Abstract
Background
Risk for posttraumatic stress disorder (PTSD) is thought to be mediated by gene × environment (G×E) interactions that affect core cognitive processes such as fear learning. The catechol-O-methyltransferase (COMT) val158met polymorphism has been associated with risk for PTSD and impaired fear inhibition. We used a large, relatively homogenous population to: (1) replicate previous findings of poor fear inhibition in COMT Met/Met carriers with PTSD; (2) determine if COMT association with fear inhibition is moderated by childhood trauma (CT), an environmental risk factor for PTSD; and (3) determine if COMT is associated with altered fear processing after recent exposure to combat trauma.
Methods
Male Marines and Navy Corpsmen of European-American ancestry were assessed prior to (n = 714) and 4–6 months after deployment to Afghanistan (n = 452). Acquisition and extinction of fear-potentiated startle, childhood and combat trauma history, and PTSD diagnosis were assessed at both time points.
Results
Before deployment, Met/Met genotype was associated with fear inhibition deficits in participants with current PTSD; however this association was dependent on CT exposure. After deployment, combat trauma was associated with a modest reduction in fear extinction in Met/Met compared to Val/Val carriers. There were no associations of COMT genotype with fear extinction within healthy and non-traumatized individuals.
Conclusions
These findings support the hypothesis that G×E interactions underlie associations of COMTval158met with fear inhibition deficits. These studies confirm that Met/Met carriers with PTSD have poor fear inhibition, and support further research in understanding how this polymorphism might impact response to extinction-based therapies.
Keywords: COMT polymorphism, PTSD, trauma, childhood trauma, fear extinction, Marines
Introduction
Posttraumatic stress disorder (PTSD) affects 7–8% of the American population and up to 20% of military veterans (Pace & Heim, 2011). The risk of developing PTSD, which is characterized by intrusive re-experiencing of a traumatic event, avoidance of trauma-related stimuli and hyperarousal, is close to 40% in individuals who endorsed a sufficiently high number of traumatic events (Kolassa, Kolassa, Ertl, Papassotiropoulos, & De Quervain, 2010; Neuner et al., 2004). In the US, 61% of men and 51% of women endorsed at least one trauma in their lifetime (Kessler, Ruscio, Shear, & Wittchen, 2010; Kessler, Sonnega, Bromet, Hughes, & Nelson, 1995). Because only a fraction of those that experience trauma go on to develop chronic PTSD symptoms, understanding the biological risk factors that contribute to PTSD is important and may lead to development of novel prophylactic and treatment targets.
In humans, a commonly carried single nucleotide polymorphism (SNP) in the coding sequence for COMT, with valine (Val) being substituted by methionine (Met) at amino acid residue 158, results in a 40% reduction in COMT enzymatic activity in Met/Met carriers, leading to increased catecholamine tone in Met/Met carriers, particularly in the cortex (Chen et al., 2004). This SNP (rs4680) has been associated with risk for several neuropsychiatric disorders, although these associations are controversial and inconsistent (Almli, Fani, Smith, & Ressler, 2013; Clark et al., 2013; Goenjian et al., 2014). Some human studies reported that Met/Met carriers are at higher risk to develop PTSD by exhibiting impaired affective function, reduced fear extinction, and exaggerated potentiated startle reflex and anxiety (Enoch, Xu, Ferro, Harris, & Goldman, 2003; Lonsdorf et al., 2009; Montag et al., 2008; Zubieta et al., 2003). Nevertheless, the relationship between COMTval158met polymorphism and PTSD remains inconclusive (Li et al., 2016), suggesting the importance of gene × environment interactions in the development of PTSD. Although several studies have shown COMTval158met polymorphism × trauma interactions on risk for PTSD, it remains unclear how the SNP interacts with other PTSD risk factors such as early life stress or recent trauma (Boscarino, Erlich, Hoffman, & Zhang, 2012; Kolassa et al., 2010; Valente et al., 2011).
PTSD is associated with increased fear responding, reduced safety signal learning, and poor fear extinction (Acheson et al., 2015b; Jovanovic & Norrholm, 2011). Although previous studies have indicated that homozygous Met carriers similarly exhibit increased conditioned fear, reduced safety signal learning, and/or impaired fear extinction (Lonsdorf et al., 2009; Norrholm et al., 2013; Wendt et al., 2015), the results are inconsistent as to whether these alterations are found in healthy subjects or in subjects with current PTSD symptoms only. Childhood trauma is a strong predictor of PTSD in adulthood, and is well known to interact with genes to modulate PTSD risk (Mehta & Binder, 2012). However, it is not clear how early life stress may modify COMT associations with fear learning processes or if these processes are altered by recent trauma exposure. In the present study we used a large, relatively homogenous population to: (1) replicate previous findings of altered fear processes in COMT Met/Met carriers with and without PTSD; (2) determine if COMT associations with fear learning and inhibition are moderated by childhood trauma, a strong environmental risk factor for PTSD; and (3) determine if COMT is associated with altered fear processing after exposure to recent combat trauma.
Methods
Study design and participants
The second phase of the Marine Resiliency study (MRS-II; Baker et al., 2012) involved a prospective longitudinal study of ≈ 1200 U.S. Marines and Navy Corpsmen that were deployed to Afghanistan. The U.S. Marines were from infantry battalions tested between October 2011 and October 2013, at bases in Southern California. Participants of European-American ancestry were assessed prior to (n = 714) and approximately 4–6 months after deployment to Afghanistan (n = 452) (see below for exclusion details). The study, for which all participants provided written informed consent, was approved by the institutional review boards of the University of California San Diego, Veterans Affairs San Diego Research Service, and Naval Health Research Center. Only males were included in the study, since infantry battalions did not include females at the time of assessment.
Genotyping
COMTval158met SNP (rs4680) was genotyped as previously described (Nievergelt et al., 2015). Briefly, blood samples were collected at the first visit and blood leukocytes were isolated. Genomic DNA was prepared from blood leukocytes at the Biomedical Genomics Microarray (BioGeM) Core facility (University of California San Diego, La Jolla, CA, USA) using the automated AutoGenFlex Star System, quantified with the Nanodrop 1000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA). Genotyping was performed by RUCDR Infinite Biologics (Piscataway, NJ, USA) with the human OmniExpress Exome array (Illumina Inc., San Diego, CA, USA). The assay call and reproducibility rates were > 99%. SNPs for control samples (identified as those with no PTSD diagnosis) respected the Hardy-Weinberg Equilibrium (p < 5 × 10−8). To control for race/ethnicity we used ancestry identification using genetic markers as previously described (Nievergelt et al., 2013), to identify 4 possible ancestry categories: African-American, Hispanic/Native American, European-American and East Asian/other. All ancestries other than European-American were excluded to reduce population stratification. Within European-Americans a principal component analysis (PCA) (Nievergelt et al., 2015), implemented in EIGENSTRAT (Price et al., 2006) was performed to control for additional genetic background heterogeneity. PCAs were then used as covariates in all statistical models.
Childhood Trauma Questionnaire
Before deployment, participants completed a modified 34-item (25–170 range) Childhood Trauma Questionnaire (CTQ; Bernstein et al., 1994) to assess traumatic experiences (emotional, physical and sexual abuse and emotional and physical neglect) during childhood. Presence of childhood trauma was determined based on cut-off scores as previously described (Agorastos et al., 2014): emotional abuse: ≥13; physical abuse: ≥10; sexual abuse: ≥8; emotional neglect ≥15; physical neglect: ≥10. It has been shown that risk for PTSD and depression symptoms were similar for both single and multiple childhood traumatic event type (Agorastos et al., 2014). Therefore, we divided the childhood traumatic experiences in two categories: 0 (no childhood trauma) or 1 (at least one type of childhood trauma).
