Abstract
Aims
To assess whether individuals trying to quit smoking who have high depressive symptoms (HD), compared with low-depressive-symptom (LD) symptoms: 1) report more frequent stressful events (SEs), 2) are more likely to smoke after SEs, 3) experience greater acute or persistent changes in affect after a SE, and 4) are at greater risk of smoking following affective changes.
Design
Smoking cessation data were analyzed using multilevel path modeling to examine the moderating effects of depressive symptoms on relations among SEs, subsequent affect, and smoking.
Setting
An academic research center in Central New Jersey, USA.
Participants
Seventy-one adult treatment-seeking daily smokers recruited from 2010 to 2012.
Measurements
Baseline depressive symptoms (HD: Center for Epidemiological Studies Depression Scale [CES-D] ≥ 16 vs. LD: CES-D < 16); and real-time ecological momentary assessment (EMA) reports of SEs, affect, and smoking assessed over 21 days post-quit.
Findings
Multilevel models indicated that HD smokers were more likely than LD smokers to report stressful events (OR = 2.32, p = .009), but had similar post-stress acute affective changes (negative affect: b = - .12, p = .137, positive affect: b = .02, p = .805). Only HD smokers reported increased negative affect (NA) (b = .20, p = .030) and decreased positive affect (PA) up to 12 hours later (b = -.22, p = .021), and greater lapse risk up to 24 hours after an SE (OR = 3.21, p = .017). The persistence of elevated NA and suppressed PA was partially explained by increased odds of subsequent SEs among HD smokers. However, the heightened stress-lapse association over 24 hours found in HD smokers was not fully explained by sustained aversive affect or subsequent SEs.
Conclusions
Depressed and non-depressed smokers trying to quit appear to experience similar acute affective changes following stress: however, depressed smokers experience higher rates of exposure to stress, longer-lasting post-stress affective disturbance, and greater risk of smoking lapse 12-24 hours after a stressful event than non-depressed smoker.
Keywords: smoking cessation, affect, stress reactivity, depressive symptoms, ecological momentary assessment
Introduction
Although smoking prevalence in the US has declined to 15% in the general population (1), smoking rates among individuals with mental illness remain above 35% (2, 3). Individuals with depressive symptoms smoke at higher rates than those without such symptoms (4, 5), and elevated pre-cessation depressive symptoms are predictive of poor smoking cessation outcomes (6-12). Individuals with depression report greater exposure to various types of stressful events (13-15), which may increase smoking risk (16). Pre-cessation depressive symptoms are also associated with greater negative affect and lower positive affect during cessation (17-20), both of which predict near-term smoking lapse (21-24). Pre-cessation depressive symptoms impede successful quitting, in part, by modulating affect during cessation.
Pre-quit depressive symptoms may also impede cessation by increasing reactivity to negative life events (25, 26) or reducing distress tolerance (35-38), both of which are associated with failure to quit (10, 27, 28) and account for depression-substance use relations (29, 30). Yet it is unknown if depressive symptoms are associated with heightened affective reactivity and smoking lapse risk during cessation.
Examining the associations among pre-quit depressive symptoms, real-time stress, and later smoking may shed light on particular challenges to abstinence experienced by smokers high in depressive symptoms. No studies to date have investigated whether pre-cessation depressive symptoms moderate the acute or persistent impact of stressful events on smoking, affect, and affect-smoking relations during a quit attempt. Studies examining relations between smoking and stress reactivity often employ laboratory paradigms (31-33) to examine these constructs in a controlled environment. However, these methods do not provide information regarding the differential effects of post-stress experience on smoking behavior in natural contexts or during ongoing change efforts. Ecological Momentary Assessment (EMA), on the other hand, captures smokers' affective reactions to stressful events and relations between acute changes in affect and smoking in a natural context.
The current study examines relations among stressful events (SEs), affect, and smoking in real-time during a quit attempt using EMA in 20 smokers high (Center for Epidemiological Studies Depression Scale [CES-D] scores ≥ 16) and 51 smokers low (CES-D < 16) in baseline depressive symptoms. We first assessed whether, during the first 21 days of a quit attempt, 1) high-depressive-symptoms (HD) and low-depressive-symptom (LD) smokers differed in the likelihood of reporting SEs. Next, we examined whether affective and smoking responses to stressful events differed by depressive symptoms by assessing whether HD smokers were more likely than LD smokers to: 2) smoke after a stressful event (moderated c path), 3) experience increases in negative affect and/or decreases in positive affect after a stressful event (moderated a paths), and 4) smoke following post-stress changes in affect (moderated b path) (Figure 1). In order to examine both acute and persistent affective reactivity to a stressor, these relations were examined both in short-term (12-hour) and longer-term (24-hour) models. In addition, we also explored the role of subsequent SEs in persistent effects of stressful events on affect and smoking.
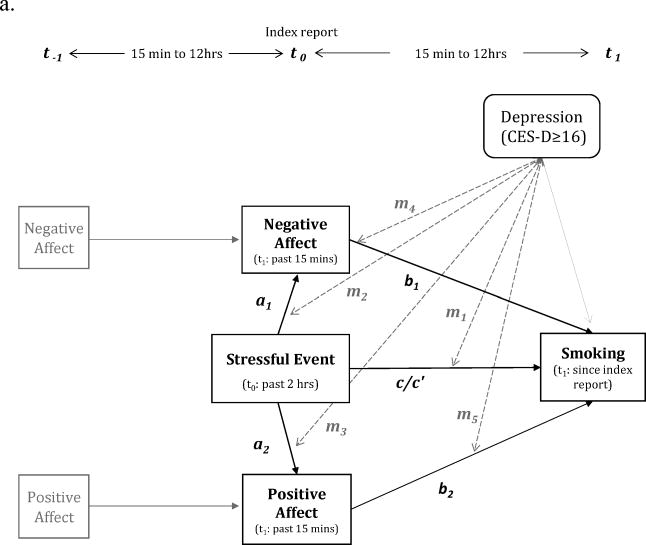
Figure 1. Simplified short-term and long-term models.
a. Short-term models (over 2-12 hours)
b. Long-term models (over 12-24 hours)
Covariates in the model are not depicted in the figures (see Table 2 & 4). Dichotomized depressive symptom level (HD vs. LD) was entered as a level-2 moderator.
Note: m (dotted gray lines): moderating effects of baseline depressive symptoms (HD: CES-D ≥ 16 vs. LD: CES-D < 16)
Method
Design
Secondary analyses of data from a smoking cessation study (34) prospectively examined baseline depressive symptoms as a moderator of relations among stressful events, affective changes, and smoking risk assessed via EMA during the first 21 days of a quit attempt. All participants received standard smoking cessation counseling and a 12-week course of nicotine lozenge treatment starting on the target quit day. Participants carried electronic diaries for 31 days, 10 days pre-quit to 21 days post-quit.
Participants
Seventy-one (53% out of 134) participants enrolled in the parent study (34) who completed a minimum of 3 consecutive post-quit reports and reported at least 1 self-perceived significant stressful event were included in analyses. There were no significant differences between those included or excluded on predictors of interest. Participants were adult daily smokers (≥10 cigarettes per day) motivated to quit, and without bipolar or psychotic disorders, recruited in central New Jersey from April 17, 2010 to November 9, 2012. Detailed inclusion/exclusion criteria are provided in supporting information. Demographic characteristics are summarized in Table 1. In total, participants provided 4,528 random post-quit reports (M= 63.49 reports/person).
Table 1.
