Abstract
Necroptosis is a form of cell death associated with inflammation, however the biological consequences of chronic necroptosis are unknown. Necroptosis is mediated by RIPK1, RIPK3 and MLKL kinases but in hematopoietic cells RIPK1 has anti-inflammatory roles and functions to prevent necroptosis. Here we interrogate the consequences of chronic necroptosis on immune homeostasis by deleting Ripk1 in mouse dendritic cells (DC). We demonstrate that deregulated necroptosis results in systemic inflammation, tissue fibrosis and autoimmunity. We show that inflammation and autoimmunity are prevented upon expression of kinase inactive RIPK1 or deletion of RIPK3 or MLKL. We provide evidence that the inflammation is not driven by microbial ligands, but depends on the release of danger-associated molecular patterns (DAMPs) and MyD88-dependent signaling. Importantly, whilst the inflammation is independent of type I interferon and the nucleic acid sensing TLRs, blocking these pathways rescues the autoimmunity. These mouse genetic studies reveal that chronic necroptosis may underlie human fibrotic and autoimmune disorders.
INTRODUCTION
Receptor-interacting protein kinase 1 (RIPK1) is a key component of the necroptotic and apoptotic cell death pathways, and is important for the optimal activation of the NF-κB and MAPK pathways. TNF normally induces NF-κB and MAP kinase activation, but under certain conditions can induce apoptosis or when caspases are inhibited, stimulate necroptosis. Necroptosis is an inflammatory form of cell death triggered by death ligands such as TNF, FasL, TRAIL, type I and type II interferons (IFN) or by activation of pathogen recognition receptors including Toll-like receptors (TLR) 3 or 4 (1). RIPK1 initiates the necroptotic kinase cascade by phosphorylating and activating RIPK3, which then activates the pseudo-kinase mixed lineage kinase domain-like (MLKL) (2, 3). MLKL phosphorylation results in its translocation to the plasma membrane and changes in membrane permeability (4), resulting in the release of danger-associated molecular patterns (DAMPs) such as HMGB1, ATP and mitochondrial DNA (5). These DAMPs activate TLRs on macrophages and dendritic cells (DCs) to induce and amplify pro-inflammatory cytokine and chemokine production. In some cell types, RIPK1 kinase activity is crucial for the activation of necroptosis, as the kinase inhibitor Necrostatin-1 prevents necroptosis (6) and RIPK1 kinase inactive mice, Ripk1D138N, are resistant to necroptosis and TNF-induced shock in vivo (7, 8). RIPK1 has also been shown to have essential kinase independent scaffold functions that mediate cell survival due to effects on the canonical (9) or non-canonical NF-κB pathways, depending on the cell type (10).
Complete RIPK1 deficiency results in postnatal lethality (9) driven by an increased sensitivity to both RIPK3-dependent necroptosis and Caspase-8 dependent apoptosis, whereby compound deletion of both Caspase-8 and Ripk3 or Caspase-8 and Mlkl or the deletion of Ripk3 and TNF receptor type 1 (Tnfr1) rescues RIPK1 associated-lethality (11–13). Whether RIPK1 triggers apoptosis and/or necroptosis is cell-type and context-dependent. For example, RIPK1 is essential for necroptosis in murine embryonic fibroblasts and bone marrow derived macrophages (BMDM) (8, 14) but functions to negatively regulate RIPK3 and MLKL in hematopoietic stem and progenitor cells and keratinocytes (11, 15, 16). Ripk1 deletion in intestinal epithelial cells sensitizes to both TNF-mediated apoptosis and necroptosis (16). These findings reveal that RIPK1 can positively or negatively regulate necroptosis or apoptosis depending on cellular context.
DCs are critical to maintain immune homeostasis and to generate successful responses to infection. Given the important roles DCs have in maintaining tolerance, we examined the in vivo consequences of DC necroptosis on immune homeostasis by deleting Ripk1 in DC. We found that Ripk1-deficient DCs exhibit normal responses to TNF- and FasL-induced apoptosis, but have an increased sensitivity to necroptosis in vitro. Mice with a DC RIPK1-deficiency develop inflammation and autoimmunity, characterized by splenomegaly, lymphadenopathy, tissue fibrosis and production of anti-nuclear autoantibodies (ANAs). We demonstrate that inflammation and autoimmunity associated with a DC RIPK1 deficiency are rescued by expression of kinase inactive RIPK1 or an absence of RIPK3, MLKL or the TLR adapter MyD88; thereby implicating necroptosis as the mediator of inflammation, and MyD88-dependent TLR signaling as an amplifier of DAMP signaling. Importantly, autoantibody production but not inflammation, was prevented by an absence of the type I interferon receptor or UNC93B1-dependent TLR 3, 7 and 9 signaling; thereby genetically separating signals that trigger DAMP release, inflammation and fibrosis from those responsible for autoimmunity. This study provides genetic evidence that cell death and inflammation precede the development of autoimmunity and suggests that necroptosis kinase inhibitors may be useful in at-risk individuals to prevent autoimmune disease.
MATERIALS AND METHODS
Mice
Ripk1 conditional mice (Ripk1fl/fl) (16) were crossed with CD11cCre (Itgax-cre) (17), Ripk3−/− mice (a gift from Vishva Dixit, Genentech, San Francisco), Mlkl−/− (4), Ifngr1−/− (18), Tnfr1−/− (19), MyD88−/− (20), Ifnar1−/− (21) and Unc93b3d/3d (22) mice. B6.Cg-Tg(TcraTcrb)425Cbn/J (OT-II) and Gt(ROSA)26Sortm9(CAG-tdTomato)Hze mice were obtained from Jackson Laboratory. All animal procedures used in this study were approved by The University of Massachusetts Medical School Institutional Animal Care and Use Committee. For antibiotic treatment, Ampicillin (1 mg/ml), Neomycin (1 mg/ml), Ciprofloxacin (0.5 mg/ml), Meropenem (0.5 mg/ml) and Grape Kool-aid (20 mg/ml) were added to drinking water from 2 days after birth. Following weaning, ciprofloxacin was substituted with vancomycin (0.5 mg/ml). When littermate controls were not used, sex-matched control mice carrying the CD11cCre transgene were co-housed with experimental mice. For LPS-induced endotoxic shock experiments, age and sex-matched mice were intraperitoneally injected with 5 mg/kg LPS from E. coli (Sigma) and re-extracted using phenol chloroform as previously described (23).
Cell cultures
Bone marrow-derived dendritic cells (BMDCs) were generated by culturing bone marrow cell suspensions in 20ng/ml recombinant GM-CSF (Peptrotech) for 10 days. For necroptosis assays, BMDCs were treated with 0.1 μM Smac mimetic (ChemieTek) and 10 μM zVAD (Enzo). For apoptosis assays, BMDCs were treated with cyclohexmide (0.5 μg/ml), TNFα (10 ng/ml), IFNγ (10 ng/ml) or with FasL and control vesicles purified from N2-mFasL and N2-neo cell supernatant (diluted 1/40), as previously described (24). Splenic DC were isolated from mice following treatment of the Flt3L producing melanoma line B16, using a CD11c positive selection kit (Stemcell Technologies).
To examine T cell proliferation, purified CD11c+ splenic DC from CD11cCre and Ripk1DC KO mice were incubated with OVA323–339 or control OVA257–264 peptide for 1 h. CD4+ T cells were isolated from spleens of OT-II mice using CD4 positive selection beads (Invitrogen). Isolated CD4+ cells were labeled with 0.5 μM CFSE (Invitrogen) and incubated with splenic DCs for 72 h. CFSE staining was examined in viable CD4+ cells by flow cytometry.
Detection of autoantibodies
Anti-nuclear Abs (ANAs) were detected by immunofluorescence on HEp-2 slides (Antibodies, Inc.) as previously described (27).
Histology
Tissues were fixed in 10% formalin (Fisher Scientific). Slides were stained with H&E or Masson’s trichrome. Images were taken on an Olympus BX41 microscope using an Evolution MP 5.0 Mega-Pixel Camera (MediaCybernetics) and QCapture Pro software (QImaging).
Flow Cytometry
Single-cell suspensions were stained with cell-surface antibodies and DAPI (Molecular Probes) was used to distinguish between live and dead cells. Samples were run on a BD LSRII flow cytometer (BD Bioscience) and analyzed using FlowJo software (Tree Star). Cell populations were determined as follows: erythrocytes, CD71− Ter119+; erythroblasts, CD71+ Ter119+; neutrophils CD11b+ Ly6Cint Gr-1+; monocytes, CD11b+, Ly-6Chi and Gr1lo; CD4 T lymphocytes, CD4+ CD8−; CD8 T lymphocytes CD8+ CD4−; B lymphocytes, B220+ CD4− CD8−; germinal center B cells, B220+ Fas+ GL7+; progenitors, lineage- c-Kit+. Dendritic cells were gated according to (25).
Cytokine analysis
Serum cytokines were measured using an 11-plex protein/peptide multiplex analysis (Luminex Technology) conducted by the National Mouse Metabolic Phenotyping Center at the University of Massachusetts Medical School. Chemokines and cytokines below the level of detection were assigned the value of zero. Serum Flt3L levels were measured by ELISA (R&D Systems). IFN-β ELISA kit was previously described (26).
Gene-Expression Analysis
RNA was prepared using TRIzol (Invitrogen) or with the RNeasy Mini kit (Qiagen). Each RNA sample was adjusted to contain the same quantity using the Nanodrop ND-1000 spectrophotometer (Thermo Scientific). RNA was used for quantitative RT-PCR using Power SYBR Green PCR Master Mix (Applied Biosystems) with the following primer pairs: β-actin, sense, 5′-TGG CAT AGA GGT CTT TAC GGA-3′, antisense, 5′-TTG AAC ATG GCA TTG TTA CCA A-3′; Iκbα, Sense 5′-TGA AGG ACG AGG AGT ACG AGC-3′; Antisense, 5′-TTC GTG GAT GAT TGC CAA GTG-3′; A20, sense, 5′-CTT TCT TCA TGT CCG TGA ACA CT-3′, antisense, 5′-TTC AGG GCC TAG CTT CGA GT-3′; cIAP1, sense, 5′-TGT GGC CTG ATG TTG GAT AAC-3′, antisense, 5′-GGT GAC GAA TGT GCA AAT CTA CT-3′; cIAP2, sense, 5′-GCT GTG GCC TAA TGC TAG ACA-3′, antisense, 5′-GGA CAA TCT TGA TTT GCT CGG AA-3′; XIAP, sense, 5′-CGA GCT GGG TTT CTT TAT ACC G-3′, antisense, 5′-GCA ATT ATG GGA TAT TCT CCT GT-3′.
