Abstract
Background
In individuals with a low diastolic blood pressure (DBP), potential benefits or risks of intensive systolic blood pressure (SBP) lowering are unclear.
Methods
The Systolic Blood Pressure Intervention Trial was a randomized, controlled trial that compared the effects of intensive (target <120 mm Hg) versus standard (target <140 mm Hg) SBP control in 9361 older adults with high blood pressure at increased risk of cardiovascular disease (CVD). The primary outcome was a composite of CVD events. All-cause death and incident CKD were secondary outcomes. This post-hoc analysis examined whether the effects of the SBP intervention differed by baseline DBP.
Results
Mean baseline SBP and DBP were 139.7 ± 15.6 and 78.1 ± 11.9 mm Hg, respectively. Irrespective of the randomized treatment, baseline DBP had a U-shaped association with the hazard of the primary CVD outcome. However, the effects of the intensive SBP intervention on the primary outcome was not influenced by baseline DBP level (p for interaction 0.83). The primary outcome hazard ratio for intensive versus standard treatment was 0.78 (95% CI 0.57 to 1.07) in the lowest DBP quintile (mean baseline DBP 61 ± 5 mm Hg) and 0.74 (95% CI 0.61 to 0.90) in the upper four DBP quintiles (mean baseline DBP 82 ± 9 mm Hg), with an interaction p-value = 0.78. Results were similar for all-cause death and kidney events.
Conclusions
Low baseline DBP was associated with increased risk of CVD events, but there was no evidence that the benefit of the intensive SBP lowering differed by baseline DBP.
Clinical Trial Registration
URL: https://clinicaltrials.gov Unique Identifier: NCT01206062
Keywords: hypertension, J-curve, Diastolic blood pressure
Elevated blood pressure (BP) is an important risk factor for cardiovascular disease (CVD)1, 2, end-stage kidney disease (ESKD)3, 4 and all-cause mortality2, 5. Beginning in the 1960s, randomized controlled trials demonstrated the value of treating high diastolic BP (DBP) and subsequently high systolic BP (SBP)6, 7. Recently, the Systolic Blood Pressure Intervention Trial (SPRINT) demonstrated that intensive SBP lowering (SBP target <120 versus <140 mm Hg) improved CVD outcomes and all-cause mortality in adults at high risk for CVD events8, even in those ≥ 75 years.9
Despite the documented value of traditional treatment in adults with a high DBP6, intensive therapy to low levels of DBP is controversial. Nearly 30 years ago, a J-shaped relationship was observed between on-treatment DBP and death from myocardial infarction, with the risk being lowest in those with an achieved DBP between 85–90 mm Hg and higher at achieved DBP levels on either side of this range10, 11.
We examined the hypothesis that low baseline DBP adversely modifies the effect of intensive SBP lowering on CVD, kidney disease and all-cause mortality in SPRINT8. In addition, we examined whether baseline pulse pressure (PP) or mean arterial pressure (MAP) modified the effects of the SPRINT intervention.
Methods
Limited SPRINT data are available through NHLBI at https://biolincc.nhlbi.nih.gov/studies/sprint_pop for reproducing/ replicating the results of this analysis. Statistical methods section and supplemental material provide details of analytical procedures.SPRINT was a randomized, controlled, open-label trial that compared the effects of intensive (SBP target < 120 mm Hg) versus standard (SBP target < 140 mm Hg) BP control in 9361 participants from the US and Puerto Rico8. Details of the SPRINT protocol have been published12, 13. Institutional review boards at each of the participating study sites approved the protocol and all participants provided informed consent.
Study population
Participants had to be ≥ 50 years with an SBP 130 to 180 mm Hg and an increased risk of CVD (defined as having at least one of the following: clinical or subclinical CVD other than stroke; 10-year risk of CVD ≥15%, based on the Framingham global risk indicator14; age ≥75 years; or estimated glomerular filtration rate (eGFR) 20 to < 60 ml/min/1.73 m2). Major exclusion criteria included diabetes, prior stroke, advanced CKD (eGFR <20 ml/min/1.73m2), proteinuria >1 g/d, polycystic kidney disease, congestive heart failure, dementia or residence in a nursing home.
Intervention, follow-up and measurements
Participants were randomly assigned to intensive or standard SBP control, stratified by clinical site. Details of the SPRINT intervention algorithm and medication formulary are provided elsewhere12, 13. Participants were seen monthly for 3 months and quarterly thereafter for standardized study visits by trained study staff following protocol requirements. An automated measurement system (Model 907XL, Omron Healthcare) was used to record BP at the clinic visit after the participant had been seated for 5 minutes of quiet rest. The mean of three BP readings, each one minute apart, was used to estimate BP.
Medications were adjusted to target a SBP <120 mm Hg in the intensive-treatment group and a SBP of 135 to 139 mm Hg in the standard treatment group. Blood specimens were obtained at each visit for the first three months and quarterly thereafter for measurement of serum creatinine. The four-variable MDRD equation was used to estimate GFR15. Event ascertainment and safety assessments were performed per protocol. 12, 13
SPRINT outcomes
The primary outcome was a composite of non-fatal myocardial infarction, acute coronary syndrome not resulting in myocardial infarction, stroke, acute decompensated heart failure, or death from CVD. Death from any cause was a predefined secondary outcome in SPRINT. All outcome events were adjudicated by a committee blinded to treatment assignment. In the current analysis, we also explored a composite CVD outcome that excluded stroke.
The main secondary kidney outcome was a composite of ≥ 50% decrease in eGFR or development of ESRD in participants with baseline CKD (eGFR <60ml/min/1.73m2). A secondary kidney outcome was incident CKD defined as >30% decrease in eGFR (with a value <60 ml/min/1.73m2, confirmed at the next available SPRINT blood draw) in participants without CKD at baseline. In addition, we monitored for serious adverse events as reported earlier8. A decision to discontinue the SPRINT BP intervention was made on August 20, 2015 after interim analyses showed the primary outcome had exceeded preset monitoring boundaries on two consecutive occasions.8 Our analysis is based on information provided in the SPRINT public access BioLINCC database16. It includes events that occurred on or before the trial was stopped on August 20, 2015 and were recognized using a data freeze date of October 14th, 2015.
Statistical methods
We performed all analyses in STATA version MP 14.0 or SAS version 9.4, and used a 2-sided α=0.05 for hypothesis testing, without adjustment for multiple comparisons. We compared baseline characteristics between DBP quintiles using 1-way analysis of variance (ANOVA) for numeric variables (following log transformation for the albumin to creatinine ratio) and used chi-square tests for categorical variables.
We computed the mean follow-up DBP for each patient by averaging their BP measurements from month 3 to the last reading. We used boxplots to display the patients’ mean follow-up DBP values by quintile of baseline DBP within the intensive and standard groups, and applied 2-sample tests to compare mean follow-up DBP between the lowest and highest quintile of baseline DBP.
We analyzed the association of baseline DBP with the primary and secondary outcomes by fitting a Cox regression model with the randomized SBP intervention and cubic spline terms in baseline DBP as predictor variables, with covariable adjustment for age, sex and race. We then performed three types of analyses based on Cox proportional hazards regression to investigate whether the effects of intensive SBP intervention on the primary and secondary outcomes differed depending on baseline level of DBP. The primary analysis of the interaction between the intensive SBP intervention and baseline DBP, which was specified prior to initiation of these post-hoc analyses, compared the hazard ratio for the effect of intensive SBP intervention on the primary CVD composite outcome between the lowest baseline DBP quintile and the upper four quintiles. Second, we investigated the possibility of a more general interaction by fitting a Cox regression for the primary outcome with main effects for the SBP intervention and cubic spline terms for baseline DBP, plus multiplicative interactions between the SBP intervention and the cubic spline terms. In the absence of evidence of a nonlinear interaction (indicated by an interaction p > 0.10), we refit the Cox regression using a linear interaction between the SBP intervention and baseline DPB. Third, we provided hazard ratios with 95% confidence intervals to compare the intensive vs. usual SBP goals within each baseline DPB quintile. These hazard ratios are presented to provide a comprehensive presentation of the results; however, it is important to note much or all of the reported variation in hazard ratios between the quintile subgroups is due to chance, and that in the absence of a statistically significant interactions the best estimate of the effect of the intervention is given by the study-wide effect estimate, including all patients.
We performed similar analyses of the interaction between the SBP intervention and baseline DBP for the secondary outcomes, except that we categorized baseline DBP by a median split rather than quintiles for the kidney composite outcome due to the small number of events for this outcome.
We repeated each of these three analyses to evaluate interactions of the BP intervention with baseline MAP and baseline PP. We also provided hazard ratios from Cox regressions comparing the intensive vs. usual SBP interventions by baseline DBP quintile for the safety outcomes: all serious adverse events, hypotension, syncope, electrolyte abnormality and acute kidney injury or acute kidney failure.
In participants with and without baseline clinical/subclinical cardiovascular disease, we performed additional sensitivity analyses in separate Cox Models to evaluate the linear interaction of the SPRINT intervention with baseline DBP.
Additional details of the Cox regression models are provided in supplemental material.
Results
Mean age of the study population (N = 9361) was 67.9 ± 9.4 years, with 35.6 % being women and 31.5 % Black. Means (± SD) baseline SBP and DBP were 139.7 ± 15.6 and 78.1 ± 11.9 mm Hg, respectively. Baseline demographic, clinical and laboratory characteristics of the study population by DBP quintile are summarized in Table 1. In general, participants with lower DBP tended to be older, have a higher baseline prevalence of CVD and CKD, be on more antihypertensive medications, have a lower baseline SBP and MAP and a higher PP, and have a lower estimated GFR.
Table 1.