Demographic and Baseline Characteristics for Final Sample by Baseline Depressive Symptoms (High vs. Low).
| High CES-D ≥ 16 (n=20) | Low CES-D < 16 (n=51) | p | |
|---|---|---|---|
|
| |||
| n (%) | n (%) | ||
| Female | 10 (50.00%) | 26 (50.98%) | 1.00 |
| White | 14 (70.00%) | 36 (70.59%) | 1.00 |
| Education | |||
| < High school graduate | 0 (0.00%) | 1 (1.96%) | 0.22 |
| High school graduate | 8 (40.00%) | 9 (17.65%) | |
| Some college | 6 (30.00%) | 24 (47.06%) | |
| College degree | 6 (30.00%) | 17 (33.33%) | |
| Household Income | |||
| < $ 25,000 | 11 (57.89%) | 13 (25.49%) | 0.06 |
| $25,000 - $34,999 | 0 (0.00%) | 3 (5.88%) | |
| $25,000 - $49,999 | 3 (15.79%) | 8 (15.69%) | |
| $50,000 - $74,999 | 4 (21.05%) | 10 (19.60%) | |
| > $75,000 | 1 (5.26%) | 17 (33.33%) | |
|
| |||
| Mean (SD) | Mean (SD) | ||
|
| |||
| Age | 44.30 (9.98) | 44.35 (12.28) | 0.99 |
| Age at first cigarette | 13.65 (3.70) | 15.20 (2.76) | 0.10 |
| Cigarettes smoked per day | 19.75 (8.19) | 18.41 (6.44) | 0.52 |
| Previous quit attempts | 7.70 (21.57) | 3.22 (2.66) | 0.37 |
| Baseline FTCD score | 5.70 (2.03) | 5.18 (1.89) | 0.33 |
| Baseline CES-D score | 24.20 (6.90) | 7.59 (3.77) | 0.00** |
FTCD: Fagerström Test for Cigarette Dependence
CES-D: Center for Epidemiological Studies Depression Scale
p < .001
Measures
At baseline, participants self-reported demographics, smoking history, nicotine dependence (using the Fagerström Test for Cigarette Dependence: FTCD) (35) and depressive symptoms (using the Center for Epidemiological Studies Depression Scale: CES-D) (36). Depressive symptoms were dichotomized using a frequently employed cutoff of 16 for clinical depression (Radloff, 1977). While a higher cutoff score has been proposed (37), this cutoff of 16 provided a clear distinction between HD vs. LD groups in this study (CES-D: M = 24.2 vs. 7.59, respectively).
EMA Measures
Participants received four daily EMA prompts (random times within four equal intervals during participants' waking hours that were scheduled at least 30 minutes apart) to complete reports on stressful events, affect, and smoking behavior. Only reports collected in the first 21 days following the target quit day were included in analyses.
Stressful events in the past 2 hours (Yes/No) were assessed by asking participants to report any events that they perceived as significantly stressful. If endorsed, participants reported the type of stressful event (i.e., Interpersonal, Work/School, Financial, Health, Trauma, and Other) and rated event stress level on a scale of 1 (Not at all) to 10 (Extremely). Participants were allowed to select multiple types of stressful events (maximum of 12 types: 6 new and 6 ongoing stressors).
Momentary affect (for the past 15 minutes) was assessed with items derived from the Positive and Negative Affect Scale (PANAS: (38)) and the Wisconsin Smoking Withdrawal Scale (WSWS: (39)) using a 5-point response ranging from 1 (very slightly or not at all) to 5 (extremely) for the PANAS items and 1 (disagree) to 5 (agree) for the WSWS items. Negative affect items included “tense or anxious,” “sad or depressed,” “impatient” “distressed” and “upset” which load on a single factor (40). Positive affect items included “enthusiastic,” and “interested”.
Smoking was assessed as the number of cigarettes smoked during the last two hours and since the last report. Report of any smoking (at least 1 cigarette) since the last report was considered a lapse.
Analytic Plan
First, t-test and multilevel models assessed whether stress exposure during a quit attempt differed across HD and LD smokers. Next, multilevel path analyses tested the hypothesized relations in both short-term (up to 12-hour, Figure 1a) and long-term (up to 24-hour, Figure 1b) models using R and package “glmmADMB” with Laplace approximation. Reports of stressful events, positive and negative affect, and smoking made up level-1 data nested within individuals at level 2. We lagged the data in order to build models where stressful events reported in the index reports (t0) predicted smoking and affect reported in subsequent reports (t1 up to 12 hours later, or t2 up to 24-hours later), controlling for smoking or affect in the preceding reports (t-1). The elapsed time (in minutes) between index and subsequent reports was included as a level-1 (within-subject) covariate. Short-term models examined: c path relations between stressful events reported at t0 and smoking behavior (a dichotomous outcome: smoke free = 0, any smoking = 1) in the ensuing (t1) 15 minutes to 12 hours (M[SD] = 271.6[170] minutes); a path relations between stressful events and positive and negative affect (t0); b path positive and negative affect (t0) relations with t1 smoking, and residual c' path relations between t0 stressful events and t1 smoking. For longer-term models, the paths c and a cross longer timeframes (i.e., affect changes up to 12 hours (t1) and smoking over 12 to 24 hours (t2) following stressful events). The a path models included 4457 (short-term) and 3426 (long-term) reports and the c & b path models included 3426 (short-term) and 3258 (long-term) reports. On average, 3 of 4 prompted reports per day (75%) were completed during the 21 days post-quit period.
Dichotomized depressive symptom level was entered as a level-2 moderator of paths a, b and c. Non-significant level-2 control variables (e.g., gender, age, minority status, income, education) were pruned from the models for parsimony without affecting the direction or significance of findings. None of the level-2 variables had missing data and 71 participants were included in all the models. Regression coefficients for covariates were allowed to vary across individuals, if doing so improved model fit, indicated by significant reduction in deviance (log likelihood) (41). Within-subject centering was used in order to separate within- and between-subject effects of stressful events on affect and smoking. A detailed data analytic plan is provided in supporting information.
Results
Stressful Event Reports
We first examined whether the likelihood of stressful event occurrence differed across HD and LD smokers over 21 days post-quit. Descriptive statistics of stressful event reports are shown in Table 2 and Figure 2 for those high and low in depressive smokers. An independent t-test showed that the proportion of reports with a stressful event during a quit attempt was significantly higher among HD smokers (M[SD] = 15.4% [21.8%]) than LD smokers (M[SD] = 6.4% [5.6%]) (t(67) = 2.77, p = .007). A follow-up multilevel analysis confirmed that HD smokers had twice the odds of endorsing stressful event(s) (OR = 2.32, 95% CI = 1.23, 4.37, p = .009). Moreover, HD smokers were significantly more likely than LD individuals to report stressful events in two consecutive reports (OR = 3.16, 95% CI = 1.31, 7.65, p = .011), even though the odds of experiencing a stressful event were similar for HD and LD smokers following a non-stress report (OR = 1.56, 95% CI = 0.97, 2.49, p = .065). These findings suggest that HD smokers are more likely to experience stressful events overall and more likely to report continuing or repeat stressful events during a quit attempt, compared to LD smokers, although average stress severity ratings did not differ between HD and LD smokers (t(69)= 1.07, p = .288).
Table 2.
