Abstract
An important entraining signal for the endogenous circadian clock, independent of light, is food intake. The circadian and immune systems are linked; forced desynchrony of the circadian clock via nighttime light exposure or genetic ablation of core clock components impairs immune function. The timing of food intake affects various aspects of the circadian clock, but its effects on immune function are unknown. We tested the hypothesis that temporal desynchrony of food intake alters innate immune responses. Adult male Swiss Webster mice were provided with food either during the night, the day, or ad libitum for four weeks, followed by administration of lipopolysaccharide (LPS) either prior to the onset of the active (ZT12: Experiment 1) or inactive (ZT0: Experiment 2) phase. Three hours after LPS administration, blood was collected and serum was tested for bacteria killing capacity against Escherichia coli, as a functional assay of immune function. Additionally, cytokine expression was examined in the serum (protein), spleen, and hypothalamus (mRNA). Day-fed mice suppressed bacteria killing capacity and serum cytokine responses to LPS during the active phase (ZT12). Night-fed mice increased bactericidal capacity, as well as serum and hypothalamic mRNA responses of certain pro-inflammatory cytokines during the active phase. Only Day-fed mice enhanced serum cytokine responses when LPS challenge occurred during the inactive phase (ZT0), this did not result in enhanced bactericidal capacity. These data suggest that mistimed feeding has functional relevance for immune function, and provides further evidence for the integration of the circadian, metabolic, and immune systems.
Keywords: Cytokines, LPS, spleen & lymph nodes, circadian dysregulation, biological rhythms, timed-feeding, circadian
Introduction
The circadian and immune systems are fundamentally connected; most cells of the immune system display autonomous circadian oscillations in gene expression (1, 2) and core clock genes regulate several key immune transcription factors driving rhythmic expression of cytokines, cell proliferation, and immune receptor expression and function (2–7). Additionally, circadian clock proteins regulate inflammatory responses, immune cell trafficking, and phenotype (4, 5, 7–10). Therefore, optimal immune function is dependent on a functional circadian clock.
Light is the most potent entraining cue for the circadian clock, acting via signal transduction in the suprachiasmatic nuclei (SCN) of the hypothalamus. The SCN convey timing information to the immune system via autonomic and humoral pathways, ‘setting’ peripheral clocks and governing daily variations in immune function (11). Disruption of the circadian clock markedly deregulates inflammatory responses. Specifically, chronic jetlag enhances LPS-induced increases in serum cytokine concentrations (12). Both chronic jetlag and exposure to light at night enhance hypothalamic cytokine expression in response to LPS (12, 13). Shortening of the circadian period dampens serum cytokine concentrations in response to LPS (14). These changes in immune response occur independent from effects of sleep loss or stress (12, 14–17).
Timing of food intake can also act as an entraining signal to the circadian clock; timed feeding synchronizes rhythmic clock gene expression in the SCN of mice housed in constant darkness and restores rhythmicity in mice housed in constant light (18, 19). Hypocaloric feeding (~50% daily food intake) can alter the phase of clock gene expression in the SCN independent of the light/dark cycle (20, 21). Additionally, timed feeding regimens can phase shift clock gene expression in the liver independent of SCN phase or light/dark cycle (22, 23). Metabolic homeostasis requires robust circadian gene expression in the liver. Indeed, liver-specific clock gene knockout and disruption, through high fat diet and feeding during the inactive phase, disrupt metabolic homeostasis (24–32).
Due to the association between circadian timing and metabolic homeostasis, several research groups have used time-restricted-feeding paradigms to tease apart circadian contributions to metabolic diseases such as diabetes and obesity (32, 33). Most individuals in the developed world live in a ‘24/7’ society, with access to food at all times of the day; obesity and metabolic disease are frequently related to environmental circadian disruption (34, 35) and a chronic inflammatory state (36–38). Obese patients exhibit increases in pro-inflammatory cytokines, acute phase proteins, and chemokines that are reduced with weight loss and positively correlate with comorbidities such as insulin resistance (39–43). The interaction between the timing of food intake and immune responses, however, remains unspecified.
To investigate interactions among these systems, we employed a time-restricted feeding protocol allowing adult male Swiss Webster mice access to food during the day, during the night, or ad libitum for four weeks before administering an endotoxin challenge at either the onset of the active phase (ZT12: Experiment 1) or the onset of the inactive phase (ZT0: Experiment 2). We hypothesized that time-restricted feeding alters immune responses to an endotoxin challenge. If true, then we predicted that day-restricted feeding would impair bacteria killing capacity and cytokine responses to LPS compared to night-fed or ad libitum fed mice.
Methods
Animals
60 adult male (>8 wks old) Swiss Webster (Charles River Laboratories, Kingston, NY) mice were used for each experiment; a separate set of 36 mice was used for the nighttime bacteria killing assay. Mice were individually housed and allowed to recover for 1 week after arriving to our facility to entrain to a 12:12 light/dark cycle (09:00–21:00 EST) and recover from shipping. Food (Harlan, Teklad #7912) and filtered tap water was provided ad libitum. The following week all mice were habituated to twice daily cage switching (32). Mice were then randomly assigned to one of three groups: Day-fed, Night-fed, or ad libitum-fed (AdLib). Day-fed mice were allowed access to chow during the 12 h light phase, after which they were transferred to a second cage which contained only water and no food. After the 12 h restriction period, mice were placed back into their food-containing cage. This was done to prevent ‘crumbling ‘or ‘hoarding’ behavior which may allow animals to eat outside of the restricted time frame. Night-fed animals were similarly only allowed food during the 12 h dark phase, and transferred to their second cage with water at the beginning of the light phase. Ad lib animals were allowed access to food at all times, but also experienced twice-daily cage switching to control for any possible stress of this manipulation. All procedures and experiments were approved by the Ohio State University Institutional Animal Care and Use Committee (IACUC).
