Abstract
Purpose
To compare the upgrading rate obtained by re-sampling precise spots of prostate cancer (tracking biopsy) vs conventional systematic re-sampling, during follow-up of men in active surveillance.
Materials and Methods
Subjects were all 352 men, from 2009 to 2017, with Gleason 3+3 (n=268) or Gleason 3+4 (n=84) prostate cancer at initial MRI/ultrasound fusion biopsy and who subsequently had a second fusion biopsy. At first biopsy session, all men underwent 12-core systematic biopsies and, when MRI-visible lesions were present, targeted biopsies. All cancerous sites were recorded electronically. During active surveillance, at a second fusion-biopsy session 6–18 months later, both tracking and systematic non-tracking samples were obtained. Primary outcome measure was an increase in Gleason Score (upgrading) at follow-up sampling, stratified by biopsy method.
Results
Overall, 91 of 352 men (25.9%) experienced upgrading at second biopsy, during an 11-month median interval. Upgrade rates for Gleason 3+3 and Gleason 3+4 groups were 26.9% and 22.6%, respectively. Mean number of cores taken at second biopsy was 12.2 +/−3.3 for those who upgraded and 12.4 +/− 4.1 for those who remained stable (p= NS). Men with MRI targets of grade 0–4 all upgraded at approximately the same rate (20–30%) (p=NS); but, 58.8% of men with grade 5 MRI targets upgraded. 48 of 91 upgrades (53%) were detected only by tracking.
Conclusions
The tracking function of MRI/US fusion biopsy warrants further study, since re- sampling specific sites, when used in men undergoing active surveillance of prostate cancer, leads to detection of upgrading more often than non-tracking biopsy.
Keywords: Prostate cancer, MRI, MRI/US Fusion, Prostate Biopsy
INTRODUCTION
Biopsy site tracking, a method to revisit and resample a specific locus of cancer, was approved in 2008 as the initial indication for prostate image-fusion devices (1). However, compared to the lesion-targeting function of such devices, the biopsy-site tracking function has been studied but little. Early-on, an operator using one such device demonstrated, under ideal circumstances, ability to return to the site of a prior prostate biopsy within a few mm (2). Using ERG expression as a marker, Palapattu and colleagues confirmed recently that precise resampling of a prostate cancer (CaP) site one year after initial sampling is possible using an image-fusion device (3).
A potential value of biopsy site tracking for men in Active Surveillance (AS) programs was suggested in preliminary studies from our institution and others (4–10). Sonn et al. reported that when initial cancer core length was ≥4 mm within a multi-parametric magnetic resonance imaging (mpMRI) target, more than 80% of follow-up tracking biopsies were also positive (4). Felker et al. reported an incremental value of serial MRI in predicting results of follow-up biopsy, which included tracking (11). Frye et al showed that MRI progression predicted Gleason Score (GS) upgrading in men undergoing repeat fusion biopsy, though ‘tracking’ was not described (5). In addition, GS upgrading has been detected outside of MRI-visible lesions (7). The above notwithstanding, a systematic evaluation of biopsy site tracking, including re-sampling of tumorous sites both within and apart from MRI-visible targets, is currently lacking.
Herein we evaluate biopsy-site tracking in a large group of men who were eligible for AS after undergoing MRI-ultrasound fusion biopsy. All men had a baseline 12-core systematic biopsy and when MRI-visible lesions were present, targeted biopsy. Tumors were found both within and apart from MRI-visible lesions; then all tumors were specifically re-sampled by tracking biopsy during follow-up. The findings appear to confirm the preliminary studies: tracking biopsy provides increased detection of clinically-significant prostate cancer (csCaP) in men undergoing AS.
