Abstract
Autologous bone marrow mononuclear cell (BM-MNC) therapy for patients with ST-segment elevation myocardial infarction (STEMI) has produced inconsistent results, possibly due to BM-MNC product heterogeneity. Patient-specific cardiovascular risk factors (CRFs) may contribute to variations in BM-MNC composition. We sought to identify associations between BM-MNC subset frequencies and specific CRFs in STEMI patients. Bone marrow was collected from 191 STEMI patients enrolled in the CCTRN TIME and LateTIME trials. Relationships between BM-MNC subsets and CRFs were determined with multivariate analyses. An assessment of CRFs showed that hyperlipidemia and hypertension were associated with a higher frequency of CD11b+ cells (P = 0.045 and P = 0.016, respectively). In addition, we found that females had lower frequencies of CD11b+ (P = 0.018) and CD45+CD14+ (P = 0.028) cells than males, age was inversely associated with the frequency of CD45+CD31+ cells (P = 0.001), smoking was associated with a decreased frequency of CD45+CD31+ cells (P = 0.013), glucose level was positively associated with the frequency of CD45+CD3+ cells, and creatinine level (an indicator of renal function) was inversely associated with the frequency of CD45+CD3+ cells (P = 0.015). In conclusion, the frequencies of monocytic, lymphocytic, and angiogenic BM-MNCs varied in relation to patients’ CRFs. These phenotypic variations may affect cell therapy outcomes and might be an important consideration when selecting patients for and reviewing results from autologous cell therapy trials.
Keywords: Bone marrow mononuclear cells, Ischemic heart disease, Cardiovascular risk factors, ST-segment elevation myocardial infarction, Autologous cell therapy
Introduction
End-stage heart failure results in the deaths of more than 60,000 patients annually in the US. In the western world, the leading cause of heart failure is ischemic heart disease [59]. Several approaches have been proposed for the treatment of patients with ischemic heart disease (IHD), one of which is stem cell therapy. Although stem cell therapies have shown promise in preclinical studies as a treatment for IHD [22, 25, 33, 40], clinical trials have produced inconsistent results [1, 10, 13, 16, 20, 51, 52, 58]. Thus, efforts are underway to determine ways to optimize these therapies.
Patients with IHD have a wide range of cardiovascular risk factors (CRFs). In a study by Nauta et al. [32], 69.3% of patients with acute myocardial infarction (MI) had at least one CRF; from those, 39.2% had a single CRF, 21.7% had 2, and 8.4% had 3 or 4. These CRFs have been shown to correlate with changes in the frequencies of particular cell types in the blood. Recently, it was reported that hypertension is associated with an increase in the level of circulating CD11b+ cells [38], advanced age and smoking are associated with a decrease in circulating CD31+ leukocytes [14], age is inversely associated with the level of CD34+ cells [30], and diabetes is associated with a decrease in circulating endothelial progenitor cells [26].
Changes in the cellular composition of the blood and bone marrow (BM) could affect the outcomes of IHD. For example, Cogle et al. [8] showed a negative correlation between the percentage of CD11b+ cells in the BM and post-infarct left ventricular ejection fraction (LVEF) in patients with ST-segment elevation myocardial infarction (STEMI), regardless of whether they received BM mononuclear cells (BM-MNCs) or placebo. In addition, Schutt et al. [41] found that infarct size reduction after STEMI was greater in patients who had a higher percentage of CD31+ mononuclear cells in the BM.
We hypothesized that a patient’s CRFs may affect the frequencies of specific angiogenic, lymphocytic, monocytic, and hematopoietic cells within the BM, which could, in turn, impact the efficacy of autologous BM-MNC therapies. To test this hypothesis, we assessed the relationships between the frequencies of BM-MNCs expressing CD34, CD31, CD3, CD14, CD11b, CD19, CD45, and C-X-C chemokine motif receptor 4 (CXCR4) and 8 CRFs in patients with STEMI.
Methods
For this retrospective analysis, our study cohort comprised patients who participated in the Cardiovascular cell therapy research network (CCTRN) transplantation in myocardial infarction evaluation (TIME) and LateTIME trials and provided consent to have their remaining BM-MNC product further analyzed at the CCTRN Biorepository [51–54, 62]. The CCTRN TIME and LateTIME trials had similar inclusion criteria but differed in the timing of BM aspiration and cell delivery after STEMI. Institutional Review Boards at each clinical site approved the protocols for both trials, and all participants provided written informed consent.
Flow cytometry
BM-MNC samples collected at baseline (i.e., before treatment) were immunophenotyped via polychromatic flow cytometry to determine the frequency of hematopoietic, lymphocytic, monocytic, and angiogenic precursors in the BM, as previously described [51, 53]. Briefly, 1–5 million BM-MNCs incubated with antibodies against CD34, CD11b, CD31, CD45, CD3, CD14, CD19, and CXCR4 for 20 min at 4 °C in the dark, washed twice in 2.5% phosphate-buffered saline (PBS), and then resuspended to a final volume of 1 mL in 2.5% PBS for flow cytometry analysis. Samples were analyzed with an LSR II flow cytometer (Becton–Dickinson, Franklin Lakes, NJ, USA), and the data were analyzed with the FlowJo software (Tree Star, Inc., Ashland, OR, USA). All analyses were performed on gated lymphocytes or monocytes or using the International Society of Hematotherapy and Graft Engineering (ISHAGE) gating strategy [45]. Figures 1 and 2 show examples of the gating strategies used to determine the frequencies of specific lymphocyte, monocyte, and hematopoietic stem cell populations in the BM samples.
Fig. 1.
