Abstract
Connectivity of the prefrontal cortex (PFC) matures through adolescence, coinciding with emergence of adult executive function and top-down inhibitory control over behavior. Alcohol exposure during this critical period of brain maturation may affect development of PFC and frontolimbic connectivity. Adult rats exposed to adolescent intermittent ethanol (AIE; 5 g/kg ethanol, 25% v/v in water, i.g., 2-day-on, 2-day-off, postnatal day 25–54) or water control underwent resting-state functional MRI to test the hypothesis that AIE induces persistent changes in frontolimbic functional connectivity under baseline and acute alcohol conditions (2 g/kg ethanol or saline, i.p., administered during scanning). Data were acquired on a Bruker 9.4T MR scanner with rats under dexmedetomidine sedation in combination with isoflurane. Frontolimbic network regions-of-interest (ROIs) for data analysis included: PFC [prelimbic (PrL), infralimbic (IL) and orbitofrontal cortex (OFC) portions], nucleus accumbens (NAc), caudate putamen (CPu), dorsal hippocampus, ventral tegmental area, amygdala, and somatosensory forelimb used as a control region. AIE decreased baseline resting-state connectivity between PFC subregions (PrL-IL and IL-OFC) and between PFC-striatal regions (PrL-NAc, IL-CPu, IL-NAc, OFC-CPu and OFC-NAc). Acute ethanol induced negative BOLD changes within all ROIs examined, along with significant increases in functional connectivity in control, but not AIE animals. Together these data support the hypothesis that binge-like adolescent alcohol exposure causes persistent decreases in baseline frontolimbic (particularly, frontostriatal) connectivity and alters sensitivity to acute ethanol-induced increases in functional connectivity in adulthood.
Keywords: adolescence, ethanol, fcMRI, prefrontal cortex, Sprague-Dawley, striatum
Introduction
The prefrontal cortex (PFC) and frontolimbic pathways undergo structural and functional connectivity changes through adolescence in humans (e.g., Asato et al., 2010; Liston et al., 2006; Rubia 2013; Supekar et al., 2010) and rodents (Cunningham et al., 2002; Cressman et al., 2010; Markham et al., 2007), which may underlie the development of cognitive control. Indeed, adolescents’ relative immature frontolimbic circuitry is thought to contribute to adolescent-typical behaviors such as impulsivity, risk-taking and drug use (Casey & Jones, 2010; Ernst, 2014), with alcohol being the most widely used drug at that age. In the 2014 Monitoring the Future Survey, 11%, 30% and 50% of 8th, 10th and 12th graders reported having been “drunk” in their lifetime, and 19% of 12th graders reported binge drinking (5+ drinks in a row) within the past two weeks (Johnston et al., 2015). Despite the prevalence of adolescent alcohol use, scientists are still in the early stages of identifying the potential long-term impact that binge alcohol exposure might impose on the developing adolescent brain. One finding is that an individual may be four times more likely to develop an alcohol use disorder (AUD) in adulthood when drinking is initiated at an early age (Grant & Dawson, 1998). Several rodent studies have also found increased alcohol (ethanol) consumption in adulthood following adolescent intermittent ethanol (AIE) exposure (see Crews et al., 2016 for review), consistent with adolescent alcohol exposure increasing risks for AUD.
The mechanisms underlying susceptibility for AUD after adolescent alcohol exposure are unknown. One hypothesis is that alcohol exposure during adolescence may perpetuate a lack of top-down inhibitory control, consistent with the notion of a “lock-in” of an adolescent phenotype (Spear & Swartzwelder, 2014). Indeed, several human imaging studies have suggested the PFC may be particularly vulnerable to adolescent alcohol use, with evidence for decreased PFC volume (De Bellis et al., 2005; Medina et al., 2008; Squeglia et al., 2015) and alterations in frontal white matter tracts (Bava et al. 2013; Jacobus et al. 2013a; Jacobus et al. 2013b; McQueeny et al. 2009). Interestingly, decreased frontolimbic white matter integrity is associated with increased likelihood of risk taking in heavy substance-using adolescents (Jacobus et al., 2013c), and white matter integrity was shown to be further decreased by heavy alcohol use during the interscan interval in adolescent users (Bava et al., 2013). However, it is difficult to disentangle the contribution of alcohol exposure per se versus potential pre-existing conditions and other environmental factors (e.g., multisubstance use) in human studies.
Animal models of AIE exposure (Crews et al., 2016), which can control these extraneous factors, have likewise found that adolescent rats are more affected by the degenerative effects of alcohol on frontal brain regions (Crews et al., 2000). A recent study found persistent alterations in cortical thickness in frontal brain regions of adult rats that were exposed to AIE (Vetreno et al., 2016). Further, adult rodents with a history of AIE also show behavioral changes consistent with alterations in PFC function, like deficits in set-shifting (Gass et al., 2014), reversal learning (Coleman et al., 2011, 2014; Vetreno et al., 2012) and extinction (Gass et al., 2014). In response to an acute alcohol challenge, adult rats exposed to AIE also show altered c-fos activity, a surrogate marker for neuronal activation, with a blunted response in PFC and enhanced activation in nucleus accumbens (NAc) compared to controls (Liu & Crews, 2015). Together these studies suggest persistent changes in PFC function after AIE exposure under baseline and acute ethanol conditions. However, it is unknown how frontolimbic functional connectivity may be persistently altered by AIE.
Resting-state functional connectivity can be assessed with MRI (rs-fcMRI) by correlating fluctuations in blood-oxygen-level-dependent (BOLD) signal, a surrogate marker of neural activity, between and across brain regions while the subject is at rest (i.e., in the absence of any task; Biswal et al., 1995; Chen et al., 2009; Pan et al., 2015; Pawela et al., 2009). Importantly, humans, other primates, and rats share similar resting-state networks of connectivity (Lu et al., 2012a), and these connectivity networks are sensitive to changes in neurodevelopmental stage (Rubia et al., 2013) and psychological disorders, including alcoholism (Muller-Oehring et al., 2015). The purpose of this study was to use rs-fcMRI in rats to test the hypothesis that AIE induces persistent changes in PFC and frontolimbic functional connectivity under baseline and acute alcohol conditions in adulthood. We hypothesized that AIE would decrease frontolimbic connectivity under baseline conditions, consistent with deficits in PFC control of top-down inhibition. In response to an acute ethanol challenge, we anticipated that AIE would alter evoked BOLD response in a region-specific manner (Liu & Crews, 2015), resulting in AIE-related alterations in functional connectivity after acute ethanol.
Materials and Methods
Subjects
A total of 20 male Sprague-Dawley rats bred and reared in-house were used in this experiment (breeders purchased from Envigo, previously Harlan Laboratories, in Dublin, VA). Only males were used in this study since the aforementioned AIE rodent studies used males. On the day after birth, postnatal day (P) 1, litters were culled to 8–10 pups, with a sex ratio of 6 males and 4 females retained whenever possible. Pups were housed with their mother until pair-housed at weaning (P21). Animals were maintained in a temperature- and humidity-controlled vivarium on a 12:12-h light: dark cycle (lights on 0700) with ad libitum access to food and water. All animals were maintained and treated in accordance with the Guide for the Care and Use of Laboratory Animals established by the National Institutes of Health (8th Ed), using protocols approved the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill.
Exposure
A well-established model of adolescent binge ethanol exposure was used (Crews et al., 2016). Animals were given 5 g/kg ethanol (25% v/v; AIE group, n=10) or water (CTRL group, n=10) intragastrically (i.g.) on a 2-day-on, 2-day-off regimen during adolescence (P25–54) for a total of 16 exposures. Blood samples were collected from the tail 30 min after gavage on P33 or 34 (5th or 6th exposure) and blood ethanol concentrations (BECs) were measured using an Analox-AM1 (Analox Technologies, Atlanta, GA, USA). This exposure produced average BECs of 127 ± 24 mg/dl, in the range of the National Institute of Alcohol and Alcohol Abuse (NIAAA) definition of binge level alcohol exposure (i.e., > 80 mg/dl). Animals were housed with same-sex littermates at weaning, and each pair of animals received the same exposure (either EtOH or water) throughout adolescence. After the exposure period, animals were left undisturbed aside from routine animal care until MRI procedures in adulthood (~P100–110; see Figure 1A for experiment timeline).
Figure 1.