METHODS
Study Design
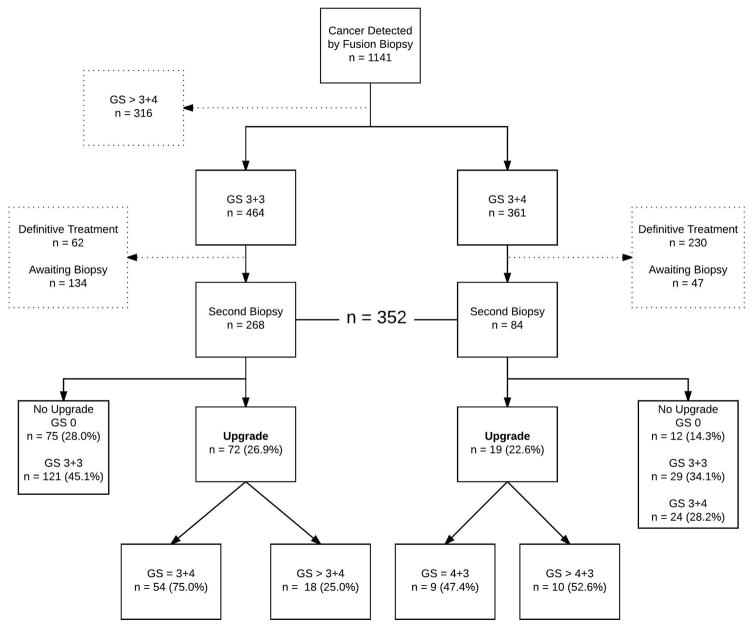
Figure 1 illustrates the patient selection process. From the larger group of patients who had cancer detected on fusion biopsy during the study period of 2009 to 2017 (n=1141), those inappropriate for AS were eliminated (GS>3+4, n=316). Others were eliminated if they received treatment (n=292) or have not yet had a second biopsy (n=181). The remaining 352 men, all candidates for AS, represent the study sample. All 352 men had either GS 3+3 (n=268) or GS 3+4 (n=84) detected by fusion biopsy; and all underwent follow-up fusion biopsy within 6 to 18 months. Long-term follow-up of a portion of these patients was reported previously (8,11).
Figure 1.
Flow diagram showing how patients were selected for study. Note that 28% of men with Gleason 6 and 14% of men with Gleason 7 were found at second biopsy to contain no cancer (GS 0), even with targeting; these men may represent an especially low-risk group (17).
The primary outcome was a Gleason Score at second biopsy higher than at first biopsy, i.e., upgrading. If the initial positive fusion biopsy showed a maximum GS 3+3, then GS ≥ 3+4 on subsequent fusion biopsy was considered an upgrade. If the initial maximum GS was 3+4, then GS ≥ 4+3 was considered an upgrade. All data collection was performed in a UCLA registry approved by the institutional review board.
Fusion Biopsy and Tracking (Figure 2)
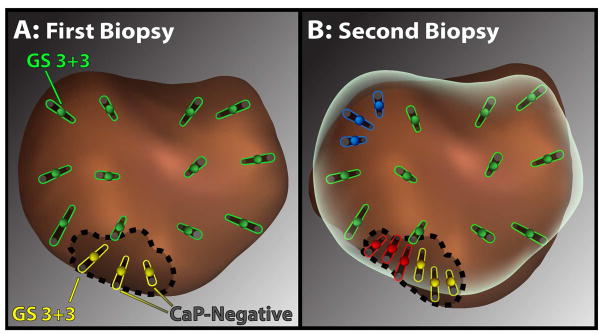
Figure 2.
Diagram showing an example of the biopsy methods used. First biopsy (A) consisted of 12 systematic cores (green), following the built-in template, and targeted cores (yellow) taken from the MRI visible lesion (dotted line). Second biopsy (B), which shows preliminary fusion of the first and second prostate models, consisted of systematic cores (green), MRI-targeted cores (yellow), and tracking cores taken from systematic cancer sites (blue) or cancerous sites within MRI-lesions (red). Using this scheme, resampling of all initial cancerous sites was achieved, both within and apart from MRI-visible lesions.