Proteinuria
An anti-mouse albumin ELISA was used to measure urine protein (Bethyl Laboratories).
Immunoblotting
Cell lysates were prepared in RIPA buffer (150 mM NaCl, 50 mM Tris-HCl (pH 7.5), 1% NP40, 0.25% deoxycholate, 0.1% SDS, 1 mM EDTA), supplemented with protease inhibitors (Roche Applied Science), 1 mM dithiothreitol (DTT), 1 mM Na3VaO4 and 1 mM phenylmethylsulfonyl fluoride (PMSF) and boiled with SDS reducing sample buffer. Lysates were run on 4%–12% Bis-Tris gels (Invitrogen). Membranes were probed with phospho-MLKL (Abcam), cIAP1 (Enzo Life Sciences), cIAP2 (R&D Systems), RIPK1 (BD Transduction), RIPK3 (Pro-sci), XIAP (MBL), β-Actin (Sigma-Aldrich), Erk 1/2 (Cell Signaling Technology), MLKL (a gift from Jiahuai Han) or phospho-IκBα (Cell Signaling Technology) antibodies.
Statistical Measures
Statistical analyses were performed using GraphPad Prism software, version 6.0. Kaplan–Meier survival curves were analyzed using a log rank test with a 95% confidence interval. A two-sided P < 0.05 was considered statistically significant for Student’s t tests and two-way ANOVA tests. * p<0.05; **p<0.01
RESULTS
RIPK1-deficient dendritic cells are more sensitive to necroptosis
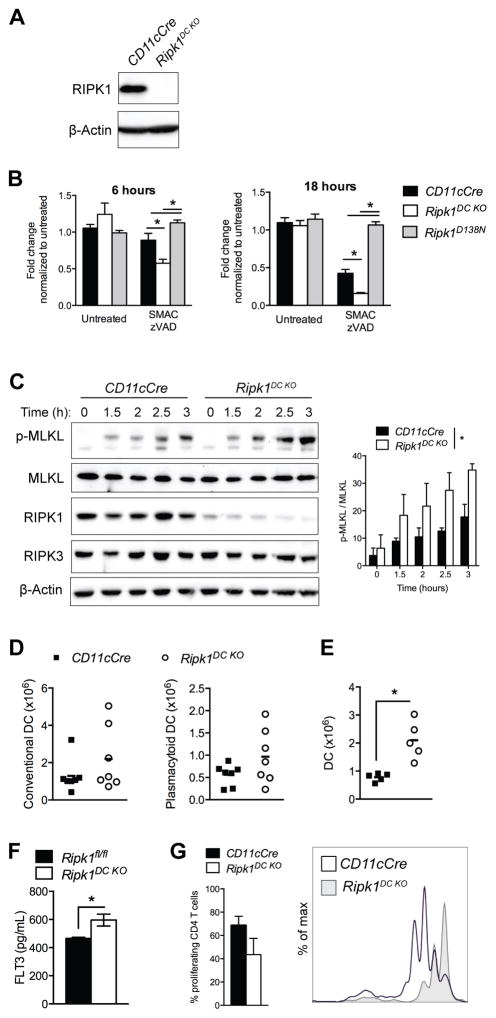
To determine the effects of DC necroptosis on immune homeostasis, mice containing a conditional allele of Ripk1 (16) were crossed to CD11cCre transgenic mice (hereafter referred to as Ripk1DC KO). Ripk1DC KO mice were born at the expected Mendelian ratios and developed to adulthood normally. These mice exhibit a specific loss of RIPK1 expression in CD11c+-enriched splenic DCs by western blot analysis (Fig. 1A).
Figure 1. RIPK1-deficient dendritic cells are more sensitive to necroptosis.
(A) Western blot depicting RIPK1 expression in CD11c+ enriched splenic DCs from CD11cCre and Ripk1DC KO mice. (B) RIPK1-deficient DCs are more susceptible to necroptosis. BMDCs were treated with 0.1 μM SMAC mimetics and 10 μM zVAD-fmk. Effects on cell viability were measured at 6 and 18h using Cell Titre Glo. N=6–8 independent samples. (C) Western blot depicting MLKL phosphorylation and total MLKL, RIPK1 and RIPK3 levels in BMDCs treated with SMAC mimetics and zVAD-fmk. Quantification of pMLKL relative to MLKL from 3 western blots is shown. (D) Number of splenic conventional and plasmacytoid DCs. (E) Number of DCs in the bone marrow. (F) Serum Flt3L levels in 4 week old mice. (G) DCs were incubated with OVA323–339 peptide for 1 hour then incubated with CFSE-labeled CD4+ OT-II T cells for 3 days. T cell proliferation was measured by flow cytometry. The percentage of proliferating CD4 T cells is shown from 5 independent samples. Error bars, means ± SEM. Unpaired two-tailed Student’s t test (B, D–G). * p<0.05.
To determine whether RIPK1 deletion altered DC survival, bone-marrow-derived dendritic cells (BMDCs) were treated in vitro with necroptotic and apoptotic stimuli. Treatment with zVAD-fmk and SMAC mimetics primes cells for necroptosis by inhibiting caspase activation and inducing IAP degradation, respectively. Consistent with its negative regulatory roles in hematopoietic cells (11, 15), RIPK1-deficient DCs are more sensitive than controls to necroptotic death (Fig. 1B). In contrast, RIPK1 kinase inactive (Ripk1D138N) DCs remained resistant to zVAD and SMAC treatment, revealing a requirement for the kinase activity of RIPK1 in TNF-mediated necroptosis in DC.
Necroptosis is thought to be mediated by a serial phosphorylation cascade whereby RIPK1 phosphorylates RIPK3, which then phosphorylates the effector pseudo-kinase MLKL (2–4). To confirm that necroptosis was induced in RIPK1-deficient DC, we examined DC cultures for evidence of MLKL phosphorylation. MLKL phosphorylation was detected in RIPK1-deficient DCs treated with zVAD-fmk and SMAC-mimetics at early time points, indicating that a RIPK1 deficiency predisposes DCs to necroptotic death (Fig. 1C). RIPK3 overexpression has been shown to bypass the requirement for RIPK1 to drive necroptotic death (28), however no differences in RIPK3 or MLKL expression were observed in RIPK1-deficient DCs (Fig. 1C). In fact, we demonstrate that increasing concentrations of zVAD-fmk, in the absence of SMAC-mimetics or exogenous death ligands, was sufficient to induce necroptosis in Ripk1-deficient DCs (Supplemental Fig. 1A). DCs deficient for both RIPK1 and RIPK3 were not susceptible to zVAD-fmk-induced cell death, confirming that caspase inhibition alone was sufficient to trigger necroptotic death in RIPK1-deficient DCs (Supplemental Fig. 1A). In contrast, RIPK1-deficient BMDCs responded normally to apoptotic stimuli including exposure to FasL, IFNγ or TNFα with cycloheximide (Supplemental Fig. 1B–C).
The fact that necroptosis was induced by treatment with zVAD-fmk and that SMAC mimetics were not required suggested that IAP/XIAP expression may be reduced in RIPK1-deficient DCs. Reduced cIAP1/2 and XIAP expression has been shown previously to sensitize antigen presenting cells to necroptosis (29–31). We found cIAP2 and XIAP expression reduced in the absence of RIPK1, however no significant changes in cIAP1 levels were observed (Supplemental Fig. 1D). Gene expression analysis revealed no differences in cIAP1/2 or XIAP mRNA levels in Ripk1DC KO DC (Supplemental Fig. 1E). Therefore, these data suggest that a RIPK1 deficiency may lead to cIAP2/XIAP protein degradation and predispose DCs to necroptosis.
RIPK1 is not required for dendritic cell development or function
Since RIPK1-deficient DCs exhibited increased susceptibility to necroptosis in vitro (Fig. 1B), decreased numbers of DCs were expected in Ripk1DC KO mice. However, the loss of RIPK1 did not lead to alterations in the number of splenic conventional DCs (cDCs; CD11c+ MHC class II+) or plasmacytoid DCs (pDCs; CD11clo MHC class II+ Siglec H+) (Fig. 1D), even at 1 year of age (Supplemental Fig. 1F). Furthermore, no significant differences in the expression of activation markers CD80, CD86 or MHC class II were observed in cDCs or pDCs from Ripk1DC KO mice compared to CD11cCre controls (Supplemental Fig. 1G–H). However, significant increases in bone marrow cDCs were observed (Fig. 1E) as well as increases in serum Flt3L levels (Fig. 1F), suggesting that DC progenitor activity may be stimulated in response to DC loss in vivo (32). The loss of RIPK1 had no detectable effects on BMDC production, as normal numbers of BMDC were generated from the bone marrow in vitro, indicating that RIPK1 is not required for DC proliferation and/or differentiation (Supplemental Fig. 1I).
We treated RIPK1-deficient and control DCs with TNF to examine NF-κB activation. Although not statistically significant, the IκBα phosphorylation in response to TNF was consistently less robust in RIPK1-deficient DCs than controls (Supplemental Fig. 1J). Similarly, IκBα expression was slightly dampened in TNF-treated RIPK1-deficient DCs (Supplemental Fig. 1K). We then examined the ability of RIPK1-deficient DCs to induce CD4 T cell proliferation. We found that RIPK1-deficient DCs induced proliferation of CD4 T cells, although slightly fewer T cells proliferated in response to antigen-stimulated RIPK1-deficient DCs (Fig. 1G). These data demonstrate that RIPK1-deficient DCs are able to respond to TNF and to antigen stimulation.
A Caspase 8-deficiency has been shown to enhance the assembly and activation of the NLRP3 inflammasome, a signaling complex that mediates the processing of pro-inflammatory cytokines such as IL-1β (33). IL-1β secretion generally requires exposure to two stimuli: first, a priming agent such as LPS, which triggers pro-IL-1β synthesis; and a second signal such as ATP, which processes pro-IL-1β to its active form (34). RIPK1 deletion in macrophages, as well cIAP, XIAP or Caspase-8 depletion in DCs, has been shown to stimulate IL-1β production in response to LPS without the need for a second signal (11, 31, 35). We therefore examined IL-1β production in RIPK1-deficient DCs stimulated with LPS only or in combination with ATP and found that IL-1β processing was not significantly altered in LPS-primed RIPK1-deficient DCs in vitro (Supplemental Fig. 1L).