Descriptions of Stressful Events by Smokers High vs. Low in Depressive Symptoms.
| Baseline Depressive Symptoms | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Categories of Stressful Events | High (n=20) (CES-D≥16) | Low (n=51) (CES-D<16) | Total (N=71) | p-value* | |||
| Mean | SD | Mean | SD | Mean | SD | ||
| Stressful Ratings (1-10) | 8.24 | 1.46 | 7.84 | 1.40 | 7.95 | 1.42 | 0.30 |
| Mean # of New Stressful Events (per stress report) | 0.89 | 0.56 | 0.86 | 0.51 | 0.86 | 0.52 | 0.84 |
| Mean # of Ongoing Stressful Events (per stress report) | 0.70 | 0.78 | 0.38 | 0.50 | 0.47 | 0.61 | 0.10 |
| Mean # of reports | % across all reports | Mean # of reports | % across all reports | Mean # of reports | % across all reports | ||
|
| |||||||
| Any Stressful Event (Yes) | 9.55 | 15.4% | 3.45 | 6.4% | 5.17 | 8.9% | 0.01** |
| New Stressful Events Only (Yes) | 2.80 | 5.6% | 2.08 | 3.9% | 2.28 | 4.4% | 0.35 |
| Ongoing Stressful Events Only (Yes) | 2.25 | 3.6% | 1.14 | 2.1% | 1.45 | 2.5% | 0.28 |
| Both New & Ongoing Stressful Events (Yes) | 4.55 | 6.3% | 0.24 | 0.3% | 1.45 | 2.0% | 0.21 |
| Mean # of reports | % across SE reports | Mean # of reports | % across SE reports | Mean # of reports | % across SE reports | ||
|
| |||||||
| New Stressful Events | |||||||
| Interpersonal | 1.35 | 24.1% | 0.82 | 25.7% | 0.97 | 25.3% | 0.87 |
| Work/School | 0.50 | 12.9% | 0.37 | 17.3% | 0.42 | 16.0% | 0.56 |
| Financial | 0.50 | 4.4% | 0.18 | 4.9% | 0.27 | 4.7% | 0.91 |
| Health | 1.25 | 10.0% | 0.18 | 9.0% | 0.47 | 9.3% | 0.86 |
| Others | 6.00 | 37.1% | 1.16 | 28.7% | 2.52 | 31.1% | 0.43 |
| Ongoing Stressful Events | |||||||
| Interpersonal | 3.50 | 29.7% | 0.59 | 12.2% | 1.30 | 17.1% | 0.08 |
| Work/School | 0.65 | 10.1% | 0.45 | 10.6% | 0.54 | 10.5% | 0.94 |
| Financial | 4.80 | 14.6% | 0.16 | 5.1% | 1.46 | 7.8% | 0.17 |
| Health | 0.80 | 6.5% | 0.24 | 5.7% | 0.39 | 6.0% | 0.88 |
| Others | 0.70 | 9.0% | 0.16 | 4.4% | 0.31 | 5.7% | 0.49 |
CES-D: Center for Epidemiological Studies Depression Scale
Findings from independent t-tests between high and low depressive symptoms smokers.
p < .01
Figure 2.
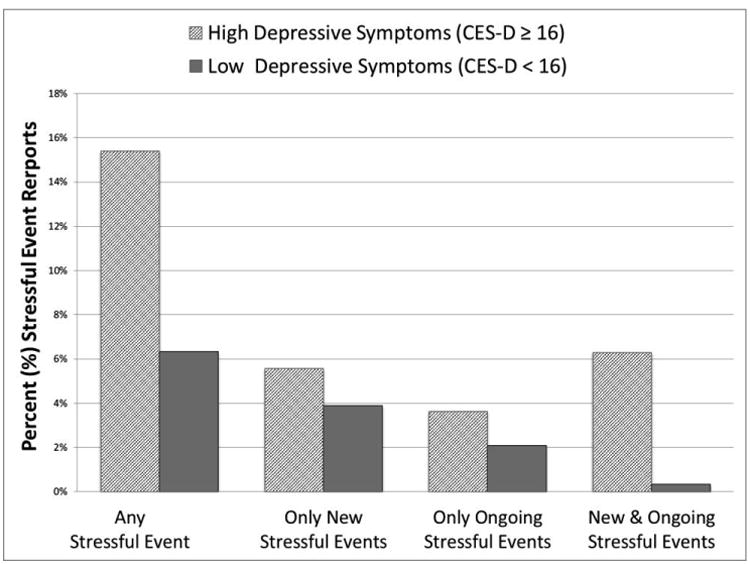
Percentage of reports where stressful events were reported by High vs. Low baseline depressive symptoms.
“Any Stress Report” reflects a report that new, ongoing, or both new and ongoing stressful events were endorsed.
Short-term Models
Stressful Events and Later Lapse (c path: up to 12 hours)
We examined whether the occurrence of a recent (past 2 hours) stressful event at the index report predicted lapse risk up to 12 hours later (Figure 1: c path). The model controlled for negative and positive affect and smoking status at the previous report (t-1), smoking in the past 2 hours (t0), and minutes between t0 and t1 reports (M =271.6, SD =170.0) at the report level (level 1), with nicotine dependence (assessed by FTCD), depressive symptoms (HD vs. LD), and the proportion of reports with a stressful event at the individual level (level 2). As predicted, the occurrence of a stressful event significantly predicted lapse risk within 12 hours. However, non-significant cross-level interactions (Table 3a, middle panel) indicated that stressful events predicted similar short-term increases in lapse risk in both HD and LD smokers.
Table 3.
Multilevel Analysis of the Moderating Effects of Baseline Depressive Symptoms on Relations between 1) Stress and Lapse Risk, 2) Stress and Affect, and 3) Affect and Lapse Relationship (Short Timeframe: 2-12 hours).
| a. The direct effect of stressful event and lapse risk within 12 hours (c path) and the effect of negative and positive affect on lapse risk (b path) | |||
|---|---|---|---|
|
| |||
| Level-1, Report-Level Coefficient | Odds Ratio | 95% CI | p-value |
| Level-2, Individual-Level & Cross-Level Interaction Coefficient | |||
| Model of t1 Lapse Risk 15 minutes to 12 hours after Index Report (c path) | |||
|
| |||
| Random Intercept: Mean Person-level Probability of Smoking at t1 a | 0.064 | (0.037, 0.112) | 0.000** |
| Fagerström Test for Cigarette Dependence | 1.198 | (0.955, 1502) | 0.118 |
| High Depressive Symptoms (vs. Low) | 3.682 | (1.338, 10.13) | 0.012* |
| Proportion of Stressful Event Reports | 1.019 | (0.038, 27.50) | 0.268 |
| t0 (Index Report) Stressful Event (Y/N) | 2.231 | (1.240, 4.014) | 0.007** |
| t0 Stressful Event X High Depressive Symptoms (vs. Low) | 0.836 | (0.345, 2.027) | 0.692 |
| t-1 Negative Affect 15-min. to 12 hrs. pre-index | 0.927 | (0.782, 1.099) | 0.382 |
| t-1 Positive Affect 15-min. to 12 hrs. pre-index | 0.930 | (0.780, 1.108) | 0.417 |
| t0 (Index Report) Recent Smoking (within 2hrs. pre-index) Random Slope (Y/N) | 4.081 | (2.629, 6.336) | 0.000** |
| t-1 Previous Smoking Status (Y/N) | 1.526 | (1.144, 2.036) | 0.004** |
| Minutes between t0 and t1 Reports Random Slope | 1.002 | (1.001, 1.003) | 0.000** |
|
| |||
| b path - entered into c path model aboveb | |||
|
| |||
| t0 (Index Report) Negative Affect - main effect | 1.264 | (1.050, 1.522) | 0.013* |
| t0 Negative Affect X High Depressive Symptoms (vs. Low) | 1.175 | (0.824, 1.674) | 0.373 |
| t0 (Index Report) Positive Affect - main effect | 1.139 | (0.937, 1.370) | 0.168 |
| t0 Positive Affect X High Depressive Symptoms (vs. Low) | 0.932 | (0.644, 1.348) | 0.707 |
| b. The effect of stressful event on negative affect within 2 hours (a path) | |||
|---|---|---|---|
|
| |||
| Level-1, Report-Level Coefficient | Estimate | Standard Error | p-value |
| Level-2, Individual-Level & Cross-Level Interaction Coefficient | |||
| Model of t0 (Index Report) Negative Affect (a path) | |||
|
| |||
| Random Intercept: Mean Negative Affect at t0 (Index Report)a | -0.056 | 0.057 | 0.323 |
| High Depressive Symptoms (vs. Low) | 0.285 | 0.113 | 0.012* |
| Proportion of Stressful Event Reports | 0.012 | 0.004 | 0.003** |
| t0 (Index Report) Stressful Event (Y/N) | 0.809 | 0.049 | 0.000** |
| t0 Stressful Event X High Depressive Symptoms (vs. Low) | -0.117 | 0.079 | 0.137 |
| t1 Negative Affect 15-min. to 12 hrs. pre-index | 0.332 | 0.014 | 0.000** |
| t0 (Index Report) Recent Smoking (within 2hrs. pre-index) Random Slope (Y/N) | 0.074 | 0.043 | 0.085 |
| t1 Previous Smoking Status Random Slope (Y/N) | -0.003 | 0.040 | 0.934 |
| c. The effect of stressful event on positive affect within 2 hours (a path) | |||
|---|---|---|---|
|
| |||
| Level-1, Report-Level Coefficient | Estimate | Standard Error | p-value |
| Level-2, Individual-Level & Cross-Level Interaction Coefficient | |||
| Model of t0 (Index Report) Positive Affect (a path) | |||
|
| |||
| Random Intercept: Mean Positive Affect at t0 (Index Report)a | 0.095 | 0.081 | 0.254 |
| High Depressive Symptoms (vs. Low) | -0.266 | 0.160 | 0.096 |
| Proportion of Stressful Event Reports | -0.004 | 0.006 | 0.428 |
| t0 (Index Report) Stressful Event (Y/N) | -0.406 | 0.051 | 0.000** |
| t0 Stressful Event X High Depressive Symptoms (vs. Low) | 0.020 | 0.081 | 0.805 |
| t-1 Positive Affect 15-min. to 12 hrs. pre-index | 0.204 | 0.015 | 0.000** |
| t0 (Index Report) Recent Smoking (within 2hrs. pre-index) (Y/N) | -0.017 | 0.032 | 0.601 |
| t-1 Previous Smoking Status (Y/N) | -0.032 | 0.230 | 0.249 |
Note:
The variables that are left aligned are Level-1 (report: within-subject) variables and those that are right aligned are Level-2 (individual: between-subject) variables and cross-level interaction terms. Proportion of Stressful Event.
