Abstract
Background and Purpose:
To assess the clinical feasibility of time-resolved 3D phase contrast (4D Flow) MRI assessment of the ophthalmic artery (OphA) flow in patients with internal carotid artery stenosis (ICS).
Materials and Methods:
Twenty-one consecutive patients with unilateral ICS were recruited. 4D Flow MRI and acetazolamide-stress brain perfusion single photon emission computed tomography (SPECT) were performed. The flow direction on the affected-side OphA was categorized into native flow (anterograde or unclear) and non-native flow (retrograde flow) based on 4D Flow MRI. In the affected-side middle cerebral artery (MCA) territory, the ratio of rest cerebral blood flow to normal control (RCBFMCA) and cerebral vascular reserve (CVRMCA) were calculated from SPECT dataset. High-risk patients were defined based on the previous large cohort study (RCBFMCA < 80% and CVRMCA < 10%).
Results:
Eleven patients had native OphA flow (4 anterograde, 7 unclear) and the remaining 10 had non-native OphA flow. RCBFMCA and CVRMCA each were significantly lower in non-native flow group (84.9 ± 18.9% vs. 69.8 ± 7.3%, P < 0.05; 36.4 ± 20.6% vs. 17.0 ± 15.0%, P < 0.05). Four patients in the non-native flow group and none in the native flow group were confirmed as high-risk (Sensitivity/Specificity, 1.00/0.65).
Conclusion:
The 6 min standard 4D Flow MRI assessment of OphA in patients with ICS can predict intracranial hemodynamic impairment.
Keywords: time-resolved three-dimensional phase contrast, magnetic resonance angiography, ophthalmic artery, Single Photon Emission Computed Tomography, atherosclerosis
Introduction
Internal carotid artery stenotic disease (ICS) is a common condition in patients with atherosclerosis and considered a risk factor for transient ischemic disease (TIA) or cerebral infarction (CI).1 The prognosis of patients with ICS correlates not only with the severity of stenosis itself but also with how the collateral pathway compensates for the decrease in blood flow from the internal carotid artery (ICA).2,3 Among these collateral pathways, the retrograde flow through the affected-side ophthalmic artery (OphA) has been considered a sign of inadequate circulation because it reflects the reduction of arterial pressure in the distal ICA.3–6 Previous studies have confirmed this relationship using catheter angiography and ultrasonography.4–7 However, this sign is seldom used in the clinical setting because these procedures are not practical. Side effects from catheter angiography are inevitable, and ultrasonography for OphA requires a skilled operator.8 One alternative candidate for the assessment of OphA flow is time-resolved 3D phase contrast (4D Flow) MRI. This imaging modality acquires quantitative flow information in each voxel, which can generate qualitative and quantitative flow images.9–14 From a clinical point of view, this method is promising. Though dedicated software is needed for the analysis, clinical 4D Flow sequence itself has been installed in most of commercial MR scanners and become used in the clinical setting. Acceleration techniques reduce the scan time of 4D Flow MRI by approximately 5 min and subsequently enable 4D Flow MRI to be combined with a clinical MRI workup, i.e., MRA, diffusion weighted image (DWI), and Fluid attenuated inversion recovery (FLAIR).15,16 In healthy subjects, a static 3D phase contrast MRI and time-resolved 2D phase contrast MRI can clearly depict the OphA.17,18 However, there is no study to perform 4D Flow MRI for the OphA or to assess its clinical feasibility for the evaluation of OphA flow in patients with ICS.
The purposes of this study were to assess the feasibility of 4D Flow MRI assessment of OphA flow in ICS patients by comparing with single photon emission computed tomography (SPECT) data combined with acetazolamide challenge.
Materials and Methods
This prospective study followed the institutional ethics guideline approved by the institutional review board. All subjects provided signed informed consent prior to the examinations.
For a preliminary study, we recruited sixteen healthy volunteers (12 men, 4 women; mean age 33.3 years, range 26 to 56 years) with no history of neurological disease. They underwent a time of flight (TOF)- MRA and 4D Flow MRI.
For the clinical study, we enrolled 22 consecutive patients (17 men, 5 women; mean age, 66.8 years, range 41–75) from January 2010 to April 2014. The inclusion criteria were severe stenosis (> 70%) of the unilateral ICA and a referral to the department of neurological surgery at our institution.19 Exclusion criteria were bilateral ICA stenosis, contraindication to MR imaging, large brain infarction spread widely over the territory of a main arterial trunk, and other brain lesions, i.e., malignant tumor or trauma. Unilateral ICS had been confirmed by at least one objective imaging modality, computed tomography angiography (CTA) and MRA. All patients underwent clinical MRI, i.e., TOF-MRA, DWI and FLAIR, followed by 4D Flow MRI with a 6 min scan. In addition to these MRI examinations, an N-isopropyl-p-(123I)-iodoamphetamine (IMP) SPECT study with acetazolamide challenge was performed within 120 days to evaluate cerebrovascular perfusion. The average interval from SPECT to 4D Flow MRI was 31 days (range 14 days before to 105 days after).