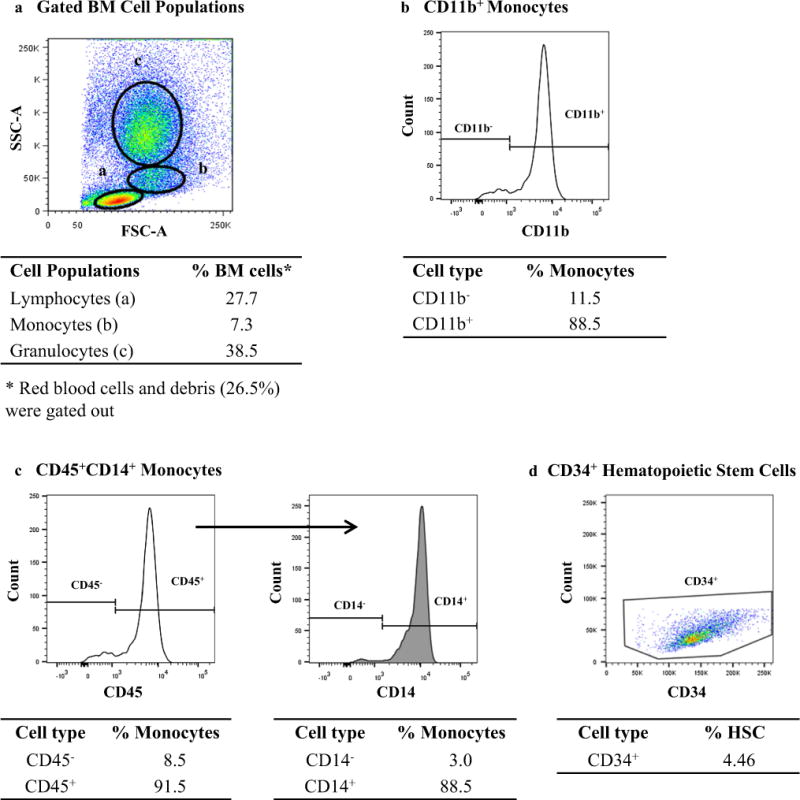
Gating strategy used for analyzing CD11b+ and CD45+−CD14+ monocyte subsets and CD34+ hematopoietic stem cells (HSC) in the bone marrow (BM). a Representative dot plot showing the gates used to identify bone marrow mononuclear cell (BM-MNC) populations based on forward scatter (FSC-A) and side scatter (SSC-A). b Representative histogram showing CD11b+ cells within the monocyte gate. c Representative histogram showing the CD14+ cells (right panel) gated from CD45+ cells (left panel) within the monocyte gate. d Representative dot plot showing the CD45dimCD31+ SSClow HSCs using the ISHAGE gating strategy (not shown). Percentages shown in b and c are based on the total monocyte population. Percentage shown in d is based on the CD45+ cells. All data presented are from a single patient
Fig. 2.
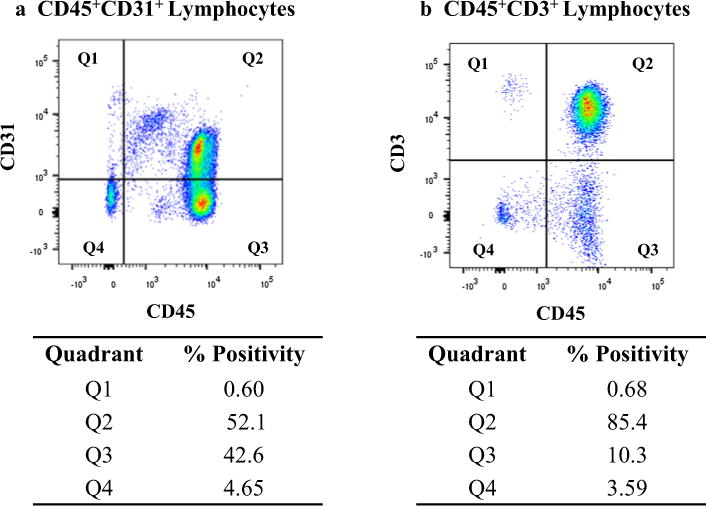
Gating strategy used for analyzing CD45+CD31+ and CD45+CD3+ lymphocyte subsets. a Representative dot plot showing CD45+CD31+ cells (Q2) within the lymphocyte gate. b Representative dot plot showing the CD45+CD3+ cells (Q2) within the lymphocyte gate. Percentages shown in a and b are based on the total lymphocyte population. All data presented are from a single patient
Statistical analyses
Patients from the TIME and LateTIME studies were combined into a single cohort for all analyses. Demographic data are shown as counts and percentages for dichotomous and polychotomous variables and as means and standard deviations for continuous variables. We calculated the associations between the frequencies of eight BM-MNC subsets (Table 1); specifically, each of the subsets was the dependent variable in a multiple regression model that contained one of eight CRFs (self-reported hypertension, hyperlipidemia, diabetes mellitus, and smoking, as well as sex, age, creatinine level, and blood glucose level) plus a dichotomous variable reflecting the study (TIME or LateTIME). In this exploratory analysis, no corrections made for multiplicity. All analyses conducted using SAS 9.3 (SAS Institute, Cary, NC, USA).
Table 1.
Identification of bone marrow cell subsets according to phenotype
| Phenotype | BM-MCSs Type | Involvement in CVD |
|---|---|---|
| CD34+ | Hematopoietic stem cells | Angiogenesis and attenuation of negative left ventricular remodeling [21, 22, 35, 50] |
| CD11b+ | Monocytes | Worsening of LVEF after AMI [8] |
| CD34+CD31+ | Angiogenic cells | Angiogenesis in ischemic vascular disease and reduction in infarct size [36, 41, 60] |
| CD45+CD31+ | Lymphocytes (T cell) | Angiogenesis and vasculogenesis [23] |
| CD45+CD3+ | Lymphocytes (T-cell precursor) | Immunoregulatory and cytotoxic effects [2, 4] |
| CD45+CD14+ | Monocytes | Vascular inflammation in atherosclerosis [29, 57] |
| CD45+CD19+ | Lymphocytes (B cell) | Protective immunity during atherosclerosis [7] |
| CD19+CXCR4+ | Lymphocytes (B cell) | Improvement in heart function [34, 48, 49] |
AMI acute myocardial infarction, LVEF left ventricular ejection fraction
Results
Baseline clinical characteristics
The current study included 191 patients who provided consent for BM analysis by the CCTRN Biorepository. Demographic and CRF data for the current study cohort are reported in Table 2. The mean age of the population analyzed was 56 years (standard deviation, 11), and only 15% (n = 28) of the participants were female.
Table 2.
