Abstract
Inflammatory monocyte (iMOs) recruitment to the brain is a hallmark of many neurological diseases. Prior to entering the brain, iMOs must first egress into the blood from the bone marrow through mechanism which for known encephalitic viruses is CCR2-dependent. Here, we show that during La Crosse Virus (LACV)-induced encephalitis, egress of iMOs was surprisingly independent of CCR2, with similar percentages of iMOs in the blood and brain of heterozygous and CCR2−/− mice following infection. Interestingly, CCR2 was required for iMO trafficking, from perivascular areas to sites of virus infection within the brain. Thus, CCR2 was not essential for iMO trafficking to the blood or the brain, but was essential for trafficking within the brain parenchyma. Analysis of other Orthobunyaviruses showed that Jamestown Canyon virus also induced CCR2-independent iMO egress to the blood. These studies demonstrate that the CCR2-requirement for iMO egress to the blood is not universal for all viruses.
Introduction
Recruitment of classically activated “inflammatory monocytes” (iMOs, defined as CCR2+, CD45+, CD11b+ and Ly6Chi) into tissues is a hallmark of inflammation during viral infections. iMOs originate from the bone marrow (BM) and egress to the blood prior to entering infected tissues where they further differentiate into tissue macrophages(1). iMOs are crucial for host defense(2), however, iMOs can also promote damage to the central nervous system (CNS)(3, 4). Thus, determining the mechanisms controlling iMO recruitment during encephalitic disease is important for understanding pathogenesis and developing therapeutics.
iMO recruitment from BM to blood is mediated by the chemokine CCL2 and its receptor CCR2(1, 5–7). CCL2 expression is produced at very low basal levels, but highly upregulated during viral encephalitis(2, 8). CCL2 binds to CCR2 on iMOs causing desensitization of CXCL12-CXCR4 signaling(9), leading to decreased expression of the adhesion molecule VLA-4 thus allowing iMOs egress from BM to blood(10). Inhibition of CCR2 stops iMO from entering the blood or inflamed tissues(3, 11). CCR2-deficient mice lack normal levels of iMO in the blood, and do not show recruitment of iMOs in response to West Nile Virus (WNV), Herpes Simplex Virus (HSV-1) or experimental autoimmune encephalitis (EAE)(2, 12).
La Crosse virus (LACV) belongs to the California Serogroup of Orthobunyaviruses and is a primary cause of pediatric encephalitis in the United States(13). In both humans and mice, LACV disease is associated with infection of neurons and recruitment of leukocytes in the brain. Immunohistochemical (IHC) analysis showed monocyte/macrophage lineage cells in areas of virus infection in the brain, suggesting these cells may contribute to pathogenesis(14). To understand the role of iMOs in LACV disease, we analyzed iMO recruitment and migration to the CNS. We found significant iMO recruitment to the brain during LACV disease. Surprisingly, these iMOs were recruited to the blood independently of CCR2. Analysis of other CNS viruses, including Jamestown Canyon (JCV) and Tahyna virus (TAHV) showed that these viruses have different mechanisms of monocyte recruitment.
Materials and Methods
Virus stocks
LACV, JCV and TAHV were previously described(14–16). LACV and McKrae HSV1 (William Halford) virus stocks were made using Vero cells, while JTC, TAHV and WNV NY99 stocks were made using C636 cells (ATCC) and tittered using plaque assay(17).
Mice
Studies were completed under NIH/RML Institutional Animal Care and Use Committee approved protocols 2012–047 and 2015–023. Ccr2R/R, Macrophage Fas-Induced Apopotsis (MaFIA), and C57BL/6 mice were purchased from Jackson Laboratories and maintained in breeding colonies at RML. Ccr2+/R mice were F1 progeny of Ccr2R/R and C57BL/6 crosses. 3 week old male and female mice were inoculated i.p. with 103 plaque forming units (PFU) of LACV, JCV or TAHV or 107 PFU HSV-1 or s.c. with 102 PFU WNV. Supernatant from Vero or C636 cells were used for mock controls. Mice were observed daily for neurological signs as previously described(18). For generation of chimeric mice, 7-day old MaFIA were given 200 rad and then given 107 fetal liver cells from Ccr2R/R mice, i.p. After 2 weeks, mice were infected with virus.
