Abstract
Purpose
To investigate the utility of an advanced MRS protocol in the clinical setting, and to compare the localization accuracy, spectral quality and quantification repeatability between this advanced and the conventional vendor-provided MRS protocol on a clinical 3T platform.
Methods
Proton spectra were measured from the posterior cingulate cortices in 30 healthy elderly subjects by clinical MR technologists using a vendor-provided (PRESS with advanced 3D-GRE B0 shimming) and an advanced (semi-LASER with FAST(EST)MAP (FM) shimming) protocol, in random order. Spectra were quantified with LCModel using standard pipelines for the clinical and research settings, respectively.
Results
The advanced protocol outperformed the vendor-provided protocol in localization accuracy (chemical-shift-displacement error: 2.0%/ppm, semi-LASER vs. 11.6%/ppm, PRESS), spectral quality (water linewidth: 6.1±1.8Hz, FM vs. 10.5±3.7Hz, 3D-GRE; P<7e-6; residual water: 0.08±0.12%, VAPOR vs. 0.45±0.50%, WET; P<2e-5) and within-session repeatability of metabolite concentrations, particularly of low SNR data with 2–8 averages (test-retest coefficients-of-variance of metabolite concentrations, P<0.01). Concentrations of J-coupled metabolites such as GABA and glutamate were biased when using the default pipeline with simulated macromolecules.
Conclusion
Quality of MRS data can be improved using advanced acquisition and analysis protocols on standard 3T hardware in the clinical setting, which can facilitate robust applications in CNS diseases.
Keywords: 3T, sLASER, PRESS, FAST(EST)MAP, chemical shift displacement, linewidth
Introduction
Proton magnetic resonance spectroscopy (1H MRS) provides a wealth of biochemical and metabolic information complementary to conventional structural MRI as it enables non-invasive and regional quantification of endogenous neurochemicals. The international MRS Consensus Group has recently documented the clinical utility of MRS, for diagnostic and prognostic purposes, in common disorders of the central nervous system (1). The group also emphasized that lack of quality assurance is a current impediment to widespread diagnostic and prognostic use in the clinical setting. Thus, compromised data quality obtained with standard clinical MRS packages may result in poor reproducibility of neurochemical concentrations, thereby limiting clinical utility. Meanwhile, an increasing volume of high-quality MRS data is being generated in the research setting from centers that specialize in MR methods development, as well as at sites that obtain advanced MRS protocols via customer-to-customer sequence transfer agreements. However, the feasibility of utilizing such advanced MRS protocols, including non-standard adjustments such as voxel-based B0 and B1 calibrations (2,3), has not been evaluated in the clinical setting. Furthermore, the potential benefits of utilizing an advanced MRS protocol over a conventional protocol for data quality have not been evaluated systematically by parallel acquisitions in the same MR session.
For clinical 1H MRS, STEAM (STimulated Echo Acquisition Mode) (4) and PRESS (Point RESolved Spectroscopy) (5) are the standard pulse sequences provided in commercial MRS packages. Although STEAM provides good localization due to high bandwidth of the 90° pulses, requires low RF power, and allows ultra-short echo-time (TE), it only produces half the signal compared to a spin-echo sequence. PRESS, in comparison, retains full signal intensity and achieves relatively short TE (~30 ms). However, due to increased spectral dispersion at 3T and above, chemical shift displacement error (CSDE) becomes unacceptable with PRESS (6).
On the other hand, several highly optimized pulse sequences, such as STEAM, SPECIAL and semi-LASER, have been utilized to generate high quality MRS data from healthy and diseased brain in the research setting (3,7–17). Of these, the semi-LASER (sLASER) sequence (13,14) provides single-shot full-intensity signal with clean localization and minimal CSDE due to high bandwidth adiabatic full-passage (AFP) pulses. Pairs of AFP pulses in sLASER further suppress J-evolution and prolong apparent transverse relaxation times (T2) (18). sLASER, when combined with voxel-based B0 and B1 calibration routines, was shown to provide neurochemical profiles with high within- (2) and between-site reproducibility at 3 and 7 T (3,12). Of note, 5 major metabolites (N-acetylaspartate (NAA), total creatine (tCr), total choline (tCho), glutamate (Glu) and myo-inositol (Ins)) were quantified with a test-retest coefficient-of-variance (CV) ≤5% from spectra averaged over 5 minutes (2).
Therefore, here we chose to compare the vendor-provided full intensity MRS protocol utilizing the PRESS sequence with an advanced protocol that utilizes the sLASER sequence (14) that has been validated for within- and between-site reproducibility (3,12). The aim of the study was to 1) evaluate the feasibility of executing an advanced MRS protocol in the clinical setting with rotating MR technologists and 2) compare the spectral quality and quantification precision between the standard vendor-provided and the advanced protocols on a 3T scanner. We studied the posterior cingulate cortex (PCC) in a healthy elderly cohort due to its role in age-related neurodegenerative diseases such as Alzheimer’s disease. Moreover, the technologists who participated in the study were well-trained to position this voxel based on anatomical landmarks. Finally, healthy elderly presented a clinically relevant cohort with smaller brain volumes than a young cohort, and associated challenges for MRS such as compromised SNR.
Methods
Subjects
Clinically normal elderly participants (N=30; 15 males, age=80 ± 5 years) were recruited from the Mayo Clinic Study of Aging (19) and enrolled after giving informed consent approved by the institutional review board. Participants were excluded if they had cognitive impairment or abnormal MRI findings, such as traumatic brain injury, intracranial tumor, large hemispheric infarct, epilepsy, or normal pressure hydrocephalus.
MR Acquisitions
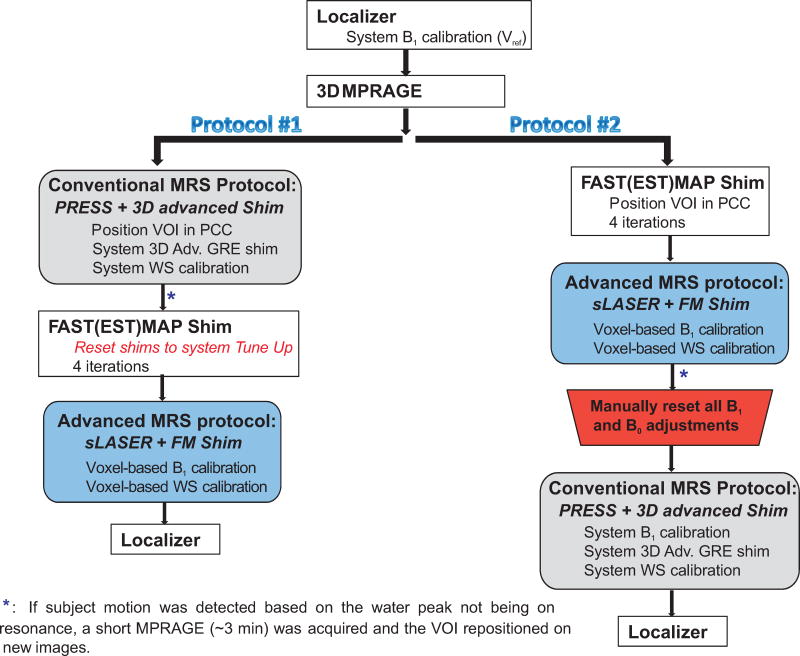
Data were acquired on a 3T clinical Verio Siemens scanner (Siemens Medical Solutions, Erlangen, Germany) running syngo VB17A at the Mayo Clinic Rochester by three rotating neuroradiology MR technologists. The technologists had at least 10 years of experience in the MR field (both in MRI and MRS acquisitions on GE and Siemens scanners). Each technologist followed detailed written instructions on how to run the randomized MRS acquisition protocols (Figure 1). In addition, the first three examinations by each of the technologists were performed under the supervision of a spectroscopist (DKD). Body coil was used for transmission and the 32-channel head coil was used for signal reception.
