Highlights
-
•
Neuroendocrine tumors (NETs) frequently occur in the lungs or the gastrointestinal tract; they are uncommon in the ovary.
-
•
The mammalian target of rapamycin (mTOR) pathway has been reported as a treatment for advanced NETs.
-
•
We describe a patient with an aggressive primary ovarian NET, successfully treated with everolimus (an mTOR inhibitor).
Keywords: Carcinoid, Everolimus, Multiple metastases, Neuroendocrine tumor, Ovary
Abbreviations: NETs, neuroendocrine tumors; FIGO, International Federation of Gynecology and Obstetrics; mTOR, mammalian target of rapamycin; MRI, magnetic resonance imaging; CT, computed tomography; CA-125, carbohydrate antigen 125; BEP, bleomycin, etoposide, and cisplatin; CD56, cluster of differentiation 56; EMA, epithelial membrane antigen; CDX2, caudal-type homeobox transcription factor 2
1. Background
Neuroendocrine tumors (NETs) are a heterogeneous group of neoplasms, ranging from aggressive small cell cancers to the relatively slow-growing, well-differentiated carcinoid tumors. It is uncommon for an NET to occur in the gynecologic tract; they are usually seen in the lungs or the gastrointestinal tract. Ovarian NETs account for 0.5% of all carcinoid tumors (Modlin et al., 2003) and 0.1% of all ovarian cancers (Talerman, 1997). One-third of patients with ovarian NETs show clinical neuroendocrine symptoms such as facial flushing, diarrhea, and abdominal cramping (Robboy et al., 1975). The differential diagnosis of NET in the ovary includes germ cell tumors, sex-cord and granulosa cell cancers, other gynecologic cancers, and metastatic neoplasms (Reed et al., 2014). The accurate classification and grading of NETs is generally difficult because the characteristics of the tumor differ depending on the tissue subtype and the site of origin. Ovarian NETs are managed using surgical resection, ideally with negative margins. The Society of Gynecologic Oncology (Gardner et al., 2011) and the Gynecologic Cancer InterGroup (Reed et al., 2014) have published documents covering the diagnosis and management of gynecologic NETs. Most primary ovarian carcinoids are International Federation of Gynecology and Obstetrics (FIGO) stage I, often very slow growing and infrequently associated with metastatic disease.
There are only a few case reports describing primary ovarian carcinoid tumors with aggressive histology such as a loss of the neuroendocrine growth pattern, frequent mitotic figures, and foci of coagulative tumor necrosis (Kim et al., 2015) (Vora et al., 2016). A new targeting agent that affects the mammalian target of rapamycin (mTOR) pathway has been used to treat advanced NETs (Chan and Kulke, 2014) (Cives and Strosberg, 2017). We describe a patient with an aggressive primary ovarian NET, successfully treated with everolimus (an mTOR inhibitor). We describe the histomorphology and clinical behavior of this rare disease.
