Abstract
Purpose
Inflammation is key risk factor for many adverse outcomes in older people. Whilst diet is a potential source of inflammation, little is known about the impact of inflammatory diet on fractures. Thus, we investigated whether higher Dietary Inflammatory Index (DII)™ scores are associated with fractures in a cohort of North American people.
Methods
This longitudinal with a follow-up of 8 years included 3,648 participants (1,577 Males and 2,071 Females; mean age: 60.6 years) with/at risk of knee osteoarthritis participating to the Osteoarthritis Initiative. DII scores were calculated using the validated Block Brief 2000 Food-Frequency Questionnaire, categorized into sex-specific quintiles. Information on fractures was obtained through self-reported history of fractures at hip, spine and forearm. The relationship between baseline DII score and incident fracture was assessed through a Cox’s regression analysis, adjusted for potential baseline confounders, and reported as hazard ratios (HRs).
Results
During 8 years of follow-up, 560 individuals developed fractures (=15.4%). Adjusting for 10 potential confounders, women in the highest DII score quintile (i.e. most proinflammatory diet) had a significantly higher risk for fractures (HR: 1.46; 95% CI: 1.02–2.11) compared to women in the lowest quintile. An increase in one standard deviation of DII scores significantly predicted fracture onset in women (adjusted HR=1.14; 95%CI: 1.02–1.27). The association between DII score and fractures was not significant among men or in the sample as whole.
Conclusion
Pro-inflammatory diet is associated with a higher incidence of fractures in women but not men.
Keywords: aged, dietary inflammatory index, osteoporosis, fracture, inflammation
INTRODUCTION
Worldwide, osteoporosis is the leading risk factor for over 8 million fractures annually, equating to an osteoporotic fracture every 3 seconds.[1] Osteoporosis and fractures are associated with several negative outcomes, such as increased risk of cardiovascular disease[2], hospitalization[3], poor quality of life[4], increased healthcare costs[4] and premature mortality.[4] Thus, elucidating the risk factors for osteoporosis and fractures is a public health priority, and key public health bodies (such as the World Health Organization) have developed strategies to prevent and manage poor bone health and fractures.[5]
Several factors are recognized as important for fractures. Physical inactivity and a sedentary lifestyle[6] are important factors for developing fragility fractures, as well as female gender[7], heavy smoking[8], alcohol drinking[9], low weight[10], psychiatric disorders (such as anorexia nervosa[11]) and the use of some medications.[12, 13]
The interest in inflammation as a potential risk factor is increasing. It is established that markers of inflammation linearly increase with age (a phenomenon commonly called “inflammaging”).[14] Several observational longitudinal studies examined the potential association between serum inflammatory markers and osteoporotic fractures, finding that higher inflammatory levels are associated with a higher risk of fractures.[15–20] These longitudinal studies have advanced our knowledge regarding this topic, but a number of limitations persist. In particular, most included only older women, and most of these subjects were White, and thus generalizability is limited.
Diet is an important source of inflammation.[21] The Dietary Inflammatory Index (DII) is a literature-derived dietary tool, which has been validated to assessed the overall inflammatory potential of individual’s diet.[21] Previous research has found that higher DII scores (indicating a more pro-inflammatory diet) are significantly associated with serum inflammatory markers (such as C-reactive protein), suggesting a close relationship between this index and bio-humoral inflammatory parameters.[22] To the best of our knowledge, two studies have been conducted to examine the relationship of DII scores and fractures. One conducted in a large sample of American women reported finding that high DII scores, indicating a more inflammatory diet, was associated with increased hip fracture risk, although this finding was limited only to White women.[23] Another, more recent, case-control study in China, substantially confirmed these findings, in both genders.[24] However, it is unclear if a more pro-inflammatory diet is associated with fractures in men because only the Chinese included both sexes.[24] Knowing about a difference by sex could be important for tailoring intervention messages related to modulating diet to reduce inflammation.
Given this background, the purpose of this study was to investigate whether pro-inflammatory diets, as measured by the DII scores, are associated with increased incidence of fractures, using the data from a large cohort of North American adults. An important goal in using this cohort is to identify potential sex differences in the association between DII and fractures.
MATERIALS AND METHODS
Data source and subjects
Data were included from the Osteoarthritis Initiative (OAI) database. The OAI is freely available (http://www.oai.ucsf.edu/). Within the OAI, potential participants were recruited across four clinical sites in the United States of America (Baltimore, MD; Pittsburgh, PA; Pawtucket, RI; and Columbus, OH) between February 2004 and May 2006. In this database, people were included if they: (1) had knee OA with knee pain for a 30-day period in the past 12 months or (2) were at high risk of developing knee OA[25] with data collected during baseline and screening evaluations in November 2008.
All participants provided informed written consent. The OAI study was given full ethical approval by the institutional review board of the OAI Coordinating Center of the University of California at San Francisco.
Dietary data and Dietary inflammatory index (exposure)
Dietary intake was assessed using a validated tool, the Block Brief 2000 Food Frequency Questionnaire (FFQ) during the baseline visit.[26] Seventy items were assessed to determine an individual’s typical food and beverage consumption over the past year. The frequency of consumption was reported at nine levels of intake from “never” to “every day”. In addition, seven dietary behavior questions were asked regarding food preparation methods and fat intake, one question on fiber intake, and 13 questions on vitamin and mineral intakes.