Endotoxin Administration and Tissue Collection
After four weeks of timed food restriction, mice received an I.P. injection of 0.5 mg/kg LPS (serotype 0111:B4; Sigma Aldrich, St. Louis, MO) in sterile saline or the saline alone one hour prior to the onset of the dark phase (ZT11–12: Experiment 1) or the light phase (ZT 23-0: Experiment 2). Three hours later (ZT15 and ZT3), mice were lightly anesthetized with isoflurane and rapidly decapitated and blood and tissues were collected. Blood was centrifuged at 4°C and serum was removed, aliquoted, and stored at −80°C until bacteria killing and multiplex assays. Spleens, adrenal glands, and brains were also collected. Spleens and adrenals were weighed and immediately flash frozen on dry ice. Brains were placed into RNALater reagent (Qiagen) on ice and hypothalami were later dissected, flash frozen, and stored at −80°C until qPCR analysis.
Bacteria Killing Assay (BKA)
The bacteria killing assay is an ex vivo assessment of innate immunity mediated by complement proteins and natural antibodies. Samples were immediately centrifuged at 4°C for 25 min at 4000xg and serum aliquots were stored at −80°C until assayed. Under a laminar flow hood, serum samples were diluted 1:20 in CO2-independent media (Gibco, Carlsbad, CA, USA). A standard number of colony-forming units (CFUs) of Escherichia coli (Epower 0483E7, Fisher Scientific) were added to each serum sample in a ratio of 1:10. Serum-bacteria mixtures were then incubated for 30 min at 37°C, and plated in duplicate onto tryptic-soy agar plates (Teknova, Hollister, CA, USA) using sterile technique. Two plates were spread with diluted bacteria alone as positive controls, and two were spread with media alone as negative controls. All plates were incubated at 37°C overnight, and then total CFUs were quantified by an experimenter unaware of group assignments. Total CFUs were averaged across the duplicates for each animal and then compared with the average of the positive control plates to calculate the per cent of bacteria killed. Neither negative control plates contained CFUs.
Serum Multiplex Assay
To examine serum cytokine protein profiles, a 10-plex cytokine (V-Plex proinflammatory panel 1, Meso-Scale Discovery (MSD)) panel was performed according to the manufacturer’s instructions. This kit measures protein levels of IFN-γ, IL-10, IL-12p70, IL-1β, IL-2, IL-4, IL-5, IL-6, KC/GRO (CXCL1), and TNF-α.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
RNA was extracted using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. RNA quality and quantity were determined using a spectrophotometer (NanoDrop), and cDNA was synthesized using M-MLV reverse transcription. 40 ng of cDNA/reaction was used in subsequent PCR. Taqman Fast advanced master mix (Life Technologies) containing AmpliTaq Fast DNA polymerase was used in a 20 μL duplex reaction with one of the primer/probe pairs listed below and a primer-limited primer/probe for the endogenous control eukaryotic 18s rRNA. Gene expression was assayed for tnf-α (Mm00443260_g1), il-6 (Mm00446190_m1), and il-1β (Mm00434228_m1). The 2-step real-time PCR cycling conditions used were: 95°C for 20 s, 40 cycles of 95°C for 3 s, and then 60°C for 30 s. Relative gene expression was calculated using the Pfaffl method(44). Data are expressed as fold-change from saline-injected animals on the same feeding regimen.
Statistics
Differences in body mass were analyzed using a one-way ANOVA. Two way ANOVAs, assessing the effects of time of feeding, injection, and interactions, were conducted on other somatic measures, bacteria killing capacity, nighttime serum IL-10, -12, -1β, -6, TNFα, splenic tnf-α expression, as well as daytime serum IFN-γ, IL-1β, -5, and TNF-α. If the data did not meet the assumptions of normality or equal variance, then nonparametric tests (Mann-Whitney and Kruskal-Wallis tests) were conducted, followed by a Dunn’s post-hoc test. Data that fell into this latter category were nighttime serum IFN-γ, IL-2, -4, -5, CXCL1, splenic il-1β, il-6, hypothalamic tnf-α, il-1β, and il-6, as well as daytime serum IL-10, -12, -2, -4, -6, CXCL1, daytime splenic and hypothalamic gene expression. Samples were excluded by Z-score analysis (> 2 SD) and gene expression analysis if 18S CT value was greater than 15. Mean differences were considered statistically significant when mean differences were p ≤ 0.05 for all analyses. Statistical analyses were conducted using SPSS Statistics v 22 (IBM; Armonk, NY) and visualized using Prism 7.0 (GraphPad Software; La Jolla, CA).
Results
Experiment 1: Nighttime LPS
Somatic Measures
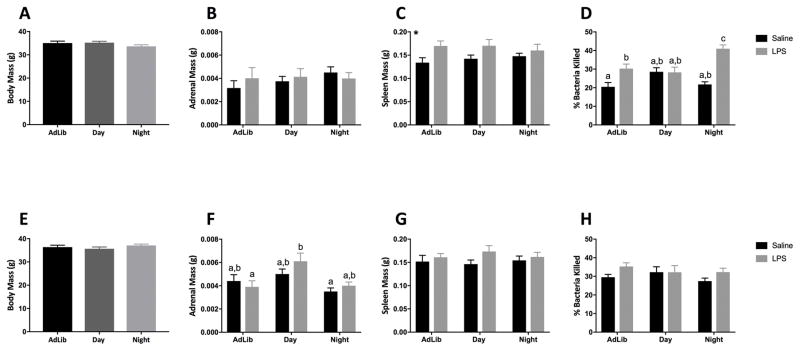
Neither body nor adrenal mass was affected by four weeks of timed food restriction at ZT15 (p > 0.05: Fig 1A and 1B, respectively). Spleen mass was increased in mice that received LPS (F1,53 = 8.48; p < 0.01; Fig 1C).
Figure 1.
Night-fed mice enhanced, while Day-fed mice eliminated, bactericidal capacity in response to a nighttime LPS challenge. Graphs depict data from nighttime (A–D) and daytime (E–H) LPS administration in timed-fed mice. (A,E) Body mass after 4 weeks of time-restricted feeding. (B,F) Adrenal and (C,G) spleen mass 3h post LPS injection (D,H). N= 5–10/feeding group/injection; error bars represent SEM. Bars sharing the same letter are not statistically significant from one another. *, p < 0.05 Saline vs LPS.