Baseline characteristics by baseline quintiles of diastolic blood pressure (N=9361)
| 1st quintile | 2nd quintile | 3rd quintile | 4th quintile | 5th quintile | |
|---|---|---|---|---|---|
| < 68 mm Hg | 68 – 74 | 75 – 80 | 81 – 87 | ≥ 88 | |
| (N=1749) | (N=1874) | (N=1816) | (N=1934) | (N=1988) | |
| Diastolic blood pressure, (mmHg) | 61 ± 5 | 71 ± 2 | 78 ± 2 | 84 ± 2 | 95 ± 6 |
| Age, (year) | 74.7 ± 8.2 | 70.3 ± 8.8 | 68.0 ± 8.5 | 65.2 ± 8.3 | 62.3 ± 8.3 |
| Female sex, (%) | 39.5 | 36.0 | 35.8 | 32.3 | 34.9 |
| Black race, (%) | 23.2 | 25.1 | 29.7 | 33.6 | 44.4 |
| History of cardiovascular disease, (%) | 29.1 | 24.1 | 18.0 | 15.3 | 14.9 |
| Chronic kidney disease, (%) | 42.3 | 29.8 | 27.6 | 23.2 | 20.1 |
| Framingham 10-year CVD risk score ≥15%, (%) | 60.3 | 59.5 | 60.3 | 60.8 | 67.2 |
| Never smoked, (%) | 43.2 | 43.6 | 45.5 | 44.6 | 43.3 |
| Antihypertensive agents, (no./patient) | 2.1 ± 1.0 | 1.9 ± 1.0 | 1.8 ± 1.0 | 1.7 ± 1.0 | 1.6 ± 1.1 |
| Systolic blood pressure, (mmHg)* | 131 ± 15 | 134 ± 13 | 138 ± 13 | 142 ± 13 | 152 ± 15 |
| Pulse pressure, (mmHg) | 70 ± 15 | 63 ± 14 | 61 ± 13 | 58 ± 13 | 57 ± 13 |
| Mean arterial pressure, (mmHg) | 85 ± 6 | 92 ± 5 | 98 ± 5 | 103 ± 5 | 114 ± 8 |
| Body-mass index, (kg/m2) | 28.3 ± 5.3 | 29.4 ± 5.7 | 30.0 ± 5.7 | 30.5 ± 5.8 | 30.8 ± 6.0 |
| Estimated GFR, (ml/min/1.73 m2) | 65 ± 20 | 70 ± 20 | 72 ± 20 | 75 ± 20 | 76 ± 21 |
| Urine ACR, (mg/g) | 10.7 (6.2,24.8) | 9.4 (5.6,20.3) | 8.5 (5.2,18.7) | 8.9 (5.4,20.5) | 10.2 (6.1,24.6) |
Results are presented as percents for binary variables and as mean ± SDs for continuous variables other than ACR or as median (interquartile range) for ACR.
Systolic blood pressure at screening visit was used to determine trial eligibility. Baseline visit values are presented in this table.
For comparison of differences between the quintiles, all p values <0.001, except for “never smoked” (p=0.57). CVD = cardiovascular disease; ACR = albumin-to-creatinine ratio; GFR = glomerular filtration rate.
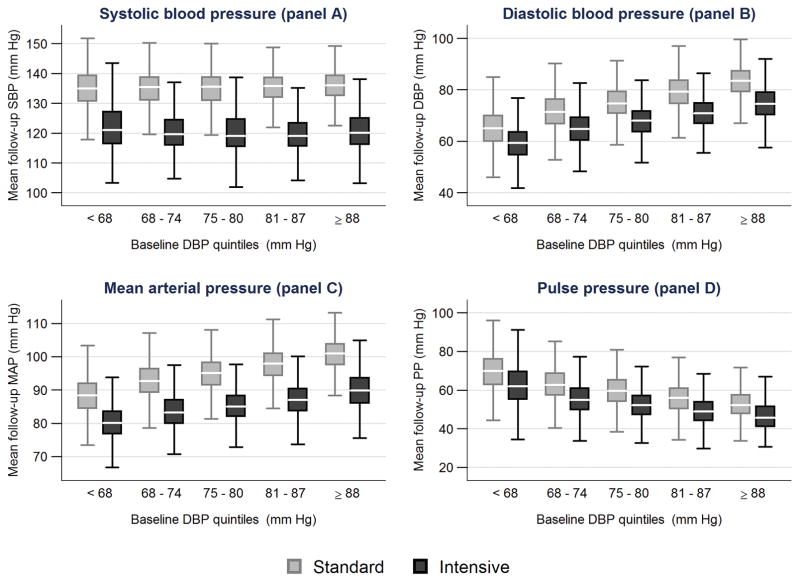
Boxplots displaying the medians, 25th and 75th percentiles of mean follow-up SBP, DBP, PP and MAP levels by baseline DBP quintile for participants in the intensive and standard arms are presented in Figure 1. Because the intervention targeted SBP, irrespective of baseline DBP, the distribution of achieved mean follow-up SBP in the intensive arm was similar across baseline quintiles of DBP (Figure 1, panel A). Similar findings for achieved SBP across baseline quintiles of DBP were noted in the standard arm (Figure 1, panel A). However, the achieved mean follow-up DBP was significantly lower among participants in the lowest compared to the highest quintile of baseline DBP within both the intensive (59.5 ± 6.9 versus 74.9 ± 7.0 mm Hg, p <0.001) and standard groups (65.0 ± 7.6 versus 83.3 ± 6.5 mm Hg, p <0.001) (Figure 1, panel B). Within each baseline quintile of DBP, achieved DBP was lower in the intensive compared to the standard group (Figure 1, panel B). Achieved MAP mirrored the pattern noted for achieved DBP (Figure 1, panel C). Achieved PP was the highest in the lowest baseline DBP quintile in both the intensive and standard groups (Figure 1 panel D).
Figure 1.
The boxplots display the median, 25th and 75th percentiles of the patients’ mean follow-up values for systolic blood pressure (panel A), diastolic blood pressure (panel B), mean arterial pressure (panel C) and pulse pressure (panel D), by randomized SBP intervention and quintile of baseline DBP (N=9119). 242 of 9361 subjects (2.6%) (140 in the standard group and 102 in the intensive group) had missing blood pressure measurements after month 2 and are not included.
SBP = systolic blood pressure; DBP = diastolic blood pressure.
In the entire cohort, there were 562 primary outcome events over 29,278 person-years of follow-up, and 365 all-cause deaths over 30,158 person-years of follow-up. In the subgroup with CKD at baseline, there were 29 kidney composite outcomes over 8,490 person-years of follow-up. In the subgroup of participants without CKD at baseline, there were 164 incident CKD events over 21,155 person-years of follow-up.
Adjusted for age, sex, race and the intervention arm, there was a U shaped association of baseline DBP with the primary outcome, all-cause deaths and incident CKD in cubic spline regression analyses (Supplemental Figure S1).
Interactions of baseline DBP and SBP intervention for pre-specified outcomes
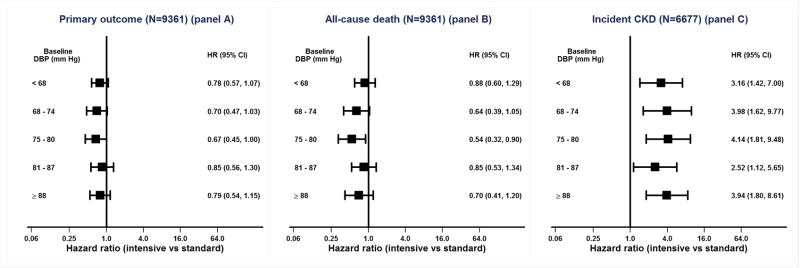
In our primary assessment of the interaction between the intensive SBP intervention treatment effect and baseline DBP (Table 2), the hazard ratio for the primary outcome was 0.78 (95% CI 0.57 to 1.07) within the lowest DBP quintile and 0.74 (95% CI 0.61 to 0.90) within the upper four DBP quintiles (interaction p-value = 0.78). Similarly, there was no evidence of an interaction between intensive SBP intervention and baseline DBP for all-cause death, composite kidney outcome or incident CKD events (Table 2).
Table 2.
Effects of intensive SBP control on the primary and secondary outcomes in the lowest DBP quintile compared to the upper four quintiles of baseline DBP, based on intention-to-treat analysis.
| Intensive vs standard in lowest DBP quintileHR (95%CI) | Intensive vs standard in upper 4 DBP quintilesHR (95%CI) | Interaction p value* | |
|---|---|---|---|
| Primary CVD outcome (N = 9361) | 0.78 (0.57, 1.07) | 0.74 (0.61, 0.90) | 0.78 |
| All-cause death (N = 9361) | 0.88 (0.60, 1.29) | 0.68 (0.53, 0.87) | 0.29 |
| Composite kidney outcome in CKD subgroup (N = 2646) | 1.17 (0.36, 3.84) | 0.79 (0.31, 2.00) | 0.61 |
| Incident CKD in non-CKD subgroup (N = 6677) | 3.16 (1.42, 7.00) | 3.58 (2.37, 5.41) | 0.79 |
Hazard ratios comparing the intensive vs. standard SBP interventions are presented for patients in the lowest baseline DBP quintile subgroup (left) and for patients in the upper 4 baseline DBP quintiles (right). Interaction p-values evaluate if the hazard ratios differed between the two baseline DBP subgroups, and were computed using likelihood ratio tests for the interaction between the randomized SBP intervention and baseline DBP subgroup in Cox regressions with separate baseline hazards for the two baseline DBP subgroups.
DBP = diastolic blood pressure; SBP = systolic blood pressure; HR = hazard ratio; CI =confidence interval; CVD = cardiovascular disease; CKD = chronic kidney disease.
