Abstract
Objective
The objective of this brief review is to provide an overview of the expression and function of KV channels in VSMC of resistance arteries and arterioles.
Methods and Results
A review of the literature revealed that arterioles and resistance arteries express a diverse array of KV channels with members of the KV1, KV2 and KV7 families being particularly important. Members of the KV channel family: 1.) are highly expressed in VSMCs; 2.) are active at the resting membrane potential of VSMCs in vivo (-45 to −30 mV); 3.) contribute to the negative feedback regulation of VSMC membrane potential and myogenic tone; 4.) are activated by cAMP-related vasodilators, hydrogen sulfide and hydrogen peroxide; 5.) are inhibited by increases in intracellular Ca2+ and vasoconstrictors that signal through Gq-coupled receptors; 6.) are involved in the proliferative phenotype of VSMC; and 7.) are modulated by diseases such as hypertension, obesity, the metabolic syndrome and diabetes.
Conclusions
KV channels participate in every aspect of the regulation of VSMC function in both health and disease.
Keywords: KV channels, potassium channels, vascular smooth muscle, resistance arteries, arterioles, vasoconstriction, vasodilation, microcirculation, blood flow
Introduction
Arterioles in the microcirculation contribute to vascular resistance and control of blood pressure and blood flow to and within tissues and organs [47]. Vascular smooth muscle cells (VSMCs) are the effectors of changes in vascular resistance; their contraction state, or tone, determines arteriolar internal diameters that strongly impacts the hydraulic resistance offered by these microvessels. Arteriolar tone, in turn, depends on VSMC intracellular Ca2+ concentration ([Ca2+]in) and the Ca2+ sensitivity of the VSMC contractile proteins [108]. The electrical potential across the plasma membrane (membrane potential) of VSMCs importantly impacts arteriolar tone by controlling the activity of voltage-gated Ca2+ channels (VGCC), a major source of activator Ca2+ in these microvessels [146]. Membrane potential also modulates Ca2+ release from internal stores and the Ca2+ sensitivity of the contractile machinery [48,57–59,117,122,132,154,186,191–193]. Thus, understanding the regulation of VSMC membrane potential is central to understanding the regulation of arteriolar tone and hence, blood pressure and blood flow control.
Potassium channels in VSMC plasma membranes play an important role in the control and regulation of VSMC membrane potential and arteriolar tone. They are the dominant plasmalemmal ion conductance in VSMCs, as in all cells, and their activity is modulated by membrane potential, vasodilators and vasoconstrictors such that these ion channels participate in all aspects of the regulation of arteriolar tone and control of microvascular perfusion [184]. At physiological ion concentrations (4–5 mM K+ extracellular, 140 mM K+ intracellular), the driving force (the electrochemical gradient) for diffusion of K+ through K+ channels is outward at physiological membrane potentials (-45 to −30 mV [22,53,112,169,188]). Thus, opening of K+ channels leads to membrane hyperpolarization and vasodilation, whereas closure of K+ channels results in membrane depolarization and vasoconstriction (Figure 1). Furthermore, because the membrane resistance of VSMCs is high (~1–10 GΩ [146]), only a few K+ channels have to open or close to produce significant effects on membrane potential and arteriolar tone. Vascular SMCs express four or more classes of K+ channels [184]. The remainder of this brief review will focus on voltage-gated K+ (KV) channels expressed by VSMCs and their role in the regulation of arteriolar and resistance artery tone and VSMC proliferation.
Figure 1.
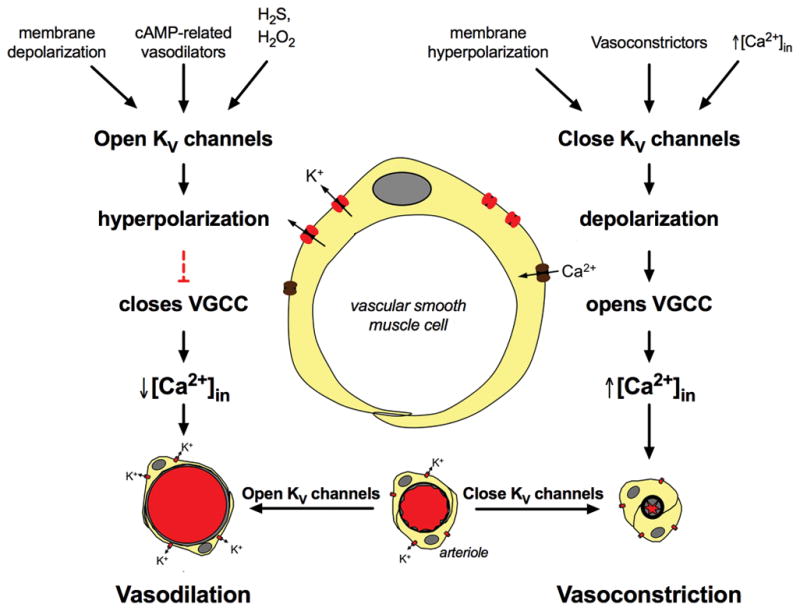
KV channels and the regulation of arteriolar tone. Left side of the diagram shows several factors that activate KV channels in VSMCs (see text for more information and references) leading to K+ -efflux through these channels, membrane hyperpolarization and ultimately, vasodilation. Right side of diagram shows factors that inhibit KV channel activity (see text for details and references), leading to membrane depolarization and vasoconstriction.
Discovery of KV channels
Hodgkin and Huxley were the first to report currents through KV channels using squid giant axons [80,81]. Currents through VSMC KV channels were first reported by Beech and Bolton [13,14] and Okabe et al. [153] and confirmed by numerous investigators in every VSMC studied [39,66,87,89,96,131,147]. Membrane depolarization activates KV channels; maintained depolarization results in a variable degree of channel inactivation [64,93,147]. However, KV channel properties vary within and among tissues, suggesting diversity of expressed channels [64,70,93,147]. This functional diversity reflects molecular diversity of KV channels and associated accessory subunits: there are 40 genes that code for mammalian KV channels. This results in 12 distinct families of KV channels (KV1–12) [64,70]: Members of the KV1–4, 7 and 10–12 form functional channels as homomers, whereas KV5, 6, 8 and 9 must co-assemble with KV2 or 3 subunits to form functional channels [64]. Vascular SMCs have been reported to express members of the KV1 [39], KV2 [39], KV3 [39], KV4 [39], KV6 [145], KV7 [66,96,131], KV9 [39] and KV11 [12] families of KV channels, with KV1, 2 and 7 being particularly important (see KV channels and myogenic tone, below).
Structure of KV channels
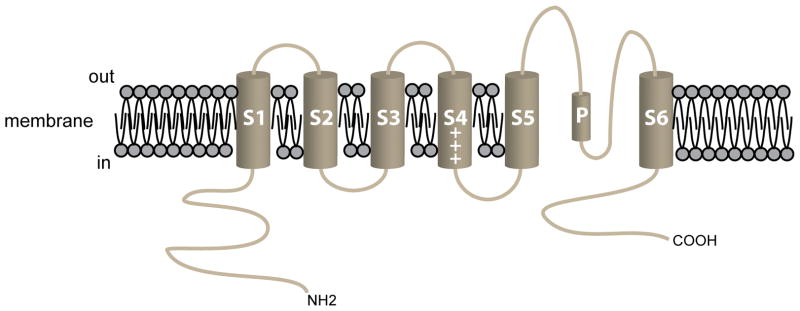
Functional KV channels consist of homo- or heterotetramers of six transmembrane-domain (S1–S6), pore-forming α-subunits [64,93]. The pore of the channel is formed by the P-loop between S5 and S6 along with S6 from each α-subunit [11,64,92,116,147,156]. Positively charged amino acids in membrane spanning domain S4 detect changes in membrane potential and serve as the voltage sensor in these channels [11,64,92,116,147,156] (Figure 2). Fast, N-type inactivation involves the α-subunit N-terminus [82,83,92,116,196], whereas slow (C-type) inactivation involves the C-terminal domain and the channel’s pore [83,116]. Accessory subunits interact with the pore-forming α-subunits and modulate channel function and interactions with scaffolding and other proteins in macromolecular signaling complexes [70,183]. Heterogeneity in the function of expressed KV channels arises from the type of α-subunit(s) expressed, heteromultimerization of different α-subunits, the presence (or absence) of modifier subunits, alternative splicing and post-translational modifications [64,70].
Figure 2.
The pore-forming α-subunit of KV channels. Schematic diagram of the 6 membrane spanning domains of a typical KV channel. The length and composition of the amino (NH2) and the carboxy (COOH) termini vary among the large number of KV channel isoforms expressed in VSMCs. For KV1.5, the NH2-terminus in the full-length version of the channel is 247 amino acids (AAs) and the COOH-terminus is 89 AAs in length. In KV1.5, the selectivity filter (AAs 480–485) follows the 9 AA, pore helix (AAs 468–479), labeled “P” in the diagram. Modulatory KVβ-subunits interact with residues in the NH2-terminus of the α-subunit. Post-synaptic density (PSD)-95 interacts with PDZ domains in the COOH-terminus of KV channels.
Pharmacology of VSMC KV channels
Pharmacological dissection of the function of individual KV channels in VSMCs is challenging, because: 1.) VSMCs express a large number of KV channel isoforms, and 2.) the pharmacology of KV channels is diverse (Table 1). This difficulty is compounded in isolated vessel and in vivo experiments where the readout is only membrane potential, vessel diameter or isometric tension. Nonetheless, a pharmacological “finger print” of the functional expression of KV channel isoforms can be established by the use of a combination of the more selective compounds, analysis of current kinetics in patch clamp experiments and functional assays using isolated vessels [41,42]. For example, Correolide, at micromolar concentrations [55], or psoralen derivatives (Psora-4 [187] and PAP-1 [167], Table 1) can be used to inhibit KV 1 family members. While difficult to use in vivo, toxins, such as stromotoxin-1 (KV 2.1) [54] or phrixotoxins (KV 4) [51] can be used in patch clamp and isolated vessel experiments. There are a number of compounds that block various members of the KV7 family (Table 1), and the availability of agonists for these channels provides a working armamentarium to study the function of this family of KV channels. As with all drugs, care must be taken to use the lowest concentration possible to achieve channel blockade. For example, while PAP-1 is selective for KV1 channels at nanomolar concentrations, this drug inhibits most other KV channels at micromolar concentrations (Table 1).
Table 1.