Bacteria Killing
Night-fed mice injected with LPS killed more bacteria than all other groups in a serum bactericidal assay (F2,28= 7.96; p < 0.05; Tukey’s, p < 0.05: Fig 1D). Day-fed mice did not display a bactericidal response to LPS (Tukey’s, p > 0.05).
Serum
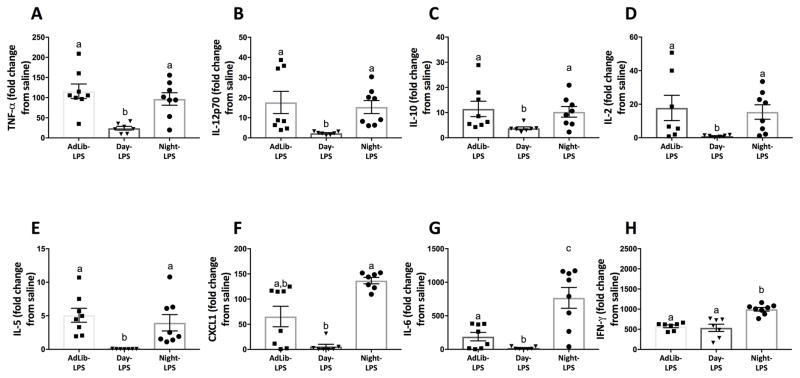
Day-fed mice decreased serum TNFα, IL-12p70, IL-10, IL-2, IL-5, CXCL1, and IL-6 concentrations in response to LPS relative to Night-fed and AdLib mice (Tukey’s, p < 0.05; Fig 2A–G). Night-fed mice increased serum IL-6 and IFN-γ concentrations in response to LPS relative to Day- and AdLib-fed mice (Tukey’s, p < 0.05; Fig 2G–H). The factorial main effects of LPS and timed feeding, as well as interactions are presented in Table 1.
Figure 2.
Serum cytokines following a nighttime (ZT12) endotoxin challenge. Four weeks of time-restricted feeding blunted serum TNF-α (A), IL-12p70 (B), IL-10 (C), IL-2 (D), IL-5 (E), CXCL1 (F), and IL-6 (G) responses to LPS in Day-fed mice. Night-fed mice increased IL-6 (G) and IFN-γ (H) responses to LPS. N=6–10/feeding group. Error bars represent SEM for parametric data and 95% confidence intervals for non-parametric data. Bars sharing the same letter are not statistically significant from one another.
Table 1.
Serum cytokine concentrations following LPS injection at ZT12 in timed fed mice.
| Cytokine | LPS | Timed Feeding | Interaction |
|---|---|---|---|
| TNF-α | p < 0.001, U= 0.00 | p = 0.41, X2= 1.79 | p < 0.01, X2= 39.49 |
| IL-1β | p < 0.001, U= 0.00 | p = 0.58, X2= 1.11 | p < 0.01, X2= 37.64 |
| IL-6 | p < 0.001, U= 7.00 | p = 0.18, X2= 3.47 | p < 0.01, X2= 38.22 |
| IFN-γ | p < 0.001, U= 0.00 | p = 0.166, X2= 3.59 | p < 0.01, X2= 37.68 |
| IL-12p70 | p < 0.001, U= 16.00 | p = 0.31, X2= 2.34 | p < 0.01, X2= 31.63 |
| IL-2 | p < 0.001, U= 96.00 | p = 0.19, X2= 3.32 | p < 0.01, X2= 21.77 |
| IL-4 | p = 0.09, U= 118.00 | p = 0.39, X2= 1.88 | P = 0.28, X2= 6.32 |
| IL-5 | p < 0.05, U= 150.00 | p < 0.01, X2= 32.32 | p < 0.01, X2= 41.94 |
| IL-10 | p < 0.001, U= 0.00 | p = 0.45, X2= 1.61 | p < 0.01, X2= 38.56 |
| CXCL1 | p < 0.001, U= 90.00 | p < 0.05, X2= 7.25 | p < 0.001, X2= 26.36 |
Table represents main effects and interactions of results from a cytokine multiplex. For parametric tests, p and F values are reported. For non-parametric tests, p and U values are reported for the Mann-Whitney U test, and p and X2 are presented for the Kruskal-Wallis test. Bolding indicates statistical significance.
Spleen
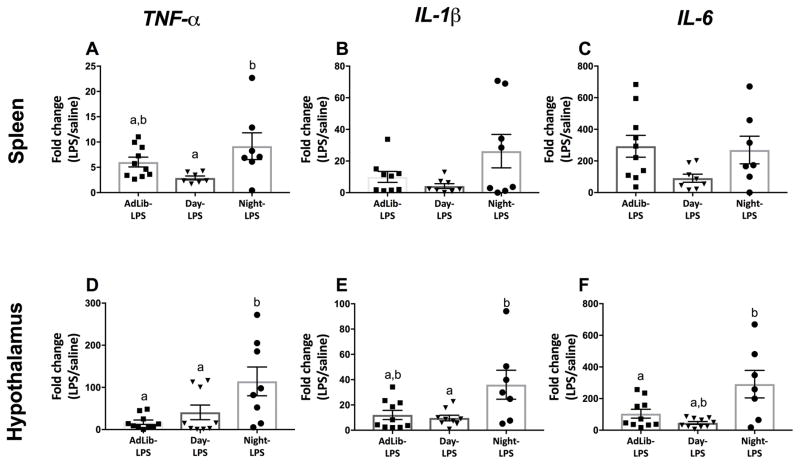
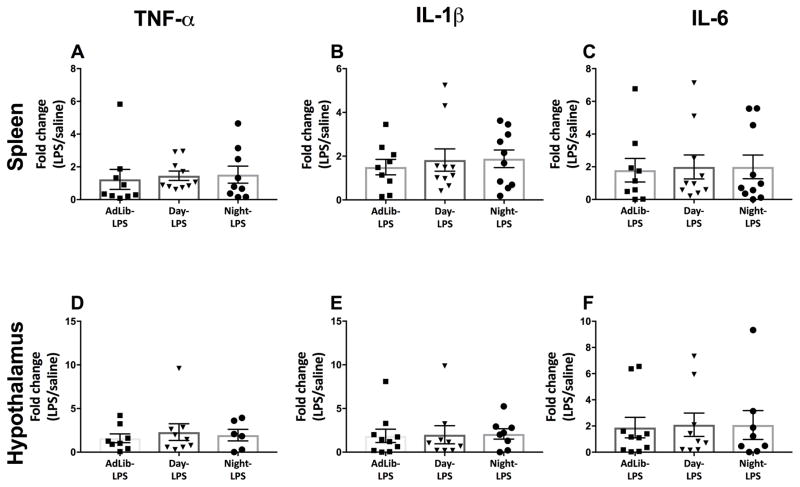
Night-fed mice elevated splenic gene expression of tnf-α 3 hrs post LPS relative to other timed feeding groups (F2,21 = 3.70; p < 0.05; Fig. 3A). No statistical differences were observed in splenic il-1β, and il-6 expression (p > 0.05; Fig. 3B–C). Timed feeding did not affect expression of tnf-α, il-1β, or il-6 in the spleens of saline-treated mice regardless of feeding group (p > 0.05).