Reports: this variable was multiplied by 100 before being entered into the model in order to interpret the changes in estimate or odds ratio per 1% increment in the proportion of the stressful event reports. Radom Slope: Specified as a random coefficient. All other predictors were treated as fixed to facilitate model convergence.
t-1 = Report 15 minutes and 12 hours preceding index report
t0 = Index report
t1 = Report 15 minutes to 12 hours after index report
Mean affect when all variables in the model are at zero (for the continuous variables, zero reflects the overall mean).
In order to test the b paths, negative affect and positive affect at index were first entered into the model (presented as main effects) and then cross-level interactions between depressive symptoms (level 2) and affect (level 1) were entered into the model. For simplicity, the other variables in the model are not presented.
p<.05,
p <.001
Overall, HD smokers had a significantly higher likelihood of smoking than did LD smokers when all other variables were set to zero (sample mean for continuous variables). Nicotine dependence and the proportion of stressful event reports did not predict smoking risk. Thus, baseline depressive symptoms predict lapse risk above and beyond nicotine dependence and the frequency of stressful events during a quit attempt.
Stressful Events and Affect (a paths: up to 2 hours)
Positive and negative affect (t0; past 15 minutes) were regressed (separately) on the occurrence of stressful events (t0; past 2 hours) at the same report (Figure 1: a paths). In order to assess changes in affect following stressful events, previous (t-1) levels of positive or negative affect were included as a control variable in the corresponding model. Other covariates included smoking status at the previous report (t-1) and recent smoking (t0: past 2 hours) at level 1 and depressive symptoms and the proportion of reports with a stressful event at level 2. Stressful events predicted subsequent increases in negative affect and decreases in positive affect. Non-significant cross-level interactions indicated that relations between stressful events and both negative and positive affect (within 2 hours) did not differ across HD vs. LD smokers, contrary to our hypotheses.
Significant level-2 variables for the intercept indicated that high baseline depressive symptoms (HD) and more frequent stressful events predicted greater average negative affect during a quit attempt, but did not predict average positive affect (Table 3b & 3c, middle panel).
Affect and Lapse (b paths: up to 12 hours)
Positive and negative affect at the index report (t0) were entered simultaneously, controlling for the same covariates as described above in the c path models. Results revealed a significant effect of changes in negative affect, but not positive affect, on later lapse risk, such that increases in negative affect following a stressful event predicted greater smoking risk. Non-significant cross-level interactions showed that affect-lapse relations (negative or positive affect) did not differ across HD and LD smokers (Table 3a, bottom panel). Inclusion of positive and negative affect did not change the significant relation between stressful events at t0 and lapse risk at t1 (c' path) although the magnitude of SE-lapse relation was slightly reduced (c' path OR = 2.11, 95%CI = 1.14, 3.93, p = .018). Overall lapse risk was still significantly greater among HD (vs. LD) smokers when negative and positive (t0) affect were entered in the model.
Longer-term Models
Stressful Events and Persistent Lapse Risk (c path: up to 24 hours)
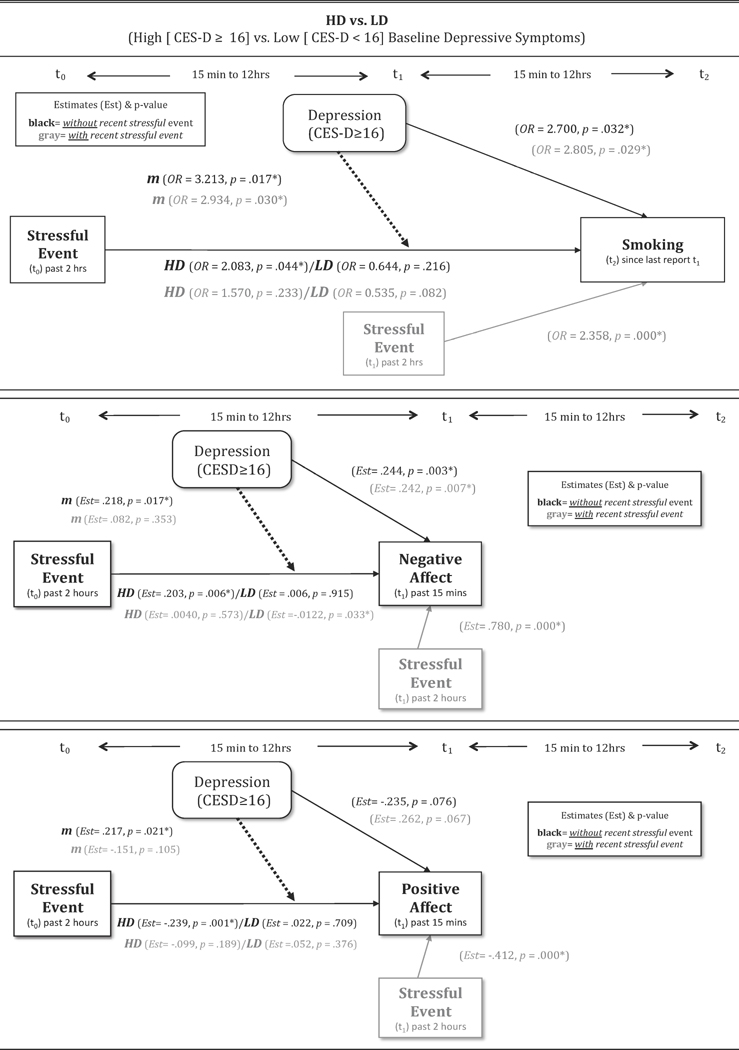
First, we tested whether relations between stressful events (t0) and lapse risk within 24 hours later (t2) varied across HD and LD smokers. Time-varying covariates included negative and positive affect reported at index and t-1, smoking status reported at t-1, t0, and t1, and minutes between the index and t2 reports (M =760.5, SD =334.2). While there was no significant main effect of stressful events on lapse risk within 24 hours (OR =1.11, 95%CI = .68, 1.83, p = .671), baseline depressive symptoms (HD vs. LD) significantly moderated the stress-lapse relation such that stressful events (t0) significantly predicted increased lapse risk (t2) only among HD smokers (Table 4a, top panel). That is, HD smokers, but not LD smokers, continued to be at risk of smoking lapse up to 24 hours after a stressful event, even after controlling for the initial (acute) affective response (t0) following a stressful event (Figure 3).
