did find a small, but reliable difference in the visual detail ratings between the categories. Participants rated the most visual details for neutral images, followed by highly arousing negative images, and then minimally arousing negative images. Subsequent memory accuracy was greatest for stimuli that participants rated as having high visual detail during encoding, but this pattern was similar across the neutral, high, and low arousal negative categories. Thus, in our study, the memory advantage for highly arousing negative stimuli cannot be explained by their greater visual complexity.
Consistent with previous studies, participants, across groups, had better memory performance for the highly arousing negative images than the minimally arousing negative images and neutral images. We also found no difference in memory accuracy between minimally arousing negative and neutral images. Emotional arousal increases activity in the amygdala and hippocampus (Denkova, Dolcos, & Dolcos, 2012; Dolcos, LaBar, & Cabeza, 2004a; Dolcos et al., 2004b). This enhanced activity is suggested to modulate memory consolidation (Dolcos et al., 2004b), resulting in better memory performance for emotionally arousing material relative to neutral (Dolcos, Denkova, & Dolcos, 2012; Dolcos et al., 2004b; Kensinger & Corkin, 2004). These findings add to the body of literature that suggests emotional materials are better remembered than non-emotional materials and that this effect is driven by arousal (Dolcos et al., 2004b; Kensinger & Corkin, 2003, 2004).
We found no difference in memory performance between the stress and the control groups. The literature examining the effect of acute stress on memory performance is mixed, with some studies finding improvements and some finding impairments (for review, LaBar & Cabeza, 2006; McGaugh, 2000; Shields et al., 2016). Existing literature suggests that the effect of acute stress on memory depends on the three factors previously discussed in the introduction: the timing of the stressor (for review, Wolf, 2009), the type of material being encoded (Cahill & Alkire, 2003; Cahill et al., 2003; Smeets et al., 2008) and the strength of the stressor (Akirav et al., 2004; Diamond et al., 1992; Sandi et al., 1997). In relationship to timing, we induced stress immediately after encoding. Given that numerous human studies have consistently found memory benefits when stress was induced during consolidation (Beckner et al., 2006; Cahill et al., 2003; Preuss & Wolf, 2009; Smeets et al., 2008) it is unlikely that the time of the stress induction is the reason for the negative result here.
In relationship to the type of material encoded, previous studies have found various stressors generally improve memory performance for emotionally arousing stimuli, specifically negative stimuli, relative to neutral stimuli (Cahill & Alkire, 2003; Cahill et al., 2003; Smeets et al., 2008). As previously discussed we found that across groups, participants remembered more highly arousing negative images than minimally arousing negative or neutral images. Animal research has demonstrated that acute stress modulates activity in the BLA, an area important for memory consolidation (for review, McGaugh & Roozendaal, 2002; Wolf, 2008). This stress response is predicted to result in highly arousing images being better remembered than minimally arousing negative or neutral images. One possible explanation for the lack of effect of stress on memory performance is that we used an item recognition task but did not assess recollection, specifically. Recollection, to a greater extent than item recognition, is dependent on the hippocampus (for review, Diana, Yonelinas, & Ranganath, 2007; Eichenbaum, Yonelinas, & Ranganath, 2007; Ranganath, 2010). Stress elevates levels of glucocorticoids that in turn induce LTP in the hippocampus, which is rich in glucocorticoid receptors (McEwen & Sapolsky, 1995). Consequently, stress may be more likely to affect recollection than item recognition or familiarity (McCullough et al., 2015). However, there is abundant evidence that the hippocampus also contributes to item recognition for emotional images (Keightley, Chiew, Anderson, & Grady, 2011; Mackiewicz, Sarinopoulos, Cleven, & Nitschke, 2006; Mickley Steinmetz, Schmidt, Zucker, & Kensinger, 2012; Strange & Dolan, 2004). In addition, previous stress studies have shown that psychological and physical stressors affect item recognitions (Keightley et al., 2011; Mackiewicz et al., 2006; Mickley Steinmetz et al., 2012; Strange & Dolan, 2004).