2. Case presentation
2.1. Presentation and course
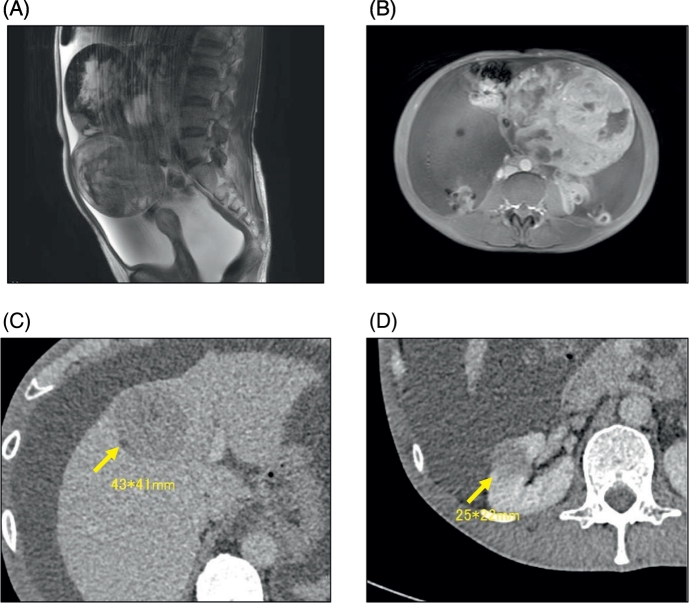
A 54-year-old woman, gravida 2 para 2, with lower abdominal distension but no other gastrointestinal symptoms consulted a nearby gynecologist's office. She was referred to our hospital when ultrasonography revealed an ovarian mass. Physical examination demonstrated a solid tumor, 20 cm in diameter, with a large amount of ascites in the pelvis. Magnetic resonance imaging of the pelvis showed a solid ovarian mass with a low signal on T2-weighted imaging and significant enhancement with gadolinium contrast (Fig. 1A). There was massive ascites present in the pelvis, and T1-weighted imaging showed a mixed signal consistent with bleeding inside the tumor and significant enhancement with gadolinium contrast (Fig. 1B). Computed tomography (CT) revealed multiple hepatic masses with a mottled contrast effect (Fig. 1C) and a marginally irregular tumor in the right kidney (Fig. 1D). There was also a shadow on the dorsal side of the right adrenal gland and swelling in the peripheral and para-aortic and left superior supraclavicular lymph nodes. These findings were suspicious for the presence of multiple metastases in the liver, right kidney, right adrenal gland, and multiple lymph nodes.
Fig. 1.
Imaging studies.
(A) T2-weighted magnetic resonance imaging (MRI), sagittal section. (B) T1-weighted, enhanced MRI, horizontal section. (C) Computed tomography (CT) showing metastasis to the liver; (D) CT showing metastasis to the right kidney.
Testing for serum tumor markers revealed that the carbohydrate antigen (CA)-125 level was increased to 348 U/mL. A slight increase in hormone levels was observed: the testosterone level was 90 ng/dL and estradiol was 24.1 pg/mL. All other gynecologic tumor markers were within normal limits. We decided to remove the pelvic tumor to make a histological diagnosis and relieve the worsening compression symptoms caused by the tumor and ascites.
On laparotomy, we observed a right ovarian tumor along with 4700 mL of ascitic fluid. The surface of the tumor was smooth (Fig. 2A) and there was no adhesion. The tumor was mainly solid, with some cystic components that were bleeding (Fig. 2B). There was no macroscopic evidence of dissemination in the peritoneal cavity. We performed total hysterectomy with bilateral salpingo-oophorectomy and partial resection of the omentum. After surgery was completed, upper and lower gastrointestinal endoscopy and an ultrasound-guided liver biopsy were performed. No neoplastic lesions were detected. The initial histopathological diagnosis was a Sertoli-Leydig cell tumor of the ovary. We administered chemotherapy consisting of bleomycin, etoposide, and cisplatin (BEP). After 3 courses of BEP, repeat CT examination revealed that the liver metastasis was increasing in size; suspected bone metastases were also seen. We performed an ultrasound-guided liver biopsy that revealed small tumor cells exhibiting a cord-like sequence, similar to the ovarian tumor. The ovarian mass was then pathologically re-examined with the addition of immunohistochemical staining. The final diagnosis was an atypical carcinoid of the ovary, according to the pulmonary carcinoid criteria, and a NET (grade G2) according to the gastrointestinal neuroendocrine tumor criteria. We administered everolimus according to the chemotherapy for an advanced pancreatic NETs. The patient's disease has been stable for more than 7 months.
Fig. 2.
Pathological findings.
