Abstract
Osteoarthritis (OA) is a low-grade chronic inflammatory joint disease. Innate immunity contributes to OA progression, mediated by toll-like receptors (TLR2 and TLR4). We evaluated the role of CD44, a transmembrane glycoprotein, in regulating TLR2-linked macrophage activation and resultant proinflammatory responses. TLR2 stimulation was performed on differentiated THP-1 macrophages in the presence or absence of a CD44-specific antibody or hyaluronan (HA). Nuclear factor kappa B (NFκB) nuclear translocation; interleukin-1 beta (IL-1β) and tumor necrosis factor alpha (TNF-α) gene expression and protein concentrations were determined. Anti-CD44 antibody and HA treatments reduced NFκB translocation, IL-1β and TNF-α expression and production (p<0.001). Inhibition of proinflammatory response in macrophages by HA was mediated by CD44. Protein phosphatase 2A (PP2A) mediated the reduction in NFκB translocation by HA. CD44 Knockdown reduced NFκB nuclear translocation and downstream IL-1β and TNF-α protein production following TLR2 receptor stimulation (p<0.001). CD44+/+ murine bone marrow derived macrophages (BMDMs) produced higher TNF-α compared to CD44−/− macrophages following TLR2 stimulation (p<0.01). HA dose-dependently inhibited TLR2 induced TNF-α production by murine BMDMs (p<0.001). OA synovial fluids (SF) stimulated TLR2 and TLR4 receptors and induced NFκB translocation in THP-1 macrophages. Anti-CD44 antibody treatment significantly reduced macrophage activation by OA SF (p<0.01). CD44 regulated TLR2 responses in human macrophages, whereby a reduction in CD44 levels or engagement of CD44 by its ligand (HA) or a CD44-specific antibody reduced NFκB translocation and downstream proinflammatory cytokine production. A CD44-specific antibody reduced macrophage activation by OA synovial fluids and CD44 is a potentially novel target in OA treatment.
Keywords: Toll-like receptor 2, CD44, hyaluronan, macrophages, arthritis
Introduction
Osteoarthritis (OA) is a degenerative joint disease involving joint structures e.g. the articular cartilage, synovium and subchondral bone (1, 2). Risk factors for OA pathogenesis include age, obesity, traumatic joint injury, and low-grade chronic inflammation (2–7). Inflammation of the synovial tissue is recognized as an active contributor to OA pathogenesis with multiple studies showing a correlation between the extent of synovitis and pain, cartilage erosion and disease progression (8–12). The synovium is a soft tissue that is comprised of a surface layer, the intima and an underlying subintima (12, 13). The intima of normal synovium is 1–3 cell layers thick, with two cell types: fibroblast-like synoviocytes and macrophages (13, 14). Chronically inflamed synovium, as seen in OA, is characterized by synovial intimal hyperplasia, immune cell infiltration, subintimal fibrosis and neovascularization (15, 16).
Infiltrating immune cells detected most frequently in OA synovial tissues include macrophages, T cells and to a lesser extent mast cells and B cells (16–18). Synovial macrophages play an important role in driving OA pathogenesis due to their significantly higher production of proinflammatory cytokines e.g. interleukin-1 beta (IL-1β) and tumor necrosis factor alpha (TNF-α) compared to fibroblast-like synoviocytes (19, 20). Activation of synovial macrophages was shown to promote synovial thickening, osteophyte formation and cartilage degeneration in experimental OA models (21, 22). As a component of the innate immune system, macrophages can be activated by damage-associated molecular patterns (DAMPs) through interaction with pattern recognition receptors, e.g. toll-like receptors (TLRs) on the surface of macrophages (23). Examples of DAMPs in OA include extracellular matrix breakdown products e.g. biglycan, fibronectin fragments, low molecular weight hyaluronic acid, plasma proteins e.g. α1 and α2 macroglobulins, intracellular alarmins e.g. high mobility group box 1 (HMGB1) and crystals e.g. uric acid (24).
Hyaluronan (HA) is produced by fibroblast-like synoviocytes and is a major component of the synovial fluid. HA exerts important functions in the joint and its biological effects are mediated by its binding to its transmembrane receptor, cluster determinant 44 (CD44) (25). In OA synoviocytes, HA suppresses IL-1β mediated nuclear factor kappa B (NFκB) activation and downstream expression of matrix metalloproteinases (MMPs) in a CD44-dependent manner (26, 27). Additionally, HA’s anti-inflammatory effects may be mediated by its ability to modulate TLR2 and TLR4 cartilage expression in experimental arthritis (28).
In this work, we aimed to evaluate the role of CD44 in regulating NFκB activation and proinflammatory cytokine production in response to TLR2 receptor activation in human macrophages, using a combination of CD44 receptor knockdown, TNF-α production by macrophages from CD44 wildtype and knockout mice, and CD44 receptor engagement by CD44 neutralizing antibody and HA treatments. We hypothesized that CD44 functions to regulate NFκB activation and downstream IL-1β and TNF-α gene expression and production in response to TLR2 stimulation of a human macrophage cell line. Finally, we studied TLR2 and TLR4 receptor activation by OA synovial fluids and examined the effect of CD44 targeting in suppressing the activation of macrophages by these fluids.
Materials and Methods
Differentiation of THP-1 monocytes into macrophages
THP-1 monocytes were obtained from American Type Culture Collection (ATCC, USA). Cells were cultured to a density of 1.5 × 106 cells/mL in 75 cm flask in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 10mM HEPES, 2mM glutamine, 100U/L penicillin and 100μg/mL streptomycin and maintained at 37°C under 5% CO2. In sterile 12 well plates (Corning, Sigma Aldrich, USA), 600,000 cells in 2 ml RPMI 1640 media were differentiated into macrophages by incubation with phorbol 12-myristate-13-acetate (PMA; Sigma Aldrich) to a final concentration of 5 ng/ml for 48 hours (29). Subsequently, media supernatants were removed and wells were washed three times with sterile PBS to remove unattached cells and new RPMI 1640 media was added.
CD44, TLR2 and TLR4 expression in THP-1 macrophages
THP-1 macrophages were incubated with CD44-specific, TLR2-specific or TLR4-specific antibody (Abcam, USA) at a final concentration of 1μg per 600,000 cells for 1 hour at 4°C. Cells were subsequently pelleted and the cell pellet was washed three times with PBS. THP-1 cells were subsequently incubated with DyLight® 488 goat anti-mouse IgG (Abcam) at 1:500 dilution for 30 min at 4°C. Following cell pelleting and washing, 500 μL of 4% paraformaldehyde was added and cell-associated fluorescence was determined by flow cytometry using BD FACSVerse (BD Biosciences, USA).
Impact of TLR2 and TLR4 receptor activation on IL-1β and TNF-α production by THP-1 macrophages
THP-1 macrophages (600,000 cells per well) were treated with Pam3CSK4 (Pam; Invivogen, USA), a TLR2 ligand (30), at a concentration range between 0.1 and 10 ng/ml for 24 hours. Similarly, THP-1 macrophages (600,000 cells per well) were treated with lipopolysaccharide (LPS; Invivogen, USA), a TLR4 ligand, at a concentration range between 0.1 and 10 ng/ml for 24 hours. Media supernatants were collected and IL-1β and TNF-α concentrations were determined using commercially available ELISA kits (R&D systems, USA).
HA binding to recombinant human CD44 and TLR2 receptors
High-binding microtiter plates (Corning, Sigma Aldrich) were used to coat recombinant human CD44 Fc chimeric protein or recombinant human TLR2 (R&D systems) overnight at 4°C. Each well received 100μL of CD44 or TLR2 protein (1 ng/ml each) in PBS. Wells that were coated with CD44 or TLR2 were blocked with 2% bovine serum albumin (BSA; 300μL per well) for 2 hours at room temperature. Biotinylated HA (MW 1,500 kDa, Creative PEGWorks, USA) was added to the plate in serial dilutions and incubated for 1 hour at room temperature. Following washing with PBS+0.1% Tween 20, streptavidin-HRP (R&D systems) was added at 1:10,000 dilution and incubated for 1 hour at room temperature. Following washing with PBS+0.1% tween 20, the assay was developed using 1-step Turbo TMB ELISA reagent (Lifetechnologies, USA) and the absorbance was measured at 450 nm. Data is presented as percent maximal binding. Data represents the mean ± standard deviation (S.D.) of three independent experiments with duplicate wells per group.
