Abstract
Mu-opioid agonists are clinically effective analgesics, but also produce undesirable effects such as sedation and abuse potential that limit their clinical utility. Glutamatergic systems also modulate nociception, and N-Methyl D-Aspartate (NMDA) receptor antagonists have been proposed as one useful adjunct to enhance the therapeutic effects and/or attenuate undesirable effects of mu-opioid agonists. Whether NMDA antagonists enhance the antiallodynic effects of mu agonists in preclinical models of thermal hypersensitivity (i.e. capsaicin-induced thermal allodynia) are unknown. The present study determined the behavioral effects of racemic ketamine, (+)-MK-801, (–)-nalbuphine, and (–)-oxycodone alone and in fixed-proportion mixtures in assays of capsaicin-induced thermal allodynia and schedule-controlled responding in rhesus monkeys. Ketamine, nalbuphine and oxycodone produced dose-dependent antiallodynia. MK-801 was inactive up to doses that produced undesirable effects. Ketamine, but not MK-801, enhanced the potency of mu agonists to decrease rates of operant responding. Ketamine and nalbuphine interactions were additive in both procedures. Ketamine and oxycodone interactions were additive or sub-additive depending upon the mixture. Furthermore, oxycodone and MK-801 interactions were sub-additive on anti-allodynia and additive on rate suppression. These results do not support the broad clinical utility of NMDA receptor antagonists as adjuncts to mu-opioid agonists for thermal allodynic pain states.
Keywords: Mu-opiod agonists, NMDA antagonists, capsaicin, thermal allodynia, response rate, monkey
INTRODUCTION
Pain states pose a major public health challenge in the United States and around the world; one recent estimate suggest that over one-third of Americans reported pain symptoms within the past three months (Nahin, 2015). Mu-opioid agonists, such as hydrocodone and oxycodone, are increasingly being prescribed for pain management and from 2000 to 2010 there was a 4-fold increase in opioid prescriptions (Comer et al., 2013). However, mu-opioid agonists are limited in their clinical utility due to undesirable effects such as sedation and abuse liability. One drug development approach may be to combine mu agonists with adjuncts targeting other receptor systems to enhance the therapeutic effects (e.g. antinociception) and/or attenuate undesirable effects (e.g. sedation) (Dietis et al., 2009).
One receptor system that might be a biological target of interest is the glutamatergic system. Three lines of evidence support the evaluation of N-Methyl-D-Aspartate (NMDA) receptor antagonists as adjunctions to mu-opioid agonists. First, anatomical studies have demonstrated the presence of NMDA receptors at both the spinal level within the dorsal horn and supraspinal level in nociceptive pathways (Rodriguez-Munoz et al., 2012; Bourbia et al., 2014). Second, NMDA receptor antagonists, such as ketamine and dizocilpine (MK-801), produce antinociception in some, but not all, preclinical models of pain utilizing mice (Malec et al., 2008), rats (Hillhouse and Negus, 2016), and monkeys (France et al., 1989; Allen and Dykstra, 2001; Banks et al., 2010a) as research subjects. Lastly, preclinical studies in rodents have suggested these NMDA receptor antagonists may also enhance the antiallodynic and antihyperalgesic effects mu agonists in rodents depending upon the noxious stimulus (Holtman Jr et al., 2008; Pascual et al., 2010). Furthermore, these preclinical studies are supported by some clinical evidence suggesting that although ketamine has undesirable effects, it also may serve as a useful adjunct to mu agonists under certain clinical conditions (for review and recent meta-analysis, see (McGuinness et al., 2011; Lee and Lee, 2016)). Overall, both preclinical and clinical studies support the further consideration of NMDA antagonists as adjuncts to mu agonists.
The goal of the present study was to determine whether mu-opioid agonist efficacy was a determinant of opioid/NMDA interactions in male rhesus macaques using previously described procedures for opioid interaction assessment (Stevenson et al., 2003; Banks et al., 2010a). Antiallodynic interactions were assessed using an assay of capsaicin-induced thermal allodynia for two main reasons. First, ketamine may have anti-inflammatory properties (De Kock et al., 2013; Wang et al., 2013), attenuates capsaicin-induced allodynia in humans (Park et al., 1995) and monkeys (Butelman et al., 2003), and we have previously shown that ketamine alone does not produce antinociception in monkeys using a warm water tail-withdrawal procedure (Banks et al., 2010a). Thus, one potential reason for the lack of a synergistic interaction between ketamine and fentanyl in our previous monkey study (Banks et al., 2010a) could be the preclinical nociception procedure. For example, delta-opioid agonist and mu agonist antinociceptive interactions were found to be synergistic using a strict thermal noxious stimulus (i.e. warm water tail-withdrawal), but additive under thermal allodynia (i.e. capsaicin-induced thermal allodynia) conditions (Stevenson et al., 2003; Banks et al., 2010a; Negus et al., 2012). Second, opioid/NMDA antagonist interactions have not been previously assessed in preclinical allodynia models using nonhuman primates as research subjects and under experimental conditions using fixed-proportion mixtures and dose-addition analysis. Drug interactions were also evaluated in an assay of schedule-controlled responding for the following two reasons. First, nalbuphine, oxycodone, ketamine, and MK-801 alone produced dose-dependent effects in this procedure, and data from this procedure could be used to quantify the relative potencies of fixed-proportions in drug mixtures if one of the drugs (e.g. MK-801) was inactive in the assay of capsaicin-induced thermal allodynia. Second, drug or drug mixture effects on schedule-controlled responding provide one dependent measure of behavioral depression that may confound measures of antiallodynia in pain-stimulated behaviors (i.e. tail withdrawal). Therefore, potency comparisons of drug or drug mixture effects in assays of allodynia and schedule-controlled responding may provide an experimental index of therapeutic effect selectivity. Based on the preclinical literature, we hypothesized that NMDA antagonists would selectively enhance mu agonist-induced antiallodynia vs. mu agonist-induced rate suppression.
METHODS
Subjects
A total of 7 adult male rhesus macaques (Macaca mulatta) of either Indian or Chinese origin and weighing between 10–14 kg served as subjects. Three monkeys were used in studies of schedule-controlled responding, and 4 monkeys were used in studies of capsaicin-induced thermal allodynia. All animals had prior experimental histories consisting of opioids, cocaine, and NMDA antagonist exposure. The diet consisted of laboratory monkey chow (#5049, Purina, Framingham, MA), and was supplemented daily with fresh fruits and/or nuts. Monkeys were individually housed with free access to water under a 12-h light/12-h dark cycle (lights on from 06:00 until 18:00 h). The facility was licensed by the United States Department of Agriculture and accredited by AAALAC International. Both research and enrichment protocols were approved by the Institutional Animal Care and Use Committee and in accordance with the 8th edition of the Guide for the Care and Use of Laboratory Animals (Council, 2011). Environmental enrichment included: music, movies, puzzle feeders, and chew toys. Furthermore, monkeys were afforded opportunities to interact socially using olfactory and auditory cues; mirrors provided visual interaction.
