Abstract
Background
To investigate whether histologic subtyping from biopsies can predict local recurrence after thermal ablation for lung adenocarcinoma.
Methods
Patients treated with CT guided thermal ablation for lung adenocarcinoma that had pre-ablation needle biopsy with analysis of histological components were identified. Age, gender, smoking status, treatment indication (primary stage 1 tumor versus salvage), histologic subtype, ground glass radiographic appearance, tumor size, ablation modality, and ablation margin were evaluated in relation to time to local recurrence (TTLR). Cumulative incidence of recurrence (CIR) was calculated using competing risks analysis and compared across groups using Fine and Grey method with clustering. Multivariate analysis was conducted with stepwise regression.
Results
There were 53 patients with 57 tumors diagnosed as adenocarcinoma on pre-ablation biopsy and with histologic subtype analysis. Of these, 19% (11) had micropapillary components, 14% (8) had solid components, and 26% (15) had micropapillary and/or solid components. In the univariate analysis, solid (subdistribution hazard ratio [sHR]=4.04, p=0.0051, 95% confidence interval [CI]=1.52–10.7), micropapillary (sHR=3.36, p=0.01, CI=1.33–8.47), and micropapillary and/or solid components (SHR=5.85, p=0.00038, CI=2.21–15.5) were significantly correlated with shorter TTLR. On multivariate analysis, presence of micropapillary and/or solid component (sHR 11.4., p=0.00021, CI: 3.14–41.3) was the only independent predictor of TTLR. The 1, 2, and 3-year CIR in patients with micropapillary and/or solid components was 33%, 49%, and 66% compared to 5%, 14% and 18% in patients with no micropapillary or solid components on biopsy specimens.
Conclusion
Micropapillary and/or solid histological components identified in pre-ablation biopsy are associated with shorter TTLR after thermal ablation of lung adenocarcinoma.
Introduction
Lung cancer is the most frequent cause of cancer-related deaths worldwide.(1) Image guided thermal ablation offers an important therapeutic alternative for surgically ineligible patients as well as those who have lung metastasis or tumor recurrence after surgical treatment.(2, 3) A recent prospective trial of thermal ablation in medically inoperable early stage non small cell lung cancer demonstrated comparable overall survival rates to stereotactic body radiotherapy (SBRT) and surgery (4), though high rates of local recurrence (7–55%) remain a major critique.(5, 6) Evaluation of determinants of ablation success have to date largely focused on technique-associated parameters, such as tumor size and treatment margin size.(7) An association between presence of Ki67+ tumor cells and local tumor progression after ablation of lung tumors has been demonstrated.(8) Recent work has also shown an association between KRAS mutation status and local recurrence after ablation of lung adenocarcinoma.(9)
In 2011, the International Association for the Study of Lung Cancer (IASLC), the American Thoracic Society (ATS), and the European Respiratory Society (ERS) proposed a new classification system for lung adenocarcinoma.(10) Invasive lung adenocarcinoma tumor was subdivided into lepidic (LEP) predominant, acinar (ACI) predominant, papillary (PAP) predominant, micropapillary (MIP) predominant, and solid (SOL) predominant, and invasive mucinous adenocarcinoma (MUC). Multiple studies have since investigated the prognostic and predictive utility of this classification with respect to recurrence patterns and post-recurrence survival after surgery.(11–13) Most of these studies have pointed to the role of micropapillary and solid histological subtypes as independent predictors of higher local recurrence rates, distant metastasis and poorer overall prognosis even in completely resected early-stage lung adenocarcinoma. The prognostic potential of the IASLC/ATS/ERS classification system following thermal ablation remains unknown. This was a retrospective study to evaluate the association between different histological subtypes of lung adenocarcinoma identified in pre-ablation biopsies and local recurrence after ablation.
Materials and methods
This study was approved by the institutional review board with informed consent waived and was compliant with the Health Insurance Portability and Accountability Act.
