Abstract
Background
Liver transplantation remains the primary treatment for primary sclerosing cholangitis (PSC). Mdr2−/− mice provide a reliable in vivo model of PSC and develop characteristic biliary inflammation and fibrosis. We tested the hypothesis that the tumor suppressor protein menin is implicated in the progression of liver fibrosis and that menin expression can be regulated in the liver via microRNA-24 (miR-24).
Materials and methods
Menin expression was measured in human PSC and Mdr2−/− mice. Twelve-week-old FVB/NJ wild-type (WT) and Mdr2−/− mice were treated with miR-24 Vivo-Morpholino to knockdown miR-24 expression levels. Liver fibrosis was evaluated by Sirius Red staining and quantitative polymerase chain reaction (qPCR) for genes associated with liver fibrosis, such as fibronectin 1, collagen type 1 alpha 1, transforming growth factor-β1 (TGF-β1), and α-smooth muscle actin. Studies were also performed in vitro using immortalized murine cholangiocyte lines treated with miR-24 hairpin inhibitor and mimic.
Results
Menin gene expression was increased in Mdr2−/− mice and late-stage human PSC samples. Treatment of FVB/NJ WT and Mdr2−/− mice with miR-24 Vivo-Morpholino increased menin expression, which correlated with increased expression of fibrosis genes. In vitro, inhibition of miR-24 also significantly increased the expression of fibrosis genes.
Conclusions
Inhibition of miR-24 increases menin and TGF-β1 expression, subsequently increasing hepatic fibrosis in FVB/NJ WT and Mdr2−/− mice. Modulation of the menin/miR-24 axis may provide novel targeted therapies to slow the progression of hepatic fibrosis into cirrhosis in PSC patients by altering TGF-β1 expression.
Keywords: Menin, miR-24, Liver, Hepatic fibrosis
Introduction
Cholangiocytes represent 3%–5% of nucleated cells within the liver and are the targets of cholangiopathies, such as primary sclerosing cholangitis (PSC).1,2 These cholangiopathies are characterized by the classical findings of cholestatic liver injury: increased intrahepatic bile duct mass, polymorphonuclear leukocytes, and the deposition of extracellular matrix that lead to portal fibrosis and biliary cirrhosis.3 PSC in particular is characterized by chronic inflammation and obliterative fibrosis of the intrahepatic and/or extrahepatic biliary tree.4 This results in bile stasis and hepatic fibrosis that will progress to cirrhosis and the need for liver transplantation.4 PSC is also associated with a 5%–10% lifetime risk for the development of cholangiocarcinoma, 160-fold higher than the general population.5 Currently, there are no medical therapies that have been proven to alter the natural course of PSC, and liver transplantation before the onset of end-stage liver disease remains the recommended treatment strategy.5,6 Improved understanding of the cellular mechanisms that lead to biliary proliferation and portal fibrosis is needed to develop novel therapeutic strategies to diminish disease progression.
Menin is the protein product of the MEN1 gene, a tumor suppressor gene located on chromosome 11q13.1.7 It is a 67 kDa nuclear protein that is ubiquitously expressed in all tissues and evolutionarily conserved, but shares little sequence homology with other proteins.8 Several studies suggest that menin acts as a scaffold protein involved in diverse cell functions including binding and regulating transcription factor activity,9 modifying histone proteins and chromatin structure,10,11 and DNA repair.12,13 Germline mutations in the MEN1 gene cause the MEN1 syndrome, a neuroendocrine tumor syndrome that predisposes patients to neoplasms of the parathyroid glands, pancreas, and the pituitary gland.7 In the setting of cholestatic liver injury, cholangiocytes represent a neuroendocrine cell population within the liver that respond to a variety of hormones, neurotransmitters, and growth factors that have been shown to regulate cholangiocyte proliferation and the ductular reaction associated with hepatic fibrosis.14,15 Because of its implications in neuroendocrine signaling, we hypothesized that MEN1 gene expression may play an important role in the progression of hepatic fibrosis.
MicroRNA-24 (miR-24) has previously been shown to bind to the 3′ untranslated region (UTR) of the MEN1 gene and regulate menin expression through a negative feedback loop in parathyroid and pancreatic tissues.16,17 In addition, menin has been shown to interact with SMAD3 to block transforming growth factor-β1 (TGF-β1) signaling.18 SMAD3 phosphorylation and TGF-β1 have previously been shown to contribute to hepatic fibrosis19; however, the role of menin in this pathway is unknown.
The multidrug resistance gene-2 knockout mouse (Mdr2−/−) is a widely used murine model of cholestatic liver disease characterized by the development of PSC with features of biliary proliferation and portal fibrosis.14,19,20 Mdr2−/− mice are deficient in a canalicular phospholipid flippase and develop liver injury due to the absence of phospholipids in bile.21 The bile ducts of these mice are characterized by tight junction and basement membrane destruction, which creates widened intracellular spaces between biliary endothelial cells and results in bile acid leakage, periductular inflammation, and fibrosis.22 Studies in these mice from 2 wk to 12 mo of age have shown that they develop chemical and histologic evidence of endothelial disruption, hepatic inflammation, and fibrosis.22,23 Mdr2−/− mice also develop hepatic malignancies with nearly 100% incidence by 16 mo of age.24 Unlike PSC, however, these tumors resemble hepatocellular carcinoma (HCC) rather than a primary biliary malignancy, such as cholangiocarcinoma. Using this model, we hypothesized that the miR-24/menin regulatory feedback loop could be manipulated to alter the progression of hepatic fibrosis.
Materials and methods
All reagents were purchased from Sigma (St. Louis, MO) unless otherwise indicated. Cell culture reagents and media components were purchased from Invitrogen Corporation (Carlsbad, CA). Total RNA and miRNA were isolated from cells and liver tissue using the mirVana miRNA isolation kit from Thermo-Fisher Scientific (Waltham, MA). Complementary DNA was generated from 1200 μg of total RNA using iScript Reverse Transcription Supermix for qPCR (Bio Rad, Hercules, CA). Primers for qPCR were purchased from Qiagen (Valencia, CA) unless otherwise indicated. The qPCR experiments were performed using SYBR Green PCR Master Mix from SABiosciences on the Agilent Technologies Mx3005P qPCR system.