The pharmacology of VSMC KV channels
| Channel | Gene | Accessory Subunits | Inhibitors/Antagonists (IC50) | Activators/Agonists (EC50) |
|---|---|---|---|---|
| KV1.1 | KCNA1 | KV β1, KV β2 | TEA (0.3 mM) [67] 4-AP (0.29 mM) [67] correolide (430 nM) [55] α-dendrotoxin (20 nM) [67] dendrotoxin-k (2.5 nM) [161] hongotoxin (31 pM) [115] margatoxin (144 pM) [115] kaliotoxin (41 nM) [67] ShK toxin (16 pM) [101] Psora-4 (62 nM) [187] PAP-1 (65 nM) [167] |
|
| KV1.2 | KCNA2 | KV β1, KV β2 | TEA (0.56 μM) [67] 4-AP (0.59 mM) [67] correolide (700 nM) [55] charybdotoxin (14 nM) [67] α-dendrotoxin (17 nM) [67] hongotoxin (170 pM) [115] margatoxin (675 pM) [115] ShK toxin (9 nM) [101] Psora-4 (49 nM) [187] PAP-1 (250 nM) [167] |
|
| KV1.3 | KCNA3 | KV β2 | TEA (10 mM) [67] 4-AP (0.195 mM) [67] correolide (86 nM) [55] α-dendrotoxin (250 nM) [67] dendrotoxin-k (2.5 nM) [161] hongotoxin (86 pM) [115] margatoxin (230 pM) [115] kaliotoxin (0.65 nM) [67] ShK toxin (11 pM) [101] Psora-4 (3 nM) [187] PAP-1 (2 nM) [167] |
|
| KV1.5 | KCNA5 | KV β1.2, KV β2, KV β3 | TEA (330 mM) [67] 4-AP (0.27 mM) [67] correolide (1.1 μM) [55] Psora-4 (7.7 nM) [187] PAP-1 (45 nM) [167] |
|
| KV1.6 | KCNA6 | KV β1, KV β2 | TEA (1.7–7 mM) [63,68,109] 4-AP (0.3 – 1.5 mM) [68,109] correolide (450 nM) [55] charybdotoxin (1 nM) [68] α-dendrotoxin (25 nM) [109] hongotoxin (6 nM) [115] margatoxin (144 pM) [115] ShK toxin (165 pM) [101] PAP-1 (62 nM) [167] |
|
| KV2.1 | KCNB1 | KV9.3, KV6.3 | TEA (4.9 mM) [75] 4-AP (18 mM) [110] Ba2+ (30 mM) [177] SsmTx-1 (41.7 nM) [28] stromotoxin-1 (12.7 nM) [54] PAP-1 (3 μM) [167] |
|
| KV3.1 | KCNC1 | TEA (0.2 mM) [67] 4-AP (29 μM) [67] PAP-1 (5 μM) [167] |
||
| KV4.1 | KCND1 | TEA (11 mM) [173] 4-AP (1 mM) [173] phrixotoxin 1 (>250 nM) [51] phrixotoxin 2 (>300 nM) [51] |
||
| KV4.2 | KCND2 | TEA (11 mM) [173] 4-AP (1 mM) [173] phrixotoxin 1 (5 nM) [51] phrixotoxin 2 (34 nM) [51] PAP-1 (1.2 μM) [167] |
||
| KV4.3 | KCND3 | TEA (~11 mM) [173] 4-AP (1.2 mM) [178] phrixotoxin 1 (28 nM) [51] phrixotoxin 2 (71 nM) [51] |
||
| KV6.3 | KCNG3 | KV2.1 | See KV2.1 above | |
| KV7.4 | KCNQ4 | KCNE1–5 | TEA (3 mM) [160] Linopirdine (14 μM) [172] XE991 (5.5 μM) [172] |
ML277 (>30 μM) [137] ML213 (0.5–0.8 μM) [20,195] Retigabine (5.3 μM) [69] |
| KV7.5 | KCNE1–5 | XE991 (65 μM) [160] | Retigabine (6.4 μM) [69] ML213 (700 nM) [20] |
|
| KV9.3 | KCNS3 | KV2.1 | See KV2.1 above | |
| KV11.1 | KCNH2 | KCNE1 (minK)KCNE2 (miRP1) | E4031 (7.7 nM) [202] Dofetilide (6.4 nM) [170] Astemizole (1 nM) [203] |
RPR260243 (2 μM) [102] ICA-105574 (0.5 μM) [61] PD-118057 (20 μM) [201] Mallotoxin (0.5 μM) [198] NS1643 (10.5 μM) [72] |
Table modified from [184], with permission.
KV channels and myogenic tone
Early studies showed that: 1) 3,4-diaminopyridine or 4-aminopyridine (4-AP), which inhibit KV1–4 channels [39], caused contraction of isolated VSMC in a number of isolated blood vessels [36,73,185], 2.) 4-AP inhibited currents around the resting membrane potential of rabbit portal vein myocytes and depolarized renal [60] and coronary [118] VSMCs, and 3.) 4-AP depolarized VSMCs and augmented myogenic tone at intraluminal pressures greater than 40 mm Hg in pressurized rabbit cerebral arteries [111]. Subsequent studies confirmed these findings in a number of arteries and arterioles [39,86–89,147] (Figure 3). These observations are consistent with the hypothesis that 4-AP-sensitive KV channels contribute to VSMC resting membrane potential and to the negative feedback regulation of myogenic tone (Figure 1).
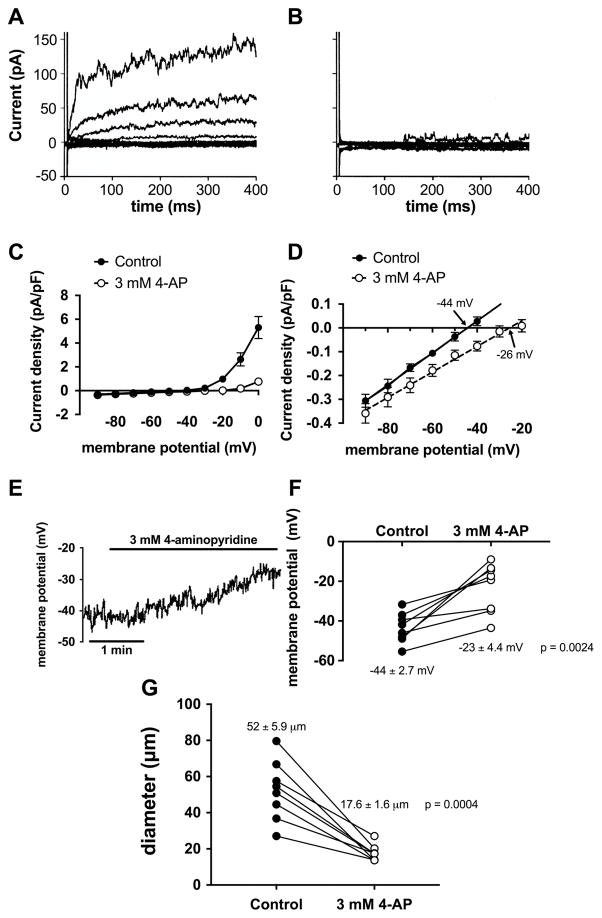
Figure 3.
KV channel block with 4-aminopyridine inhibits membrane K+ currents, depolarizes VSMCs and constricts arterioles. Panel A shows the family of outward K+ currents elicited by 10 mV depolarizing voltage steps from −90 mV to 0 mV recorded in hamster cremasteric arteriolar VSMCs using the perforated patch technique [90]. Panel B shows currents in the same cell after superfusion with 3 mM 4-aminopyridine (4-AP): outward currents are virtually abolished. Panel C shows summary means ± SEM for similar experiments showing the current-voltage relationship for the data shown in Panels A and B. Panel D shows the current-voltage relationship from Panel C on an expanded scale to show that 4-AP (3 mM) inhibits currents around the resting membrane potential of these cells (~-44 mV), and shifts the reversal potential for whole-cell currents (a measure of membrane potential) to more positive potentials. Panel E shows effects of 4-AP (3 mM) on membrane potential of an isolated hamster cremasteric arteriolar VSMC recorded in current-clamp experiments using the perforated-patch technique: 4-AP depolarized the cell from ~-40 mV to ~-25 mV, consistent with the data shown in Panel D. Panel F shows summary data for experiments as in Panel E demonstrating that 4-AP (3 mM) consistently depolarizes hamster cremasteric arteriolar VSMCs. Panel G shows data from pressure-myography experiments demonstrating that 4-AP consistently constricts isolated second-order cremasteric arterioles, consistent with VSMC depolarization (Panels D–F) due to block of outward KV currents (Panels A–C). These data show 4-AP-sensitive KV channel currents contribute to resting membrane potential and myogenic tone in arteriolar VSMCs. Data Panels A–D modified from [90], with permission. Data in Panels E–G replotted from [88], with permission.
KV1 channels in VSMCs
Members of the KV1 family are expressed and contribute substantially to whole-cell KV current in isolated VSMCs (~40% of whole-cell current at −40 mV [42]), resting VSMC membrane potential and the negative feedback regulation of myogenic tone [39,41,42,184]. Expression of KV1.2, 1.5 and 1.6 appear to predominate with likely heteromerization among these three isoforms [39,41,42,184]. Heteromeric KV channels composed of KV1.2, 1.5 and 1.6 have been proposed to underlie the delayed rectifier KV channel currents of VSMCs in rabbit portal veins [104]. In rat mesenteric [42,127,158] and cerebral arteries [7] heteromers of KV1.2 and KV1.5 have been proposed. Herteromers consisting of KV1.2 and KV1.4 have been postulated to be expressed in rat renal arteries [56].
Expression of KVβ subunits and their function with respect to KV1 channels has only been examined in a few instances [39]. For example, rat retinal arteriolar SMCs display rapidly inactivating A-type KV currents due to expression of KV1.5 and accessory KVβ1 subunits [139,140]. These currents are in contrast to the slowly inactivating KV currents that are displayed by most VSMCs (Figure 3A). Mouse coronary artery VSMCs express primarily KVβ1 and KVβ2 associated with KV1.5 α-subunits, and that KVβ2 is required for normal trafficking of KV1.5 α-subunits to the plasma membrane [150]. In rat mesenteric arteries, KVβ1.2 has been proposed to contribute to functional channel expression along with KV1.2 and KV1.5 [42].
Expression of transcripts for KV1.3 has been detected in VSMCs of a number of arteries and arterioles [7,31,34,40,138,189,190], but not in all studies [30,56,181]. Despite the presence of mRNA transcripts for KV1.3 in rat cerebral arteries, no protein was detected in lysates of these vessels [7]. Similarly, dialysis of VSMCs with KV1.3 antibodies has no effect on whole-cell-KV currents in cells from rat mesenteric artery [127]. In murine femoral artery VSMCs, currents through KV1.3 channels account for only 11% of whole-cell KV channel currents in native contractile VSMCs, but 58% of the current in proliferating VSMCs [34]. Furthermore, expression of KV1.3 mRNA and protein was substantially elevated in proliferating VSMCs, and block of KV1.3 with margatoxin or PAP-1 (Table 1) inhibited VSMC proliferation [34]. It is interesting to note that KV1.3 channels participate in the phenotypic switch of contractile VSMCs to proliferating VSMCs through a mechanism that is independent of the K+ flux-function of this protein [32,98], involves MEK/ERK and PLCγ signaling [33] and depends on the channel’s COOH-terminus [98].
KV2 channels in vascular SMCs
Vascular smooth muscle cells also express functional KV2.1 channels [39,41,42,184] that contribute to whole cell KV current (~20% at −40 mV [42]), resting membrane potential and the negative feedback regulation of myogenic tone [39,41,42,184]. Evidence from rat cerebral arteries [200] and rat mesenteric arteries [42] suggests that KV2.1 may form heteromeric channels with KV9.3. These heteromeric channels may be particularly important for regulation of VSMC resting membrane potential at low intravascular pressure [200]. In a hypertensive mouse strain, upregulation of the expression of KV6.3 and its co-expression with KV2 channels appears to account for decreased KV2-based currents and membrane depolarization [145].
KV3 and KV4 channels in VSMCs
Expression of KV3 and KV4 mRNA has been reported in porcine coronary arterioles [77], rat mesenteric artery [189], and rat tail artery [190]. In rat mesenteric artery, while mRNA for KV3 channel subunits was detected in whole-vessel lysates, expression of these KV channel subunits was not detected in isolated VSMCs [42]. These data suggest that KV3 subunits may be expressed in some other cell-type found in the wall of these resistance arteries. Protein for KV3.2 was not detected in rat mesenteric arteries [42,189]. Selective inhibitors of KV3 and KV4 channels have no effect on whole-cell currents in rat mesenteric arteries [42]. Thus, there appears to be little evidence for functional expression of these subunits related to the regulation of vascular tone. However, in human uterine arteries, KV3.4 channels play a permissive role in VSMC proliferation [141,142].
KV7 channels in VSMCs
Members of the KV7 family also contribute to whole cell currents (20% at −40 mV [42]), resting membrane potential and the negative feedback regulation of myogenic and vasoconstrictor-induced tone in several vessels; KV7.4 and 7.5 appear to play a major role [42,66,96,131]. Expression of the auxiliary subunit, KNCE4, is required for expression and function of VSMC KV7.4 in rat mesenteric arteries [94] and confers sex-linked differences in KV7.4 function and vascular reactivity in the mouse [1]. These channels also interact with G-protein βγ-subunits, that appear to be required for channel activity and which participate the in the regulation of myogenic tone in rat renal arteries [175].
KV11 channels in VSMCs
In addition to KV1.3 and KV3.4 channels mentioned above, KV11 channels also appear to participate in proliferation of VSMCs [12]. Transcripts and protein for KV11.1 were detected in VSMCs of several murine arteries [12]. However, currents through these channels were only detected in VSMCs from portal vein, but not in VSMCs isolated from carotid arteries [12]. Nonetheless, an inhibitor of KV11.1 channels, dofetilide (Table 1), suppressed, whereas an activator, NS1643 (Table 1), enhanced proliferation of VSMCs isolated from murine femoral arteries [12].