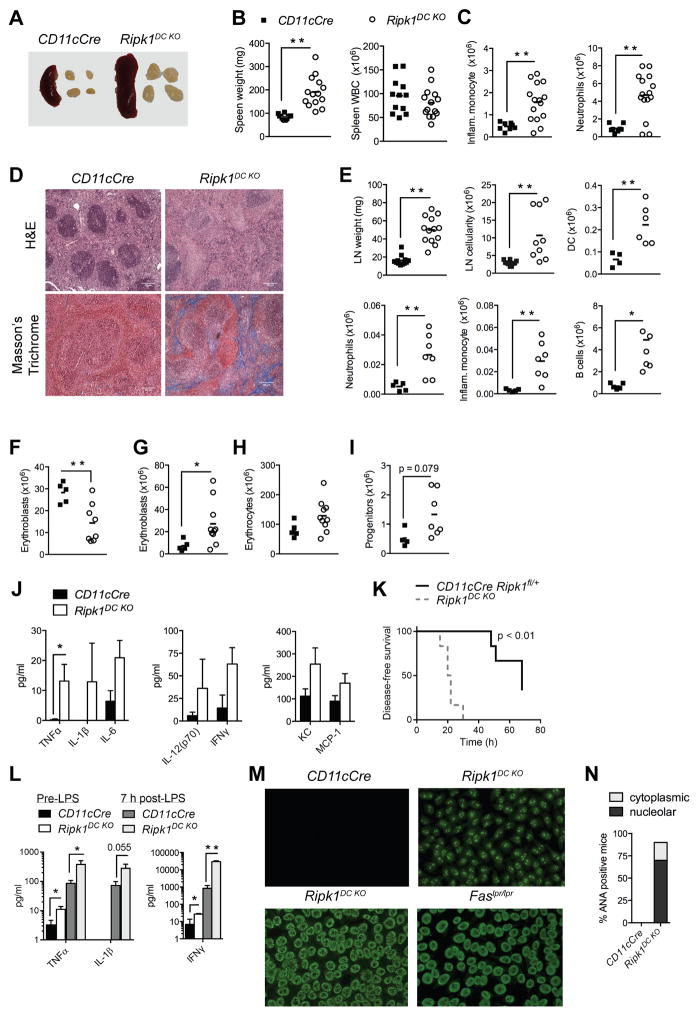
Ripk1DC KO mice develop systemic inflammation, tissue fibrosis and autoimmunity
Given the increased sensitivity of RIPK1-deficient DCs to necroptosis in vitro, we examined the in vivo effects of RIPK1 deficiency in DCs by examining Ripk1DC KO mice. Ripk1DC KO mice developed splenomegaly and lymphadenopathy by 16 weeks of age (Fig. 2A–E). Evidence of inflammation was observed in Ripk1DC KO mice, with elevated numbers of neutrophils and inflammatory monocytes in the spleens of Ripk1DC KO mice (Fig. 2C). Conversely, no differences were detected in splenic B or T lymphocyte populations (Supplemental Fig. 2B–C). Histological analysis revealed that the splenic architecture was disrupted in Ripk1DC KO mice (Fig. 2D). Masson’s Trichrome staining revealed collagen deposits in the spleen indicating fibrosis, which largely accounted for the increased spleen size. Masson’s Trichrome staining also revealed evidence of fibrosis in skin and lungs of Ripk1DC KO mice but not controls (Supplemental Fig. 2A).
Figure 2. Ripk1DC KO mice develop systemic inflammation and autoimmunity.
(A) Representative images of spleens and lymph nodes from Ripk1DC KO mice. (B) Spleen weight and cellularity. (C) Number of inflammatory monocytes and neutrophils in the spleen. (D) H&E and Masson’s trichrome staining on spleen sections from age-matched CD11cCre and Ripk1DC KO mice. (E) Lymph node weight, cellularity, with numbers of DCs, inflammatory monocytes, neutrophils and B cells in the lymph nodes. (F) Number of bone marrow erythroblasts. (G) Number of erythroblasts in the spleen. (H) Number of erythrocytes in spleen. (I) Number of lineage− cKit+ progenitors in the spleen. (J) Serum cytokine and chemokine levels in CD11cCre and Ripk1DC KO mice (n=5 per genotype). Data shown from Fig. 2 A–J are from 16 week old mice. (K) Survival curve of CD11cCre Ripk1fl/+ and Ripk1DC KO mice intraperitoneally injected with 5 mg/kg LPS. (L) Serum cytokine and chemokine levels in CD11cCre and Ripk1DC KO mice before and 7 h after intraperitoneal injection with 5 mg/kg LPS. (M) Representative images of HEp2 cells stained with serum from 6 month old mice of the indicated genotypes. (N) Proportion of CD11cCre and Ripk1DC KO mice serum positive for cytoplasmic or nucleolar ANAs. n=3–6 per genotype. Error bars, means ± SEM. Scale bars represent 100μm. Unpaired two-tailed Student’s t test (B, C, E–J, L), and log-rank test (K). * p<0.05; **p<0.01.
In contrast to the spleen, the lymph nodes exhibited increased cellularity, largely consisting of B cells, but also with increases in DCs, neutrophils and inflammatory monocytes (Fig. 2E). No increases in lymph node T cells were observed, nor was there evidence of T cell activation in spleen or lymph node (Supplemental Fig. 2B–C).
The bone marrow appeared largely normal (Supplemental Fig. 2D), however, Ripk1DC KO mice had significantly decreased numbers of erythroblasts (Fig. 2F). Conversely, increased numbers of erythroblasts and lineage-negative, c-Kit+ progenitor cells were observed in the spleen (Fig. 2G, I) indicative of extramedullary hematopoiesis potentially a result of the inflammation. Importantly, splenic erythrocyte numbers remained normal, indicating that the erythrocyte population did not contribute to the spleen enlargement (Fig. 2H).
To further assess the inflammation in Ripk1DC KO mice, we measured the levels of pro-inflammatory cytokines in the serum. A significant increase in serum TNFα and IFNγ was observed in Ripk1DC KO mice compared to control mice (Fig. 2J, L). Although not significant, increases in serum IL-1β, IL-6, IL-12, KC and MCP-1 were also observed in Ripk1DC KO mice. To determine the consequences of inflammation and elevated cytokine levels, control or Ripk1DC KO mice were given a low dose of LPS (5mg/kg). 60% of control mice succumbed to LPS administration with a median latency of 70 hours, whilst all Ripk1DC KO mice succumbed to LPS-induced endotoxic shock within 30 hours (Fig. 2K). Additionally, the levels of pro-inflammatory cytokines TNFα, IL-1β, and IFNγ were significantly elevated in the sera of Ripk1DC KO mice compared to controls (Fig. 2L). Thus, the pro-inflammatory cell death in Ripk1DC KO mice sensitizes the mice to LPS-induced endotoxic shock. Interestingly, DC loss is a clinical feature of septic patients, suggesting that DC necroptosis may underlie the hyperinflammatory syndrome and immune suppression in patients with severe sepsis (36).
The lymphadenopathy and splenomegaly observed in Ripk1DC KO mice suggested that these mice developed autoimmune disease. The B220+, CD3+, CD4−, CD8− T cells prevalent in autoimmune mouse models such as the Faslpr and Caspase-8−/− Ripk3−/− mice (37, 38) were not present in Ripk1DC KO mice (Supplemental Fig. 2E). However, anti-nuclear autoantibodies (ANAs) were detected in the serum of Ripk1DC KO mice from 6 months of age (Fig. 2M). To characterize the autoantibodies produced by these mice, we screened sera by immunofluorescent staining of HEp-2 cells. The staining intensity was similar to that observed with serum from autoimmune prone Faslpr mice. Antibodies reactive with RNA-associated autoantigens frequently exhibit a more speckled nuclear or cytoplasmic staining pattern, whereas antibodies reactive with dsDNA or other chromatin components exhibit a homogenous nuclear stain as observed with sera from FAS-deficient mice. The staining pattern of the circulating ANAs in Ripk1DC KO mice was consistent with antibodies targeting nucleolar components, with one of six mice examined exhibiting a cytoplasmic staining pattern (Fig. 2M–N). Anti-nucleolar and other speckled nuclear patterns are commonly found in TLR9-deficient or TLR7 overexpressing lupus-prone mice (39, 40). These data indicate that autoantibodies produced by Ripk1DC KO mice recognize RNA-associated autoantigens and not dsDNA.
Correlating with the presence of ANAs, we observed increased numbers of germinal center B cells in the lymph nodes of these mice (Supplemental Fig. 2F). Although ANAs were detected in young Ripk1DC KO mice, the CD11c promoter can be active in some lymphoid cells (17), therefore we mated the Ripk1DC KO and CD11cCre mice with a Rosa26-loxP-Stop-loxP-tdTomato reporter line and assessed Tomato Red expression in hematopoietic cells from spleen and lymph node (Supplemental Fig. 2G). Tomato Red expression was detected in 86% of DC and 26% of B cells isolated from spleen and LN of Ripk1DC KO but not control mice. To assess Ripk1 deletion in B cells, we sorted Tomato Red-positive and -negative B cells from control and Ripk1DC KO mice and performed genomic PCR. We detected evidence of Ripk1 deletion in Tomato Red-positive but not -negative B cells (Supplemental Fig. 2I). Notably, B cells isolated from mice with lymphadenopathy retained a floxed Ripk1 allele, indicating that these B cells likely expressed RIPK1. Based on this analysis and the fact that germinal center B cells expanded in the LN, we conclude that Ripk1 deletion in the CD11c-positive, age-associated B cell (ABC) population associated with young lupus-prone or aged wild type mice is not responsible for B cell expansion and autoantibody production in these mice (41). As is typical for mice on the C57BL/6 background (42), Ripk1DC KO mice did not develop proteinuria by 12 months of age (Supplemental Fig. 2J). Together, these data suggest that a RIPK1-deficiency in DCs leads to systemic inflammation that triggers B cell expansion and autoantibody production.
