Abstract
Despite advances in behavioral and pharmacological treatment for tobacco use and dependence, quit rates remain suboptimal. Increasing physical activity has shown some promise as a strategy for improving cessation outcomes. However, initial efficacy studies focused on intensive, highly structured exercise programs that may not be applicable to the general population of smokers. We describe the rationale and study design and report baseline participant characteristics from the Lifestyle Enhancement Program (LEAP), a two-group, randomized controlled trial. Adult smokers who engaged in low levels of leisure time physical activity were randomly assigned to treatment conditions consisting of an individualized physical activity intervention delivered by health fitness instructors in community-based exercise facilities or an equal contact wellness control. All participants received standard cognitive behavioral smoking cessation counseling combined with nicotine replacement therapy. The primary outcomes are seven-day point prevalence abstinence at seven weeks, six- and 12 months. Secondary outcomes include self-reported physical activity, dietary intake, body mass index, waist circumference, percent body fat, and nicotine withdrawal symptoms. Participants consist of 392 sedentary smokers (mean [standard deviation] age = 44.6 [10.2] = years; 62% female; 31% African American). Results reported here provide information regarding experiences recruiting smokers willing to change multiple health behaviors including smoking and physical activity.
Keywords: Smoking cessation, Physical activity, Randomized controlled trial
1. Introduction
Despite notable reductions in cigarette use, 15% of the U.S. adult population continues to smoke [1]. Empirically-supported treatments are available but rarely exceed quit rates of 30–35% at one year [2], [3]. Consequently, improving cessation strategies remains an important public health priority.
Some evidence suggests that physical activity and exercise may be beneficial to the quitting process. Physical activity refers to any movement of the body generated by skeletal muscles that leads to energy expenditure [4]. Exercise reflects a subcategory of physical activity that involves planned, structured, and repetitive activities that are engaged in for the specific purpose of improving or maintaining physical fitness [4]. Moderate-to vigorous-intensity physical activity or exercise is associated with several proximal outcomes that predict quitting success, including acute relief from nicotine withdrawal [5], [6], [7], [8], [9], [10], [11], [12], [12] and greater quitting self-efficacy [13], [14]. Exercise also attenuates post-cessation weight gain [15], a common concern among smokers [16]. Most importantly, physical activity and exercise may enhance cessation. Four prior studies [17], [18], [19], [20] demonstrated higher end-of-treatment cessation rates among smokers assigned to a physical activity or exercise intervention compared to control; however, only one study provided evidence that exercise improved long-term (12 month) abstinence [19].
Prior trials [8] were hampered by methodological limitations including small sample sizes [17], [18], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], absence of men [17], [18], [19], [25], [27], [29], [30], [31], [32], [33], [34], [35], [36], and inadequate comparison groups. In addition, interventions often suffered from insufficient “dose” of activity, poor adherence to physical activity prescriptions, and lack of sustainable programming. Studies also varied substantially in both cessation treatment (e.g., number of treatment sessions and use of pharmacotherapy) and physical activity parameters (e.g., initiation relative to quit date, intensity, instructional format and location of the exercise) [10]. Thus, the potential for physical activity to enhance cessation, and the most effective approaches to promote physical activity in the context of a quit attempt, remain unclear.
In addition, many trials relied exclusively [18], [19], [22], [25], [26], [34], [37] or primarily [20], [23], [24], [27], [33] on highly structured, supervised exercise at research-based fitness facilities, typically targeting vigorous activities (e.g., stationary bicycling) in a group setting multiple times over several weeks. Although this has allowed for carefully-controlled evaluations of the efficacy of vigorous exercise for smoking cessation, it may not be an optimal strategy to maximize treatment effectiveness in community settings [38]. Additionally, trials generally have not encouraged participants to engage in short bouts of activity outside of supervised exercise sessions as a way to cope with withdrawal symptoms and urges to smoke, although this is likely to be helpful [9]. Programs have also not usually provided the resources or skills to optimally maintain long-term adherence to physical activity goals. Finally, access to exercise resources typically has been offered for a short duration and is terminated once the intervention is completed.
Given the potential to more effectively disseminate physical activity programming as an aid to smoking cessation in community settings [36], [38], LEAP was designed to evaluate whether the efficacy of structured leisure-time moderate-intensity physical activity can be enhanced by using a more flexible treatment approach of longer duration (one year). The physical activity intervention included individual fitness instruction, cognitive-behavioral skills training, and delivery of the physical activity intervention in convenient and accessible community-based facilities (YMCAs). Physical activity programming was integrated as an adjunct to standard smoking cessation treatment (behavioral counseling and nicotine patch) and was compared to a wellness intervention that was matched on contact frequency. Here, we describe the methodological approach, recruitment flow, and baseline sample characteristics.
2. Methods
The trial was registered at www.ClinicalTrials.gov (Identifier NCT00403312). Protocols and consent documents were approved by The University of Memphis and The University of Tennessee Health Science Center Institutional Review Boards and reviewed by an independent Data and Safety Monitoring Board.
2.1. Design
The study is a two-group, randomized controlled trial comparing different adjuncts to standard smoking cessation treatment including a 1) physical activity intervention or 2) frequency matched wellness contacts. The primary outcome is seven-day point prevalence smoking cessation measured at the end of treatment (seven weeks) and both six- and 12 months. Secondary outcomes include changes in physical activity, dietary intake, body mass index (BMI), waist circumference, percent body fat, and nicotine withdrawal symptoms.
2.2. Study participants
Participants include adults 18–65 years of age who smoked at least five cigarettes per day for one or more years and were interested in quitting. To be eligible, individuals were required to be sedentary or engaging in only low levels of leisure-time physical activity for the past six months, defined as ≤ three days per week of 30 min of moderate-intensity leisure-time physical activity (equivalent to brisk walking) and ≤ one day per week of 30 min of vigorous-intensity leisure-time physical activity (equivalent to running), as measured by a brief, two-item screen that was created for the study. Prior to randomization, participants completed a medical screen to ensure they were healthy enough to engage in physical activity (described below). Eligibility and exclusion criteria are presented in Table 1. Smokers were excluded due to inability to understand consent procedures, contraindications to NRT use (known contraindication or sensitivity to nicotine replacement therapy, or currently pregnant, lactating, or intending to become pregnant, recent history of a cardiac event or procedure), history of a serious illness that might limit longevity or ability to participate in the study (e.g., significant renal disease, liver disease, cancer with life expectancy less than one year, or current substance abuse), and any of several health conditions that might be contraindications for initiating a physical activity program such as extremely elevated blood pressure, positive exercise tolerance test, uncontrolled arrhythmia or hyperthyroidism, symptomatic peripheral artery disease, 2nd or 3rd degree AV block on EKG, or congestive heart failure.
Table 1.
Inclusion and exclusion criteria.
| Inclusion criteria |
|
|
| Exclusion criteria |
|
|
|
|
|
|
|
|
|
|
|
|
2.3. Recruitment and screening
Participants were recruited through several traditional strategies commonly used for community-based clinical trials [36]. These included paid advertisements and public service announcements in local newspapers and on radio and television, free university media including telephone “on hold” announcements and stories in employee newsletters, physician referral, and “word of mouth.” Potential participants contacted the project office by telephone to receive information about the study. Individuals interested in participating completed a brief pre-screen by telephone to determine whether they met basic study requirements based on self-reported age, smoking status, physical activity level, health status, plans to remain in the area for the next year, and current pregnancy or plans to attempt pregnancy. Recruitment was initiated in June of 2004 and continued until May of 2007. Enrollment was stopped at the point when there would not be sufficient time in the funding cycle to complete follow-up assessments. The top three sources for recruiting eligible randomized participants were newspaper ads (n = 134), television (n = 71), and word-of-mouth referrals (n = 71).
Those who passed the phone screen were scheduled for the first of two in-person baseline screening visits. Study procedures were explained, informed consent was obtained, and a baseline assessment was completed, which included demographics and several self-report instruments. Because smoking cessation treatment included nicotine replacement therapy (both groups) and one intervention condition involved physical activity, a medical history and physical examination were performed by a study physician at the second screening visit. The examination included assessment of vital signs and an electrocardiogram. A urine pregnancy test was given to any eligible female participant who had the potential to be pregnant. All participants then underwent a maximal, symptom-limited exercise tolerance test (ETT) to screen for occult coronary artery disease. Individuals with positive ETT results [39] (n = 26) were deemed ineligible and were referred to their personal physician for follow-up. Positive ETT results included ≥1 mm ST segment depression or elevation in one or more leads during maximum exercise or during the recovery period, angina pectoris during exercise, arrhythmia, syncope, or an abnormal blood pressure response to exercise such as hypotension or severe hypertension.
