Abstract
Background
Hidradenitis supppurativa (HS) is a chronic inflammatory disease of the apocrine sweat glands affecting 1–4% of the population. While surgical excision is a mainstay of therapy, lesions often recur. Biologic therapies, including tumor necrosis factor-α and IL-12/23 inhibitors are effective for mild to moderate HS. However, longitudinal studies investigating biologic therapy in conjunction with surgery are limited. The purpose of this analysis was to investigate impact of surgery and biologic therapy on HS disease activity.
Methods
Data from 68 HS patients were analyzed. Outcome measures included Hidradenitis Suppurativa Sartorius Score (HSS), Active Nodule (AN) count, Hurley stage, and probability of achieving 75% reduction in active nodule count (AN75).
Results
Mean age was 40±14years, 66% were female and 72% were African American. Mean disease duration was 10 years, and Hurley stage III disease was seen in 63% of patients. Patients who received biologics had a larger drop in HSS and AN count than those who never received biologics (p=0.002). Biologic treatment was associated with average reduction of 22 (15–29) HSS points (p<0.0001). The effect of biologics was greater in patients who also underwent surgery (p=0.013). Timing of biologics relative to surgery did not impact efficacy. Patients who received HS surgery with biologic therapy were most likely to achieve the AN75 (p=0.017).
Conclusions
In this diverse cohort of patients with severe HS, biologic therapy was associated with a more rapid decline in disease activity, with the greatest effect in patients who also underwent HS surgery.
Keywords: Hidradenitis Suppurativa, tumor necrosis factor-α inhibitor, IL12/23 inhibitor, ustekinumab, adalimumab, infliximab, Surgery
INTRODUCTION
Hidradenitis suppurativa (HS) is a chronic, recurrent, inflammatory disease of the apocrine sweat glands, characterized by recurrent abscessing inflammation1. Patients with HS develop inflammatory nodules, abscesses and sinus tracts around the apocrine glands. The prevalence of HS is estimated at 1–4% in young adults2–5. Women are affected more commonly than men (with a female to male ratio of 3:1), and the disease is more common in African Americans6.
Surgery has been a mainstay of HS management for some time, and is often used for patients with extensive Hurley stage III disease7. The best results are achieved with wide local excision8–11, but the disease often recurs, and this has led to a recent interest in the use of targeted biologic therapy in the management of HS12–14.
Several recent studies have shown efficacy of tumor necrosis factor-α (TNF-α) inhibitors in mild to moderate HS15,16 and two recent large clinical trials demonstrated efficacy of the humanized monoclonal anti-TNF-α antibody adalimumab17,18 leading to orphan drug designation for this indication. Other biologic agents that have shown promise for HS include the IL-12/23 inhibitor ustekinumab15,19,20. Clinical trials evaluating efficacy of TNF-α inhibitors in HS have not investigated combining biologic therapy as an adjunct to surgical interventions17,21. One of the reasons given for excluding these patients from clinical trials is the potential confounding variable of pain and opioid exposure. Patients with HS often have significant pain and are prescribed opioid-based medications for symptom control22–24. In chronic wounds25 and in the postoperative setting26 opioid exposure may contribute to delayed healing; however, the impact of opioids on HS disease activity has not been studied in a robust longitudinal analysis.
The purpose of this study was to investigate predictors of HS disease activity scores including surgical interventions, biologic medications, and opioids using a longitudinal and diverse cohort of patients with HS.
METHODS
Setting, Population and Cohort Selection
The Wound Etiology and Healing (WE-HEAL) study (IRB 041408, NCT 01352078) is a longitudinal prospective observational biospecimen and data repository that recruits subjects with chronic wounds and HS. All subjects gave written informed consent for longitudinal collection of their data. This analysis was conducted utilizing data from subjects with confirmed diagnosis of HS27. At the time of data lock, there were 568 patients enrolled in the WE-HEAL study, and 68 had confirmed HS.
Data management for WE-HEAL study
Data for the WE-HEAL study were abstracted from the electronic health record (EHR) and stored using REDCap28. Demographic data, baseline medical comorbidities (including diabetes, autoimmune disease, cardiovascular and renal disease, and smoking exposure), and laboratory data were abstracted at enrollment. Clinical follow-up data were collected at each visit, including disease activity scores (Hurley stage, active nodule (AN) count, modified Hidradenitis Sartorius Score (HSS)), surgical interventions and medication exposures.
Hurley Stage
The Hurley staging system was used to assess HS disease severity at baseline and each subsequent visit. In this staging system lesions with single or multiple abscesses without sinus tracts or scaring are classified as stage I; lesions with recurrent abscesses with sinus tract formation and scarring are classified as stage II; and lesions with diffuse involvement and multiple interconnected sinus tracts are classified as stage III29.
Active Nodule (AN) Count
The total number of abscesses and inflammatory nodules (AN) were assessed at baseline and each visit. AN count is associated with patient-reported quality of life scores and pain level30,31. The probability of achieving AN count reduction of 75% (AN75) is a validated outcome measure used in clinical trials of HS that was assessed in this study.
Modified Hidradenitis Sartorius Score
The modified Hidradenitis Sartorius Score (HSS) is a validated measure of HS activity and was used to assess disease activity at baseline and each visit17,32,33. A score of 3 points was assigned for each anatomic region involved; 1 point per region was given for presence of nodules and 6 points for fistulae; the longest distance between lesions or size of the lesion was scored categorically (<5cm (1 point), 5–10cm (3 points) and >10cm (9 points)); and whether lesions were separated by normal skin (0 points) or not (9 points). Regional scores are summed to achieve a total modified HSS.