Dendritic cell necroptosis underlies the systemic inflammation and autoimmunity in Ripk1DC KO mice
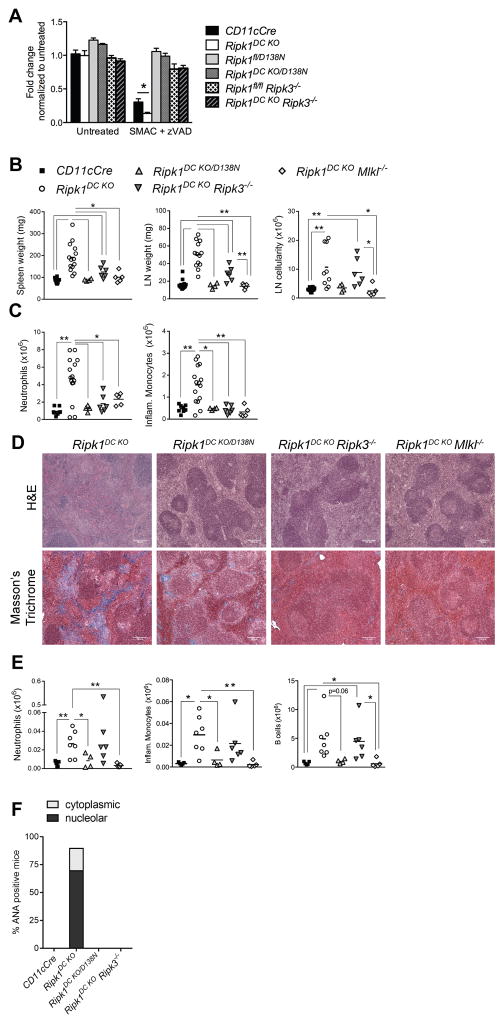
Given the in vitro sensitivity of RIPK1-deficient DCs to necroptosis (Fig. 1B) and the lack of reliable and sensitive measures to detect necroptotic death in mice, we used a genetic approach to determine whether aberrant DC necroptosis is responsible for the inflammation and autoimmunity in Ripk1DC KO mice. To address the specific role of RIPK1 kinase-dependent cell death, Ripk1DC KO mice were crossed to mice expressing the kinase-inactive RIPK1 (D138N) allele (8) to generate CD11cCre Ripk1fl/D138N (Ripk1DC KO/D138N) mice. Since the kinase activity of RIPK1 is not required for necroptosis in certain cell types (43, 44), we also generated Ripk1DC KO Ripk3−/− and Ripk1DC KO Mlkl−/− mice to provide additional genetic evidence that DC necroptosis results in chronic inflammation and a break in tolerance.
Introduction of the Ripk1D138N allele or deletion of Ripk3 protected RIPK1-deficient DCs from necroptosis in vitro (Fig. 3A). Similarly, the introduction of the Ripk1D138N allele or deletion of Ripk3 or Mlkl ameliorated the inflammatory and autoimmune disease associated with a RIPK1 DC deficiency, resulting in reductions in spleen and LN weight (Fig. 3B). Significant decreases in neutrophil and inflammatory monocyte infiltration were also observed in the spleens of these mice indicating that inflammation was reduced (Fig. 3C). Importantly, the splenic architecture was maintained in these mice with no detectable signs of fibrosis (Fig. 3D, Supplemental Fig. 3A). Interestingly, some differences were observed in Ripk1DC KO/D138N, Ripk1DC KO Ripk3−/− and Ripk1DC KO Mlkl−/− mice. For example, whilst Ripk1DC KO/D138N and Ripk1DC KO Mlkl−/− mice mice showed a complete rescue of the lymphadenopathy, Ripk1DC KO Ripk3−/− mice displayed only a partial reduction in LN weight and cellularity, with increased numbers of inflammatory monocytes, neutrophils and B cells in the LN (Fig. 3E). Collectively, these data demonstrate that chronic necroptosis drives inflammation in Ripk1DC KO mice. However, these data are also consistent with published work that reveals necroptosis-independent functions of RIPK3 (33, 35, 45).
Figure 3. Dendritic cell necroptosis underlies the systemic inflammation in Ripk1DC KO mice.
(A) DCs from CD11cCre Ripk1fl/D138N (Ripk1DC KO/D138N) and Ripk1DC KO Ripk3−/− mice are protected from necroptosis in vitro. BMDCs were treated with SMAC mimetics and zVAD-fmk. Effects on cell viability were measured at 18h using Cell Titre Glo. N=3–6 samples. (B) Spleen and lymph node weights and lymph node cellularity from CD11cCre, Ripk1DC KO, Ripk1DC KO/D138N, Ripk1DC KO Ripk3−/− and Ripk1DC KO Mlkl−/− mice. (C) Neutrophil and inflammatory monocyte numbers in the spleens of CD11cCre, Ripk1DC KO, Ripk1DC KO/D138N, Ripk1DC KO Ripk3−/− and Ripk1DC KO Mlkl−/− mice. (D) H&E and Masson’s Trichrome staining of spleens from Ripk1DC KO, Ripk1DC KO/D138N, Ripk1DC KO Ripk3−/− and Ripk1DC KO Mlkl−/− mice. (E) Neutrophils, inflammatory monocytes and B cells are elevated in the lymph nodes of Ripk1DC KO and Ripk1DC KO Ripk3−/− mice but not Ripk1DC KO/D138N or Ripk1DC KOMlkl−/− mice. (F) Proportion of mice serum positive for ANAs at 6 months (n=3–6 mice per genotype). Unless otherwise stated, all data shown is from 16 week old mice. CD11cCre and Ripk1DC KO mouse phenotyping data shown in Fig. 3B, C, E and F were shown in Fig. 2. Error bars, means ± SEM. Scale bars represent 100μm. Unpaired two-tailed Student’s t test (A–C, E). * p<0.05; **p<0.01.
We predicted that the release of DAMPs from necroptotic DCs drive the autoimmunity observed in Ripk1DC KO mice. Therefore, we examined autoantibody production in Ripk1DC KO/D138N, Ripk1DC KO Ripk3−/− and Ripk1DC KO Mlkl−/− mice. We were unable to detect ANAs in the serum of these mice (Fig. 3F, Supplemental Fig. 3B), suggesting that necroptotic DCs release DAMPs including RNA and other nucleolar components that trigger B cell proliferation, differentiation and autoantibody production. The ability of a RIPK1 kinase inactive D138N allele, an MLKL deficiency and to some extent a RIPK3 deficiency, to rescue disease in Ripk1DC KO mice provides genetic evidence that DC necroptosis underlies the inflammation and autoimmunity.
Ripk1DC KO mice develop lymphadenopathy that is partially TNF-dependent
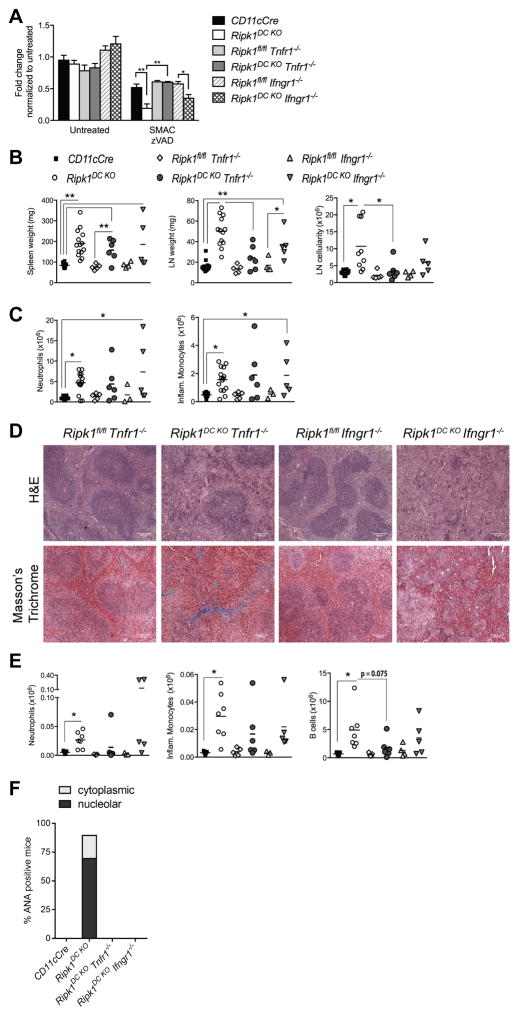
TNFα and IFNγ are known to induce necroptosis (1) and elevated levels of TNFα and IFNγ were observed in the serum of Ripk1DC KO mice (Fig. 2J), implicating TNFα and/or IFNγ as inducers of DC necroptosis in Ripk1DC KO mice. The increased sensitivity of RIPK1-deficient BMDCs to SMAC and zVAD-induced necroptosis in vitro was lost upon compound deletion of Tnfr1, but not Ifngr1 (Fig. 4A), further implicating TNFα as the DC necroptosis-inducing ligand in Ripk1DC KO mice.
Figure 4. Ripk1DC KO mice develop inflammation that is partially TNF-dependent.
(A) BMDCs from Ripk1DC KO Tnfr1−/− but not Ripk1DC KO Ifngr−/− mice are protected from necroptosis in vitro. BMDCs were treated with SMAC mimetics and zVAD-fmk. Death measured at 20 h by Cell Titre Glo. N=3. (B) Spleen and lymph node weights and lymph node cellularity of mice of the indicated genotypes. (C) Neutrophils and inflammatory monocyte numbers in the spleens of mice with the indicated genotypes. (D) H&E and Masson’s trichrome staining of spleen sections from Ripk1DC KO Tnfr1−/−, Ripk1DC KO Ifngr−/− and control mice. (E) Numbers of neutrophils, inflammatory monocytes and B cells in the lymph nodes of mice of the indicated genotypes. (F) Proportion of mice serum positive for ANAs at 6 months (n=3–6 mice per genotype). CD11cCre and Ripk1DC KO mouse phenotyping data used in Fig. 4B, C, E, F were shown in Fig. 2. Error bars, means ± SEM. Scale bars represent 100μm. Unpaired two-tailed Student’s t test (A–C, E). * p<0.05; **p<0.01.
To address the roles of TNFα and IFNγ in inflammation and autoimmunity, we generated Ripk1DC KO mice that lack either TNFR1 or IFNγR1. Surprisingly, the absence of TNFα or IFNγ signaling had little effect on spleen inflammation, with both Ripk1DC KO Tnfr1−/− and Ripk1DC KO Ifngr1−/− mice exhibiting splenic fibrosis and significant increases in inflammatory cell infiltration (Fig. 4B, C). Consistent with the accelerated TNF-mediated necroptosis observed in vitro (Fig. 4A), we found the lymphadenopathy significantly ameliorated in Ripk1DC KO Tnfr1−/− mice but not in Ripk1DC KO Ifngr1−/− mice (Fig. 4B). The decrease in lymph node size observed in Ripk1DC KO Tnfr1−/− mice appeared due to decreases in B cell number (Fig. 4E; p=0.075), indicating that TNFR1 signaling is required for optimal GC B cell expansion in LN. Serum ANAs however, were not detected in either mouse strain, indicating that loss of TNFα or IFNγ signaling is sufficient to prevent ANA development (Fig. 4F, Supplemental Fig. 3C).
