Abstract
The integration of inflammatory signals is paramount in controlling the intensity and duration of immune responses. Eicosanoids, particularly prostaglandin E2 (PGE2), are critical molecules in the initiation and resolution of inflammation and in the transition from innate to acquired immune responses. Microsomal prostaglandin E synthase 1 (mPGES1) is an integral membrane enzyme whose regulated expression controls PGE2 levels and is highly expressed at sites of inflammation. PGE2 is also associated with modulation of autoimmunity through altering the IL-23/IL-17 axis and regulatory T cell development. During a type II collagen (CII)-CFA immunization response, lack of mPGES1 impaired the numbers of CD4+ regulatory (Treg) and Th17 cells in the draining lymph nodes. Antigen-experienced mPGES1−/− CD4+ cells showed impaired IL-17A, IFNγ, and IL-6 production when re-challenged ex vivo with their cognate antigen compared to their WT counterparts. Additionally, production of PGE2 by co-cultured antigen presenting cells synergized with that of antigen-experienced CD4+ T cells, with mPGES1 competence in the APC compartment enhancing CD4+ IL-17A and IFNγ responses. However, in contrast to CD4+ cells that were antigen-primed in vivo, exogenous PGE2 inhibited proliferation and skewed IL-17A to IFNγ production under Th17 polarization of naïve T cells in vitro. We conclude that mPGES1 is necessary in vivo to mount optimal Treg and Th17 responses during an antigen-driven primary immune response. Furthermore, we uncover a coordination of autocrine and paracrine mPGES1-driven PGE2 production that impacts effector T cell IL-17A and IFNγ responses.
Keywords: Lipid mediators, Inflammation, T cells, Cytokines, Tolerance, Rheumatoid Arthritis
Introduction
Prostaglandin E2 (PGE2) is a ubiquitous eicosanoid that modulates diverse physiologic and pathologic functions. The biosynthesis of PGE2 is controlled by several constitutive (COX-1, PLA2) and inducible (COX2, mPGES1) anabolic and catabolic (15-PGDH) enzymes (1, 2). These enzymes act in concert to tightly regulate the localization and level of PGE2 concentrations during inflammation. PGE2 is the most prominent prostaglandin in many chronic inflammatory and neoplastic disorders including rheumatoid arthritis (3, 4) and many forms of cancer (5) including intestinal cancer (6). However, PGE2 can also exert immunosuppressive properties that contribute to the resolution of inflammatory events and help restore tissue homeostasis (1, 7). PGE2 has four known receptors (8) with varying expression levels in different cell types which can trigger negative feedback mechanisms to limit PGE2 concentrations (9, 10).
mPGES1 is a membrane-bound biosynthetic enzyme for PGE2 that acts downstream of COX enzymes (11). mPGES1 can become a highly rate-limiting enzyme that controls PGE2 levels due to its differential expression pattern and inducible nature during inflammation. mPGES1 deficient mice have demonstrated the relevance of mPGES1-driven PGE2 in altering several inflammatory diseases (6, 12–14). Furthermore, absence of mPGES1 can cause shunting of prostaglandins and change the characteristics of the inflammatory response (15), deficiencies in antigen-specific humoral responses that are dependent on the T cell (12), mediate collagen-induced arthritis (CIA) (3), control carcinogenesis in several cancer models (1, 16). PGE2 exacerbates arthritis development in the CIA mouse model through the inflammatory IL-23/IL-17 axis (17), and mPGES1 is required to generate inflammatory responses that result in arthritis development in this same model.
PGE2 has pleiotropic effects on many cells of the immune system, influencing both the innate and acquired immune responses. In general, PGE2 suppresses neutrophil and macrophage functions whereas it stimulates stromal and vascular endothelial cells (2). Cells belonging to the innate immune response arm rapidly react in different ways to PGE2 exposure: PGE2 can promote influx and activation of neutrophils, macrophages and mast cells (1, 18, 19), but it can also suppress NK cytolytic and granulocyte functions (20). The effects of PGE2 in dendritic cells (DC) are more complex, acting mostly on IL-12 and IL-23 production and promoting CCR7 expression, but being able to promote both proinflammatory and immunosuppressive functions (1, 15, 21–23). PGE2 can therefore serve as a regulator of APC function at many levels. Lymphocytes are also targeted by PGE2, which modulates their function not only depending on its local concentration but additional microenvironment characteristics, especially the composition of the cytokine/chemokine milieu (24, 25).
T cells can also display a multiplicity of responses to PGE2. PGE2 exerts its effect in T cells exclusively via the EP2 and EP4 receptors (9, 25, 26). PGE2 can alter the T cell subset composition in lymphoid organs and several aspects of T cell responses, with marked consequences on T cell commitment (27–29). This latter effect seems particularly relevant in the case of proinflammatory Th1 and Th17 responses, with PGE2 facilitating the expansion of Th17 cells via EP2 and EP4 differential expression when in presence of IL-1β and IL-23 (25, 30, 31). PGE2 is also capable of increasing IL-17 and reduce IFNγ production in human memory T cells (32). EP2 expression is almost absent in human Th17 cells due to binding of RORγt to ptger2 with suppressive effects, and Th17 cells from MS patients exhibit a more proinflammatory profile due to enhanced IFNγ and GM-CSF production compared to healthy individuals (33). Th1 responses can be inhibited by PGE2 (27, 34), but PGE2 can also paradoxically promote antigen-specific Th1 cells (35) and expand Th1 cells in the autoimmune EAE model in an EP4-dependent fashion (31). Many of the PGE2 Th-promoting effects are triggered by increasing production polarizing cytokines by surrounding APC or innate cells, like IL-12 or IL-23 by differently activated DCs (17, 36). PGE2 can also induce FoxP3 expression in CD4+CD25− T cells, and induced Tregs themselves can express COX2 (37). It is therefore still unclear how PGE2 precisely alters T cell commitment and T cell cytokine profiles and how the PGE2 signals are integrated in different contexts and inflammatory conditions. Moreover, the relative contribution of T cells themselves to the local PGE2 pools has been barely investigated.
The following studies were conducted to identify new roles of PGE2 on T cell function by enzymatic fine-tuning of PGE2 production using mPGES1 deficient mice. We also reconcile some of the paradoxical effects that PGE2 has been reported to have on T cells by dissecting its role in naïve and antigen-experienced/mature CD4+ populations.
Material and Methods
Mice and immunization with type-II collagen (CII)
WT and mPGES1−/− mice in a BL/6 or DBA background were bred in house and maintained under SPF conditions in the MCN II facilities at Vanderbilt University. mPGES-1 mice were obtained from Pfizer and CII-TCR transgenic mice were a kind gift of Dr. David Brand. All mice were bred in a specific pathogen-free barrier facility and used at 8–14 weeks of age. All animals were co-housed and are littermates for every experiment. The Vanderbilt University Animal Care and Use Committee approved all studies performed for the preparation of this manuscript.
Immunization with CII-CFA was performed as described by Brand et al (61). In brief, purified collagen II was emulsified with the corresponding adjuvant (IFA or CFA) and 100 μl of the emulsion were injected i.d. in the base of the tail vein as previously described (3).
Cell preparation and flow cytometry
Single cell suspensions were prepared from the spleen, inguinal, and/or popliteal lymph nodes, and stained on ice using predetermined optimal concentrations of each Ab for 20–30 min, washed, and fixed using 1.5% PFA. Cells with the light scatter properties of singlet lymphocytes were analyzed by multicolor immunofluorescence staining and a BD FACS Fortessa II flow cytometer (Becton Dickinson, San Jose, CA). Gates were always positioned to exclude ≥98% of unreactive cells or unstimulated cells.
Fc gamma receptors were blocked with mouse Fc receptor-specific mAb (2.4G2; BD PharMingen), and surface staining of cell surface markers performed. The anti-mouse mAbs used in this study included CD4 (GK1.5), Tbet (4B10), from BioLegend; CD4 (RM4-5), RORγt (Q31-378), IFNγ (XMG1.2) and Vbeta8.3 (3L2) from BD PharMingen, and FoxP3 (FJK-16s) from eBioscience. The LIVE/DEAD® fixable cell death stain kit from Invitrogen was used in all analyses to remove dead cells from all analysis and avoid background or unspecific staining of dead cells. For proliferation assays, the violet cell tracker dye from eLife Biosciences was used according to manufacturer’s instruction to load the cells prior to further culture. The proliferation index was calculated following instructions for such measures with assistance of FlowJo software. The gating strategy always followed the following hierarchy: Total events →Singlets (FSC-H/FSC-A) → Lymphocyte gate (FSC-A/SSC-A) → Live cells (Live/Dead−) → CD4+, with subsequent gating indicated in every experiment.
Intracellular staining for IFNγ and IL-17A (Biolegend, clones XMG1.2 and TC11-18H10.1) was performed after stimulation of cells, staining of surface molecules, fixation and permeabilization of cells and a final step for intracellular staining. Briefly, single cell suspensions were incubated with PMA (50 ng/ml, Sigma), ionomycin (500 ng/ml, Sigma) and monensin (2 μM, eBioScience) for 4h in vitro in complete IMDM medium (IMDM supplemented with 10% FCS, Pen/Strep, and freshly added 50 μM beta-ME). Unless indicated otherwise, the Cytofix/Cytoperm kit (BD PharMingen) or the BioLegend TrueStain TM buffer system were used to fix, following manufacturer’s instructions to permeabilize and stain cells. Once finished, Cells were resuspended in PBS and stored at 4–10°C until final analysis was carried on. For visualization of intracellular pSTAT3 and pSTAT5 cells were stimulated with 20 nM rmIL-6 (Miltenyi Biotech) in cell culture conditions for 10 minutes prior to further analysis. The staining was performed according to manufacturer’s instructions for those antibodies, and for phosphoflow antibodies from BD Pharmingen clones 4/P Stat3 (Y705) and 4/7 Stat5 (Y694), using Perm Buffer III.
Cell isolation, culture and stimulation
For all in vitro experiments IMDM medium supplemented with 10% FCS was used. Polarization assays were done in 96-well round-bottom plates and 48-well round-bottom plates were used for all APC-T cell co-cultures. In some cases, culture supernatant was collected after 2–4 days to assess cytokine production, and corresponding cells harvested for flow cytometry analysis. The specific COX-2 inhibitor NS-398 was purchased from Cayman Chemicals and stored as indicated in the manufacturer’s instructions, with reconstitution of stored aliquots before every new use.