Impact of HA and CD44-specific antibody treatments on TLR2 ligand induced nuclear factor kappa B (NFκB) nuclear translocation in THP-1 monocytes and macrophages
THP1-XBlue cells (Invivogen, USA) is a THP-1 monocyte cell line that stably expresses AP-1 and NFκB inducible secreted embryonic alkaline phosphatase (SEAP) reporter gene. Upon activation of TLR2, SEAP is secreted and its activity can be monitored using an alkaline phosphatase substrate (31). THP1-XBlue cells were cultured in RPMI 1640 media supplemented with 4.5 g/L glucose, 10% FBS, 50 U/mL penicillin, 50 μg/mL streptomycin, 100 μg/mL normocin and 2 mM L-glutamine.
THP1-XBlue cells (50,000 cells per well in HEK detection media) (100 μL per well) were treated with Pam (5 ng/ml) in the absence or presence of HA (MW >950 kDa; R&D systems) at a final concentration of 250 and 500 μg/ml, an anti-CD44 antibody (2.5 μg/ml; Abcam) or an isotype control antibody (2.5 μg/ml; Abcam) for 24 hours followed by measuring the 630 nm absorbance.
THP-1 macrophages (600,000 cells per well) were treated with Pam (5 ng/ml) in the absence or presence of HA (100, 250 and 500 μg/ml) for 1 hour followed by cell harvesting and nuclear protein extraction using a commercially available kit (Thermo Fisher Scientific, USA). Protein content in the nuclear extract was quantified in all experimental groups and a total of 3 μg of total protein was used to quantify NFκB p65 subunit nuclear levels using a DNA binding ELISA assay (Abcam). In another set of experiments, THP-1 macrophages were pre-treated with okadaic acid (5nM; Tocris Biosciences, USA), a potent inhibitor of protein phosphatase 2A, for 2 hours followed by treatment with Pam (5ng/ml) in the absence or presence of HA (250 μg/ml) for 1 hour followed by nuclear NFκB p65 subunit quantification as described above. Data is presented as detectable nuclear p65 levels normalized to control. Data represents the mean ± S.D. of three independent experiments with duplicate wells per treatment.
Impact of HA and CD44-specific antibody treatments on TLR2 ligand induced IL-1β and TNF-α gene expression and production in THP-1 macrophages
THP-1 macrophages (600,000 cells per well) were treated with Pam (1 ng/ml) in the absence or presence of HA (100, 500 and 1,000 μg/ml) for 6 hours followed by RNA extraction using TRIzol reagent (Thermo Fisher Scientific) and RNA concentrations were determined using a NanoDrop ND-2000 spectrophotometer (NanoDrop Technologies, USA). cDNA was synthesized using Transcriptor First Strand cDNA Synthesis Kit (Roche, USA). Quantitative PCR (qPCR) was performed on Applied Biosystems StepOnePlus Real-Time PCR System (Thermo Fisher Scientific) using TaqMan Fast Advanced Master Mix (Life Technologies). The cycle threshold (Ct) value of IL-1β (Hs00174097_m1; Thermo Fisher Scientific) was normalized to the Ct value of GAPDH (Hs02758991_g1; Thermo Fisher Scientific) in the same sample, and the relative expression was calculated using the 2−ΔΔCt method (32). Data is presented as fold target gene expression compared to untreated control.
In another set of experiments, THP-1 macrophages (600,000 cells per well) were treated with Pam (5ng/ml) in the absence or presence of HA (100, 500 and 1,000 μg/ml), anti-CD44 antibody (2.5 μg/ml) or isotype control antibody (2.5 μg/ml) for 24 hours. IL-1β and TNF-α media concentrations were determined using commercially available ELISA kits.
Impact of HA treatment on TLR2 ligand induced CD44 expression in THP-1 macrophages
THP-1 macrophages (600,000 cells per well) were treated with Pam (5 ng/ml) in the absence or presence of HA (100, 500 and 1,000 μg/ml) for 6 hours followed by RNA isolation, cDNA synthesis and qPCR as described above. The Ct value of CD44 (Hs01075864_m1; Thermo Fisher Scientific) was normalized to the Ct value of GAPDH in the same sample, and the relative expression was determined as described above. Data is presented as fold CD44 expression compared to untreated control.
In another set of experiments, THP-1 macrophages were treated as described above for 24 hours followed by cell harvest. THP-1 macrophages were incubated with anti-CD44 antibody (1μg per 600,000 cells) for 1 hour at 4°C. Cells were subsequently pelleted and the cell pellet was washed three times with PBS. THP-1 cells were subsequently incubated with DyLight® 488 goat anti-mouse IgG at 1:500 dilution for 30 min at 4°C. Following cell pelleting and washing, cell-associated fluorescence was determined as described above.
CD44 receptor knockdown and associated TLR2 ligand induced IL-1β and TNF-α production in THP-1 macrophages and role of HA
THP-1 macrophages (600,000 cells per well) were treated with a pre-validated CD44 small interfering RNA (siRNA) (Thermo Fisher Scientific) (25 pmoles per well) or a non-targeted negative control (NC) siRNA (25pmoles) (Thermo Fisher Scientific) for 48 hours. Transfection was performed using Lipofectamine RNAiMax (Thermo Fisher Scientific) per manufacturer’s recommendations. To confirm CD44 knockdown, CD44 gene expression was determined in CD44 siRNA and NC siRNA-treated THP-1 macrophages as described above and compared to CD44 expression in untreated control macrophages. Additionally, CD44 and TLR2 protein levels in CD44 siRNA, NC siRNA-treated and untreated control THP-1 macrophages were determined using flow cytometry as described above.
NFκB p65 subunit nuclear levels in CD44 siRNA, NC siRNA-treated and untreated control THP-1 macrophages (600,000 cells per well) following treatment with Pam (5ng/ml) for 1 hour were determined as described above. CD44 siRNA, NC siRNA-treated and untreated control THP-1 macrophages (600,000 cells per well) were treated with Pam (5ng/ml) for 24 hours. In another set of experiments, Pam treatment was performed in the absence or presence of HA (500 μg/mL). IL-1β and TNF-α media concentrations were determined as described above.
TLR2 ligand induced TNF-α production in murine bone marrow derived macrophages from CD44+/+ and CD44−/− mice and effect of HA treatment on primary murine macrophages
CD44−/− (JAX stock # 005085) and CD44+/+ (JAX stock # 00664) pathogen-free male mice (n=8 in each group) were acquired from the Jackson Laboratory (Maine, USA) (33). Animals (10–14 weeks old) were euthanized and isolation and culture of bone marrow derived macrophages (BMDMs) was performed as previously described (34). Both femurs and tibia bones were carefully dissected and bone marrows were flushed using a sterile 25G needle filled with DMEM/F12 medium (Fisher Scientific) supplemented with 10ng/ml macrophage colony-stimulating factor (M-CSF; R&D systems). Cells were subsequently cultured for 7 days with media change on day 3. On day 7, BMDMs were gently scraped and dislodged using Corning Cellstripper Solution (VWR, USA) for 5 min at 37°C. An equal volume of macrophage complete medium was added to the cells and the cells were centrifuged for 10 min at 400 × g and 4°C, and the supernatant was discarded. Subsequently, BMDMs were plated to perform TLR2 stimulation studies or to characterize surface markers Cd11b and F4/80 expression using flow cytometry.
BMDMs (400,000 cells) were treated with FITC-labeled anti-CD11b antibody (abcam) (1:500 dilution), PE-Texas Red-labeled anti- F4/80 antibody (ThermoFisher Scientific) (1:500 dilution) or FITC-labeled Rat IgG2b, kappa monoclonal - Isotype control (Abcam) for 1 hr at 37°C. Cells were subsequently pelleted and the cell pellet was washed three times with PBS. Following cell pelleting and washing, 500 μL of 4% paraformaldehyde were added and cell-associated fluorescence was determined by flow cytometry.
BMDMs from both genotypes were plated overnight in 12 well plates (200,000 cells per well). Cells were treated with Pam (1 ng/ml or 5 ng/ml) for 6 hours at 37°C and TNF-α media concentrations were determined using a commercially available ELISA (R&D Systems). In another set of experiments, CD44+/+ BMDMs were treated with Pam (5 ng/ml) in the absence or presence of HA (100, 250 and 500 μg/ml) for 6 hours at 37°C and TNF-α media concentrations were determined as above. Data is presented as the mean TNF-α concentrations ± S.D. of 4 independent experiments, with triplicate wells per treatment. All animal tissue harvests were approved by the IACUC committee at Chapman University.