MyD88 is required for the development of inflammation in Ripk1DC KO mice
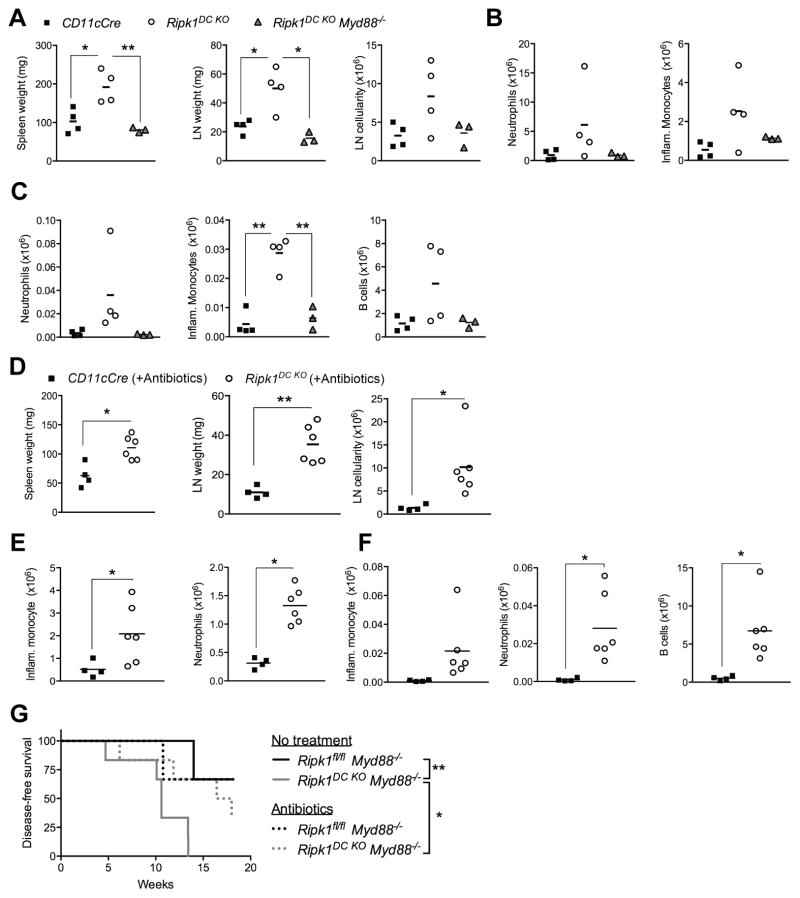
Necroptotic death is predicted to result in the release of cytokines, chemokines and DAMPs that activate TLRs and NOD-like receptors (NLRs) to promote inflammation (5). We examined the contribution of MyD88, a key component of TLR signaling (46), to the inflammatory disease observed in Ripk1DC KO mice. At 8 weeks of age Ripk1DC KO Myd88−/− mice exhibited significant decreases in spleen and LN size compared to Ripk1DC KO mice, together with decreases in neutrophil and inflammatory monocyte infiltrates in the spleen and LN (Fig. 5A–C). In addition, the number of B cells in the LN was comparable to controls. These data strongly suggest that the inflammation observed in Ripk1DC KO mice is exacerbated by MyD88-dependent TLR signaling, which is likely acting to amplify inflammation in response to the release of DAMPs from necroptotic RIPK1-deficient DCs. Notably, a TLR-MyD88 pathway also drives the B cell expansion observed in LN of Ripk1DC KO mice.
Figure 5. MyD88 is required for the development of inflammation in Ripk1DC KO mice.
(A) Spleen and lymph node weights and lymph node cellularity of Ripk1DC KO and Ripk1DC KO Myd88−/− mice at 8 weeks of age compared to CD11cCre controls. (B) Neutrophils and inflammatory monocytes are elevated in the spleens of Ripk1DC KO and Ripk1DC KO Myd88−/− mice compared to CD11cCre controls. (C) Neutrophils, inflammatory monocytes and B cells are increased in the lymph nodes of Ripk1DC KO and Ripk1DC KO Myd88−/− mice compared to CD11cCre controls. (D) Depletion of microbiota does not alter the inflammation of Ripk1DC KO mice. Spleen and lymph node weights and lymph node cellularity of Ripk1DC KO mice treated with broad-spectrum antibiotics in the drinking water for 12 weeks. (E) Neutrophils, inflammatory monocytes and B cells remain elevated in the lymph nodes of antibiotic-treated Ripk1DC KO mice. (F) Neutrophils and inflammatory monocytes remain elevated in the spleens of Ripk1DC KO mice treated with antibiotics. (G) Survival curve of mice of the indicated genotypes with or without treatment with broad-spectrum antibiotics in the drinking water. N=3–6 per group. Error bars, means ± SEM. Unpaired two-tailed Student’s t test (A–F), log-rank test (G). *p<0.05; **p<0.01.
The MyD88 dependency raised the possibility that pathogenic or commensal bacteria trigger and/or exacerbate inflammation in mice with DC RIPK1 deficiency. To examine this, we treated Ripk1DC KO mice with broad-spectrum antibiotics for 12 weeks. We found that antibiotic treatment had no detectable effect on the development of inflammatory disease in Ripk1DC KO mice (Fig. 5D–F). This suggests that the MyD88-dependent inflammation in Ripk1DC KO mice is independent of pathogenic or commensal bacteria and likely a consequence of endogenous DAMPs.
The Ripk1DC KO Myd88−/− mice began to lose weight and became moribund, succumbing with an average latency of 11.5 weeks (Fig. 5G). Treatment with broad-spectrum antibiotics significantly prolonged the survival of Ripk1DC KO Myd88−/− mice (Fig. 5G). The heightened susceptibility of Ripk1DC KO Myd88−/− mice to bacterial infection suggests that a combined total MyD88 deficiency with a RIPK1 DC deficiency interfered with the animals’ innate and adaptive anti-bacterial immune responses.
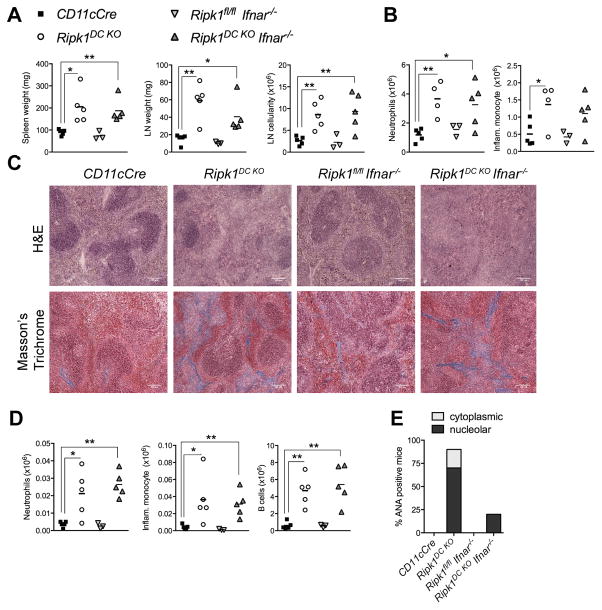
Autoimmunity, but not inflammation, in Ripk1DC KO mice is type I IFN-dependent
Given the recent data implicating type I interferons (IFN) in necroptosis and known roles for type I IFNs in ANA production and autoimmunity (47, 48), we hypothesized that type I IFN may contribute to auto-inflammation and autoimmunity observed in Ripk1DC KO mice. Although we were unable to detect IFNα or IFNβ in the serum of Ripk1DC KO mice (Table S1), we tested this hypothesis genetically by generating Ripk1DC KO Ifnar1−/− mice. The absence of the type I IFN receptor had no effect on the splenomegaly or lymphadenopathy associated with a DC RIPK1 deficiency, ruling out a critical role for type I IFN-induced necroptosis in the organ inflammation (Fig. 6). Although inflammation remained unaffected, an absence of type I IFN signaling prevented ANA development in five of six Ripk1DC KO Ifnar1−/− mice examined (Fig. 6E, Supplemental Fig. 3D). One Ripk1DC KO Ifnar1−/− mouse exhibited weak ANA staining (Supplemental Fig. 3D). Surprisingly, B cell numbers remained increased in the LNs of Ripk1DC KO Ifnar1−/− mice. Overall, these data reveal that autoimmunity but not inflammation is largely driven by or dependent on type I IFN signaling. These data provide genetic evidence that inflammation precedes autoimmunity, and suggests that type I IFNs mediate B cell differentiation into antibody secreting plasma cells.
Figure 6. Autoimmunity, but not inflammation, in Ripk1DC KO mice is type I IFN-dependent.
(A) Spleen and lymph node weights from mice of the indicated genotypes. (B) Number of splenic neutrophils and inflammatory monocytes from mice of the indicated genotypes. (C) H&E and Masson’s trichrome staining on spleen sections from mice of the indicated genotypes. (D) Number of neutrophils, inflammatory monocytes and B cells in the lymph nodes from mice of the indicated genotypes. (E) Proportion of mice that are serum positive for cytoplasmic or nucleolar ANAs. n=3–6 mice per genotype. All data shown is from 6 month old mice. Error bars, means ± SEM. Scale bars represent 100μm. Unpaired two-tailed Student’s t test (A, B, D). * p<0.05; **p<0.01.