The details of the development of DII are extensively described elsewhere. [21] Briefly, in this updated version of the DII, 1943 articles were reviewed and scored. Forty-five food parameters, including foods, nutrients, and other bioactive compounds, were identified based on their inflammatory effect on six specific inflammatory markers, including CRP, IL-1β, IL-4, IL-6, IL-10 and tumor necrosis factor (TNF)-α. A regionally representative world database representing diet surveys from 11 countries was used as a comparative standard for each of the 45 parameters (i.e. foods, nutrients, and other food components). Intake values from this database were used to calculate the DII scores. This is explained in more detail in the DII Methods paper.[21] Briefly, a standard mean for each parameter from the representative world database was subtracted from the actual individual exposure and divided by its standard deviation to generate Z scores. These Z scores were converted to proportions (thus minimizing effects of outliers/right-skewing). These values were then doubled and 1 was subtracted to achieve symmetrical distribution with values centered on 0. The resulting value was then multiplied by the corresponding inflammatory score for each food parameter and summed across all food parameters, to obtain the overall DII score. To assess construct validity, in a longitudinal cohort of ~600 subjects followed for a year multiple DII scores derived from up to 15 24-hour dietary recall interviews and up to five 7-day dietary recalls were related to up to 5 high sensitivity(hs)-CRP measurements taken over a year. It was found that the DII predicted interval changes in hs-CRP, and that the structured assessment (7-day dietary recalls) performed as well as the multiple 24HR-derived DII scores.[27] The DII was subsequently validated in four studies among different populations with a variety of inflammatory biomarkers (i.e. interleukin, IL-6, hs-CRP, fibrinogen, homocysteine and TNF-α).[22, 28–31]
For this study, from the FFQ we calculated the DII based on energy-adjusted intake of the 24 single food parameters of the 45 possible food parameters that were available from the FFQ using the energy density approach, which calculated the DII per 4184 kJ (1000 kcal) of energy.[32] The 24 food parameters available for DII calculation in this study were vitamin B12, vitamin B6, β-carotene, carbohydrate, cholesterol, fat, fiber, folic acid, iron, magnesium, monounsaturated fat acids (MUFA), niacin, protein, polyunsaturated fatty acids (PUFA), riboflavin, saturated fat acids (SFA), selenium, thiamin, vitamin A, vitamin C, vitamin E, vitamin D, zinc, niacin, and caffeine.
Outcome
The presence of fractures at baseline and during follow-up was obtained through self-reported history of fractures at the most common sites for osteoporosis, i.e. hip, spine and forearm.[33] Information regarding fractures were collected at V01 (1 year after baseline), V03 (2 years), V05 (3 years), V06 (4 years), V08 (6 years), and V10 (8 years).
Covariates
A number of variables was identified to explore the relationship between DII and incident fractures. These included: (1) race was defined as “Whites” vs. others; (2) smoking habits as “previous/current” vs. never; (3) educational level was categorized as “college” vs. others; (4) yearly income as < or ≥ $50,000 and missing data; (5) body mass index (BMI), measured by a trained nurse; (6) co-morbidities assessed through the modified Charlson comorbidity score, with higher scores indicating an increased severity of conditions[34]; (7) daily intake of vitamin D, calcium, potassium (from food and from supplements), proteins and total energy intake; and (8) physical activity, evaluated using the Physical Activity Scale for the Elderly (PASE), a validated scale for assessing physical activity level in the elderly. The scale covers 12 different activities including: walking, sports and housework, and is scored from 0 upwards, without a maximum score.[35] Moreover, data regarding the use of drugs affecting positively bone metabolism (teriparatide, bisphosphonates, hormones) were also recorded.
Statistical analyses
Because the interaction between DII and sex in predicting fracture onset at follow-up was significant (p-value for interaction<0.0001) and the mean DII significantly differed between men and women (p<0.0001), all the analyses were stratified by sex.
All continuous variables were normally distributed according to the Kolmogorov-Smirnov test. Data were presented as means and standard deviation values (SD) for quantitative measures, and frequency and percentages for all discrete variables. Levene’s test was used to test the homoscedasticity of variances and, if its assumption was violated, Welch’s ANOVA was used. P-values were calculated using the Jonckheere-Terpstra test[36] for continuous variables and the Mantel-Haenszel Chi-square test for categorical variables.
To assess the relationship between the DII score and incident fractures, a Cox’s regression analysis was conducted where incident fracture was defined as the discrete “outcome”, time-to-event was the temporal factor, and the DII score at baseline was the “exposure”. The proportional hazards assumption was checked by plotting the Schoenfeld residuals versus time and it was confirmed that there were no violations in the assumption in any of the models. People who died during follow-up were censored on their date of death. The fully adjusted model included the following covariates: age (as continuous); total energy intake (in Kcal, as continuous); race (White vs. others); BMI (as continuous); education (college vs. others); smoking habits (current vs. previous/never); yearly income (> vs. <50,000 $); Charlson co-morbidity index; use of drugs positively affecting bone metabolism (bisphosphonates, hormones); and physical activity scale for the elderly (as continuous). Only two people used teriparatide. Thus, this factor was not included as a potential confounder in the fully-adjusted model. Nutritional parameters (such as vitamin D, calcium, protein and potassium intakes) were not included in the fully adjusted model, since these estimates were already included in compute the DII score.