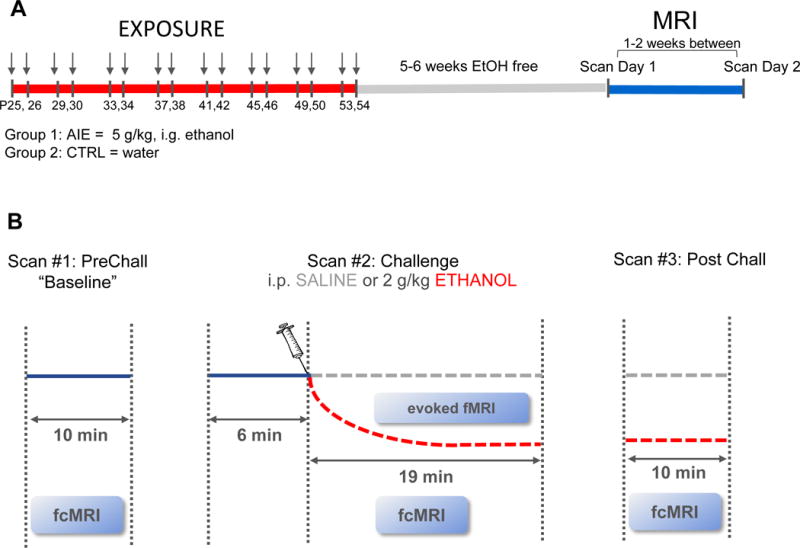
(A) Experiment timeline. Animals in Group 1 were exposed to adolescent intermittent ethanol (AIE; 5 g/kg ethanol [EtOH], 25% v/v in water, i.g., 2-day-on, 2-day-off, postnatal day [P]25-P54). Control (CTRL) animals in Group 2 received an equivalent volume of water during the same exposure regimen. In adulthood, each animal underwent two scanning days. (B) Procedure on each scan day. Three scans were acquired in immediate succession: 1) 10-minute baseline resting-state scan [PreChall], 2) 25-minute challenge scan [Chall], and 3) 10-minute post-challenge scan [PostChall]. During the Chall scan, animals were injected i.p. with either 2 g/kg EtOH (20% v/v) or an equivalent volume of saline (SAL) 6 minutes after scan start. Each animal served as its own saline control for acute EtOH challenge, and the order of injection was counterbalanced.
fMRI acquisition
In preparation for MRI procedures, rats were endotracheally intubated and mechanically ventilated using a small animal, MR-compatible ventilator (CWE Inc., MRI-1, Ardmore, PA). Anesthesia was initially maintained under constant isoflurane (2%) mixed with medical air. Next, animals were placed within a head-holder and harnessed to a custom-made, MR-compatible small animal cradle. The cradle was lined with a circulating water blanket connected to a temperature-adjustable water bath located outside the scanner room (Thermo Scientific, Waltham, MA). A rectal probe was employed and core body temperature was maintained at 37 ± 0.5°C. Mechanical ventilation volume and rate were adjusted to maintain end-tidal carbon dioxide (EtCO2) of 2.8–3.2% and peripheral capillary oxygen saturation (SpO2) above 96%, using capnometry (Surgivet, Smith Medical, Waukesha, WI) and pulse oximetry (MouseOx Plus, STARR Life Science Corp., Oakmont, PA). EtCO2 values from the capnometry system were previously calibrated against invasive sampling of arterial blood gas, reflecting a partial pressure of carbon dioxide (pCO2) level of 30–40 mm Hg (Shih et al., 2012; Shih et al., 2013).
All MR images were acquired on a 9.4-Tesla Bruker BioSpec system with a BGA-9S gradient insert (Bruker Corp., Billerica, MA). A single-loop surface coil constructed in-house with an internal diameter of 1.6 cm was used as a transceiver placed directly over the rat head. Magnetic field homogeneity was optimized first by global shim and followed by local first- and second-order shims using the FASTMAP protocol. Subsequently, anesthesia was switched from 2% isoflurane to sedation using dexmedetomidine (dexdomitor; 0.05 mg/kg/hr, i.p.) cocktailed with the paralytic agent pancuronium bromide (0.5 mg/kg/hr, i.p.). This cocktail was administered for the remaining scan duration, continuously supplemented by ~1.2% isoflurane, similar to a well-established sedation protocol for fMRI (Fukuda et al. 2013, Zhao et al., 2012; 2014; 2016). A slightly higher isoflurane regimen was used due to animal motion early on in data collection. Importantly, level of isoflurane was held constant between AIE and CTRL groups, thus any group differences detected in functional connectivity are likely associated with exposure history.
For anatomical referencing, a T2-weighted RARE pilot image was taken in the mid-sagittal plane to localize the anterior commissure; this structure is located at approximately −0.36 mm posterior to the bregma and served as a reference for anteroposterior slice positioning in subsequent anatomical and functional scans. T2-weighted anatomical images were obtained using a RARE sequence (scan parameters: TR = 2500 ms, TEeff = 33 ms, RARE factor = 8, slice thickness = 1 mm, matrix size = 256 × 256, FOV = 2.56 × 2.56 cm2). Twelve coronal slices were acquired, with the 5th slice from the anterior direction aligned with the anterior commissure (as revealed in the previous T2-weighted pilot scan in the mid-sagittal plane). BOLD fMRI scans were acquired using a multi-slice single-shot gradient echo echo-planar imaging sequence (EPI) [scan parameters: TR=1000 ms, TE=12.6 ms, bandwidth=250 kHz, slice thickness=1 mm, matrix size=80 × 80 (zero-padding to 128 × 128), and FOV=2.56 × 2.56cm2]. Image slice geometry was imported from the previously acquired T2-weighted anatomical image (12 slices).
Each animal underwent two scanning sessions, separated by at least 1 week. On each scan day, three separate EPIs were acquired in immediate succession: 1) a 10-minute baseline resting-state scan [PreChall], 2) a 25-minute challenge scan [Chall], and 3) a 10-minute post challenge scan [PostChall]. During the Chall scan, animals were injected i.p. with either 2 g/kg ethanol (20% v/v EtOH; producing an average BEC of 215 ± 20 mg/dl according to Analox analysis of tail blood samples collected 30–40 minutes after injection—i.e., immediately after the scan) or an equivalent volume of saline (SAL) 6 minutes after the start of the Chall scan (Figure 1B). Each animal served as its own saline control for acute EtOH challenge, with order counterbalanced across animals in each experimental group. In other words, half of the animals received acute EtOH on scan day one and SAL during scan day two, and the other half were given SAL on day one and EtOH on scan day two, which occurred at least one week later.
Data analysis
One animal died prior to scanning due to issues with the ventilator, and three animals died after the first scan session due to a failure to recover from the paralytic-sedative cocktail (1 from each group: AIE-EtOH, CTRL-EtOH, CTRL-SAL) leaving an n=9 and 7 for the AIE and CTRL groups, respectively, in the baseline resting-state analysis (i.e., PreChall data). Two additional animals were excluded due to substantial movement during the challenge scan, leaving an n=7 for the AIE group and an n=7 for CTRL in the analyses of acute ethanol challenge.
Functional connectivity matrix analysis
Functional scans were preprocessed using the Analysis of Functional NeuroImages software suite (AFNI v2011-12-21-1014). For functional connectivity analyses, the workflow included slice-timing correction, motion correction, alignment to a T2 template, linear detrending, spatial smoothing (Gaussian kernel FWHM = 0.3 mm), and bandpass filtering (0.01–0.08 Hz). Functional connectivity analyses were conducted using the temporal correlation method, with correlation coefficients (r-values) transformed to a standard normal distribution (Fisher-Z-scores) to allow statistical analysis between groups. Fisher-Z transformed correlation matrices were generated using the average functional time series extracted for each region-of-interest (ROI) in the template atlas. Repeated-measures analysis of variance (RM ANOVA) was used to analyze Fisher-Z transformed correlations between groups, followed by post-hoc tests when appropriate. False-discovery rate (FDR) methods were used to correct for multiple comparisons. To address the hypothesis that AIE results in altered frontolimbic functional connectivity, ROIs representing key rat PFC subregions and limbic-reward regions that have previously been shown to be particularly susceptible to AIE (Crews et al., 2016) were used: prelimbic (PrL; corresponding to human Brodmann area 32), infralimbic (IL; corresponding to human Brodmann area 25) and orbitofrontal cortex (OFC; corresponding to human Brodmann areas 11, 13 and 14) regions of the PFC, as well as nucleus accumbens (NAc), caudate putamen (CPu), dorsal hippocampus (Hipp), ventral tegmental area (VTA) and amygdala (AMY). The somatosensory forelimb (S1FL) was chosen as a control region, bringing the total number of ROIs to 9. All ROIs were manually drawn by co-registering the T2 template to the Paxinos & Watson rat brain atlas (6th Ed) (Figure 2). Left and right hemisphere (LH and RH, respectively) ROIs were analyzed separately, yielding correlation matrices detailing within and between hemisphere connectivity. However, LH and RH did not statistically differ across scans, and no significant hemisphere × exposure interactions were detected (Supplemental Figure S1); therefore, statistical results are shown on correlation matrices with LH and RH combined for each ROI (i.e., bilateral ROIs).
Figure 2.