Behavioral Procedures
Assay of Capsaicin-Induced Thermal Allodynia
Monkeys were seated in acrylic restraint chairs as described previously (Banks et al., 2010b). The monkey’s tail was shaved at least once a week 10–12 cm from the tip upward, and baseline tail-withdrawal latencies were measured from water heated to 38, 42, 46, and 50 °C. The maximal latency was 20 s, and if the monkey had not withdrawn its tail within 20 s, the experimenter removed the tail, and a latency of 20 s was assigned. Using this procedure, temperature-effect functions were determined in each monkey at the beginning of the behavioral session, and the highest temperature that failed to elicit a tail withdrawal was determined (i.e., the highest temperature to produce a tail-withdrawal latency of 20 s). Water heated to this temperature then served as the thermal stimulus for subsequent allodynia studies during that session. Allodynia was elicited by topical capsaicin application (0.3 mL of either 1.22M (n=2) or 2.44M (n=2) capsaicin) as described previously (Butelman et al., 2004; Banks et al., 2010b). After baseline tail-withdrawal latency determinations, the subject’s tail was wiped with an alcohol pad and a topical capsaicin patch was prepared as described below (see Drugs); the patch was applied to a region ~ 5 cm from the bottom of the tail for 5 min. After 5 min, the patch was removed and tail-withdrawal latencies were redetermined using the thermal stimulus identified from the baseline temperature-effect function. Initially, nalbuphine (0.032–0.32 mg/kg), oxycodone (0.01–0.32 mg/kg), ketamine (0.32–1.8 mg/kg), and MK-801 (0.0032–0.056 mg/kg) were tested alone and each dose was tested once. A single drug or drug mixture dose was administered 5 min before topical capsaicin administration and the time course of drug or drug mixture effects on tail-withdrawal latencies was determined over the course of 60 min in 15-min intervals starting 30 min post-drug or drug mixture administration. Subsequently three mixtures (1:0.33, 1:1, and 1:3 mu agonist/NMDA antagonist) of nalbuphine or oxycodone in combination with ketamine or oxycodone in combination with MK-801 were examined such that the intermediate proportion was 1:1 mu agonist/NMDA antagonist, and 3-fold lower and higher proportions were also determined. The fixed proportions for nalbuphine/ketamine and oxycodone/ketamine were based on the relative potencies of these compounds in the assay of capsaicin-induced thermal allodynia because all compounds were behaviorally active. However, fixed proportions of oxycodone/MK-801 were based on the relative potencies of these compounds in the assay of schedule-controlled responding because MK-801 did not produce > 50% maximum possible effect (MPE) in all monkeys up to doses that produced undesirable effects. Testing occurred twice weekly on Tuesdays and Fridays.
Assay of Schedule-Controlled Responding
Experiments were conducted in each monkey’s housing chamber which also served as the experimental chamber as previously described (Banks et al., 2010a). A custom-fabricated operant response panel and a food pellet dispenser (Med Associates, ENV-203-1000, St. Albans, VT) were attached to the front of the housing chamber. Panels were operated under a MED-PC interface and programmed with an IBM computer using MEDSTATE Notation (MED Associates). All behavioral training sessions were comprised of five 30-min cycles for a total session duration of 150 min. Two components were incorporated into each cycle. The first component was a 25-min time-out period during which responding was recorded, but had no scheduled consequences. The second component was a 5-min response period during which the right key was illuminated red, and subjects could respond under a fixed-ratio 30 (FR30) schedule of food pellet presentation. The response component terminated immediately and lights were extinguished if a subject earned the maximum of 10 pellets prior to completion of the 5-min period. All monkeys were trained until rates of responding were ≥ 1.0 response/s during all 5 cycles for 7 consecutive days (data not shown).
Behavioral sessions were conducted 5 days per week. Test sessions were usually conducted on Tuesdays and Fridays, and training sessions were conducted on Mondays, Wednesdays, and Thursdays. Subjects were eligible for participation in test sessions if rates of operant responding were ≥ 1.0 response/s on training days that preceded test days. On test days, test compounds were administered intramuscular (i.m.) using a cumulative dosing procedure, in which doses of the test drug or drug mixture were administered at the beginning of the 25-min time-out period, and each dose increased the total cumulative dose by one-fourth or one-half log units in 30-min intervals.
Initially, dose-effect functions were determined for nalbuphine (0.032–1.8 mg/kg), oxycodone (0.01– 1.0 mg/kg), ketamine (0.1–3.2 mg/kg) or MK-801 (0.0032–0.032 mg/kg) alone, and each drug was tested twice. Subsequently, three mixtures of ketamine in combination with nalbuphine or oxycodone were examined. In addition, three mixtures of MK-801 and oxycodone were also examined. All drug mixtures were studied across a range of three fixed-proportions (1:0.33, 1:1, and 1:3 mu agonist/NMDA antagonist) such that the intermediate proportion was 1:1 mu agonist/NMDA antagonist, and 3-fold lower and higher proportions were also determined. Each mixture was tested once, and mixtures were evaluated twice a week. All drugs and drug mixtures were tested up to doses that decreased responding >50% of the preceding training day’s response rate.
In addition, drug interactions can also be influence by their relative time courses. The relative time courses of ED80 nalbuphine, oxycodone, ketamine, and MK-801 doses were compared in the assay of schedule-controlled responding. Either saline, or a single nalbuphine (1.65 mg/kg), oxycodone (0.37 mg/kg), ketamine (1.41 mg/kg), or MK-801 (0.039 mg/kg) dose was administered, and 5 min response periods were initiated 10, 30, 100, and 300 min after drug administration.
Drugs
(–)-Oxycodone HCl was provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD). Racemic ketamine HCl was purchased from a commercial vendor as KetaVed© (Vedco, St. Joseph, MO). (–)-Nalbuphine HCl was provided by Dr. Kenner Rice (Drug Design and Synthesis Section, National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism, Bethesda, MD). (+)-MK-801 hydrogen maleate was purchased from a commercial vendor (Sigma-Aldrich, St. Louis, MO). NMDA antagonists, mu-opioid agonists, and combination fixed proportions were dissolved in sterile water. Capsaicin (M2028; Sigma-Aldrich) was dissolved in a mixture of 70% ethanol (Pharmaco-AAPER, Brookfield, CT) and 30% sterile water no more than 30 minutes before use. Dissolved capsaicin was applied transdermally via an adhesive bandage measuring 2.5 × 8.3 cm (Band-Aid, Johnson and Johnson, New Brunswick, NJ). All drug doses were expressed as the salt forms listed above and administered i.m. into the thigh.
Data Analysis
For the assay of capsaicin-induced thermal allodynia, raw tail-withdrawal latencies were converted to a percent maximum possible effect (%MPE). %MPE was defined as {(Test latency – Control latency) ÷ (20 – Control latency)*100} where “test latency” was the average latency over the 60-min test session and “control latency” was the average latency over a 60-min control session during which vehicle was administered. For the assay of schedule-controlled responding, raw rates of operant responding from each test cycle were converted to a percent control rate using the average response rate from all 5 cycles from the previous training day in that monkey.
The effective dose (ED50) that produced 50%MPE or 50% decrease in control rate of responding was determined for each mu agonist alone and in combination with either ketamine or MK-801 in each monkey for both assays. ED50 values were determined by interpolation when only two data points were available (one below and one above the 50% effect) or by linear regression when at least 3 data points were available on the linear portion of the dose-effect function. Individual ED50 values were subsequently averaged to yield mean ED50 values and 95% confidence limits. In addition, potency ratios were calculated for each individual subject by dividing the control ED50 value by the test ED50 value. These potency ratios were then averaged to yield group mean potency ratios and 95% confidence limits. Potency ratios were considered statistically significant if the 95% confidence limits of the group mean potency ratio did not include 1.