Figure 3.
Splenic and hypothalamic pro-inflammatory gene expression following a nighttime (ZT12) endotoxin challenge. Night-fed mice increased splenic tnf-α in response to LPS (A) but had no effect on il-1β, and il-6 expression (B and C). Night-fed mice increased hypothalamic tnf-α, il-1β, and il-6 expression (C,D,E; respectively). N= 7–10/feeding group; error bars represent SEM for parametric data and 95% confidence intervals for non-parametric data. Bars sharing the same letter are not statistically significant from one another.
Hypothalamus
Night-fed mice elevated hypothalamic tnf-α, il-1β, and il-6 gene expression 3 hrs post LPS relative to all other feeding groups (F2,23 = 4.07, F2,24 = 5.62, F2,24 = 4.18; p < 0.05; Fig. 3D, E, and F, respectively). Baseline gene expression of tnf-α, il-1β, and il-6 was not altered by timed feeding in the hypothalami of saline treated mice (p > 0.05).
Experiment 2: Daytime LPS
Somatic Measures
Body mass also was equivalent among groups after four weeks of timed food restriction when assessed at ZT 3 (p > 0.05: Fig 1E), although adrenal mass was altered by timed feeding at this timepoint (F2,53= 3.32; p < 0.05: Fig 1F); LPS increased adrenal mass in Day-fed mice relative to AdLib mice (p < 0.05; Fig. 1F). Spleen mass was not altered by LPS or timed feeding in daytime injected animals (p > 0.05: Fig 1G).
Bacteria Killing
Neither LPS, nor timed feeding altered serum bactericidal capacity when injected at ZT0 (p > 0.05: Fig 1H).
Serum
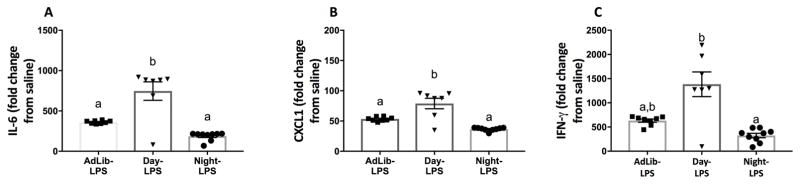
Compared to Night- and AdLib-fed mice, Day-fed mice increased concentrations of IL-6 and CXCL1 in response to LPS (Tukey’s, p < 0.05; Fig 4A–B). Day-fed mice also increased IFN-γ relative to Night-fed mice (Dunn’s, p < 0.05; Fig 4C). The factorial main effects of LPS and timed feeding, as well as interactions are presented in Table 2.
Figure 4.
Serum cytokines following a daytime (ZT0) endotoxin challenge. Daytime feeding blunted serum IL-6 (A), CXCL1 (B), and IFN-γ (C) responses to LPS. N=7–9/feeding group. Error bars represent SEM for parametric data and 95% confidence intervals for non-parametric data. Bars sharing the same letter are not statistically significant from one another.
Table 2.
Serum cytokine concentrations following LPS injection at ZT0 in timed fed mice.
| Cytokine | LPS | Timed Feeding | Interaction |
|---|---|---|---|
| TNF-α | p < 0.001, U= 26.00 | p = 0.695, X2= 4.39 | p < 0.001, X2= 1.19 |
| IL-1β | p < 0.001, U= 25.00 | p = 0.78, X2= 0.50 | p < 0.001, X2= 33.68 |
| IL-6 | p < 0.001, U= 27.00 | p = 0.088, X2= 4.87 | p < 0.001, X2= 38.87 |
| IFN-γ | p < 0.001, U= 25.00 | p = 0.07, X2= 5.31 | p < 0.001, X2= 39.02 |
| IL-12p70 | p < 0.001, U= 17.00 | p = 0.98, X2= 0.61 | p < 0.001, X2= 37.34 |
| IL-2 | p < 0.001, U= 79.00 | p = 0.78, X2= 0.50 | p < 0.001, X2= 24.40 |
| IL-4 | p < 0.001, U= 53.00 | p = 0.83, X2= 0.36 | p < 0.001, X2= 29.17 |
| IL-5 | p < 0.001, F= 44.82 | p = 0.053, F= 3.14 | p = 0.053 F= 3.14 |
| IL-10 | p < 0.001, U= 24.00 | p = 0.66, X2= 0.83 | p < 0.001, X2= 33.73 |
| CXCL1 | p < 0.001, F= 395.13 | p < 0.05, F= 4.48 | p = 0.052, F= 3.16 |
Table represents main effects and interactions of results from a cytokine multiplex. For parametric tests, p and F values are reported. For non-parametric tests, p and U values are reported for the Mann-Whitney U test, and p and X2 are presented for the Kruskal-Wallis test. Bolding indicates statistical significance.
Spleen
Timed feeding did not alter splenic cytokine response to LPS (p > 0.05; Fig. 5A, B, and C respectively). Timed feeding did not affect expression of tnf-α, il-1β, or il-6 in the spleen of saline treated mice regardless of feeding group (p > 0.05).
Figure 5.