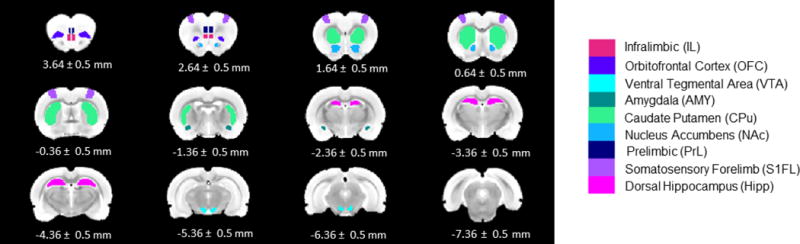
ROIs used for analyses shown on the T2 template. All ROIs were manually drawn by co-registering the T2 template to the Paxinos & Watson rat brain atlas (6th Ed). Numbers below slices indicate location from bregma.
Voxel-wise connectivity maps
Functional scans were preprocessed in the same manner as the matrix analysis, except an additional spatial smoothing step (0.6mm FWHM; about twice the voxel size) was applied to the z-score data to reduce noise (see Supplemental Figure S2). Using the same bilateral ROIs as was used in the matrix analysis, BOLD time series in each region was correlated with all other voxels in the brain, and Z-score correlation maps of whole brain connectivity were plotted for AIE and CTRL.
BOLD time course analysis
For the analysis of SAL- or EtOH-challenge BOLD signal, only slice-timing correction, motion correction, and alignment to a T2 template were employed. BOLD time courses were then extracted from each ROI and normalized to the first 20 seconds. For statistical analysis, percent change from the 6-minute baseline was calculated for each ROI per animal [(last 10 minutes − first 6 minutes) *100]. The last 10 minutes of the Chall scan was chosen to capture peak BECs after i.p. injection (Adalstenson et al., 2006).
Results
Baseline resting-state connectivity
At baseline, resting-state connectivity Z-correlations varied across brain regions. CPu connectivity to OFC was the highest Z-score among those studied in both CTRL and AIE groups. In CTRL, frontal cortical brain regions had Z-scores around 1 with each other and the striatal regions, CPu and NAc. The high Z-score correlations of frontal cortical brain regions with CPu and NAc suggest strong cortical–striatal connectivity in CTRL rats. A similar pattern of frontostriatal connectivity has also been observed under awake conditions (Liang et al., 2012).
To determine long-lasting effects of AIE exposure on resting-state connectivity in adulthood, rats were assessed 46–55 days after the last adolescent ethanol dose when they had matured to adults, e.g. P100–110. Each animal was scanned twice, and the baseline PreChall scan for both scan days was analyzed to assess persistent effects on connectivity. The 2 (adolescent exposure) × 2 (scan day) × 2 (hemisphere) × 36 (ROI pair) RM ANOVA revealed significant main effects of exposure, F (1,14) = 5.50, p< 0.05, and ROI pair, F (35, 490) = 20.05, p< 0.001, as well as an adolescent exposure × ROI pair interaction, F (35, 490) = 2.08, p< 0.001. Compared to age-matched CTRL, adult rats exposed to AIE had significantly less connectivity between PFC regions (PrL-IL and IL-OFC), as well as between PFC and striatal regions, specifically, PrL-NAc, IL-CPu, IL-NAc, OFC-CPu and OFC-NAc (Figure 3A and 3B; also see Supplemental Table S1 for Z-score and Correlation r-value means and SEMs). Thus, AIE robustly decreased frontostriatal connectivity in adulthood. For additional analyses of the PreChall data with global signal regression and partial correlation see Supplemental Figures S3 and S4, respectively.
Figure 3.
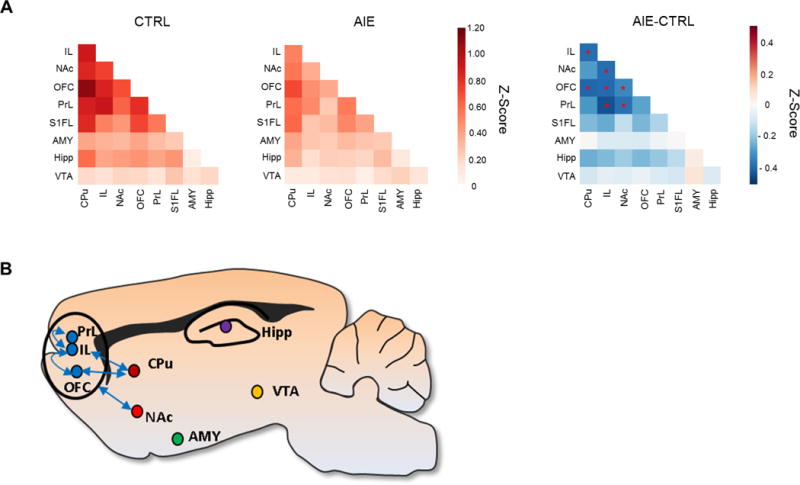
(A) Functional connectivity matrix analysis of adult baseline PreChall data following AIE is shown collapsed across scan day. Z-score correlation matrices for CTRL and AIE groups, as well as the difference between AIE and CTRL Z-scores among each ROI pair (AIE – CTRL). Red indicates increased connectivity and blue indicates decreased connectivity. AIE adults have significantly less resting-state connectivity between PFC subregions (PrL-IL and IL-OFC), as well as between PFC regions and the striatum (PrL-NAc, IL-CPu, IL-NAc, OFC-CPu and OFC-NAc). Asterisks (*) indicate statistical significance (p < 0.05). (B) Sagittal view of the rat brain showing ROI-pair connectivity that was significantly decreased in AIE adults.
A voxel-wise connectivity approach was then used to qualitatively explore differences in whole brain connectivity between AIE and CTRL. Using the same bilateral PFC ROIs that significantly differed between AIE and CTRL (i.e., PrL, IL, OFC), AIE adults showed significantly less brain-wide connectivity, consistent with the above statistical analysis (Figure 4; threshold set at Z-score = 0.5). For example, under this threshold level, PFC (PrL, IL and OFC) connectivity extends across all PFC regions as well as striatum, cingulate and parietal cortical areas in CTRL animals. In contrast, this threshold level yields much less connectivity in AIE animals compared to CTRL, with less connectivity among PFC regions and striatum, cingulate and parietal cortical regions. These results support the previous analysis that AIE decreases frontostriatal connectivity in adulthood (also see Supplementary videos showing PFC functional connectivity using a traveling seed in AIE and CTRL groups).
Figure 4.
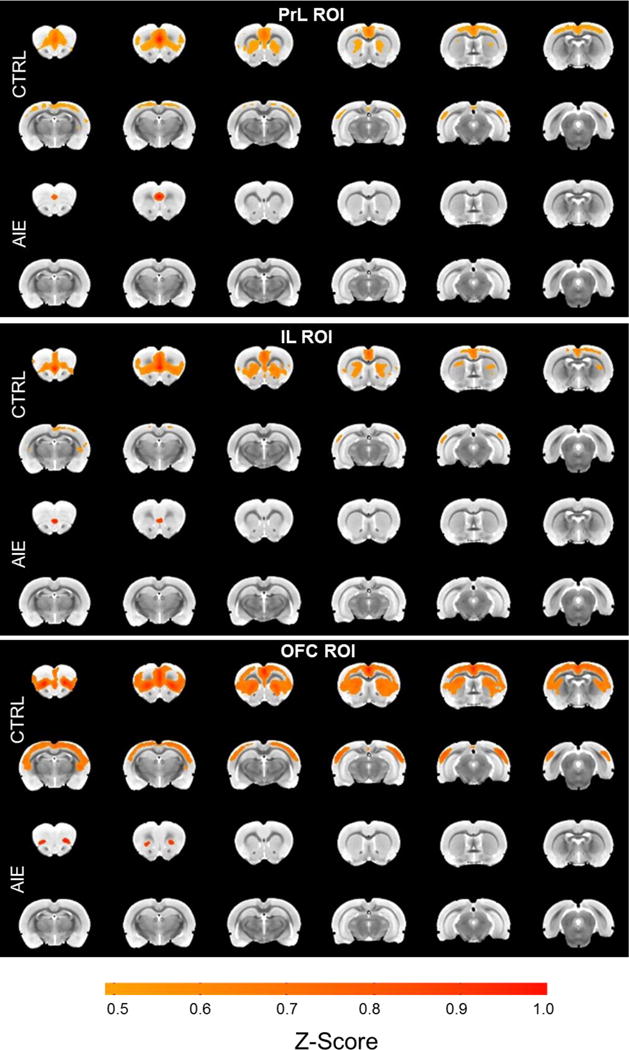
Voxel-wise correlation maps of PreChall data (threshold = 0.5 average Z-score) using the same PFC bilateral ROIs that significantly differed between AIE and CTRL in the matrix analysis. Corresponding to the results from the matrix correlation analysis, AIE rats show significantly less PrL, IL and OFC connectivity than CTRL.