To evaluate drug interactions within an assay, both graphical and statistical approaches to dose-addition analysis were utilized as described previously (Stevenson et al., 2003; Banks et al., 2010a). Graphically, data for each drug and drug mixture were plotted as isobolograms at the 50% effect level. An isobologram plotted one drug dose ± SEM in a mixture as a function of the other drug dose ± SEM in a mixture at the overall mixture dose that produced 50% effect. Statistical evaluation of drug interactions was accomplished by comparing the experimentally determined ED50 value for each mixture (Zmix) with the predicted additivity ED50 value (Zadd) as previously described (Tallarida, 2000, 2016). Zadd values were calculated for each individual monkey using the equation: , where A was the mu agonist alone ED50 value, B was the NMDA antagonist alone ED50 value, and ƒ was a fractional multiplier of A in the computation of the additive total dose. The experiments described in this study tested mixtures that yielded values of ƒ=0.25, ƒ=0.5, and ƒ=0.75, were ƒ is related to the proportion of the mu agonist in a mixture per the equation ρA=ƒ/Zadd. When mixtures were studied in the assay of capsaicin-induced thermal allodynia, where MK-801 was inactive, the additivity hypothesis predicts the inactive drug should not contribute to the mixture effect. Thus, the equation for Zadd = A/ρA. Zmix was calculated for each monkey as the total drug dose that decreased rates of responding to 50% of control or produced 50% MPE. Group mean Zmix and Zadd values were significantly different if the 95% confidence intervals did not overlap.
RESULTS
Mu-opioid agonist and ketamine interactions
Assay of capsaicin-induced thermal allodynia
The highest thermal stimulus that failed to elicit a tail-withdrawal response before capsaicin treatment was 42°C in two monkeys and 46°C in the other two monkeys throughout the study. Transdermal capsaicin application produced allodynia as indicated by reduced mean ± SEM tail withdrawal latencies at these temperatures to 2.5 ± 0.9 s, 2.0 ± 1.3 s, 2.5 ± 1.9 s, and 1.5 ± 1.0 s at 15, 30, 45, and 60 min after capsaicin treatment, respectively. Nalbuphine, oxycodone, and ketamine produced dose-dependent antiallodynia (Figure 1A). The ED50 values and 95% confidence limits for each drug alone are shown in Tables 1 and 2. Based on these ED50 values, three mixtures of nalbuphine + ketamine (1:3.3, 1:10, and 1:33 nalbuphine/ketamine) and oxycodone + ketamine (1:3.6, 1:10.7, and 1:32.1 oxycodone/ketamine) were examined. The dose ranges examined for each nalbuphine + ketamine mixture were 0.01–0.1 mg/kg nalbuphine (1:3.3), 0.01–0.1 mg/kg nalbuphine (1:10), and 0.01–0.056 mg/kg nalbuphine (1:33). The dose ranges examined for each oxycodone + ketamine mixture were 0.01–0.1 mg/kg oxycodone (1:3.6), 0.01–0.056 mg/kg oxycodone (1:10.7), and 0.0032–0.056 mg/kg oxycodone (1:32.1). Larger doses were not examined due to the emergence of undesirable effects (e.g. muscle tone loss) that impaired the monkey’s ability to maintain a sufficiently sternal posture in the chair. Tables 1 and 2 also show the ED50 values for each drug in each mixture, and Table 3 shows the predicted Zadd and experimentally determined Zmix values for nalbuphine/ketamine and oxycodone/ketamine mixtures. The dose-effect functions for nalbuphine/ketamine and oxycodone/ketamine mixtures are shown in Figure panels 2A and 3A, respectively. Isobolograms for both drug mixtures are shown in Figure panels 2C and 3C. Combining ketamine with either nalbuphine or oxycodone did not significantly alter the potency of either mu agonist to produce antiallodynia; however, ED50 values could only be determined in 2 out of 3 monkeys with the 1:10 and 1:33 nalbuphine/ketamine mixtures and the 1:32.1 oxycodone/ketamine mixture. For nalbuphine and ketamine mixtures, the 1:3.3 and 1:10 mixtures produced additive effects. In the two monkeys in which an ED50 value could be determined with the 1:33 nalbuphine/ketamine mixture, the effects were sub-additive. All oxycodone and ketamine mixtures produced antiallodynia effects consistent with additivity.
Figure 1.
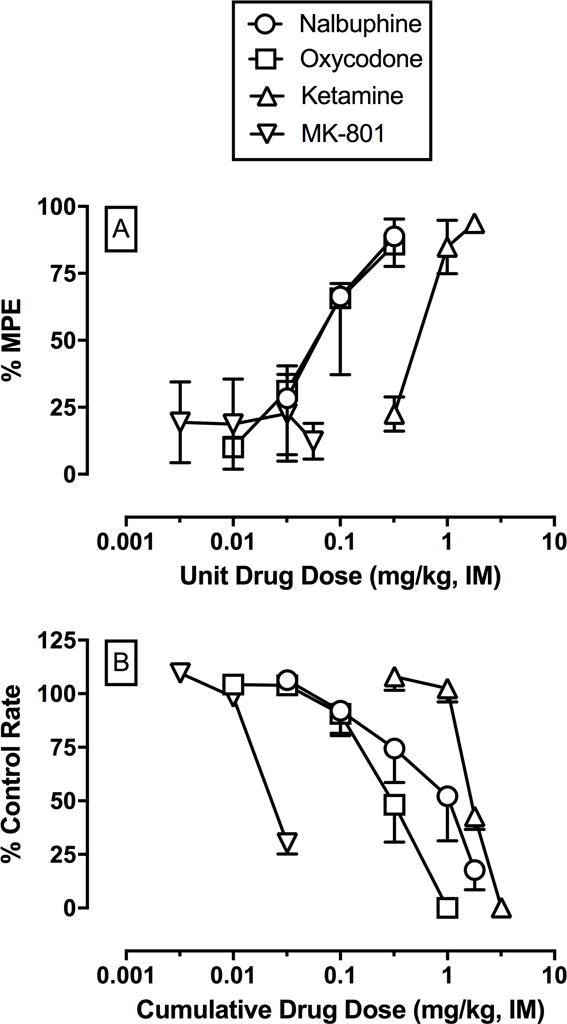
Potency of nalbuphine, oxycodone, ketamine, and MK-801 to produce anti-allodynia in an assay of capsaicin-induced thermal allodynia (Panel A; n=3–4) and decrease rates of responding in an assay of schedule-controlled responding (Panel B; n=3) in rhesus monkeys. Upper horizontal axis: unit intramuscular (i.m.) drug dose in mg/kg (log scale). Upper vertical axis: percent maximum possible effect. Lower horizontal axis: cumulative intramuscular (i.m.) drug dose in mg/kg (log scale). Lower vertical axis: percent control rate of responding. Each point shows mean ± SEM for 3–4 monkeys.
Table 1.
ED50 values (95% confidence limits) for nalbuphine, oxycodone, and ketamine alone or in combination in assays of capsaicin-induced thermal allodynia and scheduled-controlled responding in rhesus monkeys. Numbers in parentheses denote the number of subjects contributing to the ED50 value if less than the total number of monkeys tested (n=3) and the > symbol denotes a drug mixture for which an ED50 value could not be determined in all subjects tested.
| Drug or drug mixture | Nalbuphine ED50 (95% CL) |
Potency Ratio (95% CL) |
Ketamine ED50 (95% CL) |
|---|---|---|---|
| Capsaicin-induced thermal allodynia | |||
| Nalbuphine Alone | 0.05 (0, 0.36) | – | |
| Ketamine Alone | – | 0.53 (0.31, 0.91) | |
| 1:3.3 Nalbuphine/Ketamine | 0.03 (0.02, 0.04) | 1.38 (−2.91, 5.66) | 0.11 (0.08, 0.15) |
| 1:10 Nalbuphine/Ketamine (n=2) | > 0.06 (0.03, 0.1) | > 0.65 (0.1, 4.15) | |
| 1:33 Nalbuphine/Ketamine (n=2) | > 0.04 (0, 0.17) | > 1.29 (0.07, 24.12) | |
| Schedule-controlled responding | |||
| Nalbuphine Alone | 0.7 (0.25, 1.96) | – | |
| Ketamine Alone | – | 0.86 (0.70, 1.06) | |
| 1:3.3 Nalbuphine/Ketamine | 0.18 (0.09, 0.35) | 0.28 (−0.06, 0.63)a | 0.59 (0.30, 1.16) |
| 1:10 Nalbuphine/Ketamine | 0.12 (0.08, 0.17) | 0.21 (−0.24, 0.66)a | 1.15 (0.80, 1.67) |
| 1:33 Nalbuphine/Ketamine | 0.04 (0.02, 0.06) | 0.07 (−0.07, 0.20)a | 1.12 (0.71, 1.79) |
Potency shifts were considered statistically significant if the 95% confidence limits of the potency ratios did not include 1.