Patient Selection
Consecutive patients who underwent percutaneous thermal ablation of a lung nodule at our institution between 2009 and 2016 were identified. There were a total of 798 lung ablations performed. We excluded all ablations for patients that did not have a diagnosis of lung cancer (n=568), all tumors that were not biopsied (n=75), and all tumors with final pathology other than adenocarcinoma (n=35). We also excluded any tumors whose biopsy specimens did not include a description of histologic subtype (n=63). These included biopsies that were fine needle aspiration (n=38) or if pathologist was not make a judgement on the histologic subtype (n=25). The study cohort consisted of 57 treated tumors in 53 patients (4 patients underwent two ablations for two different tumors). A flowchart is provided in Figure 1. Biopsies were obtained from the same site as the targeted tumor for ablation.
Figure 1.
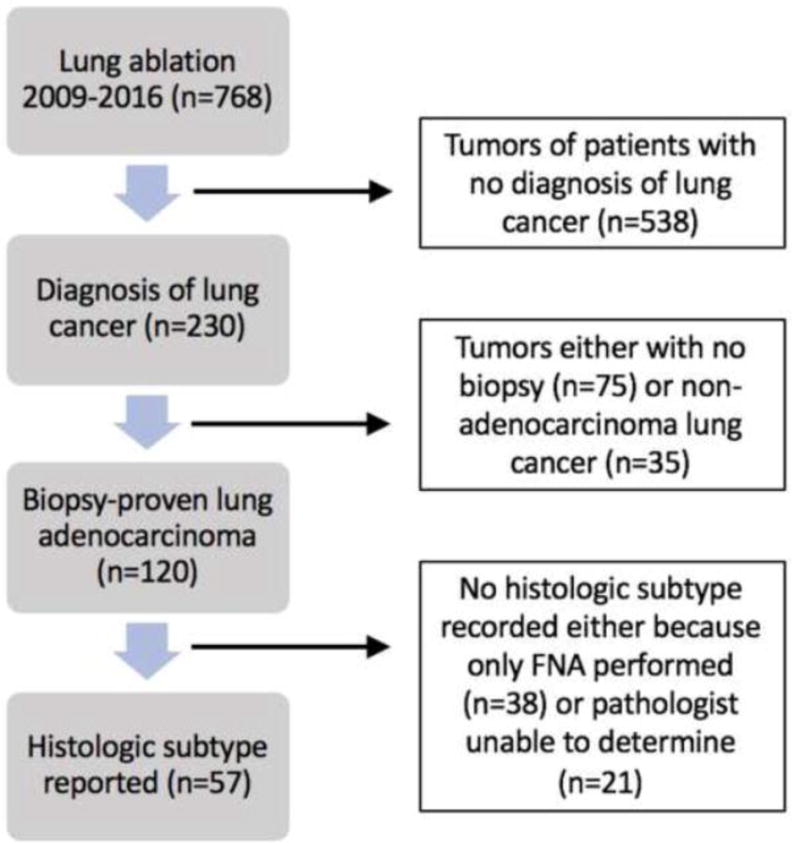
Flow diagram of patient selection and exclusion criteria.
Thermal Ablation
The decision to perform thermal ablation was made in conjunction with members of a multi-disciplinary thoracic disease management team. Computed tomography (CT) guidance was used to monitor ablation applicator placement under general anesthesia. All ablations were performed by fellowship-trained interventional radiologists. A total of 16 interventional radiologists performed the ablations with at least 8 years of experience at the beginning of the study. Ablation parameters, including modality were determined by the interventional radiologist performing the procedure according to tumor size, location, shape, adjacent structures, access route and operator preference. All ablations were performed according to the manufacturer’s protocol and with the aim of creating an ablation defect covering the entire tumor plus at least 5 mm surrounding the tumor. Immediately after every ablation procedure, a CT scan was performed to assess the ablation zone and possible complications. A minimum of 2 chest radiographs were also obtained after the procedure to exclude pneumothorax or other complications. We used the established guidelines regarding terminology and reporting.(14, 15) Technical success was based on the immediate post ablation CT at the time of procedure and defined as an ablation zone completely encompassing the tumor. Technical efficacy was based on the first post-ablation CT scan and defined as an ablation defect that completely encompassed the tumor. Failure was defined as any evidence of residual tumor within 1 cm of the ablation defect. The ablation defect at the first post ablation CT scan was considered the new baseline for future comparisons.