Activation of TLR2 and TLR4 receptors by synovial fluids from patients with OA and the role of CD44 in regulating macrophage activation in response to OA synovial fluids
Synovial fluid aspirates (SF) were collected from patients with OA (n=12) (Articular Engineering, USA) following knee replacement surgery or from donors within 24 hours of death, collected with partner site IRB approval with informed written consent from the donor or nearest relative. A total of 6 patients were female. The median age (interquartile range) of the group was 71 (60 to 82). Normal SF specimens (n=4) were obtained from subjects with no clinical history of joint disease or arthritis, and were provided by Dr. Martin Lotz from the Scripps Research Institute, USA. Screening of OA SF specimens for activation of TLR2 and TLR4 receptors was performed by incubation with TLR2-HEK and TLR4-HEK cells (25,000 cells per well in HEK Blue detection media) (Invivogen). The volume of SF aspirates was 7.5μL per well, corresponding to 3.75% dilution. The final volume in each well was 200μL. Activation of TLR2 or TLR4 in these cells results in nuclear translocation of NFκB and expression of SEAP, whose activity can be detected in the culture media. Cells were incubated with the SF samples for 48 hours at 37°C. The 630 nm absorbance was measured and normalized to the 630 nm absorbance values of untreated control cells.
THP1-XBlue monocytes (25,000 cells per well) were maintained in serum-free RPMI 1640 for 48 hours followed by differentiation of the THP-1 monocytes into macrophages as described above. SF aspirates with detectable TLR2 and TLR 4 activity were incubated with THP1-XBlue macrophages for 48 hours in HEK Blue detection media in the absence or presence a CD44-specific antibody (2.5μg/ml), TLR2-specific antibody (Abcam; 2.5 μg/ml) or isotype control (2.5 μg/ml). Normal SF specimens were used as controls. The 630 nm absorbance was subsequently measured. Data is presented as the mean 630 nm absorbance value of each SF specimen, based on two independent experiments with duplicate wells per experiment.
Statistical Analyses
Unless otherwise specified, data is presented as the mean ± S.D. of 4 independent experiments with at least duplicate wells per group. Continuous variables were tested for normality and equal variances. Variables that satisfied both assumptions were tested for statistical significance using Student’s t-test for two group comparisons. Multiple group comparisons were performed using analysis of variance (ANOVA) with Tukey’s post-hoc test. Variables that did not satisfy the normality assumption were tested using Mann-Whitney U test or ANOVA on the ranks. The level of statistical significance was a priori set at α=0.05.
Results
CD44 and TLR receptor expression in human THP-1 macrophages and proinflammatory cytokine production
A representative flow cytometry histogram depicting cell-associated fluorescence of THP-1 macrophages following probing with CD44, TLR2 or TLR4 antibodies is shown in figure 1A. Compared to unstained cells, cell associated fluorescence for CD44 and TLR2 exhibited a right shift indicating expression of CD44 and TLR2 by THP-1 macrophages. On the contrary, cell associated fluorescence for TLR4 was not qualitatively different from unstained control cells. TLR2 receptor activation resulted in a concentration-dependent elevation in media IL-1β (fig. 1B) and TNF-α (fig. 1C) concentrations. Similarly, TLR4 receptor activation resulted in detectable IL-1β and TNF-α levels. Across all ligand treatments, TLR2 activation resulted in a significantly higher (p<0.001) IL-1β and TNF-α production compared to TLR4 activation.
Fig. 1.

Toll-like receptors 2 and 4 (TLR2 and TLR4) and CD44 receptor expression in THP-1 macrophages and impact of TLR receptor stimulation on proinflammatory cytokine production. Data represents the mean ± standard deviation of 4 independent experiments. *p<0.001
A) A representative flow cytometry histogram of human THP-1 macrophages demonstrating enhanced TLR2 and CD44 receptor expression, compared to TLR4.
B) Impact of Pam3CSK4 (Pam; TLR2 ligand) and Lipopolysaccharide (LPS; TLR4 ligand) treatments on interleukin-1 beta (IL-1β) production in THP-1 macrophages.
C) Impact of Pam3CSK4 and LPS treatment on tumor necrosis factor alpha (TNF-α) production in THP-1 macrophages.
HA and CD44-specific antibody treatments reduced TLR2 ligand induced NFκB nuclear translocation in THP-1 monocytes and macrophages
HA binding to immobilized TLR2 and CD44 receptors is shown in figure 2A. HA exhibited concentration-dependent binding to CD44 with no detectable binding to TLR2. Pam treatment resulted in NFκB nuclear translocation in THP-1 monocytes compared to untreated cells (p<0.001) (fig. 2B). HA (250 and 500 μg/mL) treatment reduced NFκB nuclear translocation in THP-1 monocytes (p<0.01; p<0.001) following TLR2 receptor activation. Similarly, Anti-CD44 antibody treatment reduced NFκB nuclear translocation in THP-1 monocytes (p<0.001). In contrast, isotype control antibody treatment did not alter NFκB nuclear translocation. There was no difference in the magnitude of reduction of NFκB nuclear translocation in THP-1 monocytes with HA (500 μg/mL) or a CD44 antibody treatments.
Fig. 2.
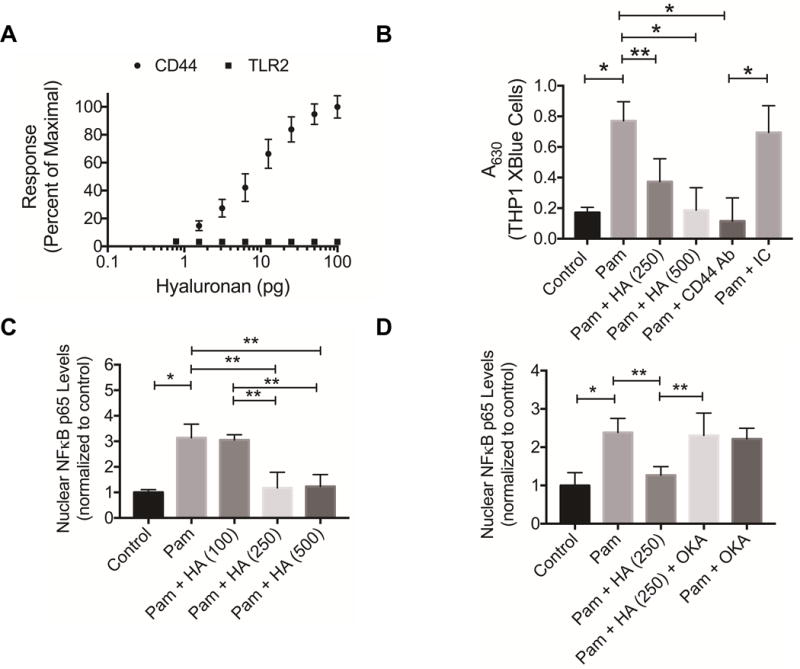
Binding of hyaluronan (HA) to recombinant human CD44 and TLR2 receptors and impact of HA treatment on TLR2 receptor stimulated nuclear translocation of nuclear factor kappa B (NFκB). TLR2 receptor stimulation was performed using Pam3CSK4 (Pam; 5 ng/mL).
A) Concentration-dependent binding of HA to immobilized CD44 or TLR2 receptors using a microplate assay format. HA exhibited a concentration-dependent binding to CD44 receptor and no significant binding to TLR2 receptor.
B) Impact of HA treatment (250 and 500 μg/mL), a CD44 antibody (CD44 Ab 2.5 μg/mL) or an isotype control (IC; 2.5 μg/mL) on TLR2 induced NFκB nuclear translocation in THP1 XBlue monocytes. HA and CD44 Ab treatments reduced TLR2 induced NFκB nuclear translocation. Data represents the mean ± standard deviation of 4 independent experiments. *p<0.001; **p<0.01.
C) Impact of HA treatment (100, 250 and 500 μg/mL) on TLR2 induced NFκB p65 subunit nuclear translocation in THP-1 macrophages. HA (250 and 500 μg/mL) treatments reduced NFκB nuclear translocation in THP-1 macrophages. Data represents the mean ± standard deviation of 4 independent experiments. *p<0.001; **p<0.01.
D) Protein phosphatase 2A (PP2A) mediates HA’s inhibition of NFκB p65 subunit nuclear translocation in THP-1 macrophages. Okadaic acid (OKA; 5 nM), a potent inhibitor of PP2A, abolished the inhibitory effect of HA (250 μg/mL) on NFκB p65 subunit translocation in THP-1 macrophages. Data represents the mean ± standard deviation of 4 independent experiments. *p<0.001; **p<0.01.
