Summary
Melanoma is the deadliest form of skin cancer and its incidence is rising, creating a costly and significant clinical problem. Exposure to ultraviolet (UV) radiation, namely UVA (315–400 nm) and UVB (280–315 nm), is a major risk factor for melanoma development. Cumulative UV radiation exposure from sunlight or tanning beds contributes to UV-induced DNA damage, oxidative stress, and inflammation in the skin. A number of factors, including hair color, skin type, genetic background, location, and history of tanning, determine the skin’s response to UV radiation. In melanocytes, dysregulation of this UV radiation response can lead to melanoma. Given the complex origins of melanoma, it is difficult to develop curative therapies and universally effective preventative strategies. Here, we describe and discuss the mechanisms of UV-induced skin damage responsible for inducing melanomagenesis, and explore options for therapeutic and preventative interventions.
Introduction
Skin cancer is the most common form of cancer, representing 40–50% of all cancers diagnosed in the US1. Approximately 3.5 million cases of skin cancer are diagnosed each year in the US alone, and that number is rising each year1. Skin cancers are broadly classified into two types: non-melanoma skin cancers (NMSCs) and melanoma. Of these, melanoma is the most aggressive and lethal form of skin cancer. Melanomas represent only 4% of all skin cancers, but they account for nearly half of all skin cancer deaths2,3. In 2017, it is expected that nearly 90,000 cases of melanoma will be diagnosed in the US, leading to nearly 10,000 melanoma-related deaths4. Recent advancements in targeted therapy (vemurafenib) and immunotherapy (pembrolizumab) for melanoma have offered improvements in survival to some patients, but most patients fail to have a sustained response5.
An estimated 60–70% of cutaneous malignant melanomas are thought to be caused by ultraviolet (UV) radiation exposure6. Two types of UV radiation are primarily responsible for causing carcinogenic skin damage: UVA (315 nm-400 nm) and UVB (280 nm-315 nm). UVA is much more abundant than UVB in sunlight, accounting for 95% of solar UV radiation7. UVA is also the primary source of light used in indoor tanning beds, and tanning beds can reach UVA doses 12-times that of the sun7. UVA penetrates more deeply into the dermis than UVB8, but is less genotoxic9.
UVB causes direct DNA damage in the form of photoproducts, including cyclobutane pyrimidine dimers (CPDs) and 6−4 photoproducts (6-4PPs)10. CPDs and 6-4PPs can be recognized and repaired by the nucleotide excision repair (NER) pathway. In this pathway, DNA damage--sensing proteins, including XPC, DDB1, DDB2, and XPA, bind to sites of DNA damage, and recruit repair machinery to the site10. Dysregulation of NER is implicated in skin carcinogenesis and defects in NER cause Xeroderma pigmentosum, a disease which increases the risk of skin cancer more than 1000-fold11.
UVA is thought to cause skin damage and ultimately tumorigenesis, primarily through oxidative stress-induced DNA damage9. UVA-induced oxidative DNA damage is recognized by 8-oxyguanine DNA glycosylase 1 (OGG1) and repaired by base excision repair (BER)12 In this review, we will summarize the mechanisms by which UVA and UVB cause melanomagenesis and progression, and their implications for therapeutic and preventative strategies.
Mechanisms of UV-induced Melanoma
UV Radiation and Melanoma
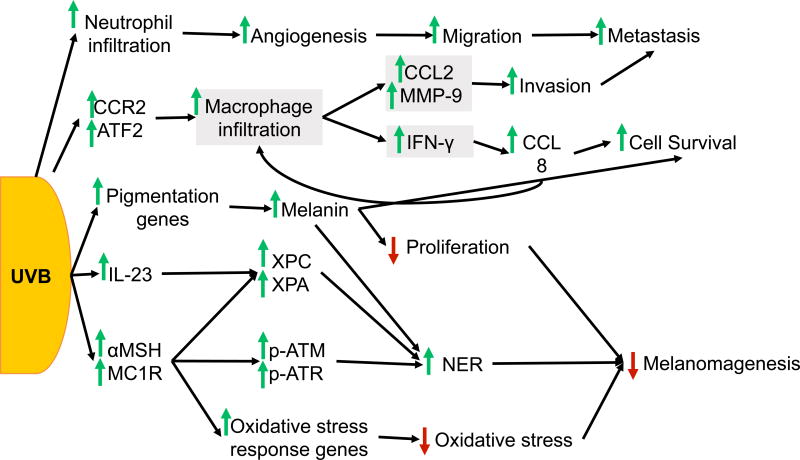
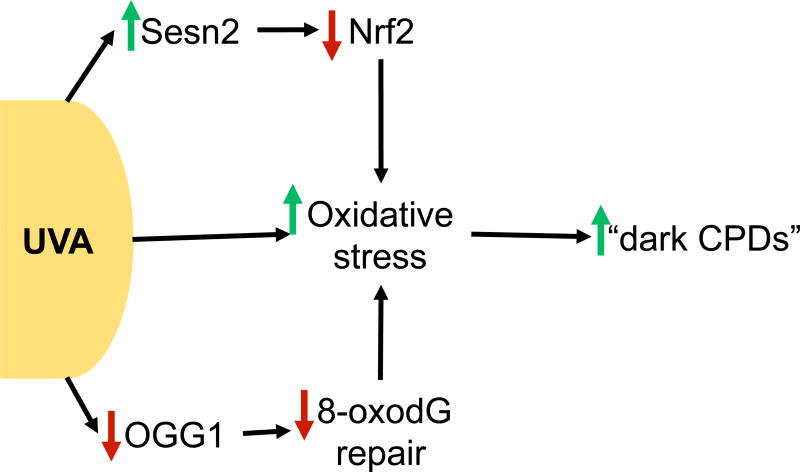
Both UVB and UVA have been shown to induce melanoma in mice. UVB radiation has a well-established role in melanomagenesis, but UVA’s contribution is controversial. In mouse models of childhood UV exposure, UVB induces melanoma formation13,14. In the same albino mouse model, perinatal UVA exposure is not sufficient to induce melanoma formation13. However, UVA was capable of inducing melanoma in pigmented C57BL/6 mice14. Furthermore, an increased risk of melanoma has also been linked to psoralen and UVA (PUVA) therapy 15. The known effects of UVB are summarized in Figure 1 and the roles of UVA in melanoma are summarized in Figure 2.
Figure 1. UVB response in melanoma.
UVB exposure triggers macrophage and neutrophil infiltration into the skin. Upregulation of CCR2 and ATF2 in melanocytes promotes recruitment of macrophages into the skin, which in turn stimulates production of CCL2, MMP-9, and IFN-γ in macrophages. IFN-γ signaling from macrophages promotes a positive feedback loop between melanocytes and macrophages, in which melanocytes upregulate CCL8, a CCR2 ligand, and promote further recruitment of macrophages. The inflammatory response created by macrophage and neutrophil recruitment promotes angiogenesis, as well as melanoma cell invasion, survival, and metastasis. UVB also independently regulates melanin production and MC1R signaling. Induction of pigmentation genes and subsequent increase in melanin production following UVB increases cell survival and NER, but decreases proliferation and ultimately, melanomagenesis. Signaling through MC1R is induced by UVB and activates DNA damage response. Signaling through αMSH and MC1R promotes phosphorylation of ATM and ATR, upregulates XPC, and promotes XPA recruitment to stimulate NER. αMSH also activates oxidative stress response genes to reduce oxidative stress in melanocytes/melanoma. UVB-induced expression of IL-23 also activates XPC and XPA to induce NER. IL-23 signaling, melanin production, and MC1R signaling can all inhibit melanomagenesis induced by UVB.
Figure 2. UVA response in melanoma.
UVA is known to directly induce oxidative stress in melanocytes, but recent work suggests that UVA perpetuates the accumulation of oxidative stress through several mechanisms. UVA induces Sestrin2, a negative regulator of Nrf2 to promote oxidative stress accumulation in melanocytes. Furthermore, UVA suppresses OGG1, impairing repair of oxidative lesions and furthering oxidative stress. Oxidative stress can ultimately lead to the accumulation of “dark CPDs” hours after UVA exposure.
UV and Genetic Alterations in Melanoma
Risk of melanoma is associated with both familial mutations and somatic mutations. Melanoma has one of the highest rates of mutation of any cancer16. Approximately 3–15% of melanomas arise due to familial genetic predisposition, in which UV-independent mutations play a significant role17. Germline mutations in CDKN2A (p16-INK4A-Arf), while rare, correlate significantly with the development of melanoma18. Other key somatic mutations in melanoma are UV-independent, including the BRAFV600E mutation found in 60% of melanomas and NRAS mutations found in 15–20% of melanomas19. While these mutations are not UV-signature mutations20, they are more common in sun-exposed skin20–23.
BRAFV600E mutation alone is often insufficient to drive malignant transformation of melanocytes24. Acquired mutations due to UV exposure can synergize with mutant BRAF to drive transformation. In mice with melanocyte-specific BRAFV600E mutations, UV exposure accelerates melanomagenesis25. 40% of the resulting tumors developed UV-signature p53 mutations, which further accelerated UV-induced melanomagenesis25. Similar UV-induced p53 mutations are seen in approximately 20% of human BRAFV600E mutant melanomas25–27. BRAFV600E mutation in melanocytes can also synergize with Arf deletion in vivo to accelerate UV-induced melanoma development28.
Recent work indicates that UV-induced mutations accumulate as melanocytic nevi transform into melanoma, including driver mutations in CDKN2A, TP53, NF1, RAC1, and PTEN25,29. One study linked UV-induced DNA damage signatures to approximately 46% of driver mutations30. Melanomas from UV-exposed areas exhibited higher mutation load than melanomas developing in protected areas27. Whole-genome sequencing of melanoma patients has identified a number of additional mutations that are significant to melanoma development, many of which can be linked to UV-induced DNA damage. A detailed review of key mutations in melanoma has been summarized elsewhere31.
Skin Color and Melanoma
Melanocytes produce two types of melanin: eumelanin and pheomelanin. Eumelanin is the most common type in dark skin and dark hair, and is synthesized upon binding of α-melanocyte-stimulating hormone (αMSH) to melanocortin-1 receptor (MC1R)32. In individuals with red hair and freckles, a loss-of-function mutation in MC1R prevents eumelanin production, leading to a higher proportion of pheomelanin33. Eumelanin reduces the accumulation of UV-induced photoproducts34, while pheomelanin may actually contribute to UV-induced DNA damage by inducing free radical formation after UV34. Total melanin levels dictate UV response in melanocytes, independent of MC1R signaling. Higher melanin levels correlate with reduced UV-induced photoproduct formation, proliferation, and apoptosis independent of MC1R function in melanocytes35,36.
