Abstract
After reviewing previously published methods, we developed a practical approach to adjusting insulin doses based on insulin sensitivity for adult patients with diabetes using rtCGM trend arrow data.
Keywords: continuous glucose monitoring, diabetes, fine-tuning, insulin dose adjustment, insulin dosing, trend arrows
As the accuracy of continuous glucose monitoring (CGM) devices has improved and the benefits are better understood, their use has greatly increased. For patients with diabetes, CGM does more than provide additional data points; it uses trend arrow data to give context to current glucose values. With this level of insight, real-time CGM (rtCGM) has been demonstrated to improve glycemic control without increasing hypoglycemia in patients with type 1 diabetes [1–3] as well as in insulin-treated patients with type 2 diabetes [4, 5].
In December 2016, the US Food and Drug Administration approved the Dexcom G5 Mobile CGM system (Dexcom G5, Dexcom, Inc., San Diego, CA) for nonadjunctive insulin dosing. Aside from the required twice daily calibrations, patients using the Dexcom G5 can now dose insulin without confirmatory fingerstick glucose monitoring in most situations, further improving usability. However, there is sparse guidance for how individuals should act on the trend arrow data.
Notably, there are four previously published methods for using trend arrow data to adjust insulin doses [6–9]. However, each method has various limitations in its complexity, utility, and applicability. Our approach focuses on the Dexcom G5 system—the first Food and Drug Administration-approved system for nonadjunctive insulin dosing and the system that we have the most clinical experience using in this manner to date.
Our goal is to offer a safe, practical approach for using CGM trend arrow data to adjust insulin dosing. We based this approach on previous algorithms [6,10,11], our clinical experiences as endocrinologists, our personal experiences as people living with diabetes and using CGM, and guidance from other diabetes specialists [8, 12]. Notably, the Klonoff/Kerr formula was published following the development of our guidance and was therefore not used in our comparisons; however, we note the similarities to our own approach and differences in application. Specifically, we aim to address the needs of clinicians treating adult patients living with diabetes requiring intensive insulin therapy who use the Dexcom G5 system. Members of the extended care team, primary care physicians, patients, and caregivers will need tailored information that we plan to develop in the near future.
Despite our goal for simplicity, we recognize diabetes management is complex, and treatment decisions require contextual information with trend arrow data being a critical component. Importantly, optimal use of any CGM device relies on understanding the principles, limitations, and caveats to using CGM in the broader context of diabetes management. Lastly, our approach is based upon Dexcom G5 trend arrows and assumes the use of currently available rapid-acting insulin analogs.
We recognize that our approach has certain limitations. Currently, there are no clinical trials that have used our suggested approach. We also recognize that other CGM systems, including the recently approved FreeStyle Libre Flash Glucose Monitoring System for nonadjunctive use in adults ages 18 and older, are currently available and provide useful information based on trend arrows. We also expect that additional nonadjunctive CGM systems will likely become available in the future. However, it is important to note that there are no standard conventions for displaying rates of change information in CGM devices and device arrows vary in meaning and display.
The suggested approach is a starting point of iterative discussion on how to best use Dexcom G5 trend arrow data. We also hope our approach will be useful when discussing future CGM systems taking into consideration that the transfer, display, and meaning of trend arrow data may be specific for each brand.
1. Safe and Effective rtCGM Use: Patient Selection and Education/Training
Recognizing which patients are most likely to benefit from rtCGM and being able to provide adequate education are paramount to the success of rtCGM. For detailed discussions on selecting patients for rtCGM, we recommend the 2016 Endocrine Society practice guideline [13] and American Association of Clinical Endocrinologists consensus statement [14].
Education on using rtCGM is a lengthy topic on its own. However, we highlight the importance of teaching patients the fundamentals of sensor insertion, sensor lag time, calibration, and setting alerts/alarms as well as providing patients with realistic expectations (e.g., rtCGM will not eliminate the need for fingerstick glucose monitoring). For older adults, we stress the importance of ensuring patients can see and hear the alerts and alarms and underscore the value in educating family members and/or caretakers. For all adults, we emphasize that rtCGM relies on an individual’s ability and motivation to use the device on a near daily basis [1, 5]. We offer Table 1 as a list of considerations for recommending rtCGM and Table 2 as a suggested list of education topics [15–17]. Table 3 lists common medications containing acetaminophen, which is an important consideration when using rtCGM.
Table 1.
Considerations for Recommending rtCGM
| Patients Meeting One or More of the Following Criteria May Be Considered for rtCGM: |
|---|
| ► Patient is 2 years of age or older |
| ► Currently treated by intensive insulin therapy |
| ► Experiencing frequent hypoglycemia |
| ► Hypoglycemia unawareness |
| ► Excessive glucose variability |
| ► Varying and/or intensive activity |
| ► Desire to improve glycemic control |
| ► Understands behaviors that influence glycemic control |
| ► Willing and able to use rtCGM on a nearly daily basis |
| ► Willing and able to learn how to use device and receive ongoing |
| ► Pregnant or wants to get pregnanta |
The following is not an exhaustive list of considerations. It is based on prior publications by the Endocrine Society and American Association of Clinical Endocrinologists, as well as the clinical experience of the authors. Additional factors such as fragile and/or elderly patients, or patients at high risk for hypoglycemia-related cardiac events may also be considered. A foundation criterion is that a patient or caretaker must be willing and able to understand, use, and learn more about rtCGM.
Currently, no rtCGM system is indicated for use in pregnancy.
Table 2.
Education Checklist Prior to Using rtCGM
| The Following are Fundamental Principles and Skills That a Patient and/or Caregiver Should Learn When Using rtCGM. At the End of Training, Patients and/or Caregivers Should be Able to: |
|---|
| ❑ Describe the difference between interstitial fluid and capillary glucose and understand the meaning of lag time. |
| ❑ Recognize the importance of handwashing prior to fingerstick monitoring. |
| ❑ Summarize the calibration procedure and explain when calibration is needed.a |
| ❑ Summarize the limitations in rtCGM data accuracy within the first 24 hours following insertion and beyond the manufacturer’s recommended wear time.b |
| ❑ Demonstrate the procedures for setting alarms/alerts.c |
| ❑ Explain the significance of alarms/alerts, glucose trend data, and trend arrows in making treatment decisions.c |
| ❑ Explain how to use trend arrows in individualized treatment decisions. |
| ❑ Explain the dangers associated with frequent insulin dosages following meals (i.e., “stacking”). |
| ❑ Explain how to use rtCGM during sick days or illness.d |
| ❑ Explain individualized monitoring and treatment strategies when exercising (e.g., temporary basal rates, insulin adjustment, carbohydrate adjustment, adjusting for trend arrows). |
| ❑ Demonstrate sensor insertion procedure and list appropriate insertion sites.b |
| ❑ When share functions are available: Demonstrate the procedure for uploading the rtCGM data (e.g., via Dexcom G5 Mobile app or Dexcom Clarity) and with others (e.g., Dexcom Follow app or Dexcom Clarity for clinics).e |
The Dexcom G5 device should be calibrated twice daily according to the manufacturer’s instructions. Persons who check fingersticks frequently should be informed to not enter every fingerstick value. It is important that patients use the proper fingerstick monitoring technique (e.g., thoroughly washing hands with soap and water before checking). Dexcom G5 calibration is reliant on a properly functioning and accurate blood glucose meter. Therefore, we recommend patients use blood glucose meters with proven accuracy and performance.
Dexcom recommends that sensors be placed in subcutaneous tissue on the abdomen and upper buttock (including lipohypertrophic areas); however, a recent study found comparable accuracy with placement on the back of the arm [15]. Importantly, patients should be instructed not to rely solely on their rtCGM data during first 24 hours after inserting the sensor.