TLR2 receptor activation resulted in NFκB p65 subunit nuclear translocation in THP-1 macrophages compared to untreated cells (p<0.001) (fig. 2C). HA (250 and 500 μg/mL) treatments reduced p65 subunit translocation (p<0.01) in THP-1 macrophages following TLR2 receptor activation. Okadaic acid treatment did not alter NFκB p65 subunit translocation following TLR2 receptor activation (fig. 2D). NFκB p65 subunit nuclear levels were significantly higher in the Pam + HA + Okadiac acid group compared to Pam + HA group (p<0.01).
HA and CD44-specific antibody treatments reduced TLR2 ligand stimulated proinflammatory cytokine expression and production in THP-1 macrophages
TLR2 activation induced IL-1β gene expression and production in THP-1 macrophages (p<0.001) (fig. 3A and fig. 3C). HA (500 and 1,000 μg/mL) treatment reduced IL-1β gene expression in THP-1 macrophages following TLR2 activation (p<0.05; p<0.01) (fig. 3A). Correspondingly, HA (500 and 1,000 μg/mL) treatment reduced IL-1β production by THP-1 macrophages (p<0.001) (fig. 3C). TLR2 activation induced TNF-α gene expression and production in THP-1 macrophages (p<0.001) (fig. 3B and fig. 3D). HA (500 and 1,000 μg/mL) treatment reduced TNF-α gene expression in THP-1 macrophages following TLR2 activation (p<0.01) (fig. 3B). Similarly, HA (500 and 1,000 μg/mL) treatment reduced TNF-α production by THP-1 macrophages (p<0.001) (fig. 3D). CD44 antibody treatment reduced IL-1β and TNF-α media supernatant concentrations following TLR2 activation (p<0.001) (Fig. 3E and fig. 3F). In contrast, IL-1β and TNF-α media concentrations were not significantly different between Pam alone and Pam + isotype control (IC) antibody groups.
Fig. 3.

Impact of hyaluronan (HA) (100, 500 and 1,000 μg/mL), CD44 antibody (CD44 Ab; 2.5 μg/mL) or isotype control (IC; 2.5 μg/mL) treatments on TLR2 receptor induced interleukin-1 beta (IL-1β) or tumor necrosis factor alpha (TNFα) gene expression and production in THP-1 macrophages. Data represents the mean ± standard deviation of 4 independent experiments. *p<0.001; **p<0.01; ***p<0.05.
A) HA (500 and 1,000 μg/mL) treatments reduced TLR2 induced IL-1β gene expression.
B) HA (500 and 1,000 μg/mL) treatments reduced TLR2 induced TNF-α gene expression.
C) HA (500 and 1,000 μg/mL) treatments reduced TLR2 induced IL-1β production.
D) HA (500 and 1,000 μg/mL) treatments reduced TLR2 induced TNF-α production.
E) CD44 Ab treatment reduced TLR2 induced IL-1β production.
F) CD44 Ab treatment reduced TLR2 induced TNF-α production.
HA reduced CD44 expression following TLR2 receptor activation in THP-1 macrophages
TLR2 activation induced CD44 gene expression in THP-1 macrophages (p<0.001) (fig. 4A). HA (100, 500 and 1,000 μg/mL) treatment reduced CD44 expression in THP-1 macrophages following TLR2 activation (p<0.01). A dose-response for HA treatment was observed as CD44 expression in the Pam + HA (1,000 μg/mL) group was significantly lower than CD44 gene expression in the Pam + HA (500 μg/mL) and Pam + HA (100 μg/mL) (p<0.01; p<0.001). A representative flow cytometry histogram depicting cell-associated fluorescence of THP-1 macrophages following probing with CD44 antibody is presented in figure 4B. TLR2 activation resulted in a right shift of the cell population indicating increased CD44 protein levels on macrophages. HA treatments showed a qualitative reduction in mean cell associated fluorescence, indicating a reduction in CD44 receptor levels on macrophages. Semi-quantitative analysis of mean cell-associated fluorescence is shown in figure 4C. Mean fluorescence in the Pam alone group was significantly higher compared to untreated control group (p<0.001). Mean cell-associated fluorescence in the Pam + HA (1,000 μg/mL) and Pam + HA (500 μg/mL) were significantly lower than in the Pam group (p<0.001). Similarly, mean cell-associated fluorescence in the Pam + HA (1,000 μg/mL) and Pam + HA (500 μg/mL) were significantly lower than in the Pam + HA (100 μg/mL) group (p<0.01).
Fig. 4.
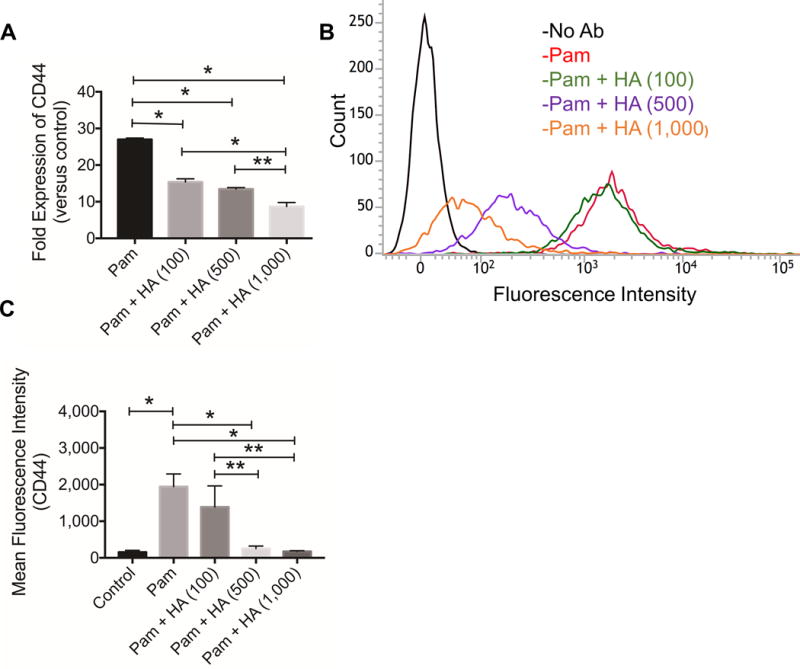
Impact of toll-like receptor 2 (TLR2) receptor stimulation on CD44 gene expression and CD44 levels in THP-1 macrophages and the role of hyaluronan (HA). TLR2 receptor stimulation was performed using Pam3CSK4 (Pam; 5ng/mL) for 24 hours. Data represents the mean ± standard deviation of 4 independent experiments. *p<0.001; **p<0.01.
A) HA (100, 500 and 1,000 μg/mL) treatments reduced TLR2 induced CD44 gene expression in THP-1 macrophages.
B) A representative flow cytometry histogram demonstrating increased CD44 protein levels in TLR2 stimulated THP-1 macrophages. HA (500 and 1,000 μg/mL) treatments reduced CD44 levels in TLR2 stimulated THP-1 macrophages.
C) Semi-quantitative analysis of mean fluorescence intensities of CD44 receptor following TLR2 stimulation in the absence or presence of HA (100, 500 or 1,000 μg/mL). TLR2 stimulation increased CD44 protein in THP-1 macrophages and HA (500 and 1,000 μg/mL) treatments reduced CD44 protein.
Role of CD44 in regulating downstream responses of TLR2 activation in THP-1 macrophages
The impact of CD44 silencing on CD44 gene expression and protein is shown in figures 5A and 5B. CD44 silencing was achieved with approximately 85% reduction in CD44 gene expression and 60% reduction in CD44 protein. CD44 knockdown did not alter TLR2 expression in THP-1 macrophages (fig. 5C). CD44 knockdown resulted in a significant reduction in NFκB p65 subunit nuclear levels following TLR2 activation compared to NC siRNA-treated or control THP-1 macrophages (p<0.001) (fig. 5D). CD44 knockdown resulted in a significant reduction in IL-1β and TNF-α production following TLR2 activation compared to NC siRNA-treated or control THP-1 macrophages (p<0.001) (fig. 5E and 5F). There was no significant difference in Pam-stimulated IL-1β or TNF-α media concentrations between NC siRNA-treated and control THP-1 macrophages. HA (500 μg/mL) treatment reduced IL-1β and TNF-α media concentrations following TLR2 receptor activation in NC siRNA treated and control THP-1 macrophages (p<0.001) (fig. 6A and 6B). In contrast, HA (500 μg/mL) treatment did not significantly alter IL-1β and TNF-α media concentrations following TLR2 receptor activation in CD44 siRNA-treated THP-1 macrophages.