2.4. Randomization
Eligible participants next attended a Randomization Visit during which laboratory measures (height, weight, body fat, blood pressure) were collected along with a seven-day physical activity recall [40], which was obtained by interview. Participants were randomly assigned to standard smoking cessation treatment combined with either the physical activity or wellness interventions. Assignments were conducted based on a 1:1 ratio by the study biostatistician, who was not involved in assessment or intervention delivery. Randomization was accomplished using a computer-generated uniform random number sequence. Participants were informed of their group assignment by the project coordinator or health educator.
2.5. Interventions
2.5.1. Overview
Participants in both treatment conditions received behavioral smoking cessation counseling combined with nicotine replacement therapy (NRT) in the form of the transdermal nicotine patch. Sessions were delivered by bachelors-level health and fitness instructors (HFI) with backgrounds in exercise science or health promotion who were cross-trained to deliver both interventions and trained in cognitive-behavioral counseling strategies. The HFIs were supervised weekly by study co-investigators and monitored for quality control by review of audiotaped sessions.
Participants assigned to the physical activity condition received a combined smoking cessation/physical activity intervention which included 16 face-to-face counseling sessions targeting increased physical activity, 11 telephone counseling sessions, and 11 supportive mailings, for a total of 38 intervention contacts. Except for the seven-week and six-month visits, which were conducted in a university setting, all in-person visits occurred at the YMCA. Participants in the wellness condition received the same smoking cessation intervention plus a general wellness program which included eight face-to-face sessions, 12 telephone calls, and 18 follow up mailings, for a total of 38 intervention contacts, all occurring in a university setting. Although the physical activity and wellness interventions were equated with regard to the number of contacts (38), it is important to note that the number of face-to-face contacts differed between conditions (16 versus eight). To the extent that the type of contact may be associated with the success of the intervention, this could impact study outcomes. Face-to-face sessions lasted 60–75 min during the initial four weeks of the program and 60 min subsequently. Phone counseling sessions lasted approximately 20 min.
The face-to-face counseling sessions were conducted on a relapse sensitive schedule and occurred more frequently early in the program with progressively longer intervals between sessions over the course of the year. As shown in the Supplementary Table, early sessions occurred twice weekly (weeks one and two of the program), then weekly (weeks 3–8), bi-monthly (9–12), monthly (weeks 13–18), and bi-monthly (weeks 19–42). The smoking cessation and physical activity/wellness interventions were started simultaneously. The target quit date was scheduled three weeks after the physical activity or wellness intervention began to allow participants to begin mastering one set of behavioral skills before a second set was introduced.
The primary purpose of the telephone contacts was to provide ongoing follow-up in a cost-effective manner by reviewing progress, providing support, and assisting the participant with problem solving and establishing new goals. HFIs used the calls to identify and intervene early on smoking relapse, assess nicotine patch adherence and side effects, provide cognitive-behavioral coping skills, and offer on-going feedback regarding physical activity or wellness goals and to review materials and information delivered during the face-to-face sessions.
Mailings sent to both treatment groups included a quarterly project newsletter as well as pamphlets about smoking cessation topics. Each group also received mailings addressing content specific to their intervention condition. The quarterly newsletter also included general content that was appropriate for both groups.
2.5.2. Theoretical framework
The smoking cessation, physical activity, and wellness interventions were based largely on Social Cognitive Theory (SCT) [41]. Each was designed to enhance self-efficacy by engaging participants in a series of graded success experiences for multiple behavior change. Self-efficacy for physical activity and smoking cessation are highly related, and a change in self-efficacy for one behavior is likely to positively enhance the other [13]. Additional person-level factors predictive of smoking cessation and physical activity include mood, perceived benefits of engaging in the behavior, and barriers to achieving behavioral goals [42], [43], [44], [45], [46].
Social environmental factors central to SCT that have been found to influence the initiation and maintenance of physical activity include social support and access to facilities and other resources [42], [43]. Intervention components designed to target these factors included enlisting community support for physical activity by providing access to YMCA facilities for exercise sessions, modeling of proper physical activity by the HFI, and social support for all targeted behavioral changes through one-on-one counseling and supervised activity with a HFI. Participants were also encouraged to elicit the help of individuals in their social networks.
The Self-Regulation Model of Behavior Change [47], which is derived from SCT, also informed all interventions. Specifically, participants were taught to use self-monitoring, self-evaluation, and self-reinforcement through goal setting, self-talk, and problem solving to enhance their ability to quit smoking, integrate more physical activity into their daily lives, and improve wellness behaviors.
2.5.3. Smoking cessation intervention
The standard smoking cessation intervention provided to both treatment conditions included nicotine replacement therapy and behavioral counseling. Participants received six weeks of transdermal nicotine, commencing on the date of their quit attempt. A gradual dose tapering strategy was used, with the initial patch dose based on baseline cigarette consumption. The behavioral cessation counseling was adapted from interventions developed at the University of Memphis [48], [49], [50] and involved four primary phases: (1) Preparing to quit; (2) Going through the quitting process; (3) Maintaining short-term smoking abstinence; and (4) Relapse prevention and long-term maintenance [51]. The cessation intervention was delivered primarily during four face-to-face sessions, with brief follow-ups occurring during other in-person and telephone sessions. The first smoking cessation session (during Week 3) occurred one week before the scheduled quit date and focused on preparing to quit and managing the first days as a nonsmoker. In addition to quitting rituals and building support, using the “Five A's” (Anticipate [high risk situations], Avoid, Alter, Alternatives, and Active) were emphasized, as well as self-monitoring of cigarette smoking. The second smoking cessation counseling session (Week 4) occurred one week after the quit attempt and involved providing support and addressed both problem solving and coping with negative emotions. A telephone counseling call occurred approximately four days after the quit attempt to provide support during the acute cessation period. The third counseling session occurred during Week 5 and included problem solving, assessment of patch use, and coping with negative cognitions. The fourth smoking cessation session, which occurred at Week 7 (three weeks after the target quit date), included a review of participants' progress, an assessment patch use, and relapse prevention. Additional topics included in these sessions were medication issues (e.g., adherence, side effects), assertiveness, and building social support. All other face-to-face and phone sessions included brief follow-up and problem solving related to smoking cessation. Additional information about the smoking cessation intervention is provided in a Supplementary Table.
2.5.4. Physical activity intervention
The physical activity intervention included developing an individualized activity plan for each participant based on personal preferences and following recommended guidelines for moderate and vigorous physical activity [52], [53]. The physical activity intervention included 1) supervised/planned leisure-time exercise sessions, 2) “lifestyle activity” (activity which occurs in the context of working, transportation, raising children, etc.), and 3) short bouts (≥10 min) of physical activity, as needed, to manage urges to smoke and other withdrawal symptoms. Each of these types of activity was included to promote cessation through different mechanisms. For example, structured exercise promotes overall fitness levels, which was hypothesized to reduce smoking and facilitate cessation, while lifestyle activity increases overall activity levels, which was similarly thought to aid quitting. Finally, brief bouts of activity were included as a strategy for managing acute cigarette cravings. As stated previously, the program was offered at convenient locations in the community (YMCAs) where participants received a free one-year membership.
HFIs worked with each participant to develop an individualized physical activity plan to achieve these goals. Initial levels and progression of activity dosage followed American College of Sports Medicine [54] recommendations beginning with a goal of 30 + minutes of activity on three days per week at a moderate intensity (60–74% maximal heart rate). The target frequency of activity sessions was gradually increased, eventually reaching the goal of five to six times per week, which translates into 150–180 min per week of activity. Participants also had the option of meeting their activity goal by exercising at a vigorous intensity (75–90% maximal heart rate) less frequently (three days/week), or accumulating brief (e.g., ≥10 min) periods of “lifestyle” activities rather than single 30-min sessions. Furthermore, participants could accumulate activity toward their goal through any combination of supervised/planned exercise sessions, lifestyle activity, or short bouts of physical activity.
The behavioral counseling component of the intervention utilized a cognitive-behavioral approach, based on SCT, and an adaptation of the “Project Active” program [55], [56]. Each face-to-face session focused on a specific theme. Further details regarding the content and timing of the physical activity sessions are included in the Supplementary Table.
2.5.5. Wellness (control) intervention
Participants in the comparison condition received an individually-tailored general wellness curriculum that covered the multiple dimensions of wellness and how to achieve balance in one's life. The combined smoking cessation/wellness intervention was delivered by HFIs in a university laboratory setting located within the community. Because participants in the physical activity condition received a free fitness center membership, wellness group participants were given small gift certificates (e.g., movie passes, discount store certificates) to help equate the incentive value for the two groups. Additional information related to the wellness information is available in the Supplementary Table.