Vasoconstrictors and KV channels
Vasoconstrictors that act at G-protein coupled receptors and depolarize VSMCs activate KV channels [23,30,31,36,71,136,155,168]. This activation blunts the vasoconstriction and is part of the negative-feedback regulation of vascular tone, essentially preventing vasospasm. Membrane depolarization has also been shown to selectively traffic KV1.5 channels to the plasma membrane of VSMCs, also contributing to the negative feedback regulation of VSMC membrane potential and vascular tone [107]. Nonetheless, there is evidence that KV channel closure contributes to the mechanism of action of the same vasoconstrictors including phenylephrine [143], 5-HT [10,113,176] and angiotensin II [35]. These agents may act via protein kinases or by Ca2+-mediated inhibition of KV channels. Activation of protein kinases (PK) such as PKC [2,35,74,106,113], c-SRC [176], Rho-kinase [128,129] inhibits currents through 4-AP-sensitive KV channels, contributing to vasoconstrictor-induced VSMC depolarization (Figure 3). Angiotensin II has been shown to selectively decrease the surface expression of KV1.5 channels via PKC-dependent degradation of these K+ channels [106]. Protein kinase C also inhibits KV7 channels [131]. Agonist-induced increases in intracellular Ca2+ concentration also inhibit 4-AP-sensitive KV channels [45,60,85]. Thus, agonist-induced activation of kinases and increases in intracellular Ca2+ have the potential to close KV channels, reduce their surface expression and provide a positive feedback signal to support depolarization, activation of VGCC and vasoconstriction (Figure 1).
Vasodilators and KV channels
cAMP-PKA-mediated activation of KV channels
Vasodilators that act through GS-coupled receptors and the cAMP signaling cascade activate 4-AP-sensitive KV channels (Figure 1) [3–5,17,49,52,76,78,119,165]. Protein kinase A phosphorylates KV1.2 at serine 449 in the C-terminus to increase channel activity [99]. Vasodilators that act through the cAMP and cGMP signaling cascades also may act, in part, by antagonizing the Rho-kinase-mediated KV channel downregulation [130]. In vessels where heteromers of KV1.2 and 1.5 or 1.6 predominate [7,29,104,158,181], phosphorylation of KV1.2 may underlie the 4-AP-sensitive effects of cAMP-PKA-related vasodilators. In rat cerebral artery VSMCs, PKA is targeted to KV1.2 by the scaffolding protein PSD95, which binds to the COOH-terminus of these channels [144]. The beta receptor agonist, isoproterenol, and the adenylate cyclase activator, forskolin, activate KV7.4 channels in renal VSMCs. Adenosine-induced dilation of coronary arteries [105] and CGRP-induced dilation of cerebral arteries [23] also involves KV7.4. However, isoproterenol-dependent activation of KV7.4 involves G-protein βγ subunits in renal arteries [175]. Data from A7R5 cells suggest that cAMP-PKA targets primarily channels containing KV7.5 [133].
NO-cGMP-PKG-mediated activation of KV channels
Vasodilators that act through the NO and cGMP signaling pathways also have been suggested to activate KV channels [184]. Vasodilation induced by endothelium-derived NO, NO-donors and cGMP analogs are inhibited by 4-AP in rat basilar arteries [171]. Nitric oxide and atrial natriuretic peptide activate KV2.1 channels in A7R5 cells and relaxation of rat aortas by these dilators can be inhibited by 4-AP [179]. Coreolide inhibits dilation of the canine coronary circulation to an NO donor suggesting a role for KV1 channels [49]. Currents through KV7 channels are also activated by NO and cGMP in rat renal and aortic VSMCs [174]. However, there may be species differences as SNP-induced relaxation of porcine coronary arteries is unaffected by KV7 channel blockade [79].
Other dilators that activate KV channels
Hydrogen sulfide (H2S) [27,134,166] and hydrogen peroxide (H2O2) [157,162,163] also act, in part, by activation of VSMC KV channels. For H2S, both 4-AP-sensitive KV channels [27] and KV7 channels are the targets [134,166]. Hydrogen peroxide activates VSMC 4-AP-sensitive KV channels through a process that involves thiol oxidation [157,162,163]. In rat mesenteric arteries, S-glutathionylation of KV2.1 has been proposed to underlie the effects of H2O2 [157]. In murine coronary arteries, vasodilation induced by H2O2 requires expression of VSMC KV1.5 channels [151]. In human coronary arteries, KV1.5 also is the target for H2O2-mediated vasodilation [149].
Hypoxia and acidosis may also activate VSMC KV channels. Hypoxia activates currents through KV7 channels in SMCs from porcine coronary arteries and mediates hypoxia-induced relaxation [79]. Acidosis activates 4-AP-sensitive KV channels in coronary VSMCs [15].
Perivascular adipose tissue (PVAT) release anti-contractile substances (adipocyte-derived relaxing factors - ADRFs) that activate KV7 channels [180]. In rat gracilis resistance artery VSMCs, ADRFs activate KV7.4 [197].
Functional vasodilation and KV channels
Blood flow in most tissues is proportional to the tissue’s metabolic activity, with increases in metabolism being mirrored by increases in blood flow (functional vasodilation, or functional hyperemia) [47]. Vascular KV channels have been postulated to mediate functional vasodilation in the heart based on the effects of blockers like 4-AP [16,164] and coreolide [65], for example. However, these studies are difficult to interpret, because the site of action of the KV channels affected by the drugs (VSMCs, cardiac myocytes, nerves, etc.) is not known. In murine hearts, VSMC-specific knockout of KV1.5 inhibits functional vasodilation, an effect that can be rescued by VSMC expression of KV1.5 [151]. These data support a major role for VSMC KV1.5 in matching blood flow to metabolism in mouse hearts. A role for KV1.3 in coronary functional hyperemia in the mouse also has been proposed [152]. However, because a global KV1.3 knockout was used, the location of the KV1.3 in the signaling pathway that couples cardiac metabolism to blood flow was not established.
KV channels and pathophysiology
The role played by KV channels in hypertension [38,46,100,114], obesity and the metabolic syndrome [16,50,97,113,148,194] and aging [62,103] remains unclear. In hypertension, for example, patch-clamp studies have reported increased [40,43], decreased [18,19,37,44,123,135,182] or no change [124,125] in KV current density. Similarly, expression of KV channel subunits has been reported to be increased [40,43], decreased [8,9,18,24,95,121,145,182,197,199], or unchanged [9,43,145,197] in SMCs from some vessels in various models of hypertension. Regional differences in the impact of hypertension on KV channel expression and function; differences in the KV isoform studied; differences in the model of hypertension used; and differences in the severity and duration of hypertension may explain the lack of consensus reported in the literature.
Voltage-gated K+ channel expression and function are decreased in experimental models of Type 1 diabetes [21,25,26,114,120,126,159]. Mechanisms involving nitration of KV channels [120], reactive oxygen species [21], and/or PKC [159] account for the down regulation of KV channel function in Type 1 diabetes.
Subarachnoid hemorrhage leads to down regulation of KV channel function in several models of this disease [6,84,91]. In a rabbit model of subarachnoid hemorrhage, oxyhemoglobin-induced activation of tyrosine kinases leads to endocytosis of KV1.5 channels and reduced KV function [84].
Conclusions and Perspective
Voltage-gated K+ channels are highly expressed in VSMCs and clearly contribute to the negative feedback regulation of myogenic and agonist-induced tone in resistance arteries and arterioles. What remains unclear is why there are so many isoforms expressed: is this a matter of redundancy or do the different KV channel isoforms play specific roles that we have yet to unravel? Study of the role played by different KV isoforms in VSMCs is complex, because there is substantial regional and species differences in channel expression, function and regulation. Thus, information from vessels in one tissue or organ cannot be extrapolated to vessels elsewhere in the body. Our understanding of the composition, complexity and function of protein-protein interactions among KV channels, signaling proteins and the cytoskeleton is only just beginning and should prove to be fertile ground for future studies. Functions of KV channel proteins, other than conductance of K+ flux across biological membrane, as demonstrated by KV1.3 and its role in VSMC proliferation, also remains largely unexplored. Finally, the effects of disease states on the expression and function of KV channels remains cloudy and poorly understood, particularly with regard to the signaling mechanisms involved. Future studies addressing these issues, particularly in arteriolar smooth muscle from tissues and organs around the body are welcomed.
Acknowledgments
Grant Support: HL32469 and PO1 HL070687
References
- 1.Abbott GW, Jepps TA. Kcne4 Deletion Sex-Dependently Alters Vascular Reactivity. J Vasc Res. 2016;53:138–148. doi: 10.1159/000449060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aiello EA, Clement-Chomienne O, Sontag DP, Walsh MP, Cole WC. Protein kinase C inhibits delayed rectifier K+ current in rabbit vascular smooth muscle cells. Am J Physiol. 1996;271:H109–119. doi: 10.1152/ajpheart.1996.271.1.H109. [DOI] [PubMed] [Google Scholar]
- 3.Aiello EA, Malcolm AT, Walsh MP, Cole WC. Beta-adrenoceptor activation and PKA regulate delayed rectifier K+ channels of vascular smooth muscle cells. American Journal of Physiology. 1998;275:H448–H459. doi: 10.1152/ajpheart.1998.275.2.H448. [DOI] [PubMed] [Google Scholar]
- 4.Aiello EA, Walsh MP, Cole WC. Isoproterenol and forskolin increase and PKI inhibits delayed rectifier K+ current in vascular myocytes isolated from rabbit coronary artery and portal vein. CanJPhysiolPharmacol. 1994;72:47. [Google Scholar]
- 5.Aiello EA, Walsh MP, Cole WC. Phosphorylation by protein kinase A enhances delayed rectifier K+ current in rabbit vascular smooth muscle cells. AmJPhysiolHeart CircPhysiol. 1995;268:H926–H934. doi: 10.1152/ajpheart.1995.268.2.H926. [DOI] [PubMed] [Google Scholar]
- 6.Aihara Y, Jahromi BS, Yassari R, Nikitina E, Agbaje-Williams M, Macdonald RL. Molecular profile of vascular ion channels after experimental subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2004;24:75–83. doi: 10.1097/01.WCB.0000095803.98378.D8. [DOI] [PubMed] [Google Scholar]