Fig. 5.
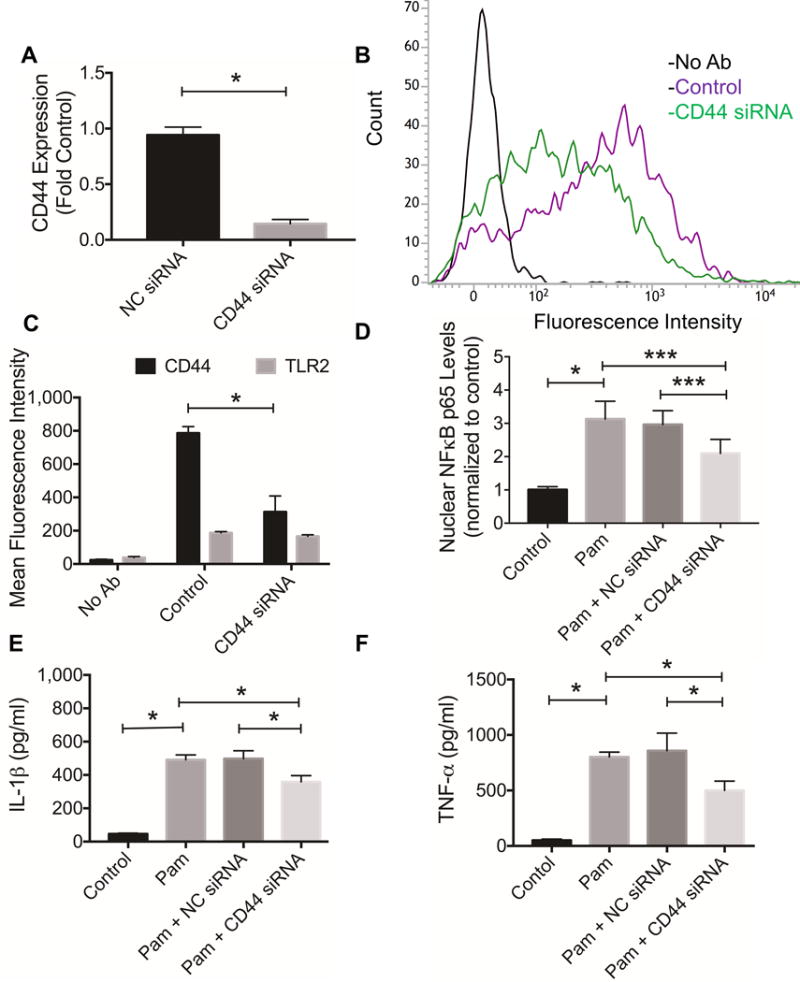
Impact of CD44 receptor knockdown on toll-like receptor 2 (TLR2) receptor stimulated proinflammatory cytokine production in THP-1 macrophages and nuclear factor kappa B (NFκB) p65 subunit nuclear translocation. TLR2 receptor stimulation was performed using Pam3CSK4 (Pam; 5ng/mL). Data represents the mean ± standard deviation of 4 independent experiments. *p<0.001. ***p<0.05.
A) CD44 siRNA treatment resulted in reduced CD44 gene expression compared to negative control (NC) siRNA treatment or control.
B) A representative flow cytometry histogram demonstrating reduced CD44 protein levels in CD44 siRNA-treated THP-1 macrophages compared to control.
C) Semi-quantitative analysis of mean fluorescence intensities of CD44 or TLR2 receptors in THP-1 macrophages following CD44 knockdown. CD44 knockdown resulted in approximately 60% reduction in CD44 levels.
D) CD44 siRNA treatment reduced TLR2 stimulated NFκB p65 subunit nuclear translocation.
E) CD44 siRNA treatment reduced TLR2 stimulated interleukin-1 beta (IL-1β) production in THP-1 macrophages.
F) CD44 siRNA treatment reduced TLR2 stimulated tumor necrosis factor alpha (TNF-α) production in THP-1 macrophages.
Fig. 6.

Impact of hyaluronan (HA) treatment on toll-like receptor 2 (TLR2) induced interleukin-1 beta (IL-1β) and tumor necrosis factor alpha (TNF-α) production in CD44 siRNA-treated, negative control (NC) siRNA-treated and untreated control THP-1 macrophages. TLR2 stimulation was performed using Pam3CSK4 (Pam; 5ng/mL) for 24 hours. HA treatment was performed at 500 μg/mL. Data represents the mean ± standard deviation of 4 independent experiments. *p<0.001; n.s.: not significant.
A) HA treatment reduced TLR2 induced IL-1β production in untreated control and NC siRNA-treated THP-1 macrophages. HA treatment did not alter IL-1β levels in CD44 siRNA-treated macrophages.
B) HA treatment reduced TLR2 induced TNF-α production in untreated control and NC siRNA-treated THP-1 macrophages. HA treatment did not alter TNF-α levels in CD44 siRNA-treated macrophages.
CD44 modulated TLR2 ligand-induced TNF-α production by murine BMDMs and the HA treatment suppressed TLR2 ligand activation of primary murine BMDMs
TNF-α production by murine BMDMs from CD44−/− and CD44+/+ mice in response to TLR2 receptor activation and the impact of HA treatment is shown in figure 7. A representative flow cytometry scatterplot showing CD11b and F4/80 probing of murine BMDMs is presented in figure 7A. Murine BMDMs exhibited strong positive staining for both CD11b and F4/80 epitopes, with typically 90% or more of the cell population positive for both surface markers. Using two-way ANOVA, we identified a significant interaction between CD44 genotype and Pam concentrations (p=0.0035). TNF-α media concentrations in the Pam (1ng/ml and 5 ng/ml) treated CD44+/+ BMDMs were significantly higher compared to Pam-treated CD44−/− BMDMs (fig. 7B) (p<0.001; p<0.01). The mean TNF-α media concentration in the Pam (1ng/ml) treated CD44−/− BMDMs group was approximately 67% lower than corresponding mean TNF-α media concentrations in the Pam-treated CD44+/+ BMDMs. Similarly, the mean TNF-α media concentration in the Pam (5ng/ml) treated CD44−/− BMDMs group was approximately 62% lower than the corresponding mean TNF-α media concentration in the Pam-treated CD44+/+ BMDMs. In CD44+/+ BMDMs, TNF-α concentrations in the Pam (1 and 5ng/ml) groups were significantly higher than control group (p<0.001). Additionally, TNF-α concentration in the Pam (5ng/ml) group was significantly higher than the Pam (1ng/ml) group (p<0.001). In CD44−/− BMDMs, TNF-α concentrations in the Pam (1ng/ml) group was not significantly different from control CD44−/− BMDMs (p=0.1207). Furthermore, TNF-α concentration in the Pam (5ng/ml) group was significantly higher than the Pam (1ng/ml) group (p<0.001).
Fig. 7.

Impact of toll-like receptor 2 (TLR2) receptor stimulation on tumor necrosis factor alpha (TNF-α) production by CD44+/+ and CD44−/− murine bone marrow derived macrophages (BMDMs) and dose-dependent effect of HA on CD44+/+ BMDMs. TLR2 stimulation was performed using Pam3CSK4 (Pam; 5ng/mL) for 6 hours. Data represents the mean ± standard deviation of 4 independent experiments. *p<0.001; **p<0.01.
A) A representative flow cytometry histogram of murine BMDMs showing positive staining for CD11b and F4/80 surface markers.
B) TLR2 receptor activation dose-dependently increased TNF-α production by murine CD44+/+ BMDMs compared to CD44−/− BMDMs.
C) HA (250 and 500 μg/mL) treatments reduced TLR2 induced TNF-α production by murine CD44+/+ BMDMs.
The impact of HA treatment on TLR2 ligand induced TNF-α production in CD44+/+ murine BMDMs is shown in figure 7C. TNF-α media concentrations in the Pam + HA (100 μg/ml) group were not significantly different from TNF-α media concentrations in the Pam alone group. TNF-α media concentrations in the Pam + HA (250 μg/ml) group were significantly lower than TNF-α media concentrations in the Pam alone and Pam + HA (100 μg/ml) groups (p<0.001). Similarly, TNF-α media concentrations in the Pam + HA (250 μg/ml) group were significantly lower than TNF-α media concentrations in the Pam alone and Pam + HA (100 μg/ml) groups (p<0.001). HA (500 μg/ml) alone treatment did not alter TNF-α production compared to untreated control cells.