Combat experiences and stress
After deployment, combat trauma and stress during deployment were measured using the Deployment Risk and Resilience Inventory-2 (DRRI-2), with high criterion validity and internal consistency (0.92) (Vogt et al., 2013). From the questionnaire, a total Combat Experience Score (CES) was calculated that encompassed all the traumatic and stressful events during deployment. To determine the role of trauma exposure (CES, 0–56 possible score), we used a validated approach (Collings, Valjee, & Penning, 2013; DeSantis et al., 2011): a median was calculated and the CES was divided in two groups: low (CES score ≤ 12) and high (CES score > 12).
Assessment of psychiatric symptoms
As previously described (Baker et al., 2012; Glenn et al., 2016), the Clinician-Administered PTSD Scale (CAPS), an interview designed to assess DSM-IV PTSD symptoms, was administered (Blake et al., 1995; Smith, Redd, DuHamel, Vickberg, & Ricketts, 1999; Weathers, Keane, & Davidson, 2001). Four CAPS subscales were assessed based on symptoms: re-experiencing (B1–5); avoidance (C1–2); numbing (C4–6); and hyperarousal (D1–5). PTSD symptom severity was measured by the CAPS total score (0–136 range). PTSD diagnosis was defined based on modified “subthreshold” guidelines, specifically at least one criterion A event, one cluster B symptom, and either three cluster C or two cluster D symptoms (Blanchard et al., 1996). Depression symptoms were assessed via self-report using the Beck Depression Inventory (BDI-II), which scores depressive symptoms as moderate to severe (> 19) within the past two weeks (Beck, Steer, Ball, & Ranieri, 1996).
Fear conditioning protocol
Fear conditioning was performed as previously described (Acheson, Eyler, Resovsky, Tsan, & Risbrough, 2015a; Orcutt et al., 2016). Briefly, a SR-HLAB Electromyography (EMG) system (San Diego Instruments, San Diego, CA, USA) was used to deliver startle pulses (108 dB, 40 ms). The air puff (250 psi) was delivered through a plastic tube positioned 2.5 cm from the center of the throat and stimuli were presented to the participant using E-Prime Software (Psychology Software Tools Inc., Sharpsburg, PA, USA). Eyeblink EMG responses were recorded through Ag/Ag 3 M Red Dot electrodes (resistance < 10 kΩ) placed on the orbicularis oculi muscle at the left eye. A reference electrode was placed at the mastoid bone behind the left ear.
The fear conditioning task was divided in two sessions: acquisition and extinction phases. Before the acquisition phase, each participant was instructed that one of two colored symbols presented was associated with an air puff. For stabilization of startle responding, each phase began with 6 startle pulses without stimuli. During the acquisition phase, 8 conditioned stimuli (CS+; danger signal; either a blue or yellow circle or square) were presented for 6 s followed by an air puff in 75% contingency (previously measured and described; Acheson et al., 2015b), and 8 non-reinforced stimuli (CS-; safety signal; either a blue or yellow circle or square) were never paired with an air puff, and 8 startle stimuli were presented in the absence of any stimuli (noise-alone trial). The noise-alone trials were used as an index of baseline startle across the phase. The startle pulses were presented 4 s after the onset of CS+ or CS- signal, and the stimuli (blue or yellow circles or squares) paired with the CS+ and CS- signals were randomly assigned across subjects. After the acquisition phase, participants rested for 5 min and were then told to remember the associations they have learned before starting the extinction phase. During the extinction phase, each stimulus (CS+, CS- and noise-alone) was presented 16 times, but no air puff was presented. Fear extinction learning in this task is relatively similar after both short (less than 10 min) or long delays (72 hrs) after fear acquisition learning (Norrholm et al., 2008).
Statistical analysis
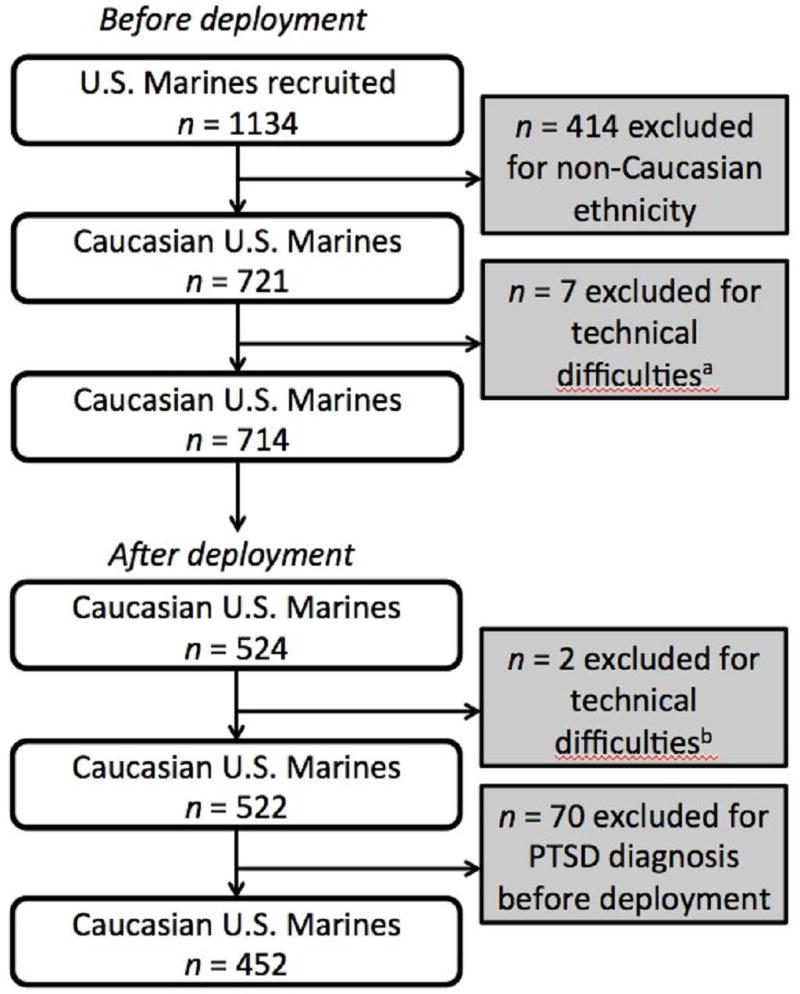
A total of 1134 U.S. Marines were assessed at pre-deployment. Only participants of European-American ancestry (EA; n = 714) were included in the study due to potential population stratification (Met allele is less frequent in African American, Hispanic, and Asian populations) (DeMille et al., 2002; Palmatier, Kang, & Kidd, 1999). Data on 7 participants were excluded from analysis due to technical difficulties during testing that resulted in low signal/noise ratio that precluded accurate signal measurement. Of the 714 EA participants analyzed in the pre-deployment phase, 524 completed all the questionnaires and fear conditioning testing at post-deployment, and 2 participants were excluded from analysis due to technical difficulties. Among the 522 participants from whom all the parameters were successfully obtained after deployment, 70 participants were excluded from the post-deployment analysis because they had been diagnosed with PTSD before deployment which could have confounded interpretation of deployment effects on fear processing (Figure 1).
Figure 1.
Chart indicating the number of participants recruited and excluded from the final analysis.
Fear conditioning data were analyzed as previously described, standard for this FPS protocol (Acheson et al., 2015b). Within each block, the noise-alone startle averages were subtracted from the CS+ and CS- averages to adjust for changes in baseline startle across the session. As in past studies using this task, to assess fear acquisition we collapsed the last half of the trials in the acquisition phase (Acheson et al., 2015b). To assess fear extinction, we followed the statistical conventions described by our group and others for this task (Acheson et al., 2015b; Norrholm et al., 2011) by collapsing each trial type into 4 equal blocks, the first block (early) consisting of the first 4 trials of the phase, the second block (mid 1) trials 5–8, the third block (mid 2) trials 9–12, and the last block (late) trials 13–16.
