Abstract
Background
We previously reported that functional recovery of rats with spinal cord contusions can occur after acute transplantation of neural stem cells distal to the site of injury. To investigate the effects of timing of administration of human neural stem cell (hNSC) distal to the site of spinal cord injury on functional outcomes in an animal model.
Methods
Thirty-six adult female Long-Evans hooded rats were randomized into three experimental and three control groups with six animals in each group. The T10 level was exposed via posterior laminectomy, and a moderate spinal cord contusion was induced by the Multicenter Animal Spinal Cord Injury Study Impactor (MASCIS, W.M. Keck Center for Collaborative Neuroscience, Piscataway, NJ, USA). The animals received either an intrathecal injection of hNSCs or control media through a separate distal laminotomy immediately, one week or four weeks after the induced spinal cord injury. Observers were blinded to the interventions. Functional assessment was measured immediately after injury and weekly using the Basso, Beattie, Bresnahan (BBB) locomotor rating score.
Results
A statistically significant functional improvement was seen in all three time groups when compared to their controls (acute, mean 9.2 vs. 4.5, P=0.016; subacute, mean 11.1 vs. 6.8, P=0.042; chronic, mean 11.3 vs. 5.8, P=0.035). Although there was no significant difference in the final BBB scores comparing the groups that received hNSCs, the group which achieved the greatest improvement from the time of cell injection was the subacute group (+10.3) and was significantly greater than the chronic group (+5.1, P=0.02).
Conclusions
The distal intrathecal transplantation of hNSCs into the contused spinal cord of a rat led to significant functional recovery of the spinal cord when injected in the acute, subacute and chronic phases of spinal cord injury (SCI), although the greatest gains appeared to be in the subacute timing group.
Keywords: Spinal cord injury (SCI), stem cells, timing
Introduction
Spinal cord injury (SCI) remains a significantly unsolved problem in medicine, and it is estimated that in the United States there are 12,000 new cases per year and 238,000–332,000 persons living with this condition (1). Motor vehicle accidents, falls, violence such as gun-shot wounds, and sport injuries constitute approximately 88.5% of the causes of SCI since 2010, and the vast majority experience some level of permanent neurologic with only 1% of persons experiencing complete neurologic recovery (2). Given its permanent neurologic sequelae, the burden of care onto patients and society is substantial and spans the duration of the patient’s life.
Stem cells for spinal cord injury have been used to modulate the inflammatory reaction, replace lost neurons and oligodendrocytes, and remyelinate damaged spinal tracts, thereby allowing for functional recovery. Stem cells are an attractive option to modulate the post-injury inflammatory reaction, replace irreparable neurons and oligodendrocytes, and provide remyelination of spinal tracts due to their pluripotent nature. Previous studies have shown the ability of neuronal stem cells to survive transplantation and develop into neuron-like cells (3,4). These cells have been shown to reconstruct damaged neuronal structures, remyelinate axons, and restore motor function.
While mounting evidence exists that transplantation of neural stem cells (NSCs) may be helpful in SCI, there is a paucity of literature regarding the optimal timing or location for transplantation. Theories abound that delayed treatment may result in a less inflammatory environment and more permissive conditions for survival of the transplanted cells (5) while others argue that the chronic phase of SCI results in an established glial scar which may limit cellular function at the site of injury (6,7). The subacute and chronic phases of SCI in the rat have been delineated by histological studies analyzing glial scar formation and neuroregenerative potential and have been determined to be 7 and >28 days respectively (8). Our laboratory has been able to demonstrate significantly improved functional outcomes in a rat model when supplying human neural stem cells (hNSCs) via distal intrathecal injection for both acute and chronic time periods (9,10).
To our knowledge, no studies thus far have been performed comparing the functional outcomes of distal intrathecal injection of hNSCs in a rat model for the acute versus subacute versus chronic phases of SCI.
Methods
The methodology for the acute and subacute administration of hNSCs in SCI have been previously described (9,11). This study compared the results of the acute, subacute, and chronic administration of hNSCs in SCI. Adult female Long-Evans hooded rats (200–350 grams; Charles River Laboratories, Wilmington, MA, USA) for all cohorts were utilized in this randomized, controlled study with approval from the Institutional Review Board and Administrative Panel on Laboratory Animal Care. A minimum of six subjects per group was required to detect a three-point difference in the locomotor scoring system between control and experimental groups for each of the cohorts based on our power analysis.
Human NSCs were collected from a single donor as previously described (9).