Signaling through αMSH and MC1R suppresses melanomagenesis by modulating UV radiation response. αMSH treatment is sufficient to reduce UV-induced oxidative stress37 and increase nucleotide excision repair (NER) in melanocytes with wild-type MC1R38. αMSH signaling activates NER by upregulating XPC and inducing the phosphorylation of DNA damage sensors Ataxia telangiectasia and Rad3-related protein (ATR) and ataxia telangiectasia mutated (ATM)38. Furthermore, αMSH signaling through MC1R increases the recruitment of XPA to UV-induced DNA damage sites by phosphorylating ATR, thus improving DNA repair39,40. Activation of NER by αMSH requires functional MC1R; mutant MC1R increases levels of UV-induced oxidative stress36–38,41.
In melanoma patients, loss-of-function mutations in MC1R are linked to enhanced sensitivity to UV-induced cytotoxicity and increased incidence of melanoma, largely independent of skin type or hair color42. A recent meta-analysis of MC1R variants and melanoma risk showed that most variants increased risk and were associated with red hair and fair skin, but two were associated with melanoma risk independent of red hair or fair skin34,43.
UVB radiation is capable of regulating MC1R signaling and pigmentation in melanocytes. UVB induces expression of a number of pigmentation-related genes in melanocytes44, including αMSH and MC1R expression32. UVB activates expression of oxidative and ER stress response genes downstream of MC1R, although this is lost in cells expressing non-functional mutant MC1R45. UVB also induces the interaction of wild-type MC1R with PTEN, stabilizing PTEN and inhibiting PI3K/AKT signaling. In MC1R mutant cell lines, this interaction is lost and increased PI3K/AKT signaling drives transformation of BRAFV600E mutant melanoma cells46. In vivo, however, UVB exposure accelerates melanomagenesis independent of MC1R mutation status and pigmentation47, suggesting that UVB-induced melanomagenesis does not require the pigmentation and MC1R signaling response. Conversely, UVA does not induce a pigmentation response44, but may require pigmentation to induce melanomagenesis14.
Downstream of MC1R, cAMP signaling activates transcription factor MITF32. MITF is a master regulator of melanocyte differentiation required for melanocyte survival48–50. MITF controls UVB-induced expression of pigmentation genes32 and DNA repair and proliferation genes in melanoma cells51,52. Deletion of MITF in melanoma cells is sufficient to increase metastasis, concurrent with increases in mesenchymal markers53 and ROCK-mediated invasion54. MITF overexpression promotes proliferation in vitro48 and in vivo53,55, in addition to reducing metastasis53. Amplification of MITF occurs in up to 20% of all melanomas, with a higher incidence in metastatic melanoma and in BRAF mutant melanomas48. MITF protein expression is suppressed by BRAFV600E in melanocytes and melanomas, however, which allows cell proliferation48,56. It is postulated that MITF amplification serves to maintain minimal MITF levels even in the presence of BRAF-mediated suppression for cells to survive the stresses of disease progression.
UV-Induced DNA Damage Repair in Melanoma
There is conflicting evidence regarding the ability of melanoma cells to respond to DNA damage compared to normal melanocytes. Some research has shown that melanoma cells exhibit reduced DNA damage repair57 and that UVB exposure further lowered their XPC, DDB1, and DDB2 expression57. UVA similarly lowered XPC expression in melanoma cells, and they also show impaired repair of UVA-induced CPDs relative to normal melanocytes58. We have found that Sestrin2, a stress-inducible protein, is induced by UVB in melanoma cells and negatively regulates DNA damage repair59. Knockdown of Sestrin2 increased UVB-induced apoptosis and decreased tumor formation in vivo60.
DNA repair is critical for suppressing melanomagenesis. Patients with xeroderma pigmentosum (XP), a disease caused by defective NER, have a 2,000-fold increased risk of melanoma11. In melanoma patients, low levels of XPC have been shown to correlate with poor survival57. In a genetically engineered mouse model of melanoma featuring deletion of Arf and expression of BRAFV600E, UVB exposure accelerated melanomagenesis by inhibiting NER28. Further analysis concluded that Arf deletion induces XPC promoter hypermethylation and repression, as well as E2F4/DP1 inhibition in this model28. BRAFV600E mutation alone was also sufficient to repress UVB-induced XPC28. Melanocyte-specific deletion of Arf alone in vivo reduced repair of UVB-induced DNA damage61. Deletion of XPC alongside Arf knockout accelerated UVB-induced melanomagenesis in vivo62.
However, other studies suggest that there are no differences in repair of UV-induced DNA damage between melanocytes and melanoma cell lines63. While several studies found an association between DNA damage response gene upregulation and melanoma progression, the upregulated genes did not include NER genes64,65. Arf-deficient mice with XPA deletion were sensitive to UVB-induced nevus formation, but developed fewer melanomas than mice with Arf deletion alone66. This work suggests that UVB-driven progression from nevus to melanoma may depend on specific NER pathways in some genetic backgrounds. Similarly, loss of cell cycle regulator RhoA led to defective repair of UV-induced DNA damage, which decreased proliferation and reduced survival of melanoma cells67.
Oxidative modification of DNA is an important mechanism of UVA-induced skin damage and carcinogenesis. Melanocytes have diminished repair of UVA-induced oxidative damage, as melanin acts as a photosensitizer to UVA68. Dysplastic nevi have increased ROS levels relative to normal melanocytes, supporting a role for ROS accumulation in melanomagenesis69. Several potential mechanisms of ROS accumulation in melanomas have been suggested. Loss-of-heterozygosity mutations in hOGG1, an enzyme that repairs oxidative DNA damage, have been demonstrated in a small number of melanomas70,71. Additionally, we have found that UVA induces Sestrin2 in melanocytes and melanoma cells, which in turn suppresses antioxidant response factor Nrf2 and increases ROS production59. Deletion of p16 could also contribute to UV-induced ROS accumulation and oxidative DNA damage in melanocytes72. However, one study has found that OGG1 is overexpressed in some metastatic melanomas73.
ROS and reactive nitrogen species (RNS) generated in melanocytes in response to UVA radiation lead to the production of “dark CPDs” hours after UVA exposure74. The accumulation of oxidatively modified DNA only in pigmented, and not albino, mice with UVA-induced melanoma 14 suggests that melanin could play a role in UVA-induced oxidative stress. Loss of MC1R reduced repair of UV-induced CPDs in melanocytes, leading to increased UV-induced apoptosis36. Accordingly, melanin content was inversely correlated with UV-induced apoptosis and CPD formation36. Melanomas featuring disruptive mutations in MC1R are associated with a 42% increase in UV-signature mutations over those in MC1R wild-type melanomas75.
The effect of antioxidants on melanomagenesis has been explored in mouse models. The antioxidant N-Acetylcysteine (NAC) has been found to delay the onset of UV-induced melanoma in vivo76. In a mouse model with BRAFV600E mutation and PTEN deletion, NAC increased metastasis, but had no effect on primary tumors77. NAC treatment also increased migration and invasion of melanoma cell lines in vitro by activating RhoA77.
UV and Autophagy in Melanoma
Autophagy has been shown to play a context-dependent role in tumorigenesis. Autophagy can suppress tumor growth by clearing oncogenic proteins and organelles damaged by oxidative or genotoxic stress. Alternatively, autophagy can provide the macromolecule building blocks needed by highly proliferative cells and allow cells to survive a range of stress conditions. In melanoma, autophagy likely has different functions at each stage of tumor progression.
Recent work suggests that malignant melanomas have increased autophagic flux relative to benign nevi78–80. Furthermore, high levels of autophagy in melanoma correlates with metastasis80, poor response to chemotherapy, and shorter overall survival78,80. Induction of autophagy has been suggested to be a pro-survival mechanism for melanoma cells79,81,82. Autohagy is also associated with proliferation, invasion, and metastasis79, as well as promoting ROS accumulation82.
BRAFV600E mutant melanomas exhibit enhanced autophagy due to chronic ER stress83 and mTOR inhibition84. Increased autophagy has been shown to increase cell survival in BRAFV600E mutant melanomas85–87. In models of BRAFV600E mutant melanoma with PTEN-deficiency, autophagy is required for tumorigenesis86. Knockdown of the essential autophagy gene Atg7 in these mice leads to accumulation of defective mitochondria and ROS, increased senescence, decreased proliferation, and increased apoptosis86.
Inhibition of BRAFV600E with vemurafenib induces autophagy by inhibition of the mTOR signaling pathway, and autophagy has been shown to promote survival of melanoma cells after vemurafenib81,85. Combined inhibition of autophagy and mTOR signaling enhances cell death78 and impairs metastasis88 in BRAFV600E mutant melanomas. Vemurafenib-resistant melanoma cells also have enhanced autophagy, although inhibition or genetic modulation of autophagy was insufficient to regain sensitivity to vemurafenib89. Combined inhibition of autophagy and MEK signaling was sufficient to restore vemurafenib sensitivity89.
Additional conflicting evidence implicates decreased autophagy in melanoma cells, sup orting dual roles for autophagy in melanoma. High levels of autophagy adaptor protein and substrate p62 constitute a prognostic marker of malignant melanoma90, although other work indicates that p62 expression increases, then decreases late in disease progression91. Atg5 expression has also been reported to decrease as melanoma progresses from benign to malignant92. Atg5 knockdown promotes proliferation, further suggesting that reduced autophagy at the early stages may contribute to tumorigenesis92. Atg5 loss of heterozygosity is found in many advanced melanomas and correlates with poor overall survival93. Atg5 LOH increases melanoma metastasis in vivo in a BRAFV600E and PTEN-deficient mouse model93.
Expression of the autophagy inducer Beclin1 decreases as melanoma progresses94,95. One study reports that BRAFV600E overexpression in melanoma cells decreases basal autophagy levels relative to BRAF wild-type cells through a Beclin1-dependent mechanism96. Furthermore, BH3-family proteins Bcl-XL and MCL-1, which disrupt the activation of autophagy by Beclin1, are upregulated in metastatic melanomas97. Interactions between BH3-only protein Noxa and MCL-1 have been shown to disrupt inhibition of Beclin1 by MCL-1 and promote autophagy98. Recent work has shown that the BH3-only protein Noxa is upregulated in melanoma cells and promotes autophagy to inhibit apoptosis99.
Expression of MITF, which correlates with increased lysosomal gene expression in melanoma cells100, decreases with disease progression97,101. LC3 has similarly been reported to decrease during melanoma disease progression92. In heavily pigmented melanoma cells, however, LC3 is highly expressed102. In these cells, LC3 regulates MITF expression and ultimately, melanin production102. As MITF plays a critical role in melanoma growth and metastasis, this link between MITF expression and autophagy may be an important link in melanoma progression.