Figure 1.
Randomized MRS protocols (that consist of the conventional and advanced acquisition schemes in alternate order) carried out by three rotating neuroradiology MR technologists at 3 T. PCC: posterior cingulate cortex; WS: water suppression; FM: FAST(EST)MAP. A localizer was acquired at the end of the study to verify that the subject has not changed their position in the scanner. The advanced MRS protocol was slightly longer by ~2 mins due to the extra steps of adjusting the B1 levels for the 90° and WS pulses.
3D T1-weighted images (MPRAGE, TR/TE = 2300/2.98 ms, slice thickness = 1.2 mm, field-of-view = 256 × 256 mm2, matrix size = 256 × 256, flip angle = 9°) were acquired to select a volume-of-interest (VOI) of 8 mL in PCC. Anterior inferior corner of the VOI was placed at the splenium of the corpus callosum and the anterior superior corner was below the cingulate sulcus (Figure 2). This VOI partially includes posterior cingulate gyri and precunei (portions of Brodmann areas 23 and 31). In the same session, single-voxel proton spectra were acquired using the vendor-provided and advanced protocols, as described below, in a randomized order in each subject (Figure 1).
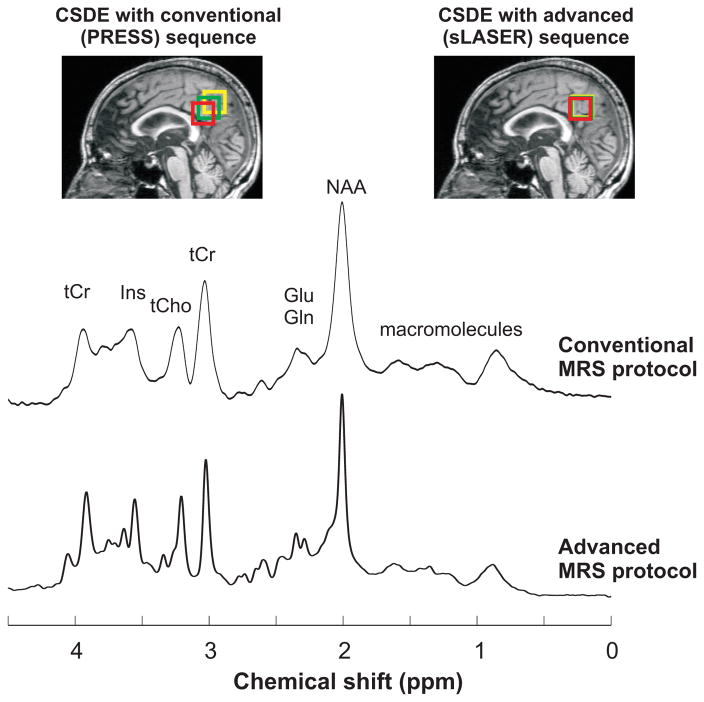
Figure 2.
PRESS (TR/TE = 5000/30 ms) and sLASER (TR/TE = 5000/28 ms) spectra (64 averages) acquired at 3T from one subject using the conventional and advanced MRS protocols, respectively. Displayed spectra were processed with 3.8 Hz Gaussian weighting. Although similar spectral patterns were observed between the different protocols, broader spectral linewidth was obtained with the Adv. 3D shim compared to FM shim. CSDE for the selected VOI (green) observed with PRESS and sLASER are shown for two resonances with a chemical shift difference of ±1.25 ppm relative to transmitter offset (red and yellow boxes) on T1-weighted images. Measured linewidth for tCr-CH3 was 12 and 4 Hz with the conventional and advanced protocols respectively. The broader linewidth observed with the conventional protocol was due to B0 shimming technique and small shot-to-shot frequency shifts.
Vendor-provided MRS Protocol
The conventional protocol consisted of a PRESS sequence (TR/TE = 5000/30 ms) that utilizes the WET technique for water suppression (WS). The protocol was executed according to vendor-provided instructions. Specifically, the system B1 RF field, i.e. reference voltage, was determined at the start of the study (i.e. during the pre-scan calibration) and the water suppression flip angle was adjusted by the system before the MRS acquisition. B0 shimming was automatically carried out using the system advanced 3D-GRE shim. No manual shim adjustments were done by the technologists.
PRESS spectra were acquired with 64 averages, 70 Hz WS bandwidth, 2048 complex data points and a spectral width of 2.5 kHz (the highest possible bandwidth currently available in the vendor’s sequence). Water reference spectra were acquired for eddy-current correction and metabolite quantification (3).
De-identified raw MRS data were transferred from Mayo Clinic to the University of Minnesota for metabolite quantification. According to standard practice for clinical MRS, eddy-current corrected, summed PRESS spectra were used for metabolite quantification (20). Metabolites were quantified with LCModel 6.3-0G (21) with the water scaling option (22,23). To enable direct comparison of metabolite concentrations obtained with the conventional and advanced protocols, basis sets for both protocols were simulated based on density-matrix formalism using the same chemical shifts and J-coupling values (24,25). To account for the large CSDE in PRESS, full 3D localized simulations (40×40×40 spatial points (26)) were performed with RF pulse shapes and TE timings used in vivo. Nineteen basis spectra were generated: alanine, ascorbate (Asc), aspartate (Asp), creatine (Cr), GABA, glucose (Glc), Glu, glutamine (Gln), glutathione (GSH), glycerophosphorylcholine (GPC), Ins, scyllo-inositol (sIns), lactate (Lac), NAA, N-acetylaspartylglutamate (NAAG), phosphocreatine (PCr), phosphocholine (PCho), phosphorylethanolamine (PE) and taurine (Tau). According to standard practice for LCModel fitting of clinical MRS spectra, mathematically estimated macromolecule (MM) and lipid signals within LCModel (i.e. setting NSIMUL = 11) and other LCModel parameters at their standard default values were used for the analysis of PRESS spectra.
Advanced MRS Protocol
The advanced protocol used the sLASER sequence (TR/TE =5000/28 ms), as described previously (3,14). Water suppression was achieved with the VAPOR technique, interleaved with outer volume suppression pulses (27). This sLASER sequence (14) is currently available as a work-in-progress (WIP) package in select versions of the Siemens software. B0 shimming inside the VOI was carried out using the adiabatic version of FAST(EST)MAP (FM) (28). Although FM is available as a work-in-progress package on Siemens (WIP # 577 on syngo VB17A), it is not routinely used in the clinical setting.
The FM shim protocol (TR/TE = 2000/56 ms, bar thickness = 5 mm, readout FOV = 300 mm) was performed in 4 iterations: the first-order shims were first corrected based on the linear 3-projection maps (evolution time, τ = 5 ms in EPI mode), followed by adjustment of second-order terms twice where the fields were mapped along six projections (τ = 5 ms in EPI mode), and finally the first-order shims were compensated again using linear six projection maps (τ = 10 ms in non-EPI mode). The total adjustment time was within 100 sec, comparable to the system advanced 3D-GRE shim routine with 3 iterations. No manual shim adjustments were performed. The RF power for the 90° asymmetric pulse and the water suppression pulses were calibrated in the selected VOI (3).
sLASER spectra were acquired with 64 averages, 70 Hz WS bandwidth, 2048 complex points and a spectral width of 6 kHz (14). Non-suppressed water scans were also obtained for eddy current correction and quantification. In addition, fully-relaxed unsuppressed water signals were acquired at twelve TEs, ranging from 28 to 4000 ms, to determine the cerebrospinal fluid (CSF) content and T2 of tissue water in the selected VOI (29).