Table 2.
ED50 values (95% confidence limits) for oxycodone and ketamine alone or in combination assays of capsaicin-induced thermal allodynia and scheduled-controlled responding and in rhesus monkeys. Numbers in parentheses denote the number of subjects contributing to the Zmix or Zadd values if less than the total number of monkeys tested (n=3) and the > symbol denotes a drug mixture for which an ED50 value could not be determined in all subjects tested.
| Drug or drug mixture | Oxycodone ED50 (95% CL) |
Potency Ratio (95% CL) |
Ketamine ED50 (95% CL) |
|---|---|---|---|
| Capsaicin-induced thermal allodynia | |||
| Oxycodone Alone | 0.05 (0.01, 0.2) | – | |
| Ketamine Alone | – | 0.53 (0.31, 0.91) | |
| 1:3.6 Oxycodone/Ketamine | 0.03 (0.01, 0.17) | 0.61 (−0.56, 1.78) | 0.11 (0.05, 0.25) |
| 1:10.7 Oxycodone/Ketamine | 0.04 (0.02, 0.07) | 0.56 (0.11, 1.02) | 0.38 (0.28, 0.51) |
| 1:32.1 Oxycodone/Ketamine (n=2) | > 0.03 (0, 0.21) | > 0.82 (0.28, 2.39) | |
| Schedule-controlled responding | |||
| Oxycodone Alone | 0.25 (0.17, 0.37) | – | |
| Ketamine Alone | – | 0.86 (0.70, 1.06) | |
| 1:3.6 Oxycodone/Ketamine | 0.21 (0.12, 0.36) | 0.83 (0.44, 1.23) | 0.75 (0.44, 1.28) |
| 1:10.7 Oxycodone/Ketamine | 0.12 (0.08, 0.16) | 0.47 (0.17, 0.76)a | 1.24 (0.88, 1.75) |
| 1:32.1 Oxycodone/Ketamine | 0.04 (0.02, 0.07) | 0.17 (0, 0.34)a | 1.29 (0.72, 2.31) |
Potency shifts were considered statistically significant if the 95% confidence limits of the potency ratios did not include 1.
Table 3.
Experimentally determined Zmix values and predicted Zadd values (95% confidence limits) for mixtures of nalbuphine or oxycodone and ketamine in assays of capsaicin-induced thermal allodynia and schedule-controlled responding in rhesus monkeys. Numbers in parentheses denote the number of subjects contributing to the Zmix or Zadd values if less than the total number of monkeys tested (n=3) and denote a drug mixture for which an ED50 value could not be determined.
| Drug or drug mixture | Zmix | Zadd |
|---|---|---|
| Capsaicin-induced thermal allodynia | ||
| 1:3.3 Nalbuphine/Ketamine | 0.15 (0.13, 0.17) | 0.19 (0.11, 0.34) |
| 1:10 Nalbuphine/Ketamine (n=2) | 0.71 (0.53, 0.95) | 0.33 (0.18, 0.61) |
| 1:33 Nalbuphine Ketamine (n=2) | 1.33 (0.85, 2.1) a | 0.43 (0.26, 0.71) |
| 1:3.6 Oxycodone/Ketamine | 0.15 (0.07, 0.32) | 0.17 (0.15, 0.20) |
| 1:10.7 Oxycodone/Ketamine | 0.41 (0.31, 0.55) | 0.25 (0.18, 0.35) |
| 1:32.1 Oxycodone/Ketamine (n=2) | 0.84 (0.29, 2.45) | 0.41 (0.28, 0.61) |
| Schedule-controlled responding | ||
| 1:3.3 Nalbuphine/Ketamine | 0.77 (0.40, 1.51) | 0.80 (0.45, 1.45) |
| 1:10 Nalbuphine/Ketamine | 1.27 (0.88, 1.84) | 0.85 (0.64, 1.14) |
| 1:33 Nalbuphine/Ketamine | 1.61 (0.73, 1.85) | 0.87 (0.79, 0.95) |
| 1:3.6 Oxycodone/Ketamine | 0.96 (0.57, 1.63) a | 0.42 (0.39, 0.44) |
| 1:10.7 Oxycodone/Ketamine | 1.36 (0.96, 1.91) a | 0.57 (0.52, 0.62) |
| 1:32.1 Oxycodone/Ketamine | 1.33 (0.74, 2.38) | 0.71 (0.61, 0.84) |
Denotes Zmix confidence limits do not overlap with Zadd confidence limits: Zmix larger than Zadd indicating sub-additivity.
Figure 2.
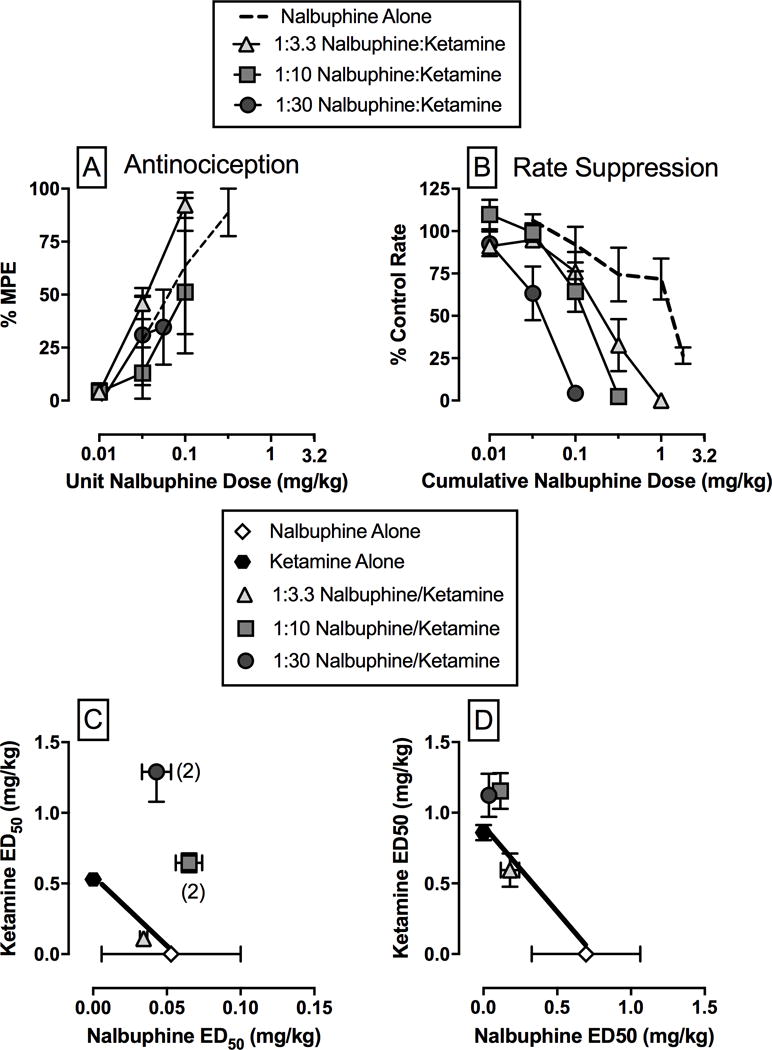
Effects of the mu-opioid agonist nalbuphine alone or in combination with the noncompetitive NMDA antagonist ketamine on capsaicin-induced thermal allodynia (left panels) and rates of schedule-controlled responding (right panels). Upper panels show dose-effect functions for nalbuphine alone or in combination with ketamine and bottom panels show isobolograms at the ED50 effect level for nalbuphine or ketamine alone or as part of a mixture. Upper horizontal axes: unit nalbuphine dose (left panel) or cumulative nalbuphine dose (right panel) in mg/kg/injection. Upper vertical axes: percent control rate of responding. Lower panels show isobolograms at the ED50 effect level for nalbuphine or ketamine alone or as part of a mixture. Lower horizontal axes: ED50 values for nalbuphine alone or in a mixture in milligrams per kilogram (linear scale). Lower vertical axes: ED50 values for ketamine alone or in a mixture in mg/kg (linear scale). Each point represents mean ± SEM of 3–4 monkeys, except when noted by the number in parentheses, which denotes an experimental condition where an ED50 value could not be determined in all subjects tested.