4D flow MRI and TOF-MRA examination
4D Flow MRI was performed using a 3.0T MRI unit (Achieva; Philips Healthcare, Best, The Netherlands). The following 4D Flow MRI parameters were as follows: TR/TE, 8.4/5.4 ms; k-space segmentation factor, 2; temporal resolution, 67.2 ms; flip angle (FA), 13 degrees; velocity encoding (VENC), 70 cm/s (VENC range, 140 cm/sec); voxel size, 0.82 × 0.82 × 1.4 mm3; 15 cardiac phases; sensitivity encoding (SENSE) factor, 2; elliptical partial k-space coverage in phase- and slice-encoding direction; and acquisition time, 6 min.16 We set the FOV and VENC to visualize not only OphA but also other major arteries; i.e., the internal carotid artery, basilar artery and middle cerebral artery. The parameters of TOF-MRA were as follows: TR/TE, 25/3.45 ms; FA, 18 degrees; and voxel size, 0.28 × 0.28 × 0.70 mm3. The parameters of 4D Flow MRI and TOF-MRA in each volunteer and patient group were identical.
Image evaluation of 4D flow MRI
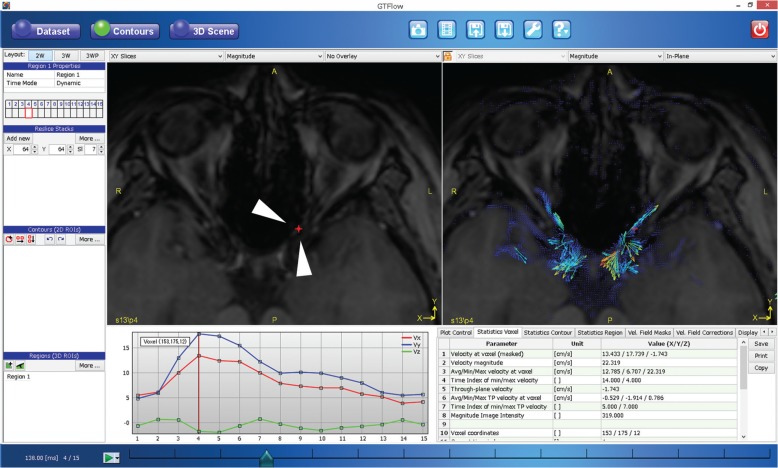
The velocity data were generated from a 4D Flow MRI dataset using the GT Flow software (GyroTools, Zurich, Switzerland). We also imported the TOF-MRA dataset, which was resliced on the MRI scanner (View Forum; Philips Medical Systems, Best, The Netherlands) to the same slice thickness and orientation as those of the 4D Flow MRI data set. Intensity thresholds were applied using MRA images. One neuroradiologist (T.S.) with 10 years of experience and who had interpreted > 100 cases of cerebral 4D Flow MRI was enlisted to evaluate 4D Flow MRI. This neuroradiologist was blinded to the clinical information, including the results of the SPECT study. On the affected-side of the OphA, the flow direction was classified as being in the antegrade and retrograde directions compared with that of the native flow. In this analysis, we investigated the vector data in each voxel based on 2D in-plane vector map, which corresponds to the vessel seen on MRA. For each direction right-to-left (RL), anteroposterior (AP), foot-to-head (FH) the flow speeds at the target voxels during the cardiac cycle were calculated as velocity-time graph on the generation time (GT) Flow software (Fig. 1). At least three voxels were chosen from the OphA. If all of them had the same flow direction during the cardiac cycle, the flow direction was determined according to these results, and if not, it was defined as “unclear”, where the to-and-fro flow was classified as “unclear”. For further analyses, the flow patterns were assigned to one of two groups: native flow pattern (antegrade or unclear) or non-native pattern (retrograde). In addition to 4D Flow MRI assessment, the flow direction of OphA on TOF-MRA was evaluated (Detailed evaluation is shown in supplemental file, which is available online.).
Fig. 1.
The analysis of time-resolved 3D phase-contrast (4D-Flow). Magnitude image (top left), in-plane vector overlay image (top right), velocity-time graph of velocity on the voxel (bottom left), and quantitative values (bottom right). We set the voxel contour at the left ophthalmic artery (red dot, arrowheads on the magnitude image) after masking the nonvascular lesion with the dedicated threshold. The in-plane vector overlying the image provides the color-coded flow direction and flow velocity at each voxel (red is fast and blue is slow). The velocity-time graph can provide the velocity along the cursor in each direction—right to left (RL), anteroposterior (AP), foot to head (FH) - during the cardiac cycle. We could then easily detect the flow direction from this information.