Multi-collinearity among covariates was assessed through variance inflation factor (VIF) [37], taking a cut-off of 2 as the criterion for exclusion. No covariates met this criterion and therefore none was excluded for this reason. Adjusted hazard ratios (HR) and 95% confidence intervals (CI) were calculated to estimate the strength of the associations between DII score (reported in quintiles) and incident fractures. P values for trend were calculated across DII groups using the Wald test, based on a score derived from the median value of each DII group. We also used standardized values of DII as a continuous exposure variable in some analyses.
A p<0.05 was deemed statistically significant. All analyses were performed using SPSS® software version 21.0 for Windows (SPSS Inc., Chicago, Illinois).
RESULTS
Sample selection
The OAI dataset initially included a total of 4,796 individuals. For 243 participants, there were insufficient data to calculate the DII score or an unreliable caloric intake was recorded (<500 or >5000 Kcal/day). At baseline, 788 participants had experienced a fracture and were excluded from the analysis. Finally, data from another 117 participants who were lost during follow-up (no sufficient data regarding fractures) also were excluded. Accordingly, 3,648 participants were included in analyses.
Descriptive characteristics
The cohort consisted of 1,577 males and 2,071 females. Mean age was 60.6 years (±SD 9.1 years; range: 45–79 years) and mean DII was −3.16 (±SD 1.68 points; range: −5.65 to 3.70). The mean DII was significantly higher in men than in women (2.82±1.76 vs. 3.43±1.57, p<0.0001).
Tables 1 (men) and Table 2 (women) illustrate the baseline characteristics by DII quintiles. Independent of gender, participants with higher DII levels were significantly younger, consumed more calories and proteins, but less potassium, than those with lower DII values (p for trend<0.0001 for all comparisons). Women with higher DII levels had less consumption of calcium and vitamin D (p for trend<0.0001 for both comparisons) (Table 1). Regarding demographic characteristics, in both sexes, people with higher DII values were more frequently obese (p for trend<0.0001), non-white (p for trend=0.001) and less educated (p for trend<0.0001). In women, people with higher DII levels were also poorer than those with lower DII values (p for trend=0.01) (Table 1). Finally, in both sexes, people with higher DII levels took less frequently bisphosphonates (Table 1), although no significant differences emerged in terms of comorbidities.
Table 1.
Participants’ characteristics by dietary inflammatory index quintiles in men, Osteoarthritis Initiative (OAI), 2004–6.
| Q1 (n=218) |
Q2 (n=260) |
Q3 (n=304) |
Q4 (n=385) |
Q5 (n=410) |
P value* |
||
|---|---|---|---|---|---|---|---|
| Age (years) | 63.5 (9.1) | 62.1 (9.3) | 61.6 (9.5) | 59.9 (9.4) | 58.0 (9.3) | <0.0001 | |
|
| |||||||
| Nutritional parameters | |||||||
| Energy intake (Kcal/day) | 1355 (499) | 1522 (572) | 1491 (515) | 1648 (622) | 1758 (708) | <0.0001 | |
| Protein intake (g/day) | 58.3 (24.6) | 66.9 (29.0) | 63.4 (23.8) | 69.6 (29.1) | 39.6 (30.8) | <0.0001 | |
| Calcium intake (mg/day) | 700 (362) | 752 (366) | 681 (318) | 713 (350) | 682 (343) | 0.41 | |
| Potassium (mg/day) | 2872 (1138) | 2868 (1159) | 2568 (935) | 2547 (1045) | 2420 (1028) | <0.0001 | |
| Vitamin D intake (mg/day) | 536 (202) | 460 (221) | 385 (214) | 377 (240) | 188 (163) | <0.0001 | |
| Alcohol intake (% of Kcal/day) | 5.9 (6.6) | 5.9 (7.2) | 5.4 (7.1) | 6.1 (7.5) | 7.0 (9.0) | 0.06 | |
| BMI (Kg/m2) | 27.3 (6.7) | 28.2 (3.8) | 28.8 (4.2) | 29.3 (3.9) | 29.5 (4.3) | <0.0001 | |
|
| |||||||
| Demographics | |||||||
| White race (n, %) | 188 (88.6) | 228 (87.7) | 260 (85.5) | 328 (85.2) | 323 (79.0) | 0.003 | |
| Smoking (previous/current, n%) | 111 (51.4) | 137 (52.9) | 150 (49.7) | 206 (53.8) | 197 (48.4) | 0.52 | |
| College (n, %) | 97 (44.5) | 113 (43.5) | 112 (36.8) | 130 (33.8) | 126 (30.9) | <0.0001 | |
| Yearly income (<50,000 $, n, %) | 154 (70.6) | 194 (74.6) | 219 (72.0) | 269 (69.9) | 274 (66.8) | 0.27 | |
|
| |||||||
| PASE (points) | 178.4 (89.1) | 188.5 (91.3) | 172.4 (92.5) | 177.5 (85.0) | 173.6 (81.2) | 0.21 | |
|
| |||||||
| Medical conditions and medications | |||||||
| Charlson co-morbidity score | 0.5 (1.1) | 0.5 (1.0) | 0.4 (0.8) | 0.4 (0.9) | 0.4 (0.8) | 0.68 | |
| Hormones (n, %) | 0 (0.0) | 4 (1.6) | 8 (2.6) | 8 (2.1) | 3 (0.7) | 0.70 | |
| Bisphosphonates (n, %) | 6 (2.8) | 6 (2.3) | 6 (2.0) | 5 (1.3) | 1 (0.2) | 0.005 | |
Abbreviations: BMI: body mass index; PASE: Physical Activity Scale for Elderly.