Effects of an EtOH challenge on BOLD response and functional connectivity
An acute EtOH challenge given 6 minutes after onset of the Chall scan decreased BOLD signal relative to saline in all ROIs examined regardless of exposure history (representative time courses from CPu are shown in Figure 5). Statistical analysis of percent change from baseline (the last 10 minutes − 6 min baseline) showed only a significant main effect of challenge, F (1,12) = 24.96, p< 0.001, in the 2 (exposure) × 2 (challenge) × 9 (ROI) RM ANOVA (Table 1). Of note, we also observed slight BOLD signal decreases following SAL injection, perhaps due to BOLD signal drift during the long scanning sessions (e.g., Yan et al., 2009). These results suggest that 2 g/kg i.p. EtOH was sufficient to produce significant decreases in BOLD compared to SAL within each region examined and this effect of EtOH did not differ by adolescent exposure history.
Figure 5.
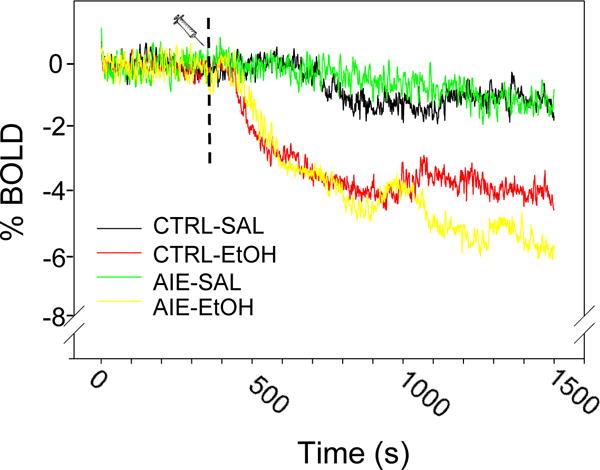
Representative BOLD time course (CPu ROI normalized to the first 20 seconds) during the 25-minute Chall scan. Dashed line indicates injection time-point (minute 6). Compared to SAL, acute EtOH decreased BOLD signal across all ROIs examined, regardless of exposure history (also see Table 1). No effect of AIE was observed.
Table 1.
Percent change in BOLD from baseline (± SEM) after saline or ethanol challenge
| Control | AIE | |||
|---|---|---|---|---|
|
|
||||
| SAL | EtOH* | SAL | EtOH* | |
| PrL | −2.5 ± 0.9 | −7.5 ± 1.4 | −3.2 ± 2.1 | −7.5 ± 1.4 |
| IL | −3.1 ± 2.4 | −7.1 ± 1.7 | −3.2 ± 1.8 | −5.3 ± 1.4 |
| OFC | −4.7 ± 0.7 | −4.3 ± 1.5 | −2.1 ± 0.8 | −6.3 ± 1.0 |
| NAc | −1.8 ± 1.1 | −4.8 ± 1.1 | −1.4 ± 0.6 | −4.3 ± 0.7 |
| VTA | −0.9 ± 0.7 | −4.4 ± 1.0 | −1.6 ± 0.9 | −3.3 ± 1.1 |
| Hipp | −0.3 ± 0.9 | −3.3 ± 0.9 | −0.7 ± 0.4 | −3.2 ± 0.8 |
| AMY | −1.1 ± 1.3 | −5.2 ± 1.1 | −3.4 ± 1.6 | −4.3 ± 1.5 |
| CPu | −1.4 ± 0.9 | −4.5 ± 1.1 | −0.9 ± 0.5 | −4.7 ± 0.7 |
| S1FL | −1.4 ± 2.0 | −4.3 ± 1.0 | −2.1 ± 0.9 | −2.4 ± 1.5 |
indicates significant difference main effect of challenge, p< 0.001
Effects of the acute ethanol challenge on functional connectivity (i.e., correlations of BOLD time series between ROI pairs) were then analyzed. Differences in functional connectivity between EtOH and SAL challenge were assessed for the last 10 minutes of the Chall scan (i.e., 10 min post EtOH) and the entire PostChall scan (i.e., 20 min post EtOH). The 2 (adolescent exposure) × 2 (challenge) × 36 (ROI pair) RM ANOVA of the 20-min post-EtOH challenge data revealed a significant main effect of challenge, F (1, 12) = 4.78, p< 0.05, and a significant adolescent exposure × challenge interaction, F (1,12) = 5.05, p< 0.05. Twenty minutes after acute EtOH, overall functional connectivity was significantly increased in CTRL, but not AIE animals (Figure 6). A similar trend was observed 10 minutes after challenge, but this did not reach significance. Thus, relative to SAL, acute EtOH significantly decreased BOLD within each ROI examined and, at the same time, increased connectivity between ROI pairs.
Figure 6.
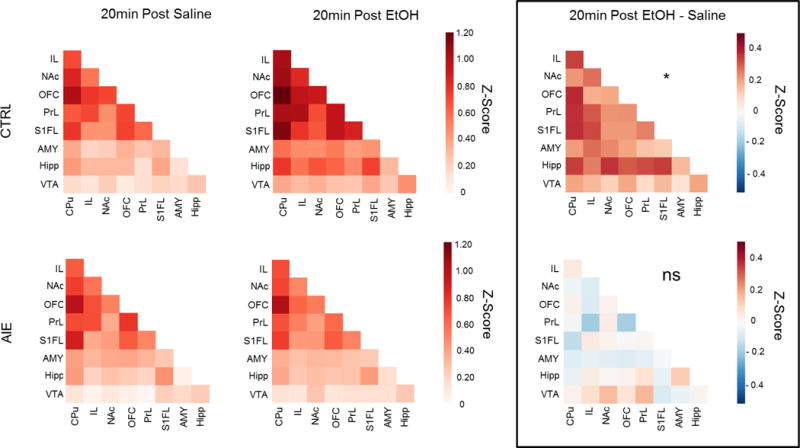
ROI-pair correlational analysis of data 20 minutes after injection of saline (SAL) or ethanol (EtOH), and the difference between SAL and EtOH (SAL-ETOH). As shown in the ROI-pair correlation matrices, EtOH significantly increased overall connectivity relative to SAL in CTRL (*), but not AIE adults (ns).
Discussion
Rodent fMRI is a valuable translational tool that allows experimental control and manipulation that cannot be achieved in human research. To our knowledge, this is the first rodent study to assess the effects of AIE and acute ethanol challenge on frontolimbic brain connectivity via rs-fcMRI. The results of this study suggest that adult rats with a history of AIE exposure have significantly less functional connectivity between subregions of the PFC (PrL-IL and IL-OFC), as well as decreased frontostriatal connectivity (PrL-NAc, IL-CPu, IL-NAc, OFC-CPu, OFC-NAc). Decreased functional connectivity may translate to decreased cognitive function as has been observed in other brain disorders (Lee et al., 2016; Liu et al., 2016; Sanganahalli et al., 2013). The results of the current study are consistent with the hypothesis that adolescent alcohol use leads to long-term disruption of PFC and frontostriatal connectivity, which may contribute to adolescent alcohol-related cognitive deficits and susceptibility for AUD in adulthood.
AIE adults showed less frontostriatal connectivity than CTRL adults. It is well known that the NAc is an integral part of the mesolimbic reward pathway and that drugs of abuse elevate dopamine in this area (e.g., Baler and Volkow, 2006), while the CPu is involved in goal-directed and habitual behavioral responses implicated in addiction. Decreased resting-state connectivity between the PFC and striatum may disrupt regulation of striatal activity (i.e., may decrease “top-down” inhibitory control), thereby promoting altered reward processing and perhaps increased alcohol consumption. Indeed, PFC dysfunction has been implicated in addiction (Goldstein & Volkow, 2011; Volkow et al., 2012; Kalivas & Volkow, 2011) and decreased fronto-accumbal functional connectivity has been shown to predict relapse in humans (Camchong et al., 2012). Further, recent studies find human heavy drinkers compared to normal controls have decreased prefrontal functional connectivity after 3 days of abstinence (Shokri-Kojori et al., 2016), whereas our rat study finds decreases lasting 25 days of abstinence. Previous studies have also found chronic alcoholics have disrupted frontostriatal network nodes implicated in response inhibition (Courtney et al., 2013; Muller-Oehring et al., 2013), with reduced connectivity being predictive of abstinent alcoholic performance on impulsivity tasks (Muller-Oehring et al., 2015), although tobacco smoking may have impacted the results. Thus, similar to the current rat study, disrupted frontostriatal connectivity has been observed in people with AUD. The current study is the first rat fMRI experiment to find that AIE induces similar connectivity alterations as human alcoholics under well-controlled experimental conditions, indicating that binge levels of ethanol exposure during adolescence are sufficient to disrupt connectivity. Further, decreased PFC functional connectivity is also consistent with previous behavioral studies reporting AIE-induced decreased behavioral flexibility and increased ethanol consumption in adulthood (Crews et al., 2016). Thus, it is plausible that AIE-induced disruption in frontostriatal connectivity may increase susceptibility for AUD.