After transfer of the de-identified raw MRS data to the University of Minnesota, single-shot sLASER spectra were processed in Matlab (20) according to a previously described protocol (2,3,30,31), namely single-shots were eddy-current, frequency and phase corrected before summation.
sLASER spectra were quantified with LCModel 6.3-0G (21) with the water scaling option (22,23) using 19 basis spectra, mentioned above, which were generated using non-localized density-matrix simulation with actual RF shapes and timings due to the minimal CSDE in sLASER (2%/ppm for AFP pulses). In addition, an experimentally measured macromolecule spectrum (obtained using the inversion-recovery technique in four healthy subjects: 928 averages and TR/TI = 2.5/0.75 s) (3) was used together with optimized LCModel parameters as described previously (2,3,12,14,27,31–36). Briefly, the fit was performed between 0.5 to 4.2 ppm, the zero- and first-order phases were fixed at zero (since phase correction between spectra is performed on the spectra during post-processing, prior to LCModel), the convolution parameter RFWHM was set to 2.5 and the knot spacing of the spline baseline, i.e. DKNTMN was set to 0.25. To account for the falx cerebri lipid signal (37) observed in several of the elderly subjects, a singlet peak was simulated at 1.45 ppm within LCModel to fit the sLASER data.
Final Concentrations
Metabolite concentrations from PRESS and sLASER were corrected for T2 relaxation of tissue water, CSF content and assuming 82% tissue water content (38), as described previously (3). A water T2 of 100 ms was used with sLASER; this value represents the mean T2 from all subjects obtained by fitting the integrals of the unsuppressed water acquired at different TEs with a biexponential fit with the T2 of CSF fixed at 740 ms and assuming that the apparent T2 of water under Carr-Purcell conditions is 1.5 times that of the measured free precession T2 (3). For PRESS, a water T2 of 80 ms was used (39). Signal loss due to T2 relaxation of metabolites was neglected, since the apparent T2 is dependent on the sequence, as well as individual metabolites.
Only metabolites quantified with Cramér-Rao lower bounds (CRLBs) ≤50% from at least half of the spectra were included in the neurochemical profiles. If the correlation coefficient between two metabolites was <−0.7 in most spectra, then only their sum was reported; e.g. tCr=Cr+PCr and tCho=GPC+PC.
Repeatability
In order to evaluate the repeatability of metabolite quantification with the two MRS protocols at varying SNR levels, we analyzed the test-retest CV of metabolite concentrations (standard deviation divided by mean concentration) obtained from subspectra, i.e. sums of every 2, 4, 8, 16 and 32 consecutive shots for all spectra. All resulting subspectra were analyzed with LCModel as described above for the conventional and advanced MRS protocols. The repeatability CV was then calculated by taking the mean of the intrasubject CVs across all subjects at each SNR level.
Statistical analysis
Water linewidth and suppression efficiency from the two MRS protocols were compared using Wilcoxon Signed-Rank Test due to the skewness of these data. Concentrations and repeatability CV between the MRS protocols were compared using paired t-tests.
Results
Feasibility, spectral pattern, quality, CSDE and RF voltage
All three MR technologists were able to successfully run the advanced spectroscopy protocol, including sLASER and FM shimming, after an average of 3 supervised sessions. A similar spectral pattern was observed between the conventional and advanced MRS protocols, while lines were typically broader with the conventional protocol (Figure 2).
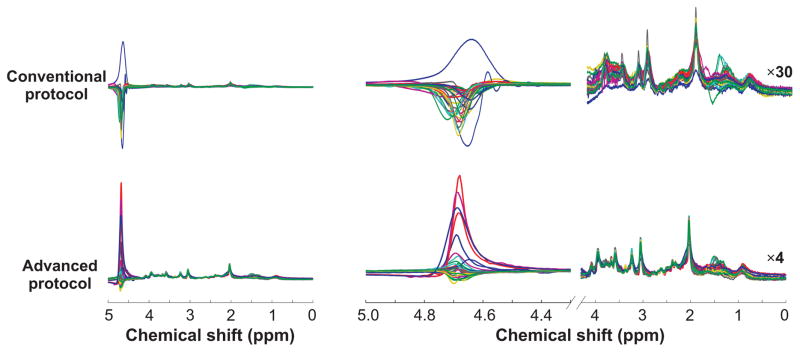
For a visual assessment of reproducibility of spectral quality between subjects, spectra from all subjects with each protocol were overlaid (Figure 3). Metabolite spectra were clearly visible when spectra were scaled by the residual water peak in sLASER data in all subjects without apparent baseline artifacts. In comparison, baseline distortions were observed downfield from 3.5 ppm in the PRESS data due to a large residual water signal. The falx cerebri lipid peak (37) at 1.45 ppm, present in some subjects, was observed in both protocols. In the 1 to 2 ppm spectral window a higher incidence of unwanted coherences was apparent in PRESS data relative to sLASER data.
Figure 3.
Overlaid spectra (exponentially line-broadened by 0.5 Hz for display purpose only) acquired from 30 subjects using the conventional and advanced protocols. The upfield region (0 to 4.1 ppm in the right panel) was appropriately scaled relative to the water residual peak (30-fold multiplication for PRESS spectra, 4-fold for sLASER spectra) to show the reproducibility of spectra between subjects.
The refocusing pulses in PRESS resulted in 11.6%/ppm CSDE, translating to ~30% CSDE between the two edges of the spectrum (over a ~2.6 ppm range), compared to 2.0%/ppm in sLASER, as depicted by the extent of the VOI displacements in Figure 2.
To evaluate the accuracy of the RF power used with the conventional protocol (based on the slice-based pre-scan calibration), we compared the system-found RF power level, as given by the transmitter reference voltage to that obtained for the selected VOI during sLASER calibration. The RF levels were identical in 16 out of the 30 subjects between the conventional PRESS and advanced sLASER protocols (Supporting Fig. S1). However, this level was underestimated in 10 subjects and overestimated in 4 subjects with the system calibration relative to the VOI-based B1 level measured with sLASER in the same subjects (Supporting Fig. S1).
B0 shimming and WS efficiency
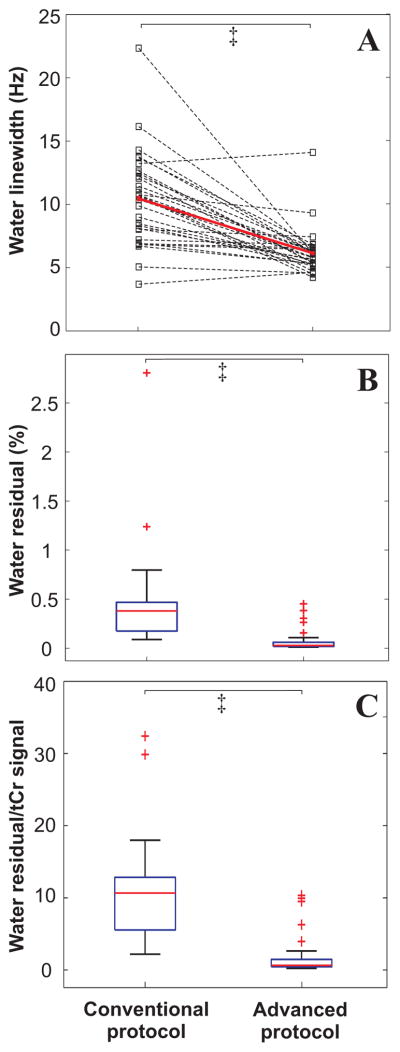
For a quantitative assessment of spectral quality, spectral linewidths and water residual signal were evaluated with each protocol. Linewidths were consistently broader with the conventional protocol utilizing the system Adv. 3D shim (10.5 ± 3.7 Hz) than with advanced protocol with FM shim (6.1 ± 1.8 Hz, Figure 4A). Seventeen out of 30 subjects had water linewidth >10 Hz with the conventional protocol compared to only 1 subject with the advanced protocol, a linewidth criterion previously used for exclusion of spectra at 3T (3).