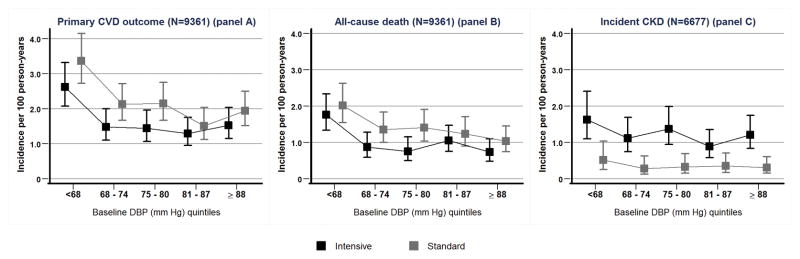
Incidence of primary outcome events, all-cause deaths and incident CKD (in participants without CKD at baseline) by quintile of baseline DBP is presented in Figure 2. Within each baseline DBP quintile, participants randomized to the intensive arm had a lower incidence of the primary outcome and all-cause death and a higher incidence of CKD. There was no suggestion of heterogeneity of the hazard ratios for intensive versus standard SBP treatment effect across DBP quintiles for the three outcomes studied (Figure 3). The p-values for interaction between treatment effect and baseline quintile of DBP were 0.92, 0.57, and 0.91 for the primary CV outcome, all-cause mortality, and incident CKD in the subgroup without CKD at baseline, respectively.
Figure 2.
Shown are incidence rates and pointwise 95% CIs for the primary CVD outcome (panel A), all-cause death (panel B) and incident CKD (panel C) in the standard and intensive SBP groups by quintile of baseline DBP. The 95% CIs were calculated for incidence rates using the quadratic approximation to the Poisson log likelihood for the log-rate parameter. Lines are drawn between the incidence rates quintiles for the different quintiles for visual clarity, and do not represent fitted regression curves. The analysis of incident CKD patients was performed for patients with baseline eGFR ≥ 60 ml/min/1.73m2. There were too few events to provide a meaningful similar analysis for the composite kidney outcome.
SBP = systolic blood pressure; DBP = diastolic blood pressure; CVD = cardiovascular disease; CKD = chronic kidney disease; eGFR = estimated glomerular filtration rate; HR = hazard ratio; CI = confidence interval.
Figure 3.
Shown are forest plots with hazard ratios for the effect of intensive vs. standard SBP intervention by quintile of baseline DBP for the primary CVD outcome (panel A), all-cause death (panel B) and incident CKD (panel C). In joint Cox regression models with separate baseline hazards for each baseline DBP quintile, likelihood ratio tests comparing the hazard ratios for the intensive vs. standard SBP interventions between the 5 baseline DBP quintiles were non-significant (primary CVD outcome interaction p = 0.92; all-cause death interaction p = 0.57; incident CKD interaction p = 0.91; composite kidney outcome interaction p = 0.71). Due to a small number of events, the interaction test for composite kidney outcome compared hazard ratios below and above the median baseline DBP instead of by baseline DBP quintile, and the HRs are not displayed in the figure. The analyses of incident CKD patients and the composite kidney outcome were performed for patients with baseline eGFR ≥ 60 ml/min/1.73m2 and baseline eGFR < 60 ml/min/1.73m2, respectively.
SBP = systolic blood pressure; DBP = diastolic blood pressure; CVD = cardiovascular disease; CKD = chronic kidney disease; eGFR = estimated glomerular filtration rate. HR = hazard ratio; CI = confidence interval
Following adjustment for baseline DBP, intensive versus standard SBP treatment had a lower hazard ratio for the primary CVD outcome, and all-cause death but a higher hazard for incident CKD (Table 3). Following adjustment for the intervention, participants with a baseline DBP of 61 mm Hg (mean DBP in the lowest baseline DBP quintile) had a higher hazard of the primary outcome, all-cause death and incident CKD compared to those with a baseline DBP of 78 mm Hg (mean baseline DBP of the entire cohort), (Table 3). There was no evidence of a nonlinear treatment by baseline DBP interaction for any of the outcomes, and the p-values for the linear treatment by baseline DBP interaction did not approach statistical significance for the primary outcome (p = 0.85), all-cause death (p = 0.37), composite kidney outcome (p = 0.57) or incident CKD events (p = 0.94) (Table 3). Similarly, within the subgroups with or without CVD at baseline, there was no evidence of interaction between the intervention and baseline DBP (Supplemental Tables S1 and S2).
Table 3.
Effects of the SBP intervention, baseline DBP, and the linear interaction between the SBP intervention and baseline DBP for the primary and secondary outcomes
| Model 1* | Model 2↑ | |||||
|---|---|---|---|---|---|---|
| Intensive versus standard | Comparison of baseline DBP=61 versus baseline DBP =78 mm Hg | Interaction term (change inintensive versus standard HR for each 5 mm Hg increase in DBP) | ||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Primary CVD outcome (N = 9361) | 0.76 (0.64, 0.89) | 0.001 | 1.27 (1.05,1.54) | 0.01 | 1.01 (0.94, 1.07) | 0.85 |
| All-cause death (N = 9361) | 0.74 (0.60, 0.92) | 0.005 | 1.17 (0.93, 1.48) | 0.18 | 0.96 (0.89, 1.04) | 0.37 |
| Composite kidney outcome in CKD subgroup (N = 2646) | 0.92 (0.44, 1.91) | 0.83 | 1.71 (0.78, 3.75) | 0.18 | 0.92 (0.69, 1.23) | 0.57 |
| Incident CKD in non-CKD subgroup (N = 6677) | 3.52 (2.44, 5.08) | <0.001 | 1.30 (0.85, 1.99) | 0.23 | 0.99 (0.87, 1.14) | 0.94 |
The 2nd to 3rd columns under Model 1 display the results of Cox regression analyses relating the primary and secondary outcomes to the randomized SBP intervention (HRs in the 2nd column) and to a cubic spline in the level of baseline DBP with knots at each baseline DBP quintile (HRs in the 4th column compare the hazards of each outcome between a baseline DBP = 61 mm Hg vs. a baseline DBP = 78 mm Hg), with covariable adjustment for age, sex and race.
The 5th and 6th columns display the proportional change in the HR comparing the intensive vs. standard SBP interventions for each 5 mm Hg increase in DBP under Model 2, which includes main effects for the randomized SBP intervention and cubic splines in baseline DBP, plus linear interactions between the randomized SBP intervention and baseline DBP. We present linear interactions between the randomized SBP intervention and baseline DBP because likelihood ratio tests evaluating interactions between the SBP intervention and cubic splines in baseline DBP indicated no evidence of nonlinear interactions (p > 0.10 for each outcome).
SBP = systolic blood pressure; DBP = diastolic blood pressure; HR = hazard ratio; CI = confidence interval; CVD = cardiovascular disease; CKD = chronic kidney disease.
Interactions of baseline DBP and SBP intervention for primary CVD endpoint excluding stroke
There were 467 non-stroke CVD outcome events over 29,434 person-years of follow-up. There was a U-shaped relation between DBP and the non-stroke CVD outcome (Supplemental Figure 2, panel A). As for the primary CVD outcome, there was no suggestion of heterogeneity of the hazard ratios for intensive versus standard SBP treatment effect across DBP quintiles when considering the non-stroke CVD outcome (Supplemental Figure 2, panel B). There was no evidence of an interaction between baseline DBP and the SBP lowering intervention.
Interactions of baseline DBP and SBP intervention for safety outcomes
Incidence of safety outcomes (any serious adverse event, and serious adverse events associated with hypotension, syncope, electrolyte abnormality, acute kidney injury or acute kidney failure) are summarized in Table 4. Those in the lowest quintile of baseline DBP had the highest incidence of these serious adverse events but there was no evidence for heterogeneity of the effects of the intervention by baseline quintile of DBP.
Table 4.
Incidence (per 100 person-years) of serious adverse events in participants randomized to the intensive and standard treatment groups, by quintile of baseline DBP (N=9361)*
| 1st quintile | 2nd quintile | 3rd quintile | 4th quintile | 5th quintile | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| < 68 mm Hg | 68 – 74 mm Hg | 75 – 80 mm Hg | 81 – 87 mm Hg | ≥ 88 mm Hg | ||||||
| Intensive | Standard | Intensive | Standard | Intensive | Standard | Intensive | Standard | Intensive | Standard | |
| Any serious adverse event | 20.53 | 19.55 | 15.67 | 15.21 | 15.39 | 14.27 | 12.99 | 12.50 | 12.60 | 12.48 |
| Serious adverse events associated with: | ||||||||||
| Hypotension | 0.79 | 0.53 | 0.98 | 0.47 | 0.75 | 0.42 | 0.62 | 0.49 | 0.66 | 0.38 |
| Syncope | 0.98 | 0.65 | 0.70 | 0.70 | 0.93 | 0.59 | 0.66 | 0.52 | 0.46 | 0.32 |
| Electrolyte abnormality | 1.45 | 1.12 | 1.27 | 0.60 | 0.86 | 0.63 | 0.85 | 0.52 | 0.63 | 0.86 |
| Acute kidney injury or acute kidney failure | 1.83 | 1.34 | 1.45 | 0.63 | 1.55 | 0.59 | 0.88 | 0.81 | 1.10 | 0.73 |
The table presents incidence rates of the indicated adverse events expressed as number of events per 100 person-years of follow-up.
In corresponding Cox regression models with separate baseline hazards for each baseline DBP quintile, likelihood ratio tests comparing the hazard ratios for the intensive vs. standard SBP intervention between the 5 baseline DBP quintiles were non-significant (serious adverse event, interaction p = 0.98; hypotension, interaction p = 0.87; syncope interaction p = 0.83; electrolyte abnormality interaction p=0.13; acute kidney injury/ acute kidney failure, interaction p = 0.12).
DBP = diastolic blood pressure.