- 7.Albarwani S, Nemetz LT, Madden JA, Tobin AA, England SK, Pratt PF, Rusch NJ. Voltage-gated K+ channels in rat small cerebral arteries: molecular identity of the functional channels. J Physiol. 2003;551:751–763. doi: 10.1113/jphysiol.2003.040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amberg GC, Rossow CF, Navedo MF, Santana LF. NFATc3 Regulates Kv2.1 Expression in Arterial Smooth Muscle. J Biol Chem. 2004;279:47326–47334. doi: 10.1074/jbc.M408789200. [DOI] [PubMed] [Google Scholar]
- 9.Amberg GC, Santana LF. Kv2 channels oppose myogenic constriction of rat cerebral arteries. Am J Physiol Cell Physiol. 2006;291:C348–356. doi: 10.1152/ajpcell.00086.2006. [DOI] [PubMed] [Google Scholar]
- 10.Bae YM, Kim A, Kim J, Park SW, Kim TK, Lee YR, Kim B, Cho SI. Serotonin depolarizes the membrane potential in rat mesenteric artery myocytes by decreasing voltage-gated K+ currents. Biochem Biophys Res Commun. 2006;347:468–476. doi: 10.1016/j.bbrc.2006.06.116. [DOI] [PubMed] [Google Scholar]
- 11.Baldwin TJ, Isacoff E, Li M, Lopez GA, Sheng M, Tsaur ML, Yan YN, Jan LY. Elucidation of biophysical and biological properties of voltage-gated potassium channels. Cold Spring Harb Symp Quant Biol. 1992;57:491–499. doi: 10.1101/sqb.1992.057.01.054. [DOI] [PubMed] [Google Scholar]
- 12.Barrese V, Cidad P, Yeung SY, Lopez-Lopez JR, McNeish AJ, Ohya S, Perez-Garcia MT, Greenwood IA. Proliferative Role of Kv11 Channels in Murine Arteries. Front Physiol. 2017;8:500. doi: 10.3389/fphys.2017.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beech DJ, Bolton TB. The effects of tetraethylammonium ions, 4-aminopyridine or quinidine on K+-currents in single smooth muscle cells of the rabbit portal vein. Biomed Biochim Acta. 1987;46:S673–676. [PubMed] [Google Scholar]
- 14.Beech DJ, Bolton TB. Two components of potassium current activated by depolarization of single smooth muscle cells from the rabbit portal vein. J Physiol. 1989;418:293–309. doi: 10.1113/jphysiol.1989.sp017841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berger MG, Vandier C, Bonnet P, Jackson WF, Rusch NJ. Intracellular acidosis differentially regulates KV channels in coronary and pulmonary vascular muscle. Am J Physiol. 1998;275:H1351–1359. doi: 10.1152/ajpheart.1998.275.4.H1351. [DOI] [PubMed] [Google Scholar]
- 16.Berwick ZC, Dick GM, Moberly SP, Kohr MC, Sturek M, Tune JD. Contribution of voltage-dependent K(+) channels to metabolic control of coronary blood flow. J Mol Cell Cardiol. 2012;52:912–919. doi: 10.1016/j.yjmcc.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berwick ZC, Payne GA, Lynch B, Dick GM, Sturek M, Tune JD. Contribution of Adenosine A2A and A2B Receptors to Ischemic Coronary Dilation: Role of KV and KATP Channels. Microcirculation. 2010;17:600–607. doi: 10.1111/j.1549-8719.2010.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bratz IN, Dick GM, Partridge LD, Kanagy NL. Reduced molecular expression of K(+) channel proteins in vascular smooth muscle from rats made hypertensive with N{omega}-nitro-L-arginine. Am J Physiol Heart Circ Physiol. 2005;289:H1277–1283. doi: 10.1152/ajpheart.01052.2004. [DOI] [PubMed] [Google Scholar]
- 19.Bratz IN, Swafford AN, Jr, Kanagy NL, Dick GM. Reduced functional expression of K(+) channels in vascular smooth muscle cells from rats made hypertensive with N{omega}-nitro-L-arginine. Am J Physiol Heart Circ Physiol. 2005;289:H1284–1290. doi: 10.1152/ajpheart.01053.2004. [DOI] [PubMed] [Google Scholar]
- 20.Brueggemann LI, Haick JM, Cribbs LL, Byron KL. Differential activation of vascular smooth muscle Kv7.4, Kv7.5, and Kv7.4/7.5 channels by ML213 and ICA-069673. Mol Pharmacol. 2014;86:330–341. doi: 10.1124/mol.114.093799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bubolz AH, Li H, Wu Q, Liu Y. Enhanced oxidative stress impairs cAMP-mediated dilation by reducing Kv channel function in small coronary arteries of diabetic rats. Am J Physiol Heart Circ Physiol. 2005;289:H1873–1880. doi: 10.1152/ajpheart.00357.2005. [DOI] [PubMed] [Google Scholar]
- 22.Burns WR, Cohen KD, Jackson WF. K+-induced dilation of hamster cremasteric arterioles involves both the Na+/K+-ATPase and inward-rectifier K+ channels. Microcirculation. 2004;11:279–293. doi: 10.1080/10739680490425985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chadha PS, Jepps TA, Carr G, Stott JB, Zhu HL, Cole WC, Greenwood IA. Contribution of kv7.4/kv7.5 heteromers to intrinsic and calcitonin gene-related peptide-induced cerebral reactivity. Arterioscler Thromb Vasc Biol. 2014;34:887–893. doi: 10.1161/ATVBAHA.114.303405. [DOI] [PubMed] [Google Scholar]
- 24.Chadha PS, Zunke F, Zhu HL, Davis AJ, Jepps TA, Olesen SP, Cole WC, Moffatt JD, Greenwood IA. Reduced KCNQ4-encoded voltage-dependent potassium channel activity underlies impaired beta-adrenoceptor-mediated relaxation of renal arteries in hypertension. Hypertension. 2012;59:877–884. doi: 10.1161/HYPERTENSIONAHA.111.187427. [DOI] [PubMed] [Google Scholar]
- 25.Chai Q, Liu Z, Chen L. Effects of streptozotocin-induced diabetes on Kv channels in rat small coronary smooth muscle cells. Chin J Physiol. 2005;48:57–63. [PubMed] [Google Scholar]
- 26.Chai Q, Xu X, Jia Q, Dong Q, Liu Z, Zhang W, Chen L. Molecular basis of dysfunctional Kv channels in small coronary artery smooth muscle cells of streptozotocin-induced diabetic rats. Chin J Physiol. 2007;50:171–177. [PubMed] [Google Scholar]
- 27.Cheang WS, Wong WT, Shen B, Lau CW, Tian XY, Tsang SY, Yao X, Chen ZY, Huang Y. 4-Aminopyridine-sensitive K+ channels contributes to NaHS-induced membrane hyperpolarization and relaxation in the rat coronary artery. Vascular Pharmacology. 2010;53:94–98. doi: 10.1016/j.vph.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Chen M, Li J, Zhang F, Liu Z. Isolation and characterization of SsmTx-I, a Specific Kv2.1 blocker from the venom of the centipede Scolopendra Subspinipes Mutilans L. Koch. Journal of peptide science : an official publication of the European Peptide Society. 2014;20:159–164. doi: 10.1002/psc.2588. [DOI] [PubMed] [Google Scholar]
- 29.Chen TT, Luykenaar KD, Walsh EJ, Walsh MP, Cole WC. Key Role of Kv1 Channels in Vasoregulation. Circulation Research. 2006;99:53–60. doi: 10.1161/01.RES.0000229654.45090.57. [DOI] [PubMed] [Google Scholar]
- 30.Cheong A, Dedman AM, Beech DJ. Expression and function of native potassium channel [K(V)alpha1] subunits in terminal arterioles of rabbit. J Physiol. 2001;534:691–700. doi: 10.1111/j.1469-7793.2001.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheong A, Dedman AM, Xu SZ, Beech DJ. K(V)alpha1 channels in murine arterioles: differential cellular expression and regulation of diameter. Am J Physiol Heart Circ Physiol. 2001;281:H1057–1065. doi: 10.1152/ajpheart.2001.281.3.H1057. [DOI] [PubMed] [Google Scholar]
- 32.Cidad P, Jimenez-Perez L, Garcia-Arribas D, Miguel-Velado E, Tajada S, Ruiz-McDavitt C, Lopez-Lopez JR, Perez-Garcia MT. Kv1.3 channels can modulate cell proliferation during phenotypic switch by an ion-flux independent mechanism. Arterioscler Thromb Vasc Biol. 2012;32:1299–1307. doi: 10.1161/ATVBAHA.111.242727. [DOI] [PubMed] [Google Scholar]
- 33.Cidad P, Miguel-Velado E, Ruiz-McDavitt C, Alonso E, Jimenez-Perez L, Asuaje A, Carmona Y, Garcia-Arribas D, Lopez J, Marroquin Y, Fernandez M, Roque M, Perez-Garcia MT, Lopez-Lopez JR. Kv1.3 channels modulate human vascular smooth muscle cells proliferation independently of mTOR signaling pathway. Pflugers Arch. 2015;467:1711–1722. doi: 10.1007/s00424-014-1607-y. [DOI] [PubMed] [Google Scholar]
- 34.Cidad P, Moreno-Dominguez A, Novensa L, Roque M, Barquin L, Heras M, Perez-Garcia MT, Lopez-Lopez JR. Characterization of ion channels involved in the proliferative response of femoral artery smooth muscle cells. Arterioscler Thromb Vasc Biol. 2010;30:1203–1211. doi: 10.1161/ATVBAHA.110.205187. [DOI] [PubMed] [Google Scholar]
- 35.Clement-Chomienne O, Walsh MP, Cole WC. Angiotensin II activation of protein kinase C decreases delayed rectifier K+ current in rabbit vascular myocytes. J Physiol. 1996;495( Pt 3):689–700. doi: 10.1113/jphysiol.1996.sp021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cook NS. Effect of Some Potassium Channel Blockers on Contractile Responses of the Rabbit Aorta. Journal of Cardiovascular Pharmacology. 1989;13:299–306. doi: 10.1097/00005344-198902000-00019. [DOI] [PubMed] [Google Scholar]
- 37.Cox RH. Comparison of K+ channel properties in freshly isolated myocytes from thoracic aorta of WKY and SHR. Am J Hypertens. 1996;9:884–894. doi: 10.1016/s0895-7061(96)00179-3. [DOI] [PubMed] [Google Scholar]
- 38.Cox RH. Changes in the expression and function of arterial potassium channels during hypertension. Vascul Pharmacol. 2002;38:13–23. doi: 10.1016/s1537-1891(02)00122-2. [DOI] [PubMed] [Google Scholar]
- 39.Cox RH. Molecular determinants of voltage-gated potassium currents in vascular smooth muscle. Cell Biochem Biophys. 2005;42:167–195. doi: 10.1385/CBB:42:2:167. [DOI] [PubMed] [Google Scholar]
- 40.Cox RH, Folander K, Swanson R. Differential expression of voltage-gated K(+) channel genes in arteries from spontaneously hypertensive and Wistar-Kyoto rats. Hypertension. 2001;37:1315–1322. doi: 10.1161/01.hyp.37.5.1315. [DOI] [PubMed] [Google Scholar]
- 41.Cox RH, Fromme S. Comparison of Voltage Gated K+ Currents in Arterial Myocytes with Heterologously Expressed K v Subunits. Cell Biochem Biophys. 2016;74:499–511. doi: 10.1007/s12013-016-0763-4. [DOI] [PubMed] [Google Scholar]
- 42.Cox RH, Fromme S. Functional Expression Profile of Voltage-Gated K(+) Channel Subunits in Rat Small Mesenteric Arteries. Cell Biochem Biophys. 2016;74:263–276. doi: 10.1007/s12013-015-0715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cox RH, Fromme SJ, Folander KL, Swanson RJ. Voltage gated K+ channel expression in arteries of Wistar-Kyoto and spontaneously hypertensive rats. Am J Hypertens. 2008;21:213–218. doi: 10.1038/ajh.2007.44. [DOI] [PubMed] [Google Scholar]
- 44.Cox RH, Lozinskaya I, Dietz NJ. Differences in K+ current components in mesenteric artery myocytes from WKY and SHR. Am J Hypertens. 2001;14:897–907. doi: 10.1016/s0895-7061(01)02145-8. [DOI] [PubMed] [Google Scholar]
- 45.Cox RH, Petrou S. Ca(2+) influx inhibits voltage-dependent and augments Ca(2+)-dependent K(+) currents in arterial myocytes. Am J Physiol. 1999;277:C51–63. doi: 10.1152/ajpcell.1999.277.1.C51. [DOI] [PubMed] [Google Scholar]
- 46.Cox RH, Rusch NJ. New expression profiles of voltage-gated ion channels in arteries exposed to high blood pressure. Microcirculation. 2002;9:243–257. doi: 10.1038/sj.mn.7800140. [DOI] [PubMed] [Google Scholar]
- 47.Davis MJ, Hill MA, Kuo L. John Wiley & Sons, Inc, editor. Local Regulation of Microvascular Perfusion. 2010. [Google Scholar]