Verbal Pain Score Evaluation
Numerical pain score based on a verbal scale of pain (0–10) was collected at each visit, prior to removing dressings. This is a valid and reproducible score that is in routine clinical use34. Baseline pain score was used as a covariate in the static multivariate models and time-to-event analyses. Pain was a time-varying covariate in the fixed-effects mixed models.
Medication exposures
Data on all immunosuppressive and opioid medication were abstracted after each visit. Medication reconciliation is a metric which is audited through the EHR and thus completed at every clinical interaction. Any discrepancies were resolved by adjudication by two investigators (SM and VKS).
Decisions on treatment, including biologic therapy, were driven entirely by clinical care. Biologic agents used in this cohort including anti-TNF-α agents (infliximab dosed IV with typical loading dose of 5mg/kg at weeks 0,2, and 6 and 5–10mg/kg every 6 weeks depending on clinical response or adalimumab dosed at 40mg subcutaneously weekly) and the IL12/23 inhibitor ustekinumab (dosed based on weight either 45mg or 90mg monthly subcutaneously). The decision to select ustekinumab over a TNF-inhibitor was typically due to presence of one or more autoantibodies, history of drug reaction from TNF-α inhibitor, or coexistent psoriasis which was felt to be more likely to respond to IL-12/23 blockade. Due to the relatively small sample size, for the purposes of analysis patients who received any biologic therapy were compared to those who did not.
Daily morphine-equivalent exposure was determined by calculating the quantity of opioid medication ordered by the milligram strength per dosage unit, then multiplying by the published opioid-specific morphine-equivalent conversion factor25,35–37. Analysis was conducted using the opioid-equivalent dose per 24 hours.
Statistical Analysis
All analysis was performed using SAS version 9.3 (Cary, NC). SAS Mixed and GLM procedures were used for multivariate analysis with p<0.05 considered significant. The analysis focused on the association of biologic medications with HS disease activity scores (HSS, AN count and Hurley stage) to investigate whether biologic therapies reduce disease activity, whether this is dependent on whether the patient also undergoes HS surgery, and timing of biologics relative to surgery. Binary variables that were present at any point during a time period were coded as positive for that period. Time-varying outcomes included HSS, AN count and Hurley stage. Predictors included time-period, time-varying age, smoking, and body mass index (BMI). The primary predictors of interest were the time varying binary indicators for HS surgery, biologic exposure, and opiates.
In mixed model regression, time points are nested within patients, in order to account for within-subject autocorrelation. In order to reduce model complexity, time was coded into quarters (3-month time periods) during the first year of follow up. During the second and subsequent years there were fewer follow up observations; therefore, year 2 of follow-up was coded as a single period and years 3 and later as a single period. This resulted in seven time periods: baseline, Q1, Q2, Q3, Q4, Yr2, and Yr3+. Not every subject had data for every time period but all available data were used in each analysis.
Analysis models
Analysis was structured around the following study questions with the three HS disease activity measures as dependent variables in the mixed regression models.
Does the slope of HS activity differ in patients who ever take biologics vs. never take biologics and does this persist after adjusting for baseline confounding patient variables, surgery and opioid exposures? We used a random effects mixed model with the predictors: time, biologic-ever, and the time × biologic-ever interaction. The primary focus of this analysis was the time × biologic-ever interaction since this investigates whether the slope of HS activity over time varies between treatment groups. To adjust for confounders we added static covariates known to show significant univariate association with ever receiving biologics, along with surgery and opioid exposures.
How strong is the effect of biologic treatment on disease activity, and does this vary with time? In this analysis, we retained any significant static predictors from the prior model and instead of using the static indicator for ever receiving biologics, we used a time-varying binary indicator for biologic treatment, so we could determine the effect of biologics during each time period. In this model, the interaction of biologics × time tells us whether the effect of biologics differs across time periods (for example, whether having biologic treatment may be associated with a larger reduction in HS activity from baseline to q3 than from baseline to Year 2).
Were there differences in HS activity between time-periods when patients did versus did not receive biologics. In this analysis, patients served as their own controls over time. Using a fixed effects mixed model, including previously significant covariates we investigated the mean within-subject improvement in HS activity for periods with versus without biologic therapy. The biologic × time interaction investigates whether this effect is stable across the follow-up period.
Did the effect of biologics differ in the patients who did versus did not receive HS surgery? To investigate whether the effect of biologic therapy depended on whether the patient also received surgery for HS, we examined the time-varying biologic exposure by surgery (ever) interaction in a random effects mixed model adjusted for baseline covariates. In this analysis a significant three-way interaction indicated that there was a difference in HSS slope over time for those who ever vs. never received biologics, which varied as a function of receiving HS surgery. Additionally, we tested whether the treatment-group × time interaction was significant comparing patients who received biologic therapy and HS surgery with those who received biologics only.
In patients who did receive HS surgery, was the effect of biologics is dependent on timing after surgery? Using a random effects mixed model adjusted for baseline covariates, we investigated only subjects who ever received surgery, and time from surgery was included as a predictor.
Time-to-event analysis for AN75
In order to account for differences in time at risk between the treatment groups, we also used time-to-event analysis for the first time point at which the patient’s AN count dropped 75% from baseline (AN75). Patients who did not reach the end-point were censored at their last visit. Kaplan-Meier analysis was used, to compare patients in four groups: no surgery with no biologics, surgery only, biologics only, surgery with biologic. Cox proportional hazard models were tested, using the same four treatment groups, adjusting for any covariates that were significant in the mixed-models.