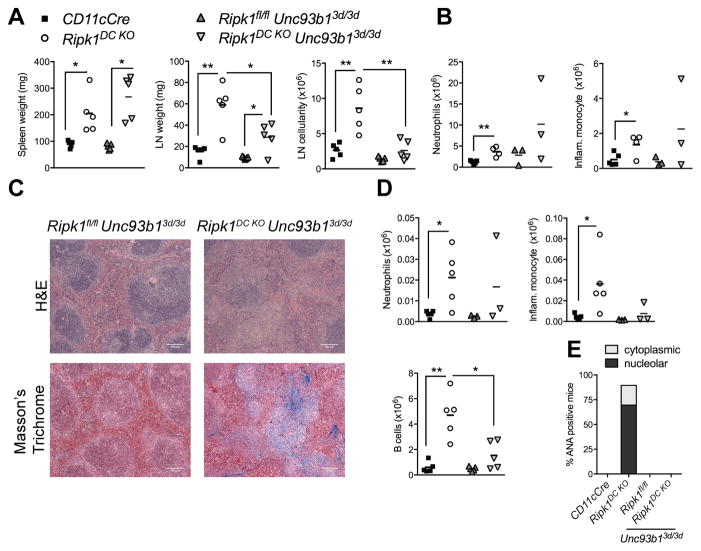
TLRs 3, 7 and 9 signaling drive B cell expansion and autoimmunity
Given that the ANAs generated in Ripk1DC KO mice largely target the nucleolar components of the cell (Fig. 2N), we examined the role of the nucleic acid sensing TLRs as drivers of the autoimmune disease. We utilized the Unc93b13d/3d mouse, which lacks functional UNC93B1 required for the intracellular trafficking of TLRs 3, 7 and 9 from the endoplasmic reticulum to the endosome (22). These TLRs sense nucleolar components such as single-stranded RNA, double-stranded RNA and unmethylated DNA and TLR 7 is required for the development of autoimmunity in lupus-prone mouse strains (22, 49, 50). An UNC93B1 deficiency had no detectable effects on splenomegaly, Masson’s Trichrome staining or on splenic inflammatory cell infiltrates, indicating that the nucleic acid TLRs do not mediate the inflammation and tissue fibrosis (Fig. 7). The size and cellularity of the lymph nodes however, were significantly reduced in Ripk1DC KO Unc93b13d/3d mice (Fig. 7A). Importantly, the UNC93B1-dependent reduction in lymph node size correlated with significant reductions in B cell number, whereas neutrophils and inflammatory monocytes were not significantly altered (Fig. 7D). No ANAs were detected in serum from Ripk1DC KO Unc93b13d/3d mice (Fig. 7E, Supplemental Fig. 3E), revealing that nucleic acid sensing TLRs are required for the generation of autoantibodies.
Figure 7. TLR 3, 7 and 9 signaling drives B cell expansion and autoimmunity in Ripk1DC KO.
(A) Spleen and lymph node weights from mice of the indicated genotypes. (B) Splenic neutrophils and inflammatory monocyte numbers from mice of the indicated genotypes. (C) H&E and Masson’s trichrome staining on spleen sections from mice of the indicated genotypes. (D) Number of neutrophils, inflammatory monocytes and B cells in the lymph nodes from mice of the indicated genotypes. (E) Proportion of mice that are serum positive for cytoplasmic or nucleolar ANAs at 6 months. n=3–6 per genotype. All data shown is from 6 month old mice. CD11cCre and Ripk1DC KO mouse phenotyping data shown in Fig. 7 were shown in Fig. 6. Error bars, means ± SEM. Scale bars represent 100μm. Unpaired two-tailed Student’s t test (A, B, D). * p<0.05; **p<0.01.
These genetic data reveal that the inflammation, tissue fibrosis and autoimmunity is dependent on the presence of RIPK1 kinase activity, RIPK3, MLKL and MyD88 (Fig. 3 and 5). Necroptotic cell death and MyD88-dependent TLRs mediate the inflammatory disease, however the nucleic acid sensing TLRs do not contribute to tissue fibrosis or inflammation. Although type I and II interferons induce necroptosis in vitro and are thought to underlie TNF-induced necroptosis in vivo (12, 51), neither IFNγ or IFNα/β are major drivers of inflammatory disease in this model. By contrast, the B cell expansion is partially TNFR1-dependent, and requires both MyD88 and UNC93B1. Surprisingly, type I IFN signaling is not required for B cell expansion in the LN, but proves essential for the differentiation of these cells into antibody secreting cells and for anti-nuclear antibody production. These in vivo genetic studies delineate the cell death and innate immune pathways as well as the temporal order of events that break tolerance and give rise to autoimmunity. These findings reveal that DC necroptosis is sufficient to give rise to inflammation and autoimmunity.
DISCUSSION
Herein we demonstrate that the loss of RIPK1 primes DCs for RIPK3- and MLKL-dependent necroptosis leading to inflammation, tissue fibrosis and autoimmunity. We show that in DCs, RIPK1 negatively regulates necroptosis but has no detectable effects on apoptosis. Genetically blocking necroptosis in DCs prevents the development of inflammation and autoimmunity in Ripk1DC KO mice, revealing a crucial role for RIPK1 in DC survival and maintenance of immune homeostasis.
The pro-inflammatory cytokine TNF is considered the classical activator of necroptosis. We show that in vitro RIPK1-deficient DCs undergo TNF-mediated necroptosis (Fig. 1B and Fig. 4A); however, an absence of TNFR1 had no detectable effects on splenic inflammation or tissue fibrosis (Figs. 4 and 6). These findings were unexpected as pulmonary fibrosis patients display elevated levels of TNFα and mice that overexpress TNF in the lung develop progressive pulmonary fibrosis (52). Splenic fibrosis was prevented by expression of kinase inactive RIPK1 or an absence of RIPK3 or MLKL (Fig. 3D), implicating necroptosis in the fibrotic response. An absence of TNFR1 signaling significantly ameliorated the lymphadenopathy in Ripk1DC KO mice (Fig. 4E), potentially due to TNFR1 effects on follicular dendritic cell networks and/or GC formation (53, 54).
Since an absence of TNF, IFNγ or type I IFN signaling had no effect on the inflammation, we speculate that ligand-independent DC necroptosis drives the inflammation observed in Ripk1DC KO mice. A RIPK1 deficiency in DC stimulates RIPK3-MLKL-dependent necroptosis in vivo, however no detectable increases in RIPK3 and/or MLKL expression were observed in vitro (Fig. 1C). The necroptotic sensitivity of Ripk1-deficient DCs could be explained by decreased expression of cIAP2 and XIAP (Supplemental Fig. 1D). Consistent with this hypothesis, cIAP1 and cIAP2 depletion in macrophages and monocytes have been show to stimulate necroptosis (29, 30). The IAP proteins regulate RIPK1 polyubiquitination and reductions in polyubiquitinated RIPK1 stimulate the formation of RIPK1-containing death complexes in the cytosol. Thus, a RIPK1 deficiency, which may result in IAP degradation (10, 16, 55), may sensitize DCs to necroptosis by unleashing RIPK3 and by promoting necrosome or complex 2b (RIPK3/MLKL) formation. XIAP loss in DC also alters RIPK1 polyubiquitination and promotes RIPK3-mediated cell death by as yet unclear mechanisms (31).
The deletion of other death receptor pathway components in DCs such as caspase-8 or FADD reveal similar but distinct disease phenotypes. For example, while the deletion of FADD sensitized the DCs to necroptosis leading to inflammation in vivo, the inflammation was driven by commensal bacteria (56). Yet, depleting commensal bacteria in Ripk1DC KO mice had no effect on disease severity or kinetics (Fig. 5D–F). A caspase-8 DC deficiency also results in chronic inflammation that was rescued upon deletion of RIPK3. However, the effects on inflammatory disease were not attributed to blocks in necroptosis, but rather to requirements for caspase-8 and RIPK3 in inflammasome activation and IL-1β production (33, 57). In contrast to caspase 8-deficient DC, we found RIPK1-deficient DCs are not primed to produce higher levels of IL-1β when stimulated with LPS in vitro (Supplemental Fig. 1L). Collectively these data suggest that commensal bacteria or inflammasome activation are not responsible for disease in Ripk1DC KO mice.
Mice deficient for caspase-8 are protected from embryonic lethality by concomitant loss of RIPK3 or MLKL, revealing that caspase-8 prevents necroptosis during development (38, 58, 59). The Caspase 8−/− Ripk3−/− and Caspase 8−/− Mlkl−/− mice are viable and fertile but develop autoimmune disease due to the expansion of CD3+, B220+, CD4−, CD8− T cells, due to impaired Fas-mediated apoptosis. We have shown that RIPK1 functions in certain cell types to prevent RIPK3 and MLKL activation however, this unusual T cell population was not detected in autoimmune Ripk1DC KO mice nor did we find any evidence of T cell activation (Supplemental Fig. 2).
Ripk1DC KO mice develop autoimmunity, which is prevented when necroptosis is blocked. We show that autoimmunity is type I IFN-dependent and that inhibiting TLR3, 7 and/or 9 signaling largely prevents ANA production. Interestingly, in our model, blocking the nucleic acid sensing TLRs (likely TLR7/9), but not type I IFNs, inhibits B cell expansion in the lymph nodes. The fact that B cell numbers were not altered in Ifnar1−/− mice was unexpected, as TLR7/9 play central roles in autoimmune disease and one of the main downstream effects of TLR7/9 signaling is IFNα/β production (60). B cells may directly respond to RNA or DNA/immune complexes through the B cell receptor (BCR) which can directly bind single-stranded RNA or double-stranded DNA and transport it to the endosome to activate TLR7 or TLR9, respectively (61, 62). Collectively, these data indicate that endosomal TLR7 and/or TLR9 cooperate with the BCR to mediate the expansion of autoreactive B cells via a pathway that does not require IFNα/β.
The frequency of Ripk1DC KO mice that produce ANAs targeting nucleolar components correlate with data showing that anti-RNA autoantibodies are common in SLE patients (63, 64). Our studies suggest that ANA production in SLE patients may also be a consequence of increased necroptosis, which likely provides a continuous source of RNA autoantigens. Finally, these data demonstrate that chronic necroptosis (in the absence of DC or T cell activation) is sufficient for GC B cell expansion and ANA production.
A theme that has emerged from the study of a wide range of autoimmune diseases is the appearance of disease-specific autoantibodies before evidence of clinical disease. In Rheumatoid Arthritis, antibodies reactive with citrullinated histones can be detected years before any evidence of joint inflammation and IFNγ levels correlate with the appearance of serum autoantibodies years prior to the clinical diagnosis of SLE (65–68). These genetic studies reveal that necroptotic cell death triggers inflammation and precedes the development of autoimmunity. Tissue fibrosis observed in the spleen, lungs and skin of Ripk1DC KO mice was prevented by expression of kinase inactive RIPK1 or an absence of RIPK3 or MLKL, implicating for the first time this form of inflammatory cell death in the fibrotic response. Fibrosis was unaffected by an absence of TNFR1, type I or II IFN signaling, but appeared rescued by a MyD88 deficiency. Our data also show that necroptosis and inflammation is not sufficient for the development of autoimmunity; ANA production requires the presence of functional nucleic acid sensing TLRs and the type I IFN receptor.