Interactions of baseline MAP or PP and SBP intervention for pre-specified outcomes and safety outcomes
With one exception (acute kidney injury by baseline PP), the results were similar for baseline quintiles of MAP (Supplemental Figure S3 and Supplemental Table S3) and PP (Supplemental Figure S4 and Supplemental Table S4). In other words, intention-to-treat analyses yielded almost no evidence for heterogeneity in the effect of SBP lowering by baseline DBP, MAP, or PP.
Discussion
The results of the current study indicate that low baseline DBP was associated with increased risk of primary CVD outcome but an intervention that actively lowered SBP consistently reduced the risk of the primary CVD outcome across baseline quintiles of DBP.
At some level of low BP, perfusion of organs must become inadequate. Based on the on-treatment reports17–23, one might expect persons with a lower DBP to be at greater risk for adverse outcomes during intensive BP lowering. Because most ventricular myocardial perfusion occurs during diastole, a lower DBP could potentially lead to myocardial hypo-perfusion and associated damage, especially in persons with left ventricular hypertrophy (which increases oxygen demand) or coronary artery disease (in which oxygen supply is already compromised). In the Atherosclerosis Risk In Communities (ARIC) cohort, lower DBP was associated with higher serum concentrations of cardiac troponin T, a marker of myocardial injury24.
Almost all SPRINT participants were being treated for hypertension at baseline. Consistent with previous on-treatment reports, our study identified a U-shaped relationship between baseline DBP and the SPRINT primary CVD composite outcome.
Use of an intention-to-treat analysis provides a better way to determine whether the beneficial effects of intensive BP control are modified by level of baseline DBP because it takes advantage of the randomized design. In SPRINT, intensive SBP lowering that also lowered DBP was beneficial rather than hazardous even for those within the lowest quintile of baseline DBP (<68 mm Hg), where the average achieved DBP during follow-up in the intensive arm was <60 mm Hg. Our findings suggest that the association of a higher CVD event rate with lower levels of DBP is more likely to be a result of the clinical characteristics associated with a lower DBP, such as age and co-morbidities, than a response to lowering of DBP per se.
Our findings are consistent with experience in the Hypertension Optimal Treatment (HOT) trial25 in which 6264 patients were randomly allocated to a target DBP ≤ 90 mm Hg, 6264 to ≤ 85 mm Hg, and 6262 to ≤ 80 mm Hg and DBP was reduced by 20.3 mm Hg, 22.3 mm Hg, and 24.3 mm Hg, respectively. An intention-to-treat analysis identified no differences in CVD events, CVD mortality or all-cause mortality between the three groups but a J-shaped relationship was noted between achieved DBP and CVD. In the African American Study of Kidney Disease and Hypertension (AASK) trial, participants with CKD were randomly assigned to a mean arterial BP (MAP) target of 102–107 mmHg or ≤92 mmHg26. While the intention-to-treat analyses by randomized groups did not show an effect of intensive BP lowering, the achieved BP analyses suggested that lower achieved MAP was associated with better kidney outcomes. Thus, analyses based on achieved BP can lead to markedly different inferences than intention-to-treat analyses, due in part to confounding as well as reverse causality.
Tissue perfusion depends upon MAP. As interventions that target lower SBP also reduce DBP, they will also decrease the MAP and hence, tissue perfusion. In theory, this might be particularly important in those with wider PP (with already lower DBP and the drop in SBP might have an even greater effect on MAP). However, we also did not find evidence of heterogeneity of the effects of intensive SBP lowering by baseline MAP and PP quintiles.
A strength of the current analysis was our ability to examine the role of baseline DBP on treatment effect using a randomized comparison. Other strengths included availability of a relatively large sample size, and a diverse population with relatively low pre-treatment levels of DBP and a high risk for CVD. In addition, SPRINT was a rigorously conducted trial with careful measurement of blood pressure and outcomes data. Weaknesses include the post-hoc nature of the analyses and lack of intermediate biomarkers of tissue damage, such as cardiac troponin. As with any subgroup analyses of randomized controlled trial data, power might be limited to definitively exclude potential harm of intensive SBP lowering on CVD outcomes in the lowest DBP quintile. As the 95% CI in the lowest DBP quintile for the primary CVD outcome ranges from 0.57 to 1.07, the potential effects of the intervention ranges from 43% reduction in primary CVD outcome up to a small 7% increase in risk of primary CVD outcome in this quintile.
In conclusion, intensive SBP lowering in SPRINT participants led to substantial reductions in DBP and MAP. Although participants with lower DBP at baseline experienced higher rates of major cardiovascular events, SBP lowering appears beneficial across the spectrum of baseline DBP, even among those in the lowest quintile of DBP at baseline. Low levels of DBP, at least within the ranges examined here, should not be an impediment to intensive treatment of hypertension.
Supplementary Material
Clinical Perspective.
What is new?
There were U-shaped relationships of baseline DBP with the primary CVD outcome and all-cause death in SPRINT.
However, the beneficial effects of intensive SBP lowering (intensive SBP goal <120 mm Hg versus standard SBP goal <140 mm Hg) on the primary CVD outcome and all-cause death were not modified by baseline level of DBP.
Increased risk of kidney events and serious adverse effects of the intervention were consistent across baseline DBP quintiles.
Therefore, there was no evidence that the benefit of the intensive SBP lowering differed by baseline DBP level.
What are the clinical implications?
Some cohort observational studies and non-randomized secondary analyses of achieved blood pressures suggested a J-curve relationship of DBP with cardiovascular events.
Results of current analyses of SPRINT data suggest that underlying processes (such as increased arterial stiffness) that lead to a decline in DBP rather than the level of DBP per se might be the reason for the observed associations of worse outcomes with lower DBP.
Low levels of DBP within the ranges examined here in SPRINT should not be an impediment to intensive treatment of hypertension, at least in those without diabetes mellitus or stroke.
Acknowledgments
All components of the SPRINT study protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. All aspects of manuscript writing and revision were carried out by the coauthors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the U.S. Department of Veterans Affairs, or the United States Government. For a full list of contributors to SPRINT, please see the supplementary material and acknowledgement list at https://www.sprinttrial.org/public/dspScience.cfm
We also acknowledge the support from the following CTSAs funded by NCATS: CWRU: UL1TR000439, OSU: UL1RR025755, U Penn: UL1RR024134& UL1TR000003, Boston: UL1RR025771, Stanford: UL1TR000093, Tufts: UL1RR025752, UL1TR000073 & UL1TR001064, University of Illinois: UL1TR000050, University of Pittsburgh: UL1TR000005, UT Southwestern: 9U54TR000017-06, University of Utah: UL1TR000105-05, Vanderbilt University: UL1 TR000445, George Washington University: UL1TR000075, University of CA, Davis: UL1 TR000002, University of Florida: UL1 TR000064, University of Michigan: UL1TR000433, Tulane University: P30GM103337 COBRE Award NIGMS.
This work is also supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK091437 and R21 DK106574) and the University of Utah Study Design and Biostatistics Center (funded in part from the Public Health Services research grant numbers UL1-RR025764 and C06-RR11234 from the National Center for Research Resources).
Sources of Funding
The Systolic Blood Pressure Intervention Trial is funded with Federal funds from the National Institutes of Health (NIH), including the National Heart, Lung, and Blood Institute (NHLBI), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute on Aging (NIA), and the National Institute of Neurological Disorders and Stroke (NINDS), under Contract Numbers HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, HHSN268200900049C, and Inter-Agency Agreement Number A-HL-13-002-001. It was also supported in part with resources and use of facilities through the Department of Veterans Affairs. The SPRINT investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals International, Inc.