Flow cytometry
Cells were isolated from brain and spleen as previously described(18) or from whole blood and analyzed by flow cytometry. Fc receptors were blocked using CD16/CD32 Fcγ III/II (BD Biosciences, clone 2.4G2). Cells were stained with a combination of CD45 (30-F11), CD11b (M1/70), Ly6C (HK1.4), Ly6G (1A8), CD11c (HL3), pDCA1 (JF05-1C2.4.1), CD8 (53-6.7), CD4 (RM4-5); CD3e (17A2) and F480 (BM8). Cells were analyzed on a LSRII (BD Biosciences) and data analyzed using FCS Express software (De Novo). iMOs were gated as CD45hi, CD11b+, CD11c− Ly6G−, Ly6Chi as shown in Supplemental Figure 1. For the CNS, iMOs are expressed as a percent of all cells isolated by Percoll gradient. For blood analysis, iMOs are expressed as a percent of CD45hi cells.
IHC and in situ hybridization (ISH)
IHC of brain tissue was performed as previously described (19) using polyclonal mouse anti-LACV or rabbit anti-CD31 (Abcam) and donkey anti-rabbit AF488 or 647 and goat anti-mouse AF488 or AF594 (Life Sciences). Slides were cover slipped with Prolong Gold mounting media containing DAPI (Life Sciences). ISH was performed as previously described(20) using digoxigenin-labeled RNA sense riboprobes targeting MCP-1 (Ccl2), which were visualized with anti-digoxigenin antibody (Roche) and Fast Red (Dako). Sections were labeled with anti-glial fibrillary acidic protein (GFAP) antibodies (Dako) and diaminobenzidine substrate and counter-stained with hematoxylin.
Results and Discussion
iMOs are the predominate CNS infiltrating immune cell during LACV-induced disease
Analysis of inflammatory cells in the CNS of LACV-infected mice at the onset of clinical signs, showed Ly6Chi iMOs as the primary immune cell infiltrating into the CNS (Fig. 1A, Supplemental Fig. 1). CD11b+, Ly6Clo MOs were the second highest cell population, with minor populations of other leukocytes. Time course analysis showed that iMOs enter the brain by 5 days post infection (dpi), prior to the onset of clinical disease at 6–7dpi (Fig. 1B) suggesting they may have a role in LACV neurological disease.
Fig 1. iMO migration from BM to blood and CNS is CCR2 independent following LACV infection.
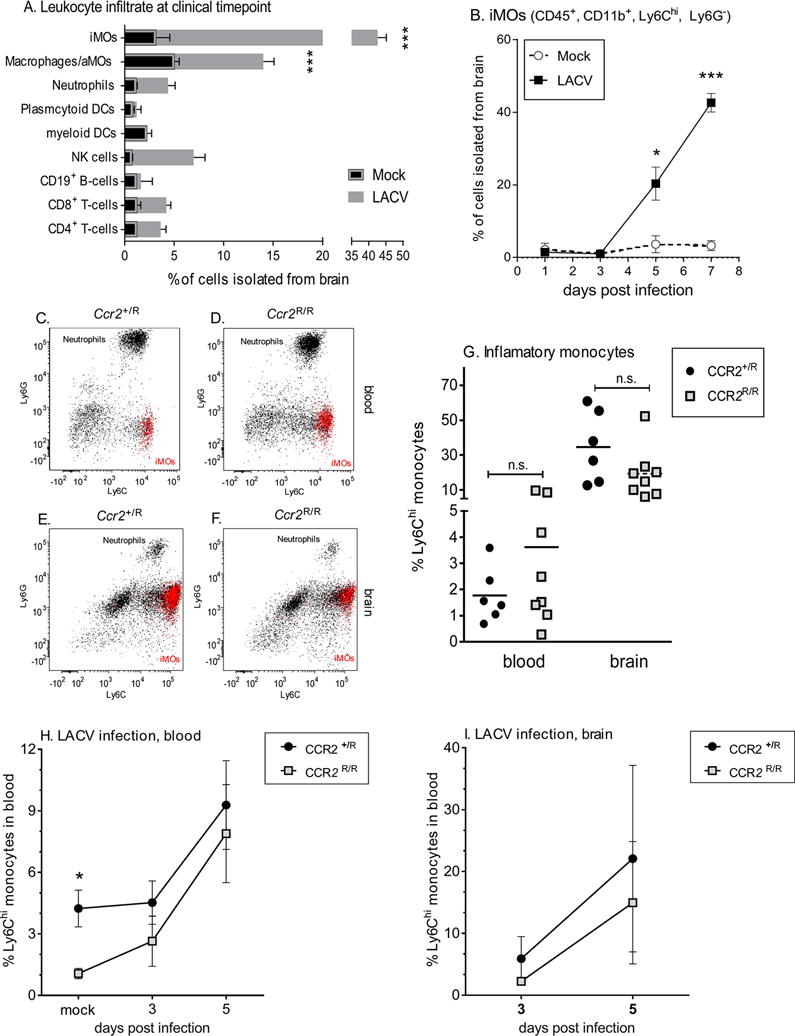