Baseline demographics and cardiovascular risk factors of patients in the study cohort
| Characteristics | |
|---|---|
| Demographics | |
| Age, mean (SD), years | 56 (11) |
| Female, n (%) | 28 (15%) |
| Cardiovascular risk factors | |
| Diabetes, n (%) | 39 (20%) |
| Hypertension, n (%) | 107 (56%) |
| Hyperlipidemia, n (%) | 130 (68%) |
| Total cholesterol, mean (SD), mg/dL | 167 (48) |
| HDL cholesterol, mean (SD), mg/dL | 37 (12) |
| LDL cholesterol, mean (SD), mg/dL, (n = 189) | 102 (45) |
| Total/HDL cholesterol ratio, mean (SD), (n = 190) | 4.6 (1.7) |
| Creatinine, mean (SD), mg/dL | 0.9 (0.2) |
| Smoking, n (%) | 115 (60%) |
| Systolic blood pressure, amean (SD), mmHg | 113 (14) |
n = 191 unless otherwise noted
HDL high-density lipoprotein, LDL low-density lipoprotein
At initial discharge
Associations between cardiovascular risk factors and BM-MNC subsets
A multivariable model was used to explore the relationships between eight CRFs (hyperlipidemia, hypertension, diabetes, sex, smoking, age, blood glucose level, and creatinine level) and the frequencies of particular BM-MNC subsets at study baseline, as determined by flow cytometry (Table 3). After adjusting for study, hyperlipidemia and hypertension were found to be positively associated with the frequency of CD11b+ monocytes. Surprisingly, diabetes was not associated with changes in any of the cell types assessed. Women were found to have lower frequencies of CD11b+ and CD45+CD14+ monocytes than men, and smokers had a lower frequency of CD45+CD31+ lymphocytes than non-smokers. Negative associations existed between age and the frequency of CD45+CD31+ lymphocytes (Fig. 3) and between creatinine level and the frequency of CD45+CD3+ cells. A positive association was found between glucose level and the frequency of CD45+CD3+ T-cell precursors. These data suggest that in our cohort of patients with STEMI, CRFs thought to influence IHD outcomes may have also affected the cellular composition of the BM, particularly the frequency of specific monocyte and lymphocyte subsets.
Table 3.
Associations between cardiovascular risk factors and BM-MNC subsets
| Phenotype | Hyperlipidemia
|
Hypertension
|
Diabetes
|
|||
|---|---|---|---|---|---|---|
| Effect size (95% CI) | P value | Effect size (95% CI) | P value | Effect size (95% CI) | P value | |
| aCD34+ | −0.25 (−1.16 to 0.65) | 0.581 | −0.72 (−1.57 to 0.12) | 0.093 | 0.37 (−0.68 to 1.41) | 0.49 |
| bCD11b+ | 4.14 (0.10 to 8.18) | 0.045 | 4.65 (0.86 to 8.49) | 0.016 | −2.71 (−7.41 to 2.00) | 0.258 |
| aCD34+CD31+ | −0.29 (−1.17 to 0.59) | 0.511 | −0.66 (−1.48 to 0.17) | 0.117 | 0.41 (−0.60 to 1.42) | 0.429 |
| CD45+CD31+ | 0.62 (−2.19 to 3.43) | 0.666 | −1.28 (−3.92 to 1.36) | 0.342 | −2.42 (−5.63 to 0.79) | 0.138 |
| CD45+CD3+ | −0.87 (−4.26 to 2.52) | 0.612 | 1.77 (−1.41 to 4.95) | 0.273 | −0.43 (−4.36 to 3.49) | 0.828 |
| bCD45+CD14+ | 2.01 (−2.51 to 6.53) | 0.381 | 4.18 (−0.05 to 8.42) | 0.053 | 0.11 (−5.13 to 5.34) | 0.968 |
| CD45+CD19+ | 0.27 (−1.18 to 1.72) | 0.712 | 0.33 (−1.03 to 1.69) | 0.634 | 0.11 (−1.57 to 1.78) | 0.901 |
| CD19+CXCR4+ | 0.25 (−1.03 to 1.52) | 0.702 | 0.32 (−0.87 to 1.52) | 0.594 | 0.37 (−1.10 to 1.84) | 0.619 |
| Phenotype | Sex (female)
|
Age
|
Smoking
|
|||
|---|---|---|---|---|---|---|
| Effect size (95% CI) | P value | Effect size (95% CI) | P value | Effect size (95% CI) | P value | |
| aCD34+ | 0.13 (−1.06 to 1.32) | 0.833 | −0.03 (0.07 to 0.01) | 0.094 | −0.52 (−1.38 to 0.35) | 0.241 |
| bCD11b+ | −6.40 (−11.70 to 1.10) | 0.018 | 0.09 (−0.08 to 0.27) | 0.299 | 1.07 (−2.84 to 4.97) | 0.591 |
| aCD34+CD31+ | 0.12 (−1.05 to 1.29) | 0.842 | −0.03 (−0.07 to 0.00) | 0.088 | −0.42 (−1.26 to 0.12) | 0.328 |
| CD45+CD31+ | 1.49 (−2.24 to 5.22) | 0.431 | −0.20 (−0.32 to −0.09) | 0.001 | −3.40 (−6.06 to 0.74) | 0.013 |
| CD45+CD3+ | 3.98 (−0.45 to 8.42) | 0.078 | −0.10 (−0.24 to 0.05) | 0.178 | 2.55 (−0.66 to 5.77) | 0.119 |
| bCD45+CD14+ | −6.61 (−12.49 to 0.72) | 0.028 | 0.00 (−0.19 to 0.20) | 0.968 | 1.92 (−2.42 to 6.27) | 0.383 |
| CD45+CD19+ | −0.92 (−2.82 to 0.99) | 0.343 | −0.02 (−0.08 to 0.04) | 0.561 | 1.33 (−0.04 to 2.7) | 0.057 |
| CD19+CXCR4+ | −0.45 (−2.13 to 1.22) | 0.594 | 0.00 (−0.05 to 0.06) | 0.952 | 0.78 (−0.42 to 1.99) | 0.203 |
| Phenotype | Creatinine
|
Glucose
|
||
|---|---|---|---|---|
| Effect size (95% CI) | P value | Effect size (95% CI) | P value | |
| aCD34+ | 1.00 (−0.90 to 2.89) | 0.301 | 0.00 (−0.01 to 0.00) | 0.399 |
| bCD11b+ | 6.65 (−1.9 to 15.19) | 0.127 | 0.02 (−0.02 to 0.05) | 0.356 |
| aCD34+CD31+ | 0.89 (−0.95 to 2.73) | 0.339 | 0.00 (−0.01 to 0.01) | 0.545 |
| CD45+CD31+ | −0.76 (−6.67 to 5.15) | 0.8 | 0.01 (−0.02 to 0.03) | 0.541 |
| CD45+CD3+ | −8.76 (−15.79 to 1.72) | 0.015 | 0.03 (0.00 to 0.06) | 0.031 |
| bCD45+CD14+ | 4.90 (−4.59 to 14.39) | 0.31 | 0.02 (−0.02 to 0.06) | 0.294 |
| CD45+CD19+ | 0.67 (−2.38 to 3.73) | 0.663 | 0.00 (−0.02 to 0.01) | 0.466 |
| CD19+CXCR4+ | 1.19 (−1.48 to 3.86) | 0.38 | 0.00 (−0.01 to 0.01) | 0.854 |
The reported effect size is the model coefficient from a multiple linear regression model with the cell type as the independent variable and the cardiovascular risk factor as the dependent variable. The reported P values are adjusted for study (TIME or LateTIME). All cells were analyzed within the lymphocyte gate unless otherwise specified
CI confidence interval
Analyzed using ISHAGE gating
Analyzed within the monocyte gate
Fig. 3.