OA SF specimens activated TLR2 and TLR4 receptors and a CD44-specific antibody reduced activation of THP-1 macrophages by OA SF specimens
Activation of TLR2 and TLR4 receptors by OA SF specimens is shown in figure 8A. A total of 8 samples demonstrated significant activation of TLR2 and TLR4 receptors (p<0.001). In contrast, 3 specimens did not exhibit activation of TLR2 or TLR4 and one sample activated TLR2 receptor but not the TLR4 receptor. OA SF specimens that activated TLR2 and TLR4 receptors also significantly stimulated NFκB nuclear translocation in THP-1 macrophages compared to normal SF specimens (p<0.001) (fig. 8B). TLR2 neutralizing antibody treatment significantly reduced OA SF activation of THP-1 human macrophages (p<0.01). Similarly, CD44 antibody treatment significantly reduced OA SF activation of THP-1 human macrophages (p<0.01).
Fig. 8.
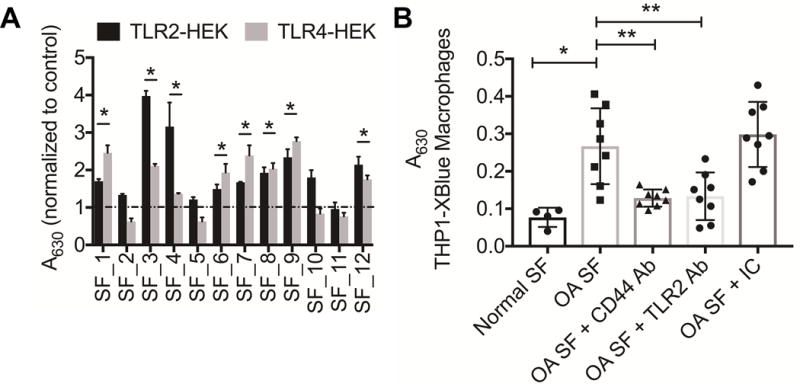
Activation of toll-like receptors 2 and (TLR2 and TLR4) by synovial fluid (SF) aspirates from patients with advanced osteoarthritis (OA) and role of CD44 in modulating THP-1 macrophages activation by OA SF. OA SF (3.75μL per well) were incubated with TLR2-HEK or TLR4-HEK cells in HEK detection media for 48 hours followed by measuring the 630 nm absorbance. THP-1XBlue macrophages were incubated with OA SF in the absence or presence of a TLR2-specific, CD44-specific, or isotype control (IC) antibody (2.5μg/mL) for 48 hours followed by measuring 630 nm absorbance. Normal SF specimens were used as controls. *p<0.001; **p<0.01.
A) Activation of TLR2 and TLR4 receptors by OA SF (n=12). A total of 8 samples activated both TLR2 and TLR4 receptors, compared to untreated controls.
B) Activation of THP-1XBlue macrophages by OA SF (n=8) and the impact of CD44-specific or TLR2-specific antibody treatments. CD44 and TLR2 antibody treatments reduced OA SF induced macrophage activation.
Discussion
The TLR family comprises ten functional receptor subtypes with TLR 1–7 and 9 being detected in the synovial tissues of patients with OA (7, 15, 35, 36). A role for TLRs, specifically TLR2 and TLR4, in the pathogenesis of OA has been suggested (37, 38). Progressive OA was associated with expression of TLR2 in cartilage and chondrocytes derived from a TLR2/TLR4 double knockout mouse showed attenuated matrix metalloproteinase-13 (MMP-13) expression in response to TLR2 stimulation (37, 38). Furthermore, evidence of macrophage activation in the synovial lining was recently reported and the extent of synovial macrophage activation was shown to be associated with OA severity and joint pain (39). In this work, we have examined macrophage activation in response to TLR2 and TLR4 activation. TLR2 and TLR4 ligands induced gene expression and production of IL-1β and TNF-α in a concentration-dependent manner. TLR2 activation produced higher levels of IL-1β and TNF-α compared to TLR4 across all ligand concentrations utilized. This may be due to the relative level of TLR2 and TLR4 expression on the surface of the macrophages. Using flow cytometry, we have detected increased TLR2 expression compared to TLR4. This finding is in line with other work that demonstrated enhanced TLR2 expression on THP-1 macrophages (40). TLR2 activation resulted in NFκB nuclear translocation in a monocyte NFκB reporter assay. In macrophages, TLR2 ligands induced the nuclear translocation of NFκB p65 subunit. In addition to the induction of proinflammatory cytokines, TLR2 activation significantly increased CD44 gene expression and increased CD44 protein levels.
CD44 is a transmembrane receptor, with various isoforms generated by extensive alternative splicing and post-translational modifications (41). In addition to its established role in mediating cell adhesion and migration, CD44 receptor has a role in regulating cell signaling pathways, by facilitating signaling protein recruitment and assembly (42). In the context of innate immunity, CD44 was found to regulate Fcγ and complement receptor 3-dependent macrophage phagocytosis (42). Additionally, CD44 may play a role in the negative regulation of TLR receptor activation (43, 44). In this present study, a CD44 antibody treatment reduced NFκB nuclear translocation and downstream IL-1β and TNF-α gene expression and production. Additionally, reducing CD44 expression in human macrophages resulted in a significant attenuation of the latter’s response to TLR2 receptor activation and downstream proinflammatory response. This attenuation is not related to the TLR receptor density on the surface of macrophages, as CD44 receptor knockdown did not modify TLR2 receptor density on THP-1 macrophages. The regulatory role of CD44 is further highlighted by a strongly attenuated proinflammatory response following TLR2 receptor stimulation of primary macrophages derived from CD44 knockout mice compared to macrophages derived from CD44 wildtype animals.
Protein phosphatase-2A (PP2A) is an abundant intracellular serine/threonine phosphatase with key roles in the regulation of many cellular functions including cellular proliferation and immune responses (45, 46). Inhibition of PP2A by okadaic acid resulted in increased nuclear translocation of NFκB and AP-1 and IL-1β expression in THP-1 macrophages (46). Additionally, CD44 engagement was shown to increase intracellular PP2A (47). In our work, HA suppressed NFκB p65 subunit nuclear translocation in response to TLR2 activation, and that effect was shown to be CD44 dependent as HA failed to demonstrate an anti-inflammatory effect in response to TLR2 activation in macrophages following CD44 knockdown. The suppressive effect of HA was confirmed in primary murine macrophages as HA treatment dose-dependently reduced TNF-α production subsequent to TLR2 receptor stimulation. Furthermore, the effect of HA was mediate by intracellular PP2A activity, as inhibition of PP2A activity reduced the inhibitory effect of HA on NFκB activation. The observed anti-inflammatory activity of HA is not due to a direct interaction between HA and the TLR2 receptor as we did not observe any significant binding of HA to recombinant TLR2 receptor. The biological effect of HA in reducing TLR2 mediated proinflammatory response in macrophages is physiologically relevant. The concentration of HA in normal SF can vary between 2 and 4 mg/ml (48). In OA SF aspirates, the concentration and molecular weight distribution of HA are significantly reduced, and this reduction was associated with TLR2 and TLR4 activation by OA SF aspirates (48, 49). This association may argue for an endogenous homeostatic role for HA in inhibiting synovial macrophage activation in response to TLR receptor stimulation by cartilage matrix degradation products.
A majority of the aspirated SF samples from patients with advanced OA that we examined activated TLR2 and TLR4 receptors. This observation is in agreement with previous reports demonstrating activation of TLR2 and TLR4 receptors by OA SF, and augmentation of TLR-mediated responses in OA fibroblast-like synoviocytes by SF from patients with early stage OA (49–51). Interestingly, SF specimens that activated TLR2 and TLR4 receptors induced NFκB nuclear translocation in macrophages, while normal SF specimens treatments failed to activate macrophages. Macrophage TLR2 receptors appeared to mediate NFκB nuclear activation as neutralization of TLR2 abrogated macrophage activation by these SF specimens. CD44 antibody treatment produced a similar effect to TLR2 neutralization, providing further support to the utility of targeting CD44 to suppress macrophage response to DAMPs present in OA SF.