We feel the most likely explanation for the lack of effect of stress on memory performance is the minimal level of stress induced by the MIST. The MIST typically increases cortisol 50–100% above baseline, whereas the TSST consistently produces increases in cortisol about 200–400% above baseline (Dedovic et al., 2005; Kirschbaum et al., 1993). In our study, we found that cortisol decreased 7.73% below baseline rather than increased, suggesting that the level of stress, as measured by cortisol, was very low. Thus, the already minimal level of stress produced following the MIST was even lower in our sample. A moderate level of stress has been found to be optimal for improved memory performance, with high levels of stress inducing an abnormal level of glucocorticoids that impair induced LTP and memory performance (for review, de Kloet et al., 2005). It is possible that mild acute stress does not elevate levels of glucocorticoids enough to induce LTP and improve memory performance. It is important to note that we chose to induce mild acute stress rather than moderate acute stress, which is commonly used in the literature, due to its real world implications. Mild acute stress is more of what we experience on a daily basis (i.e. an argument or car issues) and these daily stressors have been found to increase cortisol levels (Stawski, Cichy, Piazza, & Almeida, 2013). Yet, to the best of our knowledge, the effect these daily stressors have on memory performance and other areas of cognition are unknown.
As is often done in stress studies (Buchanan et al., 2006; Dedovic et al., 2009; Khalili-Mahani et al., 2010; Pruessner et al., 2008), we separated our stress group into responders and non-responders. Studies using the MIST have typically found 50% of the stress group participants to be responders, showing increases in cortisol levels (Dedovic et al., 2005; Pruessner et al., 2008, 2010). Thirty-three percent of the stress group were responders in our study. What might be the reason for the relatively low cortisol response in the present study? One possibility might be related to the time of the day at which cortisol was measured, which was usually around midday. Endogenous cortisol levels are highest after an individual first wakes up and decline throughout the rest of the day (Het et al., 2005; Maheu et al., 2005). Thus, MIST-induced increases in cortisol may have been too small to detect in the context of the larger circadian decline in cortisol. Another related possibility for the relatively low cortisol response is when we measured salivary cortisol levels following the MIST. Previous literature suggests that stress-induced cortisol responses are expected to be maximal 20 min after the stressor (Schwabe, Bohringer, & Wolf, 2009; Schwabe & Wolf, 2009; Schwabe et al., 2008). Thus, it is possible that we missed the maximal stress-induced cortisol response, as we took saliva samples directly after MIST completion. However, the MIST is a long duration stressor at roughly 21 min relative to other stressors such as the CPT, which takes approximately 3 min. Still, it is possible that there could have been a larger number of responders had we waited longer to measure salivary cortisol. One final possibility for the relatively low stress response in our participants is that they are unusually proficient in math. Georgia Tech undergraduates score in the upper 95th percentile of the SAT, and most students major in STEM disciplines (“Georgia Tech 2016 Freshman Profile,” 2016). The math-based MIST may not have induced as large a stress response in our participants as might be seen in a more academically diverse sample. A future comparison of different stressors and their impact on physiological stress and memory in a more diverse sample or in a population study would be very useful.
With regard to memory accuracy, we found no differences between responders, non-responders, or controls. As discussed above, there was a relatively low number of responders in this study compared to other studies using the MIST. Consequently, one reason for the null finding could be related to small sample size. A non-mutually exclusive explanation for the lack of effect of stress on memory performance may be related to the use of the MIST in a behavioral setting. To the best of our knowledge, this is the first study to use the MIST as a stressor outside of an MRI scanner. It is possible that the MRI environment and the stress associated with it are needed in order for the MIST to induce a sizeable increase in cortisol. Previous studies have found that the MRI environment even in the absence of any explicit stressor directly increases cortisol levels (Peters, Cleare, Papadopoulos, & Fu, 2011; Tessner, Walker, Hochman, & Hamann, 2006). One study, in particular, found that when the researchers administered the CPT in the MRI scanner, the increase in cortisol in participants was greater than when they administered the CPT outside of the scanner (McCullough et al., 2015). Regardless of the reasons why the MIST resulted in a minimal level of cortisol change here, the current results are consistent with findings suggesting that mild levels of cortisol do not affect memory performance (Andreano & Cahill, 2006).