Tissue Acquisition and Histologic Evaluation
Histologic diagnosis and presence of subtype of adenocarcinoma (LEP, ACI, PAP, MIP, SOL, and MUC) were extracted from pathology reports. As subtype assessment was based on needle biopsies rather than an entirely resected specimen, a “predominant” subtype was not assigned. A meta-category of micropapillary and/or histological components (MIP_SOL) was also included. The median time between biopsy and ablation was 34 days (range=0 to 333 days).
Follow Up and Assessment of Local Tumor Recurrence
Post-procedural imaging was performed according to standard guidelines(16), beginning with a baseline CT or PET/CT after 1 month. Decision to perform CT or PET/CT on all follow up examinations was based on interventional radiologist and referring oncologist and dependent on clinical scernario. Routine follow-up imaging was performed after 3, 6, and 12 months, then at yearly intervals. Successful ablation was defined as progressive reduction in size and lack of contrast enhancement in the ablation zone in relation to the 1 month baseline imaging study. Local recurrence was either biopsy proven or diagnosed based on the following imaging parameters: (1) development of new tumor adjacent to the ablation zone, (2) development of contrast enhancement within or adjacent to the ablation zone, or (3) increase in metabolic activity within or adjacent to the ablation site if a PET/CT was performed.(17) Radiology reports dictated by faculty body imaging radiologists were retrieved from the electronic medical records and reviewed. All imaging studies were prospectively assessed by the operating interventional radiologist. All studies were also independently reviewed by EZ and SG and discrepancies resolved by consensus.
Complications were categorized according to the Common Terminology Criteria for Adverse Events version 4.03 (CTCAE v4.03; National Institutes of Health, National Cancer Institute). Grade 1 or 2 adverse events were defined as minor complications and grade ≥ 3 adverse events were defined as major complications.
Covariates
Patient and clinical characteristics were recorded including age, gender, smoking status, tumor status as primary lung neoplasm or recurrence, CT appearance of predominant ground glass opacity, ablation modality (radiofrequency, microwave, or cryoablation), tumor size (length of longest dimension) and ablation margin. The ablation margin was assessed based on the intra-procedural pre and post CT examination. Similar to previously described workflow(18), anatomic landmarks surrounding the nodule were chosen and the distances from the edge of the nodule to the landmarks were measured on the pre and post intra-procedural CT scan. The subtracted distance equaled the margin at that site and the smallest value obtained was defined as the ablation margin. This was categorized as either less than or equal to 5 mm or greater than 5 mm. If a section of a nodule was immediately adjacent to a pleural surface, the margin along that section was not calculated.
Statistical Analysis
Overall survival was measured from the time of ablation to patient death or most recent follow-up, determined by review of the patient medical record. Overall survival rates were estimated using the Kaplan-Meier method. Median time to follow-up was based on the reverse Kaplan-Meier estimator.(19) A competing risks proportional hazards model was used to analyze the time to local recurrence (TTLR) after thermal ablation, with death without local recurrence considered a competing event.(20, 21) TTLR was calculated from the ablation procedure date. Patients alive without evidence of local recurrence were censored on the date of last available imaging. The cumulative incidence function was used to estimate the 1-year, 2-year, and 3-year cumulative incidence of recurrence (CIR) after ablation. Univariate analysis by histologic subtype, including the additional MIP_SOL category was performed with clustering to account for within-patient correlations. To determine which variables to include in the multivariate analysis, we tested multiple potential confounders. Covariates with significance or marginal significance (p<0.15) were subsequently included in the multivariate analysis. Backward selection with a cutoff of p=0.05 was performed to select significant predictors of outcome in the multivariate analysis. Statistical analysis was performed using R software.(22)
Results
The technical success and technical efficacy of ablation were 100%. Complications recorded were as follows. There were 13 pneumothoraces that were managed with chest tubes (23%), 2 intercostal nerve radiculitis (4%) that were managed conservatively with pain medicine, and 1 (2%) presumed recurrent laryngeal nerve injury resulting in voice changes that resolved after 2 months. Major complications included 1 pneumothorax that was treated with sclerosis (2%), 1 lung infection (2%) with cavity formation treated with a prolonged course of antibiotics, and 1 radiculitis (2%) treated with a nerve block procedure. There were no grade 4 or 5 events.