Inflammation in melanoma
Inflammation and immune response to melanomagenic conditions are critical for melanoma development and therapeutic response. UVB regulates the recruitment of inflammatory cells into the skin, including macrophages103,104 and neutrophils105,106. UVB induces macrophage infiltration of melanomas by upregulating Ccr2103 and ATF2104. Upon recruitment, IFN-γ signaling from macrophages triggers further upregulation of Ccl8, a Ccr2 ligand, in melanocytes103. This positive feedback loop increases melanoma growth in vivo by reducing melanoma cell death103. Depletion of macrophages inhibits UV-induced melanocyte proliferation in mouse skin107. Melanoma-derived factors also trigger the upregulation of CCL2 and MMP-9 in macrophages, which in turn promote invasion of melanoma cells108. UVB-mediated neutrophil recruitment further promotes melanoma metastasis by stimulating angiogenesis and increasing migration of melanoma cells toward blood vessels105.
Melanoma-associated inflammation involves multiple regulatory pathways. For example, interleukin 23 (IL-23) induces DNA damage repair in melanocytes, including XPC and XPA expression and γ-H2AX foci formation109. DNA damage repair induced by IL-23 inhibits melanomagenesis109. IL-23 also inhibited regulatory T cell expansion and limited IFN-γ production109. The IFN- γ receptor on melanocytes inhibits UV-induced apoptosis110, suggesting that suppression of IFN- γ by IL-23 suppresses melanomagenesis by clearing damaged cells.
Another inflammation and immunological regulatory pathway is Programmed cell death 1 (PD1) and its ligand PD-L1. Anti-PD-1 immunotherapy has shown efficacy in melanoma, but fewer than half of all melanoma patients treated with anti-PD-1 immunotherapy have a prolonged response5. Current efforts are aimed at understanding the differences between immunotherapy responders and non-responders. PD-L1 expression in melanoma cells is not associated with BRAFV600E mutation, and cells expressing PD-L1 recruit tumor-infiltrating lymphocytes (TILs) independent of BRAF status111. BRAF inhibitor-resistant melanoma cells increase PD-L1 expression in a MEK-dependent manner, and inhibition of MEK and BRAF increases apoptosis and decreases PD-L1 expression112. Cyclooxygenase-2 (COX-2) expression is also correlates with PD-L1 expression in primary melanomas, and inhibition of COX-2 by celecoxib downregulates PD-L1 in melanoma cells113.
Inflammation and response to immune therapy are also regulated by PTEN status. Loss of PTEN in melanoma cells allows PI3K-mediated activation of immunosuppressive cytokines114,115. PTEN expression represses PD-L1 expression, promoting an immune response against tumor cells. PTEN loss was associated with non-brisk (localized) immune response in tumors114,115. Other work shows that PTEN loss in melanoma cells inhibits both T cell recruitment into tumors and targeted killing of tumor cells by T cells115. PTEN loss is also associated with poor response to anti-PD-1 therapy115. Treatment with a PI3Kβ inhibitor improved response to immune therapies in vivo, further supporting a role for PTEN loss in immunosuppression in melanoma115.
Vitamins and Melanoma
Vitamin A
Vitamin A, which has a number of forms, including retinol, retinoic acid, and beta-carotene, is tumor suppressive in melanoma. Vitamin A inhibits growth, invasion, and metastasis of melanoma cells116–118. Vitamin A has been suggested to impair UV-induced tumorigenesis by preventing UV-induced oxidative stress accumulation118. Clinically, multiple studies have shown an inverse correlation between retinol intake and melanoma risk, while vitamin A and beta-carotene have no association119,120.
Vitamin C
Vitamin C, or ascorbic acid, has been suggested to play a dose-dependent role in melanoma growth and progression. High concentrations of vitamin C inhibit invasion and survival of melanoma cells121. Conversely, low concentrations of vitamin C promote melanoma cell growth, migration, and invasion, and protect against stress121. Another study found that ascorbic acid reduces HIF-1α activity and protein levels in metastatic melanoma, reducing invasion of melanoma cells122. Ascorbate, a reduced form of vitamin C, induces DNA damage and cell death in melanoma cell lines in vitro, and inhibits tumor growth in vivo123.
Vitamin D
UVB absorption in skin leads to the conversion of 7-dehydrocholesterol to previtamin D3, an isomer of vitamin D3124. Vitamin D3 production is induced by UVB in a dose-dependent manner125–127 and depends on melanin levels and skin type128,129. Dietary intake of vitamin D was initially explored as a promising preventative strategy for melanoma, but many studies have found no association between dietary vitamin D uptake and risk of melanoma130–133. Some controversy remains, however, as several have identified both a positive correlation134 and inverse association between Vitamin D3 intake and melanoma risk135.
Similarly, serum levels of vitamin D3 have been explored as a diagnostic target in melanoma, and results have been unclear. In several studies, normal serum levels of vitamin D3 at the time of diagnosis correlate with a better prognosis136,137. In another study, lower serum vitamin D3 levels were associated with advanced stages at diagnosis, worse disease-free survival, and poorer overall survival138. Lower serum vitamin D was associated with higher-stage melanomas139,140. Conversely, one large study found no association between serum vitamin D levels and melanoma risk141.
Vitamin D production in the skin protects against irradiation and likely suppresses melanomagenesis. Vitamin D reduces UV-induced DNA damage, including oxidative and genotoxic DNA damage, and induces DNA damage repair126,142,143. It inhibits proliferation and invasion of melanoma cells in vitro and in vivo118,144. Importantly, vitamin D synthesis is not inhibited by sunscreen use145, but strict physical avoidance of sunlight increases risk of vitamin D deficiency145,146.
Vitamin E
Vitamin E has 2 major forms, tocotrienols and tocopherols. Vitamin E succinate, a tocopherol, inhibits melanoma cell growth and induces apoptosis by blocking cell cycle progression in vitro118,147 and in vivo147. Tocotrienols similarly induce apoptosis in melanoma cells in vitro by inducing ER stress response148. Tocotrienols also induce degradation of melanosomes in the lysosome by promoting lysosomal and endosomal fusion149. δ-Tocotrienol alone reduces melanin content150, suppresses cell proliferation151, and induces apoptosis151 of melanoma cells in vitro. In vivo, tocotrienols inhibit melanomagenesis and progression148. Taken together, this work indicates that vitamin E suppresses melanoma growth and progression.
Vitamin K
Little work has explored the effects of vitamin K on melanoma cells. Several forms of vitamin K, including Vitamin K3 and K5, inhibit proliferation and increase apoptosis of melanoma cells152. In vivo, the vitamin K analog menadione inhibits growth of melanoma xenograft tumors153.
UV and Melanoma Risk
Childhood exposure to UV radiation is a major risk factor for skin cancer development, particularly at doses high enough to achieve sunburn154. Some studies suggest that childhood sunburns could as much as double the risk of melanoma155. This effect is highly dependent on skin tone, however. In red haired and freckled individuals, childhood UV exposure is a particularly potent risk factor for melanoma development156. Conversely, in light-skinned individuals prone to tanning, childhood UV exposure can be protective against melanoma157. Exposure to UV early in life is associated with the development of BRAF mutant melanomas, while NRAS mutation is more commonly associated with high UV exposure later in life158.
In addition to childhood UV exposure, ease of access to indoor tanning has provided teenagers and young adults with further opportunities to increase UV radiation exposure. Indoor tanning at a young age increases melanoma risk159. Use of indoor tanning beds increases as children enter adolescence, and this shift is accompanied by a ~50% drop in sunscreen use160. In the US, it is estimated that 40–50% of teenagers have utilized tanning beds161. Furthermore, approximately 70% of tanning salon customers are females under 30162, and indoor tanning before age 30 leads to a 75% increase in melanoma risk163. Data suggest that melanoma rates in women ages 15–39 are nearly double that of men of the same age group164.
Despite links between early age sunburn and melanoma, one study has found that melanoma risk was associated with the number of sunburns throughout life165. Some studies have found a similar dose-dependent effect of indoor tanning independent of age166, although another found that tanning increased risk for young women167. Indoor tanning also likely contributed to an epidemic of melanomas on the trunk in young Icelandic women in the early 2000s168. Furthermore, misunderstandings persist about the ability of an all-year tan to protect against melanoma155.
Sunscreen has been linked to a paradoxical increase in sun exposure and sunburns169. The sun protection factor (SPF) of sunscreen correlates with increased intentional sun exposure170. A similar paradoxical increase in risk is seen in indoor workers, who have a higher risk of melanoma than outdoor workers171. UVB exposure has also been linked to decreased rates of melanoma mortality172, and in mouse models, sunscreen is ineffective at preventing melanomagenesis173. Recent meta-analyses of published epidemiological data have found no link between sunscreen use and melanoma risk, however174,175. In the United States, use of sunscreen and physical barriers, such as clothing and sunglasses, is increasing159, but many Americans still report receiving at least one sunburn in the last year159.
Prevention of UV-induced Melanoma
Chemoprevention
A recent review from Chhabra et al. 176 has explored recent advancements in chemoprevention in-depth, and therefore we will not address it here.
Public Health/Outreach Efforts
Given the extremely high incidence of skin cancers, making significant strides in prevention will require large-scale public health campaigns. Australia implemented one such campaign in the 1980s, which has led to a shift in the behavior and attitude toward UV exposure177,178, particularly in young adults178,179. Incidence of melanoma on the trunk and shoulders, sites subject to intermittent UV exposure if left unprotected, was significantly decreased in Australian young adults179.
Recent efforts have aimed to reach teenagers and young adults via social media180,181 and texting182, in addition to determining the efficacy of positive vs negative/fear-based messaging183. Targeted messaging to parents of adolescents was effective at starting conversations between mothers and daughters about the concerns of indoor tanning184. In households receiving these messages, fewer daughters reported a desire to go indoor tanning than non-intervention households184. Fathers and sons were largely unaware of the messaging, however, suggesting that additional avenues are needed to engage men in awareness of the dangers of UV radiation exposure. A survey of young women who indoor tan indicates that, while they are overwhelmingly supportive of policies limiting indoor tanning for minors and placing stronger warnings of indoor tanning risks on tanning beds, they do not support a total ban185.
Tanning-related regulations vary across the US. Many states have age-based tanning bans, some requiring parental consent, and others simply require warning signs to be placed in tanning salons186. As of 2014, FDA regulations require displays on indoor tanning devices warning of skin cancer risk186. Similar restrictions exist worldwide, with many countries banning indoor tanning under the age of 18186. Enforcement of these laws is lax, however, and there are likely high rates of non-compliance by users and owners of tanning salons186.
A nationwide 10% tax on indoor tanning was implemented in the US in 2010 with the passing of the Affordable Care Act in an attempt to curb tanning bed use. A drop of approximately 25% in tanning salon patronage accompanied the tanning bed tax, although other salons reported customers were indifferent to the tax187.