2.6. Measures
2.6.1. Overview
Primary and secondary endpoints, predictors of treatment outcome, and possible mediators and moderators were assessed at baseline and seven weeks, six months, and 12 months after randomization. Research staff not involved with intervention delivery conducted all assessments. Measurement staff were trained at the start of the study using a common, standardized protocol. For quality assurance purposes, the staff were periodically monitored and received additional booster trainings as needed.
2.6.2. Primary endpoints
Both point prevalent and prolonged smoking abstinence were assessed. Seven-day point prevalent abstinence (PPA) was defined at each follow-up period (seven weeks, six months, and 12 months) as a self-report of no smoking whatsoever during the prior seven days, and an expired-air carbon monoxide (CO) level < 10 parts-per-million (ppm). Prolonged abstinence was defined as a self-report of no smoking throughout the follow-up period after allowing for a two week “grace period” after the quit attempt [57] and a CO level of <10 ppm at that follow-up. Because elevated CO can result from exposures other than tobacco smoke (e.g., car exhaust), participants who self-reported abstinence but who had CO levels ≥ 10 ppm provided a saliva sample for measurement of cotinine [58].
2.6.3. Secondary endpoints
2.6.3.1. Physical activity level
Physical activity was assessed using the Seven-Day Physical Activity Recall (PAR) [40]. The Compendium of Physical Activities [59] was then used to code MET intensity values for activities reported by participants. As recommended by Sallis et al. [40], occupational-related activity was differentiated from leisure activity. Because the intervention specifically targeted increases in leisure time physical activity (LTPA), and considering that participants were pre-screened for eligibility based on LTPA, analyses focused on those data.
2.6.3.2. Body composition
Several measures of body composition and adiposity were obtained including body mass index (BMI; weight [kg]/height [m]2), waist circumference, and percent body fat, which was measured using bioelectrical impedance analysis (BIA) [60].
2.6.3.3. Predictor variables and mediators/moderators
We evaluated several potential predictors of treatment outcome and mediators and moderators of the physical activity/smoking cessation association, as described below.
2.6.3.3.1. Sociodemographics
Sociodemographic characteristics of interest included gender, race, marital status, education, and age.
2.6.3.3.2. Physical and psychological functioning
Measures of physical and psychological functioning included the following. Health-related quality of life (HRQOL) was assessed using the SF-12 [61]. The SF-12 includes 12 items that are weighted and summed to provide both physical (PCS) and mental component scores (MCS) ranging from 0 to 100. Depressive symptoms were measured with the Center for Epidemiologic Studies-Depressed Mood Scale (CES-D) [62]. The CES-D was chosen due to its well-established reliability and validity and demonstrated utility for measuring depressive symptoms in a wide-range of populations. Participants indicated the occurrence and frequency of 20 depressive symptoms during the past week using a four-point Likert scale. Participants who scored at or above the established cut-off for elevated depressive symptoms (≥16) were contacted by a trained professional and offered a mental health referral. To assess changes in other types of mood, we used the Profile of Mood States (POMS) [63]. The POMS measures mood states on a five-point continuum from 0 (not at all) to 4 (extremely). Six subscales representing the following mood dimensions were assessed: tension-anxiety, depression-dejection, anger-hostility, vigor-activity, fatigue-inertia, and confusion-bewilderment. A total mood disturbance index was also derived by adding scores on the five negative mood states and subtracting the positive vigor score from the total. Participants' levels of psychosocial stress were measured using the 14-item Perceived Stress Scale (PSS) [64]. Items target thoughts and feelings experienced during the past month. Responses are provided on a five-point Likert scale ranging from 0 (never) to 4 (very often). Finally, general social support was measured using a revised version of the 12-item Perceived Social Support (PSS) scale [65]. Participants rated their level of agreement with each of 12 statements reflecting the level of support they receive from others using a six-point Likert scale with responses ranging from “very strongly agree” to “very strongly disagree.”
2.6.3.3.3. Tobacco-related variables
Variables representing smoking history and patterns included the number of cigarettes smoked per day, number of years as a smoker, and number of previous quit attempts. Baseline smoking status was based on self-report without biochemical verification. Nicotine dependence was assessed using the Fagerström Test of Nicotine Dependence (FTND) [66] and the Wisconsin Inventory of Smoking Dependence Motives (WISDM-68) [67]. Scores on the FTND rage from 0 to 10, with higher scores indicating greater dependence. The WISDM-68 is a 68-item multi-dimensional measure of dependence that yields an overall smoking dependence score, as well as subscale scores for various components of dependence, including non-physical dimensions (e.g., automaticity, and social/environmental goads). Each item is answered on a seven-point Likert scale ranging from 1 (not true of me at all) to 7 (extremely true of me). To assess nicotine withdrawal symptoms, we used the Minnesota Withdrawal Scale (MWS) [68], [69]. Participants rated the degree to which they experienced each of nine symptoms on a scale from 0 (none) to 4 (severe). A total score is computed from the responses to these items.
Smoking for the purpose of controlling body weight was assessed using the Smoking Situations Questionnaire (SSQ) [70]. The SSQ is scored using six-point Likert scales ranging from 1 (strongly disagree) to 6 (strongly agree). Support for quitting smoking was measured using the Partner Interaction Questionnaire (PIQ) [71]. The measure includes 10 positively and 10 negatively worded items representing behaviors that a partner might engage in during the course of a smoking cessation attempt. Responses are measured on a five-point Likert scale from 0 (never occurred) to 4 (occurred very often). Total positive and negative behaviors are summed. The Smoking: Self-Efficacy/Temptation (Short form) [72] was used to assess self-efficacy to remain abstinent from tobacco. The nine items are scored on a five-point Likert scale ranging from 1 (not at all tempted) to 5 (extremely tempted). The measure includes an overall total score and three sub-scale scores. The Stages of Change measure, which consists of three “yes or no” questions assessing current smoking status and, for those who are current smokers, the number of quit attempts in the past year and whether or not they are thinking about quitting in the next 30 days or six months, was used to categorize participants according to their readiness to quit smoking [73]. Finally, the Smoking Decisional Balance (SDB) (short form) [74] was used to assess decision-making related to smoking cessation. The SDB consists of six items that are scored on a five-point Likert scale ranging from 1 (not important) to 5 (extremely important). The measure is comprised of two, three-item subscales reflecting the perceived pros and cons of smoking.
2.6.3.3.4. Physical activity-related variables
Variables related to physical activity were measured as follows. Self-efficacy for exercise was assessed using the six-item version of the Exercise Self-Efficacy Scale [75]. Participants are asked to indicate their confidence in their ability to exercise in response to commonly-reported barriers to physical activity on a five-point scale ranging from 0 (not at all confident) to 4 (completely confident). Stage of change was measured by asking participants to select the statement that most accurately described their current and recent activity levels and future intentions from a list of five options representing the stages of exercise behavior change from the Transtheoretical Model [76]. Decisional balance was measured using a 10-item inventory [77] that evaluates the perceived pros and cons of engaging in exercise. Participants evaluated each item based on their personal circumstances using a five-point Likert scale with 0 indicating “not important” to 4 indicating “very important.” Social support for exercise was measured with the Social Support for Exercise Behaviors Scale [78]. Participants were asked to rate the frequency with which both friends and family engaged in 13 positive and negative supportive behaviors over the past three months on a five-point Likert scale (1 = none to 5 = very often). The Social Support for Exercise Behaviors Scale includes three subscales representing friend support for exercising together, family support involving rewards and punishments, and family support for participation and involvement in exercise. Physical activity enjoyment was measured using two scales. First, participants rated the attributes of 12 types of physical activities by level of enjoyment using a five-point Likert scale with response options ranging from 1 (don't enjoy at all) to 5 (enjoy a lot) [79]. Participants also completed five items [80] from the 18-item Physical Activity Enjoyment Scale (PACES) [81]. Participants were asked to rate their level of enjoyment of physical activity while exercising or walking on a five-point Likert scale. Finally, barriers to physical activity were measured using the Barriers to Physical Activity Scale [79] and an abbreviated version [80] of the Perceived Barriers to Exercise Scale [82], [83]. For the former, participants rated each item by the degree to which a barrier impacts their physical activity on a five-point Likert-type scale. Responses ranged from 1 (not a barrier) to 5 (very much a barrier). On the latter, participants reported the frequency that each potential barrier interfered with engaging in physical activity on a five-point Likert scale from 0 (never) to 4 (very often).