The median time between biopsy and ablation was 34 days (range 0 to 333 days). The median time to follow up was 44 months (95% CI: 33 to 52 months). The 1-, 2-, and 3-year overall survival was 93% (95% CI: 82–97%), 86% (95% CI: 72–93%), and 75% (95% CI: 59–86%), respectively. There were 16 local recurrences observed. The overall CIR after ablation was 12.7% (95%CI:5.5–23.0%) after 1-year, 24.1% (95%CI:13.1–37.0%) after 2-years, and 32.1 %(95%CI:18.8–46.1%) after 3-years.
A summary of the patient and tumor characteristics is provided in Table 1. The median tumor size was 15 mm (range 7–38 mm) and 12 (21%) nodules were greater than 2 cm. The median minimum ablation margin was 8 mm (range 2–18 mm) and 12 (21%) had ablation margin less than or equal to 5 mm. The histologic subtype components recorded in pre-ablation biopsies were as follows: 35 (61%) had ACI component, 21 (37%) had LEP component, 11 (29%) had MIP component, 11 (19%) had PAP component, and 8 (14%) had SOL component. There were 5 (9%) that had MUC features. There were 15 (26%) that were either micropapillary and/or solid (MIP_SOL).
Table 1.
Univariate analysis of local recurrence after thermal ablation. sHR (Subdistribution hazard ratio), CI (confidence interval), GGO (ground-glass opacity), ACI (acinar), LEP (lepidic), SOL (solid), PAP (papillary), MIP (micropapillary), RFA (radiofrequency ablation), MWA (microwave ablation), CRA (cryoablation). Significant variables (MIP_SOL, minimum margin) and variables with p<0.15 (LEP) were included in the multivariate analysis.
| Covariate | Competing-risks regression for clustered data | |||
|---|---|---|---|---|
| sHR | p | 95% CI | ||
| Age at ablation, years (range) | 73.9 (51.9–87.8) | 1.01 | 0.76 | 0.94–1.09 |
| Gender | 0.90 | 0.83 | 0.32–2.51 | |
| Female | 28 (53%) | |||
| Male | 25 (47%) | |||
| Smoking history | 0.84 | 0.74 | 0.31–2.33 | |
| No | 18 (34%) | |||
| Yes | 35 (66%) | |||
| Tumor status | 0.99 | 0.99 | 0.27–3.57 | |
| primary | 43 (75%) | |||
| salvage | 14 (25%) | |||
| Maximum tumor size (mm) | 0.66 | 0.45 | 0.23–1.94 | |
| >20 mm | 12 (21%) | |||
| ≤20 mm | 45 (79%) | |||
| GGO | 0.64 | 0.43 | 0.21–1.98 | |
| No | 39 (68%) | |||
| Yes | 18 (32%) | |||
| ACI | 0.69 | 0.49 | 0.25–1.94 | |
| No | 22 (39%) | |||
| Yes | 35 (61%) | |||
| LEP | 0.43 |
|
0.15–1.28 | |
| No | 36 (63%) | |||
| Yes | 21 (37%) | |||
| SOL | 4.04 |
|
1.52–10.7 | |
| No | 49 (86%) | |||
| Yes | 8 (14%) | |||
| PAP | 1.52 | 0.48 | 0.48–4.82 | |
| No | 46 (81%) | |||
| Yes | 11 (19%) | |||
| MIP | 3.36 |
|
1.33–8.47 | |
| No | 46 (81%) | |||
| Yes | 11 (19%) | |||
| MIP_SOL | 5.85 |
|
2.21–15.5 | |
| No | 42 (74%) | |||
| Yes | 15 (26%) | |||
| Thermal ablation type | 1.25 | 0.54 | 0.61–2.6 | |
| RFA | 34 (59%) | |||
| MWA | 21 (37%) | |||
| CRA | 2 (4%) | |||
| Minimum ablated margin | 0.16 |
|
0.05–0.47 | |
| < 5 mm | 6 (11%) | |||
| >= 5 mm | 51 (89% | |||
Among the histologic subtypes, SOL (subdistribution hazard ratio [sHR]=4.04, p=0.0051, confidence interval [CI]=1.52–10.70) and MIP (sHR=3.36, p=0.01, CI=1.33–8.47) were significantly correlated with shorter TTLR. The MIP_SOL category was also correlated with shorter TTLR (sHR=5.58, p=0.00038, CI=2.21–15.5). Minimum ablated margin of greater than or equal to 5 mm was significantly correlated with longer TTLR (sHR=0.16, p=0.00082, CI=0.05–0.47). Table 1 summarizes the univariate analysis of TTLR.