A) Makeup of CD45hi Percoll isolated cells from mouse brains at 7 dpi. B) Time course analysis of iMOs in the CNS. (C–F) Representative flow cytometry data of CD11b+/CD45+ gates from blood (C, D) and brain (E, F) of LACV infected Ccr2+/R and Ccr2R/R mice at 7dpi. iMOs are shown in red in all plots. G) Statistical analysis of iMOs in the blood and brain of Ccr2+/R and Ccr2R/R mice at 7 dpi using a One-way ANOVA with Tukey’s multiple comparison (n.s.= not significant). H–I) Time course of iMOs in blood (H) and brain (I) of Ccr2+/R and Ccr2R/R mice. For all experiments, N=2–4 mice for mock and 6–8 mice for LACV groups per time point. A, B, H and I) *** indicates P<0.001, * P<0.05 for Two-way ANOVA with Sidak’s multiple comparison. Error bars are standard error for all groups.
CCr2 is not necessary for iMO recruitment to the blood or CNS
Recruitment of iMOs from the BM to the blood and CNS has been shown to be dependent on CCR2 and mice deficient in Ccr2 are monocytopenic(2). To study iMO recruitment during LACV infection, we used mice containing a red fluorescence protein (RFP) cassette inserted into the open reading frame of the Ccr2 gene, resulting in Ccr2 deficient (Ccr2R/R) mice expressing RFP in the place of CCR2(21). Heterozygous mice, retaining one functional copy of Ccr2 while also expressing RFP (Ccr2+/R), were used as controls. Surprisingly, Ccr2R/R and Ccr2+/R infected with LACV had similar numbers of iMOs in their blood (Fig. 1C–D, G) and brain (Fig. 1E–G) at the clinical timepoint, suggesting iMO egress to the blood was not dependent of CCR2. To determine when iMOs were recruited to the blood during LACV infection, we examined pre-clinical time points. Following LACV infection, iMOs in the blood of CCR2R/R mice went from being monocytopenic, as observed in mock animals, to having similar iMOs levels as Ccr2+/R mice by 3 dpi through 5 dpi in both the blood (Fig. 1H) and brain (Fig. 1I). Experiments with an unrelated Ccr2−/− mice yielded similar results (Supplemental Fig 2). Thus, CCR2 was not essential for iMO egress to the blood during LACV infection which is in direct contrast to the findings for WNV and HSV-1-infections or EAE(2, 12, 21). To our knowledge, this is the first demonstration of iMO recruitment from the BM to the blood being largely CCR2-independent and suggests alternative mechanisms by which iMOs can be recruited from the BM in large quantities during infection. Understanding the mechanisms behind this response would greatly improve our knowledge about iMO development, but also be a potential therapeutic target to regulate inflammatory responses, either to limit monocyte efflux or induce large numbers of monocytes.