Appendix: SPRINT Publications Acknowledgment List
Study Leadership: Paul Whelton (Chair), Karen C. Johnson (Vice-Chair), Lawrence Fine (Project Officer), Joni Snyder (Deputy Project Officer). Program Office: National Institutes of Health, Bethesda, Maryland: Diane Bild (Project Scientist), Denise Bonds (Project Scientist), Nakela Cook (Project Scientist), Jeffrey Cutler (Project Scientist), Lawrence Fine (Project Officer), Peter Kaufmann (Project Scientist), Paul Kimmel (Project Scientist), Lenore Launer (Project Scientist), Claudia Moy (Project Scientist), William Riley (Project Scientist), Laurie Ryan (Project Scientist), Joni Snyder (Deputy Project Officer), Eser Tolunay (Project Scientist), Song Yang (Biostatistician) SPRINT Clinical Center Networks: Case Western Reserve University, Cleveland, OH: Jackson T Wright Jr (CCN PI), Mahboob Rahman (CCN Co-PI), Alan J Lerner (CCN MIND PI), Mahboob Rahman (CCN Co-PI), Carolyn Still (CCN Project Manager, Co-I), Alan Wiggers (Co-I), Sara Zamanian, (CCN Asst. Project Manager), Alberta Bee (former CCN Asst. Project Manager), Renee Dancie (former CCN Project Manager); Memphis Veteran Affairs Medical Center, Memphis, TN: William Cushman (PI), Barry Wall (Co-I), Linda Nichols (MIND PI), Robert Burns (MIND Consultant), Jennifer Martindale-Adams (MIND Consultant), Dan Berlowitz (Economic & HRQL Consultant), Elizabeth Clark (CCN Coordinator), Sandy Walsh (CCN Coordinator) Terry Geraci (CCN Coordinator) Carol Huff (Budget Analyst), Linda Shaw (CCN Research Assistant). University of Alabama, Birmingham, AL: Suzanne Oparil (PI), Cora E. Lewis (Co-PI), Virginia Bradley (MIND Co-I), David Calhoun (Co-I), Stephen Glasser (Co-I), Kim Jenkins (CCN Coordinator), Tom Ramsey (CCN Coordinator); University of Utah, Salt Lake City, UT: Alfred K. Cheung (PI), Srinivasan Beddhu (Co-I), Gordon Chelune (MIND Co-I), Jeffrey Childs (Associate Director of Operations), Lisa Gren (Director of Operations), Anne Randall (CCN Coordinator); Wake Forest University Health Sciences, Winston-Salem, NC: Michael Rocco (PI), David Goff (Co-PI), Carlos Rodriguez (Co-I), Laura Coker (Co-I), Amret Hawfield (Co-I), Joseph Yeboah (Co-I), Lenore Crago (CCN Coordinator) John Summerson (CCN Coordinator), Anita Hege (MIND Coordinator). SPRINT Central Coordinating Center: Wake Forest University Health Sciences, WinstonSalem, NC: David Reboussin (PI), Jeff Williamson (Co-PI), Walter Ambrosius (Co-I), William Applegate (Co-I), Greg Evans (Co-I), Capri Foy (Co-I), Barry I. Freedman (Co-I), Dalane Kitzman (Co-I), Mary Lyles (Co-I), Nick Pajewski (Co-I), Steve Rapp (Co-I), Scott Rushing (Co-I), Neel Shah (Co-I), Kaycee M. Sink (Co-I, Safety Officer), Mara Vitolins (Co-I), Lynne Wagenknecht (Co-I), Valerie Wilson (Co-I), Letitia Perdue (Project Coordinator), Nancy Woolard (MIND Project Coordinator), Tim Craven (Biostatistician), Katelyn Garcia (Biostatistician), Sarah Gaussoin (Biostatistician), Laura Lovato (Biostatistician), Jill Newman (Biostatistician), Bobby Amoroso (Programmer), Patty Davis (Programmer), Jason Griffin (Programmer), Darrin Harris (Programmer), Mark King (Programmer), Kathy Lane (Programmer), Wes Roberson (Programmer), Debbie Steinberg (Programmer), Donna Ashford (Project Manager), Phyllis Babcock (Project Manager), Dana Chamberlain (Project Manager), Vickie Christensen (Project Manager), Loretta Cloud (Project Manager), Christy Collins (Project Manager), Delilah Cook (Project Manager), Katherine Currie (Project Manager), Debbie Felton (Project Manager), Stacy Harpe (Project Manager), Marjorie Howard (Project Manager), Michelle Lewis (Project Manager), Pamela Nance (Project Manager), Nicole Puccinelli-Ortega (Project Manager), Laurie Russell (Project Manager), Jennifer Walker (Project Manager), Brenda Craven (former Project Coordinator), Candace Goode (Data Coordinator), Margie Troxler (Fiscal Coordinator), Janet Davis (Administrative Support), Sarah Hutchens (Administrative Support). SPRINT Central Laboratory: University of Minnesota Advanced Research and Diagnostic Laboratory: Anthony A. Killeen (PI), Anna M. Lukkari (coordinator). Pharmacy Coordinating Center: Robert Ringer (PI), Brandi Dillard (coordinator), Norbert Archibeque, (coordinator) Stuart Warren (Co-I), Mike Sather (PI), James Pontzer (programmer), Zach Taylor (programmer). SPRINT ECG Reading Center: Epidemiological Cardiology Research Center (EPICARE), Winston Salem, NC: Elsayed Z Soliman (PI), Zhu-Ming Zhang (Co-I), Yabing Li (coordinator), Chuck Campbell (coordinator), Susan Hensley (coordinator), Julie Hu (coordinator), Lisa Keasler (coordinator), Mary Barr (coordinator), Tonya Taylor (coordinator) SPRINT MRI Reading Center: University of Pennsylvania-Philadelphia, PA: R. Nick Bryan (PI), Christos Davatzikos (Co-I), Ilya Nasarallah (Co-I), Lisa Desiderio (Project Manager), Mark Elliott (MRI Physicist), Ari Borthakur (MRI Physicist), Harsha Battapady (Data Analyst), Guray Erus ( Postdoctoral Fellow), Alex Smith (Postdoctoral Fellow), Ze Wang (Research Associate), Jimit Doshi (Data Analyst). SPRINT MRI by site: University of Pennsylvania-Philadelphia, PA: Raymond Townsend (Clinic PI), Debbie Cohen (Co-I), Yonghong Huan (Co-I), Mark Duckworth (Research Coordinator), Virginia Ford (Research Coordinator), Kelly Sexton (MRI Coordinator). University Hospital Case Medical CenterCleveland, OH: Jackson T. Wright, Jr. (PI), Alan Lerner (Co-I), Mahboob Rahman (Co-I), Carolyn Still (Project Manager), Alberta Bee (Research Coordinator), Debra Lee Stokes, (MRI coordinator), Shonte Smith (MRI coordinator), Jeffrey Sunshine (Site Radiologist), Mark Clampitt (MRI Technologist). Vanderbilt University: Seth Smith (MRI Director), Brian Welch (MRI Research Manager), Manus Donahue ( MRI Physicist), Alex Dagley (Researcher Coordinator), Dave Pennell (MRI Technologist), Chris Cannistraci (Imaging Research Specialist), Kristin Merkle (MRI Research Coordinator), Julie Lewis (Clinic PI) Mohammed Sika (Research Coordinator). University of Miami: Clinton Wright (Co-I), Mohammad Sabati (MRI Director), Edward Campuzano (Chief MRI Technologist), Hector Martin (MRI Technologist), Andrea Roman (MRI Technologist), Jesus Cruz (MRI Technologist), Natalya Nagornaya (Site Radiologist). Wake Forest University: Laura Coker (Co-I), Anita Hege (Project Coordinator), Joseph Maldjian (Site Radiologist), Sandra Kaminsky (MRI Technologist), Debra Fuller (MRI Technologist), Youngkoo Jung (MRI Physicist). University of Alabama at Birmingham: Suzanne Oparil (Network PI), Beth Lewis (Co-PI), Virginia Wadley (MIND Co-I), Kim Jenkins (Project Coordinator), Tom Ramsey (Project Coordinator), William Evanochko (MRI Physicist), Glenn Roberson (Site Radiologist), Trina Corbitt (MRI Technologist), William Fisher (MRI Technologist), Cathy Clements (MRI Technologist). Boston University: Daniel Weiner (Clinic PI), Andrew Wells (Research Coordinator), Amanda Civiletto (Research Coordinator), Gerard P. Aurigemma (Clinic PI), Noelle Bodkin (Research Coordinator), Alex Norbash (Co-I,) Margaret Lavoye (Research Administrator), Andrew Ellison (MRI Technologist), Ronald Killiany (Imaging Center Director), Osama Sakai (Site Radiologist). SPRINT Sub-Committee Chairs: Ancillary Science: Alfred Cheung, Design and Analysis: Walter Ambrosius, Economic Evaluation/Health Related Quality of Life: Dan Berlowitz, Intervention: William Cushman, Measurements, Procedures and Quality Control: Beth Lewis, Mortality and Morbidity: Suzanne Oparil, Presentations and Publications: Jackson T. Wright, Jr., Recruitment, Retention and Adherence: David Goff, Safety: Kaycee Sink, SPRINT MIND: Jeff Williamson. SPRINT Clinical Centers by Network: OHIO Network: Cleveland Clinic FoundationCleveland, OH: George Thomas (PI), (Co-PI), Martin Schreiber, Jr (Co-I), Sankar Dass Navaneethan (Co-I), John Hickner (Co-I), Michael Lioudis (Co-I), Michelle Lard (Co-I), Susan Marczewski (former coordinator), Jennifer Maraschky (coordinator), Martha Colman (former coordinator) Andrea Aaby (coordinator), Stacey Payne (coordinator), Melanie Ramos, (coordinator), Carol Horner (former coordinator). Louis Stokes Cleveland VA Medical Center-Cleveland, OH: Mahboob Rahman (PI), Paul Drawz (Co-I), Pratibha P. Raghavendra (Co-I), Scott Ober (Co-I), Ronda Mourad (Co-I), Muralidhar Pallaki (Co-I), Peter Russo (Co-I), Pratibha Raghavendra, Co-I), Pual Fantauzzo (Co-I), Lisa Tucker (coordinator), Bill Schwing (coordinator). MetroHealth Medical Center-Cleveland, OH: John R. Sedor (PI), Edward J. Horwitz (Co-PI), Jeffrey R. Schellling (Co-I), John F. O’Toole (Co-I), Lisa Humbert (coordinator), Wendy Tutolo (coordinator). North East Ohio Neighborhood Health CenterCleveland, OH: Suzanne White (PI), Alishea Gay (Former Co-I), Walter Clark, Jr (former PI), Robin Hughes (coordinator). University Hospital Case Medical Center-Cleveland, OH: Mirela Dobre (PI), Jackson T. Wright, Jr. (Co-I), Carolyn H. Still (Co-I), Alberta Bee (coordinator), Monique Williams (coordinator). The Ohio State University Medical Center, Division of Nephrology and Hypertension-Columbus, OH: Udayan Bhatt (PI), Lee Hebert (former PI) Anil Agarwal (Co-PI), Melissa Brown Murphy (coordinator), Nicole Ford (former coordinator), Cynthia Stratton (coordinator), Jody Baxter (former coordinator), Alicia A. Lykins (former coordinator), Alison McKinley Neal (former coordinator) Leena Hirmath (former coordinator). The Ohio State University Medical Center, Division of Endocrine, Diabetes, and Metabolism-Columbus, OH: Osei Kwame (PI), Kyaw Soe (Co-I), William F. Miser (former Co-PI), Colleen Sagrilla (coordinator), Jan Johnston (coordinator), Amber Anaya (coordinator), Ashley Mintos (coordinator), Angel A. Howell (coordinator), Kelly Rogers (former coordinator), Sara Taylor (former Co-I). University Hospitals Landerbrook Health Center-Mayfield Height, OH: Donald Ebersbacher (PI), Lucy Long (coordinator), Beth Bednarchik (coordinator). University Hospitals Glenridge Office Park-North Royalton, OH: Alan Wiggers (PI), Lucy Long (coordinator). University Hospitals Suburban HealthCleveland, OH: Adrian Schnall (PI), Jonathan Smith (coordinator), Lori Peysha (coordinator), Lori Peysha (coordinator), Beth Bednarchik (coordinator), Lisa Leach (coordinator), Megan Tribout (coordinator). University Hospitals Otis Moss Jr. Health Center-Cleveland, OH: Carla Harwell (PI), Pinkie Ellington (coordinator). SUNY Downstate Medical Center, New York: Mary Ann Banerji (PI), Pranav Ghody (Co-I), Melissa Vahídeh Rambaud (coordinator). University of Pennsylvania-Philadelphia, PA: Raymond Townsend (PI), Debbie Cohen (CoI), Yonghong Huan (Co-I), Mark Duckworth (former coordinator), Virginia Ford (coordinator), Juliet Leshner (coordinator), Ann Davison (coordinator), Sarah Vander Veen (coordinator). Temple University-Philadelphia, PA: Crystal A Gadegbeku (PI), Avi Gillespie (Co-I), Anuradha Paranjape (Co-I), Sandra Amoroso (coordinator), Zoe Pfeffer (coordinator), Sally B. Quinn (coordinator). Tulane University-New Orleans, LA: Jiang He (PI), Jing Chen (Co-I), Eva Lustigova (coordinator), Erin Malone (coordinator). Ochsner Clinic Foundation-New Orleans, LA: Marie Krousel-Wood (PI), Richard Deichmann (Co-I), Patricia Ronney (Co-I), Susan Muery (coordinator), Donnalee Trapani (coordinator). SOUTHEAST Network: Georgia Regents University, Augusta, GA: Matt Diamond (PI), Laura Mulloy (PI), Marcella Hodges (coordinator), Michelle Collins (coordinator), Charlene Weathers (coordinator), Heather Anderson (former coordinator), Emily Stone (former coordinator), Walida Walker (former coordinator). Carolinas Medical Center, Charlotte, NC: Andrew McWilliams (PI), Michael Dulin (Co-I), Lindsay Kuhn (Co-PI), Susan Standridge (coordinator), Lindsay Lowe (coordinator), Kelly Everett (coordinator), Kelry Preston (former coordinator), Susan Norton (former coordinator), Silena Gaines (former coordinator). University of South Carolina, Columbia, SC: Ali A. Rizvi (PI), Andrew W. Sides (Co-PI), Diamond Herbert (coordinator), Matthew M. Hix (coordinator), Melanie Whitmire (former coordinator (former coordinator), Brittany Arnold (former coordinator), Philip Hutchinson (former coordinator), Joseph Espiritu (former coordinator). Duke University, Durham, NC: Mark Feinglos (PI), Eugene Kovalik (Co-PI), Georgianne Gedon-Lipscomb (coordinator), Kathryn Evans (coordinator), Connie Thacker (coordinator), Ronna Zimmer (coordinator), Mary Furst (coordinator), MaryAnn Mason (former coordinator). East Carolina University, Greenville, NC: James Powell (PI), Paul Bolin (Co-PI), Junhong Zhang (Co-PI), Mary Pinion (coordinator), Gail Davis (coordinator), Winifred Bryant (former coordinator), Presley Phelps (former coordinator), Connie Garris-Sutton (former coordinator), Beatrice Atkinson (former coordinator). University of Miami, Miami, FL: Gabriele Contreras (PI), Maritza Suarez (Co-PI), Ivonne Schulman (Co-PI), Don Koggan (coordinator), Jackie Vassallo (coordinator), Gloria Peruyera (former coordinator). Wake Forest Nephrology, Winston Salem, NC: Michael Rocco (PI), Amret Hawfield (Co-PI), Cassandra Bethea (coordinator), Sheri Mayer (coordinator), Laura Gilliam (former coordinator). Wake Forest Downtown Health Plaza, Winston Salem, NC: Carolyn Pedley (PI), Geraldine Zurek (coordinator), Miriam Baird (coordinator), Charles Herring (Pharm D), Mary Martha Smoak (former coordinator). Wake Forest Geriatrics, Winston Salem, NC: Julie Williams (PI), Samantha Rogers (Co-PI), Lindsay Gordon (coordinator), Erin Kennedy (coordinator), Beverly Belle (coordinator), Jessica McCorkle-Doomy (former coordinator), Jonathan Adams (former coordinator), Dana Chamberlain (former coordinator). University of South Florida, Tampa, FL: Ramon Lopez (PI), Juris Janavs (coordinator). Emory University, Atlanta, GA: Frederic Rahbari-Oskoui (PI), Arlene Chapman (former PI), Allen Dollar (former Co-PI), Olubunmi Williams (coordinator), Yoosun Han (former coordinator). The Mayo Clinic Jacksonville, Jacksonville, FL: William Haley (PI), Peter Fitzpatrick (Co-PI), Joseph Blackshear (Co-PI), Brian Shapiro (Co-PI), Anna Harrell (coordinator), Arta Palaj (coordinator), Katelyn Henderson (coordinator), Ashley Johnson (former coordinator), Heath Gonzalez (former coordinator), Jermaine Robinson (former coordinator). Miami VA, Miami, FL: Leonardo Tamariz (PI), Ivonne Schulman (Co-PI), Jennifer Denizard (coordinator), Rody Barakat (former coordinator), Dhurga Krishnamoorthy (former coordinator). Pennington Biomedical Research, Baton Rouge, LA: Frank Greenway (PI), Ron Monce (Co-I), Timothy Church (former PI), Chelsea Hendrick (coordinator), Aimee Yoches (coordinator), Leighanne Sones (coordinator), Markee Baltazar (former coordinator). Morehouse School of Medicine, Atlanta, GA: Priscilla Pemu (PI), Connie Jones (coordinator), Derrick Akpalu (coordinator). UTAH Network: Boston University Medical Center, Boston MA: Laura Dember (PI), Denise Soares (coordinator). Henry Ford Hospital, Detroit MI: Jerry Yee (PI), Kausik Umanath (Co-PI), Naima Ogletree (Sub-I), Schawana Thaxton (Sub-I), Karen Campana (coordinator), Dayna Sheldon (coordinator), Krista MacArthur (coordinator). Intermountain Health Care, Salt Lake City UT: J. Brent Muhlestein (PI), Nathan Allred (Co-I), Brian Clements (Co-I), Ritesh Dhar (Co-I), Kent Meredith (Co-I), Viet Le (Co-I), Edward Miner (Co-I), James Orford (Co-I), Erik R. Riessen (Co-I), Becca Ballantyne (coordinator), Ben Chisum (coordinator), Kevin Johnson (coordinator), Dixie Peeler (coordinator). Stanford University, Palo Alto CA: Glenn Chertow (PI), Manju Tamura (Co-PI), Tara Chang (Co-I), Kevin Erickson (Co-I), Jenny Shen (Co-I), Randall S. Stafford (Co-I), Gregory Zaharchuk (Co-I), Margareth Del Cid (coordinator), Michelle Dentinger (coordinator), Jennifer Sabino (coordinator), Rukmani Sahay (coordinator), Ekaterina (Katie) Telminova (coordinator). Tufts Medical Center, Boston MA: Daniel E. Weiner (PI), Mark Sarnak (Co-I), Lily Chan (coordinator), Amanda Civiletto (coordinator), Alyson Heath (coordinator), Amy Kantor (coordinator), Priyanka Jain (coordinator), Bethany Kirkpatrick (coordinator), Andrew Well (coordinator), Barry Yuen (coordinator). University of Colorado, Denver, Denver CO: Michel Chonchol (PI), Beverly Farmer (coordinator), Heather Farmer (coordinator), Carol Greenwald (coordinator), Mikaela Malaczewski (coordinator). University of Illinois, Chicago, Chicago IL: James Lash (PI), Anna Porter (Co-I), Ana Ricardo (Co-I), Robert T. Rosman (Co-I), Janet Cohan (coordinator), Nieves Lopez Barrera (coordinator), Daniel Meslar (coordinator), Patricia Meslar (coordinator). University of Pittsburgh, Pittsburgh PA: Margaret (Molly) Conroy (PI), Mark Unruh (PI), Rachel Hess (CoPI), Manisha Jhamb (Co-I), Holly Thomas (Co-I), Pam Fazio (coordinator), Elle Klixbull (coordinator), Melissa Komlos-Weimer (coordinator), LeeAnne Mandich (coordinator), Tina Vita (coordinator). University of Texas Southwestern, Dallas TX: Robert Toto (PI), Peter Van Buren (Co-I), Julia Inrig (Co-I), Martha Cruz (coordinator), Tammy Lightfoot (coordinator), Nancy Wang (coordinator), Lori Webster (coordinator). University of Utah, Salt Lake City UT: Srinivasan Beddhu (PI), Kalani Raphael (Co-I), Barry Stults (Co-I), Tahir Zaman (Co-I), Debra Simmons (Co-I), Tooran Lavasani (nurse practitioner), Rebecca Filipowicz (Sr. research analyst), Guo Wei (Sr research analyst), Gracie Mary Miller (coordinator), Jenice Harerra (coordinator), Jeff Christensen (Clinical research assistant), Ajay Giri (Clinical research assistant), Xiaorui Chen (graduate research assistant), Natalie Anderton (graduate research assistant), Arianna Jensen (undergraduate research assistant). Vanderbilt University, Nashville TN: Julia Lewis (PI), Anna Burgner (Co-I), Jamie P. Dwyer (Co-I), Gerald Schulman (Co-I), Terri Herrud (coordinator), Ewanda Leavell (coordinator), Tiffany McCray (coordinator), Edwina McNeil-Simaan (coordinator), Munmun Poudel (coordinator), Malia Reed (coordinator), Mohammed Sika (coordinator), Delia Woods (coordinator), Janice L. Zirkenbach (coordinator). George Washington University, Washington DC: Dominic S. Raj (PI), Scott Cohen (Co-I), Samir Patel (Co-I), Manuel Velasquez (Co-I), Roshni S. Bastian (coordinator), Maria Wing (coordinator) Akshay Roy-Chaudhury (Coordinator). University of California, Davis, Sacramento CA: Thomas Depner (PI), Lorien Dalyrymple (Co-I), George Kaysen (Co-I), Susan Anderson (coordinator). Salt Lake City VA, Salt Lake City UT: Srinivasan Beddhu (PI), John Nord (Co-I), Debra Simmons (Co-I), Gracie Mary Miller (coordinator), Jenice Harerra (coordinator), Ajay Giri (Clinical research assistant). Veterans Medical Research Foundation, San Diego CA: Joachim H. Ix (PI), Leonard Goldenstein (Co-PI), Cynthia M. Miracle (Co-I), Nketi Forbang (coordinator), Maja Mircic (coordinator), Brenda Thomas (coordinator), Tiffany Tran (coordinator). UCLA, Los Angeles CA: Anjay Rastogi (PI), Mihae Kim (Sub-PI), Mohamad Rashid (Co-PI), Bianca Lizarraga (coordinator), Amy Hocza (coordinator), Kristine Sarmosyan (coordinator), Jason Norris (coordinator), Tushar Sharma (coordinator), Amanda Chioy (coordinator), Eric Bernard (coordinator), Eleanore Cabrera (coordinator), Christina Lopez (coordinator), Susana Nunez (coordinator), Joseph Riad (coordinator), Suzanne Schweitzer (coordinator), Siran Sirop (coordinator), Sarah Thomas (coordinator), Lauren Wada (coordinator). Loyola University Medical Center, Chicago IL: Holly Kramer (PI), Vinod Bansal (Co-PI), Corliss E. Taylor (coordinator). University of Florida, Gainesville FL: Mark S. Segal (PI), Karen L. Hall (Co-I), Amir Kazory (Co-I), Lesa Gilbert (coordinator), Linda Owens (coordinator), Danielle Poulton (coordinator), Elaine Whidden (coordinator). University of Michigan, Ann Arbor MI: Jocelyn (Jo) Wiggins (PI), Caroline Blaum (PI), Linda Nyquist (Co-I), Lillian Min (Co-I), Tanya Gure (Co-I), Ruth Lewis (coordinator), Jennifer Mawby (coordinator), Eileen Robinson (coordinator). UAB Network: Athens Internal Medicine, Athens, AL: Nauman Qureshi (PI), Karen Ferguson (coordinator), Sumrah Haider (coordinator), Mandy James (coordinator), Christy Jones (coordinator), Kim Renfroe (coordinator), April Seay (coordinator), Carrie Weigart (coordinator). UAB - The Chronic Kidney Disease Clinic, Birmingham, AL: Denyse Thornley-Brown (PI), Dana Rizik (Co-I), Bari Cotton (coordinator), Meredith FitzGerald (coordinator), Tiffany Grimes (coordinator), Carolyn Johnson (coordinator), Sara Kennedy (coordinator), Chanel Mason (coordinator), Lesa Rosato-Burson (coordinator), Robin Willingham (coordinator). UAB - Vascular Biology and Hypertension Clinic, Birmingham, AL: David Calhoun (PI), Eric Judd (Co-I), Tonya Breaux-Shropshire (coordinator), Felice Cook (coordinator), Julia Medina (coordinator). Nephrology Associates, P.C., Birmingham, AL: James Lewis (PI), Roman Brantley (Co-I), John Brouilette (Co-I), Jeffrey Glaze (Co-I), Stephanie Hall (Co-I), Nancy Hiott (Co-I), David Tharpe (Co-I), Spencer Boddy (coordinator), Catherine Mack (coordinator). University of Tennessee Health Science Center, Memphis, TN: Karen C. Johnson (PI) Catherine Womack (Co –I), Beate Griffin (coordinator), Carol Hendrix (coordinator), Karen Johnson (coordinator), Lisa Jones (coordinator), Chelsea Towers (coordinator). Punzi Medical Center and Trinity Hypertension Research, Carrollton, TX: Henry Punzi (PI), Kathy Cassidy (coordinator), Kristin Schumacher (coordinator). Family Care Practice, Fajardo, Puerto Rico: Carmen Irizarry (PI), Ilma Colon (coordinator). Centro Cardiovascular de Caguas, El Verde, Caguas, Puerto Rico: Pedro Colon-Ortiz (PI), Pedro Colon-Hernandez (Co-I), Merari Carrasquillo (coordinator), Nivea Vazquez (coordinator). Miguel Sosa-Padilla, Private Practice San Juan, Puerto Rico: Miguel Sosa-Padilla (PI), Alex Cintron-Pinero (Co-I), Mayra Ayala (coordinator), Olga Pacheco (coordinator), Catalina Rivera (coordinator) Irma Sotomayor-Gonzalez (coordinator). Altamira Family Practice and Research Institute Center, San Juan, Puerto Rico: Jamie Claudio (PI), Jose Lazaro (coordinator), Migdalia Arce (coordinator), Lourdes Heres (coordinator), Alba Perez (coordinator). Centro Clinico San Patricio, San Juan, Puerto Rico: Jose Tavarez-Valle (PI), Ferlinda Arocho (coordinator), Mercedes Torres (coordinator), Melvaliz Vazquez (coordinator). University of Massachusetts – Worchester, MA: Gerard P. Aurigemma (PI), Rebecca TakisSmith (Co-I), Julia Andrieni (Co-I), Noelle Bodkin (coordinator), Kiran Chaudhary (coordinator), Paula Hu (coordinator). Rutgers-Robert Wood Johnson Medical School, New Brunswick, New Jersey: John Kostis (PI), William J. Kostis (Co-PI) Nora Cosgrove (coordinator), Denise Bankowski (coordinator), Monica Boleyn (coordinator), Laurie Casazza (coordinator), Victoria Giresi (coordinator), Tosha Patel (coordinator), Erin Squindo (coordinator), Yan Wu (coordinator). University of Mississippi Medical Center CRP – Jackson, MS: Marion Wofford (PI), Michael Flessner (Co-I), Cathy Adair (coordinator). Nashville Medical Group, Nashville, TN: Jordan Asher (PI), Debbie Loope (coordinator), Rita Cobb (coordinator), Reiner Venegas (coordinator). New York Irving Pavilion Research, Columbia University, New York, NY: Thomas Bigger (Director), Daniel Donovan (PI), Carlos Lopez-Jimenez (Co-I), Amilcar Tirado (coordinator). New York, Irving Pavilion Research Unit – CTSA Satellite, Columbia University, New York, NY: Thomas Bigger (Director), Asqual Getaneh (PI), Rocky Tang (coordinator), Sabrina Durant (coordinator). Clinical Cardiovascular Research Lab for the Elderly, Columbia University, New York, NY: Thomas Bigger (Director), Mathew Maurer (PI), Sergio Teruya (Co-I) Stephen Helmke (coordinator), Julissa Alvarez (coordinator). Medical University of South Carolina Nephrology, Charleston, SC: Ruth Campbell (PI), Roberto Pisoni (Co-I), Rachel Sturdivant (Co-I), Caroline Counts (coordinator), Vickie Hunt (coordinator), Lori Spillers (coordinator). Great Lakes Medical Research, Westfield, NY: Donald Brautigam (PI), Timothy Kitchen (CoI), Timothy Gorman (Co-I) Jessica Sayers (coordinator), Sarah Button (coordinator), June Chiarot (coordinator), Rosemary Fischer (coordinator), Melissa Lyon (coordinator), Maria Resnick (coordinator). VA Network: New Mexico VA Healthcare System – Albuquerque, NM: Karen Servilla (PI), Darlene Vigil (Co-I), Terry Barrett (coordinator). Atlanta VAMC – Atlanta GA: Mary Ellen Sweeney (PI), Rebecca Johnson (Co-I), Susan McConnell (Co-I), Khadijeh Shahid Salles (Co-I), Francoise Watson (Co-I), Cheryl Schenk (coordinator), Laura Whittington (coordinator), Maxine Maher (coordinator). VA Boston Healthcare System – Jamaica Plain, MA: Jonathan Williams (PI), Stephen Swartz (PI), Paul Conlin (Co-I), George Alexis (coordinator), Rebecca Lamkin (coordinator), Patti Underwood (coordinator), Helen Gomes (coordinator). James J. Peters VAMC – Bronx, NY: Clive Rosendorff (PI), Stephen Atlas (Co-I), Lawrence Kwon (Co-I), Matar Matar (coordinator). Ralph H. Johnson VAMC – Charleston, SC: Roberto Pisoni (PI), Jan Basile (PI), Joseph John (PI), Deborah Ham (coordinator), Hadi Baig (coordinator). Dayton VAMC – Dayton, OH: Mohammed Saklayen (PI), Jason Yap (Co-I), Helen Neff (coordinator), Carol Miller (coordinator), Ling Zheng-Phelan (coordinator). John D. Dingell VAMC – Detroit, MI: Saib Gappy (PI), Shiva Rau (Co-I), Arathi Raman (Co-I), Vicki Berchou (coordinator), Elizabeth Jones (coordinator), Erin Olgren (coordinator). VA New Jersey Healthcare System – East Orange, NJ: Michael Yudd (PI), Sithiporn Sastrasinh (PI), Jennine Michaud (Co-I), Jessica Fiore (coordinator), Marianne Kutza (coordinator). Malcom Randall VAMC – Gainesville, FL: Ronald Shorr (PI), Rattana Mount (Co-I), Jeremy Thoms (Co-I), Helen Dunn (coordinator), Susan Stinson (coordinator), Jessica Hunter (coordinator). Michael