Figure 3.

Effects of the mu-opioid agonist oxycodone alone or in combination with the noncompetitive NMDA antagonist ketamine on capsaicin-induced thermal allodynia (left panels) and rates of schedule-controlled responding (right panels). Upper panels show dose-effect functions for oxycodone alone or in combination with ketamine and lower panels show isobolograms at the ED50 effect level for oxycodone or ketamine alone or as part of a mixture. Upper horizontal axes: unit oxycodone dose (left panel) or cumulative oxycodone dose (right panel) in mg/kg/injection. Upper vertical axes: percent control rate of responding. Lower panels show isobolograms at the ED50 effect level for oxycodone or ketamine alone or as part of a mixture. Lower horizontal axes: ED50 values for oxycodone alone or in a mixture in mg/kg (linear scale). Lower vertical axes: ED50 values for ketamine alone or in a mixture in mg/kg (linear scale). Each point represents mean ± SEM of 3–4 monkeys. Asterisk indicates that the mixture produced a sub-additive effect in the assay of schedule-controlled responding as determined by dose-addition analysis (see Table 3).
Assay of Schedule-Controlled Responding
The average ± SEM control response rate throughout the entire study was 2.5 ± 0.1 responses/s. Nalbuphine, oxycodone, and ketamine dose-dependently decreased rates of responding (Figure 1B). The ED50 values and 95% confidence limits for each drug are shown in Tables 1 and 2. Based on the relative potencies in the assay of capsaicin-induced thermal allodynia, the same three mixtures of nalbuphine + ketamine (1:3.3, 1:10, and 1:33 nalbuphine/ketamine) and oxycodone + ketamine (1:3.6, 1:10.7, and 1:32.1 oxycodone/ketamine) were examined. The dose ranges examined for each nalbuphine + ketamine mixture were 0.01–1 mg/kg nalbuphine (1:3.3), 0.01–0.32 mg/kg nalbuphine (1:10), and 0.0032–0.1 mg/kg nalbuphine (1:33). The dose ranges examined for each oxycodone + ketamine mixture were 0.01–1 mg/kg oxycodone (1:3.6), 0.01–0.32 mg/kg oxycodone (1:10.7), and 0.0032–0.1 mg/kg oxycodone (1:32.1). The dose-effect functions for nalbuphine/ketamine and oxycodone/ketamine mixtures are shown in Figure panels 2B and 3B, respectively. Isobolograms are shown in Figure panels 2D and 3D. Tables 1 and 2 also show the ED50 values for each drug in each mixture, and Table 3 shows the predicted Zadd and experimentally determined Zmix values for the nalbuphine/ketamine and oxycodone/ketamine mixtures, respectively. Increasing fixed proportions of ketamine enhanced the potency of nalbuphine to decrease rates of responding as demonstrated by the 95% confidence limits for the potency ratios not including one. Dose-addition analysis demonstrated that all nalbuphine/ketamine mixtures produced additive effects. For oxycodone and ketamine mixtures, fixed proportions of 1:10.7 and 1:32.1 increased the potency of oxycodone to decrease rates of responding compared to oxycodone alone. Dose-addition analysis demonstrated the 1:3.6 and 1:10.7 oxycodone/ketamine mixtures produced sub-additive effects; whereas the 1:32.1 oxycodone/ketamine mixture produced additive effects.
Oxycodone and MK-801 interactions
Assay of capsaicin-induced thermal allodynia
Oxycodone alone produced dose-dependent antiallodynia, whereas MK-801 produced a maximum %MPE of 22.7 ± 17.8 at a dose of 0.032 mg/kg (Figure 1A). The ED50 values and 95% confidence limits for oxycodone alone and each drug in the drug mixture are shown in Table 4. Because MK-801 produced > 50%MPE in only one subject, the relative potencies were based on the ED50 values in the assay of schedule-controlled responding (below). The dose ranges examined for all oxycodone + MK-801 mixtures were 0.01–0.32 mg/kg oxycodone and larger doses were not examined due to the emergence of undesirable effects (e.g. muscle tone loss) that impaired the monkey’s ability to maintain a sufficiently sternal posture in the chair. The dose-effect functions for oxycodone/MK-801 mixtures are shown in Figure panels 4A. The isobologram for the three oxycodone + MK-801 mixtures are shown in Figure 4C. Increasing fixed proportions of MK-801 did not significantly alter the potency of oxycodone to produce antiallodynia (Table 4). Table 5 shows the predicted Zadd and experimentally determined Zmix values for the oxycodone/MK-801 mixtures. The 1:0.028 and 1:0.085 oxycodone/MK-801 mixture produced effects consistent with additivity, whereas the 1:0.25 oxycodone/MK-801 mixtures produced significant sub-additive effects.
Table 4.
ED50 values (95% confidence limits) for oxycodone and MK-801 alone or in combination assays of scheduled-controlled responding and capsaicin-induced thermal allodynia in rhesus monkeys.
| Drug or drug mixture | Oxycodone ED50 (95% CL) |
Potency Ratio (95% CL) |
MK-801 ED50 (95% CL) |
|---|---|---|---|
| Capsaicin-induced thermal allodynia | |||
| Oxycodone Alone | 0.05 (0.04, 0.06) | – | |
| MK-801 Alone | – | Inactive | |
| 1:0.028 Oxycodone/MK-801 | 0.13 (0.06, 0.3) | 2.64 (−1.69, 6.98) | 0.004 (0.002, 0.008) |
| 1:0.085 Oxycodone/MK-801 | 0.13 (0.05, 0.32) | 2.09 (−2.71, 6.9) | 0.011 (0.004, 0.027) |
| 1:0.25 Oxycodone/MK-801 | 0.17 (0.07, 0.4) | 2.37 (−2.12, 6.87) | 0.041 (0.017, 0.099) |
| Schedule-controlled responding | |||
| Oxycodone Alone | 0.27 (0.15, 0.51) | – | |
| MK-801 Alone | – | 0.02 (0.02, 0.03) | |
| 1:0.028 Oxycodone/MK-801 | 0.23 (0.12, 0.47) | 0.86 (0.75, 0.98)a | 0.01 (0.003, 0.013) |
| 1:0.085 Oxycodone/MK-801 | 0.20 (0.12, 0.33) | 0.74 (0.35, 1.14) | 0.02 (0.01, 0.03) |
| 1:0.25 Oxycodone/MK-801 | 0.12 (0.05, 0.28) | 0.45 (0.19, 0.71)a | 0.03 (0.01, 0.07) |
Potency shifts were considered statistically significant if the 95% confidence limits of the potency ratios did not include 1.
Figure 4.