2.6.3.3.5. Dietary intake
Dietary intake over the prior month was assessed using the National Cancer Institute's Diet History Questionnaire (DHQ) [84]. Data from the DHQ were analyzed using Diet*Calc Version 1.4.2 software to arrive at estimates of daily energy and macronutrient content. To address outliers in reporting of dietary intake, extreme high values (>3 SD above the mean) were Winsorized [85]. Implausibly low values that suggested significant underreporting of energy intake (defined as < 500 kcal/d) were converted to 500 kcal/d.
2.7. Treatment implementation quality control
Several procedures were used to enhance treatment fidelity. All HFIs were trained together at the start of the study using a series of didactic presentations, readings, role-plays, and mock intervention sessions under the observation of project investigators. HFIs were not allowed to conduct sessions with randomized participants until they achieved mastery of all intervention components. Protocols for both the physical activity and wellness interventions were manualized. HFIs completed checklists for each face-to-face and phone session documenting when and for how long the contact took place, whether each component was delivered, deviations from the protocol, and other problems or issues that arose. The project coordinator also observed intervention activities on a periodic basis to provide an independent assessment.
HFIs also received on-going clinical supervision from project investigators, which included a weekly team meeting to review cases. All face-to-face intervention sessions were audiotaped, and 10% were randomly selected, with 4–5 audiotapes reviewed by investigators and discussed at each weekly meeting. The reviewer completed a checklist for each audiotape to evaluate whether criteria for intervention delivery were met consistently across HFIs and across time for individual HFIs.
To ensure that the interventions were adequately “received,” participants completed a brief questionnaire at baseline and end-of-treatment to assess whether key points were learned and remembered, including the techniques, information, and philosophies discussed in the intervention sessions. Participation in physical activity also was assessed using activity logs.
To evaluate potential treatment contamination, use of any other product (or programs) that may have influenced cessation rates, including smoking cessation aids such as pharmacotherapy (e.g., nicotine replacement products or bupropion), behavioral interventions, and participation in other physical activity interventions was assessed at all follow-ups.
2.8. Sample size and power
Given the intensity of the smoking cessation intervention (behavioral cessation counseling delivered through numerous individual face-to-face sessions and phone contacts, and provision of nicotine patch) we estimated a 12 month point prevalent abstinence rate of 25% for the wellness (control) group, which is consistent with meta-analyses of quit rates for randomized trials of similarly intensive behavioral/pharmacological cessation programs [2]. Based on previous trials of physical activity as an aid to smoking cessation that had similar target activity goals as our study [18], [19], [23], [25]; reviewed by Ussher et al. [86], we anticipated that the physical activity intervention would increase quitting by 50%, for a 38% 12 month point prevalent abstinence rate. A sample size of 400 (200/group) was needed to achieve 80% power to detect an effect of 25% vs. 38% at a p-level of < 0.05. As noted above, however, enrollment was stopped at 392 participants in order to have sufficient time during the funding period to collect follow-up data.
2.9. Data analysis
The primary endpoints of seven-day point prevalent and prolonged abstinence will be analyzed using generalized estimating equations (GEE; SPSS GENLIN). This approach allows observations between participants to be considered as independent and observations within participants as correlated. Several baseline and time-varying variables will be included based on documented associations in the extant literature. Interactions with sex and race will also be considered as appropriate. The intention-to-treat principle in which those with missing data are treated as smokers will be used in all analyses of primary endpoints. Data will be analyzed in IBM SPSS Statistics for Windows Version 23.0 (IBM Corp, Armonk, NY: IBM Corp, Released 2015).
The secondary endpoints (physical activity and body composition) will be evaluated using linear mixed models (SPSS MIXED). This procedure will allow us to test changes in outcomes over time and between treatment levels in the face of heterogeneity of measures. Testing and parameter estimations will be done using the restricted maximum likelihood method. For activity, we will look at change from baseline to posttest, as well as both follow-ups. Participants within group will be repeated at the four time points, and participant will be treated as a random effect. As above, the model will include group, time, and a group by time interaction. Other factors that may be considered as potential covariables include baseline demographic, self-efficacy, and body composition variables.
3. Results
3.1. Recruitment and randomization
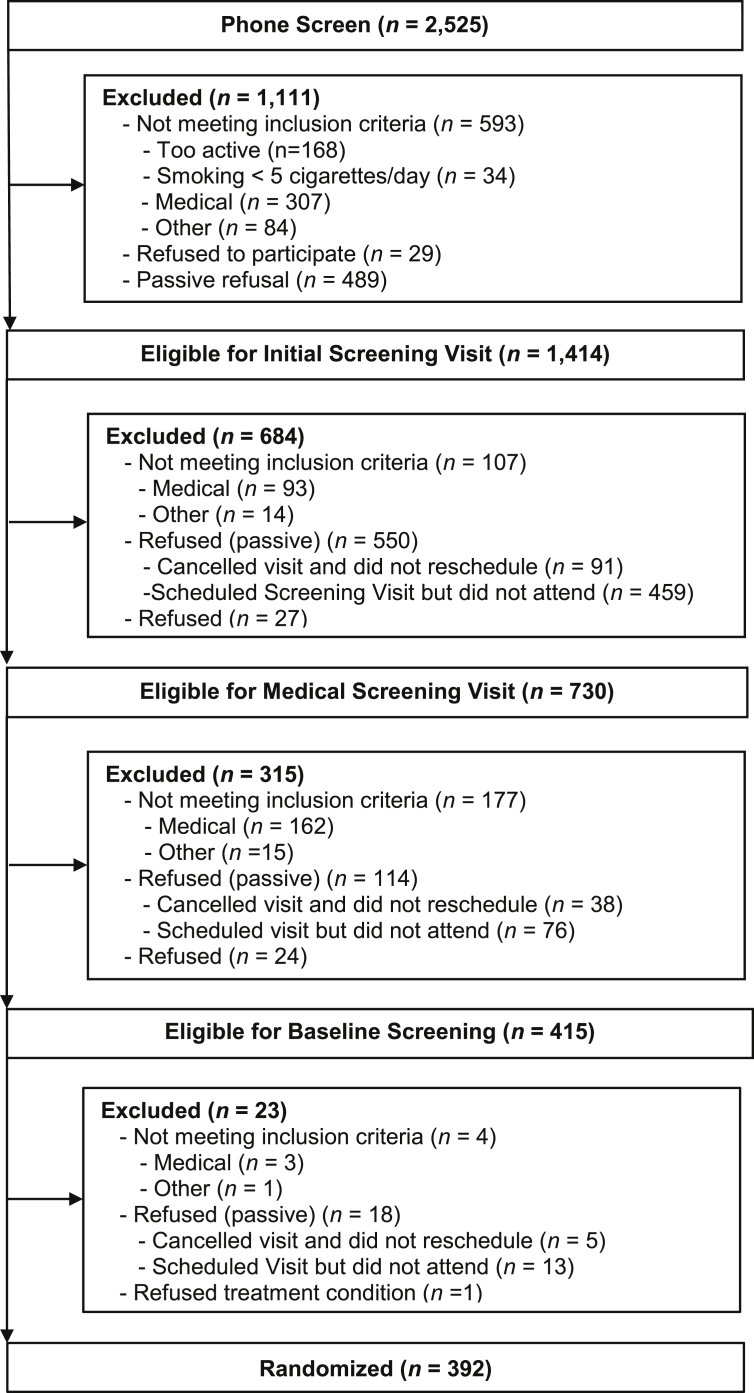
Fig. 1 shows study flow from screening to follow-up. A total of 2525 interested smokers contacted our office to learn more about the study; 80.6% (n = 2036) of these were initially pre-screened by phone. Of those, 69.4% (n = 1414) were potentially eligible based on meeting age, health, physical activity, and smoking status criteria. Ninety one percent (n = 1280) of those eligible agreed to attend a two-part face-to-face screening that involved completing self-report measures and a medical visit consisting of a physical exam, blood work, and an exercise tolerance test. Five hundred and ninety-two (46.3%) individuals attended both screening visits, of whom 32.4% (n = 415) were eligible for randomization. From these, 94.5% (n = 392) were randomized to either the physical activity (n = 199) or wellness (n = 193) conditions between August 2004 and May 2007.
Fig. 1.
Enrollment and retention.