RESULTS
Baseline demographics
There were 68 patients with HS included in this analysis. Mean (± SD) age was 40 (±14) years; 66% were female and 72% were African American (Table 1). Mean disease duration was 10 (±12) years. The majority of patients had active disease, with baseline Hurley stage III seen in 63% of the patients. At baseline the overall mean HSS score was 61 (±46). However, patients who had ever received biologics had higher baseline HSS and AN count than those who never received biologics. Patients who ever versus never received either biologics or HS surgery were more likely to also receive opioids (p=0.04 for biologics and p<0.0001 for surgery; Table 1)
Table 1. Baseline patient characteristics, stratified by ever versus never receiving biologics or surgery.
The HS cohort was representative of patients with HS in the United States with a predominantly female and African American population. The majority of patients had active disease at baseline as evidenced by Hurley stage and mHSS score at baseline.
| Patient Variable | All HS Patients (n=68) |
OPIOIDS | SURGERY | BIOLOGICS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Ever (n=39) |
Never (n=29) |
P | Ever (n=31) |
Never (n=37) |
P | Ever (n=31) |
Never (n=37) |
P | |||
|
| |||||||||||
| Mean age at baseline, years (±SD) | 40.4 (14) | 39.5 (13) | 41.6 (16) | 0.60 | 38 (13) | 41 (15) | 0.38 | 37 (12) | 42 (16) | 0.21 | |
|
| |||||||||||
| Gender, female | 45 (66%) | 23 (59%) | 22 (76%) | 0.15 | 17 (55%) | 28 (76%) | 0.07 | 18 (58%) | 27 (73%) | 0.20 | |
|
| |||||||||||
| Race | |||||||||||
| African American | 49 (72%) | 29 (74%) | 20 (69%) | 0.55 | 21 (68%) | 28 (76%) | 0.48 | 20 (65%) | 29 (78%) | 0.31 | |
| Caucasian | 18 (26%) | 9 (23%) | 9 (31.0%) | 9 (29%) | 9 (24%) | 10 (32%) | 8 (22%) | ||||
| Asian | 1 (1%) | 1 (3%) | 0 | 1 (3%) | 0 | 1 (3%) | 0 | ||||
|
| |||||||||||
| Body Mass Index (BMI, Kg/m2) | 34 (8) | 34 (9) | 35 (6) | 0.75 | 35 (9) | 33 (6) | 0.35 | 35 (8) | 34 (8) | 0.83 | |
|
| |||||||||||
| Current smoker | 15 (22%)) | 13 (33%) | 2 (7%) | 0.009 | 10 (32%) | 5 (14%) | 0.06 | 7 (23%) | 8 (22%) | 0.92 | |
|
| |||||||||||
| Diabetes Mellitus | 10 (15%) | 7 (18%) | 3 (10%) | 0.50 | 4 (13%) | 6 (16%) | 0.75 | 6 (19%) | 4 (11%) | 0.49 | |
|
| |||||||||||
| Hypertension | 17 (25%) | 13 (33%) | 4 (14%) | 0.07 | 11 (35%) | 6 (16%) | 0.07 | 7 (23%) | 10 (27%) | 0.67 | |
|
| |||||||||||
| Mean disease duration, years (±SD) | 10 (12) | 10 (11) | 11 (14) | 0.63A | 9 (9) | 12 (14) | 0.54A | 9 (7) | 12 (15) | 0.53A | |
|
| |||||||||||
| Baseline AN count | 3.4 (1.9) | 3.6 (1.6) | 3.1 (2.3) | 0.42 | 3.4 (1.7) | 3.4 (2.1) | 0.94 | 3.9 (1.8) | 2.9 (1.9) | 0.026 | |
|
| |||||||||||
| Baseline Hurley Stage | |||||||||||
| 0 | 2 (3%) | 0 | 2 (7%) | 0.004 | 0 | 2 (5%) | 0.22 | 1 (3%) | 1 (3%) | 0.24 | |
| I | 6 (9%) | 4 (10%) | 2 (7%) | 2 (6%) | 4 (11%) | 2 (6%) | 4 (11%) | ||||
| II | 13 (19%) | 2 (5%) | 11 (38%) | 4 (13%) | 9 (24%) | 3 (10%) | 10 (27%) | ||||
| III | 43 (63%) | 30 (77%) | 13 (45%) | 24 (77%) | 19 (51%) | 24 (77%) | 19 (51%) | ||||
| Post-surgical | 4 (6%) | 3 (8%) | 1 (3%) | 1 (3%) | 3 (8%) | 1 (3%) | 3 (8%) | ||||
|
| |||||||||||
| Mean baseline HSS (±SD) | 61 (46) | 71 (43) | 49 (48) | 0.02A | 66 (42) | 57 (49) | 0.42 | 75 (47) | 49 (42) | 0.014 | |
|
| |||||||||||
| Pain VAS (±SD) | 3.7 (3.5) | 4.4 (3.6) | 2.7 (3.2) | 0.07A | 4.4 (3.2) | 3.1 (3.7) | 0.07A | 4.1 (3.5) | 3.3 (3.5) | 0.46A | |
|
| |||||||||||
| Visits | 12 (9) | 15 (10) | 7 (5) | <0.0001 | 18 (10) | 6 (5) | <0.0001 | 17 (10) | 7 (6) | <0.0001 | |
(A Kruskal-Wallis test used due to skewed distributions).