SPECT imaging
All patients underwent acetazolamide-stress and rest brain perfusion SPECT in the same manner used in the Japanese Extracranial-Intracranial Bypass trial (JET).19 Cerebral blood flow (CBF) was calculated using the autoradiography (ARG) method.20 222 MBq of 123I-IMP was intravenously administered over 1 min at a constant rate, and SPECT data were acquired 15 min after the injection. An arterial blood sample was taken from the antecubital artery 10 min after the tracer injection. For the pharmacological-stress test, the tracer was injected 10 min immediately after a 15 mg/kg of acetazolamide injection, and SPECT data were acquired 15 min after the tracer injection. Arterial blood sampling was performed identically to the other test. A dual-headed gamma camera Symbia T2 (Siemens, Erlangen, Germany) and low-energy high-resolution fan-beam collimator were used for the acquisitions. Projection data were acquired via continuous rotations (3-min 360-degree circular-rotation, 5 rotations; energy peak, 157 keV with a 20% energy window; matrix size, 128). The SPECT images were reconstructed using a filtered back-projection algorithm and a Butterworth filter (cutoff frequency, 0.58 cycles/pixel; order, 8). All images were corrected for attenuation using Chan’s method (uniform linear attenuation coefficient 0.12/cm).
SPECT data analysis
Stress and rest regional CBF were measured using segmental extraction estimation JET (SEE JET).21 Using this software and the ARG method, both the acetazolamide stress and rest regional CBF were calculated. In the affected-side middle-cerebral-artery (MCA) territory, the ratio of rest CBF to the default value obtained from healthy participants (rest cerebral blood flow to normal control [RCBFMCA]) were calculated based on the SPECT data. Furthermore, the cerebral vascular reserve (CVRMCA) was calculated by dividing (stress CBF – rest CBF) by the rest CBF.
Statistics
To assess the bias related to the patients’ backgrounds, we compared each patient characteristic between patient groups with native and non-native OphA flow using Fisher’s exact test or Student’s test. In addition, a multivariable logistic regression was performed for these factors. To assess the severity of hemodynamic impairment, we compared the cerebral perfusion, RCBFMCA or CVRMCA data between the two patient groups by using Student’s t-test. These quantitative value relates to the risk for stroke recurrence in patients with ICS.19,22–24 We also defined high-risk patients, who have Stage II areas in the affected-side MCA territory, based on the criteria in the previous large cohort study (RCBFMCA < 80% and CVRMCA < 10%).19 We calculated the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy for the detection of high-risk patients by 4D Flow MRI.
For all analyses, a value of P < 0.05 was deemed to indicate significance. All statistical analyses were carried out with IBM SPSS Statistics 19.0.0 (IBM, Armonk, NY, USA).
Results
Volunteer study
4D Flow MRI examinations were completed in all but one volunteer, who did not undergo the 4D Flow MRI because of a headache and was consequently excluded from further study. All 30 OphAs were depicted on the TOF-MRA without any anomalies. Of the 30 arteries, an anterograde flow direction was observed in 29 OphAs. In one OphA, the flow direction was unclear (male, 56 years, the left OphA).
Patient study
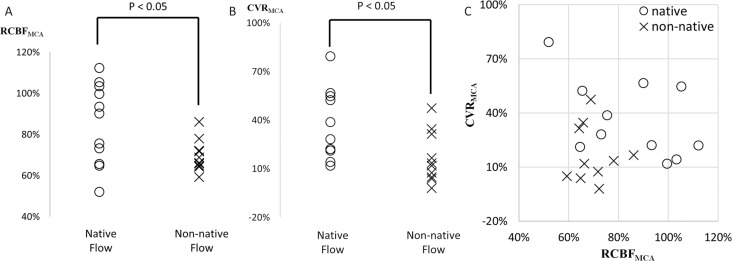
One patient was excluded because of a serious motion artifact. The other 21 patients successfully underwent 4D Flow MRI. Eleven patients had native OphA flow (four had anterograde flow and seven exhibited unclear flow), and the other 10 exhibited non-native OphA retrograde flow. Each patient’s characteristic had no bias between these two patient groups (Table 1). RCBFMCA and CVRMCA were significantly lower in the non-native flow group than in the native flow group (69.8 ± 7.3% vs. 84.9 ± 18.9%, P < 0.05; 17.0 ± 15.0% vs. 36.4 ± 20.6%, P < 0.05) (Table 2, Fig. 2A and B). Four patients in the non-native flow group and none in the native flow group were confirmed as high-risk patients (Fig. 2C). The sensitivity and specificity of 4D Flow MRI for predicting high-risk patients were 1.00 and 0.65, respectively.
Table 1.