Table 4.
Multilevel Analysis of the Moderating Effects of Baseline Depressive Symptoms on Relations between 1) Stress (t0) and Lapse Risk (t2) and 2) Stress (t0) and Affect (t1). (Long Timeframe: 12-24 hours).
| a. The direct effects of stressful event and lapse risk within 24 hours (c path) with and without recent stressful event (t1) | |||
|---|---|---|---|
|
| |||
| Level-1, Report-Level Coefficient | Odds Ratio | 95% CI | p-value |
| Level-2, Individual-Level & Cross-Level Interaction Coefficient | |||
| Model of t2 Lapse Risk 30 minutes to 24 hours after Index Report (c path) | |||
|
| |||
| Random Intercept: Mean Person-level Probability of Smoking at t2a | 0.169 | (0.079, 0.362) | 0.000** |
| Fagerström Test for Cigarette Dependence | 1.175 | (0.961, 1.436) | 0.116 |
| High Depressive Symptoms (vs. Low) | 2.700 | (1.087, 6.707) | 0.032* |
| Proportion of Stressful Event Reports | 1.03 | (0.059, 17.84) | 0.086 |
| t0 (Index Report) Stressful Event (Y/N): HD/LDb | 2.083/ 0.644 | (1.021,4.248)/ (0.326, 1.288) | 0.044* /0.216 |
| t0 Stressful Event X High Depressive Symptoms (vs. Low) | 3.213 | (1.234, 8.369) | 0.017* |
| t-1 Negative Affect 15-min. to 12 hrs. pre-index | 1.251 | (1.049, 1.493) | 0.013* |
| t-1 Positive Affect 15-min. to 12 hrs. pre-index Random Slope | 0.934 | (0.745, 1.171) | 0.556 |
| t0 (Index Report) Negative Affect | 0.800 | (0.664, 0.965) | 0.020* |
| t0 (Index Report) Positive Affect | 1.032 | (0.860, 1.238) | 0.737 |
| t1 Recent Smoking Status (between index and t1) Random Slope (Y/N) | 2.938 | (1.944, 4.441) | 0.000** |
| t0 (Index Report) Smoking within 2hrs. pre-index (Y/N) | 2.169 | (1.593, 2.954) | 0.000** |
| t-1 Previous Smoking Status (Y/N) | 1.713 | (1.401, 2.094) | 0.000** |
| Minutes between t0 and t2 Reports Random Slope | 1.000 | (0.999, 1.001) | 0.333 |
|
| |||
| b path - entered into c path model abovec | |||
|
| |||
| t1 Negative Affect 15-min. to 12 hrs. post-index- main effect | 1.400 | (1.184, 1.655) | 0.000** |
| t1 Negative Affect X High Depressive Symptoms (vs. Low) | 1.231 | (0.896, 1.691) | 0.201 |
| t1 Positive Affect 15-min. to 12 hrs. post-index - main effect | 1.125 | (0.948, 1.337) | 0.177 |
| t1 Positive Affect X High Depressive Symptoms (vs. Low) | 1.088 | (0.774, 1.529) | 0.627 |
|
| |||
| Model of t1 Lapse Risk 30 minutes to 24 hours after Index Report (c path) with stressful event at t1 | |||
|
| |||
| Random Intercept: Mean Person-level Probability of Smoking at t2a | 0.058 | (0.035, 0.096) | 0.000** |
| Fagerström Test for Cigarette Dependence | 1.176 | (0.958, 1.443) | 0.122 |
| High Depressive Symptoms (vs. Low) | 2.805 | (1.110, 7.089) | 0.029* |
| Proportion of Stressful Event Reports | 1.016 | (0.502, 2.059) | 0.278 |
| t0 (Index Report) Stressful Event: HD/LDb | 1.570/ 0.535 | (0.748, 3.296)/ (0.264, 1.084) | 0.233/ 0.082 |
| t0 Stressful Event X High Depressive Symptoms (vs. Low) | 2.934 | (1.108, 7.771) | 0.030* |
| t1 Recent stressful event (within 2 hrs. preceding t1 report: Y/N) | 2.358 | (1.483, 3.749) | 0.000** |
| t-1 Negative Affect 15-min. to 12 hrs. pre-index | 1.245 | (1.042, 1.487) | 0.016* |
| t-1 Positive Affect 15-min. to 12 hrs. pre-index Random Slope | 0.930 | (0.743, 1,165) | 0.529 |
| t0 (Index Report) Negative Affect | 0.791 | (0.654, 0.955) | 0.015* |
| t0 (Index Report) Positive Affect | 1.048 | (0.873, 1.259) | 0.615 |
| t1 Recent Smoking Status (between index and t1) Random Slope (Y/N) | 2.703 | (1.775, 4.116) | 0.000** |
| t0 (Index Report) Smoking within 2hrs. pre-index (Y/N) | 2.201 | (1.613, 3.005) | 0.000** |
| t-1 Previous Smoking Status (Y/N) | 1.731 | (1.292, 2.319) | 0.000** |
| Minutes between t0 and t2 Reports Random Slope | 1.000 | (0.999,1.001) | 0.325 |
| b. The effect of stressful event on negative affect within 2 hours (a path) with and without recent stressful event (t1) | |||
|---|---|---|---|
|
| |||
| Level-1, Report-Level Coefficient | Estimate | Standard Error | p-value |
| Level-2, Individual-Level & Cross-Level Interaction Coefficient | |||
| Model of t1 Negative Affect 15 minutes to 12 hours after Index Report (a path) | |||
|
| |||
| Random Intercept: Mean Negative Affect at t1a | 0.139 | 0.076 | 0.068 |
| High Depressive Symptoms (vs. Low) | 0.223 | 0.088 | 0.011* |
| Proportion of Stressful Event Reports | 0.010 | 0.034 | 0.005 |
| t0 (Index Report) Stressful Event (Y/N): HD/LDb | 0.206/ 0.006 | 0.073/ 0.006 | 0.005*/0.915 |
| t0 Stressful Event X High Depressive Symptoms (vs. Low) | 0.199 | 0.092 | 0.030* |
| t0 (Index Report): Negative Affect | 0.308 | 0.018 | 0.000** |
| t-1 Negative Affect 15-min. to 12 hrs. pre-index Random Slope | 0.181 | 0.021 | 0.000** |
| t1 Recent Smoking Status (between index and t1) Random Slope (Y/N) | 0.127 | 0.038 | 0.000** |
| t0 (Index Report) Smoking within 2hrs. pre-index Random Slope (Y/N) | -0.062 | 0.046 | 0.181 |
| t-1 Previous Smoking Status (Y/N) | -0.062 | 0.033 | 0.059 |
| Minutes between t0 and t1 Reports | -0.0001 | 0.000 | 0.011* |
|
| |||
| Model of t1 Negative Affect 15 minutes to 12 hours after Index Report (a path) with stressful event at t1 | |||
|
| |||
| Random Intercept: Mean Positive Affect at t1a | 0.103 | 0.078 | 0.086 |
| High Depressive Symptoms (vs. Low) | 0.242 | 0.090 | 0.007** |
| Proportion of Stressful Event Reports | 0.004 | 0.004 | 0.326 |
| t0 (Index Report) Stressful Event (Y/N): HD/LDb | 0.040/ 0.112 | 0.071/ 0.057 | 0.573/0.033* |
| t0 Stressful Event X High Depressive Symptoms (vs. Low) | 0.082 | 0.088 | 0.353 |
| t1 Recent stressful event (within 2 hrs. preceding t1 report: Y/N) | 0.780 | 0.044 | 0.000** |
| t0 (Index Report): Negative Affect | 0.296 | 0.017 | 0.000** |
| t-1 Negative Affect 15-min. to 12 hrs. pre-index Random Slope | 0.173 | 0.020 | 0.000** |
| t1 Recent Smoking Status (between index and t1) Random Slope (Y/N) | 0.047 | 0.036 | 0.197 |
| t0 (Index Report) Smoking within 2hrs. pre-index Random Slope (Y/N) | -0.048 | 0.042 | 0.262 |
| t-1 Previous Smoking Status (Y/N) | -0.048 | 0.032 | 0.131 |
| Minutes between t0 and t1 Reports | -0.0001 | 0.000 | 0.037* |
| c. Tde effect of stressful events on positive affect witdin 2 hours (a patd), witd and witdout a recent stressful event (t1) covariate | |||