When reviewing alarms/alerts, it is important to discuss how to deal with “alert fatigue,” which may prompt patients to switch them off or underutilize their rtCGM system.
rtCGM can be used during periods of illness but will require additional confirmatory fingerstick check. Importantly, patients should be cautioned about use of medications that contain acetaminophen, which can cause the rtCGM system to display false high readings for up to and beyond 6 hours following ingestion [16, 17].
If the patient chooses to use data sharing, it is important that caregivers receive training in rtCGM use, specifically, use of trend arrows, interpretation, and appropriate response.
Table 3.
Commonly Used Over-the-Counter and Prescription Medications Containing Acetaminophen
| Common Over-the-Counter Medicines Containing Acetaminophena | |||
|---|---|---|---|
| ► Actifed® | ► Dayquil® | ► Midol® | ► Sudafed® |
| ► Alka-Seltzer Plus LiquidGels® | ► Dimetapp® | ► Nyquil® | ► Theraflu® |
| ► Anacin® | ► Dristan® | ► Panadol® | ► Triaminic® |
| ► Benadryl® | ► Excedrin® | ► Robitussin® | ► TYLENOL® Brand Products |
| ► Cepacol® | ► Feverall® | ► Saint Joseph® | ► Vanquish® |
| ► Contac® | ► Equation 44® | ► Aspirin-Free Singlet® | ► Vicks® |
| ► Coricidin® | ► Goody’s® Powders | ► Sinutab® | ► Zicam® |
| ► Liquiprin | |||
| Common Prescription Medicines Containing Acetaminophena | |||
|---|---|---|---|
| ► Endocet® | ► Lortab® | ► Tylenol® with Codeine | |
| ► Fioricet® | ► Percocet® | ► Tylox® | |
| ► Hycotab | ► Phenaphen® | ► Ultracet® | |
| ► Hydrocet® | ► Sedapap® | ► Vicodin® | |
| ► Hydrocodone Bitartrate | ► Tapanol® | ► Zydone® | |
Acetaminophen is known to interfere with certain rtCGM sensors causing falsely elevated glucose readings. Patients using rtCGM are cautioned to check with the manufacturer’s information and review labels of over-the-counter medicines for acetaminophen and to ask their provider and/or pharmacist whether their prescribed medication(s) contain acetaminophen.
Includes store and other generic brands.
2. How to Use Trend Arrows to Adjust Insulin Dose
At a basic level, patients requiring intensive insulin therapy rely on their current glucose value, target glucose value, food intake (if any), and insulin dosing parameters [insulin-to-carbohydrate ratio (ICR) and correction factor (CF); also known as insulin sensitivity] to calculate their insulin dose. This standard “point in time” monitoring is limited; the glucose value used for calculating insulin dose is an isolated, static measurement.
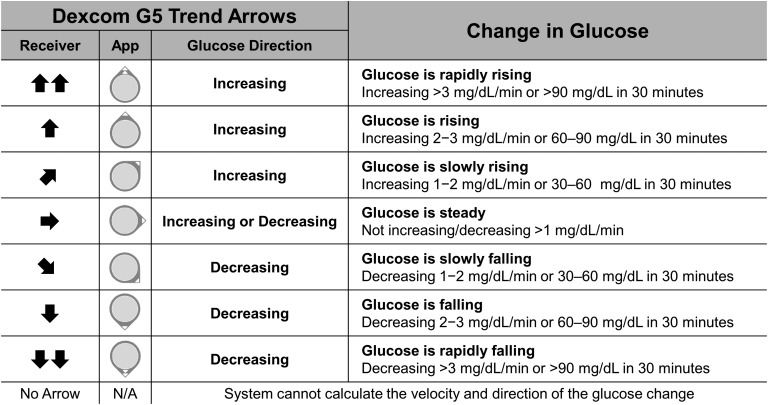
Trend arrows add context to this static measurement. The directionality of trend arrows allows individuals to “anticipate” future glucose levels. This additional information can then be used proactively to adjust insulin dose. Upward trend arrows indicate rising glucose levels and may suggest a need for additional insulin; downward trend arrows indicate falling glucose levels and may suggest a need for less insulin or corrective action with carbohydrate intake to avoid hypoglycemia. This conceptual shift from “point-in-time” monitoring to “anticipating” future glucose levels is essential to using rtCGM optimally. Figure 1 provides an example of how these trend arrows appear in Dexcom G5 displays [i.e., Dexcom Receiver and Dexcom smart device apps (Dexcom G5 Mobile and Dexcom Follow)] and the anticipated glucose change they represent.
Figure 1.
Dexcom G5 trend arrows. Dexcom G5 presents trend arrow data as icons on the Dexcom G5 Receiver and on the Dexcom G5 Mobile and Dexcom Follow apps (App) on compatible smart devices. According to the manufacturer, trend arrows indicate rates of glucose change (mg/dL per minute) and can be described as the anticipated glucose change in 30 minutes. Notably, the FLAT arrow (➡) indicates steady glucose values but does not indicate zero change. Note that trend arrows are determined by recent rtCGM measurements (generally the most recent 10 minutes of glucose values). In general, anticipated glucose may be less accurate when trying to predict changes over extended periods of time (e.g., beyond 20 to 30 minutes) due to the many factors that may influence glucose levels. Conversion: mg/dL × 0.0555 = mmol/L.
Most importantly, adjusting insulin dose using trend arrows does not replace standard calculations. Adjusting insulin dose using trend arrows is an additional step that increases or decreases the insulin dose calculated using standard parameters. There are caveats—as there are with all diabetes management approaches—and the extent of insulin adjustment may be impacted by common factors, such as meal composition, time since last meal, insulin on board, and exercise. We comment on these below.
Because adjusting insulin dose using trend arrows adds a layer of sophistication, we recommend patients wait until they are comfortable with the general application of rtCGM data and learn how their body responds to various meals (quantity/composition) and physical activity before adjusting insulin dose using trend arrows with any available approach.
3. Previous Methods to Adjust Insulin Dose Using Trend Arrows
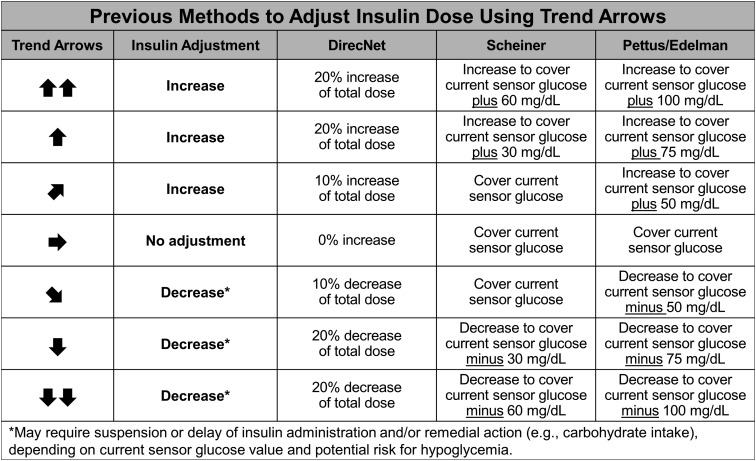
To date, there are four previously published methods to adjust insulin doses using trend arrows: DirecNet Applied Treatment Algorithm [6], Scheiner method [7], Pettus/Edelman method [8], and Klonoff/Kerr formula [9]. Notably, the DirecNet, Scheiner, and Klonoff/Kerr methods include CGM systems other than Dexcom in their approach.