Clinical characteristics in patients with native and non-native flow in affected-side OphA
| Native flow (n = 11) | Non-native flow (n = 10) | P-value | |
|---|---|---|---|
| Age | 63.1 ± 13.4 | 68.9 ± 5.4 | 0.218 |
| Male | 7 | 9 | 0.311 |
| Smoking | 8 | 4 | 0.198 |
| Hypertension | 8 | 10 | 0.214 |
| Diabetes | 4 | 4 | 1.000 |
| Hypercholesterolemia | 9 | 7 | 0.635 |
| Coronary arterial disease | 4 | 3 | 1.000 |
| Arterial fibrillation | 0 | 1 | 0.476 |
| Chronic kidney disease | 4 | 5 | 0.670 |
| Entry event type | |||
| Transient ischemic attach | 6 | 4 | 0.670 |
| Completed stroke | 5 | 6 | 0.670 |
| Imaging workup with 4D flow (within 4 months) | |||
| CT angiography | 8 | 7 | 0.633 |
| Catheter angiography | 1 | 2 | 0.462 |
| Treatment after 4D flow examination | |||
| EC-IC bypass surgery | 2 | 6 | 0.080 |
| Carotid endarterectomy | 1 | 0 | 1.000 |
| Carotid artery stenting | 1 | 0 | 1.000 |
| Recurrent stroke after 4D flow examination | 0 | 1 | 0.476 |
| Follow up duration (days) | 1222 ± 631 | 1129 ± 658 | 0.747 |
Native flow consists of anterograde or unclear, while non-native flow consists of retrograde flow. The significance of differences between two groups was evaluated using Student’s t-test for age and follow-up duration and using Fisher’s exact test for the categorical variables. CT, computed tomography; OphA, the ophthalmic artery; 4D-Flow, time-resolved 3D phase-contrast.
Table 2.
Cerebral perfusion in patients with native and non-native flow of affected-side OphA
| Native flow (n = 11) | Non-native flow (n = 10) | P-value | |
|---|---|---|---|
| RCBFMCA (%) | 84.9 ± 18.9 | 36.4 ± 20.6 | 0.034 |
| CVRMCA (%) | 36.4 ± 20.6 | 17.0 ± 15.0 | 0.029 |
| High risk patients | 0 | 4 | < 0.001 |
Rest cerebral blood flow to normal control (RCBFMCA), The ratio of rest cerebral blood flow (CBF) in the affected-side; middle cerebral artery (MCA) territory to the default value obtained from healthy participants; cerebral vascular reserve (CVRMCA), The cerebral vascular reserve in the affected-side; MCA territory calculated by dividing (stress CBF – rest CBF) by the rest CBF, High-risk patients were defined based on the previous large cohort study (RCBFMCA < 80% and CVRMCA < 10%). OphA, the ophthalmic artery.
Fig. 2.
Comparison of rest cerebral blood flow (CBF) (A), cerebral vascular reserve (CVR) (B) and both combined (C) in the territory of middle cerebral artery (MCA) between the native and non-native, retrograde, flow in the affected-side the ophthalmic artery (OphA). Rest cerebral blood flow to normal control (RCBFMCA), the ratio of rest CBF in the affected-side MCA territory to the default value obtained from healthy participants; CVRMCA, The cerebral vascular reserve in the affected-side MCA territory calculated by dividing (stress CBF – rest CBF) by the rest CBF. High-risk patients were defined based on the previous large cohort study (RCBFMCA < 80% and CVRMCA < 10%).
The representative cases are shown in Figs. 3 and 4.
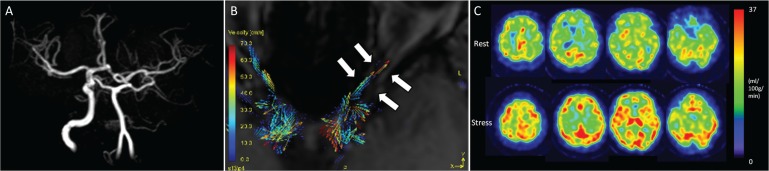
Fig. 3.
A 62-year-old woman with left internal carotid artery stenosis. Magnetic resonance angiography shows that the left internal carotid artery disappears (A). The vector map of time-resolved 3D phase-contrast (4D-Flow) MRI shows the antegrade flow of the affected-side the ophthalmic artery (OphA) (B). The rest and acetazolamide stress single photon emission computed tomography (SPECT) images show that cerebral blood flow and cerebral vascular reserve did not decrease in the territory of middle cerebral artery (MCA) (C).
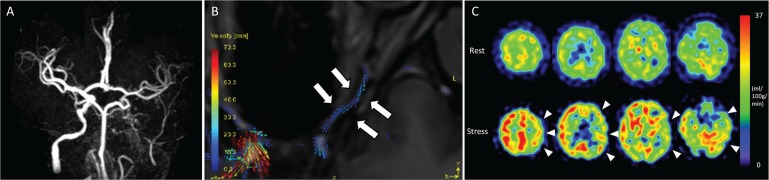
Fig. 4.