- 48.del Valle-Rodriguez A, Lopez-Barneo J, Urena J. Ca2+ channel-sarcoplasmic reticulum coupling: a mechanism of arterial myocyte contraction without Ca2+ influx. The EMBO journal. 2003;22:4337–4345. doi: 10.1093/emboj/cdg432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dick GM, Bratz IN, Borbouse L, Payne GA, Dincer UD, Knudson JD, Rogers PA, Tune JD. Voltage-dependent K+ channels regulate the duration of reactive hyperemia in the canine coronary circulation. Am J Physiol Heart Circ Physiol. 2008;294:H2371–2381. doi: 10.1152/ajpheart.01279.2007. [DOI] [PubMed] [Google Scholar]
- 50.Dick GM, Tune JD. Role of potassium channels in coronary vasodilation. Experimental Biology and Medicine. 2010;235:10–22. doi: 10.1258/ebm.2009.009201. [DOI] [PubMed] [Google Scholar]
- 51.Diochot S, Drici MD, Moinier D, Fink M, Lazdunski M. Effects of phrixotoxins on the Kv4 family of potassium channels and implications for the role of Ito1 in cardiac electrogenesis. Br J Pharmacol. 1999;126:251–263. doi: 10.1038/sj.bjp.0702283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dong H, Waldron GJ, Cole WC, Triggle CR. Roles of calcium-activated and voltage-gated delayed rectifier potassium channels in endothelium-dependent vasorelaxation of the rabbit middle cerebral artery. Br J Pharmacol. 1998;123:821–832. doi: 10.1038/sj.bjp.0701680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Emerson GG, Segal SS. Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries: role in vasomotor control. Circ Res. 2000;87:474–479. doi: 10.1161/01.res.87.6.474. [DOI] [PubMed] [Google Scholar]
- 54.Escoubas P, Diochot S, Celerier ML, Nakajima T, Lazdunski M. Novel tarantula toxins for subtypes of voltage-dependent potassium channels in the Kv2 and Kv4 subfamilies. Mol Pharmacol. 2002;62:48–57. doi: 10.1124/mol.62.1.48. [DOI] [PubMed] [Google Scholar]
- 55.Felix JP, Bugianesi RM, Schmalhofer WA, Borris R, Goetz MA, Hensens OD, Bao JM, Kayser F, Parsons WH, Rupprecht K, Garcia ML, Kaczorowski GJ, Slaughter RS. Identification and biochemical characterization of a novel nortriterpene inhibitor of the human lymphocyte voltage-gated potassium channel, Kv1.3. Biochemistry. 1999;38:4922–4930. doi: 10.1021/bi982954w. [DOI] [PubMed] [Google Scholar]
- 56.Fergus DJ, Martens JR, England SK. Kv channel subunits that contribute to voltage-gated K+ current in renal vascular smooth muscle. Pflugers Arch. 2003;445:697–704. doi: 10.1007/s00424-002-0994-7. [DOI] [PubMed] [Google Scholar]
- 57.Fernandez-Tenorio M, Gonzalez-Rodriguez P, Porras C, Castellano A, Moosmang S, Hofmann F, Urena J, Lopez-Barneo J. Short communication: genetic ablation of L-type Ca2+ channels abolishes depolarization-induced Ca2+ release in arterial smooth muscle. Circ Res. 2010;106:1285–1289. doi: 10.1161/CIRCRESAHA.109.213967. [DOI] [PubMed] [Google Scholar]
- 58.Fernandez-Tenorio M, Porras-Gonzalez C, Castellano A, Del Valle-Rodriguez A, Lopez-Barneo J, Urena J. Metabotropic regulation of RhoA/Rho-associated kinase by L-type Ca2+ channels: new mechanism for depolarization-evoked mammalian arterial contraction. Circ Res. 2011;108:1348–1357. doi: 10.1161/CIRCRESAHA.111.240127. [DOI] [PubMed] [Google Scholar]
- 59.Ganitkevich V, Isenberg G. Membrane potential modulates inositol 1,4,5-trisphosphate-mediated Ca2+ transients in guinea-pig coronary myocytes. J Physiol. 1993;470:35–44. doi: 10.1113/jphysiol.1993.sp019845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gelband CH, Ishikawa T, Post JM, Keef KD, Hume JR. Intracellular divalent cations block smooth muscle K+ channels. Circ Res. 1993;73:24–34. doi: 10.1161/01.res.73.1.24. [DOI] [PubMed] [Google Scholar]
- 61.Gerlach AC, Stoehr SJ, Castle NA. Pharmacological removal of human ether-a-go-go-related gene potassium channel inactivation by 3-nitro-N-(4-phenoxyphenyl) benzamide (ICA-105574) Mol Pharmacol. 2010;77:58–68. doi: 10.1124/mol.109.059543. [DOI] [PubMed] [Google Scholar]
- 62.Ghosh P, Mora Solis FR, Dominguez JM, 2nd, Spier SA, Donato AJ, Delp MD, Muller-Delp JM. Exercise training reverses aging-induced impairment of myogenic constriction in skeletal muscle arterioles. Journal of applied physiology (Bethesda, Md : 1985) 2015;118:904–911. doi: 10.1152/japplphysiol.00277.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gomez-Hernandez JM, Lorra C, Pardo LA, Stuhmer W, Pongs O, Heinemann SH, Elliott AA. Molecular basis for different pore properties of potassium channels from the rat brain Kv1 gene family. Pflugers Arch. 1997;434:661–668. doi: 10.1007/s004240050449. [DOI] [PubMed] [Google Scholar]
- 64.Gonzalez C, Baez-Nieto D, Valencia I, Oyarzun I, Rojas P, Naranjo D, Latorre R. K(+) channels: function-structural overview. Comprehensive Physiology. 2012;2:2087–2149. doi: 10.1002/cphy.c110047. [DOI] [PubMed] [Google Scholar]
- 65.Goodwill AG, Noblet JN, Sassoon D, Fu L, Kassab GS, Schepers L, Herring BP, Rottgen TS, Tune JD, Dick GM. Critical contribution of KV1 channels to the regulation of coronary blood flow. Basic Res Cardiol. 2016;111:56. doi: 10.1007/s00395-016-0575-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Greenwood IA, Ohya S. New tricks for old dogs: KCNQ expression and role in smooth muscle. Br J Pharmacol. 2009;156:1196–1203. doi: 10.1111/j.1476-5381.2009.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grissmer S, Nguyen AN, Aiyar J, Hanson DC, Mather RJ, Gutman GA, Karmilowicz MJ, Auperin DD, Chandy KG. Pharmacological characterization of five cloned voltage-gated K+ channels, types Kv1.1, 1.2, 1.3, 1.5, and 3.1, stably expressed in mammalian cell lines. Mol Pharmacol. 1994;45:1227–1234. [PubMed] [Google Scholar]
- 68.Grupe A, Schroter KH, Ruppersberg JP, Stocker M, Drewes T, Beckh S, Pongs O. Cloning and expression of a human voltage-gated potassium channel. A novel member of the RCK potassium channel family. EMBO J. 1990;9:1749–1756. doi: 10.1002/j.1460-2075.1990.tb08299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gunthorpe MJ, Large CH, Sankar R. The mechanism of action of retigabine (ezogabine), a first-in-class K+ channel opener for the treatment of epilepsy. Epilepsia. 2012;53:412–424. doi: 10.1111/j.1528-1167.2011.03365.x. [DOI] [PubMed] [Google Scholar]
- 70.Gutman GA, Chandy KG, Grissmer S, Lazdunski M, McKinnon D, Pardo LA, Robertson GA, Rudy B, Sanguinetti MC, Stuhmer W, Wang X. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol Rev. 2005;57:473–508. doi: 10.1124/pr.57.4.10. [DOI] [PubMed] [Google Scholar]
- 71.Hald BO, Jacobsen JC, Braunstein TH, Inoue R, Ito Y, Sorensen PG, Holstein-Rathlou NH, Jensen LJ. BKCa and KV channels limit conducted vasomotor responses in rat mesenteric terminal arterioles. Pflugers Arch. 2012;463:279–295. doi: 10.1007/s00424-011-1049-8. [DOI] [PubMed] [Google Scholar]
- 72.Hansen RS, Diness TG, Christ T, Demnitz J, Ravens U, Olesen SP, Grunnet M. Activation of human ether-a-go-go-related gene potassium channels by the diphenylurea 1,3-bis-(2-hydroxy-5-trifluoromethyl-phenyl)-urea (NS1643) Mol Pharmacol. 2006;69:266–277. doi: 10.1124/mol.105.015859. [DOI] [PubMed] [Google Scholar]
- 73.Hara Y, Kitamura K, Kuriyama H. Actions of 4-aminopyridine on vascular smooth muscle tissues of the guinea-pig. Br J Pharmacol. 1980;68:99–106. doi: 10.1111/j.1476-5381.1980.tb10704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hayabuchi Y, Standen NB, Davies NW. Angiotensin II inhibits and alters kinetics of voltage-gated K(+) channels of rat arterial smooth muscle. Am J Physiol Heart Circ Physiol. 2001;281:H2480–2489. doi: 10.1152/ajpheart.2001.281.6.H2480. [DOI] [PubMed] [Google Scholar]
- 75.He Y, Kang Y, Leung YM, Xia F, Gao X, Xie H, Gaisano HY, Tsushima RG. Modulation of Kv2.1 channel gating and TEA sensitivity by distinct domains of SNAP-25. Biochem J. 2006;396:363–369. doi: 10.1042/BJ20051478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Heaps CL, Bowles DK. Gender-specific K(+)-channel contribution to adenosine-induced relaxation in coronary arterioles. Journal of applied physiology (Bethesda, Md : 1985) 2002;92:550–558. doi: 10.1152/japplphysiol.00566.2001. [DOI] [PubMed] [Google Scholar]
- 77.Heaps CL, Jeffery EC, Laine GA, Price EM, Bowles DK. Effects of exercise training and hypercholesterolemia on adenosine activation of voltage-dependent K+ channels in coronary arterioles. Journal of applied physiology (Bethesda, Md : 1985) 2008;105:1761–1771. doi: 10.1152/japplphysiol.90958.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heaps CL, Tharp DL, Bowles DK. Hypercholesterolemia abolishes voltage-dependent K+ channel contribution to adenosine-mediated relaxation in porcine coronary arterioles. Am J Physiol Heart Circ Physiol. 2005;288:H568–576. doi: 10.1152/ajpheart.00157.2004. [DOI] [PubMed] [Google Scholar]
- 79.Hedegaard ER, Nielsen BD, Kun A, Hughes AD, Kroigaard C, Mogensen S, Matchkov VV, Frobert O, Simonsen U. KV 7 channels are involved in hypoxia-induced vasodilatation of porcine coronary arteries. Br J Pharmacol. 2014;171:69–82. doi: 10.1111/bph.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hodgkin AL, Huxley AF. Current carried by sodium and potassium ions through the membrane of the giant axon of Loligo. Journal of Physiology (London) 1952;116:449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hodgkin AL, Huxley AF, Katz B. Ionic currents underlying activity in the giant axon of the squid. Archives des Sciences Physiologiques. 1949;3:129–150. [Google Scholar]
- 82.Hoshi T, Zagotta WN, Aldrich RW. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 1990;250:533–538. doi: 10.1126/science.2122519. [DOI] [PubMed] [Google Scholar]
- 83.Hoshi T, Zagotta WN, Aldrich RW. Two types of inactivation in Shaker K+ channels: effects of alterations in the carboxy-terminal region. Neuron. 1991;7:547–556. doi: 10.1016/0896-6273(91)90367-9. [DOI] [PubMed] [Google Scholar]
- 84.Ishiguro M, Morielli AD, Zvarova K, Tranmer BI, Penar PL, Wellman GC. Oxyhemoglobin-induced suppression of voltage-dependent K+ channels in cerebral arteries by enhanced tyrosine kinase activity. Circ Res. 2006;99:1252–1260. doi: 10.1161/01.RES.0000250821.32324.e1. [DOI] [PubMed] [Google Scholar]
- 85.Ishikawa T, Hume JR, Keef KD. Modulation of K+ and Ca2+ channels by histamine H1-receptor stimulation in rabbit coronary artery cells. J Physiol. 1993;468:379–400. doi: 10.1113/jphysiol.1993.sp019777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jackson WF. Potassium channels and regulation of the microcirculation. Microcirculation. 1998;5:85–90. [PubMed] [Google Scholar]
- 87.Jackson WF. Ion channels and vascular tone. Hypertension. 2000;35:173–178. doi: 10.1161/01.hyp.35.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jackson WF. Potassium channels in the circulation of skeletal muscle. In: Archer SL, Rusch NJ, editors. Potassium channels in the cardiovascular biology. New York: Kluwer Academic/Plenum Publishers; 2001. pp. 505–522. [Google Scholar]