One performance difference we observed between stress groups was a graded pattern of memory confidence, with the responders having the lowest confidence when they were correct, and participants in the control group having the highest confidence when they were correct. There is some evidence that responders have lower self-esteem and locus of control (i.e. the extent to which people believe they have power over events in their lives) than non-responders. This can affect their stress perception and make them prone to larger increases in cortisol (Pruessner et al., 2005, 2008). If responders do in fact have lower self-esteem, it is possible that they were less confident because they perceived the MIST to be more stressful. It is important to note that we only analyzed confidence for hits as we had too few trials for misses and false alarms. Thus, we cannot conclude that low confidence is a trait-like phenomenon in responders and might be specific to successful recognition memory only.
5. Conclusion
Most stress studies have used moderate stressors to investigate the effect of stress on performance. However, we chose to induce mild stress because it is more like what we experience on a daily basis. We found that mild acute stress does not affect memory accuracy. This suggests that these daily stressors humans experience may not have detrimental effects on memory, at least in healthy younger adults. Although stress had no effect on recognition accuracy, the reduction in memory confidence for responders is consistent with the idea that greater cortisol reactivity may result, in part, from lower self-esteem and greater perceived stress. Finally, these results add to the body of literature suggesting that the memory advantage of emotional events is due to arousal rather than valence. Future work should aim to determine the effects of daily mild stressors on other areas of cognition across the lifespan.
Acknowledgments
We would like to thank all of our research participants and research assistants for their time and contribution to the study. We also thank Dr. Dedovic and Dr. Pruessner for providing us with the Montreal Imaging Stress Task.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Abercrombie HC, Kalin NH, Thurow ME, Rosenkranz MA, Davidson RJ. Cortisol variation in humans affects memory for emotionally laden and neutral information. Behavioral Neuroscience. 2003;117(3):505–516. doi: 10.1037/0735-7044.117.3.505. [DOI] [PubMed] [Google Scholar]
- Abercrombie HC, Speck NS, Monticelli RM. Endogenous cortisol elevations are related to memory facilitation only in individuals who are emotionally aroused. Psychoneuroendocrinology. 2006;31(2):187–196. doi: 10.1016/j.psyneuen.2005.06.008. http://dx.doi.org/10.1016/j.psyneuen.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Akirav I, Kozenicky M, Tal D, Sandi C, Venero C, Richter-Levin G. A facilitative role for corticosterone in the acquisition of a spatial task under moderate stress. Learning & Memory. 2004;11(2):188–195. doi: 10.1101/lm.61704. http://dx.doi.org/10.1101/lm.61704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Glucocorticoid release and memory consolidation in men and women. Psychological Science. 2006;17(6):466–470. doi: 10.1111/j.1467-9280.2006.01729.x. http://dx.doi.org/10.1111/j.1467-9280.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- Beckner VE, Tucker DM, Delville Y, Mohr DC. Stress facilitates consolidation of verbal memory for a film but does not affect retrieval. Behavioral Neuroscience. 2006;120(3):518–527. doi: 10.1037/0735-7044.120.3.518. http://dx.doi.org/10.1037/0735-7044.120.3.518. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ, editors. The International Affective Picture System (IAPS) in the study of emotion and attention. Oxford University Press; 2007. [Google Scholar]