Overall Disease Activity Scores Declined During Follow Up
Across all patients in the study, mean HSS scores, AN count, and Hurley stage declined over time (Figure 1). The decline from baseline in HSS was significant starting in quarter 2 (p<0.0001), and the mean AN count drop was significant by quarter 1 (p=0.007).
Figure 1.
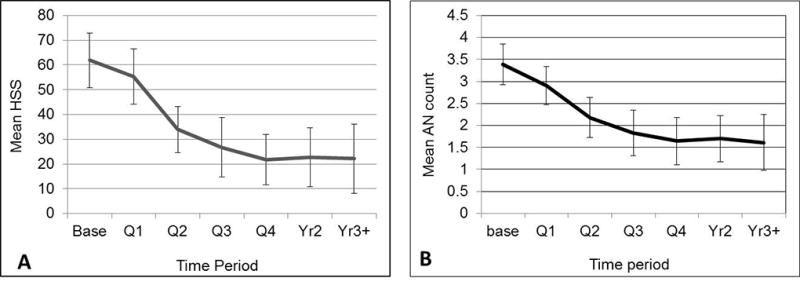
A. Mean HSS by time period. Error bars show the 95% confidence interval. The decline from baseline is significant starting at Q2 (p<0.0001). B. Mean AN count by time period. Error bars show the 95% confidence interval. Mean AN count drops significantly over time (p<0.0001). By Q1 the difference from baseline is already significant (p=0.007).
Ever receiving biologics was associated with sharper decline in HS activity than never receiving biologics
The HSS decline from baseline was larger in patients who received biologics than in those who never received biologics (p=0.06 at Q2, 0.07 at Q3, 0.04 at Q4, 0.07 at year 2, and 0.01 at year 3; Figure 2). Similarly, the decline in AN count was sharper in patients who ever versus never received biologics (p=0.002). This remained significant after adjusting for baseline disease activity. Compared with those who never received biologics, patients who received biologics demonstrated greater reduction in HSS from baseline to Q2 (p=0.047), Q3 (p=0.07), Q4 (p=0.03), Yr 2 (p=0.05) and Yr 3+ (p=0.008). This was also true for AN count (biologic-ever × time period interaction, p=0.0012), and Hurley stage (biologic-ever × time period interaction p<0.0001). When other treatments (surgery-ever and opioids-ever) were added to the mixed model for biologics-ever by HSS (still controlling for baseline disease activity) the biologics-ever × time interaction remained significant (p=0.03). Compared with patients who never received biologics, those who ever received biologics had a faster decline in HSS from baseline at Q2 (p=0.046), Q3 (p=0.07), Q4 (p=0.038), Year 2 (p=0.046) and year 3+ (p=0.005). Similar differences were seen in the AN count and Hurley stage when adjusting for surgery-ever and opioids-ever (interaction p=0.0009 and p<0.0001 respectively).
Figure 2.
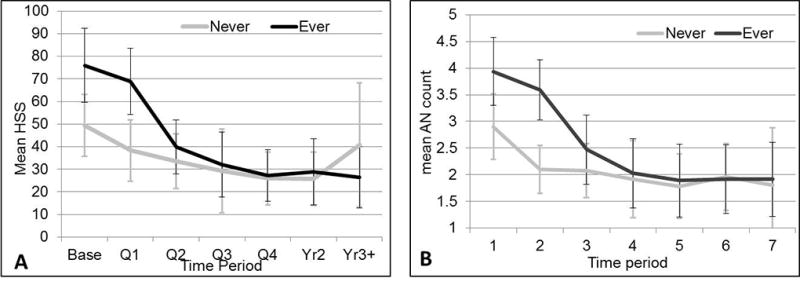
A. Mean HSS by time period, stratified by ever vs never having biologics. Error bars show the 95% confidence interval. The decline from baseline was larger in patients with biologics (p=.06 at Q2, .07 at Q3, .04 at Q4, .07 at Yr2, and .01 at Yr3+). B. Mean AN count by time, stratified by biologic-ever. Error bars show the 95% confidence interval. Mean AN count declines over time more sharply in patients who ever had biologics (p=.002).
Impact of biologic therapy does not vary based on time period during which they are received
In the mixed model using biologic-ever as a time-varying predictor, after adjusting for baseline HSS and surgery-ever, we found that biologic-ever had a significant main effect (p=0.0001) with a parameter estimate indicating that having received biologics was associated with a reduction of 35 points (95% confidence interval 13–57) in HSS score. The time-varying biologic × time interaction was not significant (p=0.18) indicating that the effect of biologics did not vary depending on the time period during which they were received. Similar differences were seen in the AN count data; when biologic-ever was used as a time-varying predictor, it had a significant effect (p=0.026) indicating that time periods with biologics present had an AN count 0.9 units lower than when biologics were absent (95% CI 0.12–1.77). The strength of the effect of biologics on AN count did not appear to vary by time period during which the patient received biologics (interaction p=0.78). A similar difference was seen in Hurley stage, with time periods when biologics were used demonstrated significantly lower Hurley stage than time-periods when biologics were not used (mean difference of 0.3 stages [0.01–0.62], p=0.045), and this effect did not differ by time period.