A 58-year-old man with left internal carotid artery stenosis. Magnetic resonance angiography shows that the left internal carotid artery disappears (A). The vector map of time-resolved 3D phase-contrast (4D-Flow) MRI shows that retrograde flow of the affected-side the ophthalmic artery (OphA) (B). The rest and acetazolamide stress single photon emission computed tomography (SPECT) images show that cerebral blood flow and cerebral vascular reserve decrease in the territory of middle cerebral artery (MCA) (arrow-heads in C).
Discussion
We assessed 16 healthy subjects and 22 patients with ICS with 4D Flow MRI. In healthy subjects, 4D Flow MRI could detect almost all of the antegrade flow direction of OphA. In half of patients with ICS, non-native flow, i.e., retrograde flow, was observed on the affected side of the OphA. The non-native flow of the affected side of the OphA was significantly related to the inadequate cerebral perfusion of affected-side MCA territory. Therefore, this study indicates that standard 4D Flow MRI with a 6 min scan can predict an intracranial hemodynamic compromise.
In this study, the flow in affected-side OphA was classified as unclear in one third of patients with ICS, while 4D Flow MRI was able to depict the flow in almost all OphAs of healthy subjects. This indicates that the unclear flow represents a slow flow or to-and-fro flow. The parameters of our protocol were approximately 1 mm voxel size, temporal resolution 67.2 ms, and VENC at 70 cm/s, respectively. Though this parameter was general setting based on previous intracranial 4D Flow MRI studies,15,16,25–28 both spatial and velocity resolution were lower than previous phase contrast MRA studies dedicated to OphA.17,18 We set the current parameter to cover not only OphA but also other major arteries; i.e., the internal carotid artery, basilar artery and middle cerebral artery. Though these major arteries were not assessed in this study, the quantitative assessment of these arteries may provide additional information for the evaluation of cerebral perfusion.2 Moreover, the aim of our study was to assess the clinical feasibility of 4D Flow MRI; therefore, we should set the scan time to a clinically acceptable 6 min. Dedicated 4D Flow MRI to OphA, lower VENC with longer TR and the acquisition with high spatial resolution make scan time prolonged and impair the flexibility in clinical use. Furthermore, patient motion is inevitable if the scan duration is prolonged,29 and even small movements significantly affect images of vessels with small diameters.
Several solutions that could avoid prolonged scanning times and consequently improve both spatial resolution and signal-to-noise ratio (SNR), such as using 7T MRI,30 combining scanning with an acceleration technique,31 using blood-pool contrast medium,32 and combining scanning with a multi-VENC sequence.33 One of these previous studies which evaluated the detectability of intracranial arteries with small diameter by 4D Flow MRI at 3T and 7T systems shows that both increasing spatial resolution and SNR improves flow visualization (five out of nine small arteries could be more clearly depicted by increasing spatial resolution from 0.8 mm to 0.5 mm at 3T, and three out of nine arteries could be done on 7T system which improves SNR, compared with 3T system).30 By applying these improvements of spatial resolutions and SNR, some unclear flow cases may be classified as non-native retrograde flow instead of native flow. However, in that situation, the strong point of our study does not change. The sensitivity for high risk patients remains high, and the decrease in specificity should be a cause for concern.
Collateral flow through the affected-side OphA has been considered a secondary collateral and is recruited only if the primary collateral circulation via the circle of Willis is insufficient.3–6 Several studies have assessed the correlation between retrograde flow in OphA and cerebral perfusion.4–7,34–37 A few studies failed to prove this relationship.34–37 Care should be taken that there are variety of methodology. Furthermore, catheter angiography was considered the gold standard in some previous studies. A variation in contrast volume and pressure during injection may cause non-physiologic hemodynamics. In the latest two sophisticated studies, which recruited more than 100 cases and performing echo assessment for the OphA, a significant correlation was described between the retrograde flow in OphA and recurrent ischemic stroke.4,5 One of these studies describes a hazard ration for the recurrent ischemic stroke of 5.2 for retrograde flow in OphA.5 Although publication bias should be considered, these results suggest that retrograde flow in OphA is a useful clinical sign for assessing the risk for stroke and intracranial hemodynamic compromise in the patients with ICS.
We classified high-risk patients using SPECT data according to stage II criteria based on the JET-2 study.19 The JET-2 study was prospective study which recruited 132 patients with 2 years follow-up. They revealed that the incidence of affected-side stroke recurrence of Stage II was significantly higher than that in the other groups which had higher RCBFMCA or higher CVRMCA values (All adverse events during 2 years follow-up; 16.6% vs. 7.0%, P < 0.02). Based on this study, patients with stage II were defined as candidates for surgical treatment.38 In the current study, 4D Flow MRI identified high-risk patients as defined based on the above SPECT data with high sensitivity (100%).