Figure 4.
Performance of voxel based B0 shimming routines (Adv. 3D versus FM) and water suppression schemes (WET versus VAPOR). FM shim resulted in narrower spectral linewidth than the Adv. 3D protocol (A) and better water suppression was achieved with VAPOR than the WET scheme (B and C). ‡ P<2.5e-5 (Wilcoxon test).
Better WS performance was achieved with VAPOR in the advanced protocol compared to WET in the conventional protocol; negligible residual water was observed with VAPOR (0.08 ± 0.12%) compared to WET (0.45 ± 0.50%, Figure 4B). Similarly, the ratio of residual water to tCr-CH3 was higher in the conventional protocol by ~10 times on average than the advanced protocol (Figure 4C, Figure 3).
Concentrations with conventional and advanced MRS protocols
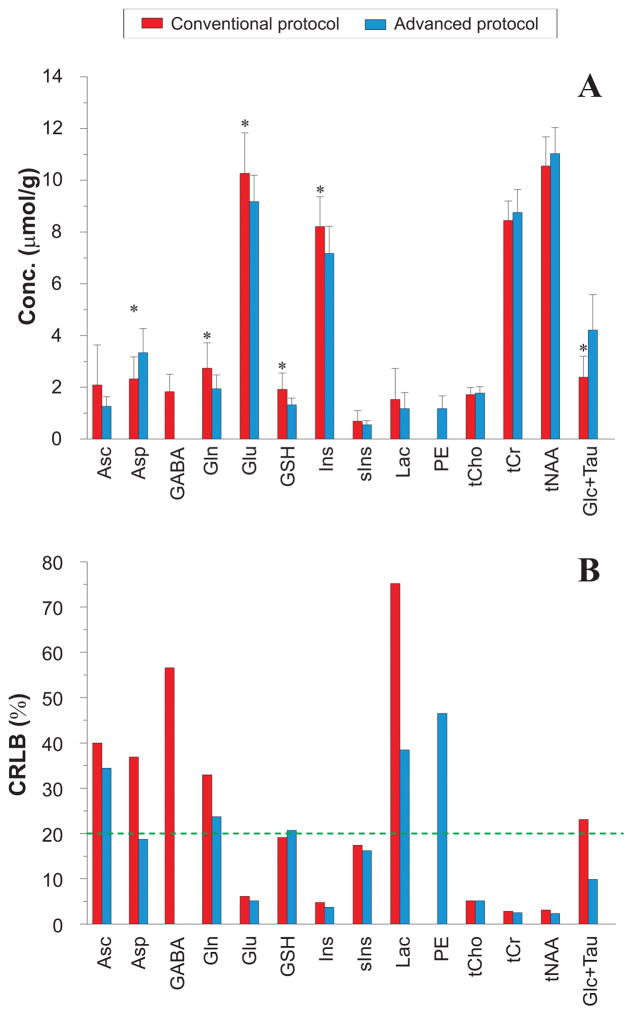
Thirteen metabolites passed the 50% CRLB criteria in both MRS protocols, although with statistically significant differences in concentrations between protocols (Figure 5). Concentrations of metabolites other than the 3 main singlet resonances (tCho, tCr and tNAA), Asc, sIns and Lac were significantly different between the conventional and advanced protocols. In particular, concentrations of J-coupled metabolites, e.g. Glu, Gln, Ins, GSH and GABA, were higher with the conventional protocol. To investigate whether these differences might result from using optimized vs. default LCModel parameters and the choice of simulated vs. measured MM, three fitting approaches were compared using the sLASER data due to their higher quality relative to the PRESS data sets (supplementary material, Supporting Fig. S2). This comparison showed that concentrations were largely comparable when using the default vs. optimized LCModel parameters, while statistically significant differences were found for most metabolites between simulated vs. measured MM. These data in particular indicated that the use of simulated vs. measured MM can introduce biases in the concentrations of the weakly represented metabolites such as GABA, Gln and GSH due to underestimation of the MM contributions, particularly in the 2–4 ppm range (Supporting Fig. S2).
Figure 5.
Mean metabolite concentrations (A) and CRLBs (B) obtained with the conventional and advanced MRS acquisition and analysis protocols. Error bars represent intersubject standard deviations and the green dotted line represents a mean CRLB cut-off of 20%. * P<0.01 (two-tailed, paired Student’s t-test) between conventional versus advanced protocol. Only differences at the level of P<0.01 are shown due to multiple comparisons. Ascorbate (Asc), aspartate (Asp), γ-aminobutyric acid (GABA), glutamine (Gln), glutamate (Glu), glutathione (GSH), myo-inositol (Ins), scyllo-inositol (sIns), lactate (Lac), phosphorylethanolamine (PE), glycerophosphorylcholine + phosphorylcholine (tCho), creatine + phosphocreatine (tCr), N-acetylaspartate + N-acetylaspartylglutamate (tNAA), glucose + taurine (Glc+Tau).
The concentrations of the 5 major metabolites measured with the advanced protocol were consistent with values recently published in PCC in elderly subjects using short TE STEAM at 7 T (40).
Quantification precision for the 5 major metabolites was similar between the conventional and advanced protocols, while the advanced protocol had a trend for lower CRLB for other metabolites (Figure 5B). We investigated if single-shot phase and frequency corrections of the PRESS data would affect the metabolite concentrations or improve quantification precision (supplementary material), and found that neither concentrations nor CRLB were different between PRESS spectra analyzed after single-shot phase and frequency correction vs. those analyzed without single-shot corrections (Supporting Fig. S3).