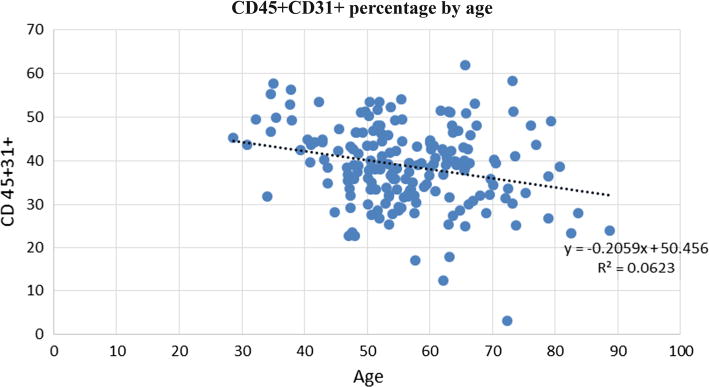
Association between the frequency of CD45+CD31+ lymphocytes and age. Dot plot showing that the percentage of CD45+CD31+ cells in the bone marrow decreased with age
Discussion
Exploratory studies conducted in conjunction with the CCTRN TIME, LateTIME, and FOCUS trials have shown associations between specific BM-MNC subsets and clinical outcomes [35, 49, 51, 52]. It has been suggested that CRFs, such as age and sex, play an important role in a patient’s response to cell therapy [47, 61]. However, to date, there has been no comprehensive assessment of how patient characteristics may alter BM composition in STEMI patients; thus, it is unknown whether or how the composition of autologous BM-MNC products may be altered by patients’ CRFs.
In the current study, we looked for associations between specific BM-MNC subsets and eight CRFs in STEMI patients enrolled in either the TIME or LateTIME trials. Hypertension, hyperlipidemia, sex, smoking, age, glucose, and creatinine levels were found to be associated with differences in BM composition in these patients. These data suggest that each patient’s BM composition differs according to the individual’s CRF profile. Thus, these attributes may affect the composition of the autologous BM-MNC therapy received and the associated outcomes.
An inflammatory process that involves the transmigration and accumulation of both innate and adaptive immune cells into the interstitium of affected tissues may play a role in hypertension [28]. Furthermore, hypertension has been associated with atherosclerosis, endothelial dysfunction, and the accumulation of monocytes within the endothelium [17]. However, the effects of hypertension on human BM monocytes are not defined. In this study, hypertension was positively associated with the frequency of CD11b+ cells in the BM. To our knowledge, our study is the first to describe an association between hypertension and the level of BM monocytes in humans. Increased expression of CD11b is suggested to stimulate the development of IHD by promoting myocyte oxidative injury and myocardial hypertrophy [12, 31]. Interestingly, Cogle et al. [8] found that an increased level of CD11b+ cells in the BM of patients at 1–3 weeks after acute MI was associated with worse LVEF at the 6-month follow-up. Our results suggest that hypertension may predispose an individual to increased levels of CD11b+ cells and, thereby, to poor outcomes after STEMI.
Hyperlipidemia, a well-known CRF, is associated with plaque formation and vessel rupture [3] by increased endothelial permeability to LDL cholesterol. In our study, we found an association between the frequency of CD11b+ cells and hyperlipidemia. Similarly, Serrano et al. [42] observed an increase in the circulating numbers of CD11b+ cells in patients with untreated hypercholesterolemia. Furthermore, they observed decreased numbers of circulating CD11b+ cells and LDL cholesterol in these patients after treatment. These findings suggest that this cell type plays an important role in the pathogenesis of atherosclerosis.
Increased age is a major contributor to endothelial dysfunction [5, 6, 11, 43, 44, 46] and an increased risk of developing IHD [39]. In our study, increased age was inversely associated with the frequency of CD45+CD31+ lymphocytes. This is similar to the findings of, both Hur et al. [19] and Ge et al. [14] who showed an inverse correlation between the level of circulating peripheral blood CD31+ T cells and age. One possible explanation for this decrease in cell number with age is age-related apoptosis. Kushner et al. [27] showed that caspase-3, a critical downstream protein involved in the execution phase of the apoptotic pathway, is higher in CD31+ T cells of middle-aged and older men than in those of younger men, supporting this correlation between age and apoptosis of CD31+ cells. Interestingly, increased age has been shown to be associated with increases in cellular apoptosis more broadly and of course with the incidence of cardiovascular disease [28]. This loss of angiogenic lymphocytes with age, or immunosenescence of multiple cell types, suggests that the BM-MNC products from elderly patients may be less effective as a cell therapy. Another cell type shown to change in frequency with age is CD34+ cells. Moresi et al. [30] found that the number of circulating CD34+ cells significantly decreased with increasing age in a population of healthy individuals (age range 16–100 years old). Although our results showed a similar trend, the association was not statistically significant. The lack of significance may have been due to differences in the sample types assessed (blood vs bone marrow) or due to differences in the age range and health status of the respective study participants. Because the patients in our study had sustained an MI recently, the CD34+ stem cells may have been released into circulation in response to the recent injury, thereby masking the effects of age on this cell population.
Furthermore, female sex has been found to be associated with a lower risk of death due to coronary artery disease after adjustment for CRFs and age [55]. In this study, female sex was associated with decreased levels of BM CD11b+ and CD45+CD14+ monocytes. Sex steroids, including estrone, progesterone, and testosterone, can modulate the ability of monocytes and platelets to adhere to endothelial cells and, therefore, can either induce or inhibit the initiation and progression of vascular lesions [9]. Estrone, which is higher in women than in men, reduces the surface expression of CD11b and decreases monocyte adhesion to endothelial cells exposed to the pro-inflammatory agent lipopolysaccharide, suggesting that it may inhibit endothelial injury under inflammatory conditions [9]. Although we did not measure the serum levels in our cohort of patients, it is known that estrone production does not stop after menopause [15]. Similar to our results, Heimbeck et al. [18] found that the level of circulating CD45+CD14+ monocytes is lower in women than in men.