The finding that PFC connectivity was altered in adults after AIE adds to the mounting evidence that adolescent alcohol may be particularly detrimental to PFC development. The implications of this decreased resting-state connectivity are unknown, but it is tempting to speculate that altered functional connectivity of PFC subregions may contribute to cognitive deficits observed after adolescent alcohol exposure (Crews et al., 2016). The subregions of the PFC in the rat differ in cytoarchitecture, connectivity with other brain regions, and functionality (Heidbreder & Groenwegen, 2003). Although it remains controversial, there is mounting evidence that rodent and human PFC regional differentiation generally overlap based on functionality (see Kesner & Churchwell, 2011; Perry et al., 2011; Wallis, 2012 for review), allowing a level of comparison across rodent and human studies. The rodent medial PFC (mPFC; corresponding to human Brodmann area 25 and 32) is comprised of PrL and IL portions and has been implicated in working memory (e.g., Yang et al., 2014) and set-shifting (Birrell & Brown, 2000; Dias et al. 1996; Floresco et al. 2006; Ragozzino et al. 1999), whereas the OFC (corresponding to human Brodmann areas 11, 13 and 14) is important for reversal learning and affective associations in rats and humans (McAlonan & Brown, 2003; O’Doherty et al. 2001, 2003; Jonker et al., 2015). Several rodent studies have found AIE-induced deficits in set-shifting (Gass et al., 2014) and reversal learning tasks (Coleman et al., 2011; Vetreno & Crews et al., 2012; Coleman et al., 2014), suggesting alterations in mPFC and OFC function after AIE. The mPFC has also been studied extensively in regards to fear conditioning, with studies implicating the PrL region in fear acquisition and expression, whereas the IL cortex regulates fear extinction (Giustino & Maren, 2015). Indeed, there is evidence to suggest that early AIE exposure (P28–42) disrupts expression of context fear and mid-late AIE exposure (P35–55) delays fear extinction, although the latter effect is also observed after a comparable adult ethanol exposure (Broadwater & Spear, 2013). Another recent study reported that adults exposed to AIE are slower to extinguish an operant response for an alcohol reward than controls (Gass et al., 2014). Therefore, numerous studies indicate that AIE disrupts PFC-mediated behaviors. Further studies are needed to determine the potential contribution of decreased PFC and frontostriatal functional connectivity on behavioral changes associated with AIE.
Recent studies have shown gross anatomical changes after AIE in some brain regions, like hippocampal, corpus callosal and hypothalamic volume (Gass et al., 2014; Vetreno et al., 2015; 2016) and altered cortical thickness in midline cingulate and insular cortex (Vetreno et al., 2016). The T2 images collected in this study are unfortunately not appropriate for gross anatomical analyses due to resolution constraints and limited brain coverage, prohibiting a direct comparison. However, AIE-induced loss of hippocampal volume is most commonly reported, and hippocampus did not show AIE-induced functional connectivity changes in the current study. Thus, it is likely that other mechanisms may better explain the functional connectivity loss after AIE, like alterations in structural connectivity (i.e., white matter tracts) or perhaps neurobiological changes (e.g., AIE-induced loss of cholinergic or serotonergic projection neurons [Crews et al., 2016]). When challenged with ethanol during scanning, ethanol significantly decreased BOLD signal compared to saline within each region examined, whereas connectivity between regions was significantly increased by ethanol relative to saline in controls. One might expect that with lower BOLD signal, it may be difficult to detect changes in functional connectivity due to a decreased signal-to-noise ratio. However, these data experimentally demonstrate that increased synchrony of the time-courses can occur during the suppression of BOLD signal at the same time-window. The reason for the differential effects on BOLD versus functional connectivity is not known. It is possible that the decreased BOLD we observed is related to ethanol-induced neural/regional inhibition, which may have contributed to increased synchrony (i.e., functional connectivity) among the brain regions. Alternatively, the BOLD decrease and functional connectivity may be separate functional measures.
Suppression of BOLD after acute ethanol may be due to ethanol-induced physiological changes, such as alcohol’s vasoconstrictive properties at high doses (Kawano, 2010), since BOLD signal relies on changes in blood oxygenation as well as volume and flow (Logothet & Pfeuffer, 2004; Shih et al., 2011). However, cocaine, another potent vasoconstrictor, has the opposite effect (i.e., increased cerebral blood volume) when administered peripherally in rats (Lu et al., 2012b; Marota et al., 2000), making this possibility less likely. Another potential reason may be alterations in blood pressure. Given that blood pressure changes can influence BOLD (e.g., Li et al., 2013; Wang et al., 2006), it is possible that decreased BOLD after acute ethanol in the current study may be associated with decreased blood pressure. A previous study found that blood pressure was not affected by a moderate dose of ethanol (1 g/kg, i.g.) in conscious male rats (El-Mas & Abdel-Rahmen, 1999), although it is possible that a higher ethanol dose, such as the 2 g/kg dose used in the current study, and/or sedation may produce different results. Although blood pressure was not measured in the current study, other physiological parameters (i.e., oxygen saturation, end-tidal CO2, temperature and heart rate) were maintained within normal ranges. Future acute ethanol studies should consider monitoring blood pressure and perhaps incorporate an ethanol dose-response curve to directly examine this possibility. Alternatively, acute ethanol-induced decreases in BOLD may at least partially be associated with neural inhibition, given that alcohol potentiates GABA and inhibits glutamate transmission (Little, 1991; Weight et al., 1992). Surprisingly, we did not detect a region-specific modulation of acute ethanol in AIE adults as was previously observed with c-fos expression (Liu & Crews, 2015). The reason for this discrepancy is unknown, but is perhaps related to timing of measurements, with BOLD assessed across time during and immediately following acute ethanol challenge, whereas c-fos was assessed at one time point two hours after ethanol administration. Further studies are needed to aid our understanding of the relationship between BOLD and c-fos measurements and to understand the mechanisms of ethanol-induced BOLD reductions.
While BOLD was significantly decreased during acute ethanol, functional connectivity was increased in control, but not AIE, adults. Previous studies in humans have likewise shown an increase in brain network connectivity after administration of GABAergic modulators like zolpidem, a positive modulator of GABAA receptors (Licata et al., 2013), the benzodiazepine midazolam (Kiviniemi et al., 2005), as well as alcohol (Esposito et al., 2010; Khalili-Mahani et al., 2011; Shokri-Kojori et al., 2016). Interestingly, enhancement of connectivity after acute ethanol in the current study was absent in AIE adults. It is possible that decreased baseline PFC functional connectivity in AIE adults contributed to the absence of connectivity changes after acute ethanol, or perhaps it may be related to decreased sensitivity to ethanol in animals exposed to AIE. Indeed, previous studies have shown that AIE adults are less sensitive to several effects of ethanol, such as sedation and motor impairment (see Crews et al., 2016 for review). In humans, low responders to alcohol (i.e., individuals with reduced alcohol sensitivity) show significantly less alcohol-induced increases in cerebral blood flow compared to high alcohol responders (Tolentino et al., 2011). Decreased sensitivity to alcohol in low alcohol responders and AIE adults may be associated with increased likelihood of developing an AUD. However, further studies are needed to understand the possible relationship between changes in functional connectivity after acute alcohol and susceptibility for AUD.
One limitation of the current study is that physiological parameters were not regressed out of the data; although this is less of a concern given that the animals were mechanically ventilated, allowing consistency in physiological parameters across animals. Global signal regression (GSR) has been used to regress out global signal from the entire brain that may, in part, be due to physiological noise. When GSR was applied to the PreChall data, we found that the AIE effect was still present, although somewhat diminished (see Supplemental Figure S3). However, caution needs to be exerted when interpreting these data, given well-established concerns that GSR may introduce spurious anticorrelations and regress out meaningful data (e.g., Murphy et al., 2009; Saad et al., 2012; Scholvinck et al., 2010), particularly for group comparisons (Gotts et al., 2013; Saad et al., 2012).
Another limitation is that anatomical (or structural) connectivity was not assessed in the current study; thus, we cannot conclude if the observed functional changes after AIE are related to anatomical connectivity alterations. Studies that compare anatomical connectivity, assessed via DTI, to functional connectivity have shown that strong anatomical connectivity typically results in high functional connectivity. Given that alcohol exposure affects white matter structural integrity (Pfefferbaum et al., 2015), which has been shown to be persistently altered after AIE exposure in some brain regions (Vetreno et al., 2015), it is possible that structural connectivity may have been altered by AIE in the current study. However, the relationship between anatomical and functional connectivity is not straightforward, as strong functional connectivity can still be observed in the absence of strong anatomical connectivity (Damoiseaux & Greicius, 2009; Honey et al., 2009; Supekar et al., 2010). Future studies will examine both anatomical and functional connectivity in the same animal to assess the contribution of structural connectivity changes to functional connectivity after AIE.