|---|---|---|---|
|
| |||
| Level-1, Report-Level Coefficient | Estimate | Standard Error | P-value |
| Level-2, Individual-Level & Cross-Level Interaction Coefficient | |||
| Model of t1 Positive Affect 15 minutes to 12 hours after Index Report (a path) | |||
|
| |||
| Random Intercept: Mean Positive Affect at t1a | 0.154 | 0.121 | 0.203 |
| High Depressive Symptoms (vs. Low) | 0.235 | 0.143 | 0.076 |
| Proportion of Stressful Event Reports | -0.002 | 0.005 | 0.688 |
| t0 (Index Report) Stressful Event (Y/N): HD/LDb | -0.239/-0.022 | 0.075/0.059 | 0.001* /0.709 |
| t0 Stressful Event X High Depressive Symptoms (vs. Low) | -0.217 | 0.094 | 0.021* |
| t0 (Index Report): Positive Affect | 0.216 | 0.018 | 0.000** |
| t-1 Positive Affect 15-min. to 12 hrs. pre-index Random Slope | 0.127 | 0.020 | 0.000** |
| t1 Recent Smoking Status (between index and t1) (Y/N) | -0.087 | 0.036 | 0.014* |
| t0 (Index Report) Smoking within 2hrs. pre-index (Y/N) | -0.028 | -0.038 | 0.448 |
| t-1 Previous Smoking Status (Y/N) | -0.003 | 0.042 | 0.942 |
| Minutes between t0 and t1 Reports | -0.0000 | 0.000 | 0.385 |
|
| |||
| Model of t1 Positive Affect 15 minutes to 12 hours after Index Report (a path) with stressful event at t1 | |||
|
| |||
| Random Intercept: Mean Positive Affect at t1 a | -0.137 | 0.121 | 0.261 |
| High Depressive Symptoms (vs. Low) | 0.262 | 0.143 | 0.067 |
| Proportion of Stressful Event Reports | 0.002 | 0.005 | 0.689 |
| t0 (Index Report) Stressful Event (Y/N): HD/LDb | -0.099/ 0.052 | 0.075/ 0.059 | 0.189/0.376 |
| t0 Stressful Event X High Depressive Symptoms (vs. Low) | -0.151 | 0.093 | 0.105 |
| t1 Recent stressful event (within 2 hrs. preceding t1report: Y/N) | -0.412 | 0.047 | 0.000** |
| t0 (Index Report): Positive Affect | 0.210 | 0.018 | 0.000** |
| t-1 Positive Affect 15-min. to 12 hrs. pre-index Random Slope | 0.125 | 0.020 | 0.000** |
| t1 Recent Smoking Status (between index and t1) (Y/N) | -0.043 | 0.036 | 0.228 |
| t0 (Index Report) Smoking within 2hrs. pre-index (Y/N) | -0.033 | 0.037 | 0.371 |
| t-1 Previous Smoking Status (Y/N) | -0.009 | 0.034 | 0.798 |
| Minutes between t0 and t1 Reports | -0.000 | 0.000 | 0.236 |
Note:
The variables that are left aligned are Level-1 (report: within-subject) variables and those that are right aligned are Level-2 (individual: between-subject) variables and cross-level interaction terms.
Proportion of stressful events: this variable was multiplied by 100 before entered into the model in order to interpret the changes in estimate or odds ratio per 1% increment in the proportion of the stressful event reports.
Radom Slope: Specified as a random coefficient. All other predictors were treated as fixed to facilitate model convergence.
t-1 = Report 15 minutes and 12 hours preceding index report t0 = Index report
t1 = Report 15 minutes to 12 hours after index report
t2 = Report 15 minutes to 12 hours after t1 report (& 30 minutes to 24 hours after index report)
Mean affect when all variables in the model are at zero (for the continuous variables, zero reflects the overall mean).
Models were run twice with different reference groups to estimate simple main effects of stress within LD or HD smokers. The coefficients for stressful events at the index report can be interpreted as the effects of stressful event on smoking within 24 hours among either HD or LD.
In order to test the b paths, negative affect and positive affect at index were first entered into the model (presented as main effects) and then cross-level interactions between depressive symptoms (level 2) and affect (level 1) were entered into the model. For simplicity, the other variables in the model are not presented.
p<.05,
p <.001
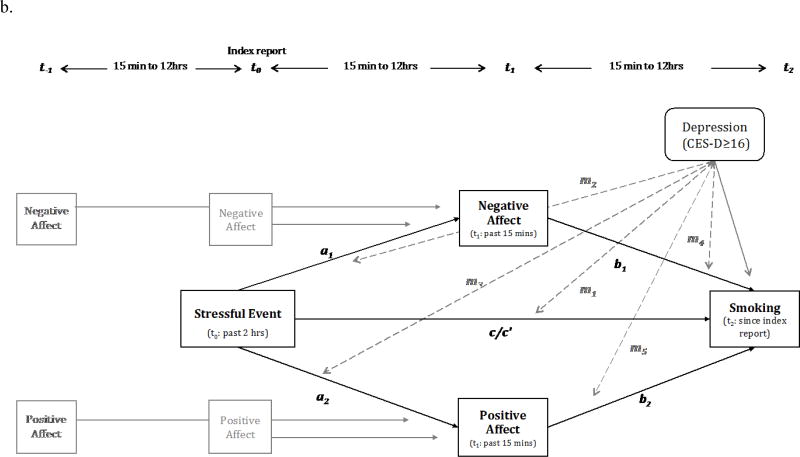
Figure 3. Simplified long-term models (over 12- 24 hours).
Covariates in the model are not depicted in the figures (see Table 4).
Two separate models with and without recent stressful events were tested in each model. The coefficients in the model with recent stressful events are depicted in gray.
Note: m (dotted lines): moderating effects of baseline depressive symptoms (HD: CES-D ≥ 16 vs. LD: CES-D < 16)
In an exploratory analysis, we assessed whether the difference between HD and LD smokers was explained by a greater frequency of subsequent stressful events among HD smokers. The moderating effect of baseline depressive symptoms on SE-lapse relations (up to 24 hours) remained significant when controlling for stressful event recurrence, although the simple main effect of stress on lapse over 24 hours was no longer statistically significant in HD smokers (p = .233) (Table 4a, bottom panel). This suggests that the SE-lapse relations observed in HD smokers was not fully explained by the greater odds of subsequent stressful events reported by HD smokers.
Stressful Events and Subsequent Affect (a paths: up to 12 hours)
Next, we examined whether baseline depressive symptoms (HD vs. LD) moderated the effect of stressful events (t1) on subsequent affect within 12 hours (t1) (Figure 3). Initial levels (t0) of positive and negative affect reported shortly after the occurrence of stressful events were included as a covariate in each model. Other time-varying covariates included: recent smoking (any smoking between index report and t1), smoking at index (t0: past 2 hours), previous smoking status (t-1), levels of corresponding affect (negative or positive affect) assessed at t-1, and minutes between the index report (t0) and report at t1.
Results showed that the occurrence of a stressful event (main effect) was significantly associated with decreases in positive affect within 12 hours (b = -.01, SE = .05 p = .025), but not with negative affect (b = .08, SE = .05, p =. 074), after controlling for earlier affect observed within 2 hours of a stressful event. However, baseline depressive symptoms (HD vs. LD) moderated these relations such that significant increases in negative affect and reductions in positive affect persisting over 12 hours after a stressful event were observed only among HD smokers (Table 4b: the top panel).