Splenic and hypothalamic pro-inflammatory gene expression following a daytime (ZT0) endotoxin challenge. Time-restricted feeding did not alter splenic (A–C) or hypothalamic (D–F) tnf-α, il-1β, and il-6 expression in response to LPS. N= 6–10/feeding group; error bars represent SEM for parametric data and 95% confidence intervals for non-parametric data. Bars sharing the same letter are not statistically significant from one another.
Hypothalamus
Timed feeding did not alter hypothalamic cytokine response to LPS (p > 0.05; Fig. 5D, E, and F respectively). Baseline expression of tnf-α, il-1β, or il-6 was not altered by timed feeding in the hypothalami of saline treated mice (p > 0.05).
Discussion
The reciprocal relationship between the immune and circadian system has been thoroughly established (7, 45). Circadian disruption, through alterations in the lighting environment, alters innate immune responses (12–14). However, the circadian system can also be entrained by non-photic factors. The liver, and in some cases the SCN, can be entrained by time-restricted feeding (20–23). With the rise of industrialization during the past century, most individuals in the developed world live in a ‘24/7’ society with continuous access to food at all times of the day. Mistimed food consumption is associated with altered circadian rhythms in the liver and disruptions in metabolic homeostasis (30–32). However, the effects of timed feeding on physiological systems outside of metabolism have not been investigated. We hypothesized that feeding during the inactive phase would alter both functional and cytokine responses to a bacterial endotoxin challenge.
Daytime-restricted feeding impaired LPS-primed bacterial killing during both the active and inactive phase (ZT12 and 0) compared to saline-treated mice (Fig. 1D and H). In contrast, Night-fed and AdLib mice showed significant enhancement of bacteria killing capacity when challenged prior to the active but not the inactive phase (Fig. 1D and H). Blood bactericidal capacity has been used in humans and animal models to test constitutive innate immunity and predict susceptibility to bacterial infections (46–48). Serum bactericidal capacity is dependent on production of acute-phase proteins and complement (49). The acute phase response (APR) is a rapid, complex, non-specific innate immune response mediated by a large set of acute phase proteins (APPs; 53). Killing of E.coli (ATCC# 0483E7) is complement dependent (47) and decreased bactericidal capacity in day-fed animals may suggest circadian disruption induced deficits in signals driving APP and complement synthesis (49, 51, 52).
IL-6 has long been identified as a major regulator of the APR (53). Diurnal variation in IL-6 and TNF-α responses to endotoxin are driven by macrophage circadian rhythms, with maximal responses during the late light/early dark phase (2). Day-fed mice eliminated serum IL-6 and TNF-α responses at ZT12 and enhanced IL-6 responses at ZT0 (Fig 2G, 2C, and 4A). Circadian variation in IL-6 and TNF-α response to LPS is driven by expression of the core clock gene REV-ERBα in macrophages (54). REV-ERBα couples circadian rhythms and fed/fasted states in order to maintain metabolic homeostasis. Indeed, daytime-restricted feeding inverses the phase of several clock genes including rev-erbα (nr1d1) in the liver (55). REV-ERBα may act as a functional integrator of metabolic state and immune function driving altered circadian phase in immune cells resulting in deregulation of innate immune responses.
In addition to direct regulation of cytokine expression, clock genes alter the expression of key transcription factors regulating immune responses including Stat3, and NF-κB (56, 57). STAT3 mediates the IL-6 driven transcriptional regulation of APP (58). Conversely, STAT3 inhibition is necessary for induction of CXCL1, to recruit and promote survival of neutrophils (59). Deletion of STAT3 results in abrogated serum IL-6, TNFα, IL-1β, and IL-10 responses to LPS (58). These responses are similar to the diminished responses to LPS in Day-fed mice when administered LPS at the onset of active phase, with the exception of IL-1β (Fig. 2A,2C,2G). Overall, this suggests that Day-fed mice may have impaired IL-6 release or downstream signaling through the JAK/STAT pathway.
Day-fed mice also decreased concentrations of IL-12p70, IL-2, and IL-5 in response to LPS during the active phase relative to their AdLib and Night-fed counterparts (Fig. 2B, 2D, and 2E), suggesting deficits in immune cell communication, specifically in differentiation and expansion of Th1 cells (60–62). IL-12p70 and IL-2 also contribute to the APR by increasing production of opsonizing and complement fixing IgG subclasses (63, 64). These data further support diminished immune responses Day-fed mice in response to LPS.
We further investigated splenic and hypothalamic cytokine responses to the endotoxin challenge. Night-fed mice increased splenic tnf-α relative to Day-fed counterparts when challenged prior to the active phase (Fig. 3A). Disruption of circadian clock function in peripheral organs through alterations to the light/dark cycle requires communication between the SCN and peripheral oscillators. Time-restricted feeding is not sufficient to alter SCN phase without caloric restriction (20, 21). Phase shifts of circadian clocks in the liver (22, 23), kidney, heart, pancreas (65), and muscle (66) have been reported in response to time restricted feeding. Within the immune system, the effect of restricted feeding has only been assessed on platelet producing cells; daytime restricted feeding phase shifts clock genes in megakaryocytes, altering gene expression of transcription factors involved in megakaryopoiesis (67, 68). These data suggest that timed feeding can modulate immunity, independent from descending input from the brain.
Activation of the innate immune system triggers not only physiological responses, such as the APR, but behavioral responses as well. Sickness responses are a host of behavioral adaptations that develop over the course of an infection to aid in survival, triggered by the production of pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 in the hypothalamus. Specifically, IL-1β, is the primary trigger of fever, anorexic and anhedonic behavior, as well as activation of the HPA axis (69–72). Night-fed mice increased hypothalamic tnf-α, il-1β, and il-6 expression when challenged prior to the inactive phase (Fig. 3D–F). Combined with increased serum IL-6 responses to LPS, these data suggest that Night-fed mice enhance both central and peripheral signaling in response to an LPS challenge during the inactive phase.
Immune function can be modulated by a variety of factors, including adiposity (73, 74). Restricting food intake to the inactive phase, daytime in nocturnal rodents, has been previously shown to increase body mass (17). In this study, we did not observe increased body mass in response to daytime feeding (Fig. 1A,E). Previous studies used a 16:8 LD cycle, where only the food hoppers, and not the cages, were switched twice daily and mice were maintained on timed food restriction for eight weeks (17). In our study, mice were maintained on a 12:12 LD cycle, cages were switched twice daily in accordance with (32), for a total of four weeks of timed food restriction. The differential response to daytime feeding in body mass may be a result of differences in methodology.