MEN1 gene expression was quantified by qPCR using RNA isolated from immortalized murine cholangiocyte lines (IMCLs), mouse and human liver tissues. Liver fibrosis was evaluated by qPCR using mouse primers for fibronectin 1 (FN1), collagen type 1 alpha 1 (COL1α1), TGF-β1, and α-smooth muscle actin (α-SMA). Proliferation was evaluated by qPCR using mouse primers for Ki-67. Glyceraldehyde-3-phosphate dehydrogenase gene expression was used as a relative control. Data are expressed as relative messenger RNA levels ± standard error of the mean (SEM).
In vitro studies
In vitro studies were performed using our IMCLs.19,25 Cell were cultured under standard conditions and treated with 75 nM of mirVana miR-24 inhibitor, mimic, or the standard control for 24 h according to the manufacturer’s protocol. Cells were collected after treatment using TrypLE solution (Gibco) and used for RNA isolation. miRNA inhibitors are single stranded, modified RNAs that bind to and inhibit endogenous function of the target miRNA. Whereas, the miRNA mimics are double stranded RNAs that mimic the functional activity of endogenous RNAs once transfected into the cell. The inhibitor and mimic controls are mismatched miRNAs that lack endogenous function. These products enable us to regulate miR-24 activity in vitro and study the downstream signaling mechanisms.
miR-24 expression was measured in cholangiocytes and murine liver tissue by qPCR. Isolation of miRNAs was performed using the mirVana RNA isolation kit. Complementary DNA was synthesized using the TaqMan microRNA Reverse Transcription Kit from Applied Biosystems (Foster City, CA). Sequence specific primers for miR-24 and U6 control were purchased from Qiagen.
The luciferase assay was performed to determine if miR-24 directly interacts with the MEN1 gene to alter its expression in cholangiocytes. Luciferase constructs were obtained from Dr Judy S. Crabtree (Louisiana State University, Baton Rouge, LA). These constructs consisted of a 1600-bp fragment of human MEN1 3′-UTR cloned into a pmirGLO vector (pmirGLO-MEN1). 5 × 105 IMCLs per assay were co-transfected with pmirGLO-MEN1 at 5 × 1 pmol/0.5 mL medium. Luciferase levels were measured after 24 h using Dual-Glo Stop & Glo per vendor’s instructions (Promega, Madison, WI), imaged using Thermo Scientific Varioskan Lux and analyzed using Thermo Scientific SkanIt 4.1 software. Data are expressed as fold-change of firefly/Renilla luminescence ± SEM.
Human samples
Control and late-stage PSC samples were obtained as a gift form Dr Invernizzi under a protocol approved by the ethics committee by the Humanitas Research Hospital (Rozzano, Italy) and also reviewed by the Central Texas Veteran’s Health Care System IRB and R&D Committee. The protocol was also approved by the Texas A&M HSC College of Medicine Institutional Review Board. Total RNA was extracted from formalin-fixed, paraffin-embedded sections using the RNeasy FFPE kit from Qiagen.19 Menin expression in control and late-stage PSC samples was verified by qPCR.
In vivo studies
All animal experiments were performed according to protocols approved by the Baylor Scott & White Institutional Animal Care and Use Committee. Male FVB/NJ wild-type (WT) mice were purchased from The Jackson Laboratory (Sacramento, CA). These mice served as the nondiseased control animals and are the background strain for the Mdr2−/− model.22 The Mdr2−/− mouse colony is established in our facility. Animals were maintained in a temperature and light controlled environment with free access to drinking water and rodent chow.
Immunohistochemistry (IHC) was used to evaluate the expression of TGF-β1 in FVB/NJ WT and Mdr2−/− liver tissues. The tissues were stained with rabbit polyclonal TGF-β1 antibody purchased from Abcam (Cambridge, MA) using a 1:200 dilution. The rabbit IgG Vectastain ABC Kit from Vector Laboratories, INC (Burlingame, CA) was used for secondary staining. Light microscopy and IHC observations were taken with a BX-40 light microscope (Olympus; Tokyo, Japan). Semiquantitative analysis of IHC images was performed using Adobe Photoshop.
Inhibition of miR-24 signaling in vivo was performed using an miR-24 Vivo-Morpholino purchased from Gene Tools, LLC (Philomath, OR).26 Similar to our in vitro studies using the miR-24 inhibitor, the miR-24 Vivo-Morpholino is a systemically stable, miRNA blocking reagent that provides quantifiable knockdown of the selected miRNA. The Vivo-Morpholino allows us to inhibit miR-24 function for our in vivo studies and measure the affects within organ systems. The purchased miR-24 Vivo-Morpholino sequence was 5′-TCCTGTTCCTGCTGAACTGAGCCAG -3. Twelve-week-old FVB/NJ WT and Mdr2−/− mice were treated with the miR-24 Vivo-Morpholino (12.5 mg/kg) via tail vein injection every other day for 1 wk. Mice were euthanized, and tissues were collected on the seventh day after the first treatment.
Liver fibrosis was evaluated by Sirius Red staining in liver sections (5 μm thick) and by qPCR of RNA isolated total liver samples for the aforementioned genes associated with liver fibrosis. Sirius Red images were taken with a BX-40 light microscope (Olympus). Semiquantitative analysis of intrahepatic collagen deposition was performed using Adobe Photoshop.
Statistical analysis
All data are expressed as mean ± SEM. Differences between groups were analyzed by Student’s unpaired t-test when two groups were analyzed and analysis of variance when more than two groups were analyzed, followed by an appropriate post hoc test. P < 0.05 was considered to be statistically significant.