Recently, the inflammation associated with a RIPK1 deficiency in keratinocytes has been shown to be depend on the RIP Homotypic Interacting Motif (RHIM) of RIPK1, MLKL and the putative nucleic acid sensor Z-DNA binding protein 1 ZBP1, also known as DNA-dependent activator of IFN-regulatory factors (DAI) (69, 70). These findings suggest that the ZBP1-RIPK3-MLKL necroptotic pathway may be constitutively activated in Ripk1DC KO mice. Although initially identified as a dsDNA sensor capable of activating the NF-κB and IRF3 transcription factors (71), ZBP1 has recently been shown to recognize RNA viruses and induce necroptosis (72). Interestingly, ZBP1 expression is increased in peripheral blood mononuclear cells of lupus patients (73) raising the intriguing possibility that ZBP1-mediated necroptosis may contribute to SLE and other autoimmune or fibrotic disorders.
Supplementary Material
Acknowledgments
The authors would like to thank Drs. Ben Croker, Shruti Sharma and Kristen Nundel for invaluable advice and discussion. We thank Dr. Dave Garlick for histopathological analysis. We also thank Jason McGowan and Stacy Cote for help with ANA experiments. We acknowledge the support of the UMass Flow Cytometry Core and the Mouse Metabolic Phenotyping Center. The authors declare that they have no competing interests.
Abbreviations used
- Ripk1DC KO
CD11cCre Ripk1fl/fl
- DAMPs
Danger-associated molecular patterns
- MLKL
mixed lineage kinase domain-like
- RIPK1
Receptor interacting protein kinase 1
- RIPK3
receptor interacting protein kinase 3
Footnotes
AUTHOR CONTRIBUTIONS
J.A.O. designed, performed and analyzed the experiments with technical assistance from J.L. J.E.R. supervised some of the flow cytometry studies. M.Z. assisted with cell death experiments, D.M. performed the IAP western blot and qPCR experiments, C.D. assisted with NF-κB activation experiments, and N.H. performed the LPS-induced shock experiments. S.L. examined the histology slides. J.A.O. and J.L. prepared the figures. M.P., K.A.F. and A.R. provided advice and reagents. M.A.K. supervised the study. J.A.O. and M.A.K. wrote the paper.
References
- 1.Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517:311–320. doi: 10.1038/nature14191. [DOI] [PubMed] [Google Scholar]
- 2.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 3.Zhao J, Jitkaew S, Cai Z, Choksi S, Li Q, Luo J, Liu ZG. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci U S A. 2012;109:5322–5327. doi: 10.1073/pnas.1200012109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy JM, Czabotar PE, Hildebrand JM, Lucet IS, Zhang JG, Alvarez-Diaz S, Lewis R, Lalaoui N, Metcalf D, Webb AI, Young SN, Varghese LN, Tannahill GM, Hatchell EC, Majewski IJ, Okamoto T, Dobson RC, Hilton DJ, Babon JJ, Nicola NA, Strasser A, Silke J, Alexander WS. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity. 2013;39:443–453. doi: 10.1016/j.immuni.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38:209–223. doi: 10.1016/j.immuni.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Degterev A, Hitomi J, Germscheid M, Ch’en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, Hedrick SM, Gerber SA, Lugovskoy A, Yuan J. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newton K, Dugger DL, Wickliffe KE, Kapoor N, de Almagro MC, Vucic D, Komuves L, Ferrando RE, French DM, Webster J, Roose-Girma M, Warming S, Dixit VM. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science. 2014;343:1357–60. doi: 10.1126/science.1249361. [DOI] [PubMed] [Google Scholar]
- 8.Polykratis A, Hermance N, Zelic M, Roderick J, Kim C, Van TM, Lee TH, Chan FKM, Pasparakis M, Kelliher MA. Cutting edge: RIPK1 Kinase inactive mice are viable and protected from TNF-induced necroptosis in vivo. J Immunol. 2014;193:1539–43. doi: 10.4049/jimmunol.1400590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity. 1998;8:297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- 10.Gentle IE, Wong WWL, Evans JM, Bankovacki A, Cook WD, Khan NR, Nachbur U, Rickard J, Anderton H, Moulin M, Lluis JM, Moujalled DM, Silke J, Vaux DL. In TNF-stimulated cells, RIPK1 promotes cell survival by stabilizing TRAF2 and cIAP1, which limits induction of non-canonical NF-kappaB and activation of caspase-8. J Biol Chem. 2011;286:13282–13291. doi: 10.1074/jbc.M110.216226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rickard JA, O’Donnell JA, Evans JM, Lalaoui N, Poh AR, Rogers T, Vince JE, Lawlor KE, Ninnis RL, Anderton H, Hall C, Spall SK, Phesse TJ, Abud HE, Cengia LH, Corbin J, Mifsud S, Di Rago L, Metcalf D, Ernst M, Dewson G, Roberts AW, Alexander WS, Murphy JM, Ekert PG, Masters SL, Vaux DL, Croker BA, Gerlic M, Silke J. RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell. 2014;157:1175–1188. doi: 10.1016/j.cell.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 12.Dillon CP, Weinlich R, Rodriguez DA, Cripps JG, Quarato G, Gurung P, Verbist KC, Brewer TL, Llambi F, Gong YN, Janke LJ, Kelliher MA, Kanneganti TD, Green DR. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell. 2014;157:1189–202. doi: 10.1016/j.cell.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaiser WJ, Daley-Bauer LP, Thapa RJ, Mandal P, Berger SB, Huang C, Sundararajan A, Guo H, Roback L, Speck SH, Bertin J, Gough PJ, Balachandran S, Mocarski ES. RIP1 suppresses innate immune necrotic as well as apoptotic cell death during mammalian parturition. Proc Natl Acad Sci U S A. 2014;111:7753–7758. doi: 10.1073/pnas.1401857111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silke J, Rickard JA, Gerlic M. The diverse role of RIP kinases in necroptosis and inflammation. Nat Immunol. 2015;16:689–697. doi: 10.1038/ni.3206. [DOI] [PubMed] [Google Scholar]
- 15.Roderick JE, Hermance N, Zelic M, Simmons MJ, Polykratis A, Pasparakis M, Kelliher Ma. Hematopoietic RIPK1 deficiency results in bone marrow failure caused by apoptosis and RIPK3-mediated necroptosis. Proc Natl Acad Sci. 2014;111:14436–14441. doi: 10.1073/pnas.1409389111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dannappel M, Vlantis K, Kumari S, Polykratis A, Kim C, Wachsmuth L, Eftychi C, Lin J, Corona T, Hermance N, Zelic M, Kirsch P, Basic M, Bleich A, Kelliher M, Pasparakis M. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature. 2014;513:90–4. doi: 10.1038/nature13608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caton ML, Smith-Raska MR, Reizis B. Notch–RBP-J signaling controls the homeostasis of CD8 − dendritic cells in the spleen. J Exp Med. 2007;204:1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel RM, Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742–5. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 19.Peschon JJ, Torrance DS, Stocking KL, Glaccum MB, Otten C, Willis CR, Charrier K, Morrissey PJ, Ware CB, Mohler KM. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol. 1998;160:943–952. [PubMed] [Google Scholar]
- 20.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted Disruption of the MyD88 Gene Results in Loss of IL-1- and IL-18-Mediated Function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 21.Müller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–21. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 22.Tabeta K, Hoebe K, Janssen EM, Du X, Georgel P, Crozat K, Mudd S, Mann N, Sovath S, Goode J, Shamel L, Herskovits AA, Portnoy DA, Cooke M, Tarantino LM, Wiltshire T, Steinberg BE, Grinstein S, Beutler B. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat Immunol. 2006;7:156–164. doi: 10.1038/ni1297. [DOI] [PubMed] [Google Scholar]
- 23.Hirschfeld M, Ma Y, Weis JHJ, Vogel SN, Weis JHJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol. 2000;165:618–622. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- 24.Hohlbaum AM, Gregory MS, Ju ST, Marshak-Rothstein A. Fas Ligand Engagement of Resident Peritoneal Macrophages In Vivo Induces Apoptosis and the Production of Neutrophil Chemotactic Factors. J Immunol. 2001;167:6217–6224. doi: 10.4049/jimmunol.167.11.6217. [DOI] [PubMed] [Google Scholar]
- 25.Dong MB, Rahman MJ, Tarbell KV. Flow cytometric gating for spleen monocyte and DC subsets: differences in autoimmune NOD mice and with acute inflammation. J Immunol Methods. 2016;432:4–12. doi: 10.1016/j.jim.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma S, Campbell AM, Chan J, Schattgen SA, Orlowski GM, Nayar R, Huyler AH, Nündel K, Mohan C, Berg LJ, Shlomchik MJ, Marshak-Rothstein A, Fitzgerald KA. Suppression of systemic autoimmunity by the innate immune adaptor STING. Proc Natl Acad Sci U S A. 2015;112:E710–7. doi: 10.1073/pnas.1420217112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bossaller L, V, Rathinam AK, Bonegio R, Chiang PI, Busto P, Wespiser AR, Caffrey DR, Li QZ, Mohan C, Fitzgerald KA, Latz E, Marshak-Rothstein A. Overexpression of membrane-bound fas ligand (CD95L) exacerbates autoimmune disease and renal pathology in pristane-induced lupus. J Immunol. 2013;191:2104–14. doi: 10.4049/jimmunol.1300341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moujalled DM, Cook WD, Okamoto T, Murphy J, Lawlor KE, Vince JE, Vaux DL. TNF can activate RIPK3 and cause programmed necrosis in the absence of RIPK1. Cell Death Dis. 2013;4:e465. doi: 10.1038/cddis.2012.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McComb S, Cheung HH, Korneluk RG, Wang S, Krishnan L, Sad S. cIAP1 and cIAP2 limit macrophage necroptosis by inhibiting Rip1 and Rip3 activation. Cell Death Differ. 2012;19:1791–1801. doi: 10.1038/cdd.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Müller-Sienerth N, Dietz L, Holtz P, Kapp M, Grigoleit GU, Schmuck C, Wajant H, Siegmund D. SMAC Mimetic BV6 Induces Cell Death in Monocytes and Maturation of Monocyte-Derived Dendritic Cells. PLoS One. 2011;6:e21556. doi: 10.1371/journal.pone.0021556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yabal M, Müller N, Adler H, Knies N, Groß CJ, Damgaard RB, Kanegane H, Ringelhan M, Kaufmann T, Heikenwälder M, Strasser A, Groß O, Ruland J, Peschel C, Gyrd-Hansen M, Jost PJ. XIAP Restricts TNF- and RIP3-Dependent Cell Death and Inflammasome Activation. Cell Rep. 2014;7:1796–1808. doi: 10.1016/j.celrep.