4D Flow MRI features several advantages for the assessment of the OphA, as described below. First, compared with angiography or ultrasonography, the 4D Flow sequence can be easily combined with a clinical MRI workup. In the patients with ICS, follow-up is mainly performed using MRI because it is non-invasive and one of the most objective methods. In addition, 4D Flow MRI can be widely performed using a clinical scanner. Second, compared with 2D phase-contrast imaging, one of the advantages of 4D Flow is that it can acquire comprehensive flow information in a large FOV, which allows operators to save the procedure for selecting the imaging slice perpendicular to the target vessel. If the target vessel, OphA, is small or complex, the examination of 2D phase-contrast imaging requires a highly skilled operator during the MRI scan, which is difficult in a clinical setting. Third, 3D spatial information with three directions of velocity information enables to reconstruct oblique plane along to the OphA which improves the visualization of the flow in the small vessel. As shown in the figures in this study, the vector map helps the investigators intuitively recognize the flow direction of OphA.
In a previous study, Uchino et al. mentioned that approximately 2% of patients harbor some OphA anomaly, e.g., persistent dorsal OphA and OphA arising from the meningeal artery.39 Such patients were not included in this study but are thought to exhibit different hemodynamics. To apply the results of the present study to a clinical evaluation, care should be taken to identify this anomaly, although most patients, approximately 98%, exhibit normal anatomy.
The limitations of our study are described below. First, the patient population was small. However, the quantitative value related to the hemodynamic impairment was significantly different between patient groups. Second, we defined impairment of cerebral perfusion using the SPECT data, not clinical outcome. The correct assessment of clinical outcome was difficult in our small sample, which featured various medication and/or surgical treatments. Only one patient experienced recurrent stroke in the present study. Furthermore, some patients were treated with surgical procedures, such as EC-IC bypass surgery or carotid artery stenting, whereas the others were treated only with medication. However, previous studies have proven CBF and CVR can correctly predict the risk of the recurrent stroke.19,22–24 Third, some of native flow patients had unclear flow. These patients may be classified into non-native retrograde flow group on another high resolution examination (e.g. dedicated 4D Flow MRI, ultrasound and so on). However, based on the result of a volunteer study that almost of all normal OphA were clearly depicted as antegrade flow, we could assume that these patients had slow or to-and-fro flow. Even if all of these patients with unclear flow were classified into non-native, retrograde flow group, 4D Flow MRI could identify high-risk patients with high sensitivity (100%) in our cohort though specificity would be decreased. Fourth, only one neuroradiologist evaluated the 4D Flow MRI. Nevertheless, previous 4D Flow studies have proven high interobserver agreement.40
Conclusion
The standard 4D Flow MRI with a 6 min scan can assess the flow in the ophthalmic artery. This assessment can predict the subsequent prognosis of the patients with internal carotid artery stenosis.
Footnotes
Conflicts of Interest
We declare that we have no conflict of interest.
References
- 1.Hendrikse J, Hartkamp MJ, Hillen B, Mali WP, van der Grond J. Collateral ability of the circle of willis in patients with unilateral internal carotid artery occlusion: border zone infarcts and clinical symptoms. Stroke 2001; 32:2768–2773. [DOI] [PubMed] [Google Scholar]
- 2.Vernieri F, Pasqualetti P, Matteis M, et al. Effect of collateral blood flow and cerebral vasomotor reactivity on the outcome of carotid artery occlusion. Stroke 2001; 32:1552–1558. [DOI] [PubMed] [Google Scholar]
- 3.Liebeskind DS. Collateral circulation. Stroke 2003; 34:2279–2284. [DOI] [PubMed] [Google Scholar]
- 4.Tsai CL, Lee JT, Cheng CA, et al. Reversal of ophthalmic artery flow as a predictor of intracranial hemodynamic compromise: implication for prognosis of severe carotid stenosis. Eur J Neurol 2013; 20:564–570. [DOI] [PubMed] [Google Scholar]