Together, our data provide evidence that non-photic circadian disruption, through mistimed feeding, impairs immune function. Decreases in serum bactericidal capacity in day-fed animals are accompanied by deficits in pro-inflammatory cytokine concentrations in serum and production in the spleen. Conversely, night restricted feeding is comparable to, and in some cases enhances, cytokine production and bacteria killing relative to AdLib mice. Future studies should address whether timed feeding disrupts clock gene expression in immune organs. Around-the-clock feeding is commonplace in modern society and its effects on metabolism have been well documented (75, 76). These data expand upon the effects of mistimed feeding and suggest that mistimed food intake contributes to deficits in immune defenses.
Acknowledgments
We thank the undergraduate research assistants that aided with daily cage switching during this experiment including Tial KaiKai Tinkai, Curtis Stegman, Reuben Don, Adam Weiss, Evan Thomas, and Anna Suresh. We further thank Jamie Tussing and the OSU Laboratory Animal Resource staff for providing excellent care to the animals in these studies.
References
- 1.Fonken LK, Frank MG, Kitt MM, Barrientos RM, Watkins LR, Maier SF. Microglia inflammatory responses are controlled by an intrinsic circadian clock. Brain Behav Immun. 2015;45:171–179. doi: 10.1016/j.bbi.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keller M, Mazuch J, Abraham U, Eom GD, Herzog ED, Volk HD, Kramer A, Maier B. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci. 2009;106:21407–21412. doi: 10.1073/pnas.0906361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silver AC, Arjona A, Walker WE, Fikrig E. The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity. 2012;36:251–261. doi: 10.1016/j.immuni.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sato S, Sakurai T, Ogasawara J, Takahashi M, Izawa T, Imaizumi K, Taniguchi N, Ohno H, Kizaki T. A circadian clock gene, Rev-erbα, modulates the inflammatory function of macrophages through the negative regulation of Ccl2 expression. J Immunol. 2013:192. doi: 10.4049/jimmunol.1301982. [DOI] [PubMed] [Google Scholar]
- 5.Narasimamurthy R, Hatori M, Nayak SK, Liu F, Panda S, Verma IM. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc Natl Acad Sci U S A. 2012;109:12662–7. doi: 10.1073/pnas.1209965109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arjona A, Sarkar DK. Circadian Oscillations of Clock Genes, Cytolytic Factors, and Cytokines in Rat NK Cells. J Immunol. 2005;174:7618–7624. doi: 10.4049/jimmunol.174.12.7618. [DOI] [PubMed] [Google Scholar]
- 7.Scheiermann C, Kunisaki Y, Frenette PS. Circadian control of the immune system. Nat Rev Immunol. 2013;13:190–8. doi: 10.1038/nri3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arjona A, Sarkar DK. Evidence supporting a circadian control of natural killer cell function. Brain Behav Immun. 2006;20:469–76. doi: 10.1016/j.bbi.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, Boyce SH, Farrow SN, Else KJ, Singh D, Ray DW, Loudon ASI. The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci. 2012;109:582–7. doi: 10.1073/pnas.1106750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS, Chawla A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science. 2013;341:1483–8. doi: 10.1126/science.1240636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Logan RW, Sarkar DK. Circadian nature of immune function. Mol Cell Endocrinol. 2012;349:82–90. doi: 10.1016/j.mce.2011.06.039. [DOI] [PubMed] [Google Scholar]
- 12.Castanon-Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, Johnson RL, Besing RC, Menaker M, Gewirtz AT, Davidson AJ. Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol. 2010;185:5796–805. doi: 10.4049/jimmunol.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fonken LK, Weil ZM, Nelson RJ. Mice exposed to dim light at night exaggerate inflammatory responses to lipopolysaccharide. Brain Behav Immun. 2013;34:159–63. doi: 10.1016/j.bbi.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Phillips DJ, Savenkova MI, Karatsoreos IN. Environmental disruption of the circadian clock leads to altered sleep and immune responses in mouse. Brain Behav Immun. 2015;47:14–23. doi: 10.1016/j.bbi.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Borniger JC, Weil ZM, Zhang N, Nelson RJ. Dim light at night does not disrupt timing or quality of sleep in mice. Chronobiol Int. 2013;30:1016–1023. doi: 10.3109/07420528.2013.803196. [DOI] [PubMed] [Google Scholar]
- 16.Fonken LK, Finy MS, Walton JC, Weil ZM, Workman JL, Ross J, Nelson RJ. Influence of light at night on murine anxiety- and depressive-like responses. Behav Brain Res. 2009;205:349–54. doi: 10.1016/j.bbr.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, Nelson RJ. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci U S A. 2010;107:18664–9. doi: 10.1073/pnas.1008734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castillo MR, Hochstetler KJ, Tavernier RJ, Greene DM, Bult-Ito A. Entrainment of the master circadian clock by scheduled feeding. Am J Physiol - Regul Integr Comp Physiol. 2004:287. doi: 10.1152/ajpregu.00247.2004. [DOI] [PubMed] [Google Scholar]
- 19.Lamont EW, Diaz LR, Barry-Shaw J, Stewart J, Amir S. Daily restricted feeding rescues a rhythm of period2 expression in the arrhythmic suprachiasmatic nucleus. Neuroscience. 2005;132:245–248. doi: 10.1016/j.neuroscience.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 20.Mendoza J, Graff C, Dardente H, Pevet P, Challet E. Feeding cues alter clock gene oscillations and photic responses in the suprachiasmatic nuclei of mice exposed to a light/dark cycle. J Neurosci. 2005:25. doi: 10.1523/JNEUROSCI.4397-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caldelas I, Feillet CA, Dardente H, Eclancher F, Malan A, Gourmelen S, Pévet P, Challet E. Timed hypocaloric feeding and melatonin synchronize the suprachiasmatic clockwork in rats, but with opposite timing of behavioral output. Eur J Neurosci. 2005;22:921–929. doi: 10.1111/j.1460-9568.2005.04284.x. [DOI] [PubMed] [Google Scholar]