We have demonstrated that CD44 receptor plays a significant role in suppressing TLR2-linked NFκB nuclear translocation and resultant proinflammatory response in macrophages, in a mechanism that involves intracellular PP2A. Neutralization of the CD44 receptor by its ligand hyaluronan, that binds to and is internalized by different cell types including macrophages (25), or by a monoclonal antibody resulted in inhibition of NFκB nuclear translocation. Absence of the CD44 receptor or its knockdown produced a similar effect to receptor neutralization by hyaluronan or an antibody. OA SF activated TLR2 and TLR4 receptors and correspondingly induced NFκB nuclear translocation in a mechanism that involves TLR2. Otherwise, a CD44-specific antibody reduced macrophage activation by these SF specimens. In conclusion, CD44 is a potentially novel target that may act to limit synovial macrophage activation by cartilage matrix degradation products in the joint.
This study expands the role of CD44 in OA pathogenesis. CD44 can bind different types of ligands and in addition to HA, CD44 was shown to bind proteoglycan-4 (PRG4), a major component of synovial fluids (52). CD44 expression was shown to be associated with enhanced proliferation of synoviocytes from patients with OA, rheumatoid arthritis (RA) as well synoviocytes from Prg4−/− mice. Targeting the CD44 receptor by HA or by the recombinant form of PRG4 reduced cytokine-induced OA and RA synoviocyte proliferation. The regulatory role of CD44 in controlling downstream effects of IL-1β was further confirmed by the ability of PRG4 to inhibit IκB phosphorylation, NFκB nuclear translocation and expression of cartilage degrading enzymes by OA synoviocytes in a CD44-dependent mechanism (53). Our findings are limited by the low number of SF aspirates that we have investigated. Furthermore, we did not ascertain the molecular identity of the DAMPs in the SF aspirates that activated TLR receptors and induced NFκB nuclear translocation in THP-1 macrophages.
Acknowledgments
The authors would like to thank Dr. Martin Lotz for providing normal synovial fluids.
Funding: This work is supported by R01AR067748 to KE and GJ.
Footnotes
Conflict of Interest: None
References
- 1.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chem D, Shen J, Zhao W, Wang T, Han L, Hamilton JL, Im HJ. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res. 2017;5:16044. doi: 10.1038/boneres.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Felson DT, Andersen JJ, Naimark A, Walker AM, Meenan RF. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med. 1988:109, 18–24. doi: 10.7326/0003-4819-109-1-18. [DOI] [PubMed] [Google Scholar]
- 4.Thijssen E, van Caam A, van der Kraan PM. Obesity and osteoarthritis, more than just wear and tear: pivotal role for inflamed adipose tissue and dyslipidemia in obesity-induced osteoarthritis. Rheumatology. 2015;54:588–600. doi: 10.1093/rheumatology/keu464. [DOI] [PubMed] [Google Scholar]
- 5.Felson DT, Zhang Y, Hannan MT, Naimark A, Weissman B, Aliabadi P, Levy D. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis Rheum. 1997;40:728–33. doi: 10.1002/art.1780400420. [DOI] [PubMed] [Google Scholar]
- 6.Roos EM. Joint injury causes knee osteoarthritis in young adults. Curr Opin Rheumatol. 2005;17:195–200. doi: 10.1097/01.bor.0000151406.64393.00. [DOI] [PubMed] [Google Scholar]
- 7.Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM, Sokolove J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12:580–92. doi: 10.1038/nrrheum.2016.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill CL, Hunter DJ, Niu J, Clancy M, Guermazi A, Genant H, Gale D, Grainger A, Conaghan P, Felson DT. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2007;66:1599–603. doi: 10.1136/ard.2006.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torres L, Dunlop DD, Peterfy C, Guermazi A, Prasad P, Hayes KW, Song J, Cahue S, Chang A, Marshall M, Sharma L. The relationship between specific tissue lesions and pain severity in persons with knee osteoarthritis. Osteoarthritis Cartilage. 2006;14:1033–40. doi: 10.1016/j.joca.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 10.Ishijima M, Watari T, Naito K, Kanek H, Futami I, Yoshimura-Ishida K, Tomonaga A, Yamaguchi H, Yamamoto T, Nagoka I, Kurosawa H, Poole RA, Kaneko K. Relationships between biomarkers of cartilage, bone, synovial metabolism and knee pain provide insights into the origins of pain in early knee osteoarthritis. Arthritis Res Ther. 2011;13:R22. doi: 10.1186/ar3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roemer FW, Guermazi A, Felson DT, Niu J, Nevitt MC, Crema MD, Lynch JA, Lewis CE, Torner J, Zhang Y. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Ann Rheum Dis. 2011;70:1804–9. doi: 10.1136/ard.2011.150243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathiessen A, Conaghan PG. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther. 2017;19:18–27. doi: 10.1186/s13075-017-1229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith MD, The normal synovium Open Rheumatol J. 2011;5:100–106. doi: 10.2174/1874312901105010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith MD, Barg E, Weedon H, Papengellis V, Smeets T, Tak PP, Kraan M, Coleman M, Ahern MJ. Microarchitecture and protective mechanisms in synovial tissue from clinically and arthroscopically normal knee joints. Ann Rheum Dis. 2003;62:303–7. doi: 10.1136/ard.62.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51:249–257. doi: 10.1016/j.bone.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Lange-Brokaar BJE, Ioan-Fascinay A, van Osch GJ, Zuurmond AM, Schoones J, Toes RE, Huizinga TW, Kloppenburg M. Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthritis Cartilage. 2012;20:1484–1499. doi: 10.1016/j.joca.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 17.Haynes MK, Hume EL, Smith JB. Phenotypic characterization of inflammatory cells from osteoarthritic synovium and synovial fluids. Clin Immunol. 2002;105:315–25. doi: 10.1006/clim.2002.5283. [DOI] [PubMed] [Google Scholar]
- 18.Saito I, Koshino T, Nakashima K, Useugi M, Saito T. Increased cellular infiltrate in inflammatory synovia of osteoarthritic knees. Osteoarthritis Cartilage. 2002;10:156–62. doi: 10.1053/joca.2001.0494. [DOI] [PubMed] [Google Scholar]
- 19.Bondeson J, Wainwright SD, Lauder S, Amos N, Hughes CE. The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases and other destructive and inflammatory responses in osteoarthritis. Arthritis Res Ther. 2006;8:R187–199. doi: 10.1186/ar2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bondeson J, Blom AB, Wainwright SD, Hughes C, Caterson B, van den Berg WB. The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis Rheum. 2010;62:647–657. doi: 10.1002/art.27290. [DOI] [PubMed] [Google Scholar]
- 21.Blom AB, van Lent PL, Libergts S, Holthuysen AE, van der Kraan PM, van Roojien N, van den Berg WB. Crucial role of macrophages in matrix metalloproteinase-mediated cartilage destruction during experimental osteoarthritis: involvement of matrix metalloproteinase 3. Arthritis Rheum. 2007;56:147–57. doi: 10.1002/art.22337. [DOI] [PubMed] [Google Scholar]
- 22.Blom AB, van Lent PL, Holthuysen AE, van der Kraas PM, Roth J, van Roojien N, van den Berg WB. Synovial lining macrophages mediate osteophyte formation during experimental osteoarthritis. Osteoarthritis Cartilage. 2004;12:627–35. doi: 10.1016/j.joca.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Orlowsky EW, Kraus VB. The role of innate immunity in osteoarthritis: when our first line of defense goes on the offensive. J Rheum. 2015;42:363–371. doi: 10.3899/jrheum.140382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. 2013;5:77–94. doi: 10.1177/1759720X12467868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cutly M, Nguyen HA, Underhill CB. The hyaluronan receptor (CD44) participates in the uptake and degradation of hyaluronan. J Cell Biol. 1992;116:1055–62. doi: 10.1083/jcb.116.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kataoka Y, Ariyoshi W, Okinaga T, Kaneuji T, Mitsugi S, Takahashi T, Nishihara T. Mechanisms involved in suppression of ADAMTS4 expression in synoviocytes by high molecular weight hyaluronic acid. Biochem Biophys Res Commun. 2013;432:580–5. doi: 10.1016/j.bbrc.2013.02.043. [DOI] [PubMed] [Google Scholar]