Next, we explored the role of subsequent stressful events in these stress–affect relations. Results showed that inclusion of the most recent stressful events (t1) reduced the moderating effects of baseline depressive symptoms on stress (t0) relations with both negative affect and positive affect to non-significance, suggesting that continued post-stress changes in affect observed in HD smokers can be explained, at least partially, by the greater odds of subsequent stressful events.
Affect and Lapse (b paths: up to 12 hours)
As in the short-term model, positive and negative affect at the index report (t1) were simultaneously entered along with the same covariates in the c models. The same pattern of results was found as in the short-term model. While post-stress increases at t1 in negative affect, but not positive affect, predicted greater lapse risk, non-significant cross-level interactions indicated that affect-lapse relations did not differ across HD and LD smokers (Table 4a, middle panel). The moderating effects of depressive symptoms on stress-lapse relation, c paths, remained significant when positive and negative affect at t1 were included in the model although the simple main effect of stress on lapse over 24 hours was no longer statistically significant in HD smokers (p = .07). That is, sustained increases in lapse risk observed only in HD smokers were not fully explained by persistent changes in affect following stressful events. This did not change when recent stressful events were included in the model.
Discussion
Analysis of real-time data collected during a quit attempt revealed that smokers high (HD) vs. low (LD) in depressive symptoms experienced more stressful events, longer-lasting affective reactivity to stressful events (due partly to increased risk of repeat or continued stressors), and greater lapse risk 12-24 hours following a stressful event. Although HD and LD smokers did not differ in acute (2 hour) affective reactivity, within-day (12 hour) lapse reactivity to stressful events, or lapse risk in the face of aversive affective states, stress may beget stress in HD smokers in ways that it does not in LD smokers, and this may contribute to the increased vulnerability to persistent affective and lapse vulnerability observed in this study.
Our findings suggest a few pathways through which pre-cessation depressive symptoms lead to poor cessation outcomes although the findings are preliminary given the sample size and number of parameters being estimated. Exposure to stress during a quit attempt appears to play an important role in smoking cessation, especially for depressed smokers, as it partly contributed to persistent post-stress aversive affective states and lapse risk found only in HD smokers. That is, stressful events during quit attempts may pose greater risk for depressed than for non-depressed smokers. Furthermore, consistent with past research (22, 23), increases in negative affect, but not changes in positive affect, predicted subsequent smoking. As such, continued increases in negative affect in the context of smoking cessation may make depressed smokers highly vulnerable to cessation failure. These results suggest the importance of managing stress and negative affect especially for depressed smokers, although neither greater sustained affective reactivity to stress nor more frequent exposure to stress fully explained the increased risk of smoking observed in HD smokers up to 24 hours after stressful events.
Several limitations to the current study should be discussed. First, the small sample and uneven number of HD and LD smokers may bias estimates in these complex models. Moreover, HD and LD smokers were categorized using dichotomized CES-D scores with a cutoff score of 16, rather than on the basis of a clinical diagnosis. Similar results were obtained when continuous CES-D scores were used as the moderator (see supporting information), however. Second, what constitutes a stressful event was subjectively defined; participants were asked to report any event that they considered stressful. Therefore, it is possible that the frequent occurrence of stressful events reported by HD smokers reflects lower thresholds for stressful event reporting rather than more stressful events of equal magnitude. However, the average intensity rating of stressful events did not significantly differ between HD and LD smokers, suggesting that both HD and LD smokers reported events of similar intensity. Third, as the affect-smoking relationship was examined only within a 12-hour timeframe, the lack of moderating effects of depressive symptoms may not extend to different timeframes. The optimal timeframes to examine the relations among stressful events, affect, and smoking are unknown. Finally, as our study design did not include randomization, no direct causal inferences can be made.
This study of real-time affective and smoking reactivity to stressful events adds to the literature on relations between depressive symptoms and smoking. Results suggested that HD smokers are more likely to experience persistent, aversive, affective states associated with frequent stressful events and extended lapse vulnerability following a stressful event. This study also underscores the importance of considering timeframe and chronicity when investigating affective reactivity to stressful events and supports previous research that suggests complex interactional pathways among stressful events, affect, and lapse risk (34, 42-44). In order to elucidate the link between depression and smoking, it is crucial to study relations among depressive symptoms and smoking risk factors in real-world contexts.
Supplementary Material
Acknowledgments
Funding: The project described was supported by Award Number RC1DA028129 from the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
Footnotes
Conflict of interest declaration: None
References
- 1.Ward BW, Clarke TC, Nugent CN, Schiller JS. Early release of selected estimates based on data from the 2015 National Health Interview Survey. Statistics NCfH, editor. 2016 [Google Scholar]
- 2.CDC. Vital signs: current cigarette smoking among adults aged ≥18 years with mental illness - United States, 2009-2011. MMWR Morbidity And Mortality Weekly Report. 2013;62(5):81–7. [PMC free article] [PubMed] [Google Scholar]
- 3.SAMHSA. Adults with mental illness or substance use disorder account for 40 percent of all cigarettes smoked. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. [Google Scholar]
- 4.Morisano D, Bacher I, Audrain-McGovern J, George TP. Mechanisms Underlying the Comorbidity of Tobacco Use in Mental Health and Addictive Disorders. The Canadian Journal of Psychiatry. 2009;54(6):356–67. doi: 10.1177/070674370905400603. [DOI] [PubMed] [Google Scholar]
- 5.Lasser K, Boyd J, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. JAMA. 2000;284(20):2606–10. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- 6.Cooper J, Borland R, McKee SA, Yong HH, Dugué PA. Depression motivates quit attempts but predicts relapse: differential findings for gender from the International Tobacco Control Study. Addiction. 2016;111(8):1438–47. doi: 10.1111/add.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook J, Spring B, McChargue D, Doran N. Effects of anhedonia on days to relapse among smokers with a history of depression: A brief report. Nicotine & Tobacco Research. 2010;12:978–82. doi: 10.1093/ntr/ntq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinnunen T, Korhonen T, Garvey AJ. Role of Nicotine GUM and Pretreatment Depressive Symptoms in Smoking Cessation: Twelve-Month Results of a Randomized Placebo Controlled Trial. The International Journal of Psychiatry in Medicine. 2008;38(3):373–89. doi: 10.2190/PM.38.3.k. [DOI] [PubMed] [Google Scholar]
- 9.Leventhal AM, Ramsey SE, Brown RA, LaChance HR, Kahler CW. Dimensions of Depressive Symptoms and Smoking Cessation. Nicotine & Tobacco Research. 2008;10(3):507–17. doi: 10.1080/14622200801901971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown RA, Lejuez CW, Strong DR, Kahler CW, Zvolensky MJ, Carpenter LL, et al. A prospective examination of distress tolerance and early smoking lapse in adult self- quitters. Nicotine & Tobacco Research. 2009;11(5):493–502. doi: 10.1093/ntr/ntp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blondal T, Gudmundsson LJ, Tomasson K, Jonsdottir D, Hilmarsdottir H, Kristjansson F, et al. The effects of fluoxetine combined with nicotine inhalers in smoking cessation-a randomized trial. Addiction. 1999;94(7):1007–15. doi: 10.1046/j.1360-0443.1999.94710076.x. [DOI] [PubMed] [Google Scholar]