All biopsies were performed by a single urologist (LSM) at UCLA Clark Urology Center using the Artemis™ platform and local anesthesia. The mpMRI and initial fusion biopsy were done as previously described, yielding cores from both the systematic (template) sites and from MRI-visible lesions (Figure 2A) (2,9,12). We defined an MRI-visible lesion as one classified as Grade 3–5, initially using a scoring system that predates and closely approximates PI-RADS™ and later on, the actual PI-RADS™ (2,13). MRIs were interpreted by two uro-radiologists, each with more than 10 years’ experience reading prostate mpMRI. Imaging was done before the initial fusion biopsy in all 352 men; 32 with a negative MRI initially had repeat MRI at time of second biopsy. In 28 of the 32 cases, a new lesion was seen; it was also sampled, contributing five cases of upgrading, but sampling of new lesions did not influence the tracking analysis. At the first biopsy session, each core was labeled with a specific identifier, allowing tracking of any cancerous biopsy site that might be found.
At follow-up biopsy (Figure 2B), the initial prostate model, which had been saved within the Artemis™ device, was displayed on the work screen showing any MRI region of interest (ROI) and all previous biopsy sites. The prostate was re-scanned and fusion of the initial model with the second model was performed, the overlay showing the ROI and all previous biopsy sites. Cores were then obtained from systematic sites (as initially), from any MRI-visible lesions (as initially), and by resampling cancerous spots found initially (tracking). A median of 4 (IQR 3–5) tracking cores were taken from each prior area of CaP, aiming at the midpoint of each positive core and at 4-quadrant adjacent areas within 2 to 3 mm of center point. Re-sampling of cancerous sites was performed, including sites within and apart from MRI-visible ROIs (Figure 2). A dedicated uro-pathologist interpreted all biopsy cores (JH). Overall upgrade rate and upgrade rate by each biopsy method were determined.
Statistical Analysis
Logistic regression was used for univariate and multivariate analyses. Log rank tests and chi-square tests were used to calculate p values in Table 1. Exact Binomial tests were used to calculate confidence intervals (CI). Statistical significance was considered at p < 0.05 for all analyses. Statistical analyses were performed by co-author FJD using Stata® (v. 13.1).
Table 1.
Baseline patient characteristics, mean + SD (n = 352)
| Gleason 3+3 | Gleason 3+4 | p | |
|---|---|---|---|
|
|
|||
| No. patients | 268 | 84 | |
| Age, years | 64 (7.4) | 64 (7.1) | - |
| PSA, ng/ml median (IQR) | 4.9 (2.8–6.6) | 6.0 (4.4–8.8) | 0.762 |
| Prostate Volume, cc | 52.0 (24.0) | 60.0 (21.0) | 0.001 |
| PSA Density, ng/ml/cc | 0.108 (0.078) | 0.164 (0.109) | 0.009 |
| Ethnicity | |||
| Caucasian | 209 (77.6%) | 57 (67.9%) | 0.376 |
| Hispanic | 12 (4.5%) | 7 (8.3%) | |
| Asian American | 11 (4.1%) | 6 (7.1%) | |
| African American | 11 (4.1%) | 4 (4.8%) | |
| Not stated | 26 (9.7%) | 10 (11.9%) | |
| Biopsy Data | |||
| Max cancer core length, mm | 2.5 (2.1) | 3.7 (2.1) | 0.001 |
| No. of cores | 15 (2.6) | 15 (3.2) | 0.977 |
| No. of positive cores | 1.9 (1.4) | 2.4 (1.3) | 0.003 |
RESULTS
In Table 1, baseline characteristics of the study cohort are presented. All men in the study were candidates for AS on the basis of GS. Of the 352 men, initial biopsy revealed 268 (76.1%) to have CaP of GS 3+3 and 84 (23.9%) to have GS 3+4. Men with GS3+4 at baseline had larger prostates, higher PSA density values, and longer cancer core lengths than men with GS 3+3.