Two days before transplantation, neurospheres were enzymatically dissociated into single cell suspensions and cultured in fresh medium. In order to perform in vivo bioluminescence imaging, the hNSCs were transfected with the cytomegalovirus (CMV) promoter/luciferase reporter gene as previously described (10).
Each animal was anesthetized and a moderate contusion injury was created at the T10 level using the Multicenter Animal Spinal Cord Injury Study Impactor (MASCIS, W.M. Keck Center for Collaborative Neuroscience, Piscataway, NJ, USA) as previously described (10). The height, velocity and acceleration of the impaction was measured with computer assistance to ensure consistency.
In the acute groups, the animals were randomized to receive control medium or hNSCs immediately after creation of the SCI. In the subacute and chronic groups, the animals were also randomized and returned for secondary procedures 7 and 28 days after SCI, respectively. Intrathecal injections of 500,000 NSC in 4 µL of media for the experimental groups or 4 µL of culture medium alone for the control groups through a separate laminotomy site in the lumbar spine were performed caudal to the level of SCI. The lead surgeon was blinded to the material injected. Injections were slowly given over a 5-minute period through 33-gauge Hamilton syringes (Hamilton Company, Reno, NV, USA) attached to a micromanipulator mounted adjacent to the operative field assisted by loupe magnification. Following injection of medium or hNSCs, surgical incisions were closed with 2-0 chromic suture.
Functional outcome was assessed using the Basso, Beattie, Bresnahan (BBB) locomotor rating score for rat hind limb motor function as described in previously published studies (12). Two trained observers, who were blinded to the experimental groups and scored independently in a noise-free environment, performed the BBB recordings. Animals were assessed during the course of a 4-minute exposure to an open-field arena consisting of a metal circular enclosure (90-cm diameter, 7-cm wall height). In order to consistently measure the effect of cellular intervention over a consistent time period, BBB scores were recorded weekly for 6 weeks after injection of cells to assess functional recovery. General categories of the rating scale are score of 0–7: isolated joint movements with little or no hindlimb movement; score of 8–13: intervals of uncoordinated stepping; and score of 14–21: forelimb and hindlimb coordination. In vivo bioluminescence measurements of signal intensity of the luciferase-expressing engrafted hNSCs was performed as previously described on a weekly basis (10). Statistical significance was determined using two-sample t-test, Tukey contrasts, and linear mixed-effects model analysis (13).
Results
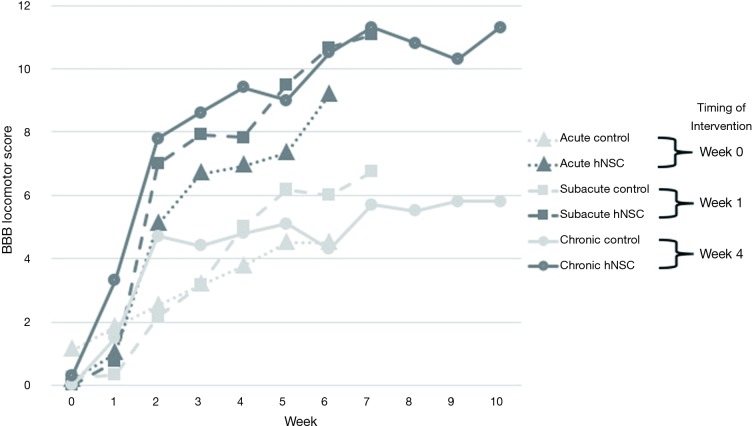
Thirty-six subjects underwent SCI and injection of either hNSCs or culture media via distal lumbar puncture: acute experimental (n=6), acute control (n=6), subacute experimental (n=6), subacute control (n=6), chronic experimental (n=6), and chronic control (n=6). At the completion of the study, the control groups and the experimental groups prior to cell injection did demonstrate spontaneous improvement in scores. All experimental groups injected with hNSCs, however, had a statistically significant improvement in function compared with their control counterparts injected with culture medium as measured with BBB scoring at final testing (acute: mean 9.2 vs. 4.5, respectively, P=0.016; subacute: mean 11.1 vs. 6.8, P=0.042; chronic: mean 11.3 vs. 5.8, P=0.035; see Figure 1).
Figure 1.
BBB locomotor scores comparing subjects receiving distal site injection of NSCs versus control medium in the acute versus subacute versus chronic time periods after spinal cord injury. BBB, Basso, Beattie, Bresnahan; hNSC, human neural stem cell.