Finally, cigarette smoking may cause apoptotic cell death and cellular senescence, and may inhibit repair functions [24]. We found that smoking was associated with a decrease in BM CD45+CD31+ lymphocytes. Similar to our findings, Ge et al. [14] found a negative association between smoking and the level of circulating CD45+−CD31dim lymphocytes in healthy men and women. Further investigation of this cell type is warranted to determine its role in the prevention or repair of cardiac injury.
Study limitations
This study had several limitations. Before the TIME and LateTIME trials began, we chose the BM-MNC phenotypes and CRFs to assess based on the current knowledge of the factors that affect the repair process after acute MI, balanced by available fiscal resources. The fields of cardiovascular regeneration and cell therapy have evolved rapidly since the design of TIME and LateTIME studies; recent studies have revealed multiple cell types that may contribute to cardiovascular outcomes. Unfortunately, some of the cell populations that are now recognized as important in this field were either not understood 8 years ago or were too costly to evaluate in the first in-depth analysis, and thus were not included in the design of TIME and LateTIME. In particular, cell populations involved in pro-inflammatory responses, such as CD14++/CD16− monocytes (“classical”, Mon1), CD14+/CD16++ monocytes (“non-classical”, Mon3), and CD14++/CD16+ monocytes (“intermediate”, Mon2), which are considered independent predictors of cardiovascular events [37], were not evaluated. We also did not assess the levels of T-cells subsets: CD3+CD4+ cells (T-helper cells), CD3+CD8+ cells (cytotoxic T cells), and other cell subsets now known to be involved in anti-inflammatory responses, (e.g., CD4+CD25+CD127low cells regulatory T cells) [56]. We identify this as a shortcoming of the study design. Unfortunately, because cell phenotypes can only be analyzed in fresh samples, we are not able to assess these newly recognized phenotypes. Despite this, we feel that our results provide a relatively comprehensive assessment of BM phenotypes in patients with STEMI. Another limitation was that the BM-MNC products were obtained from a cohort of patients with multiple risk factors, potentially making it difficult to discern associations between specific BM-MNC frequencies and individual CRFs. In addition, all the patients in this study had an STEMI event before the BM-MNCs were collected, which could have overshadowed other factors affecting the composition of the BM. Moreover, the timing between the STEMI event and BM-MNC collection varied among patients. Finally, because our study had a low number of patients in some demographic (e.g., females) and CRF groups (e.g., diabetes), the statistical power for these groups may have been too low to detect all associations between these factors and BM-MNC populations.
Conclusions
To our knowledge, this is the first study to show associations between specific CRFs and the frequencies of particular BM-MNC subsets, including monocytes and lymphocytes, in patients with STEMI. Because of the exploratory nature of this study, we cannot determine whether the CRFs directly affected the BM composition or if other factors contributed to this association. Future studies will be necessary to assess whether a cause-and-effect relationship exists. Since the BM obtained from patients who had hyperlipidemia or hypertension who were advanced in age or who smoked showed changes that would be expected to be unfavorable for cardiac repair, our results suggest that the BM product from these patients may be less effective as a cell therapy than that from healthier individuals. If this is proven to be true, patients’ CRFs may need to be considered when designing future autologous cell therapy studies and assessing clinical outcomes.
Acknowledgments
We would like to acknowledge the National Heart, Lung, and Blood Institute, and National Center for Research Resources. The CCTRN acknowledges its industry partners, Biosafe, Biologics Delivery System Group, and Cordis Corporation, for their contributions of equipment and technical support during the conduct of the trial. Finally, we would like to thank Heather Leibrecht for her assistance in the preparation of this manuscript.
Sources of funding: This work was supported by the National Heart, Lung, and Blood Institute under cooperative agreement 5 UM1 HL087318. It was also supported, in part, by National Heart, Lung, and Blood Institute contracts N01 HB 37164 and HHSN268201000008C, which were awarded to the Molecular and Cellular Therapeutics Facility, University of Minnesota, and by contracts N01 HB 37163 and HHSN268201000007C, which were awarded to the Cell Processing Facility, Baylor College of Medicine. Further funding provided by National Center for Research Resources CTSA Grant UL1 TR000064 awarded to the University of Florida. In addition, funding from the Texas State Legislature was used to assist investigators at the Texas Heart Institute, Houston, Texas.
Abbreviations
- BM
Bone marrow
- BM-MNC
Bone marrow mononuclear cell
- CCTRN
Cardiovascular cell therapy research network
- CRF
Cardiovascular risk factor
- IHD
Ischemic heart disease
- LVEF
Left ventricular ejection fraction
- MI
Myocardial infarction
- STEMI
ST-segment elevation myocardial infarction
Footnotes
Compliance with ethical standards
Conflict of interest: There are no potential conflicts of interest to disclose.
References
- 1.Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 2.Barth SD, Kaaks R, Johnson T, Katzke V, Gellhaus K, Schulze JJ, Olek S, Kuhn T. The ratio of regulatory (FOXP3+) to total (CD3+) T cells determined by epigenetic cell counting and cardiovascular disease risk: a prospective case-cohort study in non-diabetics. EBioMedicine. 2016 doi: 10.1016/j.ebiom.2016.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114:1852–1866. doi: 10.1161/CIRCRESAHA.114.302721. [DOI] [PubMed] [Google Scholar]