In brief, the DirecNet method determines insulin dose adjustment by calculating percent increase/decrease based on trend arrow directionality. Notably, the net increase/decrease will vary depending on food intake because the DirecNet method considers total insulin dose—e.g., meal and correction dose as appropriate. The Scheiner and Pettus/Edelman methods use a different approach. Both use anticipated glucose values to recommend an adjustment in insulin dose. The patients can then use their predetermined CF and the recommended corrective parameter to add or subtract insulin based on insulin sensitivity. Although the two methods have a similar approach, the Scheiner method has more conservative recommendations for correction parameters. The Klonoff/Kerr formula offers a simplified approach by using rate of change midpoints for each trend arrow scenario and extrapolating anticipated glucose [9]. Simplified insulin dose adjustments are provided as insulin units similar to our approach. To assess safety, the authors consider the impact of insulin dose adjustments using minimum total daily insulin dose and the 1500 and 1960 rules. In the Klonoff/Kerr safety assessment, it was determined that the formula is limited to a range of CF values and/or total daily insulin dose, which restricts application in more insulin-sensitive individuals. Due to the complexity of applying the Klonoff/Kerr formula across insulin sensitivities and the fact that it was not considered in the development of our approach, we have not included the formula in our illustrated comparisons. Figure 2 provides a comparison of the DirecNet, Scheiner, and Pettus/Edelman methods; the Klonoff/Kerr formula is described above.
Figure 2.
Previous methods to adjust insulin doses using trend arrows. Three previously published methods for adjusting insulin dose using rtCGM trend arrow data are compared [DirecNet (Abbott system) [6], Scheiner (Medtronic and Dexcom systems) [7], and Pettus/Edelman (Dexcom system)] [8]. The DirecNet method takes total insulin dosage including carbohydrate consumption (if any) into consideration. Scheiner and Pettus/Edelman methods are based on anticipated change in blood glucose with the Scheiner method being more conservative in insulin adjustment. Notably, the author of the Scheiner method has presented slightly modified values in recent presentations (personal communication) relative to past publication [7]. We use the more recently presented values in this comparison. All three require calculations beyond correction and carbohydrate consumption. All three assume the patient has insulin requiring diabetes and is using rapid-acting insulin for meals and correction. Note that the recently published Klonoff/Kerr formula recommends adjusting insulin doses by 1, 1.5, or 2 U supplements/decrements for rates of change of 1 to 2, 2 to 3, and >3 mg/dL/min, respectively [9]. Conversion: mg/dL × 0.0555 = mmol/L.
Despite the described differences, the insulin dose adjustments using trend arrows are relatively similar and appear to be safe among the described approaches. However, the approaches are limited by their complexity, dependence on patients’ numeracy skills, and/or lack of guidance for postmeal application. Additionally, the Scheiner and Pettus/Edelman methods indirectly ask individuals to enter information that is different from actual measurement into their records and do not take into consideration the limitation of multiple daily insulin injections (MDIs) users with minimum insulin increments/decrements of 1.0 or 0.5 U. Importantly, the DirecNet method may pose a challenge in situations where larger amounts of carbohydrates are consumed as it calculates a percentage of the total insulin dose. For the Scheiner and Pettus/Edelman methods, we recognize that bolus calculators, either as an integrated tool in an insulin pump or as a standalone app, can be used to overcome the challenge of requiring additional calculations. However, the methods still pose additional challenges for MDI users who are constrained by minimum insulin adjustment increments of 1.0 or 0.5 U. We recognize that 0.5-U increment insulin pens are generally reserved for use in highly insulin-sensitive patients, which, based on our clinical experience, is more prevalent in pediatric patients. However, it is not uncommon for adults with type 1 diabetes to also be insulin sensitive and use of 0.5-U increment insulin pens can be a practical tool for diabetes management [18].
Overall, these methods may be overly reliant on use of bolus calculators. As a related concern, clinicians should be aware of the apps their patients use to calculate insulin doses because many bolus calculator apps have not been rigorously evaluated for accuracy and performance. The Klonoff/Kerr formula takes a similar approach to our method by providing adjustments in insulin units without calculation; however, the formula has limitations in the broader range of insulin sensitivities.
4. New Approach to “Adjusting for the Arrows”
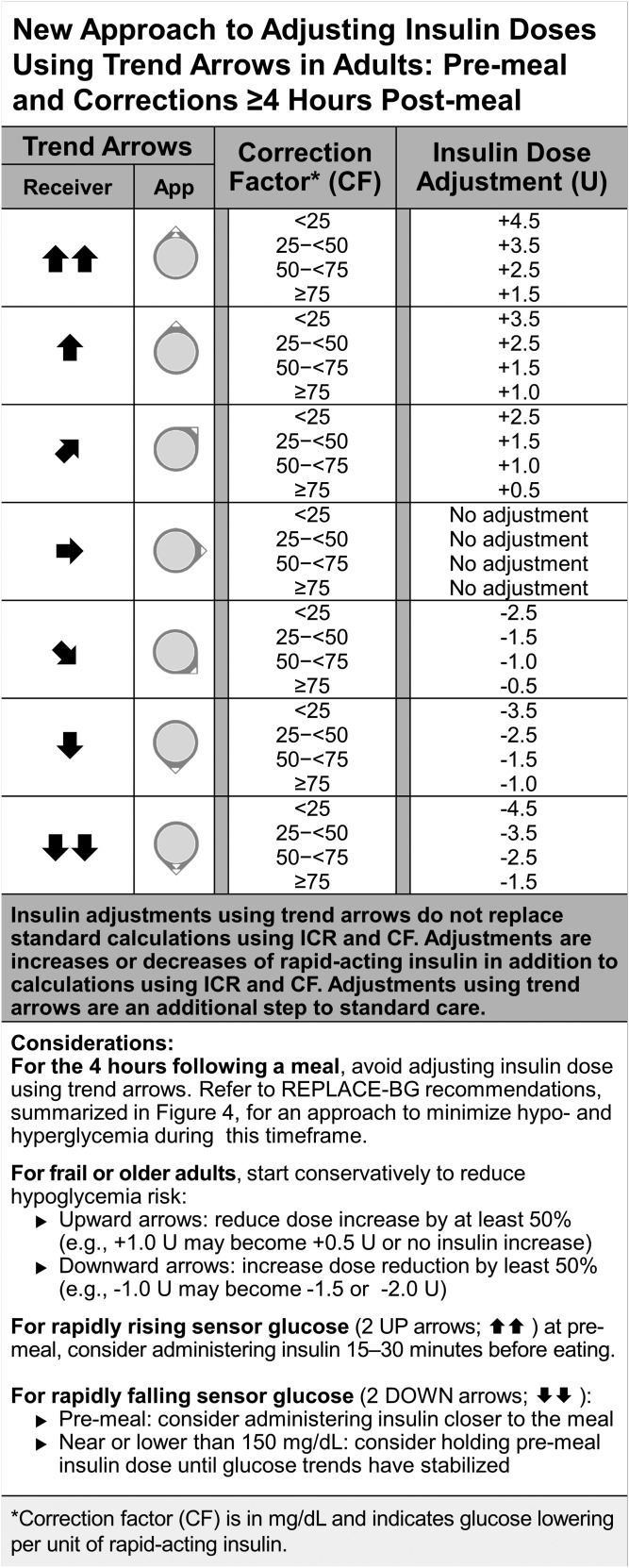
We preferred the Scheiner and Pettus/Edelman methods, which rely on insulin sensitivity, and we sought to address the limitations described above. Figure 3 outlines our approach to adjusting insulin dose using trend arrows, which is based on typical insulin sensitivity ranges for adult patients. For each insulin sensitivity range, we offer an insulin dose adjustment in insulin units. In this manner, insulin adjustments can be simply added or subtracted to standard calculations. The adjustments also take into consideration the limitations of 0.5-U increment minimums for MDI-treated individuals with substantial insulin sensitivity. Alternatively, adult patients using 1.0-U increments can round to the closest full unit. Additionally, an approach to postmeal monitoring and treatment is outlined in Fig. 4.