Total CD4+ or naïve CD4+ T cells were isolated using the StemCell EasySep magnetic cell separation method (StemCell). Subsequent isolation of CD25− and CD25+ cells was performed using CD25-PE and PE-magnetic beads in combination with Miltenyi columns following manufacturer’s instructions. Cell sorting was performed in different ways depending on the final usage. Single cell isolates were purified using cocktail mAb-coupled microbeads to sort untouched CD4+ T cells (Miltenyi Biotech) by sequential separation of first CD4+CD25− and then CD25-PE/Biotin Streptavidin-Magnetic beads CD4+CD25+ T cells. In addition, CD4+ T cell subsets were also sorted using a FACS Diva flow cytometer (Becton-Dickinson) with purities over 95%. Naïve CD4+ T cells used for polarization assays were sorted using the StemCell EasySep system.
Bone marrow dendritic cells were generated as previously described (62). In brief, total bone marrow was collected from mice tibiae and femurs, red blood cells were lysed, and the resulting mix was cultured for 3–4 days in RPMI supplemented with 10% FCS and 20 ng/ml rmGM-CSF (Miltenyi) in 100mm diameter Petri dishes at 2×106 cells/ml. Non-adherent cells were recovered then and plated in new petri dishes with same culture conditions and freshly prepared media. This process was in total repeated 3 consecutive times before collecting final BMDCs used for all experiments. Co-culture assays with BMDCs and purified CD4+ T cells were performed always at a 1:1 ratio in 48-well plates in presence of 100 μg/ml of T cell-grade bovine Collagen II (Chondrex) in a final volume of 800 μl complete IMDM. When indicated, BMDCs were stimulated prior to co-culture with E.Coli 0111:B4 LPS (Sigma) at 1 μg/ml in complete medium and incubated for 2 hours at 37°C, and then washed twice in 50 ml of medium prior to co-culture.
Th1 and Th17 polarization assays
Naïve CD4+ T cells collected from pooled spleen and lymph nodes were cultured in round-bottom 96-well plates at 1×105 cells/well. Plates were pre-coated overnight with anti-CD3ε (BD, clone 2C11), and washed with PBS before adding media and cells. All cells were cultured in IMDM media supplemented with 10% FCS (Gibco), Pen/Strep and 2 μM freshly added β-mercaptoethanol. For Th17 differentiation, this media was supplemented to achieve the following final concentrations: 1 μg/ml soluble anti-CD28 (clone 37.51, BD Pharmingen), 10 μg/ml anti-IFNγ (clone XMG1.2, BioLegend) and anti-IL-4 (clone 11B11, BioLegend), 1 ng/ml hTGFβ (Miltenyi), and 20 ng/ml rmIL-6 (Miltenyi). For Th1 polarization, the media contained instead 1 μg/ml soluble anti-CD28 (clone 37.51, BD Pharmingen), 10 μg/ml anti-IL-4 (clone 11B11, BioLegend), 1 ng/ml rmIL-2 and 10 ng/ml rmIL-12 (Miltenyi Biotec). PGE2 was obtained from Cayman chemical and stored and diluted according to manufacturer’s instructions.
Quantitation of eicosanoids by liquid chromatography-mass spectrometry
Analysis also performed by gas chromatography-mass spectrometry (LC/MS) as follows: fresh cell culture media or cell culture supernatants were incubated for 30 min in the presence of 50 mM arachidonic acid and this mixture was added to 5 ml ice-cold methanol containing 1.0 ng each of the following internal standards: [2H4]-15- F2t-isoprostane ([2H4]-8-iso-PGF2a), [2H4]-PGD2, [2H4]-PGE2, [2H3]-11- dehydro-TXB2 (11-dehydro-TXB2), and [2H4]-6-keto-PGF1a (all purchased from Cayman Chemicals). The lipids were extracted and separated from the solid particulates by centrifugation. The liquid layer was transferred to another tube and the methanol was dried under a stream of nitrogen. The residue was reconstituted in 10 ml 0.01M hydrochloric acid and applied to a C-18 Sep-Pak column (Waters) was prewashed with 5 ml methanol and 5 ml H2O (pH 3.0). For each assay, the precision was 65%, and the accuracy for each assay was 95%.
Real-time PCR
Analysis of expression levels of mRNA were performed with Taqman assays. RNA was obtained from cell culture lysates with QIAGEN mini columns and DNA digestion was performed during the process. cDNA was generated from the extracted RNA with the VILO SuperScript kit from InVitrogen. cDNA samples were then subject to RT-PCR Taqman amplification using the following probes: ptges (Mm0042105_m1), ptgs2 (Mm00478374_m1), rorc (Mm01261022_m1), tbx21 (Mm00450960_m1), tgfbr1 (Mm00436964_m1), ptger2 (Mm00436051_m1), ptger4 (Mm00436053_m1), il23r (Mm00519943_m1) and gapdh (Mm9999915_g1).
ELISA and multiplex analysis of cytokines
Mouse IL-17A and IFNγ were measured using the corresponding ELISA Max Deluxe Sets by from BioLegend. In some cases, supernatants were also evaluated for IL-17A, IFNγ, IL-6, IL-22, and TNFα using the MILLIPLEX © system from EMD Millipore in a Luminex100 system at the Hormone Assay Core at Vanderbilt University (supported by NIH grants DK059637 and DK020593).
Results
mPGES1 modulates T-cell phenotype but not proliferation following immunization with type II collagen (CII)
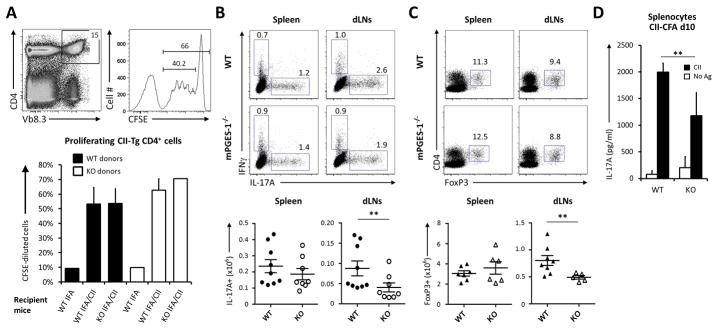
Our initial experiments aimed to understand the in vivo role for mPGES1-derived PGE2 during response to a defined antigen. To determine if mPGES1 was necessary for T cell proliferation during immunization, we transferred CFSE-labeled splenocytes from either WT or mPGES1−/− CII-TCR transgenic mice (with T cells that express CII-specific Vβ8.3+) into WT or mPGES1−/− naïve animals. The recipient mice were then injected with either IFA alone or CII-IFA and their draining lymph nodes (dLNs) were recovered and analyzed 3 days later to evaluate their proliferation by CFSE-dilution of the transferred cells. Once the dLNs were recovered and processed for flow cytometry analysis, we gated on CD4+Vb8.3+ cells in the recipient mice (Fig. 1A) and evaluated the frequencies of proliferating cells within that population. Antigen elicited CD4+ proliferation did not significantly differ between the transferred WT or mPGES1−/− donor T cells. Furthermore, the presence or absence of mPGES1 in the recipient mice did not significantly alter proliferation rates of CD4+ cells. These experiments indicate that the physiologic production of PGE2 that depends on mPGES1 does significantly impair the proliferative T cell response during a recall antigen challenge in vivo. It is known that activated CD4+ T cells release picomolar concentrations of PGE2, which are beneficial for proliferation in vitro (9), but how this autocrine PGE2 contributes to different T cell phenotypes in vivo and antigen responses is still largely unknown.
Figure 1. mPGES1 modulates T-cell phenotype but not proliferation following immunization with type II collagen (CII).
(A) Adoptive transfer of 3×106 total splenocytes labeled with CFSE from naïve CII-TCR transgenic (CII-TCRTg, Vβ3+) WT or mPGES1−/− donor mice was performed into WT or mPGES1−/− recipient mice. These recipient mice received 24 hours later either IFA alone or IFA/CII/IFA i.d. and their draining lymph nodes were recovered and analyzed 3 days later. Flow plots show the gating strategy on transferred cells, with CD4+Vb3+ cells and their corresponding CFSE dilution histograms on the indicated gate below. Graph bars show the percentage of proliferating CD4+Vb3+ cells for each group of transferred mice (n=1 for IFA only and n=4/group for the CII/IFA groups). (B) Treg and Th17 cell numbers were evaluated in day-10 CII/CFA immunized WT or mPGES1−/− mice in the indicated organs. Depicted are intracellular IL-17A and IFNγ after 4h of PMA-Ionomycin stimulation (n=8) and (C) FoxP3+ proportions within the CD4+ cells (n = 6–8) with the corresponding cell numbers on their bar graphs at the right. (D) Freshly isolated splenocytes from day-10 CFA-CII immunized WT or mPGES1−/− DBA mice (n= 7) were collected and stimulated with 100 mg/ml CII for 4 days, and the presence of IL-17A in the supernatant was measured by ELISA. ** indicates a P value <0.05 using a 2-tailed heteroscedastic Student’s T-test.
Despite the lack of altered T cell proliferation upon antigen challenge, the question of whether a qualitative and quantitative response regarding the cytokine production identity is different in the context of a pro-inflammatory response was still unresolved. To address this question, we immunized WT or mPGES1−/− animals with type-II collagen (CII)-CFA and analyzed the specific response of CD4+ cells in different lymphoid organs. Intracellular production of IL-17A and IFNγ was evaluated in the spleen and the dLNs on day-10 following immunization. dLNs of WT mice showed significantly higher proportions and numbers of IL-17A+ cells compared with mPGES1−/− mice despite the lack of differences in splenic T cells, as might be expected given the time point evaluated (Fig. 1B). This same effect was also observed on the CD4+FoxP3+ cells in the dLNs but not in the spleen (Fig. 1C). We hence conclude that mPGES1-driven PGE2 production during a proinflammatory immune response alters both Th17 and regulatory T cell responses in vivo. No significant changes were detected in CD4+IFNγ+ cells (Supl. Fig. 1).