- 4.Bjorkbacka H. Can circulating regulatory T cells predict cardiovascular disease? EBioMedicine. 2016 doi: 10.1016/j.ebiom.2016.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black MA, Cable NT, Thijssen DH, Green DJ. Impact of age, sex, and exercise on brachial artery flow-mediated dilatation. Am J Physiol Heart Circ Physiol. 2009;297:H1109–H1116. doi: 10.1152/ajpheart.00226.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black MA, Green DJ, Cable NT. Exercise prevents age-related decline in nitric-oxide-mediated vasodilator function in cutaneous microvessels. J Physiol. 2008;586:3511–3524. doi: 10.1113/jphysiol.2008.153742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caligiuri G, Nicoletti A, Poirier B, Hansson GK. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J Clin Invest. 2002;109:745–753. doi: 10.1172/JCI7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cogle CR, Wise E, Meacham AM, Zierold C, Traverse JH, Henry TD, Perin EC, Willerson JT, Ellis SG, Carlson M, Zhao DX, Bolli R, Cooke JP, Anwaruddin S, Bhatnagar A, da Graca Cabreira-Hansen M, Grant MB, Lai D, Moye L, Ebert RF, Olson RE, Sayre SL, Schulman IH, Bosse RC, Scott EW, Simari RD, Pepine CJ, Taylor DA, Cardiovascular Cell Therapy Research N Detailed analysis of bone marrow from patients with ischemic heart disease and left ventricular dysfunction: BM CD34, CD11b, and clonogenic capacity as biomarkers for clinical outcomes. Circ Res. 2014;115:867–874. doi: 10.1161/CIRCRESAHA.115.304353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cutini PH, Campelo AE, Agriello E, Sandoval MJ, Rauschemberger MB, Massheimer VL. The role of sex steroids on cellular events involved in vascular disease. J Steroid Biochem Mol Biol. 2012;132:322–330. doi: 10.1016/j.jsbmb.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 10.de Jong R, Houtgraaf JH, Samiei S, Boersma E, Duckers HJ. Intracoronary stem cell infusion after acute myocardial infarction: a meta-analysis and update on clinical trials. Circ Cardiovasc Interv. 2014;7:156–167. doi: 10.1161/CIRCINTERVENTIONS.113.001009. [DOI] [PubMed] [Google Scholar]
- 11.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- 12.Entman ML, Youker K, Shoji T, Kukielka G, Shappell SB, Taylor AA, Smith CW. Neutrophil induced oxidative injury of cardiac myocytes. A compartmented system requiring CD11b/CD18-ICAM-1 adherence. J Clin Invest. 1992;90:1335–1345. doi: 10.1172/JCI115999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez-Aviles F, San Roman JA, Garcia-Frade J, Fernandez ME, Penarrubia MJ, de la Fuente L, Gomez-Bueno M, Cantalapiedra A, Fernandez J, Gutierrez O, Sanchez PL, Hernandez C, Sanz R, Garcia-Sancho J, Sanchez A. Experimental and clinical regenerative capability of human bone marrow cells after myocardial infarction. Circ Res. 2004;95:742–748. doi: 10.1161/01.RES.0000144798.54040.ed. [DOI] [PubMed] [Google Scholar]
- 14.Ge Y, Cheng S, Larson MG, Ghorbani A, Martin RP, Klein RJ, O’Donnell CJ, Vasan RS, Shaw SY, Wang TJ, Cohen KS. Circulating CD31 + leukocyte frequency is associated with cardiovascular risk factors. Atherosclerosis. 2013;229:228–233. doi: 10.1016/j.atherosclerosis.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. Production and actions of estrogens. N Engl J Med. 2002;346:340–352. doi: 10.1056/NEJMra000471. [DOI] [PubMed] [Google Scholar]
- 16.Gyongyosi M, Wojakowski W, Lemarchand P, Lunde K, Tendera M, Bartunek J, Marban E, Assmus B, Henry TD, Traverse JH, Moye LA, Surder D, Corti R, Huikuri H, Miettinen J, Wohrle J, Obradovic S, Roncalli J, Malliaras K, Pokushalov E, Romanov A, Kastrup J, Bergmann MW, Atsma DE, Diederichsen A, Edes I, Benedek I, Benedek T, Pejkov H, Nyolczas N, Pavo N, Bergler-Klein J, Pavo IJ, Sylven C, Berti S, Navarese EP, Maurer G, Investigators A Meta-analysis of cell-based cardiac studies (ACCRUE) in patients with acute myocardial infarction based on individual patient data. Circ Res. 2015;116:1346–1360. doi: 10.1161/CIRCRESAHA.116.304346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrison DG, Marvar PJ, Titze JM. Vascular inflammatory cells in hypertension. Front Physiol. 2012;3:128. doi: 10.3389/fphys.2012.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heimbeck I, Hofer TP, Eder C, Wright AK, Frankenberger M, Marei A, Boghdadi G, Scherberich J, Ziegler-Heitbrock L. Standardized single-platform assay for human monocyte subpopulations: lower CD14+CD16++ monocytes in females. Cytometry A. 2010;77:823–830. doi: 10.1002/cyto.a.20942. [DOI] [PubMed] [Google Scholar]
- 19.Hur J, Yang HM, Yoon CH, Lee CS, Park KW, Kim JH, Kim TY, Kim JY, Kang HJ, Chae IH, Oh BH, Park YB, Kim HS. Identification of a novel role of T cells in postnatal vasculogenesis: characterization of endothelial progenitor cell colonies. Circulation. 2007;116:1671–1682. doi: 10.1161/CIRCULATIONAHA.107.694778. [DOI] [PubMed] [Google Scholar]
- 20.Jeevanantham V, Butler M, Saad A, Abdel-Latif A, Zuba-Surma EK, Dawn B. Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: a systematic review and meta-analysis. Circulation. 2012;126:551–568. doi: 10.1161/CIRCULATIONAHA.111.086074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kandala J, Upadhyay GA, Pokushalov E, Wu S, Drachman DE, Singh JP. Meta-analysis of stem cell therapy in chronic ischemic cardiomyopathy. Am J Cardiol. 2013;112:217–225. doi: 10.1016/j.amjcard.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 22.Kawamoto A, Tkebuchava T, Yamaguchi J, Nishimura H, Yoon YS, Milliken C, Uchida S, Masuo O, Iwaguro H, Ma H, Hanley A, Silver M, Kearney M, Losordo DW, Isner JM, Asahara T. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation. 2003;107:461–468. doi: 10.1161/01.cir.0000046450.89986.50. [DOI] [PubMed] [Google Scholar]
- 23.Kim H, Cho HJ, Kim SW, Liu B, Choi YJ, Lee J, Sohn YD, Lee MY, Houge MA, Yoon YS. CD31+ cells represent highly angiogenic and vasculogenic cells in bone marrow: novel role of nonendothelial CD31+ cells in neovascularization and their therapeutic effects on ischemic vascular disease. Circ Res. 2010;107:602–614. doi: 10.1161/CIRCRESAHA.110.218396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SY, Lee JH, Kim HJ, Park MK, Huh JW, Ro JY, Oh YM, Lee SD, Lee YS. Mesenchymal stem cell-conditioned media recovers lung fibroblasts from cigarette smoke-induced damage. Am J Physiol Lung Cell Mol Physiol. 2012;302:L891–L908. doi: 10.1152/ajplung.00288.2011. [DOI] [PubMed] [Google Scholar]