- 89.Jackson WF. Potassium channels in the peripheral microcirculation. Microcirculation. 2005;12:113–127. doi: 10.1080/10739680590896072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jackson WF, Huebner JM, Rusch NJ. Enzymatic isolation and characterization of single vascular smooth muscle cells from cremasteric arterioles. Microcirculation. 1997;4:35–50. doi: 10.3109/10739689709148316. [DOI] [PubMed] [Google Scholar]
- 91.Jahromi BS, Aihara Y, Ai J, Zhang ZD, Weyer G, Nikitina E, Yassari R, Houamed KM, Macdonald RL. Temporal profile of potassium channel dysfunction in cerebrovascular smooth muscle after experimental subarachnoid haemorrhage. Neurosci Lett. 2008;440:81–86. doi: 10.1016/j.neulet.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jan LY, Jan YN. Structural elements involved in specific K+ channel functions. Annu Rev Physiol. 1992;54:537–555. doi: 10.1146/annurev.ph.54.030192.002541. [DOI] [PubMed] [Google Scholar]
- 93.Jan LY, Jan YN. Voltage-gated and inwardly rectifying potassium channels. J Physiol. 1997;505( Pt 2):267–282. doi: 10.1111/j.1469-7793.1997.267bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jepps TA, Carr G, Lundegaard PR, Olesen SP, Greenwood IA. Fundamental role for the KCNE4 ancillary subunit in Kv7.4 regulation of arterial tone. J Physiol. 2015;593:5325–5340. doi: 10.1113/JP271286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jepps TA, Chadha PS, Davis AJ, Harhun MI, Cockerill GW, Olesen SP, Hansen RS, Greenwood IA. Downregulation of Kv7.4 channel activity in primary and secondary hypertension. Circulation. 2011;124:602–611. doi: 10.1161/CIRCULATIONAHA.111.032136. [DOI] [PubMed] [Google Scholar]
- 96.Jepps TA, Olesen SP, Greenwood IA. One man’s side effect is another man’s therapeutic opportunity: targeting Kv7 channels in smooth muscle disorders. British Journal of Pharmacology. 2013;168:19–27. doi: 10.1111/j.1476-5381.2012.02133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jiang J, Thoren P, Caligiuri G, Hansson GK, Pernow J. Enhanced phenylephrine-induced rhythmic activity in the atherosclerotic mouse aorta via an increase in opening of KCa channels: relation to Kv channels and nitric oxide. Br J Pharmacol. 1999;128:637–646. doi: 10.1038/sj.bjp.0702855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jimenez-Perez L, Cidad P, Alvarez-Miguel I, Santos-Hipolito A, Torres-Merino R, Alonso E, de la Fuente MA, Lopez-Lopez JR, Perez-Garcia MT. Molecular Determinants of Kv1.3 Potassium Channels-induced Proliferation. J Biol Chem. 2016;291:3569–3580. doi: 10.1074/jbc.M115.678995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Johnson RP, El-Yazbi AF, Hughes MF, Schriemer DC, Walsh EJ, Walsh MP, Cole WC. Identification and functional characterization of protein kinase A-catalyzed phosphorylation of potassium channel Kv1.2 at serine 449. J Biol Chem. 2009;284:16562–16574. doi: 10.1074/jbc.M109.010918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Joseph BK, Thakali KM, Moore CL, Rhee SW. Ion channel remodeling in vascular smooth muscle during hypertension: Implications for novel therapeutic approaches. Pharmacol Res. 2013;70:126–138. doi: 10.1016/j.phrs.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kalman K, Pennington MW, Lanigan MD, Nguyen A, Rauer H, Mahnir V, Paschetto K, Kem WR, Grissmer S, Gutman GA, Christian EP, Cahalan MD, Norton RS, Chandy KG. ShK-Dap22, a potent Kv1.3-specific immunosuppressive polypeptide. J Biol Chem. 1998;273:32697–32707. doi: 10.1074/jbc.273.49.32697. [DOI] [PubMed] [Google Scholar]
- 102.Kang J, Chen XL, Wang H, Ji J, Cheng H, Incardona J, Reynolds W, Viviani F, Tabart M, Rampe D. Discovery of a small molecule activator of the human ether-a-go-go-related gene (HERG) cardiac K+ channel. Mol Pharmacol. 2005;67:827–836. doi: 10.1124/mol.104.006577. [DOI] [PubMed] [Google Scholar]
- 103.Kang LS, Kim S, Dominguez JM, 2nd, Sindler AL, Dick GM, Muller-Delp JM. Aging and muscle fiber type alter K+ channel contributions to the myogenic response in skeletal muscle arterioles. Journal of applied physiology (Bethesda, Md : 1985) 2009;107:389–398. doi: 10.1152/japplphysiol.91245.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kerr PM, Clement-Chomienne O, Thorneloe KS, Chen TT, Ishii K, Sontag DP, Walsh MP, Cole WC. Heteromultimeric Kv1.2-Kv1.5 channels underlie 4-aminopyridine-sensitive delayed rectifier K(+) current of rabbit vascular myocytes. Circ Res. 2001;89:1038–1044. doi: 10.1161/hh2301.100803. [DOI] [PubMed] [Google Scholar]
- 105.Khanamiri S, Soltysinska E, Jepps TA, Bentzen BH, Chadha PS, Schmitt N, Greenwood IA, Olesen SP. Contribution of Kv7 channels to basal coronary flow and active response to ischemia. Hypertension. 2013;62:1090–1097. doi: 10.1161/HYPERTENSIONAHA.113.01244. [DOI] [PubMed] [Google Scholar]
- 106.Kidd MW, Bulley S, Jaggar JH. Angiotensin II reduces the surface abundance of KV 1.5 channels in arterial myocytes to stimulate vasoconstriction. J Physiol. 2017;595:1607–1618. doi: 10.1113/JP272893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kidd MW, Leo MD, Bannister JP, Jaggar JH. Intravascular pressure enhances the abundance of functional Kv1.5 channels at the surface of arterial smooth muscle cells. Sci Signal. 2015;8:ra83. doi: 10.1126/scisignal.aac5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim HR, Appel S, Vetterkind S, Gangopadhyay SS, Morgan KG. Smooth muscle signalling pathways in health and disease. Journal of cellular and molecular medicine. 2008;12:2165–2180. doi: 10.1111/j.1582-4934.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kirsch GE, Drewe JA, Verma S, Brown AM, Joho RH. Electrophysiological characterization of a new member of the RCK family of rat brain K+ channels. FEBS Lett. 1991;278:55–60. doi: 10.1016/0014-5793(91)80082-e. [DOI] [PubMed] [Google Scholar]
- 110.Kirsch GE, Shieh CC, Drewe JA, Vener DF, Brown AM. Segmental exchanges define 4-aminopyridine binding and the inner mouth of K+ pores. Neuron. 1993;11:503–512. doi: 10.1016/0896-6273(93)90154-j. [DOI] [PubMed] [Google Scholar]
- 111.Knot HJ, Nelson MT. Regulation of membrane potential and diameter by voltage-dependent K+ channels in rabbit myogenic cerebral arteries. Am J Physiol. 1995;269:H348–355. doi: 10.1152/ajpheart.1995.269.1.H348. [DOI] [PubMed] [Google Scholar]
- 112.Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol. 1998;508( Pt 1):199–209. doi: 10.1111/j.1469-7793.1998.199br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ko EA, Park WS, Firth AL, Hong DH, Choi SW, Heo HJ, Kim MH, Noh JH, Ko JH, Kim N, Earm YE, Song DK, Han J. Increased sensitivity of serotonin on the voltage-dependent K+ channels in mesenteric arterial smooth muscle cells of OLETF rats. Prog Biophys Mol Biol. 2010;103:88–94. doi: 10.1016/j.pbiomolbio.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 114.Ko EA, Park WS, Firth AL, Kim N, Yuan JX, Han J. Pathophysiology of voltage-gated K+ channels in vascular smooth muscle cells: modulation by protein kinases. Prog Biophys Mol Biol. 2010;103:95–101. doi: 10.1016/j.pbiomolbio.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 115.Koschak A, Bugianesi RM, Mitterdorfer J, Kaczorowski GJ, Garcia ML, Knaus HG. Subunit composition of brain voltage-gated potassium channels determined by hongotoxin-1, a novel peptide derived from Centruroides limbatus venom. J Biol Chem. 1998;273:2639–2644. doi: 10.1074/jbc.273.5.2639. [DOI] [PubMed] [Google Scholar]
- 116.Kukuljan M, Labarca P, Latorre R. Molecular determinants of ion conduction and inactivation in K+ channels. AmJPhysiolCell Physiol. 1995;268:C535–C556. doi: 10.1152/ajpcell.1995.268.3.C535. [DOI] [PubMed] [Google Scholar]
- 117.Kukuljan M, Rojas E, Catt KJ, Stojilkovic SS. Membrane potential regulates inositol 1,4,5-trisphosphate-controlled cytoplasmic Ca2+ oscillations in pituitary gonadotrophs. J Biol Chem. 1994;269:4860–4865. [PubMed] [Google Scholar]
- 118.Leblanc N, Wan X, Leung PM. Physiological role of Ca(2+)-activated and voltage-dependent K+ currents in rabbit coronary myocytes. Am J Physiol. 1994;266:C1523–1537. doi: 10.1152/ajpcell.1994.266.6.C1523. [DOI] [PubMed] [Google Scholar]
- 119.Li H, Chai Q, Gutterman DD, Liu Y. Elevated glucose impairs cAMP-mediated dilation by reducing Kv channel activity in rat small coronary smooth muscle cells. Am J Physiol Heart Circ Physiol. 2003;285:H1213–1219. doi: 10.1152/ajpheart.00226.2003. [DOI] [PubMed] [Google Scholar]
- 120.Li H, Gutterman DD, Rusch NJ, Bubolz A, Liu Y. Nitration and functional loss of voltage-gated K+ channels in rat coronary microvessels exposed to high glucose. Diabetes. 2004;53:2436–2442. doi: 10.2337/diabetes.53.9.2436. [DOI] [PubMed] [Google Scholar]
- 121.Li Z, Lu N, Shi L. Exercise training reverses alterations in Kv and BKCa channel molecular expression in thoracic aorta smooth muscle cells from spontaneously hypertensive rats. J Vasc Res. 2014;51:447–457. doi: 10.1159/000369928. [DOI] [PubMed] [Google Scholar]
- 122.Liu QH, Zheng YM, Korde AS, Yadav VR, Rathore R, Wess J, Wang YX. Membrane depolarization causes a direct activation of G protein-coupled receptors leading to local Ca2+ release in smooth muscle. Proc Natl Acad Sci U S A. 2009;106:11418–11423. doi: 10.1073/pnas.0813307106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu Y, Hudetz AG, Knaus HG, Rusch NJ. Increased expression of Ca2+-sensitive K+ channels in the cerebral microcirculation of genetically hypertensive rats: evidence for their protection against cerebral vasospasm. Circ Res. 1998;82:729–737. doi: 10.1161/01.res.82.6.729. [DOI] [PubMed] [Google Scholar]
- 124.Liu Y, Jones AW, Sturek M. Increased barium influx and potassium current in stroke-prone spontaneously hypertensive rats. Hypertension. 1994;23:1091–1095. doi: 10.1161/01.hyp.23.6.1091. [DOI] [PubMed] [Google Scholar]
- 125.Liu Y, Pleyte K, Knaus HG, Rusch NJ. Increased expression of Ca2+-sensitive K+ channels in aorta of hypertensive rats. Hypertension. 1997;30:1403–1409. doi: 10.1161/01.hyp.30.6.1403. [DOI] [PubMed] [Google Scholar]
- 126.Liu Y, Terata K, Rusch NJ, Gutterman DD. High glucose impairs voltage-gated K(+) channel current in rat small coronary arteries. Circ Res. 2001;89:146–152. doi: 10.1161/hh1401.093294. [DOI] [PubMed] [Google Scholar]
- 127.Lu Y, Hanna ST, Tang G, Wang R. Contributions of Kv1.2, Kv1.5 and Kv2.1 subunits to the native delayed rectifier K(+) current in rat mesenteric artery smooth muscle cells. Life Sci. 2002;71:1465–1473. doi: 10.1016/s0024-3205(02)01922-7. [DOI] [PubMed] [Google Scholar]
- 128.Luykenaar KD, Brett SE, Wu BN, Wiehler WB, Welsh DG. Pyrimidine nucleotides suppress KDR currents and depolarize rat cerebral arteries by activating Rho kinase. Am J Physiol Heart Circ Physiol. 2004;286:H1088–1100. doi: 10.1152/ajpheart.00903.2003. [DOI] [PubMed] [Google Scholar]