- 22.Hara R, Wan K, Wakamatsu H, Aida R, Moriya T, Akiyama M, Shibata S. Restricted feeding entrains liver clock without participation of the suprachiasmatic nucleus. Genes to Cells. 2001;6:269–278. doi: 10.1046/j.1365-2443.2001.00419.x. [DOI] [PubMed] [Google Scholar]
- 23.Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–3. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- 24.Oishi K, Miyazaki K, Kadota K, Kikuno R, Nagase T, Atsumi G, Ohkura N, Azama T, Mesaki M, Yukimasa S, Kobayashi H, Iitaka C, Umehara T, Horikoshi M, Kudo T, Shimizu Y, Yano M, Monden M, Machida K, Matsuda J, Horie S, Todo T, Ishida N. Genome-wide expression analysis of mouse liver reveals CLOCK-regulated circadian output genes. J Biol Chem. 2003;278:41519–27. doi: 10.1074/jbc.M304564200. [DOI] [PubMed] [Google Scholar]
- 25.Bugge A, Feng D, Everett LJ, Briggs ER, Mullican SE, Wang F, Jager J, Lazar MA. Rev-erbα and Rev-erbβ coordinately protect the circadian clock and normal metabolic function. Genes Dev. 2012;26:657–67. doi: 10.1101/gad.186858.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang EE, Liu Y, Dentin R, Pongsawakul PY, Liu AC, Hirota T, Nusinow DA, Sun X, Landais S, Kodama Y, Brenner DA, Montminy M, Kay SA. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med. 2010;16:1152–6. doi: 10.1038/nm.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu C, Li S, Liu T, Borjigin J, Lin JD. Transcriptional coactivator PGC-1a integrates the mammalian clock and energy metabolism. Nature. 2007;447:477–481. doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]
- 28.Adamovich Y, Rousso-Noori L, Zwighaft Z, Neufeld-Cohen A, Golik M, Kraut-Cohen J, Wang M, Han X, Asher G. Circadian clocks and feeding time regulate the oscillations and levels of hepatic triglycerides. Cell Metab. 2014;19:319–30. doi: 10.1016/j.cmet.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–21. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Sherman H, Genzer Y, Cohen R, Chapnik N, Madar Z, Froy O. Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J. 2012;26:3493–502. doi: 10.1096/fj.12-208868. [DOI] [PubMed] [Google Scholar]
- 31.Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014;20:991–1005. doi: 10.1016/j.cmet.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JAJ, Ellisman MH, Panda S. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–60. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eckel-Mahan K, Sassone-Corsi P. Metabolism and the circadian clock converge. Physiol Rev. 2013;93:107–35. doi: 10.1152/physrev.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fonken LK, Nelson RJ. The effects of light at night on circadian clocks and metabolism. Endocr Rev. 2014:er20131051. doi: 10.1210/er.2013-1051. [DOI] [PubMed] [Google Scholar]
- 35.McFadden E, Jones ME, Schoemaker MJ, Ashworth A, Swerdlow AJ. The relationship between obesity and exposure to light at night: cross-sectional analyses of over 100,000 women in the Breakthrough Generations Study. Am J Epidemiol. 2014;180:245–50. doi: 10.1093/aje/kwu117. [DOI] [PubMed] [Google Scholar]
- 36.Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm. 2010 doi: 10.1155/2010/289645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105:141–150. doi: 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 38.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia. 1997;40:1286–1292. doi: 10.1007/s001250050822. [DOI] [PubMed] [Google Scholar]
- 40.Bastard JP, Jardel C, Bruckert E, Blondy P, Capeau J, Laville M, Vidal H, Hainque B. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J Clin Endocrinol Metab. 2000;85:3338–3342. doi: 10.1210/jcem.85.9.6839. [DOI] [PubMed] [Google Scholar]
- 41.Haffner S, Temprosa M, Crandall J, Fowler S, Goldberg R, Horton E, Marcovina S, Mather K, Orchard T, Ratner R, Barrett-Connor E Diabetes Prevention Program Research Group. Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance. Diabetes. 2005;54:1566–72. doi: 10.2337/diabetes.54.5.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Natali A, Toschi E, Baldeweg S, Ciociaro D, Favilla S, Saccà L, Ferrannini E. Clustering of insulin resistance with vascular dysfunction and low-grade inflammation in type 2 diabetes. Diabetes. 2006;55:1133–40. doi: 10.2337/diabetes.55.04.06.db05-1076. [DOI] [PubMed] [Google Scholar]
- 43.Phillips CM, I, Perry J. Does inflammation determine metabolic health status in obese and nonobese adults? J Clin Endocrinol Metab. 2013;98:E1610–E1619. doi: 10.1210/jc.2013-2038. [DOI] [PubMed] [Google Scholar]
- 44.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:45e–45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cermakian N, Lange T, Golombek D, Sarkar D, Nakao A, Shibata S, Mazzoccoli G. Crosstalk between the circadian clock circuitry and the immune system. Chronobiol Int. 2015;30:870–888. doi: 10.3109/07420528.2013.782315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keusch GT, Douglas SD, Ugurbil K. Intracellular bactericidal Activity of leukocytes in whole blood for the diagnosis of chronic granulomatous disease of childhood. J Infect Dis. 1975;131:584–587. doi: 10.1093/infdis/131.5.584. [DOI] [PubMed] [Google Scholar]
- 47.Millet S, Bennett J, Lee KA, Hau M, Klasing KC. Quantifying and comparing constitutive immunity across avian species. Dev Comp Immunol. 2007;31:188–201. doi: 10.1016/j.dci.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 48.Martin LB, Weil ZM, Nelson RJ. Immune defense and reproductive pace of life in peromyscus mice. Ecology. 2007;88:2516–2528. doi: 10.1890/07-0060.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weinrauch Y, Abad C, Liang NS, Lowry SF, Weiss J. Mobilization of potent plasma bactericidal activity during systemic bacterial challenge. Role of group IIA phospholipase A2. J Clin Invest. 1998;102:633–8. doi: 10.1172/JCI3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cray C, Zaias J, Altman NH. Acute phase response in animals: a review. Comp Med. 2009;59:517–26. [PMC free article] [PubMed] [Google Scholar]