From the time of injection of hNSCs, the mean change in BBB scores and standard deviations (SD) were as follows: acute +9.0 (SD 3.1), subacute +10.3 (SD 2.3), and chronic +5.1 (SD 4.0). Comparing these changes over time in the hNSC groups using the Tukey contrasts, the only comparison that reached significance was a greater improvement in subacute versus chronic [mean +5.3, 95% confidence interval (CI): +0.8 to +9.8, P=0.02]. Comparing the other inter-group changes were subacute versus acute (mean +1.3, 95% CI: −3.6 to +6.3, P=0.77) and acute versus chronic (mean +3.9, 95% CI −0.6 to +8.5, P=0.09).
The final BBB scores for the hNSC groups did not show a significant difference between groups—acute (mean 9.2, SD 3.0), subacute (mean 11.1, SD 2.1), chronic (mean 11.3, SD 4.8), acute vs. subacute (P=0.65), subacute vs. chronic (P=1.00), and acute vs. chronic (P=0.54).
Using the linear mixed effects analysis to compare the slopes (or rate of change) from the time of injection of hNSCs, the difference between the acute and subacute was P=0.13, the difference between acute and chronic was P=0.0001, and the difference between subacute and chronic was P=0.0003. Significant differences in function were noted between hNSC groups prior to the injection of cells with the chronic group having a significant higher baseline function (acute: mean 0.17, SD 0.41; subacute: mean 0.75, SD 0.42; chronic: mean 6.2, SD 1.64; acute vs. subacute, P=0.65; acute vs. chronic, P<0.0001; subacute vs. chronic, P<0.0001).
In all animals, luciferase-expressing hNSCs were detected at the sites of injection immediately after application of the cells (figure not shown) (10). Because cells were not detected in any animals after one week, imaging was subsequently discontinued.
Discussion
This study is the first to demonstrate that the injection of neuronal stem cells via distal intrathecal injection can induce significant functional improvement regardless of the timing of injection. While it has been previously shown in animal models that spontaneous motor recovery can occur (14), there remained significant functional improvement in each experimental group after the application of stem cells.
We surmise that the stem cells may be more effective while they are free within the cerebral spinal fluid rather than restricted within the scar tissue of the spinal cord injury itself when applied directly to the site of injury. Cytokines and growth factors expressed by the stem cells may help in differing ways in each phase of the injury. In the acute phase, they may act as an immunosuppressant. In the subacute phase, they may help prevent cell death and scar formation. In the chronic phase, they may help stimulate surviving cells to divide and differentiate. In our study, there was a statistically significantly more rapid improvement in function in the subacute phase compared with chronic and trended towards significance comparing subacute versus acute. This finding is consistent with previous reports and may be due to the decreased inflammatory response and less toxic mediators at the site of injury (5-7). Keirstead et al. was also able to show that embryonic stem cell-derived oligodendrocyte precursor cells successfully differentiated into oligodendrocytes in subacutely injured spinal cords (15).
In a chronic model of SCI, Nishimura et al. (16) were able to demonstrate similar survival of both subacutely and chronically grafted progenitor cells. They found, however, that the cells were more limited in their functionality in the chronic setting due to the glial scar enclosure and actually differentiated into macrophages rather than neurons. In another study, Kumamaru et al. (17) showed that the cell expression of neural stem cells did not differ based on timing of transplantation. Rather, the chronic environment actually seemed to encourage the transcriptional activity of the cells more so than the acute or subacute phases. This may imply that cells injected locally in the chronic phase are capable of survival and increased activity but are restricted by surrounding scar tissue. On the other hand, cells allowed to freely flow within the cerebral spinal fluid via distal injection may be able to better provide a paracrine effect to latent oligodendrocytes at the site of injury thereby facilitating functional recovery.
A significant limitation to this study is the lack of histologic and electrophysiologic data to explain the effect of the cells on locomotor recovery. The bioluminescence imaging suggests that the cells were able to survive immediately after injection and may have provided a supportive role for injured cells prior to disappearing after one week. Future studies will focus on the immunohistochemistry, axonal counts, and electrical conductivity across the site of injury.
In summary, transplantation of hNSCs provided significant functional improvement after contusion SCI in the rat model in the acute, subacute, and chronic phases. This provides hope to patients regardless of the length of time since their injuries that their function may improve through a potentially therapeutic injection of hNSCs through a traditional lumbar puncture.
Acknowledgements
We thank Stemedica (San Diego, CA, USA) for providing the human neural stem cells for this study, the McCulloch family for their generous financial support, the Cervical Spine Research Society for the Seed Starter Research & Education Grant, and Alex Sox-Harris for his assistance with statistical analysis.
Ethical Statement: This study obtained approval from the Institutional Review Board and Administrative Panel on Laboratory Animal Care (No. 17966).