In Figure 1, patient selection and overall upgrade rate are shown. Overall, 91 of 352 men (25.9%) experienced upgrading at second biopsy, during the 11-month median interval (IQR 6–12 months). The upgrade rates for the GS 3+3 and GS 3+4 groups were 26.9% (95% CI, 0.21–0.33) and 22.6% (95% CI, 0.14–0.33) respectively. Of the upgrades in the GS 3+3 group, 75.0% (95% CI, 0.63–0.84) upgraded to GS 3+4 while 25.0% (95% CI, 0.15–0.37) upgraded to GS > 3+4. Of the upgrades in the GS 3+4 group, 47.4% (95% CI, 0.24–0.71) upgraded to GS 4+3 and 52.6% (95% CI, 0.29–0.76) upgraded to GS > 4+3.
Effect of baseline mpMRI grade
Upgrade rate per MRI grade is shown in Table 2. 21.0% (95% CI, 0.15–0.29) of men with a maximum grade 3 ROI upgraded while 29.6% (95% CI, 0.20–0.41) of men with grade 4 upgraded. 58.8% of men with a grade 5 upgraded (95% CI, 0.33–0.81)
Table 2.
Upgrade rate stratified by baseline MRI grade (n = 352)
| MRI Grade* | Upgrades | n = 352 | 95% CI |
|---|---|---|---|
| 5 | 10 (58.8%) | 17 | 0.33–0.81 |
| 4 | 24 (29.6%) | 81 | 0.20–0.41 |
| 3 | 29 (21.0%) | 138 | 0.15–0.29 |
| 0–2 | 28 (24.1%) | 116 | 0.17–0.33 |
|
| |||
| Total | 91 | 352 | 0.21–0.31 |
116 of the 352 men (32.7%) initially did not have MRI-visible targets. 32 of the 116 men with initially negative mpMRI had a repeat mpMRI before second biopsy because of increased clinical suspicion. 28 of the 116 (24.1%, 95% CI, 0.17–0.33) men upgraded. 4 of the 28 upgrades (14.2%) in this group were missed by systematic tracking but found by target biopsy of ROI seen on second mpMRI. One of the 28 upgrades (3.6%) was missed by systematic tracking but found by non-tracked systematic sites (not shown in Table 2). Two upgrades were found by multiple methods. The remaining 21 upgrades (75.0%) were found only by systematic tracking biopsy. Stated otherwise, three-quarters of upgrades in men with no MRI-visible lesions were found only by tracking of a cancer focus present on a template site at initial sampling.
Effect of sampling method
Table 3 shows the rates of cancer detection based on sampling method at the first (left panels) and second biopsy sessions (right panels). In the first session, 268 men were diagnosed with GS 3+3: 175 by systematic biopsy only; 41 by target only; and 52 by both. Upgrade rates from these groupings were 24.6%, 31.7%, and 30.8% respectively. 84 men were diagnosed with GS 3+4: 53 by systematic biopsy only, 28 by target biopsy only, and 3 by both. Upgrade rates based on these groupings were 18.9%, 25.0% and 66.7% respectively. In both groups, MRI-visible lesions were more likely to contain tumors than other areas of the prostate.
Table 3.
Effect of sampling method on detection of cancer
| First Biopsy Gleason Score1: | Second Biopsy: Upgrades/Biopsy Method2 | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| 3+3 (n=268) | Total | Upgrades | Systematic only | MRI Target only | Systematic Tracking only | MRI Target Tracking only |
| Systematic Only | 175 | 43 (24.6%) | 3 | 9 | 22 | 0 |
| Target Only | 41 | 13 (31.7%) | 0 | 4 | 0 | 7 |
| Both | 52 | 16 (30.8%) | 0 | 1 | 4 | 8 |
| 3+4 (n=84) | ||||||
| Systematic Only | 53 | 10 (18.9%) | 1 | 3 | 4 | 0 |
| Target Only | 28 | 7 (25.0%) | 1 | 2 | 0 | 3 |
| Both | 3 | 2 (66.7%) | 0 | 2 | 0 | 0 |
Systematic only: CaP only found on systematic cores. Target only: CaP only found in MRI-visible lesion. Both: CaP found on both systematic and MR-visible lesions.
Upgrades/biopsy method is mutually exclusive. Example: 22 upgrades were found in the Gleason score 3+3 group by systematic tracking only and by no other biopsy method.