The prognostic variables identified in the univariate analysis as significant, MIP_SOL and minimum ablated margin, were incorporated into a multivariate competing risks proportional hazards model along with the marginally significant, LEP (sHR=0.43, p=0.13, CI=0.15–1.28). In the multivariate analysis, only MIP_SOL remained significant (sHR=11.4, p=0.00021, CI=3.14–41.3).
Figure 2 represents the cumulative incidence of local recurrence after ablation in patients with and without MIP or SOL components on the pre-ablation biopsy. The 1-, 2-, and 3-year cumulative incidence of local recurrence in patients with no MIP_SOL components was 5% (CI: 1–15%), 14% (CI: 5–28%) and 18% (CI:7–33%); compared with 33% (Ci: 11–57%), 49% (20–72%), and 66% (CI:30–86%).
Figure 2.
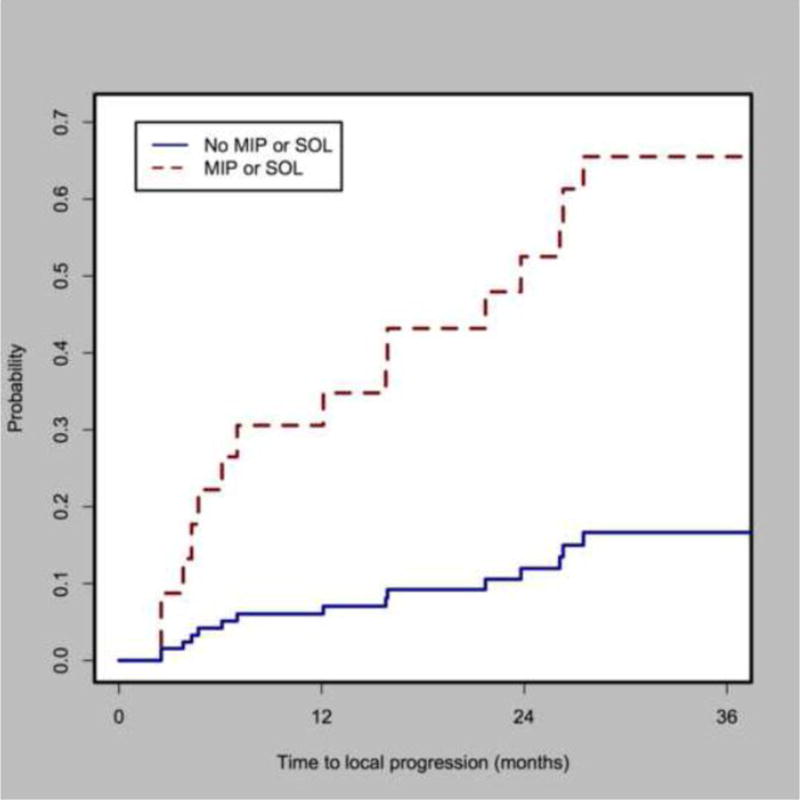
Cumulative incidence of local recurrence for tumors with death as competing risk for tumors with and without micropapillary and/or solid components (sHR=5.85, p=0.0038, CI=2.21–15.5).
Discussion
Lung adenocarcainoma histologic subtypes have distinct clinicopathologic characteristics and prognosis. MIP and SOL components, in particular, are associated with increased risk of recurrence after surgery and worse prognosis.(11–13, 23, 24) This study demonstrated the prognostic significance of histologic subtype after thermal ablation. The presence of MIP or SOL on pre-treatment biopsy specimens was an independent predictor of shorter TTLR. Similar results have recently been reported after stereotactic body radiation therapy in the setting of MIP and SOL subtypes.(25) This work may be used to improve patient selection and stratification, to define optimal follow-up strategies after ablation based on risk of recurrence, and potentially to target larger ablation margins for high risk patients. The fact that subtype identification was performed on biopsy specimens also suggests that even surgical and radiation patients may benefit from biopsies to identify high risk patients.