Even more recently there has been a push to name tanning bed use an addiction, as frequent users can exhibit many of the classic signs of addiction188,189. A study of indoor tanning users found that those who met standards for addiction to indoor tanning exhibited higher anxiety-related symptoms and substance abuse than those who did not189. This suggests that for some indoor tanning salon users, taxes and regulations will be insufficient to prevent tanning.
Discussion and Future Directions
Melanoma presents a significant clinical problem, as its incidence is rising worldwide and current therapeutic options are ineffective for many patients. The European Cancer Organization has predicted that melanoma death rates will fall by 2050, but the number of deaths will increase unless more effective treatments are developed190. Improvements in both prevention efforts and therapeutic targeting of melanomas will be necessary to reduce melanoma-related deaths.
Prevention of melanoma can be improved by optimization of sunscreen designs, as significant research suggests that sunscreen is ineffective at reducing melanoma risk. Sunscreens could also be optimized to account for improper application and duration of use. Educational programs can be optimized to target young women and to more readily engage men, two groups who are likely to ignore warnings about the negative effects of UV exposure. Furthermore, by approaching tanning as a potentially addictive behavior, techniques could be adapted from substance abuse treatment and prevention.
Recent therapeutic advancements have made significant strides toward achieving sustained progression-free survival for a subset of metastatic melanoma patients. However, there remain a number of opportunities for improving melanoma treatment. Little work has focused on understanding the role of UV exposure in response to immunotherapy. Currently, research does not indicate that vitamins will be beneficial therapeutic options for melanoma, although more work could clarify specific opportunities in melanoma. Autophagy appears to have a highly context-specific role in melanoma, but further research will determine whether careful modulation of autophagy would benefit melanoma patients. Furthermore, our overall understanding of melanoma pathogenesis is far from complete. Future investigation is required to elucidate the molecular and cellular basis by which melanocytes become cancerous melanoma cells, and the mechanism by which melanoma cells evade immune surveillance and become resistant to targeted therapy or immunotherapy. These future studies will improve our ability to prevent melanoma development and resistance to therapies.
Acknowledgments
We apologize to those investigators whose work could not be directly referenced owing to space limitations. Work in the authors’ laboratory was supported by the NIH/NIEHS grant ES024373 and ES016936 (YYH), the American Cancer Society (ACS) grant RSG-13-078-01 (YYH), the University of Chicago Cancer Research Center (P30 CA014599), the CTSA (UL1 TR000430), and the University of Chicago Friends of Dermatology Endowment Fund. We thank Dr. Ann Motten for a critical reading of the manuscript.
References
- 1.World Health Organization, Cancer Research UK. World cancer factsheet. World Heal Organ. 2014;4 doi: 10.1002/ijc.27711. [DOI] [Google Scholar]
- 2.Skin Cancer Foundation. Skin Cancer Facts & Statistics. 2015. [Google Scholar]
- 3.Guy GP, Machlin SR, Ekwueme DU, Yabroff KR. Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007–2011. Am J Prev Med. 2015;48(2):183–187. doi: 10.1016/j.amepre.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Cancer Society T. Cancer Facts & Figures. 2017. [Google Scholar]
- 5.Ribas A, Hamid O, Daud A, et al. Association of Pembrolizumab With Tumor Response and Survival Among Patients With Advanced Melanoma. JAMA. 2016;315(15):1600. doi: 10.1001/jama.2016.4059. [DOI] [PubMed] [Google Scholar]
- 6.Koh HK, Geller AC, Miller DR, Grossbart TA, Lew RA. Prevention and early detection strategies for melanoma and skin cancer - Current status. Arch Dermatol. 1996;132(4):436–443. doi: 10.1001/archderm.1996.03890280098014. [DOI] [PubMed] [Google Scholar]
- 7.van Weelden H, de Gruijl FR, van der Putte SC, Toonstra J, van der Leun JC. The carcinogenic risks of modern tanning equipment: is UV-A safer than UV-B? Arch Dermatol Res. 1988;280(5):300–307. doi: 10.1007/BF00440604. [DOI] [PubMed] [Google Scholar]
- 8.Bruls WAG, Slaper H, can der Leun JC, Berrens L. Transmission of human epidermis and stratum corneum as a function of thickness in the ultraviolet and visible wavelengths. Photochem Photobiol. 1984;40(4):485–494. doi: 10.1111/j.1751-1097.1984.tb04622.x. [DOI] [PubMed] [Google Scholar]
- 9.De Gruijl FR. Photocarcinogenesis: UVA vs. UVB Radiation. Ski Pharmacol Appl Ski Physiol. 2002;15:316–320. doi: 10.1159/000064535. 1422-2868/02/0155-0316$18.50/0. [DOI] [PubMed] [Google Scholar]
- 10.Shah P, He Y-Y. Molecular regulation of UV-induced DNA repair. Photochem Photobiol. 91(2):254–264. doi: 10.1111/php.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradford PT, Goldstein AM, Tamura D, et al. Cancer and neurologic degeneration in xeroderma pigmentosum: long term follow-up characterises the role of DNA repair. J Med Genet. 2011;48(3):168–176. doi: 10.1136/jmg.2010.083022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dahle J, Brunborg G, Svendsrud DH, Stokke T, Kvam E. Overexpression of human OGG1 in mammalian cells decreases ultraviolet A induced mutagenesis. Cancer Lett. 2008;267(1):18–25. doi: 10.1016/j.canlet.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 13.De Fabo EC, Noonan FP, Fears T, Merlino G. Ultraviolet B but not Ultraviolet A Radiation Initiates Melanoma. Cancer Res. 2004;64(18):6372–6376. doi: 10.1158/0008-5472.CAN-04-1454. [DOI] [PubMed] [Google Scholar]
- 14.Noonan FP, Raza Zaidi M, Wolnicka-Glubisz A, et al. Melanoma induction by ultraviolet A but not ultraviolet B radiation requires melanin pigment. Nat Commun. 2012;3(884) doi: 10.1038/ncomms1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krauthammer M, Kong Y, Ha BH, et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat Genet. 2012;44(9):1006–1014. doi: 10.1038/ng.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499(7457):214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Debniak T. Familial malignant melanoma - overview. Hered Cancer Clin Pract. 2004;2(3):123–129. doi: 10.1186/1897-4287-2-3-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borg A, Sandberg T, Nilsson K, et al. High frequency of multiple melanomas and breast and pancreas carcinomas in CDKN2A mutation-positive melanoma families. J Natl Cancer Inst. 2000;92(15):1260–1266. doi: 10.1093/jnci/92.15.1260. doi: https://doi.org/10.1093/jnci/92.15.1260. [DOI] [PubMed] [Google Scholar]
- 19.Ellerhorst JA, Greene VR, Ekmekcioglu S, et al. Clinical Correlates of NRAS and BRAF Mutations in Primary Human Melanoma. Clin Cancer Res. 2011;17(2):229–235. doi: 10.1158/1078-0432.CCR-10-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curtin JA, Fridlyand J, Kageshita T, et al. Distinct Sets of Genetic Alterations in Melanoma. N Engl J Med. 2005;353(20):2135–2147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 21.Maldonado JL, Fridlyand J, Patel H, et al. Determinants of BRAF mutations in primary melanomas. J Natl Cancer Inst. 2003;95(24):1878–1890. doi: 10.1093/jnci/djg123. doi: https://doi.org/10.1093/jnci/djg123. [DOI] [PubMed] [Google Scholar]
- 22.Edwards RH, Ward MR, Wu H, et al. Absence of BRAF mutations in UV-protected mucosal melanomas. J Med Genet. 2004;41(4):270–272. doi: 10.1136/jmg.2003.016667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bauer J, Büttner P, Murali R, et al. BRAF mutations in cutaneous melanoma are independently associated with age, anatomic site of the primary tumor, and the degree of solar elastosis at the primary tumor site. Pigment Cell Melanoma Res. 2011;24(2):345–351. doi: 10.1111/j.1755-148X.2011.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michaloglou C, Vredeveld LCW, Soengas MS, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436(7051):720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 25.Viros A, Sanchez-Laorden B, Pedersen M, et al. Ultraviolet radiation accelerates BRAF-driven melanomagenesis by targeting TP53. Nature. 2014;511(7510):478–482. doi: 10.1038/nature13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodis E, Watson IR, Kryukov GV, et al. A landscape of driver mutations in melanoma. Cell. 2012;150(2):251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia J, Jia P, Hutchinson KE, et al. A Meta-analysis of Somatic Mutations from Next Generation Sequencing of 241 Melanomas: A Road Map for the Study of Genes with Potential Clinical Relevance. Mol Cancer Ther. 2014;13(7):1918–1928. doi: 10.1158/1535-7163.MCT-13-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo C, Sheng J, Hu MG, et al. Loss of ARF Sensitizes Transgenic BRAF V600E Mice to UV-Induced Melanoma via Suppression of XPC. Cancer Res. 2013;73(14):4337–4348. doi: 10.1158/0008-5472.CAN-12-4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melamed RD, Aydin IT, Rajan GS, et al. Genomic Characterization of Dysplastic Nevi Unveils Implications for Diagnosis of Melanoma. J Invest Dermatol. 2017;137(4):905–909. doi: 10.1016/j.jid.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 30.Hodis E, Watson IR, Kryukov GV, et al. A landscape of driver mutations in melanoma. Cell. 2012;150(2):251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsao H, Chin L, Garraway LA, Fisher DE. Melanoma: from mutations to medicine. Genes Dev. 2012;26(11):1131–1155. doi: 10.1101/gad.191999.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilchrest BA. Molecular Aspects of Tanning. J Invest Dermatol. 2011;131:E14–E17. doi: 10.1038/skinbio.2011.6. [DOI] [PubMed] [Google Scholar]