In the right side of Table 3, upgrades based on method of the second biopsy are shown. The four methods used in the second biopsy were systematic, MRI targeted, systematic tracking, and MRI tracking. The four methods are shown and explained in Figure 2. In GS 3+3 patients, 41 of 72 upgrades (56.9%, 95% CI, 0.45–0.69) were found on tracking (systematic tracking and/or MRI target tracking) but missed by non-tracking (systematic and/or MRI target). 17 upgrades (23.6%, 95% CI, 0.14–0.35) found by non-tracking were missed by tracking. Differences were less pronounced in the GS3+4 groups, where numbers were small. Overall, when combining both GS groups, 48 of 91 upgrades (52.7%, 95% CI, 0.42–0.63) were found by tracking only. Regarding total number of cores obtained at second biopsy, no significant difference was found between men found to have upgraded (12.2 ± 3.3) vs men with a stable Gleason score (12.4 ± 4.1).
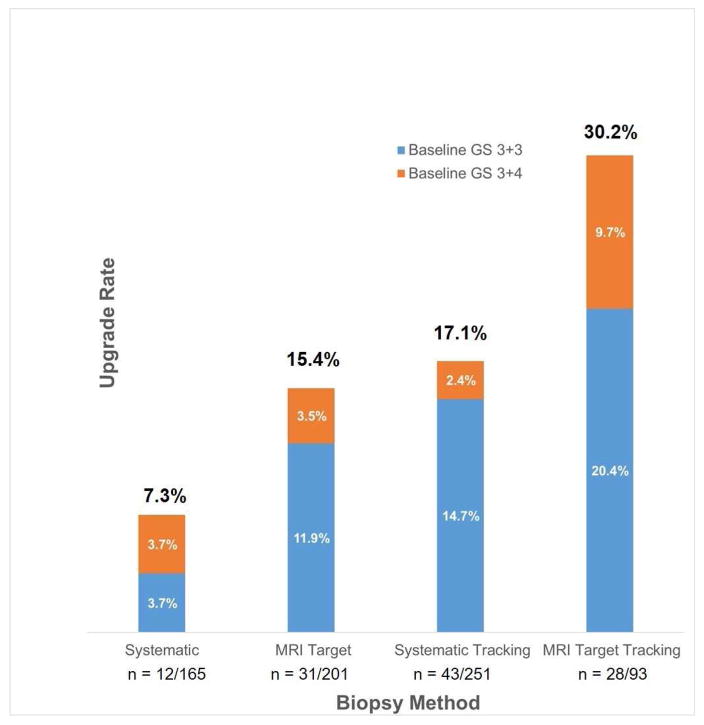
Figure 3 combines the GS 3+3 and GS 3+4 groups and shows the number of upgrades based on the four different repeat biopsy methods. Upgrade rates were 7.3% (95% CI, 0.04–0.14), 15.4% (95% CI, 0.11–0.21), 17.1% (95% CI, 0.13–0.22), and 30.2% (95% CI, 0.21–0.40) based on systematic, MRI target, systematic tracking and MRI tracking biopsy respectively.
Figure 3.
Upgrade rate to Gleason Score (GS) ≥ 3+4 on repeat biopsy, stratified by biopsy method (systematic, MRI target, systematic tracking, and MRI target tracking). Percent on top of each bar represents total upgrade rate when patients with baseline GS 3+3 and GS 3+4 are combined.
Univariate and Multivariate Analysis
Table 4 shows the univariate analysis. For initial GS 3+3 patients, PSA density (p=0.001) and greater number of positive cores on first biopsy (p=0.001) were associated with upgrade. Prostate volume remained unchanged (p=NS) and was not predictive of upgrading. For initial GS 3+4 patients, none of the variables were predictive of an upgrade.
Table 4.