Together these data support the hypothesis that binge-like adolescent alcohol exposure persistently decreases baseline PFC and frontostriatal functional connectivity, adding to the growing preclinical and clinical literature suggesting that the PFC is particularly vulnerable to the lasting effects of adolescent binge alcohol use. These data further suggest that adolescent alcohol exposure may alter PFC-mediated top-down inhibitory control, perhaps contributing to increased susceptibility for AUDs in adulthood. Future studies will examine potential mechanisms and behavioral outcomes associated with AIE-induced changes in PFC functional connectivity.
Supplementary Material
Video #1. A unilateral seed (2×2×2 voxel dimension) is shown traveling around a prefrontal brain slice in the CTRL group. Note the strong bilateral connectivity evident in the contralateral hemisphere as the seed moves around the prefrontal brain regions (threshold derived from t-tests = p>0.0001).
Supplementary Figure S1. Correlation matrices with left and right hemisphere ROIs for all scans. ANOVA table (top) shows no significant difference between left and right brain hemispheres.
Supplementary Figure S2. Two smoothing parameters were applied (0.6 versus 0.3 FWHM) to OFC voxel-wise connectivity data in the CTRL group. These data demonstrate that signal noise was reduced at the higher smoothing parameter (0.6 FWHM; about twice the voxel size) while maintaining a similar correlation pattern to the lower smoothing parameter (0.3 FWHM).
Supplementary Figure S3. Global signal regression (GSR) was applied to the PreChall data to determine effects of global signal change on the functional connectivity data. GSR decreased connectivity strength and introduced negative correlations compared to non-GSR data (i.e., Figure 3). A similar pattern to the non-GSR data emerged, with the 2 (adolescent exposure) × 2 (scan day) × 2 (hemisphere) × 36 (ROI pair) RM ANOVA revealing a significant exposure × scan day × ROI pair interaction, F (35, 490) = 1.68, p< 0.05. Post-hoc analysis yielded AIE animals showing significantly less PrL-IL and PrL-NAc functional connectivity on scan day 2. However, caution needs to be exerted when interpreting these data (see Discussion section).
Supplementary Figure S4. Partial correlation was applied to the PreChall data to assess indirect (i.e., third party ROI signal) contribution to the connectivity data. Overall, partial correlation dramatically decreased connectivity strength compared to full correlation data (see Figure 3) and, like GSR, added negative correlations. No significant effect of AIE emerged in this analysis, although this study may be underpowered to detect significant changes using partial correlation.
Video #2. A unilateral seed (2×2×2 voxel dimension) is shown traveling around a prefrontal brain slice in the AIE group. Similar to the ROI voxel-wise connectivity maps, AIE adults show less brain-wide functional connectivity than CTRL as the seed moves around the prefrontal brain regions (threshold derived from t-tests = p>0.0001).
Acknowledgments
Supported by NIH grants P60AA011605, U01AA020023-NADIA Project, U24AA020024, R01NS091236, R01MH111429 and UL1TR001111 (NC TraCS 550KR91413).
Footnotes
Author Contribution:
MAB, FTC, DLR and YYS were responsible for the study concept and design. All authors were responsible for data preparation and analyses. MAB drafted the manuscript. All authors were involved in the manuscript editing and have approved the final version for publication.
References
- Adalsteinsson E, Sullivan EV, Mayer D, Pfefferbaum A. In Vivo Quantification of Ethanol Kinetics in Rat Brain. Neuropsychopharmacology. 2006;31(12):2683–2691. doi: 10.1038/sj.npp.1301023. [DOI] [PubMed] [Google Scholar]
- Asato MR, Terwilliger R, Woo J, Luna B. White matter development in adolescence: a DTI study. Cereb Cortex. 2010;20(9):2122–2131. doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baler RD, Volkow ND. Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med. 2006;12(12):559–566. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Bava S, Jacobus J, Thayer RE, Tapert SF. Longitudinal Changes in White Matter Integrity Among Adolescent Substance Users. Alcohol Clin Exp Res. 2013;37(Suppl 1):E181–E189. doi: 10.1111/j.1530-0277.2012.01920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20(11):4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Magnetic Resonance in Medicine. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Broadwater M, Spear LP. Consequences of ethanol exposure on cued and contextual fear conditioning and extinction differ depending on timing of exposure during adolescence or adulthood. Behav Brain Res. 2013;256:10–19. doi: 10.1016/j.bbr.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, Stenger A, Fein G. Resting-state synchrony during early alcohol abstinence can predict subsequent relapse. Cereb Cortex. 2012;23(9):2086–2099. doi: 10.1093/cercor/bhs190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM. Neurobiology of the adolescent brain and behavior: implications for substance use disorders. J Am Acad Child Adolesc Psychiatry. 2010;49(12):1189–1201. doi: 10.1016/j.jaac.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Liu X, Zhu XH, Zhang N. Functional MRI study of brain function under resting and activated states. Conf Proc IEEE Eng Med Biol Soc. 2009:5333175. doi: 10.1109/IEMBS.2009.5333175. [DOI] [PubMed] [Google Scholar]
- Coleman LG, Jr, He J, Lee J, Styner M, Crews FT. Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice. Alcohol Clin Exp Res. 2011;35(4):671–688. doi: 10.1111/j.1530-0277.2010.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman LG, Jr, Liu W, Oguz I, Styner M, Crews FT. Adolescent binge ethanol treatment alters adult brain regional volumes, cortical extracellular matrix protein and behavioral flexibility. Pharmacol Biochem Behav. 2014;116:142–151. doi: 10.1016/j.pbb.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Ghahremani DG, Ray LA. Fronto-striatal functional connectivity during response inhibition in alcohol dependence. Addiction biology. 2013;18(3):593–604. doi: 10.1111/adb.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cressman VL, Balaban J, Steinfeld S, Shemyakin A, Graham P, Parisot N, et al. Prefrontal cortical inputs to the basal amygdala undergo pruning during late adolescence in the rat. J Comp Neurol. 2010;518(14):2693–2709. doi: 10.1002/cne.22359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, 3rd, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24(11):1712–1723. [PubMed] [Google Scholar]
- Crews FT, Vetreno RP, Broadwater MA, Robinson DL. Adolescent Alcohol Exposure Persistently Impacts Adult Neurobiology and Behavior. Pharmacol Rev. 2016;68(4):1074–1109. doi: 10.1124/pr.115.012138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453(2):116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Greicius MD. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain Struct Funct. 2009;213(6):525–533. doi: 10.1007/s00429-009-0208-6. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal Cortex, Thalamus, and Cerebellar Volumes in Adolescents and Young Adults with Adolescent-Onset Alcohol Use Disorders and Comorbid Mental Disorders. Alcoholism: Clinical and Experimental Research. 2005;29(9):1590–1600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci. 1996;110(5):872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Sexually dimorphic hemodynamic effects of intragastric ethanol in conscious rats. Clin Exp Hypertens. 1999;21(8):1429–1445. doi: 10.3109/10641969909070858. [DOI] [PubMed] [Google Scholar]
- Ernst M. The triadic model perspective for the study of adolescent motivated behavior. Special Issue on Reward and Regulatory Processes in Adolescence. 2014:104–111. doi: 10.1016/j.bandc.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito F, Pignataro G, Di Renzo G, Spinali A, Paccone A, Tedeschi G, et al. Alcohol increases spontaneous BOLD signal fluctuations in the visual network. Neuroimage. 2010;53(2):534–543. doi: 10.1016/j.neuroimage.2010.06.061. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MT. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology. 2006;31(2):297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Vazquez AL, Zong X, Kim SG. Effects of the alpha(2)-adrenergic receptor agonist dexmedetomidine on neural, vascular and BOLD fMRI responses in the somatosensory cortex. Eur J Neurosci. 2013;37(1):80–95. doi: 10.1111/ejn.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Glen WB, Jr, McGonigal JT, Trantham-Davidson H, Lopez MF, Randall PK, et al. Adolescent alcohol exposure reduces behavioral flexibility, promotes disinhibition, and increases resistance to extinction of ethanol self-administration in adulthood. Neuropsychopharmacology. 2014;39(11):2570–2583. doi: 10.1038/npp.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustino TF, Maren S. The Role of the Medial Prefrontal Cortex in the Conditioning and Extinction of Fear. Front Behav Neurosci. 2015;9(298):2015. doi: 10.3389/fnbeh.2015.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12(11):652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotts S, Saad Z, Jo HJ, Wallace G, Cox R, Martin A. The perils of global signal regression for group comparisons: a case study of Autism Spectrum Disorders. Frontiers in Human Neuroscience. 