Results
MEN1 expression is increased in advanced stage PSC and 66-wk Mdr2−/− mice
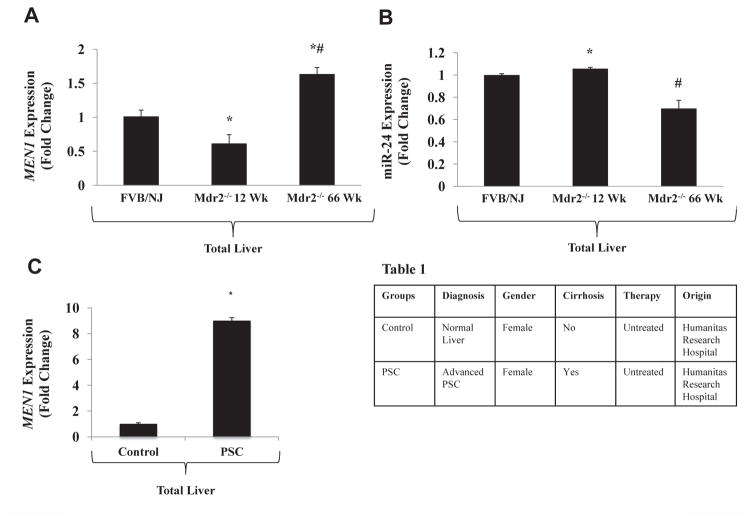
Expression of the MEN1 gene was measured by qPCR in total liver tissues from FVB/NJ WT and Mdr2−/− mice at 12 and 66 wk of age. MEN1 expression was significantly decreased in the 12-wk-old Mdr2−/− mice compared with the FVB/NJ WT control, but was significantly upregulated in the 66-wk-old Mdr2−/− mice (Fig. 1A). This corresponded with increased expression of miR-24 in total liver at 12 wk and decreased expression of miR-24 at 66 wk in the Mdr2−/− mice (Fig. 1B) compared with the FVB/NJ WT control mice. MEN1 expression measured in human liver with advanced-stage PSC was also upregulated, similar to the 66-wk-old Mdr2−/− mouse (Fig. 1C). Pathologic and descriptive data for the human samples are shown in Table 1. These data show that menin expression is variable throughout the lifespan of the Mdr2−/− mouse and is higher in mice with advanced disease.
Fig. 1.
(A) Hepatic Men1 gene expression is decreased in 12-wk Mdr2−/− mice and increased in 66-wk Mdr2−/− mice compared with the FVB/NJ WT control mice via qPCR (n = 3). (B) Hepatic miR-24 expression is significantly increased in 12-wk Mdr2−/− and decreased in 66-wk Mdr2−/− compared with FVB/NJ WT control mice via qPCR (n = 3). (C) Men1 gene expression is increased in human liver with advanced-stage PSC compared with normal control liver (n = 1). Demographic and pathologic data from human samples are shown in Table 1. (* = P < 0.05 versus FVB/NJ WT control samples; # = P < 0.05 versus 12-wk Mdr2−/− samples).
miR-24 regulates MEN1 expression in mouse cholangiocytes
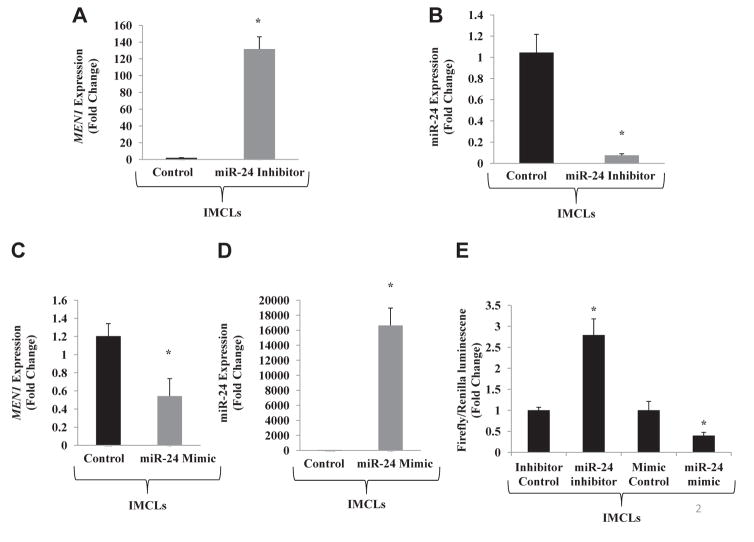
Inhibition of miR-24 in IMCLs corresponded with a significant increase in MEN1 gene expression (Fig. 2A and B). Treatment of IMCLs with miR-24 mimic resulted in downregulation of MEN1 expression (Fig. 2C and D). These results demonstrate that miR-24 participates in a negative feedback loop within cholangiocytes to alter the expression of menin. Results of the luciferase assay are shown in Figure 2E. Treatment of cholangiocytes with miR-24 inhibitor significantly increased luminescence, whereas treatment with the miR-24 mimics significantly decreased luminescence. These results confirm that miR-24 directly interacts with the 3′ UTR of MEN1 messenger RNA transcript to regulate protein expression.
Fig. 2.
(A) Inhibition of miR-24 in IMCLs increases Men1 gene expression by qPCR (n = 3). (B) Treatment of IMCLs with miR-24 inhibitor decreases expression of miR-24 by qPCR (n = 1). (C) Treatment of IMCLs with miR-24 mimic decreases Men1 gene expression by qPCR (n = 3). (D) Treatment of IMCLs with miR-24 mimic significantly increases miR-24 expression (n = 1). (E) Luciferase assay demonstrates increased luminescence when mouse cholangiocytes are treated with miR-24 inhibitor, suggesting an increase in Men1 transcription. Treatment with miR-24 mimic decreases luminescence by inhibiting Men1 transcription (n = 1; * = P < 0.05).