3.2. Demographic and health-related characteristics
Participant characteristics are presented in Table 2. Participants' mean (SD) age was 44.6 (10.2) years. Sixty two percent were female, 67% were Caucasian and 31% were African American. Approximately half of participants were married or living with a significant other, and 26% had at least some college education. Overall, 42% of participants rated their health as very good to excellent. Twenty-seven percent of participants' scores on the CES-D suggested clinically significant levels of depressive symptomatology. None of the baseline characteristics differed significantly by group.
Table 2.
Baseline participant characteristics by treatment group.
| Variable | Physical Activity (n = 199) (Mean (SD) except where stated) | Wellness (n = 193) (Mean (SD) except where stated) |
|---|---|---|
| Demographic measures | ||
| Age in years | 44.6 (9.9) | 44.6 (10.4) |
| Gender, n (%) | ||
| Male | 70 (35.2) | 79 (40.9) |
| Race, n (%) | ||
| White, non-Hispanic | 131 (65.8) | 130 (67.4) |
| African American | 64 (32.2) | 57 (29.5) |
| Other | 4 (2.0) | 6 (3.1) |
| Marital status, n (%) | ||
| Never married | 28 (14.1) | 34 (17.6) |
| Married or cohabitating | 96 (48.2) | 102 (52.9) |
| Divorced or separated | 67 (33.7) | 53 (27.5) |
| Widowed | 8 (4.0) | 4 (2.1) |
| Education, n (%) | ||
| Completed college | 43 (21.6) | 38 (19.7) |
| Some post-baccalaureate | 11 (5.5) | 11 (5.7) |
| Tobacco Use | ||
| Cigarettes smoked/day | 20.0 (9.0) | 21.9 (10.0) |
| Total years smoking | 23.7 (9.8) | 24.0 (11.6) |
| Number of prior quit attempts, n (%) | ||
| None | 19 (9.6) | 25 (13.0) |
| 1–5 | 115 (58.4) | 104 (53.9) |
| 6–10 | 30 (15.2) | 33 (17.1) |
| 11–15 | 10 (5.1) | 8 (4.1) |
| 16+ | 23 (11.7) | 23 (11.9) |
| Fagerström Test of Nicotine Dependence | 4.7 (2.3) | 5.1 (2.3) |
| Self-efficacy for Quitting Smoking | ||
| Habit | 3.5 (0.9) | 3.6 (0.9) |
| Positive social situations | 3.9 (0.8) | 3.9 (0.9) |
| Negative social situations | 4.4 (0.7) | 4.2 (0.8) |
| Confidence in quitting smokinga | 8.1 (2.1) | 8.0 (2.1) |
| Motivation to quit smokinga | 8.8 (1.5) | 8.8 (1.3) |
| Social support for quitting (positive) | 14.2 (11.4) | 13.7 (10.6) |
| Social support for quitting (negative) | 14.9 (10.8) | 13.4 (11.2) |
| Nicotine withdrawal symptoms | 0.93 (0.62) | 1.02 (0.74) |
| Smoking for weight control | 1.8 (1.1) | 1.8 (1.2) |
| Psychosocial | ||
| Perceived stress | 22.1 (7.5) | 22.4 (8.9) |
| Depressed mood (CES-D) | 11.3 (9.4) | 12.7 (10.4) |
| Social support | 66.9 (14.5) | 65.6 (14.7) |
| Total Mood Disorder (POMS) | 15.2 (29.1) | 18.5 (38.4) |
| Body Composition | ||
| Body mass index (BMI) | 28.2 (6.2) | 27.7 (5.5) |
| Percent body fat (%) | 32.9 (10.0) | 32.2 (9.2) |
| Waist circumference (cm) | 96.5 (15.2) | 96.4 (15.7) |
| Abdominal obesityb | 106 (53.3) | 108 (56.0) |
| Physical Activity | ||
| Leisure Physical Activity Only | ||
| Moderate activity (min/week) | 91.7 (138.9) | 84.2 (135.8) |
| Hard activity (min/week) | 41.9 (144.9) | 24.5 (71.7) |
| Very hard activity (min/week) | 4.7 (29.6) | 1.7 (8.6) |
| Strength Training | 6.7 (26.4) | 5.7 (20.5) |
| Self-efficacy for physical activity | 18.7 (5.9) | 17.8 (5.8) |
| Percent attaining 150 + minutes of moderate or greater intensity physical activity per week | 27.6 | 21.8 |
| Importance of decision to exercise (positive opinion) | 20.2 (4.3) | 20.3 (4.4) |
| Importance of decision to exercise (negative opinion) | 6.4 (2.1) | 6.6 (2.5) |
| Dietary Intake | ||
| Total energy intake (kcal/d) | 2285.8 (1273.4) | 2360.0 (1264.8) |
| Percent of total energy intake from carbohydrates (%)c | 47.6 (10.0) | 47.2 (10.2) |
| Percent of total energy intake from fat (%)c | 35.7 (7.6) | 36.6 (7.4) |
| Percent of total energy intake from protein (%)c | 15.1 (4.1) | 15.5 (3.6) |
| Percent of total energy intake from alcohol (%)c | 3.4 (6.3) | 2.4 (4.5) |
SD = standard deviation.
Assessed on a 0 (lowest) to 10 (highest) point scale.
Defined as > 88 cm for women and >102 cm for men.
Reflects the percentage of total calories derived from a given macronutrient.
3.3. Tobacco use history, patterns, dependence, and attitudes
Participants consumed a mean of 21 (9.5) cigarettes/day and had been smoking for approximately 23.8 (10.7) years. The majority (56.2%) had tried to quit 1–5 times in the past; only 11% had never made a quit attempt. Of those who had made a previous quit attempt, most attributed their lack of success to the urge or need to have a cigarette (73%) and the difficulty in quitting the habit (58%). Scores on the FTND indicated that, on average, participants were moderately dependent on nicotine (mean = 4.9; SD = 2.3). Eighty-six percent of participants were in the preparation stage of change for smoking cessation at the time of enrollment, indicating that they were starting to take steps to modify their smoking behavior. Participants rated their mean level of motivation to quit as 8.8 (1.5) on a 0 to 10 scale (with 10 being the highest level of motivation). The mean score for confidence to stay quit was 8.1 (2.1). Mean concern about post-cessation weight gain was 3.5 on a scale of 0–5 (with 5 indicating the highest degree of concern).
3.4. Body composition, physical activity, and dietary intake
Participants' mean (SD) BMI was 27.9 (5.9) indicating that, on average, participants were in the overweight range. Overall, 63.3% of participants met criteria for overweight, while 32.1% met criteria for obesity. Based on waist circumference, 43% of men and 62% of women exhibited abdominal obesity. Mean (SD) body fat percent was 32.5 (9.6)%. Participants' mean (SD) waist circumference was 96.5 (15.4) cm. Approximately 55% of participants met criteria for abdominal obesity, defined as waist circumference >88 cm for women and >102 cm for men [87]. Mean self-reported energy intake was 2322 kcal/d, with 36.1% of energy derived from dietary fat. The proportions of energy intake that came from carbohydrate and protein sources were 47.4% and 15.3%, respectively. Results from the Seven-Day Physical Activity Recall indicated means of 88 min of moderate-, 33 min of hard-, and 3 min of very hard-intensity leisure-`time physical activity per week. Although those assigned to the physical activity intervention had slightly higher levels of baseline activity than those in the wellness condition, differences were not statistically significant (all p's > 0.10).
4. Discussion
This study was designed to evaluate the impact of physical activity as an adjunct to standard smoking cessation treatment consisting of cognitive behavioral smoking cessation counseling and nicotine replacement therapy. Although evidence supports the benefits of exercise for alleviating acute nicotine withdrawal and craving among those undergoing temporary abstinence, and one prior trial found vigorous structured group exercise to be associated with increased long-term cessation in women, little is known about whether a more flexible and personalized approach to increasing physical activity improves treatment outcomes.
The recruitment goal for the study was largely achieved, with 98% of the targeted sample size enrolled in the trial. To achieve this sample size, however, the study required a lengthy enrollment period of approximately three years. Furthermore, a high proportion of those who initially expressed interest were excluded or dropped prior to randomization. While many (n = 881) were excluded because they did not meet all eligibility criteria, a large number were either active (n = 81) or passive refusals (i.e., cancelled or failed to show for a screening visit; n = 1171). Ultimately, only 16% of those who requested more information about the project were randomized to treatment conditions. Although the reasons for the high attrition rate are not readily apparent, the large number of required study visits and focus on multiple domains of health behavior change may have reduced interest among some smokers.
Compared to prior trials of physical activity as an aid to smoking cessation, the sample was relatively diverse with regard to gender (38% male) and race (31% African American). The sample was slightly overrepresented with middle-aged, heavy smokers with a long history of cigarette use. Participants evidenced high levels of both confidence in their ability to quit and motivation for smoking cessation.