HS activity differs between time-periods when patients do versus do not receive biologics
After excluding patients who always or never had biologic treatment, in the fixed effects model, time varying biologic treatment remained significantly associated with time-varying HSS (p<0.0001). When compared to their own HSS scores during time periods with and without biologic treatment, patients had an average reduction of 22 HSS points (95% CI 15–29) during time periods with biologic treatment, after adjusting for baseline HSS score, and for surgery-ever. In the same analysis for the AN count, time periods when biologics were present had a mean AN count 0.8 units lower than the same patients during time periods when biologics were absent (95% CI 0.49–1.09, p<0.0001). Similar findings were seen in the Hurley stage, with biologics being associated with a significant reduction in Hurley stage (by 0.33 stages [0.11–0.55], p=0.0037).
The effect of biologics was greater in patients who did versus did not receive HS surgery
We examined the interaction of time-varying biologic treatment and surgery-ever on HSS in order to determine whether the effect of biologics differed in patients who ever versus never received surgery after adjusting for baseline HSS. This interaction was significant (p=0.013). In patients who never had surgery, the HSS score dropped on average only 2 points in time periods with versus without biologics (mean HSS 45 [95% CI 36–54] versus 43 [36–51]). In contrast in patients who ever had surgery, the HSS score dropped 27 points on average in time periods with versus without biologics (42 [29–54] versus 15 [2–29]). Using the static biologic-ever predictor, comparing those who ever received biologics without surgery and those who received both biologics with surgery the difference was significant (p=0.003). Those who received biologics with surgery exhibited faster decline in HSS scores than those who received biologics without surgery (Figure 3).
Figure 3.
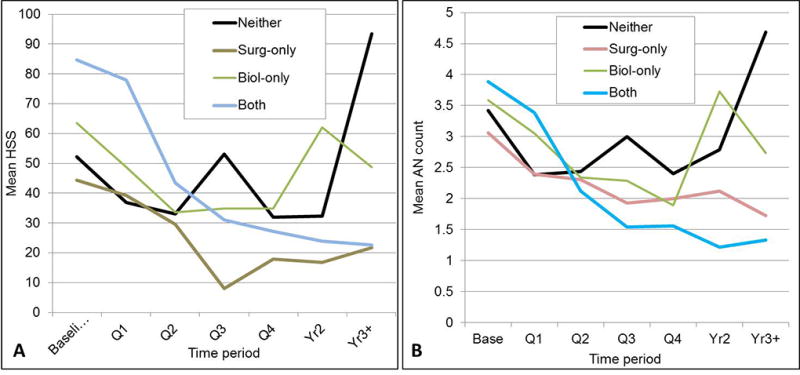
A. Mean HSS by time period, stratified by ever having biologics and/or surgery. The effect of ever having biologics on the slope of HSS score over time varied significantly, depending on whether the patient ever had surgery (p-value for Biologic × period × surgery interaction <.0001, after adjusting for baseline HSS in mixed model regression). Just comparing those who ever received biologics without ever receiving surgery (BIOL-SURG) versus those who received both, the difference was significant (p=.003): those who received both declined faster than those who received biologics only. B. Mean AN count by time period, stratified by treatment combination. The effect of ever having biologics varied significantly, depending on whether or not the patient ever had surgery (biologic-ever × time period × surgery-ever interaction p<.0001). Patients with biologics only had a rebound in AN count in Yr2 and Yr3+, while those who also had surgery continued to show decline in AN count in Yr2 and Yr3+.
The effect of biologics on AN count varied significantly, depending on whether the patient ever had HS surgery (interaction p=0.033). In patients with HS surgery, time periods with biologics present were associated with a reduction of 0.9 AN units (mean AN count 2.4 [2.2–2.7] without biologics compared to 1.5 [0.8–2.1] with biologics). In patients who never had HS surgery, time periods without biologics present were associated with a drop of 0.1 AN units (2.6 [2.2–3.0] without compared to 2.5 [2.1–2.9] with biologics). The effect of ever having biologics varied significantly, depending on whether or not the patient ever had surgery (biologic-ever × time period × surgery-ever interaction, p<0.0001). Patients with biologics-only had a rebound in AN count in Years 2 and 3+, while those who also had surgery continued to show decline in AN count in years 2 and 3+ (Figure 3B). The effect of ever having biologics on Hurley stage over time similarly varied significantly depending on whether the patient ever had HS surgery (p<0.0001).
Timing of biologics after surgery did not alter efficacy
When we added time from surgery as a predictor in the model, time-varying biologics continued to have a significant association with HSS (reducing mean HSS by 17 points [95% CI 6–28], p=0.03) but the biologic × time from surgery interaction was not significant (p=0.56) indicating that the effect of biologics did not change with recency of surgery. This was also reflected in the model for AN count, in which time-varying biologics still had a significant association with AN count (reducing mean AN count by 0.6 points [95% CI 0.1–1.2], p=0.032) but the biologic vs. time from surgery interaction was not significant indicating that the effect on AN count does not vary based on recency of surgery. Similar findings were seen in the analysis of Hurley stage.
Combination therapy with surgery and biologic treatment was associated with a higher probability of achieving AN75
The log-rank chi-square was significant for time to 75% reduction in AN count (AN75, p=0.017). Patients treated with surgery plus biologic therapies were most likely to reach an AN75 (log rank chi-square =0.01). This remained true after adjusting for baseline HSS count, AN count, age and ever having opioids (adjusted HR for both vs biologics only was 7.06 [1.16–42.92], p=0.034, in Cox regression Figure 4). After adjusting for covariates, those who received both treatments had HR 2.88 (1.02–8.13, p=0.047) for reaching AN75, compared to those who received surgery only. In other words, the combination of surgery plus biologics was significantly better than either surgery or biologics alone for achieving the AN75 outcome.