Univariate analysis for baseline predictors of upgrading. *
| Gleason 6 (n = 268) | Gleason 7 (n = 84) | |||
|---|---|---|---|---|
|
| ||||
| Odds Ratio | p | Odds Ratio | p | |
| Age, years | 1.01 | 0.440 | 1.07 | 0.080 |
| PSA, ng/ml | 1.08 | 0.079 | 1.1 | 0.130 |
| Prostate Volume, cc | 0.99 | 0.037 | 0.96 | 0.320 |
| PSA Density, ng/ml/cc | 1.34 | 0.001 | 1.24 | 0.060 |
| Max cancer core length, mm | 1.08 | 0.116 | 0.89 | 0.400 |
| No. of cores | 0.97 | 0.570 | 0.94 | 0.420 |
| No. of positive cores | 1.34 | 0.001 | 0.97 | 0.860 |
All biopsy methods combined
On multivariate analysis of the GS 3+3 patients, upgrading was associated with PSA density (p=0.002) and number of positive cores on initial biopsy (p=0.099). For the initial GS 3+4 patients, only PSA density was associated with upgrading (p=0.005).
DISCUSSION
Before image-fusion devices were available, tracking of prostate cancer foci was inexact because a practical mechanism to record biopsy sites did not exist. Han et al. showed that using ultrasound alone, even skilled and experienced urologists are often unable to match their planned core location to the actual site to be sampled within the prostate (14). With the advent of image-fusion devices, recording and later resampling of specific sites containing tumor foci became possible (5,15,16). Some tumors are found outside of MRI-visible lesions or ROIs (12); they can still be tracked. Thus, tracking may allow re-sampling of cancerous areas within the prostate, irrespective of location within or apart from a ROI (2,4). For men undergoing AS of CaP, the benefits of tracking biopsy could be considerable.
In the present study, tracking technology within the Artemis device was employed to revisit and resample all tumor foci. Of the 91 men who showed upgrading by the various biopsy methods, 48 (53%) were detected only by tracking biopsy (Table 3). Tracking delivered a substantially higher yield than conventional systematic sampling. Of special note are the other 85 men who, despite tracking biopsy, were found to have no tumor on confirmatory biopsy (Figure 1). As suggested by Ganesan et al., these men may require less vigilance than men with demonstrable tumor at follow-up (17).
Evidence that tracking, as described herein, is highly accurate was recently established using sophisticated molecular biology techniques (3). In that study, which involved the Artemis device as in the present report, accuracy for repeat sampling of a specific CaP clonal site over a 1-year interval was 96%. Using a different image-fusion device and a panel of genes to determine re-sampling accuracy, others have reported similar findings (18). Whether the upgrades reported here represent true progression or initial under-detection cannot be discerned from the present study. However, detection of the upgrades was, to a large extent, dependent on tracking biopsy.
In one large AS cohort using mostly ultrasound-guided biopsy, the upgrade rate from GS6 to GS≥7 on surveillance biopsy averaged 18.5% (19), substantially lower than that reported here. However, any comparison is complicated because in the present study (1.) MRI targeting was used initially to improve screening of men not suitable for AS, and (2.) targeting was combined with tracking biopsy at the second session. Use of image-fusion devices to reduce sampling error via targeting and tracking, thus increasing detection of clinically significant cancers, appears to be a noteworthy advance.
In early studies of AS, all biopsies–diagnostic, confirmatory, and follow-up–were US-guided systematic sampling of the organ (20,21). The present data indicate that if the initial diagnostic biopsy is performed with an image-fusion device and positive sites tracked, resampling the previously negative systematic sites has a low yield. In the present work, when tumor was found only on a systematic site (n=228), a subsequent upgrade was found in a non-tracked systematic site but four times (Table 3). Thus, re-sampling non-tracked systematic sites (outside of MRI-visible lesions) may not be necessary in AS programs, allowing reduction in number of cores needed at follow-up.
In the present work, biopsy tracking within MRI targets detected 30.2% of all upgrades (Figure 2). The importance of paying special attention to MRI-visible lesions is again confirmed. However, 23 of 93 men (24.7%) with a tumor only in a systematic site--- who had no MRI-visible lesions on initial biopsy---were found to have upgraded by tracking biopsy alone. In such instances, re-sampling of systematic biopsy sites, which were initially negative, rarely led to detection of upgrading (4/228, see above). Thus, tracking of tumorous biopsy sites provides detection of upgrading, even in men with no MRI-visible lesions, and rarely misses upgrades found in those men by repeat systematic (non-tracking) biopsy.