2013;7(356) doi: 10.3389/fnhum.2013.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age of onset of drug use and its association with DSM-IV drug abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1998;10(2):163–173. doi: 10.1016/s0899-3289(99)80131-x. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27(6):555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, et al. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A. 2009;106(6):2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Bava S, Tapert SF. White matter characterization of adolescent binge drinking with and without co-occurring marijuana use: a 3-year investigation. Psychiatry Res. 2013;214(3):374–381. doi: 10.1016/j.pscychresns.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Infante MA, Bava S, Tapert SF. White matter integrity pre- and post marijuana and alcohol initiation in adolescence. Brain Sci. 2013;3(1):396–414. doi: 10.3390/brainsci3010396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Thayer RE, Trim RS, Bava S, Frank LR, Tapert SF. White matter integrity, substance use, and risk taking in adolescence. Psychol Addict Behav. 2013;27(2):431–442. doi: 10.1037/a0028235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use: 1975–2014 Overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, The University of Michigan 2015 [Google Scholar]
- Jonker FA, Jonker C, Scheltens P, Scherder EJ. The role of the orbitofrontal cortex in cognition and behavior. Rev Neurosci. 2015;26(1):1–11. doi: 10.1515/revneuro-2014-0043. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry. 2011;16(10):974–986. doi: 10.1038/mp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y. Physio-pathological effects of alcohol on the cardiovascular system: its role in hypertension and cardiovascular disease. Hypertens Res. 2010;33(3):181–191. doi: 10.1038/hr.2009.226. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Churchwell JC. An analysis of rat prefrontal cortex in mediating executive function. Neurobiology of Learning and Memory. 2011;96(3):417–431. doi: 10.1016/j.nlm.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Khalili-Mahani N, Zoethout RM, Beckmann CF, Baerends E, de Kam ML, Soeter RP, et al. Effects of morphine and alcohol on functional brain connectivity during “resting state”: a placebo-controlled crossover study in healthy young men. Hum Brain Mapp. 2012;33(5):1003–1018. doi: 10.1002/hbm.21265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiviniemi VJ, Haanpaa H, Kantola JH, Jauhiainen J, Vainionpaa V, Alahuhta S, et al. Midazolam sedation increases fluctuation and synchrony of the resting brain BOLD signal. Magn Reson Imaging. 2005;23(4):531–537. doi: 10.1016/j.mri.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Lee ES, Yoo K, Lee YB, Chung J, Lim JE, Yoon B, et al. Default Mode Network Functional Connectivity in Early and Late Mild Cognitive Impairment: Results From the Alzheimer’s Disease Neuroimaging Initiative. Alzheimer Dis Assoc Disord. 2016;2016:2. doi: 10.1097/WAD.0000000000000143. [DOI] [PubMed] [Google Scholar]
- Li G, Shih YY, Kiel JW, De La Garza BH, Du F, Duong TQ. MRI study of cerebral, retinal and choroidal blood flow responses to acute hypertension. Exp Eye Res. 2013;112:118–124. doi: 10.1016/j.exer.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, King J, Zhang N. Anticorrelated resting-state functional connectivity in awake rat brain. Neuroimage. 2012;59(2):1190–1199. doi: 10.1016/j.neuroimage.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licata SC, Nickerson LD, Lowen SB, Trksak GH, Maclean RR, Lukas SE. The hypnotic zolpidem increases the synchrony of BOLD signal fluctuations in widespread brain networks during a resting paradigm. Neuroimage. 2013;70:211–222. doi: 10.1016/j.neuroimage.2012.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, et al. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cereb Cortex. 2006;16(4):553–560. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- Little HJ. Mechanisms that may underlie the behavioural effects of ethanol. Progress in Neurobiology. 1991;36(3):171–194. doi: 10.1016/0301-0082(91)90029-z. [DOI] [PubMed] [Google Scholar]
- Liu W, Crews FT. Adolescent intermittent ethanol exposure enhances ethanol activation of the nucleus accumbens while blunting the prefrontal cortex responses in adult rat. Neuroscience. 2015;293:92–108. doi: 10.1016/j.neuroscience.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Yang J, Chen K, Luo C, Burgunder J, Gong Q, et al. Resting-state fMRI reveals potential neural correlates of impaired cognition in Huntington’s disease. Parkinsonism Relat Disord. 2016;8020(16):30121–30123. doi: 10.1016/j.parkreldis.2016.04.017. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pfeuffer J. On the nature of the BOLD fMRI contrast mechanism. Magn Reson Imaging. 2004;22(10):1517–1531. doi: 10.1016/j.mri.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Lu H, Chefer S, Kurup PK, Guillem K, Vaupel DB, Ross TJ, et al. fMRI response in the medial prefrontal cortex predicts cocaine but not sucrose self-administration history. Neuroimage. 2012;62(3):1857–1866. doi: 10.1016/j.neuroimage.2012.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Zou Q, Gu H, Raichle ME, Stein EA, Yang Y. Rat brains also have a default mode network. Proceedings of the National Academy of Sciences. 2012;109(10):3979–3984. doi: 10.1073/pnas.1200506109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Morris JR, Juraska JM. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience. 2007;144(3):961–968. doi: 10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Marota JJA, Mandeville JB, Weisskoff RM, Moskowitz MA, Rosen BR, Kosofsky BE. Cocaine Activation Discriminates Dopaminergic Projections by Temporal Response: An fMRI Study in Rat. Neuroimage. 2000;11(1):13–23. doi: 10.1006/nimg.1999.0520. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146(1–2):97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- McQueeny T, Schweinsburg BC, Schweinsburg AD, Jacobus J, Bava S, Frank LR, et al. Altered white matter integrity in adolescent binge drinkers. Alcohol Clin Exp Res. 2009;33(7):1278–1285. doi: 10.1111/j.1530-0277.2009.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal Cortex Volumes in Adolescents With Alcohol Use Disorders: Unique Gender Effects. Alcoholism: Clinical and Experimental Research. 2008;32(3):386–394. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Oehring EM, Jung YC, Pfefferbaum A, Sullivan EV, Schulte T. The Resting Brain of Alcoholics. Cereb Cortex. 2015;25(11):4155–4168. doi: 10.1093/cercor/bhu134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Oehring EM, Jung YC, Sullivan EV, Hawkes WC, Pfefferbaum A, Schulte T. Midbrain-Driven Emotion and Reward Processing in Alcoholism. Neuropsychopharmacology. 2013;38(10):1844–1853. doi: 10.1038/npp.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44(3):893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah FA, Tay HC, Chuang KH. Detection of functional connectivity in the resting mouse brain. Neuroimage. 2014;86:417–424. doi: 10.1016/j.neuroimage.2013.10.025. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. J Neurosci. 2003;23(21):7931–7939. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4(1):95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15(5 Pt 1):3622–3639. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W-J, Billings J, Grooms J, Shakil S, Keilholz S. Considerations for resting state functional MRI and functional connectivity studies in rodents. Frontiers in Neuroscience. 2015;9(269) doi: 10.3389/fnins.2015.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawela CP, Biswal BB, Cho YR, Kao DS, Li R, Jones SR, et al. Resting-State Functional Connectivity of the Rat Brain. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2008;59(5):1021–1029. doi: 10.1002/mrm.21524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawela CP, Biswal BB, Hudetz AG, Schulte ML, Li R, Jones SR, et al. A protocol for use of medetomidine anesthesia in rats for extended studies using task-induced BOLD contrast and resting-state functional connectivity. Neuroimage. 2009;46(4):1137–1147. doi: 10.1016/j.neuroimage.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Joseph JE, Jiang Y, Zimmerman RS, Kelly TH, Darna M, et al. Prefrontal cortex and drug abuse vulnerability: translation to prevention and treatment interventions. Brain Res Rev. 2011;65(2):124–149. doi: 10.1016/j.brainresrev.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Zahr NM, Mayer D, Rohlfing T, Sullivan EV. Dynamic responses of selective brain white matter fiber tracts to binge alcohol and recovery in the rat. PLoS One. 2015;10(4):2015. doi: 10.1371/journal.pone.0124885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci. 1999;19(11):4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K. Functional brain imaging across development. Eur Child Adolesc Psychiatry. 2013;22(12):719–731. doi: 10.1007/s00787-012-0291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, et al. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012;2(1):25–32. doi: 10.1089/brain.