- Buchanan TW, Lovallo WR. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology. 2001;26(3):307–317. doi: 10.1016/s0306-4530(00)00058-5. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Tranel D, Adolphs R. Impaired memory retrieval correlates with individual differences in cortisol response but not autonomic response. Learning & Memory. 2006;13(3):382–387. doi: 10.1101/lm.206306. http://dx.doi.org/10.1101/lm.206306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Alkire MT. Epinephrine enhancement of human memory consolidation: Interaction with arousal at encoding. Neurobiology of Learning and Memory. 2003;79(2):194–198. doi: 10.1016/s1074-7427(02)00036-9. [DOI] [PubMed] [Google Scholar]
- Cahill L, Gorski L, Le K. Enhanced human memory consolidation with post-learning stress: Interaction with the degree of arousal at encoding. Learning & Memory. 2003;10(4):270–274. doi: 10.1101/lm.62403. http://dx.doi.org/10.1101/lm.62403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: From adaptation to disease. Nature Reviews Neuroscience. 2005;6(6):463–475. doi: 10.1038/nrn1683. http://dx.doi.org/10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Aerni A, Roozendaal B. Preventive effect of beta-adrenoceptor blockade on glucocorticoid-induced memory retrieval deficits. American Journal of Psychiatry. 2007;164(6):967–969. doi: 10.1176/ajp.2007.164.6.967. http://dx.doi.org/10.1176/ajp.2007.164.6.967. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Henke K, Aerni A, Treyer V, McGaugh JL, Berthold T, Hock C. Glucocorticoid-induced impairment of declarative memory retrieval is associated with reduced blood flow in the medial temporal lobe. European Journal of Neuroscience. 2003;17(6):1296–1302. doi: 10.1046/j.1460-9568.2003.02542.x. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Roozendaal B, Nitsch RM, McGaugh JL, Hock C. Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nature Neuroscience. 2000;3(4):313–314. doi: 10.1038/73873. http://dx.doi.org/10.1038/73873. [DOI] [PubMed] [Google Scholar]
- Dedovic K, Renwick R, Mahani NK, Engert V, Lupien SJ, Pruessner JC. The Montreal Imaging Stress Task: Using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. Journal of Psychiatry and Neuroscience. 2005;30(5):319–325. [PMC free article] [PubMed] [Google Scholar]
- Dedovic K, Rexroth M, Wolff E, Duchesne A, Scherling C, Beaudry T, Pruessner JC. Neural correlates of processing stressful information: An event-related fMRI study. Brain Research. 2009;1293:49–60. doi: 10.1016/j.brainres.2009.06.044. http://dx.doi.org/10.1016/j.brainres.2009.06.044. [DOI] [PubMed] [Google Scholar]
- Denkova E, Dolcos S, Dolcos F. Reliving emotional personal memories: Affective biases linked to personality and sex-related differences. Emotion. 2012;12(3):515–528. doi: 10.1037/a0026809. http://dx.doi.org/10.1037/a0026809. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Bennett MC, Fleshner M, Rose GM. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus. 1992;2(4):421–430. doi: 10.1002/hipo.450020409. http://dx.doi.org/10.1002/hipo.450020409. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: A three-component model. Trends in Cognitive Sciences. 2007;11(9):379–386. doi: 10.1016/j.tics.2007.08.001. http://dx.doi.org/10.1016/j.tics.2007.08.001. S1364-6613(07)00187-8[pii] [DOI] [PubMed] [Google Scholar]
- Dolcos F, Denkova E, Dolcos S. Neural correlates of emotional memories: A review of evidence from brain imaging studies. Psychologia. 2012;55(2):80–111. [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: An event-related fMRI study. Neuroimage. 2004a;23(1):64–74. doi: 10.1016/j.neuroimage.2004.05.015. http://dx.doi.org/10.1016/j.neuroimage.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004b;42(5):855–863. doi: 10.1016/s0896-6273(04)00289-2. S0896627304002892 [pii] [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. http://dx.doi.org/10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgia Tech. Freshman Profile 2016. 2016 Retrieved from < http://admission.gatech.edu/images/pdf/Freshman_Profile_2016.pdf>.