- 129.Luykenaar KD, El-Rahman RA, Walsh MP, Welsh DG. Rho-kinase-mediated suppression of KDR current in cerebral arteries requires an intact actin cytoskeleton. Am J Physiol Heart Circ Physiol. 2009;296:H917–926. doi: 10.1152/ajpheart.01206.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Luykenaar KD, Welsh DG. Activators of the PKA and PKG pathways attenuate RhoA-mediated suppression of the KDR current in cerebral arteries. Am J Physiol Heart Circ Physiol. 2007;292:H2654–2663. doi: 10.1152/ajpheart.01255.2006. [DOI] [PubMed] [Google Scholar]
- 131.Mackie AR, Brueggemann LI, Henderson KK, Shiels AJ, Cribbs LL, Scrogin KE, Byron KL. Vascular KCNQ potassium channels as novel targets for the control of mesenteric artery constriction by vasopressin, based on studies in single cells, pressurized arteries, and in vivo measurements of mesenteric vascular resistance. J Pharmacol Exp Ther. 2008;325:475–483. doi: 10.1124/jpet.107.135764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mahaut-Smith MP, Martinez-Pinna J, Gurung IS. A role for membrane potential in regulating GPCRs? Trends Pharmacol Sci. 2008;29:421–429. doi: 10.1016/j.tips.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 133.Mani BK, Robakowski C, Brueggemann LI, Cribbs LL, Tripathi A, Majetschak M, Byron KL. Kv7.5 Potassium Channel Subunits Are the Primary Targets for PKA-Dependent Enhancement of Vascular Smooth Muscle Kv7 Currents. Mol Pharmacol. 2016;89:323–334. doi: 10.1124/mol.115.101758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Martelli A, Testai L, Breschi MC, Lawson K, McKay NG, Miceli F, Taglialatela M, Calderone V. Vasorelaxation by hydrogen sulphide involves activation of Kv7 potassium channels. Pharmacological Research. 2013;70:27–34. doi: 10.1016/j.phrs.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 135.Martens JR, Gelband CH. Alterations in rat interlobar artery membrane potential and K+ channels in genetic and nongenetic hypertension. Circ Res. 1996;79:295–301. doi: 10.1161/01.res.79.2.295. [DOI] [PubMed] [Google Scholar]
- 136.Martinez AC, Pagan RM, Prieto D, Recio P, Garcia-Sacristan A, Hernandez M, Benedito S. Modulation of noradrenergic neurotransmission in isolated rat radial artery. J Pharmacol Sci. 2009;111:299–311. doi: 10.1254/jphs.09135fp. [DOI] [PubMed] [Google Scholar]
- 137.Mattmann ME, Yu H, Lin Z, Xu K, Huang X, Long S, Wu M, McManus OB, Engers DW, Le UM, Li M, Lindsley CW, Hopkins CR. Identification of (R)-N-(4-(4-methoxyphenyl)thiazol-2-yl)-1-tosylpiperidine-2-carboxamide, ML277, as a novel, potent and selective K(v)7.1 (KCNQ1) potassium channel activator. Bioorg Med Chem Lett. 2012;22:5936–5941. doi: 10.1016/j.bmcl.2012.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.McDaniel SS, Platoshyn O, Yu Y, Sweeney M, Miriel VA, Golovina VA, Krick S, Lapp BR, Wang JY, Yuan JX. Anorexic effect of K+ channel blockade in mesenteric arterial smooth muscle and intestinal epithelial cells. Journal of applied physiology (Bethesda, Md : 1985) 2001;91:2322–2333. doi: 10.1152/jappl.2001.91.5.2322. [DOI] [PubMed] [Google Scholar]
- 139.McGahon MK, Dawicki JM, Arora A, Simpson DA, Gardiner TA, Stitt AW, Scholfield CN, McGeown JG, Curtis TM. Kv1.5 is a major component underlying the A-type potassium current in retinal arteriolar smooth muscle. Am J Physiol Heart Circ Physiol. 2007;292:H1001–1008. doi: 10.1152/ajpheart.01003.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.McGahon MK, Dawicki JM, Scholfield CN, McGeown JG, Curtis TM. A-Type Potassium Current in Retinal Arteriolar Smooth Muscle Cells. Invest Ophthalmol Vis Sci. 2005;46:3281–3287. doi: 10.1167/iovs.04-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Miguel-Velado E, Moreno-Dominguez A, Colinas O, Cidad P, Heras M, Perez-Garcia MT, Lopez-Lopez JR. Contribution of Kv channels to phenotypic remodeling of human uterine artery smooth muscle cells. Circulation Research. 2005;97:1280–1287. doi: 10.1161/01.RES.0000194322.91255.13. [DOI] [PubMed] [Google Scholar]
- 142.Miguel-Velado E, Perez-Carretero FD, Colinas O, Cidad P, Heras M, Lopez-Lopez JR, Perez-Garcia MT. Cell cycle-dependent expression of Kv3.4 channels modulates proliferation of human uterine artery smooth muscle cells. Cardiovasc Res. 2010;86:383–391. doi: 10.1093/cvr/cvq011. [DOI] [PubMed] [Google Scholar]
- 143.Mistry DK, Garland CJ. The influence of phenylephrine outward potassium currents in single smooth muscle cells from the rabbit mesenteric artery. Gen Pharmacol. 1999;33:389–399. doi: 10.1016/s0306-3623(99)00031-2. [DOI] [PubMed] [Google Scholar]
- 144.Moore CL, Nelson PL, Parelkar NK, Rusch NJ, Rhee SW. Protein kinase A-phosphorylated KV1 channels in PSD95 signaling complex contribute to the resting membrane potential and diameter of cerebral arteries. Circ Res. 2014;114:1258–1267. doi: 10.1161/CIRCRESAHA.114.303167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Moreno-Dominguez A, Cidad P, Miguel-Velado E, Lopez-Lopez JR, Perez-Garcia MT. De novo expression of Kv6.3 contributes to changes in vascular smooth muscle cell excitability in a hypertensive mice strain. J Physiol. 2009;587:625–640. doi: 10.1113/jphysiol.2008.165217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990;259:C3–18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- 147.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268:C799–822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 148.Nieves-Cintron M, Nystoriak MA, Prada MP, Johnson K, Fayer W, Dell’Acqua ML, Scott JD, Navedo MF. Selective down-regulation of KV2.1 function contributes to enhanced arterial tone during diabetes. J Biol Chem. 2015;290:7918–7929. doi: 10.1074/jbc.M114.622811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nishijima Y, Cao S, Chabowski DS, Korishettar A, Ge A, Zheng X, Sparapani R, Gutterman DD, Zhang DX. Contribution of KV1.5 Channel to Hydrogen Peroxide-Induced Human Arteriolar Dilation and Its Modulation by Coronary Artery Disease. Circ Res. 2017;120:658–669. doi: 10.1161/CIRCRESAHA.116.309491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nystoriak MA, Zhang D, Jagatheesan G, Bhatnagar A. Heteromeric complexes of aldo-keto reductase auxiliary KVbeta subunits (AKR6A) regulate sarcolemmal localization of KV1.5 in coronary arterial myocytes. Chemico-biological interactions. 2017 doi: 10.1016/j.cbi.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ohanyan V, Yin L, Bardakjian R, Kolz C, Enrick M, Hakobyan T, Kmetz J, Bratz I, Luli J, Nagane M, Khan N, Hou H, Kuppusamy P, Graham J, Fu FK, Janota D, Oyewumi MO, Logan S, Lindner JR, Chilian WM. Requisite Role of Kv1.5 Channels in Coronary Metabolic Dilation. Circ Res. 2015;117:612–621. doi: 10.1161/CIRCRESAHA.115.306642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ohanyan V, Yin L, Bardakjian R, Kolz C, Enrick M, Hakobyan T, Luli J, Graham K, Khayata M, Logan S, Kmetz J, Chilian WM. Kv1.3 channels facilitate the connection between metabolism and blood flow in the heart. Microcirculation. 2017;24 doi: 10.1111/micc.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Okabe K, Kitamura K, Kuriyama H. Features of 4-aminopyridine sensitive outward current observed in single smooth muscle cells from the rabbit pulmonary artery. Pflugers Arch. 1987;409:561–568. doi: 10.1007/BF00584654. [DOI] [PubMed] [Google Scholar]
- 154.Okada Y, Yanagisawa T, Taira N. BRL 38227 (levcromakalim)-induced hyperpolarization reduces the sensitivity to Ca2+ of contractile elements in canine coronary artery. Naunyn-Schmiedeberg’s archives of pharmacology. 1993;347:438–444. doi: 10.1007/BF00165396. [DOI] [PubMed] [Google Scholar]
- 155.Pagan RM, Martinez AC, Martinez MP, Hernandez M, Garcia-Sacristan A, Correa C, Prieto D, Benedito S. Endothelial and potassium channel dependent modulation of noradrenergic vasoconstriction in the pig radial artery. Eur J Pharmacol. 2009;616:166–174. doi: 10.1016/j.ejphar.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 156.Pallotta BS, Wagoner PK. Voltage-dependent potassium channels since Hodgkin and Huxley. PhysiolRev. 1992;72(Suppl):S49–S67. doi: 10.1152/physrev.1992.72.suppl_4.S49. [DOI] [PubMed] [Google Scholar]
- 157.Park SW, Noh HJ, Sung DJ, Kim JG, Kim JM, Ryu SY, Kang K, Kim B, Bae YM, Cho H. Hydrogen peroxide induces vasorelaxation by enhancing 4-aminopyridine-sensitive Kv currents through S-glutathionylation. Pflugers Arch. 2015;467:285–297. doi: 10.1007/s00424-014-1513-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Plane F, Johnson R, Kerr P, Wiehler W, Thorneloe K, Ishii K, Chen T, Cole W. Heteromultimeric Kv1 channels contribute to myogenic control of arterial diameter. Circ Res. 2005;96:216–224. doi: 10.1161/01.RES.0000154070.06421.25. [DOI] [PubMed] [Google Scholar]
- 159.Rainbow RD, Hardy ME, Standen NB, Davies NW. Glucose reduces endothelin inhibition of voltage-gated potassium channels in rat arterial smooth muscle cells. J Physiol. 2006;575:833–844. doi: 10.1113/jphysiol.2006.114009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Robbins J. KCNQ potassium channels: physiology, pathophysiology, and pharmacology. Pharmacol Ther. 2001;90:1–19. doi: 10.1016/s0163-7258(01)00116-4. [DOI] [PubMed] [Google Scholar]
- 161.Robertson B, Owen D, Stow J, Butler C, Newland C. Novel effects of dendrotoxin homologues on subtypes of mammalian Kv1 potassium channels expressed in Xenopus oocytes. FEBS Lett. 1996;383:26–30. doi: 10.1016/0014-5793(96)00211-6. [DOI] [PubMed] [Google Scholar]
- 162.Rogers PA, Chilian WM, Bratz IN, Bryan RM, Jr, Dick GM. H2O2 activates redox- and 4-aminopyridine-sensitive Kv channels in coronary vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2007;292:H1404–1411. doi: 10.1152/ajpheart.00696.2006. [DOI] [PubMed] [Google Scholar]
- 163.Rogers PA, Dick GM, Knudson JD, Focardi M, Bratz IN, Swafford AN, Jr, Saitoh S, Tune JD, Chilian WM. H2O2-induced redox-sensitive coronary vasodilation is mediated by 4-aminopyridine-sensitive K+ channels. Am J Physiol Heart Circ Physiol. 2006;291:H2473–2482. doi: 10.1152/ajpheart.00172.2006. [DOI] [PubMed] [Google Scholar]
- 164.Saitoh S, Zhang C, Tune JD, Potter B, Kiyooka T, Rogers PA, Knudson JD, Dick GM, Swafford A, Chilian WM. Hydrogen peroxide: a feed-forward dilator that couples myocardial metabolism to coronary blood flow. Arterioscler Thromb Vasc Biol. 2006;26:2614–2621. doi: 10.1161/01.ATV.0000249408.55796.da. [DOI] [PubMed] [Google Scholar]