Expression of MEN1 correlates with changes in fibrosis gene expression
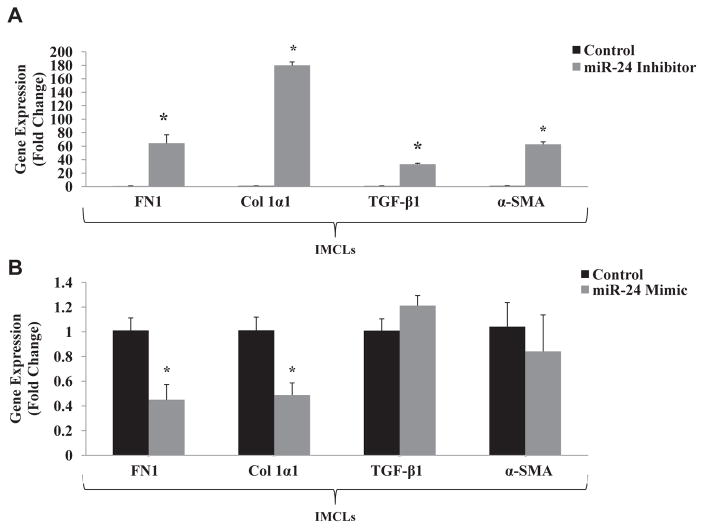
The expression of fibrotic genes was evaluated by qPCR in IMCLs treated with miR-24 inhibitor. When MEN1 expression was increased (Fig. 2A), expressions of FN1, COL1α1, TGF-β1, and α-SMA were also increased (Fig. 3A). Inhibition of MEN1 with the miR-24 mimic resulted in decreased expression of these fibrosis genes (Fig. 3B). These results demonstrate in vitro that the negative feedback loop between miR-24 and menin also contributes to the expression of genes associated with liver fibrosis.
Fig. 3.
(A) Expression of fibrotic genes (FN1, COL1a1, TGF-β1, and α-SMA) is increased in IMCLs treated with miR-24 inhibitor (n = 3). (B) Expression of fibrotic genes (FN1 and COL1α1) is decreased in mouse cholangiocytes treated with miR-24 mimic. Expression of TGF-β1 and α-SMA did not significantly change with miR-24 mimic (n = 3; * = P < 0.05).
Inhibition of miR-24 in vivo regulates expression of MEN1 and drives hepatic fibrosis
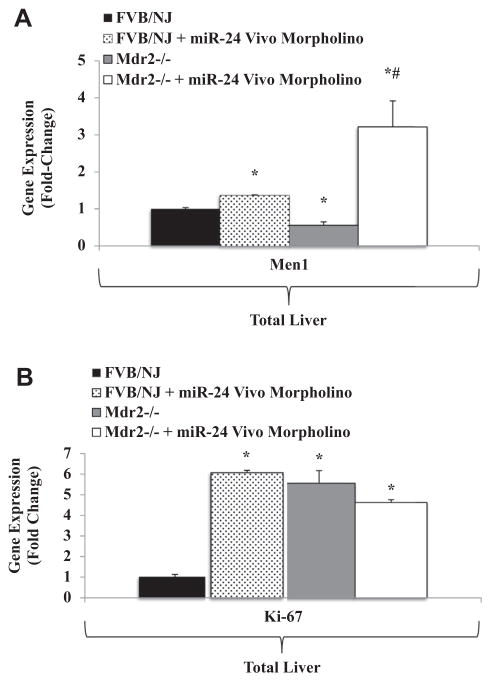
Treatment of FVB/NJ WT and Mdr2−/− mice with miR-24 Vivo-Morpholino significantly increased MEN1 expression in total liver tissues as measured by qPCR (Fig. 4A). Expression of Ki-67 significantly increased in FVB/NJ WT mice treated with miR-24 Vivo-Morpholino, but this change was not seen after treatment of the Mdr2−/− mice (Fig. 4B).
Fig. 4.
(A) Treatment of FVB/NJ WT and Mdr2−/− with miR-24 Vivo-Morpholino significantly increases hepatic Men1 expression compared with untreated FVB/NJ WT and Mdr2−/− control mice by qPCR (n = 3). (B) Ki-67 expression is significantly upregulated in FVB/NJ WT mice treated with miR-24 Vivo-Morpholino (n = 3). (* = P <0.05 versus FVB/NJ WT control samples; # = P < 0.05 versus Mdr2−/− samples).
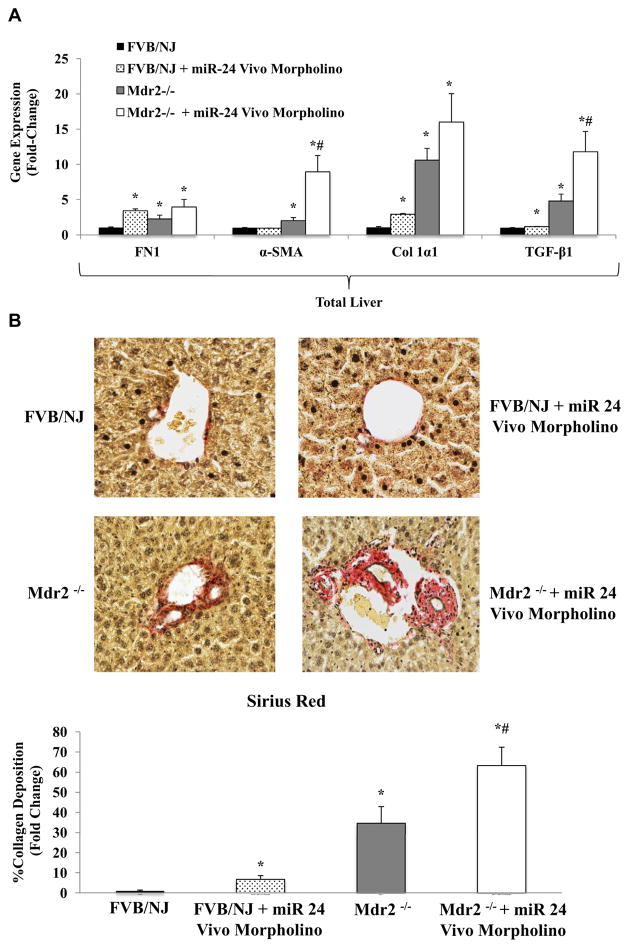
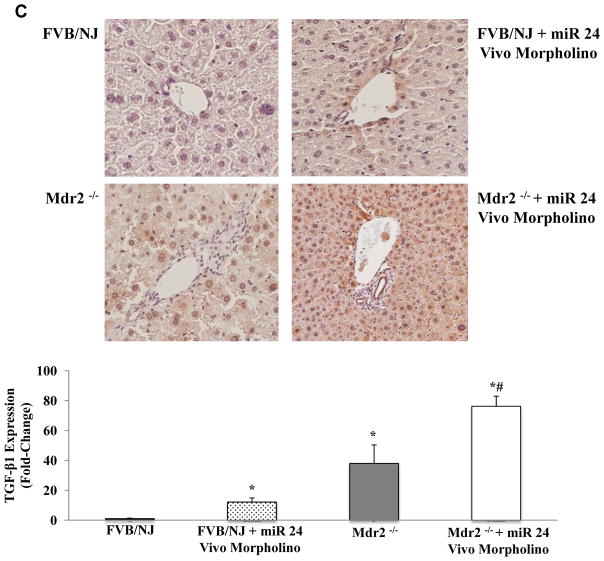
Similar to our in vitro results, inhibition of miR-24 resulted in significantly increased expression of fibrosis-related genes, including TGF-β1 in both the FVB/NJ WT and Mdr2−/− mice (Fig. 5A). Sirius Red staining and semiquantitative analysis of liver sections were performed to evaluate degree of hepatic fibrosis. FVB/NJ WT and Mdr2−/− mice treated with miR-24 Vivo-Morpholino demonstrated significantly more collagen deposition than the FVB/NJ WT and untreated Mdr2−/− mice (Fig. 5B). IHC demonstrates increased TGF-β1 staining in FVB/NJ WT and Mdr2−/− livers treated with miR-24 Vivo-Morpholino compared with the untreated control mice (Fig. 5C).
Fig. 5.