Repeatability
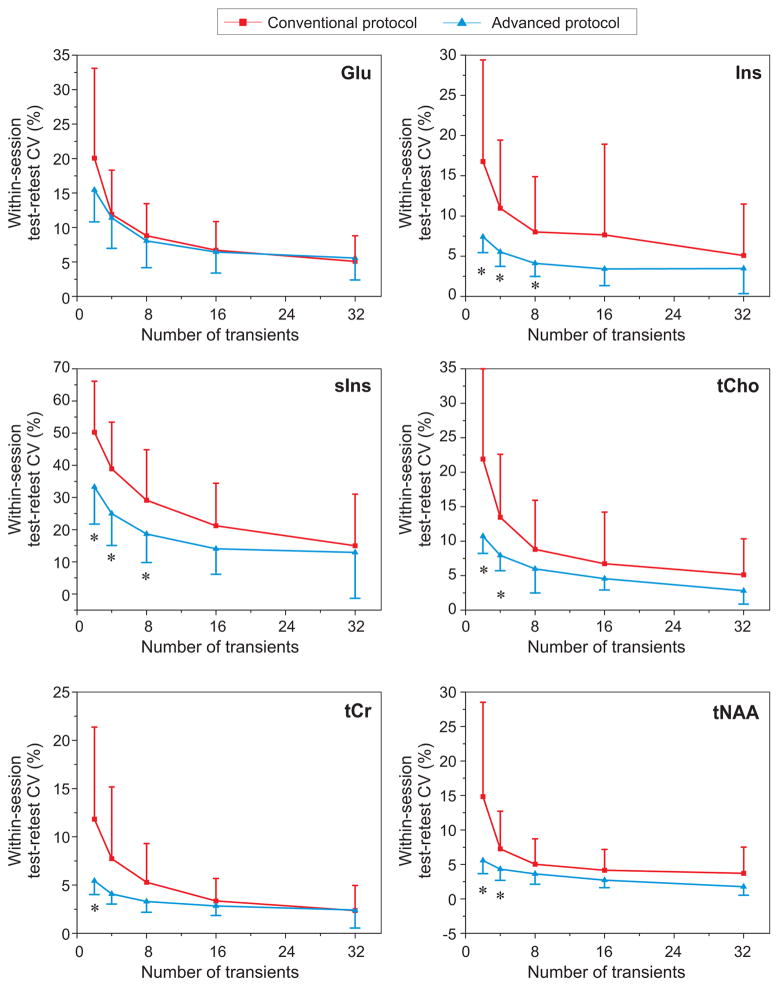
Finally, the within-session repeatability of the most reliably quantified metabolites was evaluated with each protocol at different levels of SNR. Metabolites that had mean CRLB ≤20% with both protocols (Figure 5) were selected for this analysis since it involved fitting of low SNR spectra, starting with averages of 2 single-shots. Test-retest CVs were better with MRS data acquired with the advanced compared to the conventional protocol for all metabolites except Glu, and the difference was most significant for low SNR data (averages of 2 – 8 shots) (Figure 6).
Figure 6.
Mean repeatability CVs for metabolites with mean CRLB ≤20% as determined from Figure 5. Error bars represent intersubject standard deviations. * P<0.01 (two-tailed, paired Student’s t-test) between conventional versus advanced protocols.
Discussion
This study showed the feasibility of executing an advanced MRS protocol in the clinical setting with elderly participants and rotating MR technologists. The advanced MRS protocol (sLASER with FM shim) reproducibly generated high quality MRS data with negligible CSDE and outperformed the conventional protocol (PRESS with vendor-provided shimming routine) with respect to localization accuracy, WS efficiency, spectral resolution, repeatability of metabolite concentrations and reliable quantification of low concentration coupled metabolites.
The differences in data quality we observed with the advanced vs. the vendor-provided protocol are due to many factors, including sequence optimization, voxel-based calibrations, as well as differences in post-processing steps, and it was not the goal of our study to pinpoint which factors were the most critical. Further improvements are clearly possible in the vendor-provided PRESS protocol, such as by modifying the water suppression module, incorporating outer-volume suppression and implementing voxel-based power calibrations. However, such optimization is typically not done in clinical settings where the standard vendor-provided MRS packages are utilized. Therefore, here we rather aimed to evaluate what is routinely available and executed in the clinical setting, in the absence of support from MR physicists, versus what is possible using in-house developed, advanced MRS technology, on the same standard clinical hardware. In addition, optimization of the standard PRESS sequence would not have affected the intrinsic issue with this sequence at high field, namely the CSDE. A comparison between a vendor-provided STEAM sequence vs. an optimized STEAM sequence would have avoided the CSDE issue and provide important insights, however we chose to focus on protocols that generate full signal intensity for obvious SNR advantages, especially in the elderly brain with appreciable atrophy. Therefore, here we present a practical comparison of a standard vs. non-standard full intensity protocol, with implications on how data quality can be improved for clinical CNS applications of 3T MRS. Important from a practical perspective, the advanced protocol took approximately 2 minutes longer (Figure 1), which can further be reduced by automation (as done in vendor-provided packages) and is deemed justified for the yields in higher data quality.
The main motivation to utilize a sequence like sLASER at high fields is the CSDE, which can be substantial even at 3T, especially for refocusing pulses, such that the MRS localization accuracy is compromised, consequently affecting neurochemical quantification. Generally for VOI located in large cortical areas, CSDE might not be noticeable in the spectra. However for VOI close to the ventricles or at the edge of the brain, CSDE will be visible in the spectrum (manifested as a distorted spectral pattern or contamination with extracranial lipid signals at ~1.2 ppm). AFP pulses in sLASER reduce the CSDE by ~6-fold compared to PRESS. In this study, full 3D simulation was implemented to generate accurate basis spectra for reliable spectral fitting of the PRESS data by accounting for the large CSDE (6). However, simulating the PRESS basis spectra for 19 metabolites took about 2 months even though parallel computation in Matlab was utilized on at least 2 Linux machines to reduce computational time. For optimal results, basis sets need to be generated for selected sequence parameters, however such long computation times are not practical for the clinical setting. Alternatively, these basis spectra could be experimentally measured on phantoms using the identical sequence as used in vivo under normal brain physiological temperature and pH, which might also not be feasible in the clinical setting. On the other hand, due to negligible CSDE in sLASER, non-localized simulation is sufficient to accurately generate the basis spectra and requires a computational time of less than 1 hour to simulate all metabolites. Therefore sLASER provides an advantage for computation time due to ease of generating basis spectra at any TE.
Incorrect flip angles in MRS generally result in low SNR data. In this study, the RF power for the conventional PRESS protocol was determined during the system pre-scan step on a slice at isocenter while in sLASER the required B1 field for the RF pulses was obtained in the actual VOI. This, in turn, has resulted in almost half of the PRESS datasets having under- or over-estimated RF levels, possibly due to VOI positioning outside the 10 mm slice at the magnet isocenter, where the automatic system RF calibration is conventionally performed, or due to B1 inhomogeneity within the slice. Several techniques have been proposed that allow RF field adjustment for a specific region at any location (41,42). In MRS, however, voxel-based B1 calibration using the metabolite localization sequence is ideal for accurate RF levels.
One of the critical requirements for obtaining high quality spectra is efficient B0 shimming in the selected VOI. This is ideally done by adjusting both first- and second-order shim terms using fully automated B0 field mapping techniques, either based on 3D B0 mapping or mapping along projections whereby the precision of the field mapping depends on the spatial resolution and evolution time. We obtained significantly narrower spectral linewidths with FM vs. the vendor-provided Adv. 3D shim routine due to the spatial resolution used in acquiring the B0 maps or projections: Adv. 3D shim uses a fixed resolution of ~8 mm in each direction while in this study FM shim had 1.2 mm resolution. Therefore, a typical voxel dimension of 20 mm will contain 3 pixels with Adv. 3D shim compared to 17 pixels with FM reducing the precision of the calculated shims with Adv. 3D shim. Note that all measurements in this study were done on VB17 platform. In the latest Siemens software update, i.e. syngo VD and VE platforms, the spatial resolution can be changed based on specific region-of-interest, likely improving shimming results with the vendor-provided shim routine.
The comparative analyses showed systematic differences between concentrations obtained with the conventional vs. the advanced protocols and in particular suggested that some low concentration J-coupled metabolites are overestimated with simulated MM, consistent with a prior study (43). Performing single-shot phase and frequency corrections of the conventional PRESS data did not affect the metabolite concentrations or CRLB (Supporting Fig. S3), suggesting that single-shot frequency and phase corrections of broad spectra (water linewidth of 10.5 ± 3.7 Hz for the PRESS data) will not improve the spectral quality and quantification precision. Alternatively, the reason for no improvement in CRLB with single-shot corrections of PRESS data in our study may have been that we studied a healthy and relatively cooperative cohort. Improvements in linewidth and CRLB in conventional MRS data with single shot correction are likely in less cooperative clinical cohorts with higher amounts of motion. Note however that the single shot frequency and phase correction is only acceptable for motion that does not substantially change the tissue content of the voxel. For larger (~cm level) motions, recently implemented real-time voxel tracking methods (44,45) will be necessary to acquire both conventional and advanced MRS data with accurate localization.