Intratumoral heterogeneity of lung adenocarcinomas presents a challenge for assessing histologic subtype. The presence of even a small amount of MIP (as little as 5%) is associated with risk of recurrence after surgery.(12). Targeted needle biopsies may under-sample diversity, though concordance rates between biopsy specimens and predominant subtype after surgical excision can be high when tumors are small.(26) Further work to delineate the relationship between intratumoral heterogeneity, specific histologic subtypes, and biopsy characterization may more effectively guide patient care.
Mutation status has also been associated with histologic subtype. SOL-predominant adenocarcinomas are negatively associated with EGFR mutations and positively associated with KRAS mutations.(27, 28) MIP predominant tumors are associated with EGFR mutations despite their inherent aggressive biology.(29) An association between KRAS mutations and shorter TTLR after thermal ablation was recently reported.(9) In this cohort, a substantial number (25/57 44%) of tumor specimens did not undergo KRAS mutation testing and only 8/57 (14%) of tumors were KRAS mutants. Further studies with larger sample sizes and thorough genotyping are warranted.
A circumferential GGO margin >5 mm is required to ensure complete tumor ablation.(30) Similar to prior reports, we found an association between margins < 5mm and worse outcome.(18) In a study by Nitadori et al, high-risk tumors with an MIP component of 5% or greater were significantly associated with increased risk of local recurrence when the surgical margin was less than 1cm, but had no effect when it was 1cm or more (12). This raises the possibility that certain histologic subtypes require larger ablation margins. Interestingly, the presence of micropapillary or solid subtype is associated with occult metastasis in resected lung adenocarcinomas (31). An alternative hypothesis may then be that recurrences seen in the setting of micropapillary or solid subtypes may represent instances of self-seeding (32). Local recurrence rates may then be manifestations of the degree to which tumor cells can re-establish colonies in the inflammatory environment post-surgery, post-radiation, or post-ablation. A third alternative may be that high grade tumors have inherently higher tumor doubling time, resulting in faster tumor regrowth.
Our results support the use of biopsy prior to ablation. However, there is some concern that biopsy may result in tumor seeding and possibly contribute to recurrences. At least in the surgery literature, the evidence seems to support no needle dissemination from CT-guided lung biopsies(33). Moreover, the overall rate of local recurrence in our cohort of biopsied patients was similar to published rates including studies where not all patients were biopsied. One possibility is that tumor seeding risk is also dependent on the histologic subtype, although this question would be difficult to address directly in an ablation clinical trial.
There were several limitations to this study. This was a retrospective study with a small number of patients. The results should be interpreted as exploratory and must be validated on a separate cohort. Histologic assessment was performed by needle biopsies limiting the accuracy of histologic subtype assessment. While the objective was to demonstrate the role of histologic subtype as an independent predictor of time to local recurrence after thermal ablation, the results do not negate the contribution of additional variables (tumor size, margin, KRAS mutation status) to local recurrence. Despite these limitations, these encouraging results suggest a useful framework for future prospective, large-scale clinical studies.
In conclusion, MIP and SOL histological components are associated with statistically significant increased risk of local recurrence after thermal ablation. This study supports the utility of biopsy for histologic subtype as a prognostic indicator of faster local recurrence prior to lung ablation. Furthermore, the results suggest that any studies comparing techniques should appropriately stratify patients by histologic subtype.
Acknowledgments
Funding: This work was supported by a National Institute of Health Cancer Center Support Grant (2P30CA008748-48). Dr Ziv is supported in part by a grant from the Tully Research Foundation. Dr. Solomon reports grants from GE Healthcare and personal fees from Medtronic and personal fees from AstraZeneca, outside the submitted work.