Next, WT or mPGES1-deficient mice were immunized with CII-CFA. After 10 days, total splenocytes were isolated and cultured for 4 days in the presence of CII. When re-stimulated, splenocytes from WT mice released significantly more IL-17A than splenocytes of mPGES1−/− mice into the supernatant (Fig. 1D). Taken together, these results implicate mPGES1-derived PGE2 as important to shaping the phenotype of the developing immune response, with absence of PGE2 reducing the numbers of T cells polarizing towards an IL-17 phenotype. The inverse of these observations suggests that the presence of PGE2 may facilitate polarization towards the IL-17 phenotype.
mPGES1-dependent PGE2 regulates EP2 and EP4 expression in T cells
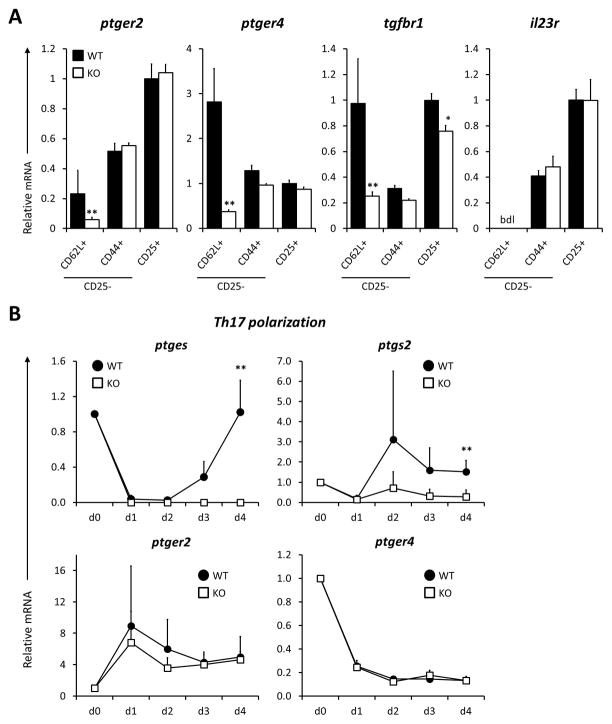
To better understand our observation of reduced IL-17A production by T cells from mPGES1 deficient animals following immunization, we analyzed the expression levels of genes that control Th17 commitment and integrate PGE2 sensing. Mice deficient in PGE2 receptors 2 or 4 (EP2 and EP4) show impairment in IFNγ and IL-17A production during inflammatory responses like contact hypersensitivity (31) or during EAE (26). However, if enzymatic control of PGE2 production alters T cell EP receptor expression levels and susceptibility for cytokine signals is unknown. In order to identify such differences in an unbiased approach in unmanipulated mice, we sorted freshly isolated splenic populations of T cells from WT and mPGES1−/− mice according to their canonical naïve, memory and Treg markers CD62L, CD44, and CD25. In WT mice, naïve T cells showed lower ptger2 but higher ptger4 expression when compared to memory or regulatory T cells (Fig. 2A). mPGES1-competent peripheral Tregs expressed a >5-fold higher level of ptger2 than naïve T cells, while their ptger4 expression level was 3-fold lower. Interestingly, absence of mPGES1 reduced ptger2, ptger4 and tgfbr1 expression levels only in naïve T cells, with mPGES1−/− naïve T cells expressing 5–10-fold lower levels of those transcripts. IL-23 is also known to regulate the maintenance and expansion of Th17 cells; however, we did not observe any difference in WT and mPGES1−/− CD4+ cells IL-23 receptor levels. These results suggest a mechanism by which absence of mPGES1 may influence the phenotype of naïve T-cells due to resistance to TGF-β and also suggest why these cells may be resistant to negative effects of PGE2 on T cell proliferation in vivo.
Fig 2. mPGES1 and ptger4 are strongly downregulated during Th17 polarization.
(A) Freshly isolated CD4+ T cells from naïve mice were sorted into the indicated naïve, memory and regulatory subsets (CD25−CD44−CD62L+, CD25−CD44+CD62L− and CD25+) and analyzed for their expression levels of ptger2, ptger4, tgfbr1 and il23r. All values are relative to the WT CD4+CD25+ T cell population. (B) Expression levels kinetics of the indicated mRNAs were evaluated at the indicated time-points (freshly isolated, days 1, 2, 3 and 4) under Th17-polarizing conditions. Results are compiled from 3 different experiments with 3–4 pooled mice cells and 4 replicates. ** indicates a statistically significant difference (P<0.01) to WT cells for each data point in a 1-way ANOVA test.
The initial differences in these receptor levels prompted us to evaluate their differences during in vitro polarization. We cultured naïve CD4+ T cells from WT and mPGES1−/− mice under Th17 polarizing conditions for 4 days. To further understand the role that autocrine PGE2 might play in Th17 responses, we examined the expression levels of the genes encoding for the key enzymes controlling PGE2 metabolism mPGES1 (ptges) and COX2 (ptgs2), and the PGE2 receptors EP2 (ptger2) and EP4 (ptger4) in WT compared with mPGES1 null cells (Fig. 2B). We found that ptges was very rapidly downregulated under Th17 polarizing conditions during the first 2 days, recovering to initial levels on day 4. This observation may explain the loss of differential IL-17A expression during in vitro polarization compared to in vivo circumstances. Similar to what has been observed in other systems (15), ptgs2 was upregulated in WT cells compared to mPGES1−/− cells, suggesting a positive feedback of PGE2 on COX-2 expression that may rely on ptger2, as this gene was gradually upregulated over the course of Th17 polarization. Expression of ptger4 decreased over time as Th17 polarization progressed, and stayed silenced over the course of 4 days. The rapid and pronounced decline in both ptges and ptger4 suggests the possibility that autocrine PGE2 signaling should be initially robust but then silenced to complete the commitment to a Th17 phenotype.
Production of PGE2 by antigen stimulated T cells is mPGES-1-dependent and acts in synergy with APCs
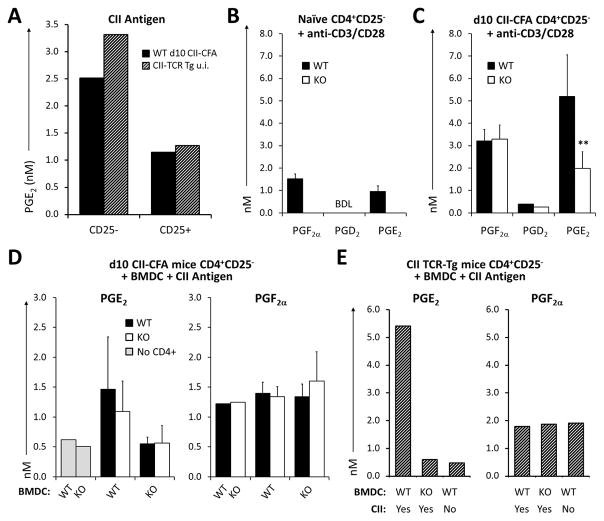
The intrinsic capacity of T cells to produce prostaglandins has been scarcely investigated. T cells are known to produce PGE2 upon strong TCR-driven stimuli (33, 38), but the enzymatic control of such PGE2 production and whether this differs within different T cell subsets upon inflammation is barely known. To explore the role of PGE2 in an antigen specific manner we isolated CD4+CD25− and CD25+ T cells from WT mice following a CII-CFA specific immunization response (d10 after immunization). We observed different levels of PGE2 production when T cells were re-challenged ex-vivo with their cognate antigen in the presence of splenic DCs acting as antigen presenting cells (APCs) (Fig. 3A), with CD4+CD25− WT mPGES1-competent cells producing nearly 3-fold more PGE2 than CD4+CD25+ cells. To corroborate the capacity to elicit antigen-specific T cell activation and PGE2 production ex-vivo, we also isolated CD4+CD25− and CD25+ cells from unimmunized CII-TCR transgenic animals and incubated them for 4 days in the presence of CII (Fig. 3A). CD4+ cells from unimmunized CII TCR-Tg CD4+ and from CII-CFA d10 CII-CFA animal responded similarly to day 10-immunized DBA mice, with CD4+CD25− CII TCR-Tg cells showing increased PGE2 production compared to CD4+CD25+ cells. These data demonstrate that PGE2 production is an integral component of an antigen-specific T cell response.
Fig. 3. Production of PGE2 by T cells is dependent on mPGES1 competence in antigen presenting cells.
(A) CD4+CD25− and CD25+ T cells from unimmunized CII-TCRTg mice or WT mice immunized with CII-CFA (day 10) were co-cultured with splenic DCs (1:1) supplemented with 100 mg/ml of the same CII antigen protein used for immunizations for 4 days and PGE2 concentrations were measured by ELISA. (B–C) CD4+CD25− and CD25+ T cells were isolated from DBA WT or mPGES1−/− naïve (B) or (C) immunized with CII-CFA for 10 days mice, and then stimulated with anti-CD3/28 for 4 days. PGE2 concentration in supernatants was evaluated by LC/MS. (D) WT or mPGES1−/− BMDCs were co-cultured with WT or KO CD4+CD25− T cells from day-10 CII CFA-immunized mice in the presence of 100 mg/ml of T cell-grade CII for 4 days then PGE2 and PGF2α were measured by LC/MS in the supernatant. (E) WT or mPGES1−/− BMDCs were also co-cultured with CD4+CD25− T cells from naive CII-TCRTg mice in presence or absence of CII. ** indicates a P value <0.01 and * correspond to <0.05 using a 2-tailed heteroscedastic Student’s T-test. Results in A+D are compiled data from 2 experiments and in B–C compiled from 3 independent experiments always with T cells pooled from 3 mice/group each time. BDL = below detection limits
Since mPGES1 is one of the terminal enzyme controlling PGE2 production, and in other systems absence of mPGES1 can lead to shunting from PGE2 to other PGs, we investigated a variety of PG during T-cell activation. Purified CD4+CD25− cells from naïve WT and mPGES1−/− unmanipulated mice were stimulated with anti-CD3/CD28 for 4 days and the supernatants analyzed for different prostaglandins. Comparing WT and mPGES1−/− T cells, PGE2, PGF2α and PGD2 were either not detected or expressed at low levels, but did not show differences between WT and mPGES1−/− mice (Fig. 3B). However, when WT CD4+CD25− cells from day-10 CII-immunized mice were re-stimulated with anti-CD3/CD28, we observed higher levels of PGF2α and PGE2 (Fig. 3B–C), and revealed a significant difference in PGE2 concentrations between WT and mPGES1−/− T cells. We did not see shunting from PGE2 to an alternate terminal PG in these conditions. These data demonstrate that CD4+CD25− T cells acquire the capacity to produce different prostaglandins during the course of a proinflammatory immune response following immunization with antigen, and that mPGES1 controls the magnitude of the corresponding increase in PGE2.