P values for trends were calculated using the Jonckheere-Terpstra test for continuous variables and the Mantel-Haenszel Chi-square test for categorical variables.
Data are means (with standard deviation) or number (and percentage) as appropriate.
Q1 indicates participants having the lowest dietary inflammatory index values; Q5 the highest.
Table 2.
Participants’ characteristics by dietary inflammatory index quintiles in women, Osteoarthritis Initiative (OAI), 2004–6.
| Q1 (n=512) |
Q2 (n=470) |
Q3 (n=425) |
Q4 (n=345) |
Q5 (n=319) |
P value* |
||
|---|---|---|---|---|---|---|---|
| Age (years) | 62.4 (8.7) | 61.8 (9.0) | 60.2 (8.4) | 60.0 (9.1) | 57.3 (8.2) | <0.0001 | |
|
| |||||||
| Nutritional parameters | |||||||
| Energy intake (Kcal/day) | 1161 (394) | 1263 (470) | 1289 (481) | 1329 (523) | 1435 (636) | <0.0001 | |
| Protein intake (g/day) | 52.8 (19.9) | 57.4 (23.6) | 57.0 (22.7) | 56.4 (23.4) | 55.8 (30.0) | 0.02 | |
| Calcium intake (mg/day) | 673 (346) | 675 (331) | 631 (303) | 628 (307) | 576 (331) | <0.0001 | |
| Potassium (mg/day) | 2625 (996) | 2522 (976) | 2278 (860) | 2169 (873) | 1977 (980) | <0.0001 | |
| Vitamin D intake (mg/day) | 582 (200) | 490 (221) | 436 (236) | 349 (231) | 167 (143) | <0.0001 | |
| Alcohol intake (% of Kcal/day) | 3.0 (4.7) | 3.4 (5.4) | 4.0 (6.5) | 4.2 (6.7) | 3.6 (6.8) | 0.03 | |
| BMI (Kg/m2) | 27.2 (4.8) | 28.1 (4.8) | 28.9 (5.4) | 28.9 (5.4) | 30.7 (5.8) | <0.0001 | |
|
| |||||||
| Demographics | |||||||
| White race (n, %) | 390 (76.3) | 362 (77.0) | 323 (76.0) | 269 (78.0) | 200 (62.7) | 0.001 | |
| Smoking (previous/current, n%) | 304 (59.7) | 261 (55.8) | 237 (56.2) | 185 (53.8) | 153 (48.1) | 0.002 | |
| College (n, %) | 160 (31.3) | 121 (25.8) | 111 (26.1) | 76 (22.0) | 54 (16.9) | <0.0001 | |
| Yearly income (<50,000 $, n, %) | 289 (56.4) | 241 (51.3) | 236 (55.5) | 188 (54.5) | 134 (42.0) | 0.01 | |
|
| |||||||
| PASE (points) | 158.0 (76.7) | 152.7 (75.8) | 150.8 (78.0) | 148.9 (73.6) | 155.0 (82.5) | 0.46 | |
|
| |||||||
| Medical conditions and medications | |||||||
| Charlson co-morbidity score | 0.3 (0.6) | 0.4 (0.8) | 0.4 (0.8) | 0.4 (0.7) | 0.4 (1.0) | 0.17 | |
| Hormones (n, %) | 8 (1.8) | 9 (2.1) | 11 (2.9) | 5 (1.7) | 4 (1.4) | 0.78 | |
| Bisphosphonates (n, %) | 108 (21.1) | 90 (19.2) | 71 (16.8) | 55 (16.0) | 26 (8.2) | <0.0001 | |
Abbreviations: BMI: body mass index; PASE: Physical Activity Scale for Elderly.
P values for trends were calculated using the Jonckheere-Terpstra test for continuous variables and the Mantel-Haenszel Chi-square test for categorical variables.
Data are means (with standard deviation) or number (and percentage) as appropriate.
Q1 indicates participants having the lowest dietary inflammatory index values; Q5 the highest.
Dietary inflammatory index and incident fractures
Over a mean follow-up of 8 years, 560 individuals (198 men and 362 women; =15.4% of the baseline population) developed a fracture for a global incidence of 24 (95%CI: 22–26) events for 1,000 persons-year.