Footnotes
Conflict of Interests: The authors otherwise declare that they have no conflicts of interest.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Does not apply.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Kwan SW, Mortell KE, Talenfeld AD, Brunner MC. Thermal ablation matches sublobar resection outcomes in older patients with early-stage non-small cell lung cancer. J Vasc Interv Radiol. 2014;25(1):1–9.e1. doi: 10.1016/j.jvir.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Ambrogi MC, Fanucchi O, Cioni R, Dini P, De Liperi A, Cappelli C, et al. Long-term results of radiofrequency ablation treatment of stage I non-small cell lung cancer: a prospective intention-to-treat study. J Thorac Oncol. 2011;6(12):2044–51. doi: 10.1097/JTO.0b013e31822d538d. [DOI] [PubMed] [Google Scholar]
- 4.Dupuy DE, Fernando HC, Hillman S, Ng T, Tan AD, Sharma A, et al. Radiofrequency ablation of stage IA non-small cell lung cancer in medically inoperable patients: Results from the American College of Surgeons Oncology Group Z4033 (Alliance) trial. Cancer. 2015;121(19):3491–8. doi: 10.1002/cncr.29507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donington JS. Radiofrequency ablation in high-risk stage I non-small cell lung cancer. Cancer. 2015;121(19):3393–4. doi: 10.1002/cncr.29501. [DOI] [PubMed] [Google Scholar]
- 6.Louie AV, Siva S, Senan S. Defining the role of radiofrequency ablation and stereotactic ablative radiotherapy in patients with high-risk, early-stage non-small cell lung cancer. Cancer. 2016;122(2):322–3. doi: 10.1002/cncr.29698. [DOI] [PubMed] [Google Scholar]
- 7.de Baere T, Palussiere J, Auperin A, Hakime A, Abdel-Rehim M, Kind M, et al. Midterm local efficacy and survival after radiofrequency ablation of lung tumors with minimum follow-up of 1 year: prospective evaluation. Radiology. 2006;240(2):587–96. doi: 10.1148/radiol.2402050807. [DOI] [PubMed] [Google Scholar]
- 8.Sofocleous CT, Garg S, Petrovic LM, Gonen M, Petre EN, Klimstra DS, et al. Ki-67 is a prognostic biomarker of survival after radiofrequency ablation of liver malignancies. Ann Surg Oncol. 2012;19(13):4262–9. doi: 10.1245/s10434-012-2461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ziv E, Erinjeri JP, Yarmohammadi H, Boas FE, Petre EN, Gao S, et al. Lung Adenocarcinoma: Predictive Value of KRAS Mutation Status in Assessing Local Recurrence in Patients Undergoing Image-guided Ablation. Radiology. 2016:160003. doi: 10.1148/radiol.2016160003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6(2):244–85. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hung JJ, Yeh YC, Jeng WJ, Wu KJ, Huang BS, Wu YC, et al. Predictive value of the international association for the study of lung cancer/American Thoracic Society/European Respiratory Society classification of lung adenocarcinoma in tumor recurrence and patient survival. J Clin Oncol. 2014;32(22):2357–64. doi: 10.1200/JCO.2013.50.1049. [DOI] [PubMed] [Google Scholar]
- 12.Nitadori J, Bograd AJ, Kadota K, Sima CS, Rizk NP, Morales EA, et al. Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2cm or smaller. J Natl Cancer Inst. 2013;105(16):1212–20. doi: 10.1093/jnci/djt166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ujiie H, Kadota K, Chaft JE, Buitrago D, Sima CS, Lee MC, et al. Solid Predominant Histologic Subtype in Resected Stage I Lung Adenocarcinoma Is an Independent Predictor of Early, Extrathoracic, Multisite Recurrence and of Poor Postrecurrence Survival. J Clin Oncol. 2015;33(26):2877–84. doi: 10.1200/JCO.2015.60.9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria—a 10-year update. Radiology. 2014;273(1):241–60. doi: 10.1148/radiol.14132958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, 3rd, Dupuy DE, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009;20(7 Suppl):S377–90. doi: 10.1016/j.jvir.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Pereira PL, Masala S. Standards of practice: guidelines for thermal ablation of primary and secondary lung tumors. Cardiovasc Intervent Radiol. 2012;35(2):247–54. doi: 10.1007/s00270-012-0340-1. [DOI] [PubMed] [Google Scholar]