Since PGE2 is the predominant PG in T cells during an inflammatory immunization we next inquired to what extent an antigen presenting cell (APC) would collaborate, and whether this response would be substantially altered by antigenic re-stimulation. To answer this question, purified CD4+CD25− cells from d10 CFA-CII immunized WT and mPGES1−/− mice were co-cultured with WT or mPGES1−/− bone marrow derived dendritic cells (BMDCs) from unimmunized mice in the presence or absence of CII, and supernatants were collected after 4 days of ex-vivo co-culture. Unstimulated BMDCs did not secrete considerable amounts of PGE2, and presence of mPGES1 on BMDCs cultured alone had no impact on their PGs profile. On the other hand, when T cells were present, PGE2 was produced in larger quantities when both the T cell and the BMDC were mPGES-1 competent, while PGF2α was again not altered, which demonstrates a lack of PG shunting (Fig. 3D). To further decipher the relative contribution of APCs to the production of PGE2, we co-cultured CII TCR-Tg CD4+CD25− cells with WT or mPGES1−/− BMDCs in the presence of CII. PGE2 concentrations where highest when BMDCs were mPGES1-competent (Fig. 3E), and absence of mPGES1 in BMDCs reduced PGE2 to unstimulated levels, which indicates a co-dependence of T cells and the concomitant APCs in their requirement for mPGES1 in order to produce maximal amounts of PGE2 during antigen-specific T cell activation.
T cell-autocrine and paracrine PGE2 sources coordinate to control antigen-specific T cell cytokine responses
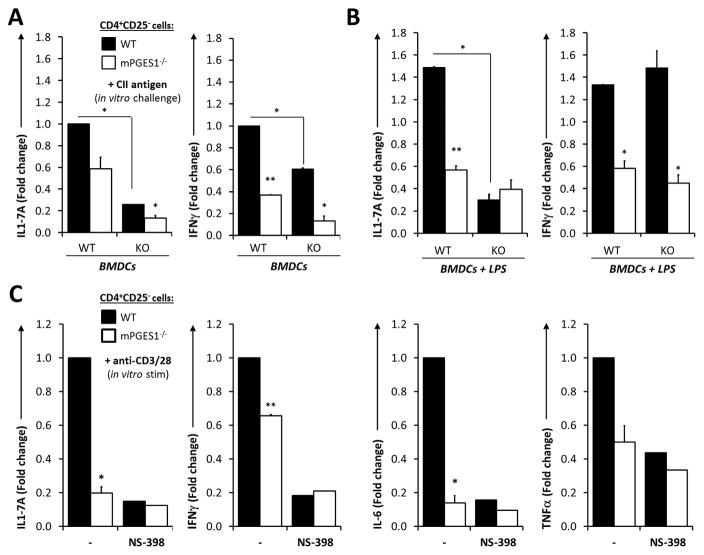
Our previous data demonstrates that PGE2 production is enhanced during immunization in T cells in vivo, and that presence of mPGES1 increased CD4+ cell IL-17 production in vivo. However, there remained uncertainty as to how PGE2 generated by T cells or by APC or other surrounding cells are integrated to control T cell cytokine phenotype during a recall response. To better delineate what is the relative contribution of both T cell autocrine and paracrine mPGES1-driven PGE2, we isolated CD4+CD25− T cells from the spleens and dLNs of day-10 CII-CFA immunized WT and mPGES1−/− mice and co-cultured them with either WT or mPGES1-deficient BMDCs in the presence of their cognate antigen (CII) for 4 additional days in vitro. Antigen re-challenge with CII induced a much larger IL-17A production in WT CD4+CD25− cells than in the mPGES1-deficient counterparts (Fig 4A). Absence of mPGES1 in both T cells and APCs resulted in almost absent IL-17A concentrations in the supernatant. mPGES1-competent BMDC induced a 7-fold higher production of IL-17A from WT T cells, while mPGES1−/− T cells IL-17A production could be only partially rescued by presence of mPGES1 in the BMDC. Production of IFNγ paralleled what was observed with IL-17A, but showed independence of mPGES1 in BMDCs. These results suggest autocrine and paracrine PGE2 may cooperate to coordinate T cell IL-17A and IFNγ when both the T cell and the APC are mPGES1-competent during a cognate antigen interaction. To enhance the strength of the T cell-APC interaction and the T cell response and PG production by BMDCs, we also stimulated the co-cultured BMDCs for 2 hours with LPS prior to activation of T cells in the same co-culture conditions. As anticipated, LPS stimulation of the APC increased cytokine production by T cells (Fig. 4 A–B). The results recapitulated what was observed without LPS stimulation, but these conditions unveiled significant differences in IL-17A production between WT and mPGES1−/− CD4+CD25− cells when the BMDCs were mPGES1-competent (Fig. 4B). These results demonstrate that IL-17A production relies more on PGE2 production by cognate APCs than IFNγ production, and suggest that APC costimulatory capacities enhance cytokine production together with PGE2.
Fig. 4. PGE2 from T cell-intrinsic and -extrinsic sources controls antigen-specific T cell cytokine responses in immunized mice.
CD4+CD25− T cells from WT or mPGES1−/− DBA mice immunized with CII-CFA were isolated on day 10. These T cells were co-cultured with WT or mPGES1−/− BMDCs for 4 days in the presence of CII. IL-17A and IFNγ production was measured in the supernatants of CD4+CD25− cells co-cultured with (A) unstimulated BMDCs or (B) BMDCs previously stimulated with LPS at 1 μg/ml for 2 hours. (C) Pooled purified CD4+CD25− from WT and mPGES1-deficient mice were isolated on day 10 of CII-CFA immunization and stimulated with anti-CD3/CD28 for 4 days in vitro. Cytokines from the supernatants were measured in the presence or absence of the COX-2 inhibitor NS398 at 10 μM. Compiled results of 3 different experiments with n=3 replicates each and expressed as fold-difference relative to WT cells. * indicates a P value <0.05 and ** correspond to <0.01 using a 2-tailed heteroscedastic Student’s T-test. All results are relative to WT T cells + WT BMDCs in A–B and to WT T cells in C–D.
To confirm that the cytokine profile that was being regulated by PGE2 during T cell activation, we stimulated WT or mPGES1-deficient purified CD4+CD25− T cells from mice immunized in the same manner as before with anti-CD3/anti-CD28 rather than cognate antigen for 3 days (Fig. 4C). The capacity of in vivo primed T cells to produce IL-17 was 5-fold larger in T cells from WT mice compared to mPGES1−/− mice, and the increase was fully abrogated by specifically inhibiting COX-2 activity with NS-398, confirming the effect is related to PGE2 biosynthesis. Furthermore, when measured by Multiplex, IL-6, IL-17A, TNFα, and IFNγ were also significantly also higher in supernatants of WT compared with KO T cells (Fig 4C). When PG production was blocked by COX-2 inhibition, levels of IL-6, IL-17A, and TNFα from WT T cells were reduced to the level of mPGES1−/− T cells in all cases. These data confirm that the changes in production of these cytokines are also due to changes in PGE2 synthesis on T cells. It is of interest to note that magnitude of reduction in IFNγ in KO T-cells was not as dramatic as IL-17A, and thus may not be as dependent on PGE2.
Altogether, our results demonstrate that T cell mPGES1-driven autocrine PGE2 controls T cell cytokine profiles and significantly enhances IL-17A, IL-6, and IFNγ production during antigen-specific and non-specific T cell activation. Additionally, IL-17A and IFNγ were differentially regulated during antigen-specific versus non-specific T cell activation, with IL-17A more strongly affected by paracrine PGE2 levels derived from BMDC.
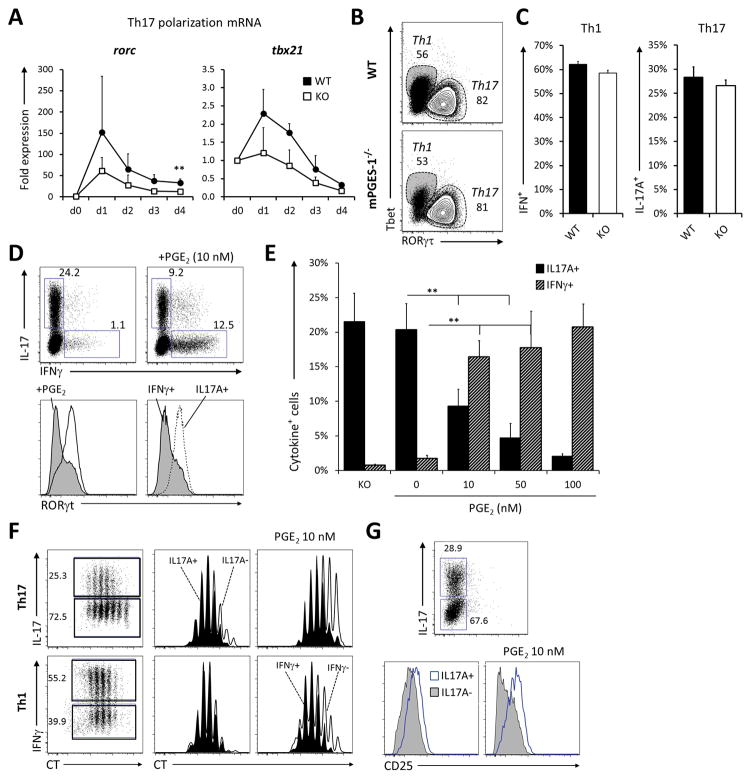
PGE2 regulates RORγt expression and promotes IFNγ production of naïve CD4+ T cells under Th1 and Th17 conditions
Previous reports have demonstrated that PGE2 can expand T cells committed to the Th17 lineage both in mice and humans, but PGE2 can also promote the proliferation of murine Th1 cells. We tested whether mPGES1 deficiency would impact either the generation or proliferation of naïve T cells undergoing Th1 or Th17 polarization in vitro. During Th17 differentiation, WT cells show a relative increase in both rorc and tbx21 expression (Fig. 5A), although they were not significant except for rorc at day 4. We then evaluated the intracellular expression of either Tbet or RORγt at day 4 of differentiation but found no significant differences between the WT and mPGES1−/− proportions of CD4+Tbet+ cells under Th1 differentiation or CD4+RORγt+ under Th17 polarizing conditions (Fig 5B). Under these conditions mPGES1 did also not significantly alter the proportions of IL-17A+ cells under Th17 polarization, or IFNγ+ in Th1 conditions (Fig. 5C). We hence concluded that T-cell autocrine PGE2 does not have the capacity to alter Th1 or Th17 commitment of naïve T cells in absence of a cognate APC interaction and/or during a non-antigen-driven T cell response. This lack of significantly lower IL-17A during in vitro polarization of naïve CD4+ T cells contrasts with our observation that mPGES1-deficient cells differentiated in vivo demonstrated significantly lower levels of IL-17A. To address this issue, we examined the effect of exogenously provided PGE2 during polarization. We polarized purified naïve CD4+ T cells under Th1 or Th17 conditions for 4 days in the presence of increasing concentrations of PGE2 and analyzed the intracellular production of IL-17A and IFNγ. Surprisingly, under Th17 polarization, gradual increase in PGE2 concentrations caused a drop in the percentage of cells capable of producing IL-17A while at the same time promoted the intracellular accumulation of IFNγ (Fig. 5D–E), although concentrations higher than 10 nM had a negative impact on overall T cell proliferation and survival (data not shown). Interestingly, intermediate concentrations of PGE2 did not alter T cell proliferation of IL-17A+ cells during the first days of in-vitro Th17 polarization (Fig. 5F), in contrast to what seems to be the case during the expansion phase of such cells (31) or human memory T cells (32). PGE2 did however inhibit proliferation of all other IL-17A− cells, and hence might provide a competitive advantage during inflammatory conditions. In the case of Th1 cells, PGE2 also favored proliferation of IFNγ+ cells, consistent with reports in mice that show a synergistic amplification of IL-12 signaling (36), but contrary to what seems to happen during re-stimulation of expanded human naïve T cells (25). PGE2 also downregulated CD25 expression selectively on IL-17A− cells under Th17 conditions (Fig. 5 G).