We first determined if our study replicated previous reports of Met/Met increases in FPS during acquisition and extinction in subjects with PTSD, using the pre-deployment data set. Repeated measures two-way (Genotype × PTSD diagnosis) analysis of variance (ANOVA), with block as within-subjects factor and childhood trauma (total CTQ score), lifetime trauma burden (Life Event Checklist, LEC; Gray, Litz, Hsu, & Lombardo, 2004), CAPS score, and 4 ancestry PCAs as covariates, was used for each CS trial separately, followed by Sidak post hoc tests, as appropriate. We separated the CS trial types because of the difficulty in interpreting and powering analyses with >3-way interactions, and because previous studies suggest that CS- responses are strongly associated with COMTval158met genotype (Norrholm et al., 2013). We next asked if childhood trauma moderates the relationship between COMT genotype and PTSD on FPS acquisition and extinction. Therefore, repeated measures three-way ANOVA (Genotype × PTSD diagnosis × childhood trauma), with block as within-subjects factor, and LEC, CAPS score and ancestry PCAs as covariates, was conducted, followed by Sidak post hoc tests. Finally, to investigate the effect of recent combat trauma exposure on FPS, repeated measures two-way ANOVA (Genotype × CES), with block as within-subjects factor, and childhood trauma, LEC, CAPS score, and ancestry PCAs as covariates, was performed, followed by Sidak post hoc tests. Interactions with block were followed by separate ANOVAs at each block.
Results
Demographic and trauma history of participants
Demographic and descriptive information of participants in MRS-II have been described previously (Acheson et al., 2015b; Minassian et al., 2015). Pre-deployment demographic and trauma information are described for each COMT genotype (Table 1). Following one-way ANOVA, no differences in age, months spent in military, childhood or lifetime trauma history (CTQ and LEC, respectively), or BDI-II score were found across COMT genotype groups before deployment (Table 1) and Chi-square test revealed no difference in marital status, education, or PTSD diagnosis (Table 1). However, a non-parametric Kruskal-Wallis test revealed a main effect of genotype was observed on CAPS score (p < 0.01), with Met/Met carriers showing higher CAPS score before deployment compared to Met/Val carriers (p < 0.01, following Dunn’s comparison test) (Table 1). In Marines not diagnosed with PTSD, a main effect of genotype was still observed before deployment on CAPS score (Kruskal-Wallis test, p = 0.01), with Met/Met carriers showing higher CAPS score compared to Met/Val carriers (p < 0.01 following Dunn’s comparison test) (data not shown). After deployment, no change was observed on combat experience score (CES), CAPS and BDI-II score, or in the percentage of participants diagnosed with PTSD across COMT genotype groups (Table 2).
Table 1.
Demographic characteristics, trauma history and psychiatric symptoms scores across COMT genotype groups before deployment
|
COMT Met/Met |
COMT Met/Val |
COMT Val/Val |
p value |
||
|---|---|---|---|---|---|
|
| |||||
| Demographics
| |||||
| n | 201 | 332 | 181 | N/A | |
|
| |||||
| Age (M, SD) a | 22.30 (2.89) | 22.11 (2.77) | 22.04 (2.61) | 0.54 | |
|
| |||||
| Marital status % b | 0.63 | ||||
| Never married | 68.66 | 71.69 | 69.61 | ||
| Married | 28.85 | 26.51 | 29.83 | ||
| Divorced | 1.00 | 1.20 | 0.56 | ||
| Separated | 1.49 | 0.60 | 0.00 | ||
|
| |||||
| Higher education % b | 0.65 | ||||
| Masters or Doctorate Degree | 0.00 | 0.30 | 0.00 | ||
| College | 24.38 | 24.40 | 28.73 | ||
| High school diploma | 74.13 | 72.29 | 66.85 | ||
| Other | 1.49 | 3.01 | 4.42 | ||
|
| |||||
| Months spent in military (M, SD) a | 31.77 (25.44) | 29.36 (24.82) | 29.06 (24.87) | 0.46 | |
|
| |||||
| Months left in enlistment (M, SD) a | 27.19 (12.96) | 27.32 (13.48) | 26.84 (13.54) | 0.95 | |
|
| |||||
| Trauma history
| |||||
| Childhood trauma (CTQ) (M, SD)a | 36.75 (12.91) | 36.68 (11.61) | 38.08 (14.81) | 0.46 | |
|
| |||||
| Lifetime trauma (LEC) (M, SD) a | 4.60 (3.17) | 4.32 (2.82) | 4.28 (2.77) | 0.44 | |
|
| |||||
| Psychiatric symptoms
| |||||
| CAPS score (M, SD) b | 14.11 (13.62)** | 11.01 (12.68) | 12.36 (12.90) | < 0.01 | |
|
| |||||
| BDI-II (M, SD) a | 5.95 (7.04) | 4.72 (5.90) | 5.14 (6.06) | 0.09 | |
|
| |||||
| PTSD diagnosis (%) c | 11.44 | 7.83 | 11.60 | 0.26 | |
BDI-II, Beck Depression Inventory II; CAPS, Clinician-Administered PTSD Scale.
One-way ANOVA performed followed by Tukey’s post hoc test;
non-parametric Kruskal-Wallis test;
Chi-squared test performed;
p < 0.05 following Dunn’s comparison test, compared to COMT Met/Val genotype
Table 2.
Combat trauma history and psychiatric symptoms across COMT genotype groups after deployment
|
COMT Met/Met |
COMT Met/Val |
COMT Val/Val |
p value |
|
|---|---|---|---|---|
| n | 127 | 236 | 124 | N/A |
| Combat experience score (M, SD) a | 11.64 (7.60) | 13.29 (8.52) | 13.45 (9.90) | 0.18 |
| CAPS score (M, SD)b | 16.91 (17.36) | 14.00 (15.69) | 15.22 (16.75) | 0.19 |
| BDI-II (M, SD) a | 3.56 (5.92) | 3.42 (5.56) | 3.24 (4.32) | 0.37 |
| PTSD diagnosis (%)c | 17.32 | 18.64 | 18.55 | 0.95 |
BDI-II, Beck Depression Inventory II; CAPS, Clinician-Administered PTSD Scale.