- 17.Ridge CA, Solomon SB, Thornton RH. Thermal ablation of stage I non-small cell lung carcinoma. Semin Intervent Radiol. 2014;31(2):118–24. doi: 10.1055/s-0034-1373786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shady W, Petre EN, Gonen M, Erinjeri JP, Brown KT, Covey AM, et al. Percutaneous Radiofrequency Ablation of Colorectal Cancer Liver Metastases: Factors Affecting Outcomes–A 10-year Experience at a Single Center. Radiology. 2016;278(2):601–11. doi: 10.1148/radiol.2015142489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17(4):343–6. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 20.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 21.Dignam JJ, Zhang Q, Kocherginsky M. The use and interpretation of competing risks regression models. Clin Cancer Res. 2012;18(8):2301–8. doi: 10.1158/1078-0432.CCR-11-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 23.Xu S, Xi J, Jiang W, Lu S, Wang Q. Solid component and tumor size correlate with prognosis of stage IB lung adenocarcinoma. Ann Thorac Surg. 2015;99(3):961–7. doi: 10.1016/j.athoracsur.2014.10.079. [DOI] [PubMed] [Google Scholar]
- 24.Cha MJ, Lee HY, Lee KS, Jeong JY, Han J, Shim YM, et al. Micropapillary and solid subtypes of invasive lung adenocarcinoma: clinical predictors of histopathology and outcome. J Thorac Cardiovasc Surg. 2014;147(3):921–8.e2. doi: 10.1016/j.jtcvs.2013.09.045. [DOI] [PubMed] [Google Scholar]
- 25.Leeman JE, Rimner A, Montecalvo J, Hsu M, Zhang Z, von Reibnitz D, et al. Histologic subtype in core lung biopsies of early-stage lung adenocarcinoma is a prognostic factor for treatment response and failure patterns after stereotactic body radiation therapy. International Journal of Radiation Oncology*Biology*Physics. 2016 doi: 10.1016/j.ijrobp.2016.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuzawa R, Kirita K, Kuwata T, Umemura S, Matsumoto S, Fujii S, et al. Factors influencing the concordance of histological subtype diagnosis from biopsy and resected specimens of lung adenocarcinoma. Lung Cancer. 2016;94:1–6. doi: 10.1016/j.lungcan.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Tsuta K, Kawago M, Inoue E, Yoshida A, Takahashi F, Sakurai H, et al. The utility of the proposed IASLC/ATS/ERS lung adenocarcinoma subtypes for disease prognosis and correlation of driver gene alterations. Lung Cancer. 2013;81(3):371–6. doi: 10.1016/j.lungcan.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 28.Rekhtman N, Ang DC, Riely GJ, Ladanyi M, Moreira AL. KRAS mutations are associated with solid growth pattern and tumor-infiltrating leukocytes in lung adenocarcinoma. Mod Pathol. 2013;26(10):1307–19. doi: 10.1038/modpathol.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Oliveira Duarte Achcar R, Nikiforova MN, Yousem SA. Micropapillary lung adenocarcinoma: EGFR, K-ras, and BRAF mutational profile. Am J Clin Pathol. 2009;131(5):694–700. doi: 10.1309/AJCPBS85VJEOBPDO. [DOI] [PubMed] [Google Scholar]
- 30.Anderson EM, Lees WR, Gillams AR. Early indicators of treatment success after percutaneous radiofrequency of pulmonary tumors. Cardiovasc Intervent Radiol. 2009;32(3):478–83. doi: 10.1007/s00270-008-9482-6. [DOI] [PubMed] [Google Scholar]
- 31.Hung JJ, Yeh YC, Jeng WJ, Wu YC, Chou TY, Hsu WH. Factors predicting occult lymph node metastasis in completely resected lung adenocarcinoma of 3 cm or smaller. Eur J Cardiothorac Surg. 2016;50(2):329–36. doi: 10.1093/ejcts/ezv485. [DOI] [PubMed] [Google Scholar]
- 32.Norton L, Massague J. Is cancer a disease of self-seeding? Nat Med. 2006;12(8):875–8. doi: 10.1038/nm0806-875. [DOI] [PubMed] [Google Scholar]
- 33.Sano Y, Date H, Toyooka S, Oto T, Yamane M, Hiraki T, et al. Percutaneous computed tomography-guided lung biopsy and pleural dissemination: an assessment by intraoperative pleural lavage cytology. Cancer. 2009;115(23):5526–33. doi: 10.1002/cncr.24620. [DOI] [PubMed] [Google Scholar]