By design, the sample was largely sedentary. To be eligible for the study, all participants at baseline were screened to engage in no more than 3 days/week of 30 min of moderate intensity activity and no more than 1 day per week of 30 min of vigorous intensity activity. Prior to intervention, and using a more sensitive assessment tool (the 7-day PAR), only 24% reported that they accumulated the recommended 150 + minutes of moderate to vigorous leisure-time activity in the prior week. Among US adults in 2005, when data for the current study were collected, 49% of adults reported getting either 150 + minutes of physical activity per week or 60 + minutes of vigorous activity [88]. Although these estimates were obtained based on different measures and are therefore not directly comparable, they do suggest that participants in the study were slightly less active overall than the general population. Nevertheless, these results indicate that nearly a quarter of participants' baseline activity levels exceeded target levels for the study, despite meeting entry criteria at the time of screening. Although this could reflect behavioral reactivity to the initial phone screen or a desire to increase one's activity in response to enrolling in the study, it also may be because the initial screening instrument consisted of a brief, two-item assessment, whereas subsequent measures were based on a more detailed physical activity interview (i.e., Seven-day Physical Activity Recall). Considering that the interview is likely to be a more valid indicator of physical activity than the brief, two-item screen, some of the participants may have been more active than the target group for this information. We chose not to exclude non-sedentary participants because this would have made our randomized treatment arms non-comparable. Future studies targeting physical activity should consider this issue when assessing eligibility.
Findings regarding the proportion of participants that met criteria for overweight, obesity, and abdominal obesity are generally consistent with characteristics of the general US population at the time the study was conducted. Considering that quitting smoking is typically associated with increases in body weight and body fat, some participants may be at elevated risk for certain health problems due to post-cessation weight gain. Whether physical activity helps to mitigate the amount of weight gain that participants experience will be investigated as a secondary outcome.
The mean score on the CES-D was 12.5 (SD = 9.9). Although within the normal range, this score represents a higher average level of depressive symptoms than is typically observed in surveys of the general adult population [89], [90]. Given the elevated rates of depression in smokers relative to non-smokers [91], [92] and the increased prevalence of smoking in patients with a history of depression [93], [94], this finding is not particularly surprising. Notably, 27% of participants scored at or above the cut-off commonly used to identify those with probable depression, a rate that is approximately double that reported in large population samples and cohort studies [89], [90], [95]. Considering that depression is associated with a reduced likelihood of quitting smoking [94], [96], [97] and poorer adherence to physical activity interventions [98], [99], [100], these findings may have important implications for treatment outcomes.
5. Summary
Despite considerable advances in the design and implementation of pharmacological and behavioral treatments for tobacco use and dependence, most quit attempts do not lead to successful long-term abstinence. Considerable evidence supports the benefits of acute bouts of physical activity for reducing craving and nicotine withdrawal, at least during periods of temporary abstinence. Furthermore, although highly structured, vigorous-intensity activity conducted in a group setting has been shown to increase cessation rates in women out to one year [19], most studies investigating the impact of physical activity have failed to demonstrate long-term effects. LEAP was specifically designed to address many of the limitations associated with prior trials. By providing smokers with personalized instruction delivered in convenient locations in their community, and allowing considerable flexibility regarding the nature, intensity, and frequency of activities that they can choose to engage in, it is hoped that physical activity adherence and maintenance will be increased, thereby enhancing the potential benefits of regular activity for smokers attempting to quit. The results of LEAP will provide important insights about how to most effectively promote physical activity as an adjunctive treatment for smokers attempting cessation.
Trial registration
ClinicalTrials.gov identifier number NCT00403312.
Acknowledgements
This study was funded by PHS Grant No. R01 HL068569 (K. Ward, PI). We wish to thank our project staff (Dawn Adair, Adam Brock, Bethany Godwin, Cody Newman, Martha Payne, John Saunders, Tom Wride, and Jennifer Skyes) for their assistance with conducting this study; Bess Marcus, PhD for her guidance in designing the study, and the members of the LEAP Data Safety and Monitoring Board (James Arrighi, MD [chair], Mary O'Toole, PhD, Jim Wan, PhD, and Bryan Williams, PhD), and the participants of LEAP for their time and commitment.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.conctc.2017.11.013.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Jamal A. Current cigarette smoking among adults - United States, 2005-2015. MMWR Morb. Mortal. Wkly. Rep. 2016;65(44):1205–1211. doi: 10.15585/mmwr.mm6544a2. [DOI] [PubMed] [Google Scholar]
- 2.Fiore M.C. 2008. Clinical Practice Guideline: Treating Tobacco Use and Dependence: 2008 Update. (Washington, DC) [Google Scholar]
- 3.Stead L.F. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst. Rev. 2016;3 doi: 10.1002/14651858.CD008286.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caspersen C.J., Powell K.E., Christenson G.M. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–131. [PMC free article] [PubMed] [Google Scholar]
- 5.Haasova M. The acute effects of physical activity on cigarette cravings: systematic review and meta-analysis with individual participant data. Addiction. 2013;108(1):26–37. doi: 10.1111/j.1360-0443.2012.04034.x. [DOI] [PubMed] [Google Scholar]
- 6.Roberts V. Effects of exercise on the desire to smoke and physiological responses to temporary smoking abstinence: a crossover trial. Psychopharmacol. Berl. 2015;232(6):1071–1081. doi: 10.1007/s00213-014-3742-8. [DOI] [PubMed] [Google Scholar]
- 7.Roberts V. The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect, and smoking behaviour: systematic review update and meta-analysis. Psychopharmacol. Berl. 2012;222(1):1–15. doi: 10.1007/s00213-012-2731-z. [DOI] [PubMed] [Google Scholar]
- 8.Taylor A.H., Ussher M.H., Faulkner G. The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect and smoking behaviour: a systematic review. Addiction. 2007;102(4):534–543. doi: 10.1111/j.1360-0443.2006.01739.x. [DOI] [PubMed] [Google Scholar]
- 9.Ussher M. Effect of a short bout of exercise on tobacco withdrawal symptoms and desire to smoke. Psychopharmacol. Berl. 2001;158(1):66–72. doi: 10.1007/s002130100846. [DOI] [PubMed] [Google Scholar]
- 10.Ussher M.H., Taylor A.H., Faulkner G.E. Exercise interventions for smoking cessation. Cochrane Database Syst. Rev. 2014;8 doi: 10.1002/14651858.CD002295.pub5. [DOI] [PubMed] [Google Scholar]
- 11.Audrain-McGovern J. Reinforcing value of smoking relative to physical activity and the effects of physical activity on smoking abstinence symptoms among young adults. Exp. Clin. Psychopharmacol. 2015;23(6):477–485. doi: 10.1037/pha0000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elibero A., Janse Van Rensburg K., Drobes D.J. Acute effects of aerobic exercise and Hatha yoga on craving to smoke. Nicotine Tob. Res. 2011;13(11):1140–1148. doi: 10.1093/ntr/ntr163. [DOI] [PubMed] [Google Scholar]
- 13.King T.K. Cognitive-behavioral mediators of changing multiple behaviors: smoking and a sedentary lifestyle. Prev. Med. 1996;25(6):684–691. doi: 10.1006/pmed.1996.0107. [DOI] [PubMed] [Google Scholar]