- 33.Nasti TH, Timares L. MC1R, eumelanin and pheomelanin: Their role in determining the susceptibility to skin cancer. Photochem Photobiol. 2015;91(1):188–200. doi: 10.1111/php.12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raimondi S, Sera F, Gandini S, et al. MC1R variants, melanoma and red hair color phenotype: A meta-analysis. Int J Cancer. 2008;122(12):2753–2760. doi: 10.1002/ijc.23396. [DOI] [PubMed] [Google Scholar]
- 35.Smit NP, Vink AA, Kolb RM, et al. Melanin offers protection against induction of cyclobutane pyrimidine dimers and 6−4 photoproducts by UVB in cultured human melanocytes. Photochem Photobiol. 2001;74(3):424–430. doi: 10.1562/0031-8655(2001)0740424MOPAIO2.0.CO2. [DOI] [PubMed] [Google Scholar]
- 36.Hauser JE, Kadekaro AL, Kavanagh RJ, et al. Melanin content and MC1R function independently affect UVR-induced DNA damage in cultured human melanocytes. Pigment Cell Res. 2006;19(4):303–314. doi: 10.1111/j.1600-0749.2006.00315.x. [DOI] [PubMed] [Google Scholar]
- 37.Kadekaro AL, Chen J, Yang J, et al. Alpha-Melanocyte-Stimulating Hormone Suppresses Oxidative Stress through a p53-Mediated Signaling Pathway in Human Melanocytes. Mol Cancer Res. 2012;10(6):778–786. doi: 10.1158/1541-7786.MCR-11-0436. [DOI] [PubMed] [Google Scholar]
- 38.Swope V, Alexander C, Starner R, Schwemberger S, Babcock G, Abdel-Malek ZA. Significance of the melanocortin 1 receptor in the DNA damage response of human melanocytes to ultraviolet radiation. Pigment Cell Melanoma Res. 2014;27(4):601–610. doi: 10.1111/pcmr.12252. [DOI] [PubMed] [Google Scholar]
- 39.Jarrett SG, Wolf Horrell EM, Boulanger MC, D’Orazio JA. Defining the Contribution of MC1R Physiological Ligands to ATR Phosphorylation at Ser435, a Predictor of DNA Repair in Melanocytes. J Invest Dermatol. 2015;135(12):3086–3095. doi: 10.1038/jid.2015.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jarrett SG, Horrell EMW, Christian PA, et al. PKA-Mediated Phosphorylation of ATR Promotes Recruitment of XPA to UV-Induced DNA Damage. Mol Cell. 2014;54(6):999–1011. doi: 10.1016/j.molcel.2014.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Kadekaro AL, Leachman S, Kavanagh RJ, et al. Melanocortin 1 receptor genotype: an important determinant of the damage response of melanocytes to ultraviolet radiation. FASEB J. 2010;24(10):3850–3860. doi: 10.1096/fj.10-158485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jhappan C, Noonan FP, Merlino G. Ultraviolet radiation and cutaneous malignant melanoma. Oncogene. 2003;22(20):3099–3112. doi: 10.1038/sj.onc.1206450. [DOI] [PubMed] [Google Scholar]
- 43.Tagliabue E, Gandini S, García-Borrón JC, et al. Association of Melanocortin-1 Receptor Variants with Pigmentary Traits in Humans: A Pooled Analysis from the M-Skip Project. 2016;136 doi: 10.1016/j.jid.2016.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi W, Miyamura Y, Wolber R, et al. Regulation of Human Skin Pigmentation in situ by Repetitive UV Exposure: Molecular Characterization of Responses to UVA and/or UVB. J Invest Dermatol. 2010;130(6):1685–1696. doi: 10.1038/jid.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.April CS, Barsh GS. Distinct Pigmentary and Melanocortin 1 Receptor-Dependent Components of Cutaneous Defense against Ultraviolet Radiation. PLoS Genet. 2007;3(1):e9. doi: 10.1371/journal.pgen.0030009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao J, Wan L, Hacker E, et al. MC1R is a potent regulator of PTEN after UV exposure in melanocytes. Mol Cell. 2013;51(4):409–422. doi: 10.1016/j.molcel.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitra D, Luo X, Morgan A, et al. An ultraviolet-radiation-independent pathway to melanoma carcinogenesis in the red hair/fair skin background. Nature. 2012;491(7424):449–453. doi: 10.1038/nature11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garraway LA, Widlund HR, Rubin MA, et al. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature. 2005;436(7047):117–122. doi: 10.1038/nature03664. [DOI] [PubMed] [Google Scholar]
- 49.Levy C, Khaled M, Fisher DE. MITF: master regulator of melanocyte development and melanoma oncogene. Trends Mol Med. 2006;12(9):406–414. doi: 10.1016/j.molmed.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 50.Hsiao JJ, Fisher DE. The roles of microphthalmia-associated transcription factor and pigmentation in melanoma. Arch Biochem Biophys. 2014;563:28–34. doi: 10.1016/j.abb.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strub T, Giuliano S, Ye T, et al. Essential role of microphthalmia transcription factor for DNA replication, mitosis and genomic stability in melanoma. Oncogene. 2011;30(20):2319–2332. doi: 10.1038/onc.2010.612. [DOI] [PubMed] [Google Scholar]
- 52.Giuliano S, Cheli Y, Ohanna M, et al. Microphthalmia-Associated Transcription Factor Controls the DNA Damage Response and a Lineage-Specific Senescence Program in Melanomas. Cancer Res. 2010;70(9):3813–3822. doi: 10.1158/0008-5472.CAN-09-2913. [DOI] [PubMed] [Google Scholar]
- 53.Cheli Y, Giuliano S, Fenouille N, et al. Hypoxia and MITF control metastatic behaviour in mouse and human melanoma cells. Oncogene. 2012;31(19):2461–2470. doi: 10.1038/onc.2011.425. [DOI] [PubMed] [Google Scholar]
- 54.Carreira S, Goodall J, Denat L, et al. Mitf regulation of Dia1 controls melanoma proliferation and invasiveness. Genes Dev. 2006;20(24):3426–3439. doi: 10.1101/gad.406406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Selzer E, Wacheck V, Lucas T, et al. The melanocyte-specific isoform of the microphthalmia transcription factor affects the phenotype of human melanoma. Cancer Res. 2002;62(7):2098–2103. [PubMed] [Google Scholar]
- 56.Wellbrock C, Marais R. Elevated expression of MITF counteracts B-RAF-stimulated melanocyte and melanoma cell proliferation. J Cell Biol. 2005;170(5):703–708. doi: 10.1083/jcb.200505059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Budden T, Davey RJ, Vilain RE, et al. Repair of UVB-induced DNA damage is reduced in melanoma due to low XPC and global genome repair. Oncotarget. 2016;7(38):60940–60953. doi: 10.18632/oncotarget.10902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murray HC, Maltby VE, Smith DW, Bowden NA. Nucleotide excision repair deficiency in melanoma in response to UVA. Exp Hematol Oncol. 2015;5(1):6. doi: 10.1186/s40164-016-0035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao B, Shah P, Qiang L, He T-C, Budanov A, He Y-Y. Distinct Role of Sesn2 in Response to UVB-Induced DNA Damage and UVA-Induced Oxidative Stress in Melanocytes. Photochem Photobiol. 2016 doi: 10.1111/php.12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao B, Shah P, Budanov AV, et al. Sestrin2 protein positively regulates AKT enzyme signaling and survival in human squamous cell carcinoma and melanoma cells. J Biol Chem. 2014;289(52):35806–35814. doi: 10.1074/jbc.M114.595397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sarkar-Agrawal P, Vergilis I, Sharpless NE, et al. Impaired processing of DNA photoproducts and ultraviolet hypermutability with loss of p16INK4a or p19ARF. J Natl Cancer Inst. 2004;96(23):1790–1793. doi: 10.1093/jnci/djh307. [DOI] [PubMed] [Google Scholar]
- 62.Yang G, Curley D, Bosenberg MW, Tsao H. Loss of Xeroderma Pigmentosum C (Xpc) Enhances Melanoma Photocarcinogenesis in Ink4a–Arf-Deficient Mice. Cancer Res. 2007;67(12):5649–5657. doi: 10.1158/0008-5472.CAN-06-3806. [DOI] [PubMed] [Google Scholar]
- 63.Gaddameedhi S, Kemp MG, Reardon JT, et al. Similar Nucleotide Excision Repair Capacity in Melanocytes and Melanoma Cells. Cancer Res. 2010;70(12):4922–4930. doi: 10.1158/0008-5472.CAN-10-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaufmann WK, Nevis KR, Qu P, et al. Defective Cell Cycle Checkpoint Functions in Melanoma Are Associated with Altered Patterns of Gene Expression. J Invest Dermatol. 2008;128(1):175–187. doi: 10.1038/sj.jid.5700935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harbst K, Staaf J, Lauss M, et al. Molecular Profiling Reveals Low- and High-Grade Forms of Primary Melanoma. Clin Cancer Res. 2012;18(15):4026–4036. doi: 10.1158/1078-0432.CCR-12-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van Schanke A, Van Venrooij GMCAL, Jongsma MJ, et al. Induction of Nevi and Skin Tumors in Ink4a/Arf Xpa Knockout Mice by Neonatal, Intermittent, or Chronic UVB Exposures. Cancer Res. 2006;66(5):2608–2615. doi: 10.1158/0008-5472.CAN-05-2476. [DOI] [PubMed] [Google Scholar]
- 67.Espinha G, Osaki JH, Costa ET, Forti FL. Inhibition of the RhoA GTPase Activity Increases Sensitivity of Melanoma Cells to UV Radiation Effects. Oxid Med Cell Longev. 2016;2016:1–14. doi: 10.1155/2016/2696952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang H-T, Choi B, Tang M-S, Setlow RB. Melanocytes are deficient in repair of oxidative DNA damage and UV-induced photoproducts. Proc Natl Acad Sci. 2010;107(27):12180–12185. doi: 10.1073/pnas.1005244107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pavel S, van Nieuwpoort F, van der Meulen H, et al. Disturbed melanin synthesis and chronic oxidative stress in dysplastic naevi. Eur J Cancer. 2004;40(9):1423–1430. doi: 10.1016/j.ejca.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 70.Zyrek-Betts J, Micale M, Lineen A, et al. Malignant blue nevus with lymph node metastases. J Cutan Pathol. 2008;35(7):651–657. doi: 10.1111/j.1600-0560.2007.00878.x. [DOI] [PubMed] [Google Scholar]
- 71.Pashaei S, Li L, Zhang H, et al. Concordant loss of heterozygosity of DNA repair gene, hOGG1, in melanoma in situ and atypical melanocytic hyperplasia. J Cutan Pathol. 2008;35(6):525–531. doi: 10.1111/j.1600-0560.2007.00865.x. [DOI] [PubMed] [Google Scholar]