One-way ANOVA performed followed by Tukey’s post hoc test;
non-parametric Kruskal-Wallis test;
Chi-squared test performed
Met/Met genotype is associated with increased fear-potentiated startle in individuals with PTSD and childhood trauma exposure
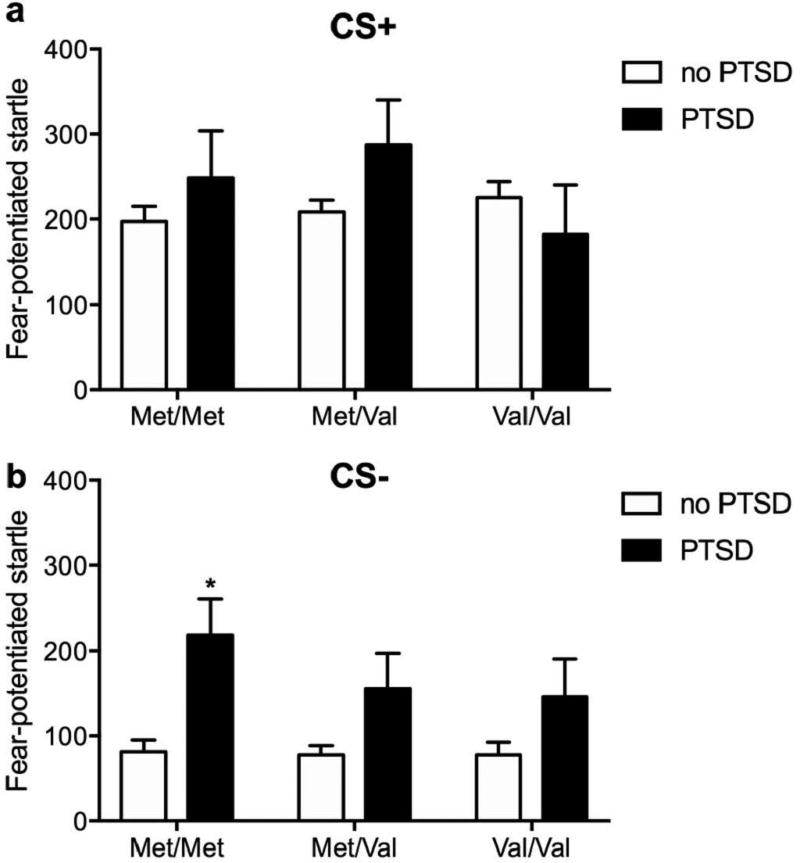
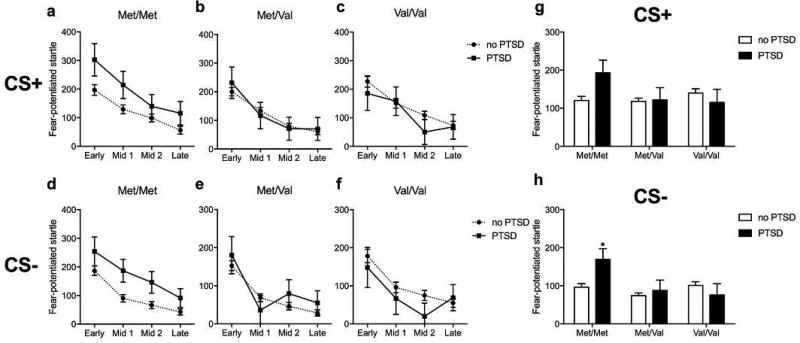
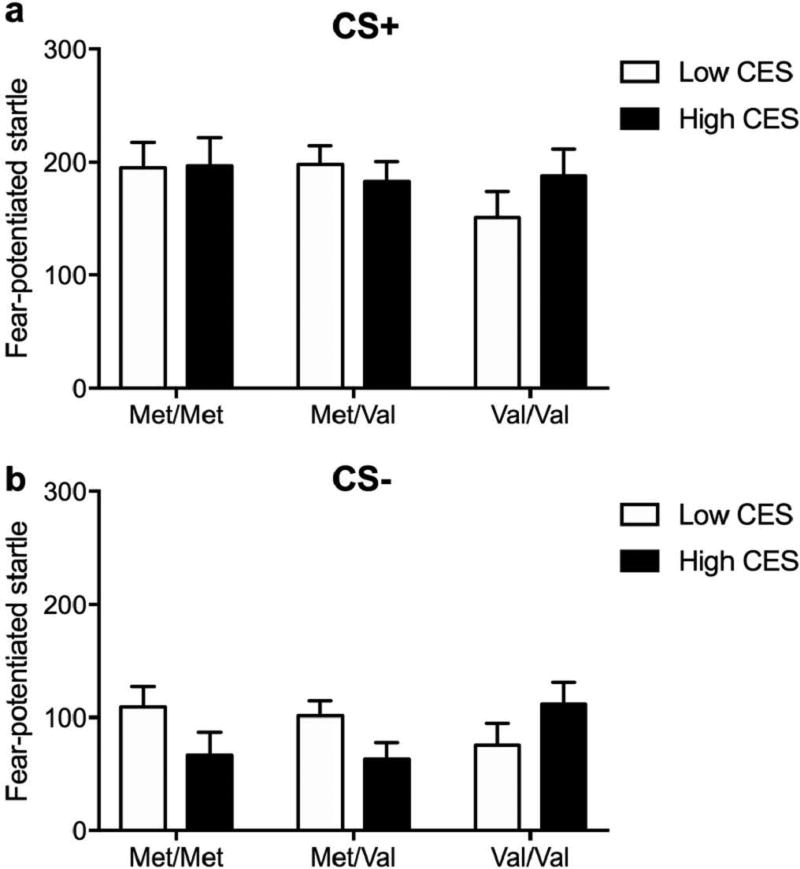
We first determined if our study replicated previous reports of Met/Met increases in FPS during acquisition and extinction in participants with PTSD when controlling for trauma burden and childhood trauma. In the acquisition phase, genotype was not associated with differences in FPS during either the CS+ or CS- (no main effects or interactions with PTSD group, Figure 2, for Table of means ± SEM see Supplementary Table 1). PTSD participants exhibited increased FPS to the CS- (F1,701 = 8.85; p < 0.01) regardless of genotype (Figure 2b). During extinction, main effects of block (F3,701 = 10.83; p < 0.001 and F3,701 = 11.07; p < 0.001) were observed on CS+ and CS- signal, respectively, but did not interact with other factors (Figure 3a–f, for Table of means ± SEM see Supplementary Table 1). There were no significant associations of genotype or PTSD with CS+ responding (Figure 3g). Across the CS- trials however, Met/Met carriers diagnosed with PTSD had increased FPS compared to Met/Met carriers without PTSD (genotype: F2,701 = 4.48, p < 0.05; genotype × PTSD: F2,701 = 3.21, p < 0.05, followed by post hoc test p < 0.05) (Figure 3h). These results replicate previous findings that Met/Met carriers with PTSD exhibit increased responses to safety signals compared to Met/Met carriers without PTSD (Norrholm et al., 2013).
Figure 2.
Effect of COMT genotype and PTSD diagnosis on fear-potentiated startle during late acquisition before deployment.
Figure 3.
Effect of COMT genotype and PTSD diagnosis on fear-potentiated startle during extinction before deployment.
We next asked if childhood trauma moderates the relationship between COMT genotype and PTSD on FPS acquisition and extinction. In the acquisition phase, three-way ANOVA (Genotype × PTSD diagnosis × childhood trauma) showed no main effects of childhood trauma or interactions with the other factors during the CS+ or CS- trials (see above). During the extinction phase, all groups showed significant reductions in potentiation to both the CS+ and CS- trials (main effects of block: F3,696 = 10.82; p < 0.001 and F3,696 = 9.51; p < 0.001) (Figure 4a–f). Whereas no genotype associations were observed with FPS during the CS+ trials (Figure 4g), a genotype × PTSD diagnosis × childhood trauma interaction was observed (F2,696 = 3.50, p < 0.05; genotype: F2,696 = 3.80, p < 0.05; genotype × PTSD: F2,696 = 2.96, p = 0.05) on FPS across the CS- trials (Figure 4h). Post hoc comparisons indicated that high FPS during the safety signal in Met/Met carriers with PTSD was primarily found in participants that endorsed childhood trauma (p < 0.05, compared to Met/Met carriers not diagnosed with PTSD, but with at least one childhood trauma) (Figure 4h), while Met/Met carriers with PTSD but without childhood trauma were not different than carriers without PTSD.
Figure 4.
Modulation by childhood trauma (CT) of the effect of COMT genotype and PTSD diagnosis on fear-potentiated startle during extinction before deployment.