We observed higher repeatability of metabolite quantification with the advanced than the conventional protocol, in particular for low SNR data, for the major metabolites except Glu (Figure 6). For Glu, both protocols provided equivalent repeatability, possibly due to the overestimated Glu concentrations with PRESS. At low SNR most significant differences in repeatability between the conventional and advanced protocols were observed for Ins and sIns. This suggests that the advanced protocol might be more sensitive to changes in preclinical Alzheimer’s disease and mild cognitive impairment due to Alzheimer’s disease (46) where elevated Ins is a very early marker of neurodegeneration, even prior to detectable amyloid pathology (47).
The advanced MRS protocol required manual B1 calibrations and the use of the non-standard FM shimming routine. When the technologists were surveyed regarding the most challenging steps in the protocol, they ranked voxel-based B1 and B0 calibrations highly. Therefore there is a need to automate these calibration steps to simplify use of the advanced protocol in the clinical setting. The technologists further emphasized that the transfer of raw data from the scanner for off-site analysis was challenging in the clinical workflow, suggesting that on-site data analysis would be beneficial in the future. Such automation of data acquisition and analysis will likely be even more critical for sites that do not have MR technologists with similar levels of MRI and MRS expertise as those who participated in the current study.
One limitation of the current study is that while the healthy elderly cohort presented a good test case for comparison of advanced vs. conventional MRS protocols, the proposed methodology needs to be further tested in neurological disease cohorts. Specifically, studies with clinical cohorts with higher levels of motion, atrophy or lipids that may occur in neurodegenerative and oncological diseases are needed to fully evaluate the performance of the advanced protocol. The protocol is expected to provide further advantages in cohorts with higher levels of motion and atrophy since the single shot correction minimizes signal loss due to small phase/frequency shifts. Presence of lipids in the VOI is not expected to affect the performance of the protocol, as we have seen with the falx cerebri lipid peak in our healthy elderly cohort. The protocol is expected to minimize lipid contributions from outside the VOI, e.g. in voxels close to the skull, due to the sharper profiles of the adiabatic RF pulses, and thereby even allow quantification of within-voxel lipids in conditions with lipid accumulation, as we have shown previously (48). Lastly, while LCModel quantification is not FDA approved for clinical diagnoses, results obtained from both MR vendor-provided packages (metabolite intensity ratios) and from LCModel have been reported in the clinical setting (49). Importantly, several other MRS quantification packages are freely available, e.g. QUEST in jMRUI (50), TARQUIN (51), VeSPA (52) and it will be beneficial to further develop and integrate any of these analysis packages in the post-processing pipelines on clinical scanners for streamlined quantification of MRS data.
Conclusion
In summary, this study showed that excellent quality MR spectra can be obtained using an advanced MRS acquisition protocol in the clinical setting. The advanced protocol outperforms the conventional MRS protocol in localization accuracy (minimal CSDE), spectral quality and reliable quantification of low concentration J-coupled metabolites. These findings warrant further automation and standardization of the advanced protocol for improved MRS data quality and reproducibility in clinical applications and multi-site clinical research studies. In conclusion, this study shows that the translation of an advanced MRS protocol into the clinical setting is feasible using standard MR hardware at 3T.
Supplementary Material
Supporting Figure S1. Comparison of measured reference voltages between the advanced (sLASER) and conventional (PRESS) protocols. For each subject, voltages used with PRESS protocol are plotted vs. the voltage used with sLASER. Sixteen out of 30 subjects had identical values between PRESS and sLASER protocols; these points lie on the identity line (red line) and 14 subjects had either under- or over- estimated levels with PRESS relative to voxel-based sLASER calibration.
Supporting Figure S2. Left panel: Mean metabolite concentrations of sLASER spectra (TE = 28 ms, 64 averages) quantified using the 3 different LCModel approaches. Note that GABA was not reported in the “Measured MM – LCModel approach 2” since it did not pass the criteria (CRLBs ≤ 50% from at least half of the spectra). Error bars represent intersubject standard deviations. * P<0.01 between Simulated MM – LCModel approach 1 vs. Measured MM – LCModel approach 2 and ◆ P<0.01 between Simulated MM – LCModel approach 2 vs. Measured MM – LCModel approach 2 (two-tailed, paired Student’s t-test). Right panel: Simulated and measured MM spectra (between-subject mean ± SD) plotted together with their respective spline baseline obtained with the 3 approaches.
Supporting Figure S3. Mean metabolite concentrations and CRLBs with PRESS obtained using the routine procedure in the clinical setting (PRESS) and advanced research analysis procedure (PRESS processed). No differences in concentration and CRLBs were observed, most likely due to broad spectral linewidth.
Acknowledgments
We would like to thank the MR technologists Vicki L Knudsen R.T., Brian Rucker R.T., and Suzanne L Carlson R.T. and Dr. Lynn Eberly for statistical consultation. This work was supported by funding from the Minnesota Partnership for Biotechnology and Medical Genomics and NIH grants R01 NS080816, P41 EB015894, P30 NS076408 and R01 AG040042.