Fig. 5. PGE2 downregulates RORγt to redirect IL-17A to IFNγ production.
(A) Freshly isolated naïve CD4+ cells from WT and mPGES1−/− (KO) mice were cultured under Th17 polarizing conditions for 4 days and the expression level of rorc and tbx21 mRNA evaluated every day. n=3 experiments (B–C) Cells were collected at day 4 of polarization and their intracellular expression of (B) RORγt (Th7 polarization) or Tbet (Th1 polarization) and (C) intracellular cytokine expression of IFNγ and IL-17A. n=5 experiments. (D–E) Naïve CD4+ cells were cultured under Th17 polarization in the presence of increasing concentrations of exogenously added PGE2. (D) A representative dot plot is depicted on the top, and histograms below show RORγt expression with or without PGE2 (left histograms) or within the IL-17A+ or IFNγ+ gates (right histograms). (E) Summary of cell percentages and cell numbers of IFNγ+ and IL-17A+ cells upon increasing PGE2 concentrations. n=4 experiments. (F) Proliferation of cells undergoing Th17 or Th1 polarization in presence or absence of 10 nM PGE2 was assessed in WT and mPGES1−/− cells. Violet cell-tracker dilution profiles of IL17A+ (filled) /IL17A− (empty) cells or IFNγ+/IFNγ− cells are shown for each condition. (G) Surface expression of CD25 was also analyzed under Th17 conditions. ** indicates a P value <0.05 and * correspond to <0.01 using a 2-tailed heteroscedastic Student’s T-test.
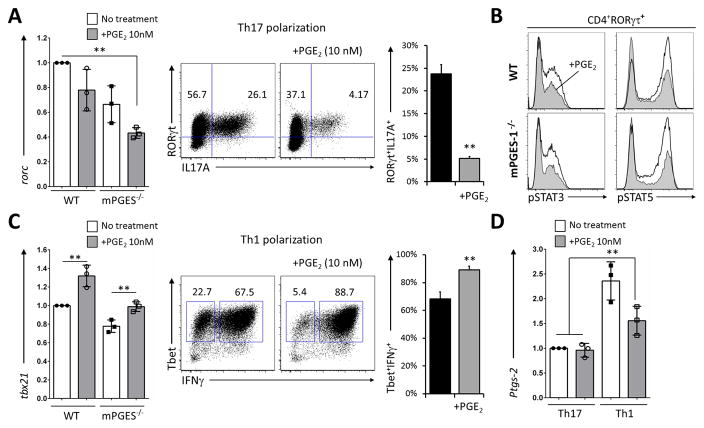
To gain insight into the mechanisms controlling the shift in IL-17A to IFNγ, we evaluated how PGE2 alters the levels of mRNA encoding for RORγt, Tbet, mPGES1 and COX-2 at the concentration that exerted the strongest shift in IL-17A to IFNγ production (10 nM) without majorly impacting proliferation or survival during Th1 and Th17 polarization. Exogenously added PGE2 decreased rorc expression when compared to untreated cells (Fig. 6A), and this downregulation was largely independent of mPGES1. Examination of the intracellular expression of RORγt and IL-17A revealed that in vitro exogenous PGE2 caused an expected decrease in RORγt+ cells, but the most accentuated inhibition took place in RORγt+IL-17A+ cells. Production of IL-17A was strictly segregated to RORγt+ cells (Fig. 6A, middle dot plots), which made us disregard the possibility of a non-Th17 concomitant population or Th cells that could be plastic enough to contribute to the pool of IL-17A+ cells. In search of a further mechanism explaining the role of PGE2 in T cell function we analyzed the phosphorylation levels of pSTAT family members involved in Th17 commitment. Exogenous addition of PGE2 during Th17 polarization downregulated the phosphorylation of both pSTAT3 and pSTAT5 in CD4+RORgt+ cells (Fig. 6B), although there was no difference due to mPGES1 competence. This suggests that exogenous PGE2 alters the activation of STAT proteins of already committed cells. Contrary to the Th17 conditions, under Th1 polarization extrinsic PGE2 increased tbx21 expression at day 2 (Fig. 6C, left), and significantly increased intracellular IFNγ+ expression in Tbet+ cells (Fig. 6C, right dot plots and bar graph). Interestingly, Th1 conditions increased ptgs-2 compared to Th17 conditions, which might contribute to reinforce the positive feedback loop that IFNγ+ exerts on Th1 cells (Fig. 6D). Expression of ptgs-2 was not altered due to initial exposure to PGE2 under Th17 differentiation, but it was decreased when PGE2 was provided under Th1 conditions. Together, this data demonstrate that PGE2 concentrations are critical in defining the commitment potential of undifferentiated cells at the exact moment that cells receive a TCR proliferative signal, and that extrinsic PGE2 supplies alter Th1 and Th17 commitment. Moreover, extrinsic PGE2 shifts IL-17A production to IFNγ production under Th17 polarization and it increases the overall IFNγ production of Th1 cells, while T cell-mPGES1 does not exert a measurable effect.
Fig. 6. PGE2 enhances IFNγ production during Th1 and 17 polarization.
(A) WT or mPGES1−/− (KO) freshly isolated naïve CD4+ cells were analyzed during Th17 polarization. Levels of rorc mRNA expression the absence or presence of 10 nM PGE2 at day 2 are shown on the bar graphs, while RORγt and IL-17A intracellular expression at day 4 are shown in representative the dot plots. The far right bar graph summarizes the double positive RORγt+IL-17A+ percentages in 4 replicate experiments, each started with a pool of naïve CD4+ cells from 3 mice. (B) The phosphorylation status of STAT3 (Y705) and STAT5 (Y694) within RORγt+ cells was also examined in presence (shaded histograms) or absence (open histograms) of PGE2. (C) Cells undergoing Th1 differentiation were analyzed for their mRNA levels of expression of tbx21 at day 2, and their intracellular expression of Tbet and IFNγ at day 4 (representative dot plots), with the right bar graph summarizing Tbet+IFNγ+ percentages in 4 replicates. (D) mRNA levels of ptgs2 under Th1 or Th17 polarization at day 2 in presence or absence of PGE2. All values are relative to WT Th17 cells, and ** indicates a P value <0.01 using a 2-tailed heteroscedastic Student’s T-test.
Discussion
In this report we demonstrate that mPGES1-driven PGE2 regulates T cell responses during an antigen-specific immune response, with the regulatory and Th17 compartments demonstrating a requirement for PGE2 to optimally expand. We also show that T cells themselves alter their PG secretion capacities in an antigen-specific manner, with PGE2 becoming a dominant PG that is dependent on mPGES1 during immune responses. Furthermore, we illustrate how mPGES1-dependent PGE2 facilitates T cells to increase their IL-17A, IFNγ and IL-6 cytokine production capacity upon re-stimulation with their cognate antigen. Finally, we show that after in-vivo priming, T cell autocrine and paracrine PGE2 act synergistically to achieve maximal IL-17A and IFNγ cytokine secretion potential. Consistent with this, exposure to intermediate levels of PGE2 during in-vitro Th1 polarization of naïve CD4+ cells increases their IFNγ production, but it surprisingly inhibits IL-17A during Th17 polarization in favor of IFNγ. Our results demonstrate that both T cell autocrine and paracrine PGE2 act differently on naïve and mature antigen-experienced T cells to regulate their IL-17A and IFNγ responses, which helps to reconcile previous conflicting results. We argue that these apparently contradicting outcomes can coexist given the differences in the initial target T cell population, the type of T cell stimulation (antigen-driven or not, type of interacting APC, etc.), the PGE2 dose and delivery (exogenously added or provided by an interacting APC), and the timing of PGE2 exposure. All these factors also depict a wide range of variety depending on the organ and microenvironment where the T cell is stimulated in vivo.
Several studies have demonstrated the relevance of PGE2 in CD4+ T cells during immune responses leading to autoimmunity, setting the path to understand how PGE2 might act in promoting disease progression. Sensing of PGE2 in T cells is paramount, as efficient T cell priming and activation require EP receptors (9). EP4 (ptger4) variants have been described as candidate risk factors in joint damage in RA patients (39) and in inflammatory bowel disease patients (40). CD4+ T cell conditional EP4 knock-out mice are protected from EAE (26), and EP2 and EP4 antagonists suppress the differentiation of Th1 and Th17 cells in vivo (31). In this report we show that in mice deficient in mPGES1, a terminal PGE2 biosynthetic enzyme, EP2 and EP4 are detected at lower levels only in the naïve CD4+ population, and EP4 is downregulated during Th17 polarization while EP2 is upregulated (Fig. 2). The net effect of PGE2 production on T cells upon an autoimmune inflammatory response is highly complex due to the diversity and variability of EP receptors expressed in different cells and tissues together with the different capacities to secrete PGE2 (41, 42). During a CII-CFA immunization, DBA mice deficient in mPGES1 show impaired generation of regulatory and IL-17A+ cells in the draining lymph nodes compared to WT mice (Fig. 1), but this is not due to a general lack of proliferative capacity of T cells. We conclude that this effect must be due to both the absence of mPGES1 in the immunized mouse, which alters the production of PGE2 by neighboring cells and APCs, as well as a T cell-intrinsic effect that is at least in part due to their altered EP receptor expression.