Figure 4.
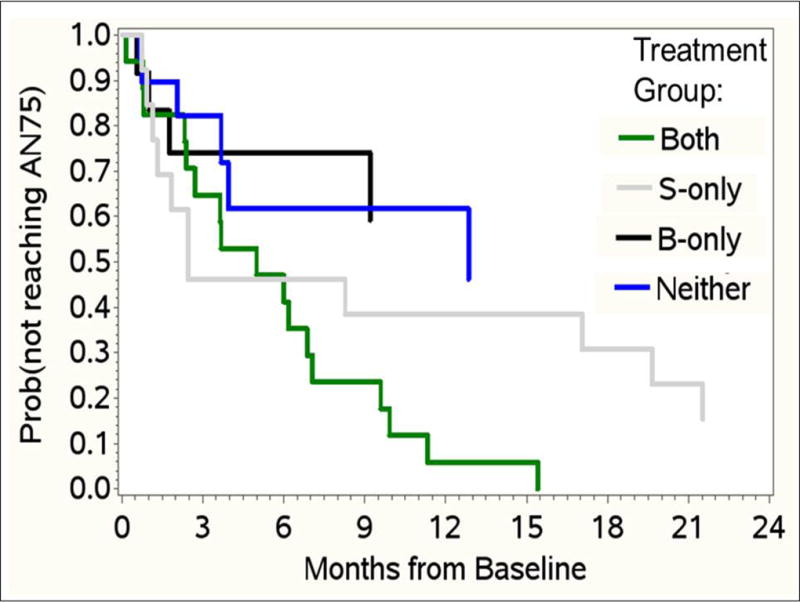
Kaplan-Meier estimates for time to AN75, stratified by treatment. In KM analysis, there was a significant treatment effect (log-rank chi-square p=.017). Patients with biologics-only were significantly less likely to have a 75% reduction in AN count from their baseline level than those who received biologics + surgery, after adjusting for baseline HSS, baseline AN count, age, and ever receiving opioids in Cox regression. HR for reaching AN75 was 7.06 (1.16–42.92, p=0.034) in patients who had biologics + surgery versus biologics only. Patients who received both treatments had adjusted HR 2.88 (1.02–8.13, p=0.047) for reaching AN75, compared with those who received surgery only. The combination of surgery + biologics was significantly better than either surgery or biologics alone, for the AN75 outcome.
DISCUSSION
One of the roadblocks limiting HS research to date is that clinical trials of immunosuppressive agents in HS typically exclude surgical patients, and tend to study patients with milder disease (of the 633 patients in the PIONEER I and II studies, approximately 70% had Hurley stage I and II disease, and patients undergoing surgery or requiring opioids for HS were excluded). Another limitation of prior of clinical trials in HS is that they study a predominantly Caucasian population (with only 18.8% of the population studied in the original adalimumab study being African American and only 14.4% of the PIONEER I and II study populations being African American). This lack of a diverse study population limits the generalizability of study findings to the US population. One of the major advantages to the WE-HEAL HS cohort is its longitudinal observational study design. This allows investigation of the relationship between surgical intervention, opioid exposures, and pain in a cohort of diverse HS patients that is more representative of the population affected in the United States (72% African American). Furthermore, the study design allows for investigation of the complex interplay of time-varying factors such as opioid exposures, smoking, and pain which play a crucial role in this disease.
The analysis presented has allowed investigation of critically important clinical outcome questions in the management of HS that are challenging to address in randomized clinical trials. Specifically, one of the most important clinical questions in HS management is whether surgery alone, or in combination with biologic therapy is the best management for HS and how best to time biologic therapy relative to surgical interventions12–14.
In this study we were able to demonstrate that patients who received biologic therapy had a more rapid decline in disease activity than those who never received biologics, and this held true after both adjusting for baseline disease activity and for other therapies including surgeries and opioid exposures. Biologic therapies were significantly associated with reduced disease activity scores regardless of the time period during which they were received. However, the effect of biologic therapies was greatest in patients who also underwent surgical intervention for their HS. The timing of biologic therapy relative to surgery did not seem to impact disease activity. Using time to event analysis, we were further able to demonstrate that patients who underwent surgical and biologic therapy had a higher probability of achieving a 75% reduction in AN count than those who received surgery or biologic therapy alone or neither.
The WE-HEAL HS observational cohort provides a unique resource for studying HS in the US population. The high prevalence and disease burden of HS in the African American population in the US means that it is crucially important to develop mechanisms to investigate therapies for this disease, but this population can be challenging to recruit to clinical trials38. The observational WE-HEAL study design has proven to be effective for investigating a diverse population of HS patients and is ideally suited for answering critically important questions in the management of this debilitating disease. While a major limitation of the study to date is the relatively small cohort size, recruitment to the WE-HEAL study is ongoing and follow-up analysis is planned.
CONCLUSION
This longitudinal observational study of a diverse US population of HS patients demonstrates that both biologic therapies and HS surgical interventions are associated with improved disease activity scores, but that the effect of biologic therapies was greatest when used as an adjunct to HS surgery.
Acknowledgments
This work was supported by award R01NR013888 from the National Institute of Nursing Research and by award number UL1 TR000075 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through the Clinical and Translational Science Awards Program (CTSA). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
The funding source had no role in the design or conduct of the study; collection, management, analysis or interpretation of the data; or preparation, review or approval of the manuscript.
