TO THE EDITOR
The extent to which Allergy and Immunology (A/I) fellowship programs in the United States (US) provide sufficient clinical training and research exposure in drug allergy to fellows-in-training is currently unknown. Antibiotic allergy has specifically been highlighted as an area of unmet need where increased resource allocation could contribute to improved individual and public health. Appropriate management of penicillin and other antibiotic allergies by Allergists and Immunologists, in conjunction with Infectious Diseases specialists and primary care providers, has been identified as an important quality intervention for antimicrobial stewardship1–3. These concerns are not isolated to the US. An Australian national survey reported approximately 18% of inpatients carried an antimicrobial allergy label which was associated with inappropriate prescriptions and an excess of prescribed antimicrobials4. However, these initiatives are complicated by a previously identified lack of consensus on beta-lactam allergy testing and management within the US5,6.
A survey conducted through the AAAAI evaluating the use of aspirin desensitization by practicing allergists and A/I trainees for aspirin-exacerbated respiratory disease (AERD) identified a lack of training during fellowship as a primary reason for not incorporating this procedure into subsequent clinical practice. The majority of responding physicians reported conducting five or less aspirin desensitizations during training while more than 20% of fellows-in-training had not performed this procedure and 36% of respondents learned the process through literature review7. Additionally, a survey of The Infectious Diseases Society of America (IDSA) Emerging Infections Network (EIN) members reported less than half of Infectious Diseases providers surveyed were aware of, or had access to, antibiotic allergy testing services—emphasizing a lack of access to these specialty services as well6.
Our survey was conducted to evaluate existing US drug allergy training opportunities for A/I fellows. A 15-question survey was distributed by the AAAAI, with local institutional review board approval, to the 78 current program directors of the US A/I fellowship training programs accredited by the Accreditation Council for Graduate Medical Education (ACGME). The online survey was distributed via email with two additional reminders in October 2016. Participation was voluntary and anonymous.
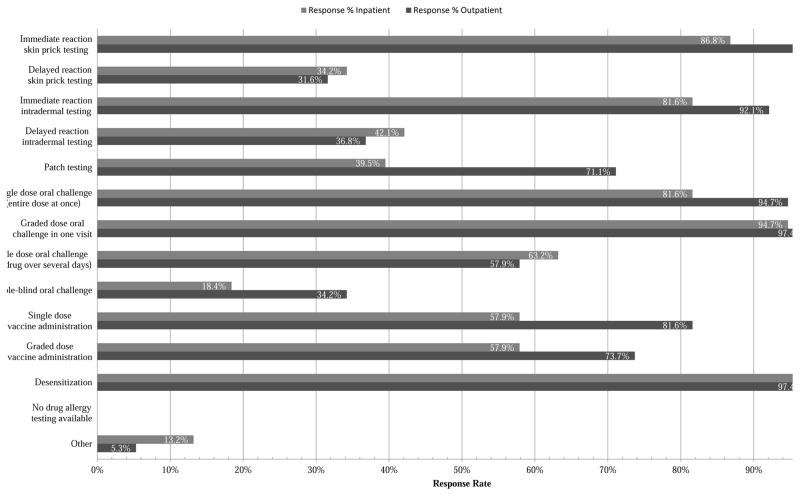
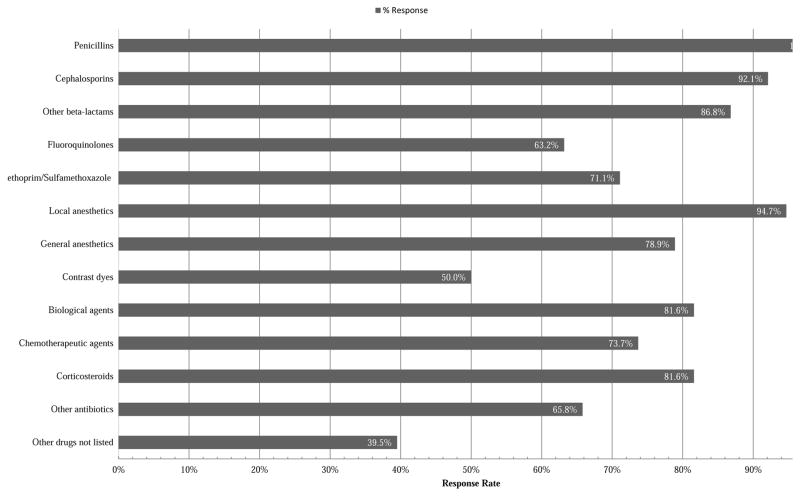
In total, 38 of 78 (49%) program directors responded, and 95% identified drug allergy training as available to fellows in their programs. Specific strategies for outpatient management of immediate drug hypersensitivity such as skin testing, oral challenge, and desensitization were each broadly available to more than 90% of programs. A comparison of available outpatient and inpatient evaluation and management methods is shown in Figure 1. In addition to penicillins (100%) and cephalosporins (92.1%), the drug classes available for hypersensitivity testing by fellows are detailed in Figure 2. Notably less exposure was available for delayed reaction testing procedures including delayed reaction intradermal testing (42%) and multiple dose oral challenge (63%). Interdisciplinary opportunities for A/I fellows to learn about drug allergy diagnosis and testing from outside services (e.g. Dermatology) were in the minority at 10.5%. Research and publication opportunities for fellows were largely limited to case reports (100%) and retrospective literature reviews (79%) with few programs offering clinical trials (13%), big data/dry laboratory exposure (16%), or basic research/wet laboratory opportunities (8%). The total inpatient and outpatient drug allergy cases involving fellows over the course of their training varied greatly with response percentages of 0% for 10 or fewer cases, 15.8% for 10–25 cases, 34.2% for 26–50 cases, 23.7% for 51–75 cases, 7.9% for 76–100 cases, and 18.4% for over 100 cases. Although more than half (52.6%) of the program directors did not identify barriers to adequate drug allergy training at their respective program, others identified deficiencies in available standardized testing methods (34.2%), insufficient logistical resources such as staff and clinic space (28.9%), a lack of a dedicated drug allergy specialist at their program (18.4%), and a too-great liability risk (2.6%). The program directors were also asked to rate their available drug allergy training on a provided scale as outstanding (42.1%), above average (44.7%), average (10.5%), or below average (2.6%).