(A) Gross appearance of the resected ovarian tumor shows a 20-cm, solid mass with an irregular, smooth surface. (B) The cut surface shows fleshy, tan-yellow, heterogeneous tissue with focal hemorrhage, necrosis, and cystic change. (C) Hematoxylin and eosin staining. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.2. Histopathological findings
The main part of the ovarian tumor specimen consisted of ribbon, cord-like tissue and formed a solid alveolar pattern of atypical cells, with hematoxylin and eosin staining showing abundant nuclear and eosinophilic cytoplasm (Fig. 2C). Tumor cells with fine granular cytoplasm proliferated in a cord shape between the abundant vascular networks that were composed of sinusoidal vessels (Fig. 2D). The differential diagnosis at this point included a Sertoli-Leydig cell tumor, endometrioid adenocarcinoma, and NET. Immnohistochemical staining was positive for synaptophysin (Fig. 3A), chromogranin A (Fig. 3B), cluster of differentiation (CD) 56, cytokeratin AE1/AE3, and epithelial membrane antigen (EMA). Staining for inhibin α (Fig. 3C) and WT-1 was negative. We suspected that the proliferative capacity of this mass was higher than that of a usual carcinoid tumor because nuclear mitosis was recognized in 3 of 10 high power fields and the Ki-67 index was high at 17% (Fig. 3D).
Fig. 3.
Immunohistochemistry.
The tumor cells are strongly positive for (A) synaptophysin and (B) chromogranin A. (C) The tumor cells are negative for inhibinα. (D) The Ki-67 index is 17%.
3. Discussion
When NETs occur in the ovary, they may present as small cell carcinomas, large cell variants, and well-differentiated carcinoids tumors (Reed et al., 2014). Primary ovarian carcinoid is subdivided into 4 categories: insular, trabecular, mucinous, and stromal (Vora et al., 2016). Since carcinoid tumor often shows a cord or tubular structure, differential diagnosis with Sertoli-Leydig tumor may be a problem in only morphological evaluation. Immunohistochemistry can be useful in the diagnosis, as synaptophysin, chromogramin, and CD56 are markers of ovarian carcinoid tumors (Reed et al., 2014). Carcinoid tumors are usually negative for EMA, estrogen receptors, progesterone receptors, and sex-cord stromal markers such as inhibin and calretinin (Reed et al., 2014). The use of immunohistochemistry is recommended if frequently observed finding in NETs, such as interposed abundant blood vessels between the cord like sequences, was seen in the tumor. It is also important to differentiate a primary ovarian NET from a metastatic gastrointestinal carcinoid in order to select appropriate therapy. Recent studies report that the immunohistochemical marker caudal-type homeobox transcription factor 2 (CDX2) may be useful for distinguishing primary ovarian carcinoid from small intestine metastasis and appendiceal NET (Desouki et al., 2013). In our patient, the primary tumor was more aggressive than we originally presumed from the pathological diagnosis of atypical carcinoid, as she had metastases to the liver and kidney and a subsequent metastasis to the bone. Kurabayashi et al. (2010) pointed out that the proliferation activity of a primary ovarian carcinoid can be a prognostic factor. Our patient had a Ki-67 positive rate of 17%, although nuclear mitotic figures were not prominent. This highly positive Ki-67 result may indicate a poor prognosis.
For patients with stage I primary ovarian carcinoid, the prognosis is excellent with more than 90% survival (Modlin et al., 2003). However, patients with more advanced stages have a poor prognosis (Robboy et al., 1975). There is no standard management of ovarian NET, as the most recent National Comprehensive Cancer Network guidelines for neuroendocrine tumor (version 3, 2017) do not include ovarian carcinoid or any ovarian NETs. According to the guidelines for gastrointestinal NETs, the primary therapeutic management is surgical resection. The most common sites of metastasis for NETs are the regional lymph nodes, liver, bones, and lungs. For patients with liver metastasis from a gastrointestinal NET, hepatic-directed therapies such as cytoreductive surgery or ablative therapies such as radiofrequency ablation or cryoablation may be considered with palliative intent (Modlin et al., 2003). For patients with bone metastasis, localized radiotherapy, with or without bisphosphonate therapy, can be considered (Gardner et al., 2011).