- 165.Satake N, Shibata M, Shibata S. The inhibitory effects of iberiotoxin and 4-aminopyridine on the relaxation induced by beta 1- and beta 2-adrenoceptor activation in rat aortic rings. Br J Pharmacol. 1996;119:505–510. doi: 10.1111/j.1476-5381.1996.tb15700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Schleifenbaum J, Kohn C, Voblova N, Dubrovska G, Zavarirskaya O, Gloe T, Crean CS, Luft FC, Huang Y, Schubert R, Gollasch M. Systemic peripheral artery relaxation by KCNQ channel openers and hydrogen sulfide. J Hypertens. 2010;28:1875–1882. doi: 10.1097/HJH.0b013e32833c20d5. [DOI] [PubMed] [Google Scholar]
- 167.Schmitz A, Sankaranarayanan A, Azam P, Schmidt-Lassen K, Homerick D, Hansel W, Wulff H. Design of PAP-1, a selective small molecule Kv1.3 blocker, for the suppression of effector memory T cells in autoimmune diseases. Mol Pharmacol. 2005;68:1254–1270. doi: 10.1124/mol.105.015669. [DOI] [PubMed] [Google Scholar]
- 168.Shimizu S, Yokoshiki H, Sperelakis N, Paul RJ. Role of voltage-dependent and Ca(2+)-activated K(+) channels on the regulation of isometric force in porcine coronary artery. J Vasc Res. 2000;37:16–25. doi: 10.1159/000025709. [DOI] [PubMed] [Google Scholar]
- 169.Siegl D, Koeppen M, Wolfle SE, Pohl U, de Wit C. Myoendothelial coupling is not prominent in arterioles within the mouse cremaster microcirculation in vivo. Circ Res. 2005;97:781–788. doi: 10.1161/01.RES.0000186193.22438.6c. [DOI] [PubMed] [Google Scholar]
- 170.Singleton DH, Boyd H, Steidl-Nichols JV, Deacon M, Groot MJ, Price D, Nettleton DO, Wallace NK, Troutman MD, Williams C, Boyd JG. Fluorescently labeled analogues of dofetilide as high-affinity fluorescence polarization ligands for the human ether-a-go-go-related gene (hERG) channel. J Med Chem. 2007;50:2931–2941. doi: 10.1021/jm0700565. [DOI] [PubMed] [Google Scholar]
- 171.Sobey CG, Faraci FM. Inhibitory effect of 4-aminopyridine on responses of the basilar artery to nitric oxide. Br J Pharmacol. 1999;126:1437–1443. doi: 10.1038/sj.bjp.0702439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sogaard R, Ljungstrom T, Pedersen KA, Olesen SP, Jensen BS. KCNQ4 channels expressed in mammalian cells: functional characteristics and pharmacology. Am J Physiol Cell Physiol. 2001;280:C859–866. doi: 10.1152/ajpcell.2001.280.4.C859. [DOI] [PubMed] [Google Scholar]
- 173.Song WJ, Tkatch T, Baranauskas G, Ichinohe N, Kitai ST, Surmeier DJ. Somatodendritic depolarization-activated potassium currents in rat neostriatal cholinergic interneurons are predominantly of the A type and attributable to coexpression of Kv4.2 and Kv4.1 subunits. J Neurosci. 1998;18:3124–3137. doi: 10.1523/JNEUROSCI.18-09-03124.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Stott JB, Barrese V, Jepps TA, Leighton EV, Greenwood IA. Contribution of Kv7 channels to natriuretic peptide mediated vasodilation in normal and hypertensive rats. Hypertension. 2015;65:676–682. doi: 10.1161/HYPERTENSIONAHA.114.04373. [DOI] [PubMed] [Google Scholar]
- 175.Stott JB, Povstyan OV, Carr G, Barrese V, Greenwood IA. G-protein betagamma subunits are positive regulators of Kv7.4 and native vascular Kv7 channel activity. Proc Natl Acad Sci U S A. 2015;112:6497–6502. doi: 10.1073/pnas.1418605112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sung DJ, Noh HJ, Kim JG, Park SW, Kim B, Cho H, Bae YM. Serotonin contracts the rat mesenteric artery by inhibiting 4-aminopyridine-sensitive Kv channels via the 5-HT2A receptor and Src tyrosine kinase. Experimental & molecular medicine. 2013;45:e67. doi: 10.1038/emm.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Taglialatela M, Drewe JA, Brown AM. Barium blockade of a clonal potassium channel and its regulation by a critical pore residue. Mol Pharmacol. 1993;44:180–190. [PubMed] [Google Scholar]
- 178.Tamargo J, Caballero R, Gomez R, Valenzuela C, Delpon E. Pharmacology of cardiac potassium channels. Cardiovasc Res. 2004;62:9–33. doi: 10.1016/j.cardiores.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 179.Tanaka Y, Tang G, Takizawa K, Otsuka K, Eghbali M, Song M, Nishimaru K, Shigenobu K, Koike K, Stefani E, Toro L. Kv channels contribute to nitric oxide- and atrial natriuretic peptide-induced relaxation of a rat conduit artery. J Pharmacol Exp Ther. 2006;317:341–354. doi: 10.1124/jpet.105.096115. [DOI] [PubMed] [Google Scholar]
- 180.Tano JY, Schleifenbaum J, Gollasch M. Perivascular adipose tissue, potassium channels, and vascular dysfunction. Arterioscler Thromb Vasc Biol. 2014;34:1827–1830. doi: 10.1161/ATVBAHA.114.303032. [DOI] [PubMed] [Google Scholar]
- 181.Thorneloe KS, Chen TT, Kerr PM, Grier EF, Horowitz B, Cole WC, Walsh MP. Molecular composition of 4-aminopyridine-sensitive voltage-gated K(+) channels of vascular smooth muscle. Circ Res. 2001;89:1030–1037. doi: 10.1161/hh2301.100817. [DOI] [PubMed] [Google Scholar]
- 182.Tobin AA, Joseph BK, Al-Kindi HN, Albarwani S, Madden JA, Nemetz LT, Rusch NJ, Rhee SW. Loss of cerebrovascular Shaker-type K(+) channels: a shared vasodilator defect of genetic and renal hypertensive rats. Am J Physiol Heart Circ Physiol. 2009;297:H293–303. doi: 10.1152/ajpheart.00991.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Torres YP, Morera FJ, Carvacho I, Latorre R. A marriage of convenience: beta-subunits and voltage-dependent K+ channels. J Biol Chem. 2007;282:24485–24489. doi: 10.1074/jbc.R700022200. [DOI] [PubMed] [Google Scholar]
- 184.Tykocki NR, Boerman EM, Jackson WF. Smooth Muscle Ion Channels and Regulation of Vascular Tone in Resistance Arteries and Arterioles. Comprehensive Physiology. 2017;7:485–581. doi: 10.1002/cphy.c160011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Uchida Y, Nakamura F, Tomaru T, Sumino S, Kato A, Sugimoto T. Phasic contractions of canine and human coronary arteries induced by potassium channel blockers. Jpn Heart J. 1986;27:727–740. doi: 10.1536/ihj.27.727. [DOI] [PubMed] [Google Scholar]
- 186.Urena J, del Valle-Rodriguez A, Lopez-Barneo J. Metabotropic Ca2+ channel-induced calcium release in vascular smooth muscle. Cell calcium. 2007;42:513–520. doi: 10.1016/j.ceca.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 187.Vennekamp J, Wulff H, Beeton C, Calabresi PA, Grissmer S, Hansel W, Chandy KG. Kv1.3-blocking 5-phenylalkoxypsoralens: a new class of immunomodulators. Mol Pharmacol. 2004;65:1364–1374. doi: 10.1124/mol.65.6.1364. [DOI] [PubMed] [Google Scholar]
- 188.Welsh DG, Jackson WF, Segal SS. Oxygen induces electromechanical coupling in arteriolar smooth muscle cells: a role for L-type Ca2+ channels. Am J Physiol. 1998;274:H2018–2024. doi: 10.1152/ajpheart.1998.274.6.H2018. [DOI] [PubMed] [Google Scholar]
- 189.Xu C, Lu Y, Tang G, Wang R. Expression of voltage-dependent K(+) channel genes in mesenteric artery smooth muscle cells. Am J Physiol. 1999;277:G1055–1063. doi: 10.1152/ajpgi.1999.277.5.G1055. [DOI] [PubMed] [Google Scholar]
- 190.Xu C, Tang G, Lu Y, Wang R. Molecular basis of voltage-dependent delayed rectifier K+ channels in smooth muscle cells from rat tail artery. Life Sci. 2000;66:2023–2033. doi: 10.1016/s0024-3205(00)00529-4. [DOI] [PubMed] [Google Scholar]
- 191.Yamagishi T, Yanagisawa T, Taira N. K+ channel openers, cromakalim and Ki4032, inhibit agonist-induced Ca2+ release in canine coronary artery. Naunyn-Schmiedeberg’s archives of pharmacology. 1992;346:691–700. doi: 10.1007/BF00168744. [DOI] [PubMed] [Google Scholar]
- 192.Yamamura H, Ohya S, Muraki K, Imaizumi Y. Involvement of inositol 1,4,5-trisphosphate formation in the voltage-dependent regulation of the Ca(2+) concentration in porcine coronary arterial smooth muscle cells. J Pharmacol Exp Ther. 2012;342:486–496. doi: 10.1124/jpet.112.194233. [DOI] [PubMed] [Google Scholar]
- 193.Yanagisawa T, Yamagishi T, Okada Y. Hyperpolarization induced by K+ channel openers inhibits Ca2+ influx and Ca2+ release in coronary artery. Cardiovascular drugs and therapy / sponsored by the International Society of Cardiovascular Pharmacotherapy. 1993;7(Suppl 3):565–574. doi: 10.1007/BF00877622. [DOI] [PubMed] [Google Scholar]
- 194.Yang Y, Jones AW, Thomas TR, Rubin LJ. Influence of sex, high-fat diet, and exercise training on potassium currents of swine coronary smooth muscle. Am J Physiol Heart Circ Physiol. 2007;293:H1553–1563. doi: 10.1152/ajpheart.00151.2007. [DOI] [PubMed] [Google Scholar]
- 195.Yu H, Wu M, Townsend SD, Zou B, Long S, Daniels JS, McManus OB, Li M, Lindsley CW, Hopkins CR. Discovery, Synthesis, and Structure Activity Relationship of a Series of N-Aryl- bicyclo[2.2.1]heptane-2-carboxamides: Characterization of ML213 as a Novel KCNQ2 and KCNQ4 Potassium Channel Opener. ACS chemical neuroscience. 2011;2:572–577. doi: 10.1021/cn200065b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zagotta WN, Hoshi T, Aldrich RW. Restoration of inactivation in mutants of Shaker potassium channels by a peptide derived from ShB. Science. 1990;250:568–571. doi: 10.1126/science.2122520. [DOI] [PubMed] [Google Scholar]
- 197.Zavaritskaya O, Zhuravleva N, Schleifenbaum J, Gloe T, Devermann L, Kluge R, Mladenov M, Frey M, Gagov H, Fesus G, Gollasch M, Schubert R. Role of KCNQ channels in skeletal muscle arteries and periadventitial vascular dysfunction. Hypertension. 2013;61:151–159. doi: 10.1161/HYPERTENSIONAHA.112.197566. [DOI] [PubMed] [Google Scholar]
- 198.Zeng H, Lozinskaya IM, Lin Z, Willette RN, Brooks DP, Xu X. Mallotoxin is a novel human ether-a-go-go-related gene (hERG) potassium channel activator. J Pharmacol Exp Ther. 2006;319:957–962. doi: 10.1124/jpet.106.110593. [DOI] [PubMed] [Google Scholar]
- 199.Zhang Y, Gao YJ, Zuo J, Lee RM, Janssen LJ. Alteration of arterial smooth muscle potassium channel composition and BKCa current modulation in hypertension. Eur J Pharmacol. 2005;514:111–119. doi: 10.1016/j.ejphar.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 200.Zhong XZ, Abd-Elrahman KS, Liao CH, El-Yazbi AF, Walsh EJ, Walsh MP, Cole WC. Stromatoxin-sensitive, heteromultimeric Kv2.1/Kv9.3 channels contribute to myogenic control of cerebral arterial diameter. J Physiol. 2010;588:4519–4537. doi: 10.1113/jphysiol.2010.196618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhou J, Augelli-Szafran CE, Bradley JA, Chen X, Koci BJ, Volberg WA, Sun Z, Cordes JS. Novel potent human ether-a-go-go-related gene (hERG) potassium channel enhancers and their in vitro antiarrhythmic activity. Mol Pharmacol. 2005;68:876–884. doi: 10.1124/mol.105.014035. [DOI] [PubMed] [Google Scholar]
- 202.Zhou Z, Gong Q, Ye B, Fan Z, Makielski JC, Robertson GA, January CT. Properties of HERG channels stably expressed in HEK 293 cells studied at physiological temperature. Biophys J. 1998;74:230–241. doi: 10.1016/S0006-3495(98)77782-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhou Z, Vorperian VR, Gong Q, Zhang S, January CT. Block of HERG potassium channels by the antihistamine astemizole and its metabolites desmethylastemizole and norastemizole. J Cardiovasc Electrophysiol. 1999;10:836–843. doi: 10.1111/j.1540-8167.1999.tb00264.x. [DOI] [PubMed] [Google Scholar]