Increased fear-potentiated startle in Met/Met carriers after deployment is moderated by combat trauma
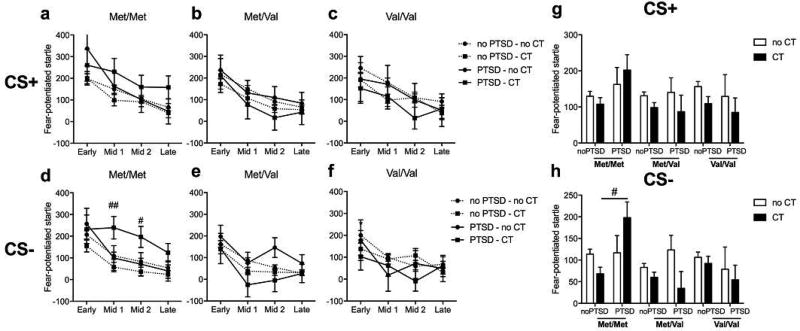
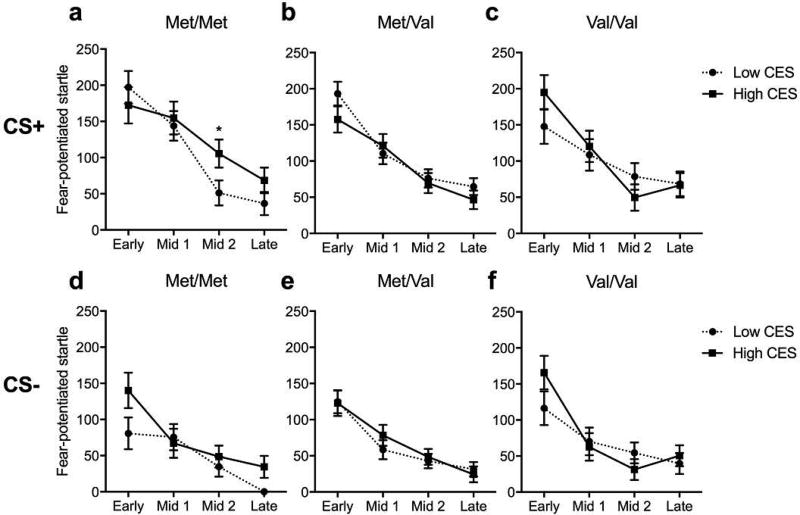
We next investigated the effect of deployment-related combat trauma exposure on FPS after return from deployment. To assess combat trauma effects, we grouped subjects into high and low trauma exposure via a median split of combat experience scores (CES) assessed by the DRRI post-deployment. To control for chronic PTSD, we excluded participants who were already diagnosed with PTSD at pre-deployment. In the acquisition phase, neither genotype nor combat trauma was associated with FPS during the CS+ and CS- signals. During the extinction phase, a main effect of block (F3,439 = 7.59; p < 0.001) and a block × genotype × CES interaction (F6,439 = 2.14; p < 0.05) were found during the CS+ trials. Thus, ANOVAs (Genotype × CES) were conducted for each block separately (Figure 6). Within the mid 2 extinction block, a strong trend for a genotype × CES interaction was observed (F2,439 = 3.28; p < 0.05). High combat exposure increased fear-potentiated response during the mid 2 CS+ trials only in Met/Met carriers, compared to Met/Val or Val/Val carriers (Figure 6a–c). During the CS- signal, a main effect of block was found (F3,439 = 5.73; p < 0.001), but no effect of either genotype or combat trauma was found on FPS (Figure 6d–f).
Figure 6.
Effect of COMT genotype and combat trauma on fear-potentiated startle during extinction after deployment.
Discussion
The present study aimed to verify whether COMTval158met polymorphism moderated impaired fear processes observed in PTSD, if these associations were further moderated by childhood trauma, and if genotype was associated with recent trauma effects on learned fear processes. We replicated previous findings that homozygous Met carriers diagnosed with PTSD displayed greater FPS to the CS- (safety signal) compared to Val carriers before deployment. Importantly, childhood trauma significantly moderated this association, since only Met/Met carriers with PTSD who endorsed childhood trauma, as compared to those who did not endorse childhood trauma, had a higher fear-potentiated response to the safety signal during extinction. After deployment, Met/Met carriers reporting high, not low, combat trauma showed modest elevations in fear-potentiated response to the CS+ (danger signal) during extinction compared to Val carriers when controlling for PTSD symptoms.
Here, the increased FPS found in Met/Met carriers diagnosed with PTSD replicated previous studies reporting poor safety signal learning and reduced fear extinction in the same genotype group (Norrholm et al., 2013). However, we showed that the elevated FPS in homozygous Met carriers was moderated by childhood trauma history, such that Met/Met carriers diagnosed with PTSD who endorsed at least one category of childhood abuse/neglect showed the lowest safety signal learning (i.e. high FPS to CS-). This pattern of results supports the theory that gene × environment interactions are important factors in the risk of PTSD development, perhaps via alterations in core fear learning processes such as fear cue generalization. Although childhood trauma has widely been described as a risk factor for PTSD in U.S. troops (Agorastos et al., 2014; Cabrera, Hoge, Bliese, Castro, & Messer, 2007; Van Voorhees et al., 2012) and has been suggested to predict impaired fear extinction in PTSD patients (Stevens et al., 2016), the development of PTSD and the severity of its symptoms have been strongly associated with gene × environment interactions (Koenen, 2007; Nievergelt et al., 2015; Norrholm & Ressler, 2009; Skelton, Ressler, Norrholm, Jovanovic, & Bradley-Davino, 2012). Among the genes that have been linked to PTSD, the COMTval158met SNP interacts with traumatic load and urban violence, with Met/Met genotype showing a higher risk of PTSD (Boscarino et al., 2012; Kolassa et al., 2010; Valente et al., 2011). To our knowledge, the findings presented here are the first to report gene × environmental risk factor interactions in disruption of fear inhibition, a behavioral characteristic linked to the fear-related symptoms of PTSD (Acheson et al., 2015b; Jovanovic & Norrholm, 2011; Jovanovic et al., 2010).
Marines with no history of PTSD symptoms before deployment, who carried the Met/Met genotype and endorsed high combat trauma showed modest increases in FPS to the CS+ during extinction compared to Met/Met carriers who experienced low trauma or were Val/Val carriers. These findings suggest that acute trauma exposure, and more importantly, current PTSD symptoms are necessary to observe COMTval158met associations with altered fear processes. Two other studies have reported modulation of fear extinction by the COMTval158met polymorphism in healthy controls (Lonsdorf et al., 2009; Wendt et al., 2015), but those studies did not control for lifetime trauma. In sum, our finding that COMT SNP-modulated response to severe combat trauma (Nievergelt et al., 2015) is in agreement with the gene × environment hypothesis of PTSD. Interestingly, impaired fear extinction has been specifically associated with PTSD, not depression or anxiety, in active-duty Marines (Acheson et al., 2015b; Jovanovic et al., 2010), suggesting that impaired fear conditioning before deployment might be a predictor of higher PTSD risk after combat.
Altogether, our data support the conclusion that the COMTval158met SNP may contribute to disrupted fear inhibition in the context of severe trauma exposure and PTSD symptom burden. Dopamine transmission in the prelimbic and infralimbic cortex in rodents (dorsal anterior cingulate cortex and ventromedial prefrontal cortex in humans) modulates fear response by maintaining and inhibiting fear response, respectively (Abraham, Neve, & Lattal, 2014; Quirk & Mueller, 2008). Although COMT is mainly expressed in the PFC, the involvement of other brain regions, such as hippocampus, should not be excluded. The hippocampus modulates contextual fear learning through dopaminergic activity (Abraham et al., 2014; Phillips & LeDoux, 1992). Interestingly, COMTval158met polymorphism has been associated with changes in hippocampal CA2/3 volumes, which is negatively correlated with trauma exposure in Met/Met (Rabl et al., 2014). Decreased hippocampal volume has been reported in civilian trauma-related PTSD (Smith, 2005; Villarreal et al., 2002; Woon, Sood, & Hedges, 2010) and combat-related PTSD (Gurvits et al., 1996), and has been shown to predict vulnerability to psychological trauma (Gilbertson et al., 2002). Therefore, COMTval158met polymorphism could alter contextual fear inhibition through changes in hippocampal function. Additionally, COMT may affect fear inhibition via modulation of norepinephrine signaling (Chen et al., 2004; Mier, Kirsch, & Meyer-Lindenberg, 2010). The COMT enzyme catabolizes norepinephrine (Tunbridge, 2010), and elevated plasma concentrations are reported in Met carriers (Jung et al., 2012). PTSD patients have higher stress-induced norepinephrine release compared to healthy individuals (O'Donnell, Hegadoren, & Coupland, 2004). The noradrenergic signaling modulates fear memory consolidation by enhancing fear response in humans (Soeter & Kindt, 2011; Visser, Kunze, Westhoff, Scholte, & Kindt, 2015), suggesting a potential role of COMT-mediated fear extinction through noradrenergic as well as dopaminergic mechanisms.