Figure 3.
New approach to adjust insulin doses using trend arrows in adults with diabetes. This figure outlines our approach to adjusting insulin dose using trend arrow data from the Dexcom G5. The approach is based on anticipated glucose change and typical insulin sensitivity ranges in adults. This simplified, practical approach provides adjustments in terms of insulin units over the range of insulin sensitivities to minimize additional calculations. It is generally recommended to start adjusting conservatively to understand how the recommendations impact individuals. The authors also recommend individuals use the REPLACE-BG study approach to minimize hypo- and hyperglycemia during the 4 hours following a meal (Fig. 4) rather than these insulin dose adjustments. It is essential to understand that adjusting insulin dose using trend arrows does not replace but adds to standard calculations using ICR and CF. The approach assumes the patient has insulin requiring diabetes, is using rapid-acting insulin for meals and correction, and is using ICR and CF factors that have been accurately determined. Conversion: mg/dL × 0.0555 = mmol/L. CF, correction factor in mg/dL indicates glucose lowering per unit of rapid-acting insulin; ICR, insulin to carbohydrate ratio; U, units of rapid-acting insulin.
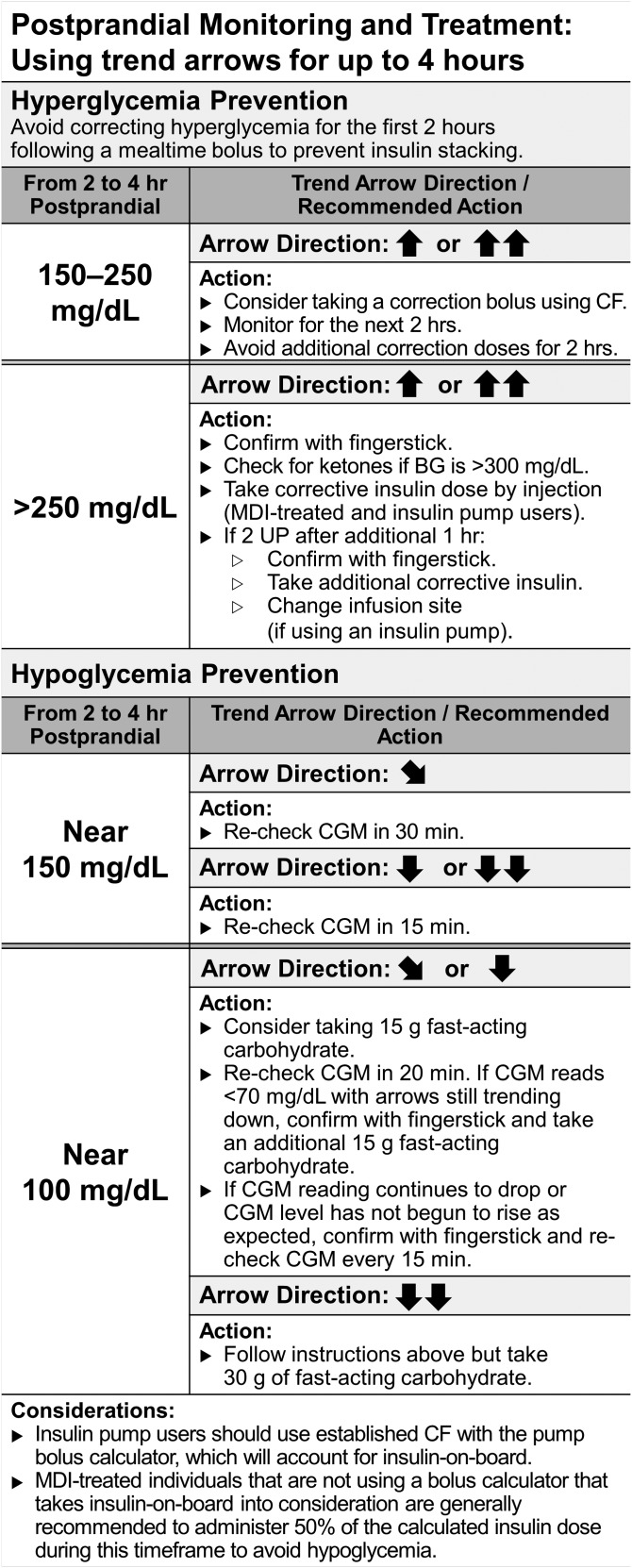
Figure 4.
Approach to postmeal monitoring and treatment using trend arrows. These suggestions are based on the REPLACE-BG trial [19], which demonstrated that the use of nonadjunctive rtCGM for insulin dose decisions was a safe and effective alternative to conventional adjunctive CGM use. In that setting, instructions were given to participants to monitor trend arrows and minimize glucose extremes following meals. It is especially important to take a standard approach to prevent insulin “stacking” and provide correction at appropriate times following meals. Importantly, these suggestions only use the patient’s CF and do not use the adjustments for trend arrows presented in Fig. 3. It is recommended that no corrective action be taken within the first 2 hours of eating to prevent glucose extremes. Recommendations serve as a guide for postprandial monitoring and correction. Beyond 4 hours, it is assumed that most, if not all, carbohydrate has entered the system and that there is no active insulin on board. In this case, the authors recommend using the trend arrows for dose adjustment (Fig. 3). Conversion: mg/dL × 0.0555 = mmol/L. CGM, continuous glucose monitor.
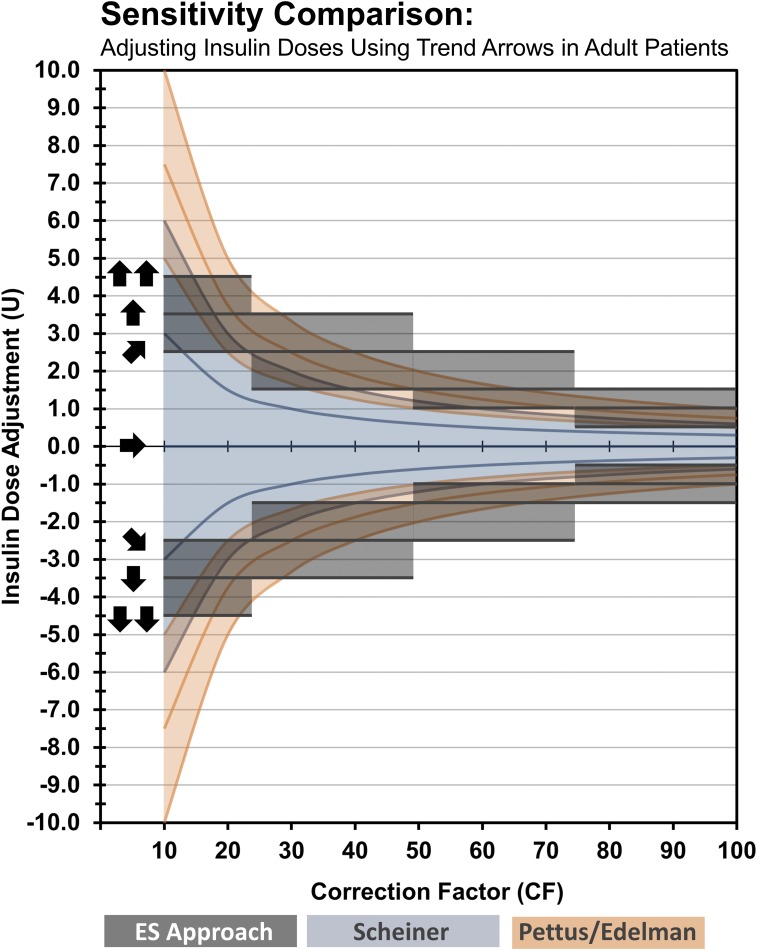
Our suggested insulin adjustments were determined by comparison of Scheiner and Pettus/Edelman methods at various insulin sensitivities and selection of conservative middle-ground insulin dosing guidance for different ranges of insulin sensitivity. Figure 5 is a visual comparison of insulin dose adjustments according to previous methods based on anticipated glucose (Scheiner and Pettus/Edelman) and our suggested approach based on insulin sensitivity ranges (Endocrine Society approach). The illustration shows that our approach aligns well with existing methods that indirectly use insulin sensitivity to adjust insulin doses while overcoming some of the limitations (e.g., a need for additional calculations and minimum increments possible for MDI-treated patients). We believe our approach allows patients to safely and effectively respond to trend arrow data by providing clinicians and patients with an easier tool to individualize insulin treatment. For example, the tool suggests insulin adjustments that may be considered more conservative (for insulin sensitive individuals) or more aggressive (for insulin resistant individuals) for each insulin sensitivity range.