- 72.Jenkins NC, Liu T, Cassidy P, et al. The p16INK4A tumor suppressor regulates cellular oxidative stress. Oncogene. 2011;30(3):265–274. doi: 10.1038/onc.2010.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kauffmann A, Rosselli F, Lazar V, et al. High expression of DNA repair pathways is associated with metastasis in melanoma patients. Oncogene. 2008;27(5):565–573. doi: 10.1038/sj.onc.1210700. [DOI] [PubMed] [Google Scholar]
- 74.Premi S, Wallisch S, Mano CM, et al. Chemiexcitation of melanin derivatives induces DNA photoproducts long after UV exposure. Science (80-) 2015;347(6224):842–847. doi: 10.1126/science.1256022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Robles-Espinoza CD, Roberts ND, Chen S, et al. Germline MC1R status influences somatic mutation burden in melanoma. Nat Commun. 2016;7:12064. doi: 10.1038/ncomms12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cotter MA, Thomas J, Cassidy P, et al. N-Acetylcysteine Protects Melanocytes against Oxidative Stress/Damage and Delays Onset of Ultraviolet-Induced Melanoma in Mice. Clin Cancer Res. 2007;13(19):5952–5958. doi: 10.1158/1078-0432.CCR-07-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Le Gal K, Ibrahim MX, Wiel C, et al. Antioxidants can increase melanoma metastasis in mice. Sci Transl Med. 2015;7(308):308re8. doi: 10.1126/scitranslmed.aad3740. [DOI] [PubMed] [Google Scholar]
- 78.Xie X, White EP, Mehnert JM. Coordinate Autophagy and mTOR Pathway Inhibition Enhances Cell Death in Melanoma. PLoS One. 2013;8(1):e55096. doi: 10.1371/journal.pone.0055096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ma X-H, Piao S, Wang D, et al. Measurements of Tumor Cell Autophagy Predict Invasiveness, Resistance to Chemotherapy, and Survival in Melanoma. Clin Cancer Res. 2011;17(10):3478–3489. doi: 10.1158/1078-0432.CCR-10-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lazova R, Camp RL, Klump V, Siddiqui SF, Amaravadi RK, Pawelek JM. Punctate LC3B Expression Is a Common Feature of Solid Tumors and Associated with Proliferation, Metastasis, and Poor Outcome. Clin Cancer Res. 2012;18(2):370–379. doi: 10.1158/1078-0432.CCR-11-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ma X-H, Piao S-F, Dey S, et al. Targeting ER stress-induced autophagy overcomes BRAF inhibitor resistance in melanoma. J Clin Invest. 2014;124(3):1406–1417. doi: 10.1172/JCI70454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rebecca VW, Massaro RR, Fedorenko IV, et al. Inhibition of autophagy enhances the effects of the AKT inhibitor MK-2206 when combined with paclitaxel and carboplatin in BRAF wild-type melanoma. Pigment Cell Melanoma Res. 2014;27(3):465–478. doi: 10.1111/pcmr.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Corazzari M, Rapino F, Ciccosanti F, et al. Oncogenic BRAF induces chronic ER stress condition resulting in increased basal autophagy and apoptotic resistance of cutaneous melanoma. Cell Death Differ. 2015;22(6):946–958. doi: 10.1038/cdd.2014.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maddodi N, Huang W, Havighurst T, Kim K, Jack Longley B, Setaluri V. Induction of Autophagy and Inhibition of Melanoma Growth In Vitro and In Vivo by Hyperactivation of Oncogenic BRAF. J Invest Dermatol. 2010;130(6):1657–1667. doi: 10.1038/jid.2010.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao Y, Wang W, Min I, et al. BRAF V600E–dependent role of autophagy in uveal melanoma. J Cancer Res Clin Oncol. 2017;143(3):447–455. doi: 10.1007/s00432-016-2317-y. [DOI] [PubMed] [Google Scholar]
- 86.Xie X, Koh JY, Price S, White E, Mehnert JM. Atg7 Overcomes Senescence and Promotes Growth of BrafV600E–Driven Melanoma. Cancer Discov. 2015;5(4):410–423. doi: 10.1158/2159-8290.CD-14-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Marino ML, Pellegrini P, Di Lernia G, et al. Autophagy Is a Protective Mechanism for Human Melanoma Cells under Acidic Stress. J Biol Chem. 2012;287(36):30664–30676. doi: 10.1074/jbc.M112.339127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Seip K, Fleten KG, Barkovskaya A, et al. Fibroblast-induced switching to the mesenchymal-like phenotype and PI3K/mTOR signaling protects melanoma cells from BRAF inhibitors. Oncotarget. 2016;7(15):19997–20015. doi: 10.18632/oncotarget.7671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Martin S, Dudek-Perić AM, Maes H, et al. Concurrent MEK and autophagy inhibition is required to restore cell death associated danger-signalling in Vemurafenib-resistant melanoma cells. Biochem Pharmacol. 2015;93(3):290–304. doi: 10.1016/j.bcp.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 90.Ellis R, Armstrong J, Kirkham N, Ness T, Horswell S, Lovat P. P62: A novel diagnostic and prognostic biomarker of malignant melanoma. J Invest Dermatol. 2012;132:S126. doi: 10.1038/jid.2013.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tang DYL, Ellis RA, Lovat PE. Prognostic Impact of Autophagy Biomarkers for Cutaneous Melanoma. Front Oncol. 2016;6:236. doi: 10.3389/fonc.2016.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu H, He Z, von Rutte T, Yousefi S, Hunger RE, Simon H-U. Down-Regulation of Autophagy-Related Protein 5 (ATG5) Contributes to the Pathogenesis of Early-Stage Cutaneous Melanoma. Sci Transl Med. 2013;5(202):202ra123–202ra123. doi: 10.1126/scitranslmed.3005864. [DOI] [PubMed] [Google Scholar]
- 93.García-Fernández M, Karras P, Checinska A, et al. Metastatic risk and resistance to BRAF inhibitors in melanoma defined by selective allelic loss of ATG5. Autophagy. 2016;12(10):1776–1790. doi: 10.1080/15548627.2016.1199301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miracco C, Cevenini G, Franchi A, et al. Beclin 1 and LC3 autophagic gene expression in cutaneous melanocytic lesions. Hum Pathol. 2010;41(4):503–512. doi: 10.1016/j.humpath.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 95.Sivridis E, Koukourakis MI, Mendrinos SE, et al. Beclin-1 and LC3A expression in cutaneous malignant melanomas. Melanoma Res. 2011;21(3):188–195. doi: 10.1097/CMR.0b013e328346612c. [DOI] [PubMed] [Google Scholar]
- 96.Armstrong JL, Corazzari M, Martin S, et al. Oncogenic B-RAF Signaling in Melanoma Impairs the Therapeutic Advantage of Autophagy Inhibition. Clin Cancer Res. 2011;17(8):2216–2226. doi: 10.1158/1078-0432.CCR-10-3003. [DOI] [PubMed] [Google Scholar]
- 97.Zhuang L, Lee CS, Scolyer RA, et al. Mcl-1, Bcl-XL and Stat3 expression are associated with progression of melanoma whereas Bcl-2, AP-2 and MITF levels decrease during progression of melanoma. Mod Pathol. 2007;20(4):416–426. doi: 10.1038/modpathol.3800750. [DOI] [PubMed] [Google Scholar]
- 98.Elgendy M, Sheridan C, Brumatti G, Martin SJ. Oncogenic Ras-Induced Expression of Noxa and Beclin-1 Promotes Autophagic Cell Death and Limits Clonogenic Survival. Mol Cell. 2011;42(1):23–35. doi: 10.1016/j.molcel.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 99.Lun Liu Y, Lai F, Wilmott JS, et al. Noxa upregulation by oncogenic activation of MEK/ERK through CREB promotes autophagy in human melanoma cells. Oncotarget. 2014;5(22):11237–11251. doi: 10.18632/oncotarget.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ploper D, Taelman VF, Robert L, et al. MITF drives endolysosomal biogenesis and potentiates Wnt signaling in melanoma cells. Proc Natl Acad Sci. 2015;112(5):E420–E429. doi: 10.1073/pnas.1424576112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nazarian RM, Prieto VG, Elder DE, Duncan LM. Melanoma biomarker expression in melanocytic tumor progression: a tissue microarray study. J Cutan Pathol. 2010;37:41–47. doi: 10.1111/j.1600-0560.2010.01505.x. [DOI] [PubMed] [Google Scholar]
- 102.Yun WJ, Kim E-Y, Park J-E, et al. Microtubule-associated protein light chain 3 is involved in melanogenesis via regulation of MITF expression in melanocytes. Sci Rep. 2016;6(1):19914. doi: 10.1038/srep19914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zaidi MR, Davis S, Noonan FP, et al. Interferon-γ links ultraviolet radiation to melanomagenesis in mice. Nature. 2011;469(7331):548–553. doi: 10.1038/nature09666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Senft D, Sorolla A, Dewing A, et al. ATF2 alters melanocyte response and macrophage recruitment in UV-irradiated neonatal mouse skin. Pigment Cell Melanoma Res. 2015;28(4):481–484. doi: 10.1111/pcmr.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bald T, Quast T, Landsberg J, et al. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature. 2014;507(7490):109–113. doi: 10.1038/nature13111. [DOI] [PubMed] [Google Scholar]
- 106.Wolnicka-Glubisz A, Damsker J, Constant S, Corn S, De Fabo E, Noonan F. Deficient inflammatory response to UV radiation in neonatal mice. J Leukoc Biol. 2007;81(6):1352–1361. doi: 10.1189/jlb.1206729. [DOI] [PubMed] [Google Scholar]
- 107.Handoko HY, Rodero MP, Boyle GM, et al. UVB-Induced Melanocyte Proliferation in Neonatal Mice Driven by CCR2-Independent Recruitment of Ly6clowMHCIIhi Macrophages. J Invest Dermatol. 2013;133(7):1803–1812. doi: 10.1038/jid.2013.9. [DOI] [PubMed] [Google Scholar]
- 108.Wang T, Ge Y, Xiao M, et al. Melanoma-derived conditioned media efficiently induce the differentiation of monocytes to macrophages that display a highly invasive gene signature. Pigment Cell Melanoma Res. 2012;25(4):493–505. doi: 10.1111/j.1755-148X.2012.01005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nasti TH, Cochran JB, Vachhani RV, et al. IL-23 Inhibits Melanoma Development by Augmenting DNA Repair and Modulating T Cell Subpopulations. J Immunol. 2017;198(2):950–961. doi: 10.4049/jimmunol.1601455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Coleman DJ, Garcia G, Hyter S, et al. Retinoid-X-Receptors (α/β) in Melanocytes Modulate Innate Immune Responses and Differentially Regulate Cell Survival following UV Irradiation. PLoS Genet. 2014;10(5):e1004321. doi: 10.1371/journal.pgen.1004321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rodi N, Anders RA, Eshleman JR, et al. PD-L1 Expression in Melanocytic Lesions Does Not Correlate with the BRAF V600E Mutation. Cancer Immunol Res. 2015;3(2):110–115. doi: 10.1158/2326-6066.CIR-14-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jiang X, Zhou J, Giobbie-Hurder A, Wargo J, Hodi FS. The Activation of MAPK in Melanoma Cells Resistant to BRAF Inhibition Promotes PD-L1 Expression That Is Reversible by MEK and PI3K Inhibition. Clin Cancer Res. 2013;19(3):598–609. doi: 10.1158/1078-0432.CCR-12-2731. [DOI] [PubMed] [Google Scholar]