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 32.Birnberg T, Bar-On L, Sapoznikov A, Caton ML, Cervantes-Barragán L, Makia D, Krauthgamer R, Brenner O, Ludewig B, Brockschnieder D, Riethmacher D, Reizis B, Jung S. Lack of Conventional Dendritic Cells Is Compatible with Normal Development and T Cell Homeostasis, but Causes Myeloid Proliferative Syndrome. Immunity. 2008;29:986–997. doi: 10.1016/j.immuni.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 33.Kang TB, Yang SH, Toth B, Kovalenko A, Wallach D. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity. 2013;38:27–40. doi: 10.1016/j.immuni.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 34.Croker BA, O’Donnell JA, Gerlic M. Pyroptotic death storms and cytopenia. Curr Opin Immunol. 2014;26:128–137. doi: 10.1016/j.coi.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Vince JE, Wong WW, Gentle I, Lawlor KE, Allam R, O’Reilly L, Mason K, Gross O, Ma S, Guarda G, Anderton H, Castillo R, Hacker G, Silke J, Tschopp J. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity. 2012;36:215–227. doi: 10.1016/j.immuni.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 36.Angus DC, van der Poll T. Severe Sepsis and Septic Shock. N Engl J Med. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 37.Laouar Y, Ezine S. In vivo CD4+ lymph node T cells from lpr mice generate CD4-CD8-B220+TCR-beta low cells. J Immunol. 1994;153:3948–55. [PubMed] [Google Scholar]
- 38.Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kono DH, Haraldsson MK, Lawson BR, Pollard KM, Koh YT, Du X, Arnold CN, Baccala R, Silverman GJ, Beutler BA, Theofilopoulos AN. Endosomal TLR signaling is required for anti-nucleic acid and rheumatoid factor autoantibodies in lupus. Proc Natl Acad Sci U S A. 2009;106:12061–6. doi: 10.1073/pnas.0905441106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christensen SR, Kashgarian M, Alexopoulou L, Flavell RA, Akira S, Shlomchik MJ. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J Exp Med. 2005:202. doi: 10.1084/jem.20050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rubtsov AV, Rubtsova K, Fischer A, Meehan RT, Gillis JZ, Kappler JW, Marrack P. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c+ B-cell population is important for the development of autoimmunity. Blood. 2011;118:1305–1315. doi: 10.1182/blood-2011-01-331462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang HC, Zuo Y, Fogo AB. Models of chronic kidney disease. Drug Discov Today Dis Models. 2010;7:13–19. doi: 10.1016/j.ddmod.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He S, Liang Y, Shao F, Wang X. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc Natl Acad Sci U S A. 2011;108:20054–20059. doi: 10.1073/pnas.1116302108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, Sehon CA, Marquis RW, Bertin J, Mocarski ES. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem. 2013;288:31268–31279. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moriwaki K, Balaji S, McQuade T, Malhotra N, Kang J, Chan FKM. The Necroptosis Adaptor RIPK3 Promotes Injury-Induced Cytokine Expression and Tissue Repair. Immunity. 2014;41:567–578. doi: 10.1016/j.immuni.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beutler B, Jiang Z, Georgel P, Crozat K, Croker B, Rutschmann S, Du X, Hoebe K. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu Rev Immunol. 2006;24:353–89. doi: 10.1146/annurev.immunol.24.021605.090552. [DOI] [PubMed] [Google Scholar]
- 47.Banchereau J, Pascual V. Type I Interferon in Systemic Lupus Erythematosus and Other Autoimmune Diseases. Immunity. 2006;25:383–392. doi: 10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 48.Thapa RJ, Nogusa S, Chen P, Maki JL, Lerro A, Andrake M, Rall GF, Degterev A, Balachandran S. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proc Natl Acad Sci U S A. 2013;110:E3109–18. doi: 10.1073/pnas.1301218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brinkmann MM, Spooner E, Hoebe K, Beutler B, Ploegh HL, Kim YM. The interaction between the ER membrane protein UNC93B and TLR3, 7, and 9 is crucial for TLR signaling. J Cell Biol. 2007;177:265–275. doi: 10.1083/jcb.200612056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim YM, Brinkmann MM, Paquet ME, Ploegh HL. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature. 2008;452:234–8. doi: 10.1038/nature06726. [DOI] [PubMed] [Google Scholar]
- 51.Legarda D, Justus S, Ang R, Rikhi N, Li W, Moran T, Zhang J, Mizoguchi E, Zelic M, Kelliher M, Magaria Blander J, Ting A. CYLD Proteolysis Protects Macrophages from TNF-Mediated Auto-necroptosis Induced by LPS and Licensed by Type I IFN. Cell Rep. 2016;15:2449–2461. doi: 10.1016/j.celrep.2016.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wynn TA. Integrating mechanisms of pulmonary fibrosis. J Exp Med. 2011:208. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune respons…. J Exp Med. 1996:184. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matsumoto M, Mariathasan S, Nahm MH, Baranyay F, Peschon JJ, Chaplin DD. Role of lymphotoxin and the type I TNF receptor in the formation of germinal centers. Science. 1996;271:1289–91. doi: 10.1126/science.271.5253.1289. [DOI] [PubMed] [Google Scholar]
- 55.Kim JY, Morgan M, Kim DG, Lee JY, Bai L, Lin Y, Liu ZG, Kim YS. TNFalpha induced noncanonical NF-kappaB activation is attenuated by RIP1 through stabilization of TRAF2. J Cell Sci. 2011;124:647–656. doi: 10.1242/jcs.075770. [DOI] [PubMed] [Google Scholar]
- 56.Young JA, He TH, Reizis B, Winoto A. Commensal Microbiota Are Required for Systemic Inflammation Triggered by Necrotic Dendritic Cells. Cell Rep. 2013;3:1932–1944. doi: 10.1016/j.celrep.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cuda CM, Misharin AV, Gierut AK, Saber R, Haines GK, Hutcheson J, Hedrick SM, Mohan C, Budinger GS, Stehlik C, Perlman H. Caspase-8 Acts as a Molecular Rheostat To Limit RIPK1- and MyD88-Mediated Dendritic Cell Activation. J Immunol. 2014;192:5548–5560. doi: 10.4049/jimmunol.1400122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alvarez-Diaz S, Dillon C, Lalaoui N, Tanzer M, Rodriguez D, Lin A, Lebois M, Hakem R, Josefsson E, O’Reilly L, Silke J, Alexander W, Green D, Strasser A. The Pseudokinase MLKL and the Kinase RIPK3 Have Distinct Roles in Autoimmune Disease Caused by Loss of Death-Receptor-Induced Apoptosis. Immunity. 2016;45:513–526. doi: 10.1016/j.immuni.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rifkin IR, Leadbetter EA, Busconi L, Viglianti G, Marshak-Rothstein A. Toll-like receptors, endogenous ligands, and systemic autoimmune disease. Immunol Rev. 2005;204:27–42. doi: 10.1111/j.0105-2896.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 61.Lau CM, Broughton C, Tabor AS, Akira S, Flavell RA, Mamula MJ, Christensen SR, Shlomchik MJ, Viglianti GA, Rifkin IR, Marshak-Rothstein A. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202:1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leadbetter EA, I, Rifkin R, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–7. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 63.Hua J, Kirou K, Lee C, Crow MK. Functional assay of type I interferon in systemic lupus erythematosus plasma and association with anti–RNA binding protein autoantibodies. Arthritis Rheum. 2006;54:1906–1916. doi: 10.1002/art.21890. [DOI] [PubMed] [Google Scholar]
- 64.Sherer Y, Gorstein A, Fritzler MJ, Shoenfeld Y. Autoantibody explosion in systemic lupus erythematosus: More than 100 different antibodies found in SLE patients. Semin Arthritis Rheum. 2004;34:501–537. doi: 10.1016/j.semarthrit.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 65.Sokolove J, Bromberg R, Deane KD, Lahey LJ, Derber LA, Chandra PE, Edison JD, Gilliland WR, Tibshirani RJ, Norris JM, Holers VM, Robinson WH. Autoantibody Epitope Spreading in the Pre-Clinical Phase Predicts Progression to Rheumatoid Arthritis. PLoS One. 2012;7:e35296. doi: 10.1371/journal.pone.0035296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, Shark KB, Grande WJ, Hughes KM, Kapur V, Gregersen PK, Behrens TW. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100:2610–5. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, Pascual V. Interferon and Granulopoiesis Signatures in Systemic Lupus Erythematosus Blood. J Exp Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kirou KA, Lee C, George S, Louca K, Peterson MGE, Crow MK. Activation of the interferon-? pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52:1491–1503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- 69.Newton K, Wickliffe KE, Maltzman A, Dugger DL, Strasser A, Pham VC, Lill JR, Roose-Girma M, Warming S, Solon M, Ngu H, Webster JD, Dixit VM. RIPK1 inhibits ZBP1-driven necroptosis during development. Nature. 2016;540:129–133. doi: 10.1038/nature20559. [DOI] [PubMed] [Google Scholar]
- 70.Lin J, Kumari S, Kim C, Van TM, Wachsmuth L, Polykratis A, Pasparakis M. RIPK1 counteracts ZBP1-mediated necroptosis to inhibit inflammation. Nature. 2016;540:124–128. doi: 10.1038/nature20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T, Lu Y, Miyagishi M, Kodama T, Honda K, Ohba Y, Taniguchi T. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–5. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 72.Thapa R, Ingram J, Ragan K, Nogusa S, Boyd D, Benitez A, Sridharan H, Kosoff R, Shubina M, Landsteiner V, Andrake M, Vogel P, Sigal L, tenOever B, Thomas P, Upton J, Balachandran S. DAI Senses Influenza A Virus Genomic RNA and Activates RIPK3-Dependent Cell Death. Cell Host Microbe. 2016;20:674–681. doi: 10.1016/j.chom.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang W, Zhou Q, Xu W, Cai Y, Yin Z, Gao X, Xiong S. DNA-dependent Activator of Interferon-regulatory Factors (DAI) Promotes Lupus Nephritis by Activating the Calcium Pathway. J Biol Chem. 2013;288:13534–13550. doi: 10.1074/jbc.M113.457218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.