- 27.Wang CT, Lin YT, Chiang BL, Lin YH, Hou SM. High molecular weight hyaluronic acid down-regulated the gene expression of osteoarthritis-associated cytokines and enzymes in fibroblast-like synoviocytes from patients with early osteoarthritis. Osteoarthritis Cartilage. 2006;14:1237–1247. doi: 10.1016/j.joca.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Campo GM, Avenoso A, Nastasi G, Micali A, Prestipino V, Vaccaro M, D’Ascola A, Calatroni A, Campo S. Hyaluronan reduces inflammation in experimental arthritis by modulating TLR-2 and TLR-4 cartilage expression. Biochimica Biophysica Acta. 2011;181:1170–1181. doi: 10.1016/j.bbadis.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Park EK, Jung HS, Yang HI, Yoo MC, Kim C, Kim KS. Optimized THP-1 differentiation is required for the detection of response to weak stimuli. Inflamm Res. 2007;56:45–50. doi: 10.1007/s00011-007-6115-5. [DOI] [PubMed] [Google Scholar]
- 30.Tsolmongyn B, Koide N, Jambalganiin U, Odkhuu E, Naiki Y, Komatsu T, Yoshida T, Yokochi T. A Toll-like receptor 2 ligand, Pam3CSK4, augments interferon-γ-induced nitric oxide production via a physical association between MyD88 and interferon-γ receptor in vascular endothelial cells. Immunology. 2013;140:352–61. doi: 10.1111/imm.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knopick PL, Bradley DS. Detecting immune responses to type III secretion systems. Methods Mol Biol. 2017;1531:165–172. doi: 10.1007/978-1-4939-6649-3_14. [DOI] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.Protin U, Schweighoffer T, Jochum W, Hilberg F. CD44-deficient mice develop normally with changes in subpopulations and recirculation of lymphocyte subsets. J Immunol. 1999;163:4917–23. [PubMed] [Google Scholar]
- 34.Zhang X, Goncalves R, Mosser D. The isolation and characterization of murine macrophages. Curr Protoc Immunol. 2008 doi: 10.1002/0471142735.im1401s83. Chapter: Unit 14.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ozawa T, Koyama K, Ando T, Ohnuma Y, Hatsushika K, Ohba T, Sugiyama H, Hamada Y, Ogawa H, Okumura K, Nakao A. Thymic stromal lymphopoietin secretion of synovial fibroblasts is positively and negatively regulated by Toll-like receptors/nuclear factor-kappa B pathway and interferon-gamma/dexamethasone. Mod Rheumatol. 2007;17:459–502. doi: 10.1007/s10165-007-0620-9. [DOI] [PubMed] [Google Scholar]
- 36.Pazar B, Ea HK, Narayan S, Kolly L, Bagnoud N, Chobaz V, Roger T, Lote F, So A, Busso N. Basic calcium phosphate crystals induce monocyte/macrophageIL-1beta secretion through the NLRP3 inflammasome in vitro. J Immunol. 2011;186:2495–502. doi: 10.4049/jimmunol.1001284. [DOI] [PubMed] [Google Scholar]
- 37.Gomez R, Villalvilla A, Largo R, Gualillo O, Herrero-Beaumont G. TLR4 signaling in osteoarthritis—finding targets for candidate DMOADs. Nat Rev Rheumatol. 2015;11:159–70. doi: 10.1038/nrrheum.2014.209. [DOI] [PubMed] [Google Scholar]
- 38.Scanzello CR, Plaas A, Crow MK. Innate immune system activation in osteoarthritis: is osteoarthritis a chronic wound? Curr Opin Rheumatol. 2008;20:565–72. doi: 10.1097/BOR.0b013e32830aba34. [DOI] [PubMed] [Google Scholar]
- 39.Kraus VB, McDaniel G, Huebner JL, Stabler TV, Pieper CF, Shipes SW, Petry NA, Low PS, Shen J, McNearney TA, Mitchell P. Direct in vivo evidence of activated macrophages in human osteoarthritis. Osteoarthritis Cartilage. 2016;24:1613–1621. doi: 10.1016/j.joca.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parker LC, Whyte M, Vogel SN, Dower SK, Sabroe I. Toll-like receptor (TLR) 2 and TLR4 agonists regulate CCR expression in human monocytic cells. J Immunol. 2004;172:4977–4986. doi: 10.4049/jimmunol.172.8.4977. [DOI] [PubMed] [Google Scholar]
- 41.Ponta H, Sherman L, Herrlich P. CD44: from adhesion molecules to signaling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 42.Amash A, Wang L, Wang Y, Bhakta V, Fairn GD, Hou M, Peng J, Sheffield WP, Lazarus AH. CD44 antibody of macrophage phagocytosis targets Fcγ receptor-and complement receptor 3-dependent mechanisms. J Immunol. 2016;196:3331–3340. doi: 10.4049/jimmunol.1502198. [DOI] [PubMed] [Google Scholar]
- 43.Kawana H, Karaki M, Higashi M, Miyazaki M, Hilberg F, Kitagawa M, Harigaya K. CD44 suppresses TLR-mediated inflammation. J Immunol. 2008;180:4235–4245. doi: 10.4049/jimmunol.180.6.4235. [DOI] [PubMed] [Google Scholar]
- 44.Liang J, Jiang D, Griffith J, Yu S, Fan J, Zhao X, Bucala R, Noble PW. CD44 is a negative regulator of acute pulmonary inflammation and lipopolysaccharide-TLR signaling in mouse macrophages. J Immunol. 2007;178:2469–2475. doi: 10.4049/jimmunol.178.4.2469. [DOI] [PubMed] [Google Scholar]
- 45.Sun L, Li AL, Pham TT, Shanely TP. Study of protein phosphatase 2A (PP2A) activity in LPS-induced tolerance using fluorescence-based and immunoprecipitation-aided methodology. Biomolecules. 2015;5:1284–301. doi: 10.3390/biom5031284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shanely TP, Vasi N, Denenberg A, Wong HR. The serine/threonine phosphatase, PP2A: endogenous regulator of inflammatory cell signaling. J Immunol. 2001;166:966–972. doi: 10.4049/jimmunol.166.2.966. [DOI] [PubMed] [Google Scholar]
- 47.Racine RR, Manalo NA, Hall JM, Dibas A, Raffel GD, Mummert ME. CD44 induced enhancement of phosphatase activity and calcium influx: modifications of EGR-1 expression and cell proliferation. Biochemistry and Biophysics Reports. 2016;6:172–178. doi: 10.1016/j.bbrep.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kosinska MK, Ludwig TE, Liebisch G, Zhang R, Siebert HC, Wilhelm J, Kaesser U, Dettmeyer RB, Klein H, Ishaque B, Rickert M, Schmitz G, Schmidt TA, Steinmeyer J. Articular joint lubricants during osteoarthritis and rheumatoid arthritis display altered levels and molecular species. PLoS One. 2015;10:e0125192. doi: 10.1371/journal.pone.0125192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alquraini A, Garguilo S, D’Souza G, Zhang LX, Schmidt TA, Jay GD, Elsaid KA. The interaction of lubricin/proteoglycan 4 (PRG4) with toll-like receptors 2 and 4: an anti-inflammatory role of PRG4 in synovial fluids. Arthritis Res Ther. 2015;17:353–365. doi: 10.1186/s13075-015-0877-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nair A, Kanda V, Bush-Joseph C, Verma N, Chubinskaya S, Mikecz K, Glant TT, Malfait AM, Crow MK, Spear GT, Finnegan A, Scanzello CR. Synovial fluid from patients with early osteoarthritis modulates fibroblast-like synoviocyte responses to TLR-4 and TLR-2 ligands via soluble CD14. Arthritis Rheum. 2012;64:2268–2277. doi: 10.1002/art.34495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sohn DH, Sokolove J, Sharpe O, Erhart JC, Chandra PE, Lahey LJ, Lindstrom TM, Hwang I, Boyer KA, Andriacchi TP, Robinson WH. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via toll-like receptor 4. Arthritis Res Ther. 2012;14:R7–20. doi: 10.1186/ar3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Al-Sharif A, Jamal M, Zhang LX, Larson K, Schmidt TA, Jay GD, Elsaid KA. Lubricin/Proteoglycan (PRG4) binding to CD44 receptor: A mechanism of the suppression of proinflammatory cytokine-induced synoviocyte proliferation by lubricin. Arthritis Rheumatol. 2015;67:1503–1513. doi: 10.1002/art.39087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alquraini A, Jamal M, Zhang LX, Schmidt TA, Jay GD, Elsaid KA. The autocrine role of proteoglycan-4 (PRG4) in modulating osteoarthritic synoviocyte proliferation and expression of matrix degrading enzymes. Arthritis Res Ther. 2017;19:89–104. doi: 10.1186/s13075-017-1301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