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanganahalli BG, Herman P, Behar KL, Blumenfeld H, Rothman DL, Hyder F. Functional MRI and neural responses in a rat model of Alzheimer’s disease. Neuroimage. 2013;79:404–411. doi: 10.1016/j.neuroimage.2013.04.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholvinck ML, Maier A, Ye FQ, Duyn JH, Leopold DA. Neural basis of global resting-state fMRI activity. Proc Natl Acad Sci U S A. 2010;107(22):10238–10243. doi: 10.1073/pnas.0913110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih YY, Li G, Muir ER, De La Garza BH, Kiel JW, Duong TQ. Pharmacological MRI of the choroid and retina: blood flow and BOLD responses during nitroprusside infusion. Magn Reson Med. 2012;68(4):1273–1278. doi: 10.1002/mrm.24112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih YY, Wang L, De La Garza BH, Li G, Cull G, Kiel JW, et al. Quantitative retinal and choroidal blood flow during light, dark adaptation and flicker light stimulation in rats using fluorescent microspheres. Curr Eye Res. 2013;38(2):292–298. doi: 10.3109/02713683.2012.756526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih YY, Wey HY, De La Garza BH, Duong TQ. Striatal and cortical BOLD, blood flow, blood volume, oxygen consumption, and glucose consumption changes in noxious forepaw electrical stimulation. J Cereb Blood Flow Metab. 2011;31(3):832–841. doi: 10.1038/jcbfm.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokri-Kojori E, Tomasi D, Wiers CE, Wang GJ, Volkow ND. Alcohol affects brain functional connectivity and its coupling with behavior: greater effects in male heavy drinkers. Mol Psychiatry. 2016;2016(29):25. doi: 10.1038/mp.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Vidaurre D, Beckmann CF, Glasser MF, Jenkinson M, Miller KL, et al. Functional connectomics from resting-state fMRI. Trends Cogn Sci. 2013;17(12):666–682. doi: 10.1016/j.tics.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Swartzwelder HS. Adolescent alcohol exposure and persistence of adolescent-typical phenotypes into adulthood: a mini-review. Neurosci Biobehav Rev. 2014;45:1–8. doi: 10.1016/j.neubiorev.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Sorg SF, Jacobus J, Brumback T, Taylor CT, Tapert SF. Structural connectivity of neural reward networks in youth at risk for substance use disorders. Psychopharmacology. 2015;232(13):2217–2226. doi: 10.1007/s00213-014-3857-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Tapert SF, Sullivan EV, Jacobus J, Meloy MJ, Rohlfing T, et al. Brain Development in Heavy-Drinking Adolescents. American Journal of Psychiatry. 2015;172(6):531–542. doi: 10.1176/appi.ajp.2015.14101249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Uddin LQ, Prater K, Amin H, Greicius MD, Menon V. Development of functional and structural connectivity within the default mode network in young children. Neuroimage. 2010;52(1):290–301. doi: 10.1016/j.neuroimage.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiihonen J, Kuikka J, Hakola P, Paanila J, Airaksinen J, Eronen M, et al. Acute ethanol-induced changes in cerebral blood flow. Am J Psychiatry. 1994;151(10):1505–1508. doi: 10.1176/ajp.151.10.1505. [DOI] [PubMed] [Google Scholar]
- Tolentino NJ, Wierenga CE, Hall S, Tapert SF, Paulus MP, Liu TT, et al. Alcohol effects on cerebral blood flow in subjects with low and high responses to alcohol. Alcohol Clin Exp Res. 2011;35(6):1034–1040. doi: 10.1111/j.1530-0277.2011.01435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Crews FT. Adolescent binge drinking increases expression of the danger signal receptor agonist HMGB1 and Toll-like receptors in the adult prefrontal cortex. Neuroscience. 2012;226:475–488. doi: 10.1016/j.neuroscience.2012.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Yaxley R, Paniagua B, Crews FT. Diffusion tensor imaging reveals adolescent binge ethanol-induced brain structural integrity alterations in adult rats that correlate with behavioral dysfunction. Addict Biol. 2015;21(4):939–953. doi: 10.1111/adb.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Yaxley R, Paniagua B, Johnson GA, Crews FT. Adult rat cortical thickness changes across age and following adolescent intermittent ethanol treatment. Addict Biol. 2016;2016(1):12364. doi: 10.1111/adb.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D. Addiction circuitry in the human brain. Annu Rev Pharmacol Toxicol. 2012;52:321–336. doi: 10.1146/annurev-pharmtox-010611-134625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis JD. Cross-species studies of orbitofrontal cortex and value-based decision-making. Nat Neurosci. 2012;15(1):13–19. doi: 10.1038/nn.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Guo Y, Myers KG, Heintz R, Peng YH, Maarek JM, et al. Exercise alters resting-state functional connectivity of motor circuits in parkinsonian rats. Neurobiol Aging. 2005;36(1):536–544. doi: 10.1016/j.neurobiolaging.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Foniok T, Wamsteeker JI, Qiao M, Tomanek B, Vivanco RA, et al. Transient blood pressure changes affect the functional magnetic resonance imaging detection of cerebral activation. Neuroimage. 2006;31(1):1–11. doi: 10.1016/j.neuroimage.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Weight FF, Aguayo LG, White G, Lovinger DM, Peoples RW. GABA- and glutamate-gated ion channels as molecular sites of alcohol and anesthetic action. Adv Biochem Psychopharmacol. 1992;47:335–347. [PubMed] [Google Scholar]
- Yan L, Zhuo Y, Ye Y, Xie SX, An J, Aguirre GK, et al. Physiological origin of low-frequency drift in blood oxygen level dependent (BOLD) functional magnetic resonance imaging (fMRI) Magnetic Resonance in Medicine. 2009;61(4):819–827. doi: 10.1002/mrm.21902. [DOI] [PubMed] [Google Scholar]
- Yang ST, Shi Y, Wang Q, Peng JY, Li BM. Neuronal representation of working memory in the medial prefrontal cortex of rats. Mol Brain. 2014;7(61):014–0061. doi: 10.1186/s13041-014-0061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Wang X, Zariwala HA, Uslaner JM, Houghton AK, Evelhoch JL, et al. fMRI study of olfaction in the olfactory bulb and high olfactory structures of rats: Insight into their roles in habituation. Neuroimage. 2016;127:445–455. doi: 10.1016/j.neuroimage.2015.10.080. [DOI] [PubMed] [Google Scholar]
- Zhao F, Welsh D, Williams M, Coimbra A, Urban MO, Hargreaves R, et al. FMRI of pain processing in the brain: A within-animal comparative study of BOLD vs. CBV and noxious electrical vs. noxious mechanical stimulation in rat. Neuroimage. 2012;59(2):1168–1179. doi: 10.1016/j.neuroimage.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Zhao F, Williams M, Bowlby M, Houghton A, Hargreaves R, Evelhoch J, et al. Qualification of fMRI as a biomarker for pain in anesthetized rats by comparison with behavioral response in conscious rats. Neuroimage. 2014;84:724–732. doi: 10.1016/j.neuroimage.2013.09.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video #1. A unilateral seed (2×2×2 voxel dimension) is shown traveling around a prefrontal brain slice in the CTRL group. Note the strong bilateral connectivity evident in the contralateral hemisphere as the seed moves around the prefrontal brain regions (threshold derived from t-tests = p>0.0001).
Supplementary Figure S1. Correlation matrices with left and right hemisphere ROIs for all scans. ANOVA table (top) shows no significant difference between left and right brain hemispheres.
Supplementary Figure S2. Two smoothing parameters were applied (0.6 versus 0.3 FWHM) to OFC voxel-wise connectivity data in the CTRL group. These data demonstrate that signal noise was reduced at the higher smoothing parameter (0.6 FWHM; about twice the voxel size) while maintaining a similar correlation pattern to the lower smoothing parameter (0.3 FWHM).
Supplementary Figure S3. Global signal regression (GSR) was applied to the PreChall data to determine effects of global signal change on the functional connectivity data. GSR decreased connectivity strength and introduced negative correlations compared to non-GSR data (i.e., Figure 3). A similar pattern to the non-GSR data emerged, with the 2 (adolescent exposure) × 2 (scan day) × 2 (hemisphere) × 36 (ROI pair) RM ANOVA revealing a significant exposure × scan day × ROI pair interaction, F (35, 490) = 1.68, p< 0.05. Post-hoc analysis yielded AIE animals showing significantly less PrL-IL and PrL-NAc functional connectivity on scan day 2. However, caution needs to be exerted when interpreting these data (see Discussion section).
Supplementary Figure S4. Partial correlation was applied to the PreChall data to assess indirect (i.e., third party ROI signal) contribution to the connectivity data. Overall, partial correlation dramatically decreased connectivity strength compared to full correlation data (see Figure 3) and, like GSR, added negative correlations. No significant effect of AIE emerged in this analysis, although this study may be underpowered to detect significant changes using partial correlation.
Video #2. A unilateral seed (2×2×2 voxel dimension) is shown traveling around a prefrontal brain slice in the AIE group. Similar to the ROI voxel-wise connectivity maps, AIE adults show less brain-wide functional connectivity than CTRL as the seed moves around the prefrontal brain regions (threshold derived from t-tests = p>0.0001).