Effects of the mu-opioid agonist oxycodone alone or in combination with the noncompetitive NMDA antagonist MK-801 on capsaicin-induced thermal allodynia (left panels) and rates of schedule-controlled responding (right panels). Upper panels show dose-effect functions for oxycodone alone or in combination with MK-801 and lower panels show isobolograms at the ED50 effect level for oxycodone or MK-801 alone or as part of a mixture. Upper horizontal axes: unit oxycodone dose (left panel) or cumulative oxycodone dose (right panel) in mg/kg/injection. Upper vertical axes: percent control rate of responding. Lower horizontal axes: ED50 values for oxycodone alone or in a mixture in mg/kg (linear scale). Lower vertical axes: ED50 values for MK-801 alone or in a mixture mg/kg (linear scale). Each point represents mean ± SEM of 3–4 monkeys. Asterisk indicates that the mixture produced a sub-additive effect in the assay of capsaicin-induced thermal allodynia as determined by dose-addition analysis (see Table 5).
Table 5.
Experimentally determined Zmix values and predicted Zadd values (95% confidence limits) for mixtures of oxycodone and MK-801 in assays of capsaicin-induced thermal allodynia and schedule-controlled responding in rhesus monkeys.
| Drug or drug mixture | Zmix | Zadd |
|---|---|---|
| Capsaicin-induced thermal allodynia | ||
| 1:0.028 Oxycodone/MK-801 | 0.14 (0.08, 0.23) | 0.06 (0.05, 0.08) |
| 1:0.085 Oxycodone/MK-801 | 0.14 (0.08, 0.24) | 0.06 (0.05, 0.08) |
| 1:0.25 Oxycodone/MK-801 | 0.21 (0.12, 0.35)a | 0.07 (0.06, 0.09) |
| Schedule-controlled responding | ||
| 1:0.028 Oxycodone/MK-801 | 0.24 (0.12, 0.48) | 0.21 (0.12, 0.39) |
| 1:0.085 Oxycodone/MK-801 | 0.22 (0.13, 0.36) | 0.15 (0.09, 0.26) |
| 1:0.25 oxycodone/MK-801 | 0.15 (0.07, 0.35) | 0.09 (0.06, 0.14) |
Denotes Zmix confidence limits do not overlap with Zadd confidence limits: Zmix larger than Zadd indicating sub-additivity.
Assay of Schedule-Controlled Responding
Oxycodone and MK-801 alone produced dose-dependent decreases in rates of responding (Figure 1B). The ED50 values and 95% confidence limits for each drug are shown in Table 4, and based on the relative potencies, three mixtures of oxycodone + MK-801 (1:0.028, 1:0.085, and 1:0.25 oxycodone/MK-801) were examined. The dose ranges examined for each oxycodone + MK-801 mixture were 0.01–1 mg/kg oxycodone (1:0.028), 0.01–0.56 mg/kg oxycodone (1:0.085), and 0.01–0.32 mg/kg oxycodone (1:0.25). The dose-effect functions for oxycodone/MK-801 mixtures are shown in Figure panel 4B. The isobologram for all three oxycodone + MK-801 mixtures are shown in Figure 4D. Fixed proportions (0.028 and 0.25) of MK-801 significantly enhanced the potency of oxycodone to decrease rates of responding (Table 4). Table 5 shows the predicted Zadd and experimentally determined Zmix values for each drug mixture. All oxycodone and MK-801 mixtures produced effects consistent with additivity.
Time Course Analysis
Figure 5 shows the time course of nalbuphine, oxycodone, ketamine, and MK-801 in the assay of schedule-controlled responding. Two-way repeated-measures analysis of variance demonstrated significant main effects of time (F3,6=164.9, p<0.001), drug (F4,8=28.9, p<0.001), and drug × time interaction (F12,24=8.5, p<0.001). All four drugs produced significant and peak rate-decreasing effects within 10–30 min post administration. MK-801 produced rate-decreasing effects that were significantly different from oxycodone and ketamine, but not nalbuphine, at the 30-min time point. Nalbuphine and MK-801 produced rate-decreasing effects that persisted to at least 100 min and were significantly longer than those of oxycodone and ketamine.
Figure 5.
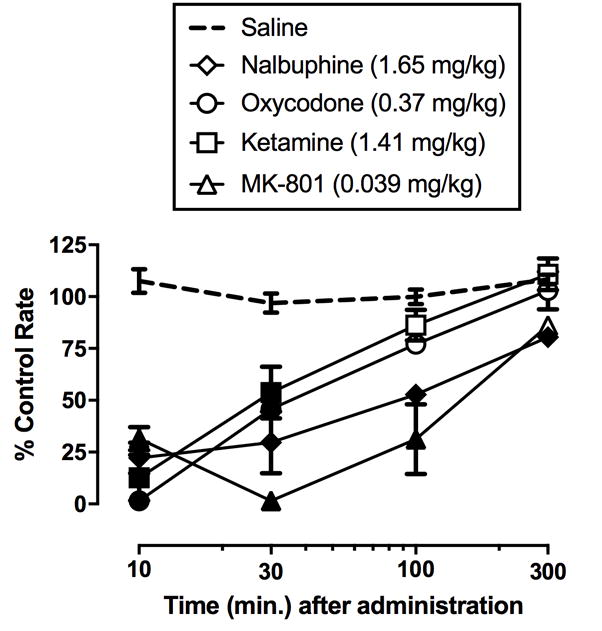
Time course of ED80 doses of nalbuphine (1.65 mg/kg), oxycodone (0.37 mg/kg), ketamine (1.41 mg/kg), and MK-801 (0.039 mg/kg) in an assay of schedule controlled responding in rhesus monkeys. Horizontal axis: time in min. post administration. Vertical axis: percent control rate of responding. Each point represents the mean ± SEM of three rhesus monkeys. Filled symbols denote statistical significance (p<0.05) compared to vehicle.
DISCUSSION
This study assessed interactions between the noncompetitive NMDA antagonists racemic ketamine and (+)-MK-801, and the low-efficacy mu agonist nalbuphine and the moderate-efficacy mu agonist oxycodone, in assays of capsaicin-induced thermal allodynia and schedule-controlled responding in rhesus monkeys. The main finding was that both racemic ketamine and (+)-MK-801 failed to enhance the antiallodynic effects of nalbuphine and oxycodone. Furthermore, ketamine selectively enhanced the potency of both nalbuphine and oxycodone to produce rate suppression. Overall, these preclinical results in monkeys do not support further consideration of noncompetitive NMDA antagonists as clinically useful adjuncts to mu-opioid agonists for the treatment of pain states associated with thermal hypersensitivity.
Effects of Mu agonists and NMDA antagonists alone
Consistent with previous studies in rodents (Emery et al., 2017), nonhuman primates (Banks et al., 2010b; Negus et al., 2012), and humans (Watson and Babul, 1998; Hoeben et al., 2012), both nalbuphine and oxycodone produced dose-dependent antiallodynia. Both nalbuphine and oxycodone dose-dependently decreased rates of operant responding and these nalbuphine results were consistent with previous nonhuman primate studies examining the rate-suppressant effects of mu-opioid agonists (Stevenson et al., 2003; Banks et al., 2010a). Furthermore, the present results extended these previous findings by determining the antiallodynic and rate-suppressant effects of the mu agonist oxycodone in nonhuman primates. Oxycodone was approximately 4 to 5-fold more potent to produce antiallodynia vs. rate suppression.