- Het S, Ramlow G, Wolf OT. A meta-analytic review of the effects of acute cortisol administration on human memory. Psychoneuroendocrinology. 2005;30(8):771–784. doi: 10.1016/j.psyneuen.2005.03.005. http://dx.doi.org/10.1016/j.psyneuen.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31(2):151–178. doi: 10.1016/j.psyneuen.2005.07.002. http://dx.doi.org/10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Keightley ML, Chiew KS, Anderson JA, Grady CL. Neural correlates of recognition memory for emotional faces and scenes. Social Cognitive and Affective Neuroscience. 2011;6(1):24–37. doi: 10.1093/scan/nsq003. http://dx.doi.org/10.1093/scan/nsq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA. Remembering the details: Effects of emotion. Emotion Review. 2009;1(2):99–113. doi: 10.1177/1754073908100432. http://dx.doi.org/10.1177/1754073908100432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Corkin S. Memory enhancement for emotional words: Are emotional words more vividly remembered than neutral words? Memory & Cognition. 2003;31(8):1169–1180. doi: 10.3758/bf03195800. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Corkin S. Two routes to emotional memory: Distinct neural processes for valence and arousal. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(9):3310–3315. doi: 10.1073/pnas.0306408101. http://dx.doi.org/10.1073/pnas.0306408101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Retrieving accurate and distorted memories: Neuroimaging evidence for effects of emotion. Neuroimage. 2005;27(1):167–177. doi: 10.1016/j.neuroimage.2005.03.038. http://dx.doi.org/10.1016/j.neuroimage.2005.03.038. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Neural processes supporting young and older adults’ emotional memories. Journal of Cognitive Neuroscience. 2008;20(7):1161–1173. doi: 10.1162/jocn.2008.20080. http://dx.doi.org/10.1162/jocn.2008.20080. [DOI] [PubMed] [Google Scholar]
- Khalili-Mahani N, Dedovic K, Engert V, Pruessner M, Pruessner JC. Hippocampal activation during a cognitive task is associated with subsequent neuroendocrine and cognitive responses to psychological stress. Hippocampus. 2010;20(2):323–334. doi: 10.1002/hipo.20623. http://dx.doi.org/10.1002/hipo.20623. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’ – A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. 119004. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: A review. Biological Psychology. 2005;69(1):113–132. doi: 10.1016/j.biopsycho.2004.11.009. http://dx.doi.org/10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Kuhlmann S, Piel M, Wolf OT. Impaired memory retrieval after psychosocial stress in healthy young men. Journal of Neuroscience. 2005;25(11):2977–2982. doi: 10.1523/JNEUROSCI.5139-04.2005. http://dx.doi.org/10.1523/JNEUROSCI.5139-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nature Reviews Neuroscience. 2006;7(1):54–64. doi: 10.1038/nrn1825. http://dx.doi.org/10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Phelps EA. Arousal-mediated memory consolidation: Role of the medial temporal lobe in humans. Psychological Science. 1998;9:490–493. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Pictures System (IAPS): Technical manual and affective ratings. Gainesville: University of Florida Center for Research in Psychophysiology; 1995. [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30(3):261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Mackiewicz KL, Sarinopoulos I, Cleven KL, Nitschke JB. The effect of anticipation and the specificity of sex differences for amygdala and hippocampus function in emotional memory. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(38):14200–14205. doi: 10.1073/pnas.0601648103. http://dx.doi.org/10.1073/pnas.0601648103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheu FS, Collicutt P, Kornik R, Moszkowski R, Lupien SJ. The perfect time to be stressed: A differential modulation of human memory by stress applied in the morning or in the afternoon. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2005;29(8):1281–1288. doi: 10.1016/j.pnpbp.2005.08.012. http://dx.doi.org/10.1016/j.pnpbp.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Maheu FS, Joober R, Beaulieu S, Lupien SJ. Differential effects of adrenergic and corticosteroid hormonal systems on human short- and long-term declarative memory for emotionally arousing material. Behavioral Neuroscience. 2004;118(2):420–428. doi: 10.1037/0735-7044.118.2.420. http://dx.doi.org/10.1037/0735-7044.118.2.420. [DOI] [PubMed] [Google Scholar]