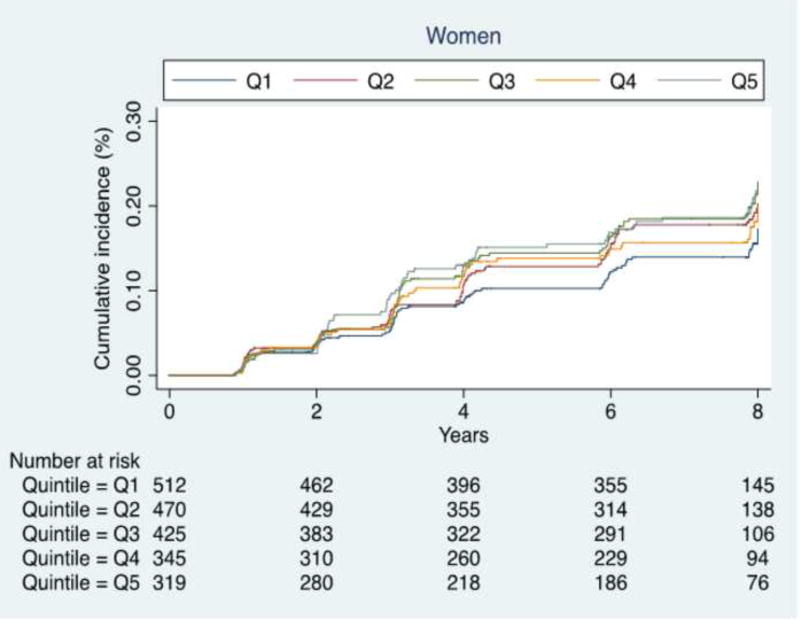
As shown in Table 3, the incidence of fractures significantly increased with increasing DII scores (indicating higher pro-inflammatory diet) in women (Cox’s regression analysis, p <0.0001, Figure 1), but not in men (p =0.82). Cox’s regression analysis, adjusting for 10 potential confounders at baseline, with the lowest DII as reference (=Q1), showed that women with the highest DII score (=Q5) had a significantly higher risk for incident fracture (HR: 1.46; 95% CI: 1.02–2.11; p=0.04; Table 3). This finding was not significant in men (Q5: HR=0.91; 95%CI: 0.54–1.54; p=0.73) or in the sample as whole (Q5: HR=1.22; 95%CI: 0.91–1.64; p=0.19).
Table 3.
Association between baseline dietary inflammatory index quintiles and incident fractures, Osteoarthritis Initiative (OAI), 2004–6.
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| DII | Cases | Subjects | Incidence rate |
HR* (95%CI) p-value |
Cases | Subjects | Incidence rate |
HR* (95%CI) p-value |
| Q1 | 25 | 218 | 17 | 1 | 77 | 77 | 24 | 1 |
| (12–25) | [reference] | (19–29) | [reference] | |||||
| Q2 | 39 | 280 | 23 | 1.21 | 81 | 470 | 28 | 1.11 |
| (17–32) | (0.72–2.04) | (22–34) | (0.81–1.53) | |||||
| 0.47 | ||||||||
| Q3 | 45 | 304 | 24 | 1.29 | 86 | 425 | 32 | 1.42 |
| (18–32) | (0.78–2.14) | (26–40) | (0.98–1.96) | |||||
| 0.33 | 0.07 | |||||||
| Q4 | 44 | 385 | 18 | 0.91 | 59 | 345 | 27 | 1.26 |
| (13–24) | (0.55–1.53) | (21–35) | (0.89–1.78) | |||||
| 0.73 | 0.20 | |||||||
| Q5 | 45 | 410 | 18 | 0.91 | 59 | 319 | 32 | 1.46 |
| (13–24) | (0.54–1.54) | (25–41) | (1.02–2.11) | |||||
| 0.73 | 0.04 | |||||||
Data are presented as hazard ratios (HRs) with correspondent 95% confidence intervals (CI).
Q1 indicates participants having the lowest dietary inflammatory index values; Q5 the highest.
Fully adjusted hazard ratios included as covariates: age (as continuous); total energy intake (in Kcal, as continuous); race (white vs. others); body mass index (as continuous); education (college vs. others); smoking habits (current vs. previous/never); yearly income (> vs. <50,000 $); Charlson co-morbidity index; use of drugs affecting positively bone metabolism (bisphosphonates, hormones); physical activity scale for the elderly (as continuous).
Figure 1. Risk of fractures in women by dietary inflammatory index quintiles at baseline, Osteoarthritis Initiative (OAI), 2004–6.
Q1 indicates participants having the lowest dietary inflammatory index values; Q5 the highest. P values shown are for trends and were calculated using the Jonckheere-Terpstra test for continuous variables.
An increase in one SD of DII (=1.68 points) increased the risk of fracture at follow-up in women (adjusted HR=1.14; 95%CI: 1.02–1.27; p=0.02), but not in men (adjusted HR=0.95; 95%CI: 0.82–1.11; p=0.54). However, the p for trend did not reach the statistical significance in either gender (p for trend=0.40 in men; p for trend=0.13 in women) (data shown only in text).
DISCUSSION
In this longitudinal study, we found that a more pro-inflammatory diet, as indicated by higher DII scores, were associated with higher incidence of fractures in women. During a follow-up period of 8 years, after adjusting for several potential confounders at baseline, women with the highest DII score (i.e. having a more pro-inflammatory diet) had a 46% higher risk of fracture. These findings, however, were not significant in men, suggesting important sex differences.