CCR2-mediated iMO egress to the blood is virus-dependent
To ensure the CCR2-independent recruitment for LACV was not due to alterations in mouse lineage or in gating strategies, we verified CCR2-dependent responses of iMOs during WNV and HSV-1 infections (Fig. 2A–B). To determine if other Orthobunyaviruses related to LACV also induced CCR2-independent iMOs, we infected Ccr2R/R mice with JCV or TAHV virus. Both viruses produce a detectable infection in mice, although only TAHV was shown to be neurovirulent(15). Interestingly, iMO frequency in blood was CCR2-independent for JCV but not for TAHV infection (Fig. 2C–D), including a decrease of iMOs in the brains of TAHV-infected Ccr2R/R mice (Fig 2E). Thus, CCR2-dependent iMO egress from the BM to the blood varied among Orthobunyaviruses and was not dependent on neurovirulence and varied even between viruses in the same family. Understanding how these viruses induce iMO responses through different mechanisms will be important not only for understanding differences in viral pathogenesis, but in determining the basic mechanisms by which iMOs can be recruited during pathogenic infections.
Fig 2. Dependence of CCR2 on iMO egress to blood is pathogen specific.
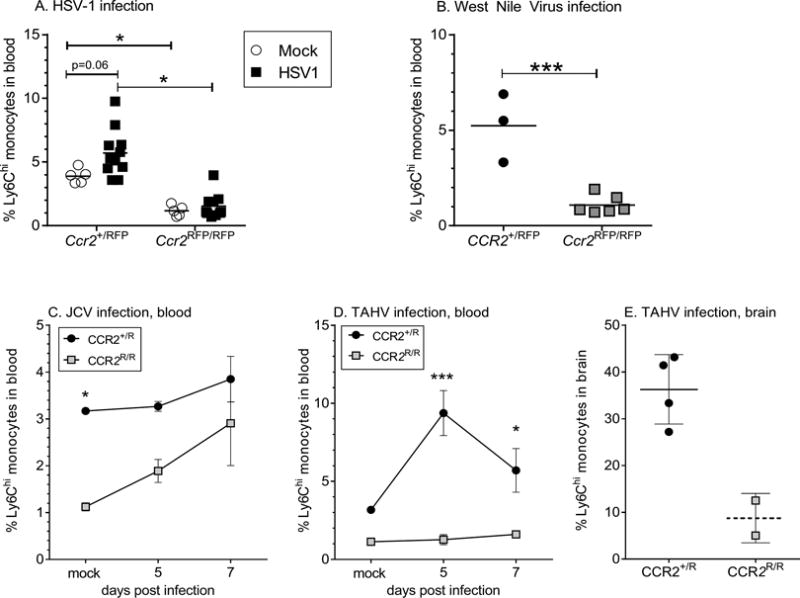
A–B) iMOs in the blood of Ccr2+/R and Ccr2R/R mice following infection with (A) HSV-1 at 7 dpi or (B) WNV and 9–13dpi, respectively. Symbols indicate individual mice. *P<0.05 using a One-way ANOVA with Tukey’s multiple comparison in (A). ***P<0.001 using unpaired t-test in (B). C–D) Time course of iMOs in the blood of Ccr2+/R and Ccr2R/R mice shown as percent of all CD45+ cells following infection with (C) JCV or (D) TAHV. n=3–8 animals per group at each time point. *** P<0.001, * P<0.05 for Two-way ANOVA analysis with Sidak’s multiple comparison. E) iMOs in brains of individual TAHV-infected Ccr2+/R and Ccr2R/R mice with clinical disease. Error bars are standard error for all graphs.
LACV-induced iMOs are BM-derived
Since iMOs may proliferate in the spleen, we analyzed iMOs in the spleen of mock and LACV infected mice by IHC and flow cytometry. Infection did not induce either focal or overall percentage increases of iMOs in the spleen (Fig. 3A–C), suggesting the spleen was not the source of virus-induced iMOs. To directly confirm that LACV-induced iMOs were not being generated from tissue-resident myeloid cells, we generated chimeric mice where BM-derived iMOs could be distinguished from resident tissue cells. In short, fetal liver cells from Ccr2R/R mice were used to repopulate partially-irradiated MaFIA mice, which express GFP under the CD115 promoter. In these chimeric mice, myeloid cells, including tissue resident cells would be GFP positive, while cells coming from the bone marrow would be CCR2+ GFP+ or CCR2− RFP+ (Fig. 3D). In mock-infected mice, only 16% of iMOs in the blood were CCR2−, correlating with the inability of CCR2− iMOs to egress from the BM (Fig. 3E). However, during LACV infection, the proportion of RFP+ CCR2− cells in the blood increased substantially (Fig. 3F), indicating that BM progenitors, not tissue resident cells, were the primary source of iMOs.