- 5.Persoon S, Luitse MJ, de Borst GJ, et al. Symptomatic internal carotid artery occlusion: a long-term follow-up study. J Neurol Neurosurg Psychiatr 2011; 82:521–526. [DOI] [PubMed] [Google Scholar]
- 6.Yamauchi H, Kudoh T, Sugimoto K, Takahashi M, Kishibe Y, Okazawa H. Pattern of collaterals, type of infarcts, and haemodynamic impairment in carotid artery occlusion. J Neurol Neurosurg Psychiatr 2004; 75:1697–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reinhard M, Müller T, Guschlbauer B, Timmer J, Hetzel A. Dynamic cerebral autoregulation and collateral flow patterns in patients with severe carotid stenosis or occlusion. Ultrasound Med Biol 2003; 29:1105–1113. [DOI] [PubMed] [Google Scholar]
- 8.Kaufmann TJ, Huston J, Mandrekar JN, Schleck CD, Thielen KR, Kallmes DF. Complications of diagnostic cerebral angiography: evaluation of 19,826 consecutive patients. Radiology 2007; 243:812–819. [DOI] [PubMed] [Google Scholar]
- 9.Markl M, Frydrychowicz A, Kozerke S, Hope M, Wieben O. 4D flow MRI. J Magn Reson Imaging 2012; 36:1015–1036. [DOI] [PubMed] [Google Scholar]
- 10.Meckel S, Leitner L, Bonati LH, et al. Intracranial artery velocity measurement using 4D PC MRI at 3 T: comparison with transcranial ultrasound techniques and 2D PC MRI. Neuroradiology 2013; 55:389–398. [DOI] [PubMed] [Google Scholar]
- 11.Nakagawa S, Murai Y, Wada T, Tateyama K. 4D flow preliminary investigation of a direct carotid cavernous fistula due to a ruptured intracavernous aneurysm. BMJ Case Rep 2015; doi: 10.1136/bcr-2014-206084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murai Y, Takagi R, Amano Y, Sekine T, Morita A, Teramoto A. 4D Flow preliminary investigation for anterior fossa dural arteriovenous fistula. Can J Neurol Sci 2014; 41:656–658. [DOI] [PubMed] [Google Scholar]
- 13.Roldan-Alzate A, Francois CJ, Wieben O, Reeder SB. Emerging applications of abdominal 4D flow MRI. AJR Am J Roentgenol 2016; 207:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terada M, Takehara Y, Isoda H, Uto T, Matsunaga M, Alley M. Low WSS and high OSI measured by 3D cine PC MRI reflect high pulmonary artery pressures in suspected secondary pulmonary arterial hypertension. Magn Reson Med Sci 2016; 15:193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bammer R, Hope TA, Aksoy M, Alley MT. Time-resolved 3D quantitative flow MRI of the major intracranial vessels: Initial experience and comparative evaluation at 1.5T and 3.0T in combination with parallel imaging. Magn Reson Med Sci 2007; 57:127–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sekine T, Amano Y, Takagi R, Matsumura Y, Murai Y, Kumita S. Feasibility of 4D flow MR imaging of the brain with either Cartesian y-z radial sampling or k-t SENSE: comparison with 4D Flow MR imaging using SENSE. Magn Reson Med Sci 2014; 13:15–24. [DOI] [PubMed] [Google Scholar]
- 17.Tsutsumi S, Yasumoto Y, Tabuchi T, Ito M. Visualization of the ophthalmic artery by phase-contrast magnetic resonance angiography: a pilot study. Surg Radiol Anat 2012; 34:833–838. [DOI] [PubMed] [Google Scholar]
- 18.Ambarki K, Hallberg P, Jóhannesson G, et al. Blood flow of ophthalmic artery in healthy individuals determined by phase-contrast magnetic resonance imaging. Invest Ophthalmol Vis Sci 2013; 54:2738–2745. [DOI] [PubMed] [Google Scholar]
- 19.Kataoka H, Miyamoto S, Ogasawara K, et al. Results of Prospective cohort study on symptomatic cerebrovascular occlusive disease showing mild hemodynamic compromise [Japanese extracranial-intracranial bypass trial (JET)-2 Study]. Neurol Med Chir (Tokyo) 2015; 55:460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iida H, Akutsu T, Endo K, et al. A multicenter validation of regional cerebral blood flow quantitation using [123I]Iodoamphetamine and single photon emission computed tomography. J Cereb Blood Flow Metab 1996; 16:781–793. [DOI] [PubMed] [Google Scholar]
- 21.Mizumura S, Nakagawara J, Takahashi M, et al. Three-dimensional display in staging hemodynamic brain ischemia for JET study: objective evaluation using SEE analysis and 3D-SSP display. Ann Nucl Med 2004; 18:13–21. [DOI] [PubMed] [Google Scholar]
- 22.Yonas H, Smith HA, Durham SR, Pentheny SL, Johnson DW. Increased stroke risk predicted by compromised cerebral blood flow reactivity. J Neurosurg 1993; 79:483–489. [DOI] [PubMed] [Google Scholar]