Although both ketamine and MK-801 produced dose-dependent decreases in rates of responding, only ketamine produced dose-dependent antiallodynia in the assay of capsaicin-induced thermal allodynia. Both the rate-suppressant and antiallodynia effects of ketamine in the present study are consistent with previous results in rhesus monkeys (Butelman et al., 2003; Banks et al., 2010a). Furthermore, the present MK-801 results are consistent with a previous study in mice (Gewehr et al., 2011). In apparent contrast to the present results demonstrating MK-801 failed to produce antiallodynia, previous nonhuman primate studies have reported antiallodynic effects of MK-801 in an assay of capsaicin-induced thermal allodynia (Butelman et al., 2003) and antinociceptive effects in an assay of thermal nociception (France et al., 1989). However, the anti-allodynic effects of MK-801 were only present at a low thermal stimulus of 38°C and MK-801 antiallodynic effects were more variable when the thermal stimulus was increased to 42°C (Butelman et al., 2003). In the present study, the baseline thermal intensities before capsaicin application were 42°C for two monkeys and 46°C for the other two monkeys. In the one monkey that did show an antiallodynic effect of MK-801, the baseline thermal intensity was 42°C. Another explanation for the differential ketamine and MK-801 results in the assay of capsaicin-induced thermal allodynia could be due to ketamine interacting with other receptor systems than NMDA. For example, racemic ketamine has been shown to have similar affinity for opioid receptors compared to the phencyclidine binding site (Hustveit et al., 1995). Overall, the general consistency of the present results with the published literature provided an empirical foundation to determine mu agonist and NMDA antagonist interactions.
Antiallodynia interactions
No fixed proportion of either NMDA antagonist selectively enhanced the antiallodynic effects of nalbuphine or oxycodone. The present additive antiallodynic results are consistent with ketamine and alfentanil analgesic interactions in humans using intradermal capsaicin (Sethna et al., 1998). Furthermore, the present additive or sub-additive interactions are consistent with and extend previous findings from our laboratory (Banks et al., 2010a) and others (Hoffmann et al., 2003; Craft and Lee, 2005; Edwards et al., 2007; Haghparast et al., 2007; Pascual et al., 2010; Lilius et al., 2015) examining NMDA antagonist and mu-opioid agonist combinations in other preclinical assays of nociception, including allodynia. However, the present results may appear in contrast to previous studies in rodents demonstrating that NMDA antagonists enhance the antiallodynic/antihyperalgesic effects of mu agonists (Holtman Jr et al., 2008; Pascual et al., 2010). There are two potential explanations for the differential results between the present study and these previous studies reporting an enhanced antinociceptive effect of the mu agonist.
First, drug interactions are dependent upon not only the relative dose, but also the time course of each drug in a mixture. Thus, differences in mu agonist and NMDA antagonist time course could have influenced drug interactions when conducting fixed proportion interaction studies. This explanation seems unlikely for the following two reasons. First, peak rate decreasing effects were at 10 min for nalbuphine, oxycodone, and ketamine and 30 min for MK-801 in the assay of schedule-controlled responding (Figure 5). Furthermore, all drugs produced significant rate-decreasing effects for at least 30 min, which was within the pretreatment time range used in assay of schedule-controlled responding. Second, statistical analyses did not demonstrate a significant main effect of time in the assay of capsaicin-induced thermal allodynia for any drug alone or drug mixture. Overall, these results suggest that modest differences in time course between the drugs do not fully explain the absence of a selective NMDA antagonist enhancement of mu agonist antiallodynia.
Second, behavioral selectivity to produce antiallodynia vs. suppression of operant responding may also explain the differential results. Pain-stimulated behaviors, such as tail withdrawal, are highly susceptible to false positive results due to drug-induced motor impairment. In paclitaxel-treated rats, ketamine and morphine combinations produced an enhanced anti-thermal hyperalgesic effect, but no interaction on mechanical allodynia and no assessment of motor activity (Pascual et al., 2010). In a rat chronic constriction nerve injury model, the ketamine metabolite norketamine enhanced the antiallodynic and antihyperalgesic effects of morphine at dose combinations that did not significantly alter behavior in a rotorod or locomotor activity procedure (Holtman Jr et al., 2008). Moreover, a recent meta-analysis of ketamine and opioid use for pain reduction found that ketamine did not generally enhance pain relief produced by opioids (only 1 out of 6 showed an enhancement) and in fact, may enhance some undesirable neurological and psychological undesirable effects (Lee and Lee, 2016). Overall, the literature suggests that NMDA antagonists may selectively enhance the antiallodynic/antihyperalgesic effects of mu agonists over a narrow range of experimental conditions that depend upon not only the research subject species, but also the type of noxious stimulus and underlying physiological state.
Comparison with other mu agonist interactions
The present mu agonist and NMDA antagonist interaction results can be compared to results that have determined fixed-proportion drug mixtures of other drug classes in combination with mu agonists under similar procedures. Two will be mentioned. First, the serotonin and norepinephrine uptake inhibitor clomipramine selectively enhanced both the antiallodynic and antinociceptive vs. rate-suppressant effects of nalbuphine in monkeys (Banks et al., 2010b). Second, the delta-opioid agonist SNC80 has also selectively enhanced the antiallodynic and antinociceptive vs. rate-suppressant effects of nalbuphine in monkeys (Stevenson et al., 2003; Negus et al., 2012). In contrast, fixed proportions of NMDA antagonists and mu agonists have thus far failed to produce a selective enhancement of antinociception vs. rate suppression in both assays of capsaicin-induced thermal allodynia (present results) and warm water tail withdrawal (Banks et al., 2010a). Overall, this literature highlights the utility of the behavioral procedures described in the present study to examine mu agonist and other drug class interactions in the development of mu opioid adjuncts for the clinical treatment of various pain states.
Acknowledgments
We appreciate the technical assistance of Ms. Crystal Reyns.
Funding: This work was supported by the National Institutes of Health, National Institute on Drug Abuse Grant R01DA037287. In addition, this research was supported, in part, by the Intramural Research Program of the National Institutes of Health, National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism.
Footnotes
Conflicts of Interest: None
References
- Allen RM, Dykstra LA. N-Methyl D-Aspartate receptor antagonists potentiate the antinociceptive effects of morphine in squirrel monkeys. J Pharmacol Exp Ther. 2001;298:288. [PubMed] [Google Scholar]
- Banks ML, Folk JE, Rice KC, Negus SS. Selective enhancement of fentanyl-induced antinociception by the delta agonist SNC162 but not by ketamine in rhesus monkeys: Further evidence supportive of delta agonists as candidate adjuncts to mu opioid analgesics. Pharmacol Biochem Behav. 2010a;97:205–212. doi: 10.1016/j.pbb.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Rice KC, Negus SS. Antinociceptive interactions between mu-opioid receptor agonists and the serotonin uptake inhibitor clomipramine in rhesus monkeys: Role of mu agonist efficacy. J Pharmacol Exp Ther. 2010b;335:497–505. doi: 10.1124/jpet.110.169276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbia N, Sagalajev B, Pertovaara A. Descending effect on spinal nociception by amygdaloid glutamate varies with the submodality of noxious test stimulation. Neurosci Lett. 2014;570:26–31. doi: 10.1016/j.neulet.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Ball JW, Harris TJ, Kreek MJ. Topical capsaicin-induced allodynia in unanesthetized primates: Pharmacological modulation. J Pharmacol Exp Ther. 2003;306:1106–1114. doi: 10.1124/jpet.103.052381. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Harris TJ, Kreek MJ. Antiallodynic effects of loperamide and fentanyl against topical capsaicin-induced allodynia in unanesthetized primates. J Pharmacol Exp Ther. 2004;311:155–163. doi: 10.1124/jpet.104.068411. [DOI] [PubMed] [Google Scholar]