Fig 3. LACV-induced CCR2-deficient iMOs are derived from BM.
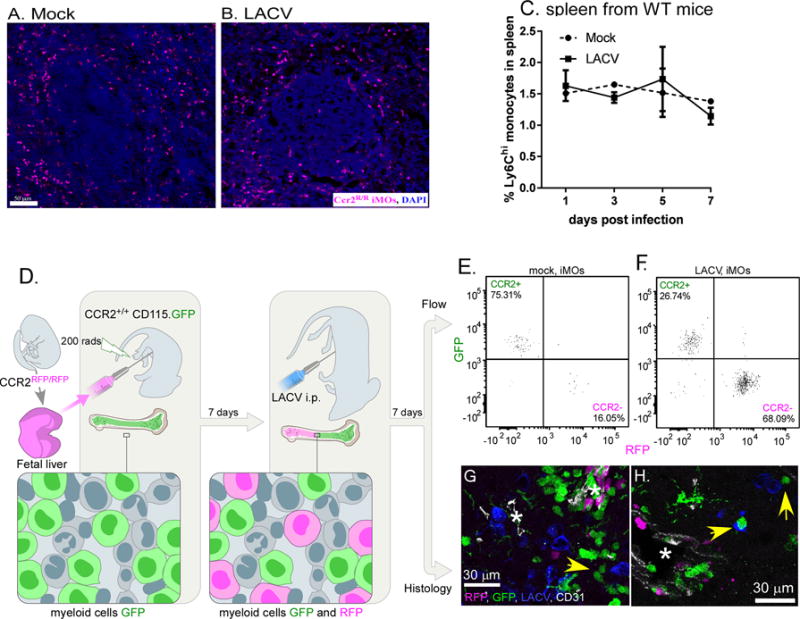
A–B) iMOs in the spleen of (A) mock and (B) LACV-infected Ccr2R/R mice at 7 dpi showing a similar staining of RFP+ iMOs (magenta) and cell nuclei (blue) around spleen follicles. C) Time course of iMOs in spleen during LACV infection in WT mice. D) Representation of transfer of Ccr2R/R fetal liver cells to irradiated MaFIA mice. E–F) Representative flow data of Ccr2+/+ (GFP) and Ccr2R/R (RFP) iMOs in blood of (E) mock and (F) LACV-infected chimeric mice at 7 dpi. G–H) IHC image of CCR2+ (GFP) and CCR2− (RFP) iMOs in the brain with staining forCD31+ BVs (white) and (H) LACV infected neurons (blue). Yellow arrows indicate GFP+ cells in close proximity to virus infected neurons (blue). Asterisk indicates blood vessel.
CCR2 is important for iMO migration away from CNS perivascular regions
Analysis of brain tissue from LACV-infected BM-chimeric mice showed both GFP+ iMOs and RFP+ iMOs in the CNS (Fig. 3G–H). However, RFP+ CCR2− iMOs stayed primarily around blood vessels (BVs), while GFP+ CCR2+ iMOs were observed away from BVs and near LACV-infected neurons (Fig. 3G–H, white arrows). Additional IHC analysis of brains from LACV-infected Ccr2+/R also showed CCR2+ iMOs localized to areas of LACV infection throughout the brain parenchyma (BP) (Fig. 4A), while CCR2− iMOs in infected Ccr2R/R mice were found primarily around BVs (Fig. 4B) and did not migrate as far from BVs (Fig. 4C). Costaining with CD31 (white staining) showed that CCR2− iMOs had crossed the brain endothelium into the perivascular space (Fig. 4B, insert). However, this lack of migration was not associated with any observable difference in infection or pathology in the brain. Because CCR2 binds CCL2, we examined whether CCL2 expressing cells were found in the brain during LACV infection. ISH for Ccl2 mRNA showed consistent staining around BVsas well as GFAP+ cells in the parenchyma, indicating that many of the Ccl2-producing cells are parenchymal astrocytes (Fig. 4D). These astrocytes may be responsible for recruiting CCR2+ iMOs into the brain parenchyma once they enter the CNS. In the absence of CCR2, there may not be signals sufficient to recruit iMOs to areas of infection within the brain. Thus, during LACV infection, CCR2 is not essential for recruitment of iMOs from the BM to the blood and CNS, but does appear to be essential for migration of iMOs to the sites of infection within the brain. These studies suggest that the role of CCR2 in iMO recruitment and function is quite complex and that it varies between pathogens and may have different role between tissues. Since the exact mechanisms of CCR2-dependent iMO egress is not known, it is difficult to determine the mechanisms of LACV-induced CCR2-independent recruitment. However, studies with mice deficient in other chemokines or chemokine receptors (CXCL10, CCR5, CCR7) or initiators of innate immune responses (MyD88, MAVS) did not alter iMO recruitment (data not shown). This suggests that the CCR2-independent mechanism of iMO recruitment may be more complex than a simple compensatory mechanism that allows iMO egress.