Systemic treatment options for patients with advanced NETs have been limited until recently. There is no recommended chemotherapeutic regimen (Kurabayashi et al., 2010), but there are some treatment options for metastatic or advanced ovarian carcinoid, such as streptozocin (Pelage et al., 2017), 5-fluorouracil (Kouvaraki et al., 2004), capecitabine (Quinn et al., 2016), and cisplatin containing regimen (Kanayama et al., 2012) from previous reports. The somatostatin analog could be another therapeutic option (Rinke et al., 2009) if the patients had carcinoid syndrome symptoms such as flushing, rash, itch, diarrhea, and wheezing. Furthermore, octreotide scan might be useful for detecting primary or metastatic lesions for the patient with those symptoms, because gastroenteropancreatic neuroendocrine tumors are reported to express high levels of somatostatin receptors (Jamar et al., 1995). Recently, altered expression in the mTOR signaling pathway has been observed in NETs, and molecular-targeted mTOR inhibitors have shown promise in treating gastrointestinal carcinoid. In a phase-III, randomized, placebo-controlled study of patients with advanced pancreatic NET, treatment with the mTOR inhibitor everolimus was associated with improved progression-free survival (Pavel et al., 2011). Based on these findings, we administered everolimus to our patient, and she maintained a stable disease state for more than 6 months without severe side effects. In addition to everolimus, sunitinib, a multi-targeted RTK inhibitor, have been recently used for gastrointestinal and pancreatic NET G1–2 (Raymond et al., 2011). It would be another therapeutic option if everolimus fail to treat this patient's tumor. Further accumulation of cases is necessary to determine the best management of women with gynecologic carcinoid tumors.
4. Conclusions
We present a patient with an aggressive primary ovarian NET, successfully treated with everolimus, an mTOR inhibitor. Immunohistochemical staining was helpful to establish the pathological diagnosis of ovarian NET.
Patient consent
Written informed consent was obtained from the patient for publication of this case report and the accompanying images.
Competing interests
The authors declare that there are no conflicts of interests.
Acknowledgement
We thank Dr. Masanobu Takahashi of the Department of Medical Oncology, Tohoku University Hospital, for providing expert advice and medical care.
Contributor Information
Michiko Kaiho-Sakuma, Email: smichiko@med.tohoku.ac.jp.
Masafumi Toyoshima, Email: m-toyo@med.tohoku.ac.jp.
Mika Watanabe, Email: mkawatan@patholo2.med.tohoku.ac.jp.
Hitoshi Niikura, Email: niikura@med.tohoku.ac.jp.
Nobuo Yaegashi, Email: myaegashi@med.tohoku.ac.jp.
References
- Chan J., Kulke M. Targeting the mTOR signaling pathway in neuroendocrine tumors. Curr. Treat. Options in Oncol. 2014;15:365–379. doi: 10.1007/s11864-014-0294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cives M., Strosberg J. Treatment strategies for metastatic neuroendocrine tumors of the gastrointestinal tract. Curr. Treat. Options in Oncol. 2017;18 doi: 10.1007/s11864-017-0461-5. [DOI] [PubMed] [Google Scholar]
- Desouki M.M., Lioyd J., Xu H., Cao D., Barner R., Zhao C. CDX2 may be a useful marker to distinguish primary ovarian carcinoid from gastrointestinal metastatic carcinoids to the ovary. Hum. Pathol. 2013;44:2536–2541. doi: 10.1016/j.humpath.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Gardner G.J., Reidy-Lagunes D., Gehrig P.A. Neuroendocrine tumors of the gynecologic tract: a Society of Gynecologic Oncology (SGO) clinical document. Gynecol. Oncol. 2011;122:190–198. doi: 10.1016/j.ygyno.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Jamar F., Fiasse R., Leners N., Pauwels S. Somatostatin receptor imaging with indium-111-pentetreotide in gastroenteropancreatic neuroendocrine tumors: safety, efficacy and impact on patient management. J. Nucl. Med. 1995;36:542–549. [PubMed] [Google Scholar]