At baseline, women having higher DII values were significantly younger than those having lower values, but they experienced a higher risk of fractures in agreement with another study regarding the same topic. [23] In this regard, our study found similar results with another large cohort study conducted among American people which did not finding any significant associations between serum markers of inflammation (such as CRP and IL-6SR) and incident fractures.[18] The findings that higher DII values at baseline significantly predicted fractures in women are in agreement with one study on DII and incident fractures made in a large cohort of American women,[23] but not with another case-control study conducted in China, which found an association between DII score and hip fracture in both sexes.[24] However, it might be difficult to directly compare the findings of a case-control study to those of a longitudinal study, since it is hard to estimate the population risk from the case-control design versus the cohort design. We can offer some hypotheses to explain these findings. First, a methodological reason; because women were more well represented than men in the OAI study, it is possible that in men the association was not significant due to a lack of power. However, it should be noted that in the sample as whole the association between DII and incident fractures was not significant suggesting that physiopathological reasons are probably more important. Second, other studies reported that inflammation has different roles and mechanisms between men and women (e.g. for cardiovascular diseases)[38]; so, it is possible that for fractures the effect of inflammation is more evident in women than in men. More studies are needed because one large cohort study reported that higher serum CRP levels are associated with a higher fracture risk in both men and women. [39] However, other large cohort studies found that the association between inflammation and fractures is stronger in women than in men indirectly confirming our results.[18–20]
Our findings substantially confirmed those already present in literature that suggest that higher serum inflammation levels can lead to higher fracture rate.[15–17, 40] Inflammation can reduce bone mass density through several mechanisms.[41] It has been reported that some pro-inflammatory cytokines might play potential critical roles in the pathogenesis of perimenopausal and late-life osteoporosis.[42] Several inflammatory cytokines seem to be involved in this process, including C reactive protein, tumor necrosis alpha, IL 1 and IL6.[41] Briefly, it was reported that the pro-inflammatory cytokines can lead to an over-activation of osteoclasts and to a reduction of osteoblasts activity. [41]
We believe that our findings might have important clinical consequences given the trend toward more pro-inflammatory dietary patterns (i.e. diets rich in refined foods, sugars, flour or starch such as diet typical of North American people) is increasing[43] and in women, in which both osteoporosis and fractures are more common than in men, can have more deleterious effects on bone metabolism. In this sense, other studies showed that healthy dietary patterns (such as Mediterranean diet) are associated with a lower risk of fracture, particularly at hip [44–47] and it is conceivable that these patterns have a reduced inflammatory power. DII is linearly associated with serum markers of inflammation[22, 27–31], and consequently the possibility of modulating inflammation with a healthier diet might provide important benefits to protect against fractures. Thus, encouraging people (particularly women) to eat healthier to prevent fractures can be of public health importance.
The findings of our research should be considered within its limitations. The principal shortcoming is that we used self-reported information for adjudicating fractures and this can underestimate the incidence of some osteoporotic fractures, such as vertebral ones.[48] Second, the comorbid medical conditions assessed in this study were self-reported, introducing a possible recall bias. Another limitation could be the non-availability of data on the remaining 21 food parameters of the DII. Some components (such as turmeric, saffron and eugenol) are not consumed in high quantity in this population, so non-availability of these food parameters may not have played major role in association. However, inclusion of parameters such as flavonoids, which are commonly consumed, may influence the results. Finally, the findings derived from the OAI include people with knee OA or at higher risk of knee OA; therefore, they may not be fully generalizable to other populations. Despite these limitations, this study is one of the few including both sexes and including not only White participants.
In conclusion, higher DII scores were associated with a higher incidence of fractures, even after considering several potentially important confounders measured at baseline, only in women. Future randomized controlled trials with diets rich in anti-inflammatory components are, however, needed to confirm establish causality and consider if such interventions can reduce the incidence of fractures.
Acknowledgments
Funding sources: The OAI is a public-private partnership comprised of five contracts(N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using an OAI public use data set and does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partners. NS and JRH were supported by the United States National Institute for Diabetes, Digestive and Kidney Diseases (grant no. R44DK103377).
Sponsor’s role: the sponsors had no role in the design, methods, subject recruitment, data collection, analysis or preparation of this paper.
Conflict of interest: Dr. JRH owns controlling interest in Connecting Health Innovations LLC (CHI), a company planning to license the right to his invention of the dietary inflammatory index (DII) from the University of South Carolina to develop computer and smart phone applications for patient counseling and dietary intervention in clinical settings. Dr. NS is an employee of CHI. Prof. Reginster received consulting fees or paid advisory boards from IBSA-GENEVRIER, MYLAN, RADIUS HEALTH, PIERRE FABRE; lecture fees when speaking at the invitation of sponsor: IBSA-GENEVRIER, MYLAN, CNIEL, DAIRY RESEARCH COUNCIL (DRC) and grant supports from IBSA-GENEVRIER, MYLAN, CNIEL, RADIUS HEALTH.