- 113.Botti G, Fratangelo F, Cerrone M, et al. COX-2 expression positively correlates with PD-L1 expression in human melanoma cells. J Transl Med. 2017;15(1):46. doi: 10.1186/s12967-017-1150-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dong Y, Richards J-A, Gupta R, et al. PTEN functions as a melanoma tumor suppressor by promoting host immune response. Oncogene. 2014;33(38):4632–4642. doi: 10.1038/onc.2013.409. [DOI] [PubMed] [Google Scholar]
- 115.Peng W, Chen JQ, Liu C, et al. Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer Discov. 2016;6(2):202–216. doi: 10.1158/2159-8290.CD-15-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wood WR, Seftor EA, Lotan D, et al. Retinoic acid inhibits human melanoma tumor cell invasion. Anticancer Res. 1990;10(2A):423–432. [PubMed] [Google Scholar]
- 117.Weinzweig J, Tattini C, Lynch S, et al. Investigation of the Growth and Metastasis of Malignant Melanoma in a Murine Model: The Role of Supplemental Vitamin A. Plast Reconstr Surg. 2003;112(1):152–158. doi: 10.1097/01.PRS.0000066008.40176.EF. [DOI] [PubMed] [Google Scholar]
- 118.Russo I, Caroppo F, Alaibac M, Richard Lee C-C. Vitamins and Melanoma. Cancers (Basel) 2015;7:1371–1387. doi: 10.3390/cancers7030841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Asgari MM, Brasky TM, White E. Association of Vitamin A and Carotenoid Intake with Melanoma Risk in a Large Prospective Cohort. J Invest Dermatol. 2012;132(6):1573–1582. doi: 10.1038/jid.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang Y-P, Chu R-X, Liu H. Vitamin A Intake and Risk of Melanoma: A Meta-Analysis. In: Williams BO, editor. PLoS One. 7. Vol. 9. 2014. p. e102527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yang G, Yan Y, Ma Y, Yang Y. Vitamin C at high concentrations induces cytotoxicity in malignant melanoma but promotes tumor growth at low concentrations. Mol Carcinog. 2017 doi: 10.1002/mc.22654. [DOI] [PubMed] [Google Scholar]
- 122.Miles SL, Fischer AP, Joshi SJ, Niles RM. Ascorbic acid and ascorbate-2-phosphate decrease HIF activity and malignant properties of human melanoma cells. BMC Cancer. 2015;15(1):867. doi: 10.1186/s12885-015-1878-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Serrano OK, Parrow NL, Violet P-C, et al. Antitumor effect of pharmacologic ascorbate in the B16 murine melanoma model. Free Radic Biol Med. 2015;87:193–203. doi: 10.1016/j.freeradbiomed.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 124.Glerup H, Mikkelsen K, Poulsen L, et al. Commonly recommended daily intake of vitamin D is not sufficient if sunlight exposure is limited. J Intern Med. 2000;247(2):260–268. doi: 10.1046/j.1365-2796.2000.00595.x. [DOI] [PubMed] [Google Scholar]
- 125.Thieden E, Jørgensen HL, Jørgensen NR, Philipsen PA, Wulf HC. Sunbed Radiation Provokes Cutaneous Vitamin D Synthesis in Humans-A Randomized Controlled Trial. Photochem Photobiol. 2008;84(6):1487–1492. doi: 10.1111/j.1751-1097.2008.00372.x. [DOI] [PubMed] [Google Scholar]
- 126.Demetriou SK, Ona-Vu K, Teichert AE, Cleaver JE, Bikle DD, Oh DH. Vitamin D Receptor Mediates DNA Repair and Is UV Inducible in Intact Epidermis but Not in Cultured Keratinocytes. J Invest Dermatol. 2012;132(8):2097–2100. doi: 10.1038/jid.2012.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bogh MKB, Schmedes AV, Philipsen PA, Thieden E, Wulf HC. Vitamin D production depends on ultraviolet-B dose but not on dose rate: A randomized controlled trial. Exp Dermatol. 2011;20(1):14–18. doi: 10.1111/j.1600-0625.2010.01201.x. [DOI] [PubMed] [Google Scholar]
- 128.Wacker M, Holick MF. Sunlight and Vitamin D. Dermatoendocrinol. 2013;5(1):51–108. doi: 10.4161/derm.24494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Armas LAG, Dowell S, Akhter M, et al. Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels: The effect of UVB dose and skin color. J Am Acad Dermatol. 2007;57(4):588–593. doi: 10.1016/j.jaad.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 130.Weinstock MA, Stampfer MJ, Lew RA, Willett WC, Sober AJ. Case-control study of melanoma and dietary vitamin D: implications for advocacy of sun protection and sunscreen use. J Invest Dermatol. 1992;98(5):809–811. doi: 10.1111/1523-1747.ep12499962. doi: https://doi.org/10.1111/1523-1747.ep12499962. [DOI] [PubMed] [Google Scholar]
- 131.Tang JY, Fu T, LeBlanc E, et al. Calcium Plus Vitamin D Supplementation and the Risk of Nonmelanoma and Melanoma Skin Cancer: Post Hoc Analyses of the Women’s Health Initiative Randomized Controlled Trial. J Clin Oncol. 2011;29(22):3078–3084. doi: 10.1200/JCO.2011.34.5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Asgari MM, Maruti SS, Kushi LH, White E. A Cohort Study of Vitamin D Intake and Melanoma Risk. J Invest Dermatol. 2009;129(7):1675–1680. doi: 10.1038/jid.2008.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Park SM, Li T, Wu S, Li W-Q, Qureshi AA, Cho E. Vitamin D Intake and Risk of Skin Cancer in US Women and Men. PLoS One. 2016;11(8):e0160308. doi: 10.1371/journal.pone.0160308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Millen AE, Tucker MA, Hartge P, et al. Diet and melanoma in a case-control study. Cancer Epidemiol Biomarkers Prev. 2004;13(6):1042–1051. [PubMed] [Google Scholar]
- 135.Vinceti M, Malagoli C, Fiorentini C, et al. Inverse Association Between Dietary Vitamin D and Risk of Cutaneous Melanoma in a Northern Italy Population. Nutr Cancer. 2011;63(4):506–513. doi: 10.1080/01635581.2011.539314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ogbah Z, Visa L, Badenas C, et al. Serum 25-hydroxyvitamin D3 levels and vitamin D receptor variants in melanoma patients from the Mediterranean area of Barcelona. BMC Med Genet. 2013;14(1):26. doi: 10.1186/1471-2350-14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Newton-Bishop JA, Beswick S, Randerson-Moor J, et al. Serum 25-Hydroxyvitamin D 3 Levels Are Associated With Breslow Thickness at Presentation and Survival From Melanoma. J Clin Oncol. 2009;27(32):5439–5444. doi: 10.1200/JCO.2009.22.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fang S, Sui D, Wang Y, et al. Association of Vitamin D Levels With Outcome in Patients With Melanoma After Adjustment For C-Reactive Protein. J Clin Oncol. 2016;34(15):1741–1747. doi: 10.1200/JCO.2015.64.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nürnberg B, Gräber S, Gärtner B, et al. Reduced serum 25-hydroxyvitamin D levels in stage IV melanoma patients. Anticancer Res. 2009;29(9):3669–3674. 0250-7005/2009 $2.00+.40. [PubMed] [Google Scholar]
- 140.Gambichler T, Bindsteiner M, Höxtermann S, Kreuter A. Serum 25-hydroxyvitamin D serum levels in a large German cohort of patients with melanoma. Br J Dermatol. 2013;168(3):625–628. doi: 10.1111/j.1365-2133.2012.11212.x. [DOI] [PubMed] [Google Scholar]
- 141.Major JM, Kiruthu C, Weinstein SJ, et al. Pre-Diagnostic Circulating Vitamin D and Risk of Melanoma in Men. PLoS One. 2012;7(4):e35112. doi: 10.1371/journal.pone.0035112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Drané P, Compe E, Catez P, Chymkowitch P, Egly J-M. Selective Regulation of Vitamin D Receptor-Responsive Genes by TFIIH. Mol Cell. 2004;16(2):187–197. doi: 10.1016/j.molcel.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 143.Gordon-Thomson C, Gupta R, Tongkao-on W, Ryan A, Halliday GM, Mason RS. 1α,25 Dihydroxyvitamin D3 enhances cellular defences against UV-induced oxidative and other forms of DNA damage in skin. Photochem Photobiol Sci. 2012;11(12):1837. doi: 10.1039/c2pp25202c. [DOI] [PubMed] [Google Scholar]
- 144.Yudoh K, Matsuno H, Kimura T. 1alpha,25-dihydroxyvitamin D3 inhibits in vitro invasiveness through the extracellular matrix and in vivo pulmonary metastasis of B16 mouse melanoma. J Lab Clin Med. 1999;133(2):120–128. doi: 10.1016/s0022-2143(99)90004-5. doi: http://doi.org/10.1016/S0022-2143(99)90004-5. [DOI] [PubMed] [Google Scholar]
- 145.Linos E, Keiser E, Kanzler M, et al. Sun protective behaviors and vitamin D levels in the US population: NHANES 2003–2006. Cancer Causes Control. 2012;23(1):133–140. doi: 10.1007/s10552-011-9862-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kuwabara A, Tsugawa N, Tanaka K, et al. High prevalence of vitamin D deficiency in patients with xeroderma pigmetosum-A under strict sun protection. Eur J Clin Nutr. 2015;69(6):693–696. doi: 10.1038/ejcn.2015.1. [DOI] [PubMed] [Google Scholar]
- 147.Malafa MP, Fokum FD, Mowlavi A, Abusief M, King M. Vitamin E inhibits melanoma growth in mice. Surgery. 2002;131:85–91. doi: 10.1067/msy.2002.119191. [DOI] [PubMed] [Google Scholar]
- 148.Montagnani Marelli M, Marzagalli M, Moretti RM, et al. Vitamin E δ-tocotrienol triggers endoplasmic reticulum stress-mediated apoptosis in human melanoma cells. Sci Rep. 2016;6(1):30502. doi: 10.1038/srep30502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Choi B, Heo JH, Kwon HJ, Lee E-S, Sohn S. Tocotrienols enhance melanosome degradation through endosome docking/fusion proteins in B16F10 melanoma cells. Food Funct. 2013;4(10):1481. doi: 10.1039/c3fo60289c. [DOI] [PubMed] [Google Scholar]
- 150.Michihara A, Ogawa S, Kamizaki Y, Akasaki K. Effect of δ-tocotrienol on melanin content and enzymes for melanin synthesis in mouse melanoma cells. Biol Pharm Bull. 2010;33(9):1471–1476. doi: 10.1248/bpb.33.1471. doi: http://doi.org/10.1248/bpb.33.1471. [DOI] [PubMed] [Google Scholar]