(A) Hepatic expression of fibrotic genes (FN1, COLα1, TGF-β1, and α-SMA) increases in FVB/NJ WT and Mdr2−/− mice treated with miR-24 Vivo-Morpholino compared with untreated FVB/NJ WT and Mdr2−/− untreated control mice (n = 3). (B) Sirius Red staining shows increased hepatic collagen deposition in FVB/NJ WT and Mdr2−/− mice treated with miR-24 Vivo-Morpholino compared with untreated FVB/NJ WT and Mdr2−/− mice. (C) IHC shows increased TGF-β1 staining in FVB/NJ livers treated with miR-24 Vivo-Morpholino compared with the untreated FVB/NJ mice. (* = P < 0.05 versus FVB/NJ WT control samples; # = P < 0.05 versus Mdr2−/− samples).
Discussion
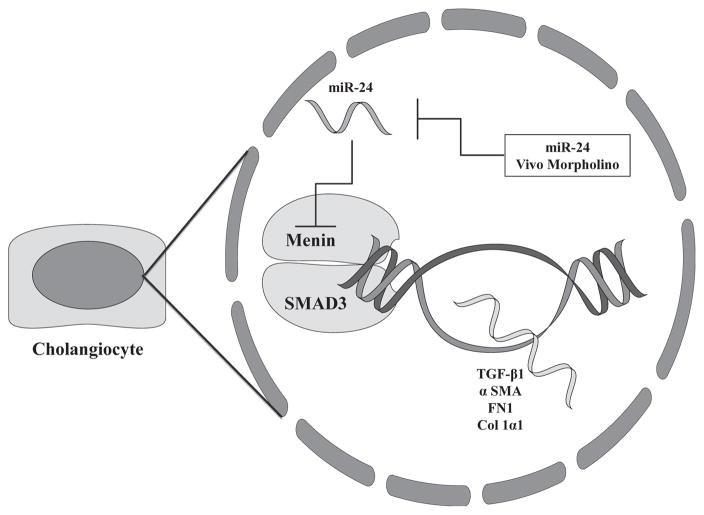
The findings of this study suggest that the miR-24/menin regulatory system may play a key role in the progression of hepatic fibrosis, in the setting of cholestasis. Figure 6 represents a working model of these results. We demonstrated in vitro that miR-24 directly interacts with the MEN1 gene in murine cholangiocytes. Also, we demonstrated that inhibition of miR-24 signaling in vitro and in vivo increases MEN1 gene expression and subsequently promotes expression of fibrotic genes in cultured cholangiocytes and liver tissues. We demonstrate increased hepatic fibrosis in Mdr2−/− mice, as well as the nondiseased FVB/NJ control strain, with miR-24 Vivo-Morpholino treatment.
Fig. 6.
Working model of miR-24/menin regulatory axis in cholangiocytes. Menin binds with SMAD3 to promote transcription of TGF-β1, α-SMA, FN1, and COL1α1, which act to promote hepatic fibrosis. miR-24 inhibits transcription of MEN1 gene, decreasing transcription of downstream fibrotic genes. Inhibition of miR-24 with miR-24 Vivo-Morpholino increases MEN1 gene expression and subsequently increases hepatic fibrosis.
These findings correlate with increased MEN1 expression in a patient with late-stage PSC and the 66-wk-old Mdr2−/− mice. Our animal data do suggest that MEN1 gene expression varies throughout the life cycle of the Mdr2−/− mouse. We saw decreased MEN1 expression at 12 wk, but increased expression at 66 wk. This correlated with increased miR-24 levels at 12 wk and decreased miR-24 levels at 66 wk. More human studies in early stage and late-stage PSC are needed to elucidate the mechanisms behind MEN1 gene transcription. Because menin is highly involved in cell cycle regulation, we hypothesize that decreased MEN1 expression at 12 wk may reflect an adaptive response to cell injury that becomes dysfunctional as the liver injury progresses. Hussein et al. demonstrated that increasing menin expression in Leydig tumor cells blocked the transition of G0/G1 into the S phase of the cell cycle and increased apoptosis.27 It is possible that similar mechanisms are occurring within the liver in advanced-stage disease. Studies have shown that cytokeratin-18 fragments (K18), a marker of apoptosis, are elevated in patients with PSC and correlate with disease severity.28 Our data add to these studies and suggest that increased menin expression may help promote apoptosis in late-stage PSC.