E. DeBakey VAMC – Houston, TX: Addison Taylor (PI), Jeffery Bates (Co-I), Catherine Anderson (coordinator). G.V. (Sonny) Montgomery VAMC – Jackson, MS: Kent Kirchner (PI), Jodi Stubbs (Co-I), Ardell Hinton (coordinator), Anita (Kaye) Spencer (coordinator). Kansas City VAMC – Kansas City, MO: Santosh Sharma (PI), Thomas Wiegmann (PI), Smita Mehta (coordinator). John L. McClellan Memorial Veterans Hospital – Little Rock, AR: Michelle Krause (PI), Kate Dishongh (coordinator). Memphis VAMC – Memphis, TN: Barry Wall (PI), Richard Childress (Co-I), William Cushman (Co-I), Geeta Gyamlani (Co-I), Atossa Niakan (Co-I), Cathy Thompson (Co-I), Janelle Moody (coordinator). Clement J. Zablocki VAMC – Milwaukee, WI: Jeffrey Whittle (PI), Gary Barnas (Co-I), Dawn Wolfgram, (Co-I), Heidi Cortese (coordinator), Jonette Johnson (coordinator). Nashville VAMC/TVHS-GRECC – Nashville, TN: Christianne Roumie (PI), Adriana Hung (Co-I), Jennifer Wharton (coordinator), Kurt Niesner (coordinator). VA New York Harbor Healthcare System – New York, NY: Lois Katz (PI), Elizabeth Richardson (coordinator), George Brock (coordinator). Northport VAMC – Northport, NY: Joanne Holland (PI), Troy Dixon (PI), Athena Zias (Co-I), Christine Spiller (coordinator). Phoenix VA Healthcare System – Phoenix, AZ: Penelope Baker (PI), James Felicetta (PI), Shakaib Rehman (Co-I), Kelli Bingham (coordinator). Portland VAMC – Portland, OR: Suzanne Watnick (PI), Jessica Weiss (Co-I), Tera Johnston (coordinator). St. Louis VA Healthcare System – St. Louis, MO: Stephen Giddings (PI), Andrew Klein (PI), Caroline Rowe (Co-I), Kristin Vargo (coordinator), Kristi Waidmann (coordinator). Washington, D.C. VAMC – Washington, D.C.: Vasilios Papademetriou (PI), Jean Pierre Elkhoury (Co-I), Barbara Gregory (coordinator), Susan Amodeo (coordinator), Mary Bloom (coordinator). West Los Angeles VA Healthcare Center/Greater Los Angeles Healthcare System – Los Angeles, CA: Dalia Goldfarb-Waysman (PI), Richard Treger (Co-I), Karen Knibloe (coordinator). Minneapolis VAMC – Minneapolis, MN: Areef Ishani (PI), Yelena Slinin (Co-I), Christine Olney (coordinator), Jacqueline Rust (coordinator). Audie L. Murphy Memorial Veterans Hospital – South Texas Veterans Healthcare System – San Antonio, TX: Paolo Fanti (PI), Shweta Bansal (Co-I), Monica Dunnam (Co-I), Christopher Dyer (Co-I), Lih-Lan Hu (coordinator), Perla Zarate-Abbott (coordinator).
Footnotes
Disclosures
WCC serves as an uncompensated consultant for Takeda Pharmaceuticals. None of the other authors have any relevant conflicts of interest for this manuscript.
References
- 1.Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, White IR, Caulfield MJ, Deanfield JE, Smeeth L, Williams B, Hingorani A, Hemingway H. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet. 2014;383:1899–1911. doi: 10.1016/S0140-6736(14)60685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 3.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, Shulman NB, Stamler J. Blood pressure and end-stage renal disease in men. N Engl J Med. 1996;334:13–18. doi: 10.1056/NEJM199601043340103. [DOI] [PubMed] [Google Scholar]
- 4.Hsu CY, McCulloch CE, Darbinian J, Go AS, Iribarren C. Elevated blood pressure and risk of end-stage renal disease in subjects without baseline kidney disease. Arch Intern Med. 2005;165:923–928. doi: 10.1001/archinte.165.8.923. [DOI] [PubMed] [Google Scholar]
- 5.Murakami Y, Hozawa A, Okamura T, Ueshima H. Relation of Blood Pressure and All-Cause Mortality in 180 000 Japanese Participants. Hypertension. 2008;51:1483. doi: 10.1161/HYPERTENSIONAHA.107.102459. [DOI] [PubMed] [Google Scholar]
- 6.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie X, Atkins E, Lv J, Bennett A, Neal B, Ninomiya T, Woodward M, MacMahon S, Turnbull F, Hillis GS, Chalmers J, Mant J, Salam A, Rahimi K, Perkovic V, Rodgers A. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2016;387:435–443. doi: 10.1016/S0140-6736(15)00805-3. [DOI] [PubMed] [Google Scholar]
- 8.SPRINT Research Group. Wright JT, Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC, Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, Fine LJ, Haley WE, Hawfield AT, Ix JH, Kitzman DW, Kostis JB, Krousel-Wood MA, Launer LJ, Oparil S, Rodriguez CJ, Roumie CL, Shorr RI, Sink KM, Wadley VG, Whelton PK, Whittle J, Woolard NF, Wright JT, Jr, Pajewski NM. Intensive vs Standard Blood Pressure Control and Cardiovascular Disease Outcomes in Adults Aged >/=75 Years: A Randomized Clinical Trial. JAMA. 2016;315:2673–2682. doi: 10.1001/jama.2016.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cruickshank J, Thorp J, Zacharias FJ. Benefits and potential harm of lowering high blood pressure. Lancet. 329:581–584. doi: 10.1016/s0140-6736(87)90231-5. [DOI] [PubMed] [Google Scholar]
- 11.Farnett L, Mulrow CD, Linn WD, Lucey CR, Tuley MR. The j-curve phenomenon and the treatment of hypertension: Is there a point beyond which pressure reduction is dangerous? JAMA. 1991;265:489–495. [PubMed] [Google Scholar]
- 12.Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, Fine LJ, Goff DC, Jr, Johnson KC, Killeen AA, Lewis CE, Oparil S, Reboussin DM, Rocco MV, Snyder JK, Williamson JD, Wright JT, Jr, Whelton PK. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT) Clin Trials. 2014;11:532–546. doi: 10.1177/1740774514537404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NHLBI. [Date accessed 8/22/17];Systolic Blood Pressure Intervention Protocol Version 4.0. https://www.sprinttrial.org/public/Protocol_Current.pdf.
- 14.D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General Cardiovascular Risk Profile for Use in Primary Care. The Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 16.National Heart, Lung and Blood Institute. [Date accessed 3/3/17];Systolic Blood Pressure Intervention Trial Primary Outcome Paper (SPRINT-POP) Data. https://biolincc.nhlbi.nih.gov/studies/sprint_pop/
- 17.Messerli FH, Mancia G, Conti C, Hewkin AC, Kupfer S, Champion A, Kolloch R, Benetos A, Pepine CJ. Dogma disputed: Can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med. 2006;144:884–893. doi: 10.7326/0003-4819-144-12-200606200-00005. [DOI] [PubMed] [Google Scholar]
- 18.Vidal-Petiot E, Ford I, Greenlaw N, Ferrari R, Fox KM, Tardif JC, Tendera M, Tavazzi L, Bhatt DL, Steg PG. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet. 2016;388:2142–2152. doi: 10.1016/S0140-6736(16)31326-5. [DOI] [PubMed] [Google Scholar]
- 19.Sim JJ, Shi J, Kovesdy CP, Kalantar-Zadeh K, Jacobsen SJ. Impact of Achieved Blood Pressures on Mortality Risk and End-Stage Renal Disease Among a Large, Diverse Hypertension Population. J Am Coll Cardiol. 2014;64:588. doi: 10.1016/j.jacc.2014.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bangalore S, Messerli FH, Wun C-C, Zuckerman AL, DeMicco D, Kostis JB, LaRosa JC. J-curve revisited: an analysis of blood pressure and cardiovascular events in the Treating to New Targets (TNT) Trial. Eur Heart J. 2010;31:2897–2908. doi: 10.1093/eurheartj/ehq328. [DOI] [PubMed] [Google Scholar]
- 21.Kang Y-Y, Wang J-G. The J-Curve Phenomenon in Hypertension. Pulse (Basel) 2016;4:49–60. doi: 10.1159/000446922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Somes GW, Pahor M, Shorr RI, Cushman WC, Applegate WB. The role of diastolic blood pressure when treating isolated systolic hypertension. Arch Intern Med. 1999;159:2004–2009. doi: 10.1001/archinte.159.17.2004. [DOI] [PubMed] [Google Scholar]
- 23.Fagard RH, Staessen JA, Thijs L, Celis H, Bulpitt CJ, de Leeuw PW, Leonetti G, Tuomilehto J, Yodfat Y. On-treatment diastolic blood pressure and prognosis in systolic hypertension. Arch Intern Med. 2007;167:1884–1891. doi: 10.1001/archinte.167.17.1884. [DOI] [PubMed] [Google Scholar]
- 24.McEvoy JW, Chen Y, Rawlings A, Hoogeveen RC, Ballantyne CM, Blumenthal RS, Coresh J, Selvin E. Diastolic Blood Pressure, Subclinical Myocardial Damage, and Cardiac Events: Implications for Blood Pressure Control. J Am Coll Cardiol. 2016;68:1713–1722. doi: 10.1016/j.jacc.2016.07.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansson L, Zanchetti A, Carruthers SG, Dahlof B, Elmfeldt D, Julius S, Menard J, Rahn KH, Wedel H, Westerling S. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755–1762. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 26.Davis EM, Appel LJ, Wang X, Greene T, Astor BC, Rahman M, Toto R, Lipkowitz MS, Pogue VA, Wright JT, Group ARC. Limitations of analyses based on achieved blood pressure: Lessons from the AASK trial. Hypertension. 2011;57:1061–1068. doi: 10.1161/HYPERTENSIONAHA.111.169367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.