Despite the large sample size of this study (714 participants), a limitation is that women were not included, because they were not part of infantry battalions at the time of assessment. Women show lower COMT activity than men, due to its estrogen-modulated downregulation (Chen et al., 2004; Hassan, Salama, Arafa, Hamada, & Al-Hendy, 2007; Tunbridge, 2010). Furthermore, sex-dependent associations between COMT polymorphism and anxiety-related traits have been reported (Stein, Fallin, Schork, & Gelernter, 2005). A previous large study showed no sex-dependent effect of COMTval158met polymorphism on impaired fear inhibition found in PTSD patients (Norrholm et al., 2013). However, further investigation will be necessary to assess the potential gene × trauma interactions in women.
These findings replicate previous studies showing that the Met/Met carriers with PTSD are more likely to show increased fear responding compared to Met/Val or Val/Val carriers with PTSD, and extend this finding to show that this association is modulated by early life trauma exposure. This study supports the suggestion that COMT could be a potential biomarker of treatment response to exposure-based cognitive-behavior therapy (CBT). Here, Met/Met carriers that endorsed a childhood or combat trauma exhibited resistance to extinction, suggesting that these individuals may show reduced response to therapy targeting the behavioral patterns associated with fear symptoms, such as exposure-based CBT (Lonsdorf & Kalisch, 2011). In addition, Met/Met carriers also exhibit increased fear responding after combat trauma, regardless of PTSD diagnosis, suggesting some acute loss of fear regulation after trauma in Met/Met carriers as a whole. These results support a role of COMT gene-environment interactions in the development of psychiatric-relevant phenotypes. Further work will be necessary to understand the mechanisms underlying the modulation of cortical signaling by the COMTval158met polymorphism and fear extinction memory impairments in PTSD pathophysiology.
Supplementary Material
Supplemental Table 1. Effect of COMT genotype and PTSD diagnosis on overall fear-potentiated startle during late acquisition and extinction before deployment
Figure 5.
Effect of COMT genotype and combat trauma on fear-potentiated startle during late acquisition after deployment.
Acknowledgments
Jessica Deslauriers, Ph.D. is recipient of a CIHR (Canadian Institutes of Health Research) postdoctoral fellowship. The MRS team includes the late Daniel O’Connor as well as members of the MRS administrative core (Anjana Patel, Andrew De La Rosa, Elin Olsson, Patricia Gorman). This study was funded by Headquarters Marine Core and NAVY BUMED in addition to individual awards from Department of Defense, Veterans Affairs (BX002558, MR141217) and the VA Center of Excellence for Stress and Mental Health, and NIH grant for GWAS (MH093500) to co-authors. We would also like to thank all the clinician-interviewers and staff for data collection and Marine and Navy Corpsmen participants.
Footnotes
Financial disclosures
Dr. Geyer holds equity interest in San Diego Instruments. Drs. Deslauriers, Acheson, Baker, Nievergelt and Risbrough, and Adam X Maihofer have no conflict of interest to declare.
References
- Abraham AD, Neve KA, Lattal KM. Dopamine and extinction: a convergence of theory with fear and reward circuitry. Neurobiology of Learning and Memory. 2014;108:65–77. doi: 10.1016/j.nlm.2013.11.007. http://doi.org/10.1016/j.nlm.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson DT, Eyler LT, Resovsky J, Tsan E, Risbrough VB. Fear extinction memory performance in an sample of stable, euthymic patients with bipolar disorder. Journal of Affective Disorders. 2015a;185(C):230–238. doi: 10.1016/j.jad.2015.06.053. http://doi.org/10.1016/j.jad.2015.06.053. [DOI] [PubMed] [Google Scholar]
- Acheson DT, Geyer MA, Baker DG, Nievergelt CM, Yurgil K, Risbrough VB MRS-II Team. Conditioned fear and extinction learning performance and its association with psychiatric symptoms in active duty Marines. Psychoneuroendocrinology. 2015b;51:495–505. doi: 10.1016/j.psyneuen.2014.09.030. http://doi.org/10.1016/j.psyneuen.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agorastos A, Pittman JOE, Angkaw AC, Nievergelt CM, Hansen CJ, Aversa LH, et al. The cumulative effect of different childhood trauma types on self-reported symptoms of adult male depression and PTSD, substance abuse and health-related quality of life in a large active-duty military cohort. Journal of Psychiatric Research. 2014;58:46–54. doi: 10.1016/j.jpsychires.2014.07.014. http://doi.org/10.1016/j.jpsychires.2014.07.014. [DOI] [PubMed] [Google Scholar]
- Almli LM, Fani N, Smith AK, Ressler KJ. Genetic approaches to understanding post-traumatic stress disorder. The International Journal of Neuropsychopharmacology / Official Scientific Journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2013;17(02):355–370. doi: 10.1017/S1461145713001090. http://doi.org/10.1017/S1461145713001090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DG, Nash WP, Litz BT, Geyer MA, Risbrough VB, Nievergelt CM, et al. Predictors of risk and resilience for posttraumatic stress disorder among ground combat Marines: methods of the Marine Resiliency Study. Preventing Chronic Disease. 2012;9:E97. doi: 10.5888/pcd9.110134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal of Personality Assessment. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. http://doi.org/10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. The American Journal of Psychiatry. 1994;151(8):1132–1136. doi: 10.1176/ajp.151.8.1132. http://doi.org/10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. Journal of Traumatic Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Hickling EJ, Taylor AE, Loos WR, Forneris CA, Jaccard J. Who develops PTSD from motor vehicle accidents? Behaviour Research and Therapy. 1996;34(1):1–10. doi: 10.1016/0005-7967(95)00058-6. [DOI] [PubMed] [Google Scholar]
- Boscarino J, Erlich Hoffman, Zhang Higher FKBP5, COMT, CHRNA5, and CRHR1 allele burdens are associated with PTSD and interact with trauma exposure: implications for neuropsychiatric research and treatment. Neuropsychiatric Disease and Treatment. 2012:131. doi: 10.2147/NDT.S29508. http://doi.org/10.2147/NDT.S29508. [DOI] [PMC free article] [PubMed]
- Cabrera OA, Hoge CW, Bliese PD, Castro CA, Messer SC. Childhood adversity and combat as predictors of depression and post-traumatic stress in deployed troops. American Journal of Preventive Medicine. 2007;33(2):77–82. doi: 10.1016/j.amepre.2007.03.019. http://doi.org/10.1016/j.amepre.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. American Journal of Human Genetics. 2004;75(5):807–821. doi: 10.1086/425589. http://doi.org/10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R, DeYoung CG, Sponheim SR, Bender TL, Polusny MA, Erbes CR, Arbisi PA. Predicting post-traumatic stress disorder in veterans: interaction of traumatic load with COMT gene variation. Journal of Psychiatric Research. 2013;47(12):1849–1856. doi: 10.1016/j.jpsychires.2013.08.013. http://doi.org/10.1016/j.jpsychires.2013.08.013. [DOI] [PubMed] [Google Scholar]
- Collings SJ, Valjee SR, Penning SL. Development and preliminary validation of a screen for interpersonal childhood trauma experiences among school-going youth in Durban, South Africa. Journal of Child and Adolescent Mental Health. 2013;25(1):23–34. doi: 10.2989/17280583.2012.722552. http://doi.org/10.2989/17280583.2012.722552. [DOI] [PubMed] [Google Scholar]