- 14.Loprinzi P.D., Wolfe C.D., Walker J.F. Exercise facilitates smoking cessation indirectly via improvements in smoking-specific self-efficacy: prospective cohort study among a national sample of young smokers. Prev. Med. 2015;81:63–66. doi: 10.1016/j.ypmed.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Farley A.C. Interventions for preventing weight gain after smoking cessation. Cochrane Database Syst. Rev. 2012;1 doi: 10.1002/14651858.CD006219.pub3. [DOI] [PubMed] [Google Scholar]
- 16.Ward K.D., Klesges R.C., Vander Weg M.W. Smoking and body weight. In: Bjorntorp J., editor. International Textbook of Obesity. John Wiley and Sons, Inc; London: 2001. [Google Scholar]
- 17.Bock B.C. Yoga as a complementary treatment for smoking cessation in women. J. Womens Health (Larchmt) 2012;21(2):240–248. doi: 10.1089/jwh.2011.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marcus B.H. Usefulness of physical exercise for maintaining smoking cessation in women. Am. J. Cardiol. 1991;68(4):406–407. doi: 10.1016/0002-9149(91)90843-a. [DOI] [PubMed] [Google Scholar]
- 19.Marcus B.H. The efficacy of exercise as an aid for smoking cessation in women: a randomized controlled trial. Arch. Intern. Med. 1999;159(11):1229–1234. doi: 10.1001/archinte.159.11.1229. [DOI] [PubMed] [Google Scholar]
- 20.Martin J.E. Prospective evaluation of three smoking interventions in 205 recovering alcoholics: one-year results of Project SCRAP-Tobacco. J. Consult. Clin. Psychol. 1997;65(1):190–194. doi: 10.1037//0022-006x.65.1.190. [DOI] [PubMed] [Google Scholar]
- 21.Abrantes A.M. A preliminary randomized controlled trial of a behavioral exercise intervention for smoking cessation. Nicotine Tob. Res. 2014;16(8):1094–1103. doi: 10.1093/ntr/ntu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ciccolo J.T. Resistance training as an aid to standard smoking cessation treatment: a pilot study. Nicotine Tob. Res. 2011;13(8):756–760. doi: 10.1093/ntr/ntr068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill J.S. Effect of a program of aerobic exercise on the smoking behaviour of a group of adult volunteers. Can. J. Public Health. 1985;76(3):183–186. [PubMed] [Google Scholar]
- 24.Hill R.D., Rigdon M., Johnson S. Behavioral smoking cessation treatment for older chronic smokers. Behav. Ther. 1993;24:321–329. [Google Scholar]
- 25.Marcus B.H. Exercise enhances the maintenance of smoking cessation in women. Addict. Behav. 1995;20(1):87–92. doi: 10.1016/0306-4603(94)00048-4. [DOI] [PubMed] [Google Scholar]
- 26.Patten C.A. Supervised, vigorous intensity exercise intervention for depressed female smokers: a pilot study. Nicotine Tob. Res. 2017;19(1):77–86. doi: 10.1093/ntr/ntw208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russell P.O. The effects of physical activity as maintenance for smoking cessation. Addict. Behav. 1988;13(2):215–218. doi: 10.1016/0306-4603(88)90016-0. [DOI] [PubMed] [Google Scholar]
- 28.Taylor C.B. Smoking cessation after acute myocardial infarction: the effects of exercise training. Addict. Behav. 1988;13(4):331–335. doi: 10.1016/0306-4603(88)90039-1. [DOI] [PubMed] [Google Scholar]
- 29.Vickers K.S. Feasibility of an exercise counseling intervention for depressed women smokers. Nicotine Tob. Res. 2009;11(8):985–995. doi: 10.1093/ntr/ntp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whiteley J.A. Commit to Quit in the YMCAs: translating an evidence-based quit smoking program for women into a community setting. Nicotine Tob. Res. 2007;9(11):1227–1235. doi: 10.1080/14622200701648334. [DOI] [PubMed] [Google Scholar]
- 31.Williams D.M. Moderate intensity exercise as an adjunct to standard smoking cessation treatment for women: a pilot study. Psychol. Addict. Behav. 2010;24(2):349–354. doi: 10.1037/a0018332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marcus B.H. The efficacy of moderate-intensity exercise as an aid for smoking cessation in women: a randomized controlled trial. Nicotine Tob. Res. 2005;7(6):871–880. doi: 10.1080/14622200500266056. [DOI] [PubMed] [Google Scholar]
- 33.Kinnunen T. Exercise as an adjunct to nicotine gum in treating tobacco dependence among women. Nicotine Tob. Res. 2008;10(4):689–703. doi: 10.1080/14622200801979043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prapavessis H. The effects of exercise and nicotine replacement therapy on smoking rates in women. Addict. Behav. 2007;32(7):1416–1432. doi: 10.1016/j.addbeh.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Prapavessis H. Exercise to enhance smoking cessation: the getting physical on cigarette randomized control trial. Ann. Behav. Med. 2016;50(3):358–369. doi: 10.1007/s12160-015-9761-9. [DOI] [PubMed] [Google Scholar]
- 36.Whiteley J.A. YMCA commit to quit: randomized trial outcomes. Am. J. Prev. Med. 2012;43(3):256–262. doi: 10.1016/j.amepre.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 37.Smits J.A. The efficacy of vigorous-intensity exercise as an aid to smoking cessation in adults with high anxiety sensitivity: a randomized controlled trial. Psychosom. Med. 2016;78(3):354–364. doi: 10.1097/PSY.0000000000000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whiteley J.A. The challenges of translating an efficacious smoking cessation program, Commit to Quit, to the community setting of YMCAs. Transl. Behav. Med. 2013;3(1):47–58. doi: 10.1007/s13142-012-0181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Medicine A.C.o.S. fifth ed. Lea and Febiger; Philadelphia, PA: 1995. Guideliens for Graded Exercise Testing and Exercise Prescription. [Google Scholar]
- 40.Sallis J.F. Physical activity assessment methodology in the Five-City Project. Am. J. Epidemiol. 1985;121(1):91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- 41.Bandura A. Prentice-Hall; Englewood Cliffs, NJ: 1986. Social Foundations of Thought and Action: a Social Cognitive Theory. [Google Scholar]
- 42.Dishman R.K., Sallis J.F., Orenstein D.R. The determinants of physical activity and exercise. Public Health Rep. 1985;100(2):158–171. [PMC free article] [PubMed] [Google Scholar]
- 43.King A.C. Determinants of physical activity and interventions in adults. w. 1992;24(6 Suppl):S221–S236. [PubMed] [Google Scholar]
- 44.Macnee C.L., Talsma A. Predictors of progress in smoking cessation. Public Health Nurs. 1995;12(4):242–248. doi: 10.1111/j.1525-1446.1995.tb00143.x. [DOI] [PubMed] [Google Scholar]
- 45.Hall S.M. Nicotine, negative affect, and depression. J. Consult Clin. Psychol. 1993;61(5):761–767. doi: 10.1037//0022-006x.61.5.761. [DOI] [PubMed] [Google Scholar]
- 46.Marcus B.H., Owen N. Motivational readiness, self-efficacy, and decision-making for exericse. J. Appl. Soc. Psychol. 1992;22(1):3–16. [Google Scholar]
- 47.Karoly P., Kanfer F.H., editors. Self-management and Behavior Change: from Theory to Practice. Pergamon; New York: 1982. [Google Scholar]
- 48.Cooper T.V. A placebo controlled randomized trial of the effects of phenylpropanolamine and nicotine gum on cessation rates and postcessation weight gain in women. Addict. Behav. 2005;30(1):61–75. doi: 10.1016/j.addbeh.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 49.Klesges R.C., DeBon M. Hunter House; Alameda, CA: 1993. How Women Can Finally Stop Smoking. [Google Scholar]
- 50.Ward K.D. Randomized trial of the effectiveness of combined behavioral/pharmacological smoking cessation treatment in Syrian primary care clinics. Addiction. 2013;108(2):394–403. doi: 10.1111/j.1360-0443.2012.04048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hatsukami D.K., Lando H. Behavioral treatment for smoking cessation. Health Val. 1993;17(2):23–40. [Google Scholar]
- 52.Pate R.R. Physical activity and public health. A recommendation from the centers for disease control and prevention and the american college of Sports medicine. JAMA. 1995;273(5):402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 53.American College of Sports Medicine position stand The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness in healthy adults. Med. Sci. Sports Exerc. 1990;22(2):265–274. [PubMed] [Google Scholar]
- 54.Haskell W.L. Physical activity and public health: updated recommendation for adults from the american college of Sports medicine and the american heart association. Med. Sci. Sports Exerc. 2007;39(8):1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 55.Blair S.N. Human Kinetics; Champaign, IL: 2001. Active Living Every Day. [Google Scholar]
- 56.Dunn A.L. Comparison of lifestyle and structured interventions to increase physical activity and cardiorespiratory fitness: a randomized trial. JAMA. 1999;281(4):327–334. doi: 10.1001/jama.281.4.327. [DOI] [PubMed] [Google Scholar]