Abbreviations
- CSDE
chemical shift displacement error
- CV
coefficient-of-variance
- FM
FAST(EST)MAP
- PCC
posterior cingulate cortex
- sLASER
semi-LASER
- WS
water suppression
References
- 1.Öz G, Alger J, Barker P, Bartha R, Bizzi A, Boesch C, Bolan P, Brindle K, Cudalbu C, Dincer A, Dydak U, Emir U, Frahm J, Gonzalez R, Gruber S, Gruetter R, Gupta R, Heerschap A, Henning A, Hetherington H, Howe F, Huppi P, Hurd R, Kantarci K, Klomp D, Kreis R, Kruiskamp M, Leach M, Lin A, Luijten P, Marjanska M, Maudsley A, Meyerhoff D, Mountford C, Nelson S, Ozduman K, Necmettin P, Pan J, Peet A, Poptani H, Posse S, Pouwels P, Ratai E, Ross B, Scheenen T, Schuster C, Soher B, Tkac I, Vigneron D, Kauppinen R The MRS Consensus Group. Clinical Proton MR Spectroscopy in Central Nervous System Disorders. Radiology. 2014;270(3):658–679. doi: 10.1148/radiol.13130531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Terpstra M, Cheong I, Lyu T, Deelchand DK, Emir UE, Bednařík P, Eberly LE, Öz G. Test-retest reproducibility of neurochemical profiles with short-echo, single-voxel MR spectroscopy at 3T and 7T. Magn Reson Med. 2016;76(4):1083–1091. doi: 10.1002/mrm.26022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deelchand DK, Adanyeguh IM, Emir UE, Nguyen T-M, Valabregue R, Henry P-G, Mochel F, Öz G. Two-site reproducibility of cerebellar and brainstem neurochemical profiles with short-echo, single-voxel MRS at 3T. Magn Reson Med. 2015;73(5):1718–1725. doi: 10.1002/mrm.25295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frahm J, Merboldt K-D, Hanicke W. Localized proton spectroscopy using stimulated echoes. J Magn Reson. 1987;72(3):502–508. doi: 10.1002/mrm.1910170113. [DOI] [PubMed] [Google Scholar]
- 5.Bottomley PA. Spatial Localization in NMR Spectroscopy in Vivo. Annals of the New York Academy of Sciences. 1987;508(1):333–348. doi: 10.1111/j.1749-6632.1987.tb32915.x. [DOI] [PubMed] [Google Scholar]
- 6.Kaiser LG, Young K, Matson GB. Numerical simulations of localized high field 1H MR spectroscopy. J Magn Reson. 2008;195(1):67–75. doi: 10.1016/j.jmr.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mekle R, Mlynárik V, Gambarota G, Hergt M, Krueger G, Gruetter R. MR spectroscopy of the human brain with enhanced signal intensity at ultrashort echo times on a clinical platform at 3T and 7T. Magn Reson Med. 2009;61(6):1279–1285. doi: 10.1002/mrm.21961. [DOI] [PubMed] [Google Scholar]
- 8.Near J, Andersson J, Maron E, Mekle R, Gruetter R, Cowen P, Jezzard P. Unedited in vivo detection and quantification of gamma-aminobutyric acid in the occipital cortex using short-TE MRS at 3 T. NMR Biomed. 2013;26(11):1353–1362. doi: 10.1002/nbm.2960. [DOI] [PubMed] [Google Scholar]
- 9.Wijtenburg SA, Gaston FE, Spieker EA, Korenic SA, Kochunov P, Hong LE, Rowland LM. Reproducibility of phase rotation STEAM at 3T: Focus on glutathione. Magn Reson Med. 2014;72(3):603–609. doi: 10.1002/mrm.24959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emir UE, Auerbach EJ, Moortele P-FVD, Marjanska M, Ugurbil K, Terpstra M, Tkac I, Oz G. Regional neurochemical profiles in the human brain measured by 1H MRS at 7T using local B1 shimming. NMR Biomed. 2012;25(1):152–160. doi: 10.1002/nbm.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marjańska M, Auerbach EJ, Valabregue R, Van de Moortele P-F, Adriany G, Garwood M. Localized 1H NMR spectroscopy in different regions of human brain in vivo at 7T: T2 relaxation times and concentrations of cerebral metabolites. NMR Biomed. 2012;25(2):332–339. doi: 10.1002/nbm.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van de Bank BL, Emir UE, Boer VO, van Asten JJA, Maas MC, Wijnen JP, Kan HE, Oz G, Klomp DWJ, Scheenen TWJ. Multi-center reproducibility of neurochemical profiles in the human brain at 7 Tesla. NMR Biomed. 2015;28(3):306–316. doi: 10.1002/nbm.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheenen TWJ, Klomp DWJ, Wijnen JP, Heerschap A. Short echo time 1H-MRSI of the human brain at 3T with minimal chemical shift displacement errors using adiabatic refocusing pulses. Magn Reson Med. 2008;59(1):1–6. doi: 10.1002/mrm.21302. [DOI] [PubMed] [Google Scholar]
- 14.Öz G, Tkac I. Short-echo, single-shot, full-intensity proton magnetic resonance spectroscopy for neurochemical profiling at 4 T: Validation in the cerebellum and brainstem. Magn Reson Med. 2011;65(4):901–910. doi: 10.1002/mrm.22708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bednařík P, Moheet A, Deelchand DK, Emir UE, Eberly LE, Bareš M, Seaquist ER, Öz G. Feasibility and reproducibility of neurochemical profile quantification in the human hippocampus at 3 T. NMR in Biomedicine. 2015;28(6):685–693. doi: 10.1002/nbm.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bednařík P, Tkáč I, Giove F, DiNuzzo M, Deelchand DK, Emir UE, Eberly LE, Mangia S. Neurochemical and BOLD Responses during Neuronal Activation Measured in the Human Visual Cortex at 7 Tesla. Journal of Cerebral Blood Flow & Metabolism. 2015;35(4):601–610. doi: 10.1038/jcbfm.2014.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boer VO, Siero JCW, Hoogduin H, van Gorp JS, Luijten PR, Klomp DWJ. High-field MRS of the human brain at short TE and TR. NMR in Biomedicine. 2011;24(9):1081–1088. doi: 10.1002/nbm.1660. [DOI] [PubMed] [Google Scholar]
- 18.Deelchand DK, Henry P-G, Marjańska M. Effect of Carr-Purcell refocusing pulse trains on transverse relaxation times of metabolites in rat brain at 9. 4 Tesla. Magn Reson Med. 2015;73(1):13–20. doi: 10.1002/mrm.25088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, Ivnik RJ, Tangalos EG, Petersen RC, Rocca WA. The Mayo Clinic Study of Aging: Design and Sampling, Participation, Baseline Measures and Sample Characteristics. Neuroepidemiology. 2008;30(1):58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MRspa. Magnetic Resonance signal processing and analysis. 2015 Sep; https://www.cmrr.umn.edu/downloads/mrspa/
- 21.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 22.Christiansen P, Henriksen O, Stubgaard M, Gideon P, Larsson HBW. In vivo quantification of brain metabolites by 1H-MRS using water as an internal standard. Magn Reson Imaging. 1993;11(1):107–118. doi: 10.1016/0730-725x(93)90418-d. [DOI] [PubMed] [Google Scholar]
- 23.Barker PB, Soher BJ, Blackband SJ, Chatham JC, Mathews VP, Bryan RN. Quantitation of proton NMR spectra of the human brain using tissue water as an internal concentration reference. NMR Biomed. 1993;6(1):89–94. doi: 10.1002/nbm.1940060114. [DOI] [PubMed] [Google Scholar]
- 24.Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13(3):129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 25.Kaiser LG, Marjanska M, Matson GB, Iltis I, Bush SD, Soher BJ, Mueller S, Young K. 1H MRS detection of glycine residue of reduced glutathione in vivo. J Magn Reson. 2010;202(2):259–266. doi: 10.1016/j.jmr.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maudsley AA, Govindaraju V, Young K, Aygula ZK, Pattany PM, Soher BJ, Matson GB. Numerical simulation of PRESS localized MR spectroscopy. J Magn Reson. 2005;173(1):54–63. doi: 10.1016/j.jmr.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 27.Tkac I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med. 1999;41(4):649–656. doi: 10.1002/(sici)1522-2594(199904)41:4<649::aid-mrm2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 28.Gruetter R, Tkac I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med. 2000;43(2):319–323. doi: 10.1002/(sici)1522-2594(200002)43:2<319::aid-mrm22>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 29.Ernst T, Kreis R, Ross BD. Absolute Quantitation of Water and Metabolites in the Human Brain. I. Compartments and Water. J Magn Reson, Series B. 1993;102(1):1–8. [Google Scholar]