- Marchewka A, Zurawski L, Jednorog K, Grabowska A. The Nencki Affective Picture System (NAPS): Introduction to a novel, standardized, wide-range, high-quality, realistic picture database. Behavior Research Methods. 2013 doi: 10.3758/s13428-013-0379-1. http://dx.doi.org/10.3758/s13428-013-0379-1. [DOI] [PMC free article] [PubMed]
- McCullough AM, Ritchey M, Ranganath C, Yonelinas A. Differential effects of stress-induced cortisol responses on recollection and familiarity-based recognition memory. Neurobiology of Learning and Memory. 2015;123:1–10. doi: 10.1016/j.nlm.2015.04.007. http://dx.doi.org/10.1016/j.nlm.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators: Central role of the brain. Progress in Brain Research. 2000;122:25–34. doi: 10.1016/s0079-6123(08)62128-7. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Sapolsky RM. Stress and cognitive function. Current Opinion in Neurobiology. 1995;5(2):205–216. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory – A century of consolidation. Science. 2000;287(5451):248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Current Opinion in Neurobiology. 2002;12(2):205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- Mickley Steinmetz KR, Schmidt K, Zucker HR, Kensinger EA. The effect of emotional arousal and retention delay on subsequent-memory effects. Cognitive Neuroscience. 2012;3(3–4):150–159. doi: 10.1080/17588928.2012.677421. http://dx.doi.org/10.1080/17588928.2012.677421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielson KA, Powless M. Positive and negative sources of emotional arousal enhance long-term word-list retention when induced as long as 30 min after learning. Neurobiology of Learning and Memory. 2007;88(1):40–47. doi: 10.1016/j.nlm.2007.03.005. http://dx.doi.org/10.1016/j.nlm.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Oei NY, Elzinga BM, Wolf OT, de Ruiter MB, Damoiseaux JS, Kuijer JP, Rombouts SA. Glucocorticoids decrease hippocampal and prefrontal activation during declarative memory retrieval in young men. Brain Imaging and Behavior. 2007;1(1–2):31–41. doi: 10.1007/s11682-007-9003-2. http://dx.doi.org/10.1007/s11682-007-9003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S, Cleare AJ, Papadopoulos A, Fu CH. Cortisol responses to serial MRI scans in healthy adults and in depression. Psychoneuroendocrinology. 2011;36(5):737–741. doi: 10.1016/j.psyneuen.2010.10.009. http://dx.doi.org/10.1016/j.psyneuen.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Preuss D, Wolf OT. Post-learning psychosocial stress enhances consolidation of neutral stimuli. Neurobiology of Learning and Memory. 2009;92(3):318–326. doi: 10.1016/j.nlm.2009.03.009. http://dx.doi.org/10.1016/j.nlm.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Baldwin MW, Dedovic K, Renwick R, Mahani NK, Lord C, Lupien S. Self-esteem, locus of control, hippocampal volume, and cortisol regulation in young and old adulthood. Neuroimage. 2005;28(4):815–826. doi: 10.1016/j.neuroimage.2005.06.014. http://dx.doi.org/10.1016/j.neuroimage.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C, Lupien S. Deactivation of the limbic system during acute psychosocial stress: Evidence from positron emission tomography and functional magnetic resonance imaging studies. Biological Psychiatry. 2008;63(2):234–240. doi: 10.1016/j.biopsych.2007.04.041. http://dx.doi.org/10.1016/j.biopsych.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Pruessner M, Lord C, Buss C, Collins L, Lupien SJ. Stress regulation in the central nervous system: Evidence from structural and functional neuroimaging studies in human populations – 2008 Curt Richter Award Winner. Psychoneuroendocrinology. 2010;35(1):179–191. doi: 10.1016/j.psyneuen.2009.02.016. http://dx.doi.org/10.1016/j.psyneuen.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Ranganath C. A unified framework for the functional organization of the medial temporal lobes and the phenomenology of episodic memory. Hippocampus. 2010;20(11):1263–1290. doi: 10.1002/hipo.20852. http://dx.doi.org/10.1002/hipo.20852. [DOI] [PubMed] [Google Scholar]
- Rimmele U, Meier F, Lange T, Born J. Suppressing the morning rise in cortisol impairs free recall. Learning & Memory. 2010;17(4):186–190. doi: 10.1101/lm.1728510. http://dx.doi.org/10.1101/lm.1728510. [DOI] [PubMed] [Google Scholar]
- Russell JA. A circumplex model of affect. Journal of Personality and Social Psychology. 1980;39(6):1161–1178. http://dx.doi.org/10.1037/H0077714. [Google Scholar]