Menin has traditionally been characterized as a nuclear tumor suppressor protein because of its involvement in MEN1 syndrome, an autosomal dominant disorder that is characterized by the development of tumors of the pituitary, parathyroid glands, and pancreatic neuroendocrine cells.29 Early studies suggested that menin inactivation contributes to parathyroid tumorigenesis through a loss of TGF-β1 signaling.30 Previous studies have also shown that menin interacts with SMAD3 to regulate TGF-β1 signaling.18 Specifically, inactivation of menin disrupts SMAD3 binding to its cognate DNA element and blocks TGF-β1 signaling.18,29 This pathway has been supported by other studies that suggest SMAD3 may also act as a tumor suppressor that regulates menin and TGF-β1 signaling in parathyroid adenomas and pancreatic endocrine tumors.31
Increased SMAD3/TGF-β1 signaling contributes to the development of hepatic fibrosis in cholestatic liver disease,19,32 but menin’s role in liver pathology has not been well defined. We have previously shown that menin expression is downregulated in cholangiocarcinoma and overexpressing menin in a xenograft model inhibits cholangiocarcinoma growth.33 We believe menin expression may become dysregulated in PSC resulting in the development of cholangiocarcinoma; however, additional studies with human PSC and cholangiocarcinoma samples are needed to establish these mechanisms.
Zindy et al. demonstrated that MEN1 expression is upregulated in HCC and in adjacent cirrhotic liver tissue.34 Particularly, menin was a key regulator of hepatic fibrosis through TGF-β1 signaling and activation of hepatic stellate cells.34 Xu et al. also demonstrated that menin expression is upregulated in HCC and expression correlates with a poor prognosis.35 Furthermore, a recent study by Cao et al. suggested that deletion of MEN1 in hepatocytes induced lipid accumulation and liver steatosis in aging mice.36 Our study contributes to growing evidence that suggests menin is involved in liver disease. We have shown that upregulation of menin increases TGF-β1 expression and promotes fibrosis in cholangiocytes and in a murine model of cholestatic liver disease. Additional research in this model is needed to further elucidate menin’s involvement and characterize the interactions between hepatocytes, cholangiocytes, and hepatic stellate cells.
This study also demonstrates that a negative feedback loop between miR-24 and menin exists within the liver and may play a role in the progression of liver disease. miR-24 was downregulated in the advanced disease 66-wk Mdr2−/− mice when MEN1 gene expression was increased. In the 12-wk-old Mdr2−/− mice, miR-24 expression was significantly increased, although not as much as we expected for the observed decrease in MEN1 gene expression. MiR-24 has previously been shown to regulate menin expression within pancreatic islet cells, which affects the cells’ overall viability and proliferation.17 This negative feedback loop has also been demonstrated within parathyroid tissues and may contribute to the tumorigenesis of MEN1 syndrome.16 The interactions between miR-24, menin, and TGF-β1 have not been previously described; however, previous studies have reported a link between miR-24 and TGF-β1 signaling. Wang et al. demonstrated that miR-24 downregulates TGF-β1 signaling and mitigates cardiac fibrosis after myocardial infarction.37 Furthermore, miR-24 has been shown to decrease TGF-β1 signaling that results from mechanical stress.38
Currently, there is limited research on the expression profile of miR-24 in liver disease. It has been shown that miR-24 is downregulated in cholangiocytes isolated from a rat model of polycystic liver disease.39 One study has also shown that miR-24 is downregulated in a model of acute cholestatic hepatitis produced by alpha-naphthylisothiocyanate (ANIT) administration.40 Our study suggests that using miR-24 to target the menin and TGF-β1 pathways may also have a therapeutic affect within the liver; however, additional studies are needed to support this hypothesis. With more research, miR-24 may be added to the growing list of miRNAs (miR-29, miR-21, and miR-122) that provide a therapeutic benefit by mitigating the progression of liver fibrosis.41
The role of miR-24 and menin in the progression of liver disease that we present in this study is a novel concept with therapeutic potential, but we must consider the limitations of the study. The amount of human data in this study is limited to one human sample because of the low availability of human tissues. The Mdr2−/− mouse is a valid model of cholestatic liver disease, but additional studies with human tissues are necessary to explore expression patterns and signaling pathways of miR-24 and menin throughout the progression of cholangiopathies, such as PSC. Our data show that MEN1 expression changes with disease progression in the Mdr2−/− mice. We hypothesize that decreased MEN1 expression at 12 wk may promote cellular proliferation in the early stages of disease and becomes dysfunctional as the hepatic injury progresses. Increased MEN1 expression at 66 wk may represent decreased cellular proliferation and a blunted response to cholestatic liver injury. Furthermore, we have shown that inhibition of miR-24 increases menin expression and drives hepatic fibrosis. The therapeutic potential of this pathway may involve upregulating miR-24 expression or inhibiting menin function. This could involve the delivery of miR-24 to liver tissues or the systemic administration of a menin inhibitor.42
We acknowledge that hepatic fibrosis is a complex process with numerous cell types involved, including cholangiocytes, hepatocytes, hepatic stellate cells, and immune cells. The goal of this article was to broadly investigate the miR-24/menin regulatory axis and its contribution to liver fibrosis; however, more work is needed to explore these pathways and the involved cell types. In addition, we need to consider other models of liver disease, such as alcohol-induced cirrhosis, nonalcoholic steatohepatitis, and viral-induced hepatitis to determine whether or not other types of liver disease may benefit from targeting this pathway.
Conclusions
In conclusion, the miR-24/menin regulatory axis regulates TGF-β1 and the progression of hepatic fibrosis in Mdr2−/− mice. This pathway has not been previously described within Hepatology literature and has therapeutic potential for the management of cholestatic liver diseases, such as PSC.