Figure 1.
Outpatient and inpatient drug allergy evaluation and management methods available to A/I fellows at programs by % of responders. (N=38 respondents)
Figure 2.
Drug classes available to A/I fellows for testing at programs by % of responders. (N=38 respondents)
The limitations of our survey results include possible bias in the selection of responding program directors and an inability to correlate responses with objective program data due to anonymization. Our survey was solely based on program directors’ assessments, and the extent to which fellows are comfortable applying drug allergy testing in their practice would be of significant interest as a follow-up measure. Overall, results suggest opportunities to create more standardized drug allergy evaluation methods, broaden clinical exposure, and increase high quality research opportunities during A/I fellowship. Penicillin allergy evaluation was universally available while the evaluation of multiple drug classes varied more widely according to program. A lack of standardized testing was identified as a barrier to adequate training. Comparing and contrasting in vitro and in vivo drug hypersensitivity diagnostic methods in Europe and North America highlights the need for additional studies and protocol standardization8. Research and publication experiences were limited with a minority offering basic science laboratory, big data, and clinical trial experiences revealing an opportunity to provides these services within other departments institutionally or interstate. Our survey results indicate US A/I fellows currently receive a high standard of drug allergy training, but continuing efforts are required on a multidisciplinary level to develop and implement strategies for evaluation and management— addressing the growing concerns of patient safety and medical costs.
Clinical Implications.
This survey of Allergy and Immunology Fellowship Program Directors has identified key opportunities to improve drug allergy education during fellowship training through development of standardized approaches to drug allergy, increasing exposure to delayed reaction testing protocols, and expansion of research opportunities.
Acknowledgments
Funding sources: Dr. Elizabeth Jane Phillips is funded by: National Institute of Health:1P50GM115305-01, 1R01AI103348-01, 1P30AI110527-01A1, 5T32AI007474-20, 1 R13AR71267-01; National Health & Medical Research Council of Australia; Australian Centre for HIV and Hepatitis Virology Research
Footnotes
Financial Disclosure:
No funding was required for this project.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2016 May 15;62(10):e51–77. doi: 10.1093/cid/ciw118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Quality Partners Playbook. National Quality Forum. 2016. May, Antibiotic Stewardship in Acute Care. [Google Scholar]
- 3.Lang DM, Castells MC, Khan DA, Macy EM, Murphy AW. Penicillin Allergy Testing Should Be Performed Routinely in Patients with Self-Reported Penicillin Allergy. J Allergy Clin Immunol Pract. 2017 Mar-Apr;5(2):333–4. doi: 10.1016/j.jaip.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Trubiano JA, Chen C, Cheng AC, Grayson ML, Slavin MA, Thursky KA. Antimicrobial allergy ‘labels’ drive inappropriate antimicrobial prescribing: lessons for stewardship. J Antimicrob Chemother. 2016 Jun;71(6):1715–22. doi: 10.1093/jac/dkw008. [DOI] [PubMed] [Google Scholar]
- 5.Gerace KS, Karlin E, McKinnon E, Phillips E. Varying penicillin allergy testing practices in the United States: a time for consensus. J Allergy Clin Immunol Pract. 2015 Sepoct;3(5):791–3. doi: 10.1016/j.jaip.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Trubiano JA, Beekmann SE, Worth LJ, Polgreen PM, Thursky KA, Slavin MA, et al. Improving antimicrobial stewardship by antibiotic allergy delabeling: evaluation of knowledge, attitude, and practices throughout the Emerging Infections Network. Open Forum Infect Dis. 2016 Sep 3;3(3):ofw153. doi: 10.1093/ofid/ofw153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waldram JD, White AA. A survey of aspirin desensitization practices among allergists and fellows in training in the United States. J Allergy Clin Immunol Pract. 2016 Novedec;4(6):1253–5. doi: 10.1016/j.jaip.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 8.Torres MJ, Romano A, Celik G, Demoly P, Khan DA, Macy E, et al. Approach to the diagnosis of drug hypersensitivity reactions: similarities and differences between Europe and North America. Clin Transl Allergy. 2017 Mar 13;7:7. doi: 10.1186/s13601-017-0144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]