- 12.Thorndike AN, Regan S, McKool K, et al. Depressive symptoms and smoking cessation after hospitalization for cardiovascular disease. Archives of Internal Medicine. 2008;168(2):186–91. doi: 10.1001/archinternmed.2007.60. [DOI] [PubMed] [Google Scholar]
- 13.Hammen C. Generation of stress in the course of unipolar depression. J Abnorm Psychol. 1991;100(4):555–61. doi: 10.1037//0021-843x.100.4.555. [DOI] [PubMed] [Google Scholar]
- 14.Cui XJ, Vaillant GE. Does depression generate negative life events? J Nerv Ment Dis. 1997;185(3):145–50. doi: 10.1097/00005053-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Assari S, Lankarani MM. Association Between Stressful Life Events and Depression; Intersection of Race and Gender. Journal Of Racial And Ethnic Health Disparities. 2016;3(2):349–56. doi: 10.1007/s40615-015-0160-5. [DOI] [PubMed] [Google Scholar]
- 16.Shiffman S, Hickcox M, Paty JA, Gnys M, Kassel JD, Richards TJ. Progression from a smoking lapse to relapse: prediction from abstinence violation effects, nicotine dependence, and lapse characteristics. J Consult Clin Psychol. 1996;64(5):993–1002. doi: 10.1037//0022-006x.64.5.993. [DOI] [PubMed] [Google Scholar]
- 17.Cook JW, Spring B, McChargue D, Hedeker D. Hedonic capacity, cigarette craving, and diminished positive mood. Nicotine & tobacco research. 2004;6(1):39–47. doi: 10.1080/14622200310001656849. [DOI] [PubMed] [Google Scholar]
- 18.Leventhal AM, Ameringer KJ, Osborn E, Zvolensky MJ, Langdon KJ. Anxiety and depressive symptoms and affective patterns of tobacco withdrawal. Drug and Alcohol Dependence. 2013;133:324–9. doi: 10.1016/j.drugalcdep.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Audrain-McGovern J, Wileyto EP, Ashare R, Cuevas J, Strasser AA. Reward and affective regulation in depression-prone smokers. Biological Psychiatry. 2014;76(9):689–97. doi: 10.1016/j.biopsych.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langdon KJ, Leventhal AM, Stewart S, Rosenfield D, Steeves D, Zvolensky MJ. Anhedonia and anxiety sensitivity: Prospective relationships to nicotine withdrawal symptoms during smoking cessation. Journal of Studies on Alcohol and Drugs. 2013;74(3):469–78. doi: 10.15288/jsad.2013.74.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.al'Absi M, Hatsukami D, Davis GL, Wittmers LE. Prospective examination of effects of smoking abstinence on cortisol and withdrawal symptoms as predictors of early smoking relapse. Drug and Alcohol Dependence. 2004;73:267–78. doi: 10.1016/j.drugalcdep.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Kenford SL, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Predicting relapse back to smoking: Contrasting affective and physical models of dependence. Journal of Consulting and Clinical Psychology. 2002;70(1):216–27. [PubMed] [Google Scholar]
- 23.Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: Within- subjects analysis of real-time reports. Journal of Consulting and Clinical Psychology. 1996;64(2):366–79. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- 24.Niaura R, Britt DM, Shadel WG, Goldstein M, Abrams D, Brown R. Symptoms of depression and survival experience among three samples of smokers trying to quit. Psychology of Addictive Behaviors. 2001;15(1):13–7. doi: 10.1037/0893-164x.15.1.13. [DOI] [PubMed] [Google Scholar]
- 25.Myin-Germeys I, Peeters F, Havermans R, Nicolson NA, deVries MW, Delespaul P, et al. Emotional reactivity to daily life stress in psychosis and affective disorder: an experience sampling study. Acta Psychiatrica Scandinavica. 2003;107(2):124–31. doi: 10.1034/j.1600-0447.2003.02025.x. [DOI] [PubMed] [Google Scholar]
- 26.Wichers MC, Nicolson NA, Peeters F, De Vries M, Mengelers R, Van Os J, et al. Reduced stress-sensitivity or increased reward experience: The psychological mechanism of response to antidepressant medication. Neuropsychopharmacology. 2009;34(4):923–31. doi: 10.1038/npp.2008.66. [DOI] [PubMed] [Google Scholar]
- 27.Steinberg ML, Williams JM, Gandhi KK, Foulds J, Epstein EE, Brandon TH. Task persistence predicts smoking cessation in smokers with and without schizophrenia. Psychology of Addictive Behaviors. 2012;26(4):850–8. doi: 10.1037/a0028375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minami H, Bloom EL, Reed KMP, Hayes SC, Brown RA. The moderating role of experiential avoidance in the relationships between internal distress and smoking behavior during a quit attempt. 2015;29(2):400–7. doi: 10.1037/adb0000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buckner JD, Keough ME, Schmidt NB. Problematic alcohol and cannabis use among young adults: The roles of depression and discomfort and distress tolerance. Addictive Behaviors. 2007;32(9):1957–63. doi: 10.1016/j.addbeh.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gorka SM, Ali B, Daughters SB. The role of distress tolerance in the relationship between depressive symptoms and problematic alcohol use. Psychology of Addictive Behaviors. 2012;26(3):621–6. doi: 10.1037/a0026386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bradford DE, Curtin JJ, Piper ME. Anticipation of smoking sufficiently dampens stress reactivity in nicotine-deprived smokers. Journal of Abnormal Psychology. 2015;124(1):128–36. doi: 10.1037/abn0000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lam CY, Robinson JD, Versace F, Minnix JA, Cui Y, Carter BL, et al. Affective reactivity during smoking cessation of never-quitters as compared with that of abstainers, relapsers, and continuing smokers. Experimental and Clinical Psychopharmacology. 2012;20(2):139–50. doi: 10.1037/a0026109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dahne J, Murphy JG, MacPherson L. Depressive Symptoms and Cigarette Demand as a Function of Induced Stress. Nicotine & Tobacco Research. 2016 doi: 10.1093/ntr/ntw145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCarthy DE, Bold KW, Minami H, Yeh VM, Rutten E, Nadkarni SG, et al. Reliability and validity of measures of impulsive choice and impulsive action in smokers trying to quit. Experimental and clinical psychopharmacology. 2016;24(2):120–30. doi: 10.1037/pha0000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerström test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. British journal of addiction. 1991;86(9):1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 36.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- 37.Haringsma R, Engels GI, Beekman AT, Spinhoven P. The criterion validity of the Center for Epidemiological Studies Depression Scale (CES-D) in a sample of self-referred elders with depressive symptomatology. Int J Geriatr Psychiatry. 2004;19(6):558–63. doi: 10.1002/gps.1130. [DOI] [PubMed] [Google Scholar]
- 38.Watson D, Clark LA, Teilegen A. Development and Validation of Brief Measures of Positive and Negative Affect: The PANAS Scales. Journal of Personality & Social Psychology. 1988;54(6):1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 39.Welsch SK, Smith SS, Jorenby DE, Fiore MC, Baker TB, Wetter DW. Development and validation of the Wisconsin smoking withdrawal scale. Experimental and Clinical Psychopharmacology. 1999;7(4):354–61. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- 40.Minami H, Yeh VM, Bold KW, Chapman GB, McCarthy DE. Relations among affect, abstinence motivation and confidence, and daily smoking lapse risk. Psychology of Addictive Behaviors. 2014;28(2):376–88. doi: 10.1037/a0034445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raudenbush SW, Bryk AS. Hierarchical linear models : applications and data analysis methods. 2nd. Thousand Oaks: Sage Publications; 2002. [Google Scholar]
- 42.Minami H, McCarthy DE, Jorenby DE, Baker TB. An Ecological Momentary Assessment analysis of relations among coping, affect and smoking during a quit attempt. Addiction. 2011;106(3):641–50. doi: 10.1111/j.1360-0443.2010.03243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piper M, Schlam T, Cook J, Sheffer M, Smith S, Loh WY, et al. Tobacco withdrawal components and their relations with cessation success. Psychopharmacology. 2011;216(4):569–78. doi: 10.1007/s00213-011-2250-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leventhal AM, Greenberg JB, Trujillo MA, Ameringer KJ, Pang RD, Lisha NE, et al. Positive and negative affect as predictors of urge to smoke: Temporal factors and mediational pathways. Psychology of Addictive Behaviors. 2013;27(1):262–7. doi: 10.1037/a0031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.