Figure 5.
Sensitivity comparison of methods to adjust insulin doses using trend arrows in adult patients. The figure is a visual comparison of insulin dose adjustments according to previous methods based on anticipated glucose (Scheiner and Pettus/Edelman) and our suggested approach based on insulin sensitivity ranges (Endocrine Society approach). The illustration shows that our approach aligns well with existing methods that indirectly use insulin sensitivity to adjust insulin doses while overcoming some of the limitations (e.g., a need for additional calculations and minimum increments possible for MDI-treated patients). When applied to lower CF ranges (e.g., <25), our approach is more conservative, whereas in the midrange (e.g., 50 to <75), it is more aggressive. However, one must consider that the conversions used in our approach are based on 30 minutes. When considering the anticipated glucose at 1 hour, our suggested dose adjustments become more conservative. For example, a single UP trend arrow indicates that glucose is rising 2 to 3 mg/dL/min. At 30 minutes, the anticipated glucose would be 60 to 90 mg/dL higher. However, the anticipated glucose could be as much as 120 to 180 mg/dL higher at 60 minutes if exposed to other perturbations. If an individual’s CF was 60, our approach would recommend adding 1.5 U of rapid-acting insulin to the premeal bolus. The additional 1.5 U of insulin would be expected to provide additional glucose lowering of 60 mg/dL over the 60 minutes. Given that the 60-minute anticipated glucose could potentially be much higher at 1-hour, our suggestion could be considered conservative. The expected glucose would be closer to target, postprandially, without overcorrecting and without increasing risk for hypoglycemia. As noted, these recommendations are starting points and should be readjusted as experience increases and responsiveness is observed and understood. Conversion: mg/dL × 0.0555 = mmol/L. CF, correction factor in mg/dL indicates glucose lowering per unit of rapid-acting insulin; U, units of rapid-acting insulin.
We also include an approach to postmeal monitoring and treatment in adults based on the guidelines used in the REPLACE-BG trial [19]. Adults using rtCGM are often frustrated when they see rapid rises in postmeal glucose values. Administering additional insulin when there is still significant insulin on board—so-called “chase bolusing” or “stacking”—can result in hypoglycemia.
The REPLACE-BG study suggested an approach to participants to minimize hypo- and hyperglycemia during the 4 hours following a meal. This is the timeframe during which most foods will impact blood glucose levels and is the activity duration commonly used for rapid-acting insulin in standard calculations for adults. Figure 4 outlines the approach. As a general rule, we recommend caution when adjusting insulin dose using trend arrows during the 4 hours following a meal due to the many variables that affect rate of glucose change during this time. Importantly, the REPLACE-BG study suggested waiting at least 2 hours after a mealtime bolus before taking any corrective action (i.e., standard corrections based on CF or insulin dose adjustments using trend arrows).
In brief, we offer an approach to adjusting insulin dose using trend arrows based on insulin sensitivity and suggest insulin units rather than corrective values. We suggest using this approach to adjust the premeal insulin dose (Fig. 3). During the 4 hours following meals, we suggest following the REPLACE-BG approach to minimize hypo- and hyperglycemia (Fig. 4). Beyond the 4 hours following meals, our approach to adjustment may be used to adjust corrective insulin doses. We provide several additional case examples to illustrate how our approach may be used in real-life scenarios in Table 4.
Table 4.
Case Examples to Put Our Approach Into Practice for Adults
| Examples Assume ICR and CF Values Have Been Accurately Determined by the Patient’s Health Care Team and That the Patient is Administering Rapid-Acting Insulin for Boluses and Corrections. Examples Assume the Patients Have Insulin-Requiring Diabetes and Are Using Dexcom G5 rtCGM. | ||||||||
|---|---|---|---|---|---|---|---|---|
| A | A 35-year-old, MDI-treated man sits down to eat a meal with 35 g carbohydrate. A SINGLE UP arrow is present. A calculated insulin dose is determined using meal and correction parameters. Due to the single UP arrow and no plan to exercise, an adjustment of +2.5 U is suggested. This increases the total insulin dose to 8.0 U. | |||||||
| Sensor Glucose | Trend Arrow | Target Glucose | Carb | Parameters | Calculated Insulin Dose | Trend Arrow Adjustment | Total Insulin Dose | |
| 180 mg/dL | ↑ | 120 mg/dL | 35 g | CF–30 | 2.0 U + 3.5 U = 5.5 U | +2.5 U | 8.0 U | |
| ICR–1:10 | ||||||||
| B | A 52-year-old woman on insulin pump therapy is eating 30 g of carbohydrate for lunch and sees a SINGLE DOWN arrow. The calculated insulin dose is determined. A negative adjustment of −1.5 U is suggested to account for the falling blood glucose and to prevent hypoglycemia. This decreases the total insulin dose to 2.5 U. (Note: An MDI-treated individual may round the total insulin dose to 2.0 U.) | |||||||
| Sensor Glucose | Trend Arrow | Target Glucose | Carb | Parameters | Calculated Insulin Dose | Trend Arrow Adjustment | Total Insulin Dose | |
| 220 mg/dL | ↓ | 120 mg/dL | 30 g | CF–50 | 2.0 U + 2.0 U = 4.0 U | −1.5 U | 2.5 U | |
| ICR–1:15 | ||||||||
| C | A 75-year-old man on MDI therapy is about to eat a meal with 50 g of carbohydrate. His sensor glucose is elevated and a SINGLE DOWN arrow is present. The calculated insulin dose is determined. A negative adjustment of −1.5 U is suggested to account for the falling blood glucose and prevent hypoglycemia. However, due to the frailty of the patient, the adjustment is further reduced by at least 50% (i.e., further reduced by at least 0.75 U in this case). Out of an abundance of caution and convenience of rounding to whole units, the man decides to reduce by 1.0 U for a total negative adjustment of −2.5 U. This decreases the total insulin dose to 2.0 U. | |||||||
| Sensor Glucose | Trend Arrow | Target Glucose | Carb | Parameters | Calculated Insulin Dose | Trend Arrow Adjustment | Total Insulin Dose | |
| 240 mg/dL | ↓ | 120 mg/dL | 50 g | CF–60 | 2.0 U + 2.5 U = 4.5 U | −2.5 U | 2.0 U | |
| ICR–1:20 | ||||||||
| D | A 28-year-old man on insulin pump therapy is noticing a mildly elevated sensor glucose and an ANGLE DOWN arrow 90 minutes after eating a meal with 45 g of carbohydrate. He is considering taking a little extra insulin. However, he follows the suggestions for postmeal monitoring and hypoglycemia prevention and determines that neither a correction nor an adjustment is needed at this time. He will recheck his sensor glucose in 30 minutes. | |||||||
| Sensor Glucose | Trend Arrow | Target Glucose | Carb | Parameters | Calculated Insulin Dose | Trend Arrow Adjustment | Total Insulin Dose | |
| 170 mg/dL | ↘ | 120 mg/dL | 0 g | CF–50 | NA | NA | NA | |
| ICR–1:10 | ||||||||