Limitations of the study, which may affect generalizability, include the following. The design was retrospective, though all data were collected in an IRB-approved registry following a set of protocols. MRI interpretation was by expert readers using state-of-the art equipment, which may not be universally available. Cognitive tracking was not tested and might be of benefit, especially in small prostates. MRI/US fusion was performed by one urologist who has had extensive experience with the procedure. And biopsy upgrading, per se, while serving to heighten awareness, may not always correlate with disease severity. Despite these limitations, the sample size was relatively large, uniform methods were used throughout the years of the study, and the results achieved statistical significance. Tracking biopsy appears to result in increased detection of csCaP with a sensitivity beyond that of conventional systematic biopsy and/or target-biopsy of MRI-visible lesions. Confirmation of this finding awaits a prospective appropriately-powered trial.
CONCLUSION
One-quarter of men, who met consensus-criteria for AS eligibility, experienced pathological upgrading on repeat fusion biopsy. Approximately half the upgrades were detected only by tracking biopsy. Tracking biopsy appears to add value to conventional follow-up biopsy in men undergoing AS for prostate cancer and deserves further study.
List of Abbreviations
- AS
active surveillance
- CaP
cancer of prostate
- csCaP
clinically-significant CaP
- CI
confidence interval
- mpMRI
multi-parametric magnetic resonance imaging
- GS
Gleason score
- PI-RADS
prostate Imaging – Reporting and Data System
- PSA
prostate specific antigen
- ROI
region of Interest
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Edward Chang, Department of Urology, David Geffen School of Medicine, University of California at Los Angeles.
Tonye A. Jones, Department of Urology, David Geffen School of Medicine, University of California at Los Angeles
Shyam Natarajan, Department of Urology, David Geffen School of Medicine, University of California at Los Angeles.
Devi Sharma, Department of Urology, David Geffen School of Medicine, University of California at Los Angeles.
Demetrios Simopoulos, Department of Urology, David Geffen School of Medicine, University of California at Los Angeles.
Daniel J. Margolis, Department of Radiology, Weill-Cornell School of Medicine, New York, N.Y
Jiaoti Huang, Department of Pathology, Duke University School of Medicine, Durham, N.C.
Frederick J. Dorey, Department of Urology, David Geffen School of Medicine, University of California at Los Angeles
Leonard S. Marks, Department of Urology, David Geffen School of Medicine, University of California at Los Angeles
References
- 1.U.S. Department of Health and Human Services. Food and Drugs. U.S. Food and Drug Administration; [online]. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K081093. [Google Scholar]
- 2.Natarajan S, Marks LS, Margolis DJA, et al. Clinical application of a 3D ultrasound-guided prostate biopsy system. Urol Oncol Semin Orig Investig. 2011 May;29(3):334–42. doi: 10.1016/j.urolonc.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palapattu G, Bratley JV, Liu C, et al. Molecular Profiling to Determine Clonality of Serial Magnetic Resonance Imaging/Ultrasound Fusion Biopsies from Men on Active Surveillance for Low Risk Prostate Cancer. Clin Cancer Res. 2017;130652:985–92. doi: 10.1158/1078-0432.CCR-16-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sonn GA, Filson CP, Chang E, et al. Initial experience with electronic tracking of specific tumor sites in men undergoing active surveillance of prostate cancer. Urol Oncol Semin Orig Investig. 2014;32(7):952–7. doi: 10.1016/j.urolonc.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frye TP, George AK, Kilchevsky A, et al. J Urol. November. Elsevier Ltd; 2016. MRI-TRUS Guided Fusion Biopsy to Detect Progression in Patients with Existing Lesions on Active Surveillance for Low and Intermediate Risk Prostate Cancer; pp. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chelluri R, Kilchevsky A, George AK, et al. Prostate Cancer Diagnosis on Repeat Magnetic Resonance Imaging-Transrectal Ultrasound Fusion Biopsy of Benign Lesions: Recommendations for Repeat Sampling. J Urol Elsevier Ltd. 2016;196(1):62–7. doi: 10.1016/j.juro.2016.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma TM, Tosoian JJ, Schaeffer EM, et al. The Role of Multiparametric Magnetic Resonance Imaging/Ultrasound Fusion Biopsy in Active Surveillance. Eur Urol European Association of Urology. 2016;71(2):174–80. doi: 10.1016/j.eururo.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Nassiri N, Margolis DJ, Natarajan S, et al. Targeted biopsy to detect Gleason score upgrading during active surveillance for men with low- vs. intermediate-risk prostate cancer. J Urol Elsevier Ltd. 2017;197(March):632–9. doi: 10.1016/j.juro.2016.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonn GA, Chang E, Natarajan S, et al. Value of targeted prostate biopsy using magnetic resonance-ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen. Eur Urol. 2014;65(4):809–15. doi: 10.1016/j.eururo.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sonn GA, Natarajan S, Margolis DJA, et al. Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. J Urol. 2013;189(1):86–91. doi: 10.1016/j.juro.2012.08.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felker ER, Wu J, Natarajan S, et al. Serial MRI in Active Surveillance of Prostate Cancer: Incremental Value. J Urol Elsevier Ltd. 2016;195(5):1421–7. doi: 10.1016/j.juro.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filson CP, Natarajan S, Margolis DJA, et al. Prostate cancer detection with magnetic resonance-ultrasound fusion biopsy: The role of systematic and targeted biopsies. Cancer. 2016:1–9. doi: 10.1002/cncr.29874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol. 2016;69(1):16–40. doi: 10.1016/j.eururo.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han M, Chang D, Kim C, et al. Geometric evaluation of systematic transrectal ultrasound guided prostate biopsy. J Urol Elsevier Inc. 2012;188(6):2404–9. doi: 10.1016/j.juro.2012.07.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scarpato KR, Barocas Da. Urol Oncol Semin Orig Investig. 7. Vol. 34. Elsevier; 2016. Use of mpMRI in active surveillance for localized prostate cancer; pp. 1–6. [DOI] [PubMed] [Google Scholar]
- 16.Schoots IG, Petrides N, Giganti F, et al. Magnetic resonance imaging in active surveillance of prostate cancer: A systematic review. Eur Urol European Association of Urology. 2015;67(4):627–36. doi: 10.1016/j.eururo.2014.10.050. [DOI] [PubMed] [Google Scholar]
- 17.Ganesan V, Dai C, Nyame YA, et al. Prognostic Significance of a Negative Confirmatory Biopsy on Reclassification among Men on Active Surveillance. Urology. 2017 Jun 15; doi: 10.1016/j.urology.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Ukimura O, Gross ME, De Castro Abreu AL, et al. A novel technique using three-dimensionally documented biopsy mapping allows precise re-visiting of prostate cancer foci with serial surveillance of cell cycle progression gene panel. Prostate. 2015;75(8):863–71. doi: 10.1002/pros.22969. [DOI] [PubMed] [Google Scholar]
- 19.Mamawala MM, Rao K, Landis P, et al. Risk prediction tool for grade reclassification in men with favourable-risk prostate cancer on active surveillance. BJU Int. 2017 Jul;120(1):25–31. doi: 10.1111/bju.13608. [DOI] [PubMed] [Google Scholar]
- 20.Klotz L, Zhang L, Lam A, et al. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2009;28(1):126–31. doi: 10.1200/JCO.2009.24.2180. [DOI] [PubMed] [Google Scholar]
- 21.Tosoian JJ, Trock BJ, Landis P, et al. Active surveillance program for prostate cancer: An update of the Johns Hopkins experience. J Clin Oncol. 2011;29(16):2185–90. doi: 10.1200/JCO.2010.32.8112. [DOI] [PubMed] [Google Scholar]