- 151.Fernandes NV, Guntipalli PK, Mo H. d-δ-Tocotrienol-mediated cell cycle arrest and apoptosis in human melanoma cells. Anticancer Res. 2010;30(12):4937–4944. 0250-7005/2010 $2.00+.40. [PubMed] [Google Scholar]
- 152.Ishibashi M, Arai M, Tanaka S, Onda K, Hirano T. Antiproliferative and apoptosis-inducing effects of lipophilic vitamins on human melanoma A375 cells in vitro. Biol Pharm Bull. 2012;35(1):10–17. doi: 10.1248/bpb.35.10. doi: http://doi.org/10.1248/bpb.35.10. [DOI] [PubMed] [Google Scholar]
- 153.Shah M, Stebbins JL, Dewing A, Qi J, Pellecchia M, Ronai ZA. Inhibition of Siah2 ubiquitin ligase by vitamin K3 (menadione) attenuates hypoxia and MAPK signaling and blocks melanoma tumorigenesis. Pigment Cell Melanoma Res. 2009;22(6):799–808. doi: 10.1111/j.1755-148X.2009.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Whiteman DC, Stickley M, Watt P, Hughes MC, Davis MB, Green AC. Anatomic site, sun exposure, and risk of cutaneous melanoma. J Clin Oncol. 2006;24(19):3172–3177. doi: 10.1200/JCO.2006.06.1325. [DOI] [PubMed] [Google Scholar]
- 155.Cress RD, Holly EA, Ahn1 DK. Cutaneous Melanoma in Women. Epidemiology. 1995;6(5):538–543. doi: 10.1097/00001648-199509000-00013. [DOI] [PubMed] [Google Scholar]
- 156.Ródenas JM, Delgado-Rodríguez M, Herranz MT, Tercedor J, Serrano S. Sun exposure, pigmentary traits, and risk of cutaneous malignant melanoma: a case-control study in a Mediterranean population. Cancer Causes Control. 1996;7(2):275–283. doi: 10.1007/BF00051303. [DOI] [PubMed] [Google Scholar]
- 157.White E, Kirkpatrick CS, Lee JA. Case-control study of malignant melanoma in Washington State. I. Constitutional factors and sun exposure. Am J Epidemiol. 1994;139(9):857–868. doi: 10.1093/oxfordjournals.aje.a117092. doi: https://doi.org/10.1093/oxfordjournals.aje.a117092. [DOI] [PubMed] [Google Scholar]
- 158.Thomas NE, Edmiston SN, Alexander A, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomarkers Prev. 2007 doi: 10.1158/1055-9965.EPI-06-1038. [DOI] [PubMed] [Google Scholar]
- 159.Centers for Disease Control and Prevention (CDC) Sunburn and sun protective behaviors among adults aged 18–29 years--United States, 2000–2010. Morb Mortal Wkly Rep. 2012;61(18):317–322. [PubMed] [Google Scholar]
- 160.Dusza SW, Halpern AC, Satagopan JM, et al. Prospective Study of Sunburn and Sun Behavior Patterns During Adolescence. Pediatrics. 2012;129(2):309–317. doi: 10.1542/peds.2011-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Doré J-F, Chignol M-C. Tanning salons and skin cancer. Photochem Photobiol Sci. 2012;11(1):30–37. doi: 10.1039/c1pp05186e. [DOI] [PubMed] [Google Scholar]
- 162.Whitmore SE, Morison WL, Potten CS, Chadwick C. Tanning salon exposure and molecular alterations. J Am Acad Dermatol. 2001;44(5):775–780. doi: 10.1067/mjd.2001.112581. [DOI] [PubMed] [Google Scholar]
- 163.Group TIA for R on CW. The association of use of sunbeds with cutaneous malignant melanoma and other skin cancers: A systematic review. Int J Cancer. 2006;120(5):1116–1122. doi: 10.1002/ijc.22453. [DOI] [PubMed] [Google Scholar]
- 164.Jemal A, Saraiya M, Patel P, et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992–2006. J Am Acad Dermatol. 2011;65(5):S17.e1–S17.e11. doi: 10.1016/j.jaad.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 165.Dennis LK, Vanbeek MJ, Beane Freeman LE, Smith BJ, Dawson DV, Coughlin JA. Sunburns and Risk of Cutaneous Melanoma: Does Age Matter? A Comprehensive Meta-Analysis. Ann Epidemiol. 2008;18(8):614–627. doi: 10.1016/j.annepidem.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lazovich D, Vogel RI, Berwick M, Weinstock MA, Anderson KE, Warshaw EM. Indoor tanning and risk of melanoma: A case-control study in a highly exposed population. Cancer Epidemiol Biomarkers Prev. 2010;19(6):1557–1568. doi: 10.1158/1055-9965.EPI-09-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ting W, Schultz K, Cac NN, Peterson M, Walling HW. Tanning bed exposure increases the risk of malignant melanoma. Int J Dermatol. 2007;46(12):1253–1257. doi: 10.1111/j.1365-4632.2007.03408.x. [DOI] [PubMed] [Google Scholar]
- 168.Hery C, Tryggvadottir L, Sigurdsson T, et al. A Melanoma Epidemic in Iceland: Possible Influence of Sunbed Use. Am J Epidemiol. 2010;172(7):762–767. doi: 10.1093/aje/kwq238. [DOI] [PubMed] [Google Scholar]
- 169.Wolf P, Quehenberger F, Müllegger R, Stranz B, Kerl H. Phenotypic markers, sunlight-related factors and sunscreen use in patients with cutaneous melanoma: an Austrian case-control study. Melanoma Res. 1998;8(4):370–378. doi: 10.1097/00008390-199808000-00012. [DOI] [PubMed] [Google Scholar]
- 170.Autier P, Boniol M, Doré J-F. Sunscreen use and increased duration of intentional sun exposure: Still a burning issue. Int J Cancer. 2007;121(1):1–5. doi: 10.1002/ijc.22745. [DOI] [PubMed] [Google Scholar]
- 171.Gandini S, Sera F, Cattaruzza MS. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur J Cancer. 2005;41(1):45–60. doi: 10.1016/j.ejca.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 172.Garland CF, Garland FC, Gorham ED. Epidemiologic Evidence for Different Roles of Ultraviolet A and B Radiation in Melanoma Mortality Rates. Ann Epidemiol. 2003;13(6):395–404. doi: 10.1016/S1047-2797(02)00461-1. [DOI] [PubMed] [Google Scholar]
- 173.Wolf P, Donawho CK, Kripke ML. Effect of Sunscreens on UV Radiation-Induced Enhancement of Melanoma Growth in Mice. J Natl Cancer Inst. 1994;86(2):99–105. doi: 10.1093/jnci/86.2.99. [DOI] [PubMed] [Google Scholar]
- 174.Xie F, Xie T, Song Q, Xia S, Li H. Analysis of association between sunscreens use and risk of malignant melanoma. Int J Cinical Exp Med. 2015;8(2):2378–2384. [PMC free article] [PubMed] [Google Scholar]
- 175.Huncharek M, Kupelnick B. Use of topical sunscreens and the risk of malignant melanoma: a meta-analysis of 9067 patients from 11 case-control studies. Am J Public Health. 2002;92(7):1173–1177. doi: 10.2105/ajph.92.7.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chhabra G, Ndiaye MA, Garcia-Peterson LM, Ahmad N. Melanoma Chemoprevention: Current Status and Future Prospects. Photochem Photobiol. 2017 doi: 10.1111/php.12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Marks R. Two decades of the public health approach to skin cancer control in Australia: why, how and where are we now? Australas J Dermatol. 1999;40(1):1–5. doi: 10.1046/j.1440-0960.1999.00307.x. [DOI] [PubMed] [Google Scholar]
- 178.Volkov A, Dobbinson S, Wakefield M, Slevin T. Seven-year trends in sun protection and sunburn among Australian adolescents and adults. Aust N Z J Public Health. 2013;37(1):63–69. doi: 10.1111/1753-6405.12012. [DOI] [PubMed] [Google Scholar]
- 179.Youl PH, Youlden DR, Baade PD. Changes in the site distribution of common melanoma subtypes in Queensland, Australia over time: implications for public health campaigns. Br J Dermatol. 2013;168(1):136–144. doi: 10.1111/j.1365-2133.2012.11064.x. [DOI] [PubMed] [Google Scholar]
- 180.Pagoto SL, Baker K, Griffith J, et al. Engaging Moms on Teen Indoor Tanning Through Social Media: Protocol of a Randomized Controlled Trial. JMIR Res Protoc. 2016;5(4):e228. doi: 10.2196/resprot.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hillhouse J, Turrisi R, Scaglione NM, Cleveland MJ, Baker K, Florence LC. A Web-Based Intervention to Reduce Indoor Tanning Motivations in Adolescents: a Randomized Controlled Trial. Prev Sci. 2017;18(2):131–140. doi: 10.1007/s11121-016-0698-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Evans WD, Mays D. Design and Feasibility of a Text Messaging Intervention to Prevent Indoor Tanning Among Young Adult Women: A Pilot Study. JMIR mHealth uHealth. 2016;4(4):e137. doi: 10.2196/mhealth.6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mays D, Zhao X. The influence of framed messages and self-affirmation on indoor tanning behavioral intentions in 18- to 30-year-old women. Heal Psychol. 2016;35(2):123–130. doi: 10.1037/hea0000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lazovich D, Choi K, Rolnick C, Jackson JM, Forster J, Southwell B. An Intervention to Decrease Adolescent Indoor Tanning: A Multi-Method Pilot Study. J Adolesc Heal. 2013;52(5):S76–S82. doi: 10.1016/j.jadohealth.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mays D, Murphy SE, Bubly R, Atkins MB, Tercyak KP. Support for indoor tanning policies among young adult women who indoor tan. Transl Behav Med. 2016;6(4):613–621. doi: 10.1007/s13142-016-0432-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Le Clair MZ, Cockburn MG. Tanning bed use and melanoma: Establishing risk and improving prevention interventions. Prev Med Reports. 2016;3:139–144. doi: 10.1016/j.pmedr.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jain N, Rademaker A, Robinson JK. Implementation of the federal excise tax on indoor tanning services in Illinois. Arch Dermatol. 2012;148(1):122–124. doi: 10.1001/archderm.148.1.122. [DOI] [PubMed] [Google Scholar]
- 188.Petit A, Lejoyeux M, Reynaud M, Karila L. Excessive indoor tanning as a behavioral addiction: a literature review. Curr Pharm Des. 2014;20(25):4070–4075. doi: 10.2174/13816128113199990615. 1381-6128/14 $58.00+.00. [DOI] [PubMed] [Google Scholar]
- 189.Mosher CE, Danoff-Burg S. Addiction to Indoor Tanning. Arch Dermatol. 2010;146(4):412–417. doi: 10.1001/archdermatol.2009.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.European Cancer Organization T. Experts predict melanoma death rates will fall by 2050, but numbers of deaths will increase unless effective treatments are developed. ECCO2017 NEWS. http://www.eccocongress.org/Global/News/ECCO2017-News/2017/01/ECCO2017-NEWS-Experts-predict-melanoma-death-rates-will-fall-by-2050. Published 2017.