| E | A 23-year-old woman on MDI therapy is experiencing hyperglycemia 2.5 hours after eating a meal with 45 g of carbohydrate. At the time of eating, she took the appropriate amount of insulin and did not need a correction. Now she is seeing DOUBLE UP arrows. Because she is within 4 hours of eating, she will follow the suggestions for postmeal monitoring and hyperglycemia prevention and will not use the adjustment table. Also, she is an MDI-treated patient who is not using a bolus calculator that accounts for insulin-on-board. Therefore, she reduces the calculated correction insulin dose of 2.0 U by 50% to prevent overcorrection. This reduces the total insulin dose to 1.0 U. She will recheck in 1 hour. | |||||||
| Sensor Glucose | Trend Arrow | Target Glucose | Carb | Parameters | Calculated Insulin Dose | Trend Arrow Adjustment | Total Insulin Dose | |
| 240 mg/dL | ↑↑ | 120 mg/dL | 0 g | CF–60 | 2.0 U × 50% = 1.0 U | NA | 1.0 U | |
| ICR–1:10 | ||||||||
| F | A 49-year-old man on insulin pump therapy is working late into a stressful afternoon. Lunch was nearly 5 hours ago. He notices his sensor glucose is elevated with an ANGLE UP arrow. He calculates his insulin dose for correction using predetermined values and then uses the adjustment table to account for the rising blood glucose. He uses the adjustment table because he is beyond 4 hours from his last meal and there shouldn’t be any active insulin-on-board from his mealtime bolus. He adds the adjustment of 1.5 U, which increases his total insulin dose to 3.8 U. | |||||||
| Sensor Glucose | Trend Arrow | Target Glucose | Carb | Parameters | Calculated Insulin Dose | Trend Arrow Adjustment | Total Insulin Dose | |
| 180 mg/dL | ↗ | 100 mg/dL | 0 g | CF–35 | 2.3 U | +1.5 U | 3.8 U | |
| ICR–1:8 | ||||||||
| G | A 35-year-old woman has been struggling with a headache all morning. She takes 1 g of acetaminophen. After 1 hour, she notices a high sensor glucose value and DOUBLE UP arrows. However, she recalls that the Dexcom G5 can yield falsely high readings when acetaminophen is used. She checks her blood glucose by fingerstick and does not use the trend arrow data or adjustment table. The fingerstick reading shows she is actually at 115 mg/dL. No correction is needed. She will continue to use fingerstick monitoring until 6 hours has passed since ingesting the acetaminophen. | |||||||
| Sensor Glucose | Trend Arrow | Target Glucose | Carb | Parameters | Calculated Insulin Dose | Trend Arrow Adjustment | Total Insulin Dose | |
| 243 mg/dL | ↑↑ | 120 mg/dL | 0 g | CF–70 | 0.0 U | NA | 0.0 U | |
| ICR–1:20 | ||||||||
| H | A 38-year-old woman on MDI therapy has had to walk much more than usual during her midday work. She managed to stay near her target glucose; however, as she prepares for her lunch, she sees DOUBLE DOWN arrows. She calculates the insulin dose a slight correction and her meal with 45 g carbohydrate. Then she uses the adjustment table, which suggests a negative adjustment of −3.5 U. After rounding, this is a net total insulin dose of 0.0 U. She will eat without taking an insulin dose and carefully monitor the rest of the afternoon. (Note: If she was consuming less carbohydrate or was below target glucose, a negative total insulin dose may result, in which case, 15 g of fast-acting carbohydrate and close monitoring until trend arrows stabilize may be more appropriate than consuming her typical lunch.) | |||||||
| Sensor Glucose | Trend Arrow | Target Glucose | Carb | Parameters | Calculated Insulin Dose | Trend Arrow Adjustment | Total Insulin Dose | |
| 160 mg/dL | ↓↓ | 130 mg/dL | 45 g | CF–45 | 0.7 U + 3.0 U = 3.7 U | −3.5 U | 0.0 U | |
| ICR–1:15 | ||||||||
Calculated Insulin Dose includes insulin needed to cover carbohydrate intake and correction to reach target glucose. The calculations use the predetermined ICR and CF values and assume these values have been accurately determined by the patient’s health care team and that the patient is using rapid-acting insulin for carbohydrate intake and correction.
Abbreviations: CF, correction factor in mg/dL indicates glucose lowering per unit of rapid-acting insulin; U, units of rapid-acting insulin. Conversion: mg/dL × 0.0555 = mmol/L.
It is worth reiterating that our approach to insulin dose adjustment does not replace standard calculations. It should also not be used in the event of missed meal boluses or miscalculations. It is more prudent to calculate insulin dose based on the carbohydrate consumed and CGM value at the time of the meal. The insulin dose should be taken as soon as the missed bolus is recognized, preferably within 2 hours of the missed dose. Adjusting insulin doses using trend arrows should also be avoided in cases of underestimating carbohydrate intake (i.e., miscalculations) and overcorrecting for hypoglycemia with fast-acting carbohydrate. At these times, the trend arrows can serve an important role as reminders to patients of a missed dose or a miscalculation. For other unplanned situations, more specific strategies for the use of trend arrows should be established between patients and their health care providers.
5. Important Considerations When “Adjusting for the Arrows”
A. Sick Day Management and Medication Considerations
During illness, there is increased risk of hyperglycemia, diabetic ketoacidosis, and acetaminophen ingestion (intentional or inadvertent). A significant concern with rtCGM is falsely elevated glucose readings due to acetaminophen interference [16, 17]. rtCGM users should be aware of each manufacturer’s recommendations regarding acetaminophen interference. A recent study reported significant differences between rtCGM and fingerstick glucose readings for up to 6 hours after acetaminophen ingestion [17]. Case example (G) in Table 4 illustrates a real-life example. Notably, acetaminophen interference will likely be eliminated in future CGM device models.
CGM likely offers some benefit in patients with acute illness, but special precautions must be considered. It is advisable to check glucose by confirmatory fingerstick every 2 to 4 hours and to consider insulin correction every 2 to 3 hours (with appropriate ketone testing) during illness. Additionally, patients should consider using fingerstick monitoring for treatment decisions when glucose is >250 mg/dL and ketones (blood or urine) are present and when glucose is <70 mg/dL or symptoms of hypoglycemia are present.
Patients should be counseled to carefully read labels of all medications they are taking, and if they choose a medication that contains acetaminophen, they should base all treatment decisions on fingerstick glucose values for the 6 hours following ingestion. A list of common medications that contain acetaminophen is included in Table 3.
B. Elderly and Frail Adults
Elderly patients with diabetes are at notably higher risk for severe hypoglycemia due to age, duration of diabetes, duration of insulin therapy, and greater prevalence of hypoglycemia unawareness [20–24]. The increased risk is compounded by cognitive and physical impairments and other comorbidities. Frail individuals may also have a higher risk of complications, comorbid conditions (e.g., cognitive deficits, renal disease, joint disease, osteoporosis, fracture, and/or cardiovascular disease), and often require assisted care, which can complicate treatment regimens. Elderly and/or frail individuals are also more likely to be prescribed medications that contain acetaminophen and have complications such as compromised renal function that exacerbate and extend the impact of acetaminophen on CGM readings.