- DeMille MMC, Kidd JR, Ruggeri V, Palmatier MA, Goldman D, Odunsi A, et al. Population variation in linkage disequilibrium across the COMT gene considering promoter region and coding region variation. Human Genetics. 2002;111(6):521–537. doi: 10.1007/s00439-002-0809-0. http://doi.org/10.1007/s00439-002-0809-0. [DOI] [PubMed] [Google Scholar]
- DeSantis SM, Baker NL, Back SE, Spratt E, Ciolino JD, Moran-Santa Maria M, et al. Gender differences in the effect of early life trauma on hypothalamic-pituitary-adrenal axis functioning. Depression and Anxiety. 2011;28(5):383–392. doi: 10.1002/da.20795. http://doi.org/10.1002/da.20795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch M-A, Xu K, Ferro E, Harris CR, Goldman D. Genetic origins of anxiety in women: a role for a functional catechol-O-methyltransferase polymorphism. Psychiatric Genetics. 2003;13(1):33–41. doi: 10.1097/00041444-200303000-00006. http://doi.org/10.1097/01.ypg.0000054709.85338.c3. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature Neuroscience. 2002;5(11):1242–1247. doi: 10.1038/nn958. http://doi.org/10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn DE, Acheson DT, Geyer MA, Nievergelt CM, Baker DG, Risbrough VB MRS Team. High and low threshold for startle reactivity associated with PTSD symptoms but not PTSD risk: evidence from a prospective study of active duty Marines. Depression and Anxiety. 2016 doi: 10.1002/da.22475. http://doi.org/10.1002/da.22475. [DOI] [PubMed]
- Goenjian AK, Noble EP, Steinberg AM, Walling DP, Stepanyan ST, Dandekar S, Bailey JN. Association of COMT and TPH-2 genes with DSM-5 based PTSD symptoms. Journal of Affective Disorders. 2014;172C(C):472–478. doi: 10.1016/j.jad.2014.10.034. http://doi.org/10.1016/j.jad.2014.10.034. [DOI] [PubMed] [Google Scholar]
- Gray MJ, Litz BT, Hsu JL, Lombardo TW. Psychometric properties of the life events checklist. Assessment. 2004;11(4):330–341. doi: 10.1177/1073191104269954. http://doi.org/10.1177/1073191104269954. [DOI] [PubMed] [Google Scholar]
- Gurvits TV, Shenton ME, Hokama H, Ohta H, Lasko NB, Gilbertson MW, et al. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biological Psychiatry. 1996;40(11):1091–1099. doi: 10.1016/S0006-3223(96)00229-6. http://doi.org/10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan MH, Salama SA, Arafa HMM, Hamada FMA, Al-Hendy A. Adenovirus-mediated delivery of a dominant-negative estrogen receptor gene in uterine leiomyoma cells abrogates estrogen- and progesterone-regulated gene expression. The Journal of Clinical Endocrinology and Metabolism. 2007;92(10):3949–3957. doi: 10.1210/jc.2007-0823. http://doi.org/10.1210/jc.2007-0823. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD. Neural mechanisms of impaired fear inhibition in posttraumatic stress disorder. Frontiers in Behavioral Neuroscience. 2011;5:44. doi: 10.3389/fnbeh.2011.00044. http://doi.org/10.3389/fnbeh.2011.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, Ressler KJ. Impaired fear inhibition is a biomarker of PTSD but not depression. Depression and Anxiety. 2010;27(3):244–251. doi: 10.1002/da.20663. http://doi.org/10.1002/da.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y-H, Kang D-H, Byun MS, Shim G, Kwon SJ, Jang G-E, et al. Influence of brain-derived neurotrophic factor and catechol O-methyl transferase polymorphisms on effects of meditation on plasma catecholamines and stress. Stress (Amsterdam, Netherlands) 2012;15(1):97–104. doi: 10.3109/10253890.2011.592880. http://doi.org/10.3109/10253890.2011.592880. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Ruscio AM, Shear K, Wittchen H-U. Epidemiology of anxiety disorders. Current Topics in Behavioral Neurosciences. 2010;2:21–35. [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52(12):1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Koenen KC. Genetics of posttraumatic stress disorder: Review and recommendations for future studies. Journal of Traumatic Stress. 2007;20(5):737–750. doi: 10.1002/jts.20205. http://doi.org/10.1002/jts.20205. [DOI] [PubMed] [Google Scholar]
- Kolassa S, Kolassa, Ertl V, Papassotiropoulos A, De Quervain DJF. The Risk of Posttraumatic Stress Disorder After Trauma Depends on Traumatic Load and the Catechol-O-Methyltransferase Val. Biological Psychiatry. 2010;67(4):304–308. doi: 10.1016/j.biopsych.2009.10.009. http://doi.org/10.1016/j.biopsych.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Li L, Bao Y, He S, Wang G, Guan Y, Ma D, et al. The Association Between Genetic Variants in the Dopaminergic System and Posttraumatic Stress Disorder: A Meta-Analysis. Medicine. 2016;95(11):e3074. doi: 10.1097/MD.0000000000003074. http://doi.org/10.1097/MD.0000000000003074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf TB, Kalisch R. A review on experimental and clinical genetic associations studies on fear conditioning, extinction and cognitive-behavioral treatment. Translational Psychiatry. 2011;1(9):e41–13. doi: 10.1038/tp.2011.36. http://doi.org/10.1038/tp.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf TB, Weike AI, Nikamo P, Schalling M, Hamm AO, Ohman A. Genetic gating of human fear learning and extinction: possible implications for gene-environment interaction in anxiety disorder. Psychological Science. 2009;20(2):198–206. doi: 10.1111/j.1467-9280.2009.02280.x. http://doi.org/10.1111/j.1467-9280.2009.02280.x. [DOI] [PubMed] [Google Scholar]
- Mehta D, Binder EB. Gene × environment vulnerability factors for PTSD: the HPA-axis. Neuropharmacology. 2012;62(2):654–662. doi: 10.1016/j.neuropharm.2011.03.009. http://doi.org/10.1016/j.neuropharm.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Mier D, Kirsch P, Meyer-Lindenberg A. Neural substrates of pleiotropic action of genetic variation in COMT: a meta-analysis. Molecular Psychiatry. 2010;15(9):918–927. doi: 10.1038/mp.2009.36. http://doi.org/10.1038/mp.2009.36. [DOI] [PubMed] [Google Scholar]
- Minassian A, Maihofer AX, Baker DG, Nievergelt CM, Geyer MA, Risbrough VB. Association of Predeployment Heart Rate Variability With Risk of Postdeployment Posttraumatic Stress Disorder in Active-Duty Marines. JAMA Psychiatry. 2015;72(10):979. doi: 10.1001/jamapsychiatry.2015.0922. http://doi.org/10.1001/jamapsychiatry.2015.0922. [DOI] [PubMed] [Google Scholar]
- Montag C, Buckholtz JW, Hartmann P, Merz M, Burk C, Hennig J, Reuter M. COMT genetic variation affects fear processing: psychophysiological evidence. Behavioral Neuroscience. 2008;122(4):901–909. doi: 10.1037/0735-7044.122.4.901. http://doi.org/10.1037/0735-7044.122.4.901. [DOI] [PubMed] [Google Scholar]
- Neuner F, Schauer M, Karunakara U, Klaschik C, Robert C, Elbert T. Psychological trauma and evidence for enhanced vulnerability for posttraumatic stress disorder through previous trauma among West Nile refugees. BMC Psychiatry. 2004;4:34. doi: 10.1186/1471-244X-4-34. http://doi.org/10.1186/1471-244X-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nievergelt CM, Maihofer AX, Mustapic M, Yurgil KA, Schork NJ, Miller MW, et al. Genomic predictors of combat stress vulnerability and resilience in U.S. Marines: A genome-wide association study across multiple ancestries implicates. Psychoneuroendocrinology. 2015;51:459–471. doi: 10.1016/j.psyneuen.2014.10.017. http://doi.org/10.1016/j.psyneuen.2014.10.017. [DOI] [PubMed] [Google Scholar]