The contribution of IL-17A and Th17 cells to many autoimmune and inflammatory diseases is widely documented, with promising results in treatment of psoriatic arthritis and RA in targeted therapies like the anti-IL-17 mAb secukinumab (43–46). IL-17A is known to be increased in the synovial fluid of RA patients, being more prevalent in ACPA+ RA (47). PGE2 can promote both human and mouse Th17 proliferation and expansion. Kofler et al. demonstrated that human Th17 cells selectively downregulate EP2 while all other T cell induced fates did not alter it (33), which contrasts with our results in mouse Th17 cells (Fig. 2). However, it remains unresolved whether this is due to the distinct source and nature of naïve T cell populations (secondary lymphoid organs versus blood) or if it is a species-specific issue. In either case, our data support the notion that sensing of locally available PGE2 upon activation of naïve T cells is critical for Th17 differentiation. Additionally, EP2 and EP4 control Th17 cell expansion and activity (25, 31). However, most of these studies show how sensing of PGE2 alters responses in cells that are already committed to a certain phenotype, like Th17 polarized cells, and use EP2 and EP4 germline/conditional T cell knockouts or administration of antagonists of EP2–EP4 to investigate how PGE2 functions. We show that T cells undergoing Th17 polarization first suppress expression of ptges to recover over time, while ptgs-2 is upregulated in absence of mPGES1. We also show how ptger2 is gradually upregulated while ptger4 is starkly and consistently suppressed (Fig. 2 B).
Inflammatory signals can induce PGE2 release in many different cell types (1). Activation of T cells also involves autocrine production of PGE2, although this has been previously studied only regarding the involvement of COX2 (38). We demonstrate that stimulation of CD4+ cells induces PGE2 production in an mPGES1-dependent fashion and is antigen-dependent (Fig. 3). More importantly, CD4+CD25− cells alter their PG profile during immunization, with a large increase in PGE2 production that relies on mPGES1, with no concomitant differences in PGD2 and PGFα2 (Fig. 3 B–C).
PGE2 acts synergistically with IL-23 to favor human Th17 expansion (30), and TCR triggering in the presence of PGE2 increases IL-17 and reduces IFNγ production by freshly PBMC-isolated human memory T cells or T-cell clones (32). In agreement with our results, BMDC that produce PGE2 can favor ex vivo Th1 and Th17 responses while being detrimental for Th2 responses, in large extent due to an imbalance in their IL-12/IL-23 secretory profile caused by PGE2 (22, 48). All these results suggest that non-lymphoid paracrine PGE2 governs Th17 expansion. Dissecting the relative contribution of PGE2 by different cell types allowed us to determine that mPGES1 competence is necessary in both cell types during cognate APC-CD4+ interactions. We demonstrate that presence of mPGES1 is strikingly important to mount IL-17A and IFNγ antigen-specific responses upon antigen challenge in BMDC/CD4+CD25− co-culture assays (Fig. 4). Most interestingly, requirement for mPGES1 revealed both an autocrine and paracrine role, with mPGES1 presence acting synergistically on APC and CD4+ cells to achieve optimal generation of IL-17A and IFNγ. This is consistent with the capacity mPGES1 in BMDCs to specifically enhance IL-12 production (15). Stimulation of WT BMDCs with LPS prior to incubation increased IL-17A production in a synergistic manner with WT T cells (Fig. 4B), effect that was not observed for IFNγ or in the absence of mPGES1 in BMDCs. Moreover, autocrine cytokine production in absence of APC proved again to be largely dependent on mPGES1, with 5-fold lower IL-17A and IL-6 responses in mPGES1−/− cells (Fig. 4C). IFNγ responses were however different, as they showed less dependency on mPGES1 competence in BMDCs, while it was necessary on T cells (Fig. 4). Importantly, cytokine production in all these instances was fully abolished by specific inhibition of COX-2, demonstrating the requirement of upstream PGH2 generation and the specificity of mPGES1 activity, as blockade of COX-2 generated cytokine values virtually identical to those from mPGES1−/− CD4+CD25− cells.
Although the transcriptional control of CD4+ T cell commitment to determined cytokine-biased fates has been vastly studied, much less is known about how PGE2 affects the molecular mechanisms behind it. RORγt binds the ptger2 promoter and represses EP2 expression in mouse and human CD4+ cells. Additionally, Th17 cells from patients with MS exhibited reduced RORC binding to the ptger2 promoter region, which promotes IFNγ and GM-CSF production in such cells (33). EP2 expression can be partially restored by increasing TCR signal strength as well in RORC+ cells. Surprisingly, it has been recently reported that Tbet or continued RORγt expression is not strictly required for Th17-associated immunopathology in mouse models of H. hepaticus-induced intestinal inflammation or EAE (49). We report that in naïve CD4+ cells, exogenous PGE2 downregulates RORγt and IL-17A expression during Th17 polarization and at the same time shifts production of IL-17A to IFNγ. Expression of IFNγ does not seem to happen through a re-conversion of previously IL-17A+ cells, as we did not observe any differences in double IL-17A+IFNγ+ cells. On the other hand, during Th1 polarization PGE2 did not alter expression of Tbet, but it increased proliferation of IFNγ+ cells (Fig. 5E–F) and IFNγ production (Fig. 6C), demonstrating that Tbet and RORγt-expressing cells are differentially sensitive to PGE2 concentrations. Our results also implicate that the effects of PGE2 provided exogenously or produced in an autocrine manner by T cells cannot be considered equivalent. Early exposure of naïve CD4+ T cells to high PGE2 concentrations in the 50–100 nM range indiscriminately affect T cell proliferation as well as function (Supl. Fig. 2). At intermediate concentrations (10 nM), PGE2 has the capacity to provide competitive proliferative advantage to IL-17A+ or IFNγ+ cells (Fig. 5F–G) and suppression of IL-17A together with enhancement of IFNγ responses (Figs. 5 and 6). In contrast, gradual accumulation of PGE2 during T cell proliferation in vivo enhances Th17 but not Th1 (Figs 1–4). This might be reconciled when considering that EP4, which has a higher affinity for PGE2 than EP2 (50), is strongly downregulated during Th17 polarization (Fig. 2C), and hence will render T cells less responsive to newly generated PGE2. Alternatively, it is possible that the Th17 bias imposed by PGE2 is subordinated to strong T cell activation accompanied by IL-2, so that when PGE2 concentrations inhibit proliferation this effect is subverted, and Th1 cells have a competitive advantage. Our data is therefore consistent with previous reports that PGE2 can favor a Th1 response under certain circumstances (36), as it has been shown to be the case for promoting IFNγ-producing Th17 cells in MS patients (33).
Differentiation of T cell phenotypes that rely on TGFβ and IL-2 like Tregs and Th17 cells require a TCR signal that is less robust than in other T effector responses (51, 52). Phosphorylation at Tyr705 in STAT3 is induced by IL-6 and IL-23, involves the PI3K-Akt-mTORC1 pathway and controls Th17 commitment and function (53, 54). In contrast, during T cell priming, IL2-induced STAT5 restricts Th17 commitment, and STAT3 induces aiolos to silence the il2 locus (55, 56) while it generally promotes regulatory T cell expansion (51, 57). However, STAT5 phosphorylation is also reduced by IL-2 under Th17 polarizing conditions (58). Reconciliation of how STAT3 and STAT5 signals are integrated to result in such a diversity of outcomes in T cell responses is still needed. Inflammatory cytokines like IL-1β, IL-6 and IL-23, together with other environmental signals like retinoic acid (59) also highly modify STAT3 and STAT5-mediated signaling (57). A balance-tipping antagonism between regulatory T cell and Th17 cell commitment due to the integration of IL-1 and retinoic acid signals was recently unveiled by Basu et al., demonstrating that STAT3 and STAT5 antagonize each other by binding reciprocally similar sites on the Foxp3 and il-17a/IL-17f regulatory regions (59). We show that PGE2 can downregulate phosphorylation of both STAT3 and STAT5 under Th17 polarizing conditions (Fig. 5B), which might partially explain its capacity to inhibit IL-17A while promoting IFNγ. However the precise molecular mechanisms that explain how these phenomena are achieved remain to be resolved.
Our results reveal an added layer of interactivity among APCs and T cells during immune responses that is co-dependent on these cells capacities to regulate PGE2 through mPGES1. In lieu of the evidence for T cell fate and cytokine potential plasticity (60) and our results, it is tempting to suggest that PGE2 might constitute a significant modulator of regulatory to effector T cell transitions during later phases of inflammatory responses, which we are currently studying. The concomitant production and sensing of PGE2 by different cell types might contribute to T plasticity and how this attunes with our knowledge of the properties of PGE2 in mounting but also resolving inflammatory responses. The relevance of T cell-derived PGE2 might be as well highlighted in lymphoid organs or infiltrates, where T cell density is high and IL-2 becomes limiting during proliferative responses, and is also able to influence the effector versus regulatory fate.
In conclusion, mPGES1 deficiency results in loss of induced PGE2 expression but preservation of other PGs. Our in vivo data demonstrating that mPGES1 deficiency inhibits the capacity of CD4+ cells to become Th17 cells is consistent with previous data showing that increased PGE2 promotes Th17 responses in vivo. Our research supports the notion that Th17 cells are more significantly affected by PGE2 generated by cognate interactions in the in vivo microenvironment than are Th1 cells. Our results also highlight the fact that there is both autocrine (T cell) and paracrine (APC or stromal cells) mPGES1-dependent PGE2 during CD4+ cell stimulation, of different nature and magnitude but with synergistic enhancing effects on cytokine production. We also demonstrate that the variations are not solely due to sensing of PGE2 (EP2 /EP4 genetic deficiency and/or agonist drug studies) or upstream generalized COX-PG blockade, but derive from responses that are controlled by the inducible enzyme mPGES1 and are hence highly PGE2-specific and physiologically relevant during inflammatory responses. Our research provides therefore a rationale for understanding how different concentrations of PGE2 can fine-tune T cell commitment into regulatory, Th1 or Th17 types, and hence locally re-shape the pro- and anti-inflammatory T cell landscape during inflammatory responses.