References
- 1.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2006;17:1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 2.Veronese N, Stubbs B, Crepaldi G, et al. Relationship Between Low Bone Mineral Density and Fractures With Incident Cardiovascular Disease: A Systematic Review and Meta-Analysis. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2017 doi: 10.1002/jbmr.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iqbal MM. Osteoporosis: epidemiology, diagnosis, and treatment. Southern medical journal. 2000;93:2–18. doi: 10.1097/00007611-200093010-00002. [DOI] [PubMed] [Google Scholar]
- 4.Lips P, van Schoor NM. Quality of life in patients with osteoporosis. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2005;16:447–455. doi: 10.1007/s00198-004-1762-7. [DOI] [PubMed] [Google Scholar]
- 5.Organization WH. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organization technical report series. 1994;843:1–129. [PubMed] [Google Scholar]
- 6.Nguyen TV, Sambrook PN, Eisman JA. Bone loss, physical activity, and weight change in elderly women: the Dubbo Osteoporosis Epidemiology Study. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1998;13:1458–1467. doi: 10.1359/jbmr.1998.13.9.1458. [DOI] [PubMed] [Google Scholar]
- 7.Cawthon PM. Gender differences in osteoporosis and fractures. Clinical orthopaedics and related research. 2011;469:1900–1905. doi: 10.1007/s11999-011-1780-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanis JA, Johnell O, Oden A, et al. Smoking and fracture risk: a meta-analysis. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2005;16:155–162. doi: 10.1007/s00198-004-1640-3. [DOI] [PubMed] [Google Scholar]
- 9.Kanis JA, Johansson H, Johnell O, Oden A, De Laet C, Eisman JA, Pols H, Tenenhouse A. Alcohol intake as a risk factor for fracture. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2005;16:737–742. doi: 10.1007/s00198-004-1734-y. [DOI] [PubMed] [Google Scholar]
- 10.De Laet C, Kanis JA, Oden A, et al. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2005;16:1330–1338. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 11.Solmi M, Veronese N, Correll CU, Favaro A, Santonastaso P, Caregaro L, Vancampfort D, Luchini C, De Hert M, Stubbs B. Bone mineral density, osteoporosis, and fractures among people with eating disorders: a systematic review and meta-analysis. Acta psychiatrica Scandinavica. 2016 doi: 10.1111/acps.12556. [DOI] [PubMed] [Google Scholar]
- 12.Rizzoli R, Cooper C, Reginster JY, et al. Antidepressant medications and osteoporosis. Bone. 2012;51:606–613. doi: 10.1016/j.bone.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 13.Vestergaard P, Rejnmark L, Mosekilde L. Anxiolytics, sedatives, antidepressants, neuroleptics and the risk of fracture. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2006;17:807–816. doi: 10.1007/s00198-005-0065-y. [DOI] [PubMed] [Google Scholar]
- 14.Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing research reviews. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbour KE, Lui LY, Ensrud KE, Hillier TA, LeBlanc ES, Ing SW, Hochberg MC, Cauley JA. Inflammatory markers and risk of hip fracture in older white women: the study of osteoporotic fractures. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2014;29:2057–2064. doi: 10.1002/jbmr.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eriksson AL, Movérare-Skrtic S, Ljunggren Ö, Karlsson M, Mellström D, Ohlsson C. High-Sensitivity CRP Is an Independent Risk Factor for All Fractures and Vertebral Fractures in Elderly Men: The MrOS Sweden Study. Journal of Bone and Mineral Research. 2014;29:418–423. doi: 10.1002/jbmr.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishii S, Cauley JA, Greendale GA, Crandall CJ, Danielson ME, Ouchi Y, Karlamangla AS. C-Reactive Protein, Bone Strength, and Nine-Year Fracture Risk: Data From the Study of Women’s Health Across the Nation (SWAN) Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2013;28 doi: 10.1002/jbmr.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cauley JA, Barbour KE, Harrison SL, Cloonan YK, Danielson ME, Ensrud KE, Fink HA, Orwoll ES, Boudreau R. Inflammatory Markers and the Risk of Hip and Vertebral Fractures in Men: the Osteoporotic Fractures in Men (MrOS) J Bone Miner Res. 2016;31:2129–2138. doi: 10.1002/jbmr.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ing SW, Orchard TS, Lu B, LaMonte MJ, Barbour KE, Cauley JA, Jackson RD. TNF Receptors Predict Hip Fracture Risk in the WHI Study and Fatty Acid Intake Does Not Modify This Association. J Clin Endocrinol Metab. 2015;100:3380–3387. doi: 10.1210/JC.2015-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbour KE, Boudreau R, Danielson ME, et al. Inflammatory markers and the risk of hip fracture: the Women's Health Initiative. J Bone Miner Res. 2012;27:1167–1176. doi: 10.1002/jbmr.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public health nutrition. 2014;17:1689–1696. doi: 10.1017/S1368980013002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tabung FK, Steck SE, Zhang J, et al. Construct validation of the dietary inflammatory index among postmenopausal women. Annals of epidemiology. 2015;25:398–405. doi: 10.1016/j.annepidem.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orchard T, Yildiz V, Steck SE, et al. Dietary Inflammatory Index, Bone Mineral Density, and Risk of Fracture in Postmenopausal Women: Results From the Women's Health Initiative. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2016 doi: 10.1002/jbmr.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang ZQ, Cao WT, Shivappa N, Hebert JR, Li BL, He J, Tang XY, Liang YY, Chen YM. Association Between Diet Inflammatory Index and Osteoporotic Hip Fracture in Elderly Chinese Population. Journal of the American Medical Directors Association. 2017;18:671–677. doi: 10.1016/j.jamda.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 25.Eby GA, Eby KL. Rapid recovery from major depression using magnesium treatment. Medical Hypotheses. 2006;67:362–370. doi: 10.1016/j.mehy.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 26.Block G, Hartman AM, Naughton D. A reduced dietary questionnaire: development and validation. Epidemiology (Cambridge, Mass) 1990;1:58–64. doi: 10.1097/00001648-199001000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Shivappa N, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, Tabung F, Hebert JR. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS) Public health nutrition. 2014;17:1825–1833. doi: 10.1017/S1368980013002565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wirth MD, Shivappa N, Davis L, et al. Construct validation of the Dietary Inflammatory Index among African Americans. J Nutr Health Aging. 2016:1–5. doi: 10.1007/s12603-016-0775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wirth MD, Burch J, Shivappa N, et al. Association of a Dietary Inflammatory Index With Inflammatory Indices and Metabolic Syndrome Among Police Officers. J Occup Environ Med. 2014;56:986–989. doi: 10.1097/JOM.0000000000000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramallal R, Toledo E, Martinez-Gonzalez MA, Hernandez-Hernandez A, Garcia-Arellano A, Shivappa N, Hebert JR, Ruiz-Canela M. Dietary Inflammatory Index and Incidence of Cardiovascular Disease in the SUN Cohort. PloS one. 2015;10 doi: 10.1371/journal.pone.0135221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shivappa N, Hebert JR, Rietzschel ER, De Buyzere ML, Langlois M, Debruyne E, Marcos A, Huybrechts I. Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study. The British journal of nutrition. 2015;113:665–671. doi: 10.1017/S000711451400395X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. The American journal of clinical nutrition. 1997;65:1220S–1228S. doi: 10.1093/ajcn/65.4.1220S. discussion 1229S-1231S. [DOI] [PubMed] [Google Scholar]
- 33.Veronese N, Stubbs B, Solmi M, Noale M, Vaona A, Demurtas J, Maggi S. Dietary magnesium intake and fracture risk: data from a large prospective study. The British journal of nutrition. 2017;117:1570–1576. doi: 10.1017/S0007114517001350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Medical care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The physical activity scale for the elderly (PASE): evidence for validity. Journal of clinical epidemiology. 1999;52:643–651. doi: 10.1016/s0895-4356(99)00049-9. [DOI] [PubMed] [Google Scholar]
- 36.Jonckheere AR. A distribution-free k-sample test against ordered alternatives. Biometrika. 1954;41:133–145. [Google Scholar]
- 37.Miles J. Tolerance and variance inflation factor. Wiley StatsRef: Statistics Reference Online 2009 [Google Scholar]
- 38.Fairweather D. Sex Differences in Inflammation During Atherosclerosis. Clinical Medicine Insights Cardiology. 2014;8:49–59. doi: 10.4137/CMC.S17068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dahl K, Ahmed LA, Joakimsen RM, Jorgensen L, Eggen AE, Eriksen EF, Bjornerem A. High-sensitivity C-reactive protein is an independent risk factor for non-vertebral fractures in women and men: The Tromso Study. Bone. 2015;72:65–70. doi: 10.1016/j.bone.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 40.Pasco JA, Kotowicz MA, Henry MJ, et al. High-sensitivity c-reactive protein and fracture risk in elderly women. Jama. 2006;296:1349–1355. doi: 10.1001/jama.296.11.1353. [DOI] [PubMed] [Google Scholar]
- 41.Ginaldi L, Di Benedetto MC, De Martinis M. Osteoporosis, inflammation and ageing. Immunity & ageing : I & A. 2005;2:14–14. doi: 10.1186/1742-4933-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. The New England journal of medicine. 1995;332:305–311. doi: 10.1056/NEJM199502023320506. [DOI] [PubMed] [Google Scholar]
- 43.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Current opinion in lipidology. 2002;13:3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Fung TT, Feskanich D. Dietary patterns and risk of hip fractures in postmenopausal women and men over 50 years. Osteoporosis International. 2015;26:1825–1830. doi: 10.1007/s00198-015-3081-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haring B, Crandall CJ, Wu C, et al. Dietary Patterns and Fractures in Postmenopausal Women. JAMA internal medicine. 2016 doi: 10.1001/jamainternmed.2016.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feart C, Lorrain S, Coupez VG, Samieri C, Letenneur L, Paineau D, Barberger-Gateau P. Adherence to a Mediterranean diet and risk of fractures in French older persons. Osteoporosis International. 2013;24:3031–3041. doi: 10.1007/s00198-013-2421-7. [DOI] [PubMed] [Google Scholar]
- 47.Romero Pérez A, Rivas Velasco A. Adherence to Mediterranean diet and bone health. Nutricion hospitalaria. 2014;29 doi: 10.3305/nh.2014.29.5.7332. [DOI] [PubMed] [Google Scholar]
- 48.R S. Report assessing vertebral fractures. Journal of Bone and Mineral Research. 1995;10:518–523. doi: 10.1002/jbmr.5650100403. [DOI] [PubMed] [Google Scholar]