- Nievergelt CM, Maihofer AX, Shekhtman T, Libiger O, Wang X, Kidd KK, Kidd JR. Inference of human continental origin and admixture proportions using a highly discriminative ancestry informative 41-SNP panel. Investigative Genetics. 2013;4(1):113. doi: 10.1186/2041-2223-4-13. http://doi.org/10.1186/2041-2223-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Ressler KJ. Genetics of anxiety and trauma-related disorders. Neuroscience. 2009;164(1):272–287. doi: 10.1016/j.neuroscience.2009.06.036. http://doi.org/10.1016/j.neuroscience.2009.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, Ressler KJ. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biological Psychiatry. 2011;69(6):556–563. doi: 10.1016/j.biopsych.2010.09.013. http://doi.org/10.1016/j.biopsych.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Smith AK, Binder E, Klengel T, Conneely K, et al. Differential Genetic and Epigenetic Regulation of catechol-O-methyltransferase is Associated with Impaired Fear Inhibition in Posttraumatic Stress Disorder. Frontiers in Behavioral Neuroscience. 2013;7:130. doi: 10.3389/fnbeh.2013.00030. http://doi.org/10.3389/fnbeh.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Vervliet B, Jovanovic T, Boshoven W, Myers KM, Davis M, et al. Timing of extinction relative to acquisition: a parametric analysis of fear extinction in humans. Behavioral Neuroscience. 2008;122(5):1016–1030. doi: 10.1037/a0012604. http://doi.org/10.1037/a0012604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell T, Hegadoren KM, Coupland NC. Noradrenergic mechanisms in the pathophysiology of post-traumatic stress disorder. Neuropsychobiology. 2004;50(4):273–283. doi: 10.1159/000080952. http://doi.org/10.1159/000080952. [DOI] [PubMed] [Google Scholar]
- Orcutt HK, Hannan SM, Seligowski AV, Jovanovic T, Norrholm SD, Ressler KJ, McCanne T. Fear-Potentiated Startle and Fear Extinction in a Sample of Undergraduate Women Exposed to a Campus Mass Shooting. Frontiers in Psychology. 2016;7:2031. doi: 10.3389/fpsyg.2016.02031. http://doi.org/10.3389/fpsyg.2016.02031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TWW, Heim CM. A short review on the psychoneuroimmunology of posttraumatic stress disorder: from risk factors to medical comorbidities. Brain, Behavior, and Immunity. 2011;25(1):6–13. doi: 10.1016/j.bbi.2010.10.003. http://doi.org/10.1016/j.bbi.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Palmatier MA, Kang AM, Kidd KK. Global variation in the frequencies of functionally different catechol-O-methyltransferase alleles. Biological Psychiatry. 1999;46(4):557–567. doi: 10.1016/s0006-3223(99)00098-0. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience. 1992;106(2):274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nature Genetics. 2006;38(8):904–909. doi: 10.1038/ng1847. http://doi.org/10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 2008;33(1):56–72. doi: 10.1038/sj.npp.1301555. http://doi.org/10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabl U, Meyer BM, Diers K, Bartova L, Berger A, Mandorfer D, et al. Additive gene-environment effects on hippocampal structure in healthy humans. Journal of Neuroscience. 2014;34(30):9917–9926. doi: 10.1523/JNEUROSCI.3113-13.2014. http://doi.org/10.1523/JNEUROSCI.3113-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton K, Ressler KJ, Norrholm SD, Jovanovic T, Bradley-Davino B. PTSD and gene variants: new pathways and new thinking. Neuropharmacology. 2012;62(2):628–637. doi: 10.1016/j.neuropharm.2011.02.013. http://doi.org/10.1016/j.neuropharm.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Bilateral hippocampal volume reduction in adults with post-traumatic stress disorder: a meta-analysis of structural MRI studies. Hippocampus. 2005;15(6):798–807. doi: 10.1002/hipo.20102. http://doi.org/10.1002/hipo.20102. [DOI] [PubMed] [Google Scholar]
- Smith, Redd W, DuHamel K, Vickberg SJ, Ricketts P. Validation of the PTSD Checklist-Civilian Version in survivors of bone marrow transplantation. Journal of Traumatic Stress. 1999;12(3):485–499. doi: 10.1023/A:1024719104351. http://doi.org/10.1023/A:1024719104351. [DOI] [PubMed] [Google Scholar]
- Soeter M, Kindt M. Noradrenergic enhancement of associative fear memory in humans. Neurobiology of Learning and Memory. 2011;96(2):263–271. doi: 10.1016/j.nlm.2011.05.003. http://doi.org/10.1016/j.nlm.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Stein MB, Fallin MD, Schork NJ, Gelernter J. COMT polymorphisms and anxiety-related personality traits. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 2005;30(11):2092–2102. doi: 10.1038/sj.npp.1300787. http://doi.org/10.1038/sj.npp.1300787. [DOI] [PubMed] [Google Scholar]
- Stevens JS, Ely TD, Sawamura T, Guzman D, Bradley B, Ressler KJ, Jovanovic T. Childhood maltreatment predicts reduced inhibition-related activity in the rostral anterior cingulate in PTSD, but not in trauma-exposed controls. Depression and Anxiety. 2016 doi: 10.1002/da.22506. http://doi.org/10.1002/da.22506. [DOI] [PMC free article] [PubMed]
- Tunbridge EM. The catechol-O-methyltransferase gene: its regulation and polymorphisms. International Review of Neurobiology. 2010;95:7–27. doi: 10.1016/B978-0-12-381326-8.00002-8. http://doi.org/10.1016/B978-0-12-381326-8.00002-8. [DOI] [PubMed] [Google Scholar]
- Valente NLM, Vallada H, Cordeiro Q, Bressan RA, Andreoli SB, Mari JJ, Mello MF. Catechol-O-methyltransferase (COMT) val158met polymorphism as a risk factor for PTSD after urban violence. Journal of Molecular Neuroscience : MN. 2011;43(3):516–523. doi: 10.1007/s12031-010-9474-2. http://doi.org/10.1007/s12031-010-9474-2. [DOI] [PubMed] [Google Scholar]
- Van Voorhees EE, Dedert EA, Calhoun PS, Brancu M, Runnals J, Beckham JC VA Mid-Atlantic MIRECC Workgroup. Childhood trauma exposure in Iraq and Afghanistan war era veterans: implications for posttraumatic stress disorder symptoms and adult functional social support. Child Abuse & Neglect. 2012;36(5):423–432. doi: 10.1016/j.chiabu.2012.03.004. http://doi.org/10.1016/j.chiabu.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Villarreal G, Hamilton DA, Petropoulos H, Driscoll I, Rowland LM, Griego JA, et al. Reduced hippocampal volume and total white matter volume in posttraumatic stress disorder. Biological Psychiatry. 2002;52(2):119–125. doi: 10.1016/s0006-3223(02)01359-8. [DOI] [PubMed] [Google Scholar]
- Visser RM, Kunze AE, Westhoff B, Scholte HS, Kindt M. Representational similarity analysis offers a preview of the noradrenergic modulation of long-term fear memory at the time of encoding. Psychoneuroendocrinology. 2015;55:8–20. doi: 10.1016/j.psyneuen.2015.01.021. http://doi.org/10.1016/j.psyneuen.2015.01.021. [DOI] [PubMed] [Google Scholar]
- Vogt D, Smith BN, King LA, King DW, Knight J, Vasterling JJ. Deployment risk and resilience inventory-2 (DRRI-2): an updated tool for assessing psychosocial risk and resilience factors among service members and veterans. Journal of Traumatic Stress. 2013;26(6):710–717. doi: 10.1002/jts.21868. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: a review of the first ten years of research. Depression and Anxiety. 2001;13(3):132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- Wendt J, Neubert J, Lindner K, Ernst FD, Homuth G, Weike AI, Hamm AO. Genetic influences on the acquisition and inhibition of fear. International Journal of Psychophysiology : Official Journal of the International Organization of Psychophysiology. 2015;98(3 Pt 2):499–505. doi: 10.1016/j.ijpsycho.2014.10.007. http://doi.org/10.1016/j.ijpsycho.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Woon FL, Sood S, Hedges DW. Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: a meta-analysis. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2010;34(7):1181–1188. doi: 10.1016/j.pnpbp.2010.06.016. http://doi.org/10.1016/j.pnpbp.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Zubieta J-K, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, et al. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science (New York, NY) 2003;299(5610):1240–1243. doi: 10.1126/science.1078546. http://doi.org/10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Effect of COMT genotype and PTSD diagnosis on overall fear-potentiated startle during late acquisition and extinction before deployment