Supplementary Material
Acknowledgments
This work was supported by an R01 grant to Dr. Crofford (5R01AR049010-11) and a Career Development Award from the Crohn’s and Colitis Foundation of America to Dr. Maseda.
mPGES1 null mice were a gift from Pfizer. The authors would like to thank Drs. James Thomas and Peggy Kendall for their valuable input and comments.
Bibliography
- 1.Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol (Baltimore, Md 1950) 2012;188:21–28. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park JY, Pillinger MH, Abramson SB. Prostaglandin E2 synthesis and secretion: the role of PGE2 synthases. Clin Immunol. 2006;119:229–240. doi: 10.1016/j.clim.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 3.Kojima F, Kapoor M, Yang L, Fleishaker EL, Ward MR, Monrad SU, Kottangada PC, Pace CQ, Clark JA, Woodward JG, Crofford LJ. Defective generation of a humoral immune response is associated with a reduced incidence and severity of collagen-induced arthritis in microsomal prostaglandin E synthase-1 null mice. J Immunol. 2008;180:8361–8. doi: 10.4049/jimmunol.180.12.8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westman M, Korotkova M, Af Klint E, Stark A, Audoly LP, Klareskog L, Ulfgren AK, Jakobsson PJ. Expression of microsomal prostaglandin E synthase 1 in rheumatoid arthritis synovium. Arthritis Rheum. 2004;50:1774–1780. doi: 10.1002/art.20286. [DOI] [PubMed] [Google Scholar]
- 5.Greenhough A, Smartt HJM, Moore AE, Roberts HR, Williams AC, Paraskeva C, Kaidi A. The COX-2/PGE2 pathway: Key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30:377–386. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- 6.Nakanishi M, Rosenberg DW. Multifaceted roles of PGE2 in inflammation and cancer. Semin Immunopathol. 2013;35:123–137. doi: 10.1007/s00281-012-0342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roulis M, Nikolaou C, Kotsaki E, Kaffe E, Karagianni N, Koliaraki V, Salpea K, Ragoussis J, Aidinis V, Martini E, Becker C, Herschman HR, Vetrano S, Danese S, Kollias G. Intestinal myofibroblast-specific Tpl2-Cox-2-PGE2 pathway links innate sensing to epithelial homeostasis. Proc Natl Acad Sci U S A. 2014;111:E4658–67. doi: 10.1073/pnas.1415762111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 9.Sreeramkumar V, Hons M, Punzón C, Stein JV, Sancho D, Fresno M, Cuesta N. Efficient T-cell priming and activation requires signaling through prostaglandin E2 (EP) receptors. Immunol Cell Biol. 2016;94:39–51. doi: 10.1038/icb.2015.62. [DOI] [PubMed] [Google Scholar]
- 10.Sreeramkumar V, Fresno M, Cuesta N. Prostaglandin E2 and T cells: friends or foes? Immunol Cell Biol. 2012;90:579–86. doi: 10.1038/icb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jakobsson PJ, Thorén S, Morgenstern R, Samuelsson B. Identification of human prostaglandin E synthase: a microsomal, glutathione-dependent, inducible enzyme, constituting a potential novel drug target. Proc Natl Acad Sci U S A. 1999;96:7220–5. doi: 10.1073/pnas.96.13.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kojima F, Frolov A, Matnani R, Woodward JG, Crofford LJ. Reduced T cell-dependent humoral immune response in microsomal prostaglandin E synthase-1 null mice is mediated by nonhematopoietic cells. J Immunol. 2013;191:4979–4988. doi: 10.4049/jimmunol.1301942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korotkova M, Jakobsson PJ. Microsomal prostaglandin e synthase-1 in rheumatic diseases. Front Pharmacol. 2010;1:146. doi: 10.3389/fphar.2010.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xue X, Shah YM. Hypoxia-inducible factor-2α is essential in activating the COX2/mPGES-1/PGE2 signaling axis in colon cancer. Carcinogenesis. 2013;34:163–169. doi: 10.1093/carcin/bgs313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monrad SU, Kojima F, Kapoor M, Kuan EL, Sarkar S, Randolph GJ, Crofford LJ. Genetic deletion of mPGES-1 abolishes PGE2 production in murine dendritic cells and alters the cytokine profile, but does not affect maturation or migration. Prostaglandins Leukot Essent Fat Acids. 2011;84:113–121. doi: 10.1016/j.plefa.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sasaki Y, Nakatani Y, Hara S. Role of microsomal prostaglandin E synthase-1 (mPGES-1)-derived prostaglandin E2 in colon carcinogenesis. Prostaglandins Other Lipid Mediat. 2015;121:42–45. doi: 10.1016/j.prostaglandins.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Sheibanie AF, Khayrullina T, Safadi FF, Ganea D. Prostaglandin E2 exacerbates collagen-induced arthritis in mice through the inflammatory interleukin-23/interleukin-17 axis. Arthritis Rheum. 2007;56:2608–2619. doi: 10.1002/art.22794. [DOI] [PubMed] [Google Scholar]
- 18.Duffin R, O’Connor RA, Crittenden S, Forster T, Yu C, Zheng X, Smyth D, Robb CT, Rossi F, Skouras C, Tang S, Richards J, Pellicoro A, Weller RB, Breyer RM, Mole DJ, Iredale JP, Anderton SM, Narumiya S, Maizels RM, Ghazal P, Howie SE, Rossi AG, Yao C. Prostaglandin E2 constrains systemic inflammation through an innate lymphoid cell-IL-22 axis. Science. 2016;351:1333–8. doi: 10.1126/science.aad9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacKenzie KF, Clark K, Naqvi S, McGuire VA, Nöehren G, Kristariyanto Y, van den Bosch M, Mudaliar M, McCarthy PC, Pattison MJ, Pedrioli PGA, Barton GJ, Toth R, Prescott A, Arthur JSC, Van Den Bosch M, Mudaliar M, Pierre C, Pattison MJ, Pedrioli PGA, Barton J, Toth R, Prescott A, Arthur JSC. PGE(2) induces macrophage IL-10 production and a regulatory-like phenotype via a protein kinase A-SIK-CRTC3 pathway. J Immunol. 2013;190:565–77. doi: 10.4049/jimmunol.1202462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joshi PC, Zhou XC, Cuchens M, Jones Q. Prostaglandin E-2 suppressed IL-15-mediated human NK cell function through down-regulation of common gamma-chain. J Immunol. 2001;166:885–891. doi: 10.4049/jimmunol.166.2.885. [DOI] [PubMed] [Google Scholar]
- 21.Rieser C, Bock G, Klocker H, Bartsch G, Thurnher M. Prostaglandin E2 and tumor necrosis factor alpha cooperate to activate human dendritic cells: synergistic activation of interleukin 12 production. J Exp Med. 1997;186:1603–1608. doi: 10.1084/jem.186.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khayrullina T, Yen JH, Jing H, Ganea D. In vitro differentiation of dendritic cells in the presence of prostaglandin E2 alters the IL-12/IL-23 balance and promotes differentiation of Th17 cells. J Immunol. 2008;181:721–35. doi: 10.4049/jimmunol.181.1.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muthuswamy R, Mueller-Berghaus J, Haberkorn U, Reinhart TA, Schadendorf D, Kalinski P. PGE2 transiently enhances DC expression of CCR7 but inhibits the ability of DCs to produce CCL19 and attract naive T cells. Blood. 2010;116:1454–1459. doi: 10.1182/blood-2009-12-258038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rincon M, Tugores A, Lopez-Rivas A, Silva A, Alonso M, De Landazuri MO, Lopez-Botet M. Prostaglandin E2 and the increase of intracellular cAMP inhibit the expression of interleukin 2 receptors in human T cells. Eur J Immunol. 1988;18:1791–1796. doi: 10.1002/eji.1830181121. [DOI] [PubMed] [Google Scholar]
- 25.Boniface K, Bak-Jensen KS, Li Y, Blumenschein WM, McGeachy MJ, McClanahan TK, McKenzie BS, Kastelein RA, Cua DJ, de Waal Malefyt R. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J Exp Med. 2009;206:535–48. doi: 10.1084/jem.20082293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esaki Y, Li Y, Sakata D, Yao C, Segi-Nishida E, Matsuoka T, Fukuda K, Narumiya S. Dual roles of PGE2-EP4 signaling in mouse experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2010;107:12233–8. doi: 10.1073/pnas.0915112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Betz M, Fox BS. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J Immunol. 1991;146:108–113. [PubMed] [Google Scholar]
- 28.Bryn T, Yaqub S, Mahic M, Henjum K, Aandahl EM, Tasén K. LPS-activated monocytes suppress T-cell immune responses and induce FOXP3+ T cells through a COX-2-PGE2-dependent mechanism. Int Immunol. 2008;20:235–245. doi: 10.1093/intimm/dxm134. [DOI] [PubMed] [Google Scholar]
- 29.Hilkens CM, Vermeulen H, van Neerven RJ, Snijdewint FG, Wierenga EA, Kapsenberg ML. Differential modulation of T helper type 1 (Th1) and T helper type 2 (Th2) cytokine secretion by prostaglandin E2 critically depends on interleukin-2. Eur J Immunol. 1995;25:59–63. doi: 10.1002/eji.1830250112. [DOI] [PubMed] [Google Scholar]
- 30.Chizzolini C, Chicheportiche R, Alvarez M, De Rham C, Roux-Lombard P, Ferrari-Lacraz S, Dayer JM. Prostaglandin E2 synergistically with interleukin-23 favors human Th17 expansion. Blood. 2008;112:3696–3703. doi: 10.1182/blood-2008-05-155408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao C, Sakata D, Esaki Y, Li Y, Matsuoka T, Kuroiwa K, Sugimoto Y, Narumiya S. Prostaglandin E2-EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion. Nat Med. 2009;15:633–40. doi: 10.1038/nm.1968. [DOI] [PubMed] [Google Scholar]