- 25.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 26.Kunz GA, Liang G, Cuculi F, Gregg D, Vata KC, Shaw LK, Goldschmidt-Clermont PJ, Dong C, Taylor DA, Peterson ED. Circulating endothelial progenitor cells predict coronary artery disease severity. Am Heart J. 2006;152:190–195. doi: 10.1016/j.ahj.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Kushner EJ, Weil BR, MacEneaney OJ, Morgan RG, Mestek ML, Van Guilder GP, Diehl KJ, Stauffer BL, DeSouza CA. Human aging and CD31+ T-cell number, migration, apoptotic susceptibility, and telomere length. J Appl Physiol (1985) 2010;109:1756–1761. doi: 10.1152/japplphysiol.00601.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leon LJ, Gustafsson AB. Staying young at heart: autophagy and adaptation to cardiac aging. J Mol Cell Cardiol. 2015 doi: 10.1016/j.yjmcc.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 30.Moresi R, Tesei S, Costarelli L, Viticchi C, Stecconi R, Bernardini G, Provinciali M. Age- and gender-related alterations of the number and clonogenic capacity of circulating CD34+ progenitor cells. Biogerontology. 2005;6:185–192. doi: 10.1007/s10522-005-7954-5. [DOI] [PubMed] [Google Scholar]
- 31.Mu L, Wang J, Guo X, Zheng S, Shan K, Jing C, Li L. Correlation and clinical significance of expressions of HIF-1alpha and Sema4D in colorectal carcinoma tissues. Zhonghua Wei Chang Wai Ke Za Zhi. 2014;17:388–392. [PubMed] [Google Scholar]
- 32.Nauta ST, Deckers JW, van der Boon RM, Akkerhuis KM, van Domburg RT. Risk factors for coronary heart disease and survival after myocardial infarction. Eur J Prev Cardiol. 2014;21:576–583. doi: 10.1177/2047487312460514. [DOI] [PubMed] [Google Scholar]
- 33.Orlic D, Kajstura J, Chimenti S, Bodine DM, Leri A, Anversa P. Bone marrow stem cells regenerate infarcted myocardium. Pediatr Transplant. 2003;7(Suppl 3):86–88. doi: 10.1034/j.1399-3046.7.s3.13.x. [DOI] [PubMed] [Google Scholar]
- 34.Penn MS. Importance of the SDF-1:CXCR4 axis in myocardial repair. Circ Res. 2009;104:1133–1135. doi: 10.1161/CIRCRESAHA.109.198929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perin EC, Willerson JT, Pepine CJ, Henry TD, Ellis SG, Zhao DX, Silva GV, Lai D, Thomas JD, Kronenberg MW, Martin AD, Anderson RD, Traverse JH, Penn MS, Anwaruddin S, Hatzopoulos AK, Gee AP, Taylor DA, Cogle CR, Smith D, Westbrook L, Chen J, Handberg E, Olson RE, Geither C, Bowman S, Francescon J, Baraniuk S, Piller LB, Simpson LM, Loghin C, Aguilar D, Rich-man S, Zierold C, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moye LA, Simari RD, Cardiovascular Cell Therapy Research N Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trial. JAMA. 2012;307:1717–1726. doi: 10.1001/jama.2012.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, Wang T, Gregg D, Ramaswami P, Pippen AM, Annex BH, Dong C, Taylor DA. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation. 2003;108:457–463. doi: 10.1161/01.CIR.0000082924.75945.48. [DOI] [PubMed] [Google Scholar]
- 37.Rogacev KS, Cremers B, Zawada AM, Seiler S, Binder N, Ege P, Grosse-Dunker G, Heisel I, Hornof F, Jeken J, Rebling NM, Ulrich C, Scheller B, Bohm M, Fliser D, Heine GH. CD14++CD16+ monocytes independently predict cardiovascular events: a cohort study of 951 patients referred for elective coronary angiography. J Am Coll Cardiol. 2012;60:1512–1520. doi: 10.1016/j.jacc.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 38.Rosendorff C, Black HR, Cannon CP, Gersh BJ, Gore J, Izzo JL, Jr, Kaplan NM, O’Connor CM, O’Gara PT, Oparil S, American Heart Association Council for High Blood Pressure R, American Heart Association Council on Clinical C, American Heart Association Council on E, Prevention Treatment of hypertension in the prevention and management of ischemic heart disease: a scientific statement from the American Heart Association Council for High Blood Pressure Research and the Councils on Clinical Cardiology and Epidemiology and Prevention. Circulation. 2007;115:2761–2788. doi: 10.1161/CIRCULATIONAHA.107.183885. [DOI] [PubMed] [Google Scholar]
- 39.Ross MD, Malone E, Florida-James G. Vascular ageing and exercise: focus on cellular reparative processes. Oxid Med Cell Longev. 2016;2016:15. doi: 10.1155/2016/3583956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanganalmath SK, Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res. 2013;113:810–834. doi: 10.1161/CIRCRESAHA.113.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schutt RC, Trachtenberg BH, Cooke JP, Traverse JH, Henry TD, Pepine CJ, Willerson JT, Perin EC, Ellis SG, Zhao DX, Bhatnagar A, Johnstone BH, Lai D, Resende M, Ebert RF, Wu JC, Sayre SL, Orozco A, Zierold C, Simari RD, Moye L, Cogle CR, Taylor DA, Cardiovascular Cell Therapy Research N Bone marrow characteristics associated with changes in infarct size after STEMI: a biorepository evaluation from the CCTRN TIME trial. Circ Res. 2015;116:99–107. doi: 10.1161/CIRCRESAHA.116.304710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serrano CV, Jr, Yoshida VM, Venturinelli ML, D’Amico E, Monteiro HP, Ramires JA, da Luz PL. Effect of simvastatin on monocyte adhesion molecule expression in patients with hypercholesterolemia. Atherosclerosis. 2001;157:505–512. doi: 10.1016/S0021-9150(00)00757-7. [DOI] [PubMed] [Google Scholar]
- 43.Soucy KG, Ryoo S, Benjo A, Lim HK, Gupta G, Sohi JS, Elser J, Aon MA, Nyhan D, Shoukas AA, Berkowitz DE. Impaired shear stress-induced nitric oxide production through decreased NOS phosphorylation contributes to age-related vascular stiffness. J Appl Physiol (1985) 2006;101:1751–1759. doi: 10.1152/japplphysiol.00138.2006. [DOI] [PubMed] [Google Scholar]
- 44.Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW, Muller-Delp JM. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol. 2004;556:947–958. doi: 10.1113/jphysiol.2003.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother. 1996;5:213–226. doi: 10.1089/scd.1.1996.5.213. [DOI] [PubMed] [Google Scholar]