For these reasons, it is important to start conservatively with frail or older adults when using our approach (Fig. 3). For example, with upward arrows, it is advisable to adjust the insulin dose by ~1 U less than suggested or reduce the additional insulin by at least 50%. Case example (C) in Table 4 illustrates a real-life example. Note that these modifications are not based on clinical data but are intended to help avoid hypoglycemia in these vulnerable populations.
An important feature of the Dexcom G5 is the data-sharing capability, which can be especially helpful to caregivers of elderly and frail patients. The Dexcom G5 Mobile and Dexcom Follow apps allow the patient to share real-time data with up to five designated individuals who can monitor glucose levels remotely on compatible smart devices. If the patient chooses to use this option, it is important that caregivers receive the same training in rtCGM; specifically, training on the interpretation and use of trend arrows.
C. Exercise/Physical Activity
Glycemic responses to exercise are complex and can be influenced by glucose concentrations prior to exercise, amount of active insulin, insulin infusion/injection site, and composition of previous meal, as well as type, intensity, and duration of activity [25]. Aerobic exercise (e.g., running, swimming, cycling) increases glucose uptake and insulin sensitivity leading to acute and delayed hypoglycemia, which can last up to 24 hours [26]. Anaerobic activity (e.g., strength training) on the other hand, may lead to acute hyperglycemia but can also increase the risk for nocturnal hypoglycemia.
rtCGM is an accurate and useful tool during exercise due to the ability to alert users to abrupt trend changes and the onset of hypoglycemia, which may be more rapid and less noticeable during exertion. For adults, there is a greater likelihood of scheduled exercise that allows planning for scheduled physical activity; however, in view of work schedules, this may not happen all that often.
Due to these complexities, we recommend conservative insulin dose adjustments prior to exercise and do not recommend increasing insulin doses when upward arrows are present during active exercise periods. Additionally, we recommend close monitoring of downward arrows and correction of impending hypoglycemia with carbohydrate intake as needed during exercise. For detailed reviews on exercise strategies for patients with diabetes, we recommend consulting recent publications [25, 27].
D. Environmental Factors
There are no indications that environmental factors, such as extreme temperature, altitude, or humidity, impact rtCGM accuracy, but they do affect fingerstick blood glucose monitoring [28]. Because calibration is essential to rtCGM accuracy, patients should be advised to perform device calibration prior to exposure to any of these environmental conditions.
6. Summary
Our goal is to provide a safe, practical approach to using Dexcom G5 trend arrow data. The approach we present here is based on a review of four previously published methods. Our approach focuses on typical insulin sensitivity ranges used in adults and provides a range of adjustments in discrete insulin units. We believe this simplified approach reduces numeracy requirements and the number of calculations, which will help patients improve glucose control and increase glucose time in range without hypoglycemia, while promoting clinical discussion.
Many questions concerning best practices for using rtCGM remain: How will remote monitoring impact patient care? Does using trend arrows improve diabetes management during pregnancy or in older adults with mild cognitive impairment? What modifications will be needed as new devices and new insulins (e.g., ultra-rapid-acting insulin analogs) become available? How should we use trend arrows with other types of insulin (e.g., human regular insulin and inhaled insulin)? How can we increase access to rtCGM technology? Studies are needed to address these questions and others that will likely emerge.
The use of rtCGM can be a valuable tool for reactive and predictive fine-tuning of insulin dosing when “adjusting for the arrows.” With this approach as a starting point, we hope to see more empirically based information and similar guidance developed for all currently available and emerging glucose monitoring devices.
Acknowledgments
The authors thank the Endocrine Society for convening and facilitating the writing group and Christopher G. Parkin for editorial support in developing this manuscript.
Financial Support: This publication was supported by an unrestricted educational grant to the Endocrine Society from Dexcom, Inc., San Diego, California.
Author Contributions: G.A. led the writing group as chair and facilitated development and group consensus of the methods proposed. All authors contributed equally in manuscript review, discussion of the results and implications, and commented on the manuscript at all stages.
Acknowledgments
Disclosure Summary: G.A. has served as an advisor board member for Novo Nordisk, has served as a consultant to Boehringer Ingelheim and Dexcom, and her institution has received research support from AstraZeneca. L.M.L. has served as a consultant to AstraZeneca, Boehringer Ingelheim Pharmaceuticals, Dexcom, Eli Lilly and Company, Insulet, Johnson & Johnson, MannKind Corporation, Menarini Diagnostics, Novo Nordisk, Roche Diabetes Care, and Sanofi U.S. A.J.A. has served as a consultant to Novo Nordisk, Dexcom, and Horizon CME, and his institution has received research support from Novo Nordisk, Lexicon, Dexcom, and Medtronic. I.B.H. has served as a consultant to Abbott Diabetes Care, Adocia, Bigfoot, Intarcia, and Roche Diabetes Care. D.F.K. has served on advisory boards and/or speaker bureaus for Novo Nordisk, Abbott, Eli Lilly and Company, Sanofi, Aventis, Janssen, Dexcom, Intarcia, Valeritas, AstraZeneca, Boehringer Ingelheim, and Insulet, and her institution has received research support from AstraZeneca, Eli Lilly and Company, Novo Nordisk, Dexcom, and Lexicon. A.P. has served as a consultant, advisory board member and/or speaker for Abbott Diabetes Care, Becton Dickinson, Bigfoot, Boehringer Ingelheim, Dexcom, Eli Lilly and Company, Janssen, Lexicon, Livongo, Medscape, Merck, Novo Nordisk, Omada Health, Sanofi, and Science 37. R.S.W. has participated in multicenter clinical trials sponsored by Diasome Pharmaceuticals, Calibra Medical, Mylan GmbH, and Medtronic, and has received CGM devices from Dexcom for a pilot research project. D.R.H. has no disclosures to report.
Footnotes
- CF
- correction factor
- CGM
- continuous glucose monitoring
- ICR
- insulin-to-carbohydrate ratio
- MDI
- multiple daily insulin injection
- rtCGM
- real-time continuous glucose monitoring.