- Comer SD, Metz VE, Cooper ZD, Kowalczyk WJ, Jones JD, Sullivan MA, Manubay JM, Vosburg SK, Smith ME, Peyser D, Saccone PA. Comparison of a drug versus money and drug versus drug self-administration choice procedure with oxycodone and morphine in opioid addicts. Behav Pharmacol. 2013;24:504–516. doi: 10.1097/FBP.0b013e328363d1c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council National Research. Guide for the care and use of laboratory animals. National Academies Press; Washington DC: 2011. [Google Scholar]
- Craft RM, Lee DA. NMDA antagonist modulation of morphine antinociception in female vs. male rats. Pharmacol Biochem Behav. 2005;80:639–649. doi: 10.1016/j.pbb.2005.02.003. [DOI] [PubMed] [Google Scholar]
- De Kock M, Loix S, Lavand’homme P. Ketamine and peripheral inflammation. CNS Neurosci Ther. 2013;19:403–410. doi: 10.1111/cns.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietis N, Guerrini R, Calo G, Salvadori S, Rowbotham DJ, Lambert DG. Simultaneous targeting of multiple opioid receptors: a strategy to improve side-effect profile. Br J Anaesth. 2009;103:38–49. doi: 10.1093/bja/aep129. [DOI] [PubMed] [Google Scholar]
- Edwards SR, Mather LE, Smith MT. Studies with ketamine and alfentanil following freund’s complete adjuvant-induced inflammation in rats. Clin Exper Pharmacol Physiol. 2007;34:414–420. doi: 10.1111/j.1440-1681.2007.04581.x. [DOI] [PubMed] [Google Scholar]
- Emery MA, Bates ML, Wellman PJ, Eitan S. Hydrocodone, but neither morphine nor oxycodone, is effective in suppressing burn-induced mechanical allodynia in the uninjured foot contralateral to the burn. J Burn Care Res. 2017 doi: 10.1097/BCR.0000000000000517. [DOI] [PubMed] [Google Scholar]
- France CP, Snyder AM, Woods JH. Analgesic effects of phencyclidine-like drugs in rhesus monkeys. J Pharmacol Exp Ther. 1989;250:197–201. [PubMed] [Google Scholar]
- Gewehr C, da Silva MA, dos Santos GT, Rossato MF, de Oliveira SM, Drewes CC, Pazini AM, Guerra GP, Rubin MA, Ferreira J. Contribution of peripheral vanilloid receptor to the nociception induced by injection of spermine in mice. Pharmacol Biochem Behav. 2011;99:775–781. doi: 10.1016/j.pbb.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Haghparast A, Gheitasi I-P, Lashgari R. Involvement of glutamatergic receptors in the nucleus cuneiformis in modulating morphine-induced antinociception in rats. Eur J Pain. 2007;11:855–862. doi: 10.1016/j.ejpain.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Hillhouse TM, Negus SS. Effects of the noncompetitive N-methyl-d-aspartate receptor antagonists ketamine and MK-801 on pain-stimulated and pain-depressed behaviour in rats. Eur J Pain. 2016;20:1229–1240. doi: 10.1002/ejp.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeben E, Smit JW, Upmalis D, Rusch S, Schaffler K, Reitmeir P, Mangold B. Dose-response relationship after single oral dose administrations of morphine and oxycodone using laser-evoked potentials on UVB- and capsaicin-irritated skin in healthy male subjects. Pain. 2012;153:1648–1656. doi: 10.1016/j.pain.2012.04.018. [DOI] [PubMed] [Google Scholar]
- Hoffmann VLH, Vermeyen KM, Adriaensen HF, Meert TF. Effects of NMDA receptor antagonists on opioid-induced respiratory depression and acute antinociception in rats. Pharmacol Biochem Behav. 2003;74:933–941. doi: 10.1016/s0091-3057(03)00020-0. [DOI] [PubMed] [Google Scholar]
- Holtman JR, Jr, Crooks PA, Johnson-Hardy J, Wala EP. Interaction between morphine and norketamine enantiomers in rodent models of nociception. Pharmacol Biochem Behav. 2008;90:769–777. doi: 10.1016/j.pbb.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Hustveit O, Maurset A, Øye I. Interaction of the chiral forms of ketamine with opioid, phencyclidine, σ and muscarinic receptors. Pharmacol Toxicol. 1995;77:355–359. doi: 10.1111/j.1600-0773.1995.tb01041.x. [DOI] [PubMed] [Google Scholar]
- Lee EN, Lee JH. The Effects of Low-Dose Ketamine on Acute Pain in an Emergency Setting: A Systematic Review and Meta-Analysis. PLOS ONE. 2016;11:e0165461. doi: 10.1371/journal.pone.0165461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilius TO, Jokinen V, Neuvonen MS, Niemi M, Kalso EA, Rauhala PV. Ketamine coadministration attenuates morphine tolerance and leads to increased brain concentrations of both drugs in the rat. Br J Pharmacol. 2015;172:2799–2813. doi: 10.1111/bph.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malec D, Mandryk M, Fidecka S. Interaction of memantine and ketamine in morphine- and pentazocine-induced antinociception in mice. Pharmacol Rep. 2008;60:149–155. [PubMed] [Google Scholar]
- McGuinness SK, Wasiak J, Cleland H, Symons J, Hogan L, Hucker T, Mahar PD. A systematic review of ketamine as an analgesic agent in adult burn injuries. Pain Med. 2011;12:1551–1558. doi: 10.1111/j.1526-4637.2011.01220.x. [DOI] [PubMed] [Google Scholar]
- Nahin RL. Estimates of pain prevalence and severity in adults: United States, 2012. J Pain. 2015;16:769–780. doi: 10.1016/j.jpain.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Morrissey EM, Folk JE, Rice KC. Interaction between mu and delta opioid receptor agonists in an assay of capsaicin-induced thermal allodynia in rhesus monkeys. Pain Res Treat. 2012;2012:867067. doi: 10.1155/2012/867067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KM, Max MB, Robinovitz E, Gracely RH, Bennett GJ. Effects of intravenous ketamine, alfentanil, or placebo on pain, pinprick hyperalgesia, and allodynia produced by intradermal capsaicin in human subjects. Pain. 1995;63:163–172. doi: 10.1016/0304-3959(95)00029-R. [DOI] [PubMed] [Google Scholar]
- Pascual D, Goicoechea C, Burgos E, Martín MI. Antinociceptive effect of three common analgesic drugs on peripheral neuropathy induced by paclitaxel in rats. Pharmacol Biochem Behav. 2010;95:331–337. doi: 10.1016/j.pbb.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Munoz M, Sanchez-Blazquez P, Vicente-Sanchez A, Berrocoso E, Garzon J. The mu-opioid receptor and the NMDA receptor associate in PAG neurons: Implications in pain control. Neuropsychopharmacology. 2012;37:338–349. doi: 10.1038/npp.2011.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethna NF, Liu M, Gracely R, Bennett GJ, Max MB. Analgesic and cognitive effects of intravenous ketamine-alfentanil combinations versus either drug alone after intradermal capsaicin in normal subjects. Anesth Analg. 1998;86:1250–1256. doi: 10.1097/00000539-199806000-00022. [DOI] [PubMed] [Google Scholar]
- Stevenson GW, Folk JE, Linsenmayer DC, Rice KC, Negus SS. Opioid interactions in rhesus monkeys: Effects of δ + μ and δ + κ agonists on schedule-controlled responding and thermal nociception. J Pharmacol Exp Ther. 2003;307:1054–1064. doi: 10.1124/jpet.103.056515. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. Drug synergism and dose-effect data analysis. Chapman and Hall/CRC; Boca Raton, FL: 2000. [Google Scholar]
- Tallarida RJ. Drug combinations: Tests and analysis with isoboles. Curr Protoc Pharmacol. 2016;72:9.19.11–19.19.19. doi: 10.1002/0471141755.ph0919s72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Liu F, Patterson TA, Paule MG, Slikker W. Preclinical assessment of ketamine. CNS Neurosci Ther. 2013;19:448–453. doi: 10.1111/cns.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CP, Babul N. Efficacy of oxycodone in neuropathic pain: a randomized trial in postherpetic neuralgia. Neurology. 1998;50:1837–1841. doi: 10.1212/wnl.50.6.1837. [DOI] [PubMed] [Google Scholar]