- Sandi C, Loscertales M, Guaza C. Experience-dependent facilitating effect of corticosterone on spatial memory formation in the water maze. European Journal of Neuroscience. 1997;9(4):637–642. doi: 10.1111/j.1460-9568.1997.tb01412.x. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Bohringer A, Chatterjee M, Schachinger H. Effects of pre-learning stress on memory for neutral, positive and negative words: Different roles of cortisol and autonomic arousal. Neurobiology of Learning and Memory. 2008;90(1):44–53. doi: 10.1016/j.nlm.2008.02.002. http://dx.doi.org/10.1016/j.nlm.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Bohringer A, Wolf OT. Stress disrupts context-dependent memory. Learning & Memory. 2009;16(2):110–113. doi: 10.1101/lm.1257509. http://dx.doi.org/10.1101/lm.1257509. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Romer S, Richter S, Dockendorf S, Bilak B, Schachinger H. Stress effects on declarative memory retrieval are blocked by a beta-adrenoceptor antagonist in humans. Psychoneuroendocrinology. 2009;34(3):446–454. doi: 10.1016/j.psyneuen.2008.10.009. http://dx.doi.org/10.1016/j.psyneuen.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT. Stress prompts habit behavior in humans. Journal of Neuroscience. 2009;29(22):7191–7198. doi: 10.1523/JNEUROSCI.0979-09.2009. http://dx.doi.org/10.1523/JNEUROSCI.0979-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT. Learning under stress impairs memory formation. Neurobiology of Learning and Memory. 2010;93(2):183–188. doi: 10.1016/j.nlm.2009.09.009. http://dx.doi.org/10.1016/j.nlm.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Sharot T, Phelps EA. How arousal modulates memory: Disentangling the effects of attention and retention. Cognitive, Affective & Behavioral Neuroscience. 2004;4(3):294–306. doi: 10.3758/cabn.4.3.294. [DOI] [PubMed] [Google Scholar]
- Shields GS, Sazma MA, McCullough AM, Yonelinas AP. The effects of acute stress on episodic memory: A meta-analysis and integrative review. Psychological Bulletin. 2017;143(6):636–675. doi: 10.1037/bul0000100. http://dx.doi.org/10.1037/bul0000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Sazma MA, Yonelinas AP. The effects of acute stress on core executive functions: A meta-analysis and comparison with cortisol. Neuroscience and Biobehavioral Reviews. 2016;68:651–668. doi: 10.1016/j.neubiorev.2016.06.038. http://dx.doi.org/10.1016/j.neubiorev.2016.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets T, Otgaar H, Candel I, Wolf OT. True or false? Memory is differentially affected by stress-induced cortisol elevations and sympathetic activity at consolidation and retrieval. Psychoneuroendocrinology. 2008;33(10):1378–1386. doi: 10.1016/j.psyneuen.2008.07.009. http://dx.doi.org/10.1016/j.psyneuen.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Stawski RS, Cichy KE, Piazza JR, Almeida DM. Associations among daily stressors and salivary cortisol: Findings from the National Study of Daily Experiences. Psychoneuroendocrinology. 2013;38(11):2654–2665. doi: 10.1016/j.psyneuen.2013.06.023. http://dx.doi.org/10.1016/j.psyneuen.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Dolan RJ. Beta-adrenergic modulation of emotional memory-evoked human amygdala and hippocampal responses. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(31):11454–11458. doi: 10.1073/pnas.0404282101. http://dx.doi.org/10.1073/pnas.0404282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmi D, Ziegler M, Hawksworth J, Lalani S, Herman CP, Moscovitch M. Emotional stimuli exert parallel effects on attention and memory. Cognition and Emotion. 2013;27(3):530–538. doi: 10.1080/02699931.2012.722527. http://dx.doi.org/10.1080/02699931.2012.722527. [DOI] [PubMed] [Google Scholar]
- Tessner KD, Walker EF, Hochman K, Hamann S. Cortisol responses of healthy volunteers undergoing magnetic resonance imaging. Human Brain Mapping. 2006;27(11):889–895. doi: 10.1002/hbm.20229. http://dx.doi.org/10.1002/hbm.20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm I, Born J, Kudielka BM, Schlotz W, Wust S. Is the cortisol awakening rise a response to awakening? Psychoneuroendocrinology. 2007;32(4):358–366. doi: 10.1016/j.psyneuen.2007.01.008. http://dx.doi.org/10.1016/j.psyneuen.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Wolf OT. The influence of stress hormones on emotional memory: Relevance for psychopathology. Acta Psychologica (Amst) 2008;127(3):513–531. doi: 10.1016/j.actpsy.2007.08.002. http://dx.doi.org/10.1016/j.actpsy.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Wolf OT. Stress and memory in humans: Twelve years of progress? Brain Research. 2009;1293:142–154. doi: 10.1016/j.brainres.2009.04.013. http://dx.doi.org/10.1016/j.brainres.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit-formation. Journal of Comparative Neurology and Psychology. 1908;18(5):459–482. http://dx.doi.org/10.1002/cne.920180503. [Google Scholar]