Acknowledgments
This work was supported by the Department of Surgery and the Dr Nicholas C. Hightower Centennial Chair of Gastroenterology from Baylor Scott & White Health, a VA Research Career Scientist Award, a VA Merit Award to G.A. (5I01BX000574), a VA Merit Award (5I01BX002192) to S.G., a VA Merit Award (1I01BX001724) to F.M., and the NIH grants DK58411, DK07698, and DK062975 to G.A., F.M., and S.G. This material is the result of work supported by resources at the Central Texas Veterans Health Care System. This material is the result of work supported by resources at the Central Texas Veterans Health Care System. The content is the responsibility of the author(s) alone and does not necessarily reflect the views or policies of the Department of Veterans Affairs or the United States Government.
Authors’ contributions: C.H. contributed to study design, data collection and analysis, and preparation of the article. L.E. contributed to study design, data collection, and article preparation. F.M. contributed to data analysis and article preparation. P.I. and F.B. contributed to data analysis and article preparation. T.C.L., G.A., and S.G. contributed to study design, data analysis, and article preparation.
Footnotes
Disclosure
The authors of this article do not have any conflicts of interest to disclose.
References
- 1.Kanno N, LeSage G, Glaser S, Alvaro D, Alpini G. Functional heterogeneity of the intrahepatic biliary epithelium. Hepatology. 2000;31:555–561. doi: 10.1002/hep.510310302. [DOI] [PubMed] [Google Scholar]
- 2.Lazaridis KN, LaRusso NF. The cholangiopathies. Mayo Clin Proc. 2015;90:791–800. doi: 10.1016/j.mayocp.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roskams T, Desmet V. Ductular reaction and its diagnostic significance. Semin Diagn Pathol. 1998;15:259–269. [PubMed] [Google Scholar]
- 4.LaRusso NF, Shneider BL, Black D, et al. Primary sclerosing cholangitis: summary of a workshop. Hepatology. 2006;44:746–764. doi: 10.1002/hep.21337. [DOI] [PubMed] [Google Scholar]
- 5.Singh S, Talwalkar JA. Primary sclerosing cholangitis: diagnosis, prognosis, and management. Clin Gastroenterol Hepatol. 2013;11:898–907. doi: 10.1016/j.cgh.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saadi M, Yu C, Othman MO. A review of the challenges associated with the diagnosis and therapy of primary sclerosing cholangitis. J Clin Transl Hepatol. 2014;2:45–52. doi: 10.14218/JCTH.2013.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dreijerink KM, Hoppener JW, Timmers HM, Lips CJ. Mechanisms of disease: multiple endocrine neoplasia type 1-relation to chromatin modifications and transcription regulation. Nat Clin Pract Endocrinol Metab. 2006;2:562–570. doi: 10.1038/ncpendmet0292. [DOI] [PubMed] [Google Scholar]
- 8.Chandrasekharappa SC, Guru SC, Manickam P, et al. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science. 1997;276:404–407. doi: 10.1126/science.276.5311.404. [DOI] [PubMed] [Google Scholar]
- 9.Huang J, Gurung B, Wan B, et al. The same pocket in menin binds both MLL and JUND but has opposite effects on transcription. Nature. 2012;482:542–546. doi: 10.1038/nature10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agarwal SK, Jothi R. Genome-wide characterization of menin-dependent H3K4me3 reveals a specific role for menin in the regulation of genes implicated in MEN1-like tumors. PLoS One. 2012;7:e37952. doi: 10.1371/journal.pone.0037952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim H, Lee JE, Cho EJ, Liu JO, Youn HD. Menin, a tumor suppressor, represses JunD-mediated transcriptional activity by association with an mSin3A-histone deacetylase complex. Cancer Res. 2003;63:6135–6139. [PubMed] [Google Scholar]
- 12.Jin S, Mao H, Schnepp RW, et al. Menin associates with FANCD2, a protein involved in repair of DNA damage. Cancer Res. 2003;63:4204–4210. [PubMed] [Google Scholar]
- 13.Gallo A, Agnese S, Esposito I, Galgani M, Avvedimento VE. Menin stimulates homology-directed DNA repair. FEBS Lett. 2010;584:4531–4536. doi: 10.1016/j.febslet.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 14.Hall C, Sato K, Wu N, et al. Regulators of cholangiocyte proliferation. Gene Expr. 2017;17:155–171. doi: 10.3727/105221616X692568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvaro D, Mancino MG, Glaser S, et al. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology. 2007;132:415–431. doi: 10.1053/j.gastro.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 16.Luzi E, Marini F, Giusti F, Galli G, Cavalli L, Brandi ML. The negative feedback-loop between the oncomir Mir-24-1 and menin modulates the Men1 tumorigenesis by mimicking the “Knudson’s second hit”. PLoS One. 2012;7:e39767. doi: 10.1371/journal.pone.0039767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vijayaraghavan J, Maggi EC, Crabtree JS. miR-24 regulates menin in the endocrine pancreas. Am J Physiol Endocrinol Metab. 2014;307:E84–E92. doi: 10.1152/ajpendo.00542.2013. [DOI] [PubMed] [Google Scholar]