Footnotes
Conflicts of Interest: AI Kharazi is an employee of Stemedica and served as a consultant to this project regarding cell preparation. He was otherwise not involved in any animal procedures, data acquisition or data analysis. The other authors have no conflicts of interest to declare.
References
- 1.Chen Y, He Y, DeVivo MJ. Changing Demographics and Injury Profile of New Traumatic Spinal Cord Injuries in the United States, 1972-2014. Arch Phys Med Rehabil 2016;97:1610-9. 10.1016/j.apmr.2016.03.017 [DOI] [PubMed] [Google Scholar]
- 2.Jain NB, Ayers GD, Peterson EN, et al. Traumatic spinal cord injury in the United States, 1993-2012. JAMA 2015;313:2236-43. 10.1001/jama.2015.6250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cummings BJ, Uchida N, Tamaki SJ, et al. Human neural stem cell differentiation following transplantation into spinal cord injured mice: association with recovery of locomotor function. Neurol Res 2006;28:474-81. 10.1179/016164106X115116 [DOI] [PubMed] [Google Scholar]
- 4.Iwanami A, Kaneko S, Nakamura M, et al. Transplantation of human neural stem cells for spinal cord injury in primates. J Neurosci Res 2005;80:182-90. 10.1002/jnr.20436 [DOI] [PubMed] [Google Scholar]
- 5.Parr AM, Kulbatski I, Tator CH. Transplantation of adult rat spinal cord stem/progenitor cells for spinal cord injury. J Neurotrauma 2007;24:835-45. 10.1089/neu.2006.3771 [DOI] [PubMed] [Google Scholar]
- 6.Ogawa Y, Sawamoto K, Miyata T, et al. Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. J Neurosci Res 2002;69:925-33. 10.1002/jnr.10341 [DOI] [PubMed] [Google Scholar]
- 7.Okano H, Ogawa Y, Nakamura M, et al. Transplantation of neural stem cells into the spinal cord after injury. Semin Cell Dev Biol 2003;14:191-8. 10.1016/S1084-9521(03)00011-9 [DOI] [PubMed] [Google Scholar]
- 8.Hu R, Zhou J, Luo C, et al. Glial scar and neuroregeneration: histological, functional, and magnetic resonance imaging analysis in chronic spinal cord injury. J Neurosurg Spine 2010;13:169-80. 10.3171/2010.3.SPINE09190 [DOI] [PubMed] [Google Scholar]
- 9.Cheng I, Mayle RE, Cox CA, et al. Functional assessment of the acute local and distal transplantation of human neural stem cells after spinal cord injury. Spine J 2012;12:1040-4. 10.1016/j.spinee.2012.09.005 [DOI] [PubMed] [Google Scholar]
- 10.Cheng I, Githens M, Smith RL, et al. Local versus distal transplantation of human neural stem cells following chronic spinal cord injury. Spine J 2016;16:764-9. 10.1016/j.spinee.2015.12.007 [DOI] [PubMed] [Google Scholar]
- 11.Park DY, Mayle RE, Smith RL, et al. Combined Transplantation of Human Neuronal and Mesenchymal Stem Cells following Spinal Cord Injury. Global Spine J 2013;3:1-6. 10.1055/s-0033-1337118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 1995;12:1-21. 10.1089/neu.1995.12.1 [DOI] [PubMed] [Google Scholar]
- 13.Scheff SW, Saucier DA, Cain ME. A statistical method for analyzing rating scale data: the BBB locomotor score. J Neurotrauma 2002;19:1251-60. 10.1089/08977150260338038 [DOI] [PubMed] [Google Scholar]
- 14.Basso DM, Beattie MS, Bresnahan JC. Descending systems contributing to locomotor recovery after mild or moderate spinal cord injury in rats: experimental evidence and a review of literature. Restor Neurol Neurosci 2002;20:189-218. [PubMed] [Google Scholar]
- 15.Keirstead HS, Nistor G, Bernal G, et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci 2005;25:4694-705. 10.1523/JNEUROSCI.0311-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishimura S, Yasuda A, Iwai H, et al. Time-dependent changes in the microenvironment of injured spinal cord affects the therapeutic potential of neural stem cell transplantation for spinal cord injury. Mol Brain 2013;6:3. 10.1186/1756-6606-6-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumamaru H, Saiwai H, Kubota K, et al. Therapeutic activities of engrafted neural stem/precursor cells are not dormant in the chronically injured spinal cord. Stem Cells 2013;31:1535-47. 10.1002/stem.1404 [DOI] [PubMed] [Google Scholar]