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Drs. Shanmugam and Amdur had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Shanmugam,, McNish, Amdur,
Acquisition of data. Shanmugam, Mulani, McNish, Buescher, Harris
Analysis and interpretation of data. Shanmugam, McNish, Mulani, Amdur
References
- 1.Jemec GBE. Hidradenitis Suppurativa. New England Journal of Medicine. 2012;366(2):158–164. doi: 10.1056/NEJMcp1014163. [DOI] [PubMed] [Google Scholar]
- 2.Shahi V, Alikhan A, Vazquez BG, Weaver AL, Davis MD. Prevalence of Hidradenitis Suppurativa: A Population-Based Study in Olmsted County, Minnesota. Dermatology. 2014;229(2):154–158. doi: 10.1159/000363381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vazquez BG, Alikhan A, Weaver AL, Wetter DA, Davis MD. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133(1):97–103. doi: 10.1038/jid.2012.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cosmatos I, Matcho A, Weinstein R, Montgomery MO, Stang P. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2013;69(5):819. doi: 10.1016/j.jaad.2013.06.042. [DOI] [PubMed] [Google Scholar]
- 5.Esmann S, Dufour DN, Jemec GB. Questionnaire-based diagnosis of hidradenitis suppurativa: specificity, sensitivity and positive predictive value of specific diagnostic questions. Br J Dermatol. 2010;163(1):102–106. doi: 10.1111/j.1365-2133.2010.09773.x. [DOI] [PubMed] [Google Scholar]
- 6.Vlassova N, Kuhn D, Okoye GA. Hidradenitis Suppurativa Disproportionately Affects African Americans: A Single-center Retrospective Analysis. Acta Derm Venereol. 2015;95(8):990–991. doi: 10.2340/00015555-2176. [DOI] [PubMed] [Google Scholar]
- 7.Alavi A. Hidradenitis suppurativa: Demystifying a chronic and debilitating disease. Journal of the American Academy of Dermatology. 73(5):S1–S2. doi: 10.1016/j.jaad.2015.08.061. [DOI] [PubMed] [Google Scholar]
- 8.Kohorst JJ, Baum CL, Otley CC, et al. Surgical Management of Hidradenitis Suppurativa: Outcomes of 590 Consecutive Patients. Dermatol Surg. 2016;42(9):1030–1040. doi: 10.1097/DSS.0000000000000806. [DOI] [PubMed] [Google Scholar]
- 9.Mehdizadeh A, Hazen PG, Bechara FG, et al. Recurrence of hidradenitis suppurativa after surgical management: A systematic review and meta-analysis. J Am Acad Dermatol. 2015;73(5 Suppl 1):S70–77. doi: 10.1016/j.jaad.2015.07.044. [DOI] [PubMed] [Google Scholar]
- 10.Danby FW, Hazen PG, Boer J. New and traditional surgical approaches to hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(5 Suppl 1):S62–65. doi: 10.1016/j.jaad.2015.07.043. [DOI] [PubMed] [Google Scholar]
- 11.Posch C, Monshi B, Quint T, Vujic I, Lilgenau N, Rappersberger K. The role of wide local excision for the treatment of severe hidradenitis suppurativa (Hurley grade III): Retrospective analysis of 74 patients. J Am Acad Dermatol. 2017 doi: 10.1016/j.jaad.2017.01.055. [DOI] [PubMed] [Google Scholar]
- 12.DeFazio MV, Economides JM, King KS, et al. Outcomes After Combined Radical Resection and Targeted Biologic Therapy for the Management of Recalcitrant Hidradenitis Suppurativa. Annals of plastic surgery. 2015 doi: 10.1097/SAP.0000000000000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falola RA, DeFazio MV, Anghel EL, Mitnick CD, Attinger CE, Evans KK. What Heals Hidradenitis Suppurativa: Surgery, Immunosuppression, or Both? Plast Reconstr Surg. 2016;138(3 Suppl):219S–229S. doi: 10.1097/PRS.0000000000002671. [DOI] [PubMed] [Google Scholar]
- 14.Alavi A. Discussion: What Heals Hidradenitis Suppurativa: Surgery, Immunosuppression, or Both? Plast Reconstr Surg. 2016;138(3 Suppl):230S–231S. doi: 10.1097/PRS.0000000000002711. [DOI] [PubMed] [Google Scholar]
- 15.Lee RA, Eisen DB. Treatment of hidradenitis suppurativa with biologic medications. J Am Acad Dermatol. 2015;73(5 Suppl 1):S82–88. doi: 10.1016/j.jaad.2015.07.053. [DOI] [PubMed] [Google Scholar]
- 16.Grant A, Gonzalez T, Montgomery MO, Cardenas V, Kerdel FA. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double-blind, placebo-controlled crossover trial. J Am Acad Dermatol. 2010;62(2):205–217. doi: 10.1016/j.jaad.2009.06.050. [DOI] [PubMed] [Google Scholar]
- 17.Kimball AB, Okun MM, Williams DA, et al. Two Phase 3 Trials of Adalimumab for Hidradenitis Suppurativa. N Engl J Med. 2016;375(5):422–434. doi: 10.1056/NEJMoa1504370. [DOI] [PubMed] [Google Scholar]
- 18.Ingram JR. Interventions for Hidradenitis Suppurativa: Updated Summary of an Original Cochrane Review. JAMA dermatology. 2017 doi: 10.1001/jamadermatol.2017.0432. [DOI] [PubMed] [Google Scholar]