- 18.Kaji H, Canaff L, Lebrun JJ, Goltzman D, Hendy GN. Inactivation of menin, a Smad3-interacting protein, blocks transforming growth factor type beta signaling. Proc Natl Acad Sci U S A. 2001;98:3837–3842. doi: 10.1073/pnas.061358098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu N, Meng F, Invernizzi P, et al. The secretin/secretin receptor axis modulates liver fibrosis through changes in TGF-beta1 biliary secretion. Hepatology. 2016;64:865–879. doi: 10.1002/hep.28622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fickert P, Wagner M, Marschall HU, et al. 24-norUrsodeoxycholic acid is superior to ursodeoxycholic acid in the treatment of sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2006;130:465–481. doi: 10.1053/j.gastro.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 21.Popov Y, Patsenker E, Fickert P, Trauner M, Schuppan D. Mdr2 (Abcb4)−/− mice spontaneously develop severe biliary fibrosis via massive dysregulation of pro- and antifibrogenic genes. J Hepatol. 2005;43:1045–1054. doi: 10.1016/j.jhep.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 22.Fickert P, Fuchsbichler A, Wagner M, et al. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2004;127:261–274. doi: 10.1053/j.gastro.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Katzenellenbogen M, Pappo O, Barash H, et al. Multiple adaptive mechanisms to chronic liver disease revealed at early stages of liver carcinogenesis in the Mdr2-knockout mice. Cancer Res. 2006;66:4001–4010. doi: 10.1158/0008-5472.CAN-05-2937. [DOI] [PubMed] [Google Scholar]
- 24.Katzenellenbogen M, Mizrahi L, Pappo O, et al. Molecular mechanisms of liver carcinogenesis in the mdr2-knockout mice. Mol Cancer Res. 2007;5:1159–1170. doi: 10.1158/1541-7786.MCR-07-0172. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy LL, Meng F, Venter JK, et al. Knockout of microRNA-21 reduces biliary hyperplasia and liver fibrosis in cholestatic bile duct ligated mice. Lab Invest. 2016;96:1256–1267. doi: 10.1038/labinvest.2016.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morcos PA, Li Y, Jiang S. Vivo-Morpholinos: a non-peptide transporter delivers Morpholinos into a wide array of mouse tissues. Biotechniques. 2008;45:613–614. doi: 10.2144/000113005. 6, 8 passim. [DOI] [PubMed] [Google Scholar]
- 27.Hussein N, Casse H, Fontaniere S, et al. Reconstituted expression of menin in Men1-deficient mouse Leydig tumour cells induces cell cycle arrest and apoptosis. Eur J Cancer. 2007;43:402–414. doi: 10.1016/j.ejca.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 28.Masuoka HC, Vuppalanchi R, Deppe R, et al. Individuals with primary sclerosing cholangitis have elevated levels of biomarkers for apoptosis but not necrosis. Dig Dis Sci. 2015;60:3642–3646. doi: 10.1007/s10620-015-3805-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hendy GN, Kaji H, Sowa H, Lebrun JJ, Canaff L. Menin and TGF-beta superfamily member signaling via the Smad pathway in pituitary, parathyroid and osteoblast. Horm Metab Res. 2005;37:375–379. doi: 10.1055/s-2005-870152. [DOI] [PubMed] [Google Scholar]
- 30.Sowa H, Kaji H, Kitazawa R, et al. Menin inactivation leads to loss of transforming growth factor beta inhibition of parathyroid cell proliferation and parathyroid hormone secretion. Cancer Res. 2004;64:2222–2228. doi: 10.1158/0008-5472.can-03-3334. [DOI] [PubMed] [Google Scholar]
- 31.Shattuck TM, Costa J, Bernstein M, Jensen RT, Chung DC, Arnold A. Mutational analysis of Smad3, a candidate tumor suppressor implicated in TGF-beta and menin pathways, in parathyroid adenomas and enteropancreatic endocrine tumors. J Clin Endocrinol Metab. 2002;87:3911–3914. doi: 10.1210/jcem.87.8.8707. [DOI] [PubMed] [Google Scholar]
- 32.Yang JH, Kim SC, Kim KM, et al. Isorhamnetin attenuates liver fibrosis by inhibiting TGF-beta/Smad signaling and relieving oxidative stress. Eur J Pharmacol. 2016;783:92–102. doi: 10.1016/j.ejphar.2016.04.042. [DOI] [PubMed] [Google Scholar]
- 33.Ehrlich L, Hall C, Venter J, et al. miR-24 inhibition increases menin expression and decreases cholangiocarcinoma proliferation. Am J Pathol. 2017;187:570–580. doi: 10.1016/j.ajpath.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zindy PJ, L’Helgoualc’h A, Bonnier D, et al. Upregulation of the tumor suppressor gene menin in hepatocellular carcinomas and its significance in fibrogenesis. Hepatology. 2006;44:1296–1307. doi: 10.1002/hep.21367. [DOI] [PubMed] [Google Scholar]
- 35.Xu B, Li SH, Zheng R, et al. Menin promotes hepatocellular carcinogenesis and epigenetically up-regulates Yap1 transcription. Proc Natl Acad Sci U S A. 2013;110:17480–17485. doi: 10.1073/pnas.1312022110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao Y, Xue Y, Xue L, et al. Hepatic menin recruits SIRT1 to control liver steatosis through histone deacetylation. J Hepatol. 2013;59:1299–1306. doi: 10.1016/j.jhep.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Huang W, Xu R, et al. MicroRNA-24 regulates cardiac fibrosis after myocardial infarction. J Cell Mol Med. 2012;16:2150–2160. doi: 10.1111/j.1582-4934.2012.01523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luna C, Li G, Qiu J, Epstein DL, Gonzalez P. MicroRNA-24 regulates the processing of latent TGFbeta1 during cyclic mechanical stress in human trabecular meshwork cells through direct targeting of FURIN. J Cell Physiol. 2011;226:1407–1414. doi: 10.1002/jcp.22476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masyuk T, Masyuk A, LaRusso N. MicroRNAs in cholangiociliopathies. Cell Cycle. 2009;8:1324–1328. doi: 10.4161/cc.8.9.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamaura Y, Nakajima M, Takagi S, Fukami T, Tsuneyama K, Yokoi T. Plasma microRNA profiles in rat models of hepatocellular injury, cholestasis, and steatosis. PLoS One. 2012;7:e30250. doi: 10.1371/journal.pone.0030250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kitano M, Bloomston PM. Hepatic stellate cells and microRNAs in pathogenesis of liver fibrosis. J Clin Med. 2016;5:38. doi: 10.3390/jcm5030038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He S, Malik B, Borkin D, et al. Menin-MLL inhibitors block oncogenic transformation by MLL-fusion proteins in a fusion partner-independent manner. Leukemia. 2016;30:508–513. doi: 10.1038/leu.2015.144. [DOI] [PMC free article] [PubMed] [Google Scholar]