References and Notes
- 1.Beck RW, Riddlesworth T, Ruedy K, Ahmann A, Bergenstal R, Haller S, Kollman C, Kruger D, McGill JB, Polonsky W, Toschi E, Wolpert H, Price D; DIAMOND Study Group . Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND Randomized Clinical Trial. JAMA. 2017;317(4):371–378. [DOI] [PubMed] [Google Scholar]
- 2.Battelino T, Conget I, Olsen B, Schütz-Fuhrmann I, Hommel E, Hoogma R, Schierloh U, Sulli N, Bolinder J; SWITCH Study Group . The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55(12):3155–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lind M, Polonsky W, Hirsch IB, Heise T, Bolinder J, Dahlqvist S, Schwarz E, Ólafsdóttir AF, Frid A, Wedel H, Ahlén E, Nyström T, Hellman J. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD Randomized Clinical Trial. JAMA. 2017;317(4):379–387. [DOI] [PubMed] [Google Scholar]
- 4.Beck RW, Riddlesworth TD, Ruedy KJ, Ahmann A, Haller S, Kruger D, Aronoff S, Aronson R, Toschi E, Kollman C, Bergenstal RM. Continuous glucose monitoring vs usual care in type 2 diabetes patients on multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167(6):365–374. [DOI] [PubMed] [Google Scholar]
- 5.Ruedy KJ, Parkin CG, Riddlesworth TD, Graham C; DIAMOND Study Group . Continuous glucose monitoring in older adults with type 1 and type 2 diabetes using multiple daily injections of insulin: results from the DIAMOND trial. J Diabetes Sci Technol. 2017;11(6):1138–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckingham B, Xing D, Weinzimer S, Fiallo-Scharer R, Kollman C, Mauras N, Tsalikian E, Tamborlane W, Wysocki T, Ruedy K, Beck R; Diabetes Research In Children Network (DirecNet) Study Group . Use of the DirecNet Applied Treatment Algorithm (DATA) for diabetes management with a real-time continuous glucose monitor (the FreeStyle Navigator). Pediatr Diabetes. 2008;9(2):142–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheiner G. Practical CGM: Improving Patient Outcomes Through Continuous Glucose Monitoring. 4th edAlexandria, VA: American Diabetes Association; 2015. [Google Scholar]
- 8.Pettus J, Edelman SV. Recommendations for using real-time continuous glucose monitoring (rtCGM) data for insulin adjustments in type 1 diabetes. J Diabetes Sci Technol. 2017;11(1):138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klonoff DC, Kerr D. A simplified approach using rate of change arrows to adjust insulin with real-time continuous glucose monitoring. J Diabetes Sci Technol. 2017;11(6):1063–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirsch IB, Abelseth J, Bode BW, Fischer JS, Kaufman FR, Mastrototaro J, Parkin CG, Wolpert HA, Buckingham BA. Sensor-augmented insulin pump therapy: results of the first randomized treat-to-target study. Diabetes Technol Ther. 2008;10(5):377–383. [DOI] [PubMed] [Google Scholar]
- 11.JDRF CGM Study Group JDRF randomized clinical trial to assess the efficacy of real-time continuous glucose monitoring in the management of type 1 diabetes: research design and methods. Diabetes Technol Ther. 2008;10(4):310–321. [DOI] [PubMed] [Google Scholar]
- 12.Scheiner G. Continuous glucose monitoring. Making sense of your numbers. Diabetes Self Manag. 2008;25(3):42, 44, 48–50. [PubMed] [Google Scholar]
- 13.Peters AL, Ahmann AJ, Battelino T, Evert A, Hirsch IB, Murad MH, Winter WE, Wolpert H. Diabetes technology-continuous subcutaneous insulin infusion therapy and continuous glucose monitoring in adults: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101(11):3922–3937. [DOI] [PubMed] [Google Scholar]
- 14.Fonseca VA, Grunberger G, Anhalt H, Bailey TS, Blevins T, Garg SK, Handelsman Y, Hirsch IB, Orzeck EA, Roberts VL, Tamborlane W; Consensus Conference Writing Committee . Continuous glucose monitoring: a consensus conference of the American Association of Clinical Endocrinologists and American College of Endocrinology. Endocr Pract. 2016;22(8):1008–1021. [DOI] [PubMed] [Google Scholar]
- 15.Faccioli S, Del Favero S, Visentin R, Bonfanti R, Iafusco D, Rabbone I, Marigliano M, Schiaffini R, Bruttomesso D, Cobelli C. PedArPan Study Group . Accuracy of a CGM sensor in pediatric subjects with type 1 diabetes. comparison of three insertion sites: arm, abdomen, and gluteus. J Diabetes Sci Technol. 2017;11(6):1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basu A, Veettil S, Dyer R, Peyser T, Basu R. Direct evidence of acetaminophen interference with subcutaneous glucose sensing in humans: a pilot study. Diabetes Technol Ther. 2016;18(Suppl 2):S243–S247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maahs DM, DeSalvo D, Pyle L, Ly T, Messer L, Clinton P, Westfall E, Wadwa RP, Buckingham B. Effect of acetaminophen on CGM glucose in an outpatient setting. Diabetes Care. 2015;38(10):e158–e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klonoff DC, Nayberg I, Stauder U, Oualali H, Domenger C. Half-unit insulin pens: disease management in patients with diabetes who are sensitive to insulin. J Diabetes Sci Technol. 2017;11(3):623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aleppo G, Ruedy KJ, Riddlesworth TD, Kruger DF, Peters AL, Hirsch I, Bergenstal RM, Toschi E, Ahmann AJ, Shah VN, Rickels MR, Bode BW, Philis-Tsimikas A, Pop-Busui R, Rodriguez H, Eyth E, Bhargava A, Kollman C, Beck RW; REPLACE-BG Study Group . REPLACE-BG: a randomized trial comparing continuous glucose monitoring with and without routine blood glucose monitoring in adults with well-controlled type 1 diabetes. Diabetes Care. 2017;40(4):538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinstock RS, DuBose SN, Bergenstal RM, Chaytor NS, Peterson C, Olson BA, Munshi MN, Perrin AJ, Miller KM, Beck RW, Liljenquist DR, Aleppo G, Buse JB, Kruger D, Bhargava A, Goland RS, Edelen RC, Pratley RE, Peters AL, Rodriguez H, Ahmann AJ, Lock JP, Garg SK, Rickels MR, Hirsch IB; T1D Exchange Severe Hypoglycemia in Older Adults With Type 1 Diabetes Study Group . Risk factors associated with severe hypoglycemia in older adults with type 1 diabetes. Diabetes Care. 2015;39(4):603–610. [DOI] [PubMed] [Google Scholar]
- 21.Bremer JP, Jauch-Chara K, Hallschmid M, Schmid S, Schultes B. Hypoglycemia unawareness in older compared with middle-aged patients with type 2 diabetes. Diabetes Care. 2009;32(8):1513–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Punthakee Z, Miller ME, Launer LJ, Williamson JD, Lazar RM, Cukierman-Yaffee T, Seaquist ER, Ismail-Beigi F, Sullivan MD, Lovato LC, Bergenstal RM, Gerstein HC; ACCORD Group of Investigators; ACCORD-MIND Investigators . Poor cognitive function and risk of severe hypoglycemia in type 2 diabetes: post hoc epidemiologic analysis of the ACCORD trial. Diabetes Care. 2012;35(4):787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giorda CB, Ozzello A, Gentile S, Aglialoro A, Chiambretti A, Baccetti F, Gentile FM, Lucisano G, Nicolucci A, Rossi MC; HYPOS-1 Study Group of AMD . Incidence and risk factors for severe and symptomatic hypoglycemia in type 1 diabetes. Results of the HYPOS-1 study. Acta Diabetol. 2015;52(5):845–853. [DOI] [PubMed] [Google Scholar]
- 24.Cariou B, Fontaine P, Eschwege E, Lièvre M, Gouet D, Huet D, Madani S, Lavigne S, Charbonnel B. Frequency and predictors of confirmed hypoglycaemia in type 1 and insulin-treated type 2 diabetes mellitus patients in a real-life setting: results from the DIALOG study. Diabetes Metab. 2015;41(2):116–125. [DOI] [PubMed] [Google Scholar]
- 25.Bally L, Laimer M, Stettler C. Exercise-associated glucose metabolism in individuals with type 1 diabetes mellitus. Curr Opin Clin Nutr Metab Care. 2015;18(4):428–433. [DOI] [PubMed] [Google Scholar]
- 26.Teich T, Riddell MC. The enhancement of muscle insulin sensitivity after exercise: a Rac1-independent handoff to some other player? Endocrinology. 2016;157(8):2999–3001. [DOI] [PubMed] [Google Scholar]
- 27.Riddell MC, Gallen IW, Smart CE, Taplin CE, Adolfsson P, Lumb AN, Kowalski A, Rabasa-Lhoret R, McCrimmon RJ, Hume C, Annan F, Fournier PA, Graham C, Bode B, Galassetti P, Jones TW, Millán IS, Heise T, Peters AL, Petz A, Laffel LM. Exercise management in type 1 diabetes: a consensus statement. Lancet Diabetes Endocrinol. 2017;5(5):377–390. [DOI] [PubMed] [Google Scholar]
- 28.Erbach M, Freckmann G, Hinzmann R, Kulzer B, Ziegler R, Heinemann L, Schnell O. Interferences and limitations in blood glucose self-testing: an overview of the current knowledge. J Diabetes Sci Technol. 2016;10(5):1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]