- 30.Tkáč I, Gruetter R. Methodology of 1H NMR spectroscopy of the human brain at very high magnetic fields. Appl Magn Reson. 2005;29(1):139–157. doi: 10.1007/BF03166960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfeuffer J, Tkáč I, Provencher SW, Gruetter R. Toward an in vivo neurochemical profile: quantification of 18 metabolites in short-echo-time 1H NMR spectra of the rat brain. J Magn Reson. 1999;141(1):104–120. doi: 10.1006/jmre.1999.1895. [DOI] [PubMed] [Google Scholar]
- 32.Gruetter R, Weisdorf SA, Rajanayagan V, Terpstra M, Merkle H, Truwit CL, Garwood M, Nyberg SL, Ugurbil K. Resolution improvements in in vivo 1H NMR spectra with increased magnetic field strength. J Magn Reson. 1998;135(1):260–264. doi: 10.1006/jmre.1998.1542. [DOI] [PubMed] [Google Scholar]
- 33.Mangia S, Tkac I, Gruetter R, Van De Moortele P-F, Giove F, Maraviglia B, Ugurbil K. Sensitivity of single-voxel 1H-MRS in investigating the metabolism of the activated human visual cortex at 7 T. Magn Reson Imaging. 2006;24(4):343–348. doi: 10.1016/j.mri.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 34.Tkáč I, Henry PG, Andersen P, Keene CD, Low WC, Gruetter R. Highly resolved in vivo 1H NMR spectroscopy of the mouse brain at 9. 4 T. Magn Reson Med. 2004;52(3):478–484. doi: 10.1002/mrm.20184. [DOI] [PubMed] [Google Scholar]
- 35.Deelchand DK, Moortele P-FVd, Adriany G, Iltis I, Andersen P, Strupp JP, Thomas Vaughan J, Ugurbil K, Henry P-G. In vivo 1H NMR spectroscopy of the human brain at 9. 4 T: Initial results. J Magn Reson. 2010;206(1):74–80. doi: 10.1016/j.jmr.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tkáč I, Andersen P, Adriany G, Merkle H, Ugurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at 7 T. Magn Reson Med. 2001;46(3):451–456. doi: 10.1002/mrm.1213. [DOI] [PubMed] [Google Scholar]
- 37.McIntyre DJO, Charlton RA, Markus HS, Howe FA. Long and short echo time proton magnetic resonance spectroscopic imaging of the healthy aging brain. J Magn Reson Imaging. 2007;26(6):1596–1606. doi: 10.1002/jmri.21198. [DOI] [PubMed] [Google Scholar]
- 38.Siegel GJ. Basic Neurochemistry: Molecular, Cellular and Medical Aspects. Lippincott-Raven Publishers; 1999. [Google Scholar]
- 39.Ganji SK, Banerjee A, Patel AM, Zhao YD, Dimitrov IE, Browning JD, Sherwood Brown E, Maher EA, Choi C. T2 measurement of J-coupled metabolites in the human brain at 3T. NMR Biomed. 2012;25(4):523–529. doi: 10.1002/nbm.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marjańska M, McCarten JR, Hodges J, Hemmy LS, Grant A, Deelchand DK, Terpstra M. Region-specific aging of the human brain as evidenced by neurochemical profiles measured noninvasively in the posterior cingulate cortex and the occipital lobe using 1H magnetic resonance spectroscopy at 7 T. Neuroscience. 2017;354:168–177. doi: 10.1016/j.neuroscience.2017.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Breton E, McGorty K, Wiggins GC, Axel L, Kim D. Image-guided radio-frequency gain calibration for high-field MRI. NMR Biomed. 2010;23(4):368–374. doi: 10.1002/nbm.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dowell NG, Tofts PS. Fast, accurate, and precise mapping of the RF field in vivo using the 180° signal null. Magn Reson Med. 2007;58(3):622–630. doi: 10.1002/mrm.21368. [DOI] [PubMed] [Google Scholar]
- 43.Schaller B, Xin L, Cudalbu C, Gruetter R. Quantification of the neurochemical profile using simulated macromolecule resonances at 3 T. NMR Biomed. 2013;26(5):593–599. doi: 10.1002/nbm.2896. [DOI] [PubMed] [Google Scholar]
- 44.Hess AT, Dylan Tisdall M, Andronesi OC, Meintjes EM, van der Kouwe AJW. Real-time motion and B0 corrected single voxel spectroscopy using volumetric navigators. Magn Reson Med. 2011;66(2):314–323. doi: 10.1002/mrm.22805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keating B, Deng W, Roddey JC, White N, Dale A, Stenger VA, Ernst T. Prospective motion correction for single-voxel 1H MR spectroscopy. Magn Reson Med. 2010;64(3):672–679. doi: 10.1002/mrm.22448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kantarci K, Jack CR, Xu YC, Campeau NG, O’Brien PC, Smith GE, Ivnik RJ, Boeve BF, Kokmen E, Tangalos EG, Petersen RC. Regional metabolic patterns in mild cognitive impairment and Alzheimer’s disease: A 1H MRS study. Neurology. 2000;55(2):210–217. doi: 10.1212/wnl.55.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Voevodskaya O, Sundgren PC, Strandberg O, Zetterberg H, Minthon L, Blennow K, Wahlund L-O, Westman E, Hansson O For the Swedish Bio Fsg. Myo-inositol changes precede amyloid pathology and relate to APOE genotype in Alzheimer disease. Neurology. 2016;86(19):1754–1761. doi: 10.1212/WNL.0000000000002672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Öz G, Tkáč I, Charnas LR, Choi IY, Bjoraker KJ, Shapiro EG, Gruetter R. Assessment of adrenoleukodystrophy lesions by high field MRS in non-sedated pediatric patients. Neurology. 2005;64(3):434–441. doi: 10.1212/01.WNL.0000150906.52208.E7. [DOI] [PubMed] [Google Scholar]
- 49.Kantarci K, Reynolds G, Petersen RC, Boeve BF, Knopman DS, Edland SD, Smith GE, Ivnik RJ, Tangalos EG, Jack CR. Proton MR Spectroscopy in Mild Cognitive Impairment and Alzheimer Disease: Comparison of 1. 5 and 3 T. AJNR Am J Neuroradiol. 2003;24(5):843–849. [PMC free article] [PubMed] [Google Scholar]
- 50.Ratiney H, Coenradie Y, Cavassila S, van Ormondt D, Graveron-Demilly D. Time-domain quantitation of 1H short echo-time signals: background accommodation. Magma. 2004;16(6):284–296. doi: 10.1007/s10334-004-0037-9. [DOI] [PubMed] [Google Scholar]
- 51.Wilson M, Reynolds G, Kauppinen RA, Arvanitis TN, Peet AC. A constrained least-squares approach to the automated quantitation of in vivo 1H magnetic resonance spectroscopy data. Magn Reson Med. 2011;65(1):1–12. doi: 10.1002/mrm.22579. [DOI] [PubMed] [Google Scholar]
- 52.Soher BJ, Semanchuk P, Todd D, Steinberg J, Young K. VeSPA: Integrated applications for RF pulse design, spectral simulation and MRS data analysis. Proc Intl Soc Mag Reson Med. 2011;19:1410. doi: 10.1002/mrm.29686. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Figure S1. Comparison of measured reference voltages between the advanced (sLASER) and conventional (PRESS) protocols. For each subject, voltages used with PRESS protocol are plotted vs. the voltage used with sLASER. Sixteen out of 30 subjects had identical values between PRESS and sLASER protocols; these points lie on the identity line (red line) and 14 subjects had either under- or over- estimated levels with PRESS relative to voxel-based sLASER calibration.
Supporting Figure S2. Left panel: Mean metabolite concentrations of sLASER spectra (TE = 28 ms, 64 averages) quantified using the 3 different LCModel approaches. Note that GABA was not reported in the “Measured MM – LCModel approach 2” since it did not pass the criteria (CRLBs ≤ 50% from at least half of the spectra). Error bars represent intersubject standard deviations. * P<0.01 between Simulated MM – LCModel approach 1 vs. Measured MM – LCModel approach 2 and ◆ P<0.01 between Simulated MM – LCModel approach 2 vs. Measured MM – LCModel approach 2 (two-tailed, paired Student’s t-test). Right panel: Simulated and measured MM spectra (between-subject mean ± SD) plotted together with their respective spline baseline obtained with the 3 approaches.
Supporting Figure S3. Mean metabolite concentrations and CRLBs with PRESS obtained using the routine procedure in the clinical setting (PRESS) and advanced research analysis procedure (PRESS processed). No differences in concentration and CRLBs were observed, most likely due to broad spectral linewidth.