Fig 4. CCR2 promotes iMO dispersal away from perivascular areas into sites of LACV infection.
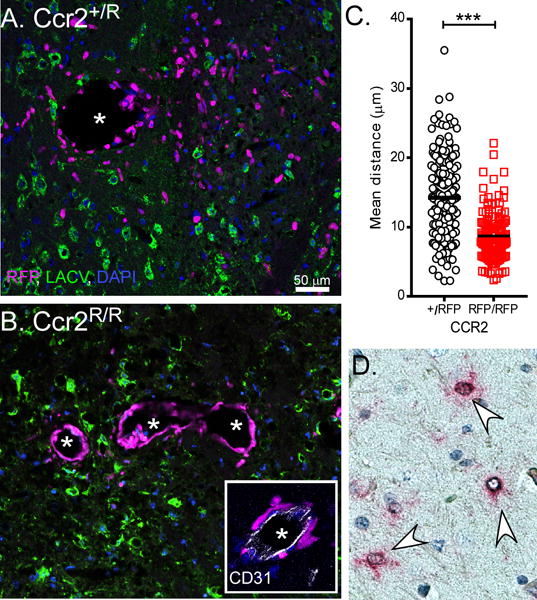
A–B) IHC labeling of iMO (magenta) infiltration into LACV (green) infected brains from Ccr2+/R (A) and Ccr2R/R (B) mice at 7 dpi. Inset in (B) shows iMOs in Ccr2R/R mice have crossed the CD31+ endothelium. DAPI=blue. Scale bar in (A) also pertains to (B). C) Quantification of distance from the center of CCR2+/R and CCR2R/R cell nuclei to closest CD31+ blood vessel. N = 2–5 mice per group with 12–19 20× fields analyzed. D) ISH of 7dpi LACV brain tissues shows Ccl2 (red) labeling in GFAP+ astrocytes (brown).
Supplementary Material
Acknowledgments
This work was supported by the NIAID Division of Intramural Research.
References
- 1.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim JK, Obara CJ, Rivollier A, Pletnev AG, Kelsall BL, Murphy PM. Chemokine receptor Ccr2 is critical for monocyte accumulation and survival in West Nile virus encephalitis. J Immunol. 2011;186:471–478. doi: 10.4049/jimmunol.1003003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Terry RL, Getts DR, Deffrasnes C, van Vreden C, Campbell IL, King NJ. Inflammatory monocytes and the pathogenesis of viral encephalitis. J Neuroinflammation. 2012;9:270. doi: 10.1186/1742-2094-9-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamasaki R, Lu H, Butovsky O, Ohno N, Rietsch AM, Cialic R, Wu PM, Doykan CE, Lin J, Cotleur AC, Kidd G, Zorlu MM, Sun N, Hu W, Liu L, Lee JC, Taylor SE, Uehlein L, Dixon D, Gu J, Floruta CM, Zhu M, Charo IF, Weiner HL, Ransohoff RM. Differential roles of microglia and monocytes in the inflamed central nervous system. J Exp Med. 2014;211:1533–1549. doi: 10.1084/jem.20132477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 6.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, Leiner I, Li MO, Frenette PS, Pamer EG. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity. 2011;34:590–601. doi: 10.1016/j.immuni.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JH, Patil AM, Choi JY, Kim SB, Uyangaa E, Hossain FM, Park SY, Lee JH, Kim K, Eo SK. CCL2, but not its receptor, is essential to restrict immune privileged central nervous system-invasion of Japanese encephalitis virus via regulating accumulation of CD11b(+) Ly-6C(hi) monocytes. Immunology. 2016;149:186–203. doi: 10.1111/imm.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung H, Mithal DS, Park JE, Miller RJ. Localized CCR2 Activation in the Bone Marrow Niche Mobilizes Monocytes by Desensitizing CXCR4. PLoS One. 2015;10:e0128387. doi: 10.1371/journal.pone.0128387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hidalgo A, Sanz-Rodriguez F, Rodriguez-Fernandez JL, Albella B, Blaya C, Wright N, Cabanas C, Prosper F, Gutierrez-Ramos JC, Teixido J. Chemokine stromal cell-derived factor-1alpha modulates VLA-4 integrin-dependent adhesion to fibronectin and VCAM-1 on bone marrow hematopoietic progenitor cells. Exp Hematol. 2001;29:345–355. doi: 10.1016/s0301-472x(00)00668-8. [DOI] [PubMed] [Google Scholar]