- Kanayama S., Yamada Y., Tanase Y., Haruta S., Nagai A., Kawaguchi R., Yoshida S., Furukawa N., Oi H., Kobayashi H. A case of early-stage ovarian carcinoid tumor metastasized to the liver. Case Rep. Obs. Gynecol. 2012;2012:961087. doi: 10.1155/2012/961087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.S., Yoon G., Jang H.I., Song S.Y., Kim B.G. Primary ovarian carcinoid tumor showing unusual histology and nuclear accumulation of β-catenin. Int. J. Clin. Exp. Pathol. 2015;8:5749–5752. [PMC free article] [PubMed] [Google Scholar]
- Kouvaraki M.A., Ajani J.A., Hoff P., Wolff R., Evans D.B., Lozano R., Yao J.C. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J. Clin. Oncol. 2004;22:4710–4719. doi: 10.1200/JCO.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Kurabayashi T., Minamikawa T., Nishijima S., Tsuneki I., Tamura M., Yanase T., Hashidate H., Shibuya H., Motoyama T. Primary strumal carcinoid tumor of the ovary with multiple bone and breast metastases. J. Obstet. Gynaecol. Res. 2010;36:567–571. doi: 10.1111/j.1447-0756.2010.01231.x. [DOI] [PubMed] [Google Scholar]
- Modlin I.M., Lye K.D., Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- Pavel M.E., Hainsworth J.D., Baudin E., Peeters M., Hörsch D., Winkler R.E., Klimovsky J., Lebwohl D., Jehl V., Wolin E.M., Öberg K., Van Cutsem E., Yao J.C. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011;378:2005–2012. doi: 10.1016/S0140-6736(11)61742-X. [DOI] [PubMed] [Google Scholar]
- Pelage J.P., Fohlen A., Mitry E., Lagrange C., Beauchet A., Rougier P. Erratum to: chemoembolization of neuroendocrine liver metastases using streptozocin and tris-acryl microspheres: embozar (EMBOsphere + ZAnosaR) study. Cardiovasc. Intervent. Radiol. 2017;40(3):394–400. doi: 10.1007/s00270-016-1535-7. (Cardiovasc. Intervent. Radiol. 40, 480. doi:10.1007/s00270-017-1573-9) [DOI] [PubMed] [Google Scholar]
- Quinn A.M., Chaturvedi A., Nonaka D. High-grade neuroendocrine carcinoma of the lung with carcinoid morphology. Am. J. Surg. Pathol. 2016;41:1. doi: 10.1097/PAS.0000000000000767. [DOI] [PubMed] [Google Scholar]
- Raymond E., Dahan L., Raoul J.-L., Bang Y.-J., Borbath I., Lombard-Bohas C., Valle J., Metrakos P., Smith D., Vinik A., Chen J.-S., Hörsch D., Hammel P., Wiedenmann B., Van Cutsem E., Patyna S., Lu D.R., Blanckmeister C., Chao R., Ruszniewski P. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011;364:501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- Reed N.S., Gomez-Garcia E., Gallardo-Rincon D., Barrette B., Baumann K., Friedlander M., Kichenadasse G., Kim J., Lorusso D., Mirza M.R., Ray-Coquard I. Gynecologic Cancer InterGroup (GCIG) consensus review for carcinoid tumors of the ovary. Int. J. Gynecol. Cancer. 2014;24:S35–41. doi: 10.1097/IGC.0000000000000265. [DOI] [PubMed] [Google Scholar]
- Rinke A., Müller H.H., Schade-Brittinger C., Klose K.J., Barth P., Wied M., Mayer C., Aminossadati B., Pape U.F., Bläker M., Harder J., Arnold C., Gress T., Arnold R. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID study group. J. Clin. Oncol. 2009;27:4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- Robboy S.J., Norris H.J., Scully R.E. Insular carcinoid primary in the ovary. A clinicopathologic analysis of 48 cases. Cancer. 1975;36:404–418. doi: 10.1002/1097-0142(197508)36:2<404::aid-cncr2820360216>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Talerman A. Germ cell tumors of the ovary. Curr. Opin. Obstet. Gynecol. 1997;9:44–47. [PubMed] [Google Scholar]
- Vora M., Lacour R.A., Black D.R., Turbat-Herrera E.A., Gu X. Neuroendocrine tumors in the ovary: histogenesis, pathologic differentiation, and clinical presentation. Arch. Gynecol. Obstet. 2016;293:659–665. doi: 10.1007/s00404-015-3865-0. [DOI] [PubMed] [Google Scholar]