- 19.Gulliver WP, Jemec GB, Baker KA. Experience with ustekinumab for the treatment of moderate to severe hidradenitis suppurativa. Journal of the European Academy of Dermatology and Venereology : JEADV. 2012;26(7):911–914. doi: 10.1111/j.1468-3083.2011.04123.x. [DOI] [PubMed] [Google Scholar]
- 20.Sharon VR, Garcia MS, Bagheri S, et al. Management of recalcitrant hidradenitis suppurativa with ustekinumab. Acta Derm Venereol. 2012;92(3):320–321. doi: 10.2340/00015555-1229. [DOI] [PubMed] [Google Scholar]
- 21.Kimball AB, Kerdel F, Adams D, et al. Adalimumab for the treatment of moderate to severe Hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med. 2012;157(12):846–855. doi: 10.7326/0003-4819-157-12-201212180-00004. [DOI] [PubMed] [Google Scholar]
- 22.Ring HC, Sorensen H, Miller IM, List EK, Saunte DM, Jemec GB. Pain in Hidradenitis Suppurativa: A Pilot Study. Acta Derm Venereol. 2016;96(4):554–556. doi: 10.2340/00015555-2308. [DOI] [PubMed] [Google Scholar]
- 23.Enamandram M, Rathmell JP, Kimball AB. Chronic pain management in dermatology: pharmacotherapy and therapeutic monitoring with opioid analgesia. J Am Acad Dermatol. 2015;73(4):575–582. doi: 10.1016/j.jaad.2014.11.038. quiz 583-574. [DOI] [PubMed] [Google Scholar]
- 24.Smith HS, Chao JD, Teitelbaum J. Painful hidradenitis suppurativa. Clin J Pain. 2010;26(5):435–444. doi: 10.1097/AJP.0b013e3181ceb80c. [DOI] [PubMed] [Google Scholar]
- 25.Shanmugam VK, Couch KS, McNish S, Amdur RL. Relationship between Opioid Treatment and Rate of Healing in Chronic Wounds. Wound Repair Regen. 2016 doi: 10.1111/wrr.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shanmugam VK, Fernandez S, Evans KK, et al. Postoperative wound dehiscence: predictors and associations. Wound Repair and Regeneration. 2015 doi: 10.1111/wrr.12268. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zouboulis CC, del Marmol V, Mrowietz U, Prens EP, Tzellos T, Jemec GBE. Hidradenitis Suppurativa/Acne Inversa: Criteria for Diagnosis, Severity Assessment, Classification and Disease Evaluation. Dermatology. 2015;231(2):184–190. doi: 10.1159/000431175. [DOI] [PubMed] [Google Scholar]
- 28.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hurley H. Hidradenitis Suppurativa. In: Roenigk RKRHJ, editor. Dermatologic Surgery: Principles and Practice. 2. New York: Marcel Dekker; 1996. pp. 623–645. [Google Scholar]
- 30.Kimball AB, Sobell JM, Zouboulis CC, et al. HiSCR (Hidradenitis Suppurativa Clinical Response): a novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo-controlled portion of a phase 2 adalimumab study. Journal of the European Academy of Dermatology and Venereology : JEADV. 2016;30(6):989–994. doi: 10.1111/jdv.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimball AB, Jemec GB, Yang M, et al. Assessing the validity, responsiveness and meaningfulness of the Hidradenitis Suppurativa Clinical Response (HiSCR) as the clinical endpoint for hidradenitis suppurativa treatment. Br J Dermatol. 2014;171(6):1434–1442. doi: 10.1111/bjd.13270. [DOI] [PubMed] [Google Scholar]
- 32.Sartorius K, Emtestam L, Jemec GBE, Lapins J. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. British Journal of Dermatology. 2009;161(4):831–839. doi: 10.1111/j.1365-2133.2009.09198.x. [DOI] [PubMed] [Google Scholar]
- 33.Sartorius K, Killasli H, Heilborn J, Jemec GB, Lapins J, Emtestam L. Interobserver variability of clinical scores in hidradenitis suppurativa is low. Br J Dermatol. 2010;162(6):1261–1268. doi: 10.1111/j.1365-2133.2010.09715.x. [DOI] [PubMed] [Google Scholar]
- 34.Gulur P, Soldinger SM, Acquadro MA. Concepts in Pain Management. Clinics in podiatric medicine and surgery. 2007;24(2):333–351. doi: 10.1016/j.cpm.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Boudreau D, Von Korff M, Rutter CM, et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiology and Drug Safety. 2009;18(12):1166–1175. doi: 10.1002/pds.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raebel MA, Newcomer SR, Reifler LM, et al. CHronic use of opioid medications before and after bariatric surgery. JAMA. 2013;310(13):1369–1376. doi: 10.1001/jama.2013.278344. [DOI] [PubMed] [Google Scholar]
- 37.Korff MV, Saunders K, Thomas Ray G, et al. De Facto Long-term Opioid Therapy for Noncancer Pain. The Clinical Journal of Pain. 2008;24(6):521–527. doi: 10.1097/AJP.0b013e318169d03b. 510.1097/AJP.1090b1013e318169d318103b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Refaie WB, Vickers SM, Zhong W, Parsons H, Rothenberger D, Habermann EB. Cancer trials versus the real world in the United States. Ann Surg. 2011;254(3):438–442. doi: 10.1097/SLA.0b013e31822a7047. discussion 442-433. [DOI] [PubMed] [Google Scholar]