- 23.Ogasawara K, Ogawa A, Yoshimoto T. Cerebrovascular reactivity to acetazolamide and outcome in patients with symptomatic internal carotid or middle cerebral artery occlusion: a xenon-133 single-photon emission computed tomography study. Stroke 2002; 33:1857–1862. [DOI] [PubMed] [Google Scholar]
- 24.Kuroda S, Houkin K, Kamiyama H, Mitsumori K, Iwasaki Y, Abe H. Long-term prognosis of medically treated patients with internal carotid or middle cerebral artery occlusion: can acetazolamide test predict it? Stroke 2001; 32:2110–2116. [DOI] [PubMed] [Google Scholar]
- 25.Sekine T, Takagi R, Amano Y, et al. 4D flow MRI assessment of extracranial-intracranial bypass: qualitative and quantitative evaluation of the hemodynamics. Neuroradiology 2016; 58:237–244. [DOI] [PubMed] [Google Scholar]
- 26.Ansari SA, Schnell S, Carroll T, et al. Intracranial 4D flow MRI: toward individualized assessment of arteriovenous malformation hemodynamics and treatment-induced changes. AJNR Am J Neuroradiol 2013; 34:1922–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hope TA, Hope MD, Purcell DD, et al. Evaluation of intracranial stenoses and aneurysms with accelerated 4D flow. Magn Reson Imaging 2010; 28:41–46. [DOI] [PubMed] [Google Scholar]
- 28.Hope MD, Purcell DD, Hope TA, et al. Complete intracranial arterial and venous blood flow evaluation with 4D flow MR imaging. AJNR Am J Neuroradiol 2009; 30:362–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ikari Y, Nishio T, Makishi Y, et al. Head motion evaluation and correction for PET scans with 18F-FDG in the Japanese Alzheimer‘s disease neuroimaging initiative (J-ADNI) multi-center study. Ann Nucl Med 2012; 26:535–544. [DOI] [PubMed] [Google Scholar]
- 30.van Ooij P, Zwanenburg JJ, Visser F, et al. Quantification and visualization of flow in the circle of Willis: time-resolved three-dimensional phase contrast MRI at 7 T compared with 3 T. Magn Reson Med 2013; 69:868–876. [DOI] [PubMed] [Google Scholar]
- 31.Pedersen H, Kozerke S, Ringgaard S, Nehrke K, Kim WY. k-t PCA: temporally constrained k-t BLAST reconstruction using principal component analysis. Magn Reson Med 2009; 62:706–716. [DOI] [PubMed] [Google Scholar]
- 32.Bock J, Frydrychowicz A, Stalder AF, et al. 4D phase contrast MRI at 3 T: effect of standard and blood-pool contrast agents on SNR, PC-MRA, and blood flow visualization. Magn Reson Med 2010; 63:330–338. [DOI] [PubMed] [Google Scholar]
- 33.Ha H, Kim GB, Kweon J, et al. Multi-VENC acquisition of four-dimensional phase-contrast MRI to improve precision of velocity field measurement. Magn Reson Med 2016:75; 1909–1919. [DOI] [PubMed] [Google Scholar]
- 34.van Everdingen KJ, Visser GH, Klijn CJ, Kappelle LJ, van der Grond J. Role of collateral flow on cerebral hemodynamics in patients with unilateral internal carotid artery occlusion. Ann Neurol 1998; 44:167–176. [DOI] [PubMed] [Google Scholar]
- 35.Rutgers DR, Klijn CJ, Kappelle LJ, van Huffelen AC, van der Grond J. A longitudinal study of collateral flow patterns in the circle of Willis and the ophthalmic artery in patients with a symptomatic internal carotid artery occlusion. Stroke 2000; 31:1913–1920. [DOI] [PubMed] [Google Scholar]
- 36.Mead GE, Wardlaw JM, Lewis SC, Dennis MS, Lothian Stroke Registry Study Group No evidence that severity of stroke in internal carotid occlusion is related to collateral arteries. J Neurol Neurosurg Psychiatr 2006; 77:729–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hofmeijer J, Klijn CJ, Kappelle LJ, Van Huffelen AC, Van Gijn J. Collateral circulation via the ophthalmic artery or leptomeningeal vessels is associated with impaired cerebral vasoreactivity in patients with symptomatic carotid artery occlusion. Cerebrovasc Dis 2002; 14:22–26. [DOI] [PubMed] [Google Scholar]
- 38.Murai Y, Mizunari T, Takagi R, et al. Analysis of ischemic cerebral lesions using 3.0-T diffusion-weighted imaging and magnetic resonance angiography after revascularization surgery for ischemic disease. Clin Neurol Neurosurg 2013; 115:1063–1070. [DOI] [PubMed] [Google Scholar]
- 39.Uchino A, Saito N, Takahashi M, et al. Persistent dorsal ophthalmic artery and ophthalmic artery arising from the middle meningeal artery diagnosed by MR angiography at 3 T. Surg Radiol Anat 2013; 35:775–782. [DOI] [PubMed] [Google Scholar]
- 40.Stankovic Z, Jung B, Collins J, et al. Reproducibility study of four-dimensional flow MRI of arterial and portal venous liver hemodynamics: Influence of spatio-temporal resolution. Magn Reson Med 2014; 72:477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]