- 11.Fujimura N, Xu B, Dalman J, Deng H, Aoyama K, Dalman RL. CCR2 inhibition sequesters multiple subsets of leukocytes in the bone marrow. Sci Rep. 2015;5:11664. doi: 10.1038/srep11664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boivin N, Menasria R, Gosselin D, Rivest S, Boivin G. Impact of deficiency in CCR2 and CX3CR1 receptors on monocytes trafficking in herpes simplex virus encephalitis. J Gen Virol. 2012;93:1294–1304. doi: 10.1099/vir.0.041046-0. [DOI] [PubMed] [Google Scholar]
- 13.Gaensbauer JT, Lindsey NP, Messacar K, Staples JE, Fischer M. Neuroinvasive arboviral disease in the United States: 2003 to 2012. Pediatrics. 2014;134:e642–650. doi: 10.1542/peds.2014-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett RS, Cress CM, Ward JM, Firestone CY, Murphy BR, Whitehead SS. La Crosse virus infectivity, pathogenesis, and immunogenicity in mice and monkeys. Virol J. 2008;5:25. doi: 10.1186/1743-422X-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett RS, Gresko AK, Murphy BR, Whitehead SS. Tahyna virus genetics, infectivity, and immunogenicity in mice and monkeys. Virol J. 2011;8:135. doi: 10.1186/1743-422X-8-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett RS, Nelson JT, Gresko AK, Murphy BR, Whitehead SS. The full genome sequence of three strains of Jamestown Canyon virus and their pathogenesis in mice or monkeys. Virol J. 2011;8:136. doi: 10.1186/1743-422X-8-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winkler CW, Myers LM, Woods TA, Messer RJ, Carmody AB, McNally KL, Scott DP, Hasenkrug KJ, Best SM, Peterson KE. Adaptive Immune Responses to Zika Virus Are Important for Controlling Virus Infection and Preventing Infection in Brain and Testes. J Immunol. 2017 doi: 10.4049/jimmunol.1601949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winkler CW, Myers LM, Woods TA, Carmody AB, Taylor KG, Peterson KE. Lymphocytes have a role in protection, but not in pathogenesis, during La Crosse Virus infection in mice. J Neuroinflammation. 2017;14:62. doi: 10.1186/s12974-017-0836-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winkler CW, Race B, Phillips K, Peterson KE. Capillaries in the olfactory bulb but not the cortex are highly susceptible to virus-induced vascular leak and promote viral neuroinvasion. Acta Neuropathol. 2015;130:233–245. doi: 10.1007/s00401-015-1433-0. [DOI] [PubMed] [Google Scholar]
- 20.Butchi NB, Woods T, Du M, Morgan TW, Peterson KE. TLR7 and TLR9 trigger distinct neuroinflammatory responses in the CNS. Am J Pathol. 2011;179:783–794. doi: 10.1016/j.ajpath.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saederup N, Cardona AE, Croft K, Mizutani M, Cotleur AC, Tsou CL, Ransohoff RM, Charo IF. Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. PLoS One. 2010;5:e13693. doi: 10.1371/journal.pone.0013693. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