- 32.Napolitani G, Acosta-Rodriguez EV, Lanzavecchia A, Sallusto F. Prostaglandin E2 enhances Th17 responses via modulation of IL-17 and IFN-γ production by memory CD4+ T cells. Eur J Immunol. 2009;39:1301–1312. doi: 10.1002/eji.200838969. [DOI] [PubMed] [Google Scholar]
- 33.Kofler DM, Marson A, Dominguez-Villar M, Xiao S, Kuchroo VK, Hafler DA. Decreased RORC-dependent silencing of Prostaglandin receptor EP2 induces autoimmune Th17 cells. J Clin Invest. 2014;124:2513–2522. doi: 10.1172/JCI72973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fabricius D, Neubauer M, Mandel B, Schütz C, Viardot A, Vollmer A, Jahrsdörfer B, Debatin KM. Prostaglandin E2 inhibits IFN-alpha secretion and Th1 costimulation by human plasmacytoid dendritic cells via E-prostanoid 2 and E-prostanoid 4 receptor engagement. J Immunol. 2010;184:677–684. doi: 10.4049/jimmunol.0902028. [DOI] [PubMed] [Google Scholar]
- 35.Bloom D, Jabrane-Ferrat N, Zeng L, Wu a, Li L, Lo D, Turck CW, An S, Goetzl EJ. Prostaglandin E2 enhancement of interferon-gamma production by antigen-stimulated type 1 helper T cells. Cell Immunol. 1999;194:21–7. doi: 10.1006/cimm.1999.1479. [DOI] [PubMed] [Google Scholar]
- 36.Yao C, Hirata T, Soontrapa K, Ma X, Takemori H, Narumiya S. Prostaglandin E2 promotes Th1 differentiation via synergistic amplification of IL-12 signalling by cAMP and PI3-kinase. Nat Commun. 2013;4:1685. doi: 10.1038/ncomms2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahic M, Yaqub S, Johansson CC, Tasken K, Aandahl EM. FOXP3+CD4+CD25+ Adaptive Regulatory T Cells Express Cyclooxygenase-2 and Suppress Effector T Cells by a Prostaglandin E2-Dependent Mechanism. J Immunol. 2006;177:246–254. doi: 10.4049/jimmunol.177.1.246. [DOI] [PubMed] [Google Scholar]
- 38.Iñiguez MA, Punzón C, Fresno M. Induction of cyclooxygenase-2 on activated T lymphocytes: Regulation of T cell activation by cyclooxygenase-2 inhibitors. J Immunol. 1999;163:111–119. [PubMed] [Google Scholar]
- 39.Rodriguez-Rodriguez L, Ivorra-Cortes J, Carmona FD, Martin J, Balsa A, van Steenbergen HW, van der Helm-van Mil AH, Gonzalez-Alvaro I, Fernandez-Gutierrez B. PTGER4 gene variant rs76523431 is a candidate risk factor for radiological joint damage in rheumatoid arthritis patients: a genetic study of six cohorts. Arthritis Res Ther. 2015;17:306. doi: 10.1186/s13075-015-0830-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, Essers J, Mitrovic M, Ning K, Cleynen I, Theatre E, Spain SL, Raychaudhuri S, Goyette P, Wei Z, Abraham C, Achkar JP, Ahmad T, Amininejad L, Ananthakrishnan AN, Andersen V, Andrews JM, Baidoo L, Balschun T, Bampton PA, Bitton A, Boucher G, Brand S, Büning C, Cohain A, Cichon S, D’Amato M, De Jong D, Devaney KL, Dubinsky M, Edwards C, Ellinghaus D, Ferguson LR, Franchimont D, Fransen K, Gearry R, Georges M, Gieger C, Glas J, Haritunians T, Hart A, Hawkey C, Hedl M, Hu X, Karlsen TH, Kupcinskas L, Kugathasan S, Latiano A, Laukens D, Lawrance IC, Lees CW, Louis E, Mahy G, Mansfield J, Morgan AR, Mowat C, Newman W, Palmieri O, Ponsioen CY, Potocnik U, Prescott NJ, Regueiro M, Rotter JI, Russell RK, Sanderson JD, Sans M, Satsangi J, Schreiber S, Simms LA, Sventoraityte J, Targan SR, Taylor KD, Tremelling M, Verspaget HW, De Vos M, Wijmenga C, Wilson DC, Winkelmann J, Xavier RJ, Zeissig S, Zhang B, Zhang CK, Zhao H, Silverberg MS, Annese V, Hakonarson H, Brant SR, Radford-Smith G, Mathew CG, Rioux JD, Schadt EE, Daly MJ, Franke A, Parkes M, Vermeire S, Barrett JC, Cho JH. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–24. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akaogi J, Nozaki T, Satoh M, Yamada H. Role of PGE2 and EP receptors in the pathogenesis of rheumatoid arthritis and as a novel therapeutic strategy. Endocr Metab Immune Disord Drug Targets. 2006;6:383–94. doi: 10.2174/187153006779025711. [DOI] [PubMed] [Google Scholar]
- 42.Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol (Baltimore, Md 1950) 2012;188:21–28. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lubberts E. The IL-23-IL-17 axis in inflammatory arthritis. Nat Rev Rheumatol. 2015;11:415–429. doi: 10.1038/nrrheum.2015.53. [DOI] [PubMed] [Google Scholar]
- 44.Sarkar S, Justa S, Brucks M, Endres J, Fox DA, Zhou X, Alnaimat F, Whitaker B, Wheeler JC, Jones BH, Bommireddy SR. Interleukin (IL)-17A, F and AF in inflammation: A study in collagen-induced arthritis and rheumatoid arthritis. Clin Exp Immunol. 2014;177:652–661. doi: 10.1111/cei.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Genovese MC, Durez P, Richards HB, Supronik J, Dokoupilova E, Aelion Ja, Lee S-H, Codding CE, Kellner H, Ikawa T, Hugot S, Ligozio G, Mpofu S. One-year Efficacy and Safety Results of Secukinumab in Patients With Rheumatoid Arthritis: Phase II, Dose-finding, Double-blind, Randomized, Placebo-controlled Study. J Rheumatol. 2014;41:414–21. doi: 10.3899/jrheum.130637. [DOI] [PubMed] [Google Scholar]
- 46.Mease PJ, I, McInnes B, Kirkham B, Kavanaugh A, Rahman P, van der Heijde D, Landewé R, Nash P, Pricop L, Yuan J, Richards HB, Mpofu S. Secukinumab Inhibition of Interleukin-17A in Patients with Psoriatic Arthritis. N Engl J Med. 2015;373:1329–1339. doi: 10.1056/NEJMoa1412679. [DOI] [PubMed] [Google Scholar]
- 47.Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N, Gillespie MT, Martin TJ, Suda T. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheibanie AF, Tadmori I, Jing H, Vassiliou E, Ganea D. Prostaglandin E2 induces IL-23 production in bone marrow-derived dendritic cells. FASEB J. 2004;18:1318–1320. doi: 10.1096/fj.03-1367fje. [DOI] [PubMed] [Google Scholar]
- 49.Veldhoen Innocentin M, Kamdar S, Withers DR, Verena Brucklacher-Waldert MC, Ferreira C, Brucklacher-Waldert V, Innocentin S, Kullberg MC, Veldhoen M. Tbet or Continued RORgt Expression Is Not Required for Th17-Associated Immunopathology. J Immunol. 2016;196:4893–4904. doi: 10.4049/jimmunol.1600137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dey I, Lejeune M, Chadee K. Prostaglandin E2 receptor distribution and function in the gastrointestinal tract. Br J Pharmacol. 2006;149:611–623. doi: 10.1038/sj.bjp.0706923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamane H, Paul WE. Cytokines of the γc family control CD4+ T cell differentiation and function. Nat Immunol. 2012;13:1037–1044. doi: 10.1038/ni.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tubo NJ, Jenkins MK. TCR signal quantity and quality in CD4+ T cell differentiation. Trends Immunol. 2014;35:591–596. doi: 10.1016/j.it.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ciofani M, Madar A, Galan C, Sellars M, MacE K, Pauli F, Agarwal A, Huang W, Parkurst CN, Muratet M, Newberry KM, Meadows S, Greenfield A, Yang Y, Jain P, Kirigin FK, Birchmeier C, Wagner EF, Murphy KM, Myers RM, Bonneau R, Littman DR. A validated regulatory network for Th17 cell specification. Cell. 2012;151:289–303. doi: 10.1016/j.cell.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim JS, Sklarz T, Banks LB, Gohil M, Waickman AT, Skuli N, Krock BL, Luo CT, Hu W, Pollizzi KN, Li MO, Rathmell JC, Birnbaum MJ, Powell JD, Jordan MS, Koretzky GA. Natural and inducible TH17 cells are regulated differently by Akt and mTOR pathways. Nat Immunol. 2013;14:611–618. doi: 10.1038/ni.2607. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O’Shea JJ. Interleukin-2 Signaling via STAT5 Constrains T Helper 17 Cell Generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 56.Quintana FJ, Jin H, Burns EJ, Nadeau M, Yeste A, Kumar D, Rangachari M, Zhu C, Xiao S, Seavitt J, Georgopoulos K, Kuchroo VK. Aiolos promotes TH17 differentiation by directly silencing Il2 expression. Nat Immunol. 2012;13:770–777. doi: 10.1038/ni.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 58.Veldhoen M, Hirota K, Christensen J, O’Garra A, Stockinger B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J Exp Med. 2009;206:43–9. doi: 10.1084/jem.20081438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Basu R, Whitley SK, Bhaumik S, Zindl CL, Schoeb TR, Benveniste EN, Pear WS, Hatton RD, Weaver CT. IL-1 signaling modulates activation of STAT transcription factors to antagonize retinoic acid signaling and control the TH17 cell–iTreg cell balance. Nat Immunol. 2015;16:286–95. doi: 10.1038/ni.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DuPage M, Bluestone JA. Harnessing the plasticity of CD4(+) T cells to treat immune-mediated disease. Nat Rev Immunol. 2016;16:149–163. doi: 10.1038/nri.2015.18. [DOI] [PubMed] [Google Scholar]
- 61.Brand DD, Latham KA, Rosloniec EF. Collagen-induced arthritis. Nat Protoc. 2007;2:1269–1275. doi: 10.1038/nprot.2007.173. [DOI] [PubMed] [Google Scholar]
- 62.Lutz MB, Kukutsch N, Ogilvie AL, Rößner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.