- 51.Reis ES, Lange T, Köhl G, Herrmann A, Tschulakow AV, Naujoks J, Born J, Köhl J. Sleep and circadian rhythm regulate circulating complement factors and immunoregulatory properties of C5a. Brain Behav Immun. 2011;25:1416–1426. doi: 10.1016/j.bbi.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 52.Perdiz P, Wacher N, Laredo-Sánchez F, Halabe Cherem J, Lifshitz A. Circadian variation of human acute phase response. Arch Med Res. 1996;27:157–63. [PubMed] [Google Scholar]
- 53.Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265:621–36. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, Boyce SH, Farrow SN, Else KJ, Singh D, Ray DW, Loudon ASI. The nuclear receptor REV-ERB mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci. 2012;109:582–587. doi: 10.1073/pnas.1106750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Damiola F. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bozek K, Relógio A, Kielbasa SM, Heine M, Dame C, Kramer A, Herzel H. Regulation of clock-controlled genes in mammals. PLoS One. 2009;4:e4882. doi: 10.1371/journal.pone.0004882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoffman AE, Zheng T, Stevens RG, Ba Y, Zhang Y, Leaderer D, Yi C, Holford TR, Zhu Y. Clock-cancer connection in non-Hodgkin’s lymphoma: a genetic association study and pathway analysis of the circadian gene cryptochrome 2. Cancer Res. 2009;69:3605–13. doi: 10.1158/0008-5472.CAN-08-4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alonzi T, Maritano D, Gorgoni B, Rizzuto G, Libert C, Poli V. Essential role of STAT3 in the control of the acute-phase response as revealed by inducible gene activation in the liver. Mol Cell Biol. 2001;21:1621–32. doi: 10.1128/MCB.21.5.1621-1632.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fielding CA, McLoughlin RM, McLeod L, Colmont CS, Najdovska M, Grail D, Ernst M, Jones SA, Topley N, Jenkins BJ. IL-6 regulates neutrophil trafficking during acute inflammation via STAT3. J Immunol. 2008:181. doi: 10.4049/jimmunol.181.3.2189. [DOI] [PubMed]
- 60.Hsieh C, Macatonia S, Tripp C, Wolf S, O’Garra A, Murphy K. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science (80- ) 1993;260:127–130. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 61.Liao W, Lin JX, Leonard WJ. IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr Opin Immunol. 2011;23:598–604. doi: 10.1016/j.coi.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Manetti R, Parronchi P, Giudizi MG, Piccinni MP, Maggi E, Trinchieri G, Romagnani S. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993;177:1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Germann T, Bongartz M, Dlugonska H, Hess H, Schmitt E, Kolbe L, Kölsch E, Podlaski FJ, Gately MK, Rüde E. Interleukin-12 profoundly up-regulates the synthesis of antigen-specific complement-fixing IgG2a, IgG2b and IgG3 antibody subclassesin vivo. Eur J Immunol. 1995;25:823–829. doi: 10.1002/eji.1830250329. [DOI] [PubMed] [Google Scholar]
- 64.Rocha AMC, Souza C, Rocha GA, de Melo FF, Clementino NCD, Marino MCA, Bozzi A, Silva ML, Martins Filho OA, Queiroz DMM. The levels of IL-17A and of the cytokines involved in Th17 cell commitment are increased in patients with chronic immune thrombocytopenia. Haematologica. 2011;96:1560–4. doi: 10.3324/haematol.2011.046417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reznick J, Preston E, Wilks DL, Beale SM, Turner N, Cooney GJ. Altered feeding differentially regulates circadian rhythms and energy metabolism in liver and muscle of rats. Biochim Biophys Acta. 2013;1832:228–38. doi: 10.1016/j.bbadis.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 67.Hartley PS, Sheward J, Scholefield E, French K, Horn JM, Holmes MC, Harmar AJ. Timed feeding of mice modulates light-entrained circadian rhythms of reticulated platelet abundance and plasma thrombopoietin and affects gene expression in megakaryocytes. Br J Haematol. 2009;146:185–192. doi: 10.1111/j.1365-2141.2009.07722.x. [DOI] [PubMed] [Google Scholar]
- 68.Hartley PS, Sheward WJ, French K, Horn JM, Holmes MC, Harmar AJ. Food-entrained rhythmic expression of PER2 and BMAL1 in murine megakaryocytes does not correlate with circadian rhythms in megakaryopoiesis. J Thromb Haemost. 2008;6:1144–1152. doi: 10.1111/j.1538-7836.2008.02978.x. [DOI] [PubMed] [Google Scholar]
- 69.Berkenbosch F, van Oers J, del Rey A, Tilders F, Besedovsky H. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science. 1987;238:524–6. doi: 10.1126/science.2443979. [DOI] [PubMed] [Google Scholar]
- 70.Romanovsky AA, Almeida MC, Aronoff DM, Ivanov AI, Konsman JP, Steiner AA, Turek VF. Fever and hypothermia in systemic inflammation: recent discoveries and revisions. Front Biosci. 2005;10:2193–216. doi: 10.2741/1690. [DOI] [PubMed] [Google Scholar]
- 71.Ericsson A, Kovács KJ, Sawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci. 1994;14:897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002;25:154–9. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- 73.Demas GE, Drazen DL, Nelson RJ. Reductions in total body fat decrease humoral immunity. Proc Biol Sci. 2003;270:905–11. doi: 10.1098/rspb.2003.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Demas GE, Sakaria S. Leptin regulates energetic tradeoffs between body fat and humoural immunity. Proc Biol Sci. 2005;272:1845–50. doi: 10.1098/rspb.2005.3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gluck ME, Venti CA, Salbe AD, Krakoff J. Nighttime eating: commonly observed and related to weight gain in an inpatient food intake study. Am J Clin Nutr. 2008;88:900–905. doi: 10.1093/ajcn/88.4.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gill S, Panda S. A smartphone app reveals Erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 2015;22:789–798. doi: 10.1016/j.cmet.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]