- 46.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- 47.Tajiri N, Duncan K, Borlongan MC, Pabon M, Acosta S, de la Pena I, Hernadez-Ontiveros D, Lozano D, Aguirre D, Reyes S, Sanberg PR, Eve DJ, Borlongan CV, Kaneko Y. Adult stem cell transplantation: is gender a factor in stemness? Int J Mol Sci. 2014;15:15225–15243. doi: 10.3390/ijms150915225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang YL, Zhu W, Cheng M, Chen L, Zhang J, Sun T, Kishore R, Phillips MI, Losordo DW, Qin G. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ Res. 2009;104:1209–1216. doi: 10.1161/CIRCRESAHA.109.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor DA, Perin EC, Willerson JT, Zierold C, Resende M, Carlson M, Nestor B, Wise E, Orozco A, Pepine CJ, Henry TD, Ellis SG, Zhao DX, Traverse JH, Cooke JP, Schutt RC, Bhatnagar A, Grant MB, Lai D, Johnstone BH, Sayre SL, Moye L, Ebert RF, Bolli R, Simari RD, Cogle CR. Identification of bone marrow cell subpopulations associated with improved functional outcomes in patients with chronic left ventricular dysfunction: an embedded cohort evaluation of the FOCUS-CCTRN TRIAL. Cell Transplant. 2015 doi: 10.3727/096368915X689901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tomita S, Li RK, Weisel RD, Mickle DA, Kim EJ, Sakai T, Jia ZQ. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation. 1999;100:II247–II256. doi: 10.1161/01.cir.100.suppl_2.ii-247. [DOI] [PubMed] [Google Scholar]
- 51.Traverse JH, Henry TD, Ellis SG, Pepine CJ, Willerson JT, Zhao DX, Forder JR, Byrne BJ, Hatzopoulos AK, Penn MS, Perin EC, Baran KW, Chambers J, Lambert C, Raveendran G, Simon DI, Vaughan DE, Simpson LM, Gee AP, Taylor DA, Cogle CR, Thomas JD, Silva GV, Jorgenson BC, Olson RE, Bowman S, Francescon J, Geither C, Handberg E, Smith DX, Baraniuk S, Piller LB, Loghin C, Aguilar D, Richman S, Zierold C, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moye LA, Simari RD, Cardiovascular Cell Therapy R Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the LateTIME randomized trial. JAMA. 2011;306:2110–2119. doi: 10.1001/jama.2011.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Traverse JH, Henry TD, Pepine CJ, Willerson JT, Zhao DX, Ellis SG, Forder JR, Anderson RD, Hatzopoulos AK, Penn MS, Perin EC, Chambers J, Baran KW, Raveendran G, Lambert C, Lerman A, Simon DI, Vaughan DE, Lai D, Gee AP, Taylor DA, Cogle CR, Thomas JD, Olson RE, Bowman S, Francescon J, Geither C, Handberg E, Kappenman C, Westbrook L, Piller LB, Simpson LM, Baraniuk S, Loghin C, Aguilar D, Richman S, Zierold C, Spoon DB, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moye LA, Simari RD, Cardiovascular Cell Therapy Research N Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the TIME randomized trial. JAMA. 2012;308:2380–2389. doi: 10.1001/jama.2012.28726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Traverse JH, Henry TD, Vaughan DE, Ellis SG, Pepine CJ, Willerson JT, Zhao DX, Piller LB, Penn MS, Byrne BJ, Perin EC, Gee AP, Hatzopoulos AK, McKenna DH, Forder JR, Taylor DA, Cogle CR, Olson RE, Jorgenson BC, Sayre SL, Vojvodic RW, Gordon DJ, Skarlatos SI, Moye LA, Simari RD, Cardiovascular Cell Therapy Research N Rationale and design for TIME: A phase II, randomized, double-blind, placebo-controlled pilot trial evaluating the safety and effect of timing of administration of bone marrow mononuclear cells after acute myocardial infarction. Am Heart J. 2009;158:356–363. doi: 10.1016/j.ahj.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Traverse JH, Henry TD, Vaughan DE, Ellis SG, Pepine CJ, Willerson JT, Zhao DX, Simpson LM, Penn MS, Byrne BJ, Perin EC, Gee AP, Hatzopoulos AK, McKenna DH, Forder JR, Taylor DA, Cogle CR, Baraniuk S, Olson RE, Jorgenson BC, Sayre SL, Vojvodic RW, Gordon DJ, Skarlatos SI, Moye LA, Simari RD, Cardiovascular Cell Therapy Research N LateTIME: a phase-II, randomized, double-blinded, placebo-controlled, pilot trial evaluating the safety and effect of administration of bone marrow mononuclear cells 2 to 3 weeks after acute myocardial infarction. Tex Heart Inst J. 2010;37:412–420. [PMC free article] [PubMed] [Google Scholar]
- 55.Vaccarino V, Krumholz HM, Berkman LF, Horwitz RI. Sex differences in mortality after myocardial infarction. Is there evidence for an increased risk for women? Circulation. 1995;91:1861–1871. doi: 10.1161/01.cir.91.6.1861. [DOI] [PubMed] [Google Scholar]
- 56.Wang H, Hou L, Kwak D, Fassett J, Xu X, Chen A, Chen W, Blazar BR, Xu Y, Hall JL, Ge JB, Bache RJ, Chen Y. Increasing regulatory T cells with interleukin-2 and interleukin-2 antibody complexes attenuates lung inflammation and heart failure progression. Hypertension. 2016;68:114–122. doi: 10.1161/HYPERTENSIONAHA.116.07084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 58.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 59. (Fact sheet no. 317).Cardiovascular diseases (CVDs) http://www.who.int/mediacentre/factsheets/fs317/en. Accessed 14 Aug 2014.
- 60.Yip HK, Chang LT, Chang WN, Lu CH, Liou CW, Lan MY, Liu JS, Youssef AA, Chang HW. Level and value of circulating endothelial progenitor cells in patients after acute ischemic stroke. Stroke. 2008;39:69–74. doi: 10.1161/STROKEAHA.107.489401. [DOI] [PubMed] [Google Scholar]
- 61.Zenovich AG, Panoskaltsis-Mortari A, Caron GJ, Kolb AG, Fremming R, Nelson WD, Taylor DA. Sex-based differences in vascular repair with bone marrow cell therapy: relevance of regulatory and Th2-type cytokines. Transplant Proc. 2008;40:641–643. doi: 10.1016/j.transproceed.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 62.Zierold C, Carlson MA, Obodo UC, Wise E, Piazza VA, Meeks MW, Vojvodic RW, Baraniuk S, Henry TD, Gee AP, Ellis SG, Moye LA, Pepine CJ, Cogle CR, Taylor DA. Developing mechanistic insights into cardiovascular cell therapy: cardiovascular Cell Therapy Research Network Biorepository Core Laboratory rationale. Am Heart J. 2011;162:973–980. doi: 10.1016/j.ahj.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]