- 57.Hughes J.R. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob. Res. 2003;5(1):13–25. [PubMed] [Google Scholar]
- 58.Jacob P., 3rd Selected ion monitoring method for determination of nicotine, cotinine and deuterium-labeled analogs: absence of an isotope effect in the clearance of (S)-nicotine-3',3'-d2 in humans. Biol. Mass Spectrom. 1991;20(5):247–252. doi: 10.1002/bms.1200200503. [DOI] [PubMed] [Google Scholar]
- 59.Ainsworth B.E. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med. Sci. Sports Exerc. 2011;43(8):1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 60.Nunez C. Bioimpedance analysis: evaluation of leg-to-leg system based on pressure contact footpad electrodes. Med. Sci. Sports Exerc. 1997;29(4):524–531. doi: 10.1097/00005768-199704000-00015. [DOI] [PubMed] [Google Scholar]
- 61.Ware J., Jr., Kosinski M., Keller S.D. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med. Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 62.Radloff L.S. The CES-D Scale: a self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977;1(385–401) [Google Scholar]
- 63.McNair D.M., Lorr M., Droppleman L.F. Educational and Industrial Testing Services; San Diego, CA: 1971. Manual for the Profile of Mood States. [Google Scholar]
- 64.Cohen S., Kamarck T., Mermelstein R. A global measure of perceived stress. J. Health Soc. Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 65.Blumenthal J.A. Social support, type a behavior, and coronary artery disease. Psychosom. Med. 1987;49(4):331–340. doi: 10.1097/00006842-198707000-00002. [DOI] [PubMed] [Google Scholar]
- 66.Heatherton T.F. The fagerstrom test for nicotine dependence: a revision of the fagerstrom tolerance questionnaire. Br. J. Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 67.Piper M.E. A multiple motives approach to tobacco dependence: the Wisconsin Inventory of Smoking Dependence Motives (WISDM-68) J. Consult. Clin. Psychol. 2004;72(2):139–154. doi: 10.1037/0022-006X.72.2.139. [DOI] [PubMed] [Google Scholar]
- 68.Hughes J.R., Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch. Gen. Psychiatry. 1986;43(3):289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 69.Hughes J.R. Symptoms of tobacco withdrawal. A replication and extension. Arch. Gen. Psychiatr. 1991;48(1):52–59. doi: 10.1001/archpsyc.1991.01810250054007. [DOI] [PubMed] [Google Scholar]
- 70.Weekley C.K., 3rd, Klesges R.C., Reylea G. Smoking as a weight-control strategy and its relationship to smoking status. Addict. Behav. 1992;17(3):259–271. doi: 10.1016/0306-4603(92)90031-p. [DOI] [PubMed] [Google Scholar]
- 71.Cohen S., Lichtenstein E. Partner behaviors that support quitting smoking. J. Consult Clin. Psychol. 1990;58(3):304–309. doi: 10.1037//0022-006x.58.3.304. [DOI] [PubMed] [Google Scholar]
- 72.Fava J.L. Annual Meeting of the American Psychological Association. 1991. Structural confirmation of short-form instruments for the transtheoretical model. San Francisco, CA. [Google Scholar]
- 73.DiClemente C.C. The process of smoking cessation: an analysis of precontemplation, contemplation, and preparation stages of change. J. Consult. Clin. Psychol. 1991;59(2):295–304. doi: 10.1037//0022-006x.59.2.295. [DOI] [PubMed] [Google Scholar]
- 74.Velicer W.F. Decisional balance measure for assessing and predicting smoking status. J. Pers. Soc. Psychol. 1985;48(5):1279–1289. doi: 10.1037//0022-3514.48.5.1279. [DOI] [PubMed] [Google Scholar]
- 75.Benisovich S.V. Development of a multidimensional measure of exercise self-efficacy. Ann. Behav. Med. 1998;20(Suppl):S190. [Google Scholar]
- 76.Marcus B.H. Self-efficacy and the stages of exercise behavior change. Res. Q. Exerc. Sport. 1992;63(1):60–66. doi: 10.1080/02701367.1992.10607557. [DOI] [PubMed] [Google Scholar]
- 77.Nigg C.R. Structure of decisional balance for exercise adoption. Ann. Behav. Med. 1998;20(Suppl):S211. [Google Scholar]
- 78.Sallis J.F. The development of scales to measure social support for diet and exercise behaviors. Prev. Med. 1987;16(6):825–836. doi: 10.1016/0091-7435(87)90022-3. [DOI] [PubMed] [Google Scholar]
- 79.Salmon J. Physical activity and sedentary behavior: a population-based study of barriers, enjoyment, and preference. Health Psychol. 2003;22(2):178–188. doi: 10.1037//0278-6133.22.2.178. [DOI] [PubMed] [Google Scholar]
- 80.Castro C.M. A prospective study of psychosocial correlates of physical activity for ethnic minority women. Psychol. Health. 1999;14(2):277–293. [Google Scholar]
- 81.Kendzierski D., DeCarlo K.J. Physical activity enjoyment scale: two validation studies. J. Sport Exerc. Psychol. 1991;13(1):50–64. [Google Scholar]
- 82.Sallis J.F. A multivariate study of determinants of vigorous exercise in a community sample. Prev. Med. 1989;18(1):20–34. doi: 10.1016/0091-7435(89)90051-0. [DOI] [PubMed] [Google Scholar]
- 83.Hovell M.F. Identifying correlates of walking for exercise: an epidemiologic prerequisite for physical activity promotion. Prev. Med. 1989;18(6):856–866. doi: 10.1016/0091-7435(89)90021-2. [DOI] [PubMed] [Google Scholar]
- 84.Thompson F.E. Cognitive research enhances accuracy of food frequency questionnaire reports: results of an experimental validation study. J. Am. Diet. Assoc. 2002;102(2):212–225. doi: 10.1016/s0002-8223(02)90050-7. [DOI] [PubMed] [Google Scholar]
- 85.Dixon W.J., Yuen K.K. Trimming and winsorization: a review. Stat. Hefte. 1974;15(2):157–170. [Google Scholar]
- 86.Ussher M.H. Does exercise aid smoking cessation? A systematic review. Addiction. 2000;95(2):199–208. doi: 10.1046/j.1360-0443.2000.9521996.x. [DOI] [PubMed] [Google Scholar]
- 87.Grundy S.M. Diagnosis and management of the metabolic syndrome: an american heart association/national heart, lung, and blood Institute scientific statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 88.CDC. BRFSS Prevalence & Trends Data [online] 2015. https://www.cdc.gov/brfss/brfssprevalence/ [cited 2017 January 6]; Available from: [Google Scholar]
- 89.Ferketich A.K. Depression as an antecedent to heart disease among women and men in the NHANES I study. National Health and Nutrition Examination Survey. Arch. Intern. Med. 2000;160(9):1261–1268. doi: 10.1001/archinte.160.9.1261. [DOI] [PubMed] [Google Scholar]
- 90.Wulsin L.R. Depressive symptoms, coronary heart disease, and overall mortality in the Framingham Heart Study. Psychosom. Med. 2005;67(5):697–702. doi: 10.1097/01.psy.0000181274.56785.28. [DOI] [PubMed] [Google Scholar]
- 91.Glassman A.H. Smoking, smoking cessation, and major depression. JAMA. 1990;264(12):1546–1549. [PubMed] [Google Scholar]
- 92.Breslau N. Psychiatric comorbidity of smoking and nicotine dependence. Behav. Genet. 1995;25(2):95–101. doi: 10.1007/BF02196920. [DOI] [PubMed] [Google Scholar]
- 93.Lasser K. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284(20):2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- 94.Smith P.H., Mazure C.M., McKee S.A. Smoking and mental illness in the U.S. population. Tob. Control. 2014;23(e2):e147–e153. doi: 10.1136/tobaccocontrol-2013-051466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Murabito J.M. Depressive symptoms are associated with visceral adiposity in a community-based sample of middle-aged women and men. Obes. (Silver Spring) 2013;21(8):1713–1719. doi: 10.1002/oby.20130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cinciripini P.M. The effects of depressed mood on smoking cessation: mediation by postcessation self-efficacy. J. Consult. Clin. Psychol. 2003;71(2):292–301. doi: 10.1037/0022-006x.71.2.292. [DOI] [PubMed] [Google Scholar]
- 97.Hitsman B. Past major depression and smoking cessation outcome: a systematic review and meta-analysis update. Addiction. 2013;108(2):294–306. doi: 10.1111/add.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Courneya K.S. Predictors of supervised exercise adherence during breast cancer chemotherapy. Med. Sci. Sports Exerc. 2008;40(6):1180–1187. doi: 10.1249/MSS.0b013e318168da45. [DOI] [PubMed] [Google Scholar]
- 99.Arikawa A.Y. Attrition and adherence of young women to aerobic exercise: lessons from the WISER study. Contemp. Clin. Trials. 2012;33(2):298–301. doi: 10.1016/j.cct.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aggarwal B., Liao M., Mosca L. Predictors of physical activity at 1 year in a randomized controlled trial of family members of patients with cardiovascular disease. J. Cardiovasc. Nurs. 2010;25(6):444–449. doi: